Abstract
Ibrutinib has superior progression-free survival compared with bendamustine plus rituximab (BR) in older CLL patients, however differences in treatment duration, 6 monthly BR cycles versus continuous ibrutinib, complicate adverse event (AE) comparisons. We introduce the AE burden score (AEsc) to compare AEs, calculated for each patient by summing over products of reporting period length and grade for each all-cause grade 1–4 AE and dividing by the length of time over which AEs are assessed. 176 patients received BR and 361 ibrutinib alone or with 6 cycles of rituximab. At 38 months median follow-up, 64% remained on ibrutinib. Median AEsc was higher with BR versus ibrutinib in the first 6 cycles (7.2 versus 4.9, p<0.0001). Within ibrutinib arms, median AEsc decreased significantly to 3.7 after 6 cycles (p<0.0001). 10% and 14% of BR and ibrutinib patients discontinued treatment for AEs. In ibrutinib arms, cumulative incidence of grade 3 or higher atrial fibrillation, hypertension, and infection (AEs of clinical interest) at 12 months was 4.5%, 17.5%, and 12.8%, respectively, and increased more slowly thereafter to 7.7%, 25.4%, and 20.5% at 36 months. Analytical tools including the AEsc and cumulative incidence of AEs can help to better characterize AE burden over time.
Introduction
Chronic lymphocytic leukemia (CLL) is a disease of older patients, with a median age of diagnosis of 72 years.1 Older patients often present with co-morbidities and have increased risk of adverse events (AEs) with therapy, leading to delays or discontinuation of treatment, and worse clinical outcomes than their younger counterparts.2,3 A common side effect with chemotherapy and chemo-immunotherapy, such as chlorambucil or bendamustine plus rituximab (BR) for older patients, is myelosuppression,4,5 which in addition to CLL-associated immune dysfunction places patients at high risk for infection.6
Ibrutinib is an oral Bruton’s tyrosine kinase (BTK) inhibitor that has altered the natural history of CLL in both younger and older patients. Specific to previously untreated patients with CLL aged ≥ 65 years and without high-risk del(17p), the RESONATE-2 study showed that ibrutinib significantly extended progression-free survival (PFS; p<0.001) and overall survival (OS; p=0.001) compared with chlorambucil, and significantly improved quality of life (p=0.0013).4,7 In the Alliance for Clinical Trials in Oncology (Alliance) A041202 study of newly diagnosed patients with CLL aged ≥ 65 years with or without high-risk del(17p), ibrutinib (with or without rituximab) significantly extended PFS compared with BR (p<0.001).5
Unlike chemotherapy and chemo-immunotherapy regimens that are administered for a fixed duration (e.g. six 28-day cycles), ibrutinib is standardly administered continuously until disease progression. Although ibrutinib is well-tolerated by most previously untreated older patients, concerns for cardiotoxicity including atrial fibrillation and hypertension and risk of serious infections has emerged. Among 135 patients on the RESONATE-2 trial and with a median time on ibrutinib of 28.5 months, grade 3 or higher all-cause atrial fibrillation was reported in 4%, hypertension in 5%, and infections in 23%. In that study, cardiotoxicity improved or resolved within days or a few weeks, rarely leading to discontinuation of therapy, and infections occurred most frequently in the first year of ibrutinib.7 On A041202 and with a median time on ibrutinib of 32 months, grade 3 or higher all-cause atrial fibrillation and hypertension rates were 7% and 32% in 361 patients versus 3% and 14% in 176 patients receiving a fixed duration of BR.5 The rates of grade 3 or higher all-cause infections were not significantly different in those receiving ibrutinib regimens versus BR (20% versus 15%).5
On A041202, differences in treatment duration for BR versus ibrutinib regimens complicated AE comparisons. Further, the common practice of reporting the highest grade of an AE and focusing on grade ≥3 AEs could mask the global AE burden of a particular therapy in which persistent but low-grade AEs are not considered, thereby potentially confounding AE comparisons. In this manuscript, additional safety analyses for patients treated on A041202 are performed, specifically by: 1) defining a global AE score and comparing the AE score across treatment groups and time; 2) providing a comprehensive assessment of the cumulative incidence of atrial fibrillation, hypertension, and infections; and 3) describing outcomes following ibrutinib discontinuation due to AEs.
Methods
A041202 Study Design, Study Population and Treatments
Alliance A041202 was a randomized, open-label, phase 3 trial in adults aged 65 years or older with previously untreated CLL for whom treatment was indicated. Patients were randomized (1:1:1) to receive six 28-day cycles of BR (arm A) followed by observation until disease progression, ibrutinib 420 mg daily until disease progression (I, arm B), or ibrutinib 420 mg daily until disease progression with rituximab for the first 6 cycles (IR, arm C). Patients randomized to arm A could cross over to receive ibrutinib within 1 year after documented disease progression. The primary endpoint was progression-free survival (PFS), defined and reported previously.5
Schedule and Assessment of Adverse Events (AEs)
AEs were routinely assessed and graded using the Common Terminology Criteria for Adverse Events version 4.0.8 Mild, moderate, severe, or life-threatening AEs were assigned grades 1 to 4, respectively, with grade 5 indicating death. Each AE had an attribution assigned ranging from definitely unrelated to definitely related to treatment. AEs were assessed every 28 days during cycles 1–6 of treatment and then every 3 cycles (84 days), either in observation or on treatment, until disease progression. The highest grade of each AE type occurring during an assessment period was captured. All-cause AEs (regardless of attribution) are used in these analyses.
