Abstract
The rapidly increasing global burden of healthcare associated infections (HAI) is resulting in proportionate increase in chemical disinfection in healthcare settings, adding an extra burden of environmental toxicity. Therefore, alternative disinfection techniques with less or no adverse side-effects need to be explored. In this regard, ayurvedic ‘dhoopan’ technique involving slow combustion of medicinal herbs, minerals and animal products hold great promise. In this study, dhoopan of a traditionally defined ayurvedic medicinal mix, ‘Vishaghn Dhoop’ (VD) has been assessed for its anti-microbial potentials against both Gram-positive and negative pathogenic bacteria, Mycobacterium and pathogenic fungus, Candida albicans. Fume generated from slow combustion of VD was subjected to physico-chemical characterization and was assessed for anti-microbial effects. VD fume contained particles of 354 ± 84 nm size, laden with anti-microbial metabolites. On agar plates, VD fumigation reduced bacterial growth by 13 - 38%. Liquid culture aeration with VD fume inhibited bacterial growth by 50 - 85%, and fungal growth by 80%. In real life settings (in vivo), un-sanitized rooms fumigated with VD fumes for 30 min reduced the environmental microbial loads by 10 folds. In addition, the safety of VD fumigation was evaluated through in vitro cytotoxicity assay on human lung epithelial (A549) cells. Cells exposed to media-collected VD fumes for 24 h exhibited normal cyto-safety profile. Collectively, these observations provide scientific evidence in support of a traditional technique of disinfection, which can be fine-tuned to have implications in clinical, healthcare and food industry where, disinfection is a prime requirement.
Keywords: healthcare associated infection, traditional ayurvedic fumigation, pathogenic bacteria and fungi, non-tuberculous mycobacteria, nosocomial pathogens
Introduction
Healthcare associated infection (HAI), according to World Health Organization (WHO) is an unmet cross-cutting problem. 1 The global burden of HAI has reached such a precipice that WHO has put it on a top priority. Disinfection and sterilization are critical requirements for preventing HAIs. Chemical disinfection through fumigation is still the most preferred mode of preventing HAIs. 2 However, chemical disinfection has several drawbacks, health and environmental toxicity and material incompatibility being the most prominent ones. 3 While fumigation is beyond doubt the most hassle-free and effective technique to ensure disinfection of large areas, the chemicals used, add an element of substitutability to it. In this conjecture, alternative techniques akin to chemical fumigation, but with less or no toxic effects will be more preferred. In this regard, unique smoking techniques of ‘dhoopan’ mentioned in the traditional Indian medicine (TIM) system of ayurveda hold great promise.
Dhoopan is the technique of generating fume through slow combustion of various medicinal herbs, herbo-minerals and medicines of animal origin for fumigation. In fact, ayurveda recommends fumigation therapy or Dhoopan chikitsya for treating pandemics (Janpadodhwansaj vicar) and infectious diseases (Auopsargika roga), 4 healing wounds/ulcers (Vrana), dysmenorrhea (Yonivyapada), ear ailments (Karna Roga), nose ailments (Nasa Roga), ailments of large intestine (Guda Roga), treating body odor (Gatra Daurgandhya), disinfecting apothecary (Bheshajagara), delivery room (Sutikagara), surgery room (Shastrakarmaghruha), nursery/neonatal room (Kumaragara) and also to sterilize ayurvedic fermented formulations, Asavas and Aristas. 5 Dhoopan is an integral part of Rakshavidhi, literally meaning protection protocol, designed to ensure protection against microbes, that has been described in detail by the ancient Indian surgeon, Suśruta in his treatise, Suśruta-saṃhitā (the Compendium of Suśruta), written in sixth century BC.5–7 Fume generated from combustion of medicinal herbs is an excellent way of purifying the environment and has been used for ages in domestic disinfection. 8 The few reviews and reported studies available in the public domain on anti-microbial property of dhoopan technique do not provide insight into the physical and chemical characteristics of the dhoop or fume and the co-relation with the disinfection function observed thereof.9–13 In this study, dhoopan of a mix of medicinal herbs, rock salt and clarified butter (Ghee), called ‘Vishaghn Dhoop’ (VD) has been assessed for its anti-microbial potentials against Gram-positive and negative pathogenic bacteria, Mycobacterium and pathogenic fungus, Candida albicans. VD consists of twelve medicinal herbs, resin from Laccifer lacca, rock salt and clarified butter (Table 1). Composition of VD is mentioned in the Compendium of Suśruta (Suśruta-saṃhitā) 14 and reports of anti-microbial efficacy of individual components of VD are available across other classical ayurvedic texts, like, Caraka-saṃhitā, Dhanwantari-nighantu, Śāligrām-nighantu, Madnādi- nighantu, Bhāvprakāsh-nighantu, Aśtanga-nighantu, Śodhāla-nighantu, Laghu-nighantu and Rāja-nighantu. However, metabolites released upon slow combustion of a mix of all these ingredients are not identified, although, the Compendium of Suśruta (Suśruta-saṃhitā) recommends the use of fume generated from burning VD in disinfection. 14 So, this study was designed to scientifically validate the claimed anti-microbial property associated with VD; and to identify the constituents present in VD fume, that could possibly be contributing to this activity.
Table 1.
Composition of Vishaghn Dhoop.
| Type of ingredient | English name | Hindi name | Scientific name of herbs | Classical Ayurvedic Reference |
|---|---|---|---|---|
| Resin | Shellac | Laksha | Laccifer lacca | Ss, Dn |
| Herbs | Turmeric | Haridra | Curcuma longa L. | Ss, Dn, Cs |
| Indian Atees | Atees | Aconitum heterophyllum Wall. Ex | Ss, Dn, Sn | |
| Indian hog plum | Haritaki | Terminalia chebula Retz. Royle | Ss, Sn, Mn | |
| Nut grass | Motha | Cyperus rotundus L. | Ss, Bn, Dn | |
| Cardamom | Elaichi | Elettaria cardamomum (L). Maton | Ss, Ln | |
| Cinnamon | Dalchini | Cinnamomum verum Lukman | Ss, Sn | |
| Crape Jasmine | Tagar | Tabernaemontana divaricata (L). R. Br. Ex Roem & Schult | Ss, As | |
| Indian Costus root | Kuth/ Kustha | Saussurea costus (Falc). Lipsch | Ss, As | |
| Sweet flag | Vacha | Acorus calamus (L) | Ss, Dn | |
| Perfumed Cherry | Priyangu | Callicarpa macrophylla Vahl | Ss, Shn | |
| Yellow mustard | Peeli sarson | Brassica campestris var. sarson Prain | Ss | |
| Indian bedellium | Guggul | Commiphora wightii (Arn) Bhandari | Ss | |
| Salt | Rock salt/ Halite | Sendha Namak | - | Ss |
| Dairy product | Clarified butter | Ghee | - | Ss |
Ss: Suśruta-samhitā, Cs: Caraka-samhitā, Dn: Dhanwantari-nighantu, Sn: Śāligrām-nighantu, Mn: Madnādi-nighantu, Bn: Bhāvprakāsh-nighantu, As: Aśtanga-nighantu, Shn: Śodhāla-nighantu, Ln: Laghu-nighantu, Rn: Rāja-nighantu.
Materials and Methods
Preparation of VD and Generation and Harvest of VD Fume
The preparation of VD was done according to the verse 12 mentioned in section 6 of Compendium of Suśruta (Suśruta-saṃhitā) which recommends air purification by burning together equal amounts of the ingredients mentioned in Table 1. 14 VD was prepared by mixing equal weights (in grams) of all these ingredients. The herbs were sun-dried before including in the mix. Mix was ground into fine powder before subjecting to slow combustion.
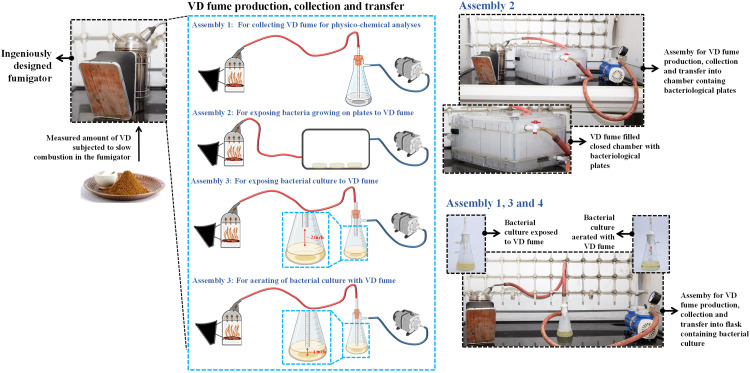
For slow combustion of VD and harvesting of the fume generated for subsequent experiments, an ingeniously designed stainless steel fumigator was used. It had a snug-fitting lid at the top for material input and prevent fume loss. A narrow pipe emanating from the lid served as the outlet for generated fume. The outlet of the fumigator was connected to either Büchner flasks with solvent for Gas chromatography-mass spectrometry (GC-MS) or actively growing microbial cultures or a closed chamber with spread plates of pathogenic microbes in it. The outlets from the Büchner flasks and the plate chamber were connected to a vacuum pump (Figure 1). The vacuum pump ensured efficient transfer of fume from the fumigator. This basic circuitry and the amount of VD being burnt were modified according to specific experimental requirements. For example, when the fume was to be collected for physico-chemical characterization, a 250 ml Büchner flask with 100 ml sterile water in it was connected to the rubber tubing from the outlet of the fumigator through a pipe inserted in its cork. The lower end of the pipe, inside flask, was submerged inside the water [for dynamic light scattering (DLS) sample)] or hydromethanolic solvent (GC-MS sample) in such a way that its tip was 1 inch above the bottom of the flask. 25 g of VD was burnt for 15 min and the solvent in the flask was aerated with the generated fume. This fume-enriched solvent was then used as sample for GC-MS analysis (Assembly 1 in Figure 1). A similar set up was used when the fume was used to aerate actively growing microbial cultures; the modifications here being culture getting aerated instead of water or hydromethanol and 5 instead of 15 g of VD was burnt for 3 instead of 15 min. This is represented as Assembly 4 in Figure 1. For exposing actively growing bacterial cultures to VD fume, instead of submerging the pipe below the culture, it was kept 2 inches above the surface of the culture (Assembly 3 in Figure 1). For evaluating the anti-microbial effect of VD fume on spread plates of different microbial pathogens, these plates were exposed to the generated fume in a closed chamber (Assembly 2 in Figure 1). VD was actually burnt on radiant hot cow dung sticks (fuel). So, as a preparatory step, cow dung sticks of uniform size (3 × 2.5 × 2.5 cm in length × breadth × height) were coated with clarified butter and slowly combusted in an open inverted pyramid shaped brass container until they caught fire from within and stopped burning as open flames. These radiant sticks were placed on the raised, meshed pseudobase fitted at the bottom of the fumigator. Measured quantities of VD was sprinkled on the radiant hot sticks and the fumigator immediately closed.
