Abstract
The coronavirus disease-2019 (COVID-19) pandemic started in early 2020 with the outbreak of a highly pathogenic human coronavirus. The world is facing a challenge and there is a pressing need for efficient drugs. Plants and natural compounds are a proven rich resource for new drug discovery. Considering the potential of natural products to manage the pandemic, this article was designed to provide an inclusive map of the stages and pathogenetic mechanisms for effective natural products on COVID-19. New drug discovery for the COVID-19 pandemic can encompass both prevention and disease management strategies. Preventive mechanisms that may be considered include boosting the immune response and hand hygiene in the preexposure phase; and blocking of virus binding and entry in the postexposure phase. Potential therapeutic target mechanisms include virus-directed therapies and host-directed therapies. Several medicinal plants and natural products, such as Withania somnifera (L.) Dunal and propolis for prevention; Tanacetum parthenium (L.) for treatment; and Ammoides verticillata (Desf.) Briq and Nigella sativa L. for both prevention and treatment have been found effective and are good targets for future research. The examples of phytochemical compounds that may be effective include aloin and terpenes as anti-septics; isothymol, dithymoquinone, and glycyrrhizin as inhibitors of virus binding and entry; glycyrrhizin, and berberine as replication suppressants; ginsenoside Rg1 and parthenolide as immunomodulators; and eriocitrin, rhoifolin, hesperidin, naringin, rutin, and veronicastroside as anti-complements. Recognizing different mechanisms of fighting against this virus can lead to a more systematic approach in finding natural products and medicinal plants for COVID-19 prevention and treatment.
Keywords: coronavirus, phytotherapy, COVID-19, angiotensin-converting enzyme, plant extracts, new drug discovery
Introduction
New respiratory infection with clinical symptoms of fever, cough, pneumonia, rhinorrhea, dyspnea, fatigue, and myalgia was first reported in Wuhan city, China in December 2019. 1 The rapid spread of COVID-19 was identified as a pandemic and public health emergency of international concern (PHEIC) by the World Health Organization (WHO) on March 11, 2020. 2 As of January 3, 2021, around 82 356 727 COVID-19 patients and over 1 815 433 deaths worldwide have been confirmed. 3 The genetic sequence of COVID-19—classified as a beta coronavirus—has similarities to other epidemic-causing viruses of this group, with more than 80% similarity to severe acute respiratory syndrome-related coronavirus (SARS-CoV) and 50% similarity to the Middle East respiratory syndrome-related coronavirus (MERS-CoV). 4 Coronaviruses belong to the large family of Coronaviridae and their genome size ranges from 26 to 32 kb. These viruses possess positive-sense single-stranded RNA ( + ssRNA) as their genome. 5 COVID-19 initiates by binding to angiotensin-converting enzyme 2 (ACE2) receptor on respiratory epithelial cells. 6 Following the attachment and cell invasion, heart damage, kidney damage, blood infection (RNAemia), and lung disease (acute respiratory distress syndrome [ARDS] and pneumonia) may occur (Jalali, 2020). There is a pressing demand to provide efficient drugs for this pandemic disaster to decrease the global vulnerability to a highly infectious coronavirus. 7
The search for healing through nature has a history as old as mankind. 8 WHO has estimated about 3.5 to 4 billion people to be relying on herbal medicines in primary care. 9 Plants, their phytochemicals, and natural products are a proven rich resource for new drug discovery. 10 Considering the higher availability, accessibility, and affordability, medicinal herbs can accelerate the drug discovery process. 11 It seems that it is an opportunity for the management of COVID-19 and a rational and scientific approach to herbal therapy and traditional medicines could help to find natural products affecting COVID-19. 12 However, a scientific approach needs to be undertaken, and risks/benefits associated with natural products should be considered.
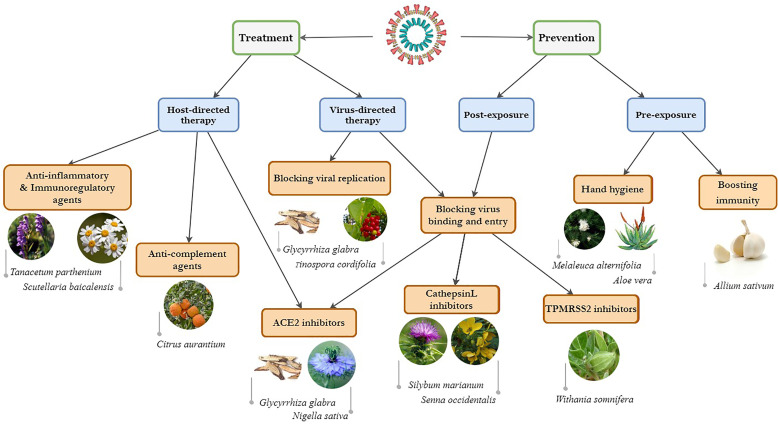
Numerous studies have been performed during the pandemic to introduce the natural products and phytochemicals effective in the management of COVID-19. Some have performed pharmacology-based reviews on medicinal plants and phytochemicals that may be effective in the management of COVID-19.13,14 Considering the potential of natural products to manage the pandemic, this article was designed to provide an inclusive map of the stages and pathogenetic mechanisms related to the disease and strategies using effective natural products on COVID-19 (Figure 1).
Figure 1.
Strategies for prevention and treatment of coronavirus disease 2019 (COVID-19) by medicinal herbs.
Materials and Methods
This article is a narrative review to provide a map of disease-making strategies using potential natural products to prevent and manage each stage of the disease. In the first stage, queries were performed in PubMed, Scopus, and Google Scholar databases to study articles discussing the prevention, pathogenesis, and treatment targets for COVID-19. Subsequently, each stage in prevention (preexposure and postexposure) and treatment (each of the virus- and host-directed therapies) were separately searched with keywords including “herbal,” “plant,” “natural,” and “traditional medicine” in combination with anti-viral, coronavirus, and COVID-19 to find instances of research conducted on the topic. The subject area was limited to medicine, pharmacology, toxicology, and pharmaceutics with no specific time restriction. All studies including clinical, in-vivo, in-vitro, and in-silico studies were investigated. All articles were studied to find the most robust evidences and instances of natural products on different stages. These evidences were used to find the mentioned map and strategies against COVID-19 based on the mechanism of action.
