Abstract
Background
A major reason for the low number of organ donors in Germany is a deficit in the recognition of patients who may have impending irreversible loss of brain function (ILBF) in hospitals capable of organ retrieval.
Methods
We used anonymized data from the German Organ Procurement Organization (Deutsche Stiftung Organtransplantation, DSO) to compare two 12-month periods (a reference period and an evaluation period) before and after the implementation of an electronic screening tool (DETECT) at the University Hospital Dresden (UKD) with four other university hospitals without tool implementation (comparative cohort). DETECT is intended to aid in the recognition of potentially impending ILBF. The study endpoints encompassed patients with potentially unrecognized ILBF, patients with recognized ILBF, organ donations performed, and reports to the DSO. Changes in absolute risk were compared with Breslow-Day tests.
Results
309 patients who died with primary or secondary brain lesions were identified in the UKD in the reference and evaluation periods (164 and 145 patients, respectively), and 1060 (529, 531) in the comparative cohort. In the UKD, the number of unrecognized cases of possibly impending ILBF was 14/164 (8.54%) in the reference period and 1/145 (0.69%) in the evaluation period, yielding an absolute reduction of 7.85% (95% confidence interval [--3.36; --12.33]); by contrast, in the comparative cohort, there was a 0.55% absolute increase between the two periods ([--2.21; 3.30]; p = 0.002 for the comparison between the two cohorts). Only minor differences in absolute risk change were seen with regard to the probability of recognized ILBF (7.09% [0.29; 13.88] vs. 2.42% [1.18; 6.01]; p = 0.234), organ donation (4.70% [--0.89; 10.28] vs. 0.55% [--2.17; 3.26]; p = 0.214), or reporting to the DSO (4.17% [--1.77; 10.11] vs. 2.22% [--1.44; 5.89; p = 0.447); these changes may have arisen by chance.
Conclusion
These findings suggest that the use of DETECT can help to reduce the deficit in the recognition of patients with impending or manifest ILBF.
Organ transplantation is considered one of the major achievements of the twentieth century. Over the decades, it has helped save many lives. Yet the supply of available organs for patients in Germany who need them has been critically low for years now, without any discernible trend toward improvement. Waiting times for organs are longer here than in any other developed western country, and several people die every day while still on the waiting list (1). Moreover, the clinical outcome of cadaveric organ transplantation (heart, lung, liver, kidney) is worse in Germany than in other countries on average because of the advanced disease stage of the recipients, who have often waited a long time for their transplant (2).
The problem of long waiting times is due to the low rate of organ donation, which, having been low for decades, dropped in 2017 to a level below 10 donors per million inhabitants for the first time ever. This puts Germany in last place among the Eurotransplant countries, some of whose organ donation and transplantation rates are three times as high (3). It must be borne in mind that each organ donation can grant its recipients many additional years of life (4, 5).
Whenever decisions regarding end-of-life–care (EOLC) have to be taken, a wide variety of obstacles can stand in the way of organ donation, which arise from the medical, judicial, ethical, and human complexity of the overall situation. Only patients who are in intensive care and demonstrate irreversible loss of brain function (ILBF) can be considered as potential organ donors. The obstacles that must be actively overcome include the following:
the absence of a legal presumption of willingness to donate unless the patient has made a prior statement of his or her willingness to do so, and the consequent absence of broad social agreement with regard to such a presumption
labor-intensiveness
doctors� shiftwork
high caseload and a simultaneous staff shortage
the focusing and restriction of medical attention to immediate cures
economic pressure on hospitals.
Studies have shown that a major reason for the low number of organ donors in Germany is a deficit in the recognition of patients who may have impending irreversible loss of brain function (ILBF) in the hospitals that are capable of organ retrieval (6– 11). The fact that there are many (1248) such hospitals with an intensive care unit in Germany makes it difficult to detect a rare event such as potential ILBF in an intensive care patient. As a result, fewer than 1000 persons become organ donors among the approximately 950,000 who die in Germany every year. Most of the hospitals that are capable of organ retrieval do not recognize or report even a single patient with potential ILBF (12).
In order to understand why many patients in intensive care are never diagnostically evaluated for ILBF, the eastern regional division of the German Organ Procurement Organization (DSO, in the states of Saxony, Saxony-Anhalt, and Thuringia) has been conducting a voluntary retrospective analysis of all patients who died with a relevant degree of brain dysfunction. The study, which began more than five years ago, makes use of a computer program called DSO Transplantcheck (which we shall call Transplantcheck in this article) followed by inspection of the medical records by the transplantation representative at the given institution, in cooperation with the responsible DSO coordinator. The data for 2016 reveal only 121 cases in which organ donation became a reality among more than 7889 cases of death due to loss of brain function (10). A retrospective analysis of individual cases identified 500 patients who died without ever having undergone diagnostic evaluation for ILBF; many of these patients may well have had ILBF without being adequately tested for it (10). Such deficits were found in all categories of hospitals with intensive care units, even though the DSO eastern region has long had one of the highest organ donation rates among all the regions of Germany.