AE Burden Score
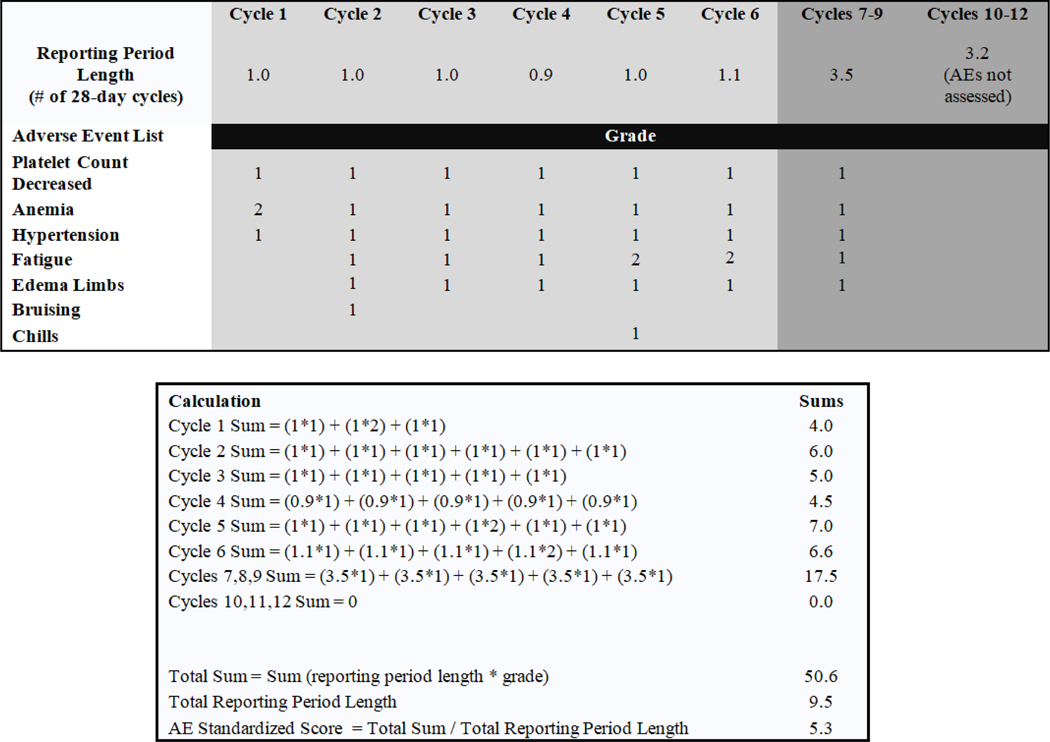
We use an exploratory approach to compare AE burden between patients treated with BR of fixed duration and patients treated with continuous ibrutinib regimens on A041202. A simple global AE score was calculated for each patient by summing over the products of reporting period length and grade recorded for each all-cause grade 1–4 AE and dividing by the length of time over which AEs were assessed. For a given patient, AEsc = , where is the length of AE assessment period and is the grade of AE during assessment period . Grade 5 AEs are not included in the AEsc calculation.
Statistical Analyses
Each patient had AEsc calculated using data from the start of treatment through all AE assessment periods, excluding AEs occurring after cross over to ibrutinib (arm A) and late AEs occurring after progression. A sample calculation is shown in Figure 1. To illustrate the comparison of AEsc across time, we calculated scores using AEs assessed during the first 6 cycles and after 6 cycles of treatment. For patients receiving ibrutinib through cycle 6, the AE assessment period for cycle 6 included cycles 7 and 8 per protocol, and we considered that assessment period to be part of the first 6 cycles of treatment. AEsc distributions were described with summary statistics and illustrated graphically with box plots and line plots. AEsc was compared between treatment groups and within treatment groups using the nonparametric Wilcoxon rank sum and signed rank tests, respectively.
Figure 1: Sample calculation of the AE score for a typical patient receiving bendamustine plus rituximab (BR).
The AE score (AESC) is calculated for an individual patient by summing over the products of reporting period length and grade recorded for each AE and dividing by the length of time over which AEs were assessed. If AEs were assessed during a particular reporting period but the grade for an AE was blank, the grade of the AE was equal to 0. If AEs were not assessed during a particular reporting period, AEs from that reporting period were not used in the calculation of the AESC. For the purposes of this study, the AE reporting length is the difference between reporting period start and end dates divided by 28, to represent the number of 28-day cycles. In general, reporting period lengths are approximately 1 for the first 5 AE assessments, 1 or 3 for AE assessment 6 depending on whether the patient received BR or ibrutinib regimens, and 3 for assessments 7 and higher.
For AEs of clinical interest (atrial fibrillation, hypertension, and infections), time to AE occurrence was measured from the first treatment date until the AE assessment start date during which an AE was first documented, censoring patients without an AE at the last AE assessment end date. Death without an AE was a competing risk, and cumulative incidence functions were estimated.9
A landmark Kaplan-Meier analysis from the date off-treatment due to AEs for subsequent PFS was performed and comparisons between groups used the log-rank test.
Data utilized for analyses coincided with the initial primary manuscript for A041202, with data cut-off date of October 4, 2018. Data quality was ensured by review of data by the Alliance Statistics and Data Management Center (SDMC) and by the study chairperson following Alliance policies. All analyses were performed by the Alliance SDMC using SAS® version 9.4. All tests were two-sided and not adjusted for multiple comparisons. Each participant signed an IRB-approved, protocol-specific informed consent document in accordance with federal and institutional guidelines.
Results
Overall, 547 patients were randomized to one of three treatment arms (BR, n=183; I, n=182; IR, n=182). Baseline characteristics and clinical outcome were previously reported.5 537 patients began treatment (BR, n=176; I, n=180; IR, n=181) and were included in AE analyses. 68% of patients receiving BR completed 6 cycles of therapy. At a median follow-up of 38 months, 64% of patients remained on I/IR. A summary of all-cause grade 3 or higher AEs, and study-chair determined cause of death for all deaths were previously reported.5 Since AE rates did not differ between the ibrutinib-containing arms (IBR), all AE analyses herein group these patients (n=361).
Adherence to Adverse Event Assessment Schedule
AE assessments were scheduled for each of the first 6 cycles of treatment, and every 3 cycles thereafter until progression. Across 537 patients, 7541 of 7880 expected AE assessments were performed (95.7%). The proportion of patients with completed AE assessments was higher in the IBR group than the BR group (99.0% versus 87.9%, p<0.0001). While on therapy during the first 6 cycles, adherence was high regardless of group (IBR 100% versus BR 99.6%). Adherence remained high for patients continuing IBR after cycle 6 (98.6%) but declined for those in observation after 6 cycles of BR (81.1%).
