Abstract
Background:
There is no study conducted on the association between disinfection byproducts (DBPs) in chlorinated drinking water and colorectal cancer (CRC) in Ethiopia. Therefore, this study aimed to determine the relation between chlorine based DBPs in drinking water and CRC in Addis Ababa, Ethiopia.
Methods:
A facility based matched case control study was conducted involving 224 cases and 448 population controls from June 2020 to May 2021. Cases were defined as histologically confirmed CRC cases. Cases were matched with controls by residence, age, and sex using frequency and individual matching. Geocoding of cases, health facility, and georeferencing of controls were carried out. Data was collected using a pretested structured questionnaire. Pearson Chi square and Fisher’s exact tests were employed to assess associations. Stratified analysis was used to detect confounding factors and effect modification. A multivariable conditional logistic regression was used to identify risk factors of CRC.
Results:
Of 214 CRC cases, 148 (69.2%) used chlorinated water whereas out of 428 controls 161 (37.6%) used chlorinated water. In the final regression model, drinking chlorinated surface water (adjusted matched odds ratio [adjusted mOR] = 2.6; 95% CI 1.7-4.0), history of swimming (adjusted mOR = 2.4; 95% CI 1.4-4.1), years at the place of current residence (adjusted mOR = 1.5; 95% CI 1.1-2.2), hot tap water use for showering (adjusted mOR; 3.8 = 95% CI 2.5-5.9) were significantly associated with CRC. The stratified analysis confirmed that smoking and meat ingestion were not effect modifiers and confounders.
Conclusion:
Drinking chlorinated water for extended years is a significant risk factor for CRC in Addis Ababa, Ethiopia. In addition, hot tap water use for showering, and swimming history are risk factors for CRC. This information is essential to design integrated interventions that consider chlorination by-products and exposure routes toward the prevention and control of CRC in Ethiopia. Initiating alternative methods to chlorine disinfection of drinking water is also essential.
Keywords: Chlorination, colorectal cancer, disinfection byproduct, drinking water, matched case-control, Ethiopia
Background
Colorectal cancer (CRC) represents nearly 10% of all cancer incidences that showed an increasing rate over the last 2 decades. 1 Studies from African and Asian countries have shown that the annual diagnosis of CRC is increasing.2,3 Evidence also indicates that the incidence of the disease has increased in most developing countries due to the emergence of potential risk factors such as smoking, obesity, an unhealthy diet, and lifetime exposure to drinking water chlorination by-products.4-7 However, high dietary intake of meat was not identified as risk factor in these countries because of the limited supply to the larger population.8-10
Chlorination has been the primary treatment process to improve the quality of drinking water. 4 However, chlorine, as a disinfectant in drinking water, is known to produce trihalomethanes (THMs). 11 THMs in chlorinated drinking water may cause health threats due to its carcinogenicity. 12 In water treatment utilities, DBPs, like chloroform, bromate, trichloroacetic acid, and bromodichloromethane are mostly formed through the reaction of chlorine with natural organic matter (NOM). The DBPs are divided into 2 categories: hydrophilic and hydrophobic. 1
THMs were regulated by the USEPA (United States Environmental Protection Authority) shortly after their discovery in disinfected drinking water, with a maximum contaminant limit (MCL) of 100 g/L for TTHMs (Total trihalomethanes). 13 The Stage 1 D-DBP Rule 14 reduced the MCL (maximum contaminant levels) for TTHM to 80 g/L and set MCLs of 60, 10, and 1000 g/L for HAAs (haloacetic acids), bromate, and chlorite respectively. Although the maximum acceptable concentrations (MACs) for TTHM and HAAs are 100 and 80 g/L, respectively, and more DBP species are controlled in Canada, 15 the standards are similar to those in the United States. The South African National Standards has set the MCLs for total THMs in drinking tap water at 300 μg/L. 16
THMs and HAAs, which occur in high amounts in drinking water, are the most commonly investigated and quantified DBPs. THMs are used as a proxy by drinking water utilities in several countries, including Southern Africa, the United States, and Canada, to monitor and control DBPs in drinking water distribution systems. 17
In Ethiopia, water treatment utilities use chlorine for the disinfection of water for public distribution. 18 However, drinking water utilities in Ethiopia in general and in Addis Ababa in particular not measured drinking water DBPs at all. There is also no monitoring and controlling system of DBPs in drinking water distribution systems in Ethiopia. In addition, the water utilities in Ethiopia not set the MAC of disinfection byproducts for total THMs and other DBPs. Therefore in the absence of historical data on the level of chlorination byproducts (DBPs), estimates of past exposure have been based on previous information about the water sources (ground and surface water sources). 19
Studies showed that people drinking chlorinated water, particularly chlorinated surface water, had increased risk of colon and rectal cancers.20,21 Several case-control studies on exposure to chlorination by-products reported positive findings for colon and rectal cancers but the interpretation was limited.22-24 Case-control studies of cases related with drinking water chlorination have the potential to overcome the limitations of the early studies, but relatively few have been conducted.25-27 Moreover, there is no available study on the association between disinfection byproducts (DBPs) in chlorinated drinking water and colorectal cancer (CRC) in Ethiopia. Hence, this study aimed to determine the effects of drinking chlorinated water on CRC in Addis Ababa, Ethiopia.
Methods
Study area and population
This study was conducted in Tikur Anbessa Specialized Hospital (TASH), College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia. TASH serves as a cancer registry and treatment center for the country. The departments in TASH are gynecology and obstetrics, internal medicine, surgery, pediatrics, radiotherapy, adult oncology, pediatric oncology/hematology, nuclear medicine, psychiatry, laboratory, orthopedics pharmacy, and others. 28 Incident CRC cases were collected from adult oncology and pediatric oncology/hematology respectively. In 2017, the Central Statistical Agency (CSA) projected the population of Addis Ababa to 3,434,000, of which 1,624,999 were males. 29 The target of this study was CRC cases and controls living in Addis Ababa, Ethiopia (Figure 1).
Figure 1.
Location of cases households (HHs), controls households, and study hospital, 2021.
