Abstract
Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) is a common side effect persisting after completion of neurotoxic chemotherapies. This observational study was designed to evaluate the effectiveness of the dietary supplement OnLife® (patented mixture of specific fatty acids and palmitoylethanolamide) in improving symptoms of CIPN in breast and colon cancer patients.
Methods
Improvement of CIPN was evaluated in adult patients, previously treated with (neo)adjuvant paclitaxel- (breast cancer) or oxaliplatin-based (colon cancer) therapies, receiving OnLife® for 3 months after completion of chemotherapy. The primary endpoint was to compare the severity of peripheral sensory neuropathy (PSN) and peripheral motor neuropathy (PMN) before and at the end of OnLife® treatment. Secondary endpoints included the assessment of patient-reported quality of life and CIPN symptoms as assessed by questionnaires.
Results
146 patients (n = 75 breast cancer patients and n = 71 colon cancer patients) qualified for analysis; 31.1% and 37.5% of breast cancer patients had an improvement of PSN and PMN, respectively. In colon cancer patients, PSN and PMN improved in 16.9% and 20.0% of patients, respectively. According to patient-reported outcomes, 45.9% and 37.5% of patients with paclitaxel-induced PSN and PMN, and 23.9% and 22.0% of patients with oxaliplatin-induced PSN and PMN experienced a reduction of CIPN symptoms, respectively.
Conclusion
OnLife® treatment confirmed to be beneficial in reducing CIPN severity and in limiting the progression of neuropathy, more markedly in paclitaxel-treated patients and also in patients with oxaliplatin-induced CIPN.
Keywords: Peripheral sensory and motor neuropathy, Chemotherapy, Oxaliplatin, Paclitaxel, Food supplement
Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) is a common side effect of many antineoplastic drugs and often results in dose limitation, switch to less efficacious agents or even therapy discontinuation. CIPN mainly affects sensory nerves, while motor or autonomic nerve injury is rare. Therefore, most patients with CIPN experience numbness, tingling, hyperesthesia, loss of vibratory perception, and burning pain. Due to the vulnerability of the long nerves, CIPN typically appears in a “stocking and glove” distribution [1]. Antineoplastic drugs that cause CIPN include platinum compounds (e.g., cisplatin and oxaliplatin), antitubulins (vinca alkaloids [e.g., vincristine], taxanes [e.g., docetaxel and paclitaxel]), proteasome inhibitors (e.g., bortezomib), and immunomodulatory drugs (e.g., lenalidomide) [2, 3].
Taxanes, which interfere with microtubule function, and platinum compounds, which form adducts with DNA, are known to cause nerve injury and provoke neuroinflammation, the 2 hallmarks of CIPN, by a variety of distinct mechanisms. However, their cellular targets are largely the same [1, 4, 5]: the dorsal root ganglions, where both drugs accumulate, leading to cell death; mitochondria, which are damaged, thereby inducing oxidative stress as a central mediator of apoptosis, neuroinflammation, metabolic disturbances, and bioenergetic failure; Schwann cells, which are impaired, resulting in numerous disturbances in molecular function and cellular structure, thus causing degeneration of the myelin sheaths; and microglia/macrophages, astrocytes, and mast cells, which are activated, evoking neuroinflammation by the attraction and activation of immune cells and by the release and elevation of pro-inflammatory cytokines. Furthermore, both drugs are known to alter the activity and the expression of ion channels, causing an acute neurotoxicity, which is characterized by a peripheral nerve hyperexcitability.
CIPN symptoms often occur during or immediately after chemotherapy and have been reported to persist from months to years after completion of chemotherapy in a large number of patients, substantially affecting their quality of life (QoL) [6]. The extent of CIPN and the degree of recovery depend on how severe the damage to peripheral nerves had been. If the damage that has developed is not yet extensive, the tissue can be completely regenerated; in case of more serious injuries, however, neuropathy can become irreversible. Efficacious pharmacological treatment options for patients with chronic CIPN are limited. So far, only duloxetine, a selective serotonin reuptake inhibitor, showed a moderate clinical benefit in patients with painful CIPN and thus is recommended for the treatment of patients with established painful CIPN [7, 8].
The present observational study has evaluated the effectiveness of OnLife®, an oral dietary supplement based on a patented fatty acids group (FAG®) [9, 10, 11, 12], in improving CIPN when administered for 3 months in breast cancer patients having finished the (neo)adjuvant paclitaxel regimen and colon cancer patients having finished (neo)adjuvant oxaliplatin-containing regimen. The FAG® contains palmitoylethanolamide (PEA) and other fatty acids like eicosapentaenoic acid, docosahexaenoic acid, alpha-linolenic acid, linoleic acid, oleic acid, palmitic acid, and stearic acid. PEA and fatty acids, in particular polyunsaturated fatty acids (PUFAs), are known to be essential in normal physiology and metabolism of the central and peripheral nervous systems. Supplementation with PEA and fatty acids has been shown to have preventive and therapeutic effects in many psychiatric [13] and neurodegenerative diseases [14], as well as toxic neuropathies like CIPN [15, 16].
