Abstract
When an individual ascends in dominance status within their social community, they often undergo a suite of behavioural, physiological and neuromolecular changes. While these changes have been extensively characterized across a number of species, we know much less about the degree to which these changes in turn influence cognitive processes like associative learning, memory and spatial navigation. Here, we assessed male Astatotilapia burtoni, an African cichlid fish known for its dynamic social dominance hierarchies, in a set of cognitive tasks both before and after a community perturbation in which some individuals ascended in dominance status. We assayed steroid hormone (cortisol, testosterone) levels before and after the community experienced a social perturbation. We found that ascending males changed their physiology and novel object recognition preference during the perturbation, and they subsequently differed in social competence from non-ascenders. Additionally, using a principal component analysis we were able to identify specific cognitive and physiological attributes that appear to predispose certain individuals to ascend in social status once a perturbation occurs. These previously undiscovered relationships between social ascent and cognition further emphasize the broad influence of social dominance on animal decision-making.
This article is part of the theme issue ‘The centennial of the pecking order: current state and future prospects for the study of dominance hierarchies’.
Keywords: social dominance, spatial maze, novel object recognition, associative learning, cortisol, testosterone
1. Introduction
Across diverse taxa individuals use cognitive processes like associative learning, memory and spatial cognition to successfully traverse their social surroundings. To display the appropriate behaviour in the relevant social context (known as social competence, [1]) often requires cognition: the mechanisms by which animals acquire, process, store and act on information from the environment [2–4]. Individual variation in behaviour, particularly along bold–shy/fast–slow/high exploration–low exploration axes, are common across taxa and can relate to cognitive performance [5–9]. From a cognitive perspective, this behavioural variation can influence how individuals approach cognitive tasks (e.g. decision speed, sampling), known as cognitive style [10]. Recent efforts have identified how cognitive style and cognitive performance correlate [10–12]. However, some studies observe a positive relationship between fast exploratory styles and learning performance [8], while others observe a negative relationship [6,13], and yet others find no relation to performance [14]. This variation can arise from social factors (such as sex, [15]), and can have significant ecological consequences: for example, a bold/fast/exploratory individual may be the first to find a food source or mating opportunity, but also may be more likely to be targeted by a predator. These frameworks are often viewed through the lens of current versus future reproductive success, where the prioritization of current reproductive success manifests as bolder/faster/more exploratory behaviour [16]. In fact, several recent studies have demonstrated that behavioural and cognitive variation affects metrics of fitness like survival [4,13,17].
Despite extensive documentation of cognitive variation at the species [18–20], individual [19,21,22] and developmental [21] level, we know less about how cognitive performance or style change across dynamic social landscapes. Variation in social dominance status, in particular, is ideally suited to examine changes in cognitive performance and style, as dominance status is often a dynamic phenotype where individuals undergo a dramatic cascade of behavioural and physiological changes when they ascend or descend in status [23,24]. Upon changing dominance status, individuals face profoundly different sets of social and ecological pressures and constraints and must adjust their behaviour to perform their new role competently—for example, an individual that may have previously fled an aggressive encounter may instead fight because it now has territory to defend. Social dominance status also has important consequences for fitness: dominant individuals can monopolize reproductive opportunities and resources like food and territories, even though their condition may be decreased [25–27] and their predation risk may be increased ([28,29], but see [30]). Subordinate individuals, conversely, may have only limited access to resources and mating opportunities, yet at the same time may be less susceptible to attacks from conspecifics or predators. Social stress such as isolation [31], chronic social defeat [32] or subordination [33] can influence a variety of behaviours and cognitive abilities, as well as mediate position in the hierarchy itself [34,35]. Successfully navigating a dominance hierarchy often requires cognitive abilities such as individual recognition, spatial learning of territories and home ranges, associative learning between aggressive encounters and individuals and transitive inference to predict fight outcomes [36–38]. Yet surprisingly few studies have examined the relationship between dominance status and cognitive performance in memory and spatial cognition tasks (but see [39] (dogs), [40] (lizards) and [41] (pheasants)).
The physiological underpinnings of social dominance have been examined in detail, especially with regards to the role of sex steroid hormones (for review see [42,43]). In fact, these hormone systems also modulate cognitive performance across species and cognitive domains (for review see [44,45]). In rodents, sex steroids like oestradiol enhance cognitive abilities [46] and testosterone modulates synaptic plasticity and improves cognitive performance across a variety of tasks [47–50]. Glucocorticoids are also critical modulators of social dominance status across species [35,51] and have similarly been shown to affect cognitive performance ([34], for review see [45]). In Florida scrub-jays, for example, associative learning and reversal learning are inversely related and depend on corticosterone exposure early in life [52]. The role of glucocorticoids both in modulation exploratory behaviour and performance on a cognitive task provides a strong mechanistic link between cognitive style and performance (as observed in sticklebacks, [53]). Exploring the shared physiological correlates of social dominance and cognition is a necessary step towards understanding the role dominance status plays in other dimensions of behaviour such as foraging, anti-predatory behaviour and dispersal [54].
Investigating how behaviour, physiology and cognition change during social ascent requires a model system that exhibits complex yet tractable social hierarchies in the laboratory and allows for experimental manipulation of social plasticity. Burton's mouthbrooder cichlid, Astatotilapia burtoni, a highly social cichlid fish from Lake Tanganyika has emerged as such a model system in social neuroscience [23,24]. In this species, males exhibit dominance hierarchies with a bi-directionally reversible dominant (colourful, territorial) and subordinate (drab, reproductively repressed gonads) phenotype [55]. Space use varies between dominant and subordinate A. burtoni, with important consequences for reproduction. When provided an opportunity to ascend in status, male A. burtoni immediately begin changing behaviour, with physiological and neuromolecular transitions completing after roughly two weeks [56]. Extensive research has described the social decision-making processes used by A. burtoni [36,57,58], but the cognitive processes outside of social learning have not been examined in detail despite their critical role in the social ecology of the species [36].
In the present study, we asked how variation in social dominance status relates to variation in cognition. Specifically, we examined whether cognitive performance and style change when individuals ascend in social status, and whether any such changes are accompanied by changes in cortisol and testosterone. We characterized changes in cognition during social ascent in A. burtoni. We first assessed non-dominant males in a suite of ecologically relevant cognitive tasks: a novel object recognition task, a spatial maze and a social competence assay. After this initial testing, we perturbed the natural social community, allowing some individuals to ascend in status. Following this perturbation, we retested the males to determine if cognitive performance and behaviour changed in concordance with social ascent. Both before and after the perturbation we assayed waterborne hormone (cortisol and testosterone) levels.
We expected that during social ascent males would exhibit cognitive, behavioural and physiological changes, whereas non-ascending males would not. We predicted ascending males would exhibit more ‘bold/exploratory/risky’ cognitive styles (detectable via space use patterns, decision latencies and neophilia) across all three tasks as they would be prioritizing current reproductive success, whereas non-ascending males would conversely prioritize future reproductive success. We expected this would be evident in our social competence assay, which was divided into a reproductive opportunity phase followed by a male challenge phase. Social competence is the ability to modify in behaviour in response to changing social contexts, so we predicted high reproductive behaviour (such as reproductive displays or relative time spent near females) in the reproductive opportunity phase, and then a change in behaviour once the male challenger was revealed. We expected the specific behavioural change would depend on the dominance status of the focal male, with males who had recently ascended in their home communities engaging in aggressive behaviour towards the challenger male, whereas non-ascenders would avoid interacting with the challenger male. Furthermore, while there is little literature on sneak copulations and alternative reproductive tactics [59] in this species (as male gonads are repressed while in a subordinate state, [60]), we hypothesized that subordinate males may show higher rates of reproductive displays and time near females during the opportunity phase.
2. Material and methods
(a) . Housing and initial community formation
Sixty-four adult male and 64 adult female A. burtoni were used from a laboratory stock derived from about 400 wild-caught fish that were collected in 1975 by Fernald & Hirata [55] in the northern part of Lake Tanganyika, near the Ruzizi River delta. Given a generation time of four to six months, we estimate that these fish have been in the laboratory for 90–130 generations. We maintained fish in mixed-sex communities in 114 l aquaria prior to testing. Both prior to and during testing, we kept fish in reverse osmosis water treated with Seachem Lake Tanganyika salt and bicarbonate buffer. Lights were on a 12 L : 12 D cycle and we fed fish once daily with Omega One Natural Protein Formula Cichlid Flakes. We tagged all individuals with coloured plastic beads for individual identification. One week prior to testing, we weighed and measured fish for standard length (average standard length of focal males: 4.78 ± 0.50 cm) and formed naturalistic communities (n = 8 replicates) consisting of eight males and eight females in a circular pool (1 m diameter, 25 cm depth, lined with a PEVA curtain liner and Spectrastone White aquarium gravel) and containing four halved terracotta pots that served as territorial shelters. We video-recorded (Alibi Security) from above each community every morning immediately after the lights turned on at 8.00 to determine male dominance status as either dominant (aggressively defending a territory and actively courting gravid females), intermediate (displaying aggressive behaviour without defending a specific area), or subordinate (shoaling behaviour, no aggressive behaviour) (electronic supplementary material, table S1).