Figure 1.
Pictorial illustration of the study plan. The entire study plan is schematically depicted here with supplementation of still pictures shot during the conduction of various parts of the experiment. Measured quantities of finely powdered mix of designated herbs constituting VD were slowly burnt in the ingeniously designed closed fumigator, the outlet of which was either connected to a chamber containing different bacterial pathogens spread on agar plates (Assembly 2) or to Büchner flask meant to collect the generated fume for physio-chemical analyses (Assembly 1), exposure of liquid bacterial cultures to VD (Assembly 3) or to aerate bacterial cultures (Assembly 4). The outlet of the fumigator was connected to plate containing chamber or Büchner flasks via rubber tubing. In case of Büchner flasks (Assemblies 1, 3 and 4) the rubber tubing was attached to a pipe inserted through the cork at the mouth of the flask. In case of Assembly 3 designed to expose bacterial cultures to VD fume, opening of the pipe inside the flask was deliberately kept 2 inches above the surface of the culture. For assemblies 1 and 4, the end of the pipe inside the flask was submerged into the solvent and culture respectively, but, kept 1 inch above the bottom of the flask. The outlets of the chamber with bacteriological plates and Büchner flasks were connected to vacuum pump to ensure efficient retrieval of VD fume from the fumigator. Still pictures of different assemblies are provided alongside the schematic describing them. Fume-filled chamber with bacteriological agar plates is shown in the outset of the still for Assembly 2. Similar outsets of Büchner flasks showing bacterial culture exposed to and aerated with VD fume are provided with the still picture showing the circuitry of Assemblies 1, 3 and 4.
Physico-Chemical Characterization of VD Fume
Field emission scanning electron microscopy (FESEM)
Field emission scanning electron microscopy (FESEM) was used to visualize the particle structures produced on combustion of VD. For FESEM imaging, combustion of 5 g of VD was carried out in the specially designed fumigator and collected as depicted in Assembly 2 of Figure 1. Instead of bacteriological plates, cover slips were placed inside the chamber. The collected fume was allowed to settle on the cover slip for 24 h, which was then dried overnight to ensure that no moisture was left behind. Cover slip with dried particles of VD fume was mounted on dedicated FESEM stub with carbon tape and gold coated for 180 s. A drop of silver paste, for improved conductivity, was put on the stub before loading it into the electron chamber. FESEM analysis was done on MIRA3 TESCAN (Tescan Orsay Holding, Kohoutovice, Czech Republic). The size and morphology of fume particles was detected using a JEOL 3010 at 10 kV.
Dynamic light scattering (DLS)
For determining the size range of the VD fume particles DLS analysis was done using Zetasizer Nano ZS (Malvern Pananlytical, Malvern, UK). For DLS, 25 g of VD was combusted for 15 min and collected in 100 ml water, since, water was to be used as dispersant during analysis. 100 µl of this suspension was subjected to DLS at a count rate of 76.4 kcps for 110 s from a distance of 4.65 mm. Data was recorded and delivered as size distribution by intensity histogram by the in-built Zetasizer ver 7.11 software (Serial Number: MAL1030285).
Gas chromatography-mass spectrometry (GC-MS) analysis of VD fume
The chemical composition of fume generated after the combustion of VD was analyzed using GC-MS/MS (7000D GC/MS triple quad with 7890B GC system) of Agilent with mass hunter software. For GC/MS/MS analysis, fume generated after the combustion of 25 g VD for 15 min in the fume assembly was extracted in hydro-methanol (50:50) solution. For extract preparation, 100 ml of hydro-methanol solution in 250 ml Büchner flask was aerated with fume for 15 min. The solution obtained was further centrifuged at 10 000 rpm for 5 min to separate any solid particles. The separation was carried out using HP-5MS capillary column (30 m × 0.25 mm, 0.25 µm). The injection volume was set to 1 μl using Helium as a gas carrier at a flow rate of 1 ml/min. The temperature of the split injector was programed at 280 °C with split ratio 20:1 with 60 °C as the initial temperature of the column (hold time 4 min) and then programmed at 3 °C /min to 135 °C (hold time 4 min), followed by 15 °C /min to 180 °C (hold time 4 min). The GC–MS ion source temperatures were set at 230 °C with an ionization potential of 70 eV. The relative percentage of each constituent present in VD fume hydromethanolic extract was calculated according to the area of the chromatographic peaks. The results were compared with the reference database using Standard Reference Data Program of the National Institute of Standards and Technology (NIST) Mass Spectral Library, version 2.2.
Bacterial Strains, Spread Plate Preparation and Assessment of Anti-Bacterial Effect of VD Fume on Bacterial Growth on agar Plate and in Culture
The bacterial strains of Staphylococcus epidermidis (MTCC 435), Staphylococcus aureus (MCC2408), Pseudomonas aeruginosa (MCC 3097), Pseudomonas fluoroscens (MTCC 2421), Salmonella enterica (MTCC 1165), Escherichia coli (MTCC 724), Streptococcus pneumonia (MCC 2425), E. coli (MTCC 729) (UPEC) and Mycobacterium spp. (NCIM 5240) were used for determination of antibacterial property of VD. S. epidermidis, P. fluoroscens, S. enterica and both E. coli strains were procured from Microbial Type Culture Collection (MTCC) in Council of Scientific & Industrial Research (CSIR)-Institute of Microbial Technology, Chandigarh, India. S. aureus, P. aeruginosa and S. pneumonia were procured from National Centre for Microbial Resource (NCMR) in National Centre for Cell Science, Pune, India. Mycobacterium spp. was obtained from National Collection of Industrial Microorganisms (NCIM) in National Chemical Laboratory, Pune, India. Total 50 µl of overnight culture of each bacterial strain containing 106 cells were spread on sterilized 60 mm nutrient agar plate. For each bacterial strain plates were prepared in two independent sets. One set was used for fume exposure and the other served as control. 5 g of VD was combusted for 3 min and fume collected as shown in Assembly 2 of Figure 1. The fume-filled chamber with bacterial plates was incubated at 37°C for 16 h, before, imaging the plates and counting the colony forming units (CFUs). In the control experiment, plates were incubated under identical conditions except for the exposure to VD. Experiment were repeated for several times (n ≥ 3) to generate the mean anti-microbial response of VD.
For assessing the anti-microbial effect of VD fume on growth kinetics of bacteria, fume generation and harvest was carried out according to either Assembly 3 or Assembly 4 plan shown in Figure 1, depending on whether the culture was to be exposed to the fume or to be aerated with the fume, respectively. This experiment was first done on pilot-scale with S. enterica, P. aeruginosa and E. coli (both gastric and UPEC) to evaluate which mode of fume treatment was more effective. After deducing that aeration was more efficient, effect of VD fume on growth kinetics of rest of the bacteria was done by aerating the actively growing cultures. Log-phase cultures with actively growing cells were used. A culture without any exposure to VD fume was included as a control. Only media aerated with VD fume was used as blank for the VD aerated cultures. In either case, 5 g of VD was combusted and fume harvested for 3 min. Secondary cultures of the bacteria, started fresh on the day of the experiment from overnight primary cultures, were allowed to grow till log-phase before treating with VD fume at 2 h from the starting of the culture. Absorbance were measured at 600 nm at given time intervals of 2, 4, 6, 8, 10, 12 and 24 h.