Materials and Methods
Patients
This observational study was conducted in 21 oncological sites (office-based medical oncologists) in Germany and included 149 patients. Two cohorts were defined: patients with colon cancer having finished a (neo)adjuvant oxaliplatin-containing regimen and patients with breast cancer having finished a (neo)adjuvant paclitaxel regimen. Patients were eligible if the end of their chemotherapy did not date back >4 months, if they were affected by CIPN (peripheral sensory neuropathy [PSN] and/or peripheral motor neuropathy [PMN]) grade 1–3 according to CTCAE v4.03 and if they agreed to the OnLife® treatment. Patients were ineligible if they suffered from sensory and/or motor disturbances due to other neurological diseases prior to the start of (neo)adjuvant chemotherapy, if they had alcohol abuse, if they had severe difficulty swallowing, or if they were intolerant to one of the ingredients of OnLife®. Pregnant or lactating women were also ineligible. All patients provided written informed consent before the start of the study.
Procedures
The treatment was 2 tablets/day (1 tablet every 12 h) of OnLife® for 3 months. Patients were required to attend 5 visits at the clinical site: before the start of OnLife® treatment (baseline, BL), 1 (visit 1, V1), 2 (visit 2, V2), and 3 (visit 3, V3) months after treatment start and 1 month after treatment discontinuation (final assessment, FA). The primary objective of the study was to evaluate the effectiveness of OnLife® in improving signs and symptoms of CIPN, determined as changes of PSN and PMN by at least 1 CTCAE v4.03 grade from BL to V3. The severity (grade) of PSN and/or PMN was evaluated by the treating physician (i.e., the treating oncologist; no neurologist was involved) through the following neuropathy assessments:
Anamnestic evaluation.
Measurement of deep tendon reflexes; examined at 4 sites (patellar reflex [left and right], Achilles reflex [left and right]) and categorized as follows: absent, diminished, normal, and increased.
Measurement of vibration sensitivity using a Rydel-Seiffer tuning fork; examined at 4 sites (foot ankle left and right [lateral or medial], hand ankle left and right [lateral]) and graded on the 8-point scale (higher values indicate better vibration perception).
Patients' QoL was evaluated with the questionnaire EORTC QLQ-C30, and patient-reported symptoms and functional limitations related to CIPN were assessed with the EORTC QLQ-CIPN20 questionnaire, as secondary objectives of the study. Concomitant medication used for the treatment of neuropathic pain was assessed and categorized as follows: analgesic (high-potency opioid, low-potency opioid, nonopioid), co-analgesic (neuroleptic, antidepressant), anticonvulsant, corticoid, vitamin, adjuvant drug, or other. All study assessments were scheduled at BL, V1, V2, V3, and FA.
Statistical Analysis
Due to the exploratory character of the study, no formal sample size calculation was performed. No hypothesis was tested. Descriptive analyses were performed for all available data. In subgroup analyses, patients with BL CTCAE PSN/PMN grade 1 versus BL grade 2/3 were analyzed separately. Sustained improvement of PSN/PMN severity was defined as CTCAE grade at V2 and V3 < CTCAE grade at BL. Sustained improvement of deep tendon reflexes/vibration sensitivity was defined as reflexes/vibration sensitivity at V2 and V3 better than at BL.
Results
Patient Population
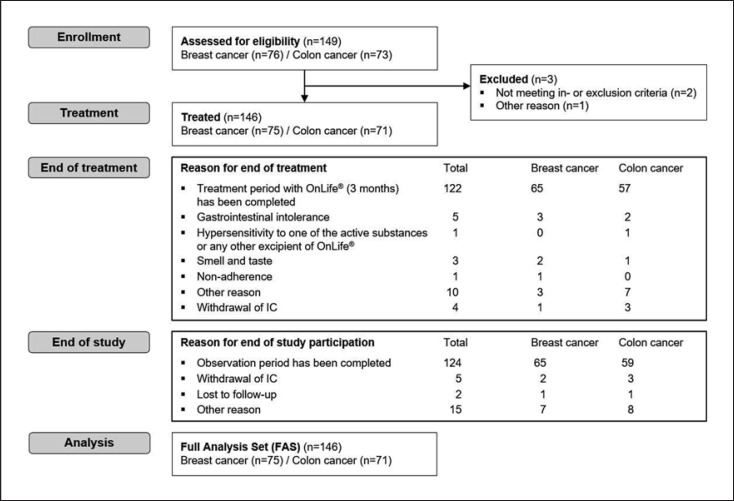
Between November 2016 and February 2018, 149 patients were assessed for eligibility, and 146 of those (75 breast cancer patients with paclitaxel-induced peripheral neuropathy [PN] and 71 colon cancer patients with oxaliplatin-induced PN) started OnLife® treatment. The majority of patients (83.6%, n = 122) completed the 3-month treatment period (shown in Fig. 1). Median cumulative dose of paclitaxel was 880.3 mg/m2 in the breast cancer cohort; 98.7% of patients (n = 74) had a PSN and 53.3% of patients (n = 40) had a PMN at BL, respectively. Median cumulative dose of oxaliplatin was 897.7 mg/m2 in the colon cancer cohort. All patients (n = 71) had a PSN and 70.4% of patients (n = 50) had a PMN at BL. Further BL patient characteristics are summarized in Table 1.