Study design and period
A hospital-based matched case-control study design was employed from June 2020 to May 2021. Histologically confirmed CRC cases were included in the present analysis (ICD-O [C18, C19, C20]). 30
Sample size determination
The sample size was calculated using a matched case-control study design 31 by considering the pitman efficiency assumption of the matched pair sample size. 32 A matched sample size of 224 pairs using individual matching (1 case-2 controls: which means 224 cases: 448 controls) was selected by taking into account (1) a type I error of 5%; (2) a probability of type II error 10%; (3) a power of 90%; (4) assumption that 30% of the control households use non-chlorinated water supply in Addis Ababa 31 ; (5) expected odds ratio of 2; and (6) a 15% non-response rate. A 50% of matched pair samples of case and control (112 cases:224 controls) was taken as the minimum requirement for exposure discordant pairs for testing disease and exposure association. 33
Inclusion and exclusion criteria
In both case and control, study participants were in the age range of 20 to 85 years; residents of Addis Ababa for at least 10 years before recruitment and lived at the time of contact and answered study questionnaire. Only CRC cases diagnosed from June 2020 to May 2021 with histological confirmation (C18, C19, C20), without previous cancer history were included.
Matching of Cases and Controls
Controls were both individual and frequency matched to cases by sex and age (±5 years) ensuring 2 controls of the same sex and a 5-year interval for each case. 32 In addition, controls were matched based on their residence location.
Case and control selection
Cases were interviewed directly after diagnosis and identified through active checkups and periodic visits to hospital departments (adult oncology and pediatric oncology/hematology).
To identify population control subjects, households randomly selected from the 10 sub cities in Addis Ababa. The matching population controls sample size (428) randomly selected from the 10 sub cities in Addis Ababa (Figure 2).
Figure 2.
Population control selection procedure of Drinking water source, chlorinated water, and colorectal cancer,Addis Ababa, Ethiopia, 2021.
Controls were selected from the general population per each sub cities (the largest city under Addis Ababa) and “woredas” (the smallest administrative unit under the sub city). The population controls randomly selected from the same residence area for the cases per each sub city. Then the allocated sample size selected from identified household in the “woredas” using matching criteria. Simple random sampling technique was used to select the study participants from selected households (per each woreda). If the household was not had the matching person after interview or refused to participate, the next households were approached. The frequency matching was done based on the age and sex distribution of cases. Person with previous cancer diagnosis was excluded from consideration as controls.
Data collection
Hospital-based survey data was collected using a standardized and pre-tested questionnaire. The questionnaire was adapted from different works of literature and/or developed from other related studies.1,34-38 Some variables signifying these factors were also coded using the operational definitions (1A).
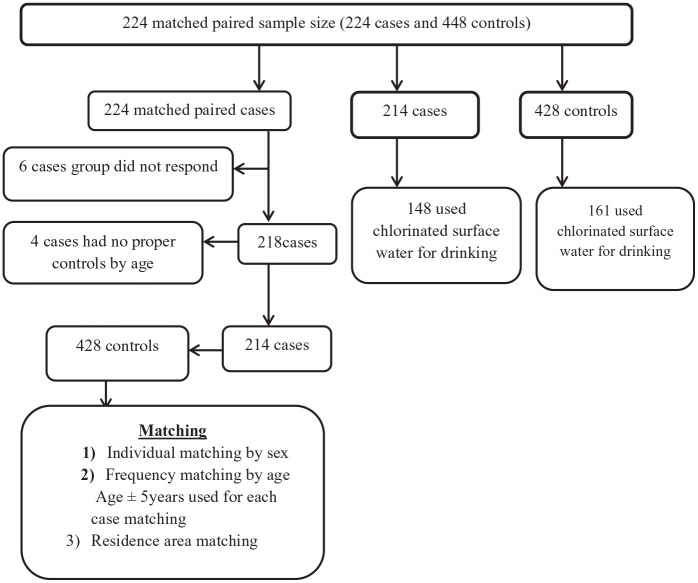
For both cases and controls, 12 trained nurse professionals (6 them were Oncology nurses) administered the survey by interviewing study participants using a pre-tested structured questionnaire. Interviewers were health professionals with BSc and above and fluent speaker of Amharic language trained on the tools. The data collectors and supervisors received three days training to ensure that study’s objectives were met. In addition COVID-19 prevention protocol training was provided. The instruments were also pretested (5% of the sample) in the health care facility (non-selected facility). The tools were validated based on the results of the pretest. The final number of total cases and controls and the water types used are described in Figure 3.
Figure 3.
Facility-based matched case-control study flow chart of Addis Ababa, Ethiopia, 2021.
Water type verification
To identify the type of water sources used by patients, geocoding was done immediately after data collection of cases using their residential information. Administrative units, roads’ lines, and spatial datasets were used for geocoding. The administrative unit data sets included administrative boundaries as areas and allocation centers as points for sub-cities (the largest administrative unit in Addis Ababa City) and woredas (the least administrative unit per sub-city) and geo-referencing of control households were also done.
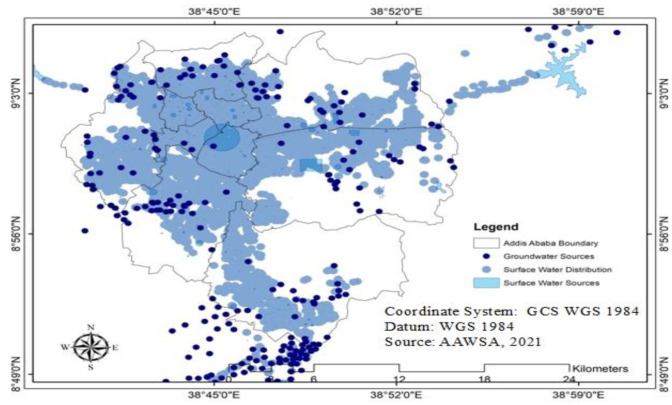
The water supply system of both cases and controls were identified using Geographic Information System (GIS) data from the Addis Ababa Water and Sewerage Authority (AAWSA) water supply network. 39 Following the water source distribution network, each household was classified either as chlorinated or non-chlorinated. Then, all cases and controls were classified into chlorinated surface water and non-chlorinated groundwater. AAWSA assisted in the identification of some households where there was a difficulty in distinguishing the source of water supply. The water supply operations in Addis Ababa contain 13 sub-systems. The chlorinated surface water sources are located in the western and eastern parts of the city. The groundwater sources (non-chlorinated) are located in the southeastern and various sites of the Addis Ababa 40 (Figure 4).
