Abstract
Background
Scabies is a skin infestation whose incidence is apparently rising.
Methods
This review is based on pertinent articles retrieved by a selective search of PubMed on diagnosis and treatment strategies.
Results
Thread-like papules (burrows), new, intense pruritus, and dermatitis guide the suspected diagnosis which is confirmed by the microscopic or dermatoscopic demonstration of scabies mites. The first line therapy is topical application of permethrin, in accordance with the current recommendations for its use. Other treatment options include systemic ivermectin and topical crotamiton or benzyl benzoate. A combination of permethrin and ivermectin is used to treat otherwise intractable cases and is generally indicated for the treatment of crusted scabies. Known causes of treatment failure include improper application of the external agents, failure of repeated treatment with ivermectin, incomplete decontamination of furnishings and clothes, failure to simultaneously treat contact persons, absence of written documents explaining treatment modalities, and the patient’s belonging to a risk group. Even though there has not yet been any direct proof of resistance of scabies mites to permethrin, there is a rising number of well-documented cases of poor response to this agent. Moxidectin is a new substance now undergoing clinical testing.
Conclusion
Treatment of scabies according to the guidelines and the additional recommendations reported here should result in effective curing, even in cases that are thought to be intractable.
Prevalence.
The diagnosis of scabies has become more common in Germany in recent years, for many possible reasons.
Faulty application.
The prevalence figures are influenced above all by the faulty use of drugs with an ensuing need for repeated treatment, as well as by reinfestation due to incomplete decontamination of the patient’s clothes and furnishings.
The diagnosis of scabies has become more common in Germany in recent years (1). The epidemiological figures are imprecise, as scabies is not reportable by German law (e1). The available data are derived from outbreaks in communal facilities and shared accommodations (which are reportable), diagnosis-related statistics from health insurance carriers, and information from pharmacies on the prescribing and dispensing of drugs against scabies (1, 2). According to German federal health reports, the number of cases of inpatient treatment for scabies (ICD-10 diagnosis code B86) rose from 960 in 2012 to 10 072 in 2019 (www.gbe-bund.de). Data from the above sources are not always reliable or representative, and they count or include some patients multiple times; they may therefore be adequate to document rising incidence, but without enabling quantification or epidemiological evaluation (1). Above all, the prevalence figures are influenced by the faulty use of drugs with an ensuing need for repeated treatment, as well as by reinfestation due to incomplete decontamination of the patient’s clothes and furnishings. Children are an underappreciated source of infection (1), for a number of reasons: scabies infections in children are often not detected early (e2) or treated thoroughly enough (3), probably involve a greater number of mites, and are more likely to be passed on through close physical contact with other persons (4). The incidence of scabies, like that of other sexually transmitted infections, rose after advances in the treatment of HIV (1, e3– e8). While it is true that the rising incidence of scabies in Germany temporally coincided with a mass migration of asylum-seekers from Arab and African countries, there is no epidemiological evidence of a causal connection (1, 5, e9). The migration of low-wage workers and nursing personnel from the European Union and economic zones outside Europe may have played a role (6, e10, e11). In general, periodic increases of scabies incidence have been observed at wide temporal intervals, with each increase accompanied by similar speculation regarding the causes as today (1).
Learning objectives
Children as a source of infection.
Children are an underappreciated source of infection, for a number of reasons: scabies infections in children are often not detected early or treated thoroughly enough, probably involve a greater number of mites, and are more likely to be passed on through close physical contact with other persons.
The pathogen and its transmission.
In typical cases of common scabies, only 10-15 adult mites are present on the skin surface, because the mites can be washed and scratched away, and because a cell-mediated immune response begins 3–6 weeks after the infestation.
This article is intended to enable the reader to:
know the pathways of transmission of scabies, and thus the situations where there is a danger of transmission, and the population groups at elevated risk;
be acquainted with dermatoscopy—the simplest, fasted method of demonstrating mites—as well as the recommended treatments and their correct modes of application;
understand the diverse reasons why treatment may fail.
The pathogen and its transmission
The pathogen that causes scabies is the host-specific human scabies mite (Sarcoptes scabiei varietas hominis). The adult female measures ca. 0.3 × 0.4 mm and is approximately twice the size as the male mite. These parasites can move approximately 2.5 cm per minute on the warm bodily surface. Pregnant females dig superficial passageways called burrows (ca. 0.5–5 mm per day) in the stratum corneum and generally stay within them for the rest of their lifetime of four to six weeks, laying two to three eggs per day (7, 8). The larvae that hatch from them two to three days later (non-ovicidal antiscabies drugs must, therefore, remain in the epidermis for at least this long) swarm out onto the cutaneous surface and go on to develop in skin wrinkles and hair follicles into nymphs and then, within 9–17 days, into sexually mature mites, which copulate there (9, 10). The male mites die shortly afterward, while the pregnant females burrow back into the skin, and the cycle recommences.
Multiple defense mechanisms eliminate scabies mites by mechanical and immunological means, which explains why, once the immune response begins, only around 11 adult female mites are found on the skin surface, instead of the otherwise expected exponential increase (9):
washing and bodily hygiene;
scratching away of mites because of the intense pruritus mediated by pruritogenic mite products and receptors of the innate immune response (e12), and later markedly increased by the specific immune response (e13);
a cell-mediated immune response to mite antigens and mite products, which arises three to six weeks after the initial infestation and one to three days after a reinfestation (9).
If these defense mechanisms are impaired or absent, the mites will become abundant. In the extreme case, crusted scabies arises (box 1), in which the number of mites in and on the skin rises into the millions:
BOX 1. Risk factors for crusted scabies*.
-
Immunosuppression
drug-induced (systemic and topical corticosteroids, immunosuppressants, cytostatic agents, biological agents), leukemia, lymphoma, HIV or HTLV-1 infection, graft-versus-host disease, congenital immune defficiencies, Down syndrome
-
chronic disease
severe autoimmune disease, diabetes mellitus, liver disease, end-stage renal failure, alcohol and drug dependency, malnutrition and undernutrition
-
sensory dysfunction of the skin
sensory neuropathy, spinal cord injury, leprosy, syringomyelia, tabes dorsalis, senile dementia
-
physical impairment
paresis, paraplegia, severe arthropathy, epidermolysis bullosa
*modified from (11)
inadequate hygiene
impaired ability to scratch (e.g., because of immobility)
absence of itch (e.g., because of immunosuppression or neurological disease)
immunosuppression (iatrogenic, e.g., after organ transplantation or as the result of intensive topical corticosteroid treatment; congenital, as in Down syndrome; or by as yet poorly understood mechanisms, e.g., in Aboriginal Australians) (e14).
The mites can survive outside the human body and remain infectious for 24 to 36 hours at normal room temperature (21° C) in relatively humid air (40–80%) (12, 13). They can survive much longer at lower temperatures and higher humidity (e15).
The transmission of a single pregnant mite, or of several larvae, suffices to infest another human host. In common scabies, this requires intensive skin-to-skin contact of at least 5–10 minutes’ duration, as occurs, for example, in the breastfeeding or cuddling of an infant, sexual intercourse, or the care of nursing-dependent persons (9, 12, 14).
Mites of all stages need somewhat less than 30 minutes to penetrate the skin; until they succeed in doing so, they can be wiped or washed away (13). There have been no more than a few scientific studies of the number of mites or of their transmission (9, 12, 13), yet the following assumptions are plausible:
The process is faster at sites where the skin is thin than at sites with a thick stratum corneum.
