Abstract
Fungal infections occurring in immunocompromised patients after immunochemotherapy treatment are often difficult to eradicate and capable of even being fatal. Systemic mycoses affecting severely immunocompromised patients often manifest acutely with rapidly progressive pneumonia, fungemia, or manifestations of extrapulmonary dissemination. Opportunistic fungal infections (mycoses) include several pathogens elements, as candidiasis, aspergillosis, mucormycosis (zygomycosis) and fusariosis. Prompt diagnosis and effective therapy are needed to improve the associated morbidity and mortality, especially in cases with non-canonical fungal localizations and not responsive to the available antifungal drugs.
Key words: Fungal infection, Candida, Isavuconazole, Caspofungin, Hematological patients, Combined antifungal therapy
Introduction
Fungal infections are an emerging problem in immunocompromised patients with hematological diseases. Fungal organisms such as Candida glabrata or tropicalis are becoming increasingly important in these areas, as they are increasingly able to acquire resistance to the common drugs and lead to the death of such patients. In this case report, we describe the excellent results obtained with the experimental combination of two antifungal agents (isavuconazole and caspofungin) in the treatment of an acute myeloid leukemia patient with disseminated Candida infection. This association is based on recent evidence (especially in vitro studies) of synergy of action against agents such as Aspergillus and Candida auris.
Case Report
We report a case of a 46-years-old-male patient affected by secondary acute myeloid leukemia (sAML), arisen from previous myelodysplastic syndrome (MDS) and treated with CPX-351 (liposomal formulation of daunorubicin and cytarabine).1 To reduce the risk of fungal infection due to profound aplasia, the patient received antifungal prophylaxis with posaconazole.2 This drug, however, was taken irregularly and not continuously, cause low compliance of the patient. During post-chemotherapy pancytopenia, the clinical course was characterized by infectious complications, with several fever peaks per day accompanied by presence of shaking chills. C-reactive protein (CRP) was elevated (388.33 mg/L, normal values 0-5 mg/L). Chest computed tomography (CT) was performed to search for any source of infection, finding multiple small parenchymal thickenings with a micronodular appearance, disseminated in apical and posterior basal segments. The patient was treated with different lines of empirical antibiotic therapy, without success. From peripheral vein blood cultures, performed during a febrile peak, candida tropicalis, sensitive to caspofungin, micafungin, voriconazole and anidulafungin, was isolated and, on blood chemistry tests, the value of 1-3-β-glucan (polysaccharide essential for the formation of fungal cell wall and directly proportional to fungal infection) was elevated (220 pg/mL, normal values <80 pg/mL).3 The patient was unsuccessfully treated with several lines of single agent antifungal drugs: caspofungin 50 mg/die for one week, voriconazole 200 mg bid for two weeks, fluconazole 400 mg/die for 10 days and amphotericin-B 240 mg for 3 weeks. Given the poor response to these therapies, the clinic’s worsening and the lung infection to instrumental examinations (‘pulmonary inflammation spread over all lobes, with prevalent interstitial involvement, micronodular pattern and groundglass level’ on re-evaluation CT) (Figure 1A), the patient underwent to a bronchoalveolar lavage (BAL) procedure, where the presence of candida tropicalis, candida glabrata and pneumocystis jirovecii was found. At the same time, the patient began to complain about progressive vision reduction in both eyes, especially in left eye. The patient underwent fundus oculi examination, where the presence of retroioloid haemorrhages at the posterior pole of both eyes and a rounded foveal cotton exudate in the left eye was found. These images, analyzed in depth with fluorangiography, were very suspicious for fungal infection due to their chorioretinal localization due to candida (Figure 1B), a potentially dangerous condition if not properly treated, as it causes extensive vitreous inflammation and possible loss of the eye.4–6
Despite the conventional antifungal drugs, the values of 1-3-β-D-glucan and CRP remained persistently elevated and febrile episodes continued to be present. We therefore decided to treat the patient with combination of caspofungin 70 mg/day in association with isavuconazole 200 mg x 3 /day.4–6 This therapy was continued for a month, with progressive improvement of symptoms and a notable reduction in the values of 1-3-β-glucan (90 pg/mL), before total recovery of blood count values. For ocular candida’s location, our therapeutic choice was intravitreal injection of 100 ug/0.1 mL of off-label voriconazole, based on experimental studies in the literature.7–9 After a single administration of voriconazole, completed without any noteworthy side effects, there was a notable clinical improvement with gradual vision recovery. Continuing the combination therapy between the two antifungals, the febrile episodes disappeared and the value of 1-3- β-glucan reduced to the point of negativization in four consecutive measurements (<80pg/mL). Thus, maintaining the complete remission from the haematological disease reached after chemotherapy and treating the infection given by candida with the combination of isavuonazole and caspofungine and the chorioretinal localization of the same mycete with the intravitreal administration of voriconazole, the patient was able to complete his therapeutic process, undergoing then a bone marrow transplant, having a complete blasts clearance.