Figure 4.
Water supply networks of Addis Ababa, Ethiopia, 2021.
Dietary history before diagnosis
Data on major recognized diets related with colorectal cancer were collected by structured interviews using semi-quantitative food frequency questionnaire (FFQ) previously used in a study in Ethiopia and other countries.28,35 Usual dietary intake of the year before the interview was assessed. The FFQ included egg, meat, fish, fat-rich food, fruits, vegetables, sweet food, and beverages. Frequency variables were labeled as “at least once a day,” “4-6 times per week,” “2-3 times per week,” “once a week,” “2-3 times per month,” “once a month,” and “never or ever in a year 36 .”
Survey of Shower Water Temperature
A survey of shower water temperature was conducted in the homes of chosen patients and controls. The study included 45 individuals from the case households and 45 people from the control households. First, the participants’ shower water temperature was adjusted as usual in their homes. The water that had been drained from the shower was then collected in a bucket. In addition, household with no facility of shower were requested to bring the type of water used for shower. Finally, the investigator took the temperature of the water with a thermometer. The sample size was proportional to the number of cases and controls (1:1).
Variables and measurements
The outcome variable was CRC. Water supply type (chlorinated surface water or non-chlorinated groundwater) was the primary exposure variable. The remaining variables were covariates (Supplemental Table 1A).
Statistical analysis
Data entry and analysis was conducted using EpiData Version 3.1 software (EpiData Association, Odense, Denmark) and STATA version 14.0 (Statistical Software: College Station, TX, USA) respectively. For continuous variables, descriptive statistics (n [%]) were produced, as well as median IQR (interquartile range). For categorical variables, Pearson Chi-square and Fisher’s exact tests were employed to assess associations. All reported P-values were 2-tailed and considered statistically significant if P < .05.
Conditional Logistic regression model was used to estimate matched odds ratios (unadjusted and adjusted odds ratios). Variables with P < .2 from bivariate analyses and variables with known biological plausibility were included in the multivariable model. Our analysis did not take account of age, sex and residence area, as all were used as matching variables.
Cochran Mantel–Haenszel stratified analyses were used to exclude cofactors, and to ascertain and designate effect modifiers. Mantel–Haenszel test of equality of stratum-specific ORs was used to detect effect modifiers. First, the equality of stratum-specific ORs was tested to confirm significant changes, indicating that the exposure and other factors were modifying the effect. The presence of confounding was next investigated if the results were not different. By correcting the effects of the third variable and the crude estimate, the Mantel–Haenszel test provided a single weighed estimate of the predictor. The presence of confounding was then determined by measuring the difference between the crude and adjusted ORs. The evidence of confounding was confirmed using a relative difference in the effect estimate with a limit of 10%. 41 The variables was set a priori to take place, if an interaction term between smoking or meat ingestion and each of the reported variables was significant.
Results
Socio-demographic characteristics of cases and controls
The response rate for fully matched paired data was 95.5% (214 matched pairs). Ten cases did not match any controls (Figure 3). Median ages of the cases were 50 years (Inter Quartile Range [IQR]: 40-61 years) and of controls 49 years (IQR: 40-60 years). The median monthly income was $125.0 (IQR: 31.3-187.5 USD) and $ 136.6 (IQR: 71.9-250.0 USD) for the case and control respectively (Table 1).
Table 1.
Characteristics of case and control of study participants in Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia, 2021.
| Variables | Case (n = 214) | Control (n = 428) |
|---|---|---|
| Age (year) (median and IQR) | 50 (41-61) | 49 (40-60) |
| Monthly income in USD a (median and IQR) | 125.0 (31.3-187.5) | 136.5 (71.8-250.0) |
| Last two years drinking water consumption (l/c/d) (median and IQR) | 1.5 (1.5-2.0) | 1.5 (1.5-2.0) |
| Years at the place of longest residence (median and IQR) | 40 (30-47) | 40 (30-50) |
| BMI (mean ± SD) | 22.54 ± 3.70 | 24.88 ± 3.24 |
Abbreviations: l/c/d, liter per capita per day; $US, United States Dollars; BMI, body mass index; IQR, interquartile range.
The average exchange rate of $ US = 38.0 ETB (Ethiopia birr) from June 2020 to May 2021.
Regarding the age distribution of cases and controls, 133 (20.7%) were between 20 and 39 years old, 403 (62.8%) between 40 and 64 years old, 83 (12.9%) between 65 and 74 years old, and 23 (3.5%) above 75 years old. Out of 214 cases, 43.9%, and out of 428 controls, 45.3% were males. About 188 (29.3%) of the cases and controls have stable source of income (Table 2).
Table 2.