An electronic screening tool called DETECT (“screening for potential brain DEath in paTiEnts with severe brain damage and clinically asCerTained loss of cerebral functions”) was developed at the University Hospital Dresden (UKD) in order to prospectively identify patients with impending or current ILBF and thereby close the detection gap.
In this cohort trial, we studied the hypothesis that the implementation of the DETECT screening tool at UKD improved detection of ILBF compared to its non-implementation at the four other university hospitals located in the eastern DSO region of Germany.
Methods
Automated screening
The electronic DETECT screening tool was implemented in the patient data management system (PDMS) of UKD in order to optimally identify patients with potential ILBF. In an initial developmental phase, simulations were conducted in which the tool was applied to retrospective data from 2016 and 2017 and cross-checked against data from the Transplantcheck software of the DSO. The program was initiated as a test from January to March 2018; from April 2018 onward, it has been used regularly for prospective screening in the intensive care units of UKD.
In order to develop an automated screening algorithm, criteria for potential ILBF that can be consistently measured and reliably documented in an intensive care unit several times per day were defined in an interdisciplinary dialogue among intensive care physicians, neurologists, and neurosurgeons. The tool primarily assessed two hard criteria:
coma, as rated on the Richmond Agitation Sedation Scale (RASS) and the Glasgow Coma Scale (GCS) (both of these are well-established neurological scores for the quantification of consciousness; a low sum indicates possible coma)
loss of the pupillary light reflex as evidence of brainstem areflexia.
These documented parameters are already included in treatment guidelines for intensive care and can thus be comprehensively ascertained (13).
Further, soft indicators can optionally be considered for a better assessment of the situation (eMethods).
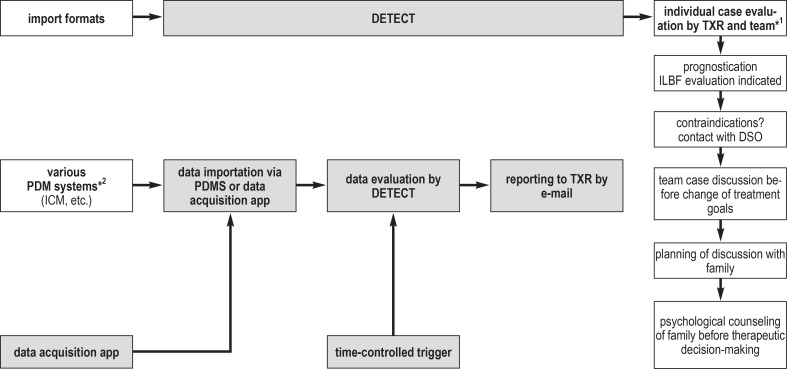
The screening process is implemented on all intensive care units of UKD except the pediatric intensive care unit (114 beds overall), without the need for any additional documentation, by way of a PDMS system called Integrated Care Manager (ICM). The hard and soft criteria for potential ILBF that are relevant to screening are retrieved periodically (every twelve hours) for all inpatients, stored, and then read into the screening tool. The tool then generates a case list, which is filtered according to defined criteria with LINQ (Language Integrated Query). If the hard criteria mentioned above are present, the screening is positive and an e-mail with detailed relevant data is sent to the hospital�s transplantation representative and to the treating intensive care physicians (figure 1).
Figure 1.
DETECT is a tool for the time-controlled evaluation of data imported from the patient data management system (PDMS) and applies certain defined criteria to identify patients with potentially impending irreversible loss of brain function (ILBF), then correspondingly sends targeted messages to a transplantation representative (TXR) in the hospital where the patient is being treated. Notification of the TXR by DETECT initiates structured further procedures in the hospital.
*1 Neuro-intensive care specialist, neurologist, neurosurgeon, ward attending; *2 patient data management systems in the intensive care unit.
DSO, German Organ Procurement Organization (Deutsche Stiftung Organtransplantation); ICM, integrated care manager; TXR, transplantation representative.
Transplantcheck
The methods used in Transplantcheck are described in eMethods (6, 10, 14).
Study design
To evaluate the adequacy of the screening tool, study endpoints measured before and after its introduction (i.e., in the reference period from January to December 2017 versus the evaluation period from April 2018 to March 2019) were subjected to a comparative analysis. These endpoints were:
the frequency of undetected potential ILBF
the determination of current ILBF (according to the guideline of the German Medical Association) (15)
effectuated organ donations
reports to the DSO without any effectuated organ donation.
The analysis was based on anonymized data from the routinely conducted Transplantcheck analyses of the university hospitals in the eastern DSO region.
These analyses of potential organ donations provided the basis for the development of a targeted prospective screening tool for the electronic detection and reporting of patients with severe brain dysfunction.
In order to reliably exclude secular trends affecting the study endpoints mentioned above independently of the introduction of the DETECT screening tool at UKD, analogous analyses were carried out in parallel in a second cohort, namely, the other four univeristy hospitals in the eastern DSO region, in which DETECT was not implemented. Patients under age 16 were excluded from the analysis, as the screening tool has not yet been applied in pediatric intensive care units.
