Abstract
Cardiac neoplasms are uncommon tumors. For epidemiological purposes, they can be divided into benign and malignant subtypes, with the former occurring at a significantly higher rate than the latter. Due to their uncommon nature, there are few data-driven studies examining the characteristics and trends of benign cardiac neoplasms. Our retrospective HCUP-NIS data review purports to illuminate some of the trends surrounding benign cardiac neoplasms and their associated co-occurrences. The data consisted of 482,872,274 weighted discharges. There were 45,568 weighted discharges that included a benign cardiac neoplasm. Benign cardiac neoplasms were more often observed in women (64.33%), and the average age was 63.8 years. The most common cardiovascular co-occurrences in patients with benign cardiac neoplasm were atrial tachyarrhythmias (28.93%), heart failure (19.61%), and embolic events such as stroke, myocardial infarct, or pulmonary embolism (19.82%). Other co-occurrences included pulmonary hypertension (7.55%), ventricular arrhythmias (3.23%), and other EKG abnormalities (3.70%). Procedures were numerous in patients with benign cardiac neoplasms. 43% of patients with this diagnosis had some form of cardiac surgery during their hospitalization. Overall, this study found low incidence of benign cardiac neoplasms in the USA during this 13-year study period. However, in the presence of benign cardiac neoplasms, our study showed that cardiovascular co-occurrences are not uncommon and may help to illuminate this otherwise rare diagnosis.
Keywords: Cardiac neoplasms, Epidemiology, USA, Cardio-oncology
Introduction
Cardiac neoplasms are rare, with varying reported incidence and few major epidemiologic studies. They can be categorized into primary (benign or malignant) and secondary (metastatic) subtypes. They may occur in any cardiac tissue, with benign and malignant tumors having generally favorable and poor outcomes, respectively.
Historical autopsy studies have determined that the overall incidence of primary cardiac neoplasms is approximately 2 in 10,000, with a majority displaying benign histologic features [1]. In adults, approximately 75% of cardiac neoplasms are of benign subtypes. In the pediatric population, up to 90% of primary cardiac tumors are benign. The commonest subtype in children is rhabdomyoma, as opposed to atrial myxomas in other age-groups [2]. Benign primary cardiac neoplasms are often asymptomatic and may arise and regress undetected throughout a person's lifetime.
Numerous complications may arise as a result of all cardiac tumor types, most notably, cardiac outflow tract obstruction leading to heart failure and embolic stroke [3, 4]. Other known complications include syncope, weight loss, anasarca, atrial arrhythmias, ventricular arrhythmias, and pulmonary hypertension [5]. Treatment for these neoplasms is dependent upon the type and location but is most often surgical in nature. Tumor removal, pacemaker implantation, and cardiac ablations are commonly performed for these patients, with generally favorable outcomes for benign neoplasms. Malignant subtypes in contrast often require multiple procedures and treatment modalities.
There are few data-driven studies examining the incidence of benign cardiac neoplasms (BCNs) in the USA, analyzing rates and types of complications, and comparing the therapeutic interventions pursued. This study aims to utilize a large database to quantify the types, characteristics, and trends of benign primary cardiac tumors in the US population of hospitalized patients.
Objective
This retrospective study used the Healthcare Utilization Projects/Nationwide Inpatient Sample database from 2002 to 2014 to evaluate the characteristics, complications, mortality, and treatments of BCNs in the USA.
Methods
The NIS is a database provided by the Healthcare Utilization Project sponsored by the Agency for Healthcare Research and Quality. The National Inpatient Sample contains discharges from the State Inpatient Databases and is designed to represent hospitals and discharges at a national level using a random sampling of discharges stratified by US census region, urban or rural location, teaching status, ownership, and size. The data collected include demographic information and diagnostic and procedural codes from the patient's hospital stay. One of the major strengths of the database is its size, with information about millions of patients each year. Limitations include the inability to assess causation or relation between different diagnoses and dependence on the coding used during the stay. Only diagnoses that were coded during the hospitalization are reflected in the data.
The NIS-HCUS database contains about 20% of all US admissions. It provides an estimate of the totality of the US discharges based on the sample, which is referred to as weighted discharges. As the database sample is 20% of all admissions, the weighted discharges are approximately 5 times more than measured number of discharges.