Prevention
Pre-exposure
One of the most important activities against COVID-19 is prevention. The strategy for preexposure preventive activities could be considered as one of the main first-line attempts against this disease. The strategies to find natural solutions for pre-exposure is listed in continue:
Immunomodulatory Agents
Management of individuals in the first phase of COVID-19 can encompass improving and modulating innate (monocyte, macrophage, and natural killers [NK]) and adaptive immune responses (T-cell and B-cell) by the use of immune-boosting food and herbal supplements. Prevention of virus entry and replication is crucial. 16 The use of natural compounds may provide alternative prophylaxis by boosting the immune response in the preexposure stage. Plant-based foods enhance and help the intestinal advantageous bacteria and the overall health of the gut microbiome that makes up to 85% of the immune system of the body. 17
Green vegetables like broccoli (Brassica oleracea), kale or leaf cabbage, mushrooms, and plants rich in omega-3 fatty acids like flax seeds (Linum usitatissimum L.) are immunity boosters that can rapidly enhance the immune system of the elderly. 17
Curcumin, the main phytochemical of Curcuma longa L. has anti-SARS-CoV-2 effects, antioxidant, and potential immune-boosting properties is a potent antioxidant and stimulates the production of interferons to activate the host innate immunity. 18 Piperine is an alkaloid compound of Piper nigrum L. that boosts innate immunity through the phosphorylation of interferon regulatory factor 3 (IRF-3), type 1 interferon (IFN) mRNA, prevents lipopolysaccharide (LPS)-induced expression of IRF-1 and IRF-7 mRNA, and down-regulates STAT-1 activity and phosphorylation of IRF-3, type 1IFN mRNA.16,19 In a hypothesis study, garlic (Allium sativum L.) was suggested as an advantageous preventive measure before infection with SARS-CoV-2, as it suppresses the production and secretion of proinflammatory cytokines and boosts immune system cells. 