Abstract
Rationale
The long-term safety and effectiveness of bronchoscopic lung volume reduction with Zephyr endobronchial valves in subjects with severe homogeneous emphysema with little to no collateral ventilation beyond 3 months have yet to be established.
Methods
Ninety-three subjects were randomized to either bronchoscopic lung volume reduction with Zephyr valves or standard of care (SoC) (1:1). Zephyr valve subjects were assessed at 3, 6, and 12 months. SoC subjects were assessed at 3 and 6 months; they were then offered crossover to Zephyr valve treatment.
Results
The mean group difference (Zephyr valve − SoC) for change in FEV<sub>1</sub> from baseline to 6 months was 16.3 ± 22.1% (mean ± SD; p < 0.001). Secondary outcomes showed the mean between-group difference for the six-minute walk distance of +28.3 ± 55.3 m (p = 0.016); St. George's Respiratory Questionnaire, −7.51 ± 9.56 points (p < 0.001); modified Medical Research Council, −0.42 ± 0.81 points (p = 0.019); BODE index, −0.85 ± 1.39 points (p = 0.006); and residual volume of −430 ± 830 mL (p = 0.011) in favor of the Zephyr valve group. At 6 months, there were significantly more responders based on the minimal clinically important difference for these same measures in the Zephyr valve versus the SoC group. The clinical benefits were persistent at 12 months. The percentage of subjects with respiratory serious adverse events was higher in the Zephyr valve group compared to SoC during the first 30 days post-procedure but not statistically different for the Zephyr valve and SoC groups from 31 days to 6 months, and stable in the Zephyr valve group out to 12 months. There were 2 deaths in the SoC group in the 31-day to 6-month period and none in the Zephyr valve group out to 12 months.
Conclusions
Bronchoscopic lung volume reduction with Zephyr valves in subjects with severe homogeneous emphysema and little to no collateral ventilation provides clinically meaningful change from baseline in lung function, quality of life, exercise capacity, dyspnea, and the BODE index at 6 months, with benefits maintained out to 12 months.
Keywords: Bronchoscopic lung volume reduction, Emphysema, Residual volume, Lung function, Quality of life
Introduction
Chronic obstructive pulmonary disease (COPD) is the fourth leading cause of death worldwide, with an estimated 328 million people worldwide with this disease [1]. Emphysema is a phenotype of COPD, and is a progressive debilitating disease characterized by irreversible destruction of the alveolar tissue [2, 3]. The resultant progressive hyperinflation and gas trapping with impaired respiratory mechanics cause patients to experience chronic dyspnea, reduced exercise tolerance, and have a poor health-related quality of life.
A minimally invasive bronchoscopic lung volume reduction technique of implanting one-way duckbill valves is now established as means of treating the hyperinflation of emphysema for a group of selected patients [4, 5, 6]. The goal of the valve deployed in the bronchial lumen is to block the inspiratory airflow into targeted, hyperinflated regions of the lung, while allowing trapped air to escape upon exhalation until the volume is decreased. The Zephyr® endobronchial valve (Pulmonx Corporation, Redwood City, CA, USA) has been previously studied in multiple prospective, randomized trials in patients with heterogeneous emphysema [7, 8, 9, 10, 11, 12, 13].
Clinical evidence indicates that by achieving lobar occlusion in the absence of collateral ventilation, significant lung volume reduction can be obtained with associated good clinical responses. The findings from retrospective analysis of data from both the US and European cohorts of the VENT study where these criteria were met suggested that meaningful responses can be achieved in both heterogeneous and homogeneous emphysema patients [14]. This was further supported by a prospective pilot study in subjects with severe homogeneous emphysema which demonstrated that Zephyr valve placement in such subjects is feasible with an acceptable safety profile [15], and from the prospective, multicenter, randomized, controlled study IMPACT, that demonstrated clinically meaningful improvements in the lung function, exercise capacity, and quality of life at 3 months [12] in patients with homogeneous emphysema treated with Zephyr valves, and the homogeneous subset in STELVIO [10]. The present report provides longer term safety and effectiveness data on Zephyr valve treatment in patients with homogeneous emphysema.
Methods
Study Subjects
Subjects had a diagnosis of homogeneous emphysema based on a difference in the emphysema destruction scores (heterogeneity index using −910 HU cutoff) between target and adjacent lobes of <15%, and an absolute difference in perfusion (using perfusion scintigraphy) between right and left lungs of ≤20% [12]. Further key inclusion criteria were age ≥40 years; a diagnosis of COPD with FEV1≥ 15% predicted and ≤45% predicted despite optimal medical therapy; total lung capacity > 100% predicted; residual volume (RV) ≥ 200% predicted; six-minute walk distance (6MWD) ≥150 m; nonsmoker >8 weeks; and little to no collateral ventilation in the target lobe (assessed with Chartis® Pulmonary Assessment System, Pulmonx Corporation, Redwood City, CA, USA).
Study Design
This was a multicenter, prospective, randomized, controlled, one-way crossover study (NCT02025205) approved by the respective Institutional Ethics Committees. All participating subjects provided informed consent. Methods were described previously by Valipour et al. [12]. Assessments were performed at 30 days, 3, 6, and 12 months (12 months Zephyr valve group only) post-enrollment. Subjects in the standard of care (SoC) control arm if eligible, were crossed over to Zephyr valve treatment after completing 6 months follow-up. Follow-up data out to 3 months were previously described [12]. Randomized results out to 6 months and single-arm results out to 12 months are reported here. Zephyr valve subjects were assessed at 6 and 12-month follow-up.
Outcome Measures
Effectiveness outcomes were assessed by comparing the between-group differences for changes from baseline to 6 months for Zephyr valve and SoC groups, and for the Zephyr valve group only at 12 months. Outcome variables assessed included percentage and absolute change in FEV1(L) and RV; absolute change in quality of life using the St. George's Respiratory Questionnaire (SGRQ), 6MWD, the dyspnea scale (modified Medical Research Council [mMRC]), the COPD assessment test (CAT) score, and the BODE index (a composite score that combines the Body mass index, FEV1[% predicted], mMRC, and 6MWD). The percent of subjects achieving the minimal clinically important difference (MCID) for FEV1, RV, SGRQ, 6MWD, mMRC, CAT, and the BODE index was also assessed at 6 and 12 months.
