Abstract
Medication-related osteonecrosis of the jaw (MRONJ) is a potentially severe adverse event affecting patients with cancer and patients with osteoporosis who have been treated with powerful antiresorptives (pARs) or angiogenesis inhibitors (AgIs). pARs, including nitrogen-containing bisphosphonates (N-BPs; e.g., zoledronic acid, alendronate) and anti-RANKL antibodies (e.g., denosumab), are used to manage bone metastases in patients with cancer or to prevent fragility fractures in patients with osteoporosis.
Though significant advances have been made in understanding MRONJ, its pathophysiology is still not fully elucidated. Multiple species have been used in preclinical MRONJ research, including the rat, mouse, rice rat, rabbit, dog, sheep, and pig. Animal research has contributed immensely to advancing the MRONJ field, particularly, but not limited to, in developing models and investigating risk factors that were first observed in humans. MRONJ models have been developed using clinically relevant doses of systemic risk factors, like N-BPs, anti-RANKL antibodies, or AgIs. Specific local oral risk factors first noted in humans, including tooth extraction and inflammatory dental disease (e.g., periodontitis, periapical infection, etc.), were then added. Research in rodents, particularly the rat, and, to some extent, the mouse, across multiple laboratories, has contributed to establishing multiple relevant and complementary preclinical models. Models in larger species produced accurate clinical and histopathologic outcomes suggesting a potential role for confirming specific crucial findings from rodent research. We view the current state of animal models for MRONJ as good. The rodent models are now reliable enough to produce large numbers of MRONJ cases that could be applied in experiments testing treatment modalities. The course of MRONJ, including stage 0 MRONJ, is characterized well enough that basic studies of the molecular or enzyme-level findings in different MRONJ stages are possible.
This review provides a current overview of the existing models of MRONJ, their more significant features and findings, and important instances of their application in preclinical research.
Keywords: MRONJ, ONJ, BRONJ, animal models, preclinical studies, bisphosphonates, RANKL-inhibitors
A. Introduction
Medication-related osteonecrosis of the jaw (MRONJ) is a potentially severe, adverse event defined as exposed bone or bone that can be probed through any fistula in the maxillofacial region that persists for more than eight weeks in patients with no history of radiation therapy or metastatic disease in the jaws, who have been treated with powerful antiresorptives (pARs) or angiogenesis inhibitors (AgIs)[1–7]. pARs, including nitrogen-containing bisphosphonates (N-BPs; e.g., zoledronate [ZOL], alendronate [ALN], pamidronate [PAM], etc.,) and anti-RANKL antibodies (e.g., denosumab), are used to manage hypercalcemia and bone metastases in patients with cancer[8–11] or to prevent fragility fractures in patients with osteoporosis[12]. Though pAR-related MRONJ is more common in patients with cancer (1.8–5% incidence) than with osteoporosis (0.01–0.03% incidence)[5, 6, 13, 14], all data suggest similar general MRONJ pathophysiology in cancer and osteoporosis patients[5, 6, 15].
Both clinical and preclinical data suggest that most MRONJ cases require systemic risk factors (e.g., pARs or AgIs) to be combined with local oral risk factors that include tooth extraction[5, 6, 15–20], inflammatory dental disease (e.g., periodontitis, periapical infection), trauma from removable oral prostheses, and potentially from placement of dental implants[5, 6, 15–18, 21–31]
In 2014, the American Association of Oral and Maxillofacial Surgeons (AAOMS) updated its 2007 guidelines[32], with significant changes in the staging and definition for MRONJ, proposing that patients be classified into five stages: “at-risk” and stages 0–3[5]. Patients at risk are asymptomatic pAR-treated patients with no exposed bone. Patients in stage 0 have no clinical evidence of necrotic bone but present with nonspecific symptoms or clinical and/or radiographic findings in the jaw. Though stage 0 in some ways appears to capture the clinical signs and symptoms of early-onset ONJ[5, 33–37], only 50% of stage 0 patients ever progress to stages 1–3[38, 39]. Conversely, all patients with stages 1–3 have necrotic bone exposed to the oral cavity or a fistula that probes to bone. Patients in stage 1 are asymptomatic and have no evidence of infection. Patients in stage 2 ONJ are symptomatic (pain, swelling, and erythema in the region surrounding the exposed necrotic bone) and have evidence of infection. Patients in stage 3 are also symptomatic with infection. They also have at least one of the following: exposed necrotic bone extending beyond the alveolar ridge, pathologic fracture, fistula, oral antral or oral-nasal communication, osteolysis extending to the sinus floor, or inferior border of the mandible.
Though significant advances have been made in understanding and treating MRONJ, its pathophysiology is still not fully elucidated. Laboratory animal experimentation has been crucial to the investigation of many human diseases. In addition, animal research has contributed immensely to advance the MRONJ field, particularly, but not limited to, developing models for future use, investigating risk factors, and testing preventive or curative modalities. In this review, we provide an overview of the existing preclinical models of MRONJ, their significant features and findings, and important instances of their use in preclinical research.
B. Animal Experimentation for MRONJ
In general, animal models are used not only to study the development and progression of diseases but also to test new treatments before they are given to humans. It is rare to find a single preclinical model that mimics all elements of a disease process. The wide range of animal models now available for MRONJ is a testament to the hard work of numerous investigators from multiple laboratories, providing a broad spectrum of opportunities to apply them in a complementary manner. Animal experimentation has also allowed investigators to perform controlled procedures that model real clinical risk factors of the disease. For example, published MRONJ studies reflect treatment with systemic risk factors, like N-BPs[19, 21–24, 26, 40–42], anti-RANKL antibodies[43, 44], and AgIs[45, 46]. MRONJ models have also combined the administration of systemic factors (pARs and AgIs) with specific oral risk factors first noted in humans, including tooth extraction, inflammatory dental disease (e.g., periodontitis, periapical infection, etc.), and also implant placement. Others modeled MRONJ scenarios combining multiple oral risk factors (e.g., periodontitis or periapical infection followed by tooth extraction) or multiple systemic risk factors (e.g., adjuvant glucocorticoid treatment in addition to pARs).
C. Clinically appropriate dosing of N-BPs in animals
Sufficient information exists in the published literature to calculate clinically relevant absorbed doses of N-BPs in animal studies. The ready availability of such data makes allometric scaling unnecessary and inherently risky. Pharmacology studies strongly suggest that the most reliable method for determining clinically relevant absorbed doses of N-BPs in any animal is first to understand the minimum absorbed dose that completely prevents bone loss in newly-ovariectomized (OVX) animals of that species. The minimum absorbed dose is also the absorbed dose of any N-BP used in adult humans to stop bone loss and treat osteoporosis. To find this dose, all major N-BPs have been tested in the OVX rat[47–52]. For example, the minimum absorbed dose of ZOL that completely prevents bone loss in adult OVX rats is 8μg/kg IV 1X/mo[47]. The minimum dose of ALN that completely prevents bone loss in adult OVX rats is 15μg/kg subcutaneously (SC) 2X/wk[48]. The minimum dose of ibandronate that completely prevents bone loss in adult OVX rats is 4μg/kg SC 2X/wk[48, 51, 52]. The minimum dose of minodronate that completely prevents bone loss in adult OVX rats is 6μg/kg SC 2X/wk[49, 50]. These are the rat “osteoporosis” doses.
The minimum dose of risedronate that completely prevents bone loss in adult OVX mice is 20μg/kg SC 2X/wk[53], or 160μg/kg/mo SC. This is the mouse “osteoporosis” dose of risedronate. The minimum dose of ALN that completely prevents bone loss in adult OVX mice is 40μg/kg every 4 days[53] or 75μg/kg SC 2X/wk[54], or 280–600 μg/kg/mo SC of ALN. This is the mouse “osteoporosis” dose of ALN. No studies to establish the minimum dose of ZOL to prevent bone loss in adult OVX mice have been published. In this case, one can use the relative N-BP potencies to predict the minimum dose that prevents bone loss in adult OVX mice for ZOL and other N-BPs (Table 1A). Considering that ZOL is 15 times more potent than ALN in stopping OVX-induced bone loss in the adult rat, we can predict that the minimum dose of ZOL that completely prevents bone loss in adults OVX mice would be ~20–40 μg/kg/mo IV. This is the predicted “osteoporosis dose of ZOL in mice.
Table 1A:
Clinical and pharmacologic features of different N-BPs
| Drug | Disease | Dose | Route | Potency (vs. ALN) | Cumulative Annual Absorbed Dose (mg) |
|---|---|---|---|---|---|
| Alendronate | Osteoporosis | 70mg/wk | Oral | 1* | 26 |
| Risedronate | Osteoporosis | 35mg/wk | Oral | 1.5 | 13 |
| Ibandronate | Osteoporosis | 150mg/mo | Oral | 4 | 8 |
| Ibandronate | Osteoporosis | 2mg/3mo | IV | 4 | 8 |
| Zoledronate | Osteoporosis | 5mg/yr | IV | 15 | 5 |
| Minodronate | Osteoporosis | 25mg/wk | Oral | 2.5 | 9 |
| Ibandronate | Cancer | 8mg/mo | IV | 4 | 96 |
| Zoledronate | Cancer | 4mg/mo | IV | 15 | 48 |
Alendronate potency arbitrarily expressed as “1”. All other BPs are more potent than alendronate[225].
The next step is to find the relevant rat or mouse “oncology dose”. To do this, one considers the oncology:osteoporosis dose ratios of a single pAR in humans. There are two examples, ZOL, and denosumab, because these two pARs are routinely used for oncology and osteoporosis patients. The ZOL dose used in oncology patients is 4mg IV 1X/mo (48mg IV/yr)[55]. The ZOL dose used in osteoporosis patients is 5mg IV 1X/yr[56]. For ZOL, the ratio of the yearly oncology dose to the yearly osteoporosis dose in humans is 9.6:1. The denosumab dose used in cancer patients is 120mg SC 1X/mo (1440mg SC/yr)[55]. The denosumab dose used in osteoporosis patients is 60mg SC 2X/yr (120mg SC/yr)[56]. For denosumab, the ratio of the yearly oncology dose to the yearly osteoporosis dose in humans is 12:1. For ZOL and denosumab, the oncology dose can be approximated as about ten-fold greater than the osteoporosis dose. To predict a reasonable rat “oncology dose” for ZOL, one then multiplies 8μg/kg IV 1X/mo, the rat “osteoporosis dose,” by ten, giving 80μg/kg ZOL IV 1X/mo as the rat “oncology dose” (Table 1B). To predict a reasonable mouse “oncology dose” for ZOL, one multiplies 20–40μg/kg IV 1X/mo, the mouse “osteoporosis dose,” by ten, giving 200–400μg/kg ZOL IV 1X/mo as the mouse “oncology dose” (Table 1B).
Table 1B:
Clinically relevant doses for zoledronate in the rat and mouse
| Species/Disease | Osteoporosis | Cancer |
|---|---|---|
| Rat | 8μg/kg/mo IVa | 80μg/kg/mo IV* |
| Mouse | ~20–40μg/kg/mo IV* | ~200–400μg/kg/mo IV* |
-actual;
-predicted; IV: intravenous administration
For species in which OVX-induced bone loss does not reliably occur, such as the dog[57, 58], the minimum dose of N-BP that completely prevents OVX-induced bone loss cannot be assessed. When the benchmark of OVX-induced bone loss cannot be established in an animal, choosing a clinically relevant dose is much more difficult.
Perspective on the use of supra-clinical doses in animals
Conventional toxicity testing in animals is designed to use proposed clinically efficacious doses of an agent to disclose previously unidentified adverse effects of an agent before they are ever seen in humans. Applying supra-clinical doses of an agent in this setting is generally used for: a) producing enough toxic events to allow their identification and characterization, and b) learning for which toxic events to search in animals receiving proposed clinical doses. However, when, as with MRONJ, a toxic event is first identified in humans, toxicity testing in animals should be streamlined. Not only does the toxic event not need to be newly identified and characterized, but its potential risk factors are also already identified. The only need is to be sure that the toxic event in animals bears a solid resemblance to what has been recorded in humans. Thus, the goal becomes creating toxic events in animals by simulating the circumstances that produce the actual toxic event in humans.
Combining supra-clinical doses with risk factors during the initial design of potential animal models is a typical first step in verifying that an animal can express the toxic event. However, since humans express the toxic event at clinical doses, testing for the toxic event in animals bearing the risk factors and utilizing clinically relevant doses is eventually essential to judging the significance of an animal model[59]. Thus, for example, when a toxic event is produced in an animal only by excessive cumulative absorbed doses, further refinement of the model should be considered because it most likely lacks one or more of the underlying conditions that cause the disease in humans. In addition, one must consider the possibility that animal models that produce the toxic event only at supra-clinical doses may cause it only through pathways/mechanisms that differ from those which cause it in humans.
D. Animal Models of MRONJ:
To update the information about the preclinical investigation of MRONJ, we performed a survey of prior research literature between March 2003 and June 2021. We utilized academic research databases including Pubmed, Europe PMC, Embase, Medline through Pubmed, ScienceDirect, and Web of Science. The following combination of search terms was used: “Osteonecrosis of the jaw” OR “ONJ” OR “BRONJ” OR “MRONJ,” AND “animals,” OR “animal models,” OR “mouse” OR “mice” OR “rat,” OR “rice rat,” OR “Oryzomys,” OR “rabbit,” OR “dog,” OR “pig,” OR “sheep.” We selected only full-text articles published in English. Reviews about animal models of MRONJ or studies presenting exclusively in vitro data were excluded. In addition, animal studies involving pARs or AgIs that investigate only the postcranial skeleton were excluded. References were managed using the software (EndNote™, Thomson Reuters) and Excel. Two independent authors (JIA, EC) reviewed all titles and abstracts. Any disagreement between the evaluators was resolved by discussion and mutual agreement. If JIA and EC did not reach a consensus, the third author (DBK) took a final decision.
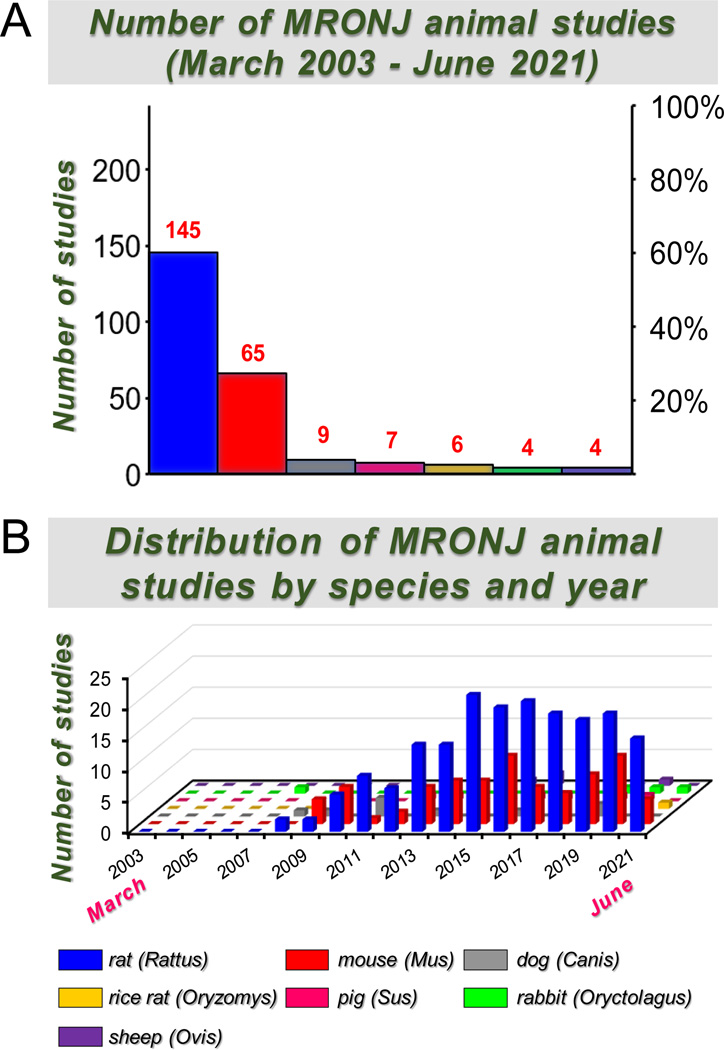
Multiple species have been used in preclinical studies associated with MRONJ, including the rat (Rattus norvegicus), mouse (Mus musculus), rice rat (Oryzomys palustris), rabbit (Oryctolagus cuniculus), dog (Canis lupus familiaris), sheep (Ovis aries), and pig (Sus scrofa domesticus) (Figure 1A). A total of 240 articles fulfilled the specified search criteria. Of these, ~60% (n= 145) utilized rats, ~27% (n= 65) mice, ~4% (n= 9) dogs, ~3% (n= 7) pigs, 2.5% (n= 6) rice rats, ~2% (n= 4) rabbits, and ~2% (n= 4) sheep (Figure 1A). The distribution of the number of MRONJ animal studies by species and year of publication is depicted in Figure 1B.
Figure 1. MRONJ animal studies between March 2003 – June 2021.
A. The number and percentage distribution of MRONJ animal studies by species are displayed. B. Distribution of MRONJ animal studies by year and species.
Small vertebrate species, such as mice and rats, have general conceptual advantages over larger vertebrate species. They do not require complex laboratory infrastructure, and they occupy less housing space per animal. Using small vertebrate species is advantageous because a broad range of specific reagents is available, including antibodies, biologics, cellular and molecular arrays, etc. Their low body weight makes treatments with rare test agents feasible because they require relatively small amounts of the drug. Furthermore, genetically engineered mice are widely available and relatively quickly made. Genetically engineered rats are rarely used, though CRISPR has made the genome editing process much more efficient and has improved prospects for transgenic rats[60]. Small vertebrate species also have disadvantages. While tooth extraction and implant placement are possible, the small size of the oral cavity and teeth themselves limits the possibility of performing intricate oral procedures and obtaining sufficient volume and size of samples, including fluids (e.g., blood, crevicular fluid, etc.), and tissues (e.g., jawbones, periodontal tissues, organs, etc.,).
Large vertebrate species, such as the rabbit, dog, sheep, and pig, have advantages over small vertebrates. These include that oral interventions and procedures commonly performed in humans are often feasible due to the larger size of the mouth and teeth. In addition, it is generally possible to obtain sufficient sample volumes and sizes to test multiple endpoints in the same animal. Another advantage of large vertebrate species is that, as in humans, the cortical bone in craniofacial and postcranial skeletons possess Haversian systems and intracortical bone remodeling.
A critical element to consider is the relative replacements emphasized in animal research concerning the 3Rs[61]. This concept states that replacing any vertebrate with a nonvertebrate species or replacing a vertebrate species with a vertebrate or nonvertebrate species is lower on the phylogenetic scale when comparable data can be obtained, should be done. Thus, for this principle of humane animal experimentation, it is preferable to limit the use of rabbits, dogs, pigs or sheep, to circumstances in which they produce unique data.
