Abstract
Background:
Few studies on outcomes after anterior cruciate ligament (ACL) reconstruction (ACLR) have provided insight into the very long-term effects of this procedure.
Purpose:
To systematically review the outcomes, failure rate, incidence, and predictors of osteoarthritis (OA) for different ACLR techniques at a minimum 20-year follow-up.
Study Design:
Systematic review; Level of evidence, 4.
Methods:
A search of the PubMed, SCOPUS, and Cochrane databases was performed on June 2020 for studies of patients who underwent ACLR and had a minimum follow-up of 20 years. We extracted data on patient and surgical characteristics, patient-reported outcomes (Lysholm score, subjective International Knee Documentation Committee [IKDC] score, Knee injury and Osteoarthritis Outcome Score [KOOS], and Tegner score), clinical outcomes (IKDC grade, pivot shift, Lachman, and KT-1000 laxity), degree of OA (Kellgren-Lawrence, Ahlbäck, and IKDC OA grading), revisions, and failures. Relative risk (RR) of OA between the operated and contralateral knees was calculated as well as the pooled rate of revisions, failures, and conversion to total knee arthroplasty (TKA).
Results:
Included were 16 studies (N = 1771 patients; mean age, 24.8 years; mean follow-up, 22.7 years); 80% of the patients underwent single-bundle bone–patellar tendon–bone (BPTB) reconstruction. The average Lysholm (89.3), IKDC (78.6), and KOOS subscale scores were considered satisfactory. Overall, 33% of patients had “abnormal” or “severely abnormal” objective IKDC grade, 6.7% had KT-1000 laxity difference of ≥5 mm, 9.4% had Lachman ≥2+, and 6.4% had pivot shift ≥2+. Signs of OA were reported in 73.3% of patients, whereas severe OA was reported in 12.8%. The operated knee had a relative OA risk of 2.8 (P < .001) versus the contralateral knee. Identified risk factors for long-term OA were male sex, older age at surgery, delayed ACLR, meniscal or cartilage injuries, BPTB autograft, lateral plasty, nonideal tunnel placement, residual laxity, higher postoperative activity, and postoperative range of motion deficits. Overall, 7.9% of patients underwent revision, and 13.4% of ACLRs were considered failures. TKA was performed in 1.1% of patients.
Conclusion:
Most patients had satisfactory subjective outcomes 20 years after ACLR; however, abnormal anteroposterior or rotatory laxity was found in nearly 10% of cases. The presence of radiographic OA was high (RR 2.8 vs uninjured knee), especially in patients with concomitant meniscal or cartilage injuries, older age, and delayed surgery; however, severe OA was present in only 12.8% of cases, and TKA was required in only 1.1%.
Keywords: knee, ACL, long-term, failures, PROMs, osteoarthritis
Anterior cruciate ligament (ACL) injuries are common in the younger population; most of those patients, especially if they want to continue sports activity, undergo ACL reconstruction (ACLR). 25 Regardless of the reconstruction technique used, the aim of the surgery is to restore native knee biomechanics in terms of correct load-bearing during movement and to increase anteroposterior and rotatory stability. 3,4
The short-term outcomes of this surgery have been well described in the literature: The clinical results are good to excellent in the vast majority of the patients, with restoration of stability, a high rate of return to sports, and a low percentage of failures. 18 However, most studies have had a short-term or midterm follow-up, which does not provide insight into the very long-term effect of ACLR. ACL injury is associated with altered joint homeostasis, and the altered kinematics can lead to knee osteoarthritis (OA) after many years; thus, long follow-ups of >10 years are required in order to investigate the predictors of OA and to assess the real incidence of this chronic process. 1,2
Over the past 40 years, ACLR has evolved considerably. Before the 1980s, the most commonly performed procedures were open repairs or reconstructions and isolated extra-articular procedures, which are no longer performed due to their poor results 11,29 ; moreover, the use of synthetic ligaments has been abandoned due to catastrophic consequences. 35 In the 1990s, arthroscopic procedures using autografts such as bone–patellar tendon–bone (BPTB) and hamstring tendon gained popularity and rapidly became the gold standard for ACLR. Moreover, since the anterolateral ligament was “rediscovered” by Claes and colleagues, 8 lateral extra-articular procedures associated with ACLR have gained renewed interest and triggered debates among surgeons. 12,32
For all of these reasons, it is now of value to investigate the very long-term results of ACLR performed between the end of the 1980s and the beginning of the 1990s, with surgical techniques that are not dissimilar to those used now. Moreover, the results after >2 decades since surgery would provide the optimal background to investigate the rates of and risk factors for knee OA.
The aim of the present article was to systematically review the clinical scores, return to sports, failure rate, incidence, and predictors of OA at a minimum of 20 years after ACLR.
Methods
This systematic review and meta-analysis was conducted according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. 21 A systematic search was conducted in PubMed, Scopus, and Cochrane databases on June 2020 with the aim of identifying all of the relevant studies that have evaluated ACLR at an average follow-up of 20 years. The gray literature was evaluated via the screening of ClinicalTrials.gov, and the reference lists of all included studies were further searched for any other relevant articles. The search was performed using the following terms, combined with the Boolean operators “AND” or “OR”: “long-term follow-up OR 20 years follow-up” AND “ACL reconstruction OR anterior cruciate ligament reconstruction.” The titles and abstracts were independently screened by 2 reviewers (A.G. and N.P.), and the full text of the relevant articles was obtained.
The inclusion criteria were (1) studies of patients who underwent ACLR; (2) studies with an average follow-up of >20 ± 1 years or a minimum follow-up of 19 years; and (3) studies that reported clinical, functional, or radiographic outcomes. The exclusion criteria were (1) studies on nonoperative treatment of ACL injury, (2) studies involving primary ACL repair, (3) studies entailing ACLR with synthetic grafts, and (4) studies of patients with a median age of <16 years. In the case of randomized controlled trials (RCTs), controlled trials, or comparative studies with multiple cohorts, only the patient series or the subgroup of patients fulfilling the inclusion criteria were included in the analysis. Studies were not excluded based on ACLR technique, type of autograft, patient characteristics, evaluation method, or language.
When any relevant studies were identified, the respective authors were contacted to obtain data on the specific patient subgroups. When we identified any small case series from the same authors and to avoid any possible overlap, only the series with larger sample sizes and longer follow-up were included. We then evaluated the reference lists of all included studies and identified any other relevant articles. When there were differences of opinion between the 2 reviewers with regard to the importance and relevance of any studies identified, a further discussion took place to find an agreement. A third reviewer (S.Z.) was used to resolve any residual difference in opinion.
Data Extraction
The information that was extracted from the original studies included patient characteristics, follow-up times and rates, graft used, and presence of lateral extra-articular plasty and meniscal lesions. Patient-reported outcome scores (Lysholm score, subjective International Knee Documentation Committee [IKDC] score, Knee injury and Osteoarthritis Outcome Score [KOOS] subscale scores, and Tegner activity level) were extracted, as were clinical outcomes (objective IKDC knee evaluation, pivot-shift test, Lachman test, and KT-1000 arthrometer side-to-side difference [SSD] in anteroposterior laxity). We recorded the number of patients with IKDC evaluations of normal (grade A), nearly normal (grade B), abnormal (grade C), and severely abnormal (grade D). For knee laxity, the mean SSD was recorded, together with the number of patients with an SSD of <3 mm, 3 to 5 mm, and >5 mm. For the Lachman and pivot-shift tests, the number of patients with grades of normal (–), nearly normal (1+), abnormal (2+), or severely abnormal (3+) was extracted.
The data on radiographic evaluations were extracted based on the Kellgren-Lawrence, Ahlbäck, and IKDC radiographic OA grading systems. The results were reported in a dichotomous manner (no OA signs vs OA signs) based on the cutoff values for the different radiological classification systems, as performed in similar meta-analyses. 16,22 Signs of OA were defined as IKDC grade B or higher, Kellgren-Lawrence grade ≥2, or Ahlbäck grade ≥1. The overall postoperative incidence of knee OA was based on the preoperative cutoff values for each study. When OA was reported for each compartment, the most severe grade was used in the statistical evaluation. In addition, we extracted the subgroup of patients with severe OA, based on the following cutoffs: IKDC grade D, Kellgren-Lawrence grade 4, or Ahlbäck grade ≥2. Finally, the radiographic assessment of the contralateral knee was extracted, when present, according to the same grading system.