Nymphs and larvae are not buried under the skin, and, in cases with many mites, some adult mites are not buried under the skin either. All organisms that are on the skin surface, rather than under it, can infest a new host more easily.
Until the specific immune response appears, and for some time afterward, the number of mites—and, therefore, the transmissibility of infestation—are relatively high, but then the number of female mites markedly declines (9). Hand-shaking, hugging, and medical examinations are not intensive enough forms of contact for the transmission of common scabies. The contacts of persons with common scabies who become infested are usually members of the same family or communal living group, or else nursing-dependent persons and those who take care of them (15). The indirect transmission of the pathogen via textiles, furniture, or articles or daily use is rare in common scabies, but not entirely negligible (9). In forms of scabies with many mites, and particularly in crusted scabies, infestation can ensue after even brief bodily contact with the patient, or by exposure to objects used by the patient, or to dandruff (8, 16). In summary, the probability of transmission of infestation depends on the number of mites on the skin and the duration and frequency of direct bodily contact (8, 9, 15, 16).
Clinical features
Immunosuppression.
If these defense mechanisms are impaired or absent, the mites will become very abundant. In the extreme case, crusted scabies arises, in which the number of mites in and on the skin rises into the thousands or millions.
Intensive skin-to-skin contact.
In common scabies (in which only a few mites are present), the pathogen is generally transmitted mainly by protracted and intensive skin-to-skin contact; in crusted scabies, it can also be transmitted by exposure to mite-bearing objects and dandruff.
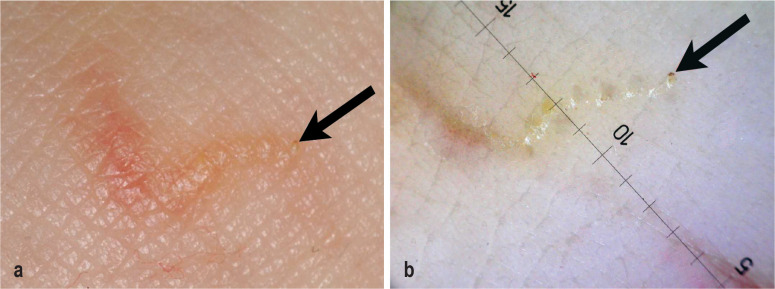
The clinical morphology of scabies can take many forms and is largely determined by the number of mites and by the patient’s age, immune status, and defensive behavior. In common scabies, a markedly pruritic papular or papulo-vesicular rash with a symmetric pattern of involvement arises 2–6 weeks after the initial infestation. In reinfestations, the interval is shortened to one or a few days. It is typical of scabies, though not specific for it, that the itch worsens in the nighttime and in the warmth of the bed (the so-called nocturnal crescendo) (e16). Whitish, straight or slightly curved mite burrows, 3–7 mm long, are characteristic; at the end of a burrow, a small vesicle, pustule, or scale may form (figure 1). Surfaces particularly prone to skin lesions include the interdigital spaces and the lateral surfaces of the fingers, the edges of the hands, the volar surfaces of the wrists, the elbows and axillae, the umbilical region, the waistline, the buttocks, the medial surface of the thighs, and the dorsa and edges of the feet; in women, the breasts, and particularly the areolae, and, in men, the penis and the scrotum, and thus any and all body regions with a thin stratum corneum and a low density of sebaceous gland follicles (15). Pruritus leads to scratching and thereby to the rapid excoriation and crusting of many lesions, resulting in a dermatitis-like appearance (16). The clinical appearance can be markedly changed by intensive hygiene (“well-groomed scabies”) or pretreatment with topical steroids (“scabies incognito”). The bothersome itch often disturbs the patient’s sleep, leading to daytime fatigue, impaired concentration, and reduced productivity. Stigmatization, social isolation, embarrassment, and depression are further potential consequences (8).
Figure 1.
a) clinical and b) dermoscopic findings of a mite burrow, with demonstration of the mite (arrow)
Clinical features.
a markedly pruritic papular or papulo-vesicular rash with a symmetric pattern of involvement that is most prominent at certain typical sites of predilection.
Nodular scabies.
Nodular scabies is characterized by inflammatory nodules that appear preferentially on the penis and scrotum and in the inguinal, perianal, and axillary regions.
Infants and toddlers often have severe, extensive, sometimes impetiginous skin changes; these frequently affect the palms and soles, and occasionally the scalp and face (“infantile scabies”) (3). In addition to papules, vesicles and pustules are more common in this age group; there are hardly any excoriations, as defensive behavior (scratching) is limited. The first skin changes can be seen as early as the end of the neonatal period (“neonatal scabies”) (e17). Atypical constellations with involvement of the head and trunk are also found more frequently in elderly persons with impaired immune defenses (8, 17). Often, in elderly, nursing-dependent persons, only clothed areas of the bodies are affected; in patients with dementia, pruritus may be entirely absent (18).
Nodular scabies particularly tends to affect small children and the elderly and is characterized by coarse, round nodules 5–20 mm in size, which are red, reddish-brown, or livid in color. The nodules appear preferentially on the penis and scrotum, in the inguinal and perianal regions, and in the region of the axillae. The nodules may be caused by deeper penetration of the scabies mite and a stronger and longer-lasting immune reaction (e18). Scabies nodules may persist for months even after successful treatment (“post-scabietic papules”) (e19). Vesicles (“bullous scabies”) are unusual and are mainly seen in elderly patients (8, e20).
In crusted scabies, the number of mites rises into the millions because of uninhibited multiplication (box 1) (18). The clinical picture is dominated by massive local or diffuse hyperkeratoses on an erythematous background, with crusting and fissures on the hands, feet, elbows, head, and neck (15). The nails are often thickened, discolored, and dystrophic. Pruritus is mild or absent because of the absence of an immune response, among other reasons. The lymph nodes are often swollen. Eosinophila is common, and an elevated IgE titer is almost always present (8, 16, 17). Because of its unusual clinical features, crusted scabies usually remains unrecognized for a long time, with the result that persons with this illness often cause outbreaks in communal facilities (18).
The most common complication of scabies is bacterial infection of the excoriations with Streptococcus pyogenes and Staphylococcus aureus, leading to contagious impetigo, ecthyma, erysipelas, furuncles, abscesses, lymphadenitis, and even bacteremia and sepsis (8, 16). Scabies mites predispose to bacterial infection by inhibiting the three pathways of the complement system (e21) as well as by inducing scratching of the skin, leading to damage of the epithelial barrier (e22). Post-streptococcal glomerulonephritis is hardly ever seen in the developed world because of good hygiene; in less well-developed countries with warm climates, it is a common enough cause of chronic renal failure and reduced life expectancy to be of health-economic relevance (6).
Diagnostic evaluation
Crusted scabies.
Crusted scabies differs from common scabies in its clinical picture, which is dominated by massive local or diffuse hyperkeratoses on an erythematous background, with crusting and fissures on the hands, feet, elbows, head, and neck.
The only proof of the diagnosis of scabies is demonstration of a scabies mite, its eggs, or feces pellets (scybala). The classic mode of establishing the diagnosis is by opening the end of an intact mite burrow with a sharp instrument and inspecting its contents in the light microscope under loupe vision (15). Dermatoscopy offers an alternative, noninvasive, faster and more sensitive mode of evaluation. In the most recent publication on the subject, the reported sensitivity and specificity of dermoscopy were 98.3% and 88.5%, respectively (19). The examiner looks for a dark triangle corresponding to the head, thorax and anterior leg pairs of the mite (“kite sign”), in connection with the air-containing intracorneal burrow system (“wake sign”) (figure 1). The edges of the mite burrow may be pigmented by melanin-containing feces (“grey-edged line sign”) (e23). Unfortunately, living and dead mites cannot be reliably distinguished from each other at the customary 10x magnification, and the method is unsuitable for use in patients with highly pigmented skin. Videodermoscopy at 70 to 1000x does enable a determination whether mites are alive (with the aid of recently described criteria such as the “hydrangea sign”) (20). Yet, for videodermatoscopy as for confocal laser microscopy and optical coherence tomography (e24), the effort and expense limit its widespread use (7, 16, 21).
