Abstract
Melanoma is the deadliest form of skin cancer in the world with a growing incidence in North America. Contemporary treatments for melanoma include surgical resection, chemotherapy, and radiotherapy. However, apart from resection in early melanoma, the prognosis of patients using these treatments is typically poor. In the past decade, there have been significant advancements in melanoma therapies. Immunotherapies such as ipilimumab and targeted therapies such as vemurafenib have emerged as a promising option for patients as seen in both scientific and clinical research. Furthermore, combination therapies are starting to be administered in the form of polychemotherapy, polyimmunotherapy, and biochemotherapy, of which some have shown promising outcomes in relative efficacy and safety due to their multiple targets. Alongside these treatments, new research has been conducted into the evidence-based use of natural health products (NHPs) and natural compounds (NCs) on melanoma which may provide a long-term and non-toxic form of complementary therapy. Nevertheless, there is a limited consolidation of the research conducted in emerging melanoma treatments which may be useful for researchers and clinicians. Thus, this review attempts to evaluate the therapeutic efficacy of current advancements in metastatic melanoma treatment by surveying new research into the molecular and cellular basis of treatments along with their clinical efficacy. In addition, this review aims to elucidate novel strategies that are currently being used and have the potential to be used in the future.
Keywords: melanoma, chemotherapy, immunotherapy, immune checkpoint inhibitor, cytokines, oncolytic virus, anti-cancer vaccine, biochemotherapy, natural health products
Introduction
Melanoma is an extremely aggressive form of cancer that originates from melanocytes found in the basal layer of the epidermis. 1 It typically presents with cutaneous origin but has been seen to arise rarely through uveal or mucosal origin.2,3 In North America, skin cancers are a common form of cancer and of these, cutaneous melanoma is the most aggressive and accounts for 6 of 7 skin cancer deaths. 4 Furthermore, melanoma is the 6th most common fatal malignancy in the United States. 4 In the next 50 years, it is expected that the incidence rate of melanoma will dramatically rise globally to make it much more common. 5 Furthermore, demographic studies have shown that melanoma incidence is correlated with lighter skin color and older age. 5 Like all skin cancers, the primary factor toward the occurrence of melanoma is exposure to UV light from the sun. 6 Exposure to UV light leads to DNA damage in cells, which in turn leads to cell cycle arrest and apoptosis of the cell if the DNA cannot be repaired. In cancer cells, apoptosis is bypassed, and cell division occurs at a high rate despite DNA damage. 7
The primary form of treatment for melanoma is surgical resection. Although surgical removal is shown to provide successful outcomes, this is only observed in pre-stage IV melanoma with minimal metastases to areas such as the lymph nodes. 8 Thus, unresectable metastatic melanoma is typically treated using systemic therapy. 9 Radiotherapy, both in local and systemic application, has been used against melanoma but has been associated with resistance and the incidence of secondary cancers such as leukemia.10,11 A more commonly used form of systemic therapy is chemotherapy, many of which target DNA and tubulin and specific biochemical pathways to induce a cytotoxic effect. 12 However, chemoresistance is an issue with the use of chemotherapy and this form of treatment can also lead to high toxicity to healthy tissues. 9 Dacarbazine, the only approved single-agent chemotherapy for melanoma, has shown low response rates in patients. 13
In recent years, immunotherapy, a form of treatment that simultaneously targets melanoma and minimizes the ability of tumor cells to escape the immune response has been promising. 14 Systemic immunotherapies lie in the classes of immune checkpoint inhibitors such as anti-cytotoxic T-lymphocyte-associated antigen (CTLA-4) antibodies, anti-programmed-death-1 (anti-PD-1) or anti-programmed-death-L (anti-PD-L) antibodies. Systemic immunotherapies also include cytokine therapy, such as interleukin-IIb and interferon-alfa2b which typically increase the ability for T-cells to recognize tumors via antigen-presenting-cells (APC). 15 Although these systemic treatments have been promising, side effects have been observed with their administration such as dermatitis, colitis, and hepatotoxicity. 16 In addition to immunotherapies, targeted therapies such as V-Raf Sarcoma Viral Oncogene Homolog B (BRAF) mutant enzyme inhibitors and MEK inhibitors have been recently developed and have shown encouraging results in patients with specific mutations.17,18
Alongside single-agent therapies, combination therapies such as polychemotherapy (e.g. dacarbazine, cisplatin, paclitaxel), polyimmunotherapy (e.g. ipilimumab with nivolumab), and biochemotherapy (e.g. dacarbazine with ipilimumab) are being researched in clinical trials due to their potential to have higher efficacy since targeting multiple molecular targets may have synergistic effects in inhibiting tumor progression. 19
Despite considerable research being conducted, there is minimal information on the scientific basis of natural health products (NHPs) and their clinical potential. NHPs have been traditionally used in many cultures and some such as dandelion root extract have shown they are non-toxic and can be used long-term. 20 With additional research, NHPs and natural compounds (NCs) have the potential to be used in an evidence-based manner as a complementary treatment alongside standard chemotherapeutics. NHPs have shown through in-vitro models and in mouse xenograft models that they can provide higher efficacy through inducing apoptosis or sensitizing cells, while also providing lower toxicity by having a protective effect for healthy cells in some cases. 21 Since metastatic melanoma is a notoriously difficult cancer to treat systemically, there is an interest in the use of NHPs/NCs on melanoma, including their interactions with standard drugs.
Thus, since there has been substantial research conducted in the past decade, we aim to outline the current advancements in the treatment of metastatic melanoma such as the emerging classes of immunotherapies. Moreover, another purpose of this review is to provide details on novel strategies that are being currently used or may be used in the future, such as the early phase trials of polyimmunotherapy, biochemotherapy, and research into NHPs/NCs.
Chemotherapeutic Monotherapies
Dacarbazine
Currently, there is only one FDA approved single-agent chemotherapy for clinical use in treating metastatic melanoma—dacarbazine (DTIC). Dacarbazine was approved by the FDA in 1975. 22 The drug has been associated with inducing cytotoxicity to inhibit the progression of melanoma, 23 however, the mechanism of action is not clear. It is speculated that dacarbazine may act as a DNA-alkylating agent by methylating purine bases. 23 Unfortunately, phase III clinical trials of dacarbazine have shown limited success with response rates of 10% to 20%. 13 Furthermore, due to its non-specific cytotoxicity, it has been associated with significant side effects such as suppressed hematopoiesis leading to anemia. 13 Other commonly reported adverse effects are nausea, vomiting, and fatigue. 24 Some research has been done on adjuvant uses of dacarbazine with chemotherapies, immunotherapies, and NHPs/NCs as described in later sections. Dacarbazine is administered intravenously at a dose of 800 to 1000 mg/m2 every 3 to 4 weeks. 24 Once in the body, in-vivo studies have shown that it is metabolically activated via the liver cytochromes: CYP1A1, CYP1A2, and CYP2E1. 25 Once metabolized, 5-[3-methyl-triazen-1-yl]-imidazole-4-carboxamide (MTIC) is formed which initiates apoptosis by methylating guanine bases of DNA. 26 A proposed explanation for the low observed therapeutic efficacy of dacarbazine is the lowered activity of cytochromes P450 in humans relative to rodents. 25
Temozolomide and Fotemustine
Temozolomide (TMZ) is an alkylating agent that is an analog of dacarbazine. Both DTIC and TMZ are converted into MTIC in-vivo.25,27 Although not currently FDA-approved for use in the metastatic melanoma indication, it has been evaluated through phase I to III clinical trials. In a randomized phase III clinical trial, it has been shown to provide similar efficacy as DTIC as it provides an objective response rate (ORR) of 14% as compare to 12%, while being well tolerated and minimizing the reduction to quality of life (QOL). 28 Interestingly, TMZ has shown high oral bioavailability which may provide a convenient form of treatment for melanoma patients in comparison to DTIC. 29 Currently, temozolomide is used as an adjuvant therapy for glioblastoma due to its ability to cross the blood-brain barrier. 27 Like temozolomide, fotemustine (FM) is a chemotherapeutic reagent used in metastatic melanoma that has spread to the central nervous system. Fotemustine is a DNA alkylating agent in the nitrosourea group. It functions similarly to DTIC and TMZ by adding chloroethyl groups to guanine bases. 29 FM is not currently approved by the FDA for use in metastatic melanoma, but has shown similar or slightly higher response rates to dacarbazine in phase III trials.30,31
Clinical Status of Other Chemotherapeutic Regimens
Cisplatin (CDDP) and carboplatin (CBDCA) are 2 platinum analogs used widely in the treatment of metastatic cancers. These 2 drugs are DNA-alkylating agents that bind to purine residues. 32 It is speculated that the main mechanism of inducing apoptosis for platinum analogs is by inhibiting RNA transcription. 32 In a phase II clinical trial, CBDCA was shown to produce response rates in the range of 10% to 25% which is similar to DTIC. 33 In a study administering a combination of cisplatin and carboplatin in 15 dacarbazine-resistant metastatic melanoma patients, the overall response rate achieved was 26.4% and the median overall survival (OS) was 12.5 months. 34 Paclitaxel is a commonly used general chemotherapeutic. Paclitaxel’s main mechanism of action is to promote tubulin polymerization within cells, leading to cell cycle inhibition within the G2/M phase and thus the triggering of apoptosis. 35 In a phase II trial assessing the use of paclitaxel (dose of 250 mg/m2) in untreated metastatic melanoma patients, the overall response rate was 12% with 4 patients achieving a partial response. 35 Regression of pulmonary metastases resulting from melanoma were also seen in 4 patients with a significant duration of 11 months. 35 Another chemotherapeutic, tamoxifen (TAM), is often used in patients with aggressive estrogen receptor (ER)-positive breast cancer, as well as in ER-negative breast cancer. 36 In 1 study, 46% of patients with metastatic melanoma had high-affinity cytoplasmic estrogen receptors. 37 Thus, TAM was evaluated for use as a single-agent in metastatic melanoma as its mechanism of action is to bind estrogen receptors and block estrogen-mediated proliferation. 38 However, various phase II clinical trials have shown poor response rates ranging from a median of 4.9% to 7%. 39 The well-known chemotherapy drug doxorubicin blocks the topoisomerase-2 enzyme used in DNA replication, leading to the activation of apoptosis due to cell cycle inhibition. It has also been associated with inducing apoptosis via the generation of reactive oxygen species (ROS). 40 In various cancers including metastatic melanoma, chemoresistance has been an issue with doxorubicin treatment which has been theorized to be a result of the efflux activity of the ATP-binding Cassette B-5 (ABCB5) P-glycoprotein. 41 Thus, novel administration strategies have been utilized for doxorubicin such as liposomal delivery 42 and prodrug delivery.41,43 However, various phase II clinical trials studying different formulations of doxorubicin have shown very limited clinical efficacy in chemotherapy-naive (CN) metastatic melanoma with response rates ranging from 0% to 10%. 43 A summary of studied single-agent and combination chemotherapeutics for use in metastatic melanoma is described in Table 1.
Table 1.
Summary of Current Chemotherapies.
| Reagent | Cancers | Mechanism | Single-agent use | Observed response rates |
|---|---|---|---|---|
| Dacarbazine (DTIC) | Melanoma, Hodgkin’s Lymphoma | DNA alkylating agent (methylates purine bases) | Yes; FDA approved (1975) | 10% to 20% 13 |
| Temozolomide (TMZ) | Melanoma, Glioblastoma | DNA alkylating agent (methylates purine bases) | No | 14% 28 |
| Fotemustine (FM) | Melanoma, Glioblastoma | DNA alkylating agent (methylates purine bases) | No | 15.2% (disseminated melanoma)30,31 |
| Carboplatin/Cisplatin | Several | DNA alkylating agent | No | 26.4% (combination), (phase II) 34 |
| Paclitaxel | Several | Tubulin stabilizer | No | 21.6%, (phase II) 35 |
| Tamoxifen | ER-positive or ER-negative breast cancer | Inhibits estrogen-mediated cell proliferation | No | 4.9% to 7% 39 |
| Doxorubicin | Several | Topoisomerase-2 inhibition, free radical generation | No | 0% to 10% (phase II) 43 |
Challenges with Chemotherapeutic Efficacy
Low response rates are seen with single-agent use of DTIC in both clinical trials and real-world clinical use. Similarly, with TMZ and RM use in clinical trials, poor prognosis is seen. It is theorized that DNA-alkylating agent use in metastatic melanoma may lead to increasing chemoresistance due to the activity of O(6)-methylguanine DNA-methyltransferase (MGMT), which removes DNA adducts created by alkylating agents, thus interfering with apoptosis. 29
Immunotherapeutic Monotherapies
In recent times, immunotherapies have emerged as a viable treatment for a variety of cancers. The basis of immunotherapies relies on the molecular interaction between cell surface receptors on cancer cells and the host’s adaptive immune system via cytotoxic T-cells. 44 For metastatic melanoma, systemic immunotherapies can be divided into cytokines, which activate an immune response, and immune checkpoint inhibitors, which are monoclonal antibodies that reduce the ability for cancer cells to evade immunosurveillance by promoting T-cell activation. 14 Furthermore, anti-cancer vaccines are available as a therapeutic option to enhance the immune response and oncolytic virotherapy is a relatively new option that induces a localized effect. A summary of immunotherapies studied for use in metastatic melanoma is described in Table 2.
