Abstract
Cough is a main symptom in cystic fibrosis (CF). We aim to validate a Spanish version of the Leicester Cough Questionnaire (LCQ-Sp) to measure the impact of cough in CF bronchiectasis. A prospective longitudinal multicentre study was performed. Internal consistency and score changes over a 15-day period in stable state were assessed to analyse reliability. Concurrent validity was analysed by correlation with Saint George’s Respiratory Questionnaire (SGRQ) and convergent validity by assessing the association with clinical variables. Changes in scores between stable state and the first exacerbation were assessed to analyse responsiveness. 132 patients (29.73 ± 10.52 years) were enrolled in four hospitals. Internal consistency was high for the total score and good for the three domains (Cronbach’s α 0.81–0.93). The test–retest reliability showed an intraclass correlation coefficient of 0.86 for the total score. The correlation between LCQ-Sp and SGRQ scores was −0.74. The LCQ-Sp score negatively correlated with sputum volume, and the mean score decreased at the beginning of exacerbations (16.04±3.81 vs 13.91±4.29) with a large effect size. The LCQ-Sp is a reliable, repeatable and responsive instrument to assess the impact of cough in CF bronchiectasis and is responsive to change in the event of exacerbations.
Keywords: cystic fibrosis, cough, quality of life, bronchiectasis, symptoms, respiratory disease
Introduction
The dysfunction of the cystic fibrosis (CF) transmembrane conductance regulator (CFTR) of epithelial cells causes thickened and viscous mucus that decreases mucociliary clearance and leads to mucus obstruction, persistent bacterial infection, inflammation, and progressive bronchial damage in the lungs of patients with CF. Patients suffer from chronic cough, daily expectoration, and frequent exacerbations. The main objectives in treating lung disease are, apart from correcting the basic defect in CFTR protein, to control the respiratory symptoms and to delay progression. 1
Pulmonary function tests have been widely used as outcome measures in clinical trials, especially in children. However, with improvements in treatment, the rate of decline in lung function has decreased 2 and these tests may now be less sensitive to changes. The lung clearance index is a new measure of ventilation distribution but is currently only available in some reference centres for use in clinical trials. 3 Clinical monitoring may detect minor changes, but these measurements are more subjective. Health-related quality of life (HRQoL) questionnaires allow the impact of disease or of certain symptoms to be measured and are increasingly being used as outcome variables in clinical trials.
Two disease-specific HRQoL questionnaires for CF that evaluate general daily life have been validated: the CF Quality of Life (CFQoL) questionnaire 4 and the CF Questionnaire for teenagers and adults (CFQ 14+),5,6 which has been used as an outcome variable in clinical trials.7,8 However, these questionnaires are not sufficiently focused on respiratory symptoms to assess their impact on health status, a task that requires respiratory- or symptom-specific tools.
Two HRQoL questionnaires that were initially designed for other diseases – the Saint George’s Respiratory Questionnaire (SGRQ) 9 and the Leicester Cough questionnaire (LCQ) 10 – have been validated in both CF11,12 and non-CF bronchiectasis.13,14,15 The SGRQ was designed for chronic obstructive lung disease 9 and only the Spanish version has been validated in CF. 11 The LCQ is a 19-item questionnaire that measures the impact of coughing on the quality of life. It was designed for chronic cough and although it had not been validated in CF patients at the time, it was used as a primary outcome measure with gastroesophageal reflux surgery in CF. 16 More recently, the LCQ has appeared to be valid, reliable and responsive after an exacerbation in CF adults in a preliminary study 12 and a Spanish version has also been found to be reliable in CF children, 17 although neither study included a calculation of the sample size and both had less than 60 patients.
We have previously translated and validated a Spanish version of the LCQ (LCQ-Sp) in adult patients with non-CF bronchiectasis. 15 Given that cough is one of the most frequent symptoms of adult patients with CF bronchiectasis, we aimed to validate the LCQ-Sp in these patients.