Failure was evaluated according to different criteria. “Revision” was defined as the need for further ipsilateral ACLR or a rerupture (if nonoperative management was not explicitly specified). Based on the definition provided in each study, “clinical failure” was considered as nonoperated reruptured ACL, KT-1000 arthrometer laxity >5 mm, high-grade Lachman or pivot shift (3+), or subjective reports of instability. “Overall failure” was considered as revisions plus clinical failures.
A nonideal tunnel placement was defined as a sagittal tibial tunnel outside the range of 40% to 50% back from the anterior tibial cortex, a sagittal femoral tunnel outside the range of 80% to 90% posteriorly along the Blumensaat line, and a coronal graft inclination >17%. 30,31
From each study we extracted the risk factors for ACL failure and OA, measured with subgroup comparison, direct correlation, multivariate analysis, odd ratios (ORs), or hazard ratios (HRs).
Level of Evidence and Methodological Assessment
The selected articles were assessed by an author (N.P.) for level of evidence and method using a modification of the original Coleman methodology score per Brown et al. 5 The modified Coleman methodology score is composed of parts A (60 points) and B (40 points), for a total possible score of 100.
Statistical Analysis
Statistical analysis was performed using MedCalc software. The pooled mean was calculated for continuous measures. We conducted a random-effects meta-analysis to calculate the pooled rate with 95% CIs for the following: IKDC grade D or grades C/D; KT-1000 SSD >3 mm or >5 mm; Lachman grades ≥1+ or ≥2+; pivot shift ≥1+, ≥2+, or 3+; revisions; clinical failures; and overall failures. Pooled rates of postoperative signs of OA and of severe OA (based on the predefined cutoff values) were calculated using a random-effects meta-analysis as well. The relative risk (RR) with 95% CI of the risk of OA between the operated and contralateral knees was calculated. The random-effects model was used to reduce bias from the potential systematic error of the included studies. 16 Values with P < .05 were considered statistically significant.
Results
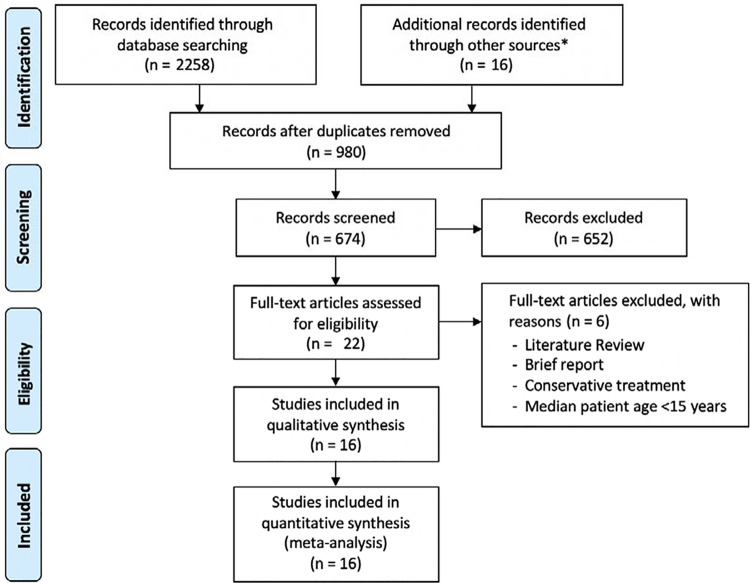
After the application of inclusion and exclusion criteria, a total of 16 studies that presented the results of ACLR at a follow-up of 20 years were included in the systematic review (Figure 1). All articles were written in English. Three studies were RCTs, comparing ACLR with or without lateral plasty, 6 ACLR with or without synthetic augmentation, 11 or ACLR with primary repair 29 ; thus, patients with primary ACL repair and those with synthetic graft augmentation were excluded from the analysis. Two other studies were prospective case-control studies comparing different autografts 31 or comparing nonoperative versus operative treatment 33 ; in the latter case, patients treated nonoperatively were not included in the analysis. A further 6 studies were prospective case series, 17,19,26 –28,37 and 5 studies were retrospective case series. 9,10,20,23,36 The 74 patients who received hamstring tendon grafts in the comparative study by Thompson et al 31 were excluded from the analysis because they were already accounted for in another study from the same authors evaluating 200 ACLRs with hamstring tendons 27 ; however, data from Thompson et al 31 were used if not available in the complete series. The characteristics of the included studies are summarized in Table 1.
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flowchart of study inclusion. *Reference lists of the included studies and ClinicalTrials.gov database.
Table 1.
Summary of Study Characteristics and Quality Evaluation of the Included Studies a
| Modified Coleman Methodology Score | ||||||
|---|---|---|---|---|---|---|
| Lead Author | Year | Design | Series | Part A (40 points) | Part B (60 points) | Total (100 points) |
| Castoldi 6 | 2020 | RCT | Series 1 (BPTB) Series 2 (BPTB+LP) |
42 | 29 | 71 |
| Costa-Paz 9 | 2019 | Retrospective | Single series | 34 | 31 | 65 |
| Curado 10 | 2020 | Retrospective | Single series | 38 | 24 | 62 |
| Elveos 11 | 2018 | RCT | Single series | 47 | 26 | 73 |
| Hagemans 17 | 2020 | Prospective | Single series | 28 | 27 | 55 |
| Lindanger 19 | 2019 | Prospective | Series 1 (early R) Series 2 (late R) |
48 | 21 | 69 |
| Marcacci 20 | 2015 | Retrospective | Single series | 30 | 31 | 61 |
| Pernin 23 | 2010 | Retrospective | Single series | 43 | 27 | 70 |
| Risberg 26 | 2016 | Prospective | Single series | 44 | 27 | 71 |
| Salmon 27 | 2018 | Prospective | Single series | 50 | 25 | 75 |
| Shelbourne 28 | 2017 | Prospective | Single series | 46 | 24 | 70 |
| Sporsheim 29 | 2019 | RCT | Single series | 27 | 25 | 53 |
| Thompson 31 | 2016 | Case-control | Series 1 (BPTB) Series 2 (HT) |
50 | 25 | 75 |
| van Yperen 33 | 2018 | Case-control | Single series | 34 | 19 | 53 |
| Yamaguchi 36 | 2006 | Retrospective | Single series | 29 | 29 | 58 |
| Zaffagnini 37 | 2017 | Prospective | Single series | 37 | 32 | 69 |
a BPTB, bone–patellar tendon–bone; HT, hamstring tendon; LP, lateral plasty; R, reconstruction; RCT, randomized controlled trial.
The average modified Coleman methodology score was 65.6 ± 6.5, with “type of study,” “study size,” and “description of postoperative rehabilitation” as the items that most affected the overall quality (Table 1).
Patients and Surgical Characteristics
Overall, 1771 patients were evaluated in the 16 studies, at an average follow-up of 22.7 years (range, 19-30 years). Most of the patients were male (60%), and the patients’ average age at surgery was 24.8 years (range, 13-52 years). Based on the data reported, at the time of surgery, 31% of patients had a medial meniscal lesion, 23% had a lateral meniscal lesion, and 5% had concomitant medial and lateral meniscal lesions (Table 2). Data regarding the surgical characteristics and grafts used are reported in Table 2.
Table 2.