An expert panel recently developed consensus criteria for the standardized establishment of the diagnosis of scabies (box 2) (21). A definitive diagnosis requires the demonstration of mites, eggs, and feces by light microscopy, dermoscopy, or another high-resolution imaging method. “Clinical scabies” and “suspected scabies” can be diagnosed from the history and physical examination alone; this should only be done if other elements of the differential diagnosis seem less likely.
BOX 2. Consensus criteria for the diagnosis of scabies, issued by the International Alliance for the Control of Scabies (21).
| A | Confirmed scabies meets at least one of the following criteria: |
| A1 | mites, eggs or feces on light microscopy of skin samples |
| A2 | mites, eggs or feces visualized on an individual using a high-powered imaging device |
| A3 | mite visualized on an individual using dermoscopy |
| B | Clinical scabies meets at least one of the following criteria: |
| B1 | scabies burrows |
| B2 | typical lesions affecting male genitalia |
| B3 | typical lesions in a typical distribution and two history features |
| C | Suspected scabies meets one of the following criteria: |
| C1 | typical lesions in a typical distribution and one history feature |
| C2 | atypical lesions or atypical distribution and two history features |
History features
itch
positive contact history
The diagnosis can be made at one of the three levels (A, B or C). A diagnosis of clinical or suspected scabies should only be made if other differential diagnoses are considered less likely than scabies.
Treatment
The most common complication.
Bacterial superinfection with group A beta-hemolytic streptococci and with Staphylococcus aureus is the most common complication of scabies.
As the typical symptoms of the disease are directly associated with infestation by scabies mites, the elimination of the organisms is the main goal of treatment (e25– e28). The drugs used for this purpose are classified as either acaricidal (mite-killing) or ovicidal (egg-killing). They are either topically applied or ingested for systemic use. The mode of correct topical application is more complex than usually thought and is not always described in the accompanying information for physicians.
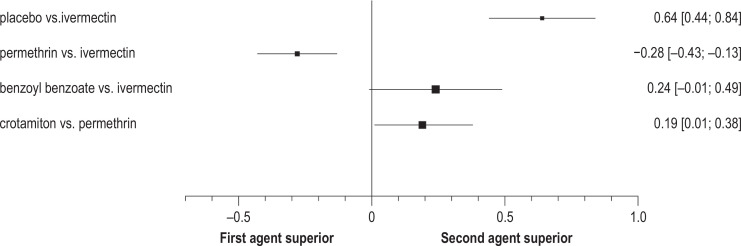
In Germany, the Federal Institute for Drugs and Medical Devices (BfArM) approved permethrin on the basis of a single-armed, multicenter trial (22), and it approved oral ivermectin in 2016 in an accelerated approval procedure. The treatment recommendations are, therefore, oriented toward randomized, controlled trials, independently of the approval data or pertinent meta-analyses (23, 24). The evidence for the drug therapies used in Germany is presented in Figure 2 (23).
Diagnostic evaluation.
Dermoscopy is the simplest, fastest method of demonstrating the pathogen. It cannot reliably determine the viability of mites, at usual magnifications of 10x or 20x, but at higher magnifications.
Treatment I.
Topical permethrin, topical crotamiton, topical benzyl benzoate, and ivermectin are the main drugs for the elimination of scabies. The recommendations for application must be followed in detail for the treatment to be successful.
The treatment of first choice is the topical application of permethrin in lipophilic vesicles at a concentration of 5% (23– 25). Permethrin is a so-called open channel blocker that exerts both acaricidal and ovicidal effects by inducing the dysregulation of voltage-dependent sodium channels in neurons (e29). According to the accompanying information for physicians, it should be applied once externally on the patient’s entire integument in cases of common scabies (e30). For an adult patient, at least 25–30 g should be applied meticulously and evenly (for children aged 6–12, up to 15 g; for children aged 2 months to 5 years, up to 7.5 g) (26). The skin should kept dry for at least 30 minutes beforehand, as permethrin is lipophilic, and hydration of the stratum corneum by washing, showering, or bathing lessens its cutaneous bioavailability. After application, the preparation should be allowed to soak into the skin for 5–10 minutes before the skin is covered with clothing; washing should be avoided for at least 8 and preferably 12 hours (26). Mild pruritus or paresthesia can arise after application, particularly in markedly eczematous skin. Close bodily contact should be avoided for 36 hours (1). Repeated application in 7–10 days is recommended by us (but not in the formal information for physicians), as there have been many reports of treatment failures; recurrences should always be treated with repeated application (1, 15, 27). Because inadequate or incomplete treatment of the entire skin is common, low-viscosity extemporaneous preparations with the same permethrin concentration may be useful, particularly for patients with dense body hair (28). An alternative with comparable efficacy (but one that requires more effort) is the topical application of crotamiton 5% or 10% on three to five consecutive days, or of benzyl benzoate 25% (10% for children) on three consecutive days (23). Both of these substances are acaricidal and ovicidal; crotamiton also counteracts itch. Both substances can irritate the skin.
Ivermectin for topical use (1%) is not approved for the treatment of scabies in Germany and cannot be obtained with a prescription (e32).
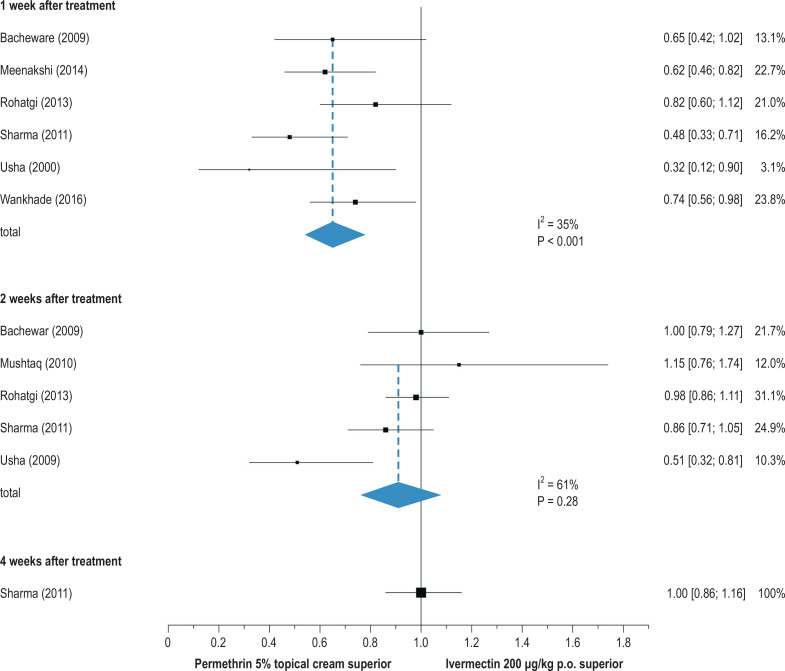
Ivermectin tablets (3 mg) are approved and available for the systemic treatment of scabies. The recommended dose is 200 µg/kg of body weight, from a body weight of 15 kg and up (thus, for a 75-kg patient, five 3-mg tablets) (15, 29, e33). As ivermectin is meanwhile considered essentially harmless for children under 15 kg as well (29, 30, e34, e35), a syrup containing ivermectin (400 µg/mL) was recently developed as an extemporaneous preparation for this age group (31). Ivermectin exerts its effect by blocking the neurotransmitter gamma-aminobutyric acid (GABA); it interacts with glycine, histamine, and nicotinic acetylcholine receptors (e36). Ivermectin is only acaricidal and not ovicidal; thus, repeated treatment after 7 to 14 days is necessary, so that all larvae that were still unhatched at the time of the initial treatment can be killed before attaining reproductive maturity. For pregnant and nursing women, permethrin can be given topically, and in some cases ivermectin, as an off-label drug (i.e., despite the lack of official approval), after meticulous evaluation of the benefits and risks and with the patient’s informed consent (15, e37, e38). The evidence for topical first-line treatment with permethrin is derived mainly from its greater efficacy compared to systemic ivermectin in comparative trials (figure 3) (24).