Adverse Events and Safety
Adverse events solicited at each visit were used to assess safety. Severity and relatedness to the device/procedure were assessed by the investigator. Pneumothorax, an anticipated adverse event following valve placement, was managed according to published guidelines [16].
Statistical Analyses
All data are reported for intention-to-treat population with imputation of missing data using the “Last Observation Carried Forward” method. The differences in absolute and percentage change from baseline at 6 months for the Zephyr valve and SoC groups were analyzed by the t test. Comparison of absolute and percent change from baseline to 6 and 12 months was analyzed by the paired sample t test. Responder data at 6 months were analyzed by the χ2 test, and responder rates were analyzed by the McNemar's p value. The Fisher's exact test was used to compare adverse events between groups.
Results
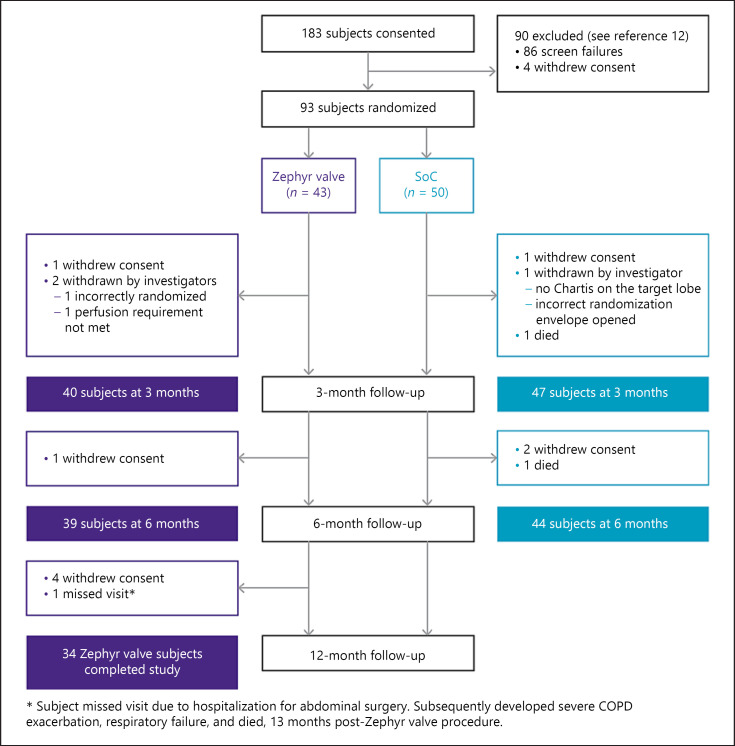
A total of 93 subjects were randomized, using a 1:1 randomization into the IMPACT study (43 Zephyr valve, and 50 SoC; see CONSORT diagram, Fig. 1) between August 2014 and January 2016. Following the 6-month evaluation, 41 SoC subjects were crossed over to Zephyr valve treatment. At 12 months, 34 of the initial Zephyr valve cohorts (6 withdrew consent, 2 withdrawn by investigator, and 1 missed visit) were evaluated.
Fig. 1.
Consort diagram.
Both groups were well matched for all baseline demographics and clinical characteristics [12]: the mean age, 64.3 versus 63.2 years; smoking history, 41.5 versus 42.5 pack years; post-bronchodilator FEV1, 28.4 versus 29.9% of predicted; RV, 277.3 versus 273.7% of predicted; total lung capacity, 144.9 versus 144.2% of predicted; 6MWD, 308 versus 328 m; and the SGRQ score, 63.2 versus 59.3 points, respectively for the Zephyr valve and SoC groups.
Verification of technical success of valve placement in the Zephyr valve-treated subjects was performed at 30 days post-implantation. Twenty-one subjects showed no evidence of functional benefits (i.e., >12% increase in FEV1, and/or >10% reduction in RV) and/or signs of volume reduction on the follow-up chest X-ray. A secondary bronchoscopy for valve adjustment was performed in 6 of these subjects. A repeat bronchoscopy was performed in 4 subjects, but no adjustments were made, and in 11 subjects the physician opted not to perform a repeat bronchoscopy for valve adjustment.
Effectiveness Outcomes
Six-Month Evaluation of Zephyr Valve and SoC Groups
Between-group differences for the changes from baseline for the key effectiveness outcomes to 6 months are summarized in Table 1. The mean group difference (∆ Zephyr valve − SoC) for change in FEV1from baseline to 6 months for subjects was 120.0 ± 150.0 mL (mean ± SD; p < 0.001) reflecting a mean percent difference of 16.3 ± 22.1% (p < 0.001). The key secondary outcome measures at 6 months were also in favor of the Zephyr valve group with statistically significant improvements for RV, 6MWD, and the SGRQ score, whereas mMRC and CAT did not exceed the threshold for statistical significance. The multidimensional BODE index showed a significant improvement in the Zephyr valve group compared to SoC (between-group difference of −1.17 ± 1.31 points at 3 months [12] and −0.85 ± 1.39 points at 6 months [p < 0.001, p = 0.006], respectively).
Table 1.
