Abstract
Type B lactic acidosis is a rare complication of non-tissue perfusion abnormalities caused by solid tumors or hematologic malignancies. Herein, we present the case of a 42-year-old man with type B lactic acidosis and hypoglycemia who was found to have a diffuse large B-cell lymphoma. The cause of lactic acidosis and/or hypoglycemia is thought to be the Warburg effect, which is when the metabolic rate of a rapidly growing malignant tumor is very high and dominated by glycolysis. Systemic damage from type B lactic acidosis can occur when the increased rate of glycolysis exceeds the normal muscle and liver lactic acid clearance rate. The Warburg effect is a rare but serious condition that needs to be recognized, not only in diffuse large B-cell lymphoma, but also in other malignancies. The prognosis of lactic acidosis in patients with malignant tumors is very poor. Currently, effective chemotherapy seems to be the only hope for survival.
Keywords: Type B lactic acidosis, Warburg effect, hypoglycemia, diffuse large B-cell lymphoma, tumor metabolism, glycolysis
Introduction
Lactic acid is an important intermediate in the metabolism of carbohydrates and non-essential amino acids. When lactic acid production exceeds its clearance, it accumulates and causes lactic acidosis. Lactic acidosis is defined as a pH below 7.35 and blood lactic acid levels >5 mmol/L. Type A lactic acidosis is associated with tissue insufficiency in the context of sepsis, circulatory failure, hypovolemia, or severe hypoxemia. In contrast, type B lactic acidosis does not show any signs of organ dysfunction and is associated with disturbances in cellular metabolism. The most common cause of type B lactic acidosis is hematologic malignancies, especially lymphomas, in which type B lactic acidosis is often a sign of poor prognosis. 1 Herein, we report a case of type B lactic acidosis for which the final pathology was confirmed to be diffuse large B-cell lymphoma. The mechanism of the hematologic malignancy complicated with type B lactic acidosis can be explained by the Warburg effect.
Case report
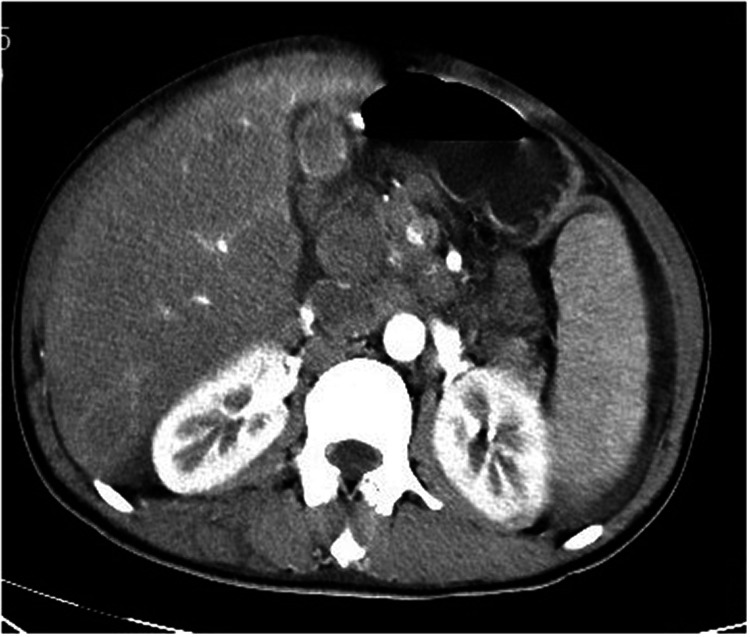
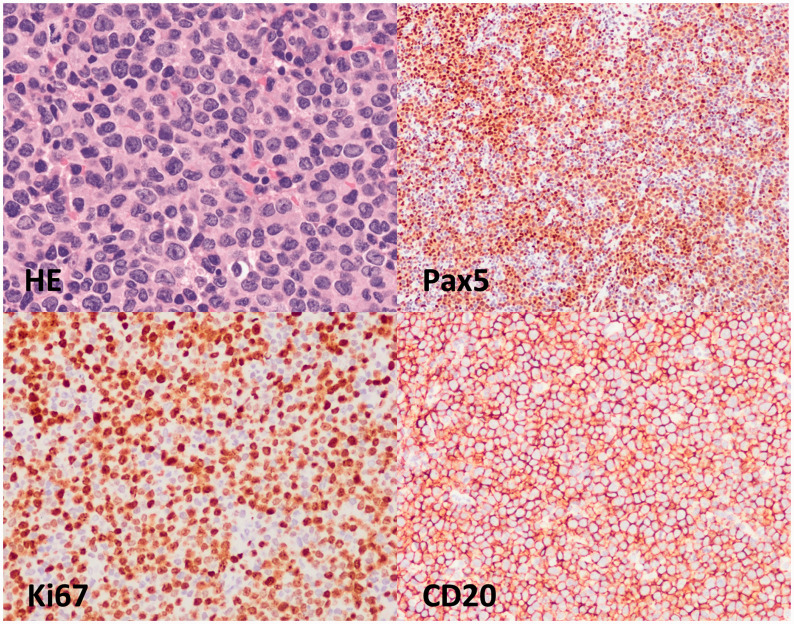
