Abstract
Virtual Reality (VR) technologies have increasingly been considered potentially valuable tools in dementia-related research and could serve as non-pharmacological therapy to improve quality of life (QoL) and wellbeing for persons with dementia (PwD). In this scoping review, we summarize peer-reviewed articles published up to Jan-21, 2021, on the use of VR to promote wellbeing in PwD. Eighteen manuscripts (reporting on 19 studies) met the inclusion criteria, with a majority published in the past 2 years. Two reviewers independently coded the articles regarding A) intended clinical outcomes and effectiveness of the interventions, B) study sample (characteristics of the participants), C) intervention administration (by whom, what setting), D) experimental methods (design/instruments), and E) technical properties of the VR-systems (hardware/devices and software/content). Emotional outcomes were by far the most common objectives of the interventions, reported in seventeen (89.5%) of the included articles. Outcomes addressing social engagement and personhood in PwD have not been thoroughly explored using VR. Based on the positive impact of VR, future opportunities lie in identifying special features and customization of the hardware/software to afford the most benefit to different sub-groups of the target population. Overall, this review found that VR represents a promising tool for promoting wellbeing in PwD, with positive or neutral impact reported on emotional, social, and functional aspects of wellbeing.
Keywords: Virtual reality, wellbeing, quality of life, dementia, iADL, ADL, HMD, headset
Introduction
Virtual Reality (VR) technologies have increasingly been considered as potentially valuable tools in dementia-related research and care, for use in cognitive and physical assessments, and therapeutic interventions. 1 VR based interventions have been used as health promotion tools to improve mobility, prevent falls, and train cognitive abilities in those with dementia and those who are at-risk of developing dementia. 2 VR has also been proposed as a prospective tool to identify early markers of cognitive decline 3 and to enhance clinicians’ and carers’ understanding and attitudes towards persons with dementia (PwD), by exposing them to experiences that simulate what it is like to live with dementia, thereby promoting empathy.4,5 Several published reviews comment on the potential clinical effectiveness of VR for these types of dementia-focused applications and highlight methodological gaps that remain in the evaluation of VR technology with this population.6,7 More recently, it has been suggested that VR could serve as a non-pharmacological therapy to improve quality of life (QoL) and wellbeing for PwD. 8 In this scoping review, we summarize current literature on the use of VR as an intervention to promote wellbeing in PwD, and discuss potential areas for future research.
VR is defined as computer-simulated versions of real locations/objects or imagined 3D graphically rendered environments that enable users to experience the sensation of being present in a different physical place. The virtual environment is updated in real time based on the real-world movements and actions of the user.6,8 VR is often considered qualitatively different from other technologies in its ability to provide a sensation of “presence,” the subjective feeling of “being there,” 4 which is highly dependent on the immersiveness of the system and the fidelity of the simulated sensory inputs. 4 A system’s immersiveness is influenced by, for example, display resolution, field of view, movement degrees of freedom, number of senses stimulated (hearing, vision, touch, and proprioception), the ability to track and update user inputs, and the ability to isolate the user from stimuli in the real world. 9 These properties of VR make it an appealing potential tool to promote wellbeing in PwD by providing them with a sense of autonomy, enhancing training and educational tools, stimulating reminiscence, and providing an escape from constrained mobility and/or pain.
Background
Dementia and wellbeing
The World Health Organization defines health as “a state of complete physical, mental, and social wellbeing and not merely the absence of disease or infirmity.” 10 Preserving and enhancing wellbeing in PwD can promote health and QoL. Although not perfectly delineated, wellbeing can be considered along a spectrum of outcomes from physical to cognitive, including functional [activities of daily living (ADL) and instrumental activities of daily living (iADL)] to social (ability to communicate, interact and maintain relationships and a sense of personhood), to emotional [feelings and mood, pain, and neuropsychiatric symptoms/behavioral and psychological symptoms of dementia (BPSD)]. When Canadians affected by dementia were asked to identify what they believe are the most important research questions related to living with dementia, emotional wellbeing was ranked second only to stigma. 11
Functional changes
Dementia is an umbrella term encompassing different subtypes, such as Alzheimer’s disease (AD), Frontotemporal dementia, Lewy-body dementia, vascular dementia, and mixed dementia. While there is variation in subtypes and associated symptoms, dementia generally negatively affects the ability to perform functional tasks and live independently, which in early stages of decline may consist of difficulties with iADLs, operationalized as shopping, managing finances, preparing meals, maintaining a home, and managing medication and personal care. In more advanced stages, dementia may lead to difficulties conducting tasks that are essential in order to live comfortably, known as ADLs, such as, mobility, toileting, dressing, and language skills. Unsurprisingly, difficulties in conducting iADLs and ADLs are strongly associated with poorer QoL. 12
VR has been used to assess and enhance the performance of iADLs in PwD. For example, Allain et al. 13 designed a non-immersive VR task where participants were asked to prepare a virtual cup of coffee using a virtual coffee machine and found the tool sensitive for the detection of everyday action impairments in persons with AD. Atkins et al. 14 designed the Virtual Reality Functional Capacity Assessment Tool (VRFCAT) to assess an individual’s ability to complete instrumental activities associated with a shopping trip, including searching the pantry at home, making a shopping list, taking the correct bus to the grocery store, shopping in the store, paying for groceries, and returning home. They found that the VRFCAT provides a sensitive tool for evaluating iADL functioning in individuals with subjective cognitive decline.
Emotional changes
PwD often experience changes in their emotional responses, including a loss of control over their feelings and how they express them. 15 In a recent feasibility study, we measured the effect of exposure to natural environments in VR on changes in emotions 16 and found that most participants had positive feedback, feeling, for example, more relaxed and adventurous, with 76% wanting to try VR again. Similarly, in their pilot study, Moyle et al. 17 described the effect of exposing PwD to a Virtual Reality Forest on engagement, apathy, and mood states and found that it was perceived by residents, family members, and staff to have a positive effect with residents experiencing more pleasure and a greater level of alertness than those previously established for PwD in an activity context.
Another aspect of emotional wellbeing refers to BPSD, also known as neuropsychiatric symptoms, which represent a heterogeneous group of non-cognitive symptoms and behaviors that include agitation, aberrant motor behavior, anxiety, elation, irritability, depression, apathy, disinhibition, delusions, hallucinations, and sleep or appetite changes. BPSDs present in up to 90% of PwD at some stage in the progression of the condition. 18 Data suggest that BPSD are more strongly associated with poorer QoL than declines in cognition or functional limitations. 19 BPSD have also been identified by caregivers as some of the most challenging and distressing aspects of care, with physical aggression being cited as a main contributor to long term care (LTC) admission.20,21 Given that current pharmacological approaches to managing BPSD are limited in their efficacy and are associated with negative adverse side effects, some researchers and clinicians are eager to explore the potential of VR with the hope that it may prove to be a less expensive, non-invasive, and ethically acceptable means of engaging and distracting PwD and managing their BPSD. 22 We recently conducted a randomized controlled trial at an acute care hospital, evaluating the effects of VR on BPSD including the need for sitters (people who provide supervision of challenging patients), refusal of care, and use of drugs.23,24 We found that VR-therapy had a statistically significant effect on reducing the cluster of “aggressiveness” as well as trended towards reducing length of stay.
Social changes
Social connectedness is influenced by one’s ability to communicate and maintain meaningful relationships. Many PwD experience significant declines in communication abilities as their symptoms progress. Barriers in communication can impair relationships with caregivers, family, and friends leading to increased social isolation and feelings of loneliness, which are associated with more a rapid cognitive decline, increased BPSD, and reduced QoL. 25 Therefore, optimizing strategies for communication and increasing exposure to new experiences and opportunities for social interaction may limit the ensemble of associated negative effects 26 and improve wellbeing.
VR has the strong potential to create person-centered experiences and to enhance social interactions. For example, VR technologies have been suggested as an aid in reminiscence therapy and to enhance communication in PwD. 27 VR has been shown to enhance the sense of control, 17 maintain autonomy, and articulate values, preferences, and choices in PwD. 28 Respect for these decisions has been correlated with improved QoL and deemed an ethical priority for PwD. 29 Moreover, VR has the ability to transport the viewer into socially engaging settings 16 in a physically safe setting, absent of possible stigma from others. In one study, participants were more talkative and provided more details regarding their dementia in a VR-simulated meeting than in the real-world scenario, suggesting that VR may facilitate more verbal interactions and improved social-emotional behavior. 30
Overall, there is still much that is unknown and that can be learned about how to use VR to promote the various aspects of wellbeing in PwD. For example, how can it best be administered (by whom and in what setting) so that it is sustainable and scalable, what evaluation methodologies are appropriate for assessing impact, and what technological properties (hardware/devices and software/content) work best with this population. As approaches to research and outcome measures differ widely, there lacks a general framework about how the following factors should be chosen and applied.