Changes from baseline for outcome measures (intention-to-treat)
| Outcome | Change from baseline to 6 months |
Change from baseline to 12 months |
p value† comparing change at 6 months to change at 12 months | ||||
|---|---|---|---|---|---|---|---|
| Zephyr valve (n) | SoC (n) | ∆ Zephyr valve − SoC | p value | Zephyr valve (n) | p value | ||
| FEV1(absolute) | 80±180 (43) | −40±120 (50) | 120±150 | <0.001 | 50±180 (41) | 0.092 | 0.060 |
| FEV1, mL responders, MCID ≥ +100 mL | 30.2% | 8.0% | 22.2% | 0.006 | 29.3% | ||
| FEV1, % | 11.54±28.58 (43) | −4.73±14.38 (50) | 16.3±22.1 | <0.001* | 8.29±28.42 (41) | 0.069* | 0.090 |
| FEV1, mL responders, MCID ≥ +12% | 30.2% | 10.0% | 20.2% | 0.014# | 29.3% | ||
| RV, mL | −480±890 (43) | −60±770 (50) | −430±830 | 0.015* | −460±1,000 (42) | 0.005* | 0.598 |
| RV, mL responders, MCID ≤ −310 mL | 53.5% | 30.0% | 23.5% | 0.022# | 50.0% | ||
| 6MWD, m | 21.3±57.5 (42) | −7.1±53.4 (50) | 28.3±55.3 | 0.016* | 3.7±74.6 (40) | 0.759* | 0.059 |
| 6MWD responders, MCID ≥ +26 m | 45.2% | 22.0% | 23.2% | 0.018# | 32.5% | ||
| SGRQ total score (points) | −6.84±9.76 (36) | 0.63±9.42 (48) | −7.51±9.56 | <0.001* | −4.01 ± 10.14 (40) | 0.017* | 0.036 |
| SGRQ responders, MCID ≤ −4 points | 63.9% | 31.3% | 32.8% | 0.003# | 52.5% | ||
| CAT total score (points) | −1.57±5.05 (42) | −0.87±3.93 (45) | −0.70±4.51 | 0.468* | −0.35±4.96 (43) | 0.647* | 0.110 |
| CAT responders, MCID ≤ −2 points | 52.4% | 37.8% | 14.6% | 0.171# | 39.5% | ||
| mMRC dyspnea scale (points) | −0.24±0.89 (41) | 0.17±0.74 (46) | −0.42±0.81 | 0.019** | −0.10±1.03 (42) | 0.553* | 0.359 |
| mMRC responders, MCID ≤ −1 point | 34.1% | 10.9% | 23.2% | 0.009# | 28.6% | ||
| BODE index (points) | −0.50±1.62 (40) | 0.35±1.16 (46) | −0.85±1.39 | 0.006** | −0.05±1.84 (38) | 0.861* | 0.096 |
| BODE index responders, MCID ≤ −1 point | 50.0% | 17.4% | 32.6% | 0.001# | 31.6% | ||
Values are means ± SD. SoC, standard of care; RV, residual volume; 6MWD, six-minute walk distance; SGRQ, St. George's Respiratory Questionnaire; mMRC, modified Medical Research Council; CAT, COPD assessment test; MCID, minimal clinically important difference.
t test p value.
Wilcoxon p value.
χ2 p value.
Paired sample t test p value.
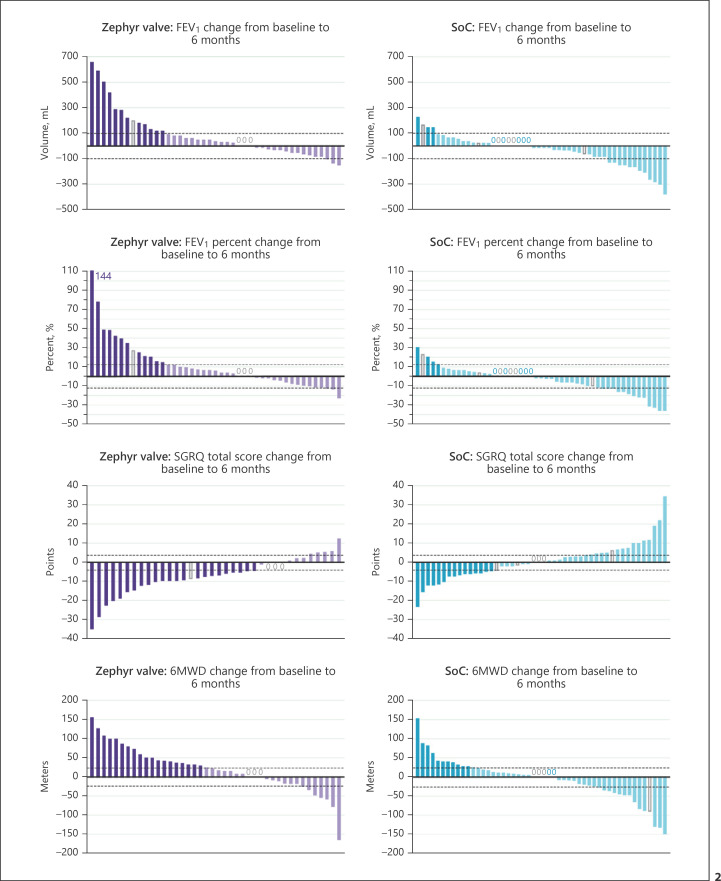
The responders (percent of subjects) who met or exceeded the MCID for each of the outcome measures of FEV1, RV, SGRQ, 6MWD, mMRC, and the BODE index were significantly greater in the Zephyr valve versus the SoC group at 6 months (Table 1). The individual subject changes from baseline to 6 months for FEV1(absolute and percent change), SGRQ, and 6MWD are provided in the waterfall plots in Figure 2. The responder rate for FEV1(improvement of ≥100 mL) in the Zephyr valve versus the SoC group was 30.2 and 8.0%, respectively; for SGRQ was 63.9 and 31.3%, respectively; and for 6MWD was 45.2 and 22.0%, respectively. For each of these measures, more subjects in the Zephyr valve group compared to the SoC group experienced an improvement that met and exceeded the MCID. Conversely, more subjects in the SoC group experienced a worsening in the measure over and above the MCID threshold.
Fig. 2.