Descriptive analysis was used to assess the frequency of BCN and patient characteristics. The proportion (95% confidence interval) of BCN, as indicated by inclusion criteria diagnosis codes, is reported for the study period, 2002–2014. Study group inclusion criteria consisted of the presence of the ICD-9 code for BCN (212.7).
SAS 9.4 was used to perform all statistical analyses via the survey procedures, to account for discharge weights and sampling methodology of the NIS database. Descriptive tables were created for each variable with quantitative variables being represented as mean ± standard deviation and categorical variables represented as frequency (%).
The prevalence of BCN was assessed by calculating a 95% confidence interval of the observed proportion. To assess the trend frequency over time, 2-sided Cochran-Armitage Trend Tests were performed to assess whether the frequency of BCN increased or decreased over time.
The co-diagnoses considered were pulmonary hypertension, stroke, atrial tachyarrhythmias, sinus node dysfunction, severe AV node dysfunction (3rd- or 2nd-degree type 1 AV block), other EKG abnormalities (bundle branch block and 1st-degree AV block), syncope, ventricular arrhythmias, cardiac arrest, acute coronary syndrome, pulmonary embolism, other forms of arterial embolization (peripheral, retinal, bowel, or renal), weight loss, edema, fever, endocarditis, and heart failure. Descriptive frequency tables were calculated for the variable mortality, costs, and treatments. No inferences were drawn from these variables. Due to NIS confidentiality concerns, all cell counts that had a frequency <10 will be represented with a dash.
Results
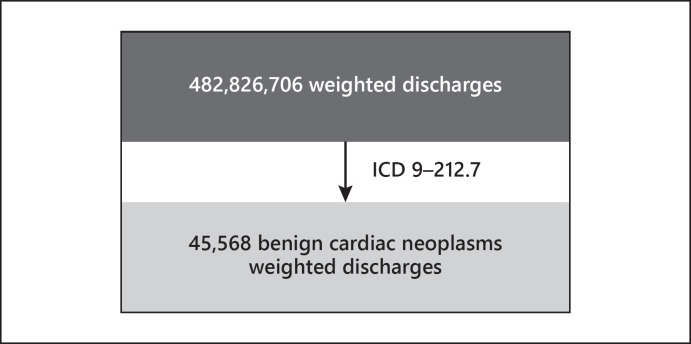
These data available during the study period consisted of 100,790,900 discharges accounting for 482,826,706 weighted discharges. The data contain 45,568 weighted discharges of BCN or a prevalence of 0.94 per 10,000 discharges (Fig. 1).
Fig. 1.
Healthcare Utilization Projects/Nationwide Inpatient Sample hospital discharges 2002–2014.
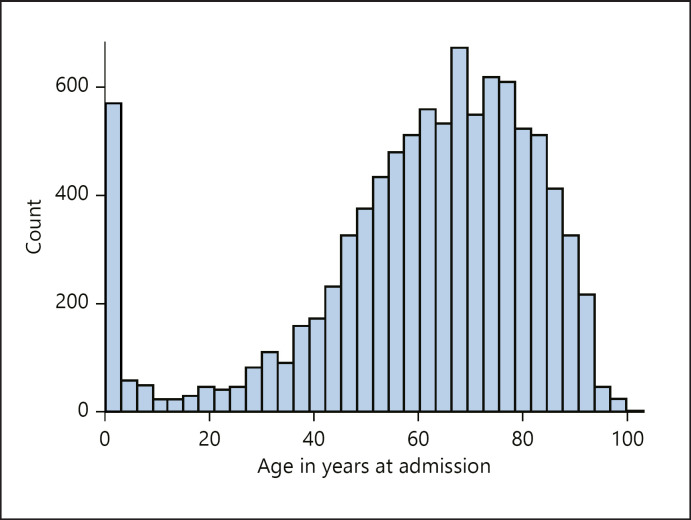
Patient age distribution (Fig. 2), median age, length of stay, occurrence per US region, mortality, length of stay, costs, income, and ethnicity are summarized in Table 1. A total of 1,267 patients died during the hospitalization.
Fig. 2.
Histogram of the age.
Table 1.