Intervention administration
As research increasingly evaluates the outcomes of VR-based applications for PwD, it is useful to compare across studies and form basic guidelines or best practices about the ways in which these applications can and should be administered. Aspects such as (1) who administers the intervention (e.g., care partner, healthcare provider, researcher, or the individual themselves) and (2) in what settings it is delivered (e.g., hospitals, long-term-care, or personal residences) are critical factors that can affect the design, sustainability, and scalability of VR-based applications. For VR-based interventions targeted towards improving wellbeing, it is important to identify the optimal VR-dose regimen, such as, how frequent (e.g., times per week), for what duration (e.g., minutes per session), and over how long a time period (e.g., weeks or months) it should be administered. While it is clear that VR-based protocols will likely depend on many factors such as individual differences (e.g., personal preferences and degree of sensory/motor/cognitive impairments) and the nature of the targeted outcomes (e.g., functional, social, and emotional), these protocol-specific metrics are currently under-reported.
Experimental methods
Alongside, the nature of the outcomes measured and evaluated is the importance of how these outcomes are obtained (e.g., study design and data collection methods). Despite the enthusiasm, few VR trials in PwD targeting wellbeing have used rigorous methods and/or validated measures, and most of the observed positive outcomes are based on anecdotal evidence and small-sample studies. Understanding how protocols are designed and what metrics are used in existing research will allow us to compare and perhaps consolidate findings more accurately across studies. This will also help situate VR among the various non-pharmacological interventions available for improving wellbeing in PwD.
Virtual reality technical properties
The technical properties of VR systems include hardware and software features. Hardware features include devices used [e.g., head mounted displays (HMD), haptic devices, and headphones] and the number of senses stimulated (e.g., visual, auditory, and touch), each of which influences the degree of immersiveness and can contribute to an enhanced sense of “presence.” Software features include the nature of virtual content including, for example, whether rendered graphics or 360-degree video are used, and the degree of interaction introduced [e.g., passive exposure to scene (e.g., a beach), instructive content or narratives (e.g., a museum tour) or active task-based performance (e.g., completing a shopping task)].
Objectives of the current review
The objectives of this scoping review are to describe the current state of peer-reviewed research involving VR-based applications aimed at promoting wellbeing for PwD. Specifically, we consider A) intended clinical outcomes of the intervention and their reported effectiveness, B) study sample (characteristics of the population), C) intervention administration protocol (by whom, in what setting), D) experimental methods (design and instruments), and E) technical properties of the VR-system (hardware/devices and software/content). Addressing these aims also helps to identify the gaps in current knowledge and suggest targeted goals for future VR-based studies aimed at promoting wellbeing in PwD.
Methods
Scoping review
This scoping review followed the protocol outlined by Clay et al. 6 The methodological framework by Arksey and O’Malley 31 was used to guide a literature search of all relevant research, regardless of study design, by iterative and reflexive means.
Search strategies
A systematic search of the following databases was conducted from their inception up to 21 Jan 2021: Ovid MEDLINE, Ovid MEDLINE Epub Ahead of Print and In-Process and Other Non-Indexed Citations, Ovid Embase, Cochrane Database of Systematic Reviews (Ovid), Cochrane Central Register of Controlled Trials (Ovid), PsycINFO (Ovid), CINAHL with Full Text (EBSCO), Health Technology Assessment (OVID), and PubMed (supplementary for non-Medline records). We also searched ALOIS, the Specialized Register for the Cochrane Dementia and Cognitive Improvement Group (CDCIG). Key phrases were determined in conjunction with an experienced research librarian (Ani Orchanian-Cheff, AO) with the search terms optimized for each database. Key terms are listed below and a copy of the full search strategy is available from the authors on request. Search items (also heading searches) included “Virtual Realit*” OR “VR” OR “computer AND (simulation* OR generat* OR therap* OR treatment*)” and “Alzheimer*.”
Eligibility criteria
Only peer-reviewed empirical articles that could be sourced in full-text and in English were included. No restrictions were set regarding study type or the publication date. Systems were considered VR if they, at a minimum, produced a 3D environment that could be manipulated by a user. Table 1 outlines the inclusion and exclusion criteria across the relevant study elements.
Table 1.
Inclusion and exclusion criteria.
| Study element | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Participants | Persons with dementia (any subtype included) | Study excludes people with MCI unless they were also considered “high risk” of developing into dementia |
| Technological features | Use of a 3D virtual reality environment | Virtual reality was not used, for example, 2D tablet/conventional computer screen display |
| Outcome | Any study design including quantitative or qualitative outcomes of wellbeing (e.g., QoL, communication, interaction, personhood, ADL, IADL, BPSD) as a primary, secondary or tertiary study objective | Interventions targeting ONLY cognitive or memory improvement, physical rehabilitation, reviews/technology appraisals |
Selection process
Step 1: Initial database extraction: Search results were uploaded into Endnote Online Systematic Review Management Software for initial screening. Duplicates were removed.
Step 2: Title/abstract screening: The remaining titles and abstracts were screened by four team members (LA, SA, TN, and KM), two independently reviewing each to identify studies that met the eligibility criteria. All disputes were resolved through discussion until an agreement was reached between the two screening members.
Step 3: Full text eligibility review: The full text of the remaining studies was assessed for eligibility by three members (SA, TN, and KM), two independently reviewing each study. A fourth author (LA) resolved disputes. Reasons for exclusion were noted.
Step 4: Final screening during data extraction: Detailed analyses of papers for data extraction included final review of eligibility for inclusion.
Data extraction
A data extraction form for the included studies was developed in SurveyMonkey, based on Clay et al., 6 but was expanded to include additional categories relevant to answering the research questions (e.g., more detail about the types of hardware and software that made up the VR systems). Three reviewers (SA, TN, and KM) extracted the data independently. Any disagreement between the reviewers was resolved through discussion with a fourth reviewer (ZP). The data extraction form used is available from the authors upon request. Table 2 describes our coding framework and extracted data points.
Table 2.
Data extraction themes.
| Objective | Extracted data | Details |
|---|---|---|
| A | Publication information | Authors, title of the article/journal, year of publication, countries where the study was conducted |
| B | Outcomes of wellbeing | Functional (ADL, iADL), social (personhood, communication/interaction/relationship), emotional (BPSD, pain, and QoL). Reported effectiveness of intervention on outcomes |
| C | Study sample | Criteria for diagnosis of dementia, dementia status (mild, moderate, or severe) and subtypes, and any comorbidities listed as inclusion or exclusion criteria |
| D | Intervention administration | Setting, administering person, frequency (sessions per week), duration (of each session), and length of the full intervention (days, weeks, months) |
| E | Experimental methods | Reported sample size, study design, data collection methods (observation, survey, interview, etc.), validated instruments used, types of data collected (subjective/objective), caregiver/PwD feedback, comparison therapy/arm |
| F | VR technical properties | Device(s) used, manufacturer or brand, product name, degrees of freedom, senses stimulated, content, virtual environment (passive vs active), feelings of presence, dementia-related adjustments |
Data Analysis
Resolved nominal observations were analyzed by examining the frequency of responses among included citations. Descriptive statistics were calculated for numeric responses including, for example, the number of minutes spent per session, frequency, and duration of the sessions, and the number of enrolled PwD (mean, median, mode, standard deviation, and range). Open-ended responses were analyzed by observing for recurring themes and items.
Results
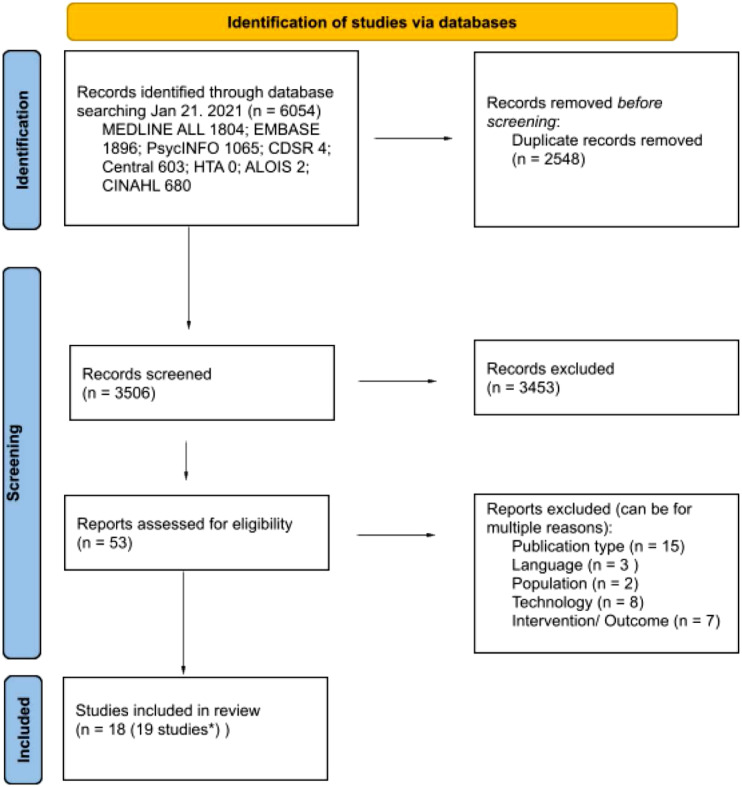
A total of 6054 research citations were found across the database search conducted on the 21 January 2021, (Figure 1 for the PRISMA flow diagram). After a review based on title and abstract, 53 research articles were selected for a full paper review. Each article was independently reviewed in full by two reviewers, who further excluded 35 citations. There were 18 citations agreed upon by consensus to be included in the review and to undergo data extraction. Below, we present the results based on the identified coded categories: Publication, Study samples, Intervention, Outcomes, and Methods (Supplementary Table 1: Complete Results).
