Abstract
Background.
Shigella infections are an important cause of diarrhea in young children and can result in severe complications. Disparities in Shigella infections are well documented among US adults. Our objective was to characterize disparities in incidence and severity of Shigella infections among US children.
Methods.
We analyzed laboratory-diagnosed Shigella infections reported to FoodNet, an active, population-based surveillance system in 10 US sites, among children during 2009–2018. We calculated the incidence rate stratified by sex, age, race/ethnicity, Shigella species, and disease severity. Criteria for severe classification were hospitalization, bacteremia, or death. The odds of severe infection were calculated using logistic regression.
Results.
During 2009–2018, 10 537 Shigella infections were reported in children and 1472 (14.0%) were severe. The incidence rate was 9.5 infections per 100 000 child-years and the incidence rate of severe infections was 1.3 per 100 000 child-years. Incidence was highest among children aged 1–4 years (19.5) and lowest among children aged 13–17 years (2.3); however, children aged 13–17 years had the greatest proportion of severe infections (21.2%). Incidence was highest among Black (16.2 total; 2.3 severe), Hispanic (13.1 total; 2.3 severe), and American Indian/Alaska Native (15.2 total; 2.5 severe) children. Infections caused by non-sonnei species had higher odds of severity than infections caused by Shigella sonnei (adjusted odds ratio 2.58; 95% confidence interval 2.12–3.14).
Conclusions.
The incidence and severity of Shigella infections among US children vary by age, race/ethnicity, and Shigella species, warranting investigation of unique risk factors among pediatric subpopulations.
Keywords: gastrointestinal infections, health disparities, pediatric, Shigella, shigellosis
Shigella infections are a major cause of diarrhea in young children [1]. Although Shigella infections are usually self-limited, they can result in severe complications including dehydration, sepsis, and invasive extraintestinal infections [2]. Complications also include seizures, which are reported most often in young children with fever or metabolic alterations [3]. In the United States, an estimated 500 000 Shigella infections occur each year and children under the age of 5 years represent an estimated 13% of infections, 22% of hospitalizations, and 24% of deaths [1]. Shigella sonnei is the predominant Shigella species in the United States; in 2016, S. sonnei represented approximately 80% of laboratory-confirmed Shigella infections, followed by S. flexneri at approximately 13% [4]. Infections caused by S. dysenteriae and S. boydii are rare in the United States [4]. Although S. dysenteriae type 1 is known to cause severe epidemic dysentery, any Shigella species can cause severe illness, particularly in individuals with immunocompromising conditions [5].
Shigella outbreaks in the United States are primarily associated with person-to-person contact in childcare settings [6]. Documented risk factors for sporadic (non-outbreak-associated) infections include age (1–4 years), ethnicity (Hispanic), international travel, contact with an ill person, and attending or working in childcare [7]. Racial and ethnic disparities in shigellosis incidence appear to be influenced by poverty and crowding, particularly among children [8]. Analysis of pediatric cases in California identified disparities by race/ethnicity and poverty level; incidence was highest in children under the age of 5 years and those of Hispanic ethnicity, and incidence increased with census tract poverty [9]. Risk factors for severe Shigella infection among children have not been well characterized in the US context; however, among US adults, prior analyses of surveillance data indicated that severe infections were associated with men 18–49 years old, S. flexneri species, and Black race [10]. To expand on these findings, we sought to characterize disparities in the incidence and severity of Shigella infections among a larger pediatric sample in the United States.
METHODS
We analyzed sporadic Shigella infections reported to the Foodborne Diseases Active Surveillance Network (FoodNet) during 2009–2018 in children (defined as persons <18 years old); cases reported to be associated with an outbreak were excluded from the analysis. FoodNet is a collaboration between the US Centers for Disease Control and Prevention, 10 state health departments, the US Department of Agriculture’s Food Safety and Inspection Service, and the US Food and Drug Administration. FoodNet conducts active, population-based surveillance for laboratory-diagnosed infections caused by pathogens, including Shigella, in 10 sites covering 15% of the US population. FoodNet collects demographic, clinical, and epidemiologic data, including the specimen source, hospitalization, and death [11]. Hospitalizations occurring within 7 days before or after specimen collection are attributed to the infection, as is the patient’s vital status at hospital discharge or 7 days after specimen collection if the patient was not hospitalized. Since 2011, FoodNet has included infections diagnosed by culture-independent diagnostic tests (CIDTs) in addition to culture-confirmed infections.
To account for missingness in race and ethnicity data, we used a composite race/ethnicity variable: cases with Hispanic ethnicity were classified in the race/ethnicity category “Hispanic” regardless of reported race, and cases with non-Hispanic or unknown ethnicity were classified in race/ethnicity categories of non-Hispanic White (“White”), non-Hispanic Black (“Black”), non-Hispanic Asian or Pacific Islander (“Asian/Pacific Islander”), non-Hispanic American Indian or Alaska Native (“American Indian/Alaska Native”), and non-Hispanic multiple/other race (“multiple/other race”) corresponding to their reported race. Sensitivity analyses, in which cases with unknown ethnicity were excluded from White, Black, Asian/Pacific Islander, and American Indian/Alaska Native race/ethnicity categories, were conducted to assess possible misclassification in the composite variable.