20 Garlic, which has also been suggested in prophylaxis, can ameliorate symptoms in infected patients. It stimulates NK cells, lymphocytes, eosinophils, and macrophages by modulation of immunoglobulin synthesis, phagocytosis, and macrophage activation, and cytokine secretion. 21 Also, garlic demonstrated immune system boosting capability by significantly increasing cluster of differentiation (CD4)+T cells and total white blood cell count.18,22 Target mechanisms of herbal drug discovery for COVID-19 with example studies are mentioned in Table 1.
Table 1.
Target Mechanisms of Drug Discovery From Natural Resources for COVID-19 With Example Studies.
| Source | Phytochemical | Structure of Phytochemicals | Study design | Mechanism | Ref | |
|---|---|---|---|---|---|---|
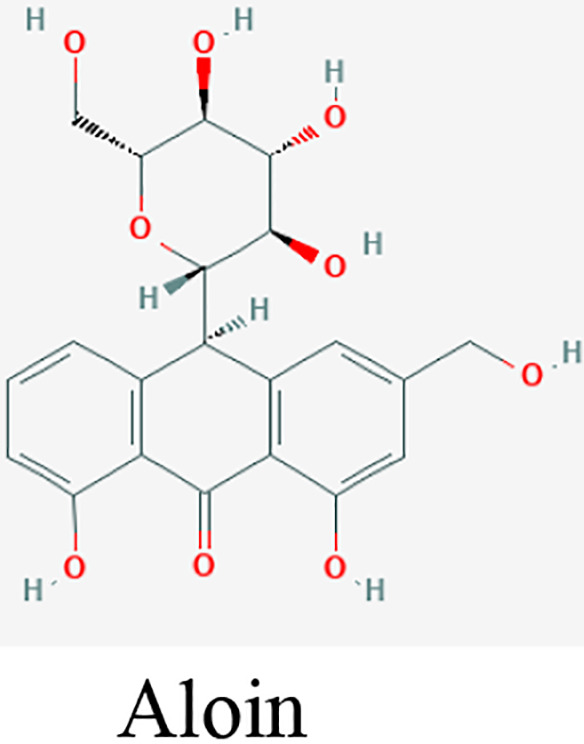
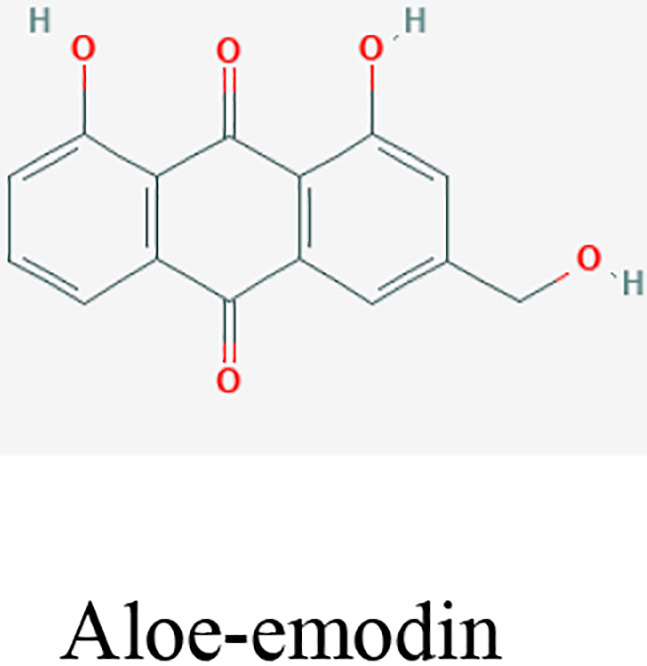
| Hand hygiene | Aloe vera (L.) Burm.f. | Aloin and aloe- emodin |
|
In vitro | Inhibiting the viral replication or destructing the virus lipid envelope | 26 |
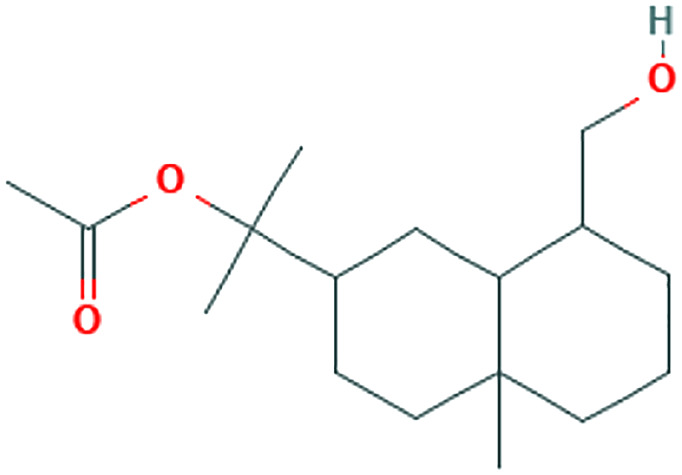
| Melaleuca alternifolia (Maiden & Betche) Cheel. | Terpenes |
|
In vitro | Disrupting the viral membrane fusion procedure and viral replication | 25 | |
| Pre-exposure immunomodulation | Allium sativum L. (garlic) | Animal (Rat) | Boosting immune system cells and suppressing production of proinflammatory cytokines | 20, 22 | ||
| Virus binding and entry | Ammoides verticillata (Desf.) Briq. | Isothymol |
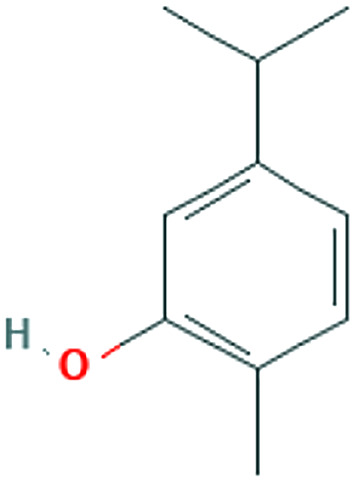
|
In silico | ACE inhibitors | 30 |
| Nigella sativa L. | Dithymoquinone (DTQ) |
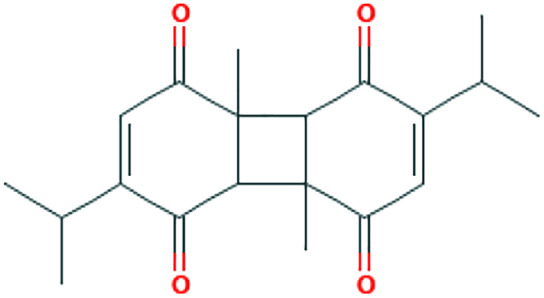
|
In silico | ACE2 inhibitor | 33 | |
| Glycyrrhiza glabra L. | Glycyrrhizin |
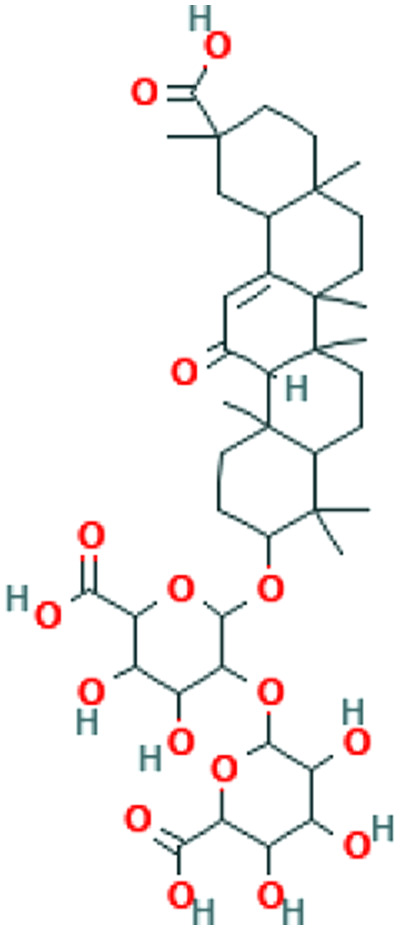
|
In silico | ACE2 inhibitor | 85 | |
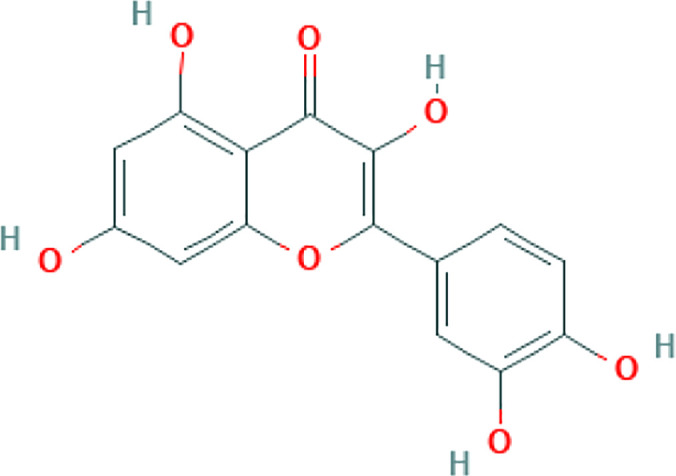
| Propolis | Myricetin, Caffeic acid phenethyl ester, Hesperetin, Pinocembrin |
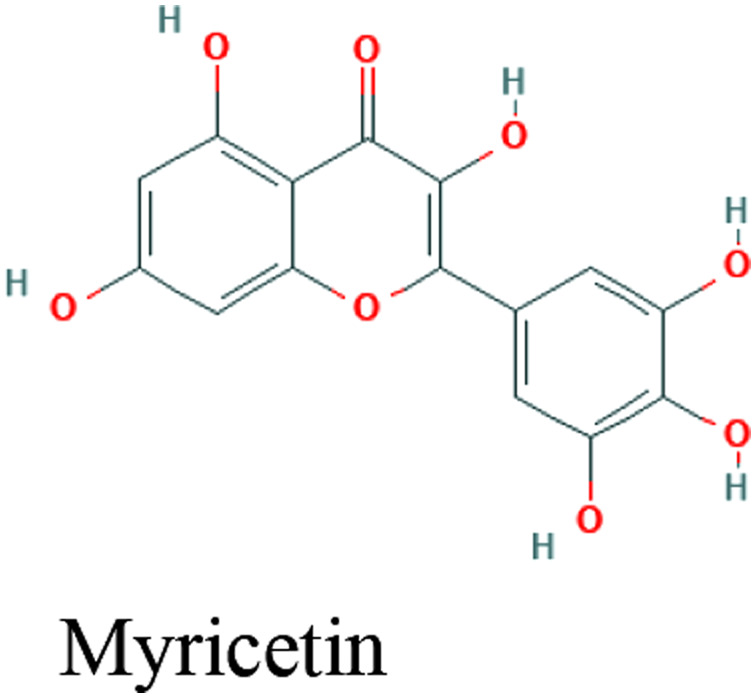
|
In silico | Inhibitory potential with high binding energy to ACE2 (-8.97 kcal/mol) | 86 | |
| Kaempferol |

|
In silico | Inhibitory potential with high binding energy to ACE2 (-7.5 kcal/mol) | 87 | ||
| Quercetin |
|
In silico | Inhibitory potential with high binding energy to ACE2 (-10.4 kcal/mol) | 88 | ||