AE Scores by Treatment Group
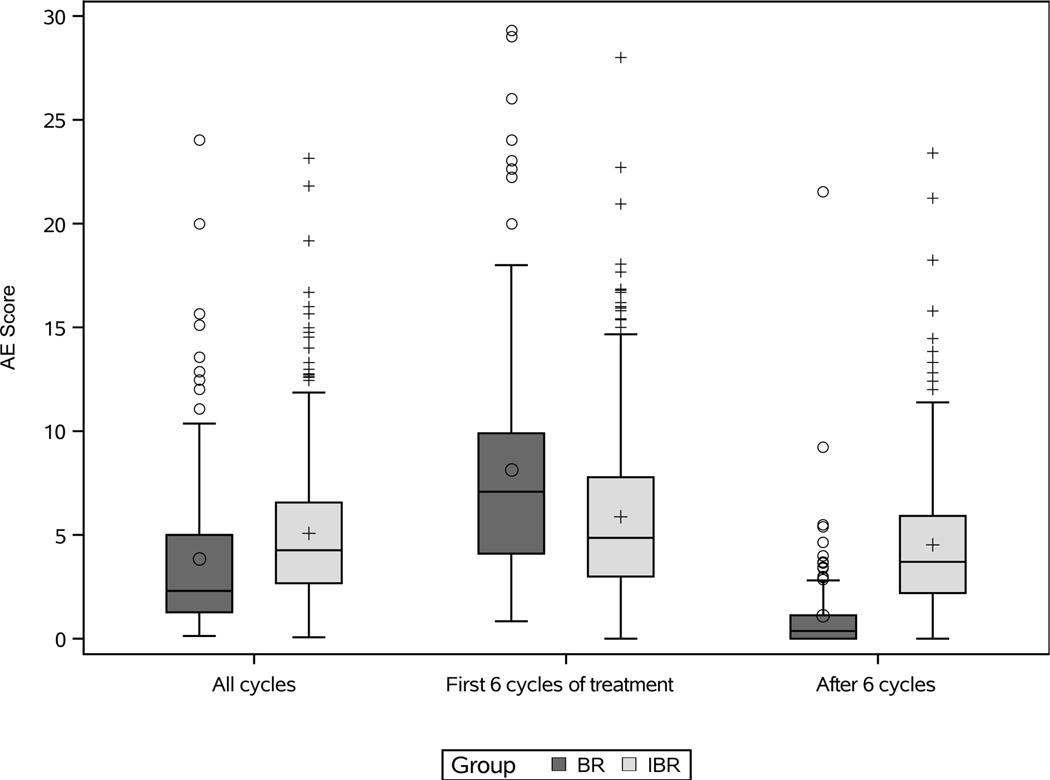
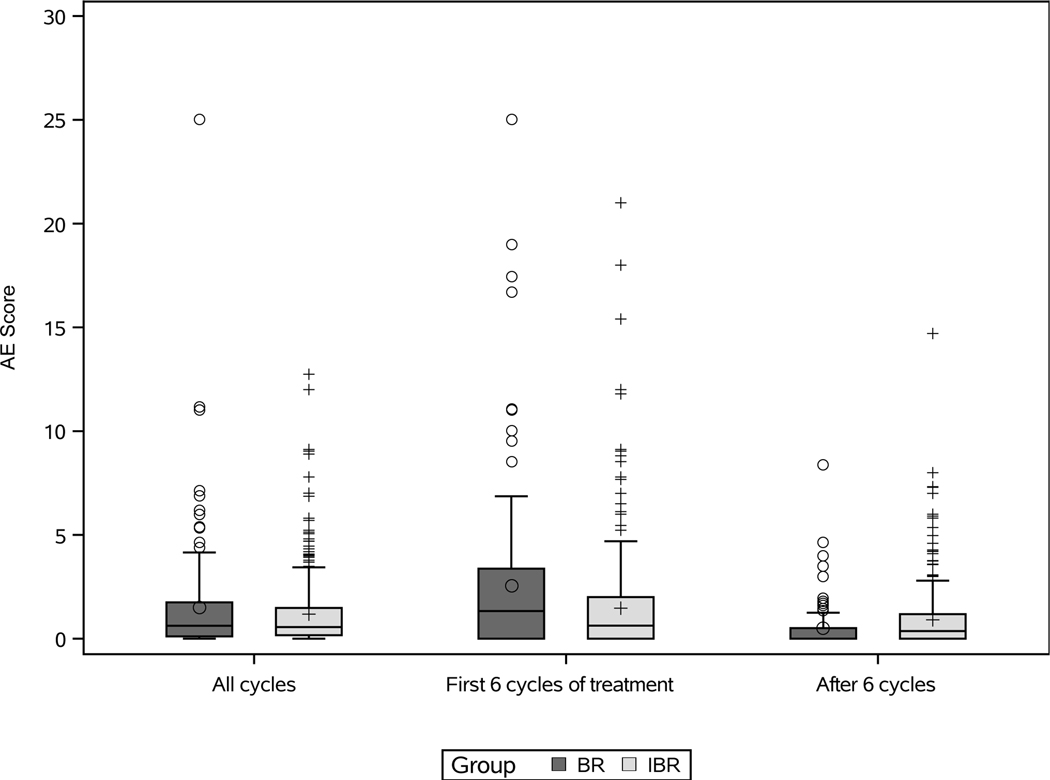
Global AE scores (AEsc) are shown by treatment group, across all AE assessments, while on treatment during the first 6 cycles, and after 6 cycles of treatment (Figure 2). Across all AE assessments, the median AEsc was 2.3 (IQR 1.3–5.1) in the BR group and 4.3 (IQR 2.7–6.6) in the IBR group (p<0.0001). In the first 6 cycles of treatment, the median AEsc was significantly higher in the BR group (7.2; IQR 4.2–10.2) compared with the IBR group (4.9; IQR 3.2–8.7) (p<0.0001). Among 325 patients who completed 6 cycles of IBR, the median AEsc decreased significantly from 4.7 (IQR 2.9–7.0) in the first 6 cycles to 3.7 (IQR 2.2–5.9) after 6 cycles (p<0.0001). In observation after BR, median AEsc was 0.4 (IQR 0–1.1) and no AEs were reported for 72% of AE assessments. Line plots with median AEsc every 3 cycles up to 24 cycles show the AE experience for BR and IBR groups in more detail (Supplementary Figure). When limiting AEsc to grade 3/4 AEs across all AE assessments, there was no significant difference in AEsc between treatment groups (p=0.46) (Figure 3), but AEsc remained significantly higher in the first 6 cycles of treatment with BR than IBR; among patients treated with IBR AEsc tended to decrease after 6 cycles (Figure 3).