(b) . Experimental timeline
For the first week we allowed the communities to acclimate and form stable dominance hierarchies. During the second week of the experiment, we periodically removed individuals from their community to undergo baseline behavioural and physiological testing. At the end of the second week (day 15), the community was socially altered (perturbation)—we removed the largest four males (note: their behavioural and physiological data is not reported here). In cases where the community had fewer than eight total males, fewer males were removed (to always ensure the post-perturbation community had four remaining males). While the removal of the dominant males influenced the sex ratio and total number of individuals in the community, we emphasize that in this species it is the number of available territories (which did not change over the course of our experiment) rather than shoal size that most strongly influences male dominance behaviour. We chose this social perturbation design to minimize disruption to the social community via new individual introduction. We resumed behavioural and physiological testing on the third day after the social perturbation (day 18) and proceeded through to the end of the experiment (day 22) (electronic supplementary material, figure S1). The focal males analysed in this experiment were only those individuals who remained in the community following the removal of the four largest males, and no focal male was dominant prior to the social perturbation.
(c) . Novel object recognition task
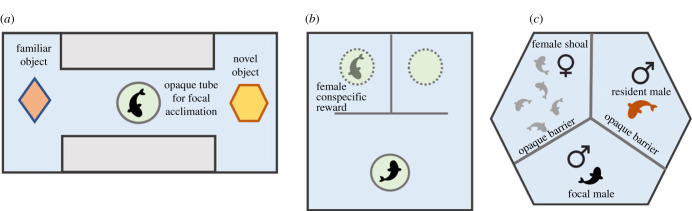
A novel object recognition (NOR) task requires an individual to discriminate between a previously encountered object and a novel one. This paradigm is commonly used in the rodent literature to assess memory [61,62], but additionally can assess exploration and neophobia [63]. The ability to remember previously encountered objects is relevant for A. burtoni, as they frequently use objects as territorial shelters. In the NOR task conducted here, we assessed individuals for relative preference (physical proximity) to a familiarized object versus a novel object in a 114 l acrylic aquarium filled to a depth of 20 cm with a central alleyway and two object regions (figure 1a—and see the electronic supplementary material, figure S2 for dimensions). We used four objects as stimuli in the task. Object pairs and their roles (familiar versus novel) were counter-balanced across communities. Prior to testing, we familiarized the entire community at once (to maintain daily consistency in the timing of the experimental design) to two identical objects (coined the ‘familiar object’) for 1 h (e.g. A + A). Approximately 24 h later, we presented each fish individually with a familiar object and a novel object at the opposite ends of the aquarium (the side of the familiar object, left or right, was counter-balanced across communities) (e.g. A + B). Variation in the delay between familiarization and testing did not influence object preference before (Wilcoxon rank sum test, W26,25 = 298.5, n = 26, p = 0.621, Cohen's D = 0.123) or after (Wilcoxon rank sum test, W4,16 = 34, n = 16, p = 0.887, Cohen's D = 0.011) the perturbation. During the trial, we acclimated individuals in an opaque PVC tube for 1 min then allowed them to swim freely in the apparatus for 12 min. In this apparatus, individuals made an average of 6.5 entries into the central alley (s.d. = 5.9) and spent 8.7% of the trial in the central alley (s.d. = 13.4). We calculated relative preference for the familiar object (NOR familiar preference) as the relative proportion of time spent at the familiar object to time spent at the novel object, where a score of 1 means the entirety of time spent at an object was at the familiar object, a score of 0.5 means equal preference and of 0 means the entirety of time spent at an object was at the novel object (see [64]). Because previous literature in this species has shown that male NOR preference is strongest during the first minute of the task [64], we additionally calculated preference specifically during the first minute of the task.
Figure 1.
General experimental designs of the novel object recognition task (a), spatial task (b) and social opportunity and challenge assay (c). (Online version in colour.)
(d) . Spatial task
Spatial tasks (mazes) have been extensively used to assess spatial learning in fishes: see [65] (goldfish), [66] (guppy), [8] (guppy), [67] (electric fish) and [68] (cichlid). Spatial learning is highly relevant for A. burtoni, as males defend spatially defined territories. We conducted a spatial task (SPA) in a 57 l acrylic aquarium filled to a depth of 20 cm. In this task individuals had to detour around an opaque barrier in one of two directions (left or right) to reach a social reward consisting of a female in a clear plastic tube behind one side of the barrier (figure 1b; see the electronic supplementary material, figure S2 for dimensions), with an empty plexiglass tube behind the other side as a control stimulus. No visual or olfactory cues were present, meaning individuals had to learn and remember which side housed the reward stimulus. Transit between the rewarded and unrewarded side required an individual to navigate back out in front of the barrier, thus creating a penalty for errors. For all trials, we initially placed individuals in an opaque PVC tube for 1 min to acclimate to the aquarium, then we allowed them to swim freely for 12 min. We performed an initial apparatus habituation trial (using two control stimuli) the day prior to the reward trials. Following this habituation trial, we assayed individuals in four rewarded trials over 2 days (in order to adhere to the social perturbation schedule), with the reward stimulus present behind either the left or right barrier, counter-balanced across individuals. Across these trials we used the same reward female stimulus per individual (excluding rare experimental limitations), and stimulus did not significantly influence any of the SPA metrics reported. To assess performance we used a previously employed learning criterion for this assay [64]. The criterion required successful learners to first approach the rewarded stimulus in all rewarded trials after the first trial (i.e. trials 2–4), thus the criterion was independent of behavioural metrics such as latency, motivation or exploration. Assessment of the criterion was based on the binomial probability of this result occurring by chance [64]. We additionally calculated relative preference for the rewarded stimuli (reward preference) as the relative proportion of time spent at the rewarded stimulus to time spent at the unrewarded stimulus, where a score of 1 means the entirety of time spent near a stimulus was at the rewarded stimulus, a score of 0.5 means equal preference and of 0 means the entirety of time spent at an object was at the unrewarded stimulus (see [64]).
(e) . Social opportunity and challenge assay
Social competence is the ability to employ appropriate behaviour in social contexts and to appropriately adjust behaviour in response to a change in social context [1]. Multiple experimental designs assessing social competence have been used specifically in A. burtoni [69–72]. Here, we developed a novel assay to both assess social competence and to validate dominance status categorizations based on the naturalistic community observations. The assay was derived from the resident-intruder paradigm but circumvented the need to remove the focal individual from the community for an extended period of time to establish a resident territory. This novel ‘social opportunity and challenge assay (SOCA)’ assessed focal male social behaviour in three contexts: a nonsocial habituation phase (3 min), a reproductive opportunity phase (30 min) and a resident challenge (30 min) (figure 1c; see the electronic supplementary material, figure S2 for dimensions). In the resident challenge phase, the focal male is the intruder and is smaller than the resident male, therefore, the focal male is unlikely to win an aggressive encounter. The SOCA apparatus, constructed of clear plexiglass, was a hexagon with each side measuring 32 cm long. We divided the apparatus into three compartments via removable barriers: a focal compartment (with PVC pipe shelter), a female shoal compartment housing four females varying in reproductive status and a resident male compartment housing one large male with a terra-cotta pot to serve as a territorial shelter. We housed both the female shoal and resident male in the apparatus to ensure the resident male was dominant and territorial in the apparatus. During the habituation phase, we separated all three compartments with opaque (black) plexiglass barriers. During the opportunity phase, we replaced the opaque plexiglass barrier between the focal compartment and female shoal compartment with a transparent one, allowing visual access between the two compartments. During the challenge phase, we replaced the opaque plexiglass barrier between the focal compartment and the resident compartment with a transparent one, allowing the focal male to see both the female shoal compartment and the resident challenger male compartment. To record the videos, we placed a camera level with the apparatus behind the focal male compartment (i.e. front facing). We recorded focal location as well as reproductive displays, aggression and activity directly at the barriers between compartments to determine if individuals were displaying appropriate social behaviour during the two social contexts (reproductive opportunity and aggressive challenge) (electronic supplementary material, table S2). The same resident challenger male was presented to the focal male before and after the perturbation, and which resident challenger male stimulus a focal received did not influence the behavioural metrics reported.
(f) . Hormone collection and analysis
We collected waterborne hormone measurements from all individuals immediately prior to the community perturbation (day 15) and on the third day after the perturbation (day 18) following the procedure described by Kidd et al. [73]. Briefly, to collect hormone samples, we removed the foal male from the home community and placed him in an autoclaved glass beaker filled with 200 ml of neutral water. We then covered the beaker with a box, which minimizes visual stimuli and has a calming effect on the animals. After 30 min, we removed the individual from the beaker and the sample was stored at −20°C. Prior literature has shown that confinement to a beaker (which is required in this collection procedure as the animal must be in a finite amount of water) induces a stress response that is habituated to in 3–4 trials [74], suggesting that this habituation would not impact our two-trial design. We prepared samples for enzyme-linked immunosorbent assay (ELISA) analysis via solid phase extraction using a vacuum manifold (ThermoFisher) and Sep-Pak C-18 cartridges (Waters). During the extraction, we equilibrated cartridges with 4 ml of methanol then activated with 4 ml of MilliQ water. Samples were refrozen at −20°C following extraction. We then eluted samples via 4 ml of ethyl acetate and stored them at 4°C. We then dried the samples using nitrogen on an Evap-O-Rac (Cole-Parmer) and stored them at −20°C. Following sample preparation, we quantified hormone level using Cayman ELISA kits (cortisol: 500360, testosterone: 582701) and standard protocols. Waterborne hormone levels are correlated with circulating levels [73,74].