Figure 1.
Prisma flow diagram.
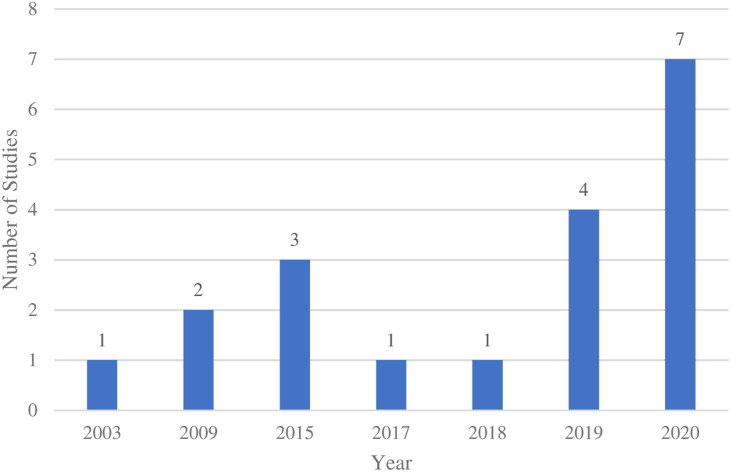
Years and countries of publication
The majority (11, 57.9%) of studies were published in the past 2 years (Figure 2) and primarily in middle-to high-income countries where English is the primary spoken language (13, 68.4%).
Figure 2.
Frequency of publications by year*. *Years are presented based on dates in which papers were published and are not equally distanced.
Outcomes related to aspects of wellbeing
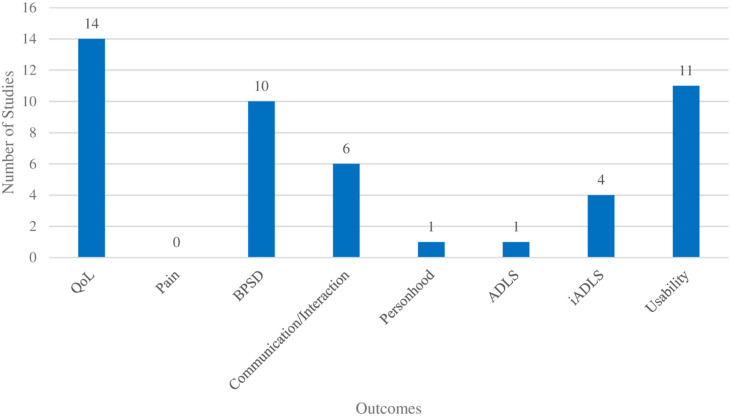
Of the objectives targeting wellbeing, emotional outcomes were by far the most common, reported in seventeen (89.5%) of the included studies, specifically fourteen (73.7%) studies targeted QoL (either through validated measures that included evaluations of feelings or specific metrics capturing mood states) and ten (52.6%) targeting BPSD. Quality of life factors (such as emotional state) were most frequently evaluated using the Observed Emotion Rating Scale (OERS) as was the case in four (21.1%) studies. The Music in Dementia Assessment Scales (MiDAS), EUROHIS-QoL-8, Alzheimer’s Disease Related QoL-French, QUALID, QoL-AD, and EuroQoL 5 Dimension Questionnaire were all used in six (31.6%) of the included papers, the remaining five (26.3%) studies used generic questions that asked about participants’ emotions. Of the studies that measured BPSD, all used validated tools including the Neuropsychiatric Inventory (NPI) and the Person Environment Apathy Rating Scale (PEARS) in two (10.5%) studies each. The Beck Depression Inventory, the Cornell Scale for Depression in Dementia, the Bedford Alzheimer Nursing Scale-Severity, the Cohen-Mansfield Agitation Inventory, the Montgomery Asberg Depression Scale, the Overt Aggression Scale-Modified for Neurorehabilitation, the Stress Symptom Rating Questionnaire, and the Behavioral Observation Scale for Intramural Psychogeriatrics Subscale were each used once. Social outcomes were the second most common category in seven (36.8%) of the studies, with six (31.6%) dedicated to communication/interactions and relationships broadly, and one (5.3%) focusing on personhood. Finally, functional outcomes were mentioned in four (21.1%) studies; iADLs were assessed in four (21.1%) studies, while ADLS were captured in one (5.3%) study. Nearly all (17, 89.5%) of the studies targeted more than one outcome of interest, and it is worth noting that several outcomes were commonly evaluated together, such as BPSD and social factors (4 studies), and BPSD and usability (6 studies). Usability, which was not a direct objective of this review, as it does not directly relate to wellbeing, was still coded for as it appeared to be a common secondary objective accompanying many of the other outcomes, such as social interaction, personhood, ADLs, and iADLs. Figure 3 shows the frequency of outcomes related to aspects of wellbeing across the included studies.
Figure 3.
Outcomes related to aspects of wellbeing.
Supplementary Table 2: Reported Impact of VR on Outcomes of Wellbeing summarizes the outcomes of interest in each study as they relate to promoting wellbeing (conceptualized under the framework of emotional, social, or functional aspects) and their reported effectiveness (having either a positive, neutral, or negative impact on the outcome). Emotional outcomes were the most frequent objective, with 17 outcomes positively impacted (e.g., increase in enjoyment and decrease in anxiety), five with neutral impact, and two with negative impact (e.g., increase in anxiety). Social outcomes were always reported as being positively impacted by VR (e.g., increasing autonomy and instigation of conversation), and impact on functional wellbeing was reported as positive in two (10.5%) studies and neutral in three (15.7%) studies.
Study samples (characteristics of the participants)
Ten (52.6%) of the studies did not report on the severity of dementia in the participants. Of the remaining studies, seven (36.8%) were conducted with participants who had mild dementia, eight (42.1%) with those who had moderate dementia, three (15.7%) with those who had severe dementia, and one (5.5%) study reported “other.”
Of the ten (55%) studies that reported the dementia sub-type(s) of the participants, nine (47.4%) were conducted on people with AD. Vascular and frontotemporal dementia subtypes were included in four (21.5%) studies each. Other unspecified or unknown subtypes were included in three (15.7%) studies, followed by two (10.5%) on mixed dementia and 1 (5.3%) on Lewy-body.
A diagnosis of dementia in the study participants was identified through consultation of clinical/medical files (7, 36.8%) or through administration of the following tests (some of which are screening tools but were used as diagnostic measures in the studies): the Mini-Mental State Examination (MMSE) (5, 26.3%), Montreal Cognitive Assessment (MoCA) (2, 10.5%), and CDR (2, 10.5%), and four (21.5%) studies used other methods such as the Global Deterioration Scale (GDS) rating. Three (15.8%) studies did not report on how participants were screened or diagnosed.
Eligibility criteria for participants in the studies varied; in some cases, the same comorbidities (e.g., mobility impairments) were inclusion criteria for some studies, and exclusion for others. Comorbidities were listed as inclusion criteria in five (26.3%) studies and as exclusion criteria in ten (52.6%) studies, their details are provided in Table 3.
Table 3.
