Abstract
Across species, animals organize into social dominance hierarchies that serve to decrease aggression and facilitate survival of the group. Neuroscientists have adopted several model organisms to study dominance hierarchies in the laboratory setting, including fish, reptiles, rodents and primates. We review recent literature across species that sheds light onto how the brain represents social rank to guide socially appropriate behaviour within a dominance hierarchy. First, we discuss how the brain responds to social status signals. Then, we discuss social approach and avoidance learning mechanisms that we propose could drive rank-appropriate behaviour. Lastly, we discuss how the brain represents memories of individuals (social memory) and how this may support the maintenance of unique individual relationships within a social group.
This article is part of the theme issue ‘The centennial of the pecking order: current state and future prospects for the study of dominance hierarchies’.
Keywords: social rank, animal models, neural circuits, social learning
1. Introduction
Dominance hierarchies are an important form of social organization found in numerous social species, yet little is known about the neural mechanisms facilitating the establishment of social rank. While the neural mechanisms of aggression—an important feature of dominance—have been explored and reviewed elsewhere [1,2], less is known as to how social rank is represented in the brain.
There is extensive behavioural evidence that animals are aware of their rank and the ranks of other group members. For example, attention hierarchies, or the monitoring of more dominant individuals by subordinates, have been observed in species ranging from humans to fish to facilitate avoidance of aggression, recognition of opportunities to rise in rank, and observational learning from successful individuals [3–9]. Importantly, in many species social ranks are not inherited and animals establish social ranks via social experience, suggesting learning mechanisms are necessary for social hierarchy formation [10–13]. An animal's ability to evaluate the social rank of nearby conspecifics is a crucial first step in the contextually appropriate expression of dominant or subordinate behaviour, and in turn the maintenance of stable social ranks. In this review, we explore the neural circuits supporting the evaluation and comprehension of social rank among social species ranging from fish, reptiles, rodents and primates (figure 1), with emphasis on mechanisms with conserved function across these taxa. We first examine hypothalamic, mesolimbic and cortical circuits involved in the perception of status signals used to rapidly assess rank in a generalizable manner. We then present evidence that signal-detecting brain regions overlap with those involved in more general social learning processes and speculate as to how they may support the learning of social rank relationships, a relatively understudied process. Lastly, we explore evidence for individual-based recognition of social rank and the mechanisms that may support more fine-tuned and cognitively complex representations of social relationships among group-living species.
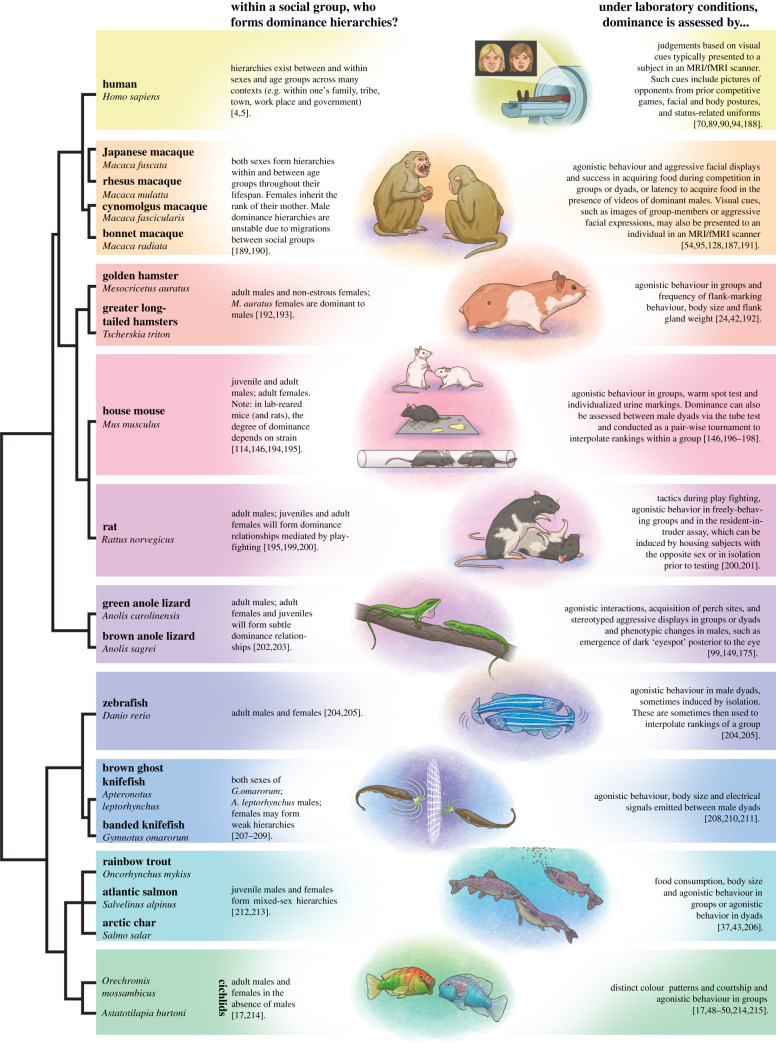
Figure 1.
Common species used to study social dominance in the laboratory. Fish, lizards, rodents and primates have been used to study the neural basis of dominant and subordinate behaviour in the laboratory setting. On the left, the life stage and sex in which hierarchies have been documented are listed. On the right, how dominance is assessed in the laboratory conditions is noted. Figure art by Amy Cao.
2. Representation of status signals
The expression of dominant or subordinate behaviour does not always require physical competition, but rather can be based on conspecific cues about individuals' competitive ability known, as status signals (see Tibbetts et al. [14]). It has been theorized that status signals evolved to convey crucial information between conspecifics, such that these signals provide a dominant individual with priority access to territory, resources and mates while also facilitating avoidance of aggression by subordinate individuals [8,15–17]. These signals are often associated with a cost to the signaller, such as androgen production or an increased risk of predation to reinforce their honesty [16]. Importantly, status signals are not any social stimuli of a given conspecific of known social rank, rather they are species-specific features that convey competitive ability regardless of familiarity with conspecifics. Status signals have been extensively documented in a variety of species, come in a wide variety of forms ranging from pheromones to complex behavioural displays, and often signallers adopt more than one signalling modality. Thus, the perception of social status is usually more complex than detecting a single signal and involves the integration of multiple features [18]. Although there is limited understanding of how the brain processes status signals, several brain regions and circuits have been implicated in processing status signals through experiments involving presentations of chemical and visual status signals (figure 2). Interestingly, several of these brain regions are also relevant in general avoidance and reward learning and memory processes, thus we also speculate as to whether the representation of status signals is innate or learned through prior social experiences.
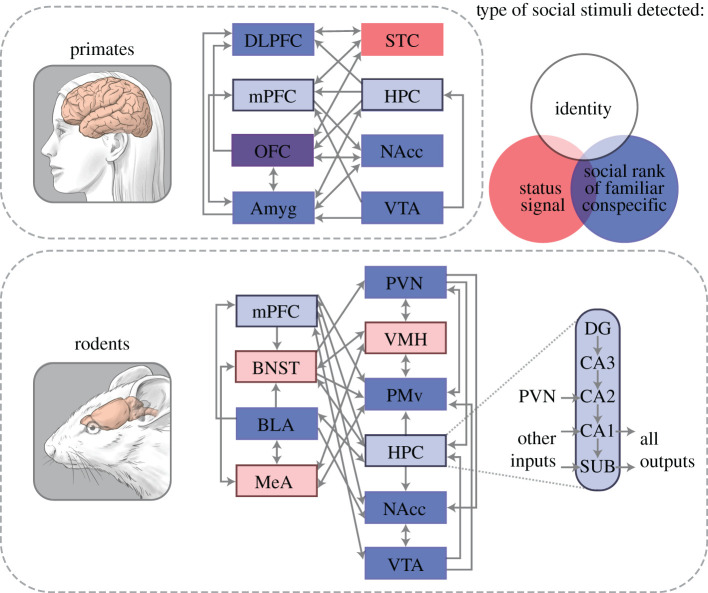
Figure 2.
Brain systems involved in the representation of a dominance hierarchy. Brain regions downstream of primary sensory processing regions (e.g. olfactory regions) involved in representing social rank are colour coded by whether local activity has been shown to represent status signals, social rank of familiar conspecifics, and social identity, as well as any combination of those types of conspecific stimuli in primates (top) and rodents (bottom). Hippocampal subregions in the rodent brain and their input and output locations are displayed in the blow-out diagram. Grey lines represent anatomical connectivity across regions. CA1, cornu ammonis 1; CA2, cornu ammonis 2; CA3, cornu ammonis 3; DG, dentate gyrus; DLPFC, dorsolateral prefrontal cortex; mPFC, medial prefrontal cortex; OFC, orbitofrontal cortex; Amyg, amygdala; STC, superior temporal cortex; HPC, hippocampus; NAcc, nucleus accumbens; VTA, ventral tegmental area; BNST, bed nuclei terminalis; BLA, basolateral amygdala; MeA, medial amygdala; PVN paraventricular hypothalamus; SUB, subiculum; VMH, ventromedial hypothalamus; PMv, ventral premammillary nucleus.
(a) . Insights from chemical signalling
(i) . Extended amygdala and hypothalamic circuits process chemical signals in rodents
Chemical signals, such as pheromones and urinary proteins, are perhaps the most common mode of communication used by social species to convey identifying information including sex, species, status and individual identity [19–23]. The use of chemical signals may be especially important in territorial species, such a rodents [20,24]. Neural circuits that detect social odours are well characterized in small mammals that use both main olfactory (volatile odours) and vomeronasal/accessory (non-volatile odours) systems. The vomeronasal organ (VNO) neurons project primarily to regions implicated in social behaviour and the canonical view is that the vomeronasal system is specialized to detect species-specific chemical signals that carry information about sex, reproductive or dominance status, but there is some evidence that the main olfactory system also processes social chemicals [25]. In male mice (Mus musculus), specific receptors in the VNO have been identified as necessary for sex cue discrimination and expression of sexual and aggressive behaviour [26,27]. In both female and male mice, downstream targets of the VNO exhibit greater activation upon sniffing urine from dominant males compared to urine from subordinate males, as evidenced by number of cfos immunoreactive cells, a proxy of neuronal excitation [28,29]. This activity could represent the detection of higher levels of major urinary proteins (MUPs), some of which are crucial for territory marking [30]. Furthermore, in rodents specific MUPs can signal dominance [31,32].
One of these downstream regions is the medial amygdala (MeA), which receives input from both vomeronasal and main olfactory systems and is an early node in the odour processing stream across vertebrates [33–35]. The MeA is also a highly sexually dimorphic nucleus rich in sex steroid hormone receptors [36] and has been implicated in sexual and aggressive behaviours in rodents, fish and lizards [37,38]. Sexual dimorphisms also appear to influence how the MeA represents social information. Calcium imaging in the MeA of male and female mice revealed that Ca2+ dynamics of neuronal ensembles and individual neurons differentiate between conspecific cues in a sex-specific manner, suggesting that biological relevance shapes social odour representations in the MeA [39]. Furthermore, the neuropeptide oxytocin (OT), which is known to mediate a variety of social behaviours, facilitates the MeA's role in sexual recognition as male animals become sexually mature, suggesting that OT in the MeA serves to encode biologically relevant odours [39–41]. How OT modulates social status signal processing in MeA remains unknown, but we hypothesize that OT facilitates the encoding of relevant social status odour cues in MeA. Interestingly, in male mice, the MeA has more neurons with cfos immunoreactivity (cfos-ir) after subjects are exposed to urine from an alpha (i.e. the most dominant individual in a hierarchy) compared to a subordinate conspecific, and this does not depend on the subject's own social rank or their familiarity with the cue source, although these factors may influence the biological relevance of the cue [29]. Activity in the MeA is also increased in both dominant and subordinate greater long-tailed hamsters (Tscheskia triton) following agonistic encounters and is not correlated to expression of aggression or defensive behaviour [42]. These findings may suggest that activity in this region represents sensory input or arousal in a general manner and that it may not be crucial for the expression of contextually appropriate dominant or subordinate behaviour. However, there is some evidence that the role of the MeA in chemosensory processing is more fine-tuned, as OT transmission in the MeA may be required for encoding memories of individual conspecifics (see §4b).