Table 2.
Summary of Current Immunotherapies.
| Reagent | Cancers | Class | FDA-approved indications for melanoma | Observed efficacy |
|---|---|---|---|---|
| Ipilimumab | • Melanoma | Anti-CTLA4 antibody | Unresectable/metastatic melanoma and as an adjuvant (2011) | 12.3%-28.4% 5-year OS rate 49 ; 65.4% 5-year OS rate as adjuvant 47 (phase III) |
| • Renal cell Carcinoma | ||||
| Tremelimumab | • Melanoma | Anti-CTLA4 Antibody | Not approved | 12.6 months OS; 10.7% ORR (ORR) (phase III) 51 |
| Nivolumab | • Melanoma | Anti-PD-1/PD-L Antibody | Unresectable/metastatic melanoma and as an adjuvant (2014) | ~40% ORR |
| • Lung cancer (non-small cell) | 72.9% 1-year OS rate | |||
| • Renal carcinoma | 5.1 months PFS, (phase III) 55 | |||
| • Hodgkin’s Lymphoma | ||||
| Pembrolizumab | • Melanoma | Anti-PD-1/PD-L antibody | Unresectable/metastatic melanoma and as an adjuvant (2014) | 74.1% 12-month OS rate 57 ; 58.2% ORR; 34% 5-year OS rate 58 (phase III) |
| • Lung cancer (non-small cell) Hodgkin’s Lymphoma | ||||
| • Several others | ||||
| Aldesleukin | • Metastatic melanoma | Cytokine activation of T-Cells (IL-2) | Metastatic or unresectable melanoma (1998) | 11.4 months OS; 16% ORR (phase III) 63 |
| • Renal cell carcinoma | ||||
| Interferon-alfa2b and pegylated-interferon-alpha2b | • Melanoma | Cytokine activation of T-cells | Adjuvant alongside surgical resection (gross nodal melanoma) (2011) | Non-significant OS; 45.6% PFS (PEG-IFNα-2b) (phase III) 65 |
| • Follicular lymphoma | ||||
| • AIDS-related Kaposi Sarcoma | ||||
| • Several others | ||||
| T-VEC | • Melanoma | Oncolytic virus | Unresectable metastatic melanoma (2015) | 26% ORR (phase III) 70 |
Immune Checkpoint Inhibitors
Anti-CTLA-4 antibodies
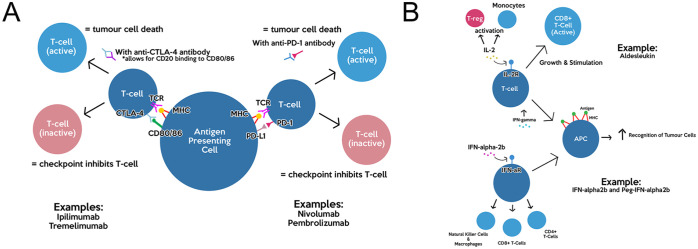
This class of immune checkpoint inhibitors focuses on preventing the binding of CD80/CD86 ligands on the cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) immune checkpoint receptor. 45 (Figure 1A) Once T-cells are partially activated by the binding of their receptors to tumor-associated-antigens (TAAs) presented on the major histocompatibility complex (MHC) of antigen-presenting cells (APC), the ligands on the APC can either bind to the CD28 receptor or CTLA-4. CTLA-4 are typically found on regulatory T-cells (Treg), resulting in inactivation of T-cells and an escape from the immune response. 45 Thus, anti-CTLA-4 antibodies prevent the binding of the ligands to CTLA-4 receptors, resulting in higher CD28 binding and thus higher T-cell activation. 45
Figure 1.
Summary of immunotherapy mechanism of action: (A) mechanism and immune targets of anti-CTLA-4 antibodies and anti-PD-1 antibodies and (B) mechanisms of cytokine drugs Aldesleukin and IFN-α2b.
Ipilimumab
This drug is an anti-CTLA-4 monoclonal antibody (IgG1 class), which as mentioned above, prevents the ability for tumor cells to escape the immune response. 46 Once administered, ipilimumab can block CTLA-4 and allow for cytotoxic T-cell activation to eliminate tumors. In a phase III study comparing ipilimumab as an adjuvant (10 mg/kg dose) to placebo in patients with resected stage III melanoma, median 5-year OS rate was found to be 65.4% as compared to 54.4% in placebo. 47 Additionally, 5-year recurrence-free survival was found to be 40.8% versus 30.3%. 47 In another phase III study evaluating the combination of ipilimumab with the gp100 vaccine versus respective monotherapies, ipilimumab given alone (dose of 3 mg/kg) was shown to increase survival in HLA-A*0201-positive patients with metastatic melanoma when compared to gp100 alone at the 1-year and 2-year mark showing 45.6% and 23.5% survival, respectively. 46 Common adverse effects (AE) found within phase II studies were related to the breakage of immune tolerance resulting from the mechanism of action. The most serious adverse effects (grade 3 and grade 4) observed with ipilimumab use were dermatitis and colitis. 46 As an adjuvant to resection at 10 mg/kg, ipilimumab was associated with 54.1% of patients experiencing grade 3 or 4 AEs. 47 This toxicity rate was lower when given at 3 mg/kg in another study at 10% to 15%. 46 Lower grade and more common side effects as observed in a phase III study were nausea, diarrhea, and an increased in liver enzyme levels. 48 Recently, in long-term studies, it was found that the 5-year OS of patients given ipilimumab monotherapy (doses 0.3-10 mg/kg) was 12.3% to 28.4% in all patients (including treatment-naive). 49 In retreated patients given 10 mg/kg ipilimumab, it was observed that 34% experienced a grade 3 or 4 AE (including 3.8% dermatologic, 3.8% gastrointestinal, and 3.8% hepatic). 49
Tremelimumab
Similar to ipilimumab, this drug is an anti-CTLA4 antibody. However, it is of the class IgG2. 50 In a randomized phase III clinical trial, tremelimumab was given as a single-agent therapy (dosage of 15 mg/kg every 90 days) in comparison to DTIC. The results of this study showed a modest response rate but no statistically significant advantage for tremelimumab administration over standard of care chemotherapy (OS: 12.6 months vs 10.7 months; response rate: 10.7% vs 9.8%). 51 Similar grade 3 and grade 4 AEs were found for tremelimumab treatment as compared to ipilimumab; this is likely because they share the same mechanism of action. 51 Tremelimumab is not FDA-approved for use in treating metastatic melanoma.
Anti-PD-1 antibodies
This class of immune checkpoint inhibitors focuses on blocking the binding of the neoplastic programmed-death-L (PD-L) ligand found on APC to the PD-1 immune checkpoint receptor (Figure 1A). It achieves this by using a monoclonal antibody that binds to PD-1. If this intervention is not used, the binding of PD-1 to PD-L results in immunosuppression via lowered proliferation of T-cells and their apoptosis.52,53
Nivolumab
This drug is an IgG4 antibody that works toward blocking the PD-1/PD-L pathway via blocking the binding of PD-1 to PD-L antigens that are expressed on APC that digest neoplastic tumor cells. 52 In a randomized, double-blind phase III trial evaluating combination ipilimumab and nivolumab to their respective monotherapies in unresectable metastatic melanoma patients, single-agent nivolumab was shown to have higher progression-free survival (6.9 months vs 2.9 months) and ORR (43.7% vs 19.0%) compared to single-agent ipilimumab as the standard of care. 54 In this same phase III study, 82.1% of patients taking nivolumab experienced any AE with 16.3% experiencing an AE of grade 3 or grade 4 severity. 54 Moreover, both of these measures were lower than the patient cohort treated with ipilimumab alone (86.2% total with 27.3% grade 3 or grade 4). 54 Thus, nivolumab may be slightly more tolerable for patients. In another study, nivolumab monotherapy was compared to DTIC in patients with untreated BRAF-negative melanoma. The results of this study indicated a higher 1-year OS rate, ORR, and PFS in the nivolumab arm (1-year OS rate: 72.9% vs 42.1%; ORR: 40% vs 13.9%; PFS: 5.1 months vs 2.1 months). 55 Nivolumab demonstrated a similar safety profile to DTIC, however, with less frequent severe AE (74.3% total with 11.7% grade or 4% vs 75.6% with 17.6% grade 3 or 4). 55
Pembrolizumab
Similar to nivolumab, pembrolizumab is an IgG4 antibody that blocks the PD-1/PD-L checkpoint. It was the first FDA-approved PD-1 antibody for advanced melanoma in 2014, which led to the approval of nivolumab shortly after. 56 In a phase III trial comparing pembrolizumab (dose of 10 mg/kg every 2 weeks) to standard of care ipilimumab, it was found that 6-month progression-free survival was higher for pembrolizumab at 47.3% versus 26.5% for ipilimumab. 57 Furthermore, 12-month OS rate and response rates were also higher in the pembrolizumab group at 74.1% and 33.7% respectively versus 58.2% and 11.9% for ipilimumab. 57 When comparing AEs, modestly severe side effects such as fatigue, nausea, and rash were common in both pembrolizumab and ipilimumab treatments. However, grade 3 and 4 AEs were not as frequently seen in pembrolizumab treatment. 57 In a recent follow-up to a phase 1b trial, it was found that the 5-year OS of patients given pembrolizumab (doses 2 mg/kg or 10 mg/kg) was 34%, 58 which is a significantly higher amount than the 12.3% to 28.4% 5-year OS rate of ipilimumab as previously mentioned in another study. 49
Cytokines
Aldesleukin
As one of the earliest immunotherapies, this drug was approved in 1998 for use in metastatic melanoma. 59 Aldesleukin contains a high-dose recombinant interleukin-2 cytokine which is administered to activate monocytes and cytotoxic T-cells. 60 IL-2 has also been shown to increase expression of interferon-gamma (IFN-γ) which leads to increased expression of MHC molecules on APC, resulting in the ability for T-cells to recognize tumors at a higher capacity 60 (Figure 1B). However, there may be the possibility for paradoxical activity as IL-2 has also been shown to promote regulatory T-cell expansion that results in protumor activity via cytokine concentration reductions. 60 It is typically given in intravenous (IV) doses of 600 000 IU/kg every 8 hours. 60 In phase III studies evaluating the efficacy of aldesleukin, it was found that the response rate was 16% and the OS was 11.4 months. 61 In phase II and phase III trials, common AEs of aldesleukin have been nausea, fatigue, fever, and chills. 61 More severe AEs seen have been shock, sepsis, and hepatotoxicity. 62 Expanding upon the latter, when patients are given a high dose of aldesleukin, mild liver enzyme serum elevation, cholestasis, and rare liver injury are observed. 62 Thus, due to the potential for significant toxicity reported with aldesleukin, it is not typically used as a first-line therapy for metastatic melanoma due to the emergence of checkpoint inhibitors and targeted therapies that are more tolerable. 61
Interferon alfa-2b and pegylated interferon alfa-2b
These 2 drugs are cytokines that directly activate signaling pathways for anti-tumor activity, activate CD4+ and CD8+ T-cells along with natural killer cells, and increase expression of class I MHC molecules to assist in T-cell migration to tumor cells 63 (Figure 1B). Furthermore, in-vitro studies showed that deficiency in interferon-alfa (IFN-alfa) led to resumption of melanoma progression despite initial senescence activated by oncogenes. 64 In the late 1990s, interferon alfa-2b (IFNα-2b) was evaluated under phase III clinical trials. The results typically showed that low dose IFNα-2b was not effective, whereas high dose IFNα-2b showed improvement in OS but was poorly tolerated with significant toxicity (e.g. grade 3 or 4 fatigue up to 24%, liver toxicity of 29%).65,66 Although IFNα-2b was approved for malignant melanoma in 1995, it is not typically considered for use due to its high toxicity and modest improvement to survival.66,67 More recently, pegylated interferon alfa-2b (PEG-IFNα-2b) was discovered as a covalent conjugate of IFNα-2b and has been evaluated as an adjuvant therapy to surgical removal of melanoma. 67 In a longitudinal phase III trial evaluating adjuvant PEG-IFNα-2b in patients with resected stage III metastatic melanoma, PEG-IFNα-2b treatment groups (initial dose of 6 µg/kg/week for 8 weeks, then maintenance 3 µg/kg/week for 5 years) showed statistically significant 45.6% 4-year recurrence-free survival (RFS) versus 38.9% in patients with no treatment. 65 However, OS was not statistically different between the 2 groups. 65 An interesting result from this study is that a significant increase in RFS (absolute difference of 12.3%) and decrease in death rate was seen in patients with microscopic nodal disease or tumor involvement limited to 1 lymph node, suggesting that adjuvant PEG-IFNα-2b intervention may provide higher benefits in proportion to risks in early stage-III metastatic melanoma with low tumor volume.63,65 AEs found within the aforementioned study and other phase II/III trials were fatigue (grade 3-4 15%), depression (grade 3 6%), and liver toxicity (grade 3 10%) when compared to patients given no treatment.65,68 Despite the considerable toxicity seen with PEG-IFNα-2b, this treatment seems to be more tolerable than IFNα-2b and can be reversed with treatment interruption. PEG-IFNα-2b became indicated for use in metastatic melanoma as an adjuvant in 2011 by the FDA. 68
Vaccines and oncolytic virotherapy