Summary of Patient Characteristics and Surgical Details of the Included Studies a
| Patient Characteristics | Surgical Details | |||||||
|---|---|---|---|---|---|---|---|---|
| Study | Knees, n | Follow-up Rate, % | Follow-up, y | Sex, M/F, n | Age at Surgery, y b | Graft | Lateral Plasty | Meniscal Lesions (Medial/Lateral/Bilateral), n |
| Castoldi 6 (BPTB) | 42 | 69 | 19.4 | 56/24 | 25.9 (16-40) | BPTB | None | 22/8/0 |
| Castoldi 6 (BPTB+LP) | 38 | 63 | 19.4 | 56/24 | 26.2 (15-40) | BPTB+LP | ITB (mod Lemaire) | 11/11/0 |
| Costa-Paz 9 | 76 | 95 | 22.0 | 59/13 | 30.0 (22-36) | BPTB | None | 30/9/4 |
| Curado 10 | 182 | 100 | 22.0 | 67/115 | 26.0 ± 7.0 | Mixed (>90% BPTB) | None | 58/36/0 |
| Elveos 11 | 48 | 94 | 25.0 | NA | 25.0 (16-42) | BPTB | None | NA |
| Hagemans 17 | 48 | 51 | 21.0 | 31/17 | 31.0 (26-39) | HT | None | 11/6/6 |
| Lindanger 19 (Early R) | 139 | 93 | 26.0 | 65/74 | 23.0 ± 5.9 | BPTB | None | 41/57/0 |
| Lindanger 19 (Late R) | 78 | 93 | 26.0 | 36/42 | 7.3 ± 6.6 | BPTB | None | 48/23/0 |
| Marcacci 20 | 15 | 100 | 26.8 | 10/5 | 24.3 ± 6.3 | BPTB+LP | ITB (Bousquet) | NA |
| Pernin 23 | 100 | 68 | 24.5 | NA | 25.1 (14-43) | BPTB+LP | ITB (mod Lemaire) | NA |
| Risberg 26 | 168 | 80 | 20.0 | 95/73 | 25.2 ± 9.1 | Mixed (>85% BPTB) | None | NA |
| Salmon 27 | 200 | 90 | 20.0 | 100/100 | 25.8 (13-52) | HT | None | 24/16/0 |
| Shelbourne 28 | 423 | 100 | 22.5 | 287/136 | 23.2 ± 6.9 | BPTB | None | 114/55/52 |
| Sporsheim 29 | 35 | 68 | 30.0 | NA | 29.0 (16-50) | BPTB | None | NA |
| Thompson 31 (BPTB) | 89 | 89 | 20.0 | 48/32 | 25.0 (14-42) | BPTB | None | 18/34/0 |
| Thompson 31 (HT) | 90 | 82 | 20.0 | 47/28 | 24.0 (13-52) | HT | None | 20/43/0 |
| van Yperen 33 | 25 | 100 | 21.2 | 19/6 | 25.8 ± 6.4 | BPTB | None | 15/6/3 |
| Yamaguchi 36 | 26 | 58 | 24.0 | 18/8 | 24.8 (16-42) | ITB+LP | ITB (Yamaguchi) | 15/1/2 |
| Zaffagnini 37 | 52 | 87 | 24.0 | 41/1 | 25.5 ± 7.6 | HT+LP | HT (Marcacci) | 8/8/0 |
a BPTB, bone–patellar tendon–bone; HT, hamstring tendon; ITB, iliotibial band; LP, lateral plasty; mod, modified; NA, not assessed; R, reconstruction.
b Expressed as mean ± SD or mean (range).
Subjective Outcomes
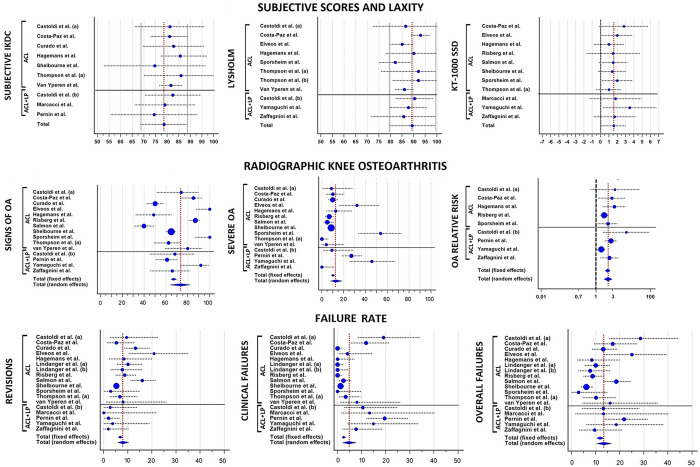
The mean subjective IKDC score (9 studies ‡ ; 1140 patients) was 78.6, whereas the mean Lysholm score (9 studies § ; 503 patients) was 89.3 (Figure 2). The mean KOOS scores (8 studies 9 –11,23,26,28,33,37 ; 674 patients) were 89.4 for the Pain subscale, 76.8 for the Symptoms subscale, 94.8 for the Activities of Daily Living subscale, 76.6 for the Sport subscale, and 75.3 for the Quality of Life subscale. The mean Tegner score (8 studies ∥ ; 437 patients) was 4. No substantial differences were present among the mean values of the different series, which all ranged within ±10 points from the overall mean value of both subjective IKDC and Lysholm score and ±1 point for the Tegner score.
Figure 2.
Forest plots for the pooled means of subjective International Knee Documentation Committee score, Lysholm score, and KT-1000 arthrometer side-to-side difference (SSD) in anteroposterior laxity. Blue circles represent the mean values, the size of the circle represents the weight in the calculation, horizontal dotted lines indicated the SDs, and vertical red dotted lines indicate the overall average value of all studies. (a) indicates series 1 and (b) indicates series 2. ACL, anterior cruciate ligament; LP, lateral plasty; OA, osteoarthritis.
Objective Outcomes
The objective IKDC assessment (8 studies ¶ ; 1011 patients) was rated as normal (grade A) in 37% of patients (95% CI, 27%-48%), nearly normal (grade B) in 31% (95% CI, 22%-43%), abnormal (grade C) in 19% (95% CI, 13%-26%), and severely abnormal (grade D) in 13% (95% CI, 6%-23%). The overall rate of patients with “abnormal” or “severely abnormal” knees was 33% (95% CI, 20%-47%).
Laxity outcomes were reported in 14 studies # including 1296 patients. The average KT-1000 arthrometer SSD was 1.6 mm (11 studies**; 1083 patients) (Figure 2). In total, 25.3% of patients (95% CI, 15.5%-36.5%) had a value ≥3 mm (10 studies †† ; 686 patients), and only 6.7% (95% CI, 1.1%-16.3%) had a value ≥5 mm (5 studies 11,20,28,36,37 ; 504 patients). According to the different cutoff values for the Lachman test, 30.0% (95% CI, 18.3%-43.2%) of patients were rated as ≥1+ (4 studies 20,31,36,37 ; 179 patients), and 9.4% (95% CI, 3.3%-18.3%) were rated as ≥2+ (8 studies 6,11,20,23,29,31,36,37 ; 348 patients). According to the different cutoff values for the pivot-shift evaluation, 15.8% (95% CI, 8.1%-27.0%) of patients were rated as ≥1+ (5 studies 20,23,27,31,37 ; 255 patients); 6.4% (95% CI, 0.9%-16.2%), as ≥2+ (6 studies 11,20,23,29,31,37 ; 278 patients); and only 2.9% (95% CI, 0.7%-6.7%), as 3+ (5 studies 6,20,23,31,37 ; 310 patients) (Table 3).
Table 3.
Summary of Laxity Outcomes a
| Study | Knees Evaluated, n | Lachman, n | Pivot Shift, n | KT-1000 Arthrometer SSD |
|---|---|---|---|---|
| Castoldi 6 (BPTB) | 42 | 39 (– and +), 3 (++) | 41 (–, +, and ++), 1 (+++) | NA |
| Castoldi 6 (BPTB+LP) b | 38 | 37 (– and +), 1 (++) | 38 (–, +, and ++), 0 (+++) | NA |
| Costa-Paz 9 | 76 | NA | NA | Mean: 2.8 mm |
| Elveos 11 | 48 | 22 (– and +), 0 (++ and +++) | 20 (– and +), 2 (++ and +++) | Mean: 2.0 mm No. <3 mm/3-5 mm/>5 mm: 16/5/1 |
| Hagemans 17 | 39 | NA | NA | Mean: 1.0 mm No. <3 mm/≥3 mm: 32/7 |
| Marcacci 20 b | 15 | 9 (–), 2 (+), 2 (++) | 12 (–), 3 (+), 0 (++), 0 (+++) | Mean ± SD: 1.8 ± 3.1 mm No. <3 mm/3-5 mm/>5 mm: 6/7/0 |
| Pernin 23 b | 92 | 53 (– and +), 13 (++) | 71 (–), 0 (+), 17 (++), 6 (+++) | NA |
| Risberg 26 | 168 | NA | NA | Mean ± SD: 1.8 ± 3.2 mm |
| Salmon 27 | 163 | 134 (–) | 147 (–) | Mean ± SD: 1.5 ± 1.7 mm No. <3 mm/≥3 mm: 120/43 |
| Shelbourne 28 | 423 | NA | NA | Mean ± SD: 1.4 ± 1.7 mm No. <3 mm/3-5 mm/>5 mm: 376/42/5 |
| Sporsheim 29 | 26 | 23 (– and +), 3 (++ and +++) | 25 (– and +), 1 (++ and +++) | Mean: 2.0 mm No. <3 mm/≥3 mm: 11/15 |
| Thompson 31 (BPTB) | 43 | 36 (–), 7 (+), 0 (++) | 41 (–), 1 (+), 0 (++), 0 (+++) | Mean ± SD: 1.0 ± 1.5 mm No. <3 mm/≥3 mm: 37/6 |
| Thompson 31 (HT) | 49 | 37 (–), 12 (+), 0 (++) | 44 (–), 5 (+), 0 (++), 0 (+++) | Mean ± SD: 1.6 ± 1.8 mm No. <3 mm/≥3 mm: 37/12 |
| van Yperen 33 | 25 | 10 (–), 13 (+ and ++) | 17 (–), 8 (+, ++, and +++) | Mean: NA No. <3 mm/≥3 mm: 15/10 |
| Yamaguchi 36 b | 20 | 10 (–), 6 (+), 4 (++) | NA | Mean ± SD: 3.5 ± 3.2 mm No. <3 mm/3-5 mm/>5 mm: 10/6/4 |
| Zaffagnini 37 b | 29 | 25 (–), 0 (+), 4 (++) | 25 (–), 0 (+), 0 (++), 4 (+++) | Mean ± SD: 1.7 ± 2.5 mm No. <3 mm/3-5 mm/>5 mm: 19/4/3 |
a BPTB, bone–patellar tendon–bone; HT, hamstring tendon; LP, lateral plasty; NA, not assessed; SSD, side-to-side difference.