Each bar represents an individual subject. Purple (Zephyr valve) and blue (SoC) bars represent subjects that met or exceeded MCID for FEV1(absolute change) of 100 mL and (percent change) of ≥12% improvement in FEV1, SGQR (−4 points), and 6MWD (+26 m). The lighter colored bars represent subjects who did not meet the MCID. Dotted line represents the MCID. The open grey bars and grey “0” represent the subject with imputed data using the “Last Observation Carried Forward” method. SoC, standard of care; 6MWD, six-minute walk distance; MCID, minimal clinically important difference; SGRQ, St. George's Respiratory Questionnaire.
Twelve-Month Evaluation of the Zephyr Valve Group Only
Changes from baseline to 12 months for the initial Zephyr valve cohort are also summarized in Table 1. The reduction in RV from baseline was significant (∆ = −460 ± 1000 mL; p = 0.004), as was the improvement in SGRQ (∆ = −4.01 ± 10.14 points; p = 0.017), while the change from baseline in FEV1did not achieve statistical significance (p = 0.092).
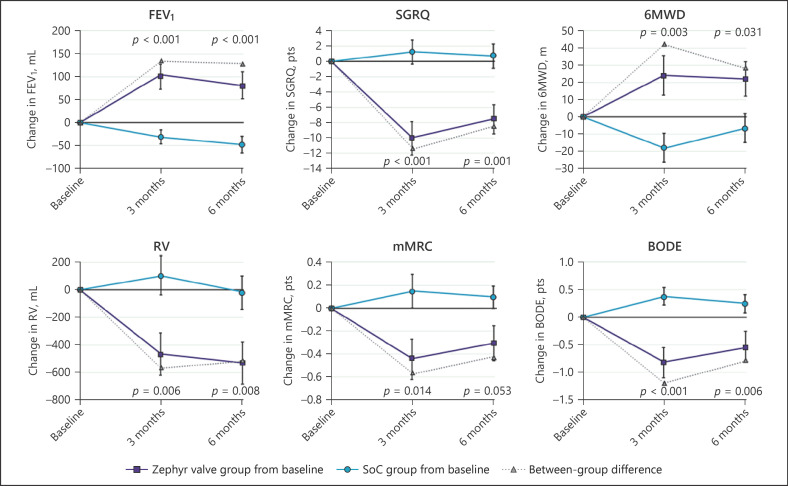
Durability of effect from 6 to 12 months was investigated by comparing the subject-level response for subjects with data at both time points (Table 1). Of the subjects who were FEV1responders at 6 months (≥ +12% change from baseline), 69.2% continued to be responders at 12 months, while 30.8% became nonresponders. In addition, 10.7% of FEV1nonresponders at 6 months became responders at 12 months (McNemar's p value, NS). Similarly, for RV, 78.2% of subjects who were responders at 6 months (≤ −310 mL change from baseline) remained RV-responders with 21.8% becoming nonresponders at 12 months, and 15.8% RV nonresponders at 6 months were RV-responders at 12 months (McNemar's p value, NS). The other outcomes showed a similar pattern from 6 to 12 months. Figure 3 shows graphically the changes from baseline to 3 and 6 months for the key effectiveness outcomes for the Zephyr valve and SoC groups, demonstrating the overall durability of effectiveness of the Zephyr valves.
Fig. 3.
Date are presented as means ± SEM at each time point for the Zephyr valve group (■) and SoC group (o). The difference between group means is represented by (∆). Within each variable, data for the same subjects are reported at each time point; this represents the completed cases at 6 months for each measure with imputations at 3 months for any subject with a missing value. Imputations were performed using the “Last Observation Carried Forward” method. p values for FEV1, SGRQ, 6MWD, and RV from the Student's t test, and for mMRC and the BODE index from the Wilcoxon test. SoC, standard of care; RV, residual volume; 6MWD, six-minute walk distance; SGRQ, St. George's Respiratory Questionnaire; mMRC, modified Medical Research Council.
Safety
During the first 6 months, there were 111 respiratory adverse events in the Zephyr valve group, of which 64% were non-serious; and 54 events in the SoC group, of which 67% were non-serious. The common respiratory non-serious adverse events included COPD exacerbations, common cold, cough, pulmonary infection, and thoracic pain which occurred in both groups. The short-term safety of the Zephyr valve procedure compared to SoC out to 3 months has previously been reported [12]. There is a higher frequency of respiratory adverse events in the short-term Zephyr valve group compared to the SoC group. The most significant serious adverse event (SAE) in the Zephyr valve group being pneumothorax and COPD exacerbations. Table 2 shows the SAEs for the treatment period (day of procedure to 30 days), the period from 31 days to 6 months (both groups), and from >6 to 12 months (Zephyr valve group only; SoC subjects were excited for crossover option at 6 months). During the treatment period, 44.1% subjects in the Zephyr valve group compared to 1.0% subjects in the SoC group reported one or more respiratory SAEs. From 31 days to 6 months, 35% subjects in the Zephyr valve group and 26% subjects in the SoC group reported one or more respiratory SAE (Table 2). During the period from >6 to 12 months, the percentage of subjects with respiratory SAEs in the Zephyr valve group remained stable (30%). Beyond the 31-day treatment period, the most frequently observed respiratory SAE was COPD exacerbation requiring hospitalization, which occurred at similar rates in the Zephyr valve and SoC groups during the 31-day to 6-month period (19 vs. 20%, respectively). COPD exacerbations requiring hospitalization were also stable in the Zephyr valve group out to 12 months (16%). Two subjects (4.0%) in the SoC group died as a result of respiratory failure during the 6-month period. There were no deaths in the Zephyr valve group out to 12 months.
Table 2.