Vaccines are an early form of immunotherapy in cancer with the first vaccine being created in 1990. 69 They rely on the adaptive immune response where, depending on the type of antigen, they activate certain immune cells like CD8+ T-cells, CD4+ T-cells, or B-cells in order to prevent tumor cells from escaping immune recognition. 69 Vaccines against melanoma and other cancers typically target neoantigens, including tumor-associated antigens (TAAs) and tumor-specific-antigens (TSAs) that are expressed on tumor cells. 69 Therapeutic anti-cancer vaccines can be categorized into broad classes such as autologous, allogeneic, and peptide. Autologous vaccines utilize neoantigens from the specific tumor of the patient, thus minimizing the risk for graft versus host disease. It has been observed that there is antigenic diversity in tumors present for patients, making autologous vaccines effective. 69 On the other hand, allogeneic vaccines utilize the neoantigens present on stock human melanoma cell lines such as A375 and G361. Allogeneic vaccines can be produced prior to patient need, however, there is a higher risk for graft versus host disease. 69 Peptide vaccines such as gp100 are easily produced for patients and utilize TAAs in the form of peptides normally recognized by T-cells on MHC proteins. 69 Alongside traditional vaccine types, vaccines including oncolytic viruses have shown promising anti-cancer activity in both preclinical studies and clinical trials. These viruses are used as novel anti-tumor agents due to their ability to selectively replicate within tumor tissue and lyse cancer cells. 70 Furthermore, they have been shown to disrupt the tumor microenvironment and upregulate natural killer (NK) cell production, leading to an increased immune response. 70
T-VEC
Talimogene laherparepvec (T-VEC) is a vaccine including a modified herpes simplex type-1 virus that serves to act as an oncolytic virus against melanoma. T-VEC is a localized therapy that is injected into melanoma lesions and lyses tumor cells by selectively replicating within them, leading to the release of tumor-derived antigens (TDAs). 71 Since T-VEC expresses GM-CSF, a factor that promotes the maturation of dendritic cells, this promotes an immune cascade where dendritic cells activate T-cells through the presentation of processed TDAs. 71 In a phase III study of T-VEC on patients with advanced melanoma, it was observed that patients given T-VEC had a significantly higher DRR (durable response rate; defined as the rate of complete plus partial responses lasting greater than 6 months and beginning within the first 12 months) than control group patients given GM-CSF. 71 In addition, patients given T-VEC had an ORR of 26% as compared to patients 6% in the GM-CSF arm. 71 T-VEC was well tolerated in this study with only 36% of patients experiencing grade 3+ AEs. 71 The promising results of T-VEC in clinical trials led to its approval as an immunotherapy against unresectable metastatic melanoma in 2015. 72
Recent therapeutic vaccine developments
Due to the promising results seen with oncolytic viruses, studies are being conducted on future vaccines involving them. In particular, a study by Capasso et al 73 evaluated the efficacy of oncolytic adenovirus against melanoma. Due to the limitations in targeting and of transduction in current oncolytic viruses that express tumor-specific antigens, patient-derived tumor-specific peptides were instead loaded onto the viral capsid, allowing for co-delivery of the virus and tumor-specific epitopes. When evaluated in in-vivo models of murine melanoma and xenografted human tumors, it was observed that these modified oncolytic adenovirus significantly decreased tumor growth as compared to controls and non-modified oncolytic adenovirus vaccine formulations, while also synergistically increasing the immune response. 73
Targeted Therapies
BRAF/MEK Inhibitors
These drugs target the Mitogen-Activated Protein Kinase (MAPK)/Extracellular Signal-Regulated Kinase (ERK) pathway (Figure 2) that is involved in melanoma progression via the ultimate activation of MAPKs involved in cell proliferation and a variety of other cellular processes. 74 MAPKs include a class of ERKs that are involved in cell cycle regulation and apoptosis. In some cases of melanoma, the V-Raf Sarcoma Viral Oncogene Homolog B (BRAF) V600E/K mutation is present which results in overexpression of the intermediate MAPK enzyme BRAF. Other mutations in this pathway target the NRAS or MEK1/2 genes. 74 In general, mutations in the MAPK pathway have been associated with genomic instability and excessive proliferation of cancer cells via hyperactivation. 17 Typical BRAF inhibitors (BRAFi) are type I, where they target the ATP-binding pocket of the enzyme to stabilize it in its active form. 74 MEK inhibitors typically allosterically inactivate the enzyme and are used not only in MEK mutated melanoma, but in BRAF-mutated and NRAS-mutated states as well. The use of BRAF inhibitors has shown resistance in some cases, which is proposed to be due to paradoxical MAPK activity that is likely mediated by genetic or epigenetic causes.74,75 In cases of resistance, a combination of BRAF/MEK inhibitors has shown potential to provide sustained efficacy. 75 A summary of studied targeted therapies for use in metastatic melanoma, including BRAF/MEK inhibitors, is described in Table 3.
Figure 2.
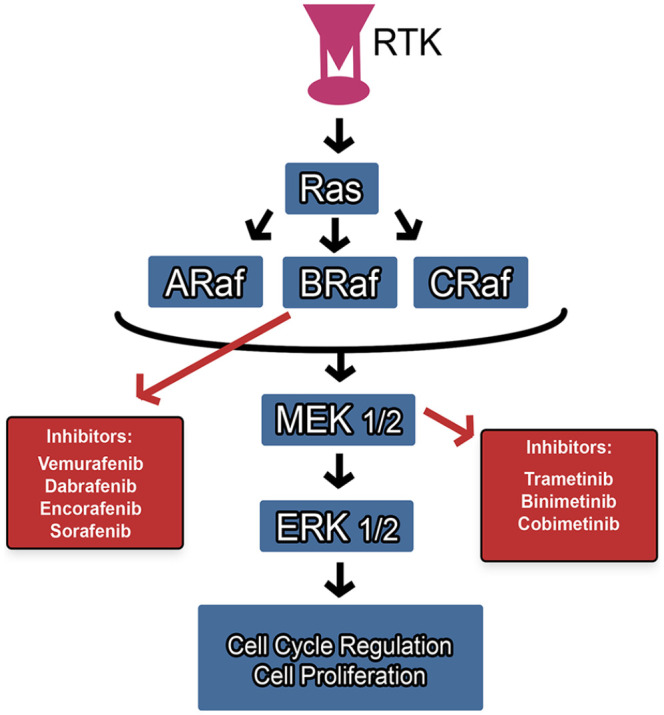
Molecular pathway and targets of BRAF/MEK inhibitors.
Table 3.
Summary of Targeted Therapies.
| Reagent | Cancers | Class | FDA-approved indications for melanoma | Observed efficacy |
|---|---|---|---|---|
| Vemurafenib | • Melanoma | BRAF Inhibitor | BRAF-V600E/V600K mutated advanced melanoma (2011) | 84% 6-month OS rate; 48% response rate (phase III) 74 |
| Trametinib | • Melanoma | MEK inhibitor | BRAF-V600E/V600K mutated advanced melanoma (2013) | 81% 6-month OS rate; 4.8 months PFS (phase III) 77 |
| • Lung cancer | ||||
| • Thyroid cancer | ||||
| Dabrafenib | • Melanoma | BRAF inhibitor | BRAF-V600E/V600K mutated advanced melanoma (2013) | 18.2 months OS; 81 10.5 months PFS, 81 12% 5-year PFS rate; 82 24% 5-year OS rate 82 (phase III) |
| • Lung cancer | ||||
| Cobimetinib | • Melanoma | MEK inhibitor | Combination with vemurafenib for BRAF-V600E/V600K mutated melanoma (2015) | 22.3 months OS; (phase III), 9.9 months PFS (combination with vemurafenib) (phase III) 83 |
| Encorafenib | • Melanoma | BRAF inhibitor | Combination with binimetinib for BRAF V600E/V600K mutated melanoma (2018) | 23.5 months median OS; 87 9.6 months PFS (phase III) 86 |
| • Colorectal cancer | ||||
| Binimetinib | • Melanoma | MEK inhibitor | Combination with encorafenib for BRAF V600E/V600K mutated melanoma (2018) | 11 months median OS; 2.5 months PFS; 15.2% ORR (phase III) 89 |
| Sorafenib | • Melanoma | BRAF inhibitor | Not approved | 2.8% response rate (phase II) 91 |
| • Liver cancer | ||||
| • Thyroid cancer | ||||
| • Kidney cancer |
Vemurafenib
This drug acts as a type I BRAFi, typically used in cases where metastatic melanoma presents with a BRAF V600 mutation. In a phase III study comparing vemurafenib (960 mg oral twice daily) to dacarbazine (1000 mg/m2 every 3 weeks), vemurafenib was associated with a 6-month OS rate of 84% (compared to 64% for DTIC), 45% response rate (compared to 5% for DTIC), 63% reduction in death, and 74% reduction in tumor progression. 76 In addition to its increased efficacy in BRAF-mutated patients, vemurafenib is generally well-tolerated, however, some AEs observed in phase III trials were grade 2 arthralgia, grade 2 rash, grade 2 fatigue, and grade 3 cutaneous squamous cell-carcinoma. 76 However, 38% of patients required dose interruption due to AEs as compared to 16% of patients that were given DTIC. Class-specific adverse events such as cutaneous squamous cell-carcinoma are suggested to be due to the paradoxical effect of BRAF inhibitors as described previously. 75 Vemurafenib was approved by the FDA as one of the earliest targeted therapies for BRAF V600 positive unresectable or metastatic melanoma in 2011. 75
Trametinib
The mechanism of action of this drug is to inhibit the mitogen-activated-protein kinases MEK1/MEK2. In pathological states, MEK1/MEK2 are intermediate kinases activated by BRAF, leading to downstream activation of enzymes in the MAP kinase (MAPK) pathway involved in tumor proliferation. 77 In a randomized phase III clinical trial comparing trametinib to standard of care chemotherapy (DTIC or paclitaxel) in BRAF-mutated melanoma patients, patients given trametinib had a median progression-free survival (PFS) of 4.8 months and 81% 6-month OS rate whereas patients given chemotherapy had 1.5 months PFS and 67% OS rate. 77 Common AEs seen with trametinib treatment were grade 2+ rash (57% of patients), diarrhea, pyrexia, fatigue, nausea, impaired ocular function (including reversible retinopathy), and impaired cardiac function (including hypertension and 7% of patients with ventricular dysfunction). 77 Similar to vemurafenib, patients given trametinib required significant dose interruption (35%) in this trial as compared to chemotherapy. 77 Trametinib was approved by the FDA for single-agent use in advanced melanoma (BRAF V600E or BRAF V600F mutations) in 2013. 78 Additionally, the combination of trametinib and dabrafenib was recently approved by the FDA in 2018 for use in metastatic melanoma as an adjuvant treatment for BRAF V600E or V600F mutations. 79
Dabrafenib
This drug is a type I BRAFi which provides therapeutic efficacy for metastatic melanoma by inhibiting cell proliferation as described previously. In a randomized phase III clinical trial comparing dabrafenib monotherapy to dacarbazine monotherapy, results showed that the median PFS for dabrafenib was higher than DTIC at 5.1 months versus 2.7. 80 Median OS was not reached in this trial, however, in the results of a 3-month follow-up to this study, it was found to be 18.2 months for dabrafenib and 15.6 months for DTIC. 81 In this update study, PFS was also re-evaluated to be 10.5 months versus 9.9 months for dabrafenib and DTIC respectively. 81 Common AEs found with dabrafenib treatment were hyperkeratosis, headache, arthralgia, and pyrexia. 81 Higher grade AEs were cutaneous squamous cell carcinoma (10%) and pyrexia (5%). 81 Long-term outcomes from phase II and phase III trials recently conducted confirmed survival benefits for patients with BRAF V600E mutated metastatic melanoma. 5-year PFS rate and OS rate in previous phase II patients (treatment naïve or previously treated) were found to be 11% and 20% respectively, whereas in previous phase III patients (previously untreated unresectable BRAF V600E mutant metastatic melanoma), they were found to be 12% and 24% respectively (as compared to DTIC where all patients progressed and had a 24% 5-year OS rate). 82 Dabrafenib was approved as a single-agent therapy for BRAF-mutated metastatic melanoma in 2013, 83 and it was also recently approved in combination with trametinib in 2018. 79
Cobimetinib
Similar to trametinib, this drug is a selective MEK inhibitor. It has been approved for use in BRAF-mutated metastatic melanoma in combination with vemurafenib in 2015. 84 In a randomized phase III clinical trial comparing cobimetinib in combination with vemurafenib (C + V) to vemurafenib alone, it was found that median PFS was higher in the C + V treatment at 9.9 months versus 6.2 months. 54 Furthermore, median OS was found to be 22.3 months in the combination treatment versus 17.4 months in vemurafenib monotherapy. 54 AEs seen under the combination treatment were found to be rash, diarrhea, fatigue, elevated creatine kinase, decreased ejection fraction, and retinopathy. 54 Grade 3 AEs were elevated in combination treatment by 12% in combination treatment versus vemurafenib monotherapy (71% combination versus 59% vemurafenib monotherapy). 54
Encorafenib
Encorafenib is a type I BRAFi which has shown positive effects in BRAF V600E mutated metastatic melanoma. Preclinical studies have been done on mouse xenografts of the A375 melanoma cell line, as well as phase I and phase II clinical studies ensuring safety and efficacy. 18 Recommended dose of encorafenib is 300 mg once daily as of phase II studies. 18 It has been suggested by a study that encorafenib may have higher safety than standard BRAF inhibitors vemurafenib and dabrafenib due to its lowered off-target effects and limited MAPK paradoxical activity. 85 Furthermore, it has been shown to have higher on-target effects and a longer half-life at 30 hours than dabrafenib (2 hours) and vemurafenib (0.5 hours), which has contributed to its observed higher efficacy. 85 Encorafenib phase III clinical trials (COLUMBUS Part I and Part II) evaluating its use in combination with binimetinib or as a monotherapy have recently concluded. In those trials, median PFS for the combination treatment was 14.9 months versus 9.6 months and 7.3 months for encorafenib alone or vemurafenib alone respectively. 86 In COLUBUS Part II, median OS was 33.6 months, 23.5 months, and 16.9 months for combination, encorafenib monotherapy, and vemurafenib monotherapy, respectively at a median follow-up of 48.6 months. 87 Thus, encorafenib monotherapy may provide improved efficacy over vemurafenib alone and in addition, enhances survival when combined with binimetinib. In 2018, the FDA approved the combination of encorafenib and binimetinib for use in BRAF-mutated metastatic melanoma. 88