Diagnostic efficacy
The diagnostic efficacy of the DETECT screening tool was evaluated by an analysis of the cases that it detected, cross-checked against the relevant cases in the Transplantcheck analysis and in relation to the overall number of patients treated in the intensive care units.
The following types of cases that are to be detected were considered relevant to the assessment of the diagnostic efficacy of the screening tool:
cases of potential ILBF that were not detected, as indicated by the Transplantcheck analysis
effectuated organ donations
reports to the DSO without any effectuated organ donations
non-consent of the patient�s family in cases of potentially imminent ILBF.
Statistical analysis
Statistical analysis was performed with the STATA program (Version 12.1, StataCorp, College Station, TX, USA). The frequency of undetected potential ILBF was the primary endpoint of this study. Further endpoints included the frequency of detected ILBF, effectuated organ donations, and reports to the DSO without any effectuated organ donation. Frequencies were reported in absolute terms and as percentages, along with 95% confidence intervals (CI). Age was reported as mean ± standard deviation (SD). Demographic data and diagnoses were comparatively analyzed with the chi-square test, Fisher�s exact test, and the t test for independent samples. Changes in risk before and after the introduction of the tool, and the associated 95% CI, were calculated for each endpoint. Changes in risk in the two cohorts (UKD and the other regional university hospitals) were tested for homogeneity with the Breslow-Day test. p-values are given as descriptive parameters.
The frequency of undetected cases of potential ILBF at UKD, as determined with the aid of the Transplantcheck analysis, was the reference standard for the calculation of sensitivity, specificity, and positive and negative predictive value as measures of the diagnostic efficacy of the automated screening tool.
Results
Comparative analyses of the observation periods within the cohorts
During the 24-month period of observation, 309 patients in the UKD who died after sustaining primary or secondary brain damage that qualified them for the defined cohorts were identified by Transplantcheck; the corresponding number of patients in the comparison hospitals was 1060. The two groups of patients did not differ with respect to age (70 ± 14 vs. 69 ± 15 years; p = 0.19), sex (59.2% vs. 58.9% male; p = 0.91), or type of brain damage (p = 0.06). Nor did they differ with respect to the numbers of cases analyzed by Transplantcheck during the two study periods (01/2017–12/2017 vs. 04/2018–03/2019) (p = 0.33).
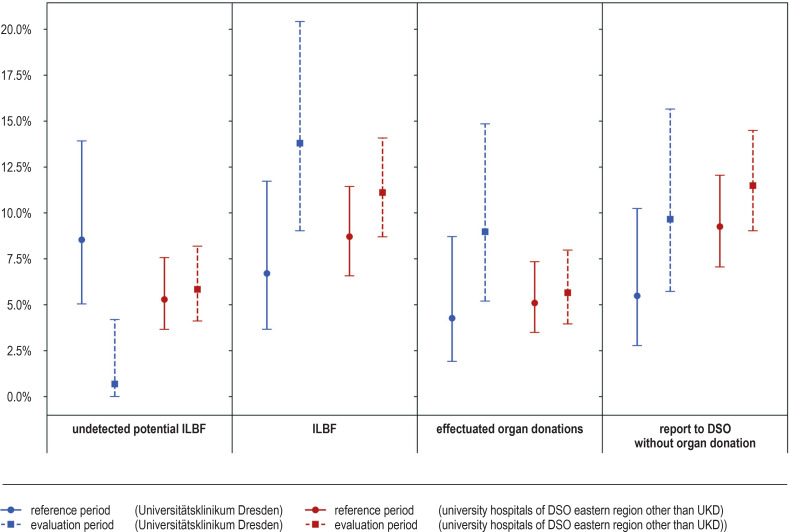
There were fewer undetected cases of potential ILBF at UKD during the evaluation period than during the reference period before the introduction of the DETECT screening tool (1/145; 0.69; 95% CI: [0.01; 4.2] vs. 14/164; 8.54; [5.05; 13.92]). In the comparison cohort of regional university hospitals, no relevant difference was seen between the two study periods with respect to undetected cases of potential ILBF (31/531; 5.84; [4.12; 8.19] vs. 28/529; 5.29; [3.66; 7.57]). The resulting absolute changes of risk (-7.85; [-3.36; -12.33] vs. 0.55; [-2.21; 3.30]) differed significantly between the two cohorts (p = 0.002).
There was a 7.09% absolute increase [0.29; 13.88] in the detection of ILBF at UKD during the evaluation period compared to the reference period, and a 2.42% increase in the cohort of other university hospitals in the region [–1.18; 6.01]. The nominal difference between these absolute risk changes may have arisen by chance (p = 0.234); the same holds for the nominal differences between risk changes for effectuated organ donations (4.70%; [-0.89; 10.28] vs. 0.55%; [-2.17; 3.26]; p = 0.214) and reports to the DSO (4.17%; [-1.77; 10.11] vs. 2.22%; [-1.44; 5.89]; p = 0.447). The odds ratios underlying the statistical tests are given in Table 1.