Criteria for severe classification were hospitalization, bacteremia (defined as blood being the source of the Shigella isolate), or death. We calculated the 10-year (2009–2018) incidence rate per 100 000 child-years stratified by sex, age, race/ethnicity, Shigella species (S. sonnei vs non-sonnei species, including S. flexneri, S. boydii, and S. dysenteriae), FoodNet site, and disease severity. We used 2018 US Census Bureau estimates (released June 20, 2019) for children in the 10 FoodNet site catchment areas. Cases with multiple/other race were excluded from analyses of incidence by race/ethnicity due to lack of population denominator estimates.
Simple logistic regression was used to estimate the odds of developing severe infection (as a binary outcome) by sex, age, race/ethnicity, and Shigella species (S. sonnei vs non-sonnei), as well as the odds of developing a non-sonnei species infection. Additionally, adjusted odds ratios (aOR) were calculated using a multivariable logistic regression model including sex, age, race/ethnicity, Shigella species, and FoodNet surveillance site. Cases with missing data for these variables were excluded from logistic regression, as were cases with multiple/other race. All analyses were performed using SAS (version 9.4; SAS Institute, Inc, Cary, NC).
RESULTS
During 2009–2018, 11 391 Shigella infections in children were reported to FoodNet, of which 10 537 (92.5%) sporadic (non-outbreak-associated) infections were included in this analysis. Of these, 1472 (14.0%) were classified as severe; 1446 patients (98.2%) were hospitalized, 43 (2.9%) were bacteremic, and 4 (0.3%) died. Patient demographics and Shigella species distributions are summarized in Table 1.
Table 1.
Demographics and Species of Total and Severe Shigella Infections Among Children—Foodborne Diseases Active Surveillance Network, 2009–2018 (n = 10 537)a
| Total Infections | Severe Infectionsb | |||
|---|---|---|---|---|
| Characteristic | No. | % | No. | % |
| All | 10 537 | 100.0 | 1472 | 100.0 |
| Sex | ||||
| Female | 5307 | 50.4 | 736 | 50.0 |
| Male | 5209 | 49.4 | 733 | 49.8 |
| Unknown | 21 | 0.2 | 3 | 0.2 |
| Age group | ||||
| <1 year | 353 | 3.4 | 44 | 3.0 |
| 1–4 years | 4658 | 44.2 | 560 | 38.0 |
| 5–8 years | 3605 | 34.2 | 523 | 35.5 |
| 9–12 years | 1205 | 11.4 | 193 | 13.1 |
| 13–17 years | 716 | 6.8 | 152 | 10.3 |
| Race/ethnicity | ||||
| Hispanic, any race | 2534 | 24.1 | 446 | 30.3 |
| American Indian/Alaska Native, non-Hispanic | 158 | 1.5 | 26 | 1.8 |
| Asian/Pacific Islander, non-Hispanic | 314 | 3.0 | 33 | 2.2 |
| Black, non-Hispanic | 3208 | 30.5 | 448 | 30.4 |
| White, non-Hispanic | 2928 | 27.8 | 414 | 28.1 |
| Multiple/other race, non-Hispanic | 258 | 2.5 | 32 | 2.2 |
| Unknown race/ethnicity | 1137 | 10.8 | 73 | 5.0 |
| FoodNet site | ||||
| California | 427 | 4.1 | 43 | 2.9 |
| Colorado | 260 | 2.5 | 46 | 3.1 |
| Connecticut | 173 | 1.6 | 44 | 3.0 |
| Georgia | 4820 | 45.7 | 612 | 41.6 |
| Maryland | 716 | 6.8 | 115 | 7.8 |
| Minnesota | 738 | 7.0 | 122 | 8.3 |
| New Mexico | 501 | 4.8 | 102 | 6.9 |
| New York | 275 | 2.6 | 45 | 3.1 |
| Oregon | 175 | 1.7 | 34 | 2.3 |
| Tennessee | 2452 | 23.3 | 309 | 21.0 |
| Species | ||||
| S. sonnei | 7875 | 74.7 | 1072 | 72.8 |
| S. flexneri | 702 | 6.7 | 212 | 14.4 |
| S. boydii | 30 | 0.3 | 9 | 0.6 |
| S. dysenteriae | 12 | 0.1 | 2 | 0.1 |
| Unknownc | 1918 | 18.2 | 117 | 12.0 |
Includes cases with a known age and not associated with an outbreak.
Criteria for severe classification were hospitalization, bacteremia, or death.
Among 1918 infections caused by unknown species, 612 (31.9%) were positive by culture but had an undetermined species and 1306 (68.1%) were positive by culture-independent diagnostic test alone.
The incidence rate of Shigella infections during 2009–2018 was 9.5 per 100 000 child-years, and the incidence rate of severe infections was 1.3 per 100 000 child-years (Table 2). Incidence rates were similar by sex. The highest incidence of total and severe Shigella infections occurred in children aged 1–4 years, with 19.5 total infections per 100 000 child-years and 2.3 severe infections per 100 000 child-years. Incidence rates were lowest in children aged 13–17 years (2.3 total; 0.5 severe); however, this age group had the greatest proportion of severe infections (21.2%).