Figure 3.
Forest plot for a comparison of efficacy (cure rates) 1, 2, and 4 weeks after treatment with either topically applied 5% permethrin cream or systemically administered ivermectin 200 µg/kg KG p.o. [95% confidence interval] (modified from [24]). p.o.. per os
Treatment II.
Oral moxidectin may become an effective single-dose treatment of scabies in the future.
In patients with crusted scabies, combined repeated treatment with permethrin cream (e.g., daily for one week, and then twice weekly till healing) and ivermectin tablets (e.g., on days 1, 2, 8, 9, and 15, and possibly also on days 22 and 29) is recommended (15, 27). Moreover, topical keratolysis with an externally applied agent containing salicylic acid or urea is required, and marked hyperkeratoses may need to be mechanically removed.
In the future, moxidectin may be a suitable treatment for scabies (e39, e40). Initial clinical data show good efficacy, particularly because this agent has a much longer half-life than ivermectin and thus may only need to be given once (32). Yet neither the safety of moxidectin nor its efficacy against scabies has yet been adequately demonstrated (e41). The development of a vaccine against scabies appears to be possible, but has not yet been accomplished (e42).
Decontamination measures
The success of treatment depends on the simultaneous treatment of all close contacts of the patient, generally including all of the members of his or her family or persons sharing close quarters (e.g., carers for small children and visiting nurses). For asymptomatic contacts, a single treatment with permethrin or ivermectin suffices (off-label use, and therefore at the contact person’s expense) (15).
Decontamination measures.
The importance of additional measures accompanying treatment is directly related to the mite burden of the individual patient and to the number of patients involved in an outbreak.
The importance of additional measures accompanying treatment (box 3) is directly related to the mite burden of the individual patient and to the number of patients involved in an outbreak. As scabies mites survive no longer than a short time away from the human host, professional pest control management of interior rooms is unnecessary. Disinfection measures are ineffective.
BOX 3. Recommended decontamination measures for scabies*.
machine washing of all recently used textiles (underwear, pyjamas, bed linen, towels) at a temperature of at least 50°C for at least 35 minutes
storage of remaining clothing and other articles with recent prolonged bodily contact (shoes, stuffed toy animals, etc.) for at least 3–4 days at a temperature of at least 21°C in a dry location in closed plastic bags, or alternatively, freezing at a temperature below -10°C for at least 5 hours (this is according to recent data [34]; earlier recommendations [15, 33] were for a temperature below -25°C)
cleaning of all contact surfaces
vacuuming of upholstered furniture, cushions, beds, mattresses, carpets, floors, and automobile seats
disposable gloves should be worn during all of these tasks
Highly contagious patients should be treated as inpatients in isolation. Personnel entering the room, and especially those coming in close contact with the patient, should wear a protective gown, head covering, mask, overshoes, and gloves. Once the patient has been discharged, the mattress and other objects that have been in intensive or protracted contact with the patient’s skin should be autoclaved or thoroughly cleaned and not used for one to two weeks (33). Disposable articles should be used preferentially. The other recommendations listed in Box 3 apply.
Procedure in case of a scabies outbreak
The procedure in case of a scabies outbreak.
The control of a scabies outbreak in a communal facility is a highly complex matter and requires coordinated measures under the direction of a leadership team.
For the last two decades, scabies outbreaks have become increasingly common in Germany in communal-living institutions, such as old people’s and nursing homes, homes for the disabled, and hospitals, in which people have frequent and close bodily contact with each other. Such outbreaks usually have their origin in a highly contagious elderly, chronically ill, or otherwise immune-compromised person (the “core transmitter”) suffering from crusted scabies that has gone unrecognized or has been misdiagnosed (36). Controlling an outbreak requires considerable expense, manpower time, and organizational effort, as summarized in Box 4 and discussed in greater detail in pertinent publications (15, 37, e43– e45).
BOX 4. The recommended procedure in case of a scabies outbreak*.
reporting to the responsible health office
formation of a leadership team: good planning, organization, and information of all participants
clarification of the absorption of costs by health insurance carriers and/or professional associations
synchronous initial examination: triage of cases into severely infested (isolation, hospitalization), definitely/possibly infested (multiple treatments), and not infested (single treatment)
synchronous treatment of all infested persons on days 1 and 8 (–15) with ivermectin, or by trained nursing staff on days 1, 2, and 8 (–15) with permethrin
synchronous individual treamtent of all contact persons on day 1, including contact persons outside the facility where the outbreak occurred
avoidance of bodily contact for at least 36 hours after treatment
intensive decontamination measures according to current recommendations
follow-up examinations 14, 28, and 42 days after treatment
The causes of treatment failure
The causes of treatment failure.
The faulty application of topical antiscabies drugs, poor compliance, and the lack of repeated treatment, decontamination measures, understandable written information, and simultaneous treatment of contact individuals are all well-documented causes of the failure of treatment for scabies.
Along with the rise in documented cases of scabies in Germany, there has also been a rise in well-documented cases of inadequate efficacy of permethrin (1, 38, e46). This suspicion of resistance to permethrin is hard to confirm without established in vitro testing, yet it is justified once there have been multiple, correctly administered, but nonetheless unsuccessful attempts at treatment, including all appropriate accompanying measures. Genetic resistance on the basis of a knock-down (kdr) mutation that neutralizes the pharmacodynamic effect of permethrin on voltage-dependent sodium channels has only been described to date in canine mites, and not in the human scabies mite (e47, e48). It seems more likely that the repeated application of permethrin in sublethal doses induces elimination enzymes in the mites, such as esterases, glutathione s-transferases, oxygenases, and CYP450, which can lead to a diminution of the permethrin effect. Metabolic resistance of this type has been shown in canine mites, but it has only been documented once, indirectly, for human mites (e47– e49). It might be combatted by giving permethrin at a higher concentration at the sites where it is needed, but this has not been studied for possible toxic effects. A polymorphism in the p-glyoprotein gene is under discussion as a possible genetic resistance factor against ivermectin (e50). In summary, there is as yet no definitive proof that the resistance mechanisms mentioned here are active in human scabies mites.
Proven causes of a lack of response to treatment also include errors in application (particularly of topical agents), the omission of repeated treatment, inadequate compliance, reinfestation because of incomplete decontamination of the patient’s environment and the lack of simultaneous treatment of contacts, along with the failure to provide written information about the measures that are required (4, 39, 40, e51). Moreover, experimental studies suggest that the cutaneous bioavailability of permethrin is lessened in children and patients with a markedly impaired barrier, with the result that the minimal effective inhibitory concentration of the drug may not be reached for a long enough time.