(g) . Video scoring
We recorded videos taken during the experiment using the Alibi Security Camera system. Except for the SOCA recordings (front facing), all observations were recorded overhead (NOR, SPA, naturalistic community). Four human observers (who had not conducted the behavioural assays) scored the videos in VideoLAN Client (VLC) media player. We scored SOCA videos via an ethogram developed for the experiment (electronic supplementary material, table S2). We classified males as either dominant, intermediate or subordinate based on behaviours observed in the naturalistic community videos (electronic supplementary material, table S1). Dominant males were categorized as those who successfully defended a territory (chasing off other individuals from a consistent location) for greater than 50% of the video. Intermediate males were categorized as those who showed sporadic defence behaviour and did not successfully defend a specific area. Subordinate males were categorized as those who showed no defence behaviour, and additionally could be distinguished by their association with the female and subordinate shoal. Only males who were successfully characterized as dominant, intermediate or subordinate both before and after the perturbation were included in the analysis (27 of 32 males). Following the community perturbation, individuals classified as ascenders were those males who had a more dominant status on day 18 than they did on day 15. Individuals classified as non-ascenders either had the same status or a lower status on day 18 relative to day 15.
We scored NOR and SPA test videos using CowLog video scoring software v. 3.0.2 [75], which is a highly consistent scoring method across multiple observers [76]. We recorded fish location across predetermined zones of the NOR and SPA experimental apparatus. For feasibility, in the SOCA assay we recorded location and behaviour during the 3 min habituation phase, the first and last 5 min of the opportunity phase and the first and last 5 min of the challenge phase.
(h) . Statistics
We performed all data analysis and visualization in R (R Core Team 2013) v. 3.6.1 (2019). We used the R package ‘cowlogdata’ to assemble score logs into summary data frames and perform initial data visualization [77]. We only analysed trials in which fish were observed for at least 10 min. For continuous data subset by categorical variables we conducted an unpaired t-test, unpaired two-sided Wilcoxon rank sum test with continuity correction, Mann–Whitney U-test or Kruskal–Wallis test, depending on the number of categories and normality (determined by a Shapiro–Wilk normality test). For entirely categorical data we conducted χ2-tests or binomial tests as appropriate. To correlate continuous data we conducted standard multiple linear regressions using the formula lm(x1 ∼ x2) using the R function ‘lm().’ We calculated effect sizes as either Cohen's D using the R function ‘cohen.d()’ in the R package ‘effsize’ [78] for categorical data, or as the Pearson correlation coefficient using the R function ‘cor()’ for continuous data. We conducted principal component analyses (PCA) on z-scored data via the R function ‘prcomp’ and subsequent hierarchical clustering analyses using the R package ‘pvclust’ [79] with distance metric: ‘correlation’, linkage metric: ‘average’, and bootstrap values set to 100. To account for collinearity, we assessed variables included in all data analysis (excluding the logistic regression models, which we conducted on a more restricted set of variables) via a covariance matrix prior to further analysis: any two variables with a Pearson correlation coefficient above 0.8 in either the baseline testing or the post-perturbation retesting were considered redundant. Thus, for subsequent analysis we only included the variable which preserved the most variables between the baseline and retesting dataset, and, if necessary, to decide between two equal variables in this regard, the variable more commonly used in previous literature. Given prior literature on social ascent in A. burtoni (e.g. [60,80]), we carried out a retrospective analysis to examine whether individuals of larger size and/or relatively high testosterone prior to the perturbation were the ones to ascend. Raw data and analysis code can be found at github.com/kellyjwallace/Wallace_Hofmann_2020_status_differences.
3. Results
(a) . Cognitive performance before the community perturbation
In the NOR task, seven males were excluded owing to technical errors. Males first approached the familiar object significantly more than expected by chance: 15 of the 20 (75%) subordinate males first approached the familiar object and five males first approached the novel object (binomial test, n = 20, p = 0.041, Cohen's D = 0.25). On the first trial of the SPA, fish first approach the rewarded and unrewarded stimuli at equal frequency (χ2-test: χ21 = 0.550, n = 23, p = 0.458, φ = 0.155), which suggests there are no cues (visual or olfactory) that informed their decision. On the test trial (trial 4), individuals still first approached the stimuli randomly (χ2-test: χ21 = 0.762, n = 24, p = 0.383, φ = 0.178). When we assessed performance based on the learning criterion employed in [64], where successful learners were those who first approached the rewarded stimulus on all trials after the first trial, only three males met the criterion. In the SOCA, 10 individuals performed at least one lateral display (a display performed during territorial competition) and eight individuals performed at least one reproductive display. One individual performed 13 lateral displays during the challenge phase. One individual engaged in five border conflicts (a highly aggressive territorial challenge behaviour) during the challenge phase. Males did not exhibit any significant differences in behaviour between the opportunity and challenge phases of the task.
(b) . Community perturbation and physiology
After the largest males were removed from the communities, 10 of the remaining males ascended in status within 1–3 days, 17 males did not. Across the eight communities, between 0 and 3 males ascended with an average 1.25 (which is similar to the natural proportion of dominant individuals in a community at 10–30%, [25]). When comparing hormone levels before and after the perturbation, circulating cortisol levels did not differ when analysed for all males (Wilcoxon rank sum test: V26,26 = 179, n = 26, p = 0.940, Cohen's D = 0.220). However, cortisol level of ascenders were significantly lower than those of non-ascenders following the perturbation (Mann–Whitney U-test: W10,16 = 118, n = 26, p = 0.047, Cohen's D = 0.876; figure 2a). Similarly, when analysed for all males, circulating levels of testosterone did not change as a consequence of the perturbation (Wilcoxon rank sum test: V26,26 = 103, n = 26, p = 0.067, Cohen's D = 0.453). However, testosterone levels increased significantly in ascending males (Wilcoxon rank sum test: V10,10 = 3, n = 10, p = 0.010, Cohen's D = 1.120; figure 2b).
Figure 2.
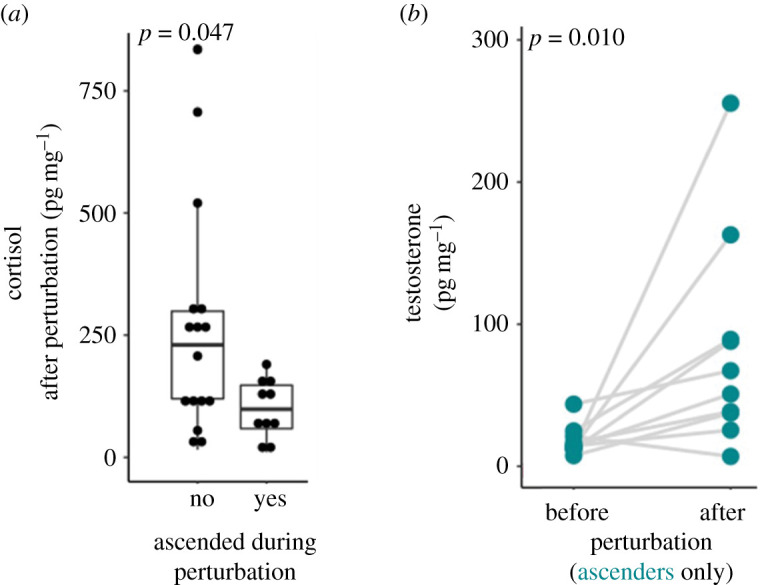
Social ascent is accompanied by changes in physiology (cortisol and testosterone). Two days following the social perturbation, ascending males exhibited significantly lower cortisol than non-ascenders (p = 0.047) (a). Testosterone increased significantly in ascending males (p = 0.010) (b). (Online version in colour.)
(c) . Changes in behaviour and cognition across the community perturbation
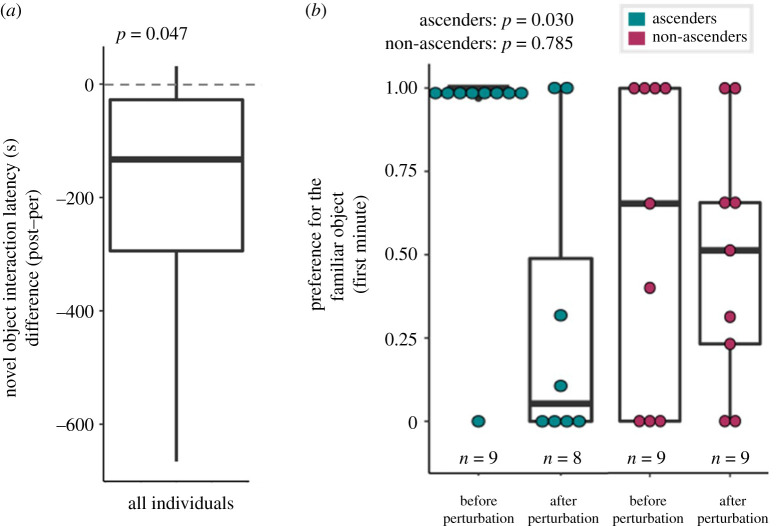
In the post-perturbation NOR task, individuals did not first choose the familiar or novel object more than expected by chance (χ2-test: χ21 = 0.921, n = 20, p = 0.337, φ = 0.215), and their object preference across time did not differ from 50% (across the entire task: t-test: t19= −0.877, n = 20, p = 0.392, Cohen's D = −0.196, across the first minute of the task; t-test: t16= −1.012, n = 17, p = 0.327, Cohen's D = −0.245). Ascending and non-ascending males did not differ in their first choice preference (χ2-test: χ21 = 1.431, n = 17, p = 0.232, φ = 0.290). Relative to their pre-perturbation baseline, all males more quickly approached the novel object (t-test: t7 = −2.412, n = 8, p = 0.047, Cohen's D = −0.853; figure 3a). Ascending males significantly decreased their preference for the familiar object in the first minute of the task (Mann–Whitney U-test: W9,8 = 57, n = 9, p = 0.030, Cohen's D = 1.500; figure 3b), whereas non-ascenders did not exhibit a change in preference (Mann–Whitney U-test: W11,9 = 44, n = 11, p = 0.785, Cohen's D = 0.178; figure 3b) relative to their pre-perturbation baseline.
