Abstract
Individuals occupying dominant and subordinate positions in social hierarchies exhibit divergent behaviours, physiology and neural functioning. Dominant animals express higher levels of dominance behaviours such as aggression, territorial defence and mate-guarding. Dominants also signal their status via auditory, visual or chemical cues. Moreover, dominant animals typically increase reproductive behaviours and show enhanced spatial and social cognition as well as elevated arousal. These biobehavioural changes increase energetic demands that are met via shifting both energy intake and metabolism and are supported by coordinated changes in physiological systems including the hypothalamic–pituitary–adrenal and hypothalamic–pituitary–gonadal axes as well as altered gene expression and sensitivity of neural circuits that regulate these behaviours. Conversely, subordinate animals inhibit dominance and often reproductive behaviours and exhibit physiological changes adapted to socially stressful contexts. Phenotypic changes in both dominant and subordinate individuals may be beneficial in the short-term but lead to long-term challenges to health. Further, rapid changes in social ranks occur as dominant animals socially ascend or descend and are associated with dynamic modulations in the brain and periphery. In this paper, we provide a broad overview of how behavioural and phenotypic changes associated with social dominance and subordination are expressed in neural and physiological plasticity.
This article is part of the theme issue ‘The centennial of the pecking order: current state and future prospects for the study of dominance hierarchies’.
Keywords: social hierarchy, dominance, plasticity, social status, aggression
1. Introduction
Social dominance hierarchies are one of the most well-understood forms of social organization and have been studied in a broad range of species, including insects, fish, birds, mammals and humans [1]. Dominance hierarchies emerge when individuals compete for access to resources (e.g. food, territory and mates), with more dominant individuals being able to monopolize access over subordinates and thereby attaining fitness benefits [2]. The individual behaviours used to establish, enforce and maintain hierarchies can vary across species but generally involve agonistic interactions and non-contact behaviours that serve as signals of social status [1,3]. Through these behaviours, dominance hierarchies form and can remain stable over time. This stability coincides with significant short- and long-term physiological, neurobiological and behavioural changes associated with social rank that allows organisms to adjust dynamically to the social environment.
In this paper, we will highlight literature that illustrates the dynamic biological and behavioural plasticity exhibited by dominant and subordinate vertebrates within a social hierarchy, including behavioural phenotypes, signals used to communicate status, changes to reproduction and altered energy metabolism. We are primarily concerned with findings from social hierarchies that consist of dominance relationships between groups of related and unrelated individuals rather than dominance relationships restricted to dyads or only family groups. Though this plasticity may support the formation and maintenance of a social hierarchy, there are costs associated with these changes that can impact health. We will consider these costs as well as the plasticity that occurs when stability of a hierarchy is disrupted through social ascent or descent of dominant individuals. Our goal with this paper is to provide a broad overview and introduction to these topics, each of which has its own substantial literature. Although it is not possible to exhaustively describe all relevant studies, overall, we emphasize the capacity of dominant and subordinate animals to behaviourally and physiologically shift in response to social and environmental changes. We provide examples across vertebrate taxa, focusing most heavily on work in primates, cichlid fish and mice to highlight the intricate associations in plasticity across various physiological and behavioural domains.
2. Plasticity in dominant animals
(a) . Biobehavioural changes
In attribute-based hierarchies (i.e. dominance ranks are determined through individual physical or behavioural qualities) and convention-based hierarchies (i.e. dominance ranks are determined through age, tenure in a group or inheritance of mother's rank), the expression of aggression is generally a defining characteristic of individuals that maintain dominant social status [4]. Levels of aggression are typically highest during dominance relationship formation [5,6], particularly in attribute-based hierarchies. As hierarchies stabilize, agonism tends to decline among all individuals resulting in energy conservation and reduction in potential for physical injury. For example, in wild geladas (Theropithecus gelada), unfamiliar conspecifics engage in a series of transitory aggressive encounters during rank formation shifting to affiliation behaviours once social relationships are established [7]. However, even in stable hierarchies, dominant animals continue to enforce their status through aggression as well as intimidation and signals [8].
The neurobiological and neuroendocrine mechanisms that support the expression of aggressive behaviours and promote dominance have been well-characterized across a number of species [9,10]. A highly conserved vertebrate subcortical core aggression circuit (CAC) comprised of four major interconnected brain nuclei: medial amygdala, bed nucleus of stria terminalis, ventrolateral region of the ventromedial hypothalamus (VMHvl) and ventral region of the premammillary nucleus (PMv), is critical to the expression of dominant behaviour across species. The CAC receives species-specific inputs from sensory pathways and promotes aggressive behaviours through outputs to midbrain premotor areas. Importantly, this CAC is under strict top-down control and can be modulated via cortical pathways. In more dominant animals, aggression-provoking sensory cues activate the CAC, whereas in more subordinate animals, the CAC is inhibited to avoid the inappropriate expression of aggression. This circuit may be up- or downregulated in the same individual depending upon the concurrent social interaction. In stable hierarchies, changes to the sensitivity of the CAC are likely to result from long-lasting modifications within neurotransmitter and steroid systems.
Variation in nonapeptide, monoamine and steroid hormone receptor distribution in the brain is associated with dominant social status [5,11–13]. The relationship between serotonergic functioning and dominance appears to be context- and species-specific. For example, enhanced serotonergic functioning increases fight durations in dominant crustaceans [11] but reduces dominance behaviours in mice (Mus musculus) [14]. Dominant male vervet monkeys (Chlorocebus pygerythrus) have elevated serotonin levels in plasma compared to subordinates [15], and pharmacologically increasing serotonergic signalling in subordinates leads to higher social rank attainment; though this is achieved by reducing aggression and increasing affiliative behaviours [16]. For other modulators, there appears to be broad cross-species agreement in the direction of effects. For example, higher oxytocinergic functioning appears to be related to ascertainment of dominance in female rhesus macaques (Macaca mulatta) [17], male Mozambique tilapia (Oreochromis mossambicus) [18] and male mice [11]. However, the precise role of oxytocin in promoting dominance is unclear. In addition to promoting aggressive behaviours, oxytocin could also regulate other behavioural systems that support social dominance including social memory, social cognition and avoidance learning (see [19]). Finally, dopaminergic signalling is upregulated in dominant individuals [12,20]. In monkeys, this upregulation emerges following hierarchy formation suggesting it is a consequence rather than the cause of dominance [12]. The involvement of dopaminergic systems may account for the aggression-seeking behaviour of dominant individuals [21]. The directionality of these correlational findings is largely supported by studies that have experimentally manipulated these systems [9,10,22], although the precise relationships are brain region, species and context specific (see [19]).
The role of steroid hormones, particularly testosterone in males, in the development and coordination of aggression has been particularly well studied across species. Experimental manipulation of androgenic signalling has demonstrated a causative role of these hormones in regulating both male and female dominance [22]. These findings highlight the importance of testosterone in activating aggressive behaviour in the short-term. For example, levels of testosterone are typically most elevated during group formation, are increased in dominant individuals following short-term aggressive interactions and can even reinforce winner effects [23,24]. Data from field studies have found positive associations between testosterone and high male social rank in several species when male–male competition is maintained via repeated aggression and tightly linked to access to reproductive opportunities or during periods of hierarchy instability characterized by elevated aggression [5,25–28]. In females, elevated androgen levels are associated with high dominance rank especially during periods of intersex competition suggesting a role in mediating aggression (e.g. ring-tailed lemurs (Lemur catta) [29], spotted hyenas (Crocuta crocuta) [30], female hybrid baboons (Papio sp) [31] and African cichlid fish (Astatotilapia burtoni) [24]). Notably, however, these relationships are not observed for all species and much remains to be understood regarding the neuroendocrine basis of social dominance.
(b) . Signalling dominant status
The maintenance of dominant social positions can be dependent on the capacity of an organism to communicate and express their high social status through a broad range of chemosensory cues that require plasticity in multiple biological systems [32] (figure 1). Status signals may be inherently linked to fighting ability such as armaments like deer antlers or unrelated to fighting but used to convey dominance information (conventional signals) (see [33] for more information). These signals are considered honest if they are energetically costly to produce [32].
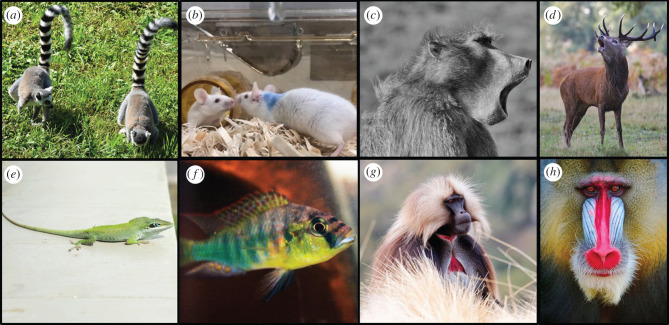
Figure 1.
Animals use a variety of modalities to signal social dominance. Some species may rely on a combination of modalities when cueing status. Ring-tailed lemurs (a) and mice (b) use scent marks to mark territories. Dominance can be signalled through vocalization as in chacma baboons (c) and red deer (d). Visual cues can reliably cue dominance such as eye spots in green anole lizards (e), eye bars and body coloration in African cichlid fish (f), red-chest patches in male geladas (g) and facial coloration in male mandrills (h). Photo credits: Maddie Dwortz, Christine Murray, Hans Hofmann.
Chemical signals are used by dominants of many species. In some species, these signals are used to directly determine dominance during physical interactions. For example, in social insects, queens indicate their dominance over workers through pheromonal cues during social exchanges [34]. Scent-marking is a common feature of territorial behaviour with individuals depositing scents within their environment to signal dominance condition. These odours are produced via secretions from integumentary glands, urine, saliva, sweat and faeces [35]. Dominant individuals tend to mark more frequently and counter-mark the scents of more subordinate individuals, and in many cases, the chemical composition of the odour differs by status. For instance, high-ranking male mandrills (Mandrillus sphinx) secrete more scents from their sternal scent-gland than low-ranking males, and it is possible to classify rank based on the relative composition of volatile components [36]. Dominant male blackbucks (Antelope cevicapra) urinate more frequently and possess specific organic compounds in their urine that cue their dominance compared to subordinates [37]. In the European rabbit (Oryctolagus cuniculus), when males become dominant they start to produce the compound 2-phenoxyethanol that is secreted via the chin gland [38]. This is a fixative that enables the slow release of other volatile odours in the secretion that communicate an individual's dominant status. Notably, such chemical cues appear to be energetically expensive to produce. The physiological plasticity associated with scent marking by dominant individuals has been best explored in mice and involves increased gene expression of major urinary proteins (MUPs) in the liver [39], stimulated by testosterone and growth hormones [40]. These metabolically costly proteins are then excreted in urine as scent marks which attract females and signal dominance status and body condition [39,41]. Dominant mice must also increase their food and water intake to support the production and excretion of these proteins [42] illustrating that plasticity in numerous central and peripheral systems is required to communicate social status (figure 2). Similar physiological changes occur in dominant male Mozambique tilapia who signal their rank via urinating dominance pheromones and develop larger, more muscular bladders to store larger volumes of these cues [43].
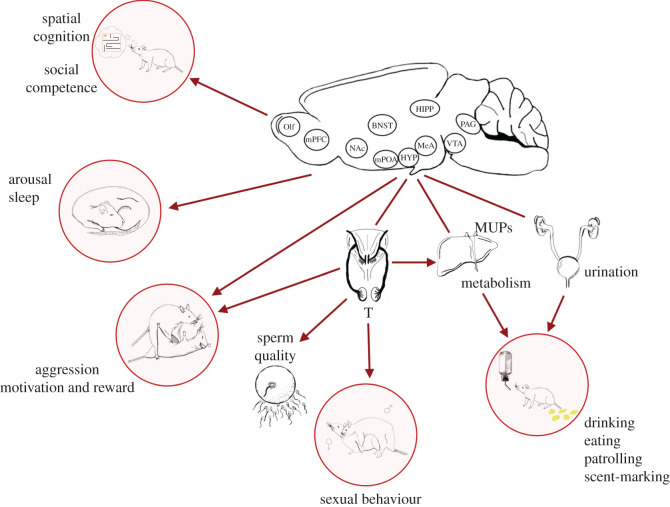
Figure 2.