Anti-Fungal Activity of VD Fume
The antifungal activity of VD fume was assessed on Candida albicans (MCC 1155), obtained from NCMR in National Centre for Cell Science, Pune, India. Experimental plan followed was same as Assembly 2 (for plate) and 4 (liquid culture) of Figure 1. 106 C. albicans cells in yeast form were spread in duplicates on sterilized 60 mm potato dextrose agar plate. One set was exposed to VD fumes whereas the other served as control. The plates were incubated at 32 °C for 24 h before imaging. The effect of VD fumes on actively growing C. albicans cells was assessed by aerating a log phase liquid culture with VD fumes. A secondary culture was initiated in potato dextrose broth with 108 cells/ml of overnight primary culture of yeast form of C. albicans, and allowed to grow for 4 h until the log phase was reached. The culture was then aerated with fumes obtained from burning 5 g of VD for 3 min. Thereafter, absorbance at 540 nm was measured at 4, 8, 12, 16, 20, 24, 32, 40 and 48 h. A log phase culture without VD aeration was included as a control. Potatao dextrose broth, without any C. albicans cells, was aerated with VD and incubated under identical conditions to serve as blank for the aerated culture.
Evaluation of Environmental Disinfecting Capacity of VD
In three independent un-sanitized rooms [sized 16 (L) × 12 (W) × 10 (H) feet], VD was generated through a set up similar to the one shown in Assembly 2 of Figure 1, except instead of collecting the medicated fumes in a closed container, they were allowed to diffuse into the room randomly. A total of 40 g of VD was slow-combusted for 30 min to fill each room with medicated fumes. Five plate count agar (PCA) plates with lids removed were placed strategically across each room and exposed to the fumigated environment for 24 h. A similar set of plates were exposed before fumigation with VD. Both sets of plates following environmental exposure were incubated at 37 °C incubator for 24 h for microbial growth. Colonies appearing on the PCA plates exposed before and after disinfecting the rooms were counted and plotted as scatter plot.
Cytotoxicity Assay
Safety of the VD was evaluated on A549 human lung epithelial cells through alamar blue assay. The A549 cells were procured from American Type Culture Collection (ATCC) recognized repository at National Centre for Cell Sciences (NCCS), Pune, Maharashtra, India. The cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) media (Catalog # 12100-038, Gibco, MA, USA) supplemented with 5% fetal bovine serum (FBS) (Catalog # RM9955, HiMedia, Mumbai, India) and 1% penicillin-streptomycin mixture (Catalog # P4333, Sigma Aldrich, Bangalore, India) at 37 °C in a humidified incubator with 5% CO2. For alamar blue assay, 10 000 A549 cells/well were plated in complete medium in 96 well plate and allowed to grow upto 70% confluency. VD aerated medium was prepared by slow-combusting different amounts of VD (0.15, 0.3, 0.6, 1.25 and 2.5 g) for 5 min, and collecting the fumes in 100 ml of media, by bubbling those independently. Subsequently, plated A549 cells were exposed to VD aerated complete growth medium and incubated for 24 h. Viable cells were stained for 3 h at 37 °C with alamar blue (15 µg/ml) (Catalog # TC235, Sigma Aldrich, Bangalore, India) prepared in serum free DMEM media. Fluorescence from each well was measured using Envision microplate reader (Perkin Elmer, MA, USA) with excitation and emission at 560 and 590 nm, respectively. Percentage cell viability was calculated with respect to untreated cells and data represented as mean ± SEM.
Quantitative Data Representation and Statistical Analysis
All quantitative data were represented through line or bar graphs plotted using GraphPad Prism Software (version 7.0) (San Diego, CA, USA). In built analysis option of the software was used to determine the statistical significance of the observed differences between various groups, and were represented with *, ** and *** for P value < .05, .01 and .001, respectively. Two-way analysis of variance (ANOVA) was used to compare two or more parameters across more than two groups, whereas, t-test was employed when a single test parameter was compared between two groups.
Results
VD Fume Production, Collection and Transfer for Subsequent Analyses
Assessing the anti-microbial potential of the fume generated from the slow combustion of traditionally defined mix of medicinal herbs required ingenious experimental designing in terms of apparatuses used and protocols employed. Figure 1 illustrates the entire experimental plan. A typical production, collection and transfer circuitry consisted of a specially designed closed fumigator made of stainless steel, the outlet of which was connected to a closed microbiological chamber or Büchner flasks through sterile rubber tubing. The outlets of the microbiological chamber and Büchner flasks were further connected to a pump to enable efficient suction of fume from the fumigator. Prior to subjecting VD to slow combustion, the constituting herbal mix was finely powdered. Constituent medicinal herbs present in VD are enlisted in Table 1. Slow combustion of measured quantities of VD was carried out in the closed fumigator to generate thick fume which was transferred to either a closed chamber with bacteriological agar plates or Büchner flasks, depending on the subsequent analysis to be carried. For physico-chemical characterization through FESEM, DLS and GC-MS, VD fume was collected in Büchner flasks containing suitable solvents, such as, water for DLS and hydro-methanol for GC-MS. This circuitry is denoted as Assembly 1 in Figure 1. Anti-bacterial potential of VD fume was evaluated on bacteria growing on agar plates as well in cultures. For bacteria growing on agar plates, VD fume was transferred from the fumigator to a closed chamber containing the spread plates of different bacterial pathogens (Assembly 2 in Figure 1). In order to assess the anti-bacterial efficacy of VD fume on bacteria growing in cultures, either the cultures were exposed to the fume (Assembly 3 in Figure 1) or they were aerated with the fume (Assembly 4 in Figure 1). In assembly 3, the pipe inserted through the cork of the Büchner flask, whose outer opening was connected to the rubber tubing from the fumigator, has its opening inside the flask adjusted 2 inches above the surface of the culture. In assembly 4, the inner opening of the pipe was submerged into the culture but kept 1 inch above the bottom of the flask. With these assemblies in place, the study began with the physico-chemical characterization of the VD fume.
Physico-Chemical Characterization of VD Fume
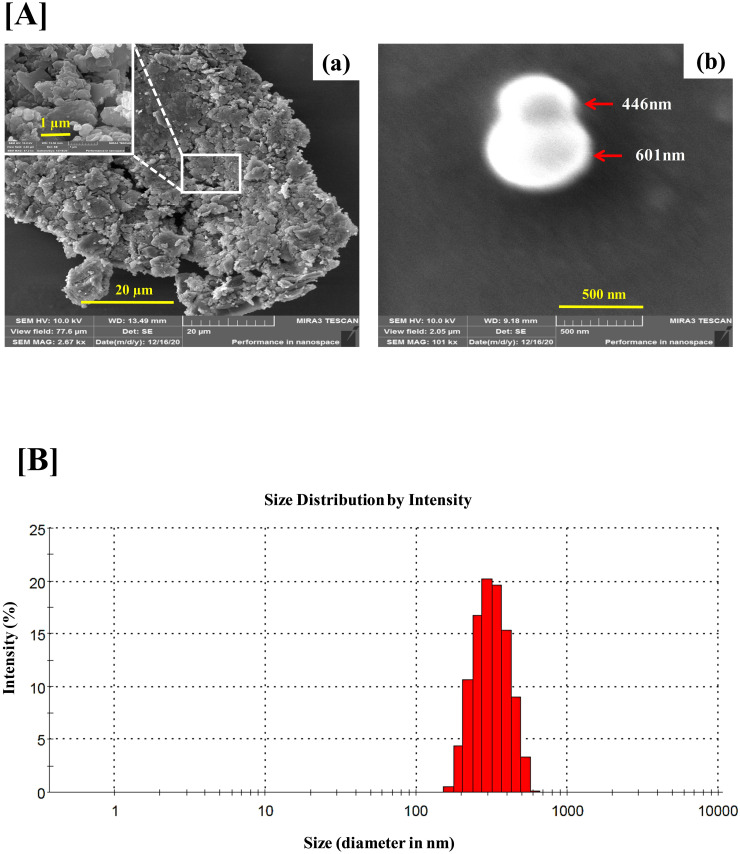
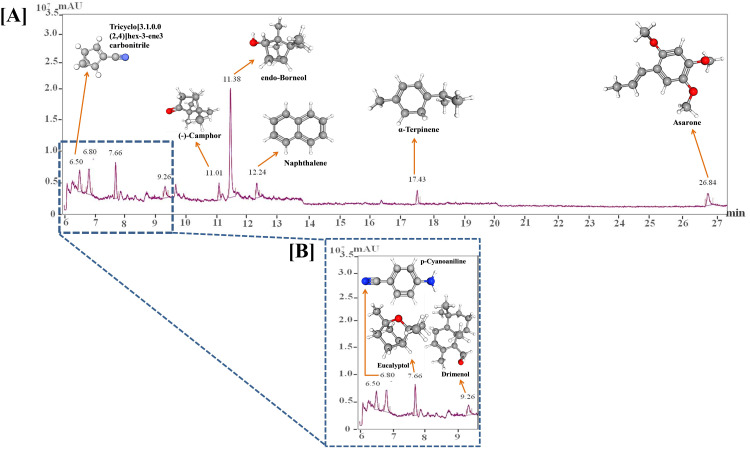
For physico-chemical characterization, VD fume was subjected to FESEM, DLS and GC-MS analyses, the first two for gaining insight into the physical nature of the fume and the last one for identifying the constituent phytochemicals present in it. Micrographs at different magnifications obtained from FESEM analysis provide a visual perception of the physical appearance of VD fume (Figure 2A). The micrograph with 20 µm scale bar shows the particulate nature of VD fume. The inset at 1 µm scale reveals that the particles do not conform to any particular shape and are below 1000 nm in diameters (Figure 2Aa). In order to have a better insight into the size range of the particles, micrograph at higher magnification corresponding to 500 nm scale was generated. The particles captured at this magnification appeared spherical with diameters measured as 441 and 601 nm (Figure 2Ab). For determining the size distribution and estimating the average size of the particles, DLS was used. From the intensity versus size histogram, the particles appeared to be between 150 to 600 nm in size with maximum being around 354 ± 84 nm (Figure 2B). Chromatogram from GC-MS analysis identified nine prominent phytoconstituents to be present in VD fume (Figure 3, Table 2). These are Tricyclo [3.1.0.0(2,4)] hex-3-ene-3-carbonitrile, p-Cyanoaniline, Eucalyptol, Drimenol, (-)-Camphor, endo-Borneol, Naphthalene, α-Terpinene and Asarone, mentioned in increasing order of their retention times (RTs), 6.50, 6.80, 7.66, 9.26, 11.01, 11.38, 12.24, 17.43 and 26. 84 min, in the column. Based on the comparison of the identified phytoconstituents with the standard metabolite library, similarity scores reveal that endo-Borneol shared the maximum score of 86.42, followed by Eucalyptol (81.88) and Asarone (79.14), whereas, rest of the compounds scored between 60 to 70, with (-)-Camphor having 68.17, α-Terpinene 67.08, Tricyclo [3.1.0.0(2,4)] hex-3-ene-3-carbonitrile 66.06, p-Cyanoaniline 65.58, Naphthalene 63.40 and Drimenol 61.96. From area %, VD fume was found to contain 41.07% endo-Borneol, 11.95% p-Cyanoaniline, 10.26% Eucalyptol, 8.30% Tricyclo [3.1.0.0(2,4)] hex-3-ene-3-carbonitrile, 7.18% Asarone, 5.86% Naphthalene, 5.74% Drimenol, 5.07% (-)-Camphor and 4.57% α-Terpinene. All the identified metabolites are known for their anti-microbial activities. Therefore, taken together, the observations from SEM, DLS and GC-MS confirm that slow combustion of VD generates ∼350 nm particles laden with a mix of nine very efficient anti-microbial metabolites. Anti-microbial potential of the VD fume was subsequently evaluated.