b Addition of a lateral plasty.
Radiographic Outcomes
OA was evaluated in 14 studies ‡‡ including 1330 patients: The radiographic IKDC score was used in 7 studies 9,10,23,27,28,31,36 ; the Kellgren-Lawrence classification, in 4 studies 17,26,33,37 ; and the Ahlbäck classification, in 3 studies 6,11,29 (Table 4).
Table 4.
Summary of Radiographic Outcomes a
| Study | Knees Evaluated, n | OA Classification | OA Grade, 0/1/2/3/4 or A/B/C/D, n | Comparison With Contralateral |
|---|---|---|---|---|
| Castoldi 6 (BPTB) | 23 | Ahlbäck | 6/15/2/0/0 | Higher lateral OA in operated knee (22%) vs contralateral knee (4%). No difference for medial and PF OA. |
| Castoldi 6 (BPTB+LP) b | 22 | Ahlbäck | 7/13/2/0/0 | Higher lateral OA in operated knee (59%) vs contralateral knee (5%). No difference for medial and PF OA. |
| Costa-Paz 9 | 60 | IKDC | 9/34/11/6 | Higher abnormal radiographs in operated knee (28%) vs contralateral knee (7%). Higher OA with meniscectomy. |
| Curado 10 | 182 | IKDC | 91/38/35/18 | NA |
| Elveos 11 | 28 | Ahlbäck | 0/19/0/8/1 | NA |
| Hagemans 17 | 39 | Kellgren-Lawrence | 9/11/3/11/5 | Higher tibiofemoral OA in operated knee (49%) vs contralateral knee (10%). |
| Pernin 23 b | 100 | IKDC | 37/7/27/27 | Pre-OA in 57% of operated knees vs normal radiographs in 85% of contralateral knees. |
| Risberg 26 | 167 c | Kellgren-Lawrence | 10/12/99/35/11 | Higher tibiofemoral and PF OA in operated knee (42% and 21%) vs contralateral knee (21% and 5%). |
| Salmon 27 | 121 | IKDC | 73/28/14/6 | NA |
| Shelbourne 28 | 423 | IKDC | 149/153/85/36 | NA |
| Sporsheim 29 | 26 | Ahlbäck | 0/12/10/4/0 | Higher tibiofemoral OA in operated knee (54%) vs contralateral knee (19%). |
| Thompson 31 (BPTB) | 61 | IKDC | 23/26/12/0 | NA |
| Thompson 31 (HT) | 61 | IKDC | 36/17/5/3 | NA |
| van Yperen 33 | 25 | Kellgren-Lawrence | 1/4/16/3/1 | NA |
| Yamaguchi 36 b | 24 | IKDC | 2/5/6/11 | Higher abnormal radiographs in operated knee (71%) vs contralateral knee (17%). Higher OA with meniscectomy. |
| Zaffagnini 37 b | 29 | Kellgren-Lawrence | 1/9/14/5/0 | Higher medial JSN in patients with meniscectomy vs contralateral. No difference in lateral JSN. |
a BPTB, bone–patellar tendon–bone; HT, hamstring tendon; IKDC, International Knee Documentation Committee; JSN, joint space narrowing; LP, lateral plasty; NA, not assessed; OA, osteoarthritis; PF, patellofemoral.
b Addition of a lateral plasty.
c One pregnant patient was not evaluated.
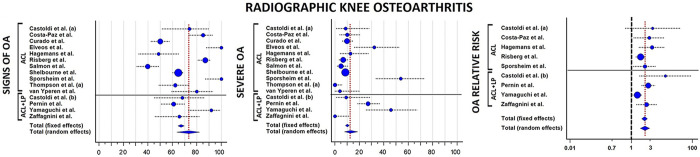
According to the predefined cutoff values, signs of OA in the operated knee were reported in 73.3% (95% CI, 63.5%-82.1%) of patients, whereas severe OA was reported in 12.8% (95% CI, 7.8%-18.6%) of the cases; the highest values of severe OA were reported in patients with concomitant ACL and lateral plasty (21%). The operated knee had an RR of 2.8 (95% CI, 2.0-4.0; P < .001) to develop OA at long-term evaluation when compared with the contralateral knee (Figure 3). The most commonly identified risk factors for long-term OA were male sex, older age at surgery, delayed ACLR, meniscal or cartilage injuries, BPTB autograft, nonideal tunnel placement, residual laxity, postoperative activity, and postoperative range of motion defects (Table 5). In the 4 studies 23,26,36,37 that stratified OA according to meniscal status, OA was present in 34 of 123 (28%) patients with no meniscal injuries and in 70 of 320 (22%) contralateral knees (P = .2118).
Figure 3.
Forest plots for the pooled rates of OA signs, severe OA, and relative risk of OA between operated and contralateral knees. Blue circles represent the mean values, the size of the circle represents the weight in the calculation, horizontal dotted lines indicate the 95% CIs, and vertical red dotted lines show the overall average value of all studies. (a) indicates series 1, and (b) indicates series 2. ACL, anterior cruciate ligament; LP, lateral plasty; OA, osteoarthritis.
Table 5.
Long-Term Risk-Factors for OA Development After Anterior Cruciate Ligament Reconstruction a
| Factor | Measure |
|---|---|
| Sex | |
| Male | OA moderate/severe: OR = 2.38
28
OA presence: P = .0026 10 |
| Age at surgery | |
| Per 1-y increase (from 23.2 ± 6.9 y) b | OA moderate/severe: OR = 1.06 28 |
| Per 1-y increase | NS 10 |
| Adolescents vs >18 y | NS 27 |
| Age OA (26.9 y) vs age non-OA (22.8 y) | OA presence: P < .001 23 |
| Age >30 y | OA presence: P < .001 10 |
| Reconstruction timing | |
| Chronic cases c | OA moderate/severe: OR = 2.01 28 |
| TTS OA vs TTS non-OA | OA presence: P = .38 23 |
| TTS >16 mo | OA presence: P = .0041 10 |
| TTS >3 mo | NS 10 |
| Medial meniscus | |
| Meniscectomy | OA moderate/severe: OR = 3.09
28
OA presence: 69% (vs 36%) 23 OA presence: 100% (vs 25%) 36 OA SSD of JSN: P = .0341 37 |
| Lateral meniscus | |
| Meniscectomy | OA presence: OR = 1.65
28
OA presence: 100% (vs 53%) 36 OA lateral: P = .04 7 NS 37 |
| Medial or lateral meniscus | |
| Meniscectomy | OA presence: P < .05 10 |
| Injury | OA presence: OR = 3.969 OA presence: 67% (vs 28%) 17 |
| Cartilage | |
| Injury | OA moderate/severe: OR = 2.76
28
OA presence: 80% (vs 49%) 23 |
| Meniscus and/or cartilage | |
| Injury | OA presence: 57% (vs 16%) 26 |
| Surgical characteristics | |
| Patellar tendon autograft | OA presence: OR = 2.4 31 |
| Lateral plasty | OA lateral: 59% (vs 22%) 6 |
| Nonideal tunnel placement | OA presence: P = .0026
10
NS 31 |
| Laxity | |
| Residual laxity >5 mm | OA presence: P < .05 10 |
| KT-1000 SSD >3 mm | NS 28 |
| Extension deficit d | OA moderate/severe: OR = 3.86 28 |
| Flexion deficit e | OA moderate/severe: OR = 3.36 28 |
| Further surgery | OA presence: OR = 2.6 31 |
| Pivoting sports | OA presence: P < .05 10 |
| Moderate/strenuous activity | OA presence: P < .05 10 |
a JSN, joint space narrowing; NS, nonsignificant; OA, osteoarthritis; OR, odds ratio; SSD, side-to-side difference; TTS, time to surgery. Superscript numbers are reference numbers.
b With respect to the mean age of the study population.
c Reports of additional giving-way episodes after the index injury and before surgery.
d More than 5° deficit compared with noninvolved knee.
e More than 2° deficit compared with noninvolved knee.