Table 2.
Incidence Rate of Total and Severe Shigella Infections and Percentage Severe Among Children—Foodborne Diseases Active Surveillance Network, 2009–2018
| Incidence Rate per 100 000 Child-Years | |||
|---|---|---|---|
| Characteristic | Total Infections | Severe Infectionsa | % Severe |
| All | 9.5 | 1.3 | 14.0 |
| Sex | |||
| Female | 9.8 | 1.4 | 13.9 |
| Male | 9.2 | 1.3 | 14.1 |
| Age group | |||
| <1 year | 6.0 | 0.8 | 12.5 |
| 1–4 years | 19.5 | 2.3 | 12.0 |
| 5–8 years | 14.7 | 2.1 | 14.5 |
| 9–12 years | 4.8 | 0.8 | 15.9 |
| 13–17 years | 2.3 | 0.5 | 21.2 |
| Race/ethnicity | |||
| Hispanic, any race | 13.1 | 2.3 | 17.6 |
| Asian/Pacific Islander, non-Hispanic | 5.5 | 0.6 | 10.5 |
| American Indian/Alaska Native, non-Hispanic | 15.2 | 2.5 | 16.5 |
| Black, non-Hispanic | 16.2 | 2.3 | 14.0 |
| White, non-Hispanic | 4.9 | 0.7 | 14.1 |
| FoodNet site | |||
| California | 6.0 | 0.6 | 10.1 |
| Colorado | 3.7 | 0.7 | 17.7 |
| Connecticut | 2.2 | 0.6 | 25.4 |
| Georgia | 19.3 | 2.5 | 12.7 |
| Maryland | 5.3 | 0.9 | 16.1 |
| Minnesota | 5.7 | 1.0 | 16.5 |
| New Mexico | 9.9 | 2.0 | 20.4 |
| New York | 3.1 | 0.5 | 16.4 |
| Oregon | 2.0 | 0.4 | 19.4 |
| Tennessee | 16.4 | 2.1 | 12.6 |
| Species | |||
| S. sonnei | 7.1 | 1.0 | 13.6 |
| Non-sonneib | 0.7 | 0.2 | 30.2 |
Criteria for severe classification were hospitalization, bacteremia, or death.
Includes S. flexneri, S. boydii, and S. dysenteriae.
Shigella incidence was highest in Black children (16.2 total infections per 100 000 child-years and 2.3 severe infections per 100 000 child-years), followed by American Indian/Alaska Native children (15.2 total; 2.5 severe) and Hispanic children (13.1 total; 2.3 severe) (Table 2). Hispanic children had a higher proportion of severe infections (17.6%) than American Indian/Alaska Native (16.5%), White (14.1%), Black (14.0%), and Asian/Pacific Islander (10.5%) children. Incidence and severity also varied by site, with highest incidence of both total and severe infections in Georgia (19.3 total; 2.5 severe) and Tennessee (16.4 total; 2.1 severe). The incidence rate of S. sonnei species infections (7.1 infections per 100 000 child-years) was 10 times higher than that of non-sonnei species (0.7); however, a higher proportion of non-sonnei species infections were severe (30.2% for non-sonnei vs 13.6% for S. sonnei).
The incidence rate of S. sonnei infections was higher than that of non-sonnei infections across all race/ethnicity groups (Figure 1); however, the distribution of S. sonnei and non-sonnei infections differed by race/ethnicity. American Indian/Alaska Native children had the highest incidence rate of S. sonnei infections, with 14.1 infections per 100 000 child-years, followed by Black (13.0), Hispanic (8.9), White (3.8), and Asian/Pacific Islander (2.4) children. Non-sonnei species infections were most common among Hispanic and Asian/Pacific Islander children (1.8 and 1.2 infections per 100 000 child-years, respectively) compared with Black (0.7), American Indian/Alaska Native (0.4), and White (0.1) children. Incidence of S. sonnei and non-sonnei species infections varied by FoodNet site (Supplementary Figure 1); however, the odds of non-sonnei species vs S. sonnei infection were higher among Asian/Pacific Islander, Hispanic, and Black children compared with White children even after adjusting for sex, age, and FoodNet site (Supplementary Table 1).
Figure 1.