D.1. Small vertebrates:
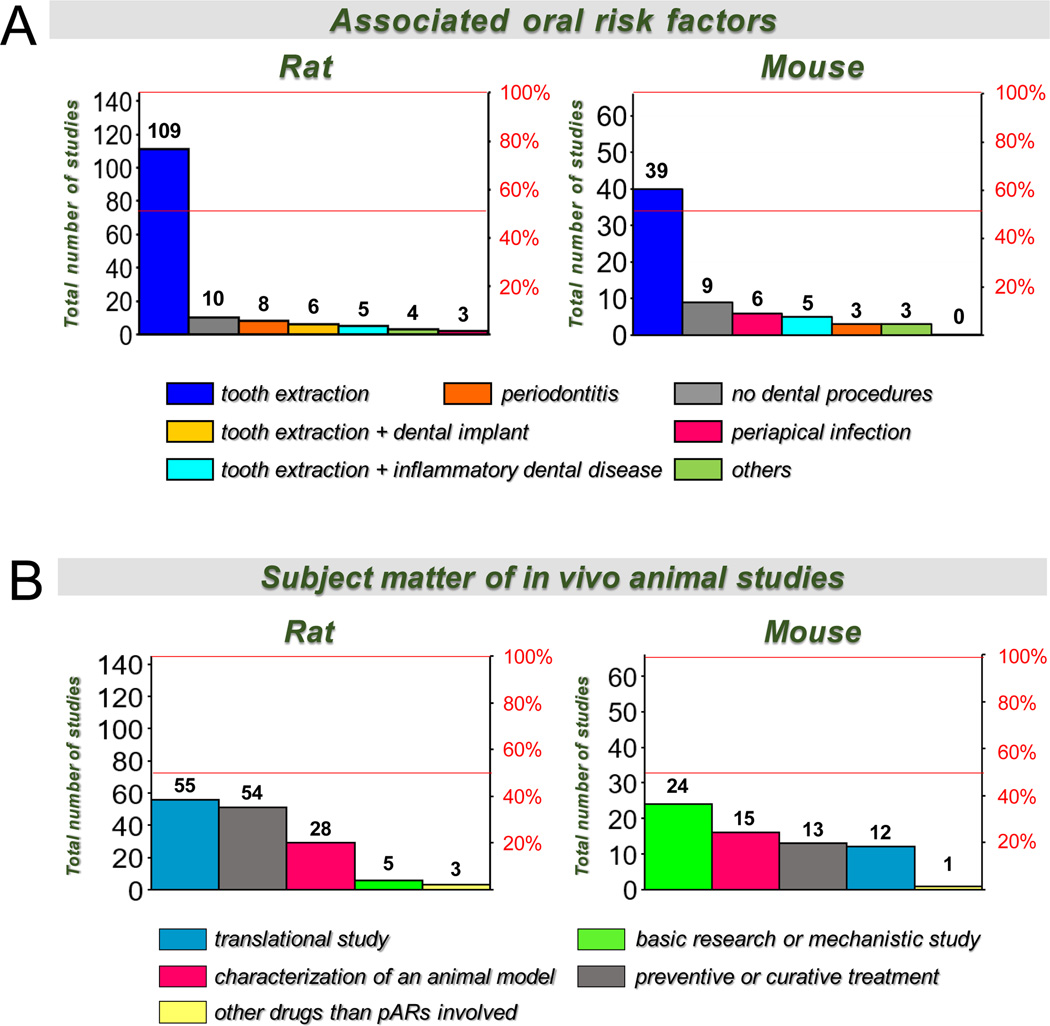
The rat (Rattus norvegicus) is the most commonly used species in preclinical MRONJ research. We identified 145 articles utilizing rats (Figure 1). Investigators purposely developed MRONJ models by associating different pARs (e.g., N-BPs, anti-RANKL antibodies) with various oral risk factors. Since tooth extraction is the most frequent oral risk factor associated with MRONJ in humans[5, 6], it is not surprising that ~75% of the in vivo rat studies (n= 109) involved tooth extraction (Figure 2A, Table 2). Extractions of one or more maxillary or mandibular molars were conducted in anesthetized rats using dental explorers, surgical curettes, and/or small forceps. While performing this procedure, researchers frequently reported that an apical portion of a root could break off and remain within the tooth socket, creating root retention. This is more common in older rats that tended to suffer dental ankylosis. The experimental outcomes (e.g., MRONJ incidence, healing, inflammation, regeneration) could be affected when root retention occurs. Thus, they have recommended that data from rats with incomplete tooth extraction be excluded.
Figure 2. Distribution of MRONJ studies conducted in rats and mice according to the utilized associated oral risk factor and the subject matter of the investigation.
A. Number and percentage distribution according to the utilized associated oral risk factor. B. Number and percentage distribution of the rat and mouse studies according to the subject matter of the investigation.
Table 2:
References of rat and mice studies distributed by the utilized associated oral risk factor
| Associated oral risk factor | Rat studies | Mouse studies |
|---|---|---|
| Tooth extractions of healthy molars | [62], [67], [226], [63],[64], [68], [227], [228], [229], [230], [65], [231], [70], [232], [71], [233], [141], [234], [235], [236], [144], [237], [238],[146], [239], [240], [241], [242], [243], [128], [244], [123], [69], [245], [246], [140], [247], [248], [249], [250], [251], [252], [133], [253], [147], [66], [254], [255], [256], [124], [257], [258], [259], [260], [129], [261], [262], [121], [263], [264], [265], [266], [267], [142], [136], [268], [130], [149], [131], [132], [269], [270], [271], [272], [273], [134], [145], [126], [274], [275], [148], [276], [277], [135], [143], [278], [279], [280], [281], [139], [282], [283], [284], [285], [286], [287], [125], [288], [289], [127], [290], [138], [122], [291], [292], [293], [294], [130], [137], [295] | [172], [19], [171], [180], [296], [297], [173], [298], [299], [300], [301], [302], [181], [303], [304], [182], [45], [174], [158], [305], [185], [306], [175], [183], [307], [308], [184], [176], [309], [310], [311], [312], [186], [178], [313], [153], [179], [314], [184], [315] |
| Periodontal disease | [80], [21], [86], [81], [82], [83], [85], [84] | [156], [155], [154] |
| Periapical infection | [87], [88], [89] | [43], [23], [152], [151], [16], [17], [150], [26] |
| Inflammatory dental disease + tooth extraction | [90], [40], [99], [91], [100] | [157], [16], [17], [158], [159] |
| Tooth extraction + dental implants | [103], [102], [101], [105], [104], [106] | None |
| Other procedures | [107], [108], [109], [110] | [161], [44], [160] |
| No Dental interventions | [120], [119], [118], [117], [116], [115], [114], [113], [112], [111], [113] | [162], [163], [164], [165], [166], [167], [168], [170], [169] |
Most studies that involved tooth extractions were conducted on healthy molars after or during treatment with pARs (n=109; Table 2;). The investigators reported either necrotic bone, though never supported by histologic evidence of exposure to the oral cavity[62–66], or absence of bone necrosis[67, 68]. For example, rats treated with clinically relevant doses of ALN (cumulative dose of 1.2 mg/kg during 14 wks) or intraoral injections with ALN (cumulative doses of 4 mg/kg during 2 wks) that underwent extraction of a healthy molar experienced a transient inhibition of healing during the early phases of socket healing. No histologic evidence of necrotic bone at any stage was observed[67, 68]. In contrast, rats that received supra-clinical doses of ALN, with cumulative doses of 12 mg/kg SC during 12 wks (10X higher than that used by[68]) and 3X higher than the cumulative dose used by[67]) developed non-exposed necrotic bone or bony sequestra, and delayed socket healing[66]. In contrast, when rats underwent a healthy molar extraction and received a pAR (ALN, ZOL, or PAM) with co-adjuvant glucocorticoid therapy, they often developed histopathologic evidence of exposed necrotic bone or bony sequestra[62, 64, 69–71].
Six percent of rat studies (n= 8) utilized experimental periodontitis as the oral risk factor (Figure 2A, Table 2). Laboratory rats and mice require sustained intervention to create mild to moderate periodontitis because, unlike humans[72–77], these species are not naturally prone to periodontal disease[63, 74, 78, 79]. In the context of MRONJ, experimental periodontitis was achieved by placement of a ligature around a molar[21, 40, 80–84], repeated injection of LPS into gingival tissues[85], or injection of bacterial pathogens into gingival tissues[82, 86].
Only two percent of rat studies (n= 3) used periapical infection alone as an oral risk factor for MRONJ[87–89]. Periapical infection was produced by exposing the pulp of one or more molars through the occlusal surface using a round bur and, in some cases, inoculating the exposed pulp with periodontal pathogens[90, 91]. Rats subjected to experimental periodontitis or periapical infection that concurrently received ZOL (66–200 μg/Kg) developed histopathological lesions compatible with osteonecrosis[21, 82, 88]. However, only one study[21] reported histopathological evidence of necrotic exposed bone and reported MRONJ prevalence (~20%). Two other studies that used rats with experimental periodontitis and were treated with ALN (1 – 2.5mg/kg SC for three or four weeks) reported histopathologic osteonecrosis[81, 85]. Still, the necrotic bone was not exposed to the oral cavity.
Interestingly, many clinical studies suggest that periodontal or periapical infection of teeth prompts the need for most tooth extractions and increases the risk for MRONJ[20, 92–98]. Thus, several investigators reported in vivo rat models (~3%; n=5) combining these oral risk factors. Inflammatory dental disease (e.g., periodontitis, periapical infection) was first induced, followed by extraction of the infected tooth[40, 90, 91, 99, 100]. In most studies, ZOL was used as the systemic risk factor. Indeed, rats treated with ZOL (200–300 μg/Kg IV 2–3x/wk) that underwent extraction of an infected molar developed more extensive alveolar bone osteonecrosis, impaired healing of the extraction socket, and persistent inflammation, compared to the extraction of uninfected teeth. Three of these studies reported exposed necrotic bone[90, 91, 100]. In addition, Hadaya et al.[91] found exposed necrotic bone in the rats that received 10mg/kg of OPG-FC SC for 9 wks.
Four percent of the studies (n=6) used implant placement as a potential oral risk factor for MRONJ[101–106] (Figure 2A, Table 2), using two phases. In the first phase, anesthetized rats were subjected to tooth extraction, usually of the first maxillary molars. In the second phase, an implant was placed in the fresh tooth extraction site or after a healing period of ~4 wks. Titanium mini-implants, titanium self-drilling screws, and/or zirconia implants were used, with 1.2–2.2 mm in diameter and 3 mm in length. One study[104] reported exposed necrotic bone lesions compatible with MRONJ associated with peri-implant bone loss areas.
A few studies (~3%; n=4) utilized models involving trauma to the jaws or gingival lesions (depicted as Others in Figure 2A; Table 2)[107–110]. One study used a mandibular angle fracture induced by unilateral mandibular osteotomy[109]. The bone segments were repositioned, and fixation was created with a stainless steel wire at the inferior border of the mandible. In other studies, a mandibular defect was made by grinding the jaw bone with a slow speed drill, which was left exposed with denuded gingiva[108, 110]. Finally, others used a curette to induce approximately 3 X 1.5 mm gingival wound in the palatal mucosa between the first molar and the great palatine canal to denude the alveolar process[107]. Two of these studies reported MRONJ but neither reported prevalence nor confirmatory histopathologic evidence of exposed necrotic bone[109, 110]. Another of these studies did not find MRONJ lesions but reported inhibitory effects of ZOL on bone healing without detrimental impact on soft tissue healing[107]. Seven percent of the studies (n= 10) were performed with no oral risk factors to directly study the effects of pARs on healthy craniofacial bones[111–120] (Figure 2A; Table 2).
Furthermore, whereas most MRONJ rat studies utilized an N-BP (ALN, ZOL, PAM) as the systemic risk factor, only two MRONJ studies in rats used RANKL inhibitors[91, 121]. Regarding the subject matter of rat studies, we found that most of the studies were mainly focused on translational research (~38%; n= 55), preventive and curative treatments (37%; n= 54), and characterizing an animal model for the disease (19%; n=28). Only a few involved basic research or mechanistic studies (~3%; n= 5) or the investigation of drugs associated with MRONJ other than pARs (2%; n= 3) (Figure 2B).
Of the 54 studies investigating preventive and curative treatments, 41 involved preventive therapies (Supplemental Table 1). Some of the investigated preventive treatments included PTH[105, 122–127], laser therapy[128–130]; photodynamic therapy[131, 132], hyperbaric oxygen therapy[133, 134], resveratrol[84, 135], local chelation of the alveolar bone matrix using cadmium or EDTA[114, 136], and local transplantation of mesenchymal stem cells (MSCs) or the application of derived products from MSCs[137–139]. Interestingly, other preventive modalities investigated the effects of discontinuation of pARS (OPG-Fc or ZOL) before or after tooth extraction[91, 140]. The curative treatments for MRONJ (n= 13) included several similar approaches to those for preventive treatments (Supplemental Table 1). These included PTH[109, 141–143], injection of platelet-rich plasma into the extraction socket[144, 145], and local transplantation of MSCs, endothelial progenitor cells, or molecular products of MSCs[146–149].
In addition, three percent of the studies (n= 5) focused on investigating mechanisms that could be involved in the pathophysiology of MRONJ. A list of these studies is presented in Supplemental Table 2, highlighting some specific details of the investigations. Finally, Supplemental Table 3 summarizes the rat studies that provided the doses of pARS used and the reported prevalence of MRONJ lesions in the experimental animals.
The mouse (Mus musculus) is the second most commonly-used species used for in vivo MRONJ studies, with 65 articles (Figure 1). As in the rat, investigators purposely developed MRONJ models by associating different systemic risk factors (pARs [e.g., N-BPs, anti-RANKL antibodies] with various oral risk factors. In 60% of the studies (n= 39), investigators utilized tooth extraction alone as the oral risk factor (Figure 2A; Table 2). Compared to rat studies, a greater percentage of mouse studies used periapical infection (~9%; n=6)[23, 26, 43, 150–153], while comparable to rat studies, only ~5% of mouse studies (n=3) used periodontitis models[154–156] (Figure 2A, Table 2).
Investigators also established murine models and performed in vivo studies by combining two oral risk factors, where inflammatory dental disease, either periodontitis or periapical infection, was first induced and then affected molars were extracted. We found five studies (~8%) that used this approach[16, 17, 157–159] (Figure 2A, Table 2). Furthermore, a few mouse studies (~5%; n= 3) used a jaw bone fracture, gingival wound, or a palatal injury as oral risk factors for MRONJ (depicted as Others in Figure 2A)[44, 160, 161]. On the other hand, as in rats, several studies were performed with no oral risk factors (n= 9; ~14%)[162–170].
MRONJ mouse studies have also shown significant outcome variations depending on the systemic and/or oral risk factors utilized in the experiments. Most MRONJ murine studies used N-BPs, particularly ZOL (alone or combined with dexamethasone [DEX] or cyclophosphamide)[16, 17, 19, 26, 150, 152, 154, 171–176] or PAM[157] as systemic risk factors. Fewer studies used RANKL inhibitors[16, 23, 43, 152, 158, 173] or angiogenesis inhibitors[45].
When ZOL was combined with the extraction of a healthy molar, mice tended to developed histopathologic oral lesions characterized by non-exposed necrotic bone[19, 171–173]. Similar findings were observed in mice treated with anti-mouse RANKL antibodies and underwent extraction of a healthy molar[173]. In contrast, when ZOL was administered in combination with dexamethasone, oral histopathologic lesions tended to be more frequently accompanied with exposed necrotic bone or sequestrum formation at the extraction sites[19, 171]. Furthermore, the combination of ZOL, dexamethasone and docetaxel (a potent chemotherapeutic agent) worsened MRONJ like-lesions and increased the prevalence of exposed bone compared to mice treated with ZOL and dexamethasone[171]. Another study showed that when mice were subjected to healthy molar extraction and treated with combination therapy of ZOL and cyclophosphamide (cytotoxic chemotherapy), the oral lesions tended to be more severe and manifest histopathologic necrotic exposed bone and/or bony sequestrum[174].
Notably, when pARs, such as ZOL[26, 150, 152], RANK-Fc, or OPG-FC[23, 43, 152] were administered to mice with natural periodontitis or experimentally induced inflammatory dental diseases (periapical or periradicular disease), exposed necrotic bone was a distinctive histopathologic feature of the oral lesions, except in one study where mice developed non-exposed necrotic bone[150] (Table 2).
The oral risk factors in other murine MRONJ models were developed combining tooth extraction and inflammatory dental disease (Figure 2A; Table 2). Indeed, a few investigators performed in vivo murine studies that combined extraction of an infected molar and ZOL or OPG-FC[16, 17] (Table 2). In both studies, extraction of the infected molar triggered extensive osteonecrosis, with impaired healing of the extraction socket and persistent inflammation. In addition, the necrotic alveolar bone was exposed in one study[16].
No murine studies in the MRONJ arena were done utilizing dental implant placement as the oral risk factor. This might be, perhaps, due to the small size of the oral cavity of mice and the technical difficulties in performing this approach compared to rats or larger vertebrates.
Regarding the subject matter of the studies, we found that compared to rats, in vivo murine studies were more focused on basic research and mechanistic studies(~37%; n=24), followed by studies for characterizing MRONJ models (~23%; n=15), preventive and curative treatments (20%; n= 13), translational research (~18%; n=12), and investigating drugs associated with MRONJ other than pARs (1.5%; n=1) (Figure 2B).
From the thirteen studies investigating preventive and curative treatments for MRONJ, six involved preventive therapy, whereas seven involved curative experimentation (Figure 2B; Supplemental Table 4). Some preventive therapy approaches included the local application of adipose-derived MSCs, to prevent or reduce the incidence of MRONJ[175, 177] and the local transplantation of BMP-2 adsorbed onto beta-tricalcium phosphate (β-TCP). The BMP-2/β-TCP compound accelerated bone formation and reduced bone necrosis in the tooth extraction socket preventing MRONJ[178]. Other tested preventive treatments included administration of recombinant human PTH before tooth extraction[179], the alternative use of 99Tm-conjugated methylene diphosphonate, instead of ALN, to reduce the risk for MRONJ[180], and the co-injection of etidronate, a non-N-BP that competes with and inhibits the entry of N-BPs (ZOL) into cells associated with inflammation and necrosis and eliminates part of the N-BPs accumulated in bone[164]. The curative treatments for MRONJ tested in murine models were similar to those utilized for preventive therapies. For example, they included the systemic transplantation of MSCs[19, 181], a stromal vascular fraction of adipose tissue[182], and peripheral blood mononuclear cells (PBMCs)[183] to induce immunomodulatory effects and acceleration of tissue repair in mice with MRONJ. Other curative treatments tested in murine models included PTH[184], intraoral injections of a low potency BP that reduced the necrotic bone area of MRONJ lesions[185], and the local injection of the tetrahedral framework of nucleic acids into the mucosa adjacent to MRONJ lesions to promote healing of the oral lesions[186].
A more significant percentage of mouse studies (~37%; n= 24), compared to rat studies (~4%; n= 24), have focused on investigating mechanisms that could be associated with MRONJ pathophysiology. A list of most of these studies is presented in Supplemental Table 5, highlighting some specific details of the investigations. Finally, Supplemental Table 6 summarizes the rat studies that provided the doses of pARS used and the reported prevalence of MRONJ lesions in the experimental animals.