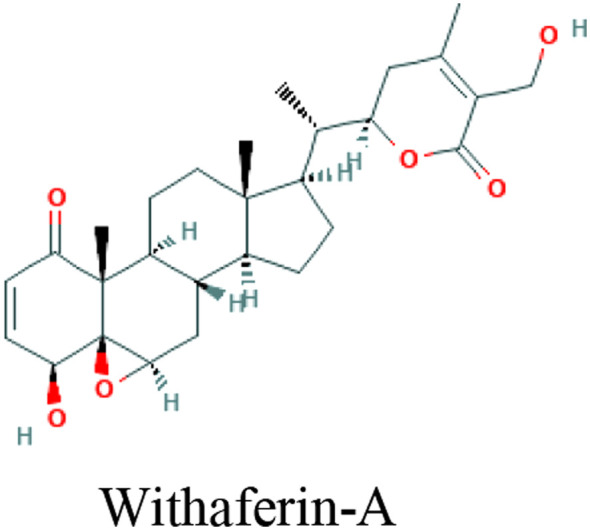
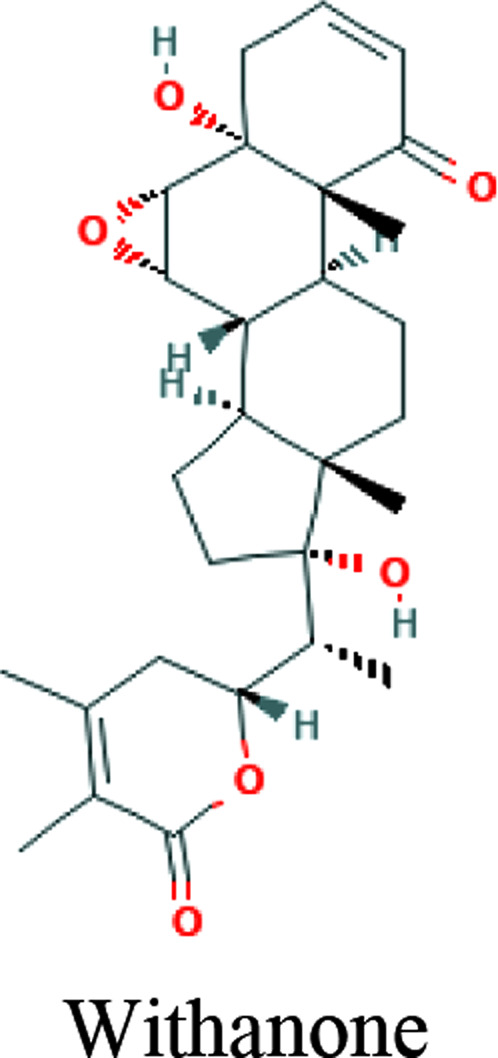
| Withania somnifera (L.) Dunal (Ashwagandha) | Withaferin-A, Withanone, Caffeic acid phenethyl ester |
|
In silico, In vitro (MCF7 cells) | TPMRSS2 inhibition | 28 | |
| Ziziphus rugosa Lam. | Rugosanine B |
|
Computational screening | Cathepsin L inhibition | 2 | |
| Senna occidentalis (L.) Link | Ararobinol |
|
Computational screening | Cathepsin L inhibition | 2 | |
| Hypecoum pendulum L. | (+)- oxoturkiyenine |
|
Computational screening | Cathepsin L inhibition | 2 | |
| Cinchona calisaya Wedd. | 3α,17α-cinchophylline |
|
Computational screening | Cathepsin L inhibition | 2 | |
| Clerodendrum trichotomum Thunb. | Trichotomine |
|
Computational screening | Cathepsin L inhibition | 2 | |
| Tectona grandis L.f. | Tectol |
|
Computational screening | Cathepsin L inhibition | 2 | |
| Silybum marianum (L.) Gaertn. | Silymonin |
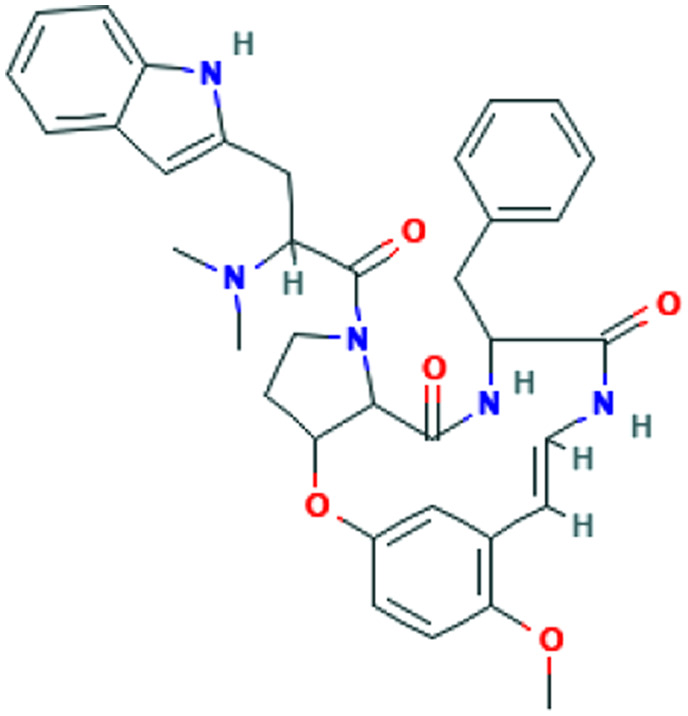
|
Computational screening | Cathepsin L inhibition | 2 | |
| Picrasma quassioides (D.Don) Benn. | Picrasidine M |
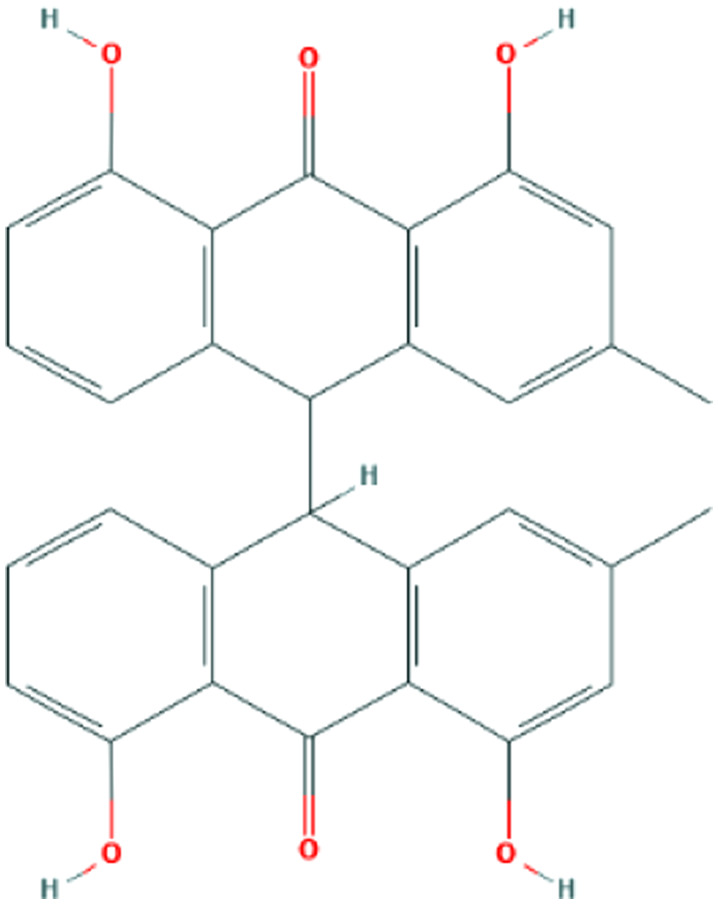
|
Computational screening | Cathepsin L inhibition | 2 | |
| Juglans regia L. | Juglone |
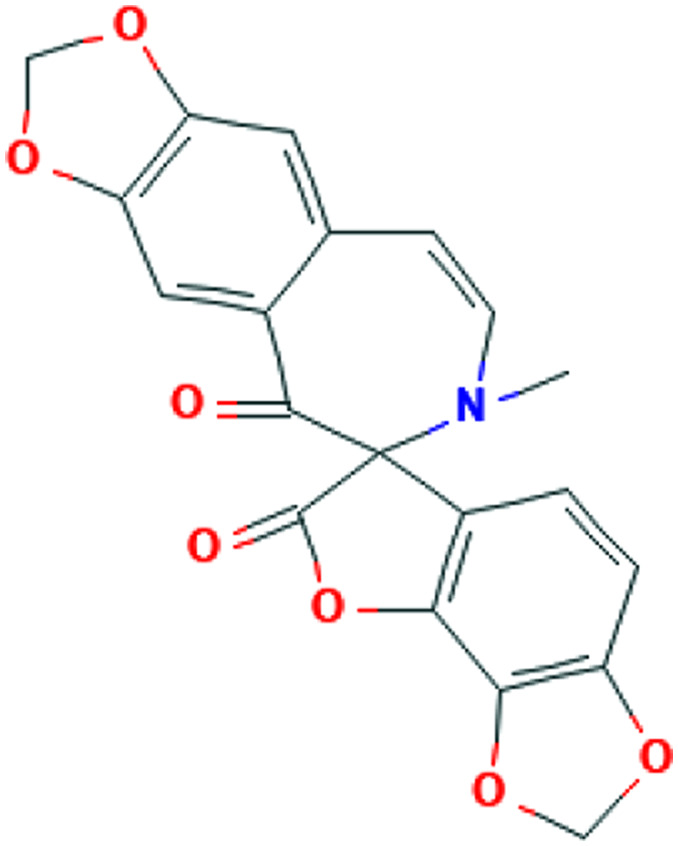
|
Computational screening | Cathepsin L inhibition | 2 | |
| Viral replication | Tinospora cordifolia (Willd.) Miers | Berberine |
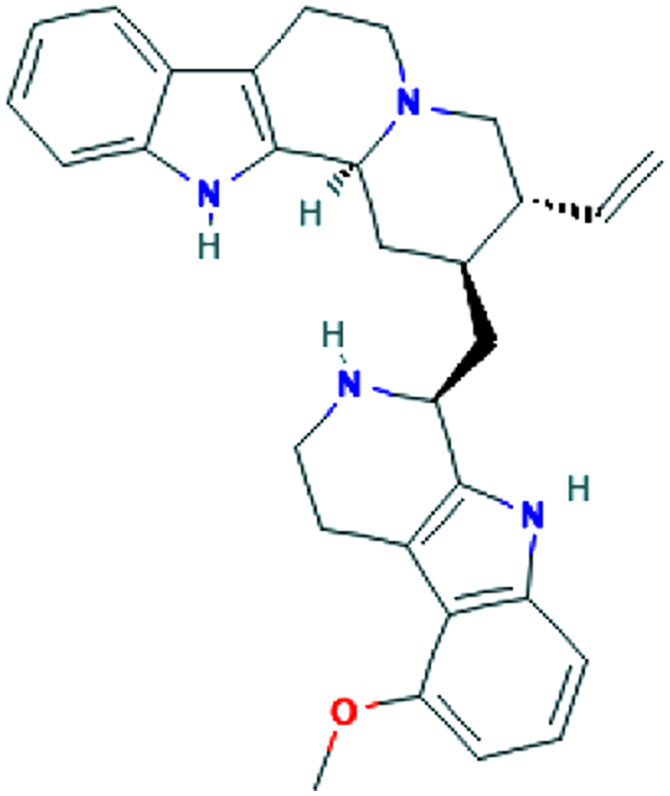
|
In silico | Inhibition of 3CLpro function | 45 |
| Anthemis hyalina DC. | In vitro (HeLa- CEACAM1a cells) | Inhibition of TRP gene expression | 57 | |||
| Asparagus racemosus Willd. | In silico | Inhibition of N protein | 48 | |||
| Glycyrrhiza glabra L. | Glycyrrhizin |
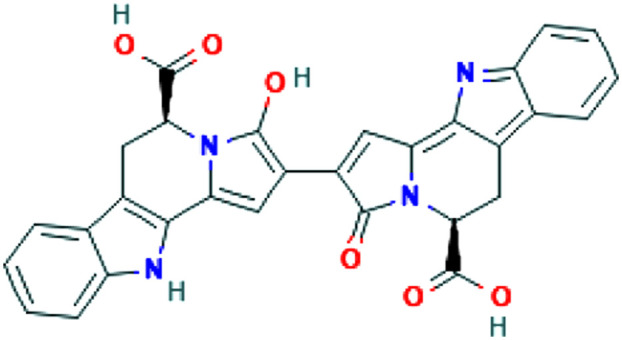
|
In vitro | Inhibition of viral replication | 56 | |
| Immunomodulation and antiinflammation | Lophatherum gracile Brongn. | In vivo | Enhancement of CD4+/CD8+ T cell ratio, inhibition of IL-Ιβ, TNF- α, and IFN-γ expression | 74 | ||
| Panax ginseng C.A.Mey. | Ginsenoside Rg1 |
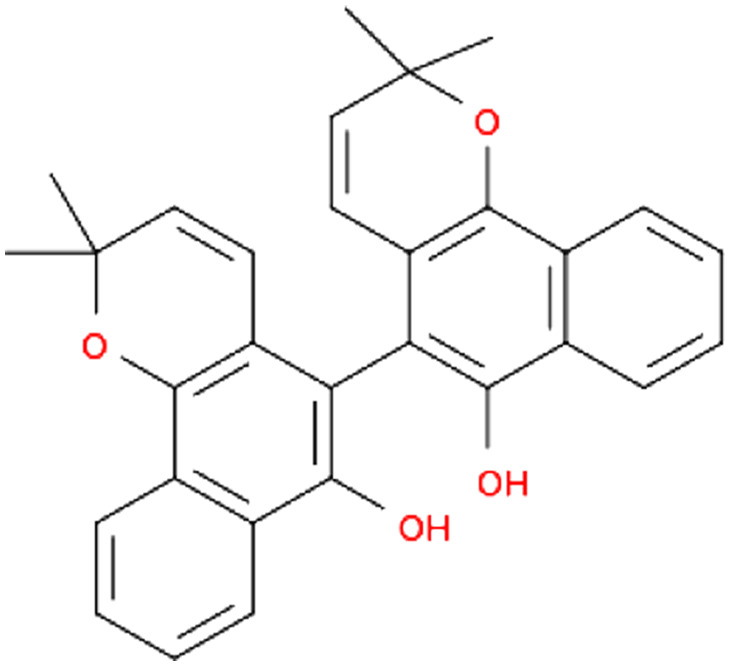
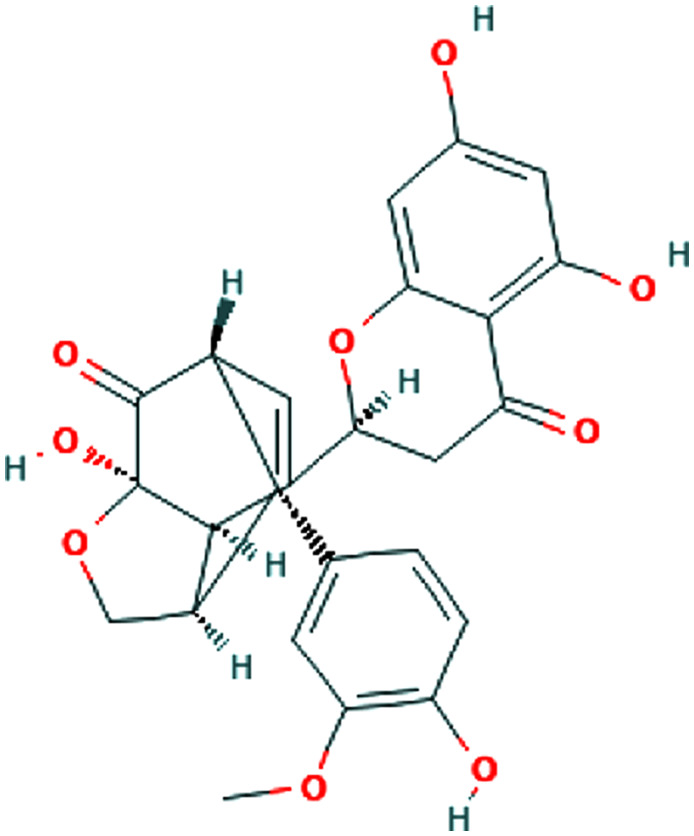
|
Enhancement of CD4+ T cell activity | 72 | ||