Figure 3.
After social perturbation, all males approached the novel object more quickly compared to pre-perturbation baselines (p = 0.047) (a). Ascending males significantly decreased preference for the familiar object when reassessed in the novel object recognition task following social ascent (p = 0.030) (b, left), whereas non-ascending males did not (p = 0.785) (b, right). (Online version in colour.)
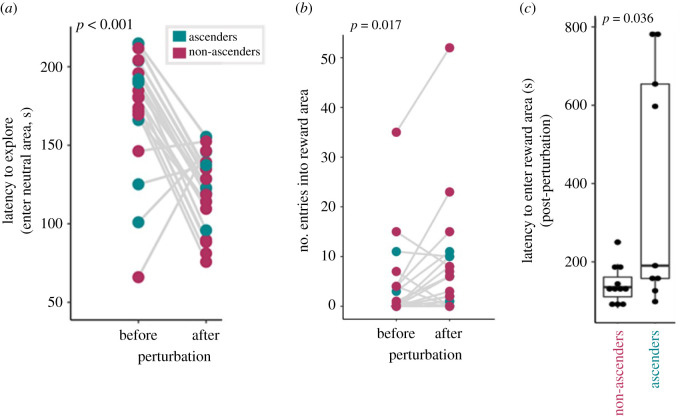
In the post-perturbation SPA individuals still did not learn, approaching the rewarded and unrewarded zones randomly (χ2-test: χ21 = 1.000, n = 25, p = 0.317, φ = 0.200). SPA first choice did not differ between ascenders and non-ascenders (χ2-test: χ21 = 1.418, n = 25, p = 0.234, φ = 0.238). Relative to their pre-perturbation behaviour, all individuals more quickly made a decision (t-test: t24 = 02.916, n = 25, p = 0.008, Cohen's D = −0.583), explored more quickly (shorter latencies to enter the neutral area, Mann–Whitney U-test: W24,25 = 540, n = 25, p = 1.7 × 10−6, Cohen's D = 1.811; figure 4a) and entered the rewarded area more (Mann–Whitney U-test: W24,25 = 184, n = 25, p = 0.017, Cohen's D = 0.369; figure 4b). While most individuals entered the reward area of the post-perturbation SPA task in under 300 s, four ascending males took much longer (greater than 500 s), which drove a significant difference between ascenders and non-ascenders in the post-perturbation SPA task (t-test: t8.4= −2.489, n = 27, p = 0.036, Cohen's D = 1.237; figure 4c). Interestingly, this variation correlated to cortisol: the four ‘slow-reward’ ascenders (reward latency: 597–786 s) exhibited significantly lower cortisol than the five ‘fast-reward’ ascenders (reward latency: 98–158 s) (Mann–Whitney U-test: W5,4 = 20, n = 9, p = 0.016, Cohen's D = 2.484; electronic supplementary material, figure S3A), but did not significantly differ in testosterone levels (Mann–Whitney U-test: W5,4 = 16, n = 9, p = 0.191, Cohen's D = 0.687) or standard length (Mann–Whitney U-test: W5,4 = 18, n = 9, p = 0.065, Cohen's D = 1.596). Because of the significant difference in cortisol levels, we hypothesized that ‘slow-reward’ ascenders may also be slower than ‘fast-reward’ ascenders to begin exploring the apparatus once the task began. However, the latency to explore the apparatus did not differ between the two groups (Mann–Whitney U-test: W5,4 = 13, n = 9, p = 0.556, Cohen's D = 0.416; electronic supplementary material, figure S3B).
Figure 4.
After social perturbation individuals were quicker to explore the apparatus in the spatial task (p < 0.001, a) and make more entries into the rewarded area (p = 0.017, b) compared to the baseline assessment before perturbation. When assessed again following perturbation, males that ascended in status during the perturbation exhibit significantly longer latencies to enter the reward area than those who did not ascend (p = 0.036, c). (Online version in colour.)
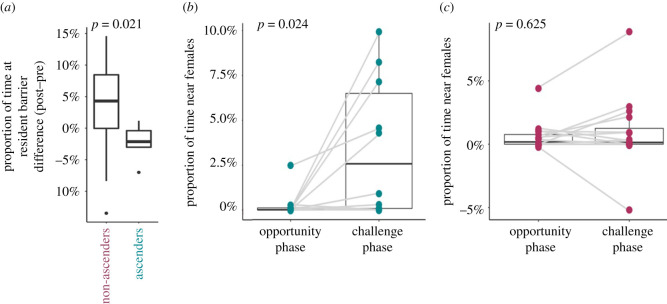
In both the pre-perturbation and post-perturbation SOCA, neither lateral nor reproductive displays were sufficiently frequent to allow for statistical testing, thus we instead assessed proportion of time spent in the various zones of the aquarium. In the SOCA following the perturbation, ascenders and non-ascenders diverged in their overall challenge behaviour relative to their pre-perturbation baselines: non-ascenders engaged more with the resident (proportion of time at the resident male barrier), whereas ascenders engaged less with the resident (t-test: t19.4 = 2.502, n = 22, p = 0.021, Cohen's D = 0.851; figure 5a). When comparing across social contexts within the task (opportunity phase to challenge phase) ascending males increased their association with females during the challenge phase relative to the opportunity phase (Wilcoxon rank sum test: V10,10 = 3 n = 10, p = 0.024, Cohen's D = 1.173; figure 5b), whereas non-ascending males did not change their female association between the opportunity and challenge phase (Wilcoxon rank sum test: V17,17 = 27, n = 17, p = 0.625, Cohen's D = 0.125; figure 5c).
Figure 5.
After the social perturbation, ascending and non-ascending males significantly diverged in their time near the resident male in the social opportunity and challenge assay, with non-ascenders increasing and ascenders decreasing relative to pre-perturbation levels (p = 0.021) (a). Ascending males also spent more time near females (% time near the female shoal) across social contexts (p = 0.024) (b), whereas non-ascending males did not (p = 0.625) (c). (Online version in colour.)
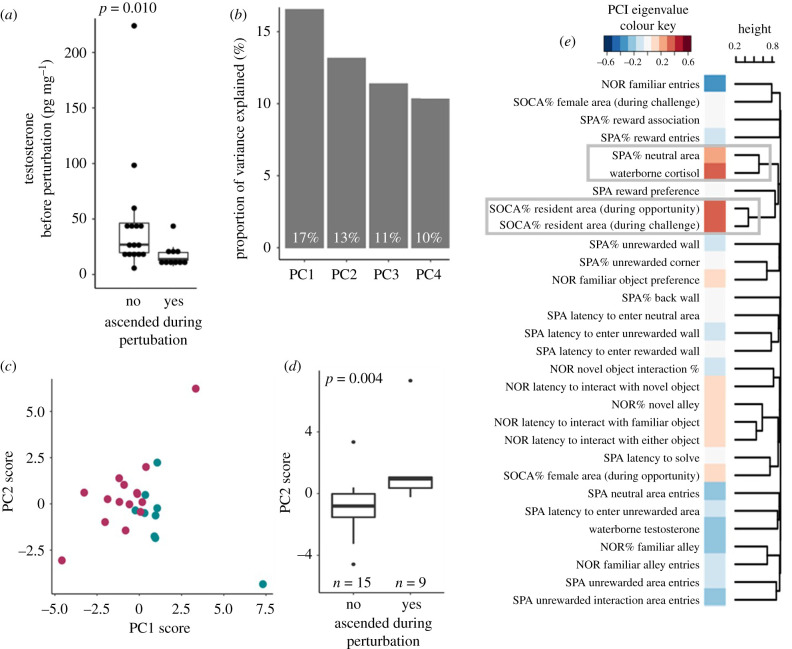
(d) . Distinguishing ascenders and non-ascenders prior to perturbation
Even though ascenders were on average larger than the non-ascenders, this difference was not significant (t-test: t21.3 = −1.863, n = 26, p = 0.076, Cohen's D = 0.727). Ascenders and non-ascenders did not differ in gonado-somatic index (GSI, the ratio of gonad mass to body mass, t-test: t15.9 = 0.190, n = 26, p = 0.851, Cohen's D = 0.081), and GSI did not significantly correlate to any physiological measure. We were surprised to find that males who ascended in social status after the perturbation had significantly lower testosterone levels prior to the perturbation (Mann–Whitney U-test: W16,10 = 128, n = 26, p = 0.010, Cohen's D = 0.666; figure 6a). Thus, to examine whether other variables distinguished ascenders from non-ascenders prior to the perturbation, we retrospectively assigned all pre-perturbation measurements to the appropriate category (that included variables from the NOR task, SPA task, SOCA task and the pre-perturbation cortisol and testosterone levels) and conducted a PCA. The PCA identified four axes that explained greater than 10% of the variance each and together explained 51.3% of the variance (figure 6b). PC1 explained 16.5% of the variance and was loaded most strongly by variables from multiple assays as well as by cortisol (centre column, figure 6e). Interestingly, PC1 scores significantly differed between ascending and non-ascending males (Mann–Whitney U-test with false discovery rate correction (4): W15,9 = 15, n = 24, p = 0.004, Cohen's D = 1.145; figure 6c,d), even though the variables that loaded most strongly on this PC were all those assessed prior to the perturbation. To identify variables that shared loading patters across the PC axes, we conducted a hierarchical clustering analysis (see dendrogram in figure 6e). This analysis identified two well-supported clusters: cortisol and SPA exploration, and SOCA challenge behaviour during the two phases of the task (highlighted in boxes, figure 6e). The analogous PCAs on the post-perturbation measurements and on the change between pre and post measurements did not yield any significantly different PC axes or well-supported clusters.