Table 1. Results of the Breslow–Day test.
| UKD | Other university hospitals (DSO east) | ||
| Study endpoints | Odds ratio [95% confidence interval) | p* | |
| Undetected potential ILBF | |||
| – Reference period | REF | REF | |
| – Evaluation period | 0.07 [0.01; 0.57] | 1.11 [0.66; 1.88] | 0.002 |
| ILBF | |||
| – Reference period | REF | REF | |
| – Evaluation period | 2.23 [1.03; 4.82] | 1.31 [0.87; 1.97] | 0.234 |
| Effectuated organ donation | |||
| – Reference period | REF | REF | |
| – Evaluation period | 2.21 [0.86; 5.7] | 1.11 [0.65; 1.9] | 0.214 |
| report to DSO without organ donation | |||
| – Reference period | REF | REF | |
| – Evaluation period | 1.84 [0.77; 4.39] | 1.27 [0.85; 1.89] | 0.447 |
*Breslow-Day test
DSO, German Organ Procurement Organization (Deutsche Stiftung Organtransplantation)
ILBF, irreversible loss of brain function; REF, reference; UKD, Universitätsklinkum Dresden
A comparative presentation of the two cohorts with respect to demographic variables, diagnoses, and study endpoints during the two study periods is given in Table 2 and supplemented in Figure 2.
Table 2. Baseline features and study endpoints in the two cohorts.
| Universitätsklinikum Dresden | University hospitals of the DSO eastern region other than the Universitätsklinikum Dresden | |||||||
|
Overall
n = 309) |
Reference period
(n = 164) |
Evaluation period
(n = 145) |
p |
Overall
(n = 1060) |
Reference period
(n = 529) |
Evaluationperiod
(n = 531) |
p | |
| Demographic variables | ||||||||
| Age (years; mean ± standard deviation) | 70.4 (14.4) |
71 (13.6) |
69.8 (15.3) |
0.48 | 69.2 (15.3) |
70.1 (15.1) |
68.3 (15.4) |
0.06 |
| Male patients (number and percent) | 183 (59.2%) |
96 (58.5%) |
87 (60%) |
0.79 | 624 (58.9%) |
316 (59.7%) |
308 (58%) |
0.57 |
| Type of brain damage (number and percent) | ||||||||
| Hemorrhagic stroke | 88 (28.5%) |
40 (24.4%) |
48 (33.1%) |
0.61 | 333 (31.4%) |
174 (32.9%) |
159 (29.9%) |
0.23 |
| Ischemic stroke | 106 (34.3%) |
58 (35.4%) |
48 (33.1%) |
318 (30%) |
160 (30.3%) |
158 (29.8%) |
||
| Trauma | 54 (17.5%) |
30 (18.3%) |
24 (16.6%) |
150 (14.2%) |
75 (14.2%) |
75 (14.1%) |
||
| Hypoxia/ischemia | 55 (17.8%) |
33 (20.1%) |
22 (15.2%) |
201 (19%) |
86 (16.3%) |
115 (21.7%) |
||
| Infection/inflammation | 2 (0.7%) |
1 (0.6%) |
1 (0.7%) |
17 (1.6%) |
9 (1.7%) |
8 (1.5%) |
||
| Other | 4 (1.3%) | 2 (1.2%) | 2 (1.4%) | 41 (3.9%) | 25 (4.7%) | 16 (3%) | ||
| Study endpoints (number, percent, 95% confidence interval) | ||||||||
| Undetected potential ILBF | 15 (4.9%) |
14 (8.5% [5.1; 13.9]) |
1 (0.7% [0.01; 4.2]) |
59 (5.6%) |
28 (5.3% [3.7;7.6]) |
31 (5.8%; [4.1; 8.2]) |
||
| ILBF | 31 (10%) |
11 (6.7% [3.7; 11.7]) |
20 (13.8% [9; 20.4]) |
105 (9.9%) |
46 (8.7% [6.6;11.4]) |
59 (11.1%; [8.7;14.1]) |
||
| Effectuated organ transplantation | 20 (6.5%) |
7 (4.3% [1.9; 8.7]) |
13 (9% [5.2; 14.9]) |
57 (5.4%) |
27 (5.1% [3.5; 7.4]) |
30 (5.7% [4;8]) |
||
| Report to DSO without organ transplantation | 23 (7.4%) |
9 (5.5% [2.8; 10.2]) |
14 (9.7% [5.7; 15.7]) |
110 (10.4%) |
49 (9.3% [7.1; 12.1]) |
61 (11.5% [9; 14.5]) |
||
DSO, German Organ Procurement Organization (Deutsche Stiftung Organtransplantation); ILBF, irreversible loss of brain function
No difference was found with respect to the determination of a current ILBF, the frequency of effectuated organ donations, or reports to the DSO (p >0.05).