On the other hand, treatment resistance may be only apparent but not real, because mites that have been killed can be distinguished only with difficulty, or not at all, from living mites with the usual dermoscopic techniques for several days after their death; high magnification is needed for this purpose (20). Moreover, the release of antigens from decomposing mites after effective treatment may lead to an increase in, or persistence of, the inflammatory reaction (e52, e53). Finally, some patients can develop psychogenic pruritus, at least transiently, which may then develop into a pathological fixation or even an isolated delusion (e54).
In our experience, the management of supposedly intractable cases of scabies in conformity with the guidelines and all of the additional expert recommendations discussed here (1, 2, 15, 27, 38) results in a cure in several cases.
Figure 2.
Forest plot for comparing the efficacy of different treatments. Differences in cure rates are shown in a direct comparison of the treatments indicated [95% confidence interval] (modified from [23]).
CME credit for this unit can be obtained via cme.aerzteblatt.de until 14 October 2022.
Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
How long can scabies mites live away from the human body at a room temperature of 21°C and 40–80% relative humidity?
30–60 minutes
8–12 hours
24–36 hours
9–17 days
4–6 weeks
Question 2
Which of the following activities with infants has the highest chance of leading to the transmission of a scabies mite?
feeding
breastfeeding
caressing
changing diapers
bathing
Question 3
Which of the following is a common cause of treatment failure?
resistance of mites to benzyl benzoate
asynchronous treatment of the infested individuals
resistance of mites to moxidectin
bodily contact with infected persons 72 hours after treatment
use of permethrin after its expiration date
Question 4
Which of the following decontamination measures is of no use in the home of one or more persons who are infested with scabies?
washing recently used bed linen at 60°C
storing recently worn slippers in a closed plastic bag for three days
disinfecting all doorknobs
vacuuming all cushions and upholstery
freezing recently used stuffed animals at -18°C for at least 5 hours
Question 5
What is the most reliable way to diagnose scabies?
the demonstration of mites by dermoscopy
a history of itch of recent onset
a positive contact history
the demonstration of burrows at sites of predilection
the demonstration of papules on the genitals
Question 6
What side effect can be expected most frequently after topical treatment with permethrin cream?
bacterial superinfection
allergic contact dermatitis
eosinophilia
worsening of itch
drug rash
Question 7
A three-month-old infant presents with marked scabies on the hands and feet, while the parents have no signs or symptoms of the disease. No treatment for scabies has yet been provided. Which of the following should be borne in mind with respect to treatment?
Permethrin has not been approved for use in infants.
Oral ivermectin therapy is the safest treatment for infants.
The parents need not be treated, as they have no signs or symptoms.
Only benzyl benzoate is approved for use up to the age of 12 months.
Permethrin must be applied on the entire body.
Question 8
A man weighing 75 kg is to be treated with permethrin. At what dose should he be treated?
a) 5 mg, b) 10 mg, c) 15 mg, d) 20 mg, e) 25 mg
Question 9
What disease is associated with an elevated risk of crusted scabies?
hypertension
diabetes mellitus
depression
atrial fibrillation
hyperthyroidism
Question 10
Who is the usual source of further infestations in outbreaks of scabies in communal living facilities?
a resident with crusted scabies
a visitor
a nurse
a member of the housekeeping staff
an external contractor working in the facility
► Participation is possible only via the Internet: cme.aerzteblatt.de
Further information on CME.
Participation in the CME certification program is possible only via the Internet: cme.aerzteblatt.de. This unit can be accessed until 14 October 2022. Submissions by letter, e-mail, or fax cannot be considered.
The completion time for all newly started CME units is 12 months. Theresults can be accessed 4 weeks following the start of the CME unit. Please note the respective submission deadline at: cme.aerzteblatt.de.
This article has been certified by the North Rhine Academy for Continuing Medical Education. CME points can be managed using the “uniform CME number” (einheitliche Fortbildungsnummer, EFN). The EFN must be stated during registration on www.aerzteblatt.de (“Mein DÄ”) or entered in “Meine Daten”, and consent must be given for results to be communicated. The 15-digit EFN can be found on the CME card (8027XXXXXXXXXXX).
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
Prof. Sunderkötter has served as a paid consultant for InfectoPharm. He has received reimbursement of travel expenses from InfectoPharm and has been paid by the same company for giving continuing medical education lectures and for performing clinical trials on its behalf.
Prof. Wohlrab has received consulting fees, lecture honoraria, reimbursement of travel and accommodation expenses, and third-party research funding from InfectoPharm, and has been paid by InfectoPharm for performing clinical trials on its behalf.
Prof. Hamm has received consulting fees, lecture honoraria, and reimbursement of travel and accommodation expenses from InfectoPharm.
References
- 1.Sunderkötter C, Aebischer A, Neufeld M, et al. Increase of scabies in Germany and development of resistant mites? Evidence and consequences. J Dtsch Dermatol Ges. 2019;17:15–23. doi: 10.1111/ddg.13706. [DOI] [PubMed] [Google Scholar]
- 2.Nenoff P, Suss A, Schulze I, et al. Skabies-Renaissance einer Ektoparasitose: Diagnostik und Therapie - Vorgehen in der Praxis. Hautarzt. 2021;72:125–136. doi: 10.1007/s00105-020-04729-6. [DOI] [PubMed] [Google Scholar]
- 3.Boralevi F, Diallo A, Miquel J, et al. Clinical phenotype of scabies by age. Pediatrics. 2014;133:e910–e916. doi: 10.1542/peds.2013-2880. [DOI] [PubMed] [Google Scholar]
- 4.Elsner E, Uhlmann T, Krause S, Hartmann R. Anstieg von Skabies und Therapierefraktärität bei Bundeswehrangehörigen: Acht-Jahre-Follow-up-Studie aus der Hautklinik des Bundeswehrkrankenhauses Berlin (2012-2019) Hautarzt. 2020;71:447–454. doi: 10.1007/s00105-020-04608-0. [DOI] [PubMed] [Google Scholar]