Respiratory SAEs by time period
| Event | Day of procedure to 30 days |
31 days to 6 months |
>6 months to 12 months |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zephyr valve |
SoC |
Fisher p value* | Zephyr valve |
SoC |
Fisher p value* | Zephyr valve |
|||||||
| No. of events | % of subjects | No. of events | %of subjects | No. of events | % of subjects | No. of events | % of subjects | No. of events | % of subjects | ||||
| Respiratory SAEs | 20 | 44.2 | 1 | 1 | <0.001 | 19 | 34.9 | 17 | 26.0 | 0.269 | 17 | 30.2 | |
| Pneumothorax | 10 | 23.3 | 0 | 0 | <0.001 | 2a | 4.7 | 0 | 0 | 0.211 | 1a | 2.3 | |
| COPD exacerbation | 6 | 14.0 | 1 | 1 | 0.046 | 12 | 18.6 | 10 | 20.0 | 1.000 | 9 | 16.3 | |
| Valve dislocation or migration | 2 | 2.3 | 0 | 0 | 0.462 | 2 | 4.6 | 0 | 0 | 0.462 | 2b | 4.6 | |
| Dyspnea | 1 | 2.3 | 0 | 0 | 0.462 | 2 | 4.7 | 0 | 0 | 0.211 | 1 | 2.3 | |
| Purulent bronchitis | 1 | 2.3 | 0 | 0 | 0.462 | 0 | 0 | 1 | 2.0 | 1.000 | 1 | 2.3 | |
| Pneumonia | 0 | 0 | 0 | 0 | N/A | 1 | 2.3 | 2 | 4.0 | 1.000 | 0 | 0 | |
| Cardiogenic pulmonary edema | 0 | 0 | 0 | 0 | N/A | 0 | 0 | 1 | 2.0 | 1.000 | 0 | 0 | |
| Hypercapnia | 0 | 0 | 0 | 0 | N/A | 0 | 0 | 3 | 6.0 | 0.246 | 0 | 0 | |
| Pulmonary infection | 0 | 0 | 0 | 0 | N/A | 0 | 0 | 0 | 0 | N/A | 1 | 2.3 | |
| Lung transplantation | 0 | 0 | 0 | 0 | N/A | 0 | 0 | 0 | 0 | N/A | 1 | 2.3 | |
Safety population includes all 43 subjects in the Zephyr valve group and 50 subjects in the SoC group. SoC, standard of care; COPD, chronic obstructive pulmonary disease; SAEs, serious adverse events.
p value comparing Zephyr valve and SoC percent of subjects at 6 months.
Pneumothorax following a recent bronchoscopy procedure for a valve “revision” procedure (1 event); and spontaneous pneumothorax, occurring at 50 days post-procedure (1 event).
One event was a valve expectoration with the valve successfully replaced.
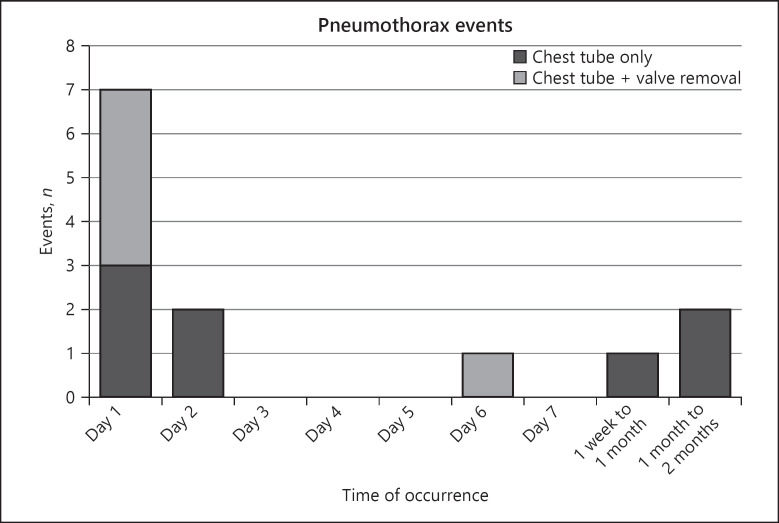
The most common post-procedural complication was pneumothorax with 13 events in 11 (25.6%) subjects in the initial EBV cohort, of which 11 (84.6%) events were severe, and 2 (15.4%) events were moderate. The time of occurrence from the most recent bronchoscopy procedure and the management of each pneumothorax is summarized in Figure 4.
Fig. 4.
Data represent time of pneumothorax occurrences after the most recent bronchoscopy procedure in the Zephyr valve group. Each bar represents the number of events per time period. Bars are color coded to represent how the pneumothorax events were managed: dark grey = chest tube only; light grey = chest tube plus valve removal. Valves were removed in 5 events to manage the pneumothorax, and were replaced at a later date in 3 cases.
Discussion
Patients with severe homogeneous emphysema with hyperinflated lungs and low lung function remain poor candidates for lung volume reduction surgery, and thus are severely limited in their treatment options [17]. The findings from the present, randomized, controlled trial demonstrate durable clinical benefits of Zephyr valve treatment over 12 months in patients with homogeneous emphysema.
The increased RV in advanced COPD as an expression of the static hyperinflation further increases disproportionately under exercise, thus resulting in a high-end expiratory lung volume followed by shortness of breath and a reduced exercise capacity of these patients [18]. Thus, for lung volume reduction procedures, RV is particularly important, as it is both a predictor for better outcome and an objective outcome measure resulting from the physiological changes achieved with Zephyr valve treatment [19]. Therapeutic strategies therefore have to aim for a significant reduction of the hyperinflation and lung volumes in order to improve respiratory mechanics, physical activity, and even symptoms [18]. Additionally, as COPD is a progressively worsening disease, particularly in patients with homogeneous disease, where the decline is rapid [20], treatment strategies that prevent or slow down further deterioration can be very impactful.
The data from this randomized, controlled trial validate the earlier findings of a smaller feasibility study [15], and subgroup analysis of the VENT [7] and STELVIO studies [10, 21], which showed the effectiveness of Zephyr valves in patients with emphysema of homogeneous distribution with benefits that are clinically significant, and extend the previous 3-month findings [12] with benefits that are maintained over a longer period. Determination of the degree of emphysema in this study was based on the voxel density at −910 HU on the CT rather than at −950 HU to be consistent with prior studies, and is unlikely to have impacted target lobe selections or outcomes.