Binimetinib
Binimetinib is a selective MEK (MEK1/MEK2) inhibitor that has been evaluated for use in BRAF-mutated and NRAS-mutated metastatic melanoma. Similar to BRAF mutations described above, NRAS mutations (specifically NRAS Q61) cause overactivation of the NRAS protein leading to hyperactivity of the MAPK pathway. 89 As shown in phase I and II clinical trials, binimetinib is the first targeted therapy to show activity against NRAS-mutated metastatic melanoma. Specifically, ORR was achieved in both BRAF-mutated patients (19.5%) and NRAS-mutated patients (20%) in phase II trials. 90 In the NEMO study, a randomized phase III clinical trial evaluating binimetinib 45 mg against DTIC 1000 mg/m2, binimetinib showed higher PFS (2.5 months vs 1.5 months), higher median OS (11.0 months vs 10.1 months), and a significantly higher ORR (15.2% vs 6.8%). 90 Common AEs reported were diarrhea, rash, fatigue, nausea, peripheral edema, and acneiform dermatitis. Serious AEs were retinal vein occlusion, decreased ejection fraction, and increased creatine phosphokinase. 90 In 2018, the FDA approved the combination of encorafenib and binimetinib for use in BRAF-mutated metastatic melanoma. 88
Sorafenib
Similar to previously discussed BRAFi, Sorafenib is a multikinase inhibitor that targets BRAF to inhibit the MAPK pathway. Specifically, it has been shown to inhibit activation of pMEK and pERK in mouse thyroid cancer xenograft models. 91 A phase II clinical trial evaluating the use of sorafenib monotherapy has shown poor results with a response rate of 2.8% and disease control rate of 11.1%. 92 These results may be explained by the low specificity of sorafenib as a BRAF inhibitor. 92 Sorafenib is not currently approved for use in melanoma.
Combination Therapies
Polychemotherapy
DTIC-based chemotherapies
In 2009, a phase I/II clinical trial was conducted evaluating the use of the Cisplatin + Paclitaxel + Dacarbazine (CPD) combination in chemotherapy-naive metastatic melanoma patients. An overall response rate of 41% and median OS of 4.3 months was observed. 93 However, dose-limiting AEs were seen in the form of myelosuppression and neuropathy with the combination. 93 In a real-world retrospective analysis, this combination was looked at in a sequential application against metastatic mucosal melanoma of the sinuses. 94 The study was designed where a combination of C + P was given sequentially after DTIC administration to 7 patients. The results of this study indicate an ORR of 14.3% and an OS of 12.5 months, suggesting this treatment plan has a slight improvement over the traditional prognosis when patients are given DTIC as a single-agent (a typical OS of 9.2 months is seen in metastatic mucosal melanoma patients). 94 However, the safety profile of this combination demonstrated some toxicity as 28.6% of patients had a grade 3 or 4 AE such as leukocytopenia and neutropenia. 94
Other chemotherapies
In 2011, a phase II trial was conducted evaluating the combination of nab-paclitaxel with carboplatin (P + CB) against chemotherapy naïve (CN) or previously treated (PT) patients with unresectable stage IV melanoma. In 73 patients, median PFS and median OS were found to be 4.5 months and 11.1 months, respectively in chemotherapy-naive patients. 95 Toxicities seen within this trial for CN patients were in the form of neutropenia (28%), fatigue (3%), nausea (3%), thrombocytopenia (5%), and leukopenia (3%). The most common grade 3 or 4 AE was neutropenia in both CN and PT patients (28% and 41%, respectively). 95 Although this study did not directly compare single-agent use to combinatory use, previous literature suggests this combination of P + CB may provide a modest improvement in anti-melanoma efficacy. 95 Similarly, BEAM, a phase II clinical trial evaluating P + CB + Bevacizumab (B) was conducted. B is an anti-angiogenic drug that targets the vascular endothelial growth factor (VEGF) protein to prevent tumor progression. In this study, the overall response rate, median OS, and PFS were all higher than in the P + CB + B arm versus the P + CB arm (PFS: 4.4 vs 2.7 months; OS: 12.3 months vs 9.2 months; overall response rate: 25.5% vs 16.4%). 96 However, PFS difference was shown to not be statistically significant. 96 The authors suggested conducting a phase III clinical trial to determine whether this combination was beneficial. 96 As previously mentioned, DTIC-resistant metastatic melanoma is difficult to treat, and thus a study was completed on the combination of cisplatin and carboplatin (C + CB). When 15 patients were treated, an overall response rate of 26.4% was achieved alongside a median OS of 12.5 months. 34 Moreover, in this study, C + CB was generally well-tolerated without discontinuation in 14 of 15 patients, although AEs such as leukopenia/thrombocytopenia (grade 1-4), anemia (grade 1-3), and gastrointestinal issues (grade 1-3) were present. 34
Polyimmunotherapy
Ipilimumab-based combinations
Ipilimumab has become the single-agent immunotherapy standard of care for metastatic melanoma in recent years. However, combination therapies with ipilimumab are being researched to further increase efficacy in various forms of metastatic melanoma. One of the emerging ipilimumab-based combinations is ipilimumab with nivolumab (I + N). The evaluation of this model was motivated by murine preclinical models where blocking both the PD-1 and CTLA-4 checkpoint molecules resulted in synergistic anti-tumor activity. 97 This synergistic activity may be a result of the inhibitory effect of PD-1 binding including dephosphorylation of CD28 components 53 In 2015, a randomized, double-blind, phase III clinical trial showed encouraging results when comparing I + N (3 mg/kg I + 1 mg/kg N for 4 doses, followed by 3 mg/kg N every 2 weeks) to N alone (3 mg/kg) and I alone (3 mg/kg). The combination treatment showed a total median PFS of 11.5 months, including a subset PFS of 11.2 months for PD-L1-negative patients. 54 Both these results were significantly higher than the 2 monotherapy arms. However, no significant difference was seen in patients with PD-L1-positive condition. Furthermore, toxicities were more frequently observed with combination treatment as 55% of patients experienced a grade 3 or 4 AE. 54 These AEs were found to be diarrhea, colitis, and increased liver enzymes. These results suggest I + N may be beneficial when negative PD-L1 tumor expression is seen, however, further research should be done in determining OS in these patients. 54 While research is continued, the FDA approved I + N for use in BRAF-mutated or unresectable metastatic melanoma in 2015. I + N was the first immunotherapy combination to be approved for use in melanoma. 98
Vaccine/oncolytic virotherapy-based combinations
The use of immune checkpoint inhibitors along with cancer vaccines has shown promising results in preclinical models. Thus, this combination has been evaluated in clinical trials. However, results have been mixed as when the peptide vaccine gp100 was combined with high dose IL-2, there was a higher median OS as compared to IL-2 alone (17.8 months vs 11.1 months), but this was only marginally significant (P = .06). 69 Similarly, anti-CTLA-4 antibody ipilimumab was evaluated with peptide vaccine gp100, was described earlier in this review. However, the median OS was 10 months in combination, which was only significantly higher than gp100 alone (6.4 months) and effectively the same as ipilimumab monotherapy (10.1 months). 46 In the past few years, preclinical models supported the beneficial effects of adding localized oncolytic virotherapy with immune checkpoint inhibition, particularly involving improved response to PD-1 inhibition.99 -101 Promising results were seen in a phase II clinical trial combining ipilimumab (3 mg/kg) with T-VEC in which it was observed that the combination arm provided a higher ORR of 39% as compared to 19% in the ipilimumab monotherapy group. 102 A similar phase 1b trial was conducted combining T-VEC with anti-PD-1 antibody pembrolizumab (200 mg every 2 weeks) in 21 patients (11 previously treated). About 61.9% overall response rate was seen in the combination arm, however, median OS and PFS were not reached as of the last follow-up. 103 About 52% of patients experienced a grade 3 or 4 AE and no dose-limiting toxicities were observed. 103 Interestingly, this initial trial demonstrated that T-VEC administration increased circulating levels of CD8+ and CD4+ T-cells which may help overcome primary resistance to PD-1 therapy, resulting in an improved response to the immune checkpoint inhibitor. In addition, there was a higher amount of PD-1 positive T-cells after T-VEC administration which may inhibit the oncolysis ability of the vaccine. Thus, the PD-1 blockade by pembrolizumab may benefit the oncolytic activity of T-VEC while the increase in CD8+ and CD4+ T-cells by the latter may increase recruitment to tumors and a systemic effect. 103 As a result, this combination may overcome resistances to both treatments as single-agent. A phase III clinical trial for T-VEC and pembrolizumab combination is currently in progress (NCT02263508). 104 Similar to T-VEC, CAVATAK is another oncolytic virus that was evaluated in a phase II clinical trial in combination with ipilimumab and pembrolizumab, showing favorable ORRs with minimal toxicity. 102
Targeted Therapy Combinations
BRAF-inhibitor-based combinations
These combination therapies are targeted toward treating patients with BRAF V600E/K-mutated metastatic melanoma. The combination of BRAFi with MEK inhibitors has shown to be particularly fruitful as it can overcome resistance to treatment in the form of paradoxical MAPK activity with BRAFi monotherapy. 75 One such combination, dabrafenib with trametinib (D + T), has shown positive results as an adjuvant and primary treatment in unresectable melanoma. As an adjuvant in the COMBI-AD study, D + T showed a median 2.8-year PFS of 58% and OS rate of 86%, which were both higher than placebo. 105 As a primary treatment, D + T showed a 3-year median PFS of 22% and 3-year OS rate of 44%, which were both higher than the monotherapy arm. 106 In the latter study, it was also seen that D + T was most effective in patients with normal lactate dehydrogenase, a marker of severe metastatic melanoma.105,106 In both trials, AE profiles were comparable to those seen with monotherapy.105,106 In 2018, D + T was approved by the FDA for use in BRAF-V600E or V600K mutation-positive metastatic melanoma. 79
Another combination studied in BRAF-mutated melanoma is vemurafenib with cobimetinib (V + C). In a phase III trial, it was found that V + C had a significantly higher PFS and 9-month OS rate when compared to control (V + placebo) (PFS; 9.9 months OS; 81%). 107 The safety profile of V + C demonstrated it was generally well-tolerated with majority grade 1 or grade 2 reactions, however, when compared to monotherapy, the combination had a significantly higher frequency of AEs such as such as nausea, fatigue, and diarrhea. In addition, MEK inhibitor-class specific AEs such as elevated creatine kinase and photosensitivity reactions were slightly higher in the combination treatment. 107 In 2015, the FDA approved the combination of V + C for use in unresectable or metastatic melanoma. 84 In 2020, igG1 monoclonal antibody atezolizumab was combined with V + C (A + V + C treatment) in a phase III trial evaluating the combinatorial use of these drugs on BRAF V600E mutation-positive advanced/metastatic melanoma. At 18.9 months follow-up, PFS was found to be higher in the A + V + C combination group versus V + C (15.1 months vs 10.6 months). 108 This combination was tolerable showing a consistent safety profile to V + C despite patients experiencing higher amounts of blood creatinine phosphokinase levels (51.3% vs 44.8%), arthralgia (39.1% vs 28.1%), and pyrexia (38.7% vs 27.4%). 108 The results of this study may suggest a tolerable and efficacious effect on BRAF V600E mutation positive melanoma when BRAF/MEK inhibitors are combined with immune checkpoint inhibitors.