The diagnostic efficacy of the DETECT screening tool
From April 2018 to March 2019, a total of 5892 patients who were treated in the intensive care units of UKD had their data registered by the automated screening tool that was implemented there. A retrospective analysis by Transplantcheck of the 145 patients who died with primary or secondary brain damage yielded 42 relevant cases with respect to screening for a potential ILBF or for the performance of a diagnostic assessment for ILBF. The sensitivity and specificity of automated screening (table 3) for the detection of a potential ILBF were 97.6% [87.4; 99.9] and 97.4% [96.9; 97.8], respectively, with an overall accuracy of 97.4% [96.9; 97.8]. The positive and negative predictive values were 21.0% [18.5; 23.9] and 99.98% [99.88; 99.99], respectively.
Table 3. 2 × 2 table: analysis of screening after implementation.
| Finding | Relevant case, potential ILBF | Not a relevant case, no potential ILBF | Overall |
|
Positive – detection by DETECT and evaluation |
41 | 154 | 195 |
|
Negative – no detection by DETECT, no evaluation |
1 | 5696 | 5697 |
| Overall | 42 | 5850 | 5892 |
PPV: (probability that a case predicted to be relevant is actually relevant): 0.21
NPV: (probability that a case predicted to be irrelevant is actually irrelevant): 0.99
Sensitivity: (probability that a relevant case is predicted to be relevant): 0.98
Specificity: (probability that an irrelevant case is predicted to be irrelevant): 0.97
ILBF: irreversible loss of brain function
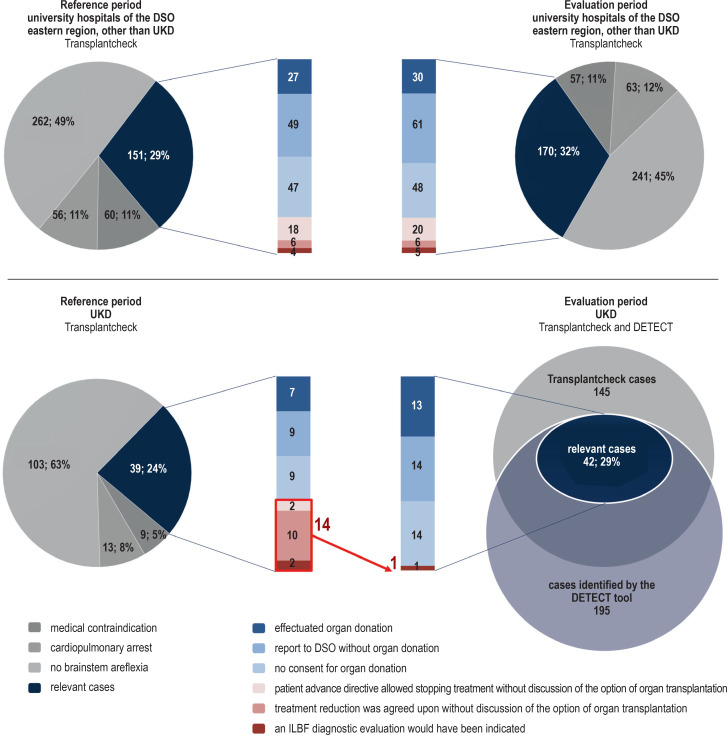
The case analyses in UKD in a comparison across the two observation periods, and in comparison with the other university clinics, are shown in the eFigure.
eFigure.
Evaluation of Transplantcheck data from the other university hospitals in the eastern region of the German Organ Procurement Organization (DSO) not including UKD (above) and from UKD (below), for the reference period (left) and the evaluation period (right).
The overlapping circles in the lower diagram (right) depict the complete prospective detection by the DETECT tool, during the evaluation period at UKD, of all cases retrospectively characterized as relevant by the Transplantcheck analysis. The detection of patients with impending ILBF by the DETECT screening tool is linked to the Transplantcheck data (circles), with demonstration of the donor detection gap on the basis of the relevant cases (vertical bars). In the Transplantcheck analysis, all cases treated in a hospital were screened and the question was retrospectively asked, for all patients who died of primary or secondary brain damage, why no diagnostic evaluation for ILBF had been performed; such patients were assigned to defined categories by individual case analysis. After the exclusion of patients with medical contraindications to organ donation, status post cardiopulmonary arrest, and absence of brainstem areflexia, the remaining cases (blue segment) were relevant to the question of identifiability by DETECT. These cases were individually analyzed and assigned to various categories: “effectuated organ donations (light blue),” “reports to the DSO without any effectuated organ donation (medium blue),” and “no consent for organ donation (light blue)” are the categories corresponding to presumably undetected cases (red region), subcategorized into “patient advance directive allowed stopping treatment without discussion of the option of organ transplantation (bright red),” “treatment reduction was agreed upon without discussion of the option of organ transplantation (medium red),” and “an ILBF diagnostic evaluation would have been indicated (dark red).” The number of cases in the last-named category was essentially the same in the two observation periods in the university hospitals of the DSO eastern region not including UKD; in contrast, the corresponding numbers in UKD were 14 in the comparison period and only 1 in the reference period.