Fig. 1.
CONSORT diagram. FAS, full analysis set.
Table 1.
Baseline characteristics
| Characteristic | Patients with paclitaxel-induced PN (N = 75) | Patients with oxaliplatin-induced PN (N = 71) |
|---|---|---|
| Age, years | ||
| Median | 61.0 | 61.4 |
| Range | 37.9–86.0 | 28.2–79.3 |
| Gender, n (%) | ||
| Female | 73 (97.3) | 32 (45.1) |
| Male | 2 (2.7) | 39 (54.9) |
| ECOG performance status, n (%) | ||
| 0 | 38 (50.7) | 43 (60.6) |
| 1 | 32 (42.7) | 23 (32.4) |
| 2 | 4 (5.3) | 2 (2.8) |
| Missing | 1 (1.3) | 3 (4.2) |
| Cumulative dose of paclitaxel/oxaliplatin, mg/m2 | ||
| Median (n) | 880.3 (72) | 897.7 (70) |
| Range | 152.6–1,080.0 | 225.0–1,275.0 |
| Dose intensity of paclitaxel/oxaliplatin, mg/m2 BSA per week | ||
| Median (n) | 78.1 (72) | 38.0 (70) |
| Range | 18.4–165.5 | 7.5–50.0 |
| PSN, n (%) | ||
| Any grade | 74 (98.7) | 71 (100.0) |
| Grade 1 | 16 (21.3) | 17 (23.9) |
| Grade 2 | 54 (72.0) | 50 (70.4) |
| Grade 3 | 4 (5.3) | 4 (5.6) |
| PMN, n (%) | ||
| Any grade | 40 (53.3) | 50 (70.4) |
| Grade 1 | 24 (32.0) | 22 (31.0) |
| Grade 2 | 13 (17.3) | 26 (36.6) |
| Grade 3 | 3 (4.0) | 2 (2.8) |
BSA, body surface area; ECOG, Eastern Cooperative Group; PMN, peripheral motor neuropathy; PN, peripheral neuropathy; PSN, peripheral sensory neuropathy.
Physician-Reported Outcomes: PN Severity Assessed by CTCAE
Peripheral Sensory Neuropathy
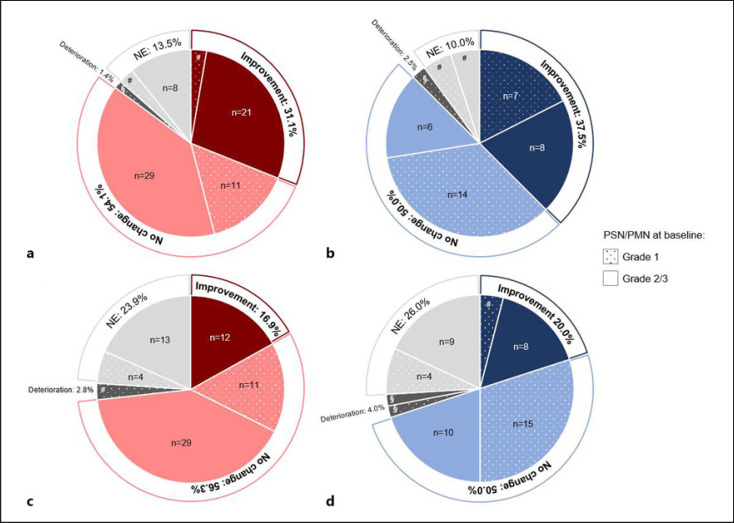
The majority of patients with paclitaxel-induced PSN (n = 74) experienced a stabilization of PSN with no further worsening (54.1%, n = 40), and almost one-third of patients had an improvement of PSN severity (31.1%, n = 23), which was evaluated as being sustained in 21.6% (n = 16) of patients (shown in online suppl. Table 1; see www.karger.com/doi/10.1159/000519000 for all online suppl. material). One patient suffered from a worsening of PSN (1.4%), and 13.5% (n = 10) of patients were not evaluable (shown in Fig. 2a; online suppl. Table 2). The benefit rate (i.e., patients with improvement or stabilization of PSN severity) was 81.3% (n = 13) in patients with BL grade 1 PSN (n = 16) compared to 86.2% (n = 50) in patients with BL grade 2/3 PSN (n = 58). And 36.2% (n = 21) of patients with BL grade 2/3 PSN had an improvement, and no patient had a deterioration (shown in online suppl. Table 2).
Fig. 2.
Changes of PSN (a, c) and PMN (b, d) by at least 1 CTCAE grade from baseline to visit 3 in patients with paclitaxel-induced PN (a, n = 74; b, n = 40) and oxaliplatin-induced PN (c, n = 71; d, n = 50); NE, not evaluable; PMN, peripheral motor neuropathy; PN, peripheral neuropathy; PSN, peripheral sensory neuropathy; §, n = 1; #, n = 2.