The present study showed clinically and statistically significant improvements in the hyperinflation (reduction in RV) and other important COPD outcomes in the Zephyr valve-treated subjects over the SoC controls at 6 months. In order to determine the efficacy of valve therapy, the statistical significance alone is not sufficient. The MCID which better describes the real clinical benefit of the patients in daily life should be considered. Therefore, thresholds have been defined in the past years for different parameters like RV, TLVR, FEV1, SGRQ, or 6MWD for patients with severe emphysema. Individual subject-level data at 6 months showed significantly more responders based on the MCID for each of the clinical outcome measures of FEV1, RV, 6MWD, SGRQ, CAT, mMRC, and the BODE index in the Zephyr valve versus the SoC group, while a larger number of subjects in the SoC group deteriorated by more than the MCID threshold as reflected in the waterfall plots for FEV1, SGRQ, and 6MWD in Figure 2. Although the protocol recommended consideration of a repeat bronchoscopy for valve adjustment in subjects who either had less than a 12% increase in FEV1, or <10% decrease in RV, or no evidence of lobar volume reduction at 30 days, this was not a mandated requirement. As such, the investigators opted not to perform a repeat bronchoscopy in 11 cases, and in 4 cases they performed a bronchoscopy but did not perform a valve adjustment; none of the patients refused a second procedure. A treatment reversal was not offered in case of a lack of response, although this is often the case in the current clinical practice.
The clinical benefits were persistent at 12 months, with the 12-month responder rates in the Zephyr valve group comparable to responder rates at 6 months. While the magnitude of change over baseline was smaller in some endpoints for the Zephyr valve-treated subjects at 12 versus 6 months, these outcomes remained improved over baseline, and for most measures were not significantly different when compared with the 6-month visit; unfortunately, without a control group, the effect size is hard to determine. This observation is consistent with previous reports from both surgical and endoscopic lung volume reduction studies [17, 18, 21]. To better appreciate the magnitude of change that was observed, it is helpful to consider the natural progression of the disease in this patient population. In NETT, at 1 year, the nonhigh-risk untreated control population that included patients with both heterogeneous and homogeneous emphysema had a decline of 40 m in the 6MWD, and an increase of 3.2 points in the SGRQ score [17]. More recent data from the RENEW study evaluating endobronchial coils similarly showed declines in homogeneous patients (RV > 225%, medical management only group), as indicated by worsening of key measures at 1 year with a 2.4% decrease in FEV1, a 13.5 m decrease in the 6MWD, and a 2.1-point increase in the SGRQ score [22]. The fact that medically managed patients with severe homogeneous emphysema are observed to deteriorate more rapidly over time provides an important context for the evaluation of new treatments in the severe homogeneous emphysema patient population. Importantly, the SGRQ data at 12 months in the Zephyr valve group continued to demonstrate an improvement with a decline of 4 points compared with baseline, indicating clinically meaningful improvements in quality of life after 1 year.
The most frequent respiratory SAE reported during the 31-day to 6-month period was COPD exacerbation, which was similar between the Zephyr valve and SoC groups, and pneumothorax that occurred in the Zephyr valve group. COPD exacerbations were also stable in the Zephyr valve group out to the 6- to 12-month period (16.3%). While the small sample size in this study is not sufficient to show statistical significance for the safety endpoints, the numerically lower incidence of typical COPD-related respiratory and cardiac events in the Zephyr valve group compared to SoC suggests that patients do not get worse. In fact, the data from the larger LIBERATE study, a RCT of Zephyr valve versus medically managed control [13] showed a reduction in COPD exacerbations in Zephyr valve-treated patients over the longer term. The timing of post-procedure pneumothoraces was similar to that seen in the LIBERATE study in heterogeneous patients with the majority of pneumothoraces occurring within the first 3 days after valve placement and a smaller number occurring late [13]. Thus, it is imperative that patients be appropriately educated at the time of discharge on the symptoms of a pneumothorax and to seek urgent medical attention if symptoms of a pneumothorax occur. Importantly, there were no deaths in the Zephyr valve group in the IMPACT study over the 12-month follow-up, while 2 deaths occurred in the SoC group who were monitored out to 6 months. Of note, some patients in the present study met the criteria for the high-risk population in the NETT trial (defined as FEV1≤ 20% predicted and either homogeneous emphysema or a carbon monoxide diffusing capacity of ≤20% predicted). In NETT, these patients experienced about a 20% death rate over 12-month follow-up [17, 23]. Eight subjects (5 Zephyr valve, 3 SoC) in the IMPACT study met the NETT high-risk definition. Of these 8 subjects, there was 1 death in the SoC group (1/3 subjects, 33%) and none in the Zephyr valve group (0/5 subjects).
The main limitation of the study was not maintaining the SoC control group out to 12 months. SoC subjects were crossed over to Zephyr valve treatment at 6 months, and thus were not available for comparison to Zephyr valve at 12 months. While important scientifically, withholding of a commercially available treatment option over a longer period for this needy patient group represented an ethical and practical challenge. Another limitation was the lack of a sham control group. Because valves are visible on CT and X-ray, a sham could not be performed. However, we believe that the results remain valid since important objective outcomes such as RV and FEV1demonstrated successful and meaningful effectiveness out to 12 months with an acceptable safety profile. In addition, the changes in subjective endpoints were consistent with those of objective outcomes.
Conclusion
The IMPACT study demonstrates that bronchoscopic lung volume reduction with the Zephyr endobronchial valve in selected patients with severe homogeneous emphysema with little to no collateral ventilation provides clinically meaningful improvement in the pulmonary function, quality of life, exercise capacity, and RV (reduction in hyperinflation) over 6 months compared to the medically managed control group. Benefits were durable out to at least 12 months when compared to the baseline levels of the Zephyr valve group with a comparable number of patients achieving the MCID for the various measures at both 6 and 12 months, with a favorable safety profile. Zephyr valves provide an acceptable treatment option for patients with severe homogeneous emphysema if they meet all the other criteria for bronchoscopic lung volume reduction.