Figure 2: AE score by treatment group, across all assessments/cycles, the first 6 cycles of treatment, and after 6 cycles of treatment.
Box-and-whisker plots show the distribution of AE scores for patients treated with bendamustine plus rituximab (BR) or ibrutinib regimens (IBR). Across all cycles and in the first 6 cycles of treatment, 176 patients in the BR group had AE scores and 361 patients in the IBR group had AE scores. Among those who completed 6 cycles of therapy, after 6 cycles 117 patients in the BR group had AE scores and 325 patients in the IBR group had AE scores. AE scores for six outliers are not shown in the plots (AE score of 59 with BR across all cycles and during the first 6 cycles of treatment, AE score of 34 with IBR during the first 6 cycles of treatment, AE score of 33 with BR across all cycles and during the first 6 cycles of treatment, and AE score of 29.3 with BR during the first 6 cycles of treatment) to better visualize the AE score distributions.
Figure 3: Limited to grade 3/4 AEs, AE score by treatment group, across all assessments/cycles, the first 6 cycles, and after 6 cycles.
Box-and-whisker plots show the distribution of AE scores for patients treated with bendamustine plus rituximab (BR) and ibrutinib regimens (IBR). Across all cycles and in the first 6 cycles of treatment, 176 patients in the BR group had AE scores and 361 patients in the IBR group had AE scores. Among those who completed 6 cycles of therapy, after 6 cycles 117 patients in the BR group had AE scores and 325 patients in the IBR group had AE scores. AE scores for two outliers are not shown in the plots (AE score of 41 with BR across all cycles and during the first 6 cycles of treatment) to better visualize the AE score distributions.
Atrial Fibrillation and Hypertension
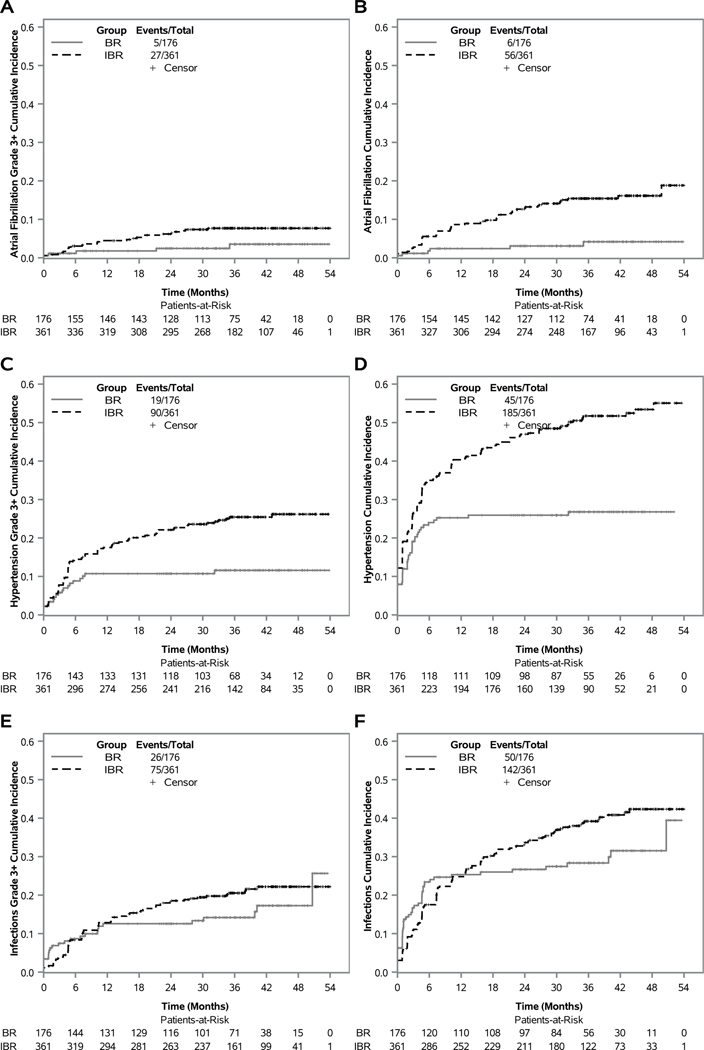
We previously reported that rates of grade 3 or higher atrial fibrillation were higher in the IBR group than in the BR group.5 Here we provide the cumulative incidence of grade 3 or higher atrial fibrillation and cumulative incidence of all grades of atrial fibrillation over time (Figures 4A-B). Cumulative incidence of grade 3 or higher atrial fibrillation in patients receiving IBR was 3.1% at 6 months and 4.5% at 12 months; cumulative incidence increased at a slower rate thereafter, with a cumulative incidence of 6.2% at 24 months and 7.7% at 36 months. Cumulative incidence of grade 3 or higher atrial fibrillation in patients receiving BR was 1.1%, 1.8%, 2.4%, and 3.5% at 6, 12, 24, and 36 months, respectively. Cumulative incidence of all grades of atrial fibrillation was higher: 5.6%, 8.6%, 12.6%, and 15.4%; and 1.8%, 2.4%, 3.1% and 4.2% respectively at 6, 12, 24, and 36 months for IBR and BR patients.
Figure 4: Cumulative incidence of atrial fibrillation, hypertension, and infection, by treatment group.
The cumulative incidence of grade 3 or higher atrial fibrillation occurring in 176 patients treated with bendamustine plus rituximab (BR) and 361 patients treated with ibrutinib regimens (IBR) is depicted (a), all grades of atrial fibrillation (b), grade 3 or higher hypertension (c), all grades of hypertension (d), grade 3 or higher infection (e), all grades of infection (f).
Rates of grade 3 or higher hypertension were previously reported and was higher in the IBR group than in the BR group.5 Hypertension was a solicited AE at baseline, and thus only new or worsening hypertension events after start of treatment were considered for this analysis, unlike in the original reporting. Among patients receiving IBR, cumulative incidence of grade 3 or higher hypertension was 14.5%, 17.5%, 22.1%, and 25.4% at 6, 12, 24, and 36 months (Figure 4C), respectively. Among patients receiving BR, cumulative incidence of grade 3 or higher hypertension was 8.8% at 6 months, but increased to only 10.7% at 12 and 24 months, and 11.6% at 36 months. Cumulative incidence of all grades of hypertension for patients receiving IBR was 35.0%, 40.3%, 47.0%, and 51.7%; and 24.0%, 25.3%, 25.9% and 26.8% in patients receiving BR at 6, 12, 24, and 36 months, respectively (Figure 4D).