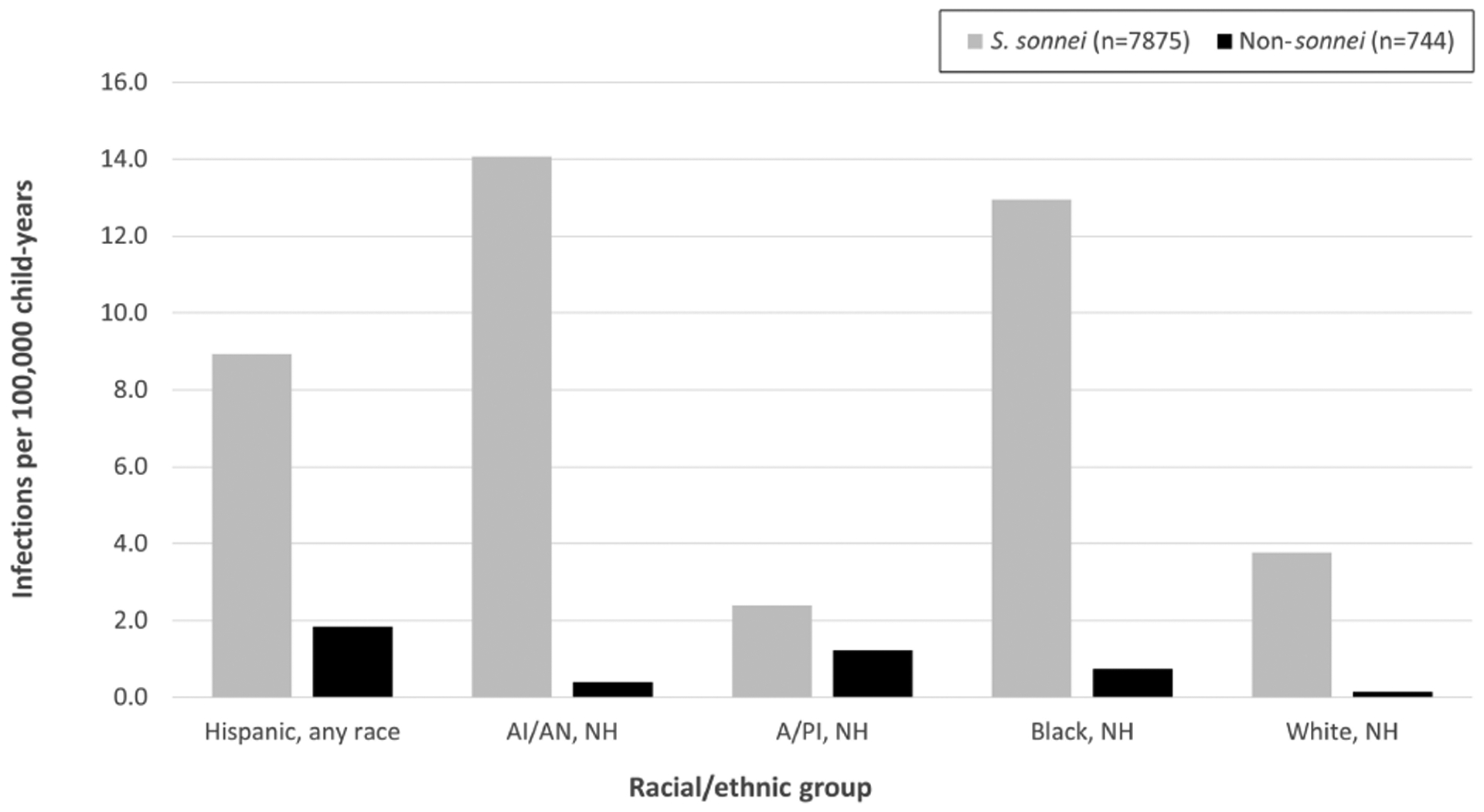
Incidence rate of Shigella sonnei and non-sonnei speciesa infections by race/ethnicity among children—Foodborne Diseases Active Surveillance Network, 2009–2018. Abbreviations: A/PI, Asian/Pacific Islander; AI/AN, American Indian/Alaska Native; NH, non-Hispanic.
aIncludes S. flexneri, S. boydii, and S. dysenteriae.
The odds of severe infection varied by age, race/ethnicity, and Shigella species (Table 3). Independently, the unadjusted odds of developing severe infection were higher among children aged 13–17 years compared with those <1 year old (OR 1.89; 95% confidence interval [CI] 1.32–2.72), Hispanic children compared with White children (OR 1.30; 95% CI 1.12–1.50), and infections caused by non-sonnei species compared with S. sonnei (OR 2.72; 95% CI 2.29–3.22). After adjusting for all other variables, the odds of developing severe infection were lower for Asian/Pacific Islander children than White children (aOR 0.57; 95% CI 0.36–0.90). Shigella species had the strongest effect on developing severe infection even after adjusting for other variables; the odds of developing severe infection were more than doubled if the infection was caused by non-sonnei species compared with S. sonnei (aOR 2.58; 95% CI 2.12–3.14).
Table 3.