Failures
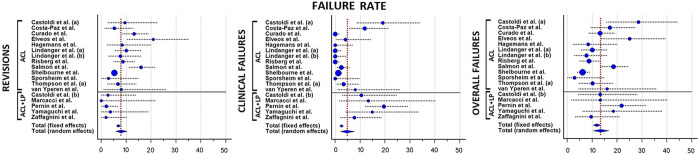
Revisions, clinical failures, and conversions to total knee arthroplasty (TKA) were analyzed in 1793 knees from the 16 included studies (Table 6). A total of 7.9% (95% CI, 5.7%-10.3%) of patients underwent an ACLR revision after a minimum of 20 years after primary ACLR. A total of 4.5% (95% CI, 2.2%-7.7%) of patients were considered to have experienced clinical failure. Finally, from the meta-analysis of the included studies, 13.4% (95% CI, 10.4%-16.7%) of ACLRs were considered as failures; 2 of 5 series of ACLR plus lateral plasty 23,36 and 5 of 13 series of isolated ACLR 6,9,11,19,27 reported failure rates above the average value (Figure 4). Identified risk factors for ACLR failure were male sex, age <18 years at index surgery, posterior tibial slope >12°, and nonideal tunnel placement (Table 7). TKA was performed in 1.1% (95% CI, 0.4%-2.1%) of patients (Figure 5).
Table 6.
Summary of Failures a
| Study | Graft | Knees Evaluated, n | Revisions, n (%) | Clinical Failures, n (%) | Definition of Clinical Failure | TKA, n (%) |
|---|---|---|---|---|---|---|
| Castoldi 6 (BPTB) | BPTB | 42 | 4 (9.8) | 8 (19) | Positive pivot shift, Lachman, or instability | 0 (0) |
| Castoldi 6 (BPTB+LP) | BPTB+LP | 38 | 1 (2.6) | 4 (10.5) | Positive pivot shift, Lachman, or instability | 0 (0) |
| Costa-Paz 9 | BPTB | 76 | 4 (5.2) | 9 (11.8) | Laxity on KT-1000, pivot shift, or Lachman | 0 (0) |
| Curado 10 | Mixed (>90% BPTB) | 182 | 24 (13.1) b | — | — | 0 (0) |
| Elveos 11 | BPTB | 48 | 10 (21) | 2 (4) | Nonoperated reruptures | 1 (2) |
| Hagemans 17 | HT | 48 | 4 (8) | — | — | 0 (0) |
| Lindanger 19 (Early R) | BPTB | 139 | 14 (10.1) | — | — | 4 (2.9) |
| Lindanger 19 (Late R) | BPTB | 78 | 6 (7.7) | — | — | 7 (8.9) |
| Marcacci 20 | BPTB+LP | 15 | 0 (0) | 2 (8) | KT-1000 laxity >5 mm | 0 (0) |
| Pernin 23 | BPTB+LP | 92 | 2 (2.0) c | 18 (19.6) | Positive pivot shift | 0 (0) |
| Risberg 26 | Mixed (>85% BPTB) | 168 | 16 (8.7) d | — | — | 1 (0.5) |
| Salmon 27 | HT | 200 | 32 (16) | 5 (2.5) | Nonoperated reruptures | 0 (0) |
| Shelbourne 28 | BPTB | 423 | 96 (5.2) e | 5 (1.2) | KT-1000 laxity >5 mm | 0 (0) |
| Sporsheim 29 | BPTB | 35 | 1 (3) | — | — | 2 (6) |
| Thompson 31 (BPTB) | BPTB | 89 | 6 (6.7) | 3 (3.4) | Nonoperated reruptures | 0 (0) |
| Thompson 31 (HT) | HT | 90 | 14 (15.6) | 2 (2.2) | Nonoperated reruptures | 0 (0) |
| van Yperen 33 | BPTB | 25 | 2 (8) | 2 (8) | Nonoperated reruptures | 1 (4) |
| Yamaguchi 36 | ITB+LP | 26 | 1 (3.7) | 4 (14.8) | KT-1000 laxity >5 mm | 0 (0) |
| Zaffagnini 37 | HT+LP | 52 | 1 (1.9) | 4 (13.8) f | KT-1000 laxity >5 mm | 0 (0) |
a BPTB, bone–patellar tendon–bone; HT, hamstring tendon; ITB; iliotibial band; LP, lateral plasty; R, reconstruction; TKA, total knee arthroplasty. Dashes indicate not assessed.
b Reruptures are assumed to have undergone revision anterior cruciate ligament reconstruction.
c Revisions assessed in the cohort of 102 patients from Ait Si Selmi et al. 1
d 15 failures that occurred within the first 15 years were included in the final count.
e Reruptures assessed in the total cohort of 1827 patients.
f Clinical failure was assessed in a subgroup (n = 29).
Figure 4.
Forest plots for the pooled rates of revisions, clinical failures, and overall failures. Blue circles represent the mean values, vertical dotted lines show the 95% CIs, and vertical red dotted lines indicate the overall average value of all studies. (a) indicates series 1, and (b) indicates series 2. ACL, anterior cruciate ligament; LP, lateral plasty.
Table 7.
Summary of Risk Factors for Anterior Cruciate Ligament Reconstruction Failure a
| Factor | Measure |
|---|---|
| Male sex | HR = 3.9 31 ; NS 27 |
| Age <18 y | HR = 4.6 31 ; HR = 3.3 27 |
| Posterior tibial slope >12° | HR = 3.0 27 |
| Family history of anterior cruciate ligament injury | NS 27,31 |
| Hamstring tendon graft | NS 31 |
| Graft diameter <7 mm | NS 27 |
| Lateral plasty | NS 6 |
| Nonideal tunnel placement | HR = 3.6 31 ; NS 27 |
| Return to preinjury sport | NS 19 |
a HR, hazard ratio; NS, nonsignificant. Superscript numbers are reference numbers.
Figure 5.
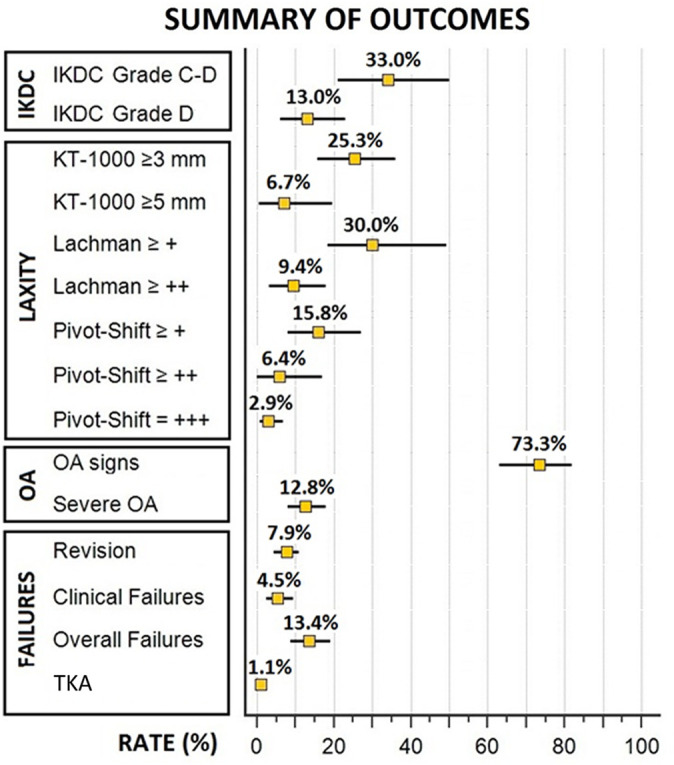
Summary of the pooled rates of objective laxity, osteoarthritis, and failure outcomes. IKDC, International Knee Documentation Committee; OA, osteoarthritis; TKA, total knee arthroplasty.