Encorafenib-based combinations have also been a topic of interest in the past few years as trials have been conducted on encorafenib with the MEK-inhibitor binimetinib (E + B). In the COLUMBUS clinical trial, E + B was compared to E and V monotherapy. In this trial, median PFS and overall response rate for E + B was 14.9 months and 63% respectively. 86 Both of these results were higher than E or V alone. In an 18-month follow-up to this study, OS was found to be 33.6 months for E + B treatment as compared to 16.9 months for V alone. 87 Common grade 3 or 4 AEs seen with this combination treatment were increased γ-glutamyltransferase, increased creatine phosphokinase, and hypertension. 87 The results of these studies led to the 2018 FDA approval of E + B as indicated for unresectable metastatic melanoma. 88 Currently, a phase I/II open-label clinical trial has made progress in evaluating the combination of E + B + anti-PD-1 antibody pembrolizumab (P) in unresectable BRAF V600-mutation positive metastatic melanoma. 109 Results for this study are expected to be available in 2023 (NCT02902042).
Biochemotherapy
An emerging field as of late has been biochemotherapy, where researchers are evaluating the clinical efficacy of combining chemotherapies, immunotherapies, and in some cases, targeted therapies. In this section, we discuss a few biochemotherapy regimens that were previously studied.
DTIC-based biochemotherapies
The most well-studied combination of dacarbazine with immunotherapies has been DTIC with ipilimumab (DTIC + I) (850 mg/m2 DTIC + 10 mg/kg ipilimumab). Through phase III clinical trials, DTIC + I has been compared to DTIC alone (with placebo). In one study, OS was significantly longer for combination treatment at 11.2 months versus 9.1 months with DTIC monotherapy. 110 However, there was a higher proportion of grade 3 or 4 AE with 56.3% of patients reporting at least 1 compared with 27.5% with DTIC combined with placebo. Although, the level of AEs observed in this study were consistent with the amount seen in previous trials on ipilimumab (10 mg/kg).47,110 Common severe AEs seen were diarrhea, colitis, and impaired hepatic function as indicated by elevated liver enzymes. 110 Thus, this combination may serve to provide a slight enhancement to efficacy with no significant impact on safety as compared to ipilimumab monotherapy. In a 5-year follow-up study, benefits to long-term survival were encouraging with combination treatment as it was seen that survival rate was significantly higher in the combination group at 18.2% versus 8.8% in the DTIC single-agent treatment group. 111 In this follow-up, immune-related AE were manageable and exclusive to the skin. 111
Similarly, another phase II clinical trial evaluated the combination of DTIC with sorafenib (DTIC + S) compared to DTIC alone against BRAF mutated metastatic melanoma. Within a cohort of 101 patients, it was found that patients in the DTIC + S arm demonstrated a higher PFS at 21.1 weeks versus 11.7 weeks for the DTIC monotherapy arm. 112 However, there was no significant difference for OS between the two arms (DTIC + S; 45.6 weeks, DTIC + placebo; 51.3 weeks). 112 There was a slightly elevated level of toxicities in combination as grade 3 or 4 AEs were observed in 69% of DTIC + S treatment group patients while 50% were seen in DTIC + placebo patients. Common AEs observed were in the form of fatigue, nausea, and hematologic events. CNS hemorrhage was observed in DTIC + S patients but not in DTIC monotherapy patients. 112
DTIC combined with interferon-2 (IFN) has also been well-studied through phase III clinical studies. In a 2016 meta-analysis of 8 studies, the overall response rate of the DTIC + IFN group was found to be significantly higher when compared to the DTIC single-agent group. 113 In this same meta-analysis, it was found that grade 3 hematologic AEs were significantly higher in DTIC + IFN treatment and that there was no significant difference in fatigue and nausea between the 2 arms. 113
Other biochemotherapies
A treatment regimen of carboplatin + paclitaxel + sorafenib (C + P + S) was studied in a phase III clinical trial; however, there was no significant improvement in PFS or OS in the C + P + S treatment group over C + P alone. 114 In another study, which involved a phase II trial on unresected chemotherapy-naive metastatic melanoma patients, C + P + I (ipilimumab) was used and showed positive results with a median OS of 16.2 months and a 3-year OS rate of 36.7%. 115 C + P + I was very well tolerated as grade 3 or 4 AEs were only seen in <10% of patients and in the form of diarrhea, neutropenia, and thrombocytopenia. 115 An interesting outcome of this study was that non-responders to treatment had higher systemic inflammation at baseline and an increase in the CD8+ PD-1+ T-cell population. This suggests that resistance to treatment may be due to immune exhaustion and that PD-1 checkpoint inhibitors may be needed in addition to CTLA-4 inhibitors. 115 Moreover, a single-arm phase II clinical trial with ipilimumab (I) + temozolomide (T) was conducted with 64 patients showing encouraging results, which may warrant a larger scale study (6-month PFS: 45%; median OS: 24.5 months). 116
A regimen of fotemustine and ipilimumab has also been studied in the NIBIT-M1 phase II clinical trial for advanced metastatic melanoma, including a significant proportion of patients with brain metastases. It was shown that 46.5% achieved disease control, the primary endpoint of the study. 117 In a 3-year follow-up to this study, median OS was found to be 12.9 months. 118 Similar to the patient population of this study, a combination of radiotherapy, temozolomide, and sorafenib has been studied for patients with glioblastomas as a result of melanoma metastasis. However, it has been shown that the addition of sorafenib has not yielded a significant benefit in prognosis for patients. 119
Potential Evidence-Based Natural Health Products and Compounds for Complementary Use
The use of NHPs to cure various diseases has been a form of treatment used by traditional healers for thousands of years, but it has only recently become a growing area of research. Specifically, many NHPs and NCs have been tested for their anti-cancer effects. Aqueous dandelion root extract is one of these more well-known products which has been shown to induce apoptosis in human pancreatic and colorectal cancer cells.120,121 Similarly, ethanolic lemongrass extract has also been an effective anti-cancer product against leukemia and lymphoma cell lines. 122 As metastatic melanoma has been difficult to treat conventionally, this review attempts to summarize current literature on the use of NHPs and NCs on melanoma and their potential for complementation when combined with contemporary therapies.
Vinblastine and Vincristine
Both of these NCs are vinca alkaloids derived from the Catharanthus roseus plant, which is more commonly referred to as Madagascar periwinkle and they have shown both anti-diabetic and anti-cancer activities. 123 The mechanism of action of these compounds is to inhibit microtubule formation by targeting tubulin dimers which ultimately causes cancer cells to undergo apoptosis. 124 In a prospective evaluation, a combination of DTIC + Cisplatin + Vinblastine (CVD) was used in metastatic melanoma and achieved a median response rate of 40% and a median OS of 9 months. 125 In comparison to the individual response rates seen with all compounds as single-agents, this combination showed higher efficacy. 125 Biochemotherapy involving these 3 drugs along with interleukin-2 and interferon alfa-2b has also been tested and this treatment has shown an enhanced response rate of 13.8% to 19.5%, but it is not recommended due to the elevated levels of toxicity that patients experience. 126
Curcumin and Curcumin Analogs
Curcumin is the major bioactive ingredient within the turmeric spice and can be isolated from the naturally occurring plant Curcuma longa. 127 Curcumin can produce a variety of effects through its anti-inflammatory and anti-oxidative pathways which allow it to selectively target melanoma cancer cells. It has been shown to increase the amount of reactive oxygen species (ROS) in this process. 127 However, when tested on in-vivo mouse models, it showed poor bioavailability and therefore, chemical analogs with similar, more stable structures were developed. 127 One of these analogs that is promising is compound A as it is far more efficacious than curcumin and therefore, it can be administered at lower dosed concentrations allowing it to better tolerated by patients. 127 In-vitro combinations were also conducted using this analog and it was shown to interact positively with tamoxifen. 127 Furthermore, it did not negatively impact either paclitaxel or cisplatin regimens. 127 Clinical studies of compound A should be conducted to see if it can be used as a complementary form of treatment along with chemotherapeutic agents such as tamoxifen.
Berberine
This compound is a natural isoquinolone alkaloid which can be extracted through the roots of plants from the genus Berberis. 128 It has been shown to inhibit the metastatic capacity of melanoma cells by affecting the signaling activity of many molecules. Berberine increases ROS levels within cells which in turn causes AMP-activated protein kinase (AMPK) activation. 129 The activation of this protein complex is responsible for the suppression of melanoma cells as it alters the activity of several signaling pathways. Signaling molecules such as ERK and p38 MAPK are downregulated and this is known to prevent the invasive effects of cancer cells. 129 A reduction in the levels of COX-2, PGE2 and PGE2 receptors is also seen which prevents cancer cells from migrating. 129 The reduction and downregulation of these molecules ultimately results in decreased metastatic potential for melanoma cancer cells. 129 Berberine has been tested on the B16F10 melanoma cell line through in-vitro and in-vivo tests in combination with doxorubicin. 130 Berberine or doxorubicin did not show significant effects on tumor growth when using in vivo models of xenografted mice when they were applied as single agent treatments. However, the combination of berberine and doxorubicin caused significant decreases in tumor volume (85%) and weight (78%) when compared to the control mice. 130
Pancratistatin
This NC is obtained from the plant Hymenocallis littoralis, which is more commonly known as the beach spider lily. 131 Pancratistatin (PST) has been shown to selectively target cancer cells. Although the exact mechanism is unknown, it is speculated that it targets mitochondrial vulnerabilities which are unique to cancer cells. In-vitro studies have confirmed that PST selectively induces apoptosis in melanoma cells when using the A375 human melanoma cell line. 131 In addition, PST was used in an in-vitro combination with tamoxifen (TAM) and this treatment was more effective than either agent used as a standalone treatment in both the A375 and G361 human melanoma cell lines. 131 TAM had no effect on the A375 melanoma cell line when used as an individual treatment, indicating it may not be inducing apoptosis on these cells directly, but could be sensitizing their mitochondria to enhance the effect of PST as both of these compounds are known to target this organelle. 131
Broccoli Sprout Extract
In 1 randomized clinical trial, 17 patients with atypical skin lesions and a prior history of melanoma were given oral broccoli sprout extract (BSE). 132 Within this extract, sulforaphane (SFN) is a compound that has shown anti-cancer effects via STAT3 activity and Nrf2 induction.132,133 It was reported that there was a reduction in the size of nevi (skin lesions) on average, with the most significant effects in the 200 µmol dose group (~700 mg/mL SFN pre-post change). 132 Along with morphological changes, there was an observed decrease in pro-inflammatory cytokines and a significant increase in decorin, a tumor suppressor protein. 132 These results show promise for the use of BSE as a preventative therapy for melanoma but are confounded by a small sample size and limited molecular marker analysis.
Paradise Tree Extract
Paradise tree extract (PTE) can be isolated from the tree Simarouba glauca, which is also referred to as Lakshmi Taru in India. Preliminary work has been completed on its effect against melanoma cancer cell lines through in-vitro tests, but this has yet to be published. However, this work has indicated that this extract can selectively induce apoptosis in melanoma cells from the A375 and G361 cell lines. In addition, it has been used in combination trials with paclitaxel, cisplatin and dacarbazine where it showed a slight enhancement of their activities with no signs of a negative interaction. Further studies on the mechanism of this extract as well as its effects on in-vivo models are currently in progress.
Considerations for Natural Health Products and Compounds
Although many in-vivo preclinical studies have been conducted on natural health compounds for melanoma, it is important to consider the effects of drug interactions. In ideal situations, complementary therapy can result in synergistic anti-cancer effects due to increased apoptosis when multiple pathways are targeted. On the other hand, certain combinations may lead to decreased efficacy. For example, it has been suggested that the compounds in St. John’s Wort may cause decreased serum dabrafenib concentration due to their induction of the CYP3A4 liver enzyme. 134 Therefore, although they may have a place in future melanoma treatment, there is a current need for natural products to be fully evaluated scientifically and clinically. Once thorough information is available, NHPs/NCs should be taken under the guidance of medical professionals.