Figure 2.
Physical analyses of VD fume. [A] Scanning electron micrograms (SEM images) of VD fume particles at different magnifications [scale bars 20 µm (a), 1 µm (inset of a) and 500 nm (b)]. [B] Size distribution of particles present in VD fume is depicted through a histogram plotted with the diameters of the particles measured through dynamic light scattering (DLS).
Figure 3.
GC-MS analysis of VD fume. [A] Chromatogram showing the compounds identified in VD fume along with their corresponding retention times (RTs) and chemical structures. [B] A portion of the chromatogram between 6 and 9.5 min has been enlarged to prominently show the peaks of the identified compounds with retention times within this range.
Table 2.
Identified Compounds in Vishaghn Dhoop.
| Name | Chemical Structure | Formula | RT | Area | Area % | Score |
|---|---|---|---|---|---|---|
| Tricyclo[3.1.0.0(2,4)]hex-3-ene-3-carbonitrile |
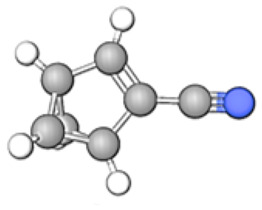
|
C7H5N | 06.50 | 15 734 408 | 8.30 | 66.06 |
| p-Cyanoaniline |
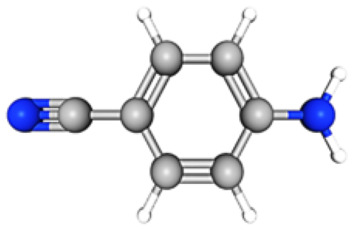
|
C7H6N2 | 06.80 | 22 657 004 | 11.95 | 65.58 |
| Eucalyptol |
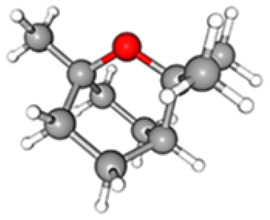
|
C10H18O | 07.66 | 19 447 494 | 10.26 | 81.88 |
| Drimenol |
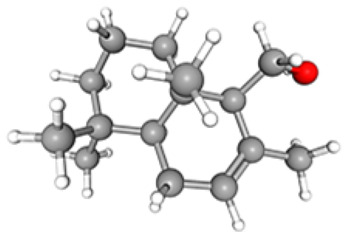
|
C15H26O | 09.26 | 10 882 929 | 05.74 | 61.96 |
| (-)-Camphor |
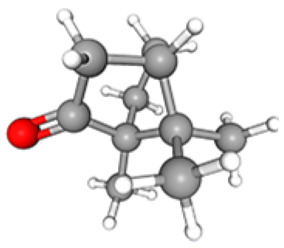
|
C10H16O | 11.01 | 9 602 885 | 05.07 | 68.17 |
| endo-Borneol |
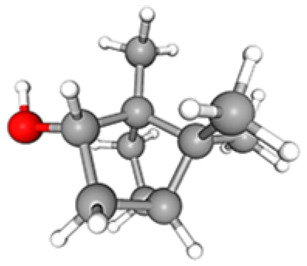
|
C10H18O | 11.38 | 77 871 535 | 41.07 | 86.42 |
| Naphthalene |
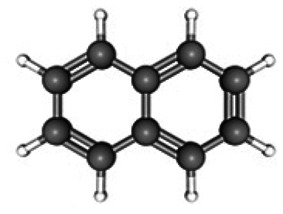
|
C10H8 | 12.24 | 11 117 785 | 05.86 | 63.40 |
| α-Terpinene |
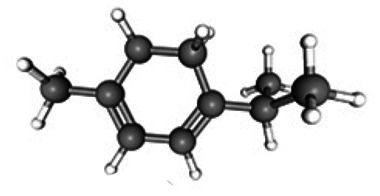
|
C10H16 | 17.43 | 8 665 689 | 04.57 | 67.08 |
| Asarone |
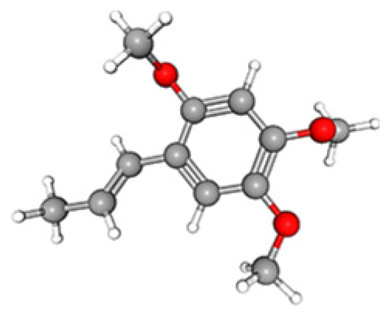
|
C12H16O3 | 26.84 | 13 606 550 | 07.18 | 79.14 |
VD Fume Reduced Bacterial Growth on agar Plates
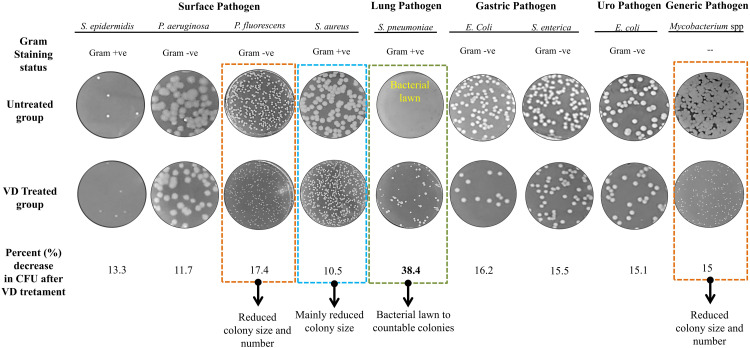
The first evaluation of the potent anti-microbial activity of VD fume was done on nine pathogenic bacteria, including both Gram-positive and Gram-negative members and belonging to different categories, such as, surface pathogens, like, S. epidermidis, P. aeruginosa, P. fluorescens and S. aureus, lung pathogen, like, S. pneumonia, gastric pathogens, like, E. coli and S. enterica, uropathogen, like, uropathogenic E. coli (UPEC), and a generic pathogen Mycobacterium spp. Actively growing cultures of these pathogenic bacteria were individually spread on agar plates before exposing to VD fume in the closed microbiological chamber, as mentioned in Figure 1 and incubated for 24 h at 37 °C. A set of corresponding spread plates of these pathogens without exposure to VD fume were incubated under identical conditions. Representative digital photographs of the bacteriological plates demonstrate that reduction in CFUs was observed in case of all the pathogens, the extent varying from pathogen to pathogen (Figure 4). Maximum percent reduction in CFU (38.4%) was observed in case of S. pneumonia, where, an entire bacterial lawn on an untreated plate was reduced to few countable CFUs on the VD treated plate (Figure 4, green dashed box). In case of P. fluorescens and Mycobacterium spp., respectively, 17.4% and 15% reductions in CFU were accompanied by visible decrease in the colony sizes (Figure 4, orange dashed box). Others, like, E. coli (gastric), S. enterica, E. coli (UPEC), S. epidermidis and P. aeruginosa showed 16.2, 15.5, 15.1, 13.3 and 11.7% decrease in CFU, respectively. Visible reduction in colony size was more pronounced in case of S. aureus (Figure 4, blue dashed box). These observations clearly demonstrated that VD fume reduced growth of all the nine pathogenic bacteria included in the study. Gram-staining status of the pathogenic bacteria did not appear to co-relate with the anti-bacterial activity of VD fume indicating that this fume treatment is likely to have a broad spectrum of targets. In fact, inhibitory effect of VD on Mycobacterium spp, known for thicker, waxy, mycolic acid rich cell wall, corroborates its broad spectrum targeting.
Figure 4.