Infection
We previously reported that the rate of grade 3 or higher infection was not different between BR and IBR.5 Based on cumulative incidence curves however, grade 3 or higher infection was initially lower in the IBR group (Figure 4E). In the IBR group, the cumulative incidence of grade 3 or higher infection was 8.3%, 12.8%, 18.5%, and 20.5% at 6, 12, 24, and 36 months, respectively. In the BR group, the cumulative incidence of grade 3 or higher infection was 8.7% at 6 months, 12.6% at 12 and 24 months, and 14.2% at 36 months. Across both groups, 101 patients (26 BR, 75 IBR) reported 139 grade 3 or higher individual infections, most commonly in the respiratory and skin systems (38% respiratory, 24% skin, 12% GU, 26% other). The causative agent was unknown in 47%, 22% were definitively not fungal, and 30% unlikely fungal. One patient receiving IBR had grade 3 lung infection with rare candida albicans on culture (likely not true infection). Two additional patients receiving IBR had mild fungal infections (one oral thrush, one tinea) and one reported aspergillus pneumonia identified when querying a sudden death at home. Seven patients had grade 5 infections (3 BR, 4 IBR); none were confirmed to be fungal. Cumulative incidence curves for all grades of infection are provided (Figure 4F). In patients receiving IBR, cumulative incidence of all grades of infection was 17.5%, 24.8%, 33.7%, and 39.2%; and 23.4%, 25.3%, 26.7% and 28.4% in patients receiving BR at 6, 12, 24, and 36 months, respectively.
Discontinuation of Therapy for Adverse Events
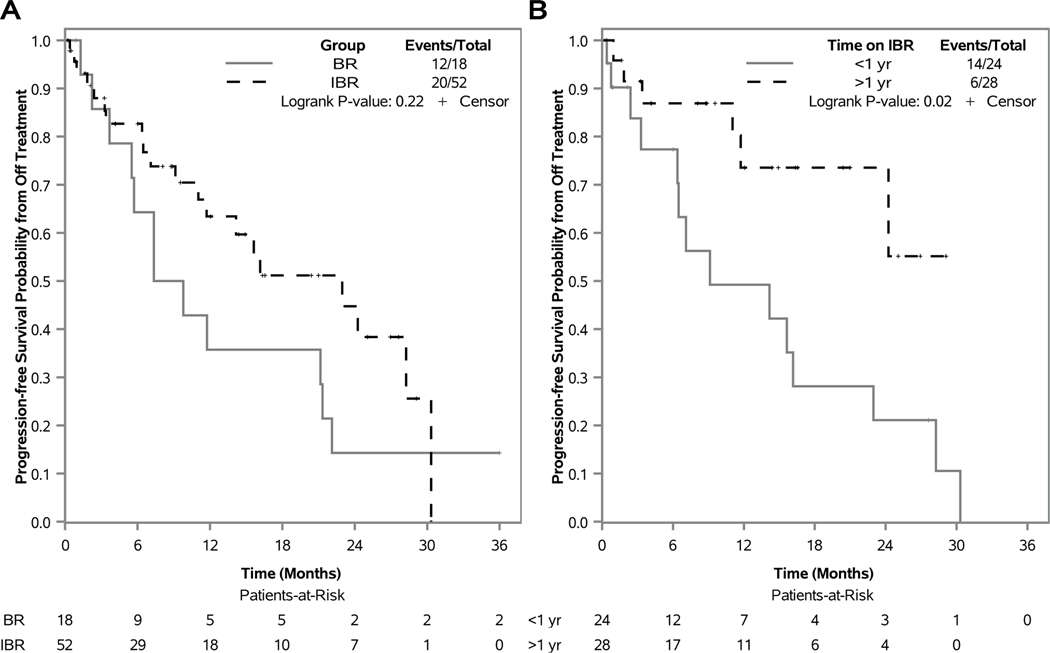
With 6 fixed cycles of BR and a median time on IBR of 32 months (range: 0–52), 18 patients (10%) discontinued BR and 52 patients (14%) discontinued IBR for AE, 35 in the first 6 cycles of IBR and 17 after 6 cycles of IBR. A variety of causes led to discontinuation of BR. Common grade 3 or higher AEs reported in at least 20% of patients in the last cycle of BR treatment corresponded to general disorders and administration site conditions (23%), injury, poisoning and procedural complications (22%), investigations (33%), metabolism and nutritional disorders (28%), and vascular disorders (39%). Common grade 3 or higher AEs reported in at least 20% of patients in the last reporting period of IBR treatment corresponded to cardiac disorders (25%). Following discontinuation of therapy for AE, the median PFS landmarked at the date off treatment was 8.6 (95% CI, 3.7–21.3) and 23.0 months (95% CI 11.0-not reached) for those who received BR and IBR, respectively (Figure 5A). Within IBR, patients who discontinued therapy for AE with less than a year on therapy had a median PFS of 9.1 (95% CI, 3.3–23.0) months, and median PFS was not reached for those who had been on therapy for more than a year (Figure 5B).
Figure 5: Progression-free survival from date of off-treatment for AE.
Kaplan-Meier curves are shown for 18 patients who discontinued treatment with bendamustine plus rituximab (BR) for AE and 52 patients who discontinued treatment with ibrutinib regimens (IBR) for AE (a), and Kaplan-Meier curves for 24 patients who received less 1 year (yr) of IBR and discontinued treatment for AE versus 28 patients who received more than 1 yr of IBR and discontinued treatment for AE (b). Differences in curves were tested using two-sided log-rank tests.
Atrial Fibrillation, Hypertension, and Infection in Patients who Crossed Over to Ibrutinib
Thirty patients progressed in the BR group and crossed over to ibrutinib. With a median follow-up of 20 months (range: 0–49) from the date of cross-over, 22 (73%) patients remain on ibrutinib and 8 patients discontinued treatment. Two patients discontinued treatment for AE (grade 2/3 anemia/platelet count decreased and grade 1 drug eruption of the skin). Although follow-up was fairly short, 5 additional patients reported atrial fibrillation following cross-over (two grade 2 and three grade 3). All 5 with atrial fibrillation had hypertension documented, two prior to cross-over and three after cross-over. Following cross-over, 7 additional patients reported hypertension (four grade 2 and three grade 3) and 6 reported worsening hypertension from time of cross-over (two from grade 1 to 2, two from grade 1 to 3, and two from grade 2 to 3). 7 patients reported grade 3/4 infections following cross-over (grade 3: lung, bronchial, upper respiratory, urinary tract, tooth; grade 4: pneumonia, sepsis).