The rice rat (Oryzomys palustris) is a rodent species from the Family Cricetidae, subfamily Sigmodontinae, and Tribe Oryzomyini, which is not commercially available. This species was first used for studying periodontitis some years ago[15, 42, 72, 187, 188]. Two models of MRONJ were developed in the rice rat linked to two different types of periodontitis: 1) a generalized form, which affects both jaws and is achieved by feeding a high sucrose-casein (HSC) diet[15, 42, 72, 187, 188]; and 2) a localized form, which affects the maxilla, and is achieved by feeding standard (STD) rodent chow[15, 25, 42]. The generalized form, similar in appearance and location to moderate/severe generalized periodontitis in humans[15, 42, 72, 187–189], was first presented many years ago[72, 73, 190]. The rice rat generalized periodontal disease model is more familiar to the field than the localized periodontitis model. The rice rat localized periodontal disease model is characterized by food/fiber impaction at the lingual aspect of the interdental space between the second and third maxillary molars. Neither form requires mechanical or microbiologic interventions to initiate or maintain the disease[72, 73, 190]. Rice rats with either generalized or localized periodontitis as an oral risk factor that simultaneously received oncologic doses of ZOL as a systemic risk factor developed MRONJ after 12–24 wks ZOL treatment[22, 24, 25]. The prevalence of MRONJ in rice rats depends on the dose and duration of exposure to ZOL, reaching 100% at ZOL oncologic doses by 18–24 weeks of ZOL treatment[22, 24, 25]. Notably, several decades before N-BPs were linked to MRONJ, the presence of “nonvital” exposed alveolar bone in rice rats treated for 12–18 wks with clodronate, a non-nitrogen-containing predecessor of the N-BPs, was described[191]. Dose-response studies in rice rats that used ZOL as a systemic risk factor contributed essential data that helped establish a causal relationship between N-BPs and MRONJ in the presence of periodontitis as a local risk factor[24, 25]. MRONJ lesions in rice rats resemble MRONJ lesions in humans. MRONJ lesions in rice rats are histopathologically characterized by areas of exposed necrotic alveolar bone, osteolysis, periodontal tissue destruction, an increased number of dead osteocytes, and fields of adjacent empty osteocyte lacunae[22, 24, 25, 41]. Rice rats with localized periodontitis treated with oncologic doses of ZOL that simultaneously receive periodic periodontal cleaning of their localized periodontal lesions showed significantly lower MRONJ prevalence than ZOL-treated rice rats with localized periodontitis that received no periodontal cleaning[41]. This finding parallels periodontal maintenance therapy outcomes in cancer patients receiving oncologic doses of ZOL[192–194]. Feeding ZOL-treated rats a nutritionally similar rodent chow that replaces the insoluble fiber of the STD diet with soluble fiber (SF) diet prevented the development of both localized periodontitis and MRONJ[41]. The SF diet makes the rice rat resistant to developing localized periodontitis, providing a convenient way to remove the main local oral risk factor for MRONJ in the rice rat.
Rabbits (Oryctolagus cuniculus):
One group[195] investigating the effects of N-BPs in the context of MRONJ found that local treatment with 2–3mg of PAM inhibited bone healing using a calvaria bony defect model[196]. Others investigated the effects of local stem cell transplantation in New Zealand white rabbits treated with ZOL (800 μg/kg) and dexamethasone (10 mg/kg) once a week for 8 wks and subjected to tooth extraction[197]. The study showed that ZOL+DEX treated rabbits that received adipose-derived stem cells had less MRONJ, a more rapid gingival healing, and bone regeneration after tooth extraction than ZOL+DEX control rabbits. Another study investigated the influence of ridge preservation on the healing of extraction sockets under ZOL treatment (50 μg/kg)[198]. The study concluded that ZOL compromised socket healing and induced MRONJ. This study showed that grafting sockets with collagen-coated natural bone mineral did not affect socket healing in ZOL-treated rabbits.
D.2. Large vertebrates:
Dogs (Canis domesticus):
The dog was among the first species used in preclinical MRONJ studies. Adult female beagle dogs with no oral risk factors were given daily oral ALN for three years (0.2 or 1.0 mg/kg/d)[199]. No animals developed exposed bone in the oral cavity. However, alveolar bone matrix necrosis was seen in the mandible in 0% of VEH dogs, 25% of ALN 0.2 dogs, and 33% of ALN 1.0 dogs (P<.04). The investigators concluded that ALN reduces alveolar bone turnover and increases the incidence of bone necrosis in dogs. These researchers also treated dogs for one or three years with oral ALN (0.2 or 1 mg/kg/d) or IV ZOL (0.06 mg/kg 2x/month)[200]. Again, none of the treatments was associated with exposed bone, but all dogs showed low bone turnover. 17–25% of one-year dogs and 25–33% of three-year ALN treated dogs showed areas of bone necrosis in the mandible and ribs, both sites of high natural bone turnover. The authors concluded that increased prevalence of mandibular bone necrosis was associated with decreased bone turnover rate. However, it was unclear whether the necrotic bone resulted from direct toxic effects of ALN on osteocytes or simply was an indirect effect caused by reduced turnover that slows the removal of all bone, including that which might contain osteocytes undergoing natural death. These investigators also studied the effects of ZOL (0.06 mg/kg IV 2x/month) on healing after extraction of healthy teeth in mature female beagle dogs for three months[201]. One of six ZOL-treated dogs developed exposed bone post-extraction, which eventually led to the formation of a sequestrum consistent with those reported in humans with MRONJ. These investigators also treated mature beagle dogs with ZOL (0.06 mg/kg IV 2x/month), DEX, or ZOL+DEX and extracted a healthy molar 7–8 months after the start of treatment[202]. Though they found no exposed bone, a few animals had severely disrupted healing in extraction sites with an intense periosteal reaction. Another study found that ZOL (0.06 mg/kg IV 2x/month) induced higher levels of apoptosis and lower levels of MMP-9 in oral epithelial cells of one-year-old dogs treated for three months, supporting the notion that N-BP treatment affects the oral mucosa[203]. Another group studied the effects of ZOL (0.1 mg/kg IV per month for four months) on bone remodeling and healing after extraction of a healthy left third premolar and placement of two orthodontic mini-implants per jaw[204]. They found no MRONJ and noted that all extraction sites in ZOL-treated dogs healed uneventfully by four months post-extraction. Others investigated a potential treatment for MRONJ using a local mesenchymal stromal cells (MSCs) transplantation approach to treat a mandibular bone defect in beagle dogs that received ZOL (0.06 mg/kg IM 2x/month) + DEX (5mg/kg IM 4x/month)[205]. Interestingly, the MSC sheet transplantation promoted healing of the wounds four weeks after surgery compared to non-MSC ZOL+DEX dogs. Others[206] assessed the effects of one-year treatment with ALN (3.5 mg/kg/wk orally) or PAM (1 mg/kg/wk IV) on implant placement and found that N-BPs, particularly PAM, hampered peri-implant bone remodeling and negatively affected osteointegration.
Dog MRONJ experiments have been helpful. They were among the earlier experiments showing that extracting healthy teeth in NBP-treated animals does not lead to consistent development of MRONJ. However, the dog model itself has never been used to investigate the crucial role of inflammatory dental disease in MRONJ. Dog experiments raised the possibility that N-BPs may cause MRONJ indirectly by slowing the removal of dying/dead bone of any origin, allowing it to accumulate in detectable amounts as MRONJ lesions. However, without consistent OVX-induced bone loss in the female beagle, it will remain difficult to determine a clinically relevant dose of BPs in the dog.
Pigs (Sus scrofa):
Minipigs possess some physiologic features that make them good animal models to study bone biology and skeletal disorders, such as the existence of non-seasonal estrus, estrogen deficiency-related bone loss, and comparable bone turnover parameters to those in humans[207, 208]. Furthermore, the anatomy of the jaws and teeth, oral microbiome, and structural properties of pig bones resemble those in humans[209–211]. A review of animal models for MRONJ[212] concluded that the minipig is a suitable animal model for MRONJ, based solely on the consistent reproducibility of the disease and the anatomical and biologic similarities of the oral bones and teeth to humans. Seven preclinical studies of MRONJ in pigs were found. The first MRONJ model in this species was published in 2012[213]. Göttingen minipigs received 50 μg/kg IV ZOL once weekly for six weeks and underwent extractions of healthy second and third premolars and first molar from both jaws and continued ZOL for another ten weeks. The investigators found that all ZOL-treated minipigs developed clinical and histopathological features of MRONJ and impaired wound healing, while such findings were never present in control animals.
Another group developed an MRONJ model by administering ZOL (~100μg/kg IV every two weeks) for 32 wks and extracting a healthy first mandibular molar after 24 weeks of ZOL[214]. Next, the investigators tested a therapeutic approach using bone marrow mesenchymal stem cells (BMMSC) transplantation. The study showed that allogeneic BMMSC transplantation enhanced mucosal and bone healing, increased Tregs, and reduced IL-17 levels in peripheral blood of minipigs with MRONJ. In another study, domestic pigs were given ZOL (~40μg/kg IV once weekly) (N=3/group)[215]. After 60 days, healthy maxillary second and mandibular third molars were extracted. No pigs developed clinical or histopathological MRONJ, though ZOL-treated pigs developed radiographic findings compatible with the disease. Another model used 20-wk-old Göttingen minipigs treated with ZOL (50 μg/kg IV weekly) for 20 weeks after extracting four healthy mandibular premolars[216]. Another group investigated preventive measures for MRONJ and the role of pAR discontinuation in the prevalence of MRONJ[217], using the above model under somewhat different experimental conditions[216]. About 80% of ZOL-treated minipigs developed MRONJ after extraction of a healthy tooth. In contrast, only 40% developed MRONJ when they received a drug holiday of 6 weeks before tooth extraction with preventive wound management plus antibiotic therapy for eight weeks. As all these measures aim to avoid local infection and alleviate the effects of remodeling suppression, the authors suggested that pAR treatment and bone infection are critical in the pathogenesis of MRONJ. Two other studies investigated different aspects of MRONJ in this species[213, 218].
Sheep (Ovis aries):
Sheep possess a healing capacity comparable to that of humans[219], making them potentially interesting for studying bone remodeling[220] and osteoporosis[221]. An MRONJ model was developed in Swiss mountain sheep by administering ZOL (75 μg/Kg IV q3wks) for 16 wks, followed by the extraction of healthy first and second mandibular molars, followed by an additional 16 weeks of ZOL[222]. Using similar protocols, the same group induced MRONJ in OVX low-calcium diet sheep by administering ZOL weekly for 16 wks[223]. Others developed a different model of MRONJ in OVX ewes[224]. Sham or OVX ewes were treated with ZOL (~100 μg/Kg IV q4wks) for one year, followed by a healthy first mandibular molar extraction. Two years later, ewes received a dental implant at the extraction site and were sacrificed 2.4 years later. One-third of the ZOL-treated sheep (2/6) developed MRONJ at the mandibular extraction site. The implants remained in place in the control SHAM and OVX ewes but were lost in all ZOL-treated SHAM and OVX ewes.
E. Conclusion
Complete parallelism of the individual in vivo animal models with human symptoms rarely exists. We view the current state of animal models for MRONJ as good. Relatively economical small animal models in laboratory rats and mice that are convenient and produce outcomes that match the tissue level behavior of human MRONJ have encouraged large numbers of investigators to do relevant experiments with substantial numbers of animals. Systematic manipulation of local oral risk factors that involve inflammatory dental disease, particularly in rodents, has made the models more relevant. Such models may eventually allow a close match of mechanisms by subsequent molecular and/or enzyme level characterization. Research in rodents, mainly the rat, and to a lesser extent the mouse, has contributed relevant preclinical models that combine systemic administration of pARs with one or more oral risk factors (e.g., tooth extraction and inflammatory dental disease) long known in humans. They produce clinical and tissue-level pathology that models MRONJ in humans in a reasonable timeframe. Models that use clinically relevant doses of ZOL as used in oncology and osteoporosis patients now exist in the presence of oral risk factors. The most pertinent models avoid using additional agents (e.g., glucocorticoids) because MRONJ routinely occurs in humans without these conditions. Experiments have been done by various approaches that demonstrate that eliminating the oral risk factors reduces the risk of MRONJ, just as eliminating oral risk factors from humans reduces the risk of MRONJ[20, 92–98]. The use of pre-clinical MRONJ models involving uninfected teeth extraction, particularly considering that most tooth extractions in humans involved infected teeth, is no longer state-of-the-art. Pre-clinical small laboratory animal models that involve inflammatory dental disease with or without tooth extraction appear to be sufficiently developed to be used for testing preventions and treatments.
Numerous studies showed that mice and rats treated systemically with a pAR with concurrent oral risk factors developed osteonecrosis. However, the osteonecrotic bone was frequently not exposed to the oral cavity. Thus, these studies may be reproducing stage 0 lesions, representing a potential opportunity to model this stage of the disease. However, further studies are required to demonstrate that exposed necrotic bone eventually occurs to confirm the existence of MRONJ.
We also observed a significant variation in the criteria used to define MRONJ, particularly among small vertebrate studies. For example, in some studies, investigators considered only gross endpoints, while in other studies, investigators utilized histologic approaches, either qualitative or quantitative endpoints, or immunohistochemical/molecular approaches.
It would be highly desirable to establishing and standardizing the criteria for defining MRONJ across the different models. In this case, similar features as human MRONJ lesions should be considered, including the presence of exposed necrotic bone and the histopathologic confirmation of oral bone areas with empty osteocyte lacunae, particularly for small vertebrates.
Models in larger species, particularly the pig, produce accurate clinical and histopathologic outcomes. Still, they have the dual disadvantages that few investigators have the facilities to use such models and the group sizes that can realistically be employed are often relatively small. In addition, the development of MRONJ does not appear to require the presence of Haversian remodeling, a skeletal feature that is often cited as an advantage of large animal models for bone. The role of large animal models may thus be to confirm specific crucial findings from rodent research conducted in multiple laboratories.
Supplementary Material
Funding
This research was supported by the National Institute of Dental and Craniofacial Research (NIDCR), R01DE023783–01A.
Abbreviations listed by order of appearance in the text
- AAOMS
American Association of Oral and Maxillofacial Surgeons
- AgIs
angiogenesis inhibitors
- ALN
alendronate
- BRONJ
Bisphosphonate-related ONJ
- BP
bisphosphonate
- DEX
Dexamethasone
- IV
Intravenously
- MRONJ
medication-related osteonecrosis of the jaw
- N-BP
nitrogen-containing BP
- ONJ
Osteonecrosis of the jaw
- PAM
pamidronate
- pAR
powerful antiresorptive
- RANK
receptor activator of nuclear factor kappa-Β
- RANKL
RANK ligand
- ZOL
zoledronate
Footnotes
Conflict of Interest
The authors have no conflicts of interest.