Discussion
The most important findings of the present systematic review and meta-analysis were that good clinical subjective and objective outcomes were present in the majority of patients after ACLR even at very long-term follow-up of >20 years. Moreover, the rates of revisions and clinical failures were low (respectively, 7.9% and 4.5%), whereas the presence of signs of OA was high (73%) with a 2.8 RR with respect to the healthy knee.
Several important considerations should be made when interpreting these results. The surgical techniques and viewpoints regarding ACLR of the very long-term studies included in this review are dated to the 1990s and slightly differ from the current standard of practice. Regarding tunnel preparation, the location of the ACL femoral tunnel has evolved since these surgeries were performed. To date, a univocal vision on the optimal ACLR technique and graft is still lacking, and surgeons around the world use both BPTB and hamstring tendon autografts, even though the most common practice for primary ACLR seems to be a single-bundle technique with hamstring tendon. 14 However, some surgeons advocate a double-bundle technique, and some favor a quadriceps tendon graft. Most of the studies included in the present review used BPTB autograft and, in many cases, lateral plasty as well. Therefore, the external validity of the results of the present study could be questioned, and caution should be used when extrapolating these results to define long-term expectations after ACLR using the actual techniques. In recent years, there has been increased interest in BPTB autograft with lateral plasty owing to reduced ACL failure rates with this procedure. 13,18,24 Therefore, the data in this review could represent a useful background for this renewed “old-fashioned” approach.
Regarding the subjective scoring, the average Lysholm (89.3), IKDC (78.6), and KOOS subscale scores can be considered completely satisfactory, especially considering the very long follow-up. Even though it is impossible to perform a sound statistical comparison between graft types and the presence or absence of lateral plasty, satisfactory subjective knee function in terms of pain, stability, and activity can be obtained after ACLR. Only a single comparative study 31 reported a significantly lower incidence of kneeling pain after using hamstring tendon (20%) compared with BPTB (38%) graft.
We noted nonoptimal results regarding sports practice, with an average Tegner score of 4, which corresponds to recreational cycling and jogging or moderately heavy work. However, this reduction in sports activity had been reported at 10 years after ACLR 15 and is considered dependent more on changes in patients’ habits due to aging rather than a decline of knee function. Moreover, male athletes after ACLR had a mean career of 10 years, with 5 years at the same level, and among those who decreased their activity level, the reason was not related to knee function in nearly 50% of cases. One of the main issues with regard to return to sports is the second ACL injury: 33% of younger athletes have been reported to sustain at least 1 further ACL injury between 3 and 5 years postoperatively. 34 The high rate of second injuries in younger patients may be one of the main factors responsible for the nonoptimal results regarding the sports participation at long-term follow-up.
Another interesting result was the overall good anteroposterior and rotatory stability, with only 5% to 10% of patients presenting pathological laxity, defined as grade 2 (2+) Lachman or pivot shift. The higher rates of increased laxity were reported only in adolescent patients aged <18 years. 27 The overall good results confirm that the restraining function of the ACL is stable over time, with only small or nonrelevant increases of laxity. In this regard, a particular mention should be reserved for the study by Thomson et al, 31 who prospectively compared 180 ACLRs with BPTB and hamstring tendon autograft at 2, 5, 7, 10, 15, and 20 years. In addition to reporting no significant differences in terms of clinical score, objective findings, and knee laxity between the 2 grafts, the authors also found stable results, with no relevant decline of outcomes along all of the follow-up points. However, 14% of their patients experienced ACL rerupture and were excluded from the analysis, thus possibly biasing the overall results.
Apart from a patient’s subjective and objective assessment, a relevant aspect when interpreting the outcomes of ACLR is the failure rate. Despite the broad definition of “failure” and the difficulty of standardizing the definition within studies that used different designs and evaluation methods, the overall failure rate of the 1793 knees included has been estimated around 13%. Specifically, when reported, ACLR revision was performed in 7.9% of patients, with no substantial differences from the rates reported at earlier follow-up points. 27,30,31 In fact, Risberg et al 26 reported that only 1 ACL rerupture in 168 patients occurred between the 15-year and 20-year follow-up evaluations. This could be attributed to decreased involvement in sports and thus a reduction of risk exposure. A similar rate of 4.5% was reported for clinical failures, which were defined in various studies as nonoperated ACL reruptures, KT-1000 arthrometer results >5 mm, high-grade Lachman (3+), high-grade pivot shift (3+), or subjective instability. Considering the patients’ average age (approaching 50 years at the 20-year follow-up) and the related decreases in knee functional demand, this percentage of patients could represent a subgroup who are not willing to undergo revision ACLR despite “clinical failure.” Several studies 6,19,27,31 investigated the long-term predictors of failure (Table 7): No role was identified for the type of graft, graft diameter, and familial history, whereas age <18 years and posterior tibial slope >12° were identified as risk factors for ACLR failure. 27,31 The worst scenario was identified when combining the 2 latter variables, with only 22% of ACLRs still intact after 20 years and a hazard ratio of 11.1 with respect to adults with normal slope. 27 The role of tunnel placement remains controversial because a nonideal placement was a risk factor in only 1 of 2 studies with similar design and overlapping patient populations. 27,31 A similar scenario was noted for male sex.
Another important technical aspect to evaluate is the use of lateral extra-articular plasty. Although 5 series used a combined intra- and extra-articular approach, no solid conclusion could be drawn based on the data from the present systematic review because of the impossibility of performing an appropriate statistical analysis. 6,20,23,36,37 According to data reported in each study, a trend for a higher revision rate seems to be present when lateral plasty is not used; however, this seems balanced by a higher rate of patients who experience clinical failures when lateral plasty is used. Thus, when we pooled revisions and clinical failures, no substantial differences between the 2 approaches could be highlighted for the “overall” failure rate. However, the specific role of lateral plasty was investigated in the RCT by Castoldi et al, 6 who compared 61 patients who underwent isolated ACLR with BPTB reconstruction and 60 patients who underwent ACLR with BPTB graft plus lateral plasty with gracilis tendon graft. The authors reported no significant differences in clinical scores and sports participation at the 20-year follow-up. However, higher rates of revisions (10% vs 3%) and clinical failures (29% vs 13%) were reported in patients with isolated ACLR versus those with combined ACLR and lateral plasty, respectively; these differences were not statistically significant (P = .1). However, the authors acknowledged that the study was underpowered to properly assess the failure-related outcomes.
Finally, the present review highlighted a high rate of knee OA 20 years after ACLR (73%) with an RR of 2.8 with respect to the contralateral knee; however, only 13% of patients had severe OA. Thus, patients undergoing ACLR should expect a nearly tripled risk of developing OA in the operated knee compared with the uninjured knee within 20 years since ACLR does not seem able to prevent the long-term development of knee OA. In this regard, we identified several recurrent risk factors (Table 5), such as male sex, older age at surgery, long delay between ACL injury and reconstruction, meniscal injuries and removal, cartilage damage, range of motion deficits, and participation in pivoting sports or strenuous activities. Specifically, Pernin et al 23 reported normal or nearly normal radiographic findings (IKDC grades A/B) in 64% of patients with no cartilage and meniscal injuries compared with 10% of patients with medial meniscectomy and damaged cartilage. Risberg et al 26 reported that radiographic OA at 20-year follow-up was present in 42% of patients—mostly with symptoms—especially in those with combined meniscal or cartilage lesions; moreover, a significant increase of radiographic OA between the 15- and 20-year follow-up points was reported in up to 22% of patients. This finding is in line with a similar meta-analysis by Claes et al, 7 who detected an increased risk of OA in patients who underwent meniscectomy at a mean of 10 years after ACLR. Perhaps a more widespread use of meniscal repair and better treatment of articular cartilage tears have the potential to reduce rates of OA in the future.