VD fume affected the growth of a variety of pathogenic bacteria on agar plate. A self-explanatory picture reporting the fold changes observed in the number of colony forming units (CFUs) upon exposure of agar spread plates of different pathogenic bacteria in comparison to untreated control plates. Representative pictures of different bacterial plates in untreated and VD treated groups are provided. Type of Gram staining taken by each of the studied pathogenic bacteria is also mentioned. Mycobacterium due to waxy cell wall is incompatible with Gram staining and hence, not identified with respect to Gram staining. Categories of these pathogens based on their area of prevalence or target organ within the body, such as, surface, lung, gastric, urogenital and generic, are mentioned. Bacterial pathogens exhibiting decrease in colony size with or without decrease in CFUs are demarcated with orange and blue dashed boxes, respectively, and identified underneath with observed specific effects. Representative plate pictures of S. pneumoniae exhibiting maximum fold change in CFU is demarcated with green dashed box.
VD Fume Inhibited Bacterial Growth in Cultures
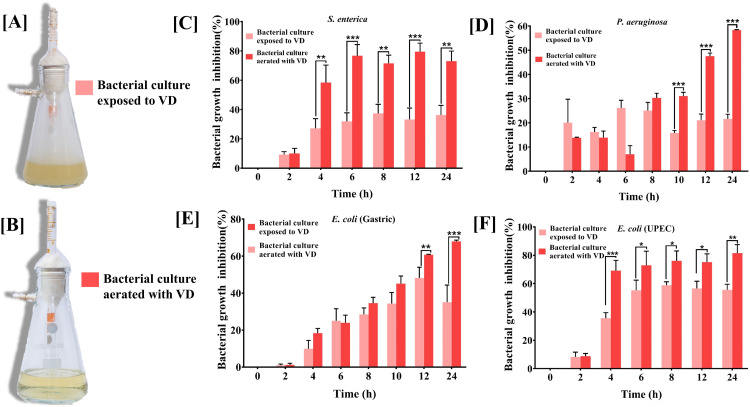
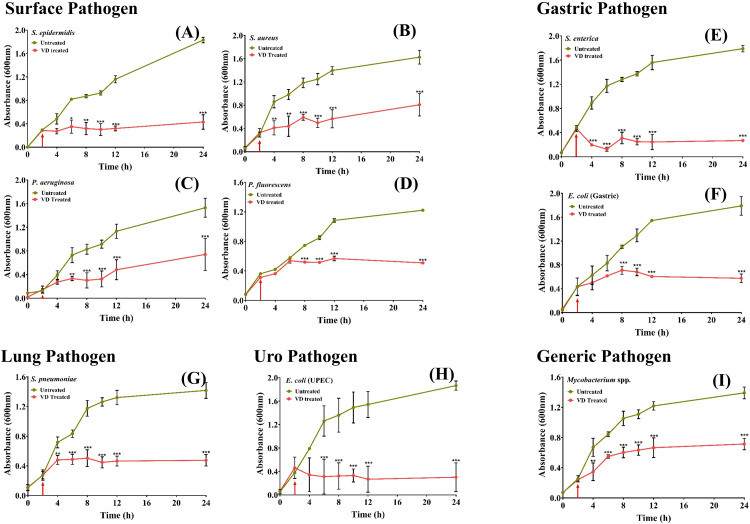
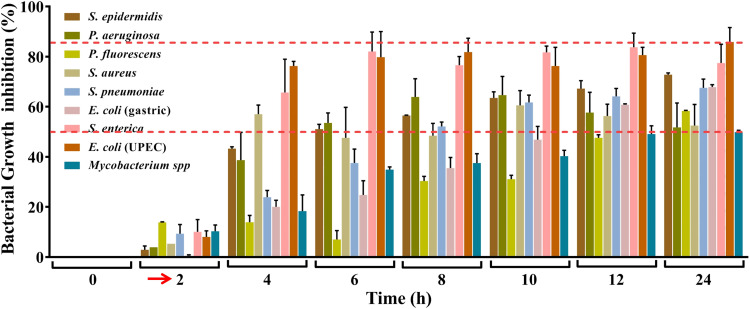
In real life situations, presence of pathogenic bacteria is not limited to solid surfaces. Moreover, given the observations that VD fume can inhibit pathogenic bacteria that affect internal organs, where the milieu is expected to be fluidy, it was pertinent to evaluate the anti-bacterial efficacy of VD on bacterial cultures. Two modes of fume treatment of bacterial cultures were first evaluated using four pathogens, namely, S. enterica, P. aeruginosa, E. coli, both gastric and UPEC. In the first set up (Assembly 3 in Figure 1), the cultures were exposed to VD fume (Figure 5A), whereas in the second set up (Assembly 4 in Figure 1), the cultures were aerated with VD fume (Figure 5B). Effect on growth kinetics of the bacteria, in both the cases, was followed by measuring the optical density (OD) of the cultures at a wavelength of 600 nm at given time intervals. Percent growth inhibition, determined relative to the untreated cultures, exhibits decrease in growth in both cases, but the effect was significantly higher when the cultures were aerated with VD fume. This observation was alike for all the four pathogens included in this study (Figure 5C-F). Therefore, in subsequent experiments involving all the nine pathogenic bacteria, actively growing cultures were aerated with VD fume. In order to provide a better visual perception of the extent of inhibition occurring in real time, the measured ODs for untreated and treated cultures are plotted over 24 h for each of the nine pathogenic bacteria, assorted under categories of surface, gastric, lung, uro and generic (Figure 6). Bacterial cultures were exposed to VD fume, according to the design depicted in Figure 1 (Assembly 4), 2 h after secondary cultures were started from overnight primary ones. This time point was decided based on the generation time of the bacteria included in the study, so that the treatment was rendered to actively growing cultures only. This had been the case as evident from the rising slopes of all the graphs, pertaining to both untreated and treated cultures (Figure 6, red arrows). All bacterial pathogens except, P. fluorescens, showed an arrest in measured OD by 2 h (that is, 4 h of secondary culture set up) of VD fume treatment (Figure 6A-C, E-I). In case of P. fluorescens, this arrest was observed to set in after 6 h of VD treatment (Figure 6D). Out of S. epidermidis, S. aureus, P. aeruginosa, S. enterica, E. coli (both gastric and UPEC), S. pneumonia and Mycobacterium, significant OD arrests by 2 h of VD treatment were evident in S. aureus, S. enterica, S. pneumoniae and Mycobacterium spp. (Figure 6B, E, G and I). OD arrests were statistically significant after 4 h of VD treatment in case of S. epidermidis, P. aeruginosa and E. coli (UPEC) (Figure 6A, C and H). E. coli (gastric) although showed arrest in OD from 2 h after VD treatment, but the observation was not statistically significant until 6 h post-treatment (Figure 6F). Similarly, P. fluorescens showed significant OD arrest 6 h post VD fume treatment until then, exhibiting a growth curve comparable to its corresponding untreated control culture (Figure 6D). With the confirmation that aeration of cultures with VD fume treatment stalls the increase in OD due to bacterial growth in all the pathogenic bacteria studied, a comparison of the percent growth inhibition among these bacteria was conducted (Figure 7). Growth of E. coli (UPEC) was maximally inhibited (85%) followed by that of S. enterica (80%). Least inhibition (50%) was observed for Mycobacterium spp. Maximum inhibitions for all other pathogens were within this 50 to 85% range. The respective maximal inhibition for each pathogen was reached by 22 h of VD treatment and maxima-minima inhibition range of 50 to 85% was clear by this time for this study with the mentioned pathogenic bacteria. Thus, these observations clearly demonstrate the efficacy of VD fume in inhibiting growth of different pathogenic bacteria, by and large, establishing its potential as an alternative option for environmental cleansing from pathogenic bacteria. Since, pathogenic bacteria are not the only source of HAIs, in the next experiment, anti-fungal activity of VD fume against one of the most prominent nosocomial fungi, pathogenic Candida albicans was assessed.
Figure 5.
Media aeration with VD fume inhibited bacterial growth more efficiently. [A, B] Pictures of Büchner flasks showing the bacterial culture exposed to VD fume (A) and aerated with VD fume (B). [C-F] Graphs showing comparisons between percent growth inhibition observed in selected pathogenic bacteria, namely, S. enterica (C), P. aeruginosa (D), E. coli (gastric) (E) and uro-pathogenic (UPEC) E. coli (F), in case of cultures exposed to VD fume (light pink bars) and those aerated with the latter (dark pink bars). Data represented is mean ± SE obtained from three independent experiments. Two-way analysis of variance (ANOVA) with Bonferroni's post-hoc tests were used run to analyse the statistical significance of the observed differences between mean growth inhibitions of the two groups at different time points. *, ** and *** denote P values < .05, < .01 and < .001, respectively.
Figure 6.
VD fume aerated cultures of different pathogenic bacteria exhibited reduced growth. Effect on growth kinetics of different actively growing pathogenic bacteria belonging to various categories represented through line graphs. Categories include surface pathogens, like, S. epidermidis (A), S. aureus (B), P. aeruginosa (C) and P. fluorescens (D), gastric pathogens, like, S. enterica (E) and E. coli (F), lung pathogen, like, S. pneumonia (G), uro-pathogen, like, E. coli (H) and a generic pathogen, Mycobacterium spp (I). Time point at which cultures were aerated with VD fume is indicated with a red arrow in each graph. Statistical significance of the differences observed between VD fume treated and untreated cultures at a given time point was analyzed using two-way analysis of variance (ANOVA) with Bonferroni's post-hoc test and denoted with *, ** and *** for P- values < .05, < .01 and < .001, respectively.