Methods
Study design and participants
A prospective longitudinal multicentre study to validate the LCQ-Sp in CF bronchiectasis was performed. Consecutive adult patients with CF diagnosed according to the current guidelines 18 attending the CF clinics of four hospitals between April 2011 and November 2012 were invited to participate in the study. Bronchiectasis was diagnosed by high-resolution computed tomography (HRCT), and for inclusion, patients had to be in stable phase (defined as no respiratory exacerbation 19 in the previous month) and to have had an HRCT in the previous 5 years. Exclusion criteria: patients who were current smokers, had previous pulmonary resection (to avoid any influence on pulmonary values, dyspnoea and affected lobes) or who were unable to respond to the questionnaire. Respiratory exacerbation was defined as an acute development and persistence (beyond normal day-to-day variations) of changes in sputum characteristics (increased volume, thicker consistency, greater purulence and haemoptysis), increased cough and increased breathlessness. 19
The study was approved by the Ethical Committee of the Dr Josep Trueta Hospital (registration number: 2011053). All patients gave written informed consent before inclusion.
Study protocol
In order to validate the LCQ-Sp, an analysis of its feasibility, validity, reliability and responsiveness was undertaken following the Aaronson et al. recommendations. 20 All patients filled in at the hospital the LCQ-Sp, the SGRQ 9 and the modified Medical Research Council (mMRC) dyspnoea scale 21 (visit 1) in order to analyse feasibility, concurrent validity and internal consistency. The variables recorded at visit 1 were demographic data, sputum colour, 22 sputum volume in the previous 24-h (using a calibrated container) and the number of exacerbations and admissions in the previous 6 months. If the patient continued in stable phase at 15 days and there had been no modification to their treatment, they were asked to fill in the LCQ-Sp again and sent by post mail to each reference hospital (visit 2) to analyse the test–retest reliability. The rest of the variables, such as sputum microbiology of the last visit, whether or not the patient has chronic bronchial colonisation, the last respiratory function test made in the stable phase during the previous 12 months, and the last HRCT studies were recorded. Patients were also asked to fill in at the hospital the LCQ-Sp again in the case that they presented an exacerbation in the following 6 months (visit 3) in order to analyse responsiveness.
Chronic bronchial colonisation was considered when there were three or more positive cultures for the same microorganism within 6 months and in samples collected at least 1 month apart.
Endpoints
The primary endpoint of the study was to assess the internal consistency (reliability) of the LCQ-Sp. Secondary endpoints were to calculate the feasibility, the test–retest (reliability), the validity and the responsiveness of the LCQ-Sp.
Questionnaires
The LCQ 10 is a 19-item questionnaire that measures the impact of coughing on the quality of life in the two previous weeks in three domains: physical (eight items), psychological (seven items) and social (four items). The total severity score ranges from 3 to 21, with a lower score indicating greater impairment of health status due to cough. The minimal clinically important difference (MCID) for the total LCQ score is 1.3. 23 The LCQ-Sp has been proved as a valid tool to assess cough impact in non-CF bronchiectasis. 15
The SGRQ 9 consists of 50 items grouped in three domains: symptoms (eight items), activity (16 items) and impacts (26 items). The total score ranges from 0 to 100, with zero indicating no impairment to the quality of life. The MCID is 4. 24
The mMRC 21 dyspnoea scale consists of five statements about perceived breathlessness.
Statistics
The sample size of internal consistency for the Cronbach’s α was calculated using Bonnett’s formula. 25 Expecting a Cronbach’s α of 0.80 for the LCQ-Sp and setting a required level of 0.70 in a two-sided test at α=0.05, power of 0.80 and assuming a 10% missing data rate, a sample size of 113 subjects would be required.
Feasibility was analysed by calculating the percentage of patients without a response for the total score and for each domain of the LCQ-Spat visit 1. The percentage of patients obtaining the lowest possible score (floor effect) and highest possible score (ceiling effect) was analysed.
For the assessment of reliability, 26 internal consistency was estimated for the total number of items and for each of the domains at visit 1 using Cronbach’s α. Test–retest reliability to analyse changes in the LCQ-Sp score between visits 1 and 2 was estimated through the intraclass correlation coefficient (ICC) using a two-way mixed effects model and type consistency. 27 The commonly accepted minimal standard for reliability coefficients is 0.70 (<0.40=poor; 0.41–0.59=fair; 0.60–0.74=good; 0.75–1=excellent). 28 A graphical analysis was also performed using the Bland and Altman method. 28 The effect size was calculated using Cohen’s 29 criteria (small=0.2; moderate=0.5; large=0.8).