Conclusion and Future Directions
Consequently, as a result of this review on available literature, it is evident there has been significant advancements in metastatic melanoma treatments in the past decade. While dacarbazine is the only FDA-approved single-agent chemotherapy indicated for metastatic melanoma, temozolomide is a similar drug that is generally well-tolerated and has shown efficacy in both melanoma and gliomas as a result of its metastases. Fotemustine can be useful in similar indications. However, with low response rates and chemoresistant melanoma, immunotherapies have been developed to mitigate the potential of tumor cells to avoid immunosurveillance. Cytokines such as aldesleukin were developed early in the 2000s in hopes to improve T-cell recognition of tumors via enhancement of MHC. Moreover, newly emerging immune checkpoint inhibitors, such as the anti-CTLA-4 antibody ipilimumab, have been thoroughly studied and have shown to be first-line therapies for metastatic melanoma due to their high observed efficacy in terms of survival, which results from their ability to activate T-cells. However, phase III trials revealed increased immune-related grade 3 or 4 toxicity with these treatments at high doses, resulting in significant amounts of patients requiring dose interruptions. Thus, patients on monoclonal antibody treatments should be given careful attention for toxicities and dose interruption/modification should be considered when severity increases. Further molecular analyses of biomarkers in metastatic melanoma has allowed for the creation of targeted therapies such as BRAF/MEK inhibitors like vemurafenib, dabrafenib, and trametinib. These targeted drugs provide encouraging and positive results in BRAF V600E-mutation positive melanoma, which may indicate that screening patients for specific biomarkers could be the future of melanoma treatment. Furthermore, the combination of BRAF/MEK inhibitors has proven to be efficacious, however, further research may be needed to determine mechanisms of resistance to these therapies and reduce paradoxical MAPK pathway activity. Moreover, while BRAF and MEK have become well-established targets, NRas currently has no specific inhibitors when mutated. Thus, the creation of an NRas inhibitor in the future may add to the diversity of targeted therapies available. 72 Nonetheless, immunotherapy and targeted therapy combinations such as ipilimumab with nivolumab and vemurafenib with cobimetinib have shown potential in both preclinical and clinical studies due to positive interactions seen with their respective molecular mechanisms. In particular, the combination of oncolytic virotherapy with immune checkpoint inhibitors is especially promising as oncolytic viruses such as T-VEC may help overcome primary resistance to monoclonal antibodies, particularly by recruiting T-cells to the tumor microenvironment. Biochemotherapies have also been a topic of recent interest as the combination may simultaneously promote intrinsic apoptosis and a T-cell activating effect, leading to an enhanced ability for the body to eliminate cancer cells. Dacarbazine combined with ipilimumab is one well-defined example of a potential biochemotherapy with benefits for patients. As future research is conducted, natural products may have an intriguing role. In the past, natural products vincristine and vinblastine were used in conjunction with standard chemotherapies and showed positive results. As more natural health products and compounds are scientifically evaluated, there may be more information revealed as to their potential role in the mechanisms exploited by current targeted therapies and immunotherapies. For example, berberine has demonstrated the ability in preclinical animal models to inhibit activation of the ERK/MAPK pathway, leading to inhibition of downstream targets that increase cellular proliferation, which is similar to BRAF inhibitors such as vemurafenib. With more research conducted on the interaction of berberine with BRAF inhibitors, it may be possible that there is an enhancement of this anti-proliferative activity. As a whole, there are much more diverse and efficacious therapeutic options available for melanoma than what would be the case a decade ago. Furthermore, it is clear that the discovery of biomarkers for melanoma (e.g. BRAF V600E) has been a breakthrough in achieving efficacy without significant toxicities for patients. Motivated by this review, we suggest that additional research is completed in the areas of identifying molecular targets and biomarkers in metastatic melanoma. By narrowing down subsets of patients most likely to benefit, treatment can become more efficient and unneeded exposure to severe toxicities can be reduced. Furthermore, research in the area of polyimmunotherapy (involving different classes) and biochemotherapy would have the potential to discover treatments that can utilize multiple anti-cancer strategies. Finally, there should be work conducted on fully evaluating natural health products as they can be non-toxic and used in a complementary fashion with standard therapies. In particular, the evaluation of natural health products and compounds with immunotherapies and/or targeted therapies may be an area of interest for the future.
Acknowledgments
The authors would like to acknowledge Mansi Arora for her contributions toward reviewing literature that was used in this paper. In addition, the authors would like to thank Darcy Wear for his effort in manuscript review.
Footnotes
Author Contributions: S.S. and S.P. were primarily responsible for the survey of literature, review design, and preparation of the manuscript. R.J. made contributions toward the review of literature and the preparation of sections within the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors would like to recognize the generous funding for this project by NSERC, the Kevin Couvillon family, and Mr. Loknath Chawla and family.
ORCID iD: Siyaram Pandey  https://orcid.org/0000-0002-7273-7154
https://orcid.org/0000-0002-7273-7154
References
- 1. Liu Y, Sheikh MS. Melanoma: molecular pathogenesis and therapeutic management. Mol Cell Pharmacol. 2014;6:31-44. doi: 10.4255/mcpharmacol.14.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yang J, Manson DK, Marr BP, Carvajal RD. Treatment of uveal melanoma: where are we now? Ther Adv Med Oncol. 2018;10:1-17. doi: 10.1177/1758834018757175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lens M, Bataille V, Krivokapic Z. Melanoma of the small intestine. Lancet Oncol. 2009;10:516-521. doi: 10.1016/S1470-2045(09)70036-1 [DOI] [PubMed] [Google Scholar]
- 4. Riker AI, Zea N, Trinh T. The epidemiology, prevention, and detection of melanoma. Ochsner J. 2010;10:56-65. Accessed June 24, 2020. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3096196/ [PMC free article] [PubMed] [Google Scholar]
- 5. Marks R. Epidemiology of melanoma. Clin Exp Dermatol. 2000;25:459-463. doi: 10.1046/j.1365-2230.2000.00693.x [DOI] [PubMed] [Google Scholar]
- 6. Autier P, Dore J-F, Group JD for E and EMC. Influence of sun exposures during childhood and during adulthood on melanoma risk. Int J Cancer. 1998;77:533-537. doi: [DOI] [PubMed] [Google Scholar]
- 7. Cadet J, Douki T. Formation of UV-induced DNA damage contributing to skin cancer development. Photochem Photobiol Sci. 2018;17:1816-1841. doi: 10.1039/c7pp00395a [DOI] [PubMed] [Google Scholar]
- 8. Rosenberg SA. Surgical treatment of malignant melanoma. Cancer Treat. Rep.1976;60:159-163. [PubMed] [Google Scholar]
- 9. Domingues B, Lopes J, Soares P, Populo H. Melanoma treatment in review. ImmunoTargets Ther. 2018;7:35-49. doi: 10.2147/itt.s134842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ballo MT, Ang KK. Radiation therapy for malignant melanoma. Surg Clin North Am. 2003;83:323-342. doi: 10.1016/S0039-6109(02)00096-8 [DOI] [PubMed] [Google Scholar]
- 11. Shimada T, Saito T, Okadome M, et al. Secondary leukemia after chemotherapy and/or radiotherapy for gynecologic neoplasia. Int J Gynecol Cancer. 2014;24:178-183. doi: 10.1097/IGC.0000000000000045 [DOI] [PubMed] [Google Scholar]
- 12. Luke JJ, Schwartz GK. Chemotherapy in the management of advanced cutaneous malignant melanoma. Clin Dermatol. 2013;31:290-297. doi: 10.1016/j.clindermatol.2012.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jiang G, Li R-H, Sun C, Liu Y-Q, Zheng J-N. Dacarbazine combined targeted therapy versus dacarbazine alone in patients with malignant melanoma: a meta-analysis. PLoS One. 2014;9:e111920. doi: 10.1371/journal.pone.0111920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. 2018;50:1-11. doi: 10.1038/s12276-018-0191-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Conlon KC, Miljkovic MD, Waldmann TA. Cytokines in the treatment of cancer. J Interf Cytokine Res. 2019;39:6-21. doi: 10.1089/jir.2018.0019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jamal S, Hudson M, Fifi-Mah A, Ye C. Immune-related adverse events associated with cancer immunotherapy: a review for the practicing rheumatologist. J Rheumatol. 2020;47:166-175. doi: 10.3899/jrheum.190084 [DOI] [PubMed] [Google Scholar]
- 17. Ascierto PA, Kirkwood JM, Grob JJ, et al. The role of BRAF V600 mutation in melanoma. J Transl Med. 2012;10:85. doi: 10.1186/1479-5876-10-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koelblinger P, Thuerigen O, Dummer R. Development of encorafenib for BRAF-mutated advanced melanoma. Curr Opin Oncol. 2018;30:125-133. doi: 10.1097/CCO.0000000000000426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smalley KSM, Eroglu Z, Sondak VK. Combination therapies for melanoma: a new standard of care? Am J Clin Dermatol. 2016;17:99-105. doi: 10.1007/s40257-016-0174-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wirngo FE, Lambert MN, Jeppesen PB. The physiological effects of dandelion (Taraxacum officinale) in type 2 diabetes. Rev Diabet Stud. 2016;13:113-131. doi: 10.1900/RDS.2016.13.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nguyen C, Mehaidli A, Baskaran K, et al. Dandelion root and lemongrass extracts induce apoptosis, enhance chemotherapeutic efficacy, and reduce tumour xenograft growth in vivo in prostate cancer. Evid-Based Complem Altern Med J. 2019;2019:12. Published July 17, 2019. Accessed June 24, 2020. https://www.hindawi.com/journals/ecam/2019/2951428/?fbclid=IwAR0EXxzbbdw5M2y_GjMAHxwo9X0MyVBItBRDpO04_0uTLebIbonAfk60w6M [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dacarbazine – FDA prescribing information, side effects and uses. Accessed June 24, 2020. https://www.drugs.com/pro/dacarbazine.html
- 23. Koprowska K, Czyz M. [Dacarbazine, a chemotherapeutic against metastatic melanoma and a reference drug for new treatment modalities]. Postepy Hig Med Dosw (Online). 2011;65:734-751. doi: 10.5604/17322693.966832 [DOI] [PubMed] [Google Scholar]
- 24. Gupta A, Gomes F, Lorigan P. The role for chemotherapy in the modern management of melanoma. Melanoma Manag. 2017;4:125-136. doi: 10.2217/mmt-2017-0003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reid JM, Kuffel MJ, Miller JK, Rios R, Ames MM. Metabolic activation of dacarbazine by human cytochromes P450: the Role of CYP1A1, CYP1A2, and CYP2E1. Clin Cancer Res. 1999;5:2192-2197. [PubMed] [Google Scholar]
- 26. Amirmostofan M, Pourahmad Jaktaji J, Soleimani Z, et al. Synthesis and molecular–cellular mechanistic study of pyridine derivative of dacarbazine. Iran J Pharm Res. 2013;12:255-265. doi: 10.22037/ijpr.2013.1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Clemente N, Ferrara B, Gigliotti CL, et al. Solid lipid nanoparticles carrying temozolomide for melanoma treatment. Preliminary in vitro and in vivo studies. Int J Mol Sci. 2018;19:255. doi: 10.3390/ijms19020255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Middleton MR, Grob JJ, Aaronson N, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000;18:158-166. doi: 10.1200/jco.2000.18.1.158 [DOI] [PubMed] [Google Scholar]
- 29. Guida M, Tommasi S, Strippoli S, et al. The search for a melanoma-tailored chemotherapy in the new era of personalized therapy: a phase II study of chemo-modulating temozolomide followed by fotemustine and a cooperative study of GOIM (Gruppo Oncologico Italia Meridionale). BMC Cancer. 2018;18:552. doi: 10.1186/s12885-018-4479-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Quéreux G, Dréno B. Fotemustine for the treatment of melanoma. Expert Opin Pharmacother. 2011;12:2891-2904. doi: 10.1517/14656566.2011.633513 [DOI] [PubMed] [Google Scholar]