Discussion
For decades now, Germany has had one of the lowest organ donation rates among all developed countries, without any trend toward improvement. Whenever decisions regarding end-of-life-care (EOLC) must be taken, multiple and complex obstacles stand in the way of organ donation, hindering the detection of potential donors in the hospitals where the retrieval of donated organs for transplantation can be carried out.
In the present proof-of-concept study, we show that the goal of bridging this donor detection gap can indeed be attained with a screening tool that automatically and prospectively identifies patients with potentially impending ILBF (as determined retrospectively with Transplantcheck). In the single year of its use, the DETECT screening tool prospectively identified 195 patients in the intensive care stations of UKD who met the defined criteria for detection by the tool; this was a modest number compared to the overall total of 5892 patients who were treated there. The retrospective analysis with Transplantcheck revealed that 42 patients were directly relevant for recognition by the tool and subsequent evaluation. Moreover, the statistical comparison of undetected cases before and after the introduction of the tool revealed a reduction of the detection gap for patients with potential ILBF at UKD. In contrast, the detection gap remained unchanged in the other four university hospitals in the eastern DSO region, where general conditions were the same but the DETECT tool had not been introduced. No comparable screening approach is known to us from the literature.
The comparison with the retrospective evaluation by Transplantcheck revealed that the tool was able to detect relevant cases of potential ILBF with a sensitivity of 97.6%, a specificity of 97.4%, a positive predictive value of 0.21, and a negative predictive value of 0.99 (table 3). There was only a single case of potential ILBF that was not considered during our study phase; this case had, in fact, been recognized by the DETECT tool and reported, yet the option of organ donation nevertheless was not appropriately evaluated thereafter. This fact merely underscores the complexity of our shared task of increasing the number of organ donations.
The screening tool helps overcome one of the major hurdles that are present at the beginning of the organ donation process, i.e., the identification of relevant patients despite their relative rarity. This interpretation of our findings is lent further weight by the fact that, beyond the reduction of the detection gap, we also found an increase in the number of detected current cases of ILBF and a trend toward an increase in the number of effectuated organ donations during the evaluation period in UKD, but not in the control institutions. The latter trend continued in 2020, with 19 organ donations effectuated in UKD up to the time of writing of this article.
We were surprised to see that two hard screening criteria sufficed for the successful establishment of this tool. Further, soft indicators were recorded as well and communicated to the transplantation representative for detailed characterization of the patients, but they were not needed for the identification of patients at risk of ILBF.
Limitations
This study is limited by the fact that DETECT was only implemented in a single university hospital over a 12-month period. Nor can we exclude a possible effect of spectrum bias on our findings, as the reference data were acquired from the Transplantcheck analyses, and therefore only patients who died were selected for our comparative analysis of the cohorts. In this context, a possible difference in the types of brain damage in the two cohorts should also be mentioned as a potential source of bias, because, for example, intracerebral hemorrhages lead to ILBF more frequently than infectious processes. A differentiated listing of the types of brain damage was not possible for 41 of the cases seen in the other regional university hospitals, as we only had access to previously coded diagnoses. Moreover, the lack of randomization of the university hospitals further restricts the generalizability of our findings, even though their internal validity is strengthened by the evaluation of the tool in a single center over two time periods.
Overview
Particularly in hospitals with a PDMS, DETECT has the advantage of not requiring any additional work for the purpose of documentation. To help make it comprehensively available, we are now working to incorporate DETECT in other PDMS systems. The percentage of hospitals with a PDMS is growing steadily and is now approximately 25% (16). We are also working to develop a universally implementable screening app with which the same parameters can be registered and analyzed without difficulty in hospitals that still use paper documents. Both variants should become available in 2021.
We are convinced that universal use of the DETECT tool in intensive care units will eliminate the detection gap for patients with impending ILBF and thereby lead to a rise in the organ donation rate in Germany. In view of the new legal measures that have been in effect in Germany since 1 April 2019 to improve organ donation (Law on Better Cooperation and Better Structures for Organ Donation, Gesetz für bessere Zusammenarbeit und bessere Strukturen bei der Organspende, GZSO), along with the many tasks that confront institutional transplantation representatives and the legal duty to report each and every potential organ donor, DETECT ought to enable the transplant representatives, whose role has been enhanced by the new legal rules, to focus their efforts more effectively on the relevant patients.
Supplementary Material
eMethods
Methods
Automated screening
Along with the hard indicators described in the article that are primarily registered by the DETECT screening tool, there are also supplementary, optional criteria that can be used in addition.
Soft indicators may optionally be considered for better assessment of the situation where these have been documented and where the two hard indicators already indicate the presence of potential irreversible loss of brain function (ILBF). The defined soft indicators include the following: intracranial pressure (ICP) >50 mm Hg/cerebral perfusion pressure (CPP) <20 mm Hg for 15 minutes of more; serum sodium concentration <130 mmol/ or >150 mmol/L or a change in the serum sodium concentration by >10 mmol/L in 24 hours; and status post cardiopulmonary resuscitation.