A previously healthy 42-year-old Asian man presented to the gastroenterology department on 3 December 2020 with epigastric swelling and pain for 14 days. The patient presented with general fatigue, loss of appetite, and weight loss of nearly 10 kg; he denied having fever, night sweats, headache, unsteady gait, visual impairment, syncope, epilepsy, palpitation, sweating, polydipsia, polyuria, or other symptoms. He had a history of viral hepatitis B (HBV) that was well controlled after taking entecavir for several years; he had no history of diabetes or insulin use. On the day of admission, his temperature was 37.1°C, respiratory rate was 18 breaths per minute, and oxygen saturation was 98% on air. His pulse was 108 beats per minute, and his lying blood pressure was 122/84 mmHg. Physical examination showed no petechiae, and cardiopulmonary examination showed no abnormalities. Remarkable findings on physical examination included distention of the right upper abdomen and tenderness accompanied with hepatosplenomegaly. Laboratory studies showed a white blood cell count of 1.43 × 109/L; neutrophils, 72.9%; lymphocytes, 16.8%; hemoglobin (HB), 136 g/L; and blood platelet count, 24 × 109/L. Levels of serum creatinine, blood urea nitrogen, serum amylase, total bilirubin, the hepatic enzyme spectrum, procalcitonin, and interleukin-6 were normal; lactate dehydrogenase levels were 697 U/L, and the HBV-DNA load was <4 × 104 IU/mL. Arterial blood gas analysis showed pH 7.318, the base excess was −12.8 mEq/L, and lactate levels were 18 mmol/L. His blood glucose level was 2.73 mmol/L. After small doses of sodium bicarbonate treatment, fluid replenishment, and maintaining his water and electrolyte balance, the lactic acid index continued to increase to 30 mmol/L. Intravenous administration of 10% glucose yielded multiple tests with blood glucose levels <5 mmol/L, and the patient did not show symptoms of hypoglycemia such as palpitations or sweating. Abdominal computed tomography imaging revealed hepatosplenomegaly and multiple lymphadenopathy in the hepatic hilar and retroperitoneum (Figure 1). A bone marrow puncture revealed suspected lymphoma cells in approximately 6% of the images, and myelodysplasia was seen. Later lymph node biopsy and immunohistochemistry showed that the atypical large cells were positive for CD20 and Pax5, with a >70% Ki67 index. Thus, the diagnosis of diffuse large B-cell lymphoma was confirmed (Figure 2). The hematology department was consulted for chemotherapy, but the patient’s family decided against treatment. The patient was discharged after 5 days in the hospital. Follow-up showed that he died 1 week after discharge.
Figure 1.
Computed tomography scan showing enlarged hilar and retroperitoneal lymph nodes.
Figure 2.
Hematoxylin and eosin staining and immunohistochemistry for Pax5, Ki67, and CD20 suggested diffuse large B-cell lymphoma.