For the majority of patients with oxaliplatin-induced PSN (n = 71), a stabilization of PSN severity was observed (56.3%, n = 40); 16.9% (n = 12) of patients experienced an improvement, which was evaluated as being sustained in 12.7% (n = 9) of patients (shown in online suppl. Table 1). Two patients (2.8%) experienced a worsening of PSN severity and 23.9% (n = 17) of patients had no evaluation at V3 (shown in Fig. 2c; online suppl. Table 2). The benefit rate was 64.7% (n = 11) in patients with BL grade 1 PSN (n = 17) with none of the patients experiencing an improvement. For 75.9% (n = 41) of patients with BL grade 2/3 PSN (n = 54) a benefit was reported, 22.2% (n = 12) of patients experienced an improvement, and no patient had a deterioration (shown in online suppl. Table 2).
Peripheral Motor Neuropathy
In patients with paclitaxel-induced PMN (n = 40), a stabilization of PNM severity was observed in 50.0% (n = 20) of patients; 37.5% (n = 15) of patients experienced an improvement, which was evaluated as being sustained in 22.5% (n = 9) of patients (shown in online suppl. Table 1). One patient (2.5%) suffered from PMN severity worsening, while 10.0% (n = 4) of patients were not evaluable (shown in Fig. 2b; online suppl. Table 2). The benefit rate was 87.5% for all patients irrespective of the BL PMN grade, with an improvement rate of 29.2% (n = 7) in patients with BL grade 1 PMN (n = 24) and 50.0% (n = 8) in patients with BL grade 2/3 PMN (n = 16). None of the patients with BL grade 2/3 PMN had a deterioration of PMN severity (shown in online suppl. Table 2).
In total, 50.0% (n = 25) of patients with oxaliplatin-induced PMN (n = 50) had a stabilization of PMN severity. An improvement was evident in 20.0% of patients (n = 10), which was evaluated as being sustained in 14.0% (n = 7) of patients (shown in online suppl. Table 1). Two patients (4.0%) experienced a worsening of PMN severity and 26.0% (n = 13) of patients had no evaluation at V3 (shown in Fig. 2d; online suppl. Table 2). The benefit rate was 77.3% (n = 17) in patients with BL grade 1 PMN (n = 22) with 9.1% (n = 2) of patients experiencing an improvement. A benefit was reported for 64.3% (n = 18) of patients with BL grade 2/3 PMN (n = 28) with 28.6% (n = 8) having an improvement. Analysis of physician-assessed severity (CTCAE) of PSN and PMN in patients with paclitaxel-induced PN and oxaliplatin-induced PN over time is shown in online suppl. Figure 1a–d.
Vibration Sensitivity and Deep Tendon Reflexes
Sustained improvement of vibration sensitivity was observed in 40.0% (n = 30) of breast cancer patients (n = 75) and 31.0% (n = 22) of colon cancer patients (n = 71). No change in vibration sensitivity was experienced by 14.7% (n = 11) and 23.9% (n = 17) of patients, respectively. For 17.3% (n = 13) of breast cancer patients and 14.1% (n = 10) of colon cancer patients, a deterioration was reported (shown in online suppl. Table 3).
With regard to deep tendon reflexes, 20.0% (n = 15) of breast cancer patients and 16.9% (n = 12) of colon cancer patients had a sustained improvement. No change in deep tendon reflexes was experienced by 42.7% (n = 32) and 33.8% (n = 24) of patients, respectively; 16.0% (n = 12) of breast cancer patients and 19.7% (n = 14) of colon cancer patients showed a deterioration of deep tendon reflexes (shown in online suppl. Table 4).
Patient-Reported Outcomes: EORTC QLQ-C30
In both, the breast cancer and colon cancer cohort, patient-reported outcome (PRO) showed a general improvement of QoL during the study treatment. The global health status/QoL and the physical status improved continuously under OnLife® treatment, represented by an increase in their mean scores of 13.1 and 15.5 points in patients with paclitaxel-induced PN (shown in online suppl. Fig. 2a) and of 11.5 and 12.2 points in patients with oxaliplatin-induced PN (shown in online suppl. Fig. 2b) at the end of OnLife® treatment, respectively. After OnLife® discontinuation, the scores remained fairly stable or declined (shown in online suppl. Fig. 2a, b).
With regard to patient-reported pain, both, breast and colon cancer patients benefited from the treatment with OnLife®, represented by a decrease in the mean pain scale score of 16.0 and 7.5 points, respectively. Upon discontinuation of OnLife®, the mean score remained fairly stable in patients in the breast cancer cohort and further decreased in patients of the colon cancer cohort (shown in online suppl. Fig. 3a, b). Of note, the symptoms nausea and vomiting, constipation, and diarrhea improved during OnLife® treatment in both cohorts (shown in online suppl. Tables 5, 6).