While the MeA plays a critical role in identifying social rank based on olfactory cues from mouse urine, it is not clear whether this process is further modified by social memory. Interestingly, several MeA hypothalamic projection targets are activated upon exposure to urine and are modulated by social rank and familiarity with the urine source [29]. For example, in the most subordinate mice from a hierarchy, differential cfos-ir within the ventromedial hypothalamic nucleus (VMH) is greater when they are exposed to alpha versus subordinate urine regardless of whether the urine is from a familiar mouse. However, in the most dominant (alpha) mice from a hierarchy, cfos-ir in the VMH is only greater when they are exposed to unfamiliar alpha urine compared to either familiar or unfamiliar subordinate urine [29]. Thus, cfos-ir in the VMH upon exposure to urinary cues is modulated by familiarity with the cue, the social rank of the animal who provided the cue, and the animal processing the cue. How the social rank of an animal modulates how it processes social cues is expanded upon in §2a(iii). Also, in both dominant and subordinate animals, the ventral premammillary nucleus (PMv) exhibits greater cfos-ir when animals are exposed to familiar alpha urine compared to familiar subordinate urine. Interestingly, this differential cfos-ir in the PMv is not observed when animals are exposed to unfamiliar alpha versus unfamiliar subordinate urine. This suggests that the PMv is not detecting features of urine cues that would necessarily qualify as status signals and that are related objectively to social rank regardless of familiarity. Rather, the PMv appears to detect features that are associated with memory of social rank.
This evidence from chemical processing of social odours in rodents suggests that various brain regions represent social rank at different scales. Some regions, such as the MeA, appear to detect status signals, as they exhibit differential cfos-ir in response to dominant versus subordinate cues regardless of familiarity with a cue. Others, such as the VMH and PMv, are modulated by additional factors, including an animal's own social rank and familiarity with the individual providing the cue, suggesting that features of learned social rank relationships or social memory are also represented in these regions.
(ii) . Chemical status signalling in other species
The neural circuits for processing social chemosensory cues are less well characterized outside of rodents, although social chemicals appear to be especially important in other taxa living in environments where vision is obscured, such as nocturnal primates and aquatic animals living in turbid environments [43,44]. Like rodents, dominant male cichlid fish (Astatotilapia burtoni and Oreochromis spp.) use urine to signal dominant status and have increased urine storage compared to subordinates [45,46]. When tank water is renewed and secreted chemicals are thus removed, pairs of fish are unable to form stable dominance relationships due to contextually inappropriate expression of aggression by subordinates towards dominant individuals [47]. Interestingly, when dominant male cichlids are exposed to a chemical status signal from another dominant male, they increase urination frequency and levels of circulating androgens and gene expression patterns in olfactory processing regions are associated with exposure to various urinary cues [48,49]. Dominant males also exhibit differential gene expression profiles in the olfactory bulb (OB) and posterior portion of the dorsal telencephalon (Dp; the putative homologue of the mammalian olfactory cortex) when they are exposed to dominant versus subordinate urinary odours [50]. Furthermore, neural recordings from the ventral nucleus of the fish ventral telencephalon (Vv; homologous to the lateral septum (LS) and striatal external globus pallidus of mammals) indicate that dominant males more robustly differentiate between sex- and food-related odours in these regions, while brain activity in subordinates only distinguished the odour of dominant males [50]. Thus, the social rank of fish exposed to social cues is a factor that influences how they process these cues, as is also observed in mice (see §2a(i)).
Unlike fish and rodents, primates are generally assumed to rely on visual information rather than chemical or olfactory information when assessing social cues [51]. However, behavioural studies in nocturnal New World monkeys (e.g. Lemuridae, Indridae, Callitrichidae and Cebidae) indicate that biological odours may be involved in activities related to dominance, such as territorial marking and reproductive behaviour [52]. Research into how the primate brain processes social olfactory cues is limited; however, studies have shown that the size of the accessory olfactory bulb (AOB) correlates with social and mating systems such that species with more dispersed social networks have greater AOB volume [53], suggesting that olfactory pathways similar to those in rodents are used to process status signals in nocturnal New World monkeys. More research is needed to determine whether primates detect social rank from chemical cues. Currently, the majority of what is known about how the primate brain represents status signals stems from experiments using visual stimuli.
(b) . Insights from visual signal processing
(i) . Amygdala processing of visual status signals in primates
Some of the earliest investigations into the neural correlates of dominance hierarchies identified the amygdala as a crucial brain region for appropriate expression of dominant and subordinate behaviour. For example, bilateral amygdala lesions given to the highest-ranking macaques (Macaca spp.) in a dominance hierarchy resulted in drastic but variable effects on dominant and subordinate behaviour and social rank changes. This included a complete reduction in aggression and loss of high status in one individual, as well as increase in aggression and a transition from typical dominant behaviour to despotic dominance in another individual [54]. Human lesion studies, such as those characterizing Klüver–Bucy syndrome [55], further support the amygdala's broad role in contextually appropriate social behaviour. Furthermore, individuals' social network complexity correlates with amygdala volume in human and non-human primates, suggesting that the amygdala serves a role in navigating the social environment [56,57].
For decades we have known that the amygdala is critical for encoding and signalling the affective valence of stimuli, including social stimuli [58–62]. Electrophysiological and neuroimaging studies have shown that the amygdala increases activity when macaques and humans view facial expressions of unfamiliar individuals who are angry or threatening [63–65]. In humans and monkeys, angry facial expressions and direct eye contact in images of single individuals are associated with dominance, as this indicates aggression directed toward the perceiver [64,66]. Conversely, fearful faces in macaques have been associated with submission, or a lack of aggression [64]. Interestingly, in humans the evoked blood-oxygen-level dependent (BOLD) signal response in the amygdala is even greater in response to fearful faces, suggesting that not knowing the source of a threat activates the amygdala more strongly [65,67]. This supports the idea that that a major role of the amygdala is to acquire salient social information in the environment.
(ii) . Amygdala's potential role in learning about social rank stimuli
The degree to which animals innately recognize status signals or must learn to appropriately interpret them is not known. Typically, the process of signal detection and response is presented as innate; however, this may not be true for all types of signals. For instance, amygdala lesions in neonatal monkeys increase social fear in social interactions despite a lack of warning facial expressions [68], but lesions in adult monkeys do not have this effect and instead lead to a reduction of cautious behaviours towards unfamiliar individuals [69]. These studies suggest that a period of socialization and learning is necessary to effectively interpret conspecific behaviour and signalling [69]. Furthermore, in the absence of status signals, activity in the amygdala is associated with social ranks of familiar individuals. For example, neuroimaging studies in humans show that BOLD signal in the amygdala increases when subjects view neutral facial expressions of familiar dominant competitors, and in macaques basolateral amygdala (BLA) firing rate correlates with social ranks of neutral familiar faces [70,71]. This suggests that the amygdala may also represent learned social rank or emotional memories associated with dominant individuals.
The role of the amygdala in fear and threat learning associations has been thoroughly studied in rodents in the context of Pavlovian conditioning. While conditioning experiments have traditionally demonstrated the learning of associations between an electric shock and an object or environmental context, it is likely that physical injury incurred through competition is associated with status signals in a similar manner. Briefly, the circuitry underlying the fear conditioning process involves the BLA, which integrates sensory information about the external environment and an animal's internal state with contextual information and memories from the hippocampus (HPC) and prefrontal cortex (PFC) [72,73]. This is where associations between fear-evoking stimuli (i.e. unconditioned stimulus; US) and neutral stimuli (i.e. conditioned stimulus; CS) are learned, and then this information is transferred to the central amygdala, which projects to midbrain regions involved in behavioural output [74]. Interestingly, this circuitry is engaged when rodents learn fearful associations directly and when they learn vicariously by observing conspecifics (i.e. vicarious learning), with additional top–down input from the PFC for sustaining attention during vicarious learning [75]. Subordinate animals may use vicarious learning via observations of agonistic interactions between group members to determine social ranks and avoid potential injuries associated with direct learning. While associative learning mechanisms that link the competitive ability of conspecifics to status signals could underlie the representation of status signals in the amygdala, this has not been empirically tested. Furthermore, the responsivity of amygdala sub-nuclei (i.e. BLA) to status signals could be further resolved to substantiate or disprove this theory. In addition, these mechanisms could also facilitate learning associations between competitive ability and individual conspecifics, rather than generalizable signals, and this will be explored further in §3a.
(iii) . Dopaminergic signalling and ventral striatum responsivity to visual status signals
Across species, the dopaminergic system is involved in processing social status signals. In cichlid fish, presentation of a dominant male is associated with increased number of dopamine (DA) neurons with c-fos immunoreactivity in the central region of the ventral telencephalon (Vc), a homologue of the mammalian striatum [48]. In green anole lizards (Anolis carolinensis), DA signalling in specific brain regions is elevated in various brain regions in response to changes in status signals. Males that viewed an opponent with a covered dominance-signalling eyespot had increased DA in the ventral tegmental area (VTA), substantia nigra (SNR), nucleus accumbens (NAcc) and hypothalamus, and became dominant. By contrast, males that viewed opponents with artificial eye-spots had increased DA in the dorsal raphe nucleus (DRN) and amygdala, and became subordinate [76].
Lesioning regions that are strongly innervated by dopaminergic neurons also appears to impair status signal detection. Lizards with unilateral lesions to the ventral striatum are unable to detect dominance signals from intruder conspecifics presented to the part of the visual field corresponding to the lesioned hemisphere, but no impairment is seen when they are presented to the intact hemisphere [77]. Interestingly, damage to the ventral striatum in humans (Homo sapiens) also impairs the ability to recognize angry unfamiliar facial expressions [78].
Beyond signal detection, the release of DA serves as a prediction-error signal alerting discrepancies in the experienced value of a cue and its expected value. This phenomenon builds on the Pavlovian learning system, such that DA signalling can assign strength and valence to CS–US associations and facilitate reinforcement learning of action patterns such as social approach and avoidance [79,80]. Similar to the representation of status signals in the amygdala, it is unclear whether dopaminergic signalling in response to status signals is innate or learned through prior social experience. There is evidence from primate studies that increased DA signalling in response to dominant stimuli is a learned response. For example, neuroimaging in humans has shown that the ventromedial striatum, which receives information about reward from dopaminergic input, exhibits greater BOLD signal when subjects are presented with the faces of familiar dominant individuals with neutral facial expressions [70]. Electrophysiological recordings in macaques also reveal a population of neurons in the medial striatum that specifically signal social information that is enhanced when viewing dominant individuals [81]. Furthermore, male macaques will sacrifice juice rewards for the opportunity to view the faces of familiar high-status monkeys [82]. Although these studies do not record dopamine signalling directly, these findings suggest that elevated DA signalling may reflect the greater value of viewing dominant group members, as these provide salient threat information. Future studies using the recently developed dopamine imaging sensors could address this hypothesis [83].
(iv) . Cortical processing of visual status signals in primates
Considering the prominent role of the PFC in social cognition and in decision making [84–86], it is not surprising that multiple prefrontal regions respond to social status signals. For instance, the orbitofrontal cortex (OFC), exhibits greater BOLD signal in response to images of conspecifics compared to non-social stimuli. Furthermore, OFC firing rate differentiates between images of familiar dominant and subordinate conspecifics in rhesus monkeys [87]. Activity within the OFC is also correlated with social image value as measured by time spent looking at the images [87]. However, it is unclear if OFC represents learned status recognition or some dominance-related facial feature, as the individuals in this study were exposed to familiar faces only. Studies in humans have shown that the OFC is important for recognizing emotion in facial expressions and voices from unfamiliar subjects [88]. Furthermore, neuroimaging studies have shown that the dorsolateral prefrontal cortex (DLPFC) also exhibits greater BOLD signal to unfamiliar individuals in postures associated with high status [89–91], and interestingly, to social status of neutral faces of contest opponents [70].
Prefrontal representations of status could also reflect attentional processes and the salience of viewing a dominant individual. This could explain why similar BOLD signal responses are observed when subjects are presented with dominant facial expressions and neutral faces of familiar dominant individuals. The PFC's role in sustaining attention has been extensively studied [92]. The elevated PFC activity observed in response to dominant signals could indicate that status signals serve to direct the attention of animals in a group towards dominant focal individuals, and this could manifest as an attention hierarchy. Interestingly, human participants who perceive themselves as low-status are more sensitive to facial dominance signals [93]. This potential skew in PFC-mediated attention could facilitate the acquisition of salient social information, i.e. which group member(s) are highly combative.