| Tanacetum parthenium (L.) | Parthenolide |
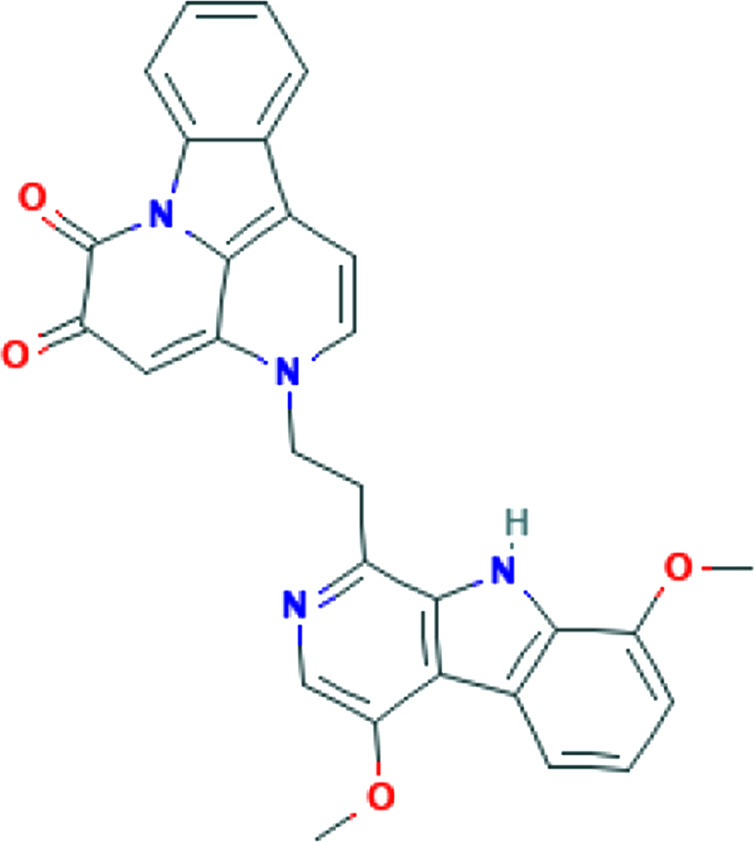
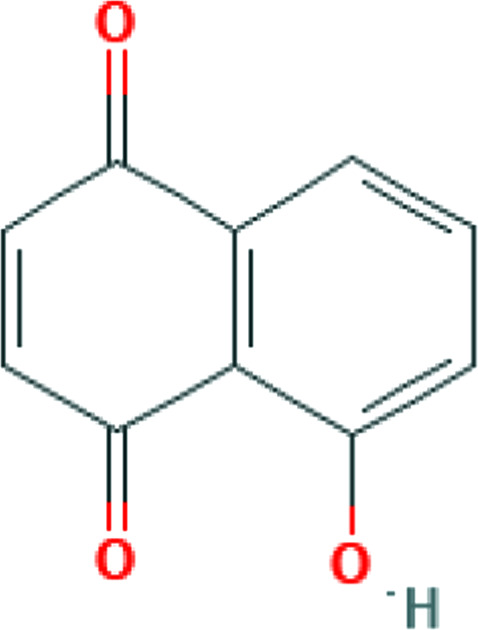
|
In vitro, In vivo | Inhibition of IL-1, IL-2, IL-6, IL-8, and TNF-α expression | 79 | |
| Sch.Bip. (feverfew) | ||||||
| Viscum album L. | In vitro | Enhancement of CD4+ T cell migration | 75 | |||
| Scutellaria baicalensis Georgi | In vivo | Inhibition of IL-1beta, IL- 2, IL-6, IL-12 and TNF-α expression | 76 | |||
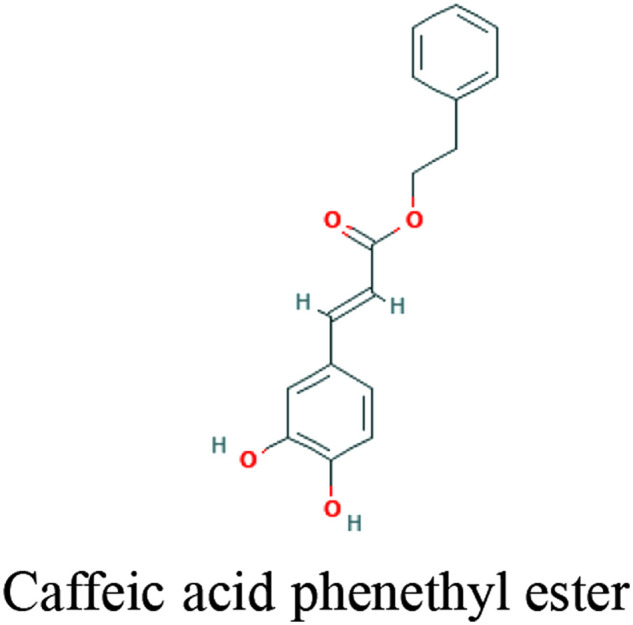
| Propolis | Caffeic acid, and Caffeic acid phenethyl ester |
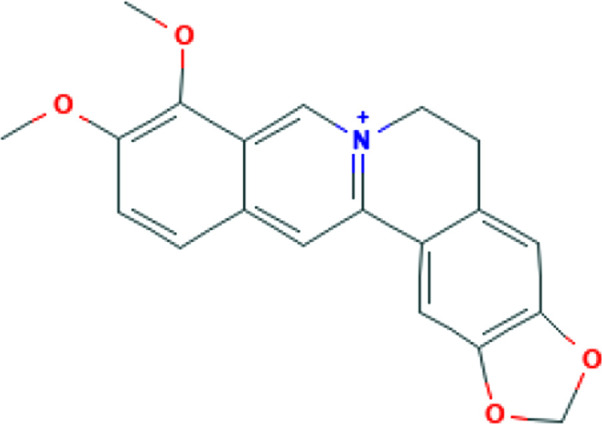
|
Downregulation and inhibition of PAK1 | 35 | ||
| Biophytum umbraculum Welw. | In vitro (RAW 264.7 cell line) | Anticomplement and antiinflammatory effects | 89 | |||
| Anticomplement | Citrus aurantium L. var. amara Engl. | Eriocitrin/ neoeriocitrin, rhoifolin, hesperidin, naringin, rutin, veronicastroside, neohesperidin, and hesperetin |
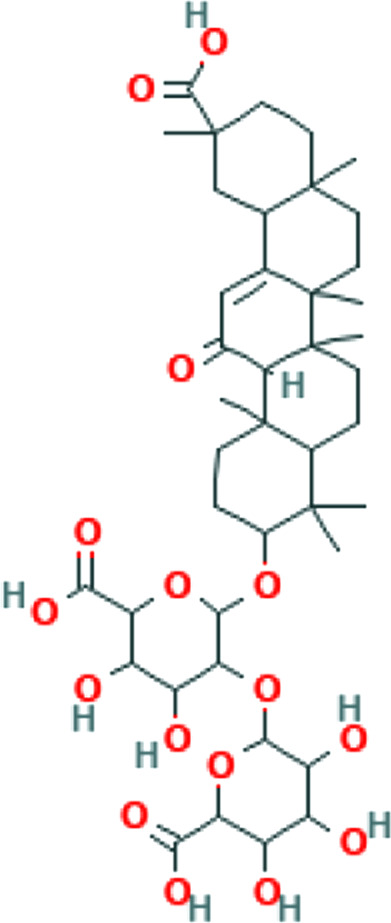
|
In vitro | Anticomplement and antiinflammatory effects | 81 |
|
||||||
|
||||||
|
||||||
|
||||||
|
||||||
|
||||||
|
||||||
|
||||||
|
||||||
|
||||||
|
||||||
|
||||||
|
Hand Hygiene
Hand hygiene includes hand cleaning with soap, alcohol-based hand rub and sanitizers. It is an important part of WHO, Centers for Disease Control and Prevention (CDC), and for Disease Prevention and Control (response for prevention and control of the spread of COVID-19 pathogens and infections in health care settings. 44 Personal hand hygiene plus plant and essential oil-based hand sanitizer could be efficient prevention for limiting the spread of viral and bacterial infections. 45 Leaves of tea tree oil (Melaleuca alternifolia [Maiden & Betche] Cheel) are a complex mixture of hydrocarbons and terpenes. Handwash formulations containing tea tree oil have been used in hospital or health care settings for many years. Several studies revealed the potent antiviral activity of this plant. Tea tree oil at a concentration of 0.02% inhibited influenza viruses from entering the host cell via disrupting the viral membrane fusion procedure and viral replication. 24