Discussion
In this analysis of older patients with previously untreated CLL, patients receiving BR had a significantly higher AE burden in the first 6 cycles of treatment compared to those receiving IBR. After 6 cycles of treatment, the AE burden within the IBR group decreased significantly. These data were supported when evaluating AEs of clinical interest, in which the cumulative incidence of grade 3 or higher atrial fibrillation, hypertension, and infections increased more slowly after the first 6–12 months of IBR. Importantly, there was no significant difference in the treatment discontinuation rate due to AEs between BR and IBR groups.
The AE score characterizing an AE burden experience is easily calculated by summing over the products of reporting period length and grade recorded for each AE and dividing by the length of time over which AEs are assessed. The AE score is flexible and can incorporate lower grade AEs, some which might persist over long time periods. It can also be used to summarize the AE burden related to certain body systems, types of AEs, or to compare the AE burden of a treatment regimen between subgroups. Standardizing the AE score by length of time over which AEs are assessed provides a simple mechanism to compare and contrast the AE burden between time periods. We used the AE score to better depict the AE burden during a period of planned active therapy for all patients. This is more desirable than the secondary AE analysis in the original publication of A041202 that included standard AE summary tables for patients during active treatment, which was confounded by the different lengths of treatment for each group.5 We also used the AE score within the IBR group to depict the decrease in AE burden between the first 6 cycles and after 6 cycles of therapy. Considering the element of time in AE reporting is advocated to help provide a more complete assessment of AEs.10
A number of AE scores have been proposed in an effort to quantify the global toxicity burden of patients treated on clinical trials. Carbini, Suárez-Fariñas, and Maki developed a weighted toxicity score (WTS),11 that correlated with dose reduction rates. Trotti and colleagues proposed a score combining AEs (TAME), but only considered high-grade AEs.12 Lee and colleagues developed a toxicity burden score (TBS) using a weighted sum to define dose-limiting toxicity in phase 1 trials.13 The novel AE score proposed by us and others is different in that it considers all grades of individual AEs and all occurrences of individual AEs, not just the highest grade of an individual AE that represents a single occurrence.14 This is relevant since a recent study in colon cancer showed that a cumulative toxicity score for all grades predicted quality of life measures better than a score considering only higher grades.15
For AE scores and AE tabulations to be useful, AE reporting must be reliable and standardized across institutions and studies. The definitions and grading of AEs in cancer clinical trials has been standardized through the use of the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE).8 However, the current 5-tier attribution system for assigning causality of study treatment to AEs (definitely related, probably related, possibly related, unlikely related, and unrelated) is subjective with great variability in assignments from person-to-person, site-to-site.16,17 For this reason, we included all-cause AEs in the calculation of AE scores and in the analysis of individual AEs. As a result of the complexity of AE data and the heterogeneous collection and reporting of AE data, a toxicity-attribution workshop convened and published guidelines, including simplification of the attribution system, reporting of all-cause AEs in addition to or in place of treatment-related AEs.18
Data from A041202 illustrated how every piece of AE data collected could be summarized and used to make comparisons in AE burden between treatment groups and across time. However, we recognize limitations in comparisons due to differences in AE collection between groups. For BR and IBR groups, decreased neutrophil or platelet count, rash maculo-papular, fatigue, cough, diarrhea, edema limbs, dizziness, dyspepsia, anemia, hypertension, and bruising were solicited, and unsolicited AEs of any grade or attribution were captured during the first 6 cycles of treatment. After 6 cycles of treatment, solicited and unsolicited AEs were captured for patients continuing ibrutinib treatment, but only unsolicited, treatment-related grade 1–2 and all-cause grade 3–4 AEs were captured for patients in observation after BR. The most meaningful comparison of AEsc is therefore between BR and IBR groups during the first 6 cycles of treatment. Within the IBR group, we showed a decrease in AEsc after 6 cycles compared to the first 6 cycles. This decrease might be more pronounced if AE start and end dates were used to calculate reporting lengths, but only AE reporting periods were available (approximately 1 cycle for each of the first 6 cycles and 3 cycles after 6 cycles) which resulted in an AE occurring after 6 cycles receiving more weight. Conversely, it is possible that patients treated with IBR for extended periods of time fail to continue to report the same AE repeatedly, which would result in a seemingly lower AEsc after 6 cycles. AEsc (grades 1–4) comparisons between BR and IBR groups after 6 cycles of treatment and overall are less meaningful, since in the BR group, few grade 1/2 events were captured after cycle 6 resulting in an AEsc biased low. This bias is likely mitigated for comparisons of AEsc (grades 3–4), since these AEs were captured in a more similar manner between groups after cycle 6, and indeed there was no significant difference in the overall AEsc (grades 3–4) between BR and IBR groups. In general, the use of AEsc may be best suited to compare the AE burden of therapies more similar in nature, such as continuous administration of BTK inhibitors, rather than the more divergent treatment regimens of fixed duration BR and continuous IBR.
With respect to AEs of clinical interest, the estimated 3-year cumulative incidence of grade 3 or higher atrial fibrillation, hypertension, and infection for patients receiving IBR (n=361) were 7.7%, 25.4%, and 20.5%, respectively. The number of patients who crossed over to ibrutinib following progression with BR was small (n=30), but the rates of grade 3 or higher atrial fibrillation, hypertension, and infection were consistent. For reasons unclear, event rates reported in A041202 are higher than in RESONATE-2 (n=135),7 but ibrutinib discontinuation rates as a result of AEs were similar, 14% in A041202 and 12% in RESONATE-2, suggesting that global AEs were likely similar between studies.
Few patients treated on A041202 had fungal infections, but one patient treated on IBR had aspergillus identified. Fungal infections, specifically aspergillus, have been seen with BTK inhibitors, highlighting the importance of macrophage BTK in clearance of aspergillus.19 Among heavily pre-treated patients in a large single-institution cohort,20 12 aspergillus infections were identified among 566 patients with a median onset of 4 months. No infections were seen in previously untreated patients. These data from A041202 support that the risk of fungal infections with ibrutinib is exceedingly low in previously untreated CLL patients.