General characteristics
| Characteristic | BCNs (confidence interval) |
|---|---|
| Age in years | 63.82 (49.52, 75.60) |
| Male, % | 35.66 |
| Median length of stay in days | 5.10 (2.89, 8.37) |
| Inhospital mortality, n (%) | 1,267 (2.78) |
| Median cost of hospitalization in 2020, | |
| USD | 51,799 (22,073; 99,302) |
| Ethnicity, % | |
| Caucasian | 73.14 |
| African American | 11.41 |
| Hispanic | 8.51 |
| Other | 3.51 |
| Asian/Pacific | 3.00 |
| Native American | 0.42 |
| Median income per zip code, % | |
| 1st quartile (lowest) | 23.97 |
| 2nd quartile | 22.83 |
| 3rd quartile | 21.04 |
| 4th quartile (higheST) | 26.81 |
| BCN per region, % | |
| South | 33.16 |
| West | 26.81 |
| Northeast | 22.36 |
| Midwest | 6.20 |
BCNs, benign cardiac neoplasms.
Co-Occurrences (Table 2)
Table 2.
Comorbidities
| Co-occurrences | BCNs, n (%) |
|---|---|
| Atrial tachyarrhythmias | 13,184 (28.93) |
| Heart failure | 8,937 (19.61) |
| Stroke | 6,730 (14.77) |
| Pulmonary hypertension | 3,440 (7.55) |
| Myocardial infarct | 1,281 (3.85) |
| Other EKG abnormalities | 1,686 (3.70) |
| Ventricular arrhythmias | 1,471 (3.23) |
| Syncope | 1,055 (2.32) |
| Sinus node dysfunction | 1,005 (2.21) |
| Severe AV node dysfunction | 930 (2.04) |
| Endocarditis | 869 (1.91) |
| Arterial embolization | 573 (1.26) |
| Pulmonary embolism | 547 (1.20) |
| Edema | 505 (1.11) |
| Cardiac arrest | 393 (0.86) |
| Fever | 383 (0.84) |
| Weight loss | 311 (0.68) |
BCNs, benign cardiac neoplasms.
Heart failure diagnostic codes were present in 8,937 discharges for BCN (19.61%), whereas atrial tachyarrhythmias were present in 13,184 (28.93%). Embolic events such as stroke, myocardial infarct, pulmonary embolism, and other arterial embolization were present in 6,730, 1,281, and 547, 573 of the weighted discharges for BCN or (14.77%, 3.85%, 1.20%, and 1.26%), respectively.
There were 3,440 weighted discharges or 7.55% of the discharges with BCN that also had a diagnostic code for pulmonary hypertension. Ventricular arrhythmias, sinus node dysfunction, severe AV node dysfunction, and other EKG abnormality codes were seen as 1,471 (3.23%), 1,005 (2.21%), 930 (2.04%), and 1,686 (3.70%) of the discharges with BCN, respectively.
Procedures
These data contained 19,637 weighted discharges with BCN that underwent any cardiac surgery or 43.09% of the admissions during the respective hospital stay. Echocardiogram and cardiac catheterization were performed during the hospitalization in 30.28% and 21.91% of the patients admitted for BCN. Pacemakers, implantable cardioverter defibrillator, and cardiac resynchronization devices were placed in 2.67%, 0.3%, and 0.1% of the discharges with BCN, respectively (Table 3). In hospital, mortality for patients who underwent a cardiac surgery during the hospitalization was 1.78% (338/19,637), which was not higher than the patients who did not undergo surgery.
Table 3.
Procedures performed
| Procedure performed | BCNs, n (%) |
|---|---|
| Cardiac surgery | 19,637 (43.09) |
| Echocardiogram | 13,796 (30.28) |
| Catheterization | 9,983 (21.91) |
| Valvular procedures | 3,797 (8.33) |
| CABG | 2,344 (5.15) |
| Pacemaker placement | 1,218 (2.67) |
| Open heart valve surgery | 3,180 (6.98) |
| ICD placement | 138 (0.30) |
| CRT placement | 44 (0.09) |
BCNs, benign cardiac neoplasms.
Discussion
NIS-HCUP is a large database with >480 million weighted discharges over 13 years. BCN has a low prevalence in the USA, with only 45,568 weighted discharges coded during the 13-year study period or approximately 1 in 10,000 discharges. Even though BCNs are important to include in a differential diagnosis for patients with suspected valvular disease, they are rare. Autopsy studies have demonstrated a prevalence of 200 BCN per 1 million autopsies [6]. Assuming that all patients with known BCN are admitted, our data are concordant with this prevalence estimate.
Studies with hospital costs of BCN did not exist. The mean hospital cost in our study was 51,799 USD, 5 times more than the national average for hospitalizations for other diagnoses over a similar length of stay [7]. Considering the hospitalization length of stay mean was 5.1 days and the cost only reflects the hospital stay, we assume the total cost is much higher since these patients often need rehabilitation, multiple outpatient visits, diagnostic testing, and long-term follow-up.