Example of the central coordination of behavioural and physiological plasticity that supports dominance status in male mice. In addition to increasing the output of aggression, dominant males also find aggression more rewarding, show higher spatial and social cognition, increase sexual behaviour, patrolling, scent-marking, drinking, eating and arousal as well as more fragmented sleep. These changes occur in concert and are driven by modulating the sensitivity of neural circuits and the upregulation of the hypothalamic–pituitary–gonadal (HPG) axis. Increased production of testosterone (T) from the gonads also stimulates increased sperm production and quality as well as major urinary protein (MUP) production in the liver which are transported to the bladder via kidneys and secreted in urine to signal dominance status. These changes are supported by altered metabolism, particularly increased fatty acid catabolism, in the liver.
Auditory cues used for dominance signalling further illustrate how social status induces differential plasticity between dominant and subordinate individuals. Modulations to several features of auditory cues including volume, calling rate, temporal structure and frequency are used to communicate social status by dominant individuals [44,45]. Testosterone can modulate the production and acoustic structure of male vocalizations across species and may mediate status-associated acoustic characteristics [25]. For instance, male Alston's singing mice (Scotinomys teguina) emit trilled songs during competitive male interactions. These songs are shorter, quieter and higher in frequency in castrated males [46]. In primates, evidence for a relationship between testosterone levels and call frequency and acoustic structure is mixed although this has been observed in some species (e.g. male chimpanzees (Pan troglodytes schweinfurthii)), with high testosterone associated with more pant-hoots with higher peak frequencies [25,47].
Visual cues are another domain in which physiology and body condition can be tightly linked to status. For example, green anole lizards (Anolis carolinensis) transition from green to black eye spots as they become dominant [48] and dominant male African cichlid fish display bright colours and prominent eye bars [5]. Increased face redness in male mandrills is associated with competitive ability and willingness to engage in conflict and is positively associated with testosterone levels [49]. In male geladas, increased redness of bare chest patches is also positively related to social rank, but is not associated with androgens, demonstrating that not all coloration changes are androgen mediated [50]. Congruently, in vervet monkeys, blue scrotal coloration serves as a signal of status and is linked to elevated serotonin, not androgen levels [51]. These observed associations between the production of visual signals and individual hormone levels are suggestive that they may be energetically expensive to produce and therefore indirectly related to body condition and fighting ability. However, it is not yet clear what other mediators may exist between visual signals and physiological condition. Indeed, although there is some good evidence that chemical, auditory and visual signals can be honest indicators of social status, this may not be universal across all species.
(c) . Dominance and cognition
Associations between social status and cognitive performance are increasingly being explored, though few studies have determined whether variation in cognitive abilities is a cause or consequence of social status. If social reasoning and learning skills are required for achieving high rank, it would be expected that dominant animals out-perform subordinates in social cognition tests. There appears to be some support for this. For example, more highly ranked domestic chickens (Gallus gallus) have higher social learning performances [52]. Dominant queen paper wasps (Polistes fuscatus) are better at memorizing and individually recognizing faces than subordinate worker wasps [53]. Alliance formation, which is required in some species to challenge for high rank, requires continuous social strategizing and high social functioning. In female carrion crows (Corvus corone), high-ranking individuals are able to actively recruit sub-dominant animals to engage in coordinated coalitionary aggression against rivals [54]. However, other studies demonstrate that subordinate animals show higher levels of social learning, particularly when solving novel tasks (e.g. chimpanzees [55], dogs (Canis lupus familiaris) [56]). This may reflect increased monitoring of more dominant individuals by subordinates, but could also indicate that both dominant and subordinate animals use social learning in different ways to solve socio-cognitive challenges.
Studies in several birds and rodents have shown that high-ranking individuals out-perform subordinates on spatial learning tasks [57–60]. Notably, in some cases, these differences in learning ability do not exist prior to dominance acquisition but can emerge following hierarchy formation suggesting that they are responses to the social environment (e.g. mice [60], pheasants (Phasianus colchicus) [58]). However, other studies have found inverse relationships between measures of learning and social status. This may occur when dominants experience stress-induced impairments in cognition. For example, high-ranking crab-eating macaques (Macaca fascicularis), who experience chronic stress while maintaining rank perform more poorly than lower ranked individuals on a series of complex problem-solving tasks requiring reversal learning [61]. Although there is increasing evidence for the association between social and spatial learning and dominance rank, more work is required to determine which other domains of cognition are preferentially used by dominant versus subordinate animals, how consistent these patterns are across species, and whether these changes occur prior to rank acquisition or as a consequence of social status.
(d) . Dominance and reproductive plasticity
Social dominance influences reproduction at behavioural and physiological levels, with both dominant males and females typically experiencing reproductive advantages. In females, the major fitness benefit for dominants are increased reproductive rates and survival, though dominant females can also gain such advantages through monopolization of resources, higher energy intake, earlier timing of conception, longer lifespan, more helpers with parental care, more copulations and stronger affiliative relationships with males, as well as by suppressing reproduction in subordinate females (see §3c) [62]. In males, dominant individuals often disproportionately sire large proportions of offspring in social groups achieving this through increased copulations with receptive females via behavioural strategies such as mate-guarding, intimidation of rivals and coercive mating. For example, 91% of Verreaux's sifakas (Propithecus verreauxi) [63], 60% of mountain gorillas (Gorilla beringei beringei) [64] and 34% of baboon (Papio cynocephalus) [65] offspring can belong to the most dominant alpha male. Even in cooperative breeding species, where male reproductive success is dependent on female choice and cooperation and subordinate males sire higher proportions of offspring, dominant males still experience reproductive benefits [62].
In some species, social status variation in reproductive behaviour is tightly regulated by the hypothalamic–pituitary–gonadal (HPG) axis. In dominant male African cichlids, elevated expression of gonadotropin mRNA in the medial preoptic area of the hypothalamus and circulating luteinizing hormone (LH) and follicle-stimulating hormone (FSH) activates the HPG axis leading to increased testes growth and release of testosterone [5]. In other species, periods of intense HPG activation by dominant individuals may be restricted to periods of increased inter-male competition such as breeding seasons. There is also evidence in some species that elevated androgens facilitate reproduction in dominant males even outside of periods of intense competition [22]. Reproductive plasticity in dominant males may also be expressed through sperm competition rather than behavioural conflict. Dominant males of several species have increased rates of spermatogenesis, higher sperm motility, larger ejaculate and larger seminal vesicle size. These changes are driven in part by upregulation of the HPG axis and higher levels of testosterone in dominant males [66–68]. Recent findings also suggest that changes in the seminal fluid proteome may also account for the increased reproductive competitiveness of dominant males in some species. For example, the seminal fluid of territorial black goby (Gobius niger) males enhances the velocity of their sperm [69]. In house mice, dominant males have higher levels of seminal vesicles, sperm production and ejaculate sizes but lower levels of protein and altered protein composition in their seminal fluid compared to subordinates [68] (figure 2). Changes to seminal fluid protein may reflect changes made by subordinate animals to adjust the efficiency of post-copulatory mating plugs to compensate for other sperm competition inequalities.
(e) . Energy regulation and metabolism
Acquiring and maintaining dominant status through expressing dominance behaviours (e.g. aggression, mate-guarding and territory defence) and status signals as well as increased reproductive output can be associated with significant increases in metabolism and energetic demands. Several studies have found status-induced variation in non-invasive physiological markers suggesting that dominants may experience generalized increased energetic costs. For example, dominant male chimpanzees excrete lower levels of C-peptide, a non-metabolized byproduct of insulin production, demonstrating that dominant animals have negative energy balance and higher energy demands [70]. Similarly, dominant male rhesus macaques have elevated levels of C-peptide during periods of food abundance and low competition, but reduced C-peptide levels following periods of reproductive competition compared to lower ranking males [71]. To meet these energetic needs, dominant animals can exhibit several behavioural and physiological changes that adjust energy intake and expenditure. In many species, dominant animals have priority access to food resources, enabling them to increase energy intake, eat higher quality foods and expend less energy in acquiring food [72,73]. Increasing basal or standard metabolic rates is another change that supports the elevated maintenance costs associated with energetically demanding behaviours. Indeed, high metabolic rates are associated with increased peripheral tissue size, elevated reproductive output, physical activity and intraspecific competition as experienced by dominant animals and dominant individuals are commonly reported to have higher metabolic rates than subordinates in fish, mammals and birds [74–76]. Importantly, these findings are largely associative and elevated metabolic rates may be a cause or a consequence of higher social status across species. More data are required from across species to determine under which contexts dominance is more strongly associated with increased energetic demands, and which strategy is used to offset these demands.
Studies that investigate tissue-specific metabolic changes may be able to better inform the dominant and subordinate individuals' contrasting energy needs. For example, shortly after hierarchy establishment in female rhesus macaques, dominant individuals were found to have higher serum concentrations of thyroid hormone T3 (triiodothyrnonine) which increases lipid metabolism and protein degradation and increases metabolic rate [77]. Following group formation, dominant male mice have increased hepatic expression of genes that promote fatty acid catabolism and energy production compared to subordinate males who show increases in fatty acid synthesis suggesting a trade-off between dominant and subordinates in energy usage [78] (figure 2). Dominant rainbow trout (Oncorhynchus mykiss) show increased AMP-activated protein kinase (AMPK) activity in skeletal muscle enhancing glucose uptake and utilization, lipid metabolism and fatty acid oxidation to meet the energetic demands of physical activity associated with territorial patrolling and aggression [79]. Conversely, subordinate rainbow trout have elevated AMPK activity in the liver, likely to support the energetic demands of chronic stress and the greatly reduced food intake experienced by subordinates. These examples illustrate the need for the coordination of multiple biological systems to support the behavioural and physiological demands of dominant and subordinate social status. Future work should aim to clarify under which contexts social status induces tissue-specific versus universal changes in those physiological pathways that support meeting the energetic requirements of dominance versus subordination.
(f) . Health effects of social dominance
Dominant social status is associated with fitness benefits, including priority access to resources, increased reproduction and increased lifespans in many species [80]. However, the behavioural and physiological plasticity that occurs to promote and maintain dominance can also have significant costs that negatively impact health outcomes. In particular, although dominant individuals tend to show reduced glucocorticoid levels (GCs) and improved hypothalamic–pituitary–adrenal (HPA) negative feedback in stable social hierarchies [81–83], dominant individuals can have higher GC levels than subordinates in hierarchies during periods of intense male–male competition. In some species, this may occur during breeding seasons [84] or changes in group membership [85], whereas in other species, these high levels of male competition may be ongoing [86]. Similar relationships between GCs and social status are also observed in females of some species during periods of intersex competition but to a considerably lesser extent than in males [82,87]. For example, female chacma baboons show no evidence of a relationship between rank and GCs under usual stable conditions; however, when an immigrant male ascends to alpha status, females show increases in GCs especially if their social rank is threatened [88]. In the short-term, such elevations in GCs can allow dominant animals to quickly mobilize energy in peripheral organs to support costly dominance maintenance behaviours. However, there is the potential that chronic elevations in GCs can result in increased risks of peripheral tissue damage leading to pathologies including cardiovascular disease, metabolic syndrome and susceptibility to infections [89]. For example, male-dominant cynomolgus macaques (Macaca fascicularis) exhibit hypercortisolemia and develop higher levels of coronary artery stenosis compared to subordinates, but only when social hierarchies are unstable and there is high male–male competition [90].