REFERENCES
- [1].Nicolatou-Galitis O, Kouri M, Papadopoulou E, Vardas E, Galiti D, Epstein JB, Elad S, Campisi G, Tsoukalas N, Bektas-Kayhan K, Tan W, Body JJ, Migliorati C, Lalla RV, Group MBS, Osteonecrosis of the jaw related to non-antiresorptive medications: a systematic review, Support Care Cancer 27(2) (2019) 383–394. [DOI] [PubMed] [Google Scholar]
- [2].Pimolbutr K, Porter S, Fedele S, Osteonecrosis of the Jaw Associated with Antiangiogenics in Antiresorptive-Naive Patient: A Comprehensive Review of the Literature, Biomed Res Int 2018 (2018) 8071579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Estilo CL, Fornier M, Farooki A, Carlson D, Bohle G 3rd, Huryn JM, Osteonecrosis of the jaw related to bevacizumab, J Clin Oncol 26(24) (2008) 4037–8. [DOI] [PubMed] [Google Scholar]
- [4].Guarneri V, Miles D, Robert N, Dieras V, Glaspy J, Smith I, Thomssen C, Biganzoli L, Taran T, Conte P, Bevacizumab and osteonecrosis of the jaw: incidence and association with bisphosphonate therapy in three large prospective trials in advanced breast cancer, Breast Cancer Res. Treat 122(1) (2010) 181–188. [DOI] [PubMed] [Google Scholar]
- [5].Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, O’Ryan F, American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw−−2014 update, J. Oral Maxillofac. Surg 72(10) (2014) 1938–1956. [DOI] [PubMed] [Google Scholar]
- [6].Khan AA, Morrison A, Hanley DA, Felsenberg D, McCauley LK, O’Ryan F, Reid IR, Ruggiero SL, Taguchi A, Tetradis S, Watts NB, Brandi ML, Peters E, Guise T, Eastell R, Cheung AM, Morin SN, Masri B, Cooper C, Morgan SL, Obermayer-Pietsch B, Langdahl BL, Al Dabagh R, Davison KS, Kendler DL, Sandor GK, Josse RG, Bhandari M, El Rabbany M, Pierroz DD, Sulimani R, Saunders DP, Brown JP, Compston J, International J. Task Force on Osteonecrosis of the, Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus, J Bone Miner Res 30(1) (2015) 3–23. [DOI] [PubMed] [Google Scholar]
- [7].Koch FP, Walter C, Hansen T, Jager E, Wagner W, Osteonecrosis of the jaw related to sunitinib, Oral Maxillofac Surg 15(1) (2011) 63–6. [DOI] [PubMed] [Google Scholar]
- [8].Van Poznak C, Somerfield MR, Barlow WE, Biermann JS, Bosserman LD, Clemons MJ, Dhesy-Thind SK, Dillmon MS, Eisen A, Frank ES, Jagsi R, Jimenez R, Theriault RL, Vandenberg TA, Yee GC, Moy B, Role of Bone-Modifying Agents in Metastatic Breast Cancer: An American Society of Clinical Oncology-Cancer Care Ontario Focused Guideline Update, J Clin Oncol 35(35) (2017) 3978–3986. [DOI] [PubMed] [Google Scholar]
- [9].Aapro M, Abrahamsson PA, Body JJ, Coleman RE, Colomer R, Costa L, Crino L, Dirix L, Gnant M, Gralow J, Hadji P, Hortobagyi GN, Jonat W, Lipton A, Monnier A, Paterson AH, Rizzoli R, Saad F, Thurlimann B, Guidance on the use of bisphosphonates in solid tumours: recommendations of an international expert panel, Ann Oncol 19(3) (2008) 420–32. [DOI] [PubMed] [Google Scholar]
- [10].Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, de Boer RH, Lichinitser M, Fujiwara Y, Yardley DA, Viniegra M, Fan M, Jiang Q, Dansey R, Jun S, Braun A, Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study, J. Clin. Oncol 28(35) (2010) 5132–5139. [DOI] [PubMed] [Google Scholar]
- [11].Van den Wyngaert T, Wouters K, Huizing MT, Vermorken JB, RANK ligand inhibition in bone metastatic cancer and risk of osteonecrosis of the jaw (ONJ): non bis in idem?, Support. Care Cancer 19(12) (2011) 2035–2040. [DOI] [PubMed] [Google Scholar]
- [12].Yu EW, Tsourdi E, Clarke BL, Bauer DC, Drake MT, Osteoporosis Management in the Era of COVID-19, J Bone Miner Res 35(6) (2020) 1009–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rugani P, Walter C, Kirnbauer B, Acham S, Begus-Nahrman Y, Jakse N, Prevalence of Medication-Related Osteonecrosis of the Jaw in Patients with Breast Cancer, Prostate Cancer, and Multiple Myeloma, Dent J (Basel) 4(4) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Coleman RE, Collinson M, Gregory W, Marshall H, Bell R, Dodwell D, Keane M, Gil M, Barrett-Lee P, Ritchie D, Bowman A, Liversedge V, De Boer RH, Passos-Coelho JL, O’Reilly S, Bertelli G, Joffe J, Brown JE, Wilson C, Tercero JC, Jean-Mairet J, Gomis R, Cameron D, Benefits and risks of adjuvant treatment with zoledronic acid in stage II/III breast cancer. 10 years follow-up of the AZURE randomized clinical trial (BIG 01/04), J Bone Oncol 13 (2018) 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wan JT, Sheeley DM, Somerman MJ, Lee JS, Mitigating osteonecrosis of the jaw (ONJ) through preventive dental care and understanding of risk factors, Bone Res 8 (2020) 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Soundia A, Hadaya D, Esfandi N, de Molon RS, Bezouglaia O, Dry SM, Pirih FQ, Aghaloo T, Tetradis S, Osteonecrosis of the jaws (ONJ) in mice after extraction of teeth with periradicular disease, Bone 90 (2016) 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Song M, Alshaikh A, Kim T, Kim S, Dang M, Mehrazarin S, Shin KH, Kang M, Park NH, Kim RH, Preexisting Periapical Inflammatory Condition Exacerbates Tooth Extraction-induced Bisphosphonate-related Osteonecrosis of the Jaw Lesions in Mice, J. Endod 42(11) (2016) 1641–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Katsarelis H, Shah NP, Dhariwal DK, Pazianas M, Infection and medication-related osteonecrosis of the jaw, J Dent Res 94(4) (2015) 534–9. [DOI] [PubMed] [Google Scholar]
- [19].Kikuiri T, Kim I, Yamaza T, Akiyama K, Zhang Q, Li Y, Chen C, Chen W, Wang S, Le AD, Shi S, Cell-based immunotherapy with mesenchymal stem cells cures bisphosphonate-related osteonecrosis of the jaw-like disease in mice, J Bone Miner. Res 25(7) (2010) 1668–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hasegawa T, Hayashida S, Kondo E, Takeda Y, Miyamoto H, Kawaoka Y, Ueda N, Iwata E, Nakahara H, Kobayashi M, Soutome S, Yamada SI, Tojyo I, Kojima Y, Umeda M, Fujita S, Kurita H, Shibuya Y, Kirita T, Komori T, Japanese M. Study Group of Co-operative Dentistry with, Medication-related osteonecrosis of the jaw after tooth extraction in cancer patients: a multicenter retrospective study, Osteoporos Int 30(1) (2019) 231–239. [DOI] [PubMed] [Google Scholar]
- [21].Aghaloo TL, Kang B, Sung EC, Shoff M, Ronconi M, Gotcher JE, Bezouglaia O, Dry SM, Tetradis S, Periodontal disease and bisphosphonates induce osteonecrosis of the jaws in the rat, J. Bone Miner. Res 26(8) (2011) 1871–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Aguirre JI, Akhter MP, Kimmel DB, Pingel JE, Williams A, Jorgensen M, Kesavalu L, Wronski TJ, Oncologic doses of zoledronic acid induce osteonecrosis of the jaw-like lesions in rice rats (Oryzomys palustris) with periodontitis, J. Bone Miner. Res 27(10) (2012) 2130–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].de Molon RS, Cheong S, Bezouglaia O, Dry SM, Pirih F, Cirelli JA, Aghaloo TL, Tetradis S, Spontaneous osteonecrosis of the jaws in the maxilla of mice on antiresorptive treatment: a novel ONJ mouse model, Bone 68 (2014) 11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Messer JG, Jiron JM, Mendieta Calle JL, Castillo EJ, Israel R, Phillips EG, Yarrow JF, Van Poznak C, Kesavalu L, Kimmel DB, Aguirre JI, Zoledronate Treatment Duration Is Linked to Bisphosphonate-Related ONJ Prevalence in Rice Rats with Generalized Periodontitis, Oral Dis 25(4) (2019) 1116–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Messer JG, Mendieta Calle JL, Jiron JM, Castillo EJ, Van Poznak C, Bhattacharyya N, Kimmel DB, Aguirre JI, Zoledronic acid increases the prevalence of medication-related osteonecrosis of the jaw in a dose dependent manner in rice rats (Oryzomys palustris) with localized periodontitis, Bone 108 (2018) 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kang B, Cheong S, Chaichanasakul T, Bezouglaia O, Atti E, Dry SM, Pirih FQ, Aghaloo TL, Tetradis S, Periapical disease and bisphosphonates induce osteonecrosis of the jaws in mice, J. Bone Miner. Res 28(7) (2013) 1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Vahtsevanos K, Kyrgidis A, Verrou E, Katodritou E, Triaridis S, Andreadis CG, Boukovinas I, Koloutsos GE, Teleioudis Z, Kitikidou K, Paraskevopoulos P, Zervas K, Antoniades K, Longitudinal cohort study of risk factors in cancer patients of bisphosphonate-related osteonecrosis of the jaw, J. Clin. Oncol 27(32) (2009) 5356–5362. [DOI] [PubMed] [Google Scholar]
- [28].Levin L, Laviv A, Schwartz-Arad D, Denture-related osteonecrosis of the maxilla associated with oral bisphosphonate treatment, J. Am. Dent. Assoc 138(9) (2007) 1218–1220. [DOI] [PubMed] [Google Scholar]
- [29].Hess LM, Jeter JM, ham-Hutchins M, Alberts DS, Factors associated with osteonecrosis of the jaw among bisphosphonate users, Am J Med 121(6) (2008) 475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kyrgidis A, Vahtsevanos K, Koloutsos G, Andreadis C, Boukovinas I, Teleioudis Z, Patrikidou A, Triaridis S, Bisphosphonate-related osteonecrosis of the jaws: a case-control study of risk factors in breast cancer patients, J. Clin. Oncol 26(28) (2008) 4634–4638. [DOI] [PubMed] [Google Scholar]
- [31].Walter C, Al-Nawas B, Wolff T, Schiegnitz E, Grotz KA, Dental implants in patients treated with antiresorptive medication - a systematic literature review, Int J Implant Dent 2(1) (2016) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].o.O AA Advisory Task Force on Bisphosphonate-Related Osteonecrosis of the Jaws, S. Maxillofacial, American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws, J Oral Maxillofac Surg 65(3) (2007) 369–76. [DOI] [PubMed] [Google Scholar]
- [33].Ruggiero SL, Dodson TB, Assael LA, Landesberg R, Marx RE, Mehrotra B, American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws−−2009 update, J. Oral Maxillofac. Surg 67(5 Suppl) (2009) 2–12. [DOI] [PubMed] [Google Scholar]
- [34].Pautke C, Bauer F, Tischer T, Kreutzer K, Weitz J, Kesting M, Holzle F, Kolk A, Sturzenbaum SR, Wolff KD, Fluorescence-guided bone resection in bisphosphonate-associated osteonecrosis of the jaws, J Oral Maxillofac Surg 67(3) (2009) 471–6. [DOI] [PubMed] [Google Scholar]
- [35].Groetz KA, Al-Nawas B, Persisting alveolar sockets-a radiologic symptom of BP-ONJ?, J Oral Maxillofac Surg 64(10) (2006) 1571–2. [DOI] [PubMed] [Google Scholar]
- [36].Fleisher KE, Welch G, Kottal S, Craig RG, Saxena D, Glickman RS, Predicting risk for bisphosphonate-related osteonecrosis of the jaws: CTX versus radiographic markers, Oral Surg Oral Med Oral Pathol Oral Radiol Endod 110(4) (2010) 509–16. [DOI] [PubMed] [Google Scholar]
- [37].Morrison A, Khan A, Tetradis S, Peters E, Osteonecrosis of the Jaw: An Update for Dentists, J Can Dent Assoc 81 (2015) f19. [PubMed] [Google Scholar]
- [38].Fedele S, Porter SR, D’Aiuto F, Aljohani S, Vescovi P, Manfredi M, Arduino PG, Broccoletti R, Musciotto A, Di Fede O, Lazarovici TS, Campisi G, Yarom N, Nonexposed variant of bisphosphonate-associated osteonecrosis of the jaw: a case series, Am J Med 123(11) (2010) 1060–4. [DOI] [PubMed] [Google Scholar]
- [39].Soundia A, Hadaya D, Mallya SM, Aghaloo TL, Tetradis S, Radiographic predictors of bone exposure in patients with stage 0 medication-related osteonecrosis of the jaws, Oral Surg Oral Med Oral Pathol Oral Radiol 126(6) (2018) 537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Soundia A, Hadaya D, Esfandi N, Gkouveris I, Christensen R, Dry SM, Bezouglaia O, Pirih F, Nikitakis N, Aghaloo T, Tetradis S, Zoledronate Impairs Socket Healing after Extraction of Teeth with Experimental Periodontitis, J. Dent. Res 97(3) (2018) 312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Castillo EJ, Messer JG, Abraham AM, Jiron JM, Alekseyenko AV, Israel R, Thomas S, Gonzalez-Perez GM, Croft S, Gohel A, Bhattacharyya N, Yarrow JF, Novince CM, Kimmel DB, Aguirre JI, Preventing or Controlling Periodontitis Reduces the Occurrence of Osteonecrosis of the Jaw (ONJ) in Rice Rats (Oryzomys palustris), Bone (2021) 115866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Messer JG, Jiron JM, Chen HY, Castillo EJ, Mendieta Calle JL, Reinhard MK, Kimmel DB, Aguirre JI, Prevalence of Food Impaction-Induced Periodontitis in Conventionally Housed Marsh Rice Rats (Oryzomys palustris), Comp Med 67(1) (2017) 43–50. [PMC free article] [PubMed] [Google Scholar]
- [43].Aghaloo TL, Cheong S, Bezouglaia O, Kostenuik P, Atti E, Dry SM, Pirih FQ, Tetradis S, RANKL inhibitors induce osteonecrosis of the jaw in mice with periapical disease, J. Bone Miner. Res 29(4) (2014) 843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kuroshima S, Al-Salihi Z, Yamashita J, Mouse anti-RANKL antibody delays oral wound healing and increases TRAP-positive mononuclear cells in bone marrow, Clin Oral Investig 20(4) (2016) 727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Akita Y, Kuroshima S, Nakajima K, Hayano H, Kanai R, Sasaki M, Sawase T, Effect of anti-angiogenesis induced by chemotherapeutic monotherapy, chemotherapeutic/bisphosphonate combination therapy and anti-VEGFA mAb therapy on tooth extraction socket healing in mice, J Bone Miner Metab 36(5) (2018) 547–559. [DOI] [PubMed] [Google Scholar]
- [46].Messer JG, Castillo EJ, Abraham AM, Jiron JM, Israel R, Yarrow JF, Thomas S, Reynolds MC, Wnek RD, Jorgensen M, Wanionok N, Van Poznak C, Bhattacharyya I, Kimmel DB, Aguirre JI, Anti-vascular endothelial growth factor antibody monotherapy causes destructive advanced periodontitis in rice rats (Oryzomys palustris), Bone 130 (2020) 115141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Gasser JA, Ingold P, Venturiere A, Shen V, Green JR, Long-term protective effects of zoledronic acid on cancellous and cortical bone in the ovariectomized rat, J Bone Miner. Res 23(4) (2008) 544–551. [DOI] [PubMed] [Google Scholar]
- [48].Seedor JG, Quartuccio HA, Thompson DD, The bisphosphonate alendronate (MK-217) inhibits bone loss due to ovariectomy in rats, J Bone Miner. Res 6(4) (1991) 339–346. [DOI] [PubMed] [Google Scholar]
- [49].Tanaka M, Mori H, Kayasuga R, Ochi Y, Kawada N, Yamada H, Kishikawa K, Long-term minodronic acid (ONO-5920/YM529) treatment suppresses increased bone turnover, plus prevents reduction in bone mass and bone strength in ovariectomized rats with established osteopenia, Bone 43(5) (2008) 894–900. [DOI] [PubMed] [Google Scholar]
- [50].Kimoto A, Tanaka M, Nozaki K, Mori M, Fukushima S, Mori H, Shiroya T, Nakamura T, Intermittent minodronic acid treatment with sufficient bone resorption inhibition prevents reduction in bone mass and strength in ovariectomized rats with established osteopenia comparable with daily treatment, Bone 55(1) (2013) 189–97. [DOI] [PubMed] [Google Scholar]
- [51].Bauss F, Lalla S, Endele R, Hothorn LA, Effects of treatment with ibandronate on bone mass, architecture, biomechanical properties, and bone concentration of ibandronate in ovariectomized aged rats, J Rheumatol 29(10) (2002) 2200–8. [PubMed] [Google Scholar]
- [52].Bauss F, Wagner M, Hothorn LH, Total administered dose of ibandronate determines its effects on bone mass and architecture in ovariectomized aged rats, J Rheumatol 29(5) (2002) 990–8. [PubMed] [Google Scholar]
- [53].Watkins MP, Norris JY, Grimston SK, Zhang X, Phipps RJ, Ebetino FH, Civitelli R, Bisphosphonates improve trabecular bone mass and normalize cortical thickness in ovariectomized, osteoblast connexin43 deficient mice, Bone 51(4) (2012) 787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Gentile MAL, Masarachia S, Carballo-Jane P., Kimmel E, B. D, Effect of alendronate in a murine model of estrogen deficiency, Journal of Bone and Mineral Research 19 (Suppl. 1) (2004) S309–S309. [Google Scholar]
- [55].Berenson JR, Zoledronic acid in cancer patients with bone metastases: results of Phase I and II trials, Semin Oncol 28(2 Suppl 6) (2001) 25–34. [DOI] [PubMed] [Google Scholar]
- [56].Lambrinoudaki I, Vlachou S, Galapi F, Papadimitriou D, Papadias K, Once-yearly zoledronic acid in the prevention of osteoporotic bone fractures in postmenopausal women, Clin Interv Aging 3(3) (2008) 445–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kimmel DB, The oophorectomized beagle as an experimental model for estrogen-deplete bone loss in the adult human, Eur Cell Mater (Suppl 1) (1991) 75–84. [Google Scholar]
- [58].Nagai SSH, Mechanical strength of bone in canine osteoporosis model: Relationship between bone mineral content and bone fragility, Journal of Orthopaedic Science 2(6) (1997) 428–433. [Google Scholar]
- [59].Klaassen CDWIJB, Casarett & Doull’s Essentials of Toxicology, Third ed., McGraw Hill Education, Lange, New York, 2021. [Google Scholar]
- [60].Neff EP, CRISPR improves prospects for transgenic rats, Lab Animal 48(6) (2019) 167–167. [Google Scholar]
- [61].Tannenbaum J, Bennett BT, Russell and Burch’s 3Rs then and now: the need for clarity in definition and purpose, J Am Assoc Lab Anim Sci 54(2) (2015) 120–32. [PMC free article] [PubMed] [Google Scholar]
- [62].Sonis ST, Watkins BA, Lyng GD, Lerman MA, Anderson KC, Bony changes in the jaws of rats treated with zoledronic acid and dexamethasone before dental extractions mimic bisphosphonate-related osteonecrosis in cancer patients, Oral Oncol 45(2) (2009) 164–172. [DOI] [PubMed] [Google Scholar]
- [63].Biasotto M, Chiandussi S, Zacchigna S, Moimas S, Dore F, Pozzato G, Cavalli F, Zanconati F, Contardo L, Giacca M, Di LR, A novel animal model to study non-spontaneous bisphosphonates osteonecrosis of jaw, J. Oral Pathol. Med 39(5) (2010) 390–396. [DOI] [PubMed] [Google Scholar]