PROs: EORTC QLQ-CIPN20
Peripheral Sensory Neuropathy
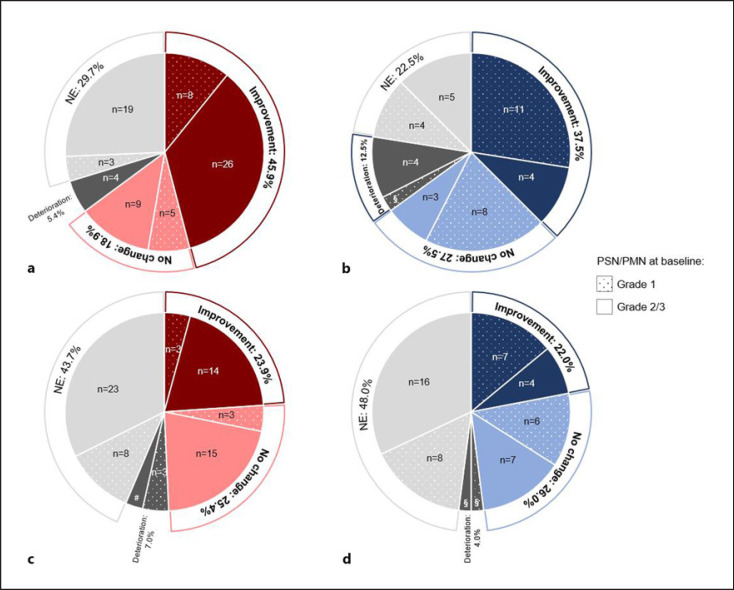
In total, 45.9% (n = 34) of patients with paclitaxel-induced PSN (n = 74) reported less symptoms and functional limitations related to PSN after OnLife® treatment. And 18.9% (n = 14) of patients reported a stabilization of symptoms, 5.4% (n = 4) reported a deterioration, and 29.7% (n = 22) were not evaluable (shown in Fig. 3a; online suppl. Table 7). The benefit rate (i.e., patients with improvement or stabilization of symptoms) was 81.3% (n = 13) in patients with BL grade 1 PSN (n = 16) compared to 60.3% (n = 35) in patients with BL grade 2/3 PSN (n = 58). None of the patients with BL grade 1 PSN reported a deterioration compared to 6.9% (n = 4) patients with BL grade 2/3 PSN (shown in online suppl. Table 7).
Fig. 3.
PROs: EORTC QLQ-CIPN20, Change in sensory scale (a, c) and motor scale (b, d) from baseline to visit 3 in patients with paclitaxel-induced PN (a, n = 74; c, n = 40) and oxaliplatin-induced PN (c, n = 71; d, n = 50); NE, not evaluable; PMN, peripheral motor neuropathy; PN, peripheral neuropathy; PRO, patient-reported outcome; PSN, peripheral sensory neuropathy; §, n = 1; #, n = 2.
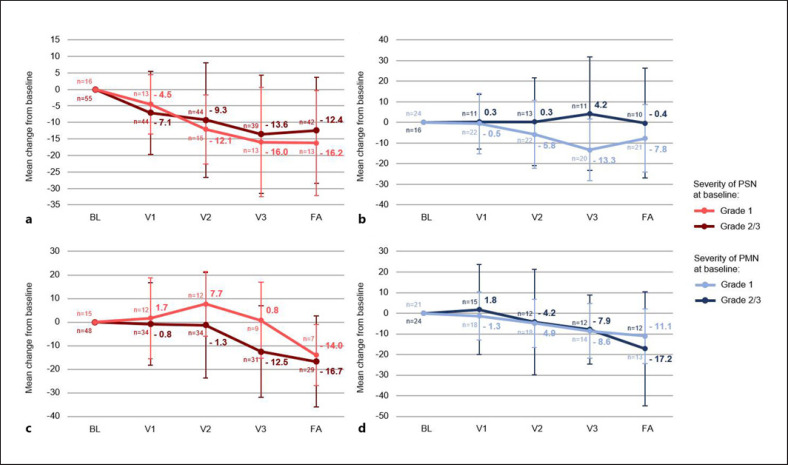
Regarding changes in the sensory scale, a continuous improvement was seen under OnLife® treatment, resulting in a decrease of the mean score of 16.0 points in patients with BL grade 1 PSN and 13.6 points in patients with BL grade 2/3 PSN after OnLife® treatment. Upon discontinuation of OnLife®, the mean scores remained stable (shown in Fig. 4a).
Fig. 4.
PROs: EORTC QLQ-CIPN20, Change from baseline in sensory and motor scale (mean, standard deviation) in patients with paclitaxel-induced PSN (grade 1, n = 16; grade 2/3, n = 58) (a), paclitaxel-induced PMN (grade 1, n = 24; grade 2/3, n = 16) (b), oxaliplatin-induced PSN (grade 1, n = 17; grade 2/3, n = 54) (c), and oxaliplatin-induced PMN (grade 1, n = 22; grade 2/3, n = 28) (d); BL, baseline; FA, final assessment; PMN, peripheral motor neuropathy; PRO, patient-reported outcome; PSN, peripheral sensory neuropathy; V1, visit 1; V2, visit 2; V3, visit 3.