Aloe vera (L.) Burm.f. gel and their key constituents, aloin and aloe-emodin are known as potential antiviral agents. These compounds eliminate the enveloped viruses, such as SARS-CoV-1, HIV viruses, and influenza viruses by inhibiting the viral replication or destructing the virus lipid envelope.23,46 These properties make Aloe an attractive choice as a key plant in a nonalcoholic hand sanitizer. 45
Post-exposure Prophylaxis
Blocking of Virus Binding and Entry
Viral spike protein interaction with cellular angiotensin-converting enzyme 2 (ACE2), host cell proteases including transmembrane protease serine 2 (TMPRSS2), and endosomal cathepsin L protease (CatL) or human airway trypsin-like protease (HAT) contribute to virus entry into host cells. An important strategy in drug discovery for COVID-19 would be to find compounds that can limit virus binding and entry into host cells via inhibiting these mechanisms. 31
Angiotensin-converting enzyme 2 Inhibitors
Coronaviruses have common proteins, including nucleocapsid (N), protuberances (S), membrane (M), envelope (E), and a special type in beta-coronavirus called hemagglutinin esterase protein (HE protein).25,47 The SARS coronavirus infection is started via the binding of spike protein-S (viral surface glycoprotein) to target membrane receptor ACE2 of a host cell. 48 Furthermore, expression of ACE2 could determine the severity of SARS-CoV-2 infection.31,49
In an in-silico study on the essential oil of Ammoides verticillata (Desf.) Briq, its major component, isothymol, has shown good results for the isothymol-ACE2 docked complex. 48 This compound is also a constituent of Ajowan essential oil isolated from aerial parts, for which antiviral and antimicrobial properties have been identified. 49 Similarly, a combination of docking, Absorption, distribution, metabolism, elimination, and toxicity (ADMET) properties calculation, molecular dynamics, and molecular mechanics/Poisson–Boltzmann or generalized Born and surface area (MM-PBSA) approaches have revealed high potential binding to ACE2 for dithymoquinone (DTQ), an active constituent of Nigella sativa L.41,50 Components of propolis, a resinous-like substance produced by bees, have also been found to have inhibitory effects on ACE2. 