- [64].Lopez-Jornet P, Camacho-Alonso F, Molina-Minano F, Gomez-Garcia F, Vicente-Ortega V, An experimental study of bisphosphonate-induced jaws osteonecrosis in Sprague-Dawley rats, J. Oral Pathol. Med 39(9) (2010) 697–702. [DOI] [PubMed] [Google Scholar]
- [65].Marino KL, Zakhary I, Abdelsayed RA, Carter JA, O’Neill JC, Khashaba RM, Elsalanty M, Stevens MR, Borke JL, Development of a Rat Model of Bisphosphonate-Related Osteonecrosis of the Jaw (BRONJ), J. Oral Implantol (2011) (S1): 511–518 (Abstract). [DOI] [PubMed] [Google Scholar]
- [66].Conte NN, Spolidorio LC, Andrade CR, Esteves JC, Marcantonio E Jr., Experimental osteonecrosis: development of a model in rodents administered alendronate, Braz Oral Res 30(1) (2016) e99. [DOI] [PubMed] [Google Scholar]
- [67].Hikita H, Miyazawa K, Tabuchi M, Kimura M, Goto S, Bisphosphonate administration prior to tooth extraction delays initial healing of the extraction socket in rats, J. Bone Miner. Metab 27(6) (2009) 663–672. [DOI] [PubMed] [Google Scholar]
- [68].Aguirre JI, Altman MK, Vanegas SM, Franz SE, Bassit AC, Wronski TJ, Effects of alendronate on bone healing after tooth extraction in rats, Oral Dis 16(7) (2010) 674–685. [DOI] [PubMed] [Google Scholar]
- [69].Jang HW, Kim JW, Cha IH, Development of animal model for Bisphosphonates-related osteonecrosis of the jaw (BRONJ), Maxillofac Plast Reconstr Surg 37(1) (2015) 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Abtahi J, Agholme F, Sandberg O, Aspenberg P, Bisphosphonate-induced osteonecrosis of the jaw in a rat model arises first after the bone has become exposed. No primary necrosis in unexposed bone, J. Oral Pathol. Med 41(6) (2012) 494–499. [DOI] [PubMed] [Google Scholar]
- [71].Abtahi J, Agholme F, Aspenberg P, Prevention of osteonecrosis of the jaw by mucoperiosteal coverage in a rat model, Int J Oral Maxillofac Surg 42(5) (2013) 632–6. [DOI] [PubMed] [Google Scholar]
- [72].Gupta O, Shaw J, Periodontal disease in the rice rat. I. Anatomic and histopathologic findings., Oral Surg. Oral Med Oral Pathol 9(6) (1956) 592–603. [DOI] [PubMed] [Google Scholar]
- [73].Ryder MI, Histological and ultrastructural characteristics of the periodontal syndrome in the rice rat. I. General light microscopic observations and ultrastructural observations of initial inflammatory changes, J Periodontal Res 15(5) (1980) 502–515. [DOI] [PubMed] [Google Scholar]
- [74].Struillou X, Boutigny H, Soueidan A, Layrolle P, Experimental animal models in periodontology: a review, Open. Dent. J 4 (2010) 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Swindle MMVGA; Fulton LK; Marini RP; Popilskis S, Laboratory Animal Medicine, in: Fox JG, Anderson LC, Loew FM, Quimby FW (Eds.), Laboratory animal medicine, American College of Laboratory Animal Medicine Series, San Diego, CA, 2002, pp. 955–1003. [Google Scholar]
- [76].Miller WA, Ripley JF, Early periodontal disease in the Syrian hamster, J. Periodontol 46(6) (1975) 368–374. [DOI] [PubMed] [Google Scholar]
- [77].Weinberg MA, Bral M, Laboratory animal models in periodontology, J. Clin. Periodontol 26(6) (1999) 335–340. [DOI] [PubMed] [Google Scholar]
- [78].Graves DT, Kang J, Andriankaja O, Wada K, Rossa C Jr., Animal models to study host-bacteria interactions involved in periodontitis, Front Oral Biol 15 (2012) 117–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Oz HS, Puleo DA, Animal models for periodontal disease, J. Biomed. Biotechnol 2011 (2011) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Cetinkaya BO, Keles GC, Ayas B, Gurgor P, Effects of risedronate on alveolar bone loss and angiogenesis: a stereologic study in rats, J Periodontol 79(10) (2008) 1950–61. [DOI] [PubMed] [Google Scholar]
- [81].Moreira MM, Bradaschia-Correa V, Marques ND, Ferreira LB, Arana-Chavez VE, Ultrastructural and immunohistochemical study of the effect of sodium alendronate in the progression of experimental periodontitis in rats, Microsc Res Tech 77(11) (2014) 902–9. [DOI] [PubMed] [Google Scholar]
- [82].Li CL, Seneviratne CJ, Huo L, Lu WW, Zheng LW, Impact of Actinomyces naeslundii on bisphosphonate-related osteonecrosis of the jaws in ovariectomized rats with periodontitis, J Craniomaxillofac Surg 43(8) (2015) 1662–9. [DOI] [PubMed] [Google Scholar]
- [83].Li CL, Lu WW, Seneviratne CJ, Leung WK, Zwahlen RA, Zheng LW, Role of periodontal disease in bisphosphonate-related osteonecrosis of the jaws in ovariectomized rats, Clin. Oral Implants. Res 27(1) (2016) 1–6. [DOI] [PubMed] [Google Scholar]
- [84].Molez AM, do Nascimento EHL, Haiter Neto F, Cirano FR, Pimentel SP, Ribeiro FV, Casati MZ, Correa MG, Effect of resveratrol on the progression of experimental periodontitis in an ovariectomized rat model of osteoporosis: Morphometric, immune-enzymatic, and gene expression analysis, J Periodontal Res 55(6) (2020) 840–849. [DOI] [PubMed] [Google Scholar]
- [85].Tanaka J, Kokuryo S, Yoshiga D, Tsurushima H, Sakaguchi O, Habu M, Nishihara T, Yoshioka I, Tominaga K, An osteonecrosis model induced by oral bisphosphonate in ovariectomised rats, Oral Dis 21(8) (2015) 969–76. [DOI] [PubMed] [Google Scholar]
- [86].Tsurushima H, Kokuryo S, Sakaguchi O, Tanaka J, Tominaga K, Bacterial promotion of bisphosphonate-induced osteonecrosis in Wistar rats, Int J Oral Maxillofac Surg 42(11) (2013) 1481–7. [DOI] [PubMed] [Google Scholar]
- [87].Franca TRT, Ramos-Perez FMM, Pontual ADA, Castro JFL, Bonan PRF, Perez D, Effects of Zoledronic Acid in Experimental Periapical Lesions in Rats: An Imaging and Histological Analysis, Braz Dent J 28(5) (2017) 566–572. [DOI] [PubMed] [Google Scholar]
- [88].Pacheco VN, Langie R, Benfica JRD, Munaretto JC, Etges A, Ponzoni D, Puricelli E, Nitrogen-containing bisphosphonate therapy-Part II: Assessment of alveolar bone tissue inflammatory response in rats-A blind randomized controlled trial, Int J Exp Pathol 99(5) (2018) 258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Hadaya D, Gkouveris I, Soundia A, Bezouglaia O, Boyce RW, Stolina M, Dwyer D, Dry SM, Pirih FQ, Aghaloo TL, Tetradis S, Clinically Relevant Doses of Sclerostin Antibody Do Not Induce Osteonecrosis of the Jaw (ONJ) in Rats with Experimental Periodontitis, J Bone Miner Res 34(1) (2019) 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Hadaya D, Soundia A, Gkouveris I, Dry SM, Aghaloo TL, Tetradis S, Development of Medication-Related Osteonecrosis of the Jaw After Extraction of Teeth With Experimental Periapical Disease, J Oral Maxillofac Surg 77(1) (2019) 71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Hadaya D, Soundia A, Gkouveris I, Bezouglaia O, Dry SM, Pirih FQ, Aghaloo TL, Tetradis S, Antiresorptive-Type and Discontinuation-Timing Affect ONJ Burden, J Dent Res (2021) 22034520986804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Epstein MS, Wicknick FW, Epstein JB, Berenson JR, Gorsky M, Management of bisphosphonate-associated osteonecrosis: pentoxifylline and tocopherol in addition to antimicrobial therapy. An initial case series, Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod 110(5) (2010) 593–596. [DOI] [PubMed] [Google Scholar]
- [93].Vandone AM, Donadio M, Mozzati M, Ardine M, Polimeni MA, Beatrice S, Ciuffreda L, Scoletta M, Impact of dental care in the prevention of bisphosphonate-associated osteonecrosis of the jaw: a single-center clinical experience, Ann. Oncol 23(1) (2012) 193–200. [DOI] [PubMed] [Google Scholar]
- [94].Hoefert S, Eufinger H, Relevance of a prolonged preoperative antibiotic regimen in the treatment of bisphosphonate-related osteonecrosis of the jaw, J. Oral Maxillofac. Surg 69(2) (2011) 362–380. [DOI] [PubMed] [Google Scholar]
- [95].Saia G, Blandamura S, Bettini G, Tronchet A, Totola A, Bedogni G, Ferronato G, Nocini PF, Bedogni A, Occurrence of bisphosphonate-related osteonecrosis of the jaw after surgical tooth extraction, J. Oral Maxillofac. Surg 68(4) (2010) 797–804. [DOI] [PubMed] [Google Scholar]
- [96].Montefusco V, Gay F, Spina F, Miceli R, Maniezzo M, Teresa AM, Farina L, Piva S, Palumbo A, Boccadoro M, Corradini P, Antibiotic prophylaxis before dental procedures may reduce the incidence of osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphonates, Leuk. Lymphoma 49(11) (2008) 2156–2162. [DOI] [PubMed] [Google Scholar]
- [97].Ripamonti CI, Maniezzo M, Campa T, Fagnoni E, Brunelli C, Saibene G, Bareggi C, Ascani L, Cislaghi E, Decreased occurrence of osteonecrosis of the jaw after implementation of dental preventive measures in solid tumour patients with bone metastases treated with bisphosphonates. The experience of the National Cancer Institute of Milan, Ann. Oncol 20(1) (2009) 137–145. [DOI] [PubMed] [Google Scholar]
- [98].Dimopoulos MA, Kastritis E, Bamia C, Melakopoulos I, Gika D, Roussou M, Migkou M, Eleftherakis-Papaiakovou E, Christoulas D, Terpos E, Bamias A, Reduction of osteonecrosis of the jaw (ONJ) after implementation of preventive measures in patients with multiple myeloma treated with zoledronic acid, Ann. Oncol 20(1) (2009) 117–120. [DOI] [PubMed] [Google Scholar]
- [99].Hidaka K, Mikuni-Takagaki Y, Wada-Takahashi S, Saita M, Kawamata R, Sato T, Kawata A, Miyamoto C, Maehata Y, Watabe H, Tani-Ishii N, Hamada N, Takahashi SS, Deguchi S, Takeuchi R, Low-Intensity Pulsed Ultrasound Prevents Development of Bisphosphonate-Related Osteonecrosis of the Jaw-Like Pathophysiology in a Rat Model, Ultrasound Med Biol 45(7) (2019) 1721–1732. [DOI] [PubMed] [Google Scholar]
- [100].Bolette A, Lecloux G, Rompen E, Albert A, Kerckhofs G, Lambert F, Influence of induced infection in medication-related osteonecrosis of the jaw development after tooth extraction: A study in rats, J Craniomaxillofac Surg 47(2) (2019) 349–356. [DOI] [PubMed] [Google Scholar]
- [101].Oh KC, Moon HS, Lee JH, Park YB, Kim JH, Effects of alendronate on the peri-implant bone in rats, Oral Dis 21(2) (2015) 248–56. [DOI] [PubMed] [Google Scholar]
- [102].Park R, Kim JH, Choi H, Park YB, Jung HS, Moon HS, Effect of alendronate on bone remodeling around implant in the rat, J Adv Prosthodont 5(4) (2013) 374–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Abtahi J, Agholme F, Sandberg O, Aspenberg P, Effect of local vs. systemic bisphosphonate delivery on dental implant fixation in a model of osteonecrosis of the jaw, J Dent Res 92(3) (2013) 279–83. [DOI] [PubMed] [Google Scholar]
- [104].Kniha K, Bock A, Peters F, Magnuska ZA, Gremse F, Mohlhenrich SC, Holzle F, Modabber A, Microstructural volumetric analysis of the jaw following dental implantation under systemic bisphosphonate delivery: An in vivo and ex vivo rat study, J Periodontol (2020). [DOI] [PubMed] [Google Scholar]
- [105].Park JY, Heo HA, Park S, Pyo SW, Enhancement of peri-implant bone formation via parathyroid hormone administration in a rat model at risk for medication-related osteonecrosis of the jaw, J Periodontal Implant Sci 50(2) (2020) 121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Inoue M, Matsumoto C, Nakajima K, Kuroshima S, Sawase T, Alendronate/dexamethasone combination therapy worsens soft and hard tissue wound healing around implants in rat maxillae, Bone 148 (2021) 115942. [DOI] [PubMed] [Google Scholar]
- [107].Yamashita J, Koi K, Yang DY, McCauley LK, Effect of zoledronate on oral wound healing in rats, Clin. Cancer Res 17(6) (2011) 1405–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Yoshioka Y, Yamachika E, Nakanishi M, Ninomiya T, Nakatsuji K, Kobayashi Y, Fujii T, Iida S, Cathepsin K inhibitor causes changes in crystallinity and crystal structure of newly-formed mandibular bone in rats, Br J Oral Maxillofac Surg 56(8) (2018) 732–738. [DOI] [PubMed] [Google Scholar]
- [109].Zandi M, Dehghan A, Amini P, Doulati S, Rezaeian L, Evaluation of the effect of teriparatide therapy on mandibular fracture healing in rats with medication-related osteonecrosis of the jaw, Clin Oral Investig 23(11) (2019) 3987–3993. [DOI] [PubMed] [Google Scholar]
- [110].Xia CW, Pan JR, Fan L, Xiao Q, Pu Y, Wang YX, The feasibility of locating the affected bone of BRONJ with indocyanine green, Oral Dis 26(5) (2020) 1086–1089. [DOI] [PubMed] [Google Scholar]
- [111].Soares MQS, Van Dessel J, Jacobs R, da Silva Santos PS, Cestari TM, Garlet GP, Duarte MAH, Imada TSN, Lambrichts I, Rubira-Bullen IRF, Zoledronic Acid Induces Site-Specific Structural Changes and Decreases Vascular Area in the Alveolar Bone, J Oral Maxillofac Surg 76(9) (2018) 1893–1901. [DOI] [PubMed] [Google Scholar]
- [112].Yoshioka Y, Yamachika E, Nakanishi M, Ninomiya T, Nakatsuji K, Matsubara M, Moritani N, Kobayashi Y, Fujii T, Iida S, Molecular alterations of newly formed mandibular bone caused by zoledronate, Int J Oral Maxillofac Surg 47(9) (2018) 1206–1213. [DOI] [PubMed] [Google Scholar]
- [113].FS DEP, Catalfamo L, Micali G, Runci M, Cutroneo G, Vermiglio G, Centofanti A, Rizzo G, Effect of bisphosphonates on the mandibular bone and gingival epithelium of rats without tooth extraction, Exp Ther Med 11(5) (2016) 1678–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Howie RN, Bhattacharyya M, Salama ME, Refaey ME, Isales C, Borke J, Daoudi A, Medani F, Elsalanty ME, Removal of pamidronate from bone in rats using systemic and local chelation, Arch Oral Biol 60(12) (2015) 1699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Sakaguchi O, Kokuryo S, Tsurushima H, Tanaka J, Habu M, Uehara M, Nishihara T, Tominaga K, Lipopolysaccharide aggravates bisphosphonate-induced osteonecrosis in rats, Int J Oral Maxillofac Surg 44(4) (2015) 528–34. [DOI] [PubMed] [Google Scholar]
- [116].Cankaya M, Cizmeci Senel F, Kadioglu Duman M, Muci E, Dayisoylu EH, Balaban F, The effects of chronic zoledronate usage on the jaw and long bones evaluated using RANKL and osteoprotegerin levels in an animal model, Int J Oral Maxillofac Surg 42(9) (2013) 1134–9. [DOI] [PubMed] [Google Scholar]
- [117].Okamoto Y, Hirota M, Monden Y, Murata S, Koyama C, Mitsudo K, Iwai T, Ishikawa Y, Tohnai I, High-dose zoledronic acid narrows the periodontal space in rats, Int J Oral Maxillofac Surg 42(5) (2013) 627–31. [DOI] [PubMed] [Google Scholar]
- [118].Hokugo A, Sun S, Park S, McKenna CE, Nishimura I, Equilibrium-dependent bisphosphonate interaction with crystalline bone mineral explains anti-resorptive pharmacokinetics and prevalence of osteonecrosis of the jaw in rats, Bone 53(1) (2013) 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Said F, Ghoul-Mazgar S, Khemiss F, El Ayeb H, Saidane D, Berdal A, Ruhin-Poncet B, The effect of etidronate on the periodontium of ovariectomized rats, J Periodontol 83(8) (2012) 1063–8. [DOI] [PubMed] [Google Scholar]
- [120].Senel FC, Kadioglu DM, Muci E, Cankaya M, Pampu AA, Ersoz S, Gunhan O, Jaw bone changes in rats after treatment with zoledronate and pamidronate, Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod 109(3) (2010) 385–391. [DOI] [PubMed] [Google Scholar]
- [121].Poubel V, Capella DL, Santos ARS, Correa M, Ruhland L, Rivero ERC, Evaluation of Mandibular Bone After Dental Extraction in Rats Treated With Antiresorptive Drugs, J Oral Maxillofac Surg 76(3) (2018) 474–482. [DOI] [PubMed] [Google Scholar]
- [122].Kuroshima S, Entezami P, McCauley LK, Yamashita J, Early effects of parathyroid hormone on bisphosphonate/steroid-associated compromised osseous wound healing, Osteoporos Int 25(3) (2014) 1141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Keskinruzgar A, Bozdag Z, Aras MH, Demir T, Yolcu U, Cetiner S, Histopathological Effects of Teriparatide in Medication-Related Osteonecrosis of the Jaw: An Animal Study, J Oral Maxillofac Surg 74(1) (2016) 68–78. [DOI] [PubMed] [Google Scholar]
- [124].Zandi M, Dehghan A, Mohammadi-Mofrad A, Amini P, Vahdatinia F, Short-term perioperative teriparatide therapy for the prevention of medication-related osteonecrosis of the jaw: A randomized, controlled preclinical study in rats, J Craniomaxillofac Surg 45(2) (2017) 275–280. [DOI] [PubMed] [Google Scholar]
- [125].Kim JY, Jang HW, Kim JI, Cha IH, Effects of pre-extraction intermittent PTH administration on extraction socket healing in bisphosphonate administered ovariectomized rats, Sci Rep 11(1) (2021) 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Erten Taysi A, Cevher E, Sessevmez M, Olgac V, Mert Taysi N, Atalay B, The efficacy of sustained-release chitosan microspheres containing recombinant human parathyroid hormone on MRONJ, Braz Oral Res 33 (2019) e086. [DOI] [PubMed] [Google Scholar]
- [127].Dayisoylu EH, Senel FC, Ungor C, Tosun E, Cankaya M, Ersoz S, Taskesen F, The effects of adjunctive parathyroid hormone injection on bisphosphonate-related osteonecrosis of the jaws: an animal study, Int J Oral Maxillofac Surg 42(11) (2013) 1475–80. [DOI] [PubMed] [Google Scholar]
- [128].Mergoni G, Vescovi P, Sala R, Merigo E, Passerini P, Maestri R, Corradi D, Govoni P, Nammour S, Bianchi MG, The effect of laser therapy on the expression of osteocalcin and osteopontin after tooth extraction in rats treated with zoledronate and dexamethasone, Support Care Cancer 24(2) (2016) 807–813. [DOI] [PubMed] [Google Scholar]
- [129].Weber JBB, Camilotti RS, Jasper J, Casagrande LCO, Maito F, Effect of low-level laser therapy on tissue repair after dental extraction in rats administered zoledronic acid and dexamethasone, J Biomed Opt 22(5) (2017) 58001. [DOI] [PubMed] [Google Scholar]