Inclusion and Exclusion criteria related to comorbidities for participants.
| Ref ID | Authors | Inclusion criteria | Exclusion criteria |
|---|---|---|---|
| 32 | Alm et al. (a) | N/A | N/A |
| 32 | Alm et al. (b) | N/A | N/A |
| 33 | Burdea et al. | Traumatic brain injury; stroke; absence of visual and upper body impairments | Severe cognitive delay |
| 34 | Coelho et al. | N/A | Severe visual deficits; unable to verbally communicate; GDS of 1–3; Lewy body dementia; late-stage dementia; schizophrenia; schizoaffective disorder; delusional disorder; non-specified psychotic disorders; bipolar disorder; major depressive disorder |
| 35 | Eisapour et al. | N/A | Moderate to severe cognitive impairment; prone to motion sickness; hearing impairment; conditions preventing exercise; prior epilepsy/seizure; use of pacemaker |
| 36 | Flynn et al. | No evidence of motion sickness; no history of epilepsy; no history of vertigo | N/A |
| 37 | Foloppe et al. | N/A | N/A |
| 30 | Mendez et al. | N/A | Aphasia; complicating medical condition; complicating psychiatric condition |
| 38 | Rohrbach et al | N/A | N/A |
| 24 | Appel et al. | N/A | Open facial wounds; cervical conditions prohibiting VR. |
| 22 | Ferreira et al. | Independent use of upper limbs; intact hearing; initial to intermediate stage of dementia | N/A |
| 39 | Dove et al. | Age-related impairments | N/A |
| 40 | Brimelow et al | N/A | Contagious conditions; ill health; receiving palliative care; bed-bound and unable sit upright |
| 41 | Masoumzadeh et al. | N/A | N/A |
| 17 | Moyle et al. | N/A | N/A |
| 42 | Padala et al. | N/A | Use of mobility device; conditions preventing exercise |
| 43 | Rose et al. | N/A | History of epilepsy; clinical discretion; visual impairments; |
| 44 | Goodall et al. | N/A | Other severe psychiatric disturbance; severe medical condition; physical disability |
| 45 | Santen et al. | N/A | Severe (terminal) condition preventing participation based on clinical discretion |
Intervention administration
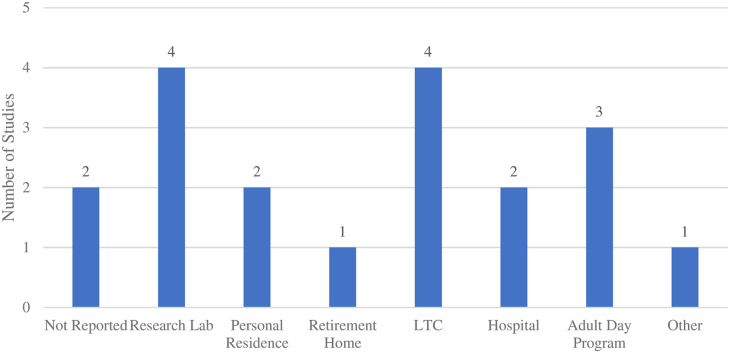
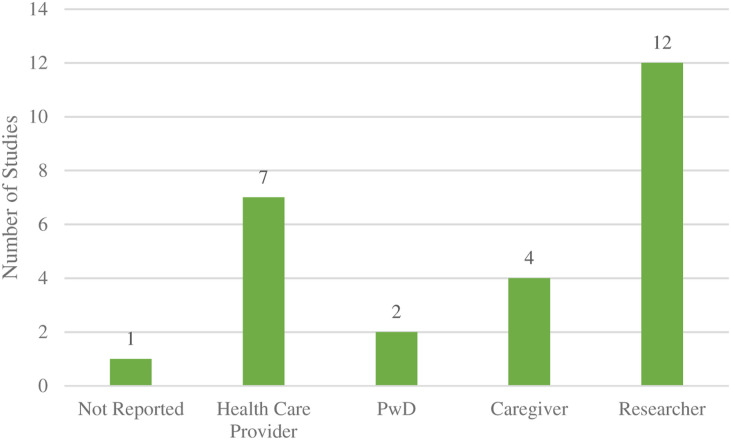
In terms of the setting where the studies were conducted, research labs and LTC were the most common (4, 21.1%), followed by personal residences and hospitals, which were each mentioned in two (10.5%) studies, and a retirement home reported in one (5.3%) study. Three (15.8%) studies were conducted in adult day programs for PwD, and one study was conducted in an “other” location (community center) (Figure 4). Nearly two thirds (12, 63.2%) of included studies were administered by a researcher, seven (36.8%) by a healthcare provider, four (21.1%) by a personal caregiver (family/friend), and two (10.5%) by the PwD. Two (10.5%) studies did not report the setting in which the intervention was assessed, and one (5.3%) did not report the person responsible for administering the intervention (Figure 5).
Figure 4.
Frequency of settings used for VR interventions.
Figure 5.
Person responsible for administering VR interventions.
In terms of the dose-regimen reported in the included studies (Table 4), across the 13 (68.4%) studies that reported on the VR intervention duration, the mean was 27 min. Across eleven (57.9%) studies that reported on the frequency of administering the VR intervention, the mean was 2.82 sessions per week. Ten (52.6%) studies reported on the longitudinal duration of the study, with a mean of 7.6 weeks. Less than half (8, 42.1%) of the studies reported on all three metrics.
Table 4.
Dose regimen: frequency, duration, and length.
| Dose | # of studies that reported | Mean | SD | Median | Mode | Range |
|---|---|---|---|---|---|---|
| Frequency of sessions (per week) | 11 | 2.82 | 1.66 | 2 | 1 | 1–5 |
| Duration per session (minutes) | 13 | 27.23 | 18.20 | 20 | 15 | 4–60 |
| Length (weeks) | 10 | 7.60 | 7.97 | 3.5 | 2 | 2–24 |
Experimental methods
Eight (42.1%) studies had sample sizes of ten or fewer participants, three (15.7%) studies had between 11–20 participants, four (21.1%) studies had between 21–30 participants, two (10.5%) studies had between 31–40 participants, and two (10.5%) studies had over 100 participants. Nine (47.4%) of the studies had multiple participant groups, for example, in addition to those participants with dementia, Moyle et al. 17 included ten family members and nine care staff, and Rose et al. 43 included 16 caregivers. Table 5 summarizes the sample size for each study and the number of PwD participants. Numbers ranged from 1–84 with a mean of 16 (SD 20.9), median of 9, and mode 10.
Table 5.
Number of participants with dementia by study.
| Ref ID | Authors | Date of publication | Study sample size | Number of participants with dementia |
|---|---|---|---|---|
| 32 | Alm et al. (a) | 2009 | 35 | 22 |
| 32 | Alm et al. (b) | 2009 | Not reported a | Not reported a |
| 33 | Burdea et al. | 2015 | 10 | 7 |
| 34 | Coelho et al. | 2020 | 9 | 9 |
| 35 | Eisapour et al. | 2020 | 6 | 6 |
| 36 | Flynn et al. | 2003 | 6 | 6 |
| 37 | Foloppe et al. | 2015 | 1 | 1 |
| 30 | Mendez et al. | 2015 | 5 | 5 |
| 38 | Rohrbach et al. | 2019 | 10 | 10 |
| 23 | Appel et al. | 2020 | 10 | 10 |
| 22 | Ferreira et al. | 2020 | 12 | 12 |
| 39 | Dove et al. | 2019 | 23 | 16 |
| 40 | Brimelow et al. | 2020 | 13 | 9 |
| 41 | Masoumzadeh et al. | 2020 | 11 | 5 |
| 17 | Moyle et al. | 2018 | 29 | 10 |
| 42 | Padala et al. | 2017 | 30 | 30 |
| 43 | Rose et al. | 2019 | 24 | 8 |
| 44 | Goodall et al. | 2019 | 55 | 55 |
| 45 | Santen et al. | 2020 | 112 | 84 |
aThe study specifies 40 individuals being consulted, however, does not indicate whether these were some/all study participants, and/or how many were PwD.
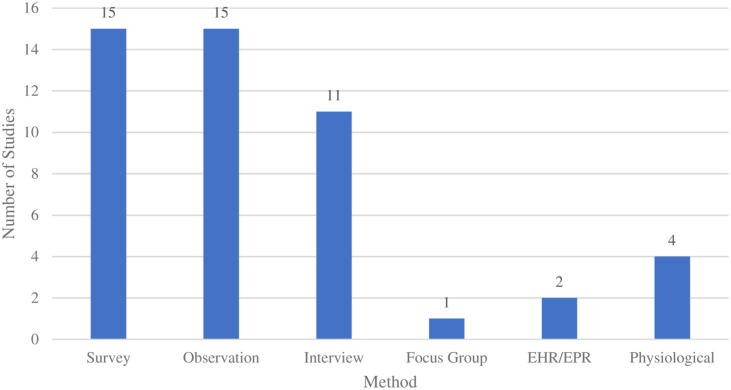
Case series were used in seven (36.8%) of the studies; cross-sectional designs were used in six (31.6%) studies; randomized controlled trials were used in five (26.3%) studies; and crossover was used in three (15.8%) studies. A case study design was used in 1 (5.3%) study. Observations and surveys were each used in fifteen (78.9%) studies, and interviews were used in eleven (57.9%) studies. Data were collected through physiological measures in four (21.1%) studies, while two (10.5%) studies used data from electronic health records. One (5.3%) study reported using focus groups (Figure 6). Fifteen (78.9%) studies used multiple data collection methods such as surveys and interviews. Observations were the most frequent data collection method (sometimes accompanied by the use of other measures such as electronic health records, focus groups, or physiological measurements). All studies collected subjective data (outcomes/reactions as determined by the researcher or participant), while fourteen (73.7%) studies reported collecting objective data (not influenced by personal feelings or opinions of any stakeholder), for example, physiological data like heart rate. 35
Figure 6.