Sociodemographic characteristics of cases and controls in Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia, 2021.
| Variables | Cases (n = 214) | Controls (n = 428) | Total | Pearson’s χ2 |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Age category (y) | ||||
| 20-39 | 38 (17.8) | 95 (22.2) | 133 (20.7) | 0.610 |
| 40-64 | 138 (64.5) | 265 (61.9) | 403 (62.8) | |
| 65-74 | 30 (14.0) | 53 (12.4) | 83 (12.9) | |
| ⩾75 | 8 (3.7) | 15 (3.5) | 23 (3.5) | |
| Sex | ||||
| Men | 94 (43.9) | 194 (45.3) | 288 (44.9) | 0.736 |
| Women | 120 (56.1) | 234 (54.7) | 354 (55.1) | |
| Educational level | ||||
| Lower a | 129 (0.58) | 244 (0.57) | 373 (0.58) | 0.428 |
| Higher | 85 (0.40) | 184 (0.43) | 269 (0.42) | |
| Marital status | ||||
| Unmarried | 85 (39.7) | 173 (40.4) | 258 (40.2) | 0.864 |
| Married | 129 (60.3) | 255 (59.6) | 384 (59.8) | |
| Stable source of income | ||||
| Yes | 59 (27.6) | 129 (30.1) | 188 (29.3) | 0.500 |
| No | 155 (72.4) | 299 (69.9) | 454 (70.7) | |
| Employment status | ||||
| Yes | 122 (57.0) | 251 (58.6) | 373 (58.1) | 0.692 |
| No | 92 (42.9) | 177 (41.4) | 269 (41.9) | |
| Average monthly income | ||||
| >126 ($US) | 87 (46.3) | 177 (51.9) | 264 (49.9) | 0.215 |
| <126 ($US) | 101 (53.7) | 164 (48.1) | 265 (50.1) | |
Includes from not capable to read or write based on self-reporting.
Water supply type, chlorination status, and residence history
Around 92% of cases and 73.4% of controls consumed water from private taps, whereas 26.6% of controls and 7.9% of cases fetched water from public taps. Similarly, about two-thirds (69.2%) of the cases and one-third of the controls (37.6%) used chlorinated water. About 96.7% of the cases and 93.2% controls used shower. In addition, 54.2% and 41.6% of the cases and controls had lived more than 40 years at their place of current residence respectively (Table 3)
Table 3.
Water supply type, chlorination status and residence history in Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia, 2021.
| Variable | Cases (n = 214) | Controls (n = 428) | Total | Pearson’s χ2 |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Piped water supply | ||||
| Private tap | 197 (92.1) | 314 (73.4) | 511 (79.6) | 0.001 |
| Public tap | 17 (7.9) | 114 (26.6) | 131 (20.4) | |
| Chlorination status | ||||
| Chlorinated surface water | 148 (69.2) | 161 (37.6) | 309 (48.1) | 0.001 |
| Non-chlorinated groundwater | 66 (30.8) | 267 (62.4) | 333 (51.9) | |
| Drinking of water (l/d) | ||||
| >1.5 | 114 (53.7) | 223 (52.1) | 337 (52.5) | 0.780 |
| <1.5 | 100 (46.7) | 205 (47.9) | 305 (47.5) | |
| Regular showering | ||||
| Yes | 207 (96.7) | 399 (93.2) | 606 (93.2) | 0.169 |
| No | 7 (3.3) | 29 (6.8) | 36 (6.7) | |
| Water used for shower | ||||
| Hot water | 86 (40.2) | 59 (13.8) | 145 (22.6) | 0.001 |
| Cold water | 128 (59.8) | 369 (86.2) | 497 (77.4) | |
| Swimming history | ||||
| Ever | 106 (49.5) | 134 (31.3) | 240 (37.4) | 0.001 |
| Never | 108 (50.5) | 294 (68.7) | 402 (62.6) | |
| Types of swimming | ||||
| Outdoor | 58 (37.9) | 95 (62.1) | 153 (63.7) | 0.567 |
| Indoor | 49 (56.3) | 38 (43.7) | 87 (36.3) | |
| Years at place of current residence | ||||
| ⩾40 | 116 (54.2) | 178 (41.6) | 294 (45.8) | 0.002 |
| <40 | 98 (45.8) | 250 (58.4) | 348 (54.2) | |
Health related characteristics and dietary information
Around 3.7% of the cases and 9.4% of the controls had high BMI, respectively. Similarly, 13.1% and 21.3% the cases and controls ever smoked cigarette, respectively. Almost two-thirds of the cases and controls drank coffee about 20 times per week. Around 22% of the cases and 27% of the controls had usual exercise at least once a week (Supplemental Table 2A).
About 82.5% of the women in the cases and 85.6% in the controls were pregnant at least once in the last 1 year. Around 50% of both cases and controls took sweet food at least once per week for the last 1 year. Almost 39% of the cases and 14% of the controls consumed meat at least once a week for last 1 year. About 57% of both cases and controls ate fruit at least once a week for last 1 year (Supplemental Table 2A).
Shower temperature
A total of 90 households (45 case households and 45 control households) were used to measure shower temperature. The mean shower temperature among cases and controls were 28.8°C and 22.5°C respectively (Supplemental Table 3A). According to one way analysis of variance (ANOVA) depicted that shower temperature does influence colorectal cancers (Supplemental Table 4A).
Mantel–Haenszel analysis for proving co-factors and interactions
The stratified analysis confirmed that there was no significant variation between stratum-specific ORs, showing no interaction between the predictors and smoking or meat consumption. Stratified analysis also depicted no variation between the pooled and adjusted ORs, indicating that both smoking and meat consumption were not co-factors for the effect of predictors on CRC.
However, smoking was a co-factor for the effects of alcohol ingestion, educational status, BMI, stable source of income, previous GIT diseases, family history of CRC, physical exercise and meat ingestion. Additional table files show the determinants of CRC by meat ingestion (Supplemental Tables 5A and 6A).
Multivariable conditional logistic regression analysis
In the multivariable analysis, we found that drinking water chlorination status, type of water source, years at place of current residence, and swimming history were significantly associated with CRC. Our main findings show that the odds of developing CRC in households with chlorinated water supply was 2.6 times the odds of non-chlorinated water supply (adjusted mOR = 2.6; 95% CI 1.7-4.0). Similarly, the odds of CRC of study participants with history of swimming were 2.4 times that of those with no history of swimming (adjusted mOR = 2.4; 95% CI 1.4-4.1). Study participants who lived for more than 40 years in their current residence had 50% higher risk of CRC (adjusted mOR = 1.5; 95% CI 1.1-2.2) compared with participants who lived for less than 40 years (Table 4).
Table 4.