- [130].Statkievicz C, Toro LF, de Mello-Neto JM, de Sá DP, Casatti CA, Issa JPM, Cintra LTA, de Almeida JM, Nagata MJH, Garcia VG, Theodoro LH, Ervolino E, Photomodulation multiple sessions as a promising preventive therapy for medication-related osteonecrosis of the jaws after tooth extraction in rats, J Photochem Photobiol B 184 (2018) 7–17. [DOI] [PubMed] [Google Scholar]
- [131].Ervolino E, Statkievicz C, Toro LF, de Mello-Neto JM, Cavazana TP, Issa JPM, Dornelles RCM, de Almeida JM, Nagata MJH, Okamoto R, Casatti CA, Garcia VG, Theodoro LH, Antimicrobial photodynamic therapy improves the alveolar repair process and prevents the occurrence of osteonecrosis of the jaws after tooth extraction in senile rats treated with zoledronate, Bone 120 (2019) 101–113. [DOI] [PubMed] [Google Scholar]
- [132].Sarkarat F, Modarresi A, Chiniforush N, Yazdanparast L, Rakhshan V, Efficacy of Photodynamic Therapy in Minimizing Bisphosphonate-Related Osteonecrosis of the Jaws After Dental Extraction: A Preliminary Animal Study, J Oral Maxillofac Surg 77(2) (2019) 307–314. [DOI] [PubMed] [Google Scholar]
- [133].Silva ML, Tasso L, Azambuja AA, Figueiredo MA, Salum FG, da Silva VD, Cherubini K, Effect of hyperbaric oxygen therapy on tooth extraction sites in rats subjected to bisphosphonate therapy-histomorphometric and immunohistochemical analysis, Clin Oral Investig 21(1) (2017) 199–210. [DOI] [PubMed] [Google Scholar]
- [134].Liu SS, Lin TY, Fu E, Hsia YJ, Chiu HC, Tu HP, Chiang CY, Immediate hyperbaric oxygen after tooth extraction ameliorates bisphosphonate-related osteonecrotic lesion in rats, J Periodontol 90(12) (2019) 1449–1456. [DOI] [PubMed] [Google Scholar]
- [135].Movahedian Attar B, Razavi SM, Daneshmand M, Davoudi A, Protective effects of resveratrol against osteonecrosis at the extraction site in bisphosphonate-treated rats, International Journal of Oral and Maxillofacial Surgery 49(11) (2020) 1518–1522. [DOI] [PubMed] [Google Scholar]
- [136].Elsayed R, Abraham P, Awad ME, Kurago Z, Baladhandayutham B, Whitford GM, Pashley DH, McKenna CE, Elsalanty ME, Removal of matrix-bound zoledronate prevents post-extraction osteonecrosis of the jaw by rescuing osteoclast function, Bone 110 (2018) 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Watanabe J, Sakai K, Urata Y, Toyama N, Nakamichi E, Hibi H, Extracellular Vesicles of Stem Cells to Prevent BRONJ, J Dent Res 99(5) (2020) 552–560. [DOI] [PubMed] [Google Scholar]
- [138].Huang J, Wang L, Tian W, Small Extracellular Vesicles Derived from Adipose Tissue Prevent Bisphosphonate-Related Osteonecrosis of the Jaw by Promoting Angiogenesis, Int J Nanomedicine 16 (2021) 3161–3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Rodríguez-Lozano FJ, Oñate-Sánchez R, Gonzálvez-García M, Vallés-Bergadá M, Martínez CM, Revilla-Nuin B, Guerrero-Gironés J, Moraleda JM, García-Bernal D, Allogeneic Bone Marrow Mesenchymal Stem Cell Transplantation in Tooth Extractions Sites Ameliorates the Incidence of Osteonecrotic Jaw-Like Lesions in Zoledronic Acid-Treated Rats, J Clin Med 9(6) (2020) 1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Zandi M, Dehghan A, Ghadermazi K, Malekzadeh H, Akbarzadeh M, Perioperative discontinuation of intravenous bisphosphonate therapy reduces the incidence and severity of bisphosphonate-related osteonecrosis of the jaw: A randomized, controlled, prospective experimental study in rats, J Craniomaxillofac Surg 43(9) (2015) 1823–8. [DOI] [PubMed] [Google Scholar]
- [141].Ersan N, van Ruijven LJ, Bronckers AL, Olgaç V, Ilgüy D, Everts V, Teriparatide and the treatment of bisphosphonate-related osteonecrosis of the jaw: a rat model, Dentomaxillofac Radiol 43(1) (2014) 20130144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Zandi M, Dehghan A, Zandipoor N, Amini P, Doulati S, Effect of different doses and durations of teriparatide therapy on resolution of medication-related osteonecrosis of the jaw: A randomized, controlled preclinical study in rats, J Craniomaxillofac Surg 46(3) (2018) 466–472. [DOI] [PubMed] [Google Scholar]
- [143].Liu J, Mattheos N, Deng C, Su C, Wang Z, Luo N, Tang H, Management of medication-related osteonecrosis of jaw: Comparison between icariin and teriparatide in a rat model, J Periodontol 92(1) (2021) 149–158. [DOI] [PubMed] [Google Scholar]
- [144].Sarkarat F, Kalantar Motamedi MH, Jahanbani J, Sepehri D, Kahali R, Nematollahi Z, Platelet-Rich Plasma in Treatment of Zoledronic Acid-Induced Bisphosphonate-related Osteonecrosis of the Jaws, Trauma Mon 19(2) (2014) e17196-e17196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Cardoso CL, Curra C, Curi MM, Matsumoto MA, Argentino CD, Franzolin SOB, Constantino D, Barbosa DN, Ferreira Júnior O, Treatment of bisphosphonate-related osteonecrosis using platelet-rich plasma: microtomographic, microscopic, and immunohistochemical analyses, Braz Oral Res 33 (2019) e050. [DOI] [PubMed] [Google Scholar]
- [146].Ogata K, Katagiri W, Osugi M, Kawai T, Sugimura Y, Hibi H, Nakamura S, Ueda M, Evaluation of the therapeutic effects of conditioned media from mesenchymal stem cells in a rat bisphosphonate-related osteonecrosis of the jaw-like model, Bone 74 (2015) 95–105. [DOI] [PubMed] [Google Scholar]
- [147].Kaibuchi N, Iwata T, Yamato M, Okano T, Ando T, Multipotent mesenchymal stromal cell sheet therapy for bisphosphonate-related osteonecrosis of the jaw in a rat model, Acta Biomater 42 (2016) 400–410. [DOI] [PubMed] [Google Scholar]
- [148].Tamari T, Elimelech R, Cohen G, Cohen T, Doppelt O, Eskander-Hashoul L, Zigdon-Giladi H, Endothelial Progenitor Cells inhibit jaw osteonecrosis in a rat model: A major adverse effect of bisphosphonate therapy, Scientific Reports 9(1) (2019) 18896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Ogata K, Matsumura M, Moriyama M, Katagiri W, Hibi H, Nakamura S, Cytokine Mixtures Mimicking Secretomes From Mesenchymal Stem Cells Improve Medication-Related Osteonecrosis of the Jaw in a Rat Model, JBMR Plus 2(2) (2018) 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Rao NJ, Wang JY, Yu RQ, Leung YY, Zheng LW, Role of Periapical Diseases in Medication-Related Osteonecrosis of the Jaws, Biomed Res Int 2017 (2017) 1560175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].de Molon RS, Hsu C, Bezouglaia O, Dry SM, Pirih FQ, Soundia A, Cunha FQ, Cirelli JA, Aghaloo TL, Tetradis S, Rheumatoid Arthritis Exacerbates the Severity of Osteonecrosis of the Jaws (ONJ) in Mice. A Randomized, Prospective, Controlled Animal Study, J Bone Miner Res 31(8) (2016) 1596–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].de Molon RS, Shimamoto H, Bezouglaia O, Pirih FQ, Dry SM, Kostenuik P, Boyce RW, Dwyer D, Aghaloo TL, Tetradis S, OPG-Fc but Not Zoledronic Acid Discontinuation Reverses Osteonecrosis of the Jaws (ONJ) in Mice, J Bone Miner Res 30(9) (2015) 1627–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Cheong S, Sun S, Kang B, Bezouglaia O, Elashoff D, McKenna CE, Aghaloo TL, Tetradis S, Bisphosphonate uptake in areas of tooth extraction or periapical disease, J Oral Maxillofac Surg 72(12) (2014) 2461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Gkouveris I, Hadaya D, Soundia A, Bezouglaia O, Chau Y, Dry SM, Pirih FQ, Aghaloo TL, Tetradis S, Vasculature submucosal changes at early stages of osteonecrosis of the jaw (ONJ), Bone 123 (2019) 234–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Hori N, Abe T, Sato T, Kokabu S, Shimamura Y, Sato T, Yoda T, Data in support of the bone analysis of NOD-SCID mice treated with zoledronic acid and prednisolone, Data Brief 7 (2016) 1486–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Pabst AM, Ziebart T, Ackermann M, Konerding MA, Walter C, Bisphosphonates’ antiangiogenic potency in the development of bisphosphonate-associated osteonecrosis of the jaws: influence on microvessel sprouting in an in vivo 3D Matrigel assay, Clin Oral Investig 18(3) (2014) 1015–22. [DOI] [PubMed] [Google Scholar]
- [157].Mawardi H, Giro G, Kajiya M, Ohta K, Almazrooa S, Alshwaimi E, Woo SB, Nishimura I, Kawai T, A role of oral bacteria in bisphosphonate-induced osteonecrosis of the jaw, J. Dent. Res 90(11) (2011) 1339–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Kim T, Kim S, Song M, Lee C, Yagita H, Williams DW, Sung EC, Hong C, Shin KH, Kang MK, Park NH, Kim RH, Removal of Pre-Existing Periodontal Inflammatory Condition before Tooth Extraction Ameliorates Medication-Related Osteonecrosis of the Jaw-Like Lesion in Mice, Am J Pathol 188(10) (2018) 2318–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Williams DW, Vuong HE, Kim S, Lenon A, Ho K, Hsiao EY, Sung EC, Kim RH, Indigenous Microbiota Protects against Inflammation-Induced Osteonecrosis, J Dent Res 99(6) (2020) 676–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Tamaoka J, Takaoka K, Hattori H, Ueta M, Maeda H, Yamamura M, Yamanegi K, Noguchi K, Kishimoto H, Osteonecrosis of the jaws caused by bisphosphonate treatment and oxidative stress in mice, Exp Ther Med 17(2) (2019) 1440–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Yu YY, Lieu S, Hu D, Miclau T, Colnot C, Site specific effects of zoledronic acid during tibial and mandibular fracture repair, PLoS One 7(2) (2012) e31771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Pozzi S, Vallet S, Mukherjee S, Cirstea D, Vaghela N, Santo L, Rosen E, Ikeda H, Okawa Y, Kiziltepe T, Schoonmaker J, Xie W, Hideshima T, Weller E, Bouxsein ML, Munshi NC, Anderson KC, Raje N, High-dose zoledronic acid impacts bone remodeling with effects on osteoblastic lineage and bone mechanical properties, Clin Cancer Res 15(18) (2009) 5829–39. [DOI] [PubMed] [Google Scholar]
- [163].Huja SS, Fernandez SA, Phillips C, Li Y, Zoledronic acid decreases bone formation without causing osteocyte death in mice, Arch Oral Biol 54(9) (2009) 851–6. [DOI] [PubMed] [Google Scholar]
- [164].Oizumi T, Funayama H, Yamaguchi K, Yokoyama M, Takahashi H, Yamamoto M, Kuroishi T, Kumamoto H, Sasaki K, Kawamura H, Sugawara S, Endo Y, Inhibition of necrotic actions of nitrogen-containing bisphosphonates (NBPs) and their elimination from bone by etidronate (a non-NBP): a proposal for possible utilization of etidronate as a substitution drug for NBPs, J Oral Maxillofac Surg 68(5) (2010) 1043–54. [DOI] [PubMed] [Google Scholar]
- [165].Kozloff KM, Volakis LI, Marini JC, Caird MS, Near-infrared fluorescent probe traces bisphosphonate delivery and retention in vivo, J Bone Miner Res 25(8) (2010) 1748–58. [DOI] [PubMed] [Google Scholar]
- [166].Bonnet N, Lesclous P, Saffar JL, Ferrari S, Zoledronate effects on systemic and jaw osteopenias in ovariectomized periostin-deficient mice, PLoS One 8(3) (2013) e58726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Vermeer J, Renders G, van Duin MA, Jansen I, Bakker LF, Kroon SA, de Vries TJ, Everts V, Bone-site-specific responses to zoledronic acid, Oral Dis 23(1) (2017) 126–133. [DOI] [PubMed] [Google Scholar]
- [168].Yamachika E, Matsui Y, Matsubara M, Matsumura T, Nakata N, Moritani N, Ikeda A, Tsujigiwa H, Ohara N, Iida S, The influence of zoledronate and teriparatide on gamma delta T cells in mice, J Dent Sci 12(4) (2017) 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Kubek DJ, Burr DB, Allen MR, Ovariectomy stimulates and bisphosphonates inhibit intracortical remodeling in the mouse mandible, Orthod Craniofac Res 13(4) (2010) 214–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Oizumi T, Yamaguchi K, Funayama H, Kuroishi T, Kawamura H, Sugawara S, Endo Y, Necrotic actions of nitrogen-containing bisphosphonates and their inhibition by clodronate, a non-nitrogen-containing bisphosphonate in mice: potential for utilization of clodronate as a combination drug with a nitrogen-containing bisphosphonate, Basic Clin Pharmacol Toxicol 104(5) (2009) 384–92. [DOI] [PubMed] [Google Scholar]
- [171].Bi Y, Gao Y, Ehirchiou D, Cao C, Kikuiri T, Le A, Shi S, Zhang L, Bisphosphonates cause osteonecrosis of the jaw-like disease in mice, Am. J Pathol 177(1) (2010) 280–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Kobayashi Y, Hiraga T, Ueda A, Wang L, Matsumoto-Nakano M, Hata K, Yatani H, Yoneda T, Zoledronic acid delays wound healing of the tooth extraction socket, inhibits oral epithelial cell migration, and promotes proliferation and adhesion to hydroxyapatite of oral bacteria, without causing osteonecrosis of the jaw, in mice, J. Bone Miner. Metab 28(2) (2010) 165–175. [DOI] [PubMed] [Google Scholar]
- [173].Williams DW, Lee C, Kim T, Yagita H, Wu H, Park S, Yang P, Liu H, Shi S, Shin KH, Kang MK, Park NH, Kim RH, Impaired bone resorption and woven bone formation are associated with development of osteonecrosis of the jaw-like lesions by bisphosphonate and anti-receptor activator of NF-kappaB ligand antibody in mice, Am. J. Pathol 184(11) (2014) 3084–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Kuroshima S, Sasaki M, Nakajima K, Tamaki S, Hayano H, Sawase T, Prevalence of bisphosphonate-related osteonecrosis of the jaw-like lesions is increased in a chemotherapeutic dose-dependent manner in mice, Bone 112 (2018) 177–186. [DOI] [PubMed] [Google Scholar]
- [175].Alonso-Rodriguez E, Gonzalez-Martin-Moro J, Cebrian-Carretero JL, Del Castillo JL, Pozo-Kreilinger JJ, Ruiz-Bravo E, Garcia-Arranz M, Hernandez-Godoy J, Burgueno M, Bisphosphonate-related osteonecrosis. Application of adipose-derived stem cells in an experimental murine model, Med Oral Patol Oral Cir Bucal 24(4) (2019) e529–e536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Elsayed R, Kurago Z, Cutler CW, Arce RM, Gerber J, Celis E, Sultan H, Elashiry M, Meghil M, Sun C, Auersvald CM, Awad ME, Zeitoun R, Elsayed R, Eldin MEM, Isales C, Elsalanty ME, Role of dendritic cell-mediated immune response in oral homeostasis: A new mechanism of osteonecrosis of the jaw, FASEB J 34(2) (2020) 2595–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Barba-Recreo P, Del JL Castillo Pardo de Vera, Georgiev-Hristov T, Ruiz Bravo-Burguillos E, Abarrategi A, Burgueno M, Garcia-Arranz M, Adipose-derived stem cells and platelet-rich plasma for preventive treatment of bisphosphonate-related osteonecrosis of the jaw in a murine model, J Craniomaxillofac Surg 43(7) (2015) 1161–8. [DOI] [PubMed] [Google Scholar]
- [178].Mikai A, Ono M, Tosa I, Nguyen HTT, Hara ES, Nosho S, Kimura-Ono A, Nawachi K, Takarada T, Kuboki T, Oohashi T, BMP-2/beta-TCP Local Delivery for Bone Regeneration in MRONJ-Like Mouse Model, Int J Mol Sci 21(19) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Jung J, Shim GJ, Kim M, Yoon Y, Kim JE, Jue SS, Al-Nawas B, Kwon YD, Effect and timing of parathyroid hormone analog administration for preventing medication-related osteonecrosis of the jaws in a murine model, J Craniomaxillofac Surg (2021). [DOI] [PubMed] [Google Scholar]
- [180].Zhao Y, Wang L, Liu Y, Akiyama K, Chen C, Atsuta I, Zhou T, Duan X, Jin Y, Shi S, Technetium-99 conjugated with methylene diphosphonate ameliorates ovariectomy-induced osteoporotic phenotype without causing osteonecrosis in the jaw, Calcif Tissue Int 91(6) (2012) 400–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Matsuura Y, Atsuta I, Ayukawa Y, Yamaza T, Kondo R, Takahashi A, Ueda N, Oshiro W, Tsukiyama Y, Koyano K, Therapeutic interactions between mesenchymal stem cells for healing medication-related osteonecrosis of the jaw, Stem Cell Res Ther 7(1) (2016) 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Kuroshima S, Sasaki M, Nakajima K, Tamaki S, Hayano H, Sawase T, Transplantation of Noncultured Stromal Vascular Fraction Cells of Adipose Tissue Ameliorates Osteonecrosis of the Jaw-Like Lesions in Mice, J Bone Miner Res 33(1) (2018) 154–166. [DOI] [PubMed] [Google Scholar]
- [183].Kuroshima S, Nakajima K, Sasaki M, Sumita TI,Y, Asahara T, Asahina I, Sawase T, Systemic administration of quality- and quantity-controlled PBMNCs reduces bisphosphonate-related osteonecrosis of jaw-like lesions in mice, Stem Cell Res Ther 10(1) (2019) 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Yu W, Su J, The effects of different doses of teriparatide on bisphosphonate-related osteonecrosis of the jaw in mice, Oral Dis 26(3) (2020) 609–620. [DOI] [PubMed] [Google Scholar]
- [185].Hokugo A, Kanayama K, Sun S, Morinaga K, Sun Y, Wu Q, Sasaki H, Okawa H, Evans C, Ebetino FH, Lundy MW, Sadrerafi K, McKenna CE, Nishimura I, Rescue bisphosphonate treatment of alveolar bone improves extraction socket healing and reduces osteonecrosis in zoledronate-treated mice, Bone 123 (2019) 115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Zhao D, Cui W, Liu M, Li J, Sun Y, Shi S, Lin S, Lin Y, Tetrahedral Framework Nucleic Acid Promotes the Treatment of Bisphosphonate-Related Osteonecrosis of the Jaws by Promoting Angiogenesis and M2 Polarization, ACS Appl Mater Interfaces 12(40) (2020) 44508–44522. [DOI] [PubMed] [Google Scholar]
- [187].Aguirre JI, Akhter M, Kimmel D, Pingel J, Xia X, Williams A, Jorgensen M, Edmonds K, Lee J, Reinhard M, Battles A, Kesavalu L, Wronski TJ, Enhanced alveolar bone loss in a model of non-invasive periodontitis in rice rats, Oral Dis 18(5) (2012) 459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Gotcher JE, Jee WS, The progress of the periodontal syndrome in the rice rat. I. Morphometric and autoradiographic studies, J Periodontal Res 16(3) (1981) 275–291. [DOI] [PubMed] [Google Scholar]
- [189].Leonard EP, Periodontitis. Animal model: periodontitis in the rice rat (Oryzomys palustris), Am. J. Pathol 96(2) (1979) 643–646. [PMC free article] [PubMed] [Google Scholar]