Data collection methods.
More than half (10, 52.6%) of the studies did not report on a comparison group or arm, one (5.3%) study included a comparison group, and eight (42.1%) studies evaluated the VR intervention against a comparator, such as conducting similar activities without the technology or comparing healthy participants to those with mild cognitive impairment and/or AD.
Virtual reality technical properties
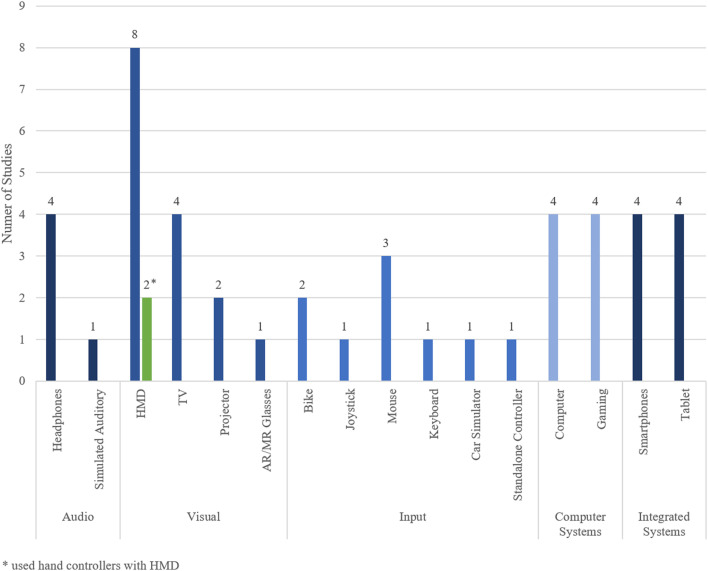
Devices used in the included studies were organized into the following five categories: audio, visual, input devices, computer systems, and integrated systems (Figure 7). Headphones were used in four (21.1%) of the studies, while a simulated auditory environment was used in one (5.3%) study. Head mounted displays (HMDs) were the most frequently used (8, 42.1%) visual device in the included studies. Of the eight studies that used HMDs, two actively made use of the controllers included with HMDs to capture motion of the upper body. Television screens were the second most used visual device (4, 21.1%), followed by projectors (2, 10.5%). Augmented Reality/Mixed Reality glasses were used in one (5.3%) study.
Figure 7.
Types of devices used.
A wide range of input devices were used, with the most prominent being computer mice (3, 15.8%), followed by bikes (2, 10.5%). Joysticks, keyboards, car simulators, and standalone controllers which did not come with an HMD were used in one (5.3%) study each. Computers, gaming consoles, smartphones, and tablets were used in four (21.1%) studies each.
All nineteen (100%) studies engaged viewers through visual stimuli, and the majority (17, 89.5%) also provided auditory stimuli of which 12 studies did not explicitly describe the devices used to present the audio; two (10.5%) included tactile interfaces and one (5.3%) olfactory. Four (21.1%) studies used proprioception, with all motions actively produced by the user (i.e., person’s own volitional head/body movements). Five (26.3%) studies implemented best practice adjustments, particularly relevant for PwD users, such as not including sudden scene changes or readjusting the straps if the PwD was in discomfort 46 and reducing the complexity of tasks to avoid unnecessary confusion. 43
Computer graphics were the most common form of content, reported in nine (47.4%) studies, followed by 360-films which were used in four (21.1%) of the studies. Three (15.8%) studies used traditional 2D videos, and three (15.8%) did not report the content design of their VR interventions. While the majority (12, 63.2%) of studies had interventions that engaged users in “active” virtual scenarios, (e.g., complete a task, activity, or game), six (31.6%) used passive-engagement, and one (5.3%) did not report the kind of experience. Seven (36.8%) studies included interventions with three-degrees of freedom (rotational head movement) versus eight studies (42.1%) that used six-degree of freedom (head rotation and translation). A minority of studies (3, 15.8%) evaluated “presence” as a distinct feature of the VR system.
Discussion
This scoping review provides a comprehensive overview of peer-reviewed research on VR interventions for PwD, with a focus on promoting wellbeing. We present details on the intended outcomes of the intervention and their reported effectiveness, intervention administration protocol, and means of evaluation and the VR-system’s technical properties. In the next section, we provide more details from some of the included studies, identifying gaps, and suggesting targeted goals for future studies.
Year of publications
Most of the studies were published in the past 2 years, which can be indicative of VR systems starting to mature in the market. This could be due to increased affordability, availability of user-friendly devices with more widely available content, availability of mobile commercial headsets which were first released in 2014, 47 and increased cohort familiarity with these technologies.
Outcomes of wellbeing
Of the studies that explicitly reported the effectiveness of VR on emotional outcomes, the results were mostly positive; however, ascertaining the effectiveness of VR therapy on emotional wellbeing is still challenging. The instruments used in most of the studies for evaluation were disparate, and the few used in multiple studies (e.g., OERS), are not validated, particularly with the target population of PwD. For example, Rose et al. 43 state in their limitations that they were “unable to rate observations of affect specifically in relation to eyes due to HMD-VR headset covering the participants’ eyes.” (44 p123). Since facial expressions and particularly eyes-gaze are frequently used to observe reactions in PwD, 48 and VR HMDs covers these features limiting an observer’s ability to gauge a user’s reactions, it would be of benefit to the field to develop standard observational tools to report on the engagement with VR with this specific population. It is worth noting that many HMDs are moving towards integrated eye tracking, 49 which could also help mitigate this, as well as present new opportunities for features in intervention design.
Our review also found no research studies on the use of VR to address (identify and alleviate) pain in PwD. Pain can cause secondary symptoms such as sleep disturbances, depression, and decreased mobility, and also frequently manifests as agitation and increased confusion, all of which contribute to decreased QoL. 50 As there is growing evidence supporting VR’s effectiveness in managing acute procedural pain and distress in many other patient populations, 51 and since VR systems appear to be tolerated and accepted by PwD, we recommend future studies explore its impact on pain-related outcomes.
Clinicians and caregivers alike are highlighting the importance of continued social stimulation and interaction, specifically the opportunity for shared experiences and communication with PwDs as a means of maintaining personhood and navigating relationships. 52 While only a few of the studies in this review had outcomes related to social wellbeing as a primary objective, their results were promising. For example, Mendez et al. 30 found that participants were more talkative and gave more elaborate answers with details when questioned by their virtual avatars when compared to a real in-person interviewer. Alm et al. 32 found that the Computer Interactive Reminiscence and Conversation Aid (CIRCA) system 53 unexpectedly restored a great deal of equality to the interaction between users and their caregivers, allowing the PwD to exercise control more easily over the direction of the conversation.
Overall, this review found that VR is a promising tool for promoting wellbeing in PwD. Its effectiveness on emotional, social, and functional aspects of wellbeing were all reported as having a positive (or neutral) impact despite some occurrences of side effects which must be taken into consideration when administering VR.
Study samples
There was no standardized method or tool used to screen for or diagnose dementia in participants across the included studies, but it is encouraging to see that most (13, 72.2%) studies recruited participants based on validated measures. Participants with AD were the most commonly sampled population, contributing to half of the studies, followed by vascular dementia (in four studies), which may be due to the fact that they are the most prevalent subtypes. 54 Since different subtypes of dementia are associated with unique symptoms, different disease progression, and require different treatments/therapies, it is important to better understand how VR design and effectiveness may best address the needs of each subtype.
Most of the studies that reported dementia severity included participants with mild to moderate dementia. These individuals are generally better able to follow instructions, communicate, and engage in activities. Moreover, there is added difficulty in recruiting participants at advanced disease stage, as usually the substitute decision maker (SDM) is required to provide consent.55,56 The few studies that did include this population23,41,44 reported a positive impact, and others recommended this as future research. Some studies actively excluded those with moderate to severe cognitive impairment, “late-stage dementia,” as well as particular subtypes of dementia, like Lewy body (due to higher probability of developing hallucinations), which identify additional areas for future research.