Chronic elevated GCs and elevated energetic demands can have broad consequences for behavioural and biological outcomes. The immune system is sensitive to GC levels and there is evidence that dominant individuals develop compromised immune systems if they trade-off energy from immune processes towards higher priority activities directly related to fitness [80,91,92]. For example, male jungle fowl (Gallus gallus) that invest heavily in sexual signals to attract females show lower levels of circulating lymphocytes [93]. Dominant individuals can also exhibit signs of chronic arousal likely related to the vigilance needed to maintain social status. High-ranking individuals have been shown to have reduced levels of deep sleep and increased sleep fragmentation in mice and baboons [94,95], but the extent to which this is common across species remains to be determined. These changes may be in part mediated by altered GC functioning, but may also occur through other mechanisms. For instance, dominant male mice display upregulated sympathetic nervous system activity that likely increases behavioural and cardiac activity but also leads to elevated systolic blood pressure and eventually aortic arteriosclerosis [96]. Oxidative stress is also elevated during intense reproductive investment and territory defence exhibited by dominants across many species including house mice [97], rhesus macaques [98] and great tits (Parus major) [99]. Increased oxidative stress in the absence of appropriate antioxidative defence can lead to peripheral tissue damage and impairments in cellular signalling and immune function [100]. Further, there is evidence that some dominant animals show accelerated epigenetic ageing (e.g. meerkats (Suricata suricatta) [101], baboons [102]) illustrating the biological costs associated with the behavioural and physiological plasticity of dominant animals.
3. Plasticity in subordinate animals
(a) . Biobehavioural changes
Subordinate animals show plasticity in behavioural and physiological systems that promote short-term survival in social hierarchies and allow for long-term increases in reproductive success. Within a social hierarchy, subordinate animals must rapidly learn to yield towards dominant animals. Depending on the social context, these behaviours include fleeing from or freezing on approach by a dominant individual [8]. Subordinates may also avoid initiating contact with dominants or show subordinate postures that typically demonstrate their vulnerability and lack of threat towards dominants. There is evidence from diverse taxa that subordinate individuals monitor the behaviour of dominant individuals more frequently than those of lower ranked individuals, establishing an attention hierarchy [103–105] (see [19]). This increased vigilance enables individuals to avoid conflict with dominants and subordinates may also inhibit their aggression towards other individuals in the presence of dominants [104,105]. In species that use opportunistic mating strategies (e.g. baboons and cichlid fish), tracking the behaviour and relationships of dominant individuals enables subordinates to achieve mating success while minimizing conflicts [104,106]. Avoidance of conflict may also lead to temporal and spatial segregation of behaviour within hierarchies and impact use of desirable foraging sites [107–109]. Social withdrawal may also emerge within subordinates as an extreme strategy for conserving energy and reducing injury risk [110]. For example, subordinate female cynomolgus and rhesus macaques show increased levels of depressive-like behaviours including reduced social interaction [111]. Alternatively, subordinates may form close social relationships with dominants especially when group-living is beneficial to all individuals (e.g. for group defence, cooperative foraging, alloparenting) [112]. For example, in many male and female non-human primates, subordinates are more tolerated when accessing resources if they have established long-term relationships with dominants [113,114].
Behavioural changes exhibited by subordinate individuals to avoid conflict and enhance survival are supported by physiological and neurobiological changes. Short-term increases in GCs in response to social stressors such as the presence of a dominant individual can redirect energy to the brain and skeletal muscle to facilitate behaviours such as vigilance, freezing and fleeing that enable the avoidance of threatening stimuli [115]. Changes to central gene expression can also support subordinate behaviours. For example, in zebrafish (Danio rerio) and rainbow trout, altered dopaminergic and serotonergic activity in several brain regions including the optic tectum, that regulates multimodal sensory integration and escape behaviour, is associated with the behavioural inhibition exhibited by subordinate fish [116,117]. Data from fish and rodents demonstrate that specific neural circuits are activated during the expression of subordinate behaviours such as freezing and escaping and are especially sensitive to social threats in subordinate individuals ([10,118], also see [19]). Social withdrawal and depressive-like behaviours are associated with the central reduction of the serotonin 5-HT1A receptor particularly in the hippocampus and hypothalamus [111,119].
(b) . Signalling subordinate social status
Communicating submissive status to dominant opponents can be advantageous in preemptively avoiding aggressive encounters. Although not as prevalent as signals of dominance, subordination can be signalled through chemical, visual or auditory subordinate-specific cues. For example, subordinate Verreaux's sifaka uses a chatter vocalization to demonstrate their submission to dominants [120]. Socially subordinate Arctic charr (Salvelinus alpinus) display a darker body colour than dominant fish that serves as a social signal to avoid receiving aggression. This increased pigmentation is related to plasma levels of α-melanocyte-stimulating hormone (αMSH) which are positively associated with central noradrenergic activity induced by the social stress experienced by subordinates [121]. How common such mechanisms are across other species is an active area of research. Subordinates may also inhibit signals that cue dominance to prevent being attacked by dominants. For example, subordinate male mice reduce the liver expression of MUPs, preventing the excretion of MUPs in their urine [39]. Reduced water intake and central gene expression changes including upregulation of corticotropin-releasing factor (CRF) in the pontine micturition centre also lead to the inhibition of urination, further reducing the potential for conflict with more dominant individuals [42,122].
(c) . Social inhibition of reproduction
In some species, socially subordinate animals inhibit reproductive output via downregulation of the HPG axis, redirecting energy into growth and other physiological systems. Delaying reproduction through this inhibitory pathway enables individuals to potentially challenge for dominance status and reproductive opportunities in the future. For example, subordinate male cichlid fish exhibit a full HPG-mediated inhibition of reproduction, including smaller gonadotropin-releasing hormone (GnRH) and arginine vasotocin (AVT) neurons and higher expression of aromatase in the preoptic area of the hypothalamus, and reduced expression of GnRH-R1, LHβ and FSHβ in the pituitary leading to lower levels of testosterone, 11-ketotestosterone (11-KT), estradiol (E2), progesterone (P), luteinizing hormone (LH) and follicle-stimulating hormone (FSH) in blood [5]. Subordinate cichlids also have reduced levels of receptors for these hormones and have reduced spermatogenesis, sperm motility and density [5,104]. In cooperative breeders, reproduction is monopolized by dominant individuals with reproductive suppression among subordinates who assist dominant reproduction via alloparental care. The amount of alloparental care ranges from low levels in facultative cooperative breeders such as black-backed jackals (Canis mesomelas) and Arctic foxes (Vulpes lagopus), to high levels in obligate cooperative breeders like meerkats and African wild dogs (Lycaon pictus) [123]. In some species where competition for resources is high, dominant females may eject subordinates from the group (e.g. cotton-top tamarins—Saguinus oedipus) or even kill their offspring (e.g. common marmosets—Callithrix jacchus) resulting in subordinates adopting an ovulatory suppression strategy [124,125].
In cooperatively breeding males and females, reproductive suppression is sometimes associated with downregulated HPG axes but also with direct behavioural interference by dominants. For example, dominant breeding African wild dogs have higher circulating estrogen and progesterone levels compared to subordinate non-breeding females [126]. Although subordinates are able to ovulate, they rarely engage in copulation likely due to intimidation from dominants [127]. Subordinate female Ethiopian wolves (Canis simensis), dwarf mongooses (Helogale parvula) and meerkats also have lower estrogen levels than dominants [128]. In meerkats, subordinates have lower levels of LH but are still physiologically capable of reproduction [129]. However, higher levels of GCs in subordinates evicted from the social group lead to increased abortion rates and decreased conception [130]. In males, lower levels of testosterone are observed in subordinates during the mating season in African wild dogs and banded mongooses (Mungos mungo) and outside the mating season in Ethiopian wolves [131–133]. However, in grey wolves (Canis lupus) and dwarf mongooses, no status differences in steroid hormones are found and subordinate reproduction appears to be behaviourally inhibited by dominants [132]. Reproductive suppression of subordinates through behavioural mechanisms is also found among some noncooperative breeders [134]. Reduced reproductive output in species with strict dominance hierarchies can also arise through reduced access to resources [135], sexual partners [136] or alloparental helpers [137], as well as delayed sexual maturity and longer interbirth intervals [138–140].
Suppression of the HPG axis and reproduction in subordinates is typically associated with chronic elevations in GCs, which act to downregulate the activity of GnRH in the brain leading to reduced release of LH and FSH and consequently reduced testosterone and estrogen release from the gonads [141]. Support for this pathway is derived from evidence of higher levels of GCs in subordinate males and females of several species that exhibit stress-induced reproductive suppression [142]. However, GC-mediated regulation of reproduction cannot explain reproductive suppression in all species, particularly among cooperative breeders [123]. An alternate pathway is stress-induced activation of the sympathetic nervous system leading to noncyclic anovulatory ovaries [141]. Energetic suppression in subordinate females can also result in reproductive dysfunction such as the reduced likelihood of implantation [143]. Collectively, these changes may ultimately contribute to reproductive success in subordinate individuals by delaying reproduction until there are sufficient resources to support fertilization, embryogenesis and postnatal investment.
(d) . Health effects of social subordination
Short-term changes made by subordinates in response to their current social environment can have long-term effects on health. Recent explorations of the relationship between social status and immune functioning highlight the complexity of the relationship between subordinate status and specific markers of health. Generally, subordinates have been reported to have lower viral resistance and clearance, impaired cell-mediated immunity, slower wound healing and higher levels of illnesses [144,145]. The relationship between parasitism and social status varies by species, although subordinate females and dominant males generally appear to have greater parasitic loads [146]. Low social status in experimentally controlled female rhesus macaques hierarchies is associated with increased expression of genes that promote inflammation such as IL-6 and IL-1b in blood cells [92,147]. Low-status females also have increased levels of binding sites for nuclear factor kB (NFkB—a regulator of inflammation) at transcriptionally active and available regions of the genome [80,147]. Alternatively, blood cells from low-status females have reduced chromatin accessibility for a glucocorticoid receptor cofactor, AP-1, that represses NFkB and promotes anti-inflammatory responses. Although this relationship appears to hold for some species, the relationship between inflammation, disease and social status is likely to be highly context-dependent, and in some cases, dominant individuals may display increased markers of immune activation and illness [91,148].
Laboratory studies implicate altered testosterone and GCs in mediating social status-associated impairments in immune function [149] but the exact roles of these hormones in wild animals still need to be fully elucidated. It is likely that there is a complex trade-off in both dominant and subordinate animals between investing energy in reproduction, somatic growth and repair, and immune investment with the particular investment being species- and context-specific. Socially subordinate animals can experience other adverse health consequences of social stress-induced hypercortisolemia. For example, male subordinate rats (Rattus norvegicus) and female long-tailed cynomolgus macaques develop increased visceral adiposity and evidence of metabolic syndrome [150]. Female subordinate cynomolgus monkeys also suffer from elevated rates of coronary artery atherosclerosis which is in part a consequence of their reduced circulating levels of atherosclerosis suppressing ovulatory estrogen [90]. These examples demonstrate how short-term plasticity in stress physiology can result in longer term detrimental health outcomes.
4. Social transitions
Within group-living organisms, individuals rarely maintain the same social rank over their entire lifetime. Individuals can ascend and descend in social status due to multiple factors including maturation, immigration/emigration, or changes in the social or ecological environment. Transitions in social rank can be dramatic or gradual, with accompanying behavioural and physiological changes occurring over multiple timescales (also see [151]). In this section, we consider the dynamic behavioural, neurobiological and physiological changes that occur during social ascent and descent.