- [190].Gupta O, Shaw J, Periodontal disease in the rice rat. II. Methods for the evaluation of the extent of periodontal disease., Oral Surg. Oral Med Oral Pathol 9(7) (1956) 727–735. [DOI] [PubMed] [Google Scholar]
- [191].Gotcher JE, Jee WS, The progress of the periodontal syndrome in the rice rat. II. The effects of a diphosphonate on the periodontium, J Periodontal Res 16(4) (1981) 441–455. [DOI] [PubMed] [Google Scholar]
- [192].Plessas A, Nonsurgical periodontal treatment: review of the evidence, Oral Health Dent Manag 13(1) (2014) 71–80. [PubMed] [Google Scholar]
- [193].Slot DE, Dorfer CE, Van der Weijden GA, The efficacy of interdental brushes on plaque and parameters of periodontal inflammation: a systematic review, Int J Dent Hyg 6(4) (2008) 253–64. [DOI] [PubMed] [Google Scholar]
- [194].Slot DE, Valkenburg C, Van der Weijden GAF, Mechanical plaque removal of periodontal maintenance patients: A systematic review and network meta-analysis, J Clin Periodontol 47 Suppl 22 (2020) 107–124. [DOI] [PubMed] [Google Scholar]
- [195].Choi JY, Kim HJ, Lee YC, Cho BO, Seong HS, Cho M, Kim SG, Inhibition of bone healing by pamidronate in calvarial bony defects, Oral Surg Oral Med Oral Pathol Oral Radiol Endod 103(3) (2007) 321–8. [DOI] [PubMed] [Google Scholar]
- [196].Draenert GF, Huetzen DO, Kammerer PW, Palarie V, Nacu V, Wagner W, Dexrazoxane shows cytoprotective effects in zoledronic acid-treated human cells in vitro and in the rabbit tibia model in vivo, J Craniomaxillofac Surg 40(8) (2012) e369–74. [DOI] [PubMed] [Google Scholar]
- [197].Zang X, He L, Zhao L, He Y, Xiao E, Zhang Y, Adipose-derived stem cells prevent the onset of bisphosphonate-related osteonecrosis of the jaw through transforming growth factor beta-1-mediated gingival wound healing, Stem Cell Res Ther 10(1) (2019) 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Schwarz F, John G, Becker J, Grotz KA, Sader R, Mihatovic I, Influence of ridge preservation procedures on extraction socket healing under antiresorptive therapy: An experimental study in rabbits, Clin Implant Dent Relat Res 22(4) (2020) 477–485. [DOI] [PubMed] [Google Scholar]
- [199].Allen MR, Burr DB, Mandible matrix necrosis in beagle dogs after 3 years of daily oral bisphosphonate treatment, J Oral Maxillofac. Surg 66(5) (2008) 987–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Burr DB, Allen MR, Mandibular necrosis in beagle dogs treated with bisphosphonates, Orthod. Craniofac. Res 12(3) (2009) 221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Allen MR, Kubek DJ, Burr DB, Ruggiero SL, Chu TM, Compromised osseous healing of dental extraction sites in zoledronic acid-treated dogs, Osteoporos. Int 22(2) (2011) 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Allen MR, Chu TM, Ruggiero SL, Absence of exposed bone following dental extraction in beagle dogs treated with 9 months of high-dose zoledronic acid combined with dexamethasone, J Oral Maxillofac Surg 71(6) (2013) 1017–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Allam E, Allen M, Chu TM, Ghoneima A, Jack Windsor L, In vivo effects of zoledronic acid on oral mucosal epithelial cells, Oral Dis 17(3) (2011) 291–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Huja SS, Mason A, Fenell CE, Mo X, Hueni S, D’Atri AM, Fernandez SA, Effects of short-term zoledronic acid treatment on bone remodeling and healing at surgical sites in the maxilla and mandible of aged dogs, J Oral Maxillofac Surg 69(2) (2011) 418–27. [DOI] [PubMed] [Google Scholar]
- [205].Kaibuchi N, Iwata T, Onizuka S, Yano K, Tsumanuma Y, Yamato M, Okano T, Ando T, Allogeneic multipotent mesenchymal stromal cell sheet transplantation promotes healthy healing of wounds caused by zoledronate and dexamethasone in canine mandibular bones, Regen Ther 10 (2019) 77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Khojasteh A, Dehghan MM, Nazeman P, Immediate implant placement following 1-year treatment with oral versus intravenous bisphosphonates: a histomorphometric canine study on peri-implant bone, Clin Oral Investig 23(4) (2019) 1803–1809. [DOI] [PubMed] [Google Scholar]
- [207].Tsutsumi H, Katagiri K, Morimoto M, Nasu T, Tanigawa M, Mamba K, Diurnal variation and age-related changes of bone turnover markers in female Gottingen minipigs, Lab Anim 38(4) (2004) 439–46. [DOI] [PubMed] [Google Scholar]
- [208].Tsutsumi H, Katagiri K, Takeda S, Nasu T, Igarashi S, Tanigawa M, Mamba K, Standardized data and relationship between bone growth and bone metabolism in female Gottingen minipigs, Exp Anim 53(4) (2004) 331–7. [DOI] [PubMed] [Google Scholar]
- [209].Laiblin C, Jaeschke G, [Clinical chemistry examinations of bone and muscle metabolism under stress in the Gottingen miniature pig--an experimental study], Berl Munch Tierarztl Wochenschr 92(6) (1979) 124–8. [PubMed] [Google Scholar]
- [210].Hickey JS, O’Neal RB, Scheidt MJ, Strong SL, Turgeon D, Van Dyke TE, Microbiologic characterization of ligature-induced peri-implantitis in the microswine model, J Periodontol 62(9) (1991) 548–53. [DOI] [PubMed] [Google Scholar]
- [211].Mosekilde L, Weisbrode SE, Safron JA, Stills HF, Jankowsky ML, Ebert DC, Danielsen CC, Sogaard CH, Franks AF, Stevens ML, et al. , Calcium-restricted ovariectomized Sinclair S-1 minipigs: an animal model of osteopenia and trabecular plate perforation, Bone 14(3) (1993) 379–82. [DOI] [PubMed] [Google Scholar]
- [212].Holtmann H, Lommen J, Kubler NR, Sproll C, Rana M, Karschuck P, Depprich R, Pathogenesis of medication-related osteonecrosis of the jaw: a comparative study of in vivo and in vitro trials, J Int Med Res 46(10) (2018) 4277–4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Pautke C, Kreutzer K, Weitz J, Knodler M, Munzel D, Wexel G, Otto S, Hapfelmeier A, Sturzenbaum S, Tischer T, Bisphosphonate related osteonecrosis of the jaw: A minipig large animal model, Bone 51(3) (2012) 592–599. [DOI] [PubMed] [Google Scholar]
- [214].Li Y, Xu J, Mao L, Liu Y, Gao R, Zheng Z, Chen W, Le A, Shi S, Wang S, Allogeneic mesenchymal stem cell therapy for bisphosphonate-related jaw osteonecrosis in Swine, Stem Cells Dev 22(14) (2013) 2047–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Mitsimponas KT, Moest T, Iliopoulos C, Rueger T, Mueller C, Lutz R, Shakib K, Neukam FW, Schlegel KA, Search for a reliable model for bisphosphonate-related osteonecrosis of the jaw: establishment of a model in pigs and description of its histomorphometric characteristics, Br J Oral Maxillofac Surg 54(8) (2016) 883–888. [DOI] [PubMed] [Google Scholar]
- [216].Nowicki B, Nehrbass D, Arens D, Stadelmann VA, Zeiter S, Otto S, Kircher P, Stoddart MJ, Medication-related osteonecrosis of the jaw in a minipig model: Parameters for developing a macroscopic, radiological, and microscopic grading scheme, J Craniomaxillofac Surg 47(7) (2019) 1162–1169. [DOI] [PubMed] [Google Scholar]
- [217].Otto S, Pautke C, Arens D, Poxleitner P, Eberli U, Nehrbass D, Zeiter S, Stoddart MJ, A Drug Holiday Reduces the Frequency and Severity of Medication-Related Osteonecrosis of the Jaw in a Minipig Model, J Bone Miner Res 35(11) (2020) 2179–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Ristow O, Nehrbass D, Zeiter S, Arens D, Moratin J, Pautke C, Hoffmann J, Freudlsperger C, Otto S, Differences between auto-fluorescence and tetracycline-fluorescence in medication-related osteonecrosis of the jaw-a preclinical proof of concept study in the mini-pig, Clin Oral Investig 24(12) (2020) 4625–4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Martini L, Fini M, Giavaresi G, Giardino R, Sheep model in orthopedic research: a literature review, Comp Med 51(4) (2001) 292–9. [PubMed] [Google Scholar]
- [220].Pearce AI, Richards RG, Milz S, Schneider E, Pearce SG, Animal models for implant biomaterial research in bone: a review, Eur Cell Mater 13 (2007) 1–10. [DOI] [PubMed] [Google Scholar]
- [221].Rodgers JB, Monier-Faugere MC, Malluche H, Animal models for the study of bone loss after cessation of ovarian function, Bone 14(3) (1993) 369–377. [DOI] [PubMed] [Google Scholar]
- [222].Voss PJ, Stoddart M, Ziebart T, Zeiter S, Nelson K, Bittermann G, Schmelzeisen R, Poxleitner P, Zoledronate induces osteonecrosis of the jaw in sheep, J Craniomaxillofac Surg 43(7) (2015) 1133–8. [DOI] [PubMed] [Google Scholar]
- [223].Voss PJ, Stoddart MJ, Bernstein A, Schmelzeisen R, Nelson K, Stadelmann V, Ziebart T, Poxleitner PJ, Zoledronate induces bisphosphonate-related osteonecrosis of the jaw in osteopenic sheep, Clin. Oral Investig 20(1) (2016) 31–38. [DOI] [PubMed] [Google Scholar]
- [224].Davison MR, Lyardet L, Preliasco M, Yaful G, Torres P, Bonanno MS, Pellegrini GG, Zeni SN, Aminobisphosphonate-treated ewes as a model of osteonecrosis of the jaw and of dental implant failure, J Periodontol 91(5) (2020) 628–637. [DOI] [PubMed] [Google Scholar]
- [225].Russell RG, Bisphosphonates: from bench to bedside, Ann N Y Acad Sci 1068 (2006) 367–401. [DOI] [PubMed] [Google Scholar]
- [226].Hokugo A, Christensen R, Chung EM, Sung EC, Felsenfeld AL, Sayre JW, Garrett N, Adams JS, Nishimura I, Increased prevalence of bisphosphonate-related osteonecrosis of the jaw with vitamin D deficiency in rats, J. Bone Miner. Res 25(6) (2010) 1337–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Maahs MP, Azambuja AA, Campos MM, Salum FG, Cherubini K, Association between bisphosphonates and jaw osteonecrosis: a study in Wistar rats, Head Neck 33(2) (2011) 199–207. [DOI] [PubMed] [Google Scholar]
- [228].Ali-Erdem M, Burak-Cankaya A, Cemil-Isler S, Demircan S, Soluk M, Kasapoglu C, Korhan-Oral C, Extraction socket healing in rats treated with bisphosphonate: animal model for bisphosphonate related osteonecrosis of jaws in multiple myeloma patients, Med. Oral Patol. Oral Cir. Bucal 16(7) (2011) e879–e883. [DOI] [PubMed] [Google Scholar]
- [229].Basi DL, Hughes PJ, Thumbigere-Math V, Sabino M, Mariash A, Lunos SA, Jensen E, Gopalakrishnan R, Matrix metalloproteinase-9 expression in alveolar extraction sockets of Zoledronic acid-treated rats, J Oral Maxillofac Surg 69(11) (2011) 2698–707. [DOI] [PubMed] [Google Scholar]
- [230].Lopez-Jornet P, Camacho-Alonso F, Martinez-Canovas A, Molina-Minano F, Gomez-Garcia F, Vicente-Ortega V, Perioperative antibiotic regimen in rats treated with pamidronate plus dexamethasone and subjected to dental extraction: a study of the changes in the jaws, J. Oral Maxillofac. Surg 69(10) (2011) 2488–2493. [DOI] [PubMed] [Google Scholar]
- [231].Cankaya AB, Erdem MA, Isler SC, Demircan S, Soluk M, Kasapoglu C, Oral CK, Use of cone-beam computerized tomography for evaluation of bisphosphonate-associated osteonecrosis of the jaws in an experimental rat model, Int. J. Med. Sci 8(8) (2011) 667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Vasconcelos AC, Berti-Couto SA, Azambuja AA, Salum FG, Figueiredo MA, da Silva VD, Cherubini K, Comparison of effects of clodronate and zoledronic acid on the repair of maxilla surgical wounds - histomorphometric, receptor activator of nuclear factor-kB ligand, osteoprotegerin, von Willebrand factor, and caspase-3 evaluation, J Oral Pathol Med 41(9) (2012) 702–12. [DOI] [PubMed] [Google Scholar]
- [233].Berti-Couto SA, Vasconcelos AC, Iglesias JE, Figueiredo MA, Salum FG, Cherubini K, Diabetes mellitus and corticotherapy as risk factors for alendronate-related osteonecrosis of the jaws: a study in Wistar rats, Head Neck 36(1) (2014) 84–93. [DOI] [PubMed] [Google Scholar]
- [234].Guevarra CS, Borke JL, Stevens MR, Bisch FC, Zakhary I, Messer R, Gerlach RC, Elsalanty ME, Vascular alterations in the sprague-dawley rat mandible during intravenous bisphosphonate therapy, J Oral Implantol 41(2) (2015) e24–9. [DOI] [PubMed] [Google Scholar]
- [235].Dayisoylu EH, Üngör C, Tosun E, Ersöz S, Kadioglu Duman M, Taskesen F, Senel F, Does an alkaline environment prevent the development of bisphosphonate-related osteonecrosis of the jaw? An experimental study in rats, Oral Surg Oral Med Oral Pathol Oral Radiol 117(3) (2014) 329–34. [DOI] [PubMed] [Google Scholar]
- [236].Jabbour Z, El-Hakim M, Henderson JE, de Albuquerque RFJ, Bisphosphonates inhibit bone remodeling in the jaw bones of rats and delay healing following tooth extractions, Oral Oncol 50(5) (2014) 485–490. [DOI] [PubMed] [Google Scholar]
- [237].Agaçayak KS, Yuksel H, Atilgan S, Koparal M, Uçan MC, Ozgöz M, Yaman F, Atalay Y, Acikan I, Experimental investigation of relationship between trauma and bisphosphonate-related osteonecrosis, Niger J Clin Pract 17(5) (2014) 559–64. [DOI] [PubMed] [Google Scholar]
- [238].Janovszky Á, Szabó A, Varga R, Garab D, Boros M, Mester C, Beretka N, Zombori T, Wiesmann HP, Bernhardt R, Ocsovszki I, Balázs P, Piffkó J, Periosteal microcirculatory reactions in a zoledronate-induced osteonecrosis model of the jaw in rats, Clin Oral Investig 19(6) (2015) 1279–88. [DOI] [PubMed] [Google Scholar]
- [239].Howie RN, Borke JL, Kurago Z, Daoudi A, Cray J, Zakhary IE, Brown TL, Raley JN, Tran LT, Messer R, Medani F, Elsalanty ME, A Model for Osteonecrosis of the Jaw with Zoledronate Treatment following Repeated Major Trauma, PLoS One 10(7) (2015) e0132520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Kim JW, Cha IH, Kim SJ, Kim MR, Biomarkers for Bisphosphonate-Related Osteonecrosis of the Jaw, Clin Implant Dent Relat Res 18(2) (2016) 281–91. [DOI] [PubMed] [Google Scholar]
- [241].Silva PG, Ferreira Junior AE, Teófilo CR, Barbosa MC, Lima Júnior RC, Sousa FB, Mota MR, Ribeiro Rde A, Alves AP, Effect of different doses of zoledronic acid in establishing of bisphosphonate-related osteonecrosis, Arch Oral Biol 60(9) (2015) 1237–45. [DOI] [PubMed] [Google Scholar]
- [242].Pacheco VN, Langie R, Etges A, Ponzoni D, Puricelli E, Nitrogen-containing bisphosphonate therapy: assessment of the alveolar bone structure in rats - a blind randomized controlled trial, Int J Exp Pathol 96(4) (2015) 255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Borke JL, McAllister B, Harris T, Neiberg M, Guevarra-Toth C, Fulzele S, Stoianovici C, Guerra C, Correlation of changes in the mandible and retina/choroid vasculature of a rat model of BRONJ, J Craniomaxillofac Surg 43(7) (2015) 1144–50. [DOI] [PubMed] [Google Scholar]
- [244].Yang H, Pan H, Yu F, Chen K, Shang G, Xu Y, A novel model of bisphosphonate-related osteonecrosis of the jaw in rats, Int. J. Clin. Exp. Pathol 8(5) (2015) 5161–5167. [PMC free article] [PubMed] [Google Scholar]
- [245].Song Z, Dong W, Yin L, Liu J, Sun H, Qi M, [Effect of thalidomide on development of bisphosphonate-related osteonecrosis of the jaws in rats], Nan Fang Yi Ke Da Xue Xue Bao 35(8) (2015) 1084–9. [PubMed] [Google Scholar]
- [246].Kim JW, Tatad JCI, Landayan MEA, Kim SJ, Kim MR, Animal model for medication-related osteonecrosis of the jaw with precedent metabolic bone disease, Bone 81 (2015) 442–448. [DOI] [PubMed] [Google Scholar]
- [247].Takaoka K, Yamamura M, Nishioka T, Abe T, Tamaoka J, Segawa E, Shinohara M, Ueda H, Kishimoto H, Urade M, Establishment of an Animal Model of Bisphosphonate-Related Osteonecrosis of the Jaws in Spontaneously Diabetic Torii Rats, PLoS One 10(12) (2015) e0144355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Jabbour Z, do Nascimento C, El-Hakim M, Henderson JE, de Albuquerque Junior RF, Bacterial profile and bone healing in rats receiving cancer therapeutic doses of bisphosphonates and corticosteroids: a pilot study, Int J Oral Maxillofac Surg 45(9) (2016) 1162–9. [DOI] [PubMed] [Google Scholar]
- [249].Kim JW, Landayan ME, Lee JY, Tatad JC, Kim SJ, Kim MR, Cha IH, Role of microcracks in the pathogenesis of bisphosphonate-related osteonecrosis of the jaw, Clin Oral Investig 20(8) (2016) 2251–2258. [DOI] [PubMed] [Google Scholar]
- [250].Zandi M, Dehghan A, Malekzadeh H, Janbaz P, Ghadermazi K, Amini P, Introducing a protocol to create bisphosphonate-related osteonecrosis of the jaw in rat animal model, J. Craniomaxillofac. Surg 44(3) (2016) 271–278. [DOI] [PubMed] [Google Scholar]
- [251].Yanik S, Aras MH, Erkilic S, Bozdag Z, Demir T, Cetiner S, Histopathological features of bisphosphonates related osteonecrosis of the jaw in rats with and without vitamin d supplementation, Arch Oral Biol 65 (2016) 59–65. [DOI] [PubMed] [Google Scholar]
- [252].Kim Y, Lee HY, Yoon HJ, Kim BS, Utility of 18F-fluorodeoxy glucose and 18F-sodium fluoride positron emission tomography/computed tomography in the diagnosis of medication-related osteonecrosis of the jaw: A preclinical study in a rat model, J Craniomaxillofac Surg 44(4) (2016) 357–63. [DOI] [PubMed] [Google Scholar]
- [253].Curra C, Cardoso CL, Ferreira OJ, Curi MM, Matsumoto MA, Cavenago BC, Santos PL, Santiago JFJ, Medication-related osteonecrosis of the jaw. Introduction of a new modified experimental model, Acta Cir Bras 31(5) (2016) 308–13. [DOI] [PubMed] [Google Scholar]
- [254].Kolpakova ME, Zubarevа AA, Artamonova TD, Lisovskaya EK, Chefu SG, Yagmurov OD, Yaremenko AI, Vlasov TD, Experimental model of osteonecrosis of the jaw in rats treated with zoledronic acid, Br J Oral Maxillofac Surg 55(2) (2017) 156–159. [DOI] [PubMed] [Google Scholar]
- [255].Zandi M, Dehghan A, Janbaz P, Malekzadeh H, Amini P, The starting point for bisphosphonate-related osteonecrosis of the jaw: Alveolar bone or oral mucosa? A randomized, controlled experimental study, J Craniomaxillofac Surg 45(1) (2017) 157–161. [DOI] [PubMed] [Google Scholar]