Figure 6.
Individuals who ascend in social status after a social perturbation display characteristic physiological and behavioural profiles already prior to the perturbation. A retrospective analysis shows that ascending males exhibited significantly lower testosterone prior to the perturbation compared to non-ascending males (p = 0.010) (a). A principal component analysis of pre-perturbation behaviour and physiology (b) differs significantly between individuals who do or do not ascend in the subsequent community perturbation (p = 0.004) (c,d). PC1 loadings (eigenvalues) for each variable are shown in the central column of (e). A hierarchical clustering analysis of the PCA eigenvalues identifies two well-supported clusters, highlighted with boxes (e). (Online version in colour.)
4. Discussion
Social dominance systems are highly dynamic environments that require individuals to make appropriate decisions (e.g. approaching or avoiding certain conspecifics, assessing risks and rewards, remembering past encounters). Here, we twice assessed male A. burtoni (a cichlid fish that exhibits dynamic dominance hierarchies) in three tasks designed to test novel object recognition, spatial learning and social competence. We administered the tasks first when the focal males were non-dominant, and then re-administered the tasks after a community perturbation in which some of the focal males had ascended in social status and some had not. Testosterone levels significantly increased in males that ascended to dominance status following a perturbation, as expected [60,80], thus validating our paradigm and behavioural characterization. We identified concordant changes in cortisol, testosterone, novel object preference, SPA cognitive style and social competence in ascending males. We additionally were able to identify individual and multivariate signatures predictive of social ascent even prior to the social perturbation. These results demonstrate that investigations of cognition outside social contexts can provide powerful and potentially even predictive insight into dominance status in this highly social species.
(a) . Novel object recognition preference in ascenders
When analysing all males prior to the perturbation, there was a significant preference in the object first approached, reflecting memory of the familiar object. Furthermore, ascending males exhibited meaningful changes in NOR preference during the social perturbation. Prior to perturbation, males that later ascended showed a strong preference for the familiar object during the first minute of the trial. However, when reassessed following the perturbation (with a new object pair), these males significantly decreased that preference, instead exhibiting a modest preference for the novel object. In raven dyads, novel object approach varies by reproductive context. In a non-reproductive context (male–male pairs) subordinate males are the first to approach a novel object, but in a reproductive context (male–female pair), it is the dominant males who first approach a novel object [81]. Similarly, we hypothesized that the change in novel object preference observed in A. burtoni may reflect a shift towards a reproductive context. In natural communities of A. burtoni, dominant males seek and aggressively defend territories by chasing other individuals away [57]. A shift in preference towards the novel object may reflect territory-seeking behaviour. The ascending males' change in preference towards the novel object in the NOR task also supports our original hypothesis that ascending males shift towards a bolder or more risky cognitive style that prioritizes current reproductive success [16], but further investigation is needed.
(b) . Cortisol and spatial task reward speed
Studies in both meadow voles [82] and pheasants [41] have found that dominant males exhibit greater spatial learning ability, though this can vary by mating system [83]. We did not observe any differences in the SPA between ascending and non-ascending A. burtoni, though this was probably owing to the task design as very few males met the learning criterion. When comparing before and after the perturbation, we did observe behavioural changes in the SPA. We had hypothesized that changes in behaviour and physiology would occur primarily in ascenders, but most of the changes in the SPA after perturbation were seen broadly across all individuals (quicker exploration, more entries into the social reward). While this broad change may be partially explained by increased familiarity with the assay, one post-perturbation SPA measurement distinguished ascenders and non-ascenders: the speed at which they entered the reward area. While most individuals entered the reward area quickly in the reassessment, a cluster of ascending males showed much longer latencies to enter the reward area. Interestingly, these same ‘slow-reward’ ascenders exhibited significantly lower cortisol than ‘fast-reward’ ascenders. Importantly, this relationship was not driven by the latency to explore the apparatus (a proxy for exploration, stress or anxiety), as the two groups did not differ in this measure. Changes in glucocorticoids influence hippocampal function across a variety of taxa [45,84,85], which provides a possible explanation as to why we observed this pattern in a spatial memory task. However, the relationship between glucocorticoids and cognition is complicated, and varies by dose, duration and development [45]. Our results provide additional insight into this relationship, suggesting glucocorticoids may influence not only cognitive performance but also cognitive style. However, without further experimentation to confirm the underlying process driving this pattern in A. burtoni (be it motivation, cognitive flexibility, general boldness/exploratory tendencies or another unidentified component), we cannot definitively contextualize our results. We highly recommend future experiments manipulate cortisol and observe effects in a spatial maze assay, as the link between spatial learning and stress has been previously explored in this species [68], and reflects the results seen in this study. More generally, the role of glucocorticoid signalling in cognition is certainly worthy of further exploration as the mechanistic link between social dominance and spatial cognitive performance/style.
(c) . Diverging social opportunity and challenge assay behaviour post-perturbation
When assessed in the SOCA task following the perturbation, ascending males exhibited social competence by changing behaviour across social contexts (i.e. the phases of the task). But non-ascenders' lack of behavioural changes in this same task may not necessarily signal a lack of social competence, rather, non-ascenders may have not engaged in any challenge behaviour whatsoever owing to a subordinate floor effect.
When viewing the SOCA task generally (rather than comparing specific phase changes), the challenge-associated behaviour of ascenders and non-ascenders diverged post-perturbation, in the opposite direction from what we originally expected. When comparing to pre-perturbation scores, non-ascenders spent more time near the resident male whereas ascending males spent less time near the resident male. One might intuitively expect that during the post-perturbation SOCA ascending males would respond vigorously to this kind of aggressive challenge. However, it is important to keep in mind that ascending males did not have any territory to defend in the assay itself, even though they were territorial in their home communities. Thus they may not have been highly motivated to engage with the resident male in this paradigm. In fact, ascending males spent more time affiliating with females during the challenge phase of the experiment, whereas non-ascenders showed no behavioural modulation. Why might ascending males, who have recently attained dominance in their home community, engage less with a resident challenger relative to their consistently subordinate counterparts? It is possible that these ascending males are prioritizing reproductive opportunities (i.e. attempting to engage in sneak copulations, [59]) over engaging with a resident dominant male. In other words, ascending males may have shifted towards behaviours that emphasize current reproductive success, focusing on reproductive-oriented though potentially risky behaviours. One additional consideration is the timing of this assay: here we are capturing changes in males at the early stages of social ascent. It is likely that assaying stable dominant males in this paradigm would yield more robust behavioural results, and we encourage the use of this novel assay in future exploration of stable dominance (as well as social descent).
(d) . Predictive signatures of social ascent prior to perturbation
Our experiment was not designed to specify a priori which individuals would ascend to dominance after social perturbation. Instead, we created a naturalistic environment where some fish spontaneously ascended and some did not. There are factors that have previously been shown in A. burtoni to ‘predispose’ individuals to ascend in social dominance under these circumstances (e.g. relative size: [58]; covert aggressive displays towards other subordinate males: [72]). Without further experiments (e.g. physiological manipulation to simulate ascension, scenarios where every focal ascends, etc.) we cannot disentangle these associated variables beyond a correlational level. In our experiment, we were surprised to find that males which ascended after the perturbation exhibited lower circulating levels of testosterone than non-ascenders prior to the perturbation. While subordinate males generally have low circulating levels of testosterone compared with dominant males (e.g. [80]; but see [86]), it is possible that the low testosterone individuals are better able to ‘capitalize’ on a social opportunity in this highly dynamic social environment, as their testosterone levels could rise relatively more before reaching a physiological maximum [43,86].
The difference in testosterone levels prior to perturbation between eventual ascenders and non-ascenders prompted us to retrospectively examine the pre-perturbation data more systematically. Indeed, PCA clearly separated eventual ascenders from non-ascenders. Importantly, this difference was driven by a broad suite of both physiological and behavioural variables across tasks. When clustering variables by their eigenvalues, we discovered a robust cluster linking cortisol and exploration in the spatial maze, again highlighting cortisol as a potential mechanistic link between social dominance and spatial cognitive performance/style. Of particular interest, we observed that males which eventually ascended in social status (after the perturbation) also displayed a very high preference for the familiar object in the NOR task. This result is consistent with our prior research showing a male preference for the familiar object [64] and additionally suggests that individuals that score high on this measure are poised to take advantage of a social opportunity. While the cognitive processes required for social dominance have been described in detail [1,36], much of the literature on cognitive predictors of social dominance focuses on predicting the outcomes of specific aggressive encounters (including an individual's own perception of the social encounter) [37,87]. Our study instead explored the cognitive and behavioural predictors of longer term social dominance structures, and in doing so we identified several novel attributes that might predispose males to become socially dominant when an opportunity presents itself.