Beyond the PFC, parietal cortical regions are also involved processing visual status signals. For example, the superior temporal cortex (STC) exhibits greater BOLD signal when human subjects are presented with dominant facial features and postures and when they use these features to make judgements regarding the relative dominance of two other individuals [89,94,95]. There is also evidence to support that the STC works in conjunction with the inferior temporal gyrus, or fusiform gyrus, and HPC to compile information about emotions and intentions reflected in faces and the individual identity of faces [96–98]. Representations of individual social memories will be further explored in §4a.
3. Representation of learned social rank in approach-avoidance circuits
Status signalling is not the only mode by which animals perceive social rank. Even species that rely on status signals appear to prioritize behavioural demonstrations of dominance in competitive interactions and memories of those interactions over status signals to guide their behavioural decisions. For example, in lizards, manipulating the dominance-signalling dark eyespot does not alter previously established dominant–subordinate relationships [99], but it does determine dominant–subordinate relationships between unfamiliar dyads [76]. Furthermore, status signals do not explain how animals are able to detect subtle rank gradations that exist in highly linear dominance hierarchies where individuals behave differentially towards their two closest-ranking group members. For instance, there are no apparent status-driven differences in the urinary odour profiles of lower-ranking mice from the same hierarchy [31]. Here, we argue that animals must also learn social ranks and how to behave appropriately with specific group members through direct experiences and interactions with competitors, observation of contests between other individuals (observational or vicarious learning), or the determination of social status based on cognitive processes such as transitive inference [12].
Notably, these learning processes and status signalling are not mutually exclusive and coexist. As mentioned in §2b(iv), elaborate status signals may serve to direct attention towards dominant conspecifics, which is crucial for learning about them. This could manifest as an attention hierarchy, which then facilitates learning about and from highly ranked group members [3]. Furthermore, a stressful event, such as presentation of a dominant signal, can elevate levels of stress hormones and neuromodulators that enhance learning and memory [100]. Stress reactivity and the neuroendocrine characteristics associated with status signalling and social rank are too exhaustive to discuss in this review (see Milewski et al. [101]). In the following sections, we discuss neural mechanisms that we speculate support associative learning between competitive ability and individuals, rather than a generalizable signal.
(a) . Amygdala circuits facilitate learning of social subordination and vigilance
We propose that the amygdala facilitates learning about social rank relationships through competitive social interactions. With the establishment of dominance relationships, subordinate animals yield to higher-ranking group members and avoid pain and injury incurred by fighting [72], a phenomenon that likely involves fear and threat learning systems. Studies in rodents using social defeat stress (SDS) paradigms may support this theory, with some caveats. During SDS, animals are continually subjected to agonistic interactions and the primary measure of successful conditioned social defeat is the animal's decreased propensity for social interaction. SDS studies have illustrated that similar neural correlates in the amygdala underlie social submission and fear learning. For example, brain derived neurotrophic factor (BDNF)-mediated plasticity and GABAergic transmission within the BLA are critical for acquisition and expression of conditioned fear as well as conditioned social defeat in rodents [102–105]. Projections to the amygdala from the medial PFC (mPFC) have also been shown to similarly modulate fear conditioning and responses to SDS [106,107]. The role of BLA homologues has also been explored in non-mammals. In ray-finned fish, the medial dorsal telencephalon (Dm) is believed to be homologous to the BLA. The Dm has been implicated in emotional learning and fear avoidance [34,108,109], and local activity measured by number of cfos immunoreactive cells increases over subsequent days of SDS [110], suggesting that it also mediates learning of social defeat and development of social avoidance.
While amygdala circuits involved in fear and social avoidance may underlie how the most low-ranking animals in a group may learn their subordinate status, it does not explain how intermediately ranked animals learn to flexibly avoid and submit to some group members and exhibit aggression towards others. Generalized social avoidance induced by fear learning of conspecifics or conditioned social defeat would prevent intermediate animals from expressing both submissive and dominant behaviour when appropriate and prevent socially enriching affiliative behaviours. Another possibility to explain flexible behaviour in intermediates is that social defeat in naturalistic settings induces an anxiety-like social state, such as social vigilance, rather than complete avoidance. Social vigilance, or monitoring of conspecifics, could enhance observational learning and selective avoidance of aggressive conspecifics. While hypervigilance is considered a maladaptive response to uncertainty and associated with higher risk for anxiety disorders [111], an appropriate degree of vigilance is considered an adaptive coping mechanism adopted by lower-ranking animals to avoid potential threats and seize opportunities to increase their rank or acquire resources [112]. Studies in mammals show that the bed nucleus of the stria terminalis (BNST), a component of the extended amygdala, signals valence of stimuli in ambiguous social contexts and thus mediates social anxiety and social vigilance [113]. In humans, activity of the BNST is greater than activity in the amygdala when participants are exposed to unpredictable associations between cues and threatening facial expressions [111].
Social vigilance also appears to be modulated by one's own social rank. In primates, rodents and fish, social vigilance is skewed, giving rise to attention hierarchies where subordinates have elevated levels of social vigilance [3–9]. In many species there is a negative association between stress hormone levels and social rank, although high levels have also been observed in dominant individuals, revealing U-shaped association between stress hormones and social rank [43,114–117]. Most studies have focused on stress responsivity to antagonistic social interactions in subordinates or socially defeated animals. Following SDS in mice, release of the stress-related neuropeptide corticotropin-releasing factor (CRF) from the paraventricular nucleus (PVN) is upregulated [118]. Interestingly, CRF antagonism specifically in the BNST blocks the conditioned social defeat in hamsters [119]. These findings support a model in which stress hormones mediate processes in the BNST required for social defeat learning.
In addition to stress hormones, OT signalling varies based on an individual's social rank and mediates social vigilance. In female macaques exposed to stress, dominant social status is associated with elevated OT in cerebrospinal fluid [120]. In male rats, OT-receptor (OTR) binding is greater in the BNST and central amygdala (CeA) in more aggressive strains [121], and in male mice, OTR binding is associated with dominant social rank in other extended amygdala nuclei such as the NAcc and LS [122]. Interestingly, OTR knockdown in the BNST of male mice prevents social stress-induced increases in social vigilance and decreases in social approach [31]. In male macaques, pharmacological delivery of OT leads to increased prosocial behaviour and decreased subject vigilance for viewing dominant faces [123]. Collectively, these data suggest that mechanisms within the BNST and social rank-associated differences in stress hormone and OT levels could drive social vigilance in low-ranking animals, and in turn, the formation of attention hierarchies.
While the function of the BNST in relation to social behaviour has not been thoroughly studied in non-mammalian species, recent evidence from fish indicates that activity in the supracommissural subdivision of the ventral telencephalon (Vs), considered homologous to a combined MeA/BNST complex, is increased during cue learning in social (i.e. group-living) compared to non-social conditions [124]. Thus, the reactivity of the BNST in social situations appears to be evolutionarily conserved.
(b) . Mesolimbic dopamine system mediates social dominance and subordination behaviours
The dopaminergic system is another associative learning system that may be involved in social rank learning. As described in §2b(ii), elevated DA signalling is observed when animals are presented with dominant signals. Further, dopamine neurons in the DRN modulate social behaviour in a social rank-dependent manner, with the impact of activating DRN DA neurons greater in more dominant mice [125,126]. Although it is not known whether this is a result of social learning processes or an innate response to the signal, there is evidence to suggest that DA signalling may be involved in learning social rank through social interactions in the absence of signals.
Studies in rodents have shown that reward learning via dopaminergic signalling mediates aggressive behaviour. In dominant animals, aggression serves as a naturally rewarding US and can reinforce behaviours that lead to attacking a submissive animal [127]. In both macaques and mice, dominant males exhibit greater binding of the D2-type DA receptor in the striatum and D2 antagonism attenuates aggression [128]. In macaques, D2 antagonism results in destabilization of dominance hierarchies when specifically administered to the dominant animals and not subordinates [128,129]. Some studies have also shown that D1 and D2 receptor antagonism can attenuate aggression in rats and mice [130–133].
While not directly tested in a dominance hierarchy context, a highly conserved dopaminergic circuit has been identified in rodents and primates (figure 2), in which dopaminergic neurons from the VTA to the NAcc (part of the ventral striatum) facilitate learning of social reward and promote social competitiveness [132,134,135]. It should be noted that other neuropeptides, serotonin and OT, are also important for social reward learning within this circuit in mice [59,60]. Similar projections have been identified between VTA and NAcc homologues in birds, reptiles, amphibians and fish that motivate social approach in mating and courtship behaviours [136–140].
It is not known whether differences in DA receptor expression pre-determine an animal's social rank or whether they are the result of acquired social rank differences. It is possible that DA signalling differences are inherent as well as mediated by experience, such that animals with initially greater motivation experience greater reinforcement of this behaviour through the rewarding aspects of winning. Elevated DA signalling has been associated with the winner effect, a phenomenon where winning fights perpetuates further winning against new individuals in the future, leading to increased social status [133,141]. Furthermore, humans highly motivated to win competitions exhibit greater ventral striatal activation when viewing images of opponents [70,142].
Interestingly, DA signalling is also involved in mediating subordinate behaviour. As discussed in §3a, SDS is similar to the repeated defeat experienced by subordinate animals in a hierarchy. DA transmission in the VTA–NAcc circuit has been implicated in social defeat learning in mice and hamsters by modulating plasticity [143,144]. Furthermore, in socially monogamous rodents, SDS induces an upregulation of D1 receptors in the MeA, an important region for processing of social information [145]. Thus, changes in DA signalling following SDS suggest that individual differences in DA transmission and DA receptor expression may be a result of acquiring a given social rank and not a pre-existing correlate of social rank. These studies also suggest that the rewarding versus aversive effects of DA signalling are brain-region specific.
In summary, dopamine circuits across species facilitate social reward learning and mediate an animal's decision to engage with or avoid certain individuals. This learning mechanism could be used as animals develop social hierarchies and learn their social ranks within that hierarchy.
4. Individual social memory
Animals living in social dominance hierarchies have to behave dynamically, such that they express dominant behaviour towards some individuals and subordinate behaviour towards others. Therefore, social memory of individual group members is required in conjunction with the associative learning processes that facilitate approach and avoidant social behaviours. In highly linear dominance hierarchies, where each individual attains a unique social rank, animals behave based on subtle rank differences. For example, dominant male mice tend to exhibit the majority of aggression towards closely ranked competitors [146], and male chimpanzees tend to groom only closely ranked males [147]. This socially selective behaviour suggests that animals have strong individual social memories of their conspecifics. Whether animals always rely on individual social memories or use generalizable features associated with status (i.e. status signals) may depend on dynamics of the social environment and selective pressures. For example, male cichlids living in highly dynamic social environments form short-term memories of dominant rivals and will only express submissive behaviour towards males with dominant morphology who have demonstrated superior fighting ability within the past 7 days [148]. Similarly, lizards form social memories from a single social interaction, but the salience of this social identity information appears to decline and is eventually forgotten as intervals between subsequent interactions increase [149]. In addition, group-housed but not single-housed mice demonstrate long-term memory of individuals even after very brief interactions, further supporting the idea that social memory is dependent on social context [150]. Below we review the neural basis of individual social recognition that may facilitate learning of individual social ranks in a dominance hierarchy.
(a) . Role of hippocampal and PFC circuits social memory in encoding and recall
Studies from a wide range of species support the hypothesis that the HPC is essential for both episodic and semantic memory [151]. More recent studies have explored the specific role of the HPC in social memory, which includes neural representations of individuals and social status relationships. Human patients with hippocampal lesions are unable to recognize familiar faces [152,153]. More specifically, studies in rodents have implicated the ventral HPC (vHPC; anterior HPC in primates; aHPC) in modulation of motivated behaviours via connections to the hypothalamus and amygdala, and for its role in valence associative learning [154]. Interconnectivity with the hypothalamus and amygdala and the ability of the vHPC to integrate valence information for memory make this neural system well poised for a role in social memory. In healthy human subjects, neurons in the aHPC are activated when presented with the same individual in a variety of postures and contexts, suggesting that distinct neuronal ensembles represent social memories of individual people [155,156]. Inhibition of the vHPC in mice can impair both memory encoding and recall of individuals, and optogenetically re-activating vHPC neurons that were active during the first social encounter can facilitate these processes [155].