Multivariable analysis of factors associated with CRCs in Addis Ababa, 2021.
| Variables | Case (n = 214 ) | Control (n = 428) | UmOR (95% CI) a | AmOR (95% CI) | P-value |
|---|---|---|---|---|---|
| Water used for shower | |||||
| Hot water | 86 | 59 | 4.3 (2.8-6.5) | 3.8 (2.5-5.9)** | .001 |
| Cold water | 128 | 369 | 1.0 | 1.0 | |
| Years at longest residence b | |||||
| ⩾40 | 116 | 178 | 1.6 (1.2-2.2) | 1.5 (1.1-2.2)** | .027 |
| <40 | 98 | 250 | 1.0 | 1.0 | |
| Coffee (drinks/wk) | |||||
| ⩾21 times | 123 | 280 | 0.7 (0.5-1.0) | 0.7 (0.50-1.0) | .058 |
| 0 | 91 | 148 | 1.0 | 1.0 | |
| Chlorination status of drinking water | |||||
| Yes | 148 | 161 | 3.8 (2.7-5.5) | 2.6 (1.7-4.0)** | .001 |
| No | 66 | 267 | 1.0 | 1.0 | |
| Swimming history | |||||
| Yes | 106 | 134 | 2.25 (1.57-3.22) | 2.37 (1.39-4.07)** | .002 |
| No | 108 | 294 | 1.0 | 1.0 | |
| Types of swimming | |||||
| Outdoor | 58 | 95 | 1.51 (0.65-3.47) | 1.30 (0.54-3.13) | .550 |
| Indoor | 49 | 38 | 1.0 | 1.0 | |
| Regular showering | |||||
| Yes | 207 | 399 | 2.2 (1.0-5.2) | 1.5 (0.6-3.9) | .920 |
| No | 7 | 29 | 1.0 | 1.0 | |
| Exercise(times/wk) | |||||
| ⩾1 | 47 | 117 | 0.7 (0.5-1.1) | 0.7 (0.4-1.0) | .065 |
| <1 | 167 | 311 | 1.0 | 1.0 | |
| Smoking | |||||
| Ever | 28 | 91 | 0.5 (0.3-0.8) | 0.4 (0.2-0.8) | .004 |
| Never | 186 | 337 | 1.0 | 1.0 | |
| Sweet food (times/wk) | |||||
| ⩾1 | 120 | 214 | 1.3 (0.9-1.8) | 1.3 (0.9-1.9) | .153 |
| <1 | 94 | 214 | 1.0 | 1.0 | |
| Alcohol (drinks/wk) | |||||
| ⩾8 times | 101 | 228 | 0.7 (0.5-1.1) | 0.7 (0.3-1.5) | .344 |
| 0 | 113 | 200 | 1.0 | 1.0 | |
| Meat consumption (times/wk) | |||||
| ⩾1 | 84 | 60 | 4.6 (2.9-7.2) | 3.5 (2.0-5.9)** | .001 |
| <1 | 130 | 368 | 1.0 | 1.0 | |
| Variables | Case (n = 214 ) | Control (n = 428) | UmOR (95% CI) a | AmOR (95% CI) | P-value |
| Eggs consumption (times/wk) | |||||
| ⩾1 | 62 | 147 | 0.7 (0.5-1.0) | 0.8 (0.5-1.2) | .340 |
| <1 | 152 | 281 | 1.0 | 1.0 | |
Abbreviations: AmOR, adjusted matched odds ratio; CI, confidence interval; UmOR, unadjusted matched odds ratio.
Denotes unadjusted mOR using 95% confidence interval from bivariate conditional logistic regression analysis in matched case-control pair.
Years at longest residence.
Statistically significant at P < .05.
The odds of developing CRCs among those who used hot water for showering were 3.8 times the odds of those who used cold water for showering (adjusted mOR = 3.8; 95% CI 2.5-5.9). Alcoholic drinks, sweet foods, eggs consumption, coffee, and usual physical exercise were not significantly associated with CRCs (P > .05) (Table 4).
Discussion
To the best of our knowledge, this study is the first of its kind to investigate the association of drinking water source, chlorination, and CRC in Addis Ababa, Ethiopia. In this facility based matched case-control study drinking water chlorination status, years at the place of longest residence, type of water used for showering and swimming history were significantly associated with CRC.
Our main findings showed that the odds of developing CRC in households with chlorinated surface water supply were higher than those with less-chlorinated groundwater supply. This finding agreed with that of studies from Ontario, Canada, 42 and Iowa, USA 43 showing that users of surface water had much greater exposure to chlorination byproducts and had a high risk of CRC than consumers of non-chlorinated ground water.
A meta-analysis performed by Morris et al 44 supported this finding by indicating chlorinated surface water has substantially larger amounts of chlorination by-products than chlorinated groundwater (medians of 50.7 and 0.8 ppb, respectively) even when the surface water is collected from protected reservoirs. Therefore consumption of surface water was found to be an indirect indicator of exposure to chlorination by-products used in different studies.45,46
In addition, other studies from USA and Taiwan showed that colon and rectal cancers were found to be more common in those who drank chlorinated water, especially chlorinated surface water.47,48 A related finding from southern Ontario Canada showed that those who drank chlorinated surface water for at least 30 years had a 49% higher risk of colorectal cancers than those who drank groundwater for at least 30 years. 42 On the contrary, a studies from Louisiana, 49 North Carolina, 25 and Iowa 47 did not find a link between chlorinated surface water and CRC . This could be due to differences in methodology, water characteristics, and population parameters.
In our study, the odd of developing CRC of study participants with long history of swimming was higher than those with no history of swimming. A similar related finding was reported from USA Environmental Protection Authority (EPA) indicated that swimming pool has been linked to a threefold increase of cancer risk (all cancers). 50 A similar consistent result was also reported from Thailand. 51
Another related study by Lévesque et al 52 supported this observation that the daily dose of chloroform (chlorine disinfection byproduct) resulting from 1-hour swimming in commonly found public swimming pools was 141 times greater than that of a 10-minutes shower and 93 times greater than that for tap water ingestion. 53 In addition, swimming is associated with higher DBP concentration than drinking water, 54 which results in an increase of genotoxicity of pool water compared with tap water.55,56
Study participants who used hot water for showering were at more risk of developing CRC in this study. This could be due to exposure to THMs that is when the water is hot it is higher due to 2 main reasons: volatility of the THMs that may increase the inhalation exposure and reduce the concentration of the same in water, and the increase diffusivity of the THMs inside the body through dermal contact. Similarly, measurement of study participants shower water temperature also showed a significant difference between case and control households.