(a) . Social ascent
Attaining higher social rank has significant growth, survival and reproductive advantages, meaning that individuals strive to socially ascend. Social ascension can happen gradually as animals mature but can also happen suddenly if more dominant individuals die, rapidly lose resource-holding power or leave a social group. This form of social ascent requires animals to constantly monitor their social environment leading to plastic shifts in neural circuits, physiology and behaviour when these social opportunities arise. One well-studied example of social ascent occurs among subordinate African cichlid fish males following experimental removal of alpha individuals from the social hierarchy (figure 3). Following removal of the dominant male, subordinate males rapidly decrease their submissive behaviour, increase aggression towards other males, display eye bars and brighter body coloration and exhibit elevations in steroid hormones (testosterone, estradiol and progestin) [5,104]. After 6 h of social ascension, these males transition from displaying bursts of aggression to being able to flexibly switch between aggressive and reproductive behaviour similar to established dominant males [152]. Within the brain, the social accent is associated with rapid activation of immediate early genes (IEGs) and increased mRNA levels of steroid hormone receptors and corticotropin-releasing factor (CRF) throughout the social behaviour brain network [153]. Changes in protein levels, neural circuitry and morphology that maintain the dominance phenotype occur over more extended periods. Ascending males have elevated pituitary mRNA and circulating levels of LH and FSH hormones within 30 min of ascent [5] and within 24 h, testes of ascenders show increased mRNA of receptors for FSH, sex steroids and GCs and increased sperm motility [154]. These findings demonstrate that social ascension can lead to dynamic physiological plasticity of the HPG axis that enables subordinate males to become reproductively active dominants.
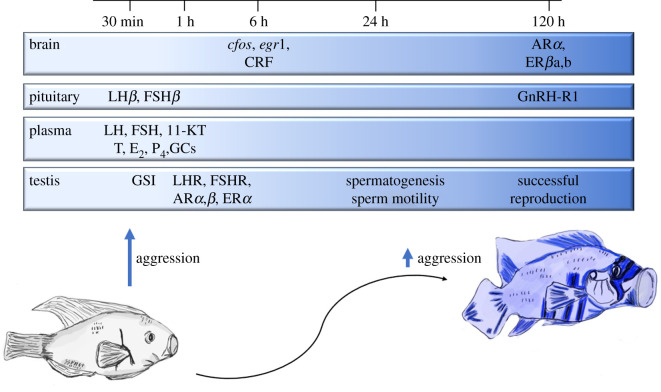
Figure 3.
Rapid physiological changes as subordinate African cichlids ascend to dominant social status. When a power vacuum emerges, subordinate males increase aggression levels towards other males within minutes. This behavioural change is supported by a rapid upregulation of the HPG axis, leading to the release of steroid hormones that facilitate aggressive behaviour. Further transition to dominance is supported via shifts in the central gene expression of immediate early and steroid hormone receptors. As social ascent is established, physical changes in coloration signal dominance status to others and males increase investment in testes size and weight, sperm motility and spermatogenesis. After dominance is established, males increase their rates of reproduction and decrease rates of aggression. (Abbreviations: CRF, corticotropin-releasing factor; ARα, androgen receptor alpha; ARβ, androgen receptor beta; ERα, estrogen receptor alpha; ERβa, estrogen receptor beta-a; ERβb, estrogen receptor beta-b; LH, luteinizing hormone; FSH, follicle-stimulating hormone; LHβ, luteinizing hormone beta; FSHβ, follicle-stimulating hormone beta; 11-KT, 11-ketotestosterone; T, testosterone; E2, estradiol; P4, progesterone; GCs, glucocorticoids; GnRH-R1, gonadotropin-releasing hormone receptor-1; GSI, gonadosomatic index; LHR, luteinizing hormone receptor; FSHR, follicle-stimulating hormone receptor).
Dynamic shifts in behaviour and physiology during social ascent are also observed in other species where reproduction is not as tightly coupled with HPG activity. When the most dominant alpha male is removed from a mouse social hierarchy, all other males increase their aggressive behaviour within minutes, and the sub-dominant beta male emerges as the new alpha male. Increased expression of medial preoptic area GnRH mRNA is also observed in both ascending sub-dominant and non-ascending subordinate males 60 min after the onset of this social opportunity, but this does not lead to an increase in circulating testosterone in subordinates [155]. Social ascension likely activates other neuroendocrine systems such as the sympathoadrenal system as evidenced by the significant rise in blood pressure of socially ascending male mice [96]. Ascending males also exhibit increased activity of IEGs throughout the social brain network (SBN) as well as dramatically elevated levels of gene activation in the prelimbic and infralimbic (prefrontal) cortices [156]. Similar to cichlids, sub-dominant mice are actively monitoring their social environment and are ready to rapidly respond to changes in social context.
In other species, social ascent by subordinates during a social opportunity may not involve increases in aggressive behaviour. In vervet monkeys, after removal of the alpha male from a group, subordinates rapidly increase their affiliative behaviour towards high-ranked females and their offspring but do not change their rates of aggression [157]. Support of these high-ranking females facilitates rank acquisition [158]. Females protect bonded males from receiving aggression from other males and direct aggression towards unbonded low-ranking males. The rates of affiliation shown by socially ascending subordinate males are far higher than those exhibited by established alpha males, indicating that the behaviours required to achieve rank are not necessarily those used to maintain high status.
Social rank takeovers, whereby sub-dominant individuals challenge and overthrow dominant individuals, are another example of social ascent. In attribute-based hierarchies, these challenges will likely be successful once individuals surpass dominants in fitness or fighting ability. Successful rank challenges can also occur via coalitionary alliances as seen in chimpanzees [159,160], Assamese macaques (Macaca assamensis) [161], rhesus macaques [162] and African wild dogs [163]. Social ascent via takeover is also fostered through strong same-sex (e.g. hyenas [4]) and between-sex alliances (e.g. white-faced capuchins (Cebus capucinus) [164] and vervet monkeys [158]). Although there are varying preceding social contexts to takeovers, rises in testosterone prior to takeover have been observed in male gelada, mandrills, chacma and yellow baboons (Papio cynocephalus anubis) [165–168]. As with social ascent in cichlid fish, other more costly phenotypic changes occur only after successful status transition and may be uncoupled from hormonal status during ascent. Male gelada red-chest patches and mandrill face colorations become redder only after acquiring dominant status [50,169,170]. It remains to be determined how consistent the role of testosterone is in promoting rapid rank acquisition across other species, or what mechanisms mediate social ascent in species that do not use aggression to ascend.
(b) . Social descent
When animals are no longer able to incur the physiological and energetic costs associated with maintaining dominance, they may be susceptible to losing social status. This may occur due to loss of the ability to physically dominate subordinates, maintain territory, successfully mate-guard or loss of coalitionary support of peers. In chimpanzees of the Tai forest, male dominance status peaks around 25 years and shows a slow decline after 35 years [171]. In other hierarchies, social descent from alpha status can be rapid and dramatic. In such cases, former alphas can quickly show behavioural and physiological changes including no longer having mating opportunities, becoming more socially isolated, ceasing to exhibit dominance displays and signals, and suffering negative health consequences including potential death [164,165,172]. For example, deposed male mandrills show reduced body mass, testicular volume, reduced red sexual skin coloration, lowered sternal gland activity and reduced social association with their group [169]. Anole lizards that lose status have reduced levels of circulating androgens, show darker brown skin coloration, are unable to secure desirable perch sites and have dramatically reduced courtship rates [173]. In cichlid fish, dominants that are socially defeated rapidly lose eye bar and body coloration, increase cortisol levels, show submissive behaviour and show increased expression of IEGs throughout the SBN [174]. Notably, these neuronal transcriptional changes are distinguishable from those that occur during social ascent indicating that distinct neural pathways are activated during social descent versus ascent to promote contextually appropriate changes in social behaviour. Finally, phenotypic and physiological changes that occur during social descent may actually be adaptive responses in some circumstances. For example, a decline in social rank by cichlid fish can lead to reinvestment of energy into growth allowing individuals to eventually be capable of overtaking a territory and socially ascending [175]. Such reversible phenotypic plasticity enables animals to rise and fall in rank within a hierarchy multiple times depending upon their current and future reproductive potential. Much remains to be understood regarding the physiological and neurobiological changes that accompany social descent, especially in species where social descent may be transient and high social status can be re-attained.
5. Conclusion
As individuals acquire dominant or subordinate status, a multitude of phenotypic changes occurs that enable animals to adjust to their social niche. These changes go beyond variation in aggression or subordinate behaviour and include plasticity in social signals, reproductive and other social behaviours, cognition, feeding and drinking, arousal and sleep cycles and are supported by shifts in energy metabolism. These phenotypic shifts are coordinated by the brain, being induced by shifts in the expression of neuropeptides, neurotransmitters, hormones and their receptors across several neural circuits leading to integrated modifications of multiple peripheral tissues and systems.
There do remain several outstanding questions regarding this plasticity. It is important to note that these findings are generalized patterns from across species. Clearly, not all dominant or subordinate animals change in response to their social environment in the same way. It is important to consider how such plasticity varies by species, sex, ecological and social contexts. Further, although there is a great deal of research into how social status shapes domains such as stress responsivity, reproduction and aggressive behaviour, much still remains to be understood about how dynamic shifts in other physiological processes and behaviours including cognition, sleep and energy metabolism manifest themselves. Much work has identified common pathways that are modulated by social status including the HPA, HPG and sympathetic nervous systems. Future studies, particularly at the molecular and genomic level, will help further delineate how variations in social status differentially impact these systems but also other peripheral tissues and how these changes are centrally coordinated and synchronized. Another common issue with many studies is identifying whether such changes are a cause versus consequence of social status. Experimental studies of dominance hierarchies can help resolve these questions, as can repeat sampling from field studies that are able to track changes in social status over the long term.
Several studies have also elegantly demonstrated plasticity in these systems during periods of social instability, particularly during social ascent. In particular, findings from the African cichlid fish have revealed the time course of changes from genomic activation to neural systems to behavioural and morphological, with relatively energetically cheaper and flexible modifications occurring first supporting energetically costly changes that occur later. Whether other species whose behaviour are not as tightly regulated by the HPG axis show similar mechanistic changes, or if they achieve social ascent through modifications of other neural and endocrine systems remains to be determined. Finally, very little research to date has focused on changes that occur during social descent. Understanding this plasticity is a topic needing further exploration.
Data accessibility
This article has no additional data.