- 31. Avril MF, Aamdal S, Grob JJ, et al. Fotemustine compared with dacarbazine in patients with disseminated malignant melanoma: a phase III study. J Clin Oncol. 2004;22:1118-1125. doi: 10.1200/JCO.2004.04.165 [DOI] [PubMed] [Google Scholar]
- 32. Johnstone TC, Park GY, Lippard SJ. Understanding and improving platinum anticancer drugs – phenanthriplatin. Anticancer Res. 2014:34:471-476. [PMC free article] [PubMed] [Google Scholar]
- 33. Evans LM, Casper ES, Rosenbluth R. Phase II trial of carboplatin in advanced malignant melanoma. Cancer Treat. Rep. 1987;71:171-172. Accessed June 24, 2020. https://books.google.ca/books?hl=en&lr=&id=acpl4uwxpQwC&oi=fnd&pg=PA171&dq=carboplatin+melanoma&ots=YeZPxpZPTD&sig=WEqCCxtHRv0MWzojbbxE8Wi_tf8#v=onepage&q=carboplatinmelanoma&f=false [PubMed] [Google Scholar]
- 34. Güven K, Kittler H, Wolff K, Pehamberger H. Cisplatin and carboplatin combination as second-line chemoth. . .: melanoma Research. Melanoma Res. 2001;11:411-415. Accessed June 12, 2020. https://journals.lww.com/melanomaresearch/Abstract/2001/08000/Cisplatin_and_carboplatin_combination_as.12.aspx [DOI] [PubMed] [Google Scholar]
- 35. Legha SS, Ring S, Papadopoulos N, Raber M, Benjamin RS. A phase II trial of taxol in metastatic melanoma. Cancer. 1990;65:2478-2481. doi: [DOI] [PubMed] [Google Scholar]
- 36. Liu CY, Hung MH, Wang DS, et al. Tamoxifen induces apoptosis through cancerous inhibitor of protein phosphatase 2A-dependent phospho-Akt inactivation in estrogen receptor-negative human breast cancer cells. Breast Cancer Res. 2014;16:431. doi: 10.1186/s13058-014-0431-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fisher RI, Neifeld JP, Lippman ME. Œstrogen receptors in human malignant melanoma. Lancet. 1976;308:337-339. doi: 10.1016/S0140-6736(76)92592-7 [DOI] [PubMed] [Google Scholar]
- 38. Sporn MB, Lippman SM. Agents for chemoprevention and their mechanism of action. Published online 2003. Accessed June 24, 2020. https://www.ncbi.nlm.nih.gov/books/NBK12522/
- 39. Mcclay EF, Eileen M, Mcclay T. Tamoxifen: is it useful in the treatment of patients with metastatic melanoma? J Clin Oncol. 1994;12:617-626. [DOI] [PubMed] [Google Scholar]
- 40. Thorn CF, Oshiro C, Marsh S, et al. Doxorubicin pathways: pharmacodynamics and adverse effects. Pharmacog Genom. 2011;21:440-446. doi: 10.1097/FPC.0b013e32833ffb56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Smylie MG, Wong R, Mihalcioiu C, et al. A phase II, open label, monotherapy study of liposomal doxorubicin in patients with metastatic malignant melanoma. Invest New Drugs. 2007;25:155-159. doi: 10.1007/s10637-006-9002-y [DOI] [PubMed] [Google Scholar]
- 42. Mansour AM, Drevs J, Esser N, et al. A new approach for the treatment of malignant melanoma: enhanced antitumor efficacy of an albumin-binding doxorubicin prodrug that is cleaved by atrix metalloproteinase 2. Cancer Res. 2003;63:4062-4066. [PubMed] [Google Scholar]
- 43. Schadendorf D, Fink W, Zimpfer-Rechner C, et al. Clinical phase II study of pegylated liposomal doxorubicin as second-line treatment in disseminated melanoma. Oncology Research and Treatment. 2004;27:540-544. doi: 10.1159/000081335 [DOI] [PubMed] [Google Scholar]
- 44. Hui E. Immune checkpoint inhibitors. J Cell Biol. 2019;218:740-741. doi: 10.1083/jcb.201810035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tarhini A, Lo E, Minor DR. Releasing the brake on the immune system: ipilimumab in melanoma and other tumors. Cancer Biother Radiopharm. 2010;25:601-613. doi: 10.1089/cbr.2010.0865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723. doi: 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Eggermont AMM, Chiarion-Sileni V, Grob J-J, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375:1845-1855. doi: 10.1056/NEJMoa1611299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. O’Day SJ, Hamid O, Urba WJ. Targeting cytotoxic T-lymphocyte antigen-4 (CTLA-4): a novel strategy for the treatment of melanoma and other malignancies. Cancer. 2007;110:2614-2627. doi: 10.1002/cncr.23086 [DOI] [PubMed] [Google Scholar]
- 49. Lebbé C, Weber JS, Maio M, et al. Survival follow-up and ipilimumab retreatment of patients with advanced melanoma who received ipilimumab in prior phase II studies. Ann Oncol. 2014;25:2277-2284. doi: 10.1093/annonc/mdu441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Millward M, Underhill C, Lobb S, et al. Phase i study of tremelimumab (CP-675 206) plus PF-3512676 (CPG 7909) in patients with melanoma or advanced solid tumours. Br J Cancer. 2013;108:1998-2004. doi: 10.1038/bjc.2013.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ribas A, Kefford R, Marshall MA, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31:616-622. doi: 10.1200/JCO.2012.44.6112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Koppolu V, Rekha Vasigala V. Checkpoint immunotherapy by nivolumab for treatment of metastatic melanoma. J Cancer Res Ther. 2018;14:1167-1175. doi: 10.4103/jcrt.JCRT_1290_16 [DOI] [PubMed] [Google Scholar]
- 53. Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol. 2018;8:86. doi: 10.3389/fonc.2018.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated Melanoma. N Engl J Med. 2015;373:23-34. doi: 10.1056/NEJMoa1504030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;3724):320-330. doi: 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- 56. Raedler LA. Keytruda (Pembrolizumab): first PD-1 inhibitor approved for previously treated unresectable or metastatic melanoma. Am Heal drug benefits. 2015;8:96-100. Accessed June 24, 2020. http://www.ncbi.nlm.nih.gov/pubmed/26629272 [PMC free article] [PubMed] [Google Scholar]
- 57. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521-2532. doi: 10.1056/NEJMoa1503093 [DOI] [PubMed] [Google Scholar]
- 58. Hamid O, Robert C, Daud A, et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol. 2019;30:582-588. Accessed June 24, 2020. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6503622/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fda, Cder. PROLEUKIN ® (Aldesleukin) for Injection, for Intravenous Infusion Rx Only. [Google Scholar]
- 60. Weiss GR, Grosh WW, Chianese-Bullock KA, et al. Molecular insights on the peripheral and intratumoral effects of systemic high-dose rIL-2 (aldesleukin) administration for the treatment of metastatic melanoma. Clin Cancer Res. 2011;17:7440-7450. doi: 10.1158/1078-0432.CCR-11-1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wargo JA, Reuben A, Cooper Z, Amaria R. Update on use of aldesleukin for treatment of high-risk metastatic melanoma. ImmunoTargets Ther. 2015;4:79. doi: 10.2147/itt.s61590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Aldesleukin . National Institute of Diabetes and Digestive and Kidney Diseases; 2012. Accessed June 5, 2020. http://www.ncbi.nlm.nih.gov/pubmed/31643421 [PubMed] [Google Scholar]
- 63. Davar D, Wang H, Chauvin JM, et al. Phase Ib/II study of pembrolizumab and pegylated-interferon alfa-2b in advanced melanoma. J Clin Oncol. 2018;36:3450-3458. doi: 10.1200/JCO.18.00632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Katlinskaya YV, Katlinski KV, Yu Q, et al. Suppression of type I interferon signaling overcomes oncogene-induced senescence and mediates melanoma sevelopment and progression. Cell Rep. 2016;15:171-180. doi: 10.1016/j.celrep.2016.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Eggermont AM, Suciu M, Santinami S, et al. Adjuvant therapy with pegylated interferon alfa-2b versus observation alone in resected stage III melanoma: final results of EORTC 18991, a randomised phase III trial. Lancet. 2008;372:117-126. doi: 10.1016/S0140-6736(08)61033-8 [DOI] [PubMed] [Google Scholar]
- 66. Ascierto PA, Palmieri G. Adjuvant therapy of cutaneous melanoma [11]. Lancet. 1999;353:328. doi: 10.1016/S0140-6736(05)74894-7 [DOI] [PubMed] [Google Scholar]
- 67. Herndon TM, Demko SG, Jiang X, et al. U.S. Food and Drug Administration Approval: peginterferon-alfa-2b for the adjuvant treatment of patients with melanoma. Oncologist. 2012;17(10):1323-1328. doi: 10.1634/theoncologist.2012-0123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sondak VK, Kudchadkar R. Pegylated interferon for the adjuvant treatment of melanoma: FDA approved, but what is its role? Oncologist. 2012;17:1223-1224. doi: 10.1634/theoncologist.2012-0368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ozao-Choy J, Lee DJ, Faries MB. Melanoma vaccines mixed past, promising future. Surg Clin North Am. 2014;94:1017-1030. doi: 10.1016/j.suc.2014.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Woller N, Gürlevik E, Ureche CI, Schumacher A, Kühnel F. Oncolytic viruses as anticancer vaccines. Front Oncol. 2014;4:188. doi: 10.3389/fonc.2014.00188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Harrington KJ, Puzanov I, Hecht JR, et al. Clinical development of talimogene laherparepvec (T-VEC): a modified herpes simplex virus type-1–derived oncolytic immunotherapy. Expert Rev Anticancer Ther. 2015;15:1389-1403. doi: 10.1586/14737140.2015.1115725 [DOI] [PubMed] [Google Scholar]
- 72. Highlights Of Prescribing Information. Accessed December 3, 2020. www.fda.gov/medwatch.
- 73. Capasso C, Hirvinen M, Garofalo M, et al. Oncolytic adenoviruses coated with MHC-I tumor epitopes increase the antitumor immunity and efficacy against melanoma. Oncoim-munology. 2016;5. doi: 10.1080/2162402X.2015.1105429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Savoia P, Fava P, Casoni F, Cremona O. Targeting the ERK signaling pathway in melanoma. Int J Mol Sci. 2019;20:1483. doi: 10.3390/ijms20061483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kakadia S, Yarlagadda N, Awad R, et al. Mechanisms of resistance to BRAF and MEK inhibitors and clinical update of us food and drug administration-approved targeted therapy in advanced melanoma. Onco Targets Ther. 2018;11:7095-7107. doi: 10.2147/OTT.S182721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516. doi: 10.1056/NEJMoa1103782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hoffner B, Benchich K. Trametinib: a targeted therapy in metastatic melanoma. J Adv Pract Oncol. 2018;9:741-745. [PMC free article] [PubMed] [Google Scholar]
- 78. Wright CJM, McCormack PL. Trametinib: first global approval. Drugs. 2013;73:1245-1254. doi: 10.1007/s40265-013-0096-1 [DOI] [PubMed] [Google Scholar]
- 79. FDA approves dabrafenib plus trametinib for adjuvant treatment of melanoma with BRAF V600E or V600K mutations and FDA. Accessed June 24, 2020. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-dabrafenib-plus-trametinib-adjuvant-treatment-melanoma-braf-v600e-or-v600k-mutations
- 80. Hauschild A, Grob JJ, Demidov LV, et al. Phase III, randomized, open-label, multicenter trial (BREAK-3) comparing the BRAF kinase inhibitor dabrafenib (GSK2118436) with dacarbazine (DTIC) in patients with BRAF V600E -mutated melanoma. J Clin Oncol. 2012;30:LBA8500-LBA8500. doi: 10.1200/jco.2012.30.18_suppl.lba8500 [DOI] [Google Scholar]
- 81. Hauschild A, Grob JJ, Demidov LV, et al. An update on BREAK-3, a phase III, randomized trial: dabrafenib (DAB) versus dacarbazine (DTIC) in patients with BRAF V600E-positive mutation metastatic melanoma (MM). J Clin Oncol. 2013;31:9013-9013. doi: 10.1200/jco.2013.31.15_suppl.9013 [DOI] [Google Scholar]
- 82. Hauschild A, Ascierto PA, Schadendorf D, et al. Long-term outcomes in patients with BRAF V600-mutant metastatic melanoma receiving dabrafenib monotherapy: analysis from phase 2 and 3 clinical trials. Eur J Cancer. 2020;125:114-120. doi: 10.1016/j.ejca.2019.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ballantyne AD, Garnock-Jones KP. Dabrafenib: first global approval. Drugs. 2013;73:1367-1376. doi: 10.1007/s40265-013-0095-2 [DOI] [PubMed] [Google Scholar]
- 84. Cobimetinib Approved for Advanced Melanoma – National Cancer Institute. Accessed June 24, 2020. https://www.cancer.gov/news-events/cancer-currents-blog/2015/cobimetinib-melanoma
- 85. Sun J, Zager JS, Eroglu Z. Encorafenib/binimetinib for the treatment of BRAF-mutant advanced, unresectable, or metastatic melanoma: design, development, and potential place in therapy. Onco Targets Ther. 2018;11:9081-9089. doi: 10.2147/OTT.S171693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Dummer R, Ascierto PA, Gogas HJ, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19:603-615. doi: 10.1016/S1470-2045(18)30142-6 [DOI] [PubMed] [Google Scholar]