A similar related finding reported from western Massachusetts, USA depicting that the concentrations of trihalomethanes (THMs), trichloroacetic acid (TCAA), and chloropicrin (CP) were substantially higher in the hot water shower than in the cold water shower. 57
In the multivariable adjustment, consumption of alcoholic drinks, sweet foods, eggs, and coffee, as well as doing the usual physical exercise were not associated with CRC. This might be due to the similarity of the socioeconomic status of case and control households. In contrast to our findings, several studies reported that alcohol intake, even in small amounts,58,59 sweet foods consumption, 60 usual physical exercise, 61 and eggs consumption 62 were significantly associated with CRC.
Our study gained its strength from using a matched case-control study where the matching helped to control for potential confounders. Matched case-control studies have minimal bias relative to unmatched case-control designs due to their internal comparison property. In addition to its being a pioneer to investigate the effect of drinking water chlorination on CRC in Ethiopia, high response rate (95.5%) of both cases and controls and broad data on potential confounders were other strengths of our study. Contrary to the previous studies of chlorination byproducts and CRC1,27,43 in which control for confounding was minimal or absent, we were able to test a number of variables as potential confounders and effect modifiers.
This study has several limitations.
Although studies measured disinfection byproducts and then showed the association with colorectal cancers27,63 and bladder cancer, 7 in this study, we were not able to measure the disinfection byproducts levels. Therefore, this study proposes further study on measurements of disinfection by products in urine and/or water for the same population in the study area.
Some households of the cases and controls received a mixed water supply system, which made it difficult to confirm whether the water source is chlorinated or not. Cases and controls might have changed their residential address in such a way that it may affect the true classification of the exposure. Case selection from health facilities can be prone to bias since patients usually do not represent the general population. Recall bias could have been introduced during historical exposure measurement.
Conclusion
Drinking chlorinated water for extended years is a significant risk factor for CRC in Addis Ababa. In addition, hot tap water use for showering, and swimming history are risk factors for CRC. This information is essential to design integrated interventions that consider chlorination by-products and exposure routes toward the prevention and control of CRC in Ethiopia. Initiating alternative methods to chlorine disinfection of drinking water is also essential.
Supplemental Material
Supplemental material, sj-docx-1-ehi-10.1177_11786302211064432 for Drinking Water Source, Chlorinated Water, and Colorectal Cancer: A Matched Case-Control Study in Ethiopia by Nebiyou Tafesse, Massimiliano Porcelli, Sirak Robele Gari and Argaw Ambelu in Environmental Health Insights
Supplemental material, sj-docx-2-ehi-10.1177_1687814020966927 for Drinking Water Source, Chlorinated Water, and Colorectal Cancer: A Matched Case-Control Study in Ethiopia by Nebiyou Tafesse, Massimiliano Porcelli, Sirak Robele Gari and Argaw Ambelu in Environmental Health Insights
Acknowledgments
We would like to Thank Tikur Anbessa Specialized Hospital (TASH), College of Health Sciences, Addis Ababa University, for giving us permission to conduct this study. We are also very grateful to the Addis Ababa Water and Sewerage Authority (AAWSA) for providing us the water supply networks (Shape file) of Addis Ababa. We strongly acknowledge the support of Zeleke Teferri, Henoke Manaye, and Altasebe Azezewe from AAWSA. We also duly acknowledge the data collectors and supervisors for their hard work, and the study participant for their patience and cooperation.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration Of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: All authors have read and approved the manuscript, and ensure that this is the case: conceptualization: NT, MP, SR, AA, data curation: NT, formal analysis: NT, funding acquisition: NT, investigation: NT, MP, SR, AA, methodology: NT, MP, SR, AA, project administration: NT, resource: NT, MP, SR, AA, software: NT, supervision: SR, MP, AA, validation: NT, MP, SR, AA, and writing original draft: NT.
Availability of Data and Materials: The datasets generated for this analysis are available from the corresponding author upon a reasonable request.
Ethics Approval and Informed Consent: Ethical approval was obtained from the Institutional Review Board of Natural and Computational Science College, Addis Ababa University. Written consent was obtained from both cases and controls prior to the interview.