Overall, 23.9% (n = 17) of patients with oxaliplatin-induced PSN (n = 71) reported less symptoms and functional limitations related to PSN after OnLife® treatment. And 25.4% (n = 18) of patients reported a stabilization of symptoms, 7.0% (n = 5) reported a deterioration, and 43.7% (n = 31) were not evaluable (shown in Fig. 3c; online suppl. Table 7). The benefit rate was 35.3% (n = 6) in patients with BL grade 1 PSN (n = 17) compared to 53.7% (n = 29) in patients with BL grade 2/3 PSN (n = 54) (shown in online suppl. Table 7). These results are also reflected by changes in the sensory scale with a decrease in the mean score of 12.5 points in patients with BL grade 2/3 PSN but even a slight increase in 0.8 points in patients with BL grade 1 PSN (shown in Fig. 4c).
Peripheral Motor Neuropathy
In all, 37.5% (n = 15) of patients with paclitaxel-induced PMN (n = 40) reported less symptoms and functional limitations related to PMN after OnLife® treatment. And 27.5% (n = 11) of patients reported a stabilization of symptoms, 12.5% (n = 5) reported a deterioration, and 22.5% (n = 9) were not evaluable (shown in Fig. 3b; online suppl. Table 7). The benefit rate was 79.2% (n = 19) in patients with BL grade 1 PMN (n = 24), and only one patient (4.2%) reported a deterioration. In patients with BL grade 2/3 PMN (n = 16), the benefit rate was 43.8% (n = 7) with 25.0% (n = 4) of patients reporting a deterioration (shown in online suppl. Table 7). Regarding changes in the motor scale, a continuous improvement was seen under OnLife® treatment for patients with BL grade 1 PSN, resulting in a decrease in the mean score of 13.3 points after OnLife® treatment. In patients with BL grade 2/3 PMN, the mean score did not decrease under OnLife® treatment (shown in Fig. 4b).
In total, 22.0% (n = 11) of patients with oxaliplatin-induced PMN (n = 50) reported less symptoms and functional limitations related to PSN after OnLife® treatment. And 26.0% (n = 13) of patients reported a stabilization of symptoms, 4.0% (n = 2) reported a deterioration, and 48.0% (n = 24) were not evaluable (shown in Fig. 3d; online suppl. Table 7). The benefit rate was 59.1% (n = 13) in patients with BL grade 1 PMN (n = 22) compared to 39.3% (n = 11) in patients with BL grade 2/3 PSN (n = 28) (shown in online suppl. Table 7).
Regarding changes in the motor scale, a continuous improvement was seen under OnLife® treatment, resulting in a decrease in the mean score of 8.6 points in patients with BL grade 1 PMN and 7.9 points in patients with BL grade 2/3 PMN after OnLife® treatment. Upon discontinuation of OnLife®, the mean score remained stable in patients with BL grade 1 PMN and further decreased in patients with BL grade 2/3 PMN (shown in Fig. 4d).
Concomitant Medication Used for the Treatment of Neuropathic Pain
At BL, 11 patients (14.7%) with paclitaxel-induced PN and 4 patients (5.6%) with oxaliplatin-induced PN received concomitant medication used for the treatment neuropathic pain. The patient numbers slightly decreased during and after OnLife® treatment with 8 (10.7%) and 7 (9.3%) patients with paclitaxel-induced PN at V3 and FA, respectively, and 3 (4.2%) and 2 (2.8%) patients with oxaliplatin-induced PN at V3 and FA, respectively (shown in online suppl. Table 8).
Discussion
CIPN is a common side effect of many antineoplastic agents and may last from months to years after chemotherapy completion, substantially affecting patients' QoL [6]. Many typologies of treatments, pharmaceutical therapies and nonpharmaceutical treatments, have been used to manage CIPN, but evidence of their clinical benefit has not been clearly determined, except for duloxetine [7, 8, 17]. The observational study STEFANO assessed the effectiveness of OnLife® in reducing the severity of CIPN in breast cancer patients with paclitaxel-induced PN and in colon cancer patients with oxaliplatin-induced PN.
The results of this analysis indicate that a dietary supplementation with OnLife® oral tablets for 3 months, starting soon after completion of the chemotherapy, can be of help in reducing or stabilizing CIPN symptoms. Overall, 85.1% and 87.5% of patients with paclitaxel-induced PSN and PMN, respectively, seemed to benefit (i.e., stabilization or improvement of PN severity) from OnLife® treatment. The benefit rates for patients with oxaliplatin-induced PSN and PMN are 73.2% and 70.0%, respectively. Also, PROs on CIPN confirmed the beneficial effect of OnLife® treatment: 64.9% and 65.0% of patients with paclitaxel-induced PSN and PMN experienced a reduction or no further worsening of CIPN symptoms, respectively. In patients with oxaliplatin-induced PSN and PMN, the patient-reported benefit rates are 49.3% and 48.0%, respectively.
The findings of the STEFANO study suggest that OnLife®, which consists of PEA and other fatty acids, mainly PUFAs, is a promising agent to manage a manifest CIPN. The endocannabinoid PEA is known to counteract paclitaxel- and oxaliplatin-induced neuroinflammation through its action on sensory neurons and on nonneuronal cells, namely, mast cells, microglia, and astrocytes [18] and to restore myelinated-fiber function [15]. Previous studies revealed that omega-3 PUFAs accelerate nerve regeneration by Schwann cell activation and prevent neuropathic pain due to their antinociceptive properties [19]. In addition, they have been shown to be protective against paclitaxel- and oxaliplatin-induced PN via their anti-neuroinflammatory properties and their ability to block voltage-gated ion channels [16, 20].