Odds Ratios for Developing a Severe Shigella Infectiona Among Children—Foodborne Diseases Active Surveillance Network, 2009–2018
| Unadjusted | Adjustedb | |
|---|---|---|
| Characteristic | Odds Ratio (95% CI) | Odds Ratio (95% CI) |
| Sex (male vs female) | 1.02 (0.91–1.14) | 1.02 (0.90–1.16) |
| Age group | ||
| <1 year | 1.00 (ref) | 1.00 (ref) |
| 1–4 years | 0.96 (0.69–1.33) | 0.80 (0.54–1.18) |
| 5–8 years | 1.19 (0.86–1.66) | 0.92 (0.62–1.37) |
| 9–12 years | 1.34 (0.94–1.90) | 1.01 (0.66–1.55) |
| 13–17 years | 1.89 (1.32–2.72) | 1.43 (0.92–2.21) |
| Race/ethnicity | ||
| Hispanic, any race | 1.30 (1.12–1.50) | 1.11 (0.93–1.31) |
| American Indian/Alaska Native, non-Hispanic | 1.20 (0.78–1.85) | 1.01 (0.62–1.64) |
| Asian/Pacific Islander, non-Hispanic | 0.71 (0.49–1.04) | 0.57 (0.36–0.90) |
| Black, non-Hispanic | 0.99 (0.85–1.14) | 0.99 (0.85–1.16) |
| White, non-Hispanic | 1.00 (ref) | 1.00 (ref) |
| Species (non-sonnei speciesc vs S. sonnei) | 2.72 (2.29–3.22) | 2.58 (2.12–3.14) |
Abbreviation: ref, reference group for odds ratio.
Criteria for severe classification were hospitalization, bacteremia, or death.
Results of multivariable model including sex, age group, race/ethnicity, Shigella species (S. sonnei vs non-sonnei species [S. flexneri, S. boydii, and S. dysenteriae]) and FoodNet site.
Includes S. flexneri, S. boydii, and S. dysenteriae.
Sensitivity analyses, in which 725 cases with unknown ethnicity were excluded from White (n = 342), Black (n = 316), American Indian/Alaska Native (n = 51), and Asian/Pacific Islander (n = 16) race/ethnicity categories, indicated no alteration of these findings (data not shown). Excluded cases represented 6.9% of total infections and 7.8% of severe infections.
DISCUSSION
FoodNet surveillance data from 2009 to 2018 demonstrated disparities in the incidence and severity of Shigella infections among children by age and race/ethnicity. Incidence was highest among children aged 1–4 years, consistent with previously described risk factors for sporadic shigellosis.[7] High incidence of Shigella infections in this age group might be driven by transmission in childcare settings and due to limited handwashing and toileting skills [12]. Adolescents aged 13–17 years had the lowest incidence of Shigella infections, likely due to less frequent exposure to childcare settings; however, adolescents had the highest proportion of severe infections among all age groups. Adolescents might have less frequent preventive health care visits than younger children (eg, for routine childhood immunizations), and may face gaps in care [13], fragmented health care services, and missed opportunities for health promotion [14], such as discussion of prevention messages for Shigella. Thus, adolescents with shigellosis might be less likely to report and seek health care for mild diarrheal symptoms than younger children, unless illness becomes severe. Underlying health conditions among this age group may further contribute to the increased severity of disease [15]. Additional formative research is needed to understand risk factors for Shigella transmission and severity among adolescents and to develop tailored prevention messaging strategies for this age group.
The incidence of Shigella infections was highest in Black, American Indian/Alaska Native, and Hispanic children; this was consistent with previous surveillance data for Shigella infection in US children and adults [7–10, 12]. In 2018, the proportion of children <18 years in the United States living in families with incomes below the federal poverty level, as defined by the US Office of Management and Budget, was greater for Black (32%), American Indian/Alaska Native (31%), and Hispanic (26%) children than White (11%) and Asian/Pacific Islander (11%) children. Poverty has been described as a risk factor for shigellosis [8, 9, 16], and factors contributing to increased risk could include greater household crowding, allowing for person-to-person spread of Shigella [8]. Poverty might also affect access to health insurance [17], influence medical care-seeking for diarrheal illness and submission of stool cultures [18], and pose economic barriers to hygiene such as lack of access to diapers for young children [19].
Racial/ethnic disparities might also be shaped by additional factors at the individual and community levels, such as access to clean water and adequate sanitation, health literacy, and childcare utilization. Race/ethnicity is a strong predictor of access to clean water and adequate sanitation (eg, complete plumbing) in the United States; American Indian/Alaska Native populations are more likely to face clean water and sanitation access issues than any other racial/ethnic group, and Black and Hispanic populations are also disproportionately affected [20]. Measures to prevent Shigella transmission, such as frequent hand hygiene, might be impaired by unsafe or inadequate water and sanitation. Furthermore, health literacy, defined as “the degree to which individuals have the capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions” [21], is a known driver of health disparities and poorer health outcomes for infectious and noninfectious conditions [22]. A recent study of US parents revealed that Black and Hispanic race/ethnicity, low income, and birth outside of the United States were significantly associated with low health literacy [23], and thus lesser reach of Shigella prevention messaging to these populations and barriers to medical care-seeking might contribute to greater incidence of infections and severe outcomes. Finally, use of childcare facilities [24, 25] and access to paid family and medical leave [26] might vary by race/ethnicity, and thus variability in Shigella transmission exposures from childcare settings might also contribute to the racial/ethnic disparities we identified.