ORCID iD: Nebiyou Tafesse  https://orcid.org/0000-0002-0314-497X
https://orcid.org/0000-0002-0314-497X
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Villanueva CM, Gracia-Lavedan E, Bosetti C, et al. Colorectal cancer and long-term exposure to trihalomethanes in drinking water: a multicenter case-control study in Spain and Italy. Environ Health Perspect. 2017;125:56-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abdulkareem FB, Abudu EK, Awolola NA, et al. Colorectal carcinoma in Lagos and Sagamu, southwest Nigeria: a histopathological review. World J Gastroenterol. 2008;14:6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. APOCPC/UICC-ARO Cancer Registration Consortium. Cancer registration literature update (2006-2008). Asian Pac J Cancer Prev. 2008;9:165-182. [PubMed] [Google Scholar]
- 4. Babaei AA, Alavi N, Hassani G, Yousefian F, Shirmardi M, Atari L. Occurrence and related risk assessment of trihalomethanes in drinking water, Ahvaz, Iran. Fresenius Environ Bull. 2015;24:4807-4815. [Google Scholar]
- 5. Fitzmaurice C, Dicker D, Pain A, et al. The global burden of cancer 2013. JAMA Oncol. 2015;1:505-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Evlampidou I, Font-Ribera L, Rojas-Rueda D, et al. Trihalomethanes in drinking water and bladder cancer burden in the European Union. Environ Health Perspect. 2020;128:17001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schmitz PM, Kavallari A. Crop plants versus energy plants—on the international food crisis. Bioorg Med Chem. 2009;17:4020-4021. [DOI] [PubMed] [Google Scholar]
- 9. Organization for Economic Co-operation and Development. OECD-FAO agricultural outlook 2017-2026. OECD Publishing; 2017. https://www.oecd-ilibrary.org/agriculture-and-food/oecd-fao-agricultural-outlook-20172026_agr_outlook-2017-en
- 10. Abou-Zeid AA, Khafagy W, Marzouk DM, Alaa A, Mostafa I, Ela MA. Colorectal cancer in Egypt. Dis Colon Rectum. 2002;45:1255-1260. [DOI] [PubMed] [Google Scholar]
- 11. Rook JJ. Formation of haloforms during chlorination of natural waters. J Water Treat Exam. 1974;23:234-243. [Google Scholar]
- 12. Kumari M, Gupta SK, Mishra BK. Multi-exposure cancer and non-cancer risk assessment of trihalomethanes in drinking water supplies – a case study of eastern region of India. Ecotoxicol Environ Saf. 2015;113:433-438. [DOI] [PubMed] [Google Scholar]
- 13. Wang X, Mao Y, Tang S, Yang H, Xie YF. Disinfection byproducts in drinking water and regulatory compliance: a critical review. Front Environ Sci Eng. 2015;9:3-15. [Google Scholar]
- 14. Brass H. Status of the drinking water standards program in the United States. Water Air Soil Pollut. 2000;123:1-9. [Google Scholar]
- 15. Health Canada. Guidelines for Canadian drinking water quality—summary table. Health Canada; 2017. [Google Scholar]
- 16. Mashau F, Ncube EJ, Voyi K. Association between exposure to drinking water disinfection byproducts and adverse pregnancy outcomes in South Africa. J Water Health. 2021;19:174-189. [Google Scholar]
- 17. Richardson S, Plewa M, Wagner E, Schoeny R, DeMarini D. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: a review and roadmap for research. Mutat Res Rev Mutat Res. 2007;636:178-242. [DOI] [PubMed] [Google Scholar]
- 18. Drinking water quality in Ethiopia results from the 2016 Ethiopia socioeconomic survey. 2017. https://microdata.worldbank.org/index.php/catalog/2783
- 19. Villanueva CM, Cordier S, Font-Ribera L, Salas LA, Levallois P. Overview of disinfection by-products and associated health effects. Curr Environ Health Rep. 2015;2:107-115. [DOI] [PubMed] [Google Scholar]
- 20. Chowdhury S, Chowdhury IR, Mazumder MAJ, Al-Suwaiyan MS. Predicting risk and loss of disability-adjusted life years (DALY) from selected disinfection byproducts in multiple water supply sources in Saudi Arabia. Sci Total Environ. 2020;737:140296. [DOI] [PubMed] [Google Scholar]
- 21. Lau SS, Wei X, Bokenkamp K, Wagner ED, Plewa MJ, Mitch WA. Assessing additivity of cytotoxicity associated with disinfection byproducts in potable reuse and conventional drinking waters. Environ Sci Technol. 2020;54:5729-5736. [DOI] [PubMed] [Google Scholar]
- 22. Oliver BG. Analysis of volatile halogenated and purgeable organics. In: Afghan BK, Chau ASY. (eds) Analysis of Trace Organics in the Aquatic Environment. CRC Press; 2017:1-29. [Google Scholar]
- 23. Lawrence CE, Taylor PR, Trock BJ, Reilly AA. Trihalomethanes in drinking water and human colorectal cancer. J Natl Cancer Inst. 1984;72:563-568. [PubMed] [Google Scholar]
- 24. Parbery GD. Chlorinated water and overall risk of cancer: a systematic review. 2016. [DOI] [PubMed] [Google Scholar]
- 25. Water CD. Epidemiologic studies of organic micropollutants in drinking water. Water Pollut. 2013;5:1. [Google Scholar]
- 26. Cragle DL. A casecontrol study of colon cancer and water chlorination in North Carolina. Water Chlor. 1984;5:153-159. [Google Scholar]
- 27. Rahman MB, Driscoll T, Cowie C, Armstrong BK. Disinfection by-products in drinking water and colorectal cancer: a meta-analysis. Int J Epidemiol. 2010;39:733-745. [DOI] [PubMed] [Google Scholar]
- 28. Abate S, Yilma Z, Assefa M, Tigeneh W. Trends of breast cancer in Ethiopia. Int J Cancer Res Mol Mech. 2016;2:1. [Google Scholar]
- 29. Population projection of Ethiopia for all regions. 2013. http://www.sciepub.com/reference/163220
- 30. Timotewos G, Solomon A, Mathewos A, et al. First data from a population based cancer registry in Ethiopia. Cancer Epidemiol. 2018;53:93-98. [DOI] [PubMed] [Google Scholar]
- 31. Schlesselman JJ. Case-Control Studies: Design, Conduct, Analysis. Oxford University Press; 1982. [Google Scholar]
- 32. Ury HK. Efficiency of case-control studies with multiple controls per case: continuous or dichotomous data. Biometrics. 1975;31:643-649. [PubMed] [Google Scholar]
- 33. McNEMAR Q. Note on the sampling error of the difference between correlated proportions or percentages. Psychometrika. 1947;12:153-157. [DOI] [PubMed] [Google Scholar]