- [256].Mada EY, Santos AC, Fonseca AC, Biguetti CC, Neves FT, Saraiva PP, Matsumoto MA, Effects of green tea and bisphosphonate association on dental socket repair of rats, Arch Oral Biol 75 (2017) 1–7. [DOI] [PubMed] [Google Scholar]
- [257].Gong X, Yu W, Zhao H, Su J, Sheng Q, Skeletal Site-specific Effects of Zoledronate on in vivo Bone Remodeling and in vitro BMSCs Osteogenic Activity, Scientific reports 7 (2017) 36129–36129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Silveira FM, Etges A, Correa MB, Vasconcelos ACU, Microscopic Evaluation of the Effect of Oral Microbiota on the Development of Bisphosphonate-Related Osteonecrosis of the Jaws in Rats, J Oral Maxillofac Res 7(4) (2016) e3–e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Vidal-Gutierrez X, Gomez-Clavel JF, Gaitan-Cepeda LA, Dental extraction following zoledronate, induces osteonecrosis in rat’s jaw, Med. Oral Patol. Oral Cir. Bucal 22(2) (2017) e177–e184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Matsumoto MA, de Abreu Furquim EM, Gonçalves A, Santiago-Júnior JF, Saraiva PP, Cardoso CL, Munerato MS, Okamoto R, Aged rats under zoledronic acid therapy and oral surgery, J Craniomaxillofac Surg 45(5) (2017) 781–787. [DOI] [PubMed] [Google Scholar]
- [261].Yalcin-Ulker GM, Cumbul A, Duygu-Capar G, Uslu Ü, Sencift K, Preventive Effect of Phosphodiesterase Inhibitor Pentoxifylline Against Medication-Related Osteonecrosis of the Jaw: An Animal Study, J Oral Maxillofac Surg 75(11) (2017) 2354–2368. [DOI] [PubMed] [Google Scholar]
- [262].Vilarinho JLP, Ferrare N, Moreira AMR, Moura HF, Acevedo AC, Chaves SB, Melo NS, Leite AF, Macedo SB, de Souza MP, Guimarães ATB, Figueiredo PT, Early bony changes associated with bisphosphonate-related osteonecrosis of the jaws in rats: A longitudinal in vivo study, Archives of Oral Biology 82 (2017) 79–85. [DOI] [PubMed] [Google Scholar]
- [263].Oliveira CC, Barros Silva PG, Ferreira AEC Jr., Gonçalves RP, Sousa FB, Mota MRL, Alves A, Effects of dexamethasone and nimesulide on bisphosphonate-related osteonecrosis of the jaw: An experimental study, Arch Oral Biol 83 (2017) 317–326. [DOI] [PubMed] [Google Scholar]
- [264].de Almeida AD, Leite FG, Chaud MV, Rebelo MA, Borges L, Viroel FJM, Hataka A, Grotto D, Safety and efficacy of hydroxyapatite scaffold in the prevention of jaw osteonecrosis in vivo, J Biomed Mater Res B Appl Biomater 106(5) (2018) 1799–1808. [DOI] [PubMed] [Google Scholar]
- [265].Liu J, Mattheos N, Su C, Deng C, Luo N, Wang Z, Tang H, The effects of icariin on wound healing of extraction sites with administration of zoledronic and dexamethasone: A rat model study, J Oral Pathol Med 47(2) (2018) 198–205. [DOI] [PubMed] [Google Scholar]
- [266].Koneski F, Popovic-Monevska D, Gjorgoski I, Krajoska J, Popovska M, Muratovska I, Velickovski B, Petrushevska G, Popovski V, In vivo effects of geranylgeraniol on the development of bisphosphonate-related osteonecrosis of the jaws, J Craniomaxillofac Surg 46(2) (2018) 230–236. [DOI] [PubMed] [Google Scholar]
- [267].Oh JS, Kim SG, Collagen sponge and rhBMP-2 improve socket healing in rats treated with zoledronic acid, Braz Oral Res 31 (2017) e99. [DOI] [PubMed] [Google Scholar]
- [268].Kün-Darbois JD, Libouban H, Mabilleau G, Pascaretti-Grizon F, Chappard D, Bone mineralization and vascularization in bisphosphonate-related osteonecrosis of the jaw: an experimental study in the rat, Clin Oral Investig 22(9) (2018) 2997–3006. [DOI] [PubMed] [Google Scholar]
- [269].Yang R, Tao Y, Wang C, Shuai Y, Jin L, Circulating microRNA Panel as a Novel Biomarker to Diagnose Bisphosphonate-Related Osteonecrosis of the Jaw, Int J Med Sci 15(14) (2018) 1694–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Toro LF, de Mello-Neto JM, Santos F, Ferreira LC, Statkievicz C, Cintra L, Issa JPM, Dornelles RCM, de Almeida JM, Nagata MJH, Garcia VG, Theodoro LH, Casatti CA, Ervolino E, Application of Autologous Platelet-Rich Plasma on Tooth Extraction Site Prevents Occurence of Medication-Related Osteonecrosis of the Jaws in Rats, Sci Rep 9(1) (2019) 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Imada M, Yagyuu T, Ueyama Y, Maeda M, Yamamoto K, Kurokawa S, Jo JI, Tabata Y, Tanaka Y, Kirita T, Prevention of tooth extraction-triggered bisphosphonate-related osteonecrosis of the jaws with basic fibroblast growth factor: An experimental study in rats, PLoS One 14(2) (2019) e0211928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [272].Kim JW, Alfafara AMD, Kim HY, Kim SY, Kim SJ, Effects of pH alteration on the pathogenesis of medication-related osteonecrosis of the jaw, Bone 122 (2019) 45–51. [DOI] [PubMed] [Google Scholar]
- [273].Mergoni G, Vescovi P, Passerini P, Maestri R, Corradi D, Sala R, Govoni P, Effects of zoledronic acid and dexamethasone on early phases of socket healing after tooth extraction in rats: A preliminary macroscopic and microscopic quantitative study, Med Oral Patol Oral Cir Bucal 24(3) (2019) e339–e345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Brierly GI, Ren J, Baldwin J, Saifzadeh S, Theodoropoulos C, Tsurkan MV, Lynham A, Hsu E, Nikolarakos D, Werner C, Woodruff MA, Hutmacher DW, Bray LJ, Investigation of Sustained BMP Delivery in the Prevention of Medication-Related Osteonecrosis of the Jaw (MRONJ) in a Rat Model, Macromol Biosci 19(11) (2019) e1900226. [DOI] [PubMed] [Google Scholar]
- [275].Gao SY, Lin RB, Huang SH, Liang YJ, Li X, Zhang SE, Ouyang DQ, Li K, Zheng GS, Liao GQ, PDGF-BB exhibited therapeutic effects on rat model of bisphosphonate-related osteonecrosis of the jaw by enhancing angiogenesis and osteogenesis, Bone 144 (2021) 115117. [DOI] [PubMed] [Google Scholar]
- [276].Wang JY, Huo L, Yu RQ, Rao NJ, Lu WW, Zheng LW, Skeletal Site-Specific Response of Jawbones and Long Bones to Surgical Interventions in Rats Treated with Zoledronic Acid, Biomed Res Int 2019 (2019) 5138175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [277].Adachi N, Ayukawa Y, Yasunami N, Furuhashi A, Imai M, Sanda K, Atsuta I, Koyano K, Preventive effect of fluvastatin on the development of medication-related osteonecrosis of the jaw, Scientific Reports 10(1) (2020) 5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [278].Paulo S, Laranjo M, Paula A, Abrantes AM, Martins J, Marto CM, Coelho A, Casalta-Lopes J, Carvalho L, Carrilho E, Serra A, Botelho MF, Marques Ferreira M, Calcium Phosphate Ceramics Can Prevent Bisphosphonate-Related Osteonecrosis of the Jaw, Materials (Basel) 13(8) (2020) 1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Göl EB, Özkan N, Bereket C, Önger ME, Extracorporeal Shock-Wave Therapy or Low-Level Laser Therapy: Which is More Effective in Bone Healing in Bisphosphonate Treatment?, J Craniofac Surg 31(7) (2020) 2043–2048. [DOI] [PubMed] [Google Scholar]
- [280].Doppelt O, Cohen G, Tamari T, Elimelech R, Sabbah N, Zigdon-Giladi H, Endothelial progenitors increase vascularization and improve fibroblasts function that prevent medication-related osteonecrosis of the jaw, Oral Dis 26(7) (2020) 1523–1531. [DOI] [PubMed] [Google Scholar]
- [281].Su Z, Li J, Bai X, Tay FR, Zhang M, Liang K, He L, Yuan H, Li J, Borate bioactive glass prevents zoledronate-induced osteonecrosis of the jaw by restoring osteogenesis and angiogenesis, Oral Dis 26(8) (2020) 1706–1717. [DOI] [PubMed] [Google Scholar]
- [282].Kosach GA, Petrosyan AL, Yaremenko AI, Zubareva AA, Kutukova SI, Yagmurov OD, Chefu SG, Molokova VA, Ignatova VD, Kosach SA, Vlasov TD, Disorders of microcirculation in the mechanism of bisphosphonate osteonecrosis: preliminary study in rats, British Journal of Oral and Maxillofacial Surgery 58(9) (2020) e38–e44. [DOI] [PubMed] [Google Scholar]
- [283].Yadegari A, Aminzadeh A, Seyyedkhamesi S, Aminian M, The effect of melatonin on prevention of bisphosphonate-related osteonecrosis of the jaw: an animal study in rats, J Korean Assoc Oral Maxillofac Surg 46(4) (2020) 266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [284].de Sousa Ferreira VC, Lopes AP, Alves NM, Sousa FRN, Pereira KMA, Gondim DV, Girão VCC, Leitão RFC, Goes P, Bisphosphonate-related osteonecrosis induced change in alveolar bone architecture in rats with participation of Wnt signaling, Clin Oral Investig 25(2) (2021) 673–682. [DOI] [PubMed] [Google Scholar]
- [285].Nakagawa T, Tsuka S, Aonuma F, Nodai T, Munemasa T, Tamura A, Mukaibo T, Kondo Y, Masaki C, Hosokawa R, Effects of metformin on the prevention of bisphosphonate-related osteonecrosis of the jaw-like lesions in rats, J Prosthodont Res (2020). [DOI] [PubMed] [Google Scholar]
- [286].Koth VS, Salum FG, de Figueiredo MAZ, Cherubini K, Morphological and immunohistochemical features of tooth extraction sites in rats treated with alendronate, raloxifene, or strontium ranelate, Clinical Oral Investigations 25(5) (2021) 2705–2716. [DOI] [PubMed] [Google Scholar]
- [287].Ferreira GZ, Zen Filho EV, Rubira-Bullen IRF, Garlet GP, Santos CF, Santos P, Delayed alveolar bone repair and osteonecrosis associated with Zoledronic Acid therapy in rats: macroscopic, microscopic and molecular analysis, J Appl Oral Sci 28 (2020) e20200204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [288].Kosach GA, Petrosyan AL, Yaremenko AI, Kutukova SI, Zubareva AA, Chefu SG, Vlasov TD, Influence of the cumulative effect of zoledronic acid on periodontal microcirculation in rats, Oral Maxillofac Surg (2021). [DOI] [PubMed] [Google Scholar]
- [289].Barba-Recreo P, JL D.C.P.d.V., Garcia-Arranz M, Yebenes L, Burgueno M, Zoledronic acid - related osteonecrosis of the jaws. Experimental model with dental extractions in rats, J. Craniomaxillofac. Surg 42(6) (2014) 744–750. [DOI] [PubMed] [Google Scholar]
- [290].de Barros Silva PG, de Oliveira CC, Brizeno L, Wong D, Lima Junior R, Goncalves RP, Sousa FB, Mota M, de Albuquerque Ribeiro R, Alves A, Immune cellular profile of bisphosphonate-related osteonecrosis of the jaw, Oral Dis 22(7) (2016) 649–57. [DOI] [PubMed] [Google Scholar]
- [291].Lee DW, Hyun H, Lee S, Kim SY, Kim GT, Um S, Hong SO, Chun HJ, Yang DH, The Effect of Polydeoxyribonucleotide Extracted from Salmon Sperm on the Restoration of Bisphosphonate-Related Osteonecrosis of the Jaw, Mar Drugs 17(1) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Monteiro CGJ, Vieira EM, Emerick C, Azevedo RS, Pascoal VAB, Homsi N, Lins RX, Ozonated oil effect for prevention of medication-related osteonecrosis of the jaw (MRONJ) in rats undergoing zoledronic acid therapy, Clin Oral Investig (2021). [DOI] [PubMed] [Google Scholar]
- [293].Razmara F, Bayat M, Shirian S, Shabankare G, Mohamadnia A, Mortazavi M, Alijani MR, Bahrami N, Application of a collagen scaffold saturated with platelet-rich plasma in prevention of bisphosphonate-related osteonecrosis of the jaw in the rat animal model, Heliyon 7(5) (2021) e06930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [294].Soundia A, Hadaya D, Chau Y, Gkouveris I, Bezouglaia O, Dry S, Pirih F, Aghaloo T, Tetradis S, Local RANKL delivery improves socket healing in bisphosphonate treated rats, Bone 148 (2021) 115945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [295].Weber M, Homm A, Muller S, Frey S, Amann K, Ries J, Geppert C, Preidl R, Most T, Kammerer PW, Kesting M, Wehrhan F, Zoledronate Causes a Systemic Shift of Macrophage Polarization towards M1 In Vivo, Int J Mol Sci 22(3) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [296].Zhang Q, Atsuta I, Liu S, Chen C, Shi S, Shi S, Le AD, IL-17-mediated M1/M2 macrophage alteration contributes to pathogenesis of bisphosphonate-related osteonecrosis of the jaws, Clin Cancer Res 19(12) (2013) 3176–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [297].Kuroshima S, Yamashita J, Chemotherapeutic and antiresorptive combination therapy suppressed lymphangiogenesis and induced osteonecrosis of the jaw-like lesions in mice, Bone 56(1) (2013) 101–109. [DOI] [PubMed] [Google Scholar]
- [298].Su J, Feng M, Han W, Zhao H, The effects of bisphosphonate on the remodeling of different irregular bones in mice, J Oral Pathol Med 44(8) (2015) 638–48. [DOI] [PubMed] [Google Scholar]
- [299].Park S, Kanayama K, Kaur K, Tseng HC, Banankhah S, Quje DT, Sayre JW, Jewett A, Nishimura I, Osteonecrosis of the Jaw Developed in Mice: Disease variants regulated by gammadelta T cells in oral mucosa barrier immunity, J. Biol. Chem 290(28) (2015) 17349–17366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [300].Zhang Q, Yu W, Lee S, Xu Q, Naji A, Le AD, Bisphosphonate Induces Osteonecrosis of the Jaw in Diabetic Mice via NLRP3/Caspase-1-Dependent IL-1beta Mechanism, J Bone Miner Res 30(12) (2015) 2300–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [301].Sun Y, Kaur K, Kanayama K, Morinaga K, Park S, Hokugo A, Kozlowska A, McBride WH, Li J, Jewett A, Nishimura I, Plasticity of Myeloid Cells during Oral Barrier Wound Healing and the Development of Bisphosphonate-related Osteonecrosis of the Jaw, J Biol Chem 291(39) (2016) 20602–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [302].Córdova LA, Guilbaud F, Amiaud J, Battaglia S, Charrier C, Lezot F, Piot B, Redini F, Heymann D, Severe compromise of preosteoblasts in a surgical mouse model of bisphosphonate-associated osteonecrosis of the jaw, J Craniomaxillofac Surg 44(9) (2016) 1387–94. [DOI] [PubMed] [Google Scholar]
- [303].Kim S, Williams DW, Lee C, Kim T, Arai A, Shi S, Li X, Shin KH, Kang MK, Park NH, Kim RH, IL-36 Induces Bisphosphonate-Related Osteonecrosis of the Jaw-Like Lesions in Mice by Inhibiting TGF-β-Mediated Collagen Expression, J Bone Miner Res 32(2) (2017) 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [304].Movila A, Mawardi H, Nishimura K, Kiyama T, Egashira K, Kim JY, Villa A, Sasaki H, Woo SB, Kawai T, Possible pathogenic engagement of soluble Semaphorin 4D produced by γδT cells in medication-related osteonecrosis of the jaw (MRONJ), Biochem Biophys Res Commun 480(1) (2016) 42–47. [DOI] [PubMed] [Google Scholar]
- [305].Zhu W, Xu R, Du J, Fu Y, Li S, Zhang P, Liu L, Jiang H, Zoledronic acid promotes TLR-4-mediated M1 macrophage polarization in bisphosphonate-related osteonecrosis of the jaw, FASEB J 33(4) (2019) 5208–5219. [DOI] [PubMed] [Google Scholar]
- [306].Biguetti CC, De Oliva AH, Healy K, Mahmoud RH, Custodio IDC, Constantino DH, Ervolino E, Duarte MAH, Fakhouri WD, Matsumoto MA, Medication-related osteonecrosis of the jaws after tooth extraction in senescent female mice treated with zoledronic acid: Microtomographic, histological and immunohistochemical characterization, PLoS One 14(6) (2019) e0214173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [307].Rao NJ, Yu RQ, Wang JY, Helm A, Zheng LW, Effect of Periapical Diseases in Development of MRONJ in Immunocompromised Mouse Model, Biomed Res Int 2019 (2019) 1271492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [308].Taniguchi N, Osaki M, Onuma K, Ishikawa M, Ryoke K, Kodani I, Okada F, Bisphosphonate-induced reactive oxygen species inhibit proliferation and migration of oral fibroblasts: A pathogenesis of bisphosphonate-related osteonecrosis of the jaw, J Periodontol 91(7) (2020) 947–955. [DOI] [PubMed] [Google Scholar]
- [309].Hayano H, Kuroshima S, Sasaki M, Tamaki S, Inoue M, Ishisaki A, Sawase T, Distinct immunopathology in the early stages between different antiresorptives-related osteonecrosis of the jaw-like lesions in mice, Bone 135 (2020) 115308. [DOI] [PubMed] [Google Scholar]
- [310].Yang X, Xu X, Chen J, Wang Q, Wang G, Ai X, Wang X, Pan J, Zoledronic acid regulates the synthesis and secretion of IL-1beta through Histone methylation in macrophages, Cell Death Discov 6 (2020) 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [311].Tamaki S, Kuroshima S, Hayano H, Nakajima K, Kakehashi H, Ishisaki A, Sawase T, Dynamic polarization shifting from M1 to M2 macrophages in reduced osteonecrosis of the jaw-like lesions by cessation of anti-RANKL antibody in mice, Bone 141 (2020) 115560. [DOI] [PubMed] [Google Scholar]
- [312].Yamashita J, Sawa N, Sawa Y, Miyazono S, Effect of bisphosphonates on healing of tooth extraction wounds in infectious osteomyelitis of the jaw, Bone 143 (2021) 115611. [DOI] [PubMed] [Google Scholar]
- [313].Soma T, Iwasaki R, Sato Y, Kobayashi T, Nakamura S, Kaneko Y, Ito E, Okada H, Watanabe H, Miyamoto K, Matsumoto M, Nakamura M, Asoda S, Kawana H, Nakagawa T, Miyamoto T, Tooth extraction in mice administered zoledronate increases inflammatory cytokine levels and promotes osteonecrosis of the jaw, J Bone Miner Metab 39(3) (2021) 372–384. [DOI] [PubMed] [Google Scholar]
- [314].Tanoue R, Koi K, Yamashita J, Effect of Alendronate on Bone Formation during Tooth Extraction Wound Healing, J Dent Res 94(9) (2015) 1251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [315].Kuroshima S, Nakajima K, Sasaki M, Hayano H, Inoue M, Kozutsumi R, Sawase T, Gene expression analysis of fresh extraction wounds prior to onset of bisphosphonate-related osteonecrosis of the jaw-like lesions in mice: A preliminary animal study, J Prosthodont Res (2021). [DOI] [PubMed] [Google Scholar]
- [316].Toro LF, de Mello-Neto JM, Santos F.F.V.d., Ferreira LC, Statkievicz C, Cintra LTÂ, Issa JPM, Dornelles RCM, de Almeida JM, Nagata MJH, Garcia VG, Theodoro LH, Casatti CA, Ervolino E, Application of Autologous Platelet-Rich Plasma on Tooth Extraction Site Prevents Occurence of Medication-Related Osteonecrosis of the Jaws in Rats, Scientific Reports 9(1) (2019) 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.