Validity was analysed using concurrent and convergent validity. For concurrent validity, the correlation of LCQ-Sp and SGRQ at visit 1 was analysed by Spearman’s rank correlation. The convergent validity was analysed comparing the statistical association between different variables suspected of being related to the cough (24-h sputum volume, sputum purulence, 22 chronic bronchial colonisation (no evidence, by microorganisms others than Pseudomonas aeruginosa or by P. aeruginosa), FEV1, mMRC dyspnoea scale and number of lobes affected) and values of the total LCQ-Sp using Kruskal–Wallis or rho Spearman, as appropriate.
For responsiveness, the mean change in total score between visit 1 and the beginning of the first exacerbation was compared using the paired Student’s t-test. The effect size was also calculated for responsiveness using Cohen’s 29 criteria.
A two-tailed p-value of <0.05 was significant. Statistical analysis was performed using SPSS for Windows, version 25.0 (IBM Corp., Armonk, New York, USA) and Stata/IC 13.1 (Stata Corp. 2013, Stata Statistical Software: Release 13, Stata Corp LP, College Station, Texas, USA).
Results
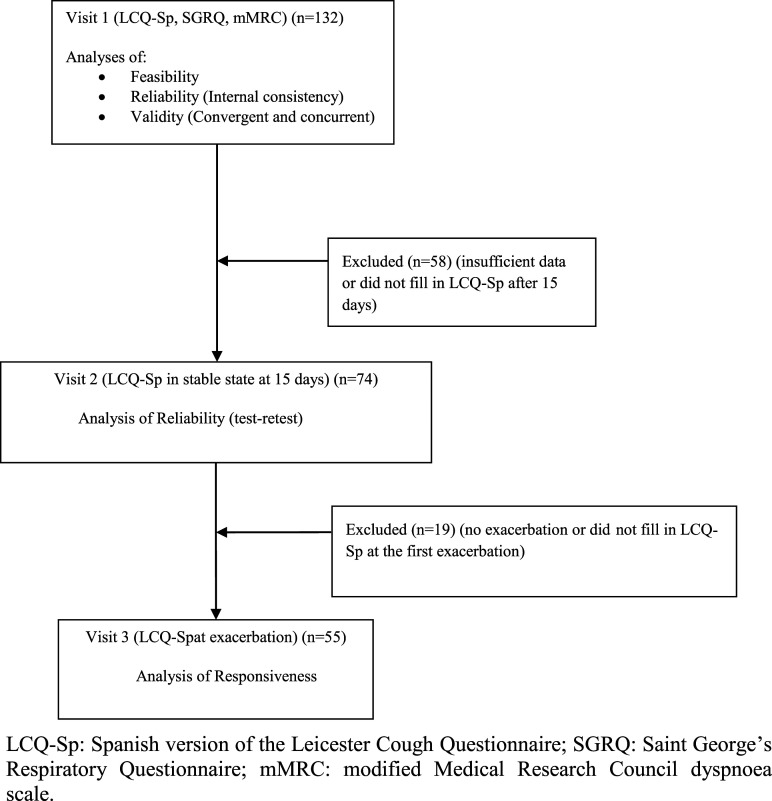
A total of 132 patients were included in the study. Baseline characteristics of the recruited patients are described in Table 1.
Table 1.