- 5.Kortas AZ, Polenz J, von Hayek J, et al. Screening for infectious diseases among asylum seekers newly arrived in Germany in 2015: a systematic single-centre analysis. Public Health. 2017;153:1–8. doi: 10.1016/j.puhe.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Karimkhani C, Colombara DV, Drucker AM, et al. The global burden of scabies: a cross-sectional analysis from the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:1247–1254. doi: 10.1016/S1473-3099(17)30483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arora P, Rudnicka L, Sar-Pomian M, et al. Scabies: a comprehensive review and current perspectives. Dermatol Ther. 2020;33 doi: 10.1111/dth.13746. e13746. [DOI] [PubMed] [Google Scholar]
- 8.Leung AKC, Lam JM, Leong KF. Scabies: a neglected global disease. Curr Pediatr Rev. 2020;16:33–42. doi: 10.2174/1573396315666190717114131. [DOI] [PubMed] [Google Scholar]
- 9.Mellanby K. The development of symptoms, parasitic infection and immunity in human scabies. Parasitology. 1944;35:197–206. [Google Scholar]
- 10.Arlian LG, Vyszenski-Moher DL. Life cycle of sarcoptes scabiei var. canis. J Parasitol. 1988;74:427–430. [PubMed] [Google Scholar]
- 11.Hamm H. Reisedermatosen. Berlin, Heidelberg, New York Springer: 2015. Skabies In: Von Stebut E (ed.): pp. 151–157. [Google Scholar]
- 12.Mellanby K. Transmission of scabies. Br Med J. 1941;2:405–406. doi: 10.1136/bmj.2.4211.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arlian LG, Runyan RA, Achar S, Estes SA. Survival and infectivity of sarcoptes scabiei var canis and var. hominis. J Am Acad Dermatol. 1984 11;(2 Pt 1):210–215. doi: 10.1016/s0190-9622(84)70151-4. [DOI] [PubMed] [Google Scholar]
- 14.Arlian LG, Runyan RA, Sorlie LB, Estes SA. Host-seeking behavior of sarcoptes scabiei. J Am Acad Dermatol. 1984 11;(4 Pt 1):594–598. doi: 10.1016/s0190-9622(84)70212-x. [DOI] [PubMed] [Google Scholar]
- 15.Sunderkötter C, Feldmeier H, Fölster-Holst R, et al. S1 guidelines on the diagnosis and treatment of scabies—short version. J Dtsch Dermatol Ges. 2016;14:1155–1167. doi: 10.1111/ddg.13130. [DOI] [PubMed] [Google Scholar]
- 16.Thomas C, Coates SJ, Engelman D, et al. Ectoparasites: scabies. J Am Acad Dermatol. 2020;82:533–548. doi: 10.1016/j.jaad.2019.05.109. [DOI] [PubMed] [Google Scholar]
- 17.Hamm H, Stoevesandt J, Sunderkötter C. Skabies im Alter. Z Gerontol Geriatr. 2019;52:795–807. doi: 10.1007/s00391-019-01650-z. [DOI] [PubMed] [Google Scholar]
- 18.Cassell JA, Middleton J, Nalabanda A, et al. Scabies outbreaks in ten care homes for elderly people: a prospective study of clinical features, epidemiology, and treatment outcomes. Lancet Infect Dis. 2018;18:894–902. doi: 10.1016/S1473-3099(18)30347-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li FZ, Chen S. Diagnostic accuracy of dermoscopy for scabies. Korean J Parasitol. 2020;58:669–674. doi: 10.3347/kjp.2020.58.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mang R, Kremer A, Lehmann P, Assmann T. Videodermoscopic clues for scabies diagnosis and assessment of therapeutic efficacy. J Dtsch Dermatol Ges. 2020;18:1022–1024. doi: 10.1111/ddg.14155. [DOI] [PubMed] [Google Scholar]
- 21.Engelman D, Yoshizumi J, Hay RJ, et al. The 2020 International Alliance for the Control of Scabies consensus criteria for the diagnosis of Scabies. Br J Dermatol. 2020;183:808–820. doi: 10.1111/bjd.18943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamm H, Beiteke U, Höger PH, Seitz CS, Thaci D, Sunderkötter C. Treatment of scabies with 5% permethrin cream: results of a German multicenter study. J Dtsch Dermatol Ges. 2006;4:407–413. doi: 10.1111/j.1610-0387.2006.05941.x. [DOI] [PubMed] [Google Scholar]
- 23.Hu S, Bigby M. Treating scabies: results from an updated Cochrane review. Arch Dermatol. 2008;144:1638–1640. doi: 10.1001/archdermatol.2008.513. [DOI] [PubMed] [Google Scholar]
- 24.Rosumeck S, Nast A, Dressler C. Evaluation of ivermectin vs permethrin for treating scabies—summary of a Cochrane review. JAMA Dermatol. 2019;155:730–732. doi: 10.1001/jamadermatol.2019.0279. [DOI] [PubMed] [Google Scholar]
- 25.Dhana A, Yen H, Okhovat JP, et al. Ivermectin versus permethrin in the treatment of scabies: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2018;78:194–198. doi: 10.1016/j.jaad.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Wohlrab J, Staubach P, Augustin M, et al. S2k-Leitlinie zum Gebrauch von Präparationen zur lokalen Anwendung auf der Haut (Topika) J Dtsch Dermatol Ges. 2018;16:376–392. doi: 10.1111/ddg.13473_g. [DOI] [PubMed] [Google Scholar]
- 27.Salavastru CM, Chosidow O, Boffa MJ, et al. European guideline for the management of scabies. J Eur Acad Dermatol Venereol. 2017;31:1248–1253. doi: 10.1111/jdv.14351. [DOI] [PubMed] [Google Scholar]
- 28.Nemecek R, Stockbauer A, Lexa M, et al. Application errors associated with topical treatment of scabies: an observational study. J Dtsch Dermatol Ges. 2020;18:554–559. doi: 10.1111/ddg.14122. [DOI] [PubMed] [Google Scholar]
- 29.Jittamala P, Monteiro W, Smit MR, et al. A systematic review and an individual patient data meta-analysis of ivermectin use in children weighing less than fifteen kilograms: Is it time to reconsider the current contraindication? PLoS Negl Trop Dis. 2021;15 doi: 10.1371/journal.pntd.0009144. e0009144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levy M, Martin L, Bursztejn AC, et al. Ivermectin safety in infants and children under 15 kg treated for scabies: a multicentric observational study. Br J Dermatol. 2020;182:1003–1006. doi: 10.1111/bjd.18369. [DOI] [PubMed] [Google Scholar]
- 31.Wohlrab J, Stadie L, Neubert RHH, Bosse K. Entwicklung eines Ivermectin-haltigen Saftes als Magistralrezeptur für Kinder zur Therapie der Skabies. Hautarzt. 2021;72:720–728. doi: 10.1007/s00105-021-04806-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mounsey KE, Bernigaud C, Chosidow O, McCarthy JS. Prospects for moxidectin as a new oral treatment for human scabies. PLoS Negl Trop Dis. 2016;10 doi: 10.1371/journal.pntd.0004389. e0004389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robert Koch-Institut. Skabies (Krätze). RKI-Ratgeber. www.rki.de/DE/Content/Infekt/EpidBull/Merkblaetter/Ratgeber_Skabies. html (last accessed on 26 March 2021) [Google Scholar]
- 34.Leeyaphan C, Pluetrattanabha N, Limphoka P, Bunyaratavej S. Scabicidal effect of heat on the in vitro survival of scabies mites and their eggs: optimal temperature and exposure time. Indian J Dermatol Venereol Leprol. 2019;85:647–649. doi: 10.4103/ijdvl.IJDVL_198_19. [DOI] [PubMed] [Google Scholar]
- 35.Bernigaud C, Fernando DD, Lu H, et al. How to eliminate scabies parasites from fomites: a high-throughput ex vivo experimental study. J Am Acad Dermatol. 2020;83:241–245. doi: 10.1016/j.jaad.2019.11.069. [DOI] [PubMed] [Google Scholar]