Shelbourne et al 28 identified several risk factors for long-term knee OA, with lack of full extension having the highest odds ratio (OR, 3.84) for both OA presence and moderate to severe OA (OR, 3.86). The role of residual postoperative laxity was controversial; one study identified a correlation with OA development, 10 whereas another study did not find any relationship. 28 Considering that these risk factors are potentially modifiable, all efforts should be made to manage them in order to mitigate the risk of OA. Patients with intact menisci had a similar rate of OA in the operated knee (28%) and contralateral knee (22%); thus, OA development could be caused also by physiological aging and sports participation and not only by ACL rupture and subsequent reconstruction.
Regarding how technical aspects could determine the long-term development of knee OA, Thompson et al 31 found that patients receiving BPTB autograft had a higher rate of abnormal radiographic results compared with patients receiving hamstring tendon graft (61% vs 41%, respectively; P = .008), especially in the patellofemoral joint, accounting for an overall increased risk of OA (OR, 2.4), even after removal of confounding variables. This is an important aspect to consider during decision-making for ACLR, especially considering that Thompson et al 31 used a modern arthroscopic technique and given the lack of other studies with similar design and follow-up. In contrast, Castoldi et al 6 conducted an RCT to investigate the role of lateral plasty in knee degeneration. The authors found a higher rate of lateral OA in patients in whom the plasty was performed (59% vs 22%; P = .02); however, the authors reported that partial lateral meniscectomy was significantly associated with lateral OA, and lateral meniscal lesions were significantly more frequent in the knees with lateral plasty than in the isolated ACLR group. Moreover, the study had a follow-up rate of 67%, which could have introduced a selection bias in the radiographic assessment, despite initial randomization. Their results should not be interpreted as an outright rejection of lateral plasty because technical aspects such as insertion point, graft type, and fixation position could be responsible for variability of results within studies. As an example, Zaffagnini et al 37 investigated the 24-year follow-up results of an over-the-top ACLR with lateral plasty using hamstring tendon graft, passing below the iliotibial band but above the lateral collateral ligament. The authors reported a similar joint space narrowing of the lateral compartment in operated and healthy knees, with a difference in anteroposterior laxity of only 0.2 ± 1.7 mm, no relevant presence of OA, and no valgus deformities, thus demonstrating that the addition of a lateral plasty did not worsen the outcome.
A point of discussion in the present review is the low rate of TKA. In fact, only 1.1% of patients underwent knee replacement in the 20 years after ACLR, representing an encouraging result, especially considering their long-term involvement in sports activity and the age of around 50 years at follow-up. The worst results were reported in high-demand athletes and in those with late ACLR performed >24 months from injury, where the TKA rate reached up to 9% of cases. 19 Moreover, it is possible that patients underwent other procedures to treat OA, such as high tibial osteotomy or unicompartmental knee arthroplasty.
The present study had several limitations, which were mostly inherent to the systematic review study design and the quality of the data of the included studies. The inclusion of studies with lower levels of evidence with respect to the RCT reduced the global level of evidence of the systematic review; however, we wanted to have the most comprehensive view on this specific topic, and we believe that this compromise was necessary. The modified Coleman methodology score highlighted shortcomings in the quality of the studies, especially regarding the type of study, sample size, and description of postoperative rehabilitation. Moreover, the heterogeneity in patient characteristics and surgical technique did not allow a sophisticated statistical analysis. The different types of graft used could have influenced the correct tunnel placement. However, only 2 studies investigated this issue, 10,31 and therefore it was not possible to derive a clear conclusion regarding this topic. Furthermore, because quadriceps tendon grafting is a more recent technique, no studies that used quadriceps tendon were included in the current systematic review.
At the time when the included studies were performed, meniscal repair in association with ACLR was not common compared with current surgical practice; this must be considered when comparing the outcomes of the studies included in the current review versus the results of more recent techniques. The different evaluations performed in the included studies led to a definition of clinical failure that may be not strict enough. Moreover, data about return to sports in the first 5 years after ACLR were not reported in the current systematic review.
Finally, the different OA radiographic scales used in the included studies required the predetermined definition of cutoff points to allow data pooling, thus possibly resulting in suboptimal precision of the outcomes.
Conclusion
Satisfactory subjective clinical outcomes were present in most patients 20 years after ACLR. We noted abnormal anteroposterior or rotatory laxity in nearly 10% of cases and an overall failure rate of nearly 13%, with a higher incidence in men who underwent reconstruction in their adolescent years. The long-term presence of OA was 73%, and the RR of OA was 2.8 with respect to the uninjured knee, especially in patients with concomitant meniscal or cartilage injuries, older age, and delayed surgery. However, severe OA was present in only 12.8% of cases, and TKA was required in only 1.1% of patients.
Footnotes
Final revision submitted August 30, 2021; accepted September 16, 2021.
The authors have declared that there are no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Aït Si Selmi T, Fithian D, Neyret P. The evolution of osteoarthritis in 103 patients with ACL reconstruction at 17 years follow-up. Knee. 2006;13(5):353–358. doi:10.1016/j.knee.2006.02.014 [DOI] [PubMed] [Google Scholar]
- 2. Amirault JD, Cameron JC, MacIntosh DL, Marks P. Chronic anterior cruciate ligament deficiency: long-term results of MacIntosh’s lateral substitution reconstruction. J Bone Joint Surg Br. 1988;70(4):622–624. [DOI] [PubMed] [Google Scholar]
- 3. Andriacchi TP, Briant PL, Bevill SL, Koo S. Rotational changes at the knee after ACL injury cause cartilage thinning. Clin Orthop Relat Res. 2006;442:39–44. [DOI] [PubMed] [Google Scholar]
- 4. Andriacchi TP, Koo S, Scanlan SF. Gait mechanics influence healthy cartilage morphology and osteoarthritis of the knee. J Bone Joint Surg Am. 2009;91(suppl 1):95–101. doi:10.2106/JBJS.H.01408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown CA, McAdams TR, Harris AHS, Maffulli C, Safran MR. ACL reconstruction in patients aged 40 years and older: a systematic review and introduction of a new methodology score for ACL studies. Am J Sports Med. 2013;41(9):2181–2190. [DOI] [PubMed] [Google Scholar]
- 6. Castoldi M, Magnussen RA, Gunst S, et al. A randomized controlled trial of bone-patellar tendon-bone anterior cruciate ligament reconstruction with and without lateral extra-articular tenodesis: 19-year clinical and radiological follow-up. Am J Sports Med. 2020;48(7):1665–1672. doi:10.1177/0363546520914936 [DOI] [PubMed] [Google Scholar]
- 7. Claes S, Hermie L, Verdonk R, Bellemans J, Verdonk P. Is osteoarthritis an inevitable consequence of anterior cruciate ligament reconstruction? A meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2013;21(9):1967–1976. doi:10.1007/s00167-012-2251-8 [DOI] [PubMed] [Google Scholar]
- 8. Claes S, Vereecke E, Maes M, Victor J, Verdonk P, Bellemans J. Anatomy of the anterolateral ligament of the knee. J Anat. 2013;223(4):321–328. doi:10.1111/joa.12087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Costa-Paz M, Garcia-Mansilla I, Marciano S, Ayerza MA, Muscolo DL. Knee-related quality of life, functional results and osteoarthritis at a minimum of 20 years’ follow-up after anterior cruciate ligament reconstruction. Knee. 2019;26(3):666–672. doi:10.1016/j.knee.2019.04.010 [DOI] [PubMed] [Google Scholar]