This study also demonstrates that after ibrutinib discontinuation for AEs, many patients will experience prolonged response durations. This has been suggested in the E1912 study of ibrutinib and rituximab versus fludarabine, cyclophosphamide, and rituximab, with similar remission duration after ibrutinib between the trials.21 This is significant because it demonstrates that after discontinuation for AEs, physicians do not need to rush to institute second-line therapy in the majority of patients. It also suggests that despite measurable disease remaining at ibrutinib discontinuation in the majority of patients, ibrutinib has likely cleared the bulk of disease-driving CLL cells, potentially leaving residual disease that does not have capacity to rapidly expand or has changed the microenvironment such that rapid progression is inhibited.
In summary, while tabulated summaries of individual AE types by grade are important in understanding AE and toxicity profiles, analytical tools such as the AE score and the cumulative incidence of AEs are also useful. The AE score is easy to calculate and incorporates all AEs, including persistent lower-grade AEs that might not be captured with standard reporting of the highest grade AE. Cumulative incidence curves show the onset of AEs, characterizing the timing of AE occurrences in a way that simple rates do not. In this post hoc analysis of older patients with CLL treated on A041202, the AE burden was significantly higher with BR than IBR during the first 6 cycles of therapy. Among patients who completed 6 cycles of therapy with IBR, AE burden subsequently decreased with continued treatment. We also show that the onset of grade 3 or higher atrial fibrillation, hypertension, and infection is highest in the first year of IBR, and diminishes thereafter based on a cumulative incidence analysis. Even though overall conclusions regarding toxicity of the treatment regimens are unchanged from the original publication of results for A041202,5 the AE score provides a standardized framework for understanding the global AE burden and can serve as an important tool in future studies to summarize and identify differences in AE data.
Supplementary Material
Acknowledgements
Support: Research reported in this publication was supported in part by National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821, U10CA180882, and U24CA196171, (to the Alliance for Clinical Trials in Oncology), UG1CA232760, UG1CA233180, UG1CA233253, UG1CA233327, UG1CA233331, R35CA198183 (JCB), and R01CA192928 (JCB/JAW), U10CA180863 (CCTG). Also supported in part by Pharmacyclics which provided ibrutinib for the clinical trial. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Those providing support to Alliance for Clinical Trials in Oncology and Alliance Foundation Trials are found at https://acknowledgments.alliancefound.org.
The authors would like to disclose conflicts of interest. ASR has acted as a consultant or advisor to Telios Pharma. WD has received clinical research support from Merck. NLB has acted as a consultant or advisor to Seattle Genetics, and has received clinical research support from ADC Therapeutics, Autolus, Bristol-Myers Squibb, Celgene, Forty Seven, Genentech, Immune Design, Janssen, Kite Pharma, Merck, Millennium, Pharmacyclics, and Seattle Genetics. DMB has received honoraria from Genentech, Abbvie, Teva, TG Therapeutics, has acted as a consultant or advisor to Genentech, Abbvie, Novartis Pharma SAS, Pharmacyclics, Teva, TG Therapeutics, has had travel and accommodation expenses paid for by Abbvie, Teva, and TG Therapeutics, and has declared other relationships with Novartis Pharma SAS. SC has stock and other ownership interests in Abbvie/Pharmacyclics, has received honoraria from Janssen Oncology and Pharmacyclics, has acted as a consultant or advisor to Abbie, Adpative Biotechnologies, Astellas Pharma, AstraZeneca, BeiGene, Celgene, Genentech/Roche, Gilead Sciences, Janssen Oncology, Novartis, and Pharmacyclics, has received clinical research support from Abbvie, Acerta Pharma/AstraZeneca, Celgene, Janssen Oncology, Pharmacyclics, and Takeda, provided expert testimony for Genentech, and has had travel and accommodation expenses paid for by Abbvie, BeiGene, Celgene, Genentech, Janssen Oncology, and Pharmacyclics. JRB has received honoraria from Abbvie and Janssen, has acted as a consultant or advisor to Astellas Pharma, AstraZeneca, Celgene, Gilead Sciences, Infinity Pharmaceuticals, Abbvie, Janssen, Pharmacyclics, Redx Pharma, Roche/Genentech, and Sun Pharma and has received clinical research support from Gilead Sciences. RAL has acted as a consultant or advisor to Novartis, Amgen, Ariad/Takeda, Astellas, Celgene/BMS, CVS/Caremark, Epizyme, and MorphoSys, and has received clinical research support from Novartis, Astellas, Celgene, Cellectis, Daiichi Sankyo, Forty Seven, Rafael Pharmaceuticals, and royalties from UpToDate. HE has acted as a consultant or advisor to Agios, Amgen, Astellas Pharma, Celgene, Daiichi Sankyo, Glycomimetics, Immunogen, Incyte, Jazz Pharmaceuticals, Macrogenics, Novartis, Pfizer, and Seattle Genetics, was on a Speakers’ Bureau for Agios, Celgene, Incyte, Jazz Pharmaceuticals, and Novartis, has received clinical research support from Abbvie, has declared other relationships with Celgene and Glycomimetics, and has declared uncompensated relationships with Daiichi Sankyo. ML has acted as a consultant or advisor to Newlink Genetics and Sanofi, and has received clinical research support from Abbvie, Abbvie/Genentech, Actinium Pharmaceuticals, Amgen, Astellas Pharma, Pluristem Therapeutics, and Tolero Pharmaceuticals. JSA has acted as a consultant or advisor to Abbvie, Allogene, Bayer, Bristol-Myers Squibb, Celgene, EMD Serono, Genentech, Gilead Sciences, Janssen, Juno Therapeutics, Karyopharm Therapeutics, Kite Pharma, Merck, MorphoSys, Novartis, and Verastem, and has received clinical research support from AI Therapeutics, Celgene, and Seattle Genetics. RMS has received honoraria from DAVA Pharmaceuticals, Medscape, Prime Oncology, and Research to Practice, has acted as a consultant or advisor to Abbvie, Actinium Pharmaceuticals, Ageios, Amgen, argenx, Arog, Astellas Pharma, AstraZeneca, Biolinerx, Celgene, Celgene/Jazz, Cornerstone Pharmaceuticals, Daiichi Sankyo, Gemoab, Macrogenics, Novartis, Otsuka, Pfizer, Roche/Genentech, Stemline Therapeutics, Syntrix, Takeda, Trovagene, and has received clinical research support from Abbvie/Genentech, Agios, and Novartis. JCB has acted as a consultant or advisor to Acerta Pharma, Genentech, Jazz Pharmaceuticals, and Pharmacyclics, and has received clinical research support from Acerta Pharma, Genentech, Janssen, and Pharmacyclics. SJM has acted as a consultant or advisor to Pfizer and Pique and has declared other relationships with BioGene. JAW has acted as a consultant or advisor to ArQule, AstraZeneca, Janssen, and Pharmacyclics, and has received clinical research support from Abbvie, Janssen, Karyopharm Therapeutics, Loxo, MorphoSys, and Verastem.