Many of the studies included comorbidities among their participant exclusion criteria, some of which concern the physical contact of the device to one’s head, such as open facial wounds (where the HMD may touch and irritate) or cervical conditions (where the HMD may be considered too heavy). Other exclusion factors such as being bed-bound, unable to sit up, having lack of active movement in either arms, or being in palliative care33,40 may not be prohibitive to VR use, but could make it more difficult and/or influence the chances that it will be effective. Some of the listed comorbidities included in our review are very common among those living with dementia (e.g., aphasia, 57 the inability to verbally communicate, 57 or having a hearing impairment 58 ), such that screening for inclusion based on the absence of these factors may actually limit the generalizability of the findings to this population. It is not uncommon to start interventional studies with the groups of participants who are most accessible, for whom the therapy is easiest to implement, and for whom the effectiveness is likely to be greatest. However, even studies that sampled the frailest individuals found the VR devices acceptable and feasible.23,41 Brimelow 40 reported that even those participants with severe cognitive impairment (half of their sample) enjoyed the experience, “further highlighting the versatility of VR” (41 p169). Finally, some characteristics, like being prone to motion sickness or having a history of seizure, are general warnings listed on device manufacturers documentation (e.g., HMDs); however, these comorbidities were not found in practice to cause major problems in the few studies for which they are reported,34,36,59 especially when precautions were taken. 60 Moreover, when symptoms of simulator sickness were observed, as were evaluated by Masoumzadeh et al., 41 they did not differ from less-invasive systems (such as TV screens), and Flynn 36 found that any observed negative side effects were comparable to the experiences of persons without cognitive impairments. This may suggest an opportunity for cautious future exploration, given that it could increase the breadth of the potential population who could benefit from VR-therapy.
Intervention administration
Research labs were the most frequent settings for conducting evaluations, and researchers were by far the most common administrators of VR-interventions; furthermore, caregivers were only involved in the administration of therapy in four studies and PwD self-administered in one study. While this may be partially because the selected literature consisted of only peer-reviewed research studies, it does present opportunities to design interventions with greater feasibility and external validity, and ones that enhance care-partner shared experiences. Future studies need to consider the practical implications of actually implementing these interventions within the real-life settings, with a focus on implementing these therapies in the community (where the majority of PwD live/reside 61 ). There is a unique opportunity to engage caregivers in the provision of the therapies in a way that could create mechanisms for novel shared experiences, thereby enhancing communication and engagement. For example, Rose et al. 43 streamed the VR content to an external flat screen, which allowed caregivers to see what the PwD was seeing in the HMD encouraging them to provide relevant prompts and reassurance during the exposure. Similarly, Flynn 36 observed that the “involvement of carers provided an invaluable source of social support that served to reduce anxiety and enhance motivation of PWD during the VR exercises” (36 p605).
Finally, a major point of interest for researchers and clinicians working with non-pharmacological interventions for dementia is to identify effective protocols regarding the dose regimen, that is, the length, frequency, and duration of the VR therapy. The studies included in this review represent a diverse set of interventions with different targeted outcomes, but collectively suggest a range of dose intervals that are tolerated and effective. The shortest exposure duration was 4–5 min per video, resulting in positive effects with increased interaction and facial expressions following VR. 40 Keeping in mind that most therapies focused on improving wellbeing can and should be tailored to the individual, this is just a first step in formalizing the use of VR as a clinically accepted intervention.
Experimental methods
Given that research of VR in dementia is still nascent and that recruiting those with dementia is challenging, it is not surprising that half of the included studies had sample sizes of ten or less participants. Future studies should strive to include larger sample sizes in order to allow for more generalizable statements about the impact of VR-therapy on this population.
It is encouraging to see that several (5, 26.3%) of the included studies were designed using a randomized controlled trial. A within-subject cross-over design is also worth exploring as this method is well equipped to compare the outcomes of VR to a comparator intervention and to the participant’s own baseline. For example, Eisapour 35 investigated whether playing games and interacting with virtual objects in VR could be a comparable alternative to (human) therapist-led exercises for PwD, using a cross-over methodology. Specifically, each participant began with 1 week of therapist-led physical exercise and were then evenly and randomly assigned to one of two virtual environments for the second week and then switched to the other virtual environment in the third week. Ferreira 22 conducted a within-subject experimental design to allow all participants to interact with all technologies, such as an HMD, tablet, mouse, augmented reality (AR), leap motion (LM), and a combination of HMD with LM, in order to determine which had the greatest ease of use.
Despite the difficulty in eliciting feedback from individuals who are not able to communicate in traditional ways, we recommend that future studies should be bolstered by methodologies that explicitly seek participants’ opinions on VR. We advise that researchers also strive to gather feedback from a proxy who knows (is very familiar with) the PwD, such as a personal caregiver; for example, Brimelow 40 relied on care staff known to the resident, to be included in the procedure.
Virtual reality technical properties
The term “VR” had been applied to an array of technological devices across the included studies. All interventions provided visual stimuli (which was required as an inclusion criterion), half of which displayed VR content on an HMD. For example, Appel et al. 23 provided participants with 360-degree films of natural scenery (e.g., peaceful beach) displayed using a HMD. Other interventions used projection systems; for example, Flynn et al. 36 invited participants into an auditorium fitted with a large 140-degree curved screen, which provides a semi-immersive view of virtual environments with surround sound. Most (61%) of the included studies provided VR content that was created using computer graphics. Foloppe. 37 for example, designed a virtual kitchen environment that included common kitchen digital objects (i.e., foods, utensils, and appliances) and asked the participant to complete 10 cooking tasks (e.g., setting the table). The fact that most of the content was created using computer graphics aligns with the finding that the majority of interventions (61%) engaged users in “active” virtual scenarios, such as task completion. Interactive tasks with cause and effect (e.g., performing an iADL like setting the table) are more easily achieved in virtual environments that are designed using computer graphics, rather than, for example, using 360-film. However, there are examples of photography/video adapted for use as interactive tools/activities, etc., see Alm et al. 32 Some senses may offer new ways of stimulating PwD. Smell, for example, has been strongly tied to memory recall and is a biomarker for AD/cognitive decline [76], but was only part of one intervention, Goodall et al.’s 44 SENSE-GARDEN, the results of which have not yet been published.
Finally, providing the sensation of “presence” is an important factor that often differentiates VR from other technologies and is expected to be a unique benefit in the provision of therapy for general wellness, 36 ; however, this subjective experience was explicitly asked of participants in only two (11.11%) studies36,43 using standardized questionnaires. Future studies could better define how presence is experienced by PwD and whether it enhances wellbeing. While many VR applications follow a “one size fits all” approach, there has been an effort to develop VR platforms in which healthcare professionals can easily customize cognitive activities (software) and deploy these on a variety of Hardware (AR, tablet, PC, and interactive table) based on the needs and user experience for PwD.62–65 Future opportunities lie in identifying special features and customization of the hardware/software to afford the most benefit to different sub-groups of the target population.
Summary
This scoping review identified 18 papers evaluating 19 studies on aspects of VR that could impact the wellbeing for PwD. Through mapping out the technical properties of the VR systems, identifying meaningful outcomes representing wellbeing, and how, by whom, and in what settings the systems were evaluated/implemented, we were able to identify gaps in the literature that could be pursued in future studies. As research in the field grows, further propelled by the adoption of digital interventions during the COVID 19 pandemic, 66 we foresee larger trials will be conducted. While much is left to be explored, this review has identified several considerations that can help research in the area of VR therapy produce meaningful findings; for example, employing well-suited study designs, having standardized validated observational tools geared to capture changes specifically in PwD, and involving/consulting with PwD and their caregivers throughout the entire design and evaluation process. Researchers should recruit participants representative of the actual PwD population, carefully considering/defining the exclusion criterion, and sampling across the dementia spectrum, including severe dementia and common comorbidities. More research should be conducted in people’s residences, treating personal caregivers as equal members in care and therapy-provision. We see value in further exploring the impact of VR on pain in PwD, as well as communication between those with dementia and their caregivers, family, and friends. As sensory technologies continue to improve, there would be great benefit in evaluating the impact of unconventional/uncommon virtual sensory experiences such as “virtual smell” for PwD, for example, as a part of VR-reminiscence therapy. Finally, future studies should aim to better understand how certain technical features (movement degrees of freedom and interactivity) affect therapeutic effectiveness, and if demographics or abilities are better suited for some VR-systems/approaches over others. Although the included studies covered a spectrum of outcomes related to emotional, social, and functional wellbeing, and used many different methods of evaluation, at face value results are encouraging with mostly positive (and few neutral) impacts of VR on these outcomes. Studies focused on enhancing the lives of PwD are crucial in moving patients and their families forward post-diagnosis, sustaining an identity, and continuing to live a life with meaning and value 67 ; VR appears to be a suitable complement to other interventions.
Supplemental Material
Supplemental Material, sj-xlsx-1-jrt-10.1177_20556683211053952 for Impact attenuation provided by older adult protective headwear products during simulated fall-related head impacts by Daniel R Martel, Michelle R Tanel and Andrew C Laing in Journal of Rehabilitation and Assistive Technologies Engineering
Supplemental Material, sj-xlsx-2-jrt-10.1177_20556683211053952 for Impact attenuation provided by older adult protective headwear products during simulated fall-related head impacts by Daniel R Martel, Michelle R Tanel and Andrew C Laing in Journal of Rehabilitation and Assistive Technologies Engineering
Acknowledgments
We would like to thank Orli Bogler, Leedan Cohen, Danjay Hill, Micaela Wiseman, and Natalie Ein for their assistance in screening articles in earlier versions of our work.