- 10. Curado J, Hulet C, Hardy P, et al. Very long-term osteoarthritis rate after anterior cruciate ligament reconstruction: 182 cases with 22-year follow-up. Orthop Traumatol Surg Res. 2020;106(3):459–463. doi:10.1016/j.otsr.2019.09.034 [DOI] [PubMed] [Google Scholar]
- 11. Elveos MM, Drogset JO, Engebretsen L, Brønn R, Lundemo TO, Gifstad T. Anterior cruciate ligament reconstruction using a bone-patellar tendon-bone graft with and without a ligament augmentation device: a 25-year follow-up of a prospective randomized controlled trial. Orthop J Sports Med. 2018;6(11):2325967118808778. doi:10.1177/2325967118808778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferretti A. Extra-articular reconstruction in the anterior cruciate ligament deficient knee: a commentary. Joints. 2014;2(1):41–47. [PMC free article] [PubMed] [Google Scholar]
- 13. Getgood AMJ, Bryant DM, Litchfield R, et al. Lateral extra-articular tenodesis reduces failure of hamstring tendon autograft anterior cruciate ligament reconstruction: 2-year outcomes from the STABILITY study randomized clinical trial. Am J Sports Med. 2020;48(2):285–297. doi:10.1177/0363546519896333 [DOI] [PubMed] [Google Scholar]
- 14. Grassi A, Carulli C, Innocenti M, et al. New trends in anterior cruciate ligament reconstruction: a systematic review of national surveys of the last 5 years. Joints. 2018;6(3):177–187. doi:10.1055/s-0038-1672157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grassi A, Macchiarola L, Lucidi GA, et al. Ten-year survivorship, patient-reported outcomes measures (PROMs) and patient acceptable symptoms state (PASS) after over-the-top hamstring ACL reconstruction with a lateral extra-articular reconstruction: analysis of 267 consecutive cases. Am J Sports Med. 2021;49(2):374–383. doi:10.1177/0363546520986875 [DOI] [PubMed] [Google Scholar]
- 16. Grassi A, Zaffagnini S, Marcheggiani Muccioli GM, et al. Revision anterior cruciate ligament reconstruction does not prevent progression in one out of five patients of osteoarthritis: a meta-analysis of prevalence and progression of osteoarthritis. J ISAKOS. 2016;1:16–24. [Google Scholar]
- 17. Hagemans FJA, Jonkers FJ, van Dam MJJ, von Gerhardt AL, van der List JP. Clinical and radiographic outcomes of anterior cruciate ligament reconstruction with hamstring tendon graft and femoral cortical button fixation at minimum 20-year follow-up. Am J Sports Med. 2020;48(12):2962–2969. doi:10.1177/0363546520951796 [DOI] [PubMed] [Google Scholar]
- 18. Li S, Chen Y, Lin Z, Cui W, Zhao J, Su W. A systematic review of randomized controlled clinical trials comparing hamstring autografts versus bone-patellar tendon-bone autografts for the reconstruction of the anterior cruciate ligament. Arch Orthop Trauma Surg. 2012;132(9):1287–1297. doi:10.1007/s00402-012-1532-5 [DOI] [PubMed] [Google Scholar]
- 19. Lindanger L, Strand T, Mølster AO, Solheim E, Inderhaug E. Return to play and long-term participation in pivoting sports after anterior cruciate ligament reconstruction. Am J Sports Med. 2019;47(14):3339–3346. doi:10.1177/0363546519878159 [DOI] [PubMed] [Google Scholar]
- 20. Marcacci M, Bonanzinga T, Grassi A, et al. Long-term clinical outcomes of combined BPTB ACL reconstruction and popliteus tendon plasty. Knee Surg Sports Traumatol Arthrosc. 2015;23(10):2930–2935. doi:10.1007/s00167-015-3673-x [DOI] [PubMed] [Google Scholar]
- 21. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Øiestad BE, Engebretsen L, Storheim K, Risberg MA. Knee osteoarthritis after anterior cruciate ligament injury: a systematic review. Am J Sports Med. 2009;37(7):1434–1443. [DOI] [PubMed] [Google Scholar]
- 23. Pernin J, Verdonk P, Si Selmi TA, Massin P, Neyret P. Long-term follow-up of 24.5 years after intra-articular anterior cruciate ligament reconstruction with lateral extra-articular augmentation. Am J Sports Med. 2010;38(6):1094–1102. doi:10.1177/0363546509361018 [DOI] [PubMed] [Google Scholar]
- 24. Rahardja R, Zhu M, Love H, Clatworthy MG, Monk AP, Young SW. Factors associated with revision following anterior cruciate ligament reconstruction: a systematic review of registry data. Knee. 2020;27(2):287–299. doi:10.1016/j.knee.2019.12.003 [DOI] [PubMed] [Google Scholar]
- 25. Rahnemai-Azar AA, Sabzevari S, Irarrázaval S, Chao T, Fu FH. Anatomical individualized ACL reconstruction. Arch Bone Jt Surg. 2016;4(4):291–297. [PMC free article] [PubMed] [Google Scholar]
- 26. Risberg MA, Oiestad BE, Gunderson R, et al. Changes in knee osteoarthritis, symptoms, and function after anterior cruciate ligament reconstruction: a 20-year prospective follow-up study. Am J Sports Med. 2016;44(5):1215–1224. doi:10.1177/0363546515626539 [DOI] [PubMed] [Google Scholar]
- 27. Salmon LJ, Heath E, Akrawi H, Roe JP, Linklater J, Pinczewski LA. 20-Year outcomes of anterior cruciate ligament reconstruction with hamstring tendon autograft: the catastrophic effect of age and posterior tibial slope. Am J Sports Med. 2018;46(3):531–543. doi:10.1177/0363546517741497 [DOI] [PubMed] [Google Scholar]
- 28. Shelbourne KD, Benner RW, Gray T. Results of anterior cruciate ligament reconstruction with patellar tendon autografts: objective factors associated with the development of osteoarthritis at 20 to 33 years after surgery. Am J Sports Med. 2017;45(12):2730–2738. doi:10.1177/0363546517718827 [DOI] [PubMed] [Google Scholar]
- 29. Sporsheim AN, Gifstad T, Lundemo TO, et al. Autologous BPTB ACL reconstruction results in lower failure rates than ACL repair with and without synthetic augmentation at 30 years of follow-up: a prospective randomized study. J Bone Joint Surg Am. 2019;101(23):2074–2081. doi:10.2106/JBJS.19.00098 [DOI] [PubMed] [Google Scholar]
- 30. Thompson S, Salmon L, Waller A, Linklater J, Roe J, Pinczewski L. Twenty-year outcomes of a longitudinal prospective evaluation of isolated endoscopic anterior cruciate ligament reconstruction with patellar tendon autografts. Am J Sports Med. 2015;43(9):2164–2174. doi:10.1177/0363546515591263 [DOI] [PubMed] [Google Scholar]
- 31. Thompson SM, Salmon LJ, Waller A, Linklater J, Roe JP, Pinczewski LA. Twenty-year outcome of a longitudinal prospective evaluation of isolated endoscopic anterior cruciate ligament reconstruction with patellar tendon or hamstring autograft. Am J Sports Med. 2016;44(12):3083–3094. doi:10.1177/0363546516658041 [DOI] [PubMed] [Google Scholar]
- 32. Tramer JS, Fidai MS, Kadri O, et al. Anterolateral ligament reconstruction practice patterns across the United States. Orthop J Sports Med. 2018;6(12):2325967118811063. doi:10.1177/2325967118811063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van Yperen DT, Reijman M, van Es EM, Bierma-Zeinstra SMA, Meuffels DE. Twenty-year follow-up study comparing operative versus nonoperative treatment of anterior cruciate ligament ruptures in high-level athletes. Am J Sports Med. 2018;46(5):1129–1136. doi:10.1177/0363546517751683 [DOI] [PubMed] [Google Scholar]
- 34. Webster KE, Feller JA, Klemm HJ. Second ACL injury rates in younger athletes who were advised to delay return to sport until 12 months after ACL reconstruction. Orthop J Sports Med. 2021;9(2):2325967120985636. doi:10.1177/2325967120985636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wredmark T, Engström B. Five-year results of anterior cruciate ligament reconstruction with the Stryker Dacron high-strength ligament. Knee Surg Sports Traumatol Arthrosc. 1993;1(2):71–75. doi:10.1007/BF01565455 [DOI] [PubMed] [Google Scholar]
- 36. Yamaguchi S, Sasho T, Tsuchiya A, Wada Y, Moriya H. Long term results of anterior cruciate ligament reconstruction with iliotibial tract: 6-, 13-, and 24-year longitudinal follow-up. Knee Surg Sports Traumatol Arthrosc. 2006;14(11):1094–1100. doi:10.1007/s00167-006-0129-3 [DOI] [PubMed] [Google Scholar]
- 37. Zaffagnini S, Marcheggiani Muccioli GM, Grassi A, et al. Over-the-top ACL reconstruction plus extra-articular lateral tenodesis with hamstring tendon grafts: prospective evaluation with 20-year minimum follow-up. Am J Sports Med. 2017;45(14):3233–3242. doi:10.1177/0363546517723013 [DOI] [PubMed] [Google Scholar]