Discussion
From the perspective of pathophysiology, lactic acidosis can be divided into the type A and type B subgroups. Type A is the most common clinical type, reflecting hypoxia due to low tissue perfusion, which is commonly caused by sepsis, cardiogenic shock, dehydration, and ischemic bowel disease. In contrast, type B lactic acidosis, which is usually associated with normal tissue perfusion, is caused by liver disease, vitamin B1 deficiency, alcoholism, metformin use, and malignancies. 2
Type B lactic acidosis is a rare but life-threatening disease that mainly occurs in patients with hematological malignancies but may also occur with solid tumors. 3 The early detection of type B lactic acidosis is of great significance to its diagnosis, treatment, and prognosis. In this case, the patient's lactic acidosis could not be explained by low perfusion status, toxins, drugs, or thiamine deficiency. These factors were excluded on the basis of full dilatation, fluid replenishment, and correction of acidosis; the patient also had severe asymptomatic hypoglycemia without a history of diabetes or insulin use. Bone marrow puncture showed suspicious lymphoma cells and reduced myelodysplasia. The patient did not have a sore throat, cough, sputum, fever, frequent urination, urgent urination, painful urination, diarrhea, petechiae, or petechiae on the skin. Physical examination indicated tenderness in the right upper abdomen, with no abnormalities in the heart or lungs. Laboratory examination showed no increase in procalcitonin, interleukin-6, white blood cells, or neutrophils. Computed tomography examination showed no obvious abnormalities in the heart or lungs. In summary, the patient did not have symptoms or laboratory results suggestive of infectious factors. Infection was not considered, so antibiotics and blood cultures were not performed. Combined with the enlarged abdominal liver, spleen, and retroperitoneal lymph nodes, the lymph node biopsy revealed diffuse large B lymphoma; thus, we considered the cause of lactic acidosis to be type B lactic acidosis secondary to the Warburg effect in malignant cells.
The Warburg effect occurs when glucose metabolism in cancer cells switches from primarily being through the oxidative pathway to the glycolytic pathway, leading to the accumulation of lactic acid and hypoglycemia. 4 In his 1923 Nobel Prize-winning study, Warburg observed that even under full oxygenation, cancer cells are characterized by accelerated glycolysis and the formation of excess lactic acid, which allows tumor cells to survive and grow in a more acidic environment that normal cells generally cannot tolerate. His discovery was named the “Warburg effect” by Racker in 1972. 5 The Warburg effect is one hallmark of malignant tumors and is more common in hematologic malignancies. 6
The association between hypoglycemia and lactic acidosis is a rare but well-documented occurrence in malignancies, especially in lymphoproliferative tumors such as lymphomas, 7 but its association with severe asymptomatic hypoglycemia is an extremely rare phenomenon. 8 Hypoglycemia is a significant feature of this patient and, similar to other reports in the literature, is considered to be the result of high-level glucose consumption by the tumor. 9 Elhomsy et al. showed that while aggressive glucose infusions do not substantially increase serum glucose levels, they instead increase lactate production, presumably by the tumor. It is possible that the intravenous glucose administration may have actually preferentially “fed” the tumor and only further worsened the lactic acidosis. 10 If lactic acidosis and/or hypoglycemia are present in patients with malignancies, we need to consider the Warburg effect, which occurs when the tumor has a high rate of proliferation. The Warburg effect may be overlooked in many cases because these changes may be considered symptoms of late-stage cancer.
In this case, marrow puncture showed suspected lymphoma cells that indicated diffuse large B-cell lymphoma with involvement of the bone marrow. The tumor was stage IV, and the International Prognostic Index score was 3. The patient died quickly after the diagnosis was confirmed. Only a few cases of diffuse large B-cell lymphoma with lactic acidosis have been reported in the literature, and the vast majority of these patients were not treated for lymphoma because of rapid disease progression. 11 No cases in the literature suggest a direct relationship between diffuse large B-cell lymphoma with bone metastasis and type B lactic acidosis, but diffuse large B-cell lymphoma with type B lactic acidosis is often the terminal state of patients. 12
Treatment for type B lactic acidosis primarily focuses on treating the primary disease, and the effect of acid correction and fluid rehydration treatment is not good. 13 It has been reported that treating lactic acidosis in patients with diffuse large B-cell lymphoma can effectively alleviate the disease and save lives.3,14 But other cases have shown that patients who exhibit the Warburg effect have a very poor prognosis. The death rate from lactic acidosis in cancer patients is >80%. 15 A previous study showed that only 2 of 28 patients with non-Hodgkin's lymphoma that showed the Warburg effect achieved complete remission, while >75% died within 1 month. 12 Elevated lactate levels are generally viewed as a late-stage event in the course of diffuse large B-cell lymphoma disease. Regrettably, this patient died after refusing follow-up treatments.
Conclusion
Clinical lactic acidosis is not uncommon, and it is important to find the etiology. If type-B lactic acidosis and/or hypoglycemia are present in patients, we need to consider the Warburg effect with malignancy, which may be overlooked because these changes may be considered to be one of the late symptoms of cancer. The Warburg Effect is a rare but serious condition that needs to be recognized.