Authors' contributions
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Drews C. 1993. The concept and definition of dominance in animal behaviour. Behaviour 125, 283-313. ( 10.1163/156853993X00290) [DOI] [Google Scholar]
- 2.Chase ID. 1982. Dynamics of hierarchy formation: the sequential development of dominance relationships. Behaviour 80, 218-239. ( 10.1163/156853982X00364) [DOI] [Google Scholar]
- 3.Schjelderup-Ebbe T. 1922. Beiträge zur sozialpsychologie des haushuhns. Z. Psychol. Physiol. Sinnesorgane 1 Z. Psychol. 88, 225-252. [Google Scholar]
- 4.Strauss ED, Holekamp KE. 2019. Social alliances improve rank and fitness in convention-based societies. Proc. Natl Acad. Sci. USA 116, 8919-8924. ( 10.1073/pnas.1810384116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maruska KP. 2014. Social regulation of reproduction in male cichlid fishes. Gen. Comp. Endocrinol. 207, 2-12. ( 10.1016/j.ygcen.2014.04.038) [DOI] [PubMed] [Google Scholar]
- 6.Lee W, Fu J, Bouwman N, Farago P, Curley JP. 2019. Temporal microstructure of dyadic social behavior during relationship formation in mice. PLoS ONE 14, e0220596. ( 10.1371/journal.pone.0220596) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunbar RIM. 1982. Structure of social relationships in a captive gelada group: a test of some hypotheses derived from studies of a wild population. Primates 23, 89-94. ( 10.1007/BF02381440) [DOI] [Google Scholar]
- 8.Dehnen T, Papageorgiou D, Nyaguthii B, Cherono W, Penndorf J, Boogert N, Farine D. 2021. Costs dictate strategic investment in dominance interactions. Phil. Trans. R. Soc. B 377, 20200447. ( 10.1098/rstb.2020.0447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lischinsky JE, Lin D. 2020. Neural mechanisms of aggression across species. Nat. Neurosci. 23, 1317-1328. ( 10.1038/s41593-020-00715-2) [DOI] [PubMed] [Google Scholar]
- 10.Zhou T, Sandi C, Hu H. 2018. Advances in understanding neural mechanisms of social dominance. Curr. Opin. Neurobiol. 49, 99-107. ( 10.1016/j.conb.2018.01.006) [DOI] [PubMed] [Google Scholar]
- 11.Lee W, Hiura LC, Yang E, Broekman KA, Ophir AG, Curley JP. 2019. Social status in mouse social hierarchies is associated with variation in oxytocin and vasopressin 1a receptor densities. Horm. Behav. 114, 104551. ( 10.1016/j.yhbeh.2019.06.015) [DOI] [PubMed] [Google Scholar]
- 12.Morgan D, et al. 2002. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat. Neurosci. 5, 169-174. ( 10.1038/nn798) [DOI] [PubMed] [Google Scholar]
- 13.Grieb ZA, et al. 2021. Sex-dependent effects of social status on the regulation of arginine-vasopressin (AVP) V1a, oxytocin (OT), and serotonin (5-HT) 1A receptor binding and aggression in Syrian hamsters (Mesocricetus auratus). Horm. Behav. 127, 104878. ( 10.1016/j.yhbeh.2020.104878) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caramaschi D, de Boer SF, Koolhaas JM. 2007. Differential role of the 5-HT1A receptor in aggressive and non-aggressive mice: an across-strain comparison. Physiol. Behav. 90, 590-601. ( 10.1016/j.physbeh.2006.11.010) [DOI] [PubMed] [Google Scholar]
- 15.Raleigh MJ, McGuire MT, Brammer GL, Yuwiler A. 1984. Social and environmental influences on blood serotonin concentrations in monkeys. Arch. Gen. Psychiatry 41, 405-410. ( 10.1001/archpsyc.1984.01790150095013) [DOI] [PubMed] [Google Scholar]
- 16.Raleigh MJ, McGuire MT, Brammer GL, Pollack DB, Yuwiler A. 1991. Serotonergic mechanisms promote dominance acquisition in adult male vervet monkeys. Brain Res. 559, 181-190. ( 10.1016/0006-8993(91)90001-C) [DOI] [PubMed] [Google Scholar]
- 17.Michopoulos V, Checchi M, Sharpe D, Wilson ME. 2011. Estradiol effects on behavior and serum oxytocin are modified by social status and polymorphisms in the serotonin transporter gene in female rhesus monkeys. Horm. Behav. 59, 528-535. ( 10.1016/j.yhbeh.2011.02.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Almeida O, Gozdowska M, Kulczykowska E, Oliveira RF. 2012. Brain levels of arginine–vasotocin and isotocin in dominant and subordinate males of a cichlid fish. Horm. Behav. 61, 212-217. ( 10.1016/j.yhbeh.2011.12.008) [DOI] [PubMed] [Google Scholar]
- 19.Dwortz MF, Curley JP, Tye KM, Padilla-Coreano N. 2021. Neural systems that facilitate the representation of social rank. Phil. Trans. R. Soc. B 377, 20200444. ( 10.1098/rstb.2020.0444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jupp B, Murray JE, Jordan ER, Xia J, Fluharty M, Shrestha S, Robbins TW, Dalley JW. 2016. Social dominance in rats: effects on cocaine self-administration, novelty reactivity and dopamine receptor binding and content in the striatum. Psychopharmacology 233, 579-589. ( 10.1007/s00213-015-4122-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golden SA, Jin M, Shaham Y. 2019. Animal models of (or for) aggression reward, addiction, and relapse: behavior and circuits. J. Neurosci. 39, 3996-4008. ( 10.1523/JNEUROSCI.0151-19.2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamilton LD, Carré JM, Mehta PH, Olmstead N, Whitaker JD. 2015. Social neuroendocrinology of status: a review and future directions. Adapt. Hum. Behav. Physiol. 1, 202-230. ( 10.1007/s40750-015-0025-5) [DOI] [Google Scholar]
- 23.Fuxjager MJ, Montgomery JL, Marler CA. 2011. Species differences in the winner effect disappear in response to post-victory testosterone manipulations. Proc. R. Soc. B. 278, 3497-3503. ( 10.1098/rspb.2011.0301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oliveira RF, Silva A, Canário AVM. 2009. Why do winners keep winning? Androgen mediation of winner but not loser effects in cichlid fish. Proc. R. Soc. B. 276, 2249-2256. ( 10.1098/rspb.2009.0132) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muller MN. 2017. Testosterone and reproductive effort in male primates. Horm. Behav. 91, 36-51. ( 10.1016/j.yhbeh.2016.09.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnston SD, Ward D, Lemon J, Gunn I, MacCallum CA, Keeley T, Blyde D. 2007. Studies of male reproduction in captive African wild dogs (Lycaon pictus). Anim. Reprod. Sci. 100, 338-355. ( 10.1016/j.anireprosci.2006.08.017) [DOI] [PubMed] [Google Scholar]
- 27.Williamson CM, Lee W, Romeo RD, Curley JP. 2017. Social context-dependent relationships between mouse dominance rank and plasma hormone levels. Physiol. Behav. 171, 110-119. ( 10.1016/j.physbeh.2016.12.038) [DOI] [PubMed] [Google Scholar]
- 28.Rincon AV, Maréchal L, Semple S, Majolo B, MacLarnon A. 2017. Correlates of androgens in wild male Barbary macaques: testing the challenge hypothesis. Am. J. Primatol. 79, e22689. ( 10.1002/ajp.22689) [DOI] [PubMed] [Google Scholar]
- 29.von Engelhardt N, Kappeler PM, Heistermann M. 2000. Androgen levels and female social dominance in Lemur catta. Proc. R. Soc. Lond. Ser. B 267, 1533-1539. ( 10.1098/rspb.2000.1175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glickman SE, Cunha GR, Drea CM, Conley AJ, Place NJ. 2006. Mammalian sexual differentiation: lessons from the spotted hyena. Trends Endocrinol. Metab. 17, 349-356. ( 10.1016/j.tem.2006.09.005) [DOI] [PubMed] [Google Scholar]
- 31.Beehner JC, Phillips-Conroy JE, Whitten PL. 2005. Female testosterone, dominance rank, and aggression in an Ethiopian population of hybrid baboons. Am. J. Primatol. 67, 101-119. ( 10.1002/ajp.20172) [DOI] [PubMed] [Google Scholar]
- 32.Fernald RD. 2014. Communication about social status. Curr. Opin. Neurobiol. 28, 1-4. ( 10.1016/j.conb.2014.04.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tibbetts EA, Pardo-Sanchez J, Weise C. 2021. The establishment and maintenance of dominance hierarchies. Phil. Trans. R. Soc. B 377, 20200450. ( 10.1098/rstb.2020.0450) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keller L, Nonacs P. 1993. The role of queen pheromones in social insects: queen control or queen signal? Anim. Behav. 45, 787-794. ( 10.1006/anbe.1993.1092) [DOI] [Google Scholar]
- 35.Eisenberg J, Kleiman D. 1972. Olfactory communication in mammals. Annu. Rev. Ecol. Systemat. 3, 1-32. ( 10.1146/ANNUREV.ES.03.110172.000245) [DOI] [Google Scholar]
- 36.Setchell JM, Vaglio S, Moggi-Cecchi J, Boscaro F, Calamai L, Knapp LA. 2010. Chemical composition of scent-gland secretions in an Old World monkey (Mandrillus sphinx): influence of sex, male status, and individual identity. Chem. Senses 35, 205-220. ( 10.1093/chemse/bjp105) [DOI] [PubMed] [Google Scholar]
- 37.Rajagopal T, Archunan G, Geraldine P, Balasundaram C. 2010. Assessment of dominance hierarchy through urine scent marking and its chemical constituents in male blackbuck Antelope cervicapra, a critically endangered species. Behav. Processes 85, 58-67. ( 10.1016/j.beproc.2010.06.007) [DOI] [PubMed] [Google Scholar]
- 38.Hayes RA, Richardson BJ, Wyllie SG. 2003. To fix or not to fix: the role of 2-phenoxyethanol in rabbit, Oryctolagus cuniculus, chin gland secretion. J. Chem. Ecol. 29, 1051-1064. ( 10.1023/a:1023836319677) [DOI] [PubMed] [Google Scholar]
- 39.Lee W, Khan A, Curley JP. 2017. Major urinary protein levels are associated with social status and context in mouse social hierarchies. Proc. R. Soc. B 284, 20171570. ( 10.1098/rspb.2017.1570) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaw PH, Held WA, Hastie ND. 1983. The gene family for major urinary proteins: expression in several secretory tissues of the mouse. Cell 32, 755-761. ( 10.1016/0092-8674(83)90061-2) [DOI] [PubMed] [Google Scholar]
- 41.Nelson AC, Cunningham CB, Ruff JS, Potts WK. 2015. Protein pheromone expression levels predict and respond to the formation of social dominance networks. J. Evol. Biol. 28, 1213-1224. ( 10.1111/jeb.12643) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee W, Yang E, Curley JP. 2018. Foraging dynamics are associated with social status and context in mouse social hierarchies. PeerJ 6, e5617. ( 10.7717/peerj.5617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keller-Costa T, Lopes OS, Almeida O, Hubbard PC, Iacovella A, Lima M, Barata EN, Canário AVM. 2012. Muscular hypertrophy of urinary bladders in dominant tilapia facilitates the control of aggression through urinary signals. Behaviour 149, 953-975. ( 10.1163/1568539X-00003023) [DOI] [Google Scholar]
- 44.Kitchen DM, Cheney DL, Engh AL, Fischer J, Moscovice LR, Seyfarth RM. 2013. Male baboon responses to experimental manipulations of loud ‘wahoo calls’: testing an honest signal of fighting ability. Behav. Ecol. Sociobiol. 67, 1825-1835. ( 10.1007/s00265-013-1592-8) [DOI] [Google Scholar]