BCNs have a classic triad of presenting signs and symptoms: thromboembolic events, intracardiac obstruction, and systemic symptoms [8]. Thromboembolic events are not uncommon in patients with BCNs. Sites of distal embolization from the left atrium and left ventricle determine the clinical presentation which can include stroke, mesenteric ischemia, splenic or renal infarction, or acute limb ischemia. Masses involving the right atrium and right ventricle can result in pulmonary embolism or, more rarely, systemic embolism in the setting of right-to-left cardiac shunting. A large majority of cardiac neoplasms are left-sided and are at greatest anatomic risk for embolism [9, 10]. In reviewing the literature, there are varying reported incidences of thromboembolic events secondary to cardiac neoplasms. In 1 retrospective study of 185 patients with a primary cardiac neoplasm, a thromboembolic event occurred in 19.8% of patients, mainly to the central nervous system (10.2%). The remainder of embolic events went to the coronary arteries (4.8%), lower limbs (4.3%), and lungs (0.5%) [11]. Our study of 45,568 patients with BCNs found embolic events such as stroke, myocardial infarct, pulmonary embolism, and other arterial embolization which were 14.77%, 3.85%, 1.20%, and 1.26%, respectively.
Cardiac neoplasms may also cause intracardiac signs and symptoms. Tumors may obstruct valvular function, blood flow, or both. In turn, this may cause valvular stenosis, valvular insufficiency, or heart failure. In the event of an obstructive mass in the right atrium or right ventricle, patients may experience symptoms of right-sided heart failure, including peripheral edema, cardiac ascites, or superior vena cava syndrome. Our study found heart failure present in 19.61% of patients and edema in 1.11%. In left atrial or left ventricular masses, shortness of breath due to pulmonary hypertension is common [12]. Our study found pulmonary hypertension present in 7.55% of patients. Tumors may also induce arrhythmias, such as atrioventricular or intraventricular blocks, as well as paroxysmal supraventricular or ventricular tachycardias due to compression or encroachment on the conduction system [13]. Arrhythmias were common in our study. Atrial tachyarrhythmias were seen in 28.93% of patients, ventricular arrhythmias in 3.23%, sinus node dysfunction in 2.21%, and severe AV node dysfunction in 2.04%. Other unclassified EKG abnormalities (that may or may not include other arrhythmias) were present in 3.70%. Overall, 36.41% of patients in our study had one or more conduction abnormality. Although rare, sudden cardiac death has also been reported as a consequence of cardiac neoplasms [14]. Our study showed cardiac arrest at a rate of 0.86% but did not differentiate between cardiac death and successful return of spontaneous circulation.
Systemic symptoms such as fever and weight loss have been reported in up to 20% and 18%, respectively, of patients with BCNs [15]. Our study found fever present in 0.84% of patients and weight loss in 0.68%. The reason for this discrepancy may reflect the difference between historic findings and objective findings. Our study relied on diagnosis codes, meaning that fever or weight loss had to be witnessed and coded for within the inpatient setting in order to be recorded. It is not clear in the above-cited study whether fever and weight loss were objective signs or historic, self-reported symptoms.
With regard to procedures for patients with BCN, our study demonstrated that a large proportion (43%) underwent some form of cardiac surgery when hospitalized with this diagnosis. With the exception of the oldest patients and those with significant comorbidities preventing cardiac surgery, we would expect a number closer to 100% of BCN patients undergoing a cardiac surgery. In our study, this number may be related to the diagnosis and surgical intervention being done during different hospital admissions. In the literature, there are few studies that quantify the rate of procedures performed on patients with a diagnosis of a BCN. However, numerous studies stress that surgical management is the superior approach for definitive management of symptomatic patients [16, 17, 18, 19]. The literature also stresses the importance of a thorough evaluation prior to consideration of surgical intervention in patients with BCN. The other cardiac procedures such as catheterization and CABG were likely performed due to comorbid coronary artery disease or another process related to cardiac ischemia, and not as sole management of a BCN. The most likely explanation for this discrepancy is that many if not most patients were discharged for outpatient evaluation and surgery at a later date.