Statement of Ethics
This was a multicenter, prospective, randomized, controlled, one-way crossover study (NCT02025205) approved by the respective Institutional Ethics Committees. All participating subjects provided informed consent.
Conflict of Interest Statement
R.E.: I have received personal fees from Olympus Europa, Pulmonx, and Broncus, outside the submitted work. D.-J.S.: I am a physician advisor, consultant, and investigator for Pulmonx, Redwood City, CA, USA. F.J.F.H.: I am a physician advisor, consultant, and investigator for Pulmonx, Redwood City, CA, USA. K.D.: I am a physician advisor, consultant, and investigator for Pulmonx, Redwood City, CA, USA. M.W.: I have received an educational grant, clinical research grant, and speaker fees from Pulmonx Corporation, outside the submitted work. J.H.F.: I have received personal fees from Pulmonx, and grants from Uptake, outside the submitted work. C.P.: I am an investigator for Pulmonx and received honoraria from Pulmonx for presentations and travel expenses, outside the submitted work. R.-H.H.: I have received personal fees and nonfinancial support from Pulmonx, outside the submitted work. F.S.: I have received fees from Pulmonx, during the conduct of the study. N.S.S.: I am an employee of Pulmonx (study sponsor) and have stock option grants. A.V.: I have received personal fees from Pulmonx, Olympus Spiration, and BTG, outside the submitted work.
Funding Sources
This study was sponsored by Pulmonx Corporation, Redwood City, CA, USA.
Author Contributions
Ralf Eberhardt, MD, Professor: R.E. was the coprincipal investigator and member of the Data and Safety Monitoring Board of this study. He recruited and treated patients within this trial, collected study data, helped with interpretation of the data, co-drafted, revised, and approved the final manuscript for submission. Dirk-Jan Slebos, MD, PhD, Professor: D.-J.S. was an investigator in this study and member of the Data and Safety Monitoring Board of this study. D.-J.S. actively recruited and treated patients in this study, collected study data, helped with interpretation of the data, and assisted in the revision and approved the final manuscript for submission. Felix J.F. Herth, MD, Professor: F.J.F.H. was an actively recruiting investigator in this study and responsible for the overall oversight of the study conduct, assisted in the revision of the manuscript, and approved the final manuscript for submission. Kaid Darwiche, MD: K.D. actively recruited and treated patients in this study, collected study data, and assisted in the revision of the manuscript, and approved the final manuscript for submission. Manfred Wagner, MD: M.W. actively recruited and treated patients in this study, collected study data, assisted in the revision of, and approved the final manuscript for submission. Joachim H. Ficker, MD, Professor: J.H.F. actively recruited and treated patients in this study, collected study data, assisted in the revision of, and approved the final manuscript for submission. Christoph Petermann, MD: C.P. actively recruited and treated patients in this study, collected study data, assisted in the revision of, and approved the final manuscript for submission. Ralf-Harto Hübner, MD: R.-H.H. actively recruited and treated patients in this study, collected study data, assisted in the revision of, and approved the final manuscript for submission. Franz Stanzel, MD: F.S. actively recruited and treated patients in this study, collected study data, assisted in the revision of, and approved the final manuscript for submission. Narinder S. Shargill, PhD: N.S.S. oversaw the trial operations, supported the analysis and interpretation of the data, developed the data tables and figures, reviewed and approved the final manuscript for submission. Arschang Valipour, MD, FCCP. Assoc. Professor: A.V. was the principal investigator and member of the Data and Safety Monitoring Board of this study. He recruited and treated patients within this trial, collected study data, helped with interpretation of the data, drafted and assisted in the revision, and approved the final manuscript for submission.
Acknowledgements
The authors thank Åsa Nilsson, PhD, and the team at Devicia AB (Mölndal, Sweden) for providing oversight and data monitoring for this study, and Anders Ljungström, BA (Progstat AB, Tumba, Sweden) for performing the statistical analyses. The IMPACT study team: Otto-Wagner-Spital, Vienna: Arschang Valipour, Marina Duller, Maria-Christine Leitgeb, and Christine Abele; UMC Groningen, Groningen: Dirk-Jan Slebos, Karin Klooster, Jorine Hartmann, Wouter van Geffen, and Nick H. Ten Hacken; Thoraxklinik-Heidelberg, Heidelberg: Ralf Eberhardt, Felix Herth, Daniela Gompelmann, Dominik Harzheim, Konstantina Kontogianni, Maren Schuhmann, and Brigitte Rump; University Clinic Essen, Essen: Kaid Darwiche, Ruediger Karpf-Wissel, Gerhard Weinreich, Jane Winantea, Diana Hartmann, Ulrike Sampel, and Jennifer Thälker; Klinikum Nürnberg/Paracelsus Medical University Nürnberg, Nuremberg: Manfred Wagner, Joachim H. Ficker, Jochen Böhm, Dieter Würflein, and Heide Wagner; Asklepios Klinikum Harburg, Hamburg: Christoph Petermann, Daniel Niemeyer, Gerd Thiemann, Andrea Möller, and Bettina John; Charite Berlin, Berlin: Ralf-Harto Hübner, Ulrike Föllmer, Melanie Wegemund, Stefanie Menge, and Diana Busch; Lungenklinik Hemer, Hemer: Franz Stanzel, Melanie Holstein, and Katja Tietze.
References
- 1.López-Campos JL, Tan W, Soriano JB. Global burden of COPD. Respirology. 2016;21((1)):14–23. doi: 10.1111/resp.12660. [DOI] [PubMed] [Google Scholar]
- 2.Bagdonas E, Raudoniute J, Bruzauskaite I, Aldonyte R. Novel aspects of pathogenesis and regeneration mechanisms in COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:995–1013. doi: 10.2147/COPD.S82518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demedts IK, Demoor T, Bracke KR, Joos GF, Brusselle GG. Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir Res. 2006;7((1)):53. doi: 10.1186/1465-9921-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.GOLD. 2017. Available from: http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd/ Accessed 2017 Oct 13.