Baseline characteristics of recruited patients.
| All patients | |
|---|---|
| n | 132 |
| Females | 72 (54.5) |
| Age (years) | 29.73 ± 10.52 |
| Ex-smokers | 8 (6.1) |
| Body mass index (Kg/m2) | 22.28 ± 3.58 |
| Exacerbations in the preceding 6 months | 1.03 ± 1.22 |
| Hospitalisations in the preceding 6 months | 0.13 ± 0.38 |
| 24-h sputum volume (mL) | 25.78±22.87 |
| Sputum colour | |
| No expectoration | 12 (9.1) |
| Mucous | 17 (12.9) |
| Mucopurulent | 49 (37.1) |
| Purulent | 54 (40.9) |
| Chronic bronchial colonisation | |
| None | 12 (9.1) |
| Other than Pseudomonas | 84 (63.6) |
| Pseudomonas aeruginosa | 36 (27.3) |
| FEV1 % predicted | 60.8 ± 23.5 |
| FEV1 (mL) | 2108.93±891.6 |
| FVC % predicted | 73.4 ± 22.2 |
| FVC (mL) | 3155.1±1098.5 |
| mMRC dyspnoea score | |
| 0 | 77 (58.3%) |
| 1 | 40 (30.3%) |
| 2 | 11 (8.3%) |
| 3 | 4 (3.0%) |
| 4 | 0 |
| Number of affected lobes | 4.48 ± 1.76 |
Data are presented as mean ±SD or n (%), where applicable. FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; mMRC: modified Medical Research Council.
Feasibility
All the patients included in the study responded to the LCQ-Sp during visit 1. The response rate of these patients was 100% both for the total score and the three domains. No floor or ceiling effect was observed (Table 2).
Table 2.
Psychometric properties of the Spanish version of the Leicester Cough Questionnaire.
| Domain | Items (n) | No response % | Mean ± SD | Floor (%) | Ceiling (%) | Cronbach’s α | ICC (95% CI) | Effect size |
|---|---|---|---|---|---|---|---|---|
| Physical | 8 | 0 | 5.32 ± 1.13 | 2.15 (0.7) | 7 (2.3) | 0.852 | 0.80 (0.71–0.87) | −0.220 |
| Psychological | 7 | 0 | 5.34 ± 1.25 | 1.28 (0.7) | 7 (8.3) | 0.858 | 0.84 (0.76–0.89) | −0.220 |
| Social | 4 | 0 | 5.69 ± 1.23 | 0.7 (0.7) | 7 (14.4) | 0.813 | 0.77 (0.66–0.85) | −0.115 |
| Total | 19 | 0 | 16.59±3.34 | 5.91 (0.7) | 21 (1.5) | 0.937 | 0.86 (0.79–0.91) | −0.225 |
SD: standard deviation, ICC: intraclass correlation coefficient.
Reliability
The internal consistency of the LCQ-Sp of the 132 patients at visit 1 was high for the total score and good for the different domains, with Cronbach’s α values ranging from 0.81 to 0.93 (Table 2).
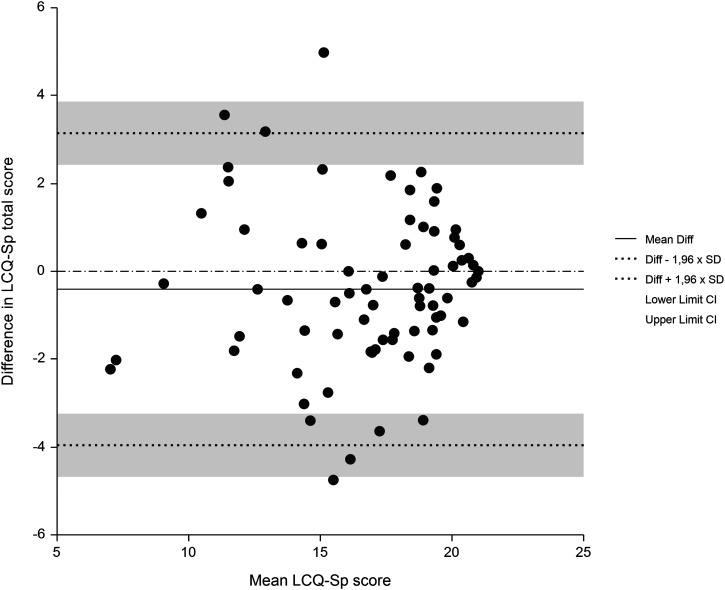
The test–retest reliability was calculated with the scores of the LCQ-Sp of the 74 patients that filled in the questionnaire again at 15 days (Figure 1). The ICC obtained indicated excellent stability for the total score and for the three domains, with values ranging from 0.77 to 0.86. Using Cohen’s criteria, a small effect size for difference in means was obtained for the total score and each domain (Table 2). Figure 2 shows a Bland–Altman plot of the difference between repeat total scores for the LCQ-Sp.