- 45.Vannoni E, McElligott AG. 2008. Low frequency groans indicate larger and more dominant fallow deer (Dama dama) males. PLoS ONE 3, e3113. ( 10.1371/journal.pone.0003113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pasch B, George AS, Hamlin HJ, Guillette LJ Jr, Phelps SM. 2011. Androgens modulate song effort and aggression in neotropical singing mice. Horm. Behav. 59, 90-97. ( 10.1016/j.yhbeh.2010.10.011) [DOI] [PubMed] [Google Scholar]
- 47.Fedurek P, Slocombe KE, Enigk DK, Emery Thompson M, Wrangham RW, Muller MN. 2016. The relationship between testosterone and long-distance calling in wild male chimpanzees. Behav. Ecol. Sociobiol. 70, 659-672. ( 10.1007/s00265-016-2087-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Korzan WJ, Summers TR, Summers CH. 2002. Manipulation of visual sympathetic sign stimulus modifies social status and plasma catecholamines. Gen. Comp. Endocrinol. 128, 153-161. ( 10.1016/S0016-6480(02)00077-1) [DOI] [PubMed] [Google Scholar]
- 49.Setchell JM, Smith T, Wickings EJ, Knapp LA. 2008. Social correlates of testosterone and ornamentation in male mandrills. Horm. Behav. 54, 365-372. ( 10.1016/j.yhbeh.2008.05.004) [DOI] [PubMed] [Google Scholar]
- 50.Bergman TJ, Ho L, Beehner JC. 2009. Chest color and social status in male geladas (Theropithecus gelada). Int. J. Primatol. 30, 791-806. ( 10.1007/s10764-009-9374-x) [DOI] [Google Scholar]
- 51.Gerald MS, McGuire MT. 2007. Secondary sexual coloration and CSF 5-HIAA are correlated in vervet monkeys (Cercopithecus aethiops sabaeus). J. Med. Primatol. 36, 348-354. ( 10.1111/j.1600-0684.2007.00227.x) [DOI] [PubMed] [Google Scholar]
- 52.Nicol CJ, Pope SJ. 1999. The effects of demonstrator social status and prior foraging success on social learning in laying hens. Anim. Behav. 57, 163-171. ( 10.1006/anbe.1998.0920) [DOI] [PubMed] [Google Scholar]
- 53.Tibbetts EA, Injaian A, Sheehan MJ, Desjardins N. 2018. Intraspecific variation in learning: worker wasps are less able to learn and remember individual conspecific faces than queen wasps. Am. Nat. 191, 595-603. ( 10.1086/696848) [DOI] [PubMed] [Google Scholar]
- 54.Holtmann B, Buskas J, Steele M, Solokovskis K, Wolf JBW. 2019. Dominance relationships and coalitionary aggression against conspecifics in female carrion crows. Sci. Rep. 9, 15922. ( 10.1038/s41598-019-52177-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kendal R, Hopper LM, Whiten A, Brosnan SF, Lambeth SP, Schapiro SJ, Hoppitt W. 2015. Chimpanzees copy dominant and knowledgeable individuals: implications for cultural diversity. Evol. Hum. Behav. 36, 65-72. ( 10.1016/j.evolhumbehav.2014.09.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pongrácz P, Vida V, Bánhegyi P, Miklósi Á. 2008. How does dominance rank status affect individual and social learning performance in the dog (Canis familiaris)? Anim. Cogn. 11, 75-82. ( 10.1007/s10071-007-0090-7) [DOI] [PubMed] [Google Scholar]
- 57.Boogert NJ, Reader SM, Laland KN. 2006. The relation between social rank, neophobia and individual learning in starlings. Anim. Behav. 72, 1229-1239. ( 10.1016/j.anbehav.2006.02.021) [DOI] [Google Scholar]
- 58.Langley EJG, Horik JO, Whiteside MA, Beardsworth CE, Madden JR. 2018. The relationship between social rank and spatial learning in pheasants, Phasianus colchicus: cause or consequence? PeerJ 6, e5738. ( 10.7717/peerj.5738) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spritzer MD, Meikle DB, Solomon NG. 2004. The relationship between dominance rank and spatial ability among male meadow voles (Microtus pennsylvanicus). J. Comp. Psychol. 118, 332-339. ( 10.1037/0735-7036.118.3.332) [DOI] [PubMed] [Google Scholar]
- 60.Barnard CJ, Luo N. 2002. Acquisition of dominance status affects maze learning in mice. Behav. Processes 60, 53-59. ( 10.1016/S0376-6357(02)00121-3) [DOI] [PubMed] [Google Scholar]
- 61.Bunnell BN, Perkins MN. 1980. Performance correlates of social behavior and organization: social rank and complex problem solving in crab-eating macaques (M. fascicularis). Primates 21, 515-523. ( 10.1007/BF02373840) [DOI] [Google Scholar]
- 62.Ellis L. 1995. Dominance and reproductive success among nonhuman animals: a cross-species comparison. Ethol. Sociobiol. 16, 257-333. ( 10.1016/0162-3095(95)00050-U) [DOI] [Google Scholar]
- 63.Kappeler PM, Schäffler L. 2008. The lemur syndrome unresolved: extreme male reproductive skew in sifakas (Propithecus verreauxi), a sexually monomorphic primate with female dominance. Behav. Ecol. Sociobiol. 62, 1007-1015. ( 10.1007/s00265-007-0528-6) [DOI] [Google Scholar]
- 64.Bradley BJ, Robbins MM, Williamson EA, Steklis HD, Steklis NG, Eckhardt N, Boesch C, Vigilant L. 2005. Mountain gorilla tug-of-war: silverbacks have limited control over reproduction in multimale groups. Proc. Natl Acad. Sci. USA 102, 9418-9423. ( 10.1073/pnas.0502019102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alberts SC, Buchan JC, Altmann J. 2006. Sexual selection in wild baboons: from mating opportunities to paternity success. Anim. Behav. 72, 1177-1196. ( 10.1016/j.anbehav.2006.05.001) [DOI] [Google Scholar]
- 66.Martinez G, Garcia C. 2020. Sexual selection and sperm diversity in primates. Mol. Cell. Endocrinol. 518, 110974. ( 10.1016/j.mce.2020.110974) [DOI] [PubMed] [Google Scholar]
- 67.Kruczek M, Styrna J. 2009. Semen quantity and quality correlate with bank vole males' social status. Behav. Processes 82, 279-285. ( 10.1016/j.beproc.2009.07.009) [DOI] [PubMed] [Google Scholar]
- 68.Bayram HL, Franco C, Brownridge P, Claydon AJ, Koch N, Hurst JL, Beynon RJ, Stockley P. 2020. Social status and ejaculate composition in the house mouse. Phil. Trans. R. Soc. B 375, 20200083. ( 10.1098/rstb.2020.0083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Poli F, Locatello L, Rasotto MB. 2018. Seminal fluid enhances competitiveness of territorial males' sperm in a fish with alternative male reproductive tactics. J. Exp. Biol. 221, 175976. ( 10.1242/jeb.175976) [DOI] [PubMed] [Google Scholar]
- 70.Emery TM, Muller MN, Wrangham RW, Lwanga JS, Potts KB. 2009. Urinary C-peptide tracks seasonal and individual variation in energy balance in wild chimpanzees. Horm. Behav. 55, 299-305. ( 10.1016/j.yhbeh.2008.11.005) [DOI] [PubMed] [Google Scholar]
- 71.Higham JP, Heistermann M, Maestripieri D. 2011. The energetics of male–male endurance rivalry in free-ranging rhesus macaques, Macaca mulatta. Anim. Behav. 81, 1001-1007. ( 10.1016/j.anbehav.2011.02.001) [DOI] [Google Scholar]
- 72.Guo S-T, et al. 2020. Male social rank and food competition in a primate multi-level society. Am. J. Phys. Anthropol. 173, 630-642. ( 10.1002/ajpa.24141) [DOI] [PubMed] [Google Scholar]
- 73.Murray CM, Mane SV, Pusey AE. 2007. Dominance rank influences female space use in wild chimpanzees, Pan troglodytes: towards an ideal despotic distribution. Anim. Behav. 74, 1795-1804. ( 10.1016/j.anbehav.2007.03.024) [DOI] [Google Scholar]
- 74.Biro PA, Stamps JA. 2010. Do consistent individual differences in metabolic rate promote consistent individual differences in behavior? Trends Ecol. Evol. 25, 653-659. ( 10.1016/j.tree.2010.08.003) [DOI] [PubMed] [Google Scholar]
- 75.Turbill C, Ruf T, Rothmann A, Arnold W. 2013. Social dominance is associated with individual differences in heart rate and energetic response to food restriction in female red deer. Physiol. Biochem. Zool. 86, 528-537. ( 10.1086/672372) [DOI] [PubMed] [Google Scholar]
- 76.Metcalfe NB, Taylor AC, Thorpe JE. 1995. Metabolic rate, social status and life-history strategies in Atlantic salmon. Anim. Behav. 49, 431-436. ( 10.1006/anbe.1995.0056) [DOI] [Google Scholar]
- 77.Jarrell H, Hoffman JB, Kaplan JR, Berga S, Kinkead B, Wilson ME. 2008. Polymorphisms in the serotonin reuptake transporter gene modify the consequences of social status on metabolic health in female rhesus monkeys. Physiol. Behav. 93, 807-819. ( 10.1016/j.physbeh.2007.11.042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee W, Milewski TM, Dwortz MF, Young RL, Gaudet AD, Fonken LK, Champagne FA, Curley JP.. 2021. Distinct inflammatory and transcriptomic profiles in dominant versus subordinate males in mouse social hierarchies. bioRxiv 458987. ( 10.1101/2021.09.04.458987) [DOI] [PubMed]
- 79.Gilmour KM, Craig PM, Dhillon RS, Lau GY, Richards JG. 2017. Regulation of energy metabolism during social interactions in rainbow trout: a role for AMP-activated protein kinase. Am. J. Physiol. Reg. Integr. Comp. Physiol. 313, R549-R559. ( 10.1152/ajpregu.00341.2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Snyder-Mackler N, et al. 2020. Social determinants of health and survival in humans and other animals. Science 368, eaax9553. ( 10.1126/science.aax9553) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abbott DH, et al. 2003. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Horm. Behav. 43, 67-82. ( 10.1016/S0018-506X(02)00037-5) [DOI] [PubMed] [Google Scholar]
- 82.Beehner JC, Bergman TJ. 2017. The next step for stress research in primates: to identify relationships between glucocorticoid secretion and fitness. Horm. Behav. 91, 68-83. ( 10.1016/j.yhbeh.2017.03.003) [DOI] [PubMed] [Google Scholar]
- 83.Cavigelli SA, Caruso MJ. 2015. Sex, social status and physiological stress in primates: the importance of social and glucocorticoid dynamics. Phil. Trans. R. Soc. B 370, 20140103. ( 10.1098/rstb.2014.0103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Muller MN, Enigk DK, Fox SA, Lucore J, Machanda ZP, Wrangham RW, Emery TM. 2021. Aggression, glucocorticoids, and the chronic costs of status competition for wild male chimpanzees. Horm. Behav. 130, 104965. ( 10.1016/j.yhbeh.2021.104965) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Setchell JM, Smith T, Wickings EJ, Knapp LA. 2010. Stress, social behaviour, and secondary sexual traits in a male primate. Horm. Behav. 58, 720-728. ( 10.1016/j.yhbeh.2010.07.004) [DOI] [PubMed] [Google Scholar]
- 86.Creel S. 2005. Dominance, aggression, and glucocorticoid levels in social carnivores. J. Mammal. 86, 255-264. ( 10.1644/BHE-002.1) [DOI] [Google Scholar]
- 87.Cavigelli SA, Dubovick T, Levash W, Jolly A, Pitts A. 2003. Female dominance status and fecal corticoids in a cooperative breeder with low reproductive skew: ring-tailed lemurs (Lemur catta). Horm. Behav. 43, 166-179. ( 10.1016/S0018-506X(02)00031-4) [DOI] [PubMed] [Google Scholar]
- 88.Engh AL, Beehner JC, Bergman TJ, Whitten PL, Hoffmeier RR, Seyfarth RM, Cheney DL. 2006. Female hierarchy instability, male immigration and infanticide increase glucocorticoid levels in female chacma baboons. Anim. Behav. 71, 1227-1237. ( 10.1016/j.anbehav.2005.11.009) [DOI] [Google Scholar]
- 89.Romero ML, Butler LK. 2007. Endocrinology of stress. Int. J. Comp. Psychol. 20, 89-95. ( 10.5070/P4202009980) [DOI] [Google Scholar]