(e) . Additional considerations
In the pre-perturbation NOR task, our subjects exhibited slight but non-significant preferences for the familiar object. In the pre-perturbation SOCA task we did not see any significant changes across social context (i.e. between the opportunity and the challenge phase). For both NOR and SOCA, we conclude that the experimental designs are the most appropriate when assessing males that vary in dominance status, but we did not have this social variation before the perturbation as all individuals were subordinate. Faster performance in the post-perturbation SPA reassessment suggests that males learned the association component of the task but did not learn the spatial discrimination aspect, probably because our training regime (designed to fit within the social perturbation schedule) provided relatively little time.
In addition to the assay optimization to facilitate spatial learning, the experiment and results described here identify many promising avenues for more targeted further analyses. Because of the timeline of this experimental design, our work focused primarily on the effects of the acquisition of dominance. This is probably why the observed testosterone levels did not (yet) significantly differ between ascenders and non-ascenders, as we were capturing the early stages of the physiological transition (similar to the findings by [60,80]). If we were instead to assess established dominant males, we probably would find more robust differences across the tasks. Additionally, our results encompassed community-level variation in dominance structure (e.g. in some communities only one male ascended, whereas in others three of the four males ascended) as well as a change in sex ratio during the perturbation (before: eight male/eight female, after: four male/eight female). Additionally, in A. burtoni males, changes in social status are accompanied by changes in gonadal and somatic growth [25]. These finer-scale community level and physiological dynamics probably shape behaviour and cognition and are critical areas of further exploration.
Lastly, social dominance is only one of many social phenotypes that may drive cognitive variation. For example, we have previously demonstrated sex differences in cognition in A. burtoni [64]. Future work should determine whether and how cognitive performance changes over the course of a female's reproductive cycle (e.g. [88]) and across the breeding season in males (e.g. [89]).
5. Conclusion
Understanding social decision-making involves simultaneously investigating changes in an animal's behaviour, physiology and environment [54]. Social ascent elicits a dramatic suite of changes across biological levels. Here, we examined how a change in social dominance influences cognition in the highly social cichlid fish A. burtoni. We assessed cognitive performance before and after social ascent. We additionally detailed behavioural and physiological characteristics. We identified a comprehensive set of relationships between social ascent, novel object preference, SPA decision speed and levels of cortisol and testosterone. We based our hypotheses and expectations on the natural history of A. burtoni, and subsequently discussed potential avenues of additional investigation. To better understand the ecological trade-offs, behavioural variation and mechanistic underpinnings that together shape cognition in a social world, it is necessary to systematically examine the relationship between social phenotype (e.g. dominance status, sex, reproductive status, mating strategy, home range size) and cognition across diverse taxa.
Acknowledgements
The authors would like to thank Emily Lessig and Shana Caro for manuscript feedback. We additionally thank all members of the Hofmann Laboratory for discussion and assistance.
Ethics
The authors certify that this work followed ethical treatment of animals outlined in their IACUC protocol (AUP-2018-00236).
Data accessibility
Raw data (csv) as well as analysis and visualization R code can be found at github.com/kellyjwallace/Wallace_Hofmann_2020_status_differences, via the Texas Data Repository (https://data.tdl.org/), or via request to the corresponding author.
Authors' contributions
H.A.H. and K.J.W. conceived of the study. K.J.W., K.H.C., L.A.K., D.H.L., M.T.L. and K.W. designed and constructed the experimental apparatus and data collection procedures and collected data. K.J.W. performed statistical analysis. H.A.H. and K.J.W. interpreted the results. K.J.W. wrote the initial draft of the manuscript, and H.A.H. provided feedback and comments during manuscript writing. All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
The authors have no competing interests.
Funding
This work was supported by the National Science Foundation (NSF) Bio/computational Evolution in Action Consortium (BEACON) Center for the Study of Evolution in Action and an NSF grant no. IOS1354942 (to H.A.H.), a Ford Foundation Predoctoral Fellowship (National Academies of Sciences, Engineering and Medicine), a UT Austin Graduate School Continuing Fellowship, The Zoology Scholarship Endowment for Excellence (Graduate School at the University of Texas at Austin) and a Department of Integrative Biology Doctoral Dissertation Improvement grant (to K.J.W.).
References
- 1.Taborsky B, Oliveira RF. 2012. Social competence: an evolutionary approach. Trends Ecol. Evol. 27, 679-688. ( 10.1016/j.tree.2012.09.003) [DOI] [PubMed] [Google Scholar]
- 2.Shettleworth SJ. 2001. Animal cognition and animal behaviour. Anim. Behav. 61, 277-286. ( 10.1006/anbe.2000.1606) [DOI] [Google Scholar]
- 3.Shettleworth SJ. 2010. Cognition, evolution, and behaviour, 2nd edn. Oxford, UK: Oxford University Press. [Google Scholar]
- 4.Snell-Rood EC, Steck MK. 2019. Behaviour shapes environmental variation and selection on learning and plasticity: review of mechanisms and implications. Special issue: cognitive ecology. Anim. Behav. 147, 147-156. ( 10.1016/j.anbehav.2018.08.007) [DOI] [Google Scholar]
- 5.Guillette LM, Reddon AR, Hurd PL, Sturdy CB. 2009. Exploration of a novel space is associated with individual differences in learning speed in black-capped chickadees, Poecile atricapillus. Behav. Processes 82, 265-270. ( 10.1016/j.beproc.2009.07.005) [DOI] [PubMed] [Google Scholar]
- 6.Guillette LM, Reddon AR, Hoeschele M, Sturdy CB. 2011. Sometimes slower is better: slow-exploring birds are more sensitive to changes in a vocal discrimination task. Proc. R. Soc. B 278, 767-773. ( 10.1098/rspb.2010.1669) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mamuneas D, Spence AJ, Manica A, King AJ. 2015. Bolder stickleback fish make faster decisions, but they are not less accurate. Behav. Ecol 26, 91-96. ( 10.1093/beheco/aru160) [DOI] [Google Scholar]
- 8.Burns JG, Rodd FH. 2008. Hastiness, brain size and predation regime affect the performance of wild guppies in a spatial memory task. Anim. Behav. 76, 911-922. ( 10.1016/j.anbehav.2008.02.017) [DOI] [Google Scholar]
- 9.Lucon-Xiccato T, Dadda M. 2016. Guppies show behavioural but not cognitive sex differences in a novel object recognition test. PLoS ONE 11, e0156589. ( 10.1371/journal.pone.0156589) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sih A, Del Giudice M. 2012. Linking behavioural syndromes and cognition: a behavioural ecology perspective. Phil. Trans. R. Soc. B 367, 2762-2772. ( 10.1098/rstb.2012.0216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carere C, Locurto C. 2011. Interaction between animal personality and animal cognition. Curr. Zool. 57, 491-498. ( 10.1093/czoolo/57.4.491) [DOI] [Google Scholar]
- 12.Guillette LM, Naguib M, Griffin AS. 2017. Individual differences in cognition and personality. Behav. Process. 134, 1-3. ( 10.1016/j.beproc.2016.12.001) [DOI] [PubMed] [Google Scholar]
- 13.Madden JR, Langley EJG, Whiteside MA, Beardsworth CE, Van Horik JO. 2018. The quick are the dead: pheasants that are slow to reverse a learned association survive for longer in the wild. Phil. Trans. R. Soc. B 373, 20170297. ( 10.1098/rstb.2017.0297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lucon-Xiccato T, Dadda M, Bisazza A. 2016. Sex differences in discrimination of shoal size in the guppy (Poecilia reticulata). Ethology 122, 481-491. ( 10.1111/eth.12498) [DOI] [Google Scholar]
- 15.Dougherty LR, Guillette LM. 2018. Linking personality and cognition:a meta-analysis. Phil. Trans. R. Soc. B 373, 20170282. ( 10.1098/rstb.2017.0282) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolf M, Van Doorn GS, Leimar O, Weissing FJ. 2007. Life-history trade-offs favour the evolution of animal personalities. Nature 447, 581-584. ( 10.1038/nature05835) [DOI] [PubMed] [Google Scholar]