Furthermore, recent studies in mice have identified intra-hippocampal circuits involved in social memory encoding and recall. The HPC consists of CA1, CA2, CA3 and dentate gyrus subregions (figure 2) and extends throughout the medial temporal lobe. Dorsal CA2 (dCA2; posterior in primates) is required to remember littermates in rodents, and projections from dCA2 to ventral CA1 (anterior in primates) encode, store and recall social memories [44,155,157]. Homologous hippocampal structures and subdivisions have been identified in fish, amphibians and reptiles that encode spatial memory similarly to mammals, but little is known about their roles in social memory [99,124,158]. However, in lizards, functional changes in CA3 are observed when status signals are manipulated such that they are incongruous with memory of and opponent's status [99]. Also, in fish, subregions of the lateral part of the dorsal telencephalon (Dl), homologue of the mammalian HPC, exhibit differential cfos immunoreactivity under social compared to non-social contexts [124]. Whether the HPC plays a role in learning social ranks in a context of social hierarchies has not been directly tested, however, one study suggests that possibility. BDNF, which enhances neural plasticity required for learning, is upregulated in the HPC of dominant mice after winning agonistic interactions [103], potentially reinforcing their dominant social status.
In mammals, the frontal cortex and its connections to the HPC also facilitate encoding and storage of memories regarding specific group members. Studies in rodents have shown that the vHPC projections to the mPFC are necessary for recalling social memory [159]. Neuroimaging studies in humans indicate that both mPFC and HPC BOLD activity are correlated with learning social ranks in a game [160]. In humans, transcranial current stimulation of rostral mPFC facilitated social rank learning [161]. Furthermore, in mice, mPFC population firing rate is predictive of relative social rank and absolute social rank when competing against conspecifics [92].
In conclusion, there is strong evidence that the role of the HPC in encoding individual social memories is conserved across social species. However, the intricacies of intra-hippocampal circuits and how they facilitate memory formation have predominantly been determined in rodents. In mammals, social memory circuitry extends to the PFC, a region that is critical for long-term memory [151,159]. Although correlative studies in humans point to a role of the HPC–mPFC pathway in social rank learning, this has yet to be demonstrated in animal model studies [160,161]. Further studies into how social memories are stored in the brains of non-mammals are required to determine whether individual recognition is a conserved requirement for stable social hierarchies.
(b) . Neuropeptides support social memory encoding
The neuropeptides OT and arginine vasopressin (AVP) are widely known to regulate social cognition and behaviour across species, with a particular role in supporting social memory. The majority of AVP and OT synthesizing neurons originate in the suprachiasmatic nucleus (SCN) and PVN of the hypothalamus and project widely throughout the brain [162]. One of the major projection targets is the HPC. In rats, hippocampal AVP-receptors are necessary for encoding memory of individual conspecifics [163] and stimulating AVP afferents to dCA2 facilitates social memory encoding [164]. In mice, OTRs in dCA2/CA3 are necessary for short-term [165] and long-term memories of conspecifics [166].
OT and AVP transmission also affect social memory by acting on components of the amygdala and extended amygdala. Studies in mice and rats indicate that the MeA, known for processing social odours, also encodes memories of individual conspecifics. Blocking OTRs in the MeA impairs social recognition in rodents and activating these receptors during an initial encounter enhances their ability to encode social memory [40,167,168]. Also in rodents, the MeA and BNST send AVP projections to the LS, where blockage or downregulation of AVP receptor 1 (V1aR) disrupts social recognition [169]. AVP projections from the MeA to the vHPC have also been confirmed in the rat brain [170], but it is unknown whether these projections are also implicated in social memory.
While evidence from rodents and primates strongly supports the function of neuropeptides in social memory, the function of the homologous neuropeptides in fish and lizards remains to be determined. Arginine vasotocin (AVT), the AVP homologue found in fish and reptiles, has a role in non-social spatial and cue learning [171], but its role in learning in social contexts and about social stimuli has not been established. Notably, AVT has been associated with increased courtship behaviour, aggression and other rank-associated behaviours such as status signalling through urine [172–175]. AVP has been associated with similar social behaviours in mammals [176–178], suggesting that its functions are largely conserved and supporting a case for studying its role in social memory across species. Similarly, isotocin and mesotocin, the OT homologues found in fish and reptiles, respectively, appear to function like OT with respect to social approach, sexual behaviour and formation of partner bonds and parenting behaviour [179–181]. Further investigation into whether AVP and OT homologues modulate memory of conspecifics is needed to know whether these neuropeptides serve a conserved role in social memory encoding across social species.
5. Conclusion and future directions
The evidence reviewed supports that social rank recognition involves the coordinated activity of highly conserved neural circuits across multiple levels of cognition, ranging from the seemingly innate perception of social status signals to more fine-tuned learning of social rank of specific individuals. Notably, the amygdala and dopaminergic neurons are involved in responding to status signals and driving learning about social rank through social interactions. While it appears that status signals serve to bypass the need for experience-based learning and prior social interactions that could incur physical injury, the extent to which status signal responses are innate or learned needs to be more thoroughly investigated. This theory, along with several other critical questions about how the brain processes social status signals, needs to be further investigated. In particular, the impact of an animal's familiarity with a social stimulus on their perception of status signals needs to be systematically studied across species. In addition, the role of an animal's own social rank in modulating how they process external status signals is largely unknown. An individual's social rank appears to influence behaviours related to acquiring social information, such as attentional postures and visual gaze direction [3–9], but how social information is differentially represented in the brains of hierarchically ranked animals is understudied. Lastly and perhaps most glaringly absent from our knowledge is how the female brain represents social rank and the neural underpinnings of how females negotiate social rank relationships. Much of the knowledge presented in this review stems from experiments conducted almost exclusively in male animals.
The technical difficulty of studying proximal mechanisms of brain function in naturalistic contexts has been a major hurdle in studying such questions and has led to our limited knowledge of the neural dynamics underlying social group behaviours. Although the species discussed in this review form dominance hierarchies, evidence for the neural systems involved in the representation of social rank typically does not come directly from animals living and behaving freely in groups. Laboratory-based neurobiological and behavioural studies have an overrepresentation of simple dyadic social interaction assays that do not directly examine the representation of social rank in groups, and traditionally measure behaviours that are exclusively expressed by males. Moreover, traditional neural recording methods, such as electrophysiology, have been hard to implement in multiple freely moving animals because of physical constraints. Several recent technological advancements have increased our ability to study the neural basis of social rank learning and memory in larger and more natural group settings. In the past few years, open-source tools have been developed to automatically track and assist in the quantification of behaviour of multiple group-living animals [182–185]. Moreover, technological advancements in light wireless neural activity recording now allow recording from multiple freely moving animals simultaneously [186]. These new developments combined will dramatically facilitate the study of neural circuits and dynamics underlying social group behaviour. We anticipate that the next decade will bring new perspectives on the neurobiology of social group behaviours that will enhance our understanding of how animals in large groups learn and represent social rank.
Data accessibility
This article has no additional data.
Authors' contributions
J.P.C., N.P.-C. and M.F.D. conceptualized the project. M.F.D. and N.P.-C. did literature review and writing, M.F.D., N.P.-C. and K.M.T. conceptualized figures and M.F.D. and N.P.-C. created figures. J.P.C. and K.M.T. edited the text.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
N.P.-C. was supported by L'Oreal For Women In Science, PDEP fellowship from Burroughs Wellcome Fund and K99 MH124435-01 (NIMH). K.M.T. is the Wylie Vale chair at Salk Institute for Biological studies and was supported by R01-MH115920 (NIMH), Pioneer Award DP1-502 AT009925 (NCCIH).
References
- 1.Chen P, Hong W. 2018. Neural circuit mechanisms of social behavior. Neuron 98, 16-30. ( 10.1016/j.neuron.2018.02.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lischinsky JE, Lin D. 2020. Neural mechanisms of aggression across species. Nat. Neurosci. 23, 1317-1328. ( 10.1038/s41593-020-00715-2) [DOI] [PubMed] [Google Scholar]
- 3.Chance MRA, Larson RR (eds). 1976. The social structure of attention. Hoboken, NJ: John Wiley & Sons Ltd. [Google Scholar]
- 4.Freniere PL, Charlesworth WR. 1983. Dominance, attention, and affiliation in a preschool group: a nine-month longitudinal study. Ethol. Sociobiol. 4, 55-67. ( 10.1016/0162-3095(83)90030-4) [DOI] [Google Scholar]
- 5.Vaughn BE, Waters E. 1981. Attention structure, sociometric status, and dominance: interrelations, behavioral correlates, and relationships to social competence. Dev. Psychol. 17, 275-288. ( 10.1037/0012-1649.17.3.275) [DOI] [Google Scholar]
- 6.Pannozzo PL, Phillips KA, Haas ME, Mintz EM. 2007. Social monitoring reflects dominance relationships in a small captive group of brown capuchin monkeys (Cebus apella). Ethology 113, 881-888. ( 10.1111/j.1439-0310.2007.01392.x) [DOI] [Google Scholar]
- 7.Curley JP. 2016. Temporal pairwise-correlation analysis provides empirical support for attention hierarchies in mice. Biol. Lett. 12, 20160192. ( 10.1098/rsbl.2016.0192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desjardins JK, Hofmann HA, Fernald RD. 2012. Social context influences aggressive and courtship behavior in a cichlid fish. PLoS ONE 7, e32781. ( 10.1371/journal.pone.0032781) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kendal R, Hopper LM, Whiten A, Brosnan SF, Lambeth SP, Schapiro SJ, Hoppitt W. 2015. Chimpanzees copy dominant and knowledgeable individuals: implications for cultural diversity. Evol. Hum. Behav. 36, 65-72. ( 10.1016/j.evolhumbehav.2014.09.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drews C. 1993. The concept and definition of dominance in animal behaviour. Behaviour 125, 283-313. ( 10.1163/156853993X00290) [DOI] [Google Scholar]