- 87. Liszkay G, Gogas H, Mandalà M, et al. Update on overall survival in COLUMBUS: a randomized phase III trial of encorafenib (ENCO) plus binimetinib (BINI) versus vemurafenib (VEM) or ENCO in patients with BRAF V600–mutant melanoma. J Clin Oncol. 2019;37:9512-9512. doi: 10.1200/jco.2019.37.15_suppl.9512 [DOI] [Google Scholar]
- 88. Shirley M. Encorafenib and binimetinib: first global approvals. Drugs. 2018;78:1277-1284. doi: 10.1007/s40265-018-0963-x [DOI] [PubMed] [Google Scholar]
- 89. Dummer R, Schadendorf D, Ascierto PA, et al. Binimetinib versus dacarbazine in patients with advanced NRAS-mutant melanoma (NEMO): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:435-445. doi: 10.1016/S1470-2045(17)30180-8 [DOI] [PubMed] [Google Scholar]
- 90. Koelblinger P, Dornbierer J, Dummer R. A review of binimetinib for the treatment of mutant cutaneous melanoma. Futur Oncol. 2017;13:1755-1766. doi: 10.2217/fon-2017-0170 [DOI] [PubMed] [Google Scholar]
- 91. Kim S, Yazici YD, Calzada G, et al. Sorafenib inhibits the angiogenesis and growth of orthotopic anaplastic thyroid carcinoma xenografts in nude mice. Mol Cancer Ther. 2007;6:1785-1792. doi: 10.1158/1535-7163.MCT-06-0595 [DOI] [PubMed] [Google Scholar]
- 92. Ott PA, Hamilton A, Min C, et al. A phase II trial of sorafenib in metastatic melanoma with tissue correlates. PLoS One. 2010;5:e15588. doi: 10.1371/journal.pone.0015588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Papadopoulos NE, Bedikian A, Ring S, et al. Phase I/II study of a cisplatin-taxol-dacarbazine regimen in metastatic melanoma. Am J Clin Oncol. 2009;32:509-514. doi: 10.1097/COC.0b013e3181942a1f [DOI] [PubMed] [Google Scholar]
- 94. Omata W, Tsutsumida A, Namikawa K, Takahashi A, Oashi K, Yamazaki N. Sequential combination chemotherapy of dacarbazine (DTIC) with carboplatin and paclitaxel for patients with metastatic mucosal melanoma of nasal cavity and paranasal sinuses. Clin Med Insights Case Reports. 2017;10:1-5. doi: 10.4137/CCRep.s39851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kottschade LA, Suman VJ, Amatruda T, et al. A phase II trial of nab-paclitaxel (ABI-007) and carboplatin in patients with unresectable stage IV melanoma. Cancer. 2011;117(8):1704-1710. doi: 10.1002/cncr.25659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kim KB, Sosman JA, Fruehauf JP, et al. BEAM: a randomized phase II study evaluating the activity of bevacizumab in combination with carboplatin plus paclitaxel in patients with previously untreated advanced melanoma. J Clin Oncol. 2012;30:34-41. doi: 10.1200/JCO.2011.34.6270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Selby M, Engelhardt J, Lu L-S, et al. Antitumor activity of concurrent blockade of immune checkpoint molecules CTLA-4 and PD-1 in preclinical models. J Clin Oncol. 2013;31:3061-3061. doi: 10.1200/jco.2013.31.15_suppl.306123569323 [DOI] [Google Scholar]
- 98. FDA approves nivolumab–ipilimumab combination for melanoma and MDedge Internal Medicine. Accessed June 24, 2020. https://www.mdedge.com/internalmedicine/article/103246/melanoma/fda-approves-nivolumab-ipilimumab-combination-melanoma
- 99. Zamarin D, Holmgaard RB, Subudhi SK, et al. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci Transl Med. 2014;6:226ra32. doi: 10.1126/scitranslmed.3008095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bommareddy PK, Aspromonte S, Zloza A, Rabkin SD, Kaufman HL. MEK inhibition enhances oncolytic virus immunotherapy through increased tumor cell killing and T cell activation. Sci Transl Med. 2018;10:eaau0417. doi: 10.1126/scitranslmed.aau0417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Feola S, Capasso C, Fusciello M, et al. Oncolytic vaccines increase the response to PD-L1 blockade in immunogenic and poorly immunogenic tumors. Oncoimmunology. 2018;7:e1457596. doi: 10.1080/2162402X.2018.1457596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kuryk L, Bertinato L, Staniszewska M, et al. From conventional therapies to immunotherapy: melanoma treatment in review. Cancers (Basel). 2020;12:1-20. doi: 10.3390/cancers12103057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ribas A, Dummer R, Puzanov I, et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2017;170:1109-1119.e10. doi: 10.1016/j.cell.2017.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Pembrolizumab with or without talimogene laherparepvec or talimogene laherparepvec placebo in unresected melanoma (KEYNOTE-034). ClinicalTrials.gov. Accessed December 6, 2020. https://clinicaltrials.gov/ct2/show/NCT02263508
- 105. Long GV, Hauschild A, Santinami M, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377:1813-1823. doi: 10.1056/NEJMoa1708539 [DOI] [PubMed] [Google Scholar]
- 106. Long GV, Flaherty KT, Stroyakovskiy D, et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol. 2019;28:1631-1639. Accessed June 18, 2020. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6927319/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Larkin J, Ascierto PA, Dréno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371:1867-1876. doi: 10.1056/NEJMoa1408868 [DOI] [PubMed] [Google Scholar]
- 108. Gutzmer R, Stroyakovskiy D, Gogas H, et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAFV600 mutation-positive melanoma (IMspire150): primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;395:1835-1844. doi: 10.1016/S0140-6736(20)30934-X [DOI] [PubMed] [Google Scholar]
- 109. Encorafenib + binimetinib + pembrolizumab in patients with unresectable or metastatic BRAF V600 mutant melanoma – ClinicalTrials.gov. Accessed June 18, 2020. https://clinicaltrials.gov/ct2/show/results/NCT02902042
- 110. Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517-2526. doi: 10.1056/NEJMoa1104621 [DOI] [PubMed] [Google Scholar]
- 111. Maio M, Grob JJ, Aamdal S, et al. Five-year survival rates for treatment-naive patients with advanced melanoma who received ipilimumab plus dacarbazine in a phase III trial. J Clin Oncol. 2015;33:1191-1196. doi: 10.1200/JCO.2014.56.6018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. McDermott DF, Sosman JA, Gonzalez R, et al. Double-blind randomized phase II study of the combination of sorafenib and dacarbazine in patients with advanced melanoma: a report from the 11715 Study Group. J Clin Oncol. 2008;26:2178-2185. doi: 10.1200/JCO.2007.14.8288 [DOI] [PubMed] [Google Scholar]
- 113. Xin Y, Huang Q, Zhang P, et al. Meta-analysis of the safety and efficacy of interferon combined with dacarbazine versus dacarbazine alone in cutaneous malignant melanoma. Med (United States). 2016;95:e3406. doi: 10.1097/MD.0000000000003406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Flaherty KT, Lee SJ, Zhao F, et al. Phase III trial of carboplatin and paclitaxel with or without sorafenib in metastatic melanoma. J Clin Oncol. 2013;31:373-379. doi: 10.1200/JCO.2012.42.1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Jamal R, Lapointe R, Cocolakis E, et al. Peripheral and local predictive immune signatures identified in a phase II trial of ipilimumab with carboplatin/paclitaxel in unresectable stage III or stage IV melanoma. J Immunother Cancer. 2017;5:83. doi: 10.1186/s40425-017-0290-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Patel SP, Kim DW, Bassett RL, et al. A phase II study of ipilimumab plus temozolomide in patients with metastatic melanoma. Cancer Immunol Immunother. 2017;66:1359-1366. doi: 10.1007/s00262-017-2030-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Di Giacomo AM, Ascierto PA, Pilla L, et al. Ipilimumab and fotemustine in patients with advanced melanoma (NIBIT-M1): an open-label, single-arm phase 2 trial. Lancet Oncol. 2012;13:879-886. doi: 10.1016/S1470-2045(12)70324-8 [DOI] [PubMed] [Google Scholar]
- 118. Di Giacomo AM, Ascierto PA, Queirolo P, et al. Three-year follow-up of advanced melanoma patients who received ipilimumab plus fotemustine in the Italian network for tumor biotherapy (NIBIT)-M1 phase II study. Ann Oncol. 2015;26:798-803. Accessed June 17, 2020. https://pubmed.ncbi.nlm.nih.gov/25538176/ [DOI] [PubMed] [Google Scholar]
- 119. Hainsworth JD, Ervin T, Friedman E, et al. Concurrent radiotherapy and temozolomide followed by temozolomide and sorafenib in the first-line treatment of patients with glioblastoma multiforme. Cancer. 2010;116:3663-3669. doi: 10.1002/cncr.25275 [DOI] [PubMed] [Google Scholar]
- 120. Ovadje P, Ammar S, Guerrero JA, Arnason JT, Pandey S. Dandelion root extract affects colorectal cancer proliferation and survival through the activation of multiple death signalling pathways. Oncotarget. 2016;7:73080-73100. doi: 10.18632/oncotarget.11485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ovadje P, Chochkeh M, Akbari-Asl P, Hamm C, Pandey S. Selective induction of apoptosis and autophagy through treatment with dandelion root extract in human pancreatic cancer cells. Pancreas. 2012;41:1039-1047. doi:10.1097/MPA.0b013e31824b22a2 [DOI] [PubMed] [Google Scholar]
- 122. Philion C, Ma D, Ruvinov I, et al. Cymbopogon citratus and Camellia sinensis extracts selectively induce apoptosis in cancer cells and reduce growth of lymphoma xenografts in vivo. Oncotarget. 2017;8:110756-110773. doi: 10.18632/oncotarget.22502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Noble RL. The discovery of the vinca alkaloids – chemotherapeutic agents against cancer. Biochem Cell Biol. 1990;68:1344-1351. doi: 10.1139/o90-197 [DOI] [PubMed] [Google Scholar]
- 124. Florian S, Mitchison TJ. Anti-microtubule drugs. Methods Mol Biol. 2016;1413:403-421. Accessed June 17, 2020. https://pubmed.ncbi.nlm.nih.gov/27193863/ [DOI] [PubMed] [Google Scholar]
- 125. Legha SS, Ring S, Papadopoulos N, Plager C, Chawla S, Benjamin R. A prospective evaluation of a triple-drug regimen containing cisplatin, vinblastine, and dacarbazine (CVD) for metastatic melanoma. Cancer. 1989;64:2024-2029. doi: [DOI] [PubMed] [Google Scholar]
- 126. Atkins MB, Hsu J, Lee S, et al. Phase III trial comparing concurrent biochemotherapy with cisplatin, vinblastine, dacarbazine, interleukin-2, and interferon alfa-2b with cisplatin, vinblastine, and dacarbazine alone in patients with metastatic malignant melanoma (E3695): a trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2008;26:5748-5754. doi: 10.1200/JCO.2008.17.5448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Parashar K, Sood S, Mehaidli A, et al. Evaluating the anti-cancer efficacy of a synthetic curcumin analog on human melanoma cells and its interaction with standard chemotherapeutics. Molecules. 2019;24:2483. doi: 10.3390/molecules24132483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Alqathama A, Prieto JM. Natural products with therapeutic potential in melanoma metastasis. Nat Prod Rep. 2015;32:1170-1182. doi: 10.1039/c4np00130c [DOI] [PubMed] [Google Scholar]
- 129. Kim HS, Kim MJ, Kim EJ, Yang Y, Lee MS, Lim JS. Berberine-induced AMPK activation inhibits the metastatic potential of melanoma cells via reduction of ERK activity and COX-2 protein expression. Biochem Pharmacol. 2012;83:385-394. doi: 10.1016/j.bcp.2011.11.008 [DOI] [PubMed] [Google Scholar]
- 130. Mittal A, Tabasum S, Singh RP. Berberine in combination with doxorubicin suppresses growth of murine melanoma B16F10 cells in culture and xenograft. Phytomedicine. 2014;21:340-347. doi: 10.1016/j.phymed.2013.09.002 [DOI] [PubMed] [Google Scholar]
- 131. Chatterjee SJ, McNulty J, Pandey S. Sensitization of human melanoma cells by tamoxifen to apoptosis induction by pancratistatin, a nongenotoxic natural compound. Melanoma Res. 2011;21:1-11. doi: 10.1097/CMR.0b013e328337abff [DOI] [PubMed] [Google Scholar]
- 132. Tahata S, Singh SV, Lin Y, et al. Evaluation of biodistribution of sulforaphane after administration of oral broccoli sprout extract in melanoma patients with multiple atypical nevi. Cancer Prev Res. 2018;11:429-437. doi: 10.1158/1940-6207.CAPR-17-0268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Wang W, Edington HD, Rao UNM, et al. STAT3 as a biomarker of progression in atypical nevi of patients with melanoma: dose-response effects of systemic IFNα therapy. J Invest Dermatol. 2008;128:1997-2002. doi: 10.1038/jid.2008.26 [DOI] [PubMed] [Google Scholar]
- 134. Gazzé G. Combination therapy for metastatic melanoma: a pharmacist’s role, drug interactions & complementary alternative therapies. Melanoma Manag. 2018;5:MMT07. doi: 10.2217/mmt-2017-0026 [DOI] [PMC free article] [PubMed] [Google Scholar]