Appendix
Acronyms Used
- ADL
Activities of Daily Living
- AD
Alzheimer’s dementia
- AR
Augmented Reality
- BPSD
Behavioral and psychological symptoms of dementia
- GDS
Global Deterioration Scale
- HMD
Head mounted displays
- iADL
Instrumental activities of daily living
- LM
Leap Motion
- LTC
Long Term Care
- MMSE
Mini-Mental State Examination
- MOCA
Montreal Cognitive Assessment
- NPI
Neuropsychiatric Inventory
- OERS
Observed Emotion Rating Scale
- PEARS
Person Environment Apathy Rating Scale
- PwD
Persons with dementia
- QoL
Quality of life
- SDM
Substitute decision maker
- VR
Virtual Reality
- VRFCAT
Virtual Reality Functional Capacity Assessment Tool
Author contributions: LA and JC conceived the study. LA, JC, AO were involved in protocol search strategy development, SA, TN, KM, and ZP conducted the data analysis. LA wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Guarantor: LA
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
L Appel https://orcid.org/0000-0002-7176-7191
A Orchanian-Cheff https://orcid.org/0000-0002-9943-2692
JL Campos https://orcid.org/0000-0002-7054-3946
References
- 1.Parsons TD, McPherson S, Interrante V. Enhancing neurocognitive assessment using immersive virtual reality. In: 2013 1st Workshop on Virtual and Augmented Assistive Technology (VAAT), Lake Buena Vista, FL, 17–17 March 2013, IEEE, pp. 27–34. [Google Scholar]
- 2.Matsangidou M, Schiza E, Hadjiaros M, et al. Dementia: I am physically fading. can virtual reality help? physical training for people with dementia in confined mental health units. In: Antona M, Stephanidis C. (eds). Universal Access in Human-Computer Interaction. Design Approaches and Supporting Technologies. Cham: Springer International Publishing; 2020, pp. 366–382. [Google Scholar]
- 3.Cavedoni S, Chirico A, Pedroli E, et al. Digital biomarkers for the early detection of mild cognitive impairment: artificial intelligence meets virtual reality. Front Hum Neurosci 2020; 14: 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.García-Betances RI, Arredondo Waldmeyer MT, Fico G, et al. A succinct overview of virtual reality technology use in alzheimer’s disease. Front Aging NeuroSci; 7. Epub Ahead of Print 2015. DOI: 10.3389/fnagi.2015.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilmartin-Thomas JF-M, McNeil J, Powell A, et al. Impact of a virtual dementia experience on medical and pharmacy students’ knowledge and attitudes toward people with dementia: a controlled study. J Alzheimers Dis JAD 2018; 62: 867–876. [DOI] [PubMed] [Google Scholar]
- 6.Clay F, Howett D, FitzGerald J, et al. Use of immersive virtual reality in the assessment and treatment of alzheimer’s disease: a systematic review. J Alzheimers Dis 2020; 75: 23–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.García-Betances R, Jimenez V, Arredondo M, et al. Using virtual reality for cognitive training of the elderly. Am J Alzheimers Dis Other Demen 2014; 30: 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benoit M, Guerchouche R, Petit P-D, et al. Is it possible to use highly realistic virtual reality in the elderly? A feasibility study with image-based rendering. Neuropsychiatr Dis Treat 2015; 11: 557–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slater M, Lotto B, Arnold MM, et al. How we experience immersive virtual environments: the concept of presence and its measurement. Anu Psicol 2009; 40: 19. [Google Scholar]
- 10.Mental Health . strengthening Our Response, Available at: https://www.who.int/news-room/fact-sheets/detail/mental-health-strengthening-our-response (accessed 24 June 2021). [Google Scholar]
- 11.Alzheimer Society of Canada . 10 priorities for dementia research in Canada. Alzheimer Society of Canada, Available at: http://alzheimer.ca/en/research/10-priorities-dementia-research-canada (accessed 24 June 2021). [Google Scholar]
- 12.Lyu W, Wolinsky FD. The onset of ADL difficulties and changes in health-related quality of life. Health Qual Life Outcomes 2017; 15: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allain P, Foloppe DA, Besnard J, et al. Detecting everyday action deficits in alzheimer’s disease using a nonimmersive virtual reality kitchen. J Int Neuropsychol Soc 2014; 20: 468–477. [DOI] [PubMed] [Google Scholar]
- 14.Atkins AS, Khan A, Ulshen D, et al. Assessment of instrumental activities of daily living in older adults with subjective cognitive decline using the virtual reality functional capacity assessment tool (VRFCAT). J Prev Alzheimers Dis 2018; 5: 216–234. [DOI] [PubMed] [Google Scholar]
- 15.Alzheimer’s Society . The Psychological and Emotional Impact of Dementia, Available at: https://www.alzheimers.org.uk/get-support/help-dementia-care/understanding-supporting-person-dementia-psychological-emotional-impact (accessed 24 June 2021). [Google Scholar]
- 16.Appel L, Appel E, Bogler O, et al. Older adults with cognitive and/or physical impairments can benefit from immersive virtual reality experiences: a feasibility study. Front Med; 6. Epub Ahead of Print 2020. DOI: 10.3389/fmed.2019.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moyle W, Jones C, Dwan T, et al. Effectiveness of a virtual reality forest on people with dementia: a mixed methods pilot study. The Gerontologist 2018; 58: 478–487. [DOI] [PubMed] [Google Scholar]
- 18.Devshi R, Shaw S, Elliott-King J, et al. Prevalence of behavioural and psychological symptoms of dementia in individuals with learning disabilities. Diagnostics 2015; 5: 564–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donaldson C, Tarrier N, Burns A. The impact of the symptoms of dementia on caregivers. Br J Psychiatry J Ment Sci 1997; 170: 62–68. [DOI] [PubMed] [Google Scholar]
- 20.Danieli E. Non-Pharmacological Assessment and Management of Behavioural and Psychological Symptoms of Dementia in Primary Care. Mount Sinai Hospital, 2013. Available at: https://www.mountsinai.on.ca/care/psych/patient-programs/geriatric-psychiatry/prc-dementia-resources-for-primary-care/dementia-toolkit-for-primary-care/responsive-behaviours-in-dementia/non-pharmacological-assessment-and-management-of-behavioural-and-psychological (accessed 24 June 2021). [Google Scholar]
- 21.Bianchetti A, Scuratti A, Zanetti O, et al. Predictors of mortality and institutionalization in Alzheimer disease patients 1 year after discharge from an Alzheimer dementia unit. Dement Basel Switz 1995; 6: 108–112. [DOI] [PubMed] [Google Scholar]
- 22.Ferreira LDA, Ferreira H, Cavaco S, et al. User experience of interactive technologies for people with dementia: comparative observational study. JMIR Serious Games 2020; 8: e17565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Appel L, Kisonas E, Appel E, et al. Introducing virtual reality therapy for inpatients with dementia admitted to an acute care hospital: learnings from a pilot to pave the way to a randomized controlled trial. Pilot Feas Stud 2020; 6: 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Appel L, Kisonas E, Appel E, et al. Administering virtual reality therapy to manage behavioral and psychological symptoms in patients with dementia admitted to an acute care hospital: results of a pilot study. JMIR Form Res 2021; 5: e22406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D’Cunha NM, Nguyen D, Naumovski N, et al. A mini-review of virtual reality-based interventions to promote well-being for people living with dementia and mild cognitive impairment. Gerontology 2019; 65: 430–440. [DOI] [PubMed] [Google Scholar]
- 26.Jao Y-L, Loken E, MacAndrew M, et al. Association between social interaction and affect in nursing home residents with dementia. Aging Ment Health 2018; 22: 778–783. [DOI] [PubMed] [Google Scholar]
- 27.Woods B, O’Philbin L, Farrell EM, et al. Reminiscence therapy for dementia. Cochrane Database Syst Rev 2018; 3: CD001120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cutler C, Hicks B, Innes A. Does digital gaming enable healthy aging for community-dwelling people with dementia? Games Cult 2016; 11: 104–129. [Google Scholar]
- 29.Wilkins JM. Dementia, decision making, and quality of life. AMA J Ethics 2017; 19: 637–639. [Google Scholar]
- 30.Mendez MF, Joshi A, Jimenez E. Virtual reality for the assessment of frontotemporal dementia, a feasibility study. Disabil Rehabil Assist Tech 2015; 10: 160–164. [DOI] [PubMed] [Google Scholar]
- 31.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005; 8: 19–32. [Google Scholar]
- 32.Alm N, Astell AJ, Gowans G, et al. Engaging multimedia leisure for people with dementia. Gerontech 2009; 8(4): 236–246. DOI: 10.4017/gt.2009.08.04.006.00. [DOI] [Google Scholar]