Non-sonnei species had higher odds of causing severe infection among children than did S. sonnei. This was consistent with prior all-age analyses from the Georgia FoodNet site indicating that the proportion of isolates cultured from blood vs feces was greater for S. flexneri than S. sonnei [27], as well as international studies identifying S. flexneri more commonly than S. sonnei in bacteremic patients [28–30]. In the United States, the incidence of both S. sonnei and S. flexneri is highest in children aged 1–4 years [12]; however, as S. sonnei is commonly transmitted in childcare settings, risk for S. sonnei infection among children may be more widely recognized by clinicians than risk for S. flexneri, which is often described among adult men [12].
Targeted outreach to pediatric clinicians regarding differences in severity by Shigella species may inform decisions regarding monitoring, supportive care, and treatment for Shigella infections. The use of CIDTs by clinical laboratories to diagnose Shigella infections has increased in recent years [31, 32]; while these tests offer advantages in ease and speed compared to traditional culture methods, CIDTs do not yield isolates that can be speciated. In fact, many infections (18.2% total; 12.0% severe) included in this analysis were caused by an unknown Shigella species, of which 68.1% were diagnosed using CIDTs alone. Cultures of specimens from children with Shigella infections identified by CIDTs (called reflex cultures) are needed to identify species, which can provide important information for clinical decision making. Additional benefits of reflex culture and sequencing of isolates include determination of antibiotic resistance and detection of outbreaks.
Non-sonnei species infections were more commonly observed in Hispanic and Asian/Pacific Islander children than in other racial/ethnic groups; this may be explained in part by international travel. Prior analysis of infections reported to FoodNet demonstrated that infections with S. dysenteriae (56.3%), S. boydii (44.3%), and S. flexneri (24.4%) were more often travel-associated than infections with S. sonnei (11.7%), and travel-associated infections occurred more commonly in individuals of Asian race or Hispanic ethnicity [33]. Outreach efforts to understand pediatric risk factors for Shigella transmission in populations with high incidence of non-sonnei species infections may facilitate the development of targeted interventions and prevention messages.
The unadjusted odds of developing a severe infection were higher in Hispanic children than in other racial/ethnic groups; this may have been driven in part by the higher incidence of non-sonnei species infections in this subpopulation. However, though Asian/Pacific Islander children also had a high incidence of non-sonnei infections, their odds of developing severe shigellosis were low. Reasons for this difference are unknown, and additional formative work is needed to understand possible contributing factors such as socioeconomic status, access to affordable health care, and cultural/behavioral norms. More granular data on the Hispanic population captured in FoodNet data, such as primary language, country of origin, and country of birth, could better elucidate risk factors for severe Shigella infection among children in Hispanic subpopulations, as they likely vary across this diverse ethnic group [34].
This analysis is subject to several limitations. Findings from the 10 FoodNet sites may not be widely generalizable, though estimates may mirror national trends. Census data from 2019 were used to estimate the population denominators for FoodNet sites and did not account for possible changes in population size during 2009–2018. Prior comparison of FoodNet demographics with US population demographics has indicated that while age and race correlate well, Hispanic ethnicity might be underrepresented in FoodNet surveillance [35]. Similarly, efforts to increase representation of American Indian/Alaska Native persons in surveillance data may assist in understanding disparities in this population [36]; small numbers limited interpretation of findings among American Indian/Alaska Native children in this analysis. Many cases had unknown race/ethnicity (10.8% total; 5.0% severe) and additional cases had unknown ethnicity despite reporting race (6.9% total; 7.8% severe). Use of a composite race/ethnicity variable allowed for inclusion of cases with unknown ethnicity if race was reported; this could have resulted in misclassification bias. However, exclusion of these cases in the sensitivity analysis did not alter results. Regardless, improving data collection for race and ethnicity can improve the quality, representativeness, and usefulness of public health surveillance for evaluation of health disparities [37].
Additionally, exclusion of infections with an unknown species may have biased results if data were not missing at random; it is plausible that differences in health care-seeking behaviors and consequent use of CIDTs could differ between racial/ethnic and age groups. Furthermore, including hospitalization to define severe infection might have included patients hospitalized for monitoring, but who never developed advanced clinical signs. Similarly, although hospitalizations within 7 days before or after specimen collection and deaths within 7 days after specimen collection were attributed to Shigella infection, it is possible that these outcomes were caused by comorbid conditions. Moreover, we were unable to assess modes of transmission, including the possibility of sexual transmission among adolescents, and were unable to assess treatment and antibiotic resistance, which may have contributed to severe infections.
Finally, we were unable to account for the complex social and environmental factors that influence health disparities, such as socioeconomic status, clean water and sanitation access, health literacy, childcare utilization, and other behavioral and social factors. Information regarding these social determinants of health is not routinely collected as part of national infectious disease surveillance systems; however, integration of case data with census tract-level data may aid in evaluating these determinants at an ecological scale [8, 9]. Racial/ethnic disparities have been previously described for other enteric pathogens including Salmonella and Campylobacter [38], and further investigation of contributing factors should be a priority for enteric disease epidemiology in the United States. Further evaluation of knowledge, attitudes, and practices related to Shigella prevention among different racial/ethnic and age groups can allow for targeted prevention messages and evaluation of interventions to reduce health disparities.