Figure 7.
Comparison between the growth inhibitory effects of VD fume on different pathogenic bacteria. Composite bar graph showing percent growth inhibitions of nine pathogenic bacteria recorded at different time points. Cultures were aerated with VD fume at 2 h which is indicated with red arrow along the X-axis. The overall maxima-minima inhibition range is demarcated with red dashed lines.
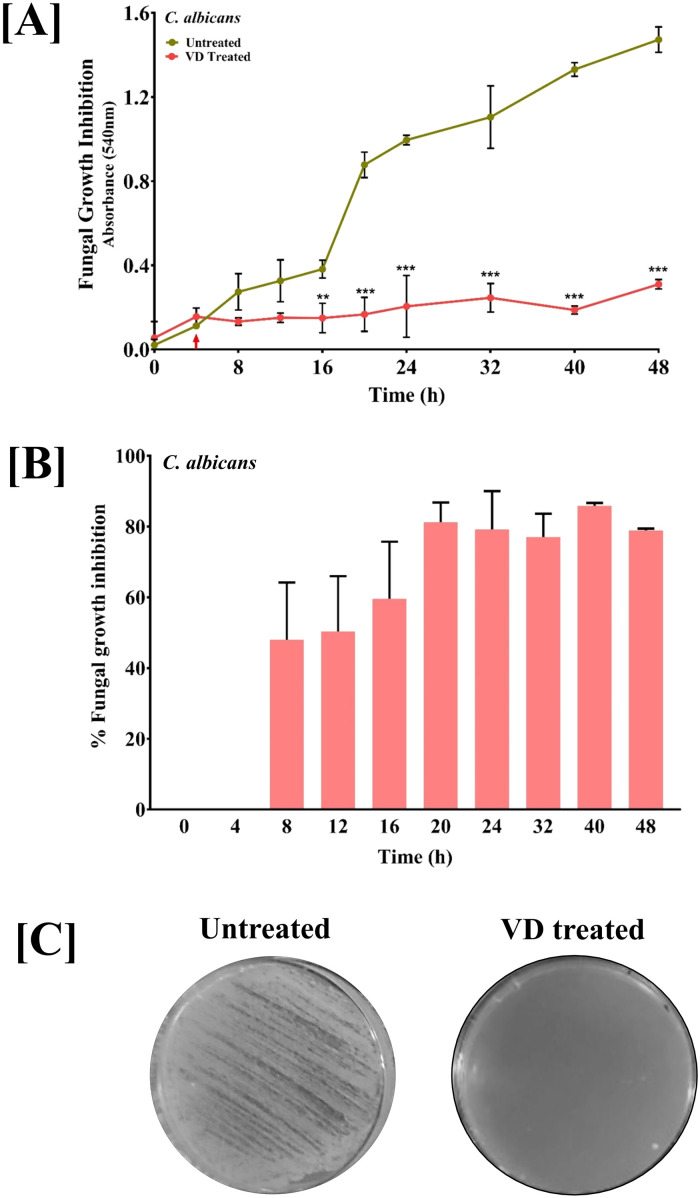
Anti-Fungal Activity of VD Fume Against Candida albicans
Employing experimental designs identical to bacterial pathogens, anti-fungal activity of VD fume on C. albicans was evaluated according to the study design depicted in Assemblies 2 and 4 of Figure 1. Actively growing culture (indicated by red arrow in Figure 8A) of C. albicans was either directly aerated with VD fume or spread on potato dextrose agar plates and then exposed to VD fume. Growth arrest in case of actively growing culture was found to set in 4 h after VD fume treatment although, statistically significant observation took another 8 h to surface. The anti-fungal activity of VD fume against C. albicans was found to be comparable to its anti-bacterial activity as evident from 80% inhibition of fungal growth by 16 h of VD treatment (Figure 8B). This effect was even more robust on agar plates (Figure 8C). Thus, VD is not only effective against pathogenic bacteria, but could also affect pathogenic fungus.
Figure 8.
Anti-fungal potential of VD fume against Candida albicans. [A] Effect of culture aeration with VD fume on growth kinetics of pathogenic C. albicans shown as line graphs. Cultures were aerated with VD fume at 4 h which is indicated with red arrow along the X-axis. Statistical significance of the observed difference between growth in untreated and VD fume aerated C. albicans cultures was analyzed using two-way analysis of variance (ANOVA) with Bonferroni's post-hoc test and indicated with *, ** and *** for P-values < .05, < .01 and < .001, respectively. [B] Inhibition of C. albicans growth subsequent to culture aeration with VD fume represented as bar graphs. [C] Representative images of plates showing the effect of VD fumes on C. albicans.
VD Reduced Environmental Microbial Load Without Affecting the Cells Adversely
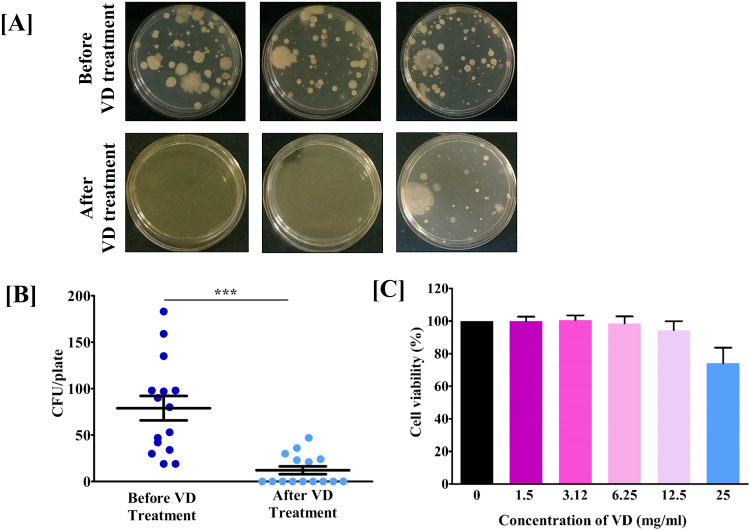
In order to test the practical applicability of VD in sterilizing indoor air, environmental monitoring of rooms was done before and after exposure to the medicated fumes. There was an appreciable visible reduction in the microbial load following fumigation of the rooms with VD for 30 min (Figure 9A), showing nearly 10-fold drop in the counts of CFUs (Figure 9B).
Figure 9.
VD reduced microbial load in indoor environment without adversely affecting the human lung cells in vitro. [A] Representative plate pictures showing the effect of VD on environmental microbial load. [B] Quantitative depiction of observation (A) as scatter plots of CFUs/plate before and after fumigating 16 × 12 × 10 ft un-sanitized rooms with VD for 30 min. Statistical significance of the observed difference between microbial load on plates incubated before and after fumigating the rooms with VD was analyzed using t-test and indicated with *** for P-values < .001. [C] Bar graph showing the effect of media-aerated various concentrations of VD, on human lung epithelial (A549) cell viability.
The other important aspect of practical application of fumigation technique is its safety. In case of VD, that was appraised through cytotoxicity assay using human lung epithelial cells, A549. The actively growing cells were exposed for 24 h to media aerated with different concentrations of VD (1.5, 3.12, 6.25, 12.5 and 25 mg/ml) bubbled in culture media, before assessing the cytotoxicity. Results show that A549 cells were viable up to the highest dose tested (25 mg/ml) of VD aerated media. (Figure 9C). Taken together, it can be concluded that VD is capable of sterilizing indoor atmosphere and is safe on human lung cells in culture.
Discussion
Burden of HAIs is increasing rapidly. 1 Chemical disinfection is the most commonly employed measure to contain HAIs.2,3 However, due to the adverse side-effects, including toxicity, carcinogenicity and high risk of environmental pollution, the HAI management system is gradually heading towards a precipice of tradeoff between prevention of HAIs and the mostly employed preventive technique. Alternative options of disinfection, with less or preferably no side-effects, will be a respite beyond comparison. With this requirement as the premise, the current study was conducted to offer preliminary scientific evidence in favor of disinfection techniques, described in ayurveda, the traditional Indian system of medicine. In this regard, the technique of dhoopan, one of the traditional disinfection methods, was selected. Dhoopan involves generating thick fume through slow combustion of individual or mix of medicinal herbs, minerals and/or animal products. 4 In this study, a specific mix of twelve medicinal herbs, resin from Laccifer lacca, rock salt and clarified butter called ‘Vishaghn Dhoop’ was used. The fume generated from VD was subjected to physico-chemical characterization and evaluation of anti-microbial activities against nine pathogenic bacteria belonging to different categories based on their site of infection, namely, surface pathogens, like, S. epidermidis, S. aureus, P. aeruginosa and P. fluorescens, gastric pathogens, like, S. enterica and E. coli, lung pathogen, like, S. pneumonia, uro-pathogen, E. coli (UPEC) and generic Mycobacterium spp. and pathogenic fungus, C. albicans. Centre for Disease Control, World Health Organization (CDC-WHO) has identified S. aureus, P. aeruginosa, E. coli and non-tuberculous Mycobacteria (NTM), the one used in this study, among others to be responsible for HAIs. 15 Likewise, C. albicans (and very recently, emerging C. auris) has been identified by CDC-WHO through Emerging Infections Program (EIP) Healthcare-Associated Infections Community (HAIC) Interface as the major fungal source of HAI. 16 Micro-organisms included in this study is a representative range involved in causing HAIs.