41 Likewise, in silico studies have proposed glycyrrhizin (the chief bioactive constituent of Glycyrrhiza glabra L. root), as an appropriate candidate of ACE2 inhibition due to reported safety, availability, and affordability. 51 Quercetin, a plant flavonol from the flavonoid group of polyphenols, is present in a variety of foodstuff including grapefruit, onions, apples, and black tea, 17 and herbs including Hypericum perforatum L. and Sambucus nigra L. 52 It is proposed as a potentially effective disruptor of virus binding. However, it should be noted that this compound is unlikely to be effective orally due to biotransformation, and is thus suggested to be used as nasal/throat spray. 53
Transmembrane protease serine 2 Inhibitors
TMPRSS2 is a serine protease on the host cell surface with which SARS-CoV-2 interacts to replicate and induce invasion. 41 TMPRSS2 is recognized in inoculation and replication of cancer, influenza virus, and SARS-CoV-1. In one study, the flavonoids baicalein and baicalin as down-regulators of the TMPRSS-2 expression were demonstrated on in-silico studies against COVID-19. 54 Another study has revealed a potential for Withania somnifera (L.) Dunal (Ashwagandha) compounds—Withaferin-A, Withanone, and caffeic acid phenethyl ester, to block SARS-CoV-2 entry into the host cells, via the binding capacity to inhibit TPMRSS2. 31
Cathepsin L Inhibitors
CatL is a lysosomal enzyme in endosomes that contributes to several physiological and pathological processes, such as extracellular matrix remodeling, antigen processing, apoptosis, invasion, inflammatory status, and viral infection. CatL is involved in degrading extracellular matrix that is a major process in binding SARS-CoV-2 spike protein and enters into host cells. 