Transplantcheck
In the eastern region of the German Organ Procurement Organization (Deutsche Stiftung Organtransplantation, DSO), retrospective analyses were performed in hospitals for a number of years to explain why no ILBF diagnostic evaluation was initiated for patients who died as the result of primary or secondary brain damage. This was done by screening all cases treated in a hospital and retrospectively determining, on the basis of the medical records of patients who died with an ICD-10 diagnosis of primary or secondary brain damage, after having received artificial ventilation and without any absolute contraindication to organ donation, why no ILBF diagnostic evaluation was performed; the individual cases were then assigned to one of seven defined categories (14). Aside from effectuated organ donations and reports to the DSO, four of the seven categories were also considered relevant to our study, and were therefore analyzed in it (efigure). These categories were as follows: lack of consent for organ donation, as well as cases of presumably unrecognized potential ILBF that, according to a retrospective analysis, did in fact sustain a potential or actual ILBF during a definable period of time. This included patients with a poor neurological prognosis whose medical care was reduced to a palliative level, in some cases according to an advance directive in which organ donation was not mentioned, without any discussion of the option of organ donation with the next of kin; it also included patients whose documented findings showed that an ILBF diagnostic evaluation would have been indicated. The analysis of individual cases based on the medical records was carried out jointly by a physician designated as the transplantation representative of the intensive care unit in question and a coordinator of the DSO. A comprehensive analysis of the organ donation potential of the eastern region of the DSO has already been published for the years 2014–2016 (6, 10).
Figure 2.
Frequency (in percent) of the study endpoints, with 95% confidence intervals
DSO, German Organ Procurement Organization (Deutsche Stiftung Organtransplantation); ILBF, irreversible loss of brain function; UKD, Universitätsklinikum Dresden
Acknowledgments
Submitted on 10 February 2021, accepted after revision on 21 July 2021.
Translated from the original German by Ethan Taub, M.D.
Acknowledgement
We are grateful to the German Organ Procurement Organization for their continuous support of this project, and in particular to its chief medical officer Dr. Rahmel and its medical executive director for the eastern region, Dr. Dittrich.
Footnotes
Conflict of interest statement
PD Dr. Barlinn has served as a paid adviser and consultant for the German Organ Procurement Organization.
Prof. Hugo has served as a paid advisor for Novartis, Chiesi, AstraZeneca, Hans BioPharma, Alexion, Boehringer Ingelheim, Bayer Vital, Hansa Chemie AG, Otsuka, Fresenius, Vifor Pharma, Ablynx, Mallinckrodt, Takeda, and MSD. He has received reimbursement of medical meeting participation fees and travel costs from Astellas, Alexion, Boehringer Ingelheim, Otsuka, and Amgen and lecture honoraria from Astellas, Alexion, Novartis, Otsuka, Chiesi, and Boehringer Ingelheim. He has received third-party research funding from Astellas, Sanofi, and Boehringer Ingelheim.
The other authors declare that they have no conflict of interest.
References
- 1.Eurotransplant. Yearly statistics - Eurotransplant, waiting list mortality in Germany, by year, by organ. www.statistics.eurotransplant.org/index.php?search_type=&search_organ=&search_region=&search_period=&search_characteristic=&search_text=mortality&search_collection= (last accessed on 16 September 2021) [Google Scholar]
- 2.CTS—Collaborative Transplant Study. Outcome Graphs, Kidney. https://www.ctstransplant.org/public/graphics/sample.shtml (last accessed on 16 September 2021) 2020 [Google Scholar]
- 3.GODT - Global Observatory on Donation and Transplantation. Newsletter Transplant. www.transplant-ob servatory.org/download/newsletter-transplant-2020-3/ (last accessed on 16 September 2021) [Google Scholar]
- 4.Schnitzler MA, Whiting JF, Brennan DC. The life-years saved by a deceased organ donor. Am J Transplant. 2005;5:2289–2296. doi: 10.1111/j.1600-6143.2005.01021.x. [DOI] [PubMed] [Google Scholar]
- 5.Nunnink L, Cook DA. Palliative ICU beds for potential organ donors: an effective use of resources based on quality-adjusted life-years gained. Crit Care Resusc. 2016;18:37–42. [PubMed] [Google Scholar]
- 6.Götze M. Analyse des Organspenderpotentials der DSO Region Ost in den Jahren 2014-2016 Dissertation, Friedrich-Schiller-Universität Jena; www.doi.org/10.22032/dbt.40832 (last accessed on 14 August 2021) 2020 [Google Scholar]