- 5.NICE . Endobronchial valve insertion to reduce lung volume in emphysema. Available from: https://www.nice.org.uk/guidance/ipg600 Accessed 2018 Sep 6. [Google Scholar]
- 6.Slebos DJ, Shah PL, Herth FJ, Valipour A. Endobronchial valves for endoscopic lung volume reduction: best practice recommendations from expert panel on endoscopic lung volume reduction. Respiration. 2017;93((2)):138–50. doi: 10.1159/000453588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sciurba FC, Ernst A, Herth FJ, Strange C, Criner GJ, Marquette CH, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med. 2010;363((13)):1233–44. doi: 10.1056/NEJMoa0900928. [DOI] [PubMed] [Google Scholar]
- 8.Herth FJ, Noppen M, Valipour A, Leroy S, Vergnon JM, Ficker JH, et al. Efficacy predictors of lung volume reduction with Zephyr valves in a European cohort. Eur Respir J. 2012;39:1334–42. doi: 10.1183/09031936.00161611. [DOI] [PubMed] [Google Scholar]
- 9.Davey C, Zoumot Z, Jordan S, McNulty WH, Carr DH, Hind MD, et al. Bronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR-HIFi study): a randomised controlled trial. Lancet. 2015;386((9998)):1066–73. doi: 10.1016/S0140-6736(15)60001-0. [DOI] [PubMed] [Google Scholar]
- 10.Klooster K, ten Hacken NH, Hartman JE, Kerstjens HA, van Rikxoort EM, Slebos DJ. Endobronchial valves for emphysema without interlobar collateral ventilation. N Engl J Med. 2015;373((24)):2325–35. doi: 10.1056/NEJMoa1507807. [DOI] [PubMed] [Google Scholar]
- 11.Kemp SV, Slebos DJ, Kirk A, Kornaszewska M, Carron K, Ek L, for the TRANSFORM Study Team A multicenter RCT of Zephyr® endobronchial valve treatment in heterogeneous emphysema (TRANSFORM) Am J Respir Crit Care Med. 2017;196:1535–43. doi: 10.1164/rccm.201707-1327OC. [DOI] [PubMed] [Google Scholar]
- 12.Valipour A, Slebos DJ, Herth F, Darwiche K, Wagner M, Ficker JH, et al. Endobronchial valve therapy in patients with homogeneous emphysema. Results from the IMPACT study. Am J Respir Crit Care Med. 2016;194((9)):1073–82. doi: 10.1164/rccm.201607-1383OC. [DOI] [PubMed] [Google Scholar]
- 13.Criner GJ, Sue R, Wright S, Dransfield M, Rivas-Perez H, Wiese T, et al. A multicenter RCT of Zephyr® endobronchial valve treatment in heterogeneous emphysema (LIBERATE) Am J Respir Crit Care Med. 2018;198((9)):1151–64. doi: 10.1164/rccm.201803-0590OC. [DOI] [PubMed] [Google Scholar]
- 14.Herth FJ, Gompelmann D, Criner GJ, Sciurba FC, Ernst A, Eberhardt R. Lung volume reduction using endobronchial valves in COPD patients with low emphysema heterogeneity scores. Am J Respir Crit Care Med. 2015;191:A1156. [Google Scholar]
- 15.Eberhardt R, Heubel CP, Kreuter M, Weinheimer O, Herth FJF. Bronchoscopic lung volume reduction in patients with severe homogeneous lung emphysema: a pilot study. Dtsch Med Wochenschr. 2009;134((11)):506–10. doi: 10.1055/s-0029-1208076. [DOI] [PubMed] [Google Scholar]
- 16.Valipour A, Slebos DJ, de Oliveira HG, Eberhardt R, Freitag L, Criner GJ, et al. Expert statement: pneumothorax associated with endoscopic valve therapy for emphysema: potential mechanisms, treatment algorithm, and case examples. Respiration. 2014;87:513–21. doi: 10.1159/000360642. [DOI] [PubMed] [Google Scholar]
- 17.Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348((21)):2059–73. doi: 10.1056/NEJMoa030287. [DOI] [PubMed] [Google Scholar]
- 18.Valipour A, Herth FJ, Burghuber OC, Criner G, Vergnon JM, Goldin J, et al. Target lobe volume reduction and COPD outcome measures after endobronchial valve therapy. Eur Respir J. 2014;43:387–96. doi: 10.1183/09031936.00133012. [DOI] [PubMed] [Google Scholar]
- 19.Gompelmann D, Hofbauer T, Gerovasili V, Eberhardt R, Lim HJ, Herth F, et al. Predictors of clinical outcome in emphysema patients with atelectasis following endoscopic valve therapy: a retrospective study. Respirology. 2016;21:1255–61. doi: 10.1111/resp.12819. [DOI] [PubMed] [Google Scholar]
- 20.Tanabe N, Muro S, Tanaka S, Sato S, Oguma T, Kiyokawa H, et al. Emphysema distribution and annual changes in pulmonary function in male patients with chronic obstructive pulmonary disease. Respir Res. 2012;13:31. doi: 10.1186/1465-9921-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klooster K, Hartman JE, ten Hacken NH, Slebos DJ. One-year follow-up after endobronchial valve treatment in patients with emphysema without collateral ventilation treated in the STELVIO trial. Respiration. 2017;93((2)):112–21. doi: 10.1159/000453529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sciurba FC, Criner GJ, Strange C, Shah PL, Michaud G, Connolly TA, et al. Effect of endobronchial coils vs usual care on exercise tolerance in patients with severe emphysema: the RENEW randomized clinical trial. JAMA. 2016;315((20)):2178–89. doi: 10.1001/jama.2016.6261. [DOI] [PubMed] [Google Scholar]
- 23.National Emphysema Treatment Trial Research Group Patients at high-risk of death after lung volume reduction surgery. New Eng J Med. 2001;345((5)):1075–83. doi: 10.1056/NEJMoa11798. [DOI] [PubMed] [Google Scholar]