Figure 1.
Flow chart of the study. LCQ-Sp: Spanish version of the Leicester Cough Questionnaire; SGRQ: Saint George’s Respiratory Questionnaire; mMRC: modified Medical Research Council dyspnoea scale.
Figure 2.
Bland–Altman plot of Spanish version of the Leicester Cough Questionnaire total score repeated over 15 days in 74 patients with stable cystic fibrosis. The solid line represents the mean change in score and the dashed line represents the limits of agreement.
Validity
In the analysis of concurrent validity, the LCQ-Sp had a significant moderate inverse correlation with the SGRQ at visit 1 for the total score and for the three domains, with scores ranging from −0.47 to −0.76 (Table 3).
Table 3.
Correlation of the Spanish version of the Leicester Cough Questionnaire with the Saint George’s Respiratory Questionnaire at visit 1, convergent validity.
| SGRQ | ||||
|---|---|---|---|---|
| LCQ-Sp | Symptoms | Activity | Impacts | Total |
| Physical | −0.59** | −0.60** | −0.65** | −0.76** |
| Psychological | −0.49** | −0.51** | −0.65** | −0.70** |
| Social | −0.51** | −0.47** | −0.63** | −0.67** |
| Total | −0.55** | −0.55** | −0.67** | −0.74** |
**Significant Spearman correlation: p<0.01 (bilateral). SGRQ: Saint George’s respiratory questionnaire. LCQ-Sp: Leicester Cough Questionnaire Spanish version.
The results of convergent validity using the variables selected show that mMRC and sputum volume had a significant negative correlation, and FEV1 had a positive significant correlation. Purulence of sputum, chronic bronchial colonisation and affected lobes had no association with the LCQ score (Table 4).
Table 4.
Convergent validity: correlation between variables suspected of being related to cough and LCQ-Sp total score.
| Variables | Rho Spearman | Median LCQ-Sp | Interquartile range (25–75%) LCQ-Sp | p |
|---|---|---|---|---|
| mMRC | <0.001 | |||
| 0 | — | 18.50 | 15.81–19.85 | |
| 1 | — | 16.26 | 13.66–18.89 | |
| 2 | — | 14.96 | 11.89–16.54 | |
| 3 | — | 12.93 | 9.78–15.01 | |
| 4 | — | 0 | 0 | |
| Sputum purulence | 0.070 | |||
| No expectoration/mucous | — | 18.88 | 14.98–20.30 | |
| Mucopurulent | — | 16.62 | 15.07–19.33 | |
| Purulent | — | 16.85 | 13.04–18.94 | |
| Chronic bronchial colonisation | 0.283 | |||
| None | — | 17.07 | 16.20–20.01 | |
| Pseudomonas aeruginosa | — | 16.43 | 13.31–19.07 | |
| Other than Pseudomonas | — | 17.21 | 13.73–19.78 | |
| FEV1% predicted | 0.210 | — | — | 0.016 |
| 24-h sputum volume (mL) | −0.328 | — | — | <0.001 |
| Number of affected lobes | 0.071 | — | — | 0.427 |
FEV1: forced expiratory volume in 1 s; LCQ: Leicester Cough Questionnaire; LCQ-Sp: Spanish version of the LCQ; mMRC: modified Medical Research Council.
Responsiveness
The mean LCQ-Sp total score at visit 1 decreased in the 55 patients that filled in the questionnaire at the beginning of the first exacerbation compared to baseline score in stable state (13.91 ± 4.29 vs 16.04 ± 3.81 respectively; mean difference of −2.15 (−2.84 and −1.47 95% CI); p < 0.001). The magnitude of the difference was higher than the MCID of 1.3. 21 The mean score of the individual domains also decreased significantly (physical 5.24 ± 1.19 vs 4.50 ± 1.43, psychological 5.27 ± 1.40 vs 4.57 ± 1.53 and social 5.34 ± 1.44 vs 4.80 ± 1.56; p < 0.001). The LCQ-Sp showed a large effect size of 0.863.