- 36.Mounsey KE, Murray HC, King M, Oprescu F. Retrospective analysis of institutional scabies outbreaks from 1984 to 2013: lessons learned and moving forward. Epidemiol Infect. 2016;144:2462–2471. doi: 10.1017/S0950268816000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stoevesandt J, Carlé L, Leverkus M, Hamm H. Control of large institutional scabies outbreaks. J Dtsch Dermatol Ges. 2012;10:637–647. doi: 10.1111/j.1610-0387.2012.07892.x. [DOI] [PubMed] [Google Scholar]
- 38.Mang R, Kremer A, Lehmann P, Assmann T. Skabies - klinische Therapieresistenz auf Permethrin: Fallbeschreibungen und eine kritische Auseinandersetzung mit den aktuellen Therapieempfehlungen. Hautarzt. 2021;72:595–599. doi: 10.1007/s00105-021-04783-8. [DOI] [PubMed] [Google Scholar]
- 39.Hackenberg B, Horvath ON, Petachti M, et al. Skabiestherapie in Deutschland: Ergebnisse einer bundesweiten Umfrage mit besonderem Fokus auf die Wirksamkeit der Erstlinientherapie mit Permethrin. Hautarzt. 2020;71:374–379. doi: 10.1007/s00105-020-04561-y. [DOI] [PubMed] [Google Scholar]
- 40.Aussy A, Houivet E, Hebert V, et al. Risk factors for treatment failure in scabies: a cohort study. Br J Dermatol. 2019;180:888–893. doi: 10.1111/bjd.17348. [DOI] [PubMed] [Google Scholar]
- E1.Kerwat K, Just M, Wulf H. Das deutsche Infektionsschutzgesetz (IfSG) Anasthesiol Intensivmed Notfallmed Schmerzther. 2009;44:182–183. doi: 10.1055/s-0029-1215548. [DOI] [PubMed] [Google Scholar]
- E2.Johnston G, Sladden M. Scabies: diagnosis and treatment. BMJ. 2005;331:619–622. doi: 10.1136/bmj.331.7517.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E3.Vyas P, Kinsey SE, Goldstone AH. Scabies infestation following autologous bone marrow transplantation. Leuk Lymphoma. 1990;3:73–74. doi: 10.3109/10428199009050978. [DOI] [PubMed] [Google Scholar]
- E4.Venning VA, Millard PR. Recurrent scabies with unusual clinical features in a renal transplant recipient. Br J Dermatol. 1992;126:204–205. [PubMed] [Google Scholar]
- E5.Ministère de la Santé, de la Famille et des Personnes Handicapées, Direction Générale de la Santé, Conseil Supérieur d‘Hygiène Publique de France Avis du Conseil Supérieur d’Hygiène Publique de France, section des maladies transmissibles, relatif à la conduite à tenir devant un cas de gale (séance du 27 juin 2003) Ann Dermatol Venereol. 2004;131:1119–1121. doi: 10.1016/s0151-9638(04)93857-4. [DOI] [PubMed] [Google Scholar]
- E6.Hubler WR Jr. Epidemic Norwegian scabies. Arch Dermatol. 1976;112:179–181. [PubMed] [Google Scholar]
- E7.Lassa S, Campbell MJ, Bennett CE. Epidemiology of scabies prevalence in the UK. from general practice records. Br J Dermatol. 2011;164:1329–1334. doi: 10.1111/j.1365-2133.2011.10264.x. [DOI] [PubMed] [Google Scholar]
- E8.Cox NH, Paterson WD. Epidemiology of scabies: the new epidemic. Lancet. 1991;337:1547–1548. doi: 10.1016/0140-6736(91)93239-6. [DOI] [PubMed] [Google Scholar]
- E9.Heukelbach J, Feldmeier H. Scabies. Lancet. 2006;367:1767–1774. doi: 10.1016/S0140-6736(06)68772-2. [DOI] [PubMed] [Google Scholar]
- E10.Vorou R, Remoudaki HD, Maltezou HC. Nosocomial scabies. J Hosp Infect. 2007;65:9–14. doi: 10.1016/j.jhin.2006.08.012. [DOI] [PubMed] [Google Scholar]
- E11.Collinson S, Ward R. A nurse-led response to unmet needs of homeless migrants in inner London. Br J Nurs. 2010;19:36–41. doi: 10.12968/bjon.2010.19.1.45910. [DOI] [PubMed] [Google Scholar]
- E12.He R, Gu X, Lai W, et al. Transcriptome-microRNA analysis of Sarcoptes scabiei and host immune response. PLoS One. 2017;12 doi: 10.1371/journal.pone.0177733. e0177733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E13.Jannic A, Bernigaud C, Brenaut E, Chosidow O. Scabies itch. Dermatol Clin. 2018;36:301–308. doi: 10.1016/j.det.2018.02.009. [DOI] [PubMed] [Google Scholar]
- E14.Aung PTZ, Cuningham W, Hwang K, et al. Scabies and risk of skin sores in remote Australian Aboriginal communities: a self-controlled case series study. PLoS Negl Trop Dis. 2018;12 doi: 10.1371/journal.pntd.0006668. e0006668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E15.Liu JM, Wang HW, Chang FW, et al. The effects of climate factors on scabies A 14-year population-based study in Taiwan. Parasite. 2016;23 doi: 10.1051/parasite/2016065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E16.Lavery MJ, Stull C, Nattkemper LA, et al. Nocturnal pruritus: prevalence, characteristics, and impact on ItchyQoL in a chronic itch population. Acta Derm Venereol. 2017;97:513–515. doi: 10.2340/00015555-2560. [DOI] [PubMed] [Google Scholar]
- E17.Lopez-Sundh AE, Gomez-Fernandez C, Marlasca-SanMartin P, et al. Neonatal scabies in times of confinement: An unexpected guest to be recognised. J Paediatr Child Health. 2020;57:1505–1507. doi: 10.1111/jpc.15238. [DOI] [PubMed] [Google Scholar]
- E18.Tesner B, Williams NO, Brodell RT. The pathophysiologic basis of scabietic nodules. J Am Acad Dermatol. 2007 57;(2 Suppl):S56–S57. doi: 10.1016/j.jaad.2007.04.006. [DOI] [PubMed] [Google Scholar]
- E19.Ruby KN, Loo EY, Mann JA, LeBlanc RE. Post-scabietic nodules: mimicker of infantile indeterminate cell histiocytosis and potential diagnostic pitfall. J Cutan Pathol. 2020;47:52–56. doi: 10.1111/cup.13557. [DOI] [PubMed] [Google Scholar]
- E20.Kokubu H, Takahashi T, Tateishi C, et al. Serological investigation of bullous scabies and review of the published work. J Dermatol. 2019;46:e324–e325. doi: 10.1111/1346-8138.14883. [DOI] [PubMed] [Google Scholar]
- E21.Swe PM, Christian LD, Lu HC, et al. Complement inhibition by Sarcoptes scabiei protects Streptococcus pyogenes—an in vitro study to unravel the molecular mechanisms behind the poorly understood predilection of S pyogenes to infect mite-induced skin lesions. PLoS Negl Trop Dis. 2017;11 doi: 10.1371/journal.pntd.0005437. e0005437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E22.Yosipovitch G, Misery L, Proksch E, et al. Skin barrier damage and itch: review of mechanisms, topical management and future directions. Acta Derm Venereol. 2019;99:1201–1209. doi: 10.2340/00015555-3296. [DOI] [PubMed] [Google Scholar]
- E23.Ueda T, Katsura Y, Sasaki A, et al. Gray-edged line sign of scabies burrow. J Dermatol. 2021;48:190–198. doi: 10.1111/1346-8138.15650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E24.Schuh S, Weins AB, Welzel J. Nichtinvasive bildgebende Diagnostik bei Hauterkrankungen im Kindesalter. Hautarzt. 2021;72:199–206. doi: 10.1007/s00105-020-04753-6. [DOI] [PubMed] [Google Scholar]
- E25.Walton SF. The immunology of susceptibility and resistance to scabies. Parasite Immunol. 2010;32:532–540. doi: 10.1111/j.1365-3024.2010.01218.x. [DOI] [PubMed] [Google Scholar]