- 11.Chase ID, Seitz K.. 2011. Chapter 4 - Self-structuring properties of dominance hierarchies: a new perspective. In Advances in genetics (eds Huber R, Bannasch DL, Brennan P), pp. 51-81. Cambridge, MA: Academic Press. [DOI] [PubMed] [Google Scholar]
- 12.Hobson EA. 2020. Differences in social information are critical to understanding aggressive behavior in animal dominance hierarchies. Curr. Opin. Psychol. 33, 209-215. ( 10.1016/j.copsyc.2019.09.010) [DOI] [PubMed] [Google Scholar]
- 13.Qu C, Ligneul R, Van der Henst J-B, Dreher J-C.. 2017. An integrative interdisciplinary perspective on social dominance hierarchies. Trends Cogn. Sci. 21, 893-908. ( 10.1016/j.tics.2017.08.004) [DOI] [PubMed] [Google Scholar]
- 14.Tibbetts EA, Pardo-Sanchez J, Weise C. 2021. The establishment and maintenance of dominance hierarchies. Phil. Trans. R. Soc. B 377, 20200450. ( 10.1098/rstb.2020.0450) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Senar JC. 2006. Bird colors as intrasexual signals of aggression and dominance. In Bird coloration: function and evolution (eds Hill GE, McGraw KJ), pp. 87-136. Cambridge, MA: Harvard University Press. [Google Scholar]
- 16.Maynard-Smith J, Harper D. 2003. Animal signals. Oxford, UK: Oxford University Press. [Google Scholar]
- 17.Maruska KP, Fernald RD. 2010. Behavioral and physiological plasticity: rapid changes during social ascent in an African cichlid fish. Horm. Behav. 58, 230-240. ( 10.1016/j.yhbeh.2010.03.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghazanfar AA, Santos LR. 2004. Primate brains in the wild: the sensory bases for social interactions. Nat. Rev. Neurosci. 5, 603-616. ( 10.1038/nrn1473) [DOI] [PubMed] [Google Scholar]
- 19.Brennan PA, Keverne EB. 2004. Something in the air? New insights into mammalian pheromones. Curr. Biol. 14, R81-R89. ( 10.1016/j.cub.2003.12.052) [DOI] [PubMed] [Google Scholar]
- 20.Hurst JL, Beynon RJ. 2004. Scent wars: the chemobiology of competitive signalling in mice. Bioessays 26, 1288-1298. ( 10.1002/bies.20147) [DOI] [PubMed] [Google Scholar]
- 21.Breithaupt T, Atema J. 2000. The timing of chemical signaling with urine in dominance fights of male lobsters (Homarus americanus). Behav. Ecol. Sociobiol. 49, 67-78. ( 10.1007/s002650000271) [DOI] [Google Scholar]
- 22.Jandt JM, Tibbetts EA, Toth AL.. 2014. Polistes paper wasps: a model genus for the study of social dominance hierarchies. Insect. Soc. 61, 11-27. ( 10.1007/s00040-013-0328-0) [DOI] [Google Scholar]
- 23.Chung M, Wang M-Y, Huang Z, Okuyama T. 2020. Diverse sensory cues for individual recognition. Dev. Growth Differ. 62, 507-515. ( 10.1111/dgd.12697) [DOI] [PubMed] [Google Scholar]
- 24.Ferris CF, Axelson JF, Shinto LH, Albers HE. 1987. Scent marking and the maintenance of dominant/subordinate status in male golden hamsters. Physiol. Behav. 40, 661-664. ( 10.1016/0031-9384(87)90114-4) [DOI] [PubMed] [Google Scholar]
- 25.Muir ER, Biju KC, Cong L, Rogers WE, Torres Hernandez E, Duong TQ, Clark RA. 2019. Functional MRI of the mouse olfactory system. Neurosci. Lett. 704, 57-61. ( 10.1016/j.neulet.2019.03.055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stowers L. 2002. Loss of sex discrimination and male–male aggression in mice deficient for TRP2. Science 295, 1493-1500. ( 10.1126/science.1069259) [DOI] [PubMed] [Google Scholar]
- 27.Mohrhardt J, Nagel M, Fleck D, Ben-Shaul Y, Spehr M. 2018. Signal detection and coding in the accessory olfactory system. Chem. Senses 43, 667-695. ( 10.1093/chemse/bjy061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veyrac A, Wang G, Baum MJ, Bakker J. 2011. The main and accessory olfactory systems of female mice are activated differentially by dominant versus subordinate male urinary odors. Brain Res. 1402, 20-29. ( 10.1016/j.brainres.2011.05.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee W, Dowd HN, Nikain C, Dwortz MF, Yang ED, Curley JP. 2021. Effect of relative social rank within a social hierarchy on neural activation in response to familiar or unfamiliar social signals. Sci. Rep. 11, 2864. ( 10.1038/s41598-021-82255-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaur AW, et al. 2014. Murine pheromone proteins constitute a context-dependent combinatorial code governing multiple social behaviors. Cell 157, 676-688. ( 10.1016/j.cell.2014.02.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee W, Khan A, Curley JP. 2017. Major urinary protein levels are associated with social status and context in mouse social hierarchies. Proc. R. Soc. B 284, 20171570. ( 10.1098/rspb.2017.1570) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo H, Fang Q, Huo Y, Zhang Y, Zhang J. 2015. Social dominance-related major urinary proteins and the regulatory mechanism in mice. Integr. Zool. 10, 543-554. ( 10.1111/1749-4877.12165) [DOI] [PubMed] [Google Scholar]
- 33.Keshavarzi S, Power JM, Albers EHH, Sullivan RKS, Sah P. 2015. Dendritic organization of olfactory inputs to medial amygdala neurons. J. Neurosci. 35, 13 020-13 028. ( 10.1523/JNEUROSCI.0627-15.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maximino C, Lima MG, Oliveira KRM, Batista E de JO, Herculano AM. 2013. ‘Limbic associative’ and ‘autonomic’ amygdala in teleosts: a review of the evidence. J. Chem. Neuroanat. 48–49, 1-13. ( 10.1016/j.jchemneu.2012.10.001) [DOI] [PubMed] [Google Scholar]
- 35.Raam T, Hong W. 2021. Organization of neural circuits underlying social behavior: a consideration of the medial amygdala. Curr. Opin Neurobiol. 68, 124-136. ( 10.1016/j.conb.2021.02.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bergan JF, Ben-Shaul Y, Dulac C. 2014. Sex-specific processing of social cues in the medial amygdala. Elife 3, e02743. ( 10.7554/eLife.02743) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Satou M, Oka Y, Kusunoki M, Matsushima T, Kato M, Fujita I, Ueda K. 1984. Telencephalic and preoptic areas integrate sexual behavior in hime salmon (landlocked red salmon, Oncorhynchus nerka): results of electrical brain stimulation experiments. Physiol. Behav. 33, 441-447. ( 10.1016/0031-9384(84)90167-7) [DOI] [PubMed] [Google Scholar]
- 38.Greenberg N, Scott M, Crews D. 1984. Role of the amygdala in the reproductive and aggressive behavior of the lizard, Anolis carolinensis. Physiol. Behav. 32, 147-151. ( 10.1016/0031-9384(84)90088-X) [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Mathis A, Grewe BF, Osterhout JA, Ahanonu B, Schnitzer MJ, Murthy VN, Dulac C. 2017. Neuronal representation of social information in the medial amygdala of awake behaving mice. Cell 171, 1176-1190.e17. ( 10.1016/j.cell.2017.10.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lukas M, Toth I, Veenema AH, Neumann ID. 2013. Oxytocin mediates rodent social memory within the lateral septum and the medial amygdala depending on the relevance of the social stimulus: male juvenile versus female adult conspecifics. Psychoneuroendocrinology 38, 916-926. ( 10.1016/j.psyneuen.2012.09.018) [DOI] [PubMed] [Google Scholar]
- 41.Yao S, Bergan J, Lanjuin A, Dulac C. 2017. Oxytocin signaling in the medial amygdala is required for sex discrimination of social cues. Elife 6, e31373. ( 10.7554/eLife.31373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan Y, Xu L, Young KA, Wang Z, Zhang Z. 2010. Agonistic encounters and brain activation in dominant and subordinate male greater long-tailed hamsters. Horm. Behav. 58, 478-484. ( 10.1016/j.yhbeh.2010.05.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DiBattista JD, Anisman H, Whitehead M, Gilmour KM. 2005. The effects of cortisol administration on social status and brain monoaminergic activity in rainbow trout Oncorhynchus mykiss. J. Exp. Biol. 208, 2707-2718. ( 10.1242/jeb.01690) [DOI] [PubMed] [Google Scholar]
- 44.Hitti FL, Siegelbaum SA. 2014. The hippocampal CA2 region is essential for social memory. Nature 508, 88-92. ( 10.1038/nature13028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maruska KP, Fernald RD. 2012. Contextual chemosensory urine signaling in an African cichlid fish. J. Exp. Biol. 215, 68-74. ( 10.1242/jeb.062794) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keller-Costa T, Saraiva JL, Hubbard PC, Barata EN, Canário AVM. 2016. A multi-component pheromone in the urine of dominant male tilapia (Oreochromis mossambicus) reduces aggression in rivals. J. Chem. Ecol. 42, 173-182. ( 10.1007/s10886-016-0668-0) [DOI] [PubMed] [Google Scholar]
- 47.Gonçalves-de-Freitas E, Teresa FB, Gomes FS, Giaquinto PC. 2008. Effect of water renewal on dominance hierarchy of juvenile Nile tilapia. Appl. Anim. Behav. Sci. 112, 187-195. ( 10.1016/j.applanim.2007.07.002) [DOI] [Google Scholar]
- 48.O'Connell LA, Rigney MM, Dykstra DW, Hofmann HA. 2013. Neuroendocrine mechanisms underlying sensory integration of social signals. J. Neuroendocrinol. 25, 644-654. ( 10.1111/jne.12045) [DOI] [PubMed] [Google Scholar]
- 49.Simões JM, Barata EN, Harris RM, O'Connell LA, Hofmann HA, Oliveira RF. 2015. Social odors conveying dominance and reproductive information induce rapid physiological and neuromolecular changes in a cichlid fish. BMC Genom. 16, 114. ( 10.1186/s12864-015-1255-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nikonov AA, Maruska KP. 2019. Male dominance status regulates odor-evoked processing in the forebrain of a cichlid fish. Sci. Rep. 9, 5083. ( 10.1038/s41598-019-41521-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meisami E, Bhatnagar KP. 1998. Structure and diversity in mammalian accessory olfactory bulb. Microsc. Res. Tech. 43, 476-499. () [DOI] [PubMed] [Google Scholar]
- 52.Schaal B, Porter RH.. 1991. ‘Microsmatic humans’ revisited: the generation and perception of chemical signals. In Advances in the study of behavior (eds Slater PJB, Rosenblatt JS, Beer C, Milinski M), pp. 135-199. Cambridge, MA: Academic Press. [Google Scholar]
- 53.Barton RA. 2006. Olfactory evolution and behavioral ecology in primates. Am. J. Primatol. 68, 545-558. ( 10.1002/ajp.20251) [DOI] [PubMed] [Google Scholar]
- 54.Rosvold HE, Mirsky AF, Pribram KH. 1954. Influence of amygdalectomy on social behavior in monkeys. J. Comp. Physiol. Psychol. 47, 173-178. ( 10.1037/h0058870) [DOI] [PubMed] [Google Scholar]
- 55.Klüver H, Bucy PC. 1939. Preliminary analysis of functions of the temporal lobes in monkeys. Arch. Neurol. Psychiatry 42, 979-1000. ( 10.1001/archneurpsyc.1939.02270240017001) [DOI] [PubMed] [Google Scholar]
- 56.Sallet J, et al. 2011. Social network size affects neural circuits in macaques. Science 334, 697-700. ( 10.1126/science.1210027) [DOI] [PubMed] [Google Scholar]
- 57.Bickart KC, Wright CI, Dautoff RJ, Dickerson BC, Barrett LF. 2011. Amygdala volume and social network size in humans. Nat. Neurosci. 14, 163-164. ( 10.1038/nn.2724) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Felix-Ortiz AC, Tye KM. 2014. Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. J. Neurosci. 34, 586-595. ( 10.1523/JNEUROSCI.4257-13.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Felix-Ortiz AC, Beyeler A, Seo C, Leppla CA, Wildes CP, Tye KM. 2013. BLA to vHPC inputs modulate anxiety-related behaviors. Neuron 79, 658-664. ( 10.1016/j.neuron.2013.06.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Janak PH, Tye KM. 2015. From circuits to behaviour in the amygdala. Nature 517, 284-292. ( 10.1038/nature14188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Putnam PT, Chang SWC. 2021. Toward a holistic view of value and social processing in the amygdala: insights from primate behavioral neurophysiology. Behav. Brain Res. 411, 113356. ( 10.1016/j.bbr.2021.113356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Troiani V, Schultz R. 2013. Amygdala, pulvinar, and inferior parietal cortex contribute to early processing of faces without awareness. Front. Hum. Neurosci. 7, 241. ( 10.3389/fnhum.2013.00241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gothard KM, Battaglia FP, Erickson CA, Spitler KM, Amaral DG. 2007. Neural responses to facial expression and face identity in the monkey amygdala. J. Neurophysiol. 97, 1671-1683. ( 10.1152/jn.00714.2006) [DOI] [PubMed] [Google Scholar]