As shown in Table 1, the measured inhospital mortality for patients in our study group with BCN was determined to be 2.78% (1,267 patients). This value is greater than most of the literature examining mortality in patients with this diagnosis. For instance, a large meta-analysis determined the overall mortality for all benign cardiac tumors to be 0.79% [20]. This disparity may be due to the characteristics of our study group, including hospitalizations for other comorbid conditions in addition to a diagnosis of BCN. In the literature, several perioperative studies for patients hospitalized for cardiac myxoma resection demonstrated mortality rates of 0–2.7% [19, 21, 22]. The patients who underwent cardiac surgeries in our study had an inhospital mortality of 1.72%. Studies with longer postsurgical follow-up times revealed mortality rates of 2–3.5% [15, 23]. These data are also challenging to directly compare to our study, as the majority of hospitalizations included in our analysis did not include surgical intervention for their BCN. It is reasonable to conclude that the mortality rate in patients who are hospitalized with BCN is higher than the mortality rate for all patients with a BCN, considering that most cases are asymptomatic for many years. Even with this consideration, our study reinforces that a diagnosis of any BCN carries a favorable prognosis, even when hospitalized.
Limitations
The NIS-HCUP database does not provide individual patient information, so we must rely on ICD-9 codes added during the hospitalization. The ICD-9 codes are often nonspecific in many medical conditions and few if any co-occurring symptoms and comorbidities outside the main problem may be coded for at all depending on the clinician. To protect the privacy of patients, the NIS database does not allow detection of cells with fewer than 10 patients. With low numbers of BCN patients, we cannot make definitive conclusions about several outcomes.
Conclusions
This study provides valuable patient demographic information for those hospitalized for BCN in the USA. BCN is rare in the USA with low mortality, however with a high morbidity and hospital cost.
Statement of Ethics
The data from NIS-HCUS database are retrospective and unidentifiable. No personal information is available. Therefore, the protocol was waived by the Western Michigan University School of Medicine Institutional Review Board and no registration had to be done.
Conflict of Interest Statement
The authors have declared that no competing interests exist.
Funding Sources
No financial support is needed.
Author Contributions
Neiberg de Alcantara Lima, Kristina Byers-Spencer, Kamil Cwikla, Thomas A. Melgar, and Nicholas Helmstetter were responsible for conception of the work, data analysis, and interpretation, drafted the article, critically revised the article, and approved the final version to be published. Cuyler Huffman and Mireya Diaz were responsible for data analysis and interpretation, drafted the article, and approved the final version to be published.
Data Availability Statement
Data are available on request from the authors.
Supplementary Material
Supplementary data
References
- 1.Reynen K. Frequency of primary tumors of the heart. Am J Cardiol. 1996;77((1)):107. doi: 10.1016/s0002-9149(97)89149-7. [DOI] [PubMed] [Google Scholar]
- 2.Uzun O, Wilson DG, Vujanic GM, Parsons JM, De Giovanni JV. Cardiac tumours in children. Orphanet J Rare Dis. 2007 Dec;2((1)):11–4. doi: 10.1186/1750-1172-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raza E, Kamal AK. Recurrent non-aneurysmal, metastatic intraparenchymal haemorrhages following resection of atrial myxoma − case report and literature review. BMJ Case Rep. 2012 Oct 26;2012:2012bcr0220125772. doi: 10.1136/bcr.02.2012.5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knepper LE, Biller J, Adams HP, Jr, Bruno A. Neurologic manifestations of atrial myxoma. A 12-year experience and review. Stroke. 1988 Nov;19((11)):1435–40. doi: 10.1161/01.str.19.11.1435. [DOI] [PubMed] [Google Scholar]
- 5.Hoffmeier A, Sindermann JR, Scheld HH, Martens S. Cardiac tumors − diagnosis and surgical treatment. Dtsch Arztebl Int. 2014 Mar 21;111((12)):205–11. doi: 10.3238/arztebl.2014.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reynen K. Frequency of primary tumors of the heart. Am J Cardiol. 1996 Jan 1;77((1)):107. doi: 10.1016/s0002-9149(97)89149-7. [DOI] [PubMed] [Google Scholar]