All the ingredients of VD, including the medicinal herbs, Curcuma longa L., Aconitum heterophyllum Wall. Ex, Terminalia chebula Retz. Royle, Cyperus rotundus L., Elettaria cardamomum (L). Maton, Cinnamomum verum Lukman, Tabernaemontana divaricata (L). R. Br. Ex Roem & Schult, Saussurea costus (Falc). Lipsch, Acorus calamus (L), Callicarpa macrophylla Vahl, Brassica campestris var. sarson Prain and Commiphora wightii (Arn) Bhandari, are known for their anti-microbial activities.17–29 Besides, the rock salt and clarified butter present in VD are also reported to have anti-microbial property. Interestingly, authoritative ayurvedic texts, like Caraka-saṃhitā, Suśruta-saṃhitā and Vāgbhata-saṃhitā, not only identify micro-organism as Krimi but classify and nomenclate them based on their visibility to naked eye [invisible (Adrishya) and visible (Drishya)] and pathogenicity [non-pathogenic or natural (Sahaja) and pathogenic (Vaikārika)]. The pathogens are further classified into external (Bāhya) and internal (Ābhyantara).30–32 In addition to the mention of the composition of VD in the Compendium of Suśruta (Suśruta-saṃhitā), 14 references for anti-microbial activity of these ingredients (that is, their ability to destroy Krimi, the ayurvedic identity of micro-organisms) are also available in other classical texts of ayurveda. For example, Dhanwantari-nighantu mentions about the anti-microbial capability of L. lacca, C. longa, A. heterophyllum, C. rotundus and A. calamus. C. rotundus is also mentioned in Bhāvprakāsh-nighantu. Similarly, Caraka-saṃhitā mentions about the anti-microbial potency of C. longa. Śāligrām-nighantu recommends A. heterophyllum, T. chebula, and C. verum as anti-microbial agents. Madnādi- nighantu identifies the anti-microbial potency of T. chebula. Laghu-nighantu and Śodhāla-nighantu mention about E. cardamomum and C. macrophylla, respectively. Anti-microbial potential of T. divaricata and S. costus are mentioned in Aśtanga-nighantu. Thus, the ingredients present in VD are not chosen randomly, rather, the anti-microbial activity of each of them is recognized in more than one classical ayurvedic texts and testified by scientific studies.
The ingenious experimental designs comprising of a basic circuitry governing the generation, collection and transfer of VD fume have been devised keeping practical reproducibility in perspective. Set-ups used for VD fume generation, plate exposure and solvents/ medium used for fume harvest for different analyses were chosen with care to ensure minimum variability in the analyzed VD fume, from the one to be generated during its intended practical application. Considering the atmospheric moisture, the VD fume for GC-MS was collected in water. Similarly, to ensure translatability of these studies to practical applications against, anti-bacterial assays were conducted on bacteria growing on agar plates as well as in liquid cultures. The observed particulate nature of the VD fume shows that it can be a potential alternative for chemical fumigation to disinfect difficult to reach niches. Metabolites identified in VD fume, endo-Borneol, p-Cyanoaniline, Eucalyptol, Tricyclo [3.1.0.0(2,4)] hex-3-ene-3-carbonitrile, Asarone, Naphthalene, Drimenol, (-)Camphor and α-Terpinene have anti-microbial activities.33–41 It is fascinating to note that while nanoparticle mediated disinfection is being considered as a promising approach to replace chemical disinfection, an ancient technique of fume disinfection, like, dhoopan, in actually capable of generating nano-sized particles laden with anti-bacterial metabolites, just by following a traditionally described protocol. The observed anti-bacterial and anti-fungal activities of VD fume provide evidence in favor of a completely natural potential alternative for chemical fumigation. Due to all-natural ingredients and the employed process being in-sync with nature, this technique likely to have no deleterious side-effects. In addition, the technique of dhoopan can be modulated and extended beyond its projected use in disinfection, through variation of medicinal herbs/ herbal mixes being burnt in combination with different respiratory exercises described in Yoga. Actually, the noteworthy ability of VD fume to reduce S. pneumoniae growth, both on plate and in culture testifies this possibility. These microbiological observations become more appreciable with real-life (in vivo) validations. In this regard, un-sanitized rooms were fumigated with VD fumes and microbial load was assessed through environmental monitoring. Air from each room was sampled on five randomly placed plates to eliminate sampling bias. The microbial loads in these rooms were visibly reduced after fumigation with VD fumes. This demonstrated the ability of VD fumes to sterilize indoor air. The cost of fumigating one of these rooms was approximately 30 INR, excluding the price of the fumigator. Therefore, sterilization with VD fumes can be cost-effective. Further validation of air sterilizing potential of VD fumes in larger enclosures and actual health care settings are warranted. Fumes are readily inhaled and therefore, are a ready source of occupational respiratory hazard. So, it was pertinent to establish the safety of VD fumes. Lungs are the primary internal targets of hazardous fumes, therefore, preliminary evaluation of the safety associated with VD fumes was conducted on deliberately chosen A549 human lung epithelial cells. Actively growing A549 cells were exposed to VD aerated growth media for 24 h, and the effect on their survival was monitored. Using the flexibility of an in vitro system, the effect of a dose dependent exposure of A549 cells to VD fumes was assessed. The VD fumes were found to be safe on these lung cells, thereby, satisfying a crucial requirement of non-toxicity. Altogether, the observations from this study provided substantial evidence to encourage developing the combination of Dhoopan technique and Vishaghn Dhoop into an acceptable disinfection technique in general healthcare set-ups. Further elaborative studies at multiple sites would re-affirm and strengthen the scientific validation of VD reported here.
Conclusions
The current study was initiated with the aim of producing scientific testimony to the efficacy of dhoopan, the ancient technique of fumigation. With an enterprising approach, the lack of prior literature on similar studies was circumvented and the evidence establishing the anti-microbial efficacy of Vishaghn Dhoop/dhoopan combination was generated. In order to develop this combination into a disinfection technique, with no potential harmful effects, further studies in healthcare settings are required. Nevertheless, the observations made here warrants for further elaborative studies at multiple sites to establish the effect of this traditional sterilization technique in hospital settings. With rapid increase of HAIs, demand for disinfection in the healthcare settings are rising proportionately. Thus, this is actually an appropriately timed study to introduce the idea of possible complementary and/or alternatives for harmful chemical fumigation in managing HAIs.
Acknowledgments
We are grateful to Prof. Partha Roy (Department of Biotechnology, Indian Institute of Technology Roorkee) for his help with DLS analysis of VD fume. We thank Dr Anamika Kumari for the experimental help. We acknowledge Mr Tarun Patel for his help with photography of the experimental assembly. We are thankful to Dr. L. P. Singh and colleagues at Central Building Research Institute, Roorkee, India for their support with the Scanning Electron Microscopy. We extend our gratitude to Ms Priyanka Kandpal, Mr Tarun Rajput, Mr Gagan Kumar and Mr Lalit Mohan for their swift administrative support. This research received no external funding. This presented work has been conducted using internal research funds from the Patanjali Research Foundation Trust, Haridwar, India.
Footnotes
Author Contributions: Conceptualization, A.B. and A.V.; Methodology, S.Y., S.V., K.S., Y.V. and S.R.; Validation, S.H.; Formal analysis, S.H.; Investigation, S.Y., S.V., K.S., Y.V. and S.R.; Resources, A.B. and A.V.; Data curation; S.H.; Writing – Original Draft, S.H.; Writing – Review & Editing, A.V. and S.H.; Visualization, S.H.; Supervision, A.V.; Project administration, S.H.; Funding Acquisition, A.B. and A.V.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work has been conducted using internal research funds from Patanjali Research Foundation Trust, Haridwar, India.