CONCLUSION
The incidence and severity of Shigella infections among US children vary by age, race/ethnicity, and Shigella species, warranting further investigation of unique risk factors among different pediatric subpopulations. Socioeconomic factors, health care-seeking practices, health literacy, access to prevention measures, and variability in transmission exposures (eg, childcare and travel-associated exposures) might drive these differences. Further evaluation of these social determinants of health is needed to guide interventions to reduce disparities in pediatric shigellosis, and intersectionality of risk factors should be further explored. Heightened awareness among pediatric clinicians of disparities in Shigella infections and targeted actions to assess and address health disparities in practice, including provision of culturally competent care [39], are important in improving health outcomes among disproportionately affected racial/ethnic groups. Improved collection of race and ethnicity in national surveillance systems may facilitate enhanced understanding of health disparities. Finally, reflex cultures of specimens from children with Shigella infections identified by CIDTs should be encouraged to identify species and provide important information for clinical decision making.
Supplementary Material
Acknowledgments.
The authors thank FoodNet partners for contributions to surveillance and Megan E. Gerdes, Waterborne Disease Prevention Branch, CDC, for assistance with data replication.
Footnotes
Supplementary Data
Supplementary materials are available at the Journal of the Pediatric Infectious Diseases Society online.
Publisher's Disclaimer: Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Scallan E, Mahon BE, Hoekstra RM, Griffin PM. Estimates of illnesses, hospitalizations and deaths caused by major bacterial enteric pathogens in young children in the United States. Pediatr Infect Dis J 2013; 32:217–21. [DOI] [PubMed] [Google Scholar]
- 2.Kotloff KL, Riddle MS, Platts-Mills JA, Pavlinac P, Zaidi AKM. Shigellosis. Lancet 2018; 391:801–12. [DOI] [PubMed] [Google Scholar]
- 3.Khan WA, Dhar U, Salam MA, Griffiths JK, Rand W, Bennish ML. Central nervous system manifestations of childhood shigellosis: prevalence, risk factors, and outcome. Pediatrics 1999; 103:E18. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC). National Shigella Surveillance Annual Report, 2016. Atlanta, GA: US Department of Health and Human Services, CDC; 2018. [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC). Shigellosis. CDC Yellow Book 2020: Health Information for International Travel. New York, NY: Oxford University Press; 2017. [Google Scholar]
- 6.Centers for Disease Control and Prevention. NORS Dashboard. Accessed May 18, 2020. https://wwwn.cdc.gov/norsdashboard/
- 7.Haley CC, Ong KL, Hedberg K, et al. Risk factors for sporadic shigellosis, FoodNet 2005. Foodborne Pathog Dis 2010; 7(7):741–7. [DOI] [PubMed] [Google Scholar]
- 8.Libby T, Clogher P, Wilson E, et al. Disparities in shigellosis incidence by census tract poverty, crowding, and race/ethnicity in the United States, FoodNet, 2004–2014. Open Forum Infect Dis 2020; 7(2):ofaa030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson R, Smith D, Tabnak F, Vugia D. Disparities of shigellosis rates among California children by race/ethnicity and census tract poverty level, 2000–2010. Pediatr Infect Dis J 2015; 34(8):843–7. [DOI] [PubMed] [Google Scholar]
- 10.McCrickard LS, Crim SM, Kim S, Bowen A. Disparities in severe shigellosis among adults - foodborne diseases active surveillance network, 2002–2014. BMC Public Health 2018; 18(1):221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tack DM, Ray L, Griffin PM, et al. Preliminary incidence and trends of infections with pathogens transmitted commonly through food - foodborne diseases active surveillance network, 10 U.S. sites, 2016–2019. MMWR Morb Mortal Wkly Rep 2020; 69(17):509–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shiferaw B, Shallow S, Marcus R, et al. Trends in population-based active surveillance for shigellosis and demographic variability in FoodNet sites, 1996–1999. Clin Infect Dis 2004; 38(Suppl 3):S175–80. [DOI] [PubMed] [Google Scholar]
- 13.Mmari K, Marshall B, Hsu T, Shon JW, Eguavoen A. A mixed methods study to examine the influence of the neighborhood social context on adolescent health service utilization. BMC Health Serv Res 2016; 16:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Research Council, Institute of Medicine. Adolescent Health Services: Missing Opportunities. Washington, DC: The National Academies Press; 2009:366. [PubMed] [Google Scholar]
- 15.Sawyer SM, Drew S, Yeo MS, Britto MT. Adolescents with a chronic condition: challenges living, challenges treating. Lancet 2007; 369:1481–9. [DOI] [PubMed] [Google Scholar]
- 16.Greene SK, Levin-Rector A, Hadler JL, Fine AD. Disparities in reportable communicable disease incidence by census tract-level poverty, New York City, 2006–2013. Am J Public Health 2015; 105(9):e27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guendelman S, Pearl M. Access to care for children of the working poor. Arch Pediatr Adolesc Med 2001; 155:651–8. [DOI] [PubMed] [Google Scholar]