- 64.Hoffman KL, Gothard KM, Schmid MC, Logothetis NK. 2007. Facial-expression and gaze-selective responses in the monkey amygdala. Curr. Biol. 17, 766-772. ( 10.1016/j.cub.2007.03.040) [DOI] [PubMed] [Google Scholar]
- 65.Pessoa L. 2010. Emotion and cognition and the amygdala: from ‘what is it?’ to ‘what's to be done?’ Neuropsychologia 48, 3416-3429. ( 10.1016/j.neuropsychologia.2010.06.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ueda Y, Yoshikawa S. 2018. Beyond personality traits: which facial expressions imply dominance in two-person interaction scenes? Emotion 18, 872. ( 10.1037/emo0000286) [DOI] [PubMed] [Google Scholar]
- 67.Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL. 2001. A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion 1, 70-83. ( 10.1037/1528-3542.1.1.70) [DOI] [PubMed] [Google Scholar]
- 68.Emery NJ, Capitanio JP, Mason WA, Machado CJ, Mendoza SP, Amaral DG. 2001. The effects of bilateral lesions of the amygdala on dyadic social interactions in rhesus monkeys (Macaca mulatta). Behav. Neurosci. 115, 515-544. ( 10.1037/0735-7044.115.3.515) [DOI] [PubMed] [Google Scholar]
- 69.Prather MD, Lavenex P, Mauldin-Jourdain ML, Mason WA, Capitanio JP, Mendoza SP, Amaral DG. 2001. Increased social fear and decreased fear of objects in monkeys with neonatal amygdala lesions. Neuroscience 106, 653-658. ( 10.1016/S0306-4522(01)00445-6) [DOI] [PubMed] [Google Scholar]
- 70.Zink CF, Tong Y, Chen Q, Bassett DS, Stein JL, Meyer-Lindenberg A. 2008. Know your place: neural processing of social hierarchy in humans. Neuron 58, 273-283. ( 10.1016/j.neuron.2008.01.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Munuera J, Rigotti M, Salzman CD. 2018. Shared neural coding for social hierarchy and reward value in primate amygdala. Nat. Neurosci. 21, 415-423. ( 10.1038/s41593-018-0082-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maren S, Phan KL, Liberzon I. 2013. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat. Rev. Neurosci. 14, 417-428. ( 10.1038/nrn3492) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. 1990. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J. Neurosci. 10, 1062-1069. ( 10.1523/JNEUROSCI.10-04-01062.1990) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.LeDoux J, Iwata J, Cicchetti P, Reis D. 1988. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J. Neurosci. 8, 2517-2529. ( 10.1523/JNEUROSCI.08-07-02517.1988) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Olsson A, Knapska E, Lindström B. 2020. The neural and computational systems of social learning. Nat. Rev. Neurosci. 21, 197-212. ( 10.1038/s41583-020-0276-4) [DOI] [PubMed] [Google Scholar]
- 76.Korzan WJ, Forster GL, Watt MJ, Summers CH. 2006. Dopaminergic activity modulation via aggression, status, and a visual social signal. Behav. Neurosci. 120, 93-102. ( 10.1037/0735-7044.120.1.93) [DOI] [PubMed] [Google Scholar]
- 77.Greenberg N, MacLean PD, Ferguson JL. 1979. Role of the paleostriatum in species-typical display behavior of the lizard (Anolis carolinensis). Brain Res. 172, 229-241. ( 10.1016/0006-8993(79)90535-3) [DOI] [PubMed] [Google Scholar]
- 78.Calder AJ, Keane J, Lawrence AD, Manes F. 2004. Impaired recognition of anger following damage to the ventral striatum. Brain 127, 1958-1969. ( 10.1093/brain/awh214) [DOI] [PubMed] [Google Scholar]
- 79.Schultz W. 2007. Behavioral dopamine signals. Trends Neurosci. 30, 203-210. ( 10.1016/j.tins.2007.03.007) [DOI] [PubMed] [Google Scholar]
- 80.Flagel SB, et al. 2011. A selective role for dopamine in stimulus–reward learning. Nature 469, 53-57. ( 10.1038/nature09588) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Klein JT, Platt ML. 2013. Social information signaling by neurons in primate striatum. Curr. Biol. 23, 691-696. ( 10.1016/j.cub.2013.03.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Deaner RO, Khera AV, Platt ML. 2005. Monkeys pay per view: adaptive valuation of social images by rhesus macaques. Curr. Biol. 15, 543-548. ( 10.1016/j.cub.2005.01.044) [DOI] [PubMed] [Google Scholar]
- 83.Patriarchi T, et al. 2018. Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors. Science 360, eaat4422. ( 10.1126/science.aat4422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nelson EE, Guyer AE. 2011. The development of the ventral prefrontal cortex and social flexibility. Dev. Cogn. Neurosci. 1, 233-245. ( 10.1016/j.dcn.2011.01.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Krawczyk DC. 2002. Contributions of the prefrontal cortex to the neural basis of human decision making. Neurosci. Biobehav. Rev. 26, 631-664. ( 10.1016/S0149-7634(02)00021-0) [DOI] [PubMed] [Google Scholar]
- 86.Euston DR, Gruber AJ, McNaughton BL. 2012. The role of medial prefrontal cortex in memory and decision making. Neuron 76, 1057-1070. ( 10.1016/j.neuron.2012.12.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Watson KK, Platt ML. 2012. Social signals in primate orbitofrontal cortex. Curr. Biol. 22, 2268-2273. ( 10.1016/j.cub.2012.10.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Adolphs R. 2002. Recognizing emotion from facial expressions: psychological and neurological mechanisms. Behav. Cogn. Neurosci. Rev. 1, 21-62. ( 10.1177/1534582302001001003) [DOI] [PubMed] [Google Scholar]
- 89.Chiao JY, Adams RB, Tse PU, Lowenthal WT, Richeson JA, Ambady N. 2008. Knowing who's boss: fMRI and ERP investigations of social dominance perception. Group Process. Intergr. Relat. 11, 201-214. ( 10.1177/1368430207088038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marsh AA, Blair KS, Jones MM, Soliman N, Blair RJR. 2009. Dominance and submission: the ventrolateral prefrontal cortex and responses to status cues. J. Cogn. Neurosci. 21, 713-724. ( 10.1162/jocn.2009.21052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Watanabe N, Yamamoto M. 2015. Neural mechanisms of social dominance. Front. Neurosci. 9, 154. ( 10.3389/fnins.2015.00154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Padilla-Coreano N, et al. 2020. A cortical-hypothalamic circuit decodes social rank and promotes dominance behavior. ( 10.21203/rs.3.rs-94115/v1) [DOI]
- 93.Re DE, Lefevre CE, DeBruine LM, Jones BC, Perrett DI. 2014. Impressions of dominance are made relative to others in the visual environment. Evol. Psychol. 12, 147470491401200130. ( 10.1177/147470491401200118) [DOI] [PubMed] [Google Scholar]
- 94.Mason M, Magee JC, Fiske ST. 2014. Neural substrates of social status inference: roles of medial prefrontal cortex and superior temporal sulcus. J. Cogn. Neurosci. 26, 1131-1140. ( 10.1162/jocn_a_00553) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Allison T, Puce A, McCarthy G. 2000. Social perception from visual cues: role of the STS region. Trends Cogn. Sci. 4, 267-278. ( 10.1016/S1364-6613(00)01501-1) [DOI] [PubMed] [Google Scholar]
- 96.Eifuku S, De Souza WC, Tamura R, Nishijo H, Ono T.. 2004. Neuronal correlates of face identification in the monkey anterior temporal cortical areas. J. Neurophysiol. 91, 358-371. ( 10.1152/jn.00198.2003) [DOI] [PubMed] [Google Scholar]
- 97.Iaria G, Fox CJ, Waite CT, Aharon I, Barton JJS. 2008. The contribution of the fusiform gyrus and superior temporal sulcus in processing facial attractiveness: neuropsychological and neuroimaging evidence. Neuroscience 155, 409-422. ( 10.1016/j.neuroscience.2008.05.046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kapur N, Friston KJ, Young A, Frith CD, Frackowiak RSJ. 1995. Activation of human hippocampal formation during memory for faces: a pet study. Cortex 31, 99-108. ( 10.1016/S0010-9452(13)80108-6) [DOI] [PubMed] [Google Scholar]
- 99.Korzan WJ, Höglund E, Watt MJ, Forster GL, Øverli Ø, Lukkes JL, Summers CH. 2007. Memory of opponents is more potent than visual sign stimuli after social hierarchy has been established. Behav. Brain Res. 183, 31-42. ( 10.1016/j.bbr.2007.05.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Summers CH, et al. 2005. Dynamics and mechanics of social rank reversal. J. Comp. Physiol. A 191, 241-252. ( 10.1007/s00359-004-0554-z) [DOI] [PubMed] [Google Scholar]
- 101.Milewski TM, Lee W, Champagne FA, Curley JP. 2021. Behavioural and physiological plasticity in social hierarchies. Phil. Trans. R. Soc. B 377, 20200443. ( 10.1098/rstb.2020.0443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rattiner LM. 2004. Brain-derived neurotrophic factor and tyrosine kinase receptor B involvement in amygdala-dependent fear conditioning. J. Neurosci. 24, 4796-4806. ( 10.1523/JNEUROSCI.5654-03.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Taylor SL, Stanek LM, Ressler KJ, Huhman KL. 2011. Differential BDNF expression in limbic brain regions following social defeat or territorial aggression. Behav. Neurosci. 125, 911-920. ( 10.1037/a0026172) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jasnow AM, Huhman KL. 2001. Activation of GABAA receptors in the amygdala blocks the acquisition and expression of conditioned defeat in Syrian hamsters. Brain Res. 920, 142-150. ( 10.1016/S0006-8993(01)03054-2) [DOI] [PubMed] [Google Scholar]
- 105.Helmstetter FJ, Bellgowan PS. 1994. Effects of muscimol applied to the basolateral amygdala on acquisition and expression of contextual fear conditioning in rats. Behav. Neurosci. 108, 1005-1009. ( 10.1037/0735-7044.108.5.1005) [DOI] [PubMed] [Google Scholar]
- 106.Dulka BN, Bagatelas ED, Bress KS, Grizzell JA, Cannon MK, Whitten CJ, Cooper MA. 2020. Chemogenetic activation of an infralimbic cortex to basolateral amygdala projection promotes resistance to acute social defeat stress. Sci. Rep. 10, 6884. ( 10.1038/s41598-020-63879-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Adhikari A, et al. 2015. Basomedial amygdala mediates top-down control of anxiety and fear. Nature 527, 179-185. ( 10.1038/nature15698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Broglio C, Gómez A, Durán E, Ocaña FM, Jiménez-Moya F, Rodríguez F, Salas C. 2005. Hallmarks of a common forebrain vertebrate plan: specialized pallial areas for spatial, temporal and emotional memory in actinopterygian fish. Brain Res. Bull. 66, 277-281. ( 10.1016/j.brainresbull.2005.03.021) [DOI] [PubMed] [Google Scholar]
- 109.Lal P, et al. 2018. Identification of a neuronal population in the telencephalon essential for fear conditioning in zebrafish. BMC Biol. 16, 45. ( 10.1186/s12915-018-0502-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Butler JM, Whitlow SM, Roberts DA, Maruska KP. 2018. Neural and behavioural correlates of repeated social defeat. Sci. Rep. 8, 6818. ( 10.1038/s41598-018-25160-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Clauss JA, Avery SN, Benningfield MM, Blackford JU. 2019. Social anxiety is associated with BNST response to unpredictability. Depress Anxiety 36, 666-675. ( 10.1002/da.22891) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wright EC, Hostinar CE, Trainor BC. 2020. Anxious to see you: neuroendocrine mechanisms of social vigilance and anxiety during adolescence. Eur. J. Neurosci. 52, 2516-2529. ( 10.1111/ejn.14628) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lebow MA, Chen A. 2016. Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol. Psychiatry 21, 450-463. ( 10.1038/mp.2016.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Williamson CM, Lee W, DeCasien AR, Lanham A, Romeo RD, Curley JP. 2019. Social hierarchy position in female mice is associated with plasma corticosterone levels and hypothalamic gene expression. Sci. Rep. 9, 7324. ( 10.1038/s41598-019-43747-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Abbott DH, et al. 2003. Are subordinates always stressed? a comparative analysis of rank differences in cortisol levels among primates. Horm. Behav. 43, 67-82. ( 10.1016/S0018-506X(02)00037-5) [DOI] [PubMed] [Google Scholar]