- 34. Villanueva CM, Cantor KP, Grimalt JO, et al. Assessment of lifetime exposure to trihalomethanes through different routes. Occup Environ Med. 2006;63:273-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Young TB, Wolf DA, Kanarek MS. Case-control study of colon cancer and drinking water trihalomethanes in Wisconsin. Int J Epidemiol. 1987;16:190-197. [DOI] [PubMed] [Google Scholar]
- 36. Regassa IF, Endris BS, Habtemariam E, Hassen HY, Ghebreyesus SH. Development and validation of food frequency questionnaire for food and nutrient intakes of adults in Butajira, southern Ethiopia. J Nutr Sci. 2021;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Morris RD, Audet A-M, Angelillo IF, Chalmers TC, Mosteller F. Chlorination, chlorination by-products, and cancer: a meta-analysis. Am J Public Health. 1992;82:955-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Crump KS, Guess HA. Drinking water and cancer: review of recent epidemiological findings and assessment of risks. Annu Rev Public Health. 1982;3:339-357. [DOI] [PubMed] [Google Scholar]
- 39. Addis Ababa water supply network. Addis Ababa Water and Sewerage Authority ( AAWSA) report. 2018. [Google Scholar]
- 40. Addis Ababa Water & Sewerage Authority AA. Ethiopia consultancy service for Addis Ababa water distribution and operation management and hydraulic modeling. 2019. [Google Scholar]
- 41. Aschengrau A, Seage GR. Essentials of Epidemiology in Public Health. Jones & Bartlett Publishers; 2013. [Google Scholar]
- 42. King WD, Marrett LD, Woolcott CG. Case-control study of colon and rectal cancers and chlorination by-products in treated water. Cancer Epidemiol Biomarkers Prev. 2000;9:813-818. [PubMed] [Google Scholar]
- 43. Jones RR, DellaValle CT, Weyer PJ, et al. Ingested nitrate, disinfection by-products, and risk of colon and rectal cancers in the Iowa Women’s health study cohort. Environ Int. 2019;126:242-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Berg JW, Burbank F. Correlations between carcinogenic trace metals in water supplies and cancer mortality. Ann N Y Acad Sci. 1972;199:249-264. [DOI] [PubMed] [Google Scholar]
- 45. Morris R, Audet A, Angelillo I, Chalmers T, Mosteller F. Chlorination, by chlorination by products, and cancer: a meta-analysis (Am J Public Health (1992) 82 (955-963)). Am J Public Health. 1993;83:1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Doyle TJ, Zheng W, Cerhan JR, et al. The association of drinking water source and chlorination by-products with cancer incidence among postmenopausal women in Iowa: a prospective cohort study. Am J Public Health. 1997;87:1168-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hsu C-H, Jeng W-L, Chang R-M, Chien L-C, Han B-C. Estimation of potential lifetime cancer risks for trihalomethanes from consuming chlorinated drinking water in Taiwan. Environ Res. 2001;85:77-82. [DOI] [PubMed] [Google Scholar]
- 48. Craun GF. Surface water supplies and health. J. 1988;80:40-52. [Google Scholar]
- 49. Gottlieb MS, Carr JK. Case-control cancer mortality study and chlorination of drinking water in Louisiana. Environ Health Perspect. 1982;46:169-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. EPA Union. Guidelines for carcinogen risk assessment US environmental protection agency. EPA/630/P-03/001F. 2005. [Google Scholar]
- 51. Panyakapo M, Soontornchai S, Paopuree P. Cancer risk assessment from exposure to trihalomethanes in tap water and swimming pool water. J Environ Sci. 2008;20:372-378. [DOI] [PubMed] [Google Scholar]
- 52. Lévesque B, Ayotte P, Tardif R, et al. Evaluation of the health risk associated with exposure to chloroform in indoor swimming pools. J Toxicol Environ Health A. 2000;61:225-243. [DOI] [PubMed] [Google Scholar]
- 53. Lévesque B, Ayotte P, LeBlanc A, et al. Evaluation of dermal and respiratory chloroform exposure in humans. Environ Health Perspect. 1994;102:1082-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Carter RAA, Allard S, Croué J-P, Joll CA. Occurrence of disinfection by-products in swimming pools and the estimated resulting cytotoxicity. Sci Total Environ. 2019;664:851-864. [DOI] [PubMed] [Google Scholar]
- 55. Manasfi T, De Méo M, Coulomb B, Di Giorgio C, Boudenne J-L. Identification of disinfection by-products in freshwater and seawater swimming pools and evaluation of genotoxicity. Environ Int. 2016;88:94-102. [DOI] [PubMed] [Google Scholar]
- 56. Dong S, Page MA, Wagner ED, Plewa MJ. Thiol reactivity analyses to predict mammalian cell cytotoxicity of water samples. Environ Sci Technol. 2018;52:8822-8829. [DOI] [PubMed] [Google Scholar]
- 57. Liu B, Reckhow DA. Disparity in disinfection byproducts concentration between hot and cold tap water. Water Res. 2015;70:196-204. [DOI] [PubMed] [Google Scholar]
- 58. Cai S, Li Y, Ding Y, Chen K, Jin M. Alcohol drinking and the risk of colorectal cancer death: a meta-analysis. Eur J Cancer Prev. 2014;23:532-539. [DOI] [PubMed] [Google Scholar]
- 59. Fedirko V, Tramacere I, Bagnardi V, et al. Alcohol drinking and colorectal cancer risk: an overall and dose-response meta-analysis of published studies. Ann Oncol. 2011;22:1958-1972. [DOI] [PubMed] [Google Scholar]
- 60. Gavrilaș LI, Ionescu C, Bălăcescu O, et al. Foods and food groups associated with colorectal cancer: a case-control study. Education. 2018;4:2-7. [Google Scholar]
- 61. Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22:191-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhang J, Zhao Z, Berkel HJ. Egg consumption and mortality from colon and rectal cancers: an ecological study. Nutr Cancer. 2003;46:158-165. [DOI] [PubMed] [Google Scholar]
- 63. El-Tawil AM. Colorectal cancers and chlorinated water. World J Gastrointest Oncol. 2016;8:402-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ehi-10.1177_11786302211064432 for Drinking Water Source, Chlorinated Water, and Colorectal Cancer: A Matched Case-Control Study in Ethiopia by Nebiyou Tafesse, Massimiliano Porcelli, Sirak Robele Gari and Argaw Ambelu in Environmental Health Insights
Supplemental material, sj-docx-2-ehi-10.1177_1687814020966927 for Drinking Water Source, Chlorinated Water, and Colorectal Cancer: A Matched Case-Control Study in Ethiopia by Nebiyou Tafesse, Massimiliano Porcelli, Sirak Robele Gari and Argaw Ambelu in Environmental Health Insights