Figure 1.
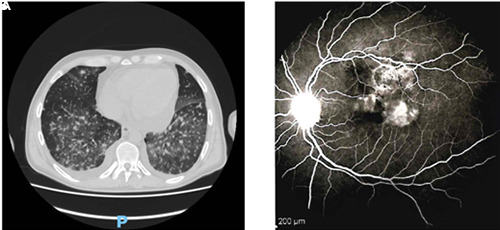
Evidence of mycotic localization in lungs (A) and eye (B), as described in text.
Discussion And Conclusions
A prompt diagnosis of fungal infection is crucial in dealing with immunosuppressed haemato-oncological patients. In cases of infections not responsive to main antifungals, it is important to look for any uncommon localization. In our experience, the localization of candida at the ocular level has been demonstrated, successfully treated with off-label intravitreal of voriconazole. Instead, the real challenge lies in the management of cases of infection not responsive to the administration of available drugs: there are little data of candida’s infections treated with antifungals in combination with each other. In particular, to the best of our knowledge, the only study about the combination between isavuconazole and caspofungin in literature is an in vitro analysis regarding the synergy between these two antifungals against aspergillus spp, where the results are not particularly suggestive of a possible strengthening of their mechanisms of action,10 and a very recent analysis of their interaction with candida auris, showing synergy of action both in vitro and in vivo tests, corresponding to safe drug concentrations and clinically achievable.11 This combination therapy, in our case, had considerable success, considering also that these drugs in single administration have not been able to obtain the desired results, allowing the patient to continue and complete his life-saving therapeutic process. Starting from this drug association, future studies are necessary to confirm the prospect of validity of this interesting therapeutic hypothesis in multidrug-resistant fungal infections.
Funding Statement
Funding: None.
References
- 1.Lancet JE, Uy GL, Cortes JE, et al. Final results of a phase III randomized trial of CPX-351 versus 7+3 in older patients with newly diagnosed high risk (secondary) AML. J Clin Oncol 2016; 34:7000. [Google Scholar]
- 2.Girmenia C, Frustaci AM, Gentile G, et al. Posaconazole prophylaxis during front-line chemotherapy of acute myeloid leukemia: a single-center, reallife experience. Haematologica 2012; 97:560-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Racil Z, Kocmanova I, Winterova J, et al. Detection of 1,3-beta-D glucan for diagnosis of invasive fungal infections in hematooncological patients: usefulness for screening of invasive mycosis and for confirmation of galactomannan positive results. Klin Mikrobiol Infekc Lek 2009;15:48-57. [PubMed] [Google Scholar]
- 4.Lingappan A, Wykoff CC., Albini TA, et al. Endogenous fungal endophthalmitis: causative organisms, management strategies, and visual acuity outcomes. Am J Ophthalmol 2012;153:162-6. [DOI] [PubMed] [Google Scholar]
- 5.Danielescu C, Anton N, Stanca HT, Munteanu M. Endogenous endophthalmitis: a review of case series published between 2011 and 2020. Journal of Ophthalmology 2020;2020:8869590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah CP, McKey J, Spirn MJ, Maguire J. Ocular candidiasis: a review. British Journal of Ophthalmology 2008;92: 466-8. [DOI] [PubMed] [Google Scholar]
- 7.Karagoz E, Ugan RA, Duzgun E, et al. A comparative study of the effects of intravitreal Anidulafungin, Voriconazole, and Amphotericin B in an experimental candida endophthalmitis model. Curr Eye Res 2017;42:225-32. [DOI] [PubMed] [Google Scholar]
- 8.Khan FA, Slain D, Khakoo RA. Candida endophthalmitis: focus on current and future antifungal treatment options. Pharmacotherapy 2007;27: 1711-21. [DOI] [PubMed] [Google Scholar]
- 9.Bienvenu AL, Aussedat M, Mathis T, et al. Intravitreal injections of voriconazole for candida endophthalmitis: a case series. Ocu Immunol Inflam. 2020;28: 471-78. [DOI] [PubMed] [Google Scholar]
- 10.Raffetin A, Courbin V, Jullien V, Dannaoui E. In vitro combination of isavuconazole with echinocandins against azole-susceptible and -resistant aspergillus spp. Antimicrob Agents Chemother 2017;62:e01382-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagy F, Toth Z, Nyikos F, et al. In vitro and in vivo interaction of caspofungin with isavuconazole against candida auris planktonic cells and biofilms. bioRxiv 2021;59:1015-23. [DOI] [PMC free article] [PubMed] [Google Scholar]