Discussion
The validation of the LCQ-Sp in adult patients with CF in this multicentre study shows that this version is reliable, valid and responsive to change for use in these patients, where cough is one of the main symptoms.
The analysis of feasibility shows that all the patients completed all of the answers, suggesting that patients do not find the questionnaire difficult to respond to. The absence of a significant floor or ceiling effect indicates that the results of the questionnaire were not affected by extreme values. In the case of reliability, good internal consistency for all the domains as well as for the total score were obtained, with a high Cronbach’s α, good repeatability, a high ICC, and a low effect size. With regard to concurrent validity, the LCQ-Sp total score had a significant moderate inverse correlation with the total SGRQ score, which means that worse impact of cough correlated with more impairment in quality of life. The correlation was only moderate probably because the LCQ and the SGRQ provide information on different aspects of the impact of bronchiectasis on the HRQoL. These results are similar to those obtained in the original version of the questionnaire 10 and in non-CF bronchiectasis. 15 In the case of convergent validity, greater 24-h sputum volume, worse dyspnoea and worse FEV1 were associated with a greater impact of cough while the number of lobes affected was not found to be associated with cough impact. Unlike our findings in non-CF bronchiectasis, no associations with sputum purulence and chronic bronchial colonisation were observed probably because CF is a more homogeneous population with more severe bronchial damage (more patients had chronic colonisation and purulent sputum). As in non-CF bronchiectasis, 15 responsiveness was analysed at the beginning of an exacerbation and a significant worsening was detected across all domains and in the total score with a large effect size. This suggests that the LCQ-Sp could be useful in confirming the presence of an exacerbation in research studies and clinical trials.
The results obtained in the present study are similar to those obtained in the preliminary study with the English version of the LCQ at a single CF adult centre 12 and those obtained in a multicentre study with another Spanish version in a CF paediatric population. 17 However, there are some differences from the current study. Neither of the two studies included a calculation of the sample size and fewer patients were included. The populations studied had better pulmonary function, which may explain why they found a ceiling effect in the psychological and social domains of the LCQ in the adult study 12 and in all the domains in the paediatric study. 17 The results for internal consistency were lower in the paediatric population probably because of the lower age of the patients. 17 The time to analyse test–retest reliability was 1 week after completing the initial questionnaire in the adult study, 12 and between 15 and 30 days afterwards in the paediatric study, where only levels >0.70 of ICC were achieved in the total LCQ score. 17 Concurrent validity was assessed in both studies with the CFQ-revised showing moderate correlations. Neither convergent validity nor variables related to bronchiectasis (sputum volume, sputum purulence, dyspnoea, the presence of chronic colonisation and the presence and extension of bronchiectasis) were reported in either study. Responsiveness, which was only assessed in the adult study, was evaluated in a different population to that used to test validity and reliability, and they measured the change from the initiation of treatment for the exacerbation to 4 weeks later. An increase in LCQ total scores by a mean of 3.6 and a large effect size ranging from 1.1 to 1.3 was reported. 12
This study has several particular strengths. First of all, it is a multicentre study, enabling a large sample size to be obtained over a relatively short period of time, improving the rigour of the methodology and increasing the representativeness of the results. Secondly, a sample size calculation was made. Finally, responsiveness was analysed at the onset of an exacerbation. Possible limitations of the present study could be the loss of patients to analysis of the test–retest reliability although this does not affect the primary endpoint, and the use of the SGRQ instead of a CF-specific HRQoL questionnaire,4,5,6 for the analysis of validity. The SGRQ was chosen for different reasons: to follow the same methodology as in the non-CF bronchiectasis study, to focus on respiratory disease 15 because the Spanish version had been validated in CF patients with a good correlation with the Spanish version of CFQR 14+ 30 and because no Spanish version of the CFQoL 4 was available. No analysis of discriminant validity was performed due to the lack of CF bronchiectasis severity scales.