Major strengths of the STEFANO study are the prospective design and the clinical importance regarding the unmet medical need of management options for patients with a manifest CIPN. However, the interpretation of results may be hampered by the single-arm setting of the study. This study design does not allow distinguishing between a direct effect of OnLife® in improving CIPN and a treatment-independent recovery from CIPN due to discontinuation of PN-inducing chemotherapy. A recent prospective, multinational study investigated the course of CIPN in patients receiving taxanes or platinum chemotherapy over time using different measures [21]. In patients treated with taxanes, physician-assessed CIPN severity and patient-reported CIPN symptoms remained stable or even increased (the so-called “coasting phenomenon”) upon therapy discontinuation with only a noticeable decrease at the 12-month follow-up. In patients treated with platinum, a decrease in CIPN severity and symptoms was seen after therapy discontinuation; however, CIPN was not completely reversible at the 12-month follow-up. These data support that the improvement of CIPN after chemotherapy discontinuation might be at least partially a direct effect of OnLife® treatment, especially in patients with paclitaxel-induced PN.
Furthermore, our data indicate a very high tolerability of the product, which is an appreciable aspect to consider in patients who have just completed chemotherapy, which is usually not free from heavy side effects. Of the 24 patients who prematurely discontinued OnLife® treatment, only 9 did it due to intolerance (gastrointestinal intolerance, hypersensitivity to the product, smell and taste intolerance).
In summary, the findings of the observational study STEFANO indicate that OnLife® is a promising approach for the treatment of established CIPN, especially for taxane-induced CIPN. Further information will be available in the future, by a consolidated use of the OnLife® dietary supplement. In addition, a prospective, randomized, multicenter trial appears warranted to further confirm the benefit of OnLife® treatment for patients with CIPN.
Statement of Ethics
Ethics approval was provided by the Ethik-Kommission bei der Landesärztekammer Baden-Württemberg on October 12, 2016. All patients provided written informed consent before start of the study.
Conflict of Interest Statement
C.H., J.H., C.V., and M.O.Z. declare that they have no competing interests. M.Z. received an honoraria from Roche, Pfizer, Vifor, and AstraZeneca and declares a consultant or advisory role for Roche, Janssen, Celgene, BMS, Astra Zeneca, and Pfizer. J.U. declares a consultant or advisory role for Roche, Amgen, Servier, MSD, Bristol-Myers Squibb, Sanofi, Merck, Celgene, Novartis, Janssen-Cilag, Boehringer-Ingelheim, and Bayer. T.D. declares a consultant or advisory role for Novartis, Roche, and Lilly. H.C.L received an honoraria from Takeda, CSL Behring, Grifols, and Alnylam; declares a consultant or advisory role for Takeda, CSL Behring, Grifols, and Alnylam; and received research funding from Sanofi Genzyme. N.M. is chief executive officer of iOMEDICO and holds shares of this company. iOMEDICO was funded by Swiss Medical Food AG for research.
Funding Sources
The study was financially supported by Swiss Medical Food AG. Swiss Medical Food AG had no role in study design, data collection and analysis, interpretation of results, or preparation of the manuscript.
Author Contributions
C.V., H.C.L., J.H., M.Z., and N.M. were involved in study conception and design. J.U., M.O.Z., M.Z., and T.D. collected the data; J.H. performed statistical analysis; C.V. and J.H. analyzed and interpreted the data; and all authors read and approved the final version of the manuscript.
Data Availability Statement
The data generated and analyzed during the current study are available from the corresponding author upon reasonable request and only with permission of the sponsor of this study (Swiss Medical Food AG).
Study Registration
The study is registered at ClinicalTrials. gov (NCT03065478).
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Acknowledgements
The authors would like to thank Melanie Frank from iOMEDICO for statistical support, Karin Potthoff from iOMEDICO for critical review of the manuscript, and Laura Michellini from Latis Srl for writing supporting services. The authors would also like to thank the participants for their contribution to the study.