- 90.Kaplan JR, Chen H, Manuck SB. 2009. The relationship between social status and atherosclerosis in male and female monkeys as revealed by meta-analysis. Am. J. Primatol. 71, 732-741. ( 10.1002/ajp.20707) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lea AJ, Akinyi MY, Nyakundi R, Mareri P, Nyundo F, Kariuki T, Alberts SC, Archie EA, Tung J. 2018. Dominance rank-associated gene expression is widespread, sex-specific, and a precursor to high social status in wild male baboons. Proc. Natl Acad. Sci. USA 115, E12 163-E12 171. ( 10.1073/pnas.1811967115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tung J, Barreiro LB, Johnson ZP, Hansen KD, Michopoulos V, Toufexis D, Michelini K, Wilson ME, Gilad Y. 2012. Social environment is associated with gene regulatory variation in the rhesus macaque immune system. Proc. Natl Acad. Sci. USA 109, 6490-6495. ( 10.1073/pnas.1202734109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zuk M, Johnsen TS, MacLarty T. 1995. Endocrine-immune interactions, ornaments and mate choice in red jungle fowl. Proc. R. Soc. Lond. B 260, 205-210. ( 10.1098/rspb.1995.0081) [DOI] [Google Scholar]
- 94.Karamihalev S, Flachskamm C, Eren N, Kimura M, Chen A. 2019. Social context and dominance status contribute to sleep patterns and quality in groups of freely-moving mice. Sci. Rep. 9, 15190. ( 10.1038/s41598-019-51375-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Noser R, Gygax L, Tobler I. 2003. Sleep and social status in captive gelada baboons (Theropithecus gelada). Behav. Brain Res. 147, 9-15. ( 10.1016/S0166-4328(03)00111-6) [DOI] [PubMed] [Google Scholar]
- 96.Ely DL. 1981. Hypertension, social rank, and aortic arteriosclerosis in CBA/J mice. Physiol. Behav. 26, 655-661. ( 10.1016/0031-9384(81)90140-2) [DOI] [PubMed] [Google Scholar]
- 97.Garratt M, McArdle F, Stockley P, Vasilaki A, Beynon RJ, Jackson MJ, Hurst JL. 2012. Tissue-dependent changes in oxidative damage with male reproductive effort in house mice. Funct. Ecol. 26, 423-433. ( 10.1111/j.1365-2435.2011.01952.x) [DOI] [Google Scholar]
- 98.Georgiev AV, Thompson ME, Mandalaywala TM, Maestripieri D. 2015. Oxidative stress as an indicator of the costs of reproduction among free-ranging rhesus macaques. J. Exp. Biol. 218, 1981-1985. ( 10.1242/jeb.121947) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Christe P, Glaizot O, Strepparava N, Devevey G, Fumagalli L. 2012. Twofold cost of reproduction: an increase in parental effort leads to higher malarial parasitaemia and to a decrease in resistance to oxidative stress. Proc. R. Soc. B. 279, 1142-1149. ( 10.1098/rspb.2011.1546) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bedard K, Krause KH. 2007. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 87, 245-313. ( 10.1152/physrev.00044.2005) [DOI] [PubMed] [Google Scholar]
- 101.Cram DL, Monaghan P, Gillespie R, Dantzer B, Duncan C, Spence-Jones H, Clutton-Brock T. 2018. Rank-related contrasts in longevity arise from extra-group excursions not delayed senescence in a cooperative mammal. Curr. Biol. 28, 2934-2939. ( 10.1016/j.cub.2018.07.021) [DOI] [PubMed] [Google Scholar]
- 102.Anderson JA, Johnston RA, Lea AJ, Campos FA, Voyles TN, Akinyi MY, Alberts SC, Archie EA, Tung J. 2021. High social status males experience accelerated epigenetic aging in wild baboons. eLife 10, e66128. ( 10.7554/eLife.66128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pannozzo PL, Phillips KA, Haas ME, Mintz EM. 2007. Social monitoring reflects dominance relationships in a small captive group of brown capuchin monkeys (Cebus apella). Ethology 113, 881-888. ( 10.1111/j.1439-0310.2007.01392.x) [DOI] [Google Scholar]
- 104.Desjardins JK, Hofmann HA, Fernald RD. 2012. Social context influences aggressive and courtship behavior in a cichlid fish. PLoS ONE 7, e32781. ( 10.1371/journal.pone.0032781) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Curley JP. 2016. Temporal pairwise-correlation analysis provides empirical support for attention hierarchies in mice. Biol. Lett. 12, 20160192. ( 10.1098/rsbl.2016.0192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Crockford C, Wittig RM, Seyfarth RM, Cheney DL. 2007. Baboons eavesdrop to deduce mating opportunities. Anim. Behav. 73, 885-890. ( 10.1016/j.anbehav.2006.10.016) [DOI] [Google Scholar]
- 107.Stone DB, Martin JA, Cohen BS, Prebyl TJ, Killmaster C, Miller KV. 2019. Intraspecific temporal resource partitioning at white-tailed deer feeding sites. Curr. Zool. 65, 139-146. ( 10.1093/cz/zoy051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Post DG, Hausfater G, McCuskey S. 1980. Feeding behavior of yellow baboons (Papio cynocephalus): relationship to age, gender and dominance rank. FPR 34, 170-195. ( 10.1159/000155954) [DOI] [PubMed] [Google Scholar]
- 109.David BO, Closs GP, Crow SK, Hansen EA. 2007. Is diel activity determined by social rank in a drift-feeding stream fish dominance hierarchy? Anim. Behav. 74, 259-263. ( 10.1016/j.anbehav.2006.08.015) [DOI] [Google Scholar]
- 110.Ike KGO, de Boer SF, Buwalda B, Kas MJH. 2020. Social withdrawal: an initially adaptive behavior that becomes maladaptive when expressed excessively. Neurosci. Biobehav. Rev. 116, 251-267. ( 10.1016/j.neubiorev.2020.06.030) [DOI] [PubMed] [Google Scholar]
- 111.Shively CA, Friedman DP, Gage HD, Bounds MC, Brown-Proctor C, Blair JB, Henderson JA, Smith MA, Buchheimer N. 2006. Behavioral depression and positron emission tomography–determined serotonin 1A receptor binding potential in cynomolgus monkeys. Arch. Gen. Psychiatry 63, 396-403. ( 10.1001/archpsyc.63.4.396) [DOI] [PubMed] [Google Scholar]
- 112.Silk JB. 2007. The adaptive value of sociality in mammalian groups. Phil. Trans. R. Soc. B 362, 539-559. ( 10.1098/rstb.2006.1994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Carne C, Wiper S, Semple S. 2011. Reciprocation and interchange of grooming, agonistic support, feeding tolerance, and aggression in semi-free-ranging Barbary macaques. Am. J. Primatol. 73, 1127-1133. ( 10.1002/ajp.20979) [DOI] [PubMed] [Google Scholar]
- 114.Tiddi B, Aureli F, di Sorrentino E Polizzi, Janson CH, Schino G. 2011. Grooming for tolerance? Two mechanisms of exchange in wild tufted capuchin monkeys. Behav. Ecol. 22, 663-669. ( 10.1093/beheco/arr028) [DOI] [Google Scholar]
- 115.Beery A, Holmes M, Lee W, Curley J. 2020. Stress in groups: lessons from non-traditional rodent species and housing models. Neurosci. Biobehav. Rev. 113, 354-372. ( 10.1016/j.neubiorev.2020.03.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Teles MC, Dahlbom SJ, Winberg S, Oliveira RF. 2013. Social modulation of brain monoamine levels in zebrafish. Behav. Brain Res. 253, 17-24. ( 10.1016/j.bbr.2013.07.012) [DOI] [PubMed] [Google Scholar]
- 117.Overli O, Harris CA, Winberg S. 1999. Short-term effects of fights for social dominance and the establishment of dominant-subordinate relationships on brain monoamines and cortisol in rainbow trout. Brain Behav. Evol. 54, 263-275. ( 10.1159/000006627) [DOI] [PubMed] [Google Scholar]
- 118.Miller TH, Clements K, Ahn S, Park C, Hye Ji E, Issa FA. 2017. Social status-dependent shift in neural circuit activation affects decision making. J. Neurosci. 37, 2137-2148. ( 10.1523/JNEUROSCI.1548-16.2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Michopoulos V, et al. 2014. Oestradiol alters central 5HT1A receptor binding potential differences related to psychosocial stress but not differences related to 5HTTLPR genotype in female rhesus monkeys. J. Neuroendocrinol. 26, 80-88. ( 10.1111/jne.12129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lewis RJ. 2019. Subordination signals improve the quality of social relationships in Verreaux's sifaka: implications for the evolution of power structures and social complexity. Am. J. Phys. Anthropol. 169, 599-607. ( 10.1002/ajpa.23876) [DOI] [PubMed] [Google Scholar]
- 121.Hoglund E, Balm PH, Winberg S. 2000. Skin darkening, a potential social signal in subordinate Arctic charr (Salvelinus alpinus): the regulatory role of brain monoamines and pro-opiomelanocortin-derived peptides. J. Exp. Biol. 203, 1711-1721. ( 10.1242/jeb.203.11.1711) [DOI] [PubMed] [Google Scholar]
- 122.Hou XH, et al. 2016. Central control circuit for context-dependent micturition. Cell 167, 73-86. ( 10.1016/j.cell.2016.08.073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Creel S. 2001. Social dominance and stress hormones. Trends Ecol. Evol. 16, 491-497. ( 10.1016/S0169-5347(01)02227-3) [DOI] [Google Scholar]
- 124.Price EC, McGrew WC. 1991. Departures from monogamy in colonies of captive cotton-top tamarins. Folia Primatol. 57, 16-27. ( 10.1159/000156559) [DOI] [Google Scholar]
- 125.Leslie JD, Wendy S. 2009. Balancing cooperation and competition in Callitrichid primates: examining the relative risk of infanticide across species. In The smallest anthropoids: the marmoset/callimico radiation (eds Ford SM, Porter LM, Davis LC), pp. 135-153. Berlin, Germany: Springer. [Google Scholar]
- 126.Creel S, Creel NM, Mills MGL, Monfort SL. 1997. Rank and reproduction in cooperatively breeding African wild dogs: behavioral and endocrine correlates. Behav. Ecol. 8, 298-306. ( 10.1093/beheco/8.3.298) [DOI] [Google Scholar]
- 127.Van den Berghe F, Paris DBBP, Van Soom A, Rijsselaere T, Van der Weyde L, Bertschinger HJ, Paris MCJ. 2012. Reproduction in the endangered African wild dog: basic physiology, reproductive suppression and possible benefits of artificial insemination. Anim. Reprod. Sci. 133, 1-9. ( 10.1016/j.anireprosci.2012.06.003) [DOI] [PubMed] [Google Scholar]
- 128.van Kesteren F, Paris M, Macdonald DW, Millar R, Argaw K, Johnson PJ, Farstad W, Sillero-Zubiri C. 2013. The physiology of cooperative breeding in a rare social canid; sex, suppression and pseudopregnancy in female Ethiopian wolves. Physiol. Behav. 122, 39-45. ( 10.1016/j.physbeh.2013.08.016) [DOI] [PubMed] [Google Scholar]
- 129.Davies CS, Smyth KN, Greene LK, Walsh DA, Mitchell J, Clutton-Brock T, Drea CM. 2016. Exceptional endocrine profiles characterise the meerkat: sex, status, and reproductive patterns. Sci. Rep. 6, 35492. ( 10.1038/srep35492) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Young AJ, Carlson AA, Monfort SL, Russell AF, Bennett NC, Clutton-Brock T. 2006. Stress and the suppression of subordinate reproduction in cooperatively breeding meerkats. Proc. Natl Acad. Sci. USA 103, 12 005-12 010. ( 10.1073/pnas.0510038103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.van Kesteren F, Sillero-Zubiri C, Millar R, Argaw K, Macdonald DW, Paris M. 2012. Sex, stress and social status: patterns in fecal testosterone and glucocorticoid metabolites in male Ethiopian wolves. Gen. Comp. Endocrinol. 179, 30-37. ( 10.1016/j.ygcen.2012.07.016) [DOI] [PubMed] [Google Scholar]