- 33.Burdea G, Polistico K, Krishnamoorthy A, et al. Feasibility study of the BrightBrainerTM integrative cognitive rehabilitation system for elderly with dementia. Disabil Rehabil Assist Tech 2015; 10: 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coelho T, Marques C, Moreira D, et al. Promoting reminiscences with virtual reality headsets: a pilot study with people with dementia. Int J Environ Res Public Health 2020; 17: E9301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eisapour M, Cao S, Boger J. Participatory design and evaluation of virtual reality games to promote engagement in physical activity for people living with dementia. J Rehabil Assist Technol Eng 2020; 7: 2055668320913770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flynn D, van Schaik P, Blackman T, et al. Developing a virtual reality-based methodology for people with dementia: a feasibility study. Cyberp Behav Imp Int Multimed Vir Real Behav Soc 2003; 6: 591–611. [DOI] [PubMed] [Google Scholar]
- 37.Foloppe DA, Richard P, Yamaguchi T, et al. The potential of virtual reality-based training to enhance the functional autonomy of Alzheimer’s disease patients in cooking activities: A single case study. Neuropsychol Rehabil 2018; 28: 709–733. [DOI] [PubMed] [Google Scholar]
- 38.Rohrbach N, Gulde P, Armstrong AR, et al. An augmented reality approach for ADL support in Alzheimer’s disease: a crossover trial. J Neuro Eng Rehabil 2019; 16: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dove E, Astell A. The kinect project: group motion-based gaming for people living with dementia. Dement Lond Eng 2019; 18: 2189–2205. [DOI] [PubMed] [Google Scholar]
- 40.Brimelow RE, Dawe B, Dissanayaka N. Preliminary research: virtual reality in residential aged care to reduce apathy and improve mood. Cyberp Behav Soc Netw 2020; 23: 165–170. [DOI] [PubMed] [Google Scholar]
- 41.Masoumzadeh S, Moussavi Z. Does practicing with a virtual reality driving simulator improve spatial cognition in older adults? A Pilot Study. Neurosci Insights 2020; 15: 2633105520967930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Padala KP, Padala PR, Lensing SY, et al. Home-based exercise program improves balance and fear of falling in community-dwelling older adults with mild alzheimer’s disease: a pilot study. J Alzheimers Dis JAD 2017; 59: 565–574. [DOI] [PubMed] [Google Scholar]
- 43.Rose V, Stewart I, Jenkins KG, et al. Bringing the outside in: The feasibility of virtual reality with people with dementia in an inpatient psychiatric care setting. Dement Lond Engl 2021; 20: 106–129. [DOI] [PubMed] [Google Scholar]
- 44.Goodall G, Ciobanu I, Taraldsen K, et al. The use of virtual and immersive technology in creating personalized multisensory spaces for people living with dementia (sense-garden): protocol for a multisite before-after trial. JMIR Res Protoc 2019; 8: e14096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Santen J, Dröes R-M, Twisk JWR, et al. Effects of exergaming on cognitive and social functioning of people with dementia: a randomized controlled trial. J Am Med Dir Assoc 2020; 21: 1958–1967. [DOI] [PubMed] [Google Scholar]
- 46.McCauley CO, Bond RB, Ryan A, et al. Evaluating user engagement with a reminiscence app using cross-comparative analysis of user event logs and qualitative data. Cyberp Behav Soc Netw 2019; 22: 543–551. [DOI] [PubMed] [Google Scholar]
- 47.Barnard D.. History of VR-Timeline of Events and Tech Development. Virtualspeech, 2019, Available at: https://virtualspeech.com/blog/history-of-vr (accessed 28 June 2021). [Google Scholar]
- 48.Molitor RJ, Ko PC, Ally BA. Eye movements in Alzheimer’s disease. J Alzheimers Dis JAD 2015; 44: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rogers S.. Seven Reasons Why Eye-Tracking Will Fundamentally Change VR. Forbes, 2019, Available at: https://www.forbes.com/sites/solrogers/2019/02/05/seven-reasons-why-eye-tracking-will-fundamentally-change-vr/ (accessed 2 July 2021). [Google Scholar]
- 50.Warden V, Hurley AC, Volicer L. Development and psychometric evaluation of the pain assessment in advanced dementia (PAINAD) scale. J Am Med Dir Assoc 2003; 4: 9–15. [DOI] [PubMed] [Google Scholar]
- 51.Sharar SR, Miller W, Teeley A, et al. Applications of virtual reality for pain management in burn-injured patients. Expert Rev Neurother 2008; 8: 1667–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vernooij-Dassen M, Moniz-Cook E, Jeon Y-H. Social health in dementia care: harnessing an applied research agenda. Int Psychogeriatr 2018; 30: 775–778. [DOI] [PubMed] [Google Scholar]
- 53.Clare L, Kudlicka A, Oyebode JR, et al. Goal-oriented cognitive rehabilitation for early-stage alzheimer’s and related dementias: the great rct. NIHR Journals Library 2019; 23(10): 1–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dementia-Symptoms and Causes. Mayo Clinic, Available at: https://www.mayoclinic.org/diseases-conditions/dementia/symptoms-causes/syc-20352013 (accessed 28 June 2021). [Google Scholar]
- 55.Burns KEA, Zubrinich C, Tan W, et al. Research recruitment practices and critically ill patients. a multicenter, cross-sectional study (the consent study). Am J Respir Crit Care Med 2013; 187: 1212–1218. [DOI] [PubMed] [Google Scholar]
- 56.Cleaver S, Ouellette-Kuntz H, Sakar A. Participation in intellectual disability research: a review of 20 years of studies. J Intellect Disabil Res 2010; 54: 187–193. [DOI] [PubMed] [Google Scholar]
- 57.Matej R, Tesar A, Rusina R. Alzheimer’s disease and other neurodegenerative dementias in comorbidity: A clinical and neuropathological overview. Clin Biochem 2019; 73: 26–31. [DOI] [PubMed] [Google Scholar]
- 58.Uhlmann RF, Larson EB, Koepsell TD. Hearing impairment and cognitive decline in senile dementia of the Alzheimer’s type. J Am Geriatr Soc 1986; 34: 207–210. [DOI] [PubMed] [Google Scholar]
- 59.Tychsen L, Thio LL. Concern of photosensitive seizures evoked by 3D video displays or virtual reality headsets in children: current perspective. Eye Brain 2020; 12: 45–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fagan K. What Happens to Your Body When You’ve Been in Virtual Reality for Too Long. Insider, 2018, Available at: https://www.businessinsider.com/virtual-reality-vr-side-effects-2018-3 (accessed 28 June 2021). [Google Scholar]
- 61.Dementia in Canada: Summary. CIHI, Available at: https://www.cihi.ca/en/dementia-in-canada/dementia-in-canada-summary (accessed 28 June 2021). [Google Scholar]
- 62.Rose V, Stewart I, Jenkins KG, et al. A Scoping Review Exploring the Feasibility of Virtual Reality Technology Use with Individuals Living with Dementia. Norrköping, Sweden: The Eurographics Association, 2018. DOI: 10.2312/egve.20181325. [DOI] [Google Scholar]
- 63.Alvseike H, Brønnick K. Feasibility of the iPad as a hub for smart house technology in the elderly; effects of cognition, self-efficacy, and technology experience. J Multidiscip Healthc 2012; 5: 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vallejo V, Tarnanas I, Yamaguchi T, et al. Usability assessment of natural user interfaces during serious games: adjustments for dementia intervention. J Pain Manag 2016; 9: 333–339. [Google Scholar]
- 65.Ferreira L, Cavaco S, Badia SB. A usability study with healthcare professionals of a customizable framework for reminiscence and music based cognitive activities for people with dementia. In: Proceedings of the 23rd Pan-Hellenic Conference on Informatics, Nicosia, Cyprus, 28–30 November 2019, New York, NY, USA: Association for Computing Machinery, pp. 16–23. [Google Scholar]
- 66.Mantovani E, Zucchella C, Bottiroli S, et al. Telemedicine and virtual reality for cognitive rehabilitation: a roadmap for the COVID-19 pandemic. Front Neurol 2020; 11: 926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yates L, Csipke E, Moniz-Cook E, et al. The development of the promoting independence in dementia (PRIDE) intervention to enhance independence in dementia. Clin Interv Aging 2019; 14: 1615–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-xlsx-1-jrt-10.1177_20556683211053952 for Impact attenuation provided by older adult protective headwear products during simulated fall-related head impacts by Daniel R Martel, Michelle R Tanel and Andrew C Laing in Journal of Rehabilitation and Assistive Technologies Engineering
Supplemental Material, sj-xlsx-2-jrt-10.1177_20556683211053952 for Impact attenuation provided by older adult protective headwear products during simulated fall-related head impacts by Daniel R Martel, Michelle R Tanel and Andrew C Laing in Journal of Rehabilitation and Assistive Technologies Engineering