References
- 1.Zajączkowska R, Kocot-Kępska M, Leppert W, Wrzosek A, Mika J, Wordliczek J. Mechanisms of chemotherapy-induced peripheral neuropathy. Int J Mol Sci. 2019 Mar 22;20((6)):E1451. doi: 10.3390/ijms20061451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han Y, Smith MT. Pathobiology of cancer chemotherapy-induced peripheral neuropathy (CIPN) Front Pharmacol. 2013 Dec 18;4:156. doi: 10.3389/fphar.2013.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park SB, Goldstein D, Krishnan AV, Lin CS, Friedlander ML, Cassidy J, et al. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin. 2013 Dec;63((6)):419–37. doi: 10.3322/caac.21204. [DOI] [PubMed] [Google Scholar]
- 4.da Costa R, Passos GF, Quintão NLM, Fernandes ES, Maia JR, Campos MM, et al. Taxane-induced neurotoxicity: pathophysiology and therapeutic perspectives. Br J Pharmacol. 2020 Jul;177((14)):3127–46. doi: 10.1111/bph.15086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Staff NP, Cavaletti G, Islam B, Lustberg M, Psimaras D, Tamburin S. Platinum-induced peripheral neurotoxicity: from pathogenesis to treatment. J Peripher Nerv Syst. 2019 Oct;24((Suppl 2)):S26–39. doi: 10.1111/jns.12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kerckhove N, Collin A, Condé S, Chaleteix C, Pezet D, Balayssac D. Long-term effects, pathophysiological mechanisms, and risk factors of chemotherapy-induced peripheral neuropathies: a comprehensive literature review. Front Pharmacol. 2017;8:86. doi: 10.3389/fphar.2017.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loprinzi CL, Lacchetti C, Bleeker J, Cavaletti G, Chauhan C, Hertz DL, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: ASCO guideline update. J Clin Oncol. 2020 Oct 1;38((28)):3325–48. doi: 10.1200/JCO.20.01399. [DOI] [PubMed] [Google Scholar]
- 8.Jordan B, Margulies A, Cardoso F, Cavaletti G, Haugnes HS, Jahn P, et al. Systemic anticancer therapy-induced peripheral and central neurotoxicity: ESMO–EONS–EANO clinical practice guidelines for diagnosis, prevention, treatment and follow-up. Ann Oncol. 2020 Oct 1;31((10)):1306–19. doi: 10.1016/j.annonc.2020.07.003. [DOI] [PubMed] [Google Scholar]
- 9.European Patent − EP 3 177 281 A1, AGAIN LIFE ITALIA Srl. 2017.
- 10.European Patent − EP 3 219 316 A1, AGAIN LIFE ITALIA Srl. 2017.
- 11.US Patent − US 9,962,355 B2, AGAIN LIFE ITALIA Srl. 2018.
- 12.US Patent − US 10,149,827 B2, AGAIN LIFE ITALIA Srl. 2018.
- 13.Mocking RJT, Assies J, Ruhé HG, Schene AH. Focus on fatty acids in the neurometabolic pathophysiology of psychiatric disorders. J Inherit Metab Dis. 2018 Jul;41((4)):597–611. doi: 10.1007/s10545-018-0158-3. [DOI] [PubMed] [Google Scholar]
- 14.Avallone R, Vitale G, Bertolotti M. Omega-3 fatty acids and neurodegenerative diseases: new evidence in clinical trials. Int J Mol Sci. 2019 Aug 30;20((17)):E4256. doi: 10.3390/ijms20174256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Truini A, Biasiotta A, Di Stefano G, La Cesa S, Leone C, Cartoni C, et al. Palmitoylethanolamide restores myelinated-fibre function in patients with chemotherapy-induced painful neuropathy. CNS Neurol Disord Drug Targets. 2011 Dec;10((8)):916–20. doi: 10.2174/187152711799219307. [DOI] [PubMed] [Google Scholar]
- 16.Ghoreishi Z, Esfahani A, Djazayeri A, Djalali M, Golestan B, Ayromlou H, et al. Omega-3 fatty acids are protective against paclitaxel-induced peripheral neuropathy: a randomized double-blind placebo controlled trial. BMC Cancer. 2012 Aug 15;12:355. doi: 10.1186/1471-2407-12-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Lustberg MB, Hu S. Emerging pharmacological and non-pharmacological therapeutics for prevention and treatment of chemotherapy-induced peripheral neuropathy. Cancers. 2021 Feb 12;13((4)):766. doi: 10.3390/cancers13040766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petrosino S, Schiano Moriello A. Palmitoylethanolamide: a nutritional approach to keep neuroinflammation within physiological boundaries − a systematic review. Int J Mol Sci. 2020 Dec 15;21((24)):9526. doi: 10.3390/ijms21249526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silva RV, Oliveira JT, Santos BLR, Dias FC, Martinez AMB, Lima CKF, et al. Long-chain omega-3 fatty acids supplementation accelerates nerve regeneration and prevents neuropathic pain behavior in mice. Front Pharmacol. 2017;8:723. doi: 10.3389/fphar.2017.00723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esfahani A, Somi MH, Ayromlou H, Nikanfar A, Jafarabadi MA, Sadat BE, et al. The effect of n−3 polyunsaturated fatty acids on incidence and severity of oxaliplatin induced peripheral neuropathy: a randomized controlled trial. Biomark Res. 2016;4:13. doi: 10.1186/s40364-016-0066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molassiotis A, Cheng HL, Lopez V, Au JSK, Chan A, Bandla A, et al. Are we mis-estimating chemotherapy-induced peripheral neuropathy? Analysis of assessment methodologies from a prospective, multinational, longitudinal cohort study of patients receiving neurotoxic chemotherapy. BMC Cancer. 2019 Feb 8;19((1)):132. doi: 10.1186/s12885-019-5302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Data Availability Statement
The data generated and analyzed during the current study are available from the corresponding author upon reasonable request and only with permission of the sponsor of this study (Swiss Medical Food AG).