CFTR modulator therapy is expected to improve mucociliary clearance and prevent the establishment of irreversible airway disease. 1 However, the LCQ can still be useful to evaluate changes in the impact of cough on HRQoL in those patients with established bronchiectasis and chronic bronchial colonisation. Validated versions in different languages allow their use in multicentre studies.
Conclusions
In conclusion, the LCQ-Sp is a valid and reliable instrument to measure changes in the impact of cough in adult patients with CF bronchiectasis and is responsive to change in the event of exacerbations.
Acknowledgements
We would like to thank Andrew Hughes for assistance in drafting the manuscript in English.
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: All authors must disclose any financial and personal relationships with other people or organisations that could inappropriately influence their work.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Gerard Muñoz https://orcid.org/0000-0002-5420-0556
Javier de Gracia https://orcid.org/0000-0003-2830-1991
References
- 1.Bell SC, Mall MA, Gutierrez H, et al. The future of cystic fibrosis care: a global perspective. Lancet Respir Med 2020; 8(1): 65–124. DOI: 10.1016/S2213-2600(19)30337-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Que C., Cullinan P, Geddes D. Improving rate of decline of FEV1 in young adults with cystic fibrosis. Thorax 2006; 61(2): 155–157. DOI: 10.1136/thx.2005.043372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kent L, Reix P, Innes JA, et al. Lung clearance index: evidence for use in clinical trials in cystic fibrosis. J Cystic Fibrosis 2014; 13(2): 123–138. DOI: 10.1016/j.jcf.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Gee L., Abbott J, Conway SP, et al. Development of a disease specific health related quality of life measure for adults and adolescents with cystic fibrosis. Thorax 2000; 55: 946–954. DOI: 10.1136/thorax.55.11.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henry B, Aussage P, Grosskopf C, et al. Development of the cystic fibrosis questionnaire (CFQ) for assessing quality of life in pediatric and adult patients. Qual Life Res 2003; 12: 63–76. DOI: 10.1023/a:1022037320039. [DOI] [PubMed] [Google Scholar]
- 6.Quittner AL, Buu A, Messer MA, et al. Development and validation of the cystic fibrosis questionnaire in the United States. Chest 2005; 128(4): 2347–2354. DOI: 10.1378/chest.128.4.2347. [DOI] [PubMed] [Google Scholar]
- 7.Moss RB, Flume PA, Elborn JS, et al. Efficacy and safety of ivacaftor in patients with cystic fibrosis who have an Arg117His-CFTR mutation: a double-blind, randomised controlled trial. Lancet Respir Med 2015; 3(7): 524–533. DOI: 10.1016/S2213-2600(15)00201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heijerman HGM, McKone EF, Downey DG, et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet (London, England) 2019; 394(19): 1940–1948. DOI: 10.1016/S0140-6736(19)32597-8. pii: S0140-6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones PW, Quirk FH, Baveystock CM, et al. A self-complete measure of health status for chronic airflow limitation: the st. george's respiratory questionnaire. Am Rev Respir Dis 1992; 145: 1321–1327. DOI: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 10.Birring SS, Prudon B, Carr AJ, et al. Development of a symptom specific health status measure for patients with chronic cough: leicester cough questionnaire (LCQ). Thorax 2003; 58: 339–343. DOI: 10.1136/thorax.58.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Padilla A, Olveira G, Olveira C, et al. Validity and reliability of the St George’s respiratory questionnaire in adults with cystic fibrosis. Archivos de Bronconeumología ((English Edition)) 2007; 43: 205–211. DOI: 10.1016/s1579-2129(07)60052-4. [DOI] [PubMed] [Google Scholar]