- E26.Mika A, Reynolds SL, Mohlin FC, et al. Novel scabies mite serpins inhibit the three pathways of the human complement system. PLoS One. 2012;7 doi: 10.1371/journal.pone.0040489. e40489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E27.Bergstrom FC, Reynolds S, Johnstone M, et al. Scabies mite inactivated serine protease paralogs inhibit the human complement system. J Immunol. 2009;182:7809–7817. doi: 10.4049/jimmunol.0804205. [DOI] [PubMed] [Google Scholar]
- E28.Han L, Dong X. Itch mechanisms and circuits. Annu Rev Biophys. 2014;43:331–355. doi: 10.1146/annurev-biophys-051013-022826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E29.Tornero-Velez R, Davis J, Scollon EJ, et al. A pharmacokinetic model of cis- and trans-permethrin disposition in rats and humans with aggregate exposure application. Toxicol Sci. 2012;130:33–47. doi: 10.1093/toxsci/kfs236. [DOI] [PubMed] [Google Scholar]
- E30.Nanda J, Juergens AL. Permethrin 2020 May 30. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. 2021 Jan- PMID: 31985943. [Google Scholar]
- E31.Pallesen K, Lassen JA, Munk NT, et al. In vitro survival of scabies mites. Clin Exp Dermatol. 2020;45:712–715. doi: 10.1111/ced.14209. [DOI] [PubMed] [Google Scholar]
- E32.Chhaiya SB, Patel VJ, Dave JN, et al. Comparative efficacy and safety of topical permethrin, topical ivermectin, and oral ivermectin in patients of uncomplicated scabies. Indian J Dermatol Venereol Leprol. 2012;78:605–610. doi: 10.4103/0378-6323.100571. [DOI] [PubMed] [Google Scholar]
- E33.Dourmishev AL, Dourmishev LA, Schwartz RA. Ivermectin: pharmacology and application in dermatology. Int J Dermatol. 2005;44:981–988. doi: 10.1111/j.1365-4632.2004.02253.x. [DOI] [PubMed] [Google Scholar]
- E34.Chosidow A, Gendrel D. Tolérance de l’ivermectine orale chez l’enfant. Arch Pediatr. 2016;23:204–209. doi: 10.1016/j.arcped.2015.11.002. [DOI] [PubMed] [Google Scholar]
- E35.Ständer S, Kirschstein DJ, Kohl-Sobania M, et al. Effectiveness and adverse events of ivermectin treatment for scabies in 30 infant patients: report from a German single centre. J Eur Acad Dermatol Venereol. 2020;34:e736–e737. doi: 10.1111/jdv.16554. [DOI] [PubMed] [Google Scholar]
- E36.Laing R, Gillan V, Devaney E. Ivermectin—old drug, new tricks? Trends Parasitol. 2017;33:463–472. doi: 10.1016/j.pt.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E37.King CL. Is ivermectin safe in pregnancy? Lancet Glob Health. 2020;8:e12–e13. doi: 10.1016/S2214-109X(19)30490-5. [DOI] [PubMed] [Google Scholar]
- E38.Van Hees S, Raimon S, Fodjo JNS, Colebunders R. Safety of ivermectin during pregnancy. Lancet Glob Health. 2020;8 doi: 10.1016/S2214-109X(19)30555-8. e338. [DOI] [PubMed] [Google Scholar]
- E39.Schraven AL, Stannard HJ, Old JM. A systematic review of moxidectin as a treatment for parasitic infections in mammalian species. Parasitol Res. 2021;120:1167–1181. doi: 10.1007/s00436-021-07092-0. [DOI] [PubMed] [Google Scholar]
- E40.Milton P, Hamley JID, Walker M, Basanez MG. Moxidectin: an oral treatment for human onchocerciasis. Expert Rev Anti Infect Ther. 2020;18:1067–1081. doi: 10.1080/14787210.2020.1792772. [DOI] [PubMed] [Google Scholar]
- E41.Cotreau MM, Warren S, Ryan JL, et al. The antiparasitic moxidectin: safety, tolerability, and pharmacokinetics in humans. J Clin Pharmacol. 2003;43:1108–1115. doi: 10.1177/0091270003257456. [DOI] [PubMed] [Google Scholar]
- E42.Liu X, Walton S, Mounsey K. Vaccine against scabies: necessity and possibility. Parasitology. 2014;141:725–732. doi: 10.1017/S0031182013002047. [DOI] [PubMed] [Google Scholar]
- E43.Buehlmann M, Beltraminelli H, Strub C, et al. Scabies outbreak in an intensive care unit with 1,659 exposed individuals—key factors for controlling the outbreak. Infect Control Hosp Epidemiol. 2009;30:354–360. doi: 10.1086/596113. [DOI] [PubMed] [Google Scholar]
- E44.Capobussi M, Sabatino G, Donadini A, et al. Control of scabies outbreaks in an Italian hospital: an information-centered management strategy. Am J Infect Control. 2014;42:316–320. doi: 10.1016/j.ajic.2013.10.006. [DOI] [PubMed] [Google Scholar]
- E45.White LC, Lanza S, Middleton J, et al. The management of scabies outbreaks in residential care facilities for the elderly in England: a review of current health protection guidelines. Epidemiol Infect. 2016;144:3121–3130. doi: 10.1017/S0950268816001746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E46.Meyersburg D, Kaiser A, Bauer JW. Loss of efficacy of topical 5% permethrin for treating scabies: an Austrian single-center study. J Dermatolog Treat 2020 1-4. doi: 10.1080/09546634.2020.1774489. doi: 10.1080/09546634.2020.1774489. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- E47.Pasay C, Arlian L, Morgan M, et al. High-resolution melt analysis for the detection of a mutation associated with permethrin resistance in a population of scabies mites. Med Vet Entomol. 2008;22:82–88. doi: 10.1111/j.1365-2915.2008.00716.x. [DOI] [PubMed] [Google Scholar]
- E48.Andriantsoanirina V, Izri A, Botterel F, et al. Molecular survey of knockdown resistance to pyrethroids in human scabies mites. Clin Microbiol Infect. 2014;20:O139–O141. doi: 10.1111/1469-0691.12334. [DOI] [PubMed] [Google Scholar]
- E49.Mounsey KE, Pasay CJ, Arlian LG, et al. Increased transcription of glutathione S-transferases in acaricide exposed scabies mites. Parasit Vectors. 2010;3 doi: 10.1186/1756-3305-3-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E50.Atif M, Smith JJ, Estrada-Mondragon A, et al. GluClR-mediated inhibitory postsynaptic currents reveal targets for ivermectin and potential mechanisms of ivermectin resistance. PLoS Pathog. 2019;15 doi: 10.1371/journal.ppat.1007570. e1007570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E51.De Sainte Marie B, Mallet S, Gaudy-Marqueste C, et al. Gales en échec de traitement: étude observationnelle. Ann Dermatol Venereol. 2016;143:9–15. doi: 10.1016/j.annder.2015.10.588. [DOI] [PubMed] [Google Scholar]
- E52.Arlian LG, Morgan MS, Paul CC. Evidence that scabies mites (Acari: Sarcoptidae) influence production of interleukin-10 and the function of T-regulatory cells (Tr1) in humans. J Med Entomol. 2006;43:283–287. doi: 10.1603/0022-2585(2006)043[0283:etsmas]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- E53.Van Neste D. Immuno-allergological aspects of scabies. A comparative study of spontaneous blastogenesis in the dermal infiltrates of common and hyperkeratotic scabies, allergic contact dermatitis and irritant dermatitis. Arch Dermatol Res. 1982;274:159–167. doi: 10.1007/BF00510369. [DOI] [PubMed] [Google Scholar]
- E54.Musalek M, Kutzer E. Psychiatrische und parasitologische Aspekte des Dermatozooenwahns. Wien Klin Wochenschr. 1989;101:153–160. [PubMed] [Google Scholar]