- 17.Sonnenberg BR, Branch CL, Pitera AM, Bridge E, Pravosudov VV. 2019. Natural selection and spatial cognition in wild food-caching mountain chickadees. Curr. Biol. 29, 670-676. ( 10.1016/j.cub.2019.01.006) [DOI] [PubMed] [Google Scholar]
- 18.Bond AB, Kamil AC, Balda RP. 2007. Serial reversal learning and the evolution of behavioral flexibility in three species of North American corvids (Gymnorhinus cyanocephalus, Nucifraga columbiana, Aphelocoma californica). J. Comp. Psychol. 121, 372-379. ( 10.1037/0735-7036.121.4.372) [DOI] [PubMed] [Google Scholar]
- 19.Lucon-Xiccato T, Bisazza A. 2017. Individual differences in cognition among teleost fishes. Behav. Process. 141, 185-195. ( 10.1016/j.beproc.2017.01.015) [DOI] [PubMed] [Google Scholar]
- 20.Agrillo C, Petrazzini MEM, Tagliapietra C, Bisazza A. 2012. Inter-specific differences in numerical abilities among teleost fish. Front. Psychol. 3, 1-9. ( 10.3389/fpsyg.2012.00483) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thornton A, Lukas D. 2012. Individual variation in cognitive performance: developmental and evolutionary perspectives. Phil. Trans. R. Soc. B 367, 2773-2783. ( 10.1098/rstb.2012.0214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Healy SD, Bacon IE, Haggis O, Harris AP, Kelley LA. 2009. Explanations for variation in cognitive ability: behavioural ecology meets comparative cognition. Behav. Processes 80, 288-294. ( 10.1016/j.beproc.2008.10.002) [DOI] [PubMed] [Google Scholar]
- 23.Hofmann HA. 2003. Functional genomics of neural and behavioral plasticity. J. Neurobiol. 54, 272-282. ( 10.1002/neu.10172) [DOI] [PubMed] [Google Scholar]
- 24.Maruska KP, Fernald RD. 2018. Astatotilapia burtoni: a model system for analyzing the neurobiology of behavior. ACS Chem. Neurosci. 9, 1951-1962. ( 10.1021/acschemneuro.7b00496) [DOI] [PubMed] [Google Scholar]
- 25.Hofmann HA, Benson ME, Fernald RD. 1999. Social status regulates growth rate: consequences for life-history strategies. Proc. Natl Acad. Sci. USA 96, 14 171-14 176. ( 10.1073/pnas.96.24.14171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snyder-Mackler N, et al. 2016. Social status alters immune regulation and response to infection in macaques. Science 354, 1041-1045. ( 10.1126/science.aah3580) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson JA, Johnston RA, Lea AJ, Campos FA, Voyles TN, Akinyi MY, Alberts SC, Archie EA, Tung J. 2021. High social status males experience accelerated epigenetic aging in wild baboons. Elife 10, e66128. ( 10.7554/eLife.66128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitaker KW, Alvarez M, Preuss T, Cummings ME, Hofmann HA. 2021. Courting danger: socially dominant fish adjust their escape behavior and compensate for increased conspicuousness to avian predators. Hydrobiologica 848, 3667-3681. ( 10.1007/s10750-020-04475-9) [DOI] [Google Scholar]
- 29.Stuart-Fox DM, Moussalli A, Marshall NJ, Owens IPF. 2003. Conspicuous males suffer higher predation risk: visual modelling and experimental evidence from lizards. Anim. Behav. 66, 541-550. ( 10.1006/anbe.2003.2235) [DOI] [Google Scholar]
- 30.Koivula K, Lahti K, Rytkionen S, Koivula MO. 1994. Do subordinates expose themselves to predation? Field experiments on feeding site selection by willow tits. J. Avian Biol. 5, 178-183. ( 10.2307/3677073) [DOI] [Google Scholar]
- 31.Barreto RE, Volpato GL, Pottinger TG. 2006. The effect of elevated blood cortisol levels on the extinction of a conditioned stress response in rainbow trout. Horm. Behav. 50, 484-488. ( 10.1016/j.yhbeh.2006.06.017) [DOI] [PubMed] [Google Scholar]
- 32.Wendelmuth M, Willam M, Todorov H, Radyushkin K, Gerber S, Schweiger S. 2020. Dynamic longitudinal behavior in animals exposed to chronic social defeat stress. PLoS ONE 15, e0235268. ( 10.1371/journal.pone.0235268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson ME. 2016. An introduction to the female macaque model of social subordination stress. In Social inequalities in health in nonhuman primates. Developments in primatology: progress and prospects (eds Shively C, Wilson ME), pp. 9-24. Cham, Switzerland: Springer. [Google Scholar]
- 34.Pravosudov VV, Mendoza SP, Clayton NS. 2003. The relationship between dominance, corticosterone, memory, and food caching in mountain chickadees. Horm. Behav. 44, 93-102. ( 10.1016/S0018-506X(03)00119-3) [DOI] [PubMed] [Google Scholar]
- 35.Creel S. 2001. Social dominance and stress hormones. Trends Ecol. Evol. 16, 491-497. ( 10.1016/S0169-5347(01)02227-3) [DOI] [Google Scholar]
- 36.Fernald RD. 2014. Cognitive skills needed for social hierarchies. Cold Spring Harb. Symp. Quant. Biol. 79, 229-236. ( 10.1101/sqb.2014.79.024752) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paz-y-Miño GC, Bond AB, Kamil AC, Balda RP. 2004. Pinyon jays use transitive inference to predict social dominance. Nature 430, 778-781. ( 10.1038/nature02723) [DOI] [PubMed] [Google Scholar]
- 38.Weitekamp CA, Hofmann HA. 2017. Neuromolecular correlates of cooperation and conflict during territory defense in a cichlid fish. Horm. Behav. 89, 145-156. ( 10.1016/j.yhbeh.2017.01.001) [DOI] [PubMed] [Google Scholar]
- 39.Pongrácz P, Vida V, Bánhegyi P, Miklósi Á. 2008. How does dominance rank status affect individual and social learning performance in the dog (Canis familiaris)? Anim. Cogn. 11, 75-82. ( 10.1007/s10071-007-0090-7) [DOI] [PubMed] [Google Scholar]
- 40.Kar F, Whiting MJ, Noble DWA. 2017. Dominance and social information use in a lizard. Anim. Cogn. 20, 805-812. ( 10.1007/s10071-017-1101-y) [DOI] [PubMed] [Google Scholar]
- 41.Van Horik JO, Langley EJG, Whiteside MA, Larker PR, Beardsworth CE, Madden JR. 2018. Group social rank is associated with performance on a spatial learning task. R. Soc. Open Sci. 285, 171475. ( 10.1098/rsos.171475) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holekamp KE, Strauss ED. 2016. Aggression and dominance: an interdisciplinary overview. Curr. Opin. Behav. Sci. 12, 44-51. ( 10.1016/j.cobeha.2016.08.005) [DOI] [Google Scholar]
- 43.Oliveira RF, Hirschenhauser K, Carneiro LA, Canario AVM. 2002. Social modulation of androgen levels in male teleost fish. Comp. Biochem. Physiol. B 132, 203-215. ( 10.1016/S1096-4959(01)00523-1) [DOI] [PubMed] [Google Scholar]
- 44.Luine VN. 2008. Sex steroids and cognitive function. J. Neuroendocrinol. 20, 866-872. ( 10.1111/j.1365-2826.2008.01710.x) [DOI] [PubMed] [Google Scholar]
- 45.Maille A, Schradin C. 2017. Ecophysiology of cognition: how do environmentally induced changes in physiology affect cognitive performance? Biol. Rev. 92, 1101-1112. ( 10.1111/brv.12270) [DOI] [PubMed] [Google Scholar]
- 46.Leuner B, Mendolia-Loffredo S, Shors TJ. 2004. High levels of estrogen enhance associative memory formation in ovariectomized females. Psychoneuroendocrinology 29, 883-890. ( 10.1016/j.psyneuen.2003.08.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leranth C, Petnehazy O, MacLusky NJ. 2003. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J. Neurosci. 23, 1588-1592. ( 10.1523/jneurosci.23-05-01588.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sandstrom NJ, Kim JH, Wasserman MA. 2006. Testosterone modulates performance on a spatial working memory task in male rats. Horm. Behav. 50, 18-26. ( 10.1016/j.yhbeh.2005.09.008) [DOI] [PubMed] [Google Scholar]
- 49.Frye CA, Seliga AM. 2001. Testosterone increases analgesia, anxiolysis, and cognitive performance of male rats. Cogn. Affect. Behav. Neurosci. 1, 371-381. ( 10.3758/CABN.1.4.371) [DOI] [PubMed] [Google Scholar]
- 50.Aubele T, Kaufman R, Montalment F, Kritzer MF. 2008. Effects of gonadectomy and hormone replacement on a spontaneous novel object recognition task in adult male rats. Horm. Behav. 54, 244-252. ( 10.1016/j.yhbeh.2008.04.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mehta PH, Josephs RA. 2010. Testosterone and cortisol jointly regulate dominance: evidence for a dual-hormone hypothesis. Horm. Behav. 58, 898-906. ( 10.1016/j.yhbeh.2010.08.020) [DOI] [PubMed] [Google Scholar]
- 52.Bebus SE, Small TW, Jones BC, Elderbrock EK, Schoech SJ. 2016. Associative learning is inversely related to reversal learning and varies with nestling corticosterone exposure. Anim. Behav. 111, 251-260. ( 10.1016/j.anbehav.2015.10.027) [DOI] [Google Scholar]