ORCID iD: Anurag Varshney https://orcid.org/0000-0001-8509-0882
References
- 1.The burden of health care-associated infection worldwide. World Health Organization. Published 2011. Accessed March 25, 2021. https://www.who.int/gpsc/country_work/summary_20100430_en.pdf
- 2.Guideline for disinfection and sterilization in healthcare facilities. Centers for Disease Control and Prevention. Published 2008. Accessed March 25, 2021. https://www.cdc.gov/infectioncontrol/guidelines/disinfection/disinfection-methods/index.html
- 3.Tables and figure: Table 4 guideline for disinfection and sterilization in healthcare facilities. Centers for Disease Control and Prevention. Published 2008. Accessed March 25, 2021. https://www.cdc.gov/infectioncontrol/guidelines/disinfection/tables/table4.html
- 4.Urkude MA. Concept of Dhoopan Chikitsa (Medicinal fumigation therapy) and its importance in prevention of air-borne diseases– a review. Int J Res Pharm Sci. 2020;11(4):5104-5107. doi: 10.26452/ijrps.v11i4.3113 [DOI] [Google Scholar]
- 5.Shrestha S, Bedarkar PB, Chaudhari SY. Dhoopana karma : a review through brihatrayi dhoopana karma : a review through brihatrayi. Int Ayurvedic Med J. 2017 ;1 (3): 316-325 [Google Scholar]
- 6.Gadag SM, Amilkanthwar RH. Concept of sterilization in ayurveda: a review. World J Pharm Med. 2019;5(5):51-53. [Google Scholar]
- 7.Loukas M, Lanteri A, Ferrauiola J, et al. Anatomy in ancient India: a focus on the Susruta Samhita. J Anat. 2010;217(6):646-650. doi: 10.1111/j.1469-7580.2010.01294.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bopate DK, Chavhan KR. Dhoopana Chikitsa as a treatment modality: a review. World J Pharm Res. 2020;9(10):163-170. doi: 10.20959/wjpr202010-18431 [DOI] [Google Scholar]
- 9.Sumitha L, Prasad BS. Evaluation of antimicrobial and antifungal property of dhoopana karma (fumigation) by “Dhup”an ayurvedic dhoopana product. Int J Pharm Sci Res. 2015;6(7):2950-2954. doi: 10.13040/IJPSR.0975-8232.6(7).2950-54 [DOI] [Google Scholar]
- 10.Celine C, Sindhu A, Muraleedharn MP. Microbial growth inhibition by aparajitha dhooma choornam. Anc Sci Life. 2007;26(3):4-8. [PMC free article] [PubMed] [Google Scholar]
- 11.Bisht LS, Brindavanam NB, Kimothic P. Comparative study of herbal agents used for fumigtation in relations to formulation(*). Anc Sci Life. 1988;8(2):125-132. [PMC free article] [PubMed] [Google Scholar]
- 12.Bhatwalkar SB, Shukla P, Srivastava RK, Mondal R, Anupam R. Validation of environmental disinfection efficiency of traditional Ayurvedic fumigation practices. J Ayurveda Integr Med. 2019;10(3):203-206. doi: 10.1016/j.jaim.2019.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamble N, Gadkari R. Application of dhoopan karma in present scenario- a review Nikita. Int J Res Indian Med. 2018;02(5):1-4. [Google Scholar]
- 14.Yagyadev S. Yagya cures epidemic diseases. Yog Sandesh. 2020; 17(7):50-52. [Google Scholar]
- 15.Healthcare-associated infections: Diseases and organisms in healthcare settings. Prevention, Centers for Disease Control. Published 2019. Accessed March 25, 2021. https://www.cdc.gov/hai/organisms/organisms.html
- 16.Healthcare-Associated Infections - Community Interface (HAIC) Candida bloodstream infections. Centers for Disease Control and Prevention. Published 2021. Accessed March 25, 2021. https://www.cdc.gov/hai/eip/candida.html
- 17.Srivastava S, RoyChowdhury A, Maurya S. Antimicrobial efficacy of methylated Lac Dye, an anthraquinone derivative. Indian J Microbiol. 2017;57(4):470-476. doi: 10.1007/s12088-017-0682-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zorofchian Moghadamtousi S, Abdul Kadir H, Hassandarvish P, Tajik H, Abubakar S, Zandi K. A review on antibacterial, antiviral, and antifungal activity of curcumin. Biomed Res Int. 2014;2014 (Article ID 186864):1-12. doi: 10.1155/2014/186864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prasad SK, Jain D, Patel DK, Sahu AN, Hemalatha S. Antisecretory and antimotility activity of Aconitum heterophyllum and its significance in treatment of diarrhea. Indian J Pharmacol. 2014;46(1):82-87. doi: 10.4103/0253-7613.125182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mostafa MG, Rahman M, Karim MM. Antimicrobial activity of Terminalia chebula. Int J Med Arom Plants. 2011;1(2):175-179. http://www.openaccessscience.com [Google Scholar]
- 21.Khalid KM, Shnawa BH, Abdullah SM. Antimicrobial activity of Cyperus rotundus Linn. Extracts and phytochemical screening. Eurasian J Sci Eng. 2017;3(2): 82-89. doi: 10.23918/eajse.v3i2p82 [DOI] [Google Scholar]
- 22.Souissi M, Azelmat J, Chaieb K, Grenier D. Antibacterial and anti-inflammatory activities of cardamom (Elettaria cardamomum) extracts: potential therapeutic benefits for periodontal infections. Anaerobe. 2020;61(xxxx):102089. doi: 10.1016/j.anaerobe.2019.102089 [DOI] [PubMed] [Google Scholar]
- 23.Yap PSX, Krishnan T, Chan KG, Lim SHE. Antibacterial mode of action of Cinnamomum verum bark essential oil, alone and in combination with piperacillin, against a multi-drug-resistant Escherichia coli strain. J Microbiol Biotechnol. 2015;25(8):1299-1306. doi: 10.4014/jmb.1407.07054 [DOI] [PubMed] [Google Scholar]
- 24.Gopinath SM, Suneetha TB, Mruganka VD, Ananda S. Evaluation of antibacterial activity of Tabernaemontana divaricata (L.) leaves against the causative organisms of Bovine mastitis. Int J Res Phytochem Pharmacol. 2011;1(4):211-213. [Google Scholar]
- 25.Abdallah EM, Qureshi KA, Ali AMH, Elhassan GO. Evaluation of some biological properties of Saussurea costus crude root extract. Biosci Biotechnol Res Commun. 2017;10(4):601-611. doi: 10.21786/bbrc/10.4/2 [DOI] [Google Scholar]
- 26.Kumar V. Antimicrobial activity of rhizome extract of Acorus calamus against different micro-organisms. Octa J Biosci. 2014;2 (1):59-63. [Google Scholar]
- 27.Yadav V, Jayalakshmi V, Singla RK, Patra A. Evaluation of antibacterial activity of Callicarpa macrophylla Vahl. Stem extracts. Webmed Cent. 2012;WMC003651:1-7. [Google Scholar]
- 28.Agrawal MK, Rathore D, Goyal S, Varma A, Varma A. Antibacterial efficacy of Brassica campestris root, stem and leaves extracts. Int J Adv Res. 2013;1(2320):131-135. http://journaldatabase.info/articles/antibacterial_efficacy_brassica.html [Google Scholar]
- 29.Singh BRAJ, Siddiqui MZ. Antimicrobial activity of Commiphora wightii gum (guggul gum) extract against gram positive and gram negative bacteria. JMAA. 2015;1(2):36-39. [Google Scholar]
- 30.Manohar R. Accounts of pathogenic organisms in the early texts of ayurveda. Indian J Hist Sci. 2012;4(October):545-559. [Google Scholar]
- 31.Saini Savita PMS. Ayurvedic aspects of Bacteria and bacterial food poisoning. Int J Pharm Sci Res. 2015;6(6):2281-2290. doi: 10.13040/IJPSR.0975-8232.6(6).2281-90 [DOI] [Google Scholar]
- 32.Deogade MS, Rama S, Kethamakka P. Krumi (microorganisms) in ayurveda - A critical review. Int J Ayurvedic Med. 2019;10 (4):297-300. [Google Scholar]
- 33.Al-Farhan KA, Warad I, Al-Resayes SI, Fouda MM, Ghazzali M. Synthesis, structural chemistry and antimicrobial activity of -(-) borneol derivative. Cent Eur J Chem. 2010;8(5):1127-1133. doi: 10.2478/s11532-010-1093-0 [DOI] [Google Scholar]
- 34.Qian W, Yuan HK, Zhang R, Fang RQ. Silver(I) complexes with halo-substituted cyanoanilines: synthesis, characterization and antibacterial activity. J Coord Chem. 2016;69(23):3593-3602. doi: 10.1080/00958972.2016.1242727 [DOI] [Google Scholar]
- 35.Hendry ER, Worthington T, Conway BR, Lambert PA. Antimicrobial efficacy of eucalyptus oil and 1,8-cineole alone and in combination with chlorhexidine digluconate against microorganisms grown in planktonic and biofilm cultures. J Antimicrob Chemother. 2009;64(6):1219-1225. doi: 10.1093/jac/dkp362 [DOI] [PubMed] [Google Scholar]
- 36.Aremu OS, Gopaul K, Kadam P, et al. Synthesis, characterization, anticancer and antibacterial activity of some novel pyrano[2,3-d]pyrimidinone carbonitrile derivatives. Anticancer Agents Med Chem. 2016;17(5):719-725. doi: 10.2174/1871520616666160813213245 [DOI] [PubMed] [Google Scholar]
- 37.Joshi N, Prakash O, Pant AK. Essential oil composition and in vitro antibacterial activity of rhizome essential oil and β-asarone from acorus calamus l. Collected from lower Himalayan region of Utarakhand. J Essent Oil-Bearing Plants. 2012;15(1):32-37. doi: 10.1080/0972060X.2012.10644016 [DOI] [Google Scholar]
- 38.Rokade YB, Sayyed RZ. Naphthalene derivatives: a new range of antimicrobials with high therapeutic value. Rasayan J Chem. 2009;2(4):972-980. [Google Scholar]
- 39.Khumalo GP, Sadgrove NJ, Van Vuuren S, Van Wyk BE. Antimicrobial activity of volatile and non-volatile isolated compounds and extracts from the bark and leaves of Warburgia salutaris (Canellaceae)against skin and respiratory pathogens. South African J Bot. 2019;122(xxxx):547-550. doi: 10.1016/j.sajb.2018.10.018 [DOI] [Google Scholar]
- 40.Guimarães AC, Meireles LM, Lemos MF, et al. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules. 2019;24(13):1-12. doi: 10.3390/molecules24132471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang L, Zhang K, Zhang K, et al. Antibacterial activity of Cinnamomum camphora essential oil on Escherichia coli during planktonic growth and biofilm formation. Front Microbiol. 2020;11(November):1-11. doi: 10.3389/fmicb.2020.561002 [DOI] [PMC free article] [PubMed] [Google Scholar]