Footnotes
Competing Interests
All other authors declare no competing interests.
ClinicalTrials.gov Identifier: NCT01886872
References
- 1.Eichhorst B, Dreyling M, Robak T, Montserrat E, Hallek M. Chronic lymphocytic leukemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011; 22(Supplement 6): vi50–vi54. [DOI] [PubMed] [Google Scholar]
- 2.Eichhorst BF, Busch R, Stilgenbauer S, Stauch M, Bergmann MA, Ritgen M et al. First-line therapy with fludarabine compared with chlorambucil does not result in a major benefit for elderly patients with advanced chronic lymphocytic leukemia. Blood. 2009; 114(16): 3382–3391. [DOI] [PubMed] [Google Scholar]
- 3.Eichhorst B, Fink AM, Bahlo J, Busch R, Math D, Kovacs G et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016. July; 17(7):928–942. [DOI] [PubMed] [Google Scholar]
- 4.Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P et al. RESONATE-2 Investigators. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N Engl J Med. 2015. December 17; 373(25):2425–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woyach JA, Ruppert AS, Heerema NA, Zhao W, Booth AM, Ding W et al. Ibrutinib Regimens versus Chemoimmunotherapy in Older Patients with Untreated CLL. N Engl J Med. 2018. December 27; 379(26):2517–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hilal T, Gea-Banacloche JC, Leis JF. Chronic lymphocytic leukemia and infection risk in the era of targeted therapies: Linking mechanisms with infections. Blood Rev. 2018. September; 32(5):387–399. [DOI] [PubMed] [Google Scholar]
- 7.Barr PM, Robak T, Owen C, Tedeschi A, Bairey O, Bartlett NL et al. Sustained efficacy and detailed clinical follow-up of first-line ibrutinib treatment in older patients with chronic lymphocytic leukemia: extended phase 3 results from RESONATE-2. Haematologica. 2018. September; 103(9):1502–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Cancer Institute: CTEP. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Bethesda, MD: National Institutes of Health; 2009. [Google Scholar]
- 9.Fine JP and Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Amer Statist Assoc. 1999; 94(446):496–509. [Google Scholar]
- 10.Thanarajasingam G, Hubbard JM, Sloan JA, Grothey A. The Imperative for a new approach to toxicity analysis in oncology clinical trials. J Natl Cancer Inst. 2015. October; 107(10). [DOI] [PubMed] [Google Scholar]
- 11.Carbini M, Suárez-Fariñas M, Maki RG. A method to summarize toxicity in cancer randomized cinical trials. Clin Cancer Res. 2018. October 15; 24(20):4968–4975. [DOI] [PubMed] [Google Scholar]
- 12.Trotti A, Pajak TF, Gwede CK, Paulus R, Cooper J, Forastiere A et al. TAME: development of a new method for summarising adverse events of cancer treatment by the Radiation Therapy Oncology Group. Lancet Oncol. 2007. May 31; 8(7):613–624. [DOI] [PubMed] [Google Scholar]
- 13.Lee SM, Hershman DL, Martin P, Leonard JP, Cheung YK. Toxicity burden score: a novel approach to summarize multiple toxic effects. Ann Oncol. 2012. February; 23(2):537–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le-Rademacher JG, Hillman S, Storrick E, Mahoney MR, Thall PF, Jatoi A et al. Adverse event burden score – a versatile summary measure for cancer clinical trials. Cancers. 2020. November 4; 12(11): 3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuurhuizen CS, Verheul HM, Braamse AM, Buffart LM, Bloemendal HJ, Dekker J et al. The predictive value of cumulative toxicity for quality of life in patients with metastatic colorectal cancer during first-line palliative chemotherapy. Cancer Manag Res. 2018. August 29; 10:3015–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sivendran S, Latif A, McBride RB, Stensland KD, Wisnivesky J, Haines L et al. Adverse event reporting in cancer clinical trial publications. J Clin Oncol. 2014. January 10; 32(2):83–89. [DOI] [PubMed] [Google Scholar]
- 17.Hillman SL, Mandrekar SJ, Bot B, DeMatteo RP, Perez EA, Ballman KV et al. Evaluation of the value of attribution in the interpretation of adverse event data: a North Central cancer treatment group and american college of surgeons oncology group investigation. J Clin Oncol. 2010. June 20; 28(18):3002–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.George GC, Barata PC, Campbell A, Chen A, Cortes JA, Hyman DM et al. Improving attribution of adverse events in oncology clinical trials. Cancer Treat Rev. 2019; 76:33–40. [DOI] [PubMed] [Google Scholar]
- 19.Bercusson A, Colley T, Shah A, Warris A, Armstrong-James D. Ibrutinib blocks Btk-dependent NF-ĸB and NFAT responses in human macrophages during Aspergillus fumigatus phagocytosis. Blood. 2018. November 1; 132(18):1985–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogers KA, Mousa L, Zhao Q, Bhat SA, Byrd JC, El Boghdadly Z et al. Incidence of opportunistic infections during ibrutinib treatment for B-cell malignancies. Leukemia. 2019. October; 33(10):2527–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shanafelt TD, Wang V, Kay NE, Hanson CA, O’Brien SM, Barrientos JC et al. Ibrutinib and rituximab provides superior clinical outcome compared to FCR in younger patients with chronic lymphocytic leukemia (CLL): extended follow-up from the E1912 trial. Blood. 2019. November 13; 134(Supplement_1):33 (abstract 642). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.