- 116.Pavlidis M, Sundvik M, Chen Y-C, Panula P. 2011. Adaptive changes in zebrafish brain in dominant–subordinate behavioral context. Behav. Brain Res. 225, 529-537. ( 10.1016/j.bbr.2011.08.022) [DOI] [PubMed] [Google Scholar]
- 117.Øverli Ø, et al. 2004. Stress coping style predicts aggression and social dominance in rainbow trout. Horm. Behav. 45, 235-241. ( 10.1016/j.yhbeh.2003.12.002) [DOI] [PubMed] [Google Scholar]
- 118.Elliott E, Ezra-Nevo G, Regev L, Neufeld-Cohen A, Chen A. 2010. Resilience to social stress coincides with functional DNA methylation of the Crf gene in adult mice. Nat. Neurosci. 13, 1351-1353. ( 10.1038/nn.2642) [DOI] [PubMed] [Google Scholar]
- 119.Jasnow AM, Davis M, Huhman KL. 2004. Involvement of central amygdalar and bed nucleus of the stria terminalis corticotropin-releasing factor in behavioral responses to social defeat. Behav. Neurosci. 118, 1052-1061. ( 10.1037/0735-7044.118.5.1052) [DOI] [PubMed] [Google Scholar]
- 120.Coplan JD, Karim A, Chandra P, St. Germain G, Abdallah CG, Altemus M. 2015. Neurobiology of maternal stress: role of social rank and central oxytocin in hypothalamic–pituitary adrenal axis modulation. Front. Psychiatry 6, 100. ( 10.3389/fpsyt.2015.00100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Calcagnoli F, de Boer SF, Beiderbeck DI, Althaus M, Koolhaas JM, Neumann ID.. 2014. Local oxytocin expression and oxytocin receptor binding in the male rat brain is associated with aggressiveness. Behav. Brain Res. 261, 315-322. ( 10.1016/j.bbr.2013.12.050) [DOI] [PubMed] [Google Scholar]
- 122.Lee W, Hiura LC, Yang E, Broekman KA, Ophir AG, Curley JP. 2019. Social status in mouse social hierarchies is associated with variation in oxytocin and vasopressin 1a receptor densities. Horm. Behav. 114, 104551. ( 10.1016/j.yhbeh.2019.06.015) [DOI] [PubMed] [Google Scholar]
- 123.Ebitz RB, Watson KK, Platt ML. 2013. Oxytocin blunts social vigilance in the rhesus macaque. Proc. Natl Acad. Sci. USA 110, 11 630-11 635. ( 10.1073/pnas.1305230110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rodriguez-Santiago M, Jordan A, Hofmann HA.. 2021. Differential neural activity patterns mediate learning across contexts in a social cichlid fish. bioRxiv, 2021.04.04.438393. ( 10.1101/2021.04.04.438393) [DOI]
- 125.Matthews GA, et al. 2016. Dorsal raphe dopamine neurons represent the experience of social isolation. Cell 164, 617-631. ( 10.1016/j.cell.2015.12.040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Matthews GA, Tye KM. 2019. Neural mechanisms of social homeostasis. Ann. NY Acad. Sci. 1457, 5-25. ( 10.1111/nyas.14016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Golden SA, Jin M, Shaham Y. 2019. Animal models of (or for) aggression reward, addiction, and relapse: behavior and circuits. J. Neurosci. 39, 3996-4008. ( 10.1523/JNEUROSCI.0151-19.2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yamaguchi Y, Lee Y-A, Kato A, Jas E, Goto Y. 2017. The roles of dopamine D2 receptor in the social hierarchy of rodents and primates. Sci. Rep. 7, 43348. ( 10.1038/srep43348) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Morgan D, et al. 2002. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat. Neurosci. 5, 169-174. ( 10.1038/nn798) [DOI] [PubMed] [Google Scholar]
- 130.Kudryavtseva NN, Lipina TV, Koryakina LA. 1999. Effects of haloperidol on communicative and aggressive behavior in male mice with different experiences of aggression. Pharmacol. Biochem. Behav. 63, 229-236. ( 10.1016/S0091-3057(98)00227-5) [DOI] [PubMed] [Google Scholar]
- 131.Fragoso VM da S, Hoppe LY, de Araújo-Jorge TC, de Azevedo MJ, Campos JD de S, Cortez CM, de Oliveira GM. 2016. Use of haloperidol and risperidone in highly aggressive Swiss Webster mice by applying the model of spontaneous aggression (MSA). Behav. Brain Res. 301, 110-118. ( 10.1016/j.bbr.2015.12.010) [DOI] [PubMed] [Google Scholar]
- 132.Couppis MH, Kennedy CH. 2008. The rewarding effect of aggression is reduced by nucleus accumbens dopamine receptor antagonism in mice. Psychopharmacology (Berl.) 197, 449-456. ( 10.1007/s00213-007-1054-y) [DOI] [PubMed] [Google Scholar]
- 133.Becker EA, Marler CA. 2015. Postcontest blockade of dopamine receptors inhibits development of the winner effect in the California mouse (Peromyscus californicus). Behav. Neurosci. 129, 205-213. ( 10.1037/bne0000043) [DOI] [PubMed] [Google Scholar]
- 134.Kohls G, et al. 2013. The nucleus accumbens is involved in both the pursuit of social reward and the avoidance of social punishment. Neuropsychologia 51, 2062-2069. ( 10.1016/j.neuropsychologia.2013.07.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.van der Kooij MA, et al. 2018. Diazepam actions in the VTA enhance social dominance and mitochondrial function in the nucleus accumbens by activation of dopamine D1 receptors. Mol. Psychiatry 23, 569-578. ( 10.1038/mp.2017.135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.O'Connell LA, Hofmann HA. 2011. The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J. Comp. Neurol. 519, 3599-3639. ( 10.1002/cne.22735) [DOI] [PubMed] [Google Scholar]
- 137.Hara E, Kubikova L, Hessler NA, Jarvis ED. 2007. Role of the midbrain dopaminergic system in modulation of vocal brain activation by social context. Eur. J. Neurosci. 25, 3406-3416. ( 10.1111/j.1460-9568.2007.05600.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hoke KL, Ryan MJ, Wilczynski W. 2007. Functional coupling between substantia nigra and basal ganglia homologues in amphibians. Behav. Neurosci. 121, 1393-1399. ( 10.1037/0735-7044.121.6.1393) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kapsimali M, Vidal B, Gonzalez A, Dufour S, Vernier P. 2000. Distribution of the mRNA encoding the four dopamine D1 receptor subtypes in the brain of the european eel (Anguilla anguilla): comparative approach to the function of D1 receptors in vertebrates. J. Comp. Neurol. 419, 320-343. () [DOI] [PubMed] [Google Scholar]
- 140.Rink E, Wullimann MF. 2001. The teleostean (zebrafish) dopaminergic system ascending to the subpallium (striatum) is located in the basal diencephalon (posterior tuberculum). Brain Res. 889, 316-330. ( 10.1016/S0006-8993(00)03174-7) [DOI] [PubMed] [Google Scholar]
- 141.Zhou T, Sandi C, Hu H. 2018. Advances in understanding neural mechanisms of social dominance. Curr. Opin Neurobiol. 49, 99-107. ( 10.1016/j.conb.2018.01.006) [DOI] [PubMed] [Google Scholar]
- 142.Ligneul R, Dreher J-C. 2017. Chapter 17 - Social dominance representations in the human brain. In Decision neuroscience (eds Dreher J-C, Tremblay L), pp. 211-224. San Diego, CA: Academic Press. [Google Scholar]
- 143.Gray CL, Norvelle A, Larkin T, Huhman KL. 2015. Dopamine in the nucleus accumbens modulates the memory of social defeat in Syrian hamsters (Mesocricetus auratus). Behav. Brain Res. 286, 22-28. ( 10.1016/j.bbr.2015.02.030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Berton O, et al. 2006. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311, 864-868. ( 10.1126/science.1120972) [DOI] [PubMed] [Google Scholar]
- 145.Tickerhoof MC, Hale LH, Butler MJ, Smith AS. 2020. Regulation of defeat-induced social avoidance by medial amygdala DRD1 in male and female prairie voles. Psychoneuroendocrinology 113, 104542. ( 10.1016/j.psyneuen.2019.104542) [DOI] [PubMed] [Google Scholar]
- 146.Williamson CM, Lee W, Curley JP. 2016. Temporal dynamics of social hierarchy formation and maintenance in male mice. Anim. Behav. 115, 259-272. ( 10.1016/j.anbehav.2016.03.004) [DOI] [Google Scholar]
- 147.Watts DP. 2000. Grooming between male chimpanzees at Ngogo, Kibale National Park. I. Partner number and diversity and grooming reciprocity. Int. J. Primatol. 21, 189-210. ( 10.1023/A:1005469302911) [DOI] [Google Scholar]
- 148.Hotta T, Takeyama T, Jordan LA, Kohda M. 2014. Duration of memory of dominance relationships in a group living cichlid. Naturwissenschaften 101, 745-751. ( 10.1007/s00114-014-1213-z) [DOI] [PubMed] [Google Scholar]
- 149.Forster GL, Watt MJ, Korzan WJ, Renner KJ, Summers CH. 2005. Opponent recognition in male green anoles, Anolis carolinensis. Anim. Behav. 69, 733-740. ( 10.1016/j.anbehav.2004.06.026) [DOI] [Google Scholar]
- 150.Kogan JH, Franklandand PW, Silva AJ. 2000. Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus 10, 47-56. () [DOI] [PubMed] [Google Scholar]
- 151.Eichenbaum H. 2017. Prefrontal–hippocampal interactions in episodic memory. Nat. Rev. Neurosci. 18, 547-558. ( 10.1038/nrn.2017.74) [DOI] [PubMed] [Google Scholar]
- 152.Smith CN, Jeneson A, Frascino JC, Kirwan CB, Hopkins RO, Squire LR. 2014. When recognition memory is independent of hippocampal function. Proc. Natl Acad. Sci. USA 111, 9935-9940. ( 10.1073/pnas.1409878111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Okuyama T. 2018. Social memory engram in the hippocampus. Neurosci. Res. 129, 17-23. ( 10.1016/j.neures.2017.05.007) [DOI] [PubMed] [Google Scholar]
- 154.Fanselow MS, Dong H-W. 2010. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65, 7-19. ( 10.1016/j.neuron.2009.11.031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Okuyama T, Kitamura T, Roy DS, Itohara S, Tonegawa S. 2016. Ventral CA1 neurons store social memory. Science 353, 1536-1541. ( 10.1126/science.aaf7003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sliwa J, Planté A, Duhamel J-R, Wirth S. 2016. Independent neuronal representation of facial and vocal identity in the monkey hippocampus and inferotemporal cortex. Cereb. Cortex 26, 950-966. ( 10.1093/cercor/bhu257) [DOI] [PubMed] [Google Scholar]
- 157.Meira T, Leroy F, Buss EW, Oliva A, Park J, Siegelbaum SA. 2018. A hippocampal circuit linking dorsal CA2 to ventral CA1 critical for social memory dynamics. Nat. Commun. 9, 4163. ( 10.1038/s41467-018-06501-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Elliott SB, Harvey-Girard E, Giassi ACC, Maler L. 2017. Hippocampal-like circuitry in the pallium of an electric fish: possible substrates for recursive pattern separation and completion. J. Comp. Neurol. 525, 8-46. ( 10.1002/cne.24060) [DOI] [PubMed] [Google Scholar]
- 159.Phillips ML, Robinson HA, Pozzo-Miller L. 2019. Ventral hippocampal projections to the medial prefrontal cortex regulate social memory. Elife 8, e44182. ( 10.7554/eLife.44182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kumaran D, Banino A, Blundell C, Hassabis D, Dayan P. 2016. Computations underlying social hierarchy learning: distinct neural mechanisms for updating and representing self-relevant information. Neuron 92, 1135-1147. ( 10.1016/j.neuron.2016.10.052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ligneul R, Obeso I, Ruff CC, Dreher J-C. 2016. Dynamical representation of dominance relationships in the human rostromedial prefrontal cortex. Curr. Biol. 26, 3107-3115. ( 10.1016/j.cub.2016.09.015) [DOI] [PubMed] [Google Scholar]
- 162.Sofroniew MV. 1980. Projections from vasopressin, oxytocin, and neurophysin neurons to neural targets in the rat and human. J. Histochem. Cytochem. 28, 475-478. ( 10.1177/28.5.7381192) [DOI] [PubMed] [Google Scholar]
- 163.van Wimersma Greidanus TjB, Maigret C.. 1996. The role of limbic vasopressin and oxytocin in social recognition. Brain Res. 713, 153-159. ( 10.1016/0006-8993(95)01505-1) [DOI] [PubMed] [Google Scholar]