Acknowledgement
We would like to thank the patient.
Author contributions: Conceptualization: Chunhua Wang
Data curation: Yanwei Zhang
Resources: Zanmei Lv
Writing-original draft: Chunhua Wang
Writing-review & editing: Chunhua Wang
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Chunhua Wang https://orcid.org/0000-0002-2352-8874
Consent for publication
Written informed consent was obtained from the next of kin for the publication of this case report.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of The 980th Hospital of the People's Liberation Army Joint Service (Bethune International Peace Hospital) (Approval number: 2020-KY-130). Written consent was obtained from the next of kin. This study is reported in accordance with the CARE guidelines. 16
References
- 1.Abdullah SY, Ali MK, Sabha MM. Type-B lactic acidosis associated with progressive multiple myeloma. Saudi Med J 2015; 36: 239–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu QS, Harji F, Jones A, et al. Type B lactic acidosis: a rare oncological emergency. BMJ Case Rep 2020; 13: e233068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wahab A, Kesari K, Smith SJ, et al. Type B lactic acidosis, an uncommon paraneoplastic syndrome. Cancer Biol Ther 2018; 19: 101–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pascale RM, Calvisi DF, Simile MM, et al. The Warburg Effect 97 Years after Its Discovery. Cancers (Basel) 2020; 12: 2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.San-Millán I, Brooks GA. Reexamining cancer metabolism: lactate production for carcinogenesis could be the purpose and explanation of the Warburg Effect. Carcinogenesis 2017; 38: 119–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Looyens C, Giraud R, Silva IN, et al. Burkitt lymphoma and lactic acidosis: A case report and review of the literature. Physiol Rep 2021; 9: e14737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedenberg AS, Brandoff DE, Schiffman FJ. Type B lactic acidosis as a severe metabolic complication in lymphoma and leukemia: a case series from a single institution and literature review. Medicine (Baltimore) 2007; 86: 225–232. [DOI] [PubMed] [Google Scholar]
- 8.Goyal I, Ogbuah C, Chaudhuri A, et al. Confirmed Hypoglycemia Without Whipple Triad: A Rare Case of Hyper-Warburgism. J Endocr Soc 2021; 5: bvaa182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brault C, Zerbib Y, Delette C, et al. The Warburg Effect as a Type B Lactic Acidosis in a Patient With Acute Myeloid Leukemia: A Diagnostic Challenge for Clinicians. Front Oncol 2018; 8: 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elhomsy GC, Eranki V, Albert SG, et al. “Hyper-warburgism,” a cause of asymptomatic hypoglycemia with lactic acidosis in a patient with non-Hodgkin's lymphoma. J Clin Endocrinol Metab 2012; 97: 4311–4316. [DOI] [PubMed] [Google Scholar]
- 11.Hwang CS, Hwang DG, Aboulafia DM. A Clinical Triad with Fatal Implications: Recrudescent Diffuse Large B-cell Non-Hodgkin Lymphoma Presenting in the Leukemic Phase with an Elevated Serum Lactic Acid Level and Dysregulation of the TP53 Tumor Suppressor Gene – A Case Report and Literature Review. Clin Med Insights Blood Disord 2021; 14: 2634853521994094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He YF, Wei W, Sun ZM, et al. Fatal lactic acidosis and hypoglycemia in a patient with relapsed natural killer/T-cell lymphoma. Adv Ther 2007; 24: 505–509. [DOI] [PubMed] [Google Scholar]
- 13.Duriseti P, Vanegas YM, Jaber BL, et al. Malignancy-induced lactic acidosis in adult lymphoma. Clin Nephrol 2021; 95: 1–21. [DOI] [PubMed] [Google Scholar]
- 14.Singh M, Ajmeri AN, Suliman MS, et al. A Challenging Case of Coexisting Type A and Type B Lactic Acidosis: A Case Report. Cureus 2019; 11: e3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez-Outschoorn UE, Whitaker-Menezes D, Valsecchi M, et al. Reverse Warburg effect in a patient with aggressive B-cell lymphoma: is lactic acidosis a paraneoplastic syndrome? Semin Oncol 2013; 40: 403–418. [DOI] [PubMed] [Google Scholar]
- 16.Gagnier JJ, Kienle G, Altman DG, et al. The CARE Guidelines: Consensus-based Clinical Case Reporting Guideline Development. Glob Adv Health Med 2013; 2: 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]