- 7.Torio CM, Moore B. Healthcare cost and utilization project (HCUP) statistical briefs [Internet] Rockville (MD): Agency for Healthcare Research and Quality (US); 2006. National inpatient hospital costs: the most expensive conditions by payer, 2013. statistical brief# 204. [PubMed] [Google Scholar]
- 8.Neragi-Miandoab S, Kim J, Vlahakes GJ. Malignant tumours of the heart: a review of tumour type, diagnosis and therapy. Clin Oncol. 2007;19((10)):748–56. doi: 10.1016/j.clon.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Dias RR, Stolf NA, Malbouisson LM, Fernandes F, Ramirez FJ, Mady C, et al. Morbidity and embolic potential of left atrial cardiac tumors. Thorac Cardiovasc Surg. 2006;54((6)):400–3. doi: 10.1055/s-2006-924091. [DOI] [PubMed] [Google Scholar]
- 10.Elbardissi AW, Dearani JA, Daly RC, Mullany CJ, Orszulak TA, Puga FJ, et al. Embolic potential of cardiac tumors and outcome after resection: a case-control study. Stroke. 2009;40((1)):156–62. doi: 10.1161/STROKEAHA.108.525709. [DOI] [PubMed] [Google Scholar]
- 11.Dias RR, Fernandes F, Ramires FJ, Mady C, Albuquerque CP, Jatene FB. Mortality and embolic potential of cardiac tumors. Arq Bras Cardiol. 2014;103((1)):13–8. doi: 10.5935/abc.20140096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silverman NA. Primary cardiac tumors. Ann Surg. 1980;191((2)):127–38. doi: 10.1097/00000658-198002000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kusano KF, Ohe T. Cardiac tumors that cause arrhythmias. Card Electrophysiol Rev. 2002;6((1–2)):174–7. doi: 10.1023/a:1017936622990. [DOI] [PubMed] [Google Scholar]
- 14.Cina SJ, Smialek JE, Burke AP, Virmani R, Hutchins GM. Primary cardiac tumors causing sudden death: a review of the literature. Am J Forensic Med Pathol. 1996;17((4)):271–81. doi: 10.1097/00000433-199612000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Pinede L, Duhaut P, Loire R. Clinical presentation of left atrial cardiac myxoma. A series of 112 consecutive cases. Medicine. 2001;80((3)):159–72. doi: 10.1097/00005792-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Yanagawa B, Chan EY, Cusimano RJ, Reardon MJ. Approach to surgery for cardiac tumors: primary simple, primary complex, and secondary. Cardiol Clin. 2019;37((4)):525–31. doi: 10.1016/j.ccl.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Yanagawa B, Mazine A, Chan EY, Barker CM, Gritti M, Reul RM, et al. Surgery for tumors of the heart. Semin Thorac Cardiovasc Surg. 2018;30((4)):385–97. doi: 10.1053/j.semtcvs.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Wang JG, Liu H, Yu WJ, Li YJ, Xin FJ. [Primary cardiac neoplasms: a clinicopathologic analysis of 81 cases] Zhonghua Bing Li Xue Za Zhi. 2012 Dec;41((12)):808–12. doi: 10.3760/cma.j.issn.0529-5807.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Karabinis A, Samanidis G, Khoury M, Stavridis G, Perreas K. Clinical presentation and treatment of cardiac myxoma in 153 patients. Medicine. 2018 Sep;97((37)):e12397. doi: 10.1097/MD.0000000000012397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rahouma M, Arisha MJ, Elmously A, El-Sayed Ahmed MM, Spadaccio C, Mehta K, et al. Cardiac tumors prevalence and mortality: a systematic review and meta-analysis. Int J Surg. 2020 Apr;76:178–89. doi: 10.1016/j.ijsu.2020.02.039. [DOI] [PubMed] [Google Scholar]
- 21.Kuroczyński W, Peivandi AA, Ewald P, Pruefer D, Heinemann M, Vahl CF. Cardiac myxomas: short- and long-term follow-up. Cardiol J. 2009;16((5)):447–54. [PubMed] [Google Scholar]
- 22.Scrofani R, Carro C, Villa L, Botta M, Antona C. Il mixoma cardiaco: risultati chirurgici e follow-up clinico a 15 anni [cardiac myxoma: surgical results and 15-year clinical follow-up] Ital Heart J Suppl. 2002 Jul;3((7)):753–8. [PubMed] [Google Scholar]
- 23.Elbardissi AW, Dearani JA, Daly RC, Mullany CJ, Orszulak TA, Puga FJ, et al. Survival after resection of primary cardiac tumors: a 48-year experience. Circulation. 2008 Sep 30;118((14 Suppl)):S7–15. doi: 10.1161/CIRCULATIONAHA.107.783126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
Data are available on request from the authors.