- 132.Montgomery TM, Pendleton EL, Smith JE. 2018. Physiological mechanisms mediating patterns of reproductive suppression and alloparental care in cooperatively breeding carnivores. Physiol. Behav. 193, 167-178. ( 10.1016/j.physbeh.2017.11.006) [DOI] [PubMed] [Google Scholar]
- 133.Newell-Fugate AE, Nöthling OJ, Bertschinger H. 2012. Seasonal changes in steroid hormone profiles, body weight, semen quality, and the reproductive tract in captive African wild dogs (Lycaon pictus) in South Africa. Gen. Comp. Endocrinol. 178, 272-281. ( 10.1016/j.ygcen.2012.05.008) [DOI] [PubMed] [Google Scholar]
- 134.Wasser SK, Starling AK. 1988. Proximate and ultimate causes of reproductive suppression among female yellow baboons at Mikumi National Park, Tanzania. Am. J. Primatol. 16, 97-121. ( 10.1002/ajp.1350160202) [DOI] [PubMed] [Google Scholar]
- 135.Pusey A. 1997. The influence of dominance rank on the reproductive success of female chimpanzees. Science 277, 828-831. ( 10.1126/science.277.5327.828) [DOI] [PubMed] [Google Scholar]
- 136.Zinner D, Schwibbe MH, Kaumanns W. 1994. Cycle synchrony and probability of conception in female hamadryas baboons Papio hamadryas. Behav. Ecol. Sociobiol. 35, 175-183. ( 10.1007/BF00167957) [DOI] [Google Scholar]
- 137.Baniel A, Cowlishaw G, Huchard E. 2018. Jealous females? Female competition and reproductive suppression in a wild promiscuous primate. Proc. R. Soc. B. 285, 20181332. ( 10.1098/rspb.2018.1332) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.van Noordwijk MA, van Schaik CP. 1999. The effects of dominance rank and group size on female lifetime reproductive success in wild long-tailed macaques, Macaca fascicularis. Primates 40, 105-130. ( 10.1007/BF02557705) [DOI] [PubMed] [Google Scholar]
- 139.Setchell JM, Dixson AF. 2002. Developmental variables and dominance rank in adolescent male mandrills (Mandrillus sphinx). Am. J. Primatol. 56, 9-25. ( 10.1002/ajp.1060) [DOI] [PubMed] [Google Scholar]
- 140.Wasser S. 1998. Ageing and social rank effects on the reproductive system of free-ranging yellow baboons (Papio cynocephalus) at Mikumi National Park, Tanzania. Hum. Reprod. Update 4, 430-438. ( 10.1093/humupd/4.4.430) [DOI] [PubMed] [Google Scholar]
- 141.Toufexis D, Rivarola MA, Lara H, Viau V. 2014. Stress and the reproductive axis. J. Neuroendocrinol. 26, 573-586. ( 10.1111/jne.12179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sanderson JL, Stott I, Young AJ, Vitikainen EIK, Hodge SJ, Cant MA. 2015. The origins of consistent individual differences in cooperation in wild banded mongooses, Mungos mungo. Anim. Behav. 107, 193-200. ( 10.1016/j.anbehav.2015.06.022) [DOI] [Google Scholar]
- 143.Wasser SK. 1996. Reproductive control in wild baboons measured by fecal steroids. Biol. Reprod. 55, 393-399. ( 10.1095/biolreprod55.2.393) [DOI] [PubMed] [Google Scholar]
- 144.Archie EA, Altmann J, Alberts SC. 2012. Social status predicts wound healing in wild baboons. Proc. Natl Acad. Sci. USA 109, 9017-9022. ( 10.1073/pnas.1206391109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cavigelli SA, Chaudhry HS. 2012. Social status, glucocorticoids, immune function, and health: can animal studies help us understand human socioeconomic-status-related health disparities? Horm. Behav. 62, 295-313. ( 10.1016/j.yhbeh.2012.07.006) [DOI] [PubMed] [Google Scholar]
- 146.Habig B, Doellman MM, Woods K, Olansen J, Archie EA. 2018. Social status and parasitism in male and female vertebrates: a meta-analysis. Sci. Rep. 8, 3629. ( 10.1038/s41598-018-21994-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Snyder-Mackler N, et al. 2016. Social status alters immune regulation and response to infection in macaques. Science 354, 1041-1045. ( 10.1126/science.aah3580) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Emery TM, Machanda ZP, Scully EJ, Enigk DK, Otali E, Muller MN, Goldberg TL, Chapman CA, Wrangham RW. 2018. Risk factors for respiratory illness in a community of wild chimpanzees (Pan troglodytes schweinfurthii). R. Soc. Open Sci. 5, 180840. ( 10.1098/rsos.180840) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Padgett DA, Glaser R. 2003. How stress influences the immune response. Trends Immunol. 24, 444-448. ( 10.1016/S1471-4906(03)00173-X) [DOI] [PubMed] [Google Scholar]
- 150.Shively CA, Register TC, Clarkson TB. 2009. Social stress, visceral obesity, and coronary artery atherosclerosis: product of a primate adaptation. Am. J. Primatol. 71, 742-751. ( 10.1002/ajp.20706) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Strauss ED, Shizuka D. 2021. The dynamics of dominance: open questions, challenges, and solutions. Phil. Trans. R. Soc. B 377, 20200445. ( 10.1098/rstb.2020.0445) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Alward BA, Hilliard AT, York RA, Fernald RD. 2019. Hormonal regulation of social ascent and temporal patterns of behavior in an African cichlid. Horm. Behav. 107, 83-95. ( 10.1016/j.yhbeh.2018.12.010) [DOI] [PubMed] [Google Scholar]
- 153.Maruska KP, Zhang A, Neboori A, Fernald RD. 2013. Social opportunity causes rapid transcriptional changes in the social behaviour network of the brain in an African cichlid fish. J. Neuroendocrinol. 25, 145-157. ( 10.1111/j.1365-2826.2012.02382.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Maruska KP. 2015. Social transitions cause rapid behavioral and neuroendocrine changes. Integr. Comp. Biol. 55, 294-306. ( 10.1093/icb/icv057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Williamson CM, Romeo RD, Curley JP. 2017. Dynamic changes in social dominance and mPOA GnRH expression in male mice following social opportunity. Horm. Behav. 87, 80-88. ( 10.1016/j.yhbeh.2016.11.001) [DOI] [PubMed] [Google Scholar]
- 156.Williamson CM, Klein IS, Lee W, Curley JP. 2019. Immediate early gene activation throughout the brain is associated with dynamic changes in social context. Soc. Neurosci. 14, 253-265. ( 10.1080/17470919.2018.1479303) [DOI] [PubMed] [Google Scholar]
- 157.Hector AK, Raleigh MJ. 1992. The effects of temporary removal of the alpha male on the behavior of subordinate male vervet monkeys. Am. J. Primatol. 26, 77-87. ( 10.1002/ajp.1350260202) [DOI] [PubMed] [Google Scholar]
- 158.Young C, McFarland R, Barrett L, Henzi SP. 2017. Formidable females and the power trajectories of socially integrated male vervet monkeys. Anim. Behav. 125, 61-67. ( 10.1016/j.anbehav.2017.01.006) [DOI] [Google Scholar]
- 159.Gilby IC, Brent LJN, Wroblewski EE, Rudicell RS, Hahn BH, Goodall J, Pusey AE. 2013. Fitness benefits of coalitionary aggression in male chimpanzees. Behav. Ecol. Sociobiol. 67, 373-381. ( 10.1007/s00265-012-1457-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hasegawa M, Kutsukake N. 2015. Bayesian competitiveness estimation predicts dominance turnover among wild male chimpanzees. Behav. Ecol. Sociobiol. 69, 89-99. ( 10.1007/s00265-014-1821-9) [DOI] [Google Scholar]
- 161.Schülke O, Bhagavatula J, Vigilant L, Ostner J. 2010. Social bonds enhance reproductive success in male macaques. Curr. Biol. 20, 2207-2210. ( 10.1016/j.cub.2010.10.058) [DOI] [PubMed] [Google Scholar]
- 162.Higham JP, Maestripieri D. 2010. Revolutionary coalitions in male rhesus macaques. Behaviour 147, 1889-1908. ( 10.1163/000579510X539709) [DOI] [Google Scholar]
- 163.de Villiers MS, Richardson PRK, van Jaarsveld AS. 2003. Patterns of coalition formation and spatial association in a social carnivore, the African wild dog (Lycaon pictus). J. Zool. 260, 377-389. ( 10.1017/S0952836903003832) [DOI] [Google Scholar]
- 164.Perry S. 1998. A case report of a male rank reversal in a group of wild white-faced capuchins (Cebus capucinus). Primates 39, 51-70. ( 10.1007/BF02557743) [DOI] [Google Scholar]
- 165.Pappano DJ, Beehner JC. 2014. Harem-holding males do not rise to the challenge: androgens respond to social but not to seasonal challenges in wild geladas. R. Soc. Open Sci. 1, 140081. ( 10.1098/rsos.140081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Beehner JC, Gesquiere L, Seyfarth RM, Cheney DL, Alberts SC, Altmann J. 2009. Testosterone related to age and life-history stages in male baboons and geladas. Horm. Behav. 56, 472-480. ( 10.1016/j.yhbeh.2009.08.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Beehner JC, Bergman TJ, Cheney DL, Seyfarth RM, Whitten PL. 2006. Testosterone predicts future dominance rank and mating activity among male chacma baboons. Behav. Ecol. Sociobiol. 59, 469-479. ( 10.1007/s00265-005-0071-2) [DOI] [Google Scholar]
- 168.Wickings EJ, Dixson AF. 1992. Testicular function, secondary sexual development, and social status in male mandrills (Mandrillus sphinx). Physiol. Behav. 52, 909-916. ( 10.1016/0031-9384(92)90370-H) [DOI] [PubMed] [Google Scholar]
- 169.Setchell JM, Dixson AF. 2001. Changes in the secondary sexual adornments of male mandrills (Mandrillus sphinx) are associated with gain and loss of alpha status. Horm. Behav. 39, 177-184. ( 10.1006/hbeh.2000.1628) [DOI] [PubMed] [Google Scholar]
- 170.Setchell JM, Wickings EJ. 2005. Dominance, status signals and coloration in male mandrills (Mandrillus sphinx). Ethology 111, 25-50. ( 10.1111/j.1439-0310.2004.01054.x) [DOI] [Google Scholar]
- 171.Boesch C, Boesch PC, Boesch-Achermann H. 2000. The chimpanzees of the Taï forest: behavioural ecology and evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 172.Georgiev AV, Christie D, Rosenfield KA, Ruiz-Lambides AV, Maldonado E, Emery Thompson M, Maestripieri D. 2016. Breaking the succession rule: the costs and benefits of an alpha-status take-over by an immigrant rhesus macaque on Cayo Santiago. Behaviour 153, 325-351. ( 10.1163/1568539X-00003344) [DOI] [Google Scholar]
- 173.Greenberg N, Crews D. 1990. Endocrine and behavioral responses to aggression and social dominance in the green anole lizard, Anolis carolinensis. Gen. Comp. Endocrinol. 77, 246-255. ( 10.1016/0016-6480(90)90309-A) [DOI] [PubMed] [Google Scholar]
- 174.Maruska KP, Becker L, Neboori A, Fernald RD. 2013. Social descent with territory loss causes rapid behavioral, endocrine and transcriptional changes in the brain. J. Exp. Biol. 216, 3656-3666. ( 10.1242/jeb.088617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hofmann HA, Benson ME, Fernald RD. 1999. Social status regulates growth rate: consequences for life-history strategies. Proc. Natl Acad. Sci. USA 96, 14 171-14 176. ( 10.1073/pnas.96.24.14171) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.