- 18.Scallan E, Jones TF, Cronquist A, et al. ; FoodNet Working Group. Factors associated with seeking medical care and submitting a stool sample in estimating the burden of foodborne illness. Foodborne Pathog Dis 2006; 3:432–8. [DOI] [PubMed] [Google Scholar]
- 19.Smith MV, Kruse A, Weir A, Goldblum J. Diaper need and its impact on child health. Pediatrics 2013; 132:253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roller Z, Gasteyer S, Nelson N, Lai W, and Shingne M. Closing the Water Access Gap in the United States: A National Action Plan. Oakland, CA: Dig Deep and U.S. Water Alliance; 2019. [Google Scholar]
- 21.U.S. Department of Health and Human Services. Healthy People 2010: Understanding and Improving Health. 2nd ed. Washington, DC: US Government Printing Office; 2000. [Google Scholar]
- 22.Berkman ND, Sheridan SL, Donahue KE, Halpern DJ, Crotty K. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med 2011; 155:97–107. [DOI] [PubMed] [Google Scholar]
- 23.Yin HS, Johnson M, Mendelsohn AL, Abrams MA, Sanders LM, Dreyer BP. The health literacy of parents in the United States: a nationally representative study. Pediatrics 2009; 124(Suppl 3):S289–98. [DOI] [PubMed] [Google Scholar]
- 24.Fram MS, Kim J. Race/ethnicity and the start of child care: a multi-level analysis of factors influencing first child care experiences. Early Child Res Q 2008; 23(4):575–90. [Google Scholar]
- 25.de Brey C, Musu L, McFarland J, et al. Status and Trends in the Education of Racial and Ethnic Groups 2018. National Center for Education Statistics. Washington, DC: U.S. Department of Education; 2019. [Google Scholar]
- 26.Bartel AP, Kim S, Nam J, Rossin-Slater M, Ruhm C, Waldfogel J. Racial and ethnic disparities in access to and use of paid family and medical leave: evidence from four nationally representative datasets. Mon Labor Rev 2019; 1–29. [Google Scholar]
- 27.Tobin-D’Angelo M, Oosmanally N, Wilson SN, Anderson EJ, Segler S, Poventud L. Shigella bacteremia, Georgia, USA, 2002–2012. Emerg Infect Dis 2020; 26(1):122–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Struelens MJ, Patte D, Kabir I, Salam A, Nath SK, Butler T. Shigella septicemia: prevalence, presentation, risk factors, and outcome. J Infect Dis 1985; 152:784–90. [DOI] [PubMed] [Google Scholar]
- 29.Greenberg D, Marcu S, Melamed R, Lifshitz M. Shigella bacteremia: a retrospective study. Clin Pediatr (Phila) 2003; 42:411–5. [DOI] [PubMed] [Google Scholar]
- 30.Davies NE, Karstaedt AS. Shigella bacteraemia over a decade in Soweto, South Africa. Trans R Soc Trop Med Hyg 2008; 102:1269–73. [DOI] [PubMed] [Google Scholar]
- 31.Marder EP, Cieslak PR, Cronquist AB, et al. Incidence and trends of infections with pathogens transmitted commonly through food and the effect of increasing use of culture-independent diagnostic tests on surveillance - foodborne diseases active surveillance network, 10 U.S. sites, 2013–2016. MMWR Morb Mortal Wkly Rep 2017; 66(15):397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tack DM, Marder EP, Griffin PM, et al. Preliminary incidence and trends of infections with pathogens transmitted commonly through food - foodborne diseases active surveillance network, 10 U.S. sites, 2015–2018. MMWR Morb Mortal Wkly Rep 2019; 68(16):369–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kendall ME, Crim S, Fullerton K, et al. Travel-associated enteric infections diagnosed after return to the United States, foodborne diseases active surveillance network (FoodNet), 2004–2009. Clin Infect Dis 2012; 54(Suppl 5):S480–7. [DOI] [PubMed] [Google Scholar]
- 34.Borak J, Fiellin M, Chemerynski S. Who is Hispanic? implications for epidemiologic research in the United States. Epidemiology 2004; 15:240–4. [DOI] [PubMed] [Google Scholar]
- 35.Hardnett FP, Hoekstra RM, Kennedy M, Charles L, Angulo FJ; Emerging Infections Program FoodNet Working Group. Epidemiologic issues in study design and data analysis related to FoodNet activities. Clin Infect Dis 2004; 38(Suppl 3):S121–6. [DOI] [PubMed] [Google Scholar]
- 36.Bertolli J, Roussel A, Harris J, et al. Surveillance of infectious diseases among American Indians and Alaska Natives. J Health Dispar Res Pract 2008; 2(2):7. [Google Scholar]
- 37.Rodriguez-Lainz A, McDonald M, Fonseca-Ford M, et al. Collection of data on race, ethnicity, language, and nativity by us public health surveillance and monitoring systems: gaps and opportunities. Public Health Rep 2018; 133:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quinlan JJ. Foodborne illness incidence rates and food safety risks for populations of low socioeconomic status and minority race/ethnicity: a review of the literature. Int J Environ Res Public Health 2013; 10:3634–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng TL, Emmanuel MA, Levy DJ, Jenkins RR. Child health disparities: what can a clinician do? Pediatrics 2015; 136:961–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