- 164.Smith AS, Williams Avram SK, Cymerblit-Sabba A, Song J, Young WS. 2016. Targeted activation of the hippocampal CA2 area strongly enhances social memory. Mol. Psychiatry 21, 1137-1144. ( 10.1038/mp.2015.189) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Raam T, McAvoy KM, Besnard A, Veenema AH, Sahay A. 2017. Hippocampal oxytocin receptors are necessary for discrimination of social stimuli. Nat. Commun. 8, 2001. ( 10.1038/s41467-017-02173-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lin Y-T, Hsieh T-Y, Tsai T-C, Chen C-C, Huang C-C, Hsu K-S. 2018. Conditional deletion of hippocampal CA2/CA3a oxytocin receptors impairs the persistence of long-term social recognition memory in mice. J. Neurosci. 38, 1218-1231. ( 10.1523/JNEUROSCI.1896-17.2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ferguson JN, Young LJ, Insel TR. 2002. The neuroendocrine basis of social recognition. Front. Neuroendocrinol. 23, 200-224. ( 10.1006/frne.2002.0229) [DOI] [PubMed] [Google Scholar]
- 168.Choleris E, Little SR, Mong JA, Puram SV, Langer R, Pfaff DW. 2007. Microparticle-based delivery of oxytocin receptor antisense DNA in the medial amygdala blocks social recognition in female mice. Proc. Natl Acad. Sci. USA 104, 4670-4675. ( 10.1073/pnas.0700670104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bielsky IF, Young LJ. 2004. Oxytocin, vasopressin, and social recognition in mammals. Peptides 25, 1565-1574. ( 10.1016/j.peptides.2004.05.019) [DOI] [PubMed] [Google Scholar]
- 170.Caffé AR, van Leeuwen FW, Luiten PGM. 1987. Vasopressin cells in the medial amygdala of the rat project to the lateral septum and ventral hippocampus. J. Comp. Neurol. 261, 237-252. ( 10.1002/cne.902610206) [DOI] [PubMed] [Google Scholar]
- 171.Cardoso SC, Bshary R, Mazzei R, Paitio JR, Oliveira RF, Soares MC. 2015. Arginine vasotocin modulates associative learning in a mutualistic cleaner fish. Behav. Ecol. Sociobiol. 69, 1173-1181. ( 10.1007/s00265-015-1931-z) [DOI] [Google Scholar]
- 172.Almeida O, Gozdowska M, Kulczykowska E, Oliveira RF. 2012. Brain levels of arginine–vasotocin and isotocin in dominant and subordinate males of a cichlid fish. Horm. Behav. 61, 212-217. ( 10.1016/j.yhbeh.2011.12.008) [DOI] [PubMed] [Google Scholar]
- 173.Carneiro LA, Oliveira RF, Canário AVM, Grober MS. 2003. The effect of arginine vasotocin on courtship behaviour in a blenniid fish with alternative reproductive tactics. Fish Physiol. Biochem. 28, 241-243. ( 10.1023/B:FISH.0000030542.31395.8a) [DOI] [Google Scholar]
- 174.Santangelo N, Bass AH. 2006. New insights into neuropeptide modulation of aggression: field studies of arginine vasotocin in a territorial tropical damselfish. Proc. R. Soc. B 273, 3085-3092. ( 10.1098/rspb.2006.3683) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hattori T, Wilczynski W. 2009. Comparison of arginine vasotocin immunoreactivity differences in dominant and subordinate green anole lizards. Physiol. Behav. 96, 104-107. ( 10.1016/j.physbeh.2008.09.010) [DOI] [PubMed] [Google Scholar]
- 176.Delville Y, Mansour KM, Ferris CF. 1996. Testosterone facilitates aggression by modulating vasopressin receptors in the hypothalamus. Physiol. Behav. 60, 25-29. ( 10.1016/0031-9384(95)02246-5) [DOI] [PubMed] [Google Scholar]
- 177.Bester-Meredith JK, Young LJ, Marler CA. 1999. Species differences in paternal behavior and aggression in Peromyscus and their associations with vasopressin immunoreactivity and receptors. Horm. Behav. 36, 25-38. ( 10.1006/hbeh.1999.1522) [DOI] [PubMed] [Google Scholar]
- 178.Rigney N, Beaumont R, Petrulis A. 2020. Sex differences in vasopressin 1a receptor regulation of social communication within the lateral habenula and dorsal raphe of mice. Horm. Behav. 121, 104715. ( 10.1016/j.yhbeh.2020.104715) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Thompson RR, Walton JC. 2004. Peptide effects on social behavior: effects of vasotocin and isotocin on social approach behavior in male goldfish (Carassius auratus). Behav. Neurosci. 118, 620-626. ( 10.1037/0735-7044.118.3.620) [DOI] [PubMed] [Google Scholar]
- 180.Kabelik D, Magruder DS. 2014. Involvement of different mesotocin (oxytocin homologue) populations in sexual and aggressive behaviours of the brown anole. Biol. Lett. 10, 20140566. ( 10.1098/rsbl.2014.0566) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.O'Connell LA, Matthews BJ, Hofmann HA. 2012. Isotocin regulates paternal care in a monogamous cichlid fish. Horm. Behav. 61, 725-733. ( 10.1016/j.yhbeh.2012.03.009) [DOI] [PubMed] [Google Scholar]
- 182.de Chaumont F, et al. 2018. Live Mouse Tracker: real-time behavioral analysis of groups of mice. bioRxiv, 345132. ( 10.1101/345132) [DOI]
- 183.Shemesh Y, Sztainberg Y, Forkosh O, Shlapobersky T, Chen A, Schneidman E. 2013. High-order social interactions in groups of mice. Elife 2, e00759. ( 10.7554/eLife.00759) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Nilsson SR, et al. 2020. Simple Behavioral Analysis (SimBA) – an open source toolkit for computer classification of complex social behaviors in experimental animals. bioRxiv, 2020.04.19.049452. ( 10.1101/2020.04.19.049452) [DOI]
- 185.Lauer J, et al. 2021. Multi-animal pose estimation and tracking with DeepLabCut. bioRxiv, 2021.04.30.442096. ( 10.1101/2021.04.30.442096) [DOI]
- 186.Marx V. 2021. Neuroscientists go wireless. Nat. Methods 18, 1150-1154. ( 10.1038/s41592-021-01281-6) [DOI] [PubMed] [Google Scholar]
- 187.Rilling JK, Winslow JT, Kilts CD. 2004. The neural correlates of mate competition in dominant male rhesus macaques. Biol. Psychiatry 56, 364-375. ( 10.1016/j.biopsych.2004.06.027) [DOI] [PubMed] [Google Scholar]
- 188.Todorov A, Engell AD. 2008. The role of the amygdala in implicit evaluation of emotionally neutral faces. Soc. Cogn. Affect. Neurosci. 3, 303-312. ( 10.1093/scan/nsn033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Datta S. 1988. The acquisition of dominance among free-ranging rhesus monkey siblings. Anim. Behav. 36, 754-772. ( 10.1016/S0003-3472(88)80159-3) [DOI] [Google Scholar]
- 190.Janus M. 1992. Interplay between various aspects in social relationships of young rhesus monkeys: dominance, agonistic help, and affiliation. Am. J. Primatol. 26, 291-308. ( 10.1002/ajp.1350260406) [DOI] [PubMed] [Google Scholar]
- 191.Kaplan JR, Manuck SB, Fontenot MB, Mann JJ. 2002. Central nervous system monoamine correlates of social dominance in cynomolgus monkeys (Macaca fascicularis). Neuropsychopharmacology 26, 431-443. ( 10.1016/S0893-133X(01)00344-X) [DOI] [PubMed] [Google Scholar]
- 192.Drickamer LC, Vandenbergh JG, Colby DR. 1973. Predictors of dominance in the male golden hamster (Mesocricetus auratus). Anim. Behav. 21, 557-563. ( 10.1016/S0003-3472(73)80016-8) [DOI] [PubMed] [Google Scholar]
- 193.Huck UW, Lisk RD, McKay MV. 1988. Social dominance and reproductive success in pregnant and lactating golden hamsters (Mesocricetus auratus) under seminatural conditions. Physiol. Behav. 44, 313-319. ( 10.1016/0031-9384(88)90031-5) [DOI] [PubMed] [Google Scholar]
- 194.Oakeshott JG. 1974. Social dominance, aggressiveness and mating success among male house mice (Mus musculus). Oecologia 15, 143-158. ( 10.1007/BF00345742) [DOI] [PubMed] [Google Scholar]
- 195.Kondrakiewicz K, Kostecki M, Szadzińska W, Knapska E. 2019. Ecological validity of social interaction tests in rats and mice. Genes Brain Behav. 18, e12525. ( 10.1111/gbb.12525) [DOI] [PubMed] [Google Scholar]
- 196.Drickamer LC. 2001. Urine marking and social dominance in male house mice (Mus musculus domesticus). Behav. Processes 53, 113-120. ( 10.1016/S0376-6357(00)00152-2) [DOI] [PubMed] [Google Scholar]
- 197.Lindzey G, Manosevitz M, Winston H. 1966. Social dominance in the mouse. Psychon Sci. 5, 451-452. ( 10.3758/BF03331044) [DOI] [Google Scholar]
- 198.Wang F, Zhu J, Zhu H, Zhang Q, Lin Z, Hu H. 2011. Bidirectional control of social hierarchy by synaptic efficacy in medial prefrontal cortex. Science 334, 693-697. ( 10.1126/science.1209951) [DOI] [PubMed] [Google Scholar]
- 199.Adams N, Boice R. 1983. A longitudinal study of dominance in an outdoor colony of domestic rats. J. Comp. Psychol. 97, 24-33. ( 10.1037/0735-7036.97.1.24) [DOI] [Google Scholar]
- 200.Blanchard RJ, Flannelly KJ, Blanchard DC. 1988. Life-span studies of dominance and aggression in established colonies of laboratory rats. Physiol. Behav. 43, 1-7. ( 10.1016/0031-9384(88)90089-3) [DOI] [PubMed] [Google Scholar]
- 201.Himmler BT, Pellis VC, Pellis SM. 2013. Peering into the dynamics of social interactions: measuring play fighting in rats. J. Vis. Exp. 71, e4288. ( 10.3791/4288) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Jenssen TA, Nunez SC. 1998. Spatial and breeding relationships of the lizard, Anolis carolinensis: evidence of intrasexual selection. Behaviour 135, 981-1003. ( 10.1163/156853998792640341) [DOI] [Google Scholar]
- 203.Lovern MB. 2000. Behavioral ontogeny in free-ranging juvenile male and female green anoles, Anolis carolinensis, in relation to sexual selection. J. Herpetol. 34, 274-281. ( 10.2307/1565424) [DOI] [Google Scholar]
- 204.Paull GC, Filby AL, Giddins HG, Coe TS, Hamilton PB, Tyler CR. 2010. Dominance hierarchies in zebrafish (Danio rerio) and their relationship with reproductive success. Zebrafish 7, 109-117. ( 10.1089/zeb.2009.0618) [DOI] [PubMed] [Google Scholar]
- 205.Oliveira RF, Silva JF, Simoes JM. 2011. Fighting zebrafish: characterization of aggressive behavior and winner–loser effects. Zebrafish 8, 73-81. ( 10.1089/zeb.2011.0690) [DOI] [PubMed] [Google Scholar]
- 206.Teles MC, Dahlbom SJ, Winberg S, Oliveira RF. 2013. Social modulation of brain monoamine levels in zebrafish. Behav. Brain Res. 253, 17-24. ( 10.1016/j.bbr.2013.07.012) [DOI] [PubMed] [Google Scholar]
- 207.Dunlap K, Oliveri L.. 2002. Retreat site selection and social organization in captive electric fish, Apteronotus leptorhynchus. J. Comp. Physiol. A: Sens. Neural Behav. Physiol. 188, 469-477. ( 10.1007/s00359-002-0319-5) [DOI] [PubMed] [Google Scholar]
- 208.Fugère V, Ortega H, Krahe R. 2011. Electrical signalling of dominance in a wild population of electric fish. Biol. Lett. 7, 197-200. ( 10.1098/rsbl.2010.0804) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hagedorn M, Heiligenberg W. 1985. Court and spark: electric signals in the courtship and mating of gymnotoid fish. Anim. Behav. 33, 254-265. ( 10.1016/S0003-3472(85)80139-1) [DOI] [Google Scholar]
- 210.Telgkamp P, Combs N, Smith GT. 2007. Serotonin in a diencephalic nucleus controlling communication in an electric fish: sexual dimorphism and relationship to indicators of dominance. Dev. Neurobiol. 67, 339-354. ( 10.1002/dneu.20356) [DOI] [PubMed] [Google Scholar]
- 211.Perrone R, Silva AC. 2018. Status-dependent vasotocin modulation of dominance and subordination in the weakly electric fish Gymnotus omarorum. Front. Behav. Neurosci. 12, 1. ( 10.3389/fnbeh.2018.00001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Øverli Ø, Harris CA, Winberg S. 1999. Short-term effects of fights for social dominance and the establishment of dominant-subordinate relationships on brain monoamines and cortisol in rainbow trout. Brain Behav. Evol. 54, 263-275. ( 10.1159/000006627) [DOI] [PubMed] [Google Scholar]
- 213.Cubitt KF, Winberg S, Huntingford FA, Kadri S, Crampton VO, Øverli Ø. 2008. Social hierarchies, growth and brain serotonin metabolism in Atlantic salmon (Salmo salar) kept under commercial rearing conditions. Physiol. Behav. 94, 529-535. ( 10.1016/j.physbeh.2008.03.009) [DOI] [PubMed] [Google Scholar]
- 214.Renn SCP, Fraser EJ, Aubin-Horth N, Trainor BC, Hofmann HA. 2012. Females of an African cichlid fish display male-typical social dominance behavior and elevated androgens in the absence of males. Horm. Behav. 61, 496-503. ( 10.1016/j.yhbeh.2012.01.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Loveland JL, Uy N, Maruska KP, Carpenter RE, Fernald RD. 2014. Social status differences regulate the serotonergic system of a cichlid fish, Astatotilapia burtoni. J. Exp. Biol. 217, 2680-2690. ( 10.1242/jeb.100685) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.