- 53.Bensky MK, Paitz R, Pereira L, Bell AM. 2017. Testing the predictions of coping styles theory in threespined sticklebacks. Behav. Process. 136, 1-10. ( 10.1016/j.beproc.2016.12.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wallace KJ, Hofmann HA. 2021b. Decision-making in a social world: integrating animal cognition and social neuroscience. Curr. Opin. Neurobiol. 68, 152-158. ( 10.1016/j.conb.2021.03.009) [DOI] [PubMed] [Google Scholar]
- 55.Fernald RD, Hirata NR. 1977. Field study of Haplochromis burtoni: quantitative behavioural observations. Anim. Behav. 25, 964-975. ( 10.1016/0003-3472(77)90048-3) [DOI] [Google Scholar]
- 56.Maruska KP. 2014. Social regulation of reproduction in male cichlid fishes. Gen. Comp. Endocrinol. 207, 2-12. ( 10.1016/j.ygcen.2014.04.038) [DOI] [PubMed] [Google Scholar]
- 57.Rodriguez-Santiago M, Nührenberg P, Derry J, Deussen O, Francisco FA, Garrison LK, Garza SF, Hofmann HA, Jordan A. 2020. Behavioral traits that define social dominance are the same that reduce social influence in a consensus task. Proc. Natl Acad. Sci. USA 117, 18 566-18 573. ( 10.1073/pnas.2000158117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alcazar RM, Hilliard AT, Becker L, Bernaba M, Fernald RD. 2014. Brains over brawn: experience overcomes a size disadvantage in fish social hierarchies. J. Exp. Biol. 217, 1462-1466. ( 10.1242/jeb.097527) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gross MR. 1996. Alternative reproductive strategies and tactics: diversity within sexes. Trends Ecol. Evol. 11, 92-98. ( 10.1016/0169-5347(96)81050-0) [DOI] [PubMed] [Google Scholar]
- 60.Maruska KP, Fernald RD. 2010. Behavioral and physiological plasticity: rapid changes during social ascent in an African cichlid fish. Horm. Behav. 58, 230-240. ( 10.1016/j.yhbeh.2010.03.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ennaceur A, Delacour J. 1988. A new one-trial test for neurobiological studies of memory in rats. 1: behavioral data. Behav. Brain Res. 31, 47-59. ( 10.1016/0166-4328(88)90157-X) [DOI] [PubMed] [Google Scholar]
- 62.Broadbent NJ, Gaskin S, LR Squire, RE Clark. 2010. Object recognition memory and the rodent hippocampus. Learn. Mem. 17, 5-11. ( 10.1101/lm.1650110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Antunes M, Biala G. 2012. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn. Process 13, 93-110. ( 10.1007/s10339-011-0430-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wallace KJ, Hofmann HA. 2021a. Equal performance but distinct behaviors: Astatotilapia burtoni sex differences in a novel object recognition task and spatial maze. Anim. Cogn. 24, 1057-1073. ( 10.1007/s10071-021-01498-0) [DOI] [PubMed] [Google Scholar]
- 65.Salas C, Rodríguez F, Vargas JP, Durán E, Torres B. 1996. Spatial learning and memory deficits after telencephalic ablation in goldfish trained in place and turn maze procedures. Behav. Neurosci. 110, 965-980. ( 10.1037/0735-7044.110.5.965) [DOI] [PubMed] [Google Scholar]
- 66.Lucon-Xiccato T, Bisazza A. 2017b. Sex differences in spatial abilities and cognitive flexibility in the guppy. Anim. Behav. 123, 53-60. ( 10.1016/j.anbehav.2016.10.026) [DOI] [Google Scholar]
- 67.Jun JJ, Longtin A, Maler L. 2016. Active sensing associated with spatial learning reveals memory-based attention in an electric fish. J. Neurophysiol. 115, 2577-2592. ( 10.1152/jn.00979.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wood LS, Desjardins JK, Fernald RD. 2011. Effects of stress and motivation on performing a spatial task. Neurobiol. Learn. Mem. 95, 277-285. ( 10.1016/j.nlm.2010.12.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fischer S, Bessert-Nettelbeck M, Kotrschal A, Taborsky B. 2015. Rearing-group size determines social competence and brain structure in a cooperatively breeding cichlid. Am. Nat. 186, 123-140. ( 10.1086/681636) [DOI] [PubMed] [Google Scholar]
- 70.Solomon-Lane TK, Hofmann HA. 2019. Early-life social environment alters juvenile behavior and neuroendocrine function in a highly social cichlid fish. Horm. Behav. 115, 104552. ( 10.1016/j.yhbeh.2019.06.016) [DOI] [PubMed] [Google Scholar]
- 71.Weitekamp CA, Nguyen J, Hofmann HA. 2017. Social context affects behavior, preoptic area gene expression, and response to D2 receptor manipulation during territorial defense in a cichlid fish. Genes Brain Behav. 16, 601-611. ( 10.1111/gbb.12389) [DOI] [PubMed] [Google Scholar]
- 72.Desjardins JK, Hofmann HA, Fernald RD. 2012. Social context influences aggressive and courtship behavior in a cichlid fish. PLoS ONE 7, e32781. ( 10.1371/journal.pone.0032781) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kidd CE, Kidd MR, Hofmann HA. 2010. Measuring multiple hormones from a single water sample using enzyme immunoassays. Gen. Comp. Endocrinol. 165, 277-285. ( 10.1016/j.ygcen.2009.07.008) [DOI] [PubMed] [Google Scholar]
- 74.Wong SC, Dykstra M, Campbell JM, Earley RL. 2008. Measuring water-borne cortisol in convict cichlids (Amatitlania Nigrofasciata): is the procedure a stressor? Behaviour 145, 1283-1305. ( 10.1163/156853908785765863) [DOI] [Google Scholar]
- 75.Hänninen L, Pastell M. 2009. CowLog: open-source software for coding behaviors from digital video. Behav. Res. Methods 41, 472-476. ( 10.3758/BRM.41.2.472) [DOI] [PubMed] [Google Scholar]
- 76.Wallace KJ, Rausch RT, Ramsey ME, Cummings ME. 2020. Sex differences in cognitive performance and style across domains in mosquitofish (Gambusia affinis). Anim. Cogn. 23, 655-669. ( 10.1007/s10071-020-01367-2) [DOI] [PubMed] [Google Scholar]
- 77.Wallace KJ. 2020. cowlogdata: analyze and visualize observations generated by the event logging software CowLog: R package version 0.1.1. See https://github.com/kellyjwallace/cowlogdata.
- 78.Torchiano M. 2020. effsize: efficient effect size computation. R package version 0.8.0. See https://CRAN.R1040project.org/package=effsize.
- 79.Suzuki R, Terada Y, Shimodaira H. 2019. pvclust: hierarchical clustering with p-values via multiscale bootstrap resampling. R package version 2.2-0. See https://cran.r-project.org/web/packages/pvclust/index.html.
- 80.Huffman LS, Mitchell MM, O'Connell LA, Hofmann HA. 2012. Rising StARs: behavioral, hormonal, and molecular responses to social challenge and opportunity. Horm. Behav. 61, 631-641. ( 10.1016/j.yhbeh.2012.02.016) [DOI] [PubMed] [Google Scholar]
- 81.Stöwe M, Bugnyar T, Loretto MC, Schloegl C, Range F, Kotrschal K. 2006. Novel object exploration in ravens (Corvus corax): effects of social relationships. Behav. Process. 73, 68-75. ( 10.1016/j.beproc.2006.03.015) [DOI] [PubMed] [Google Scholar]
- 82.Spritzer MD, Meikle DB, Solomon NG. 2004. The relationship between dominance rank and spatial ability among male meadow voles (Microtus pennsylvanicus). J. Comp. Psychol. 118, 332-339. ( 10.1037/0735-7036.118.3.332) [DOI] [PubMed] [Google Scholar]
- 83.Gray JA, Buffery AWH. 1971. Sex differences in emotion and cognitive behaviour in mammals including man: adaptive and neurla bases. Acta Psychol. 35, 89-111. ( 10.1016/0001-6918(71)90014-X) [DOI] [PubMed] [Google Scholar]
- 84.Pravosudov VV. 2003. Long-term moderate elevation of corticosterone facilitates avian food-caching behaviour and enhances spatial memory. Proc. R. Soc. B 270, 2599-2604. ( 10.1098/rspb.2003.2551) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sapolsky RM, Romero LM, Munck AU. 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 21, 55-89. ( 10.1210/er.21.1.55) [DOI] [PubMed] [Google Scholar]
- 86.Maguire SM, DeAngelis R, Dijkstra PD, Jordan A, Hofmann HA. 2021. Social network dynamics predict hormone levels and behavior in a highly social cichlid fish. Horm. Behav. 132, 104994. ( 10.1016/j.yhbeh.2021.104994) [DOI] [PubMed] [Google Scholar]
- 87.Dodge KA, et al. 2015. Hostile attributional bias and aggressive behavior in global context. Proc. Natl Acad. Sci. USA 112, 9310-9315. ( 10.1073/pnas.1418572112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shors TJ, Lewczyk C, Pacynski M, Matthew PR, Pickett J. 1998. Stages of estrus mediate the stress-induced impairment of associative learning in the female rat. Neuroreport 9, 419-423. ( 10.1097/00001756-199802160-00012) [DOI] [PubMed] [Google Scholar]
- 89.Carbia PS, Brown C. 2020. Seasonal variation of sexually dimorphic spatial learning implicates mating system in the intertidal Cocos frillgoby (Bathygobius cocosensis). Anim. Cogn. 23, 621-628. ( 10.1007/s10071-020-01366-3) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data (csv) as well as analysis and visualization R code can be found at github.com/kellyjwallace/Wallace_Hofmann_2020_status_differences, via the Texas Data Repository (https://data.tdl.org/), or via request to the corresponding author.