- 12.Ward N, Stiller K, Rowe H, et al. The psychometric properties of the leicester cough questionnaire and respiratory symptoms in CF tool in cystic fibrosis: a preliminary study. J Cystic Fibrosis 2017; 16(3): 425–432. DOI: 10.1016/j.jcf.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Wilson CB, Jones PW, O’Leary CJ, et al. Validation of the St. George’s respiratory questionnaire in bronchiectasis. Am J Respir Crit Care Med 1997; 156(2 Pt 1): 536–541. DOI: 10.1164/ajrccm.156.2.9607083. [DOI] [PubMed] [Google Scholar]
- 14.Murray MP, Turnbull K, MacQuarrie S, et al. Validation of the leicester cough questionnaire in non-cystic fibrosis bronchiectasis. Eur Respir J 2009; 34: 125–131. DOI: 10.1183/09031936.00160508. [DOI] [PubMed] [Google Scholar]
- 15.Muñoz G, Buxó M, de Gracia J, et al. Validation of a Spanish version of the leicester cough questionnaire in non-cystic fibrosis bronchiectasis. Chronic Respir Dis 2016; 13(2): 128–136. DOI: 10.1177/1479972316632005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fathi H, Moon T, Donaldson J, et al. Cough in adult cystic fibrosis: diagnosis and response to fundoplication. Cough 2009; 5: 1. DOI: 10.1186/1745-9974-5-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Del Corral T, Percegona J, López N, et al. Validación de la versión en español del Cuestionario de Tos Leicester en niños con fibrosis quística. Archivos de Bronconeumología 2016; 52(2): 63–69. DOI: 10.1016/j.arbres.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 18.O’Sullivan BP, Freedman SD. Cystic fibrosis. Lancet 2009; 373(9678): 1891–1904. DOI: 10.1016/S0140-6736(09)60327-5. [DOI] [PubMed] [Google Scholar]
- 19.Goss CH, Burns JL. Exacerbations in cystic fibrosis {middle dot} 1: epidemiology and pathogenesis. Thorax 2007; 62: 360–367. DOI: 10.1136/thx.2006.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aaronson N, Alonso J, Burnam A, et al. Assessing health status and quality-of-life instruments: attributes and review criteria. Qual Life Res 2002; 11(3): 193–205, DOI: 10.1023/a:1015291021312. [DOI] [PubMed] [Google Scholar]
- 21.Brooks SM. Divisions. Europhysics News 1982; 13: 12. [Google Scholar]
- 22.Murray MP, Pentland JL, Turnbull K, et al. Sputum colour: a useful clinical tool in non-cystic fibrosis bronchiectasis. Eur Respir J 2009; 34: 361–364. DOI: 10.1183/09031936.00163208. [DOI] [PubMed] [Google Scholar]
- 23.Raj AA, Pavord DI, Birring SS. Clinical cough IV:what is the minimal important difference for the leicester cough questionnaire?. Pharmacol Ther Cough 2009; 187: 311–320. DOI: 10.1007/978-3-540-79842-2_16. [DOI] [PubMed] [Google Scholar]
- 24.Jones PW. Interpreting thresholds for a clinically significant change in health status in asthma and COPD. Eur Respir J 2002; 19: 398–404. DOI: 10.1183/09031936.02.00063702 [DOI] [PubMed] [Google Scholar]
- 25.Bonett DG. Sample size requirements for testing and estimating coefficient alpha. J Educ Behav Stat 2002; 27: 335–340. DOI: 10.3102/10769986027004335 [DOI] [Google Scholar]
- 26.Kirshner B, Guyatt G. A methodological framework for assessing health indices. J Chronic Dis 1985; 38: 27–36. DOI: 10.1016/0021-9681(85)90005-0. [DOI] [PubMed] [Google Scholar]
- 27.McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychol Methods 1996; 1: 30–46. DOI: 10.1037/1082-989X.1.1.30 [DOI] [Google Scholar]
- 28.Martin Bland J, Altman D. Statistical methods for assessing agreement between two methods of clinical measurement. The Lancet 1986; 327: 307–310. [PubMed] [Google Scholar]
- 29.Cohen J. Statistical Power Analysis for the Behavioural Sciences. 2nd ed.. New Jersey: Lawrence Erlbaum, 1988. [Google Scholar]
- 30.Olveira G, Olveira C, Gaspar I, et al. Validation of the spanish version of the revised cystic fibrosis quality of life questionnaire in adolescents and adults (CFQR 14+ Spain). Archivos de Bronconeumología ((English Edition)) 2010; 46: 165–175. DOI: 10.1016/j.arbres.2010.01.006. [DOI] [PubMed] [Google Scholar]