- 7.Schulte K, Borzikowsky C, Rahmel A, et al. Decline in organ donation in Germany—a nationwide secondary analysis of all inpatient cases. Dtsch Arztebl Int. 2018;115:463–468. doi: 10.3238/arztebl.2018.0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schulte K, Esser G, Borzikowsky C, et al. Organ donor potential increases despite rising numbers of decompressive craniectomies. Dtsch Arztebl Int. 2020;117:542–543. doi: 10.3238/arztebl.2020.0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bendorf A, Kerridge IH, Stewart C. Intimacy or utility? Organ donation and the choice between palliation and ventilation. Crit Care. 2013;17 doi: 10.1186/cc12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brauer M, Günther A, Pleul K, et al. How many potential organ donors are there really? Retrospective analysis of why determination of irreversible loss of brain function was not performed in deceased patients with relevant brain damage. Anaesthesist. 2019;68:22–29. doi: 10.1007/s00101-018-0510-x. [DOI] [PubMed] [Google Scholar]
- 11.Esser G, Kolbrink B, Borzikowsky C, et al. Evaluation of underidentification of potential organ donors in German hospitals. PLoS One. 2020;15 doi: 10.1371/journal.pone.0242724. e0242724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deutsche Stiftung Organtransplantation. Deutsche Stiftung Organtransplantation. Frankfurt/Main: 2021. Organspende und Transplantation in Deutschland. Jahresbericht 2016 / 2017 /2018 / 2019 / 2020. [Google Scholar]
- 13.Riker RR, Fugate JE. Participants in the international multi-disciplinary consensus conference on multimodality monitoring Clinical monitoring scales in acute brain injury: assessment of coma, pain, agitation, and delirium. Neurocrit Care. 2014;21(2):S27–S37. doi: 10.1007/s12028-014-0025-5. [DOI] [PubMed] [Google Scholar]
- 14.Leitfaden zur Kategorisierung der Einzelfallanalyse von Verstorbenen mit primärer oder sekundärer Hirnschädigung. www.dso.de/SiteCollectionDocuments/Transplantcheck%20Dateien/Leitfaden%20zur%20Kategorisierung%20der%20Einzelfallanalyse.pdf (last accessed on 14 August 2021) [Google Scholar]
- 15.Richtlinie gemäß § 16 Abs. 1 S. 1 Nr. 1 TPG für die Regeln zur Feststellung des Todes nach § 3 Abs. 1 S. 1 Nr. 2 TPG und die Verfahrensregeln zur Feststellung des endgültigen, nicht behebbaren Ausfalls der Gesamtfunktion des Großhirns, des Kleinhirns und des Hirnstamms nach § 3 Abs. 2 Nr. 2 TPG. Vierte Fortschreibung Dtsch Ärztebl | 30. März 2015. www.DOI.org/10.3238/arztebl.2015.rl_hirnfunktionsausfall_01 (last accessed on 14 August 2021) [Google Scholar]
- 16.Krankenhaus-IT Online Journal. Patientendatenmanagementsysteme: Unabdinglich für eine moderne Intensivstatiaon. www.medizin-edv.de/modules/AMS/article.php?storyid=4492 (last accessed on 16 September 2021) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
Methods
Automated screening
Along with the hard indicators described in the article that are primarily registered by the DETECT screening tool, there are also supplementary, optional criteria that can be used in addition.
Soft indicators may optionally be considered for better assessment of the situation where these have been documented and where the two hard indicators already indicate the presence of potential irreversible loss of brain function (ILBF). The defined soft indicators include the following: intracranial pressure (ICP) >50 mm Hg/cerebral perfusion pressure (CPP) <20 mm Hg for 15 minutes of more; serum sodium concentration <130 mmol/ or >150 mmol/L or a change in the serum sodium concentration by >10 mmol/L in 24 hours; and status post cardiopulmonary resuscitation.
Transplantcheck
In the eastern region of the German Organ Procurement Organization (Deutsche Stiftung Organtransplantation, DSO), retrospective analyses were performed in hospitals for a number of years to explain why no ILBF diagnostic evaluation was initiated for patients who died as the result of primary or secondary brain damage. This was done by screening all cases treated in a hospital and retrospectively determining, on the basis of the medical records of patients who died with an ICD-10 diagnosis of primary or secondary brain damage, after having received artificial ventilation and without any absolute contraindication to organ donation, why no ILBF diagnostic evaluation was performed; the individual cases were then assigned to one of seven defined categories (14). Aside from effectuated organ donations and reports to the DSO, four of the seven categories were also considered relevant to our study, and were therefore analyzed in it (efigure). These categories were as follows: lack of consent for organ donation, as well as cases of presumably unrecognized potential ILBF that, according to a retrospective analysis, did in fact sustain a potential or actual ILBF during a definable period of time. This included patients with a poor neurological prognosis whose medical care was reduced to a palliative level, in some cases according to an advance directive in which organ donation was not mentioned, without any discussion of the option of organ donation with the next of kin; it also included patients whose documented findings showed that an ILBF diagnostic evaluation would have been indicated. The analysis of individual cases based on the medical records was carried out jointly by a physician designated as the transplantation representative of the intensive care unit in question and a coordinator of the DSO. A comprehensive analysis of the organ donation potential of the eastern region of the DSO has already been published for the years 2014–2016 (6, 10).