55 Therefore, plants and phytochemicals with potential CatL-inhibiting capacity could be considered a valuable therapeutic target to treat COVID-19 patients. Vivek-Ananth et al 56 have conducted a study to screen for potential CatL inhibitors and identified by computational screening 9 potential natural products such as Ararobinol (Senna occidentalis [L.] Link), ( + )-oxoturkiyenine (Hypecoum pendulum L.), Rugosanine B (Ziziphus rugosa Lam.), Trichotomine (Clerodendrum trichotomum Thunb.), Tectol (Tectona grandis L.f.), Silymonin (Silybum marianum (L.) Gaertn.), Picrasidine M (Picrasma quassioides (D.Don) Benn.), and 3α, 17α-cinchophylline (Cinchona calisaya Wedd.).
Treatment
Virus-Directed Therapies
Findings from studies using herbal preparations as a complementary treatment in patients with the beta-coronavirus infections 57 have demonstrated a positive effect. However, more trials are needed to reach conclusive results. A number of in-vitro and animal studies have been conducted to identify antiviral plants,58,59 but more research to find natural compounds that target the pathogenesis, and also clinical manifestations of COVID-19 is needed to help discover effective agents to be used as a complementary or alternative treatment.
Virus Replication
Proteins involved in the process of virus replication are an imperative target for anti-viral compounds. The most important protease enzyme in SARS-CoV is papain-like protease (PLpro) 3-chymotrypsin-like protease (3CLpro). Other nonstructural proteins (nsp) like helicase (nsp13), RdRp (nsp12/7/8), and nsp14 can also serve as drug targets. 51 A docking study has shown that the chief components of Tinospora cordifolia (Willd.) Miers, including berberine, can suppress 3CLpro function and thus inhibit viral replication. 32 Also, polyphenolic compounds have antiviral properties and can control infection via inhibiting these proteins. 60
Moreover, N protein contributes to virus replication and plays a role in forming mature virions. 61 Results of an in-silico docking study have shown this protein to be inhibited by Asparagus racemosus Willd. 34
Cyclophilin is required by many viruses including SARS-CoV for replication. 61 There is evidence of Cyclosporin A, a cyclophilin inhibitor, having the ability to suppress replication of coronaviruses. 62 To our knowledge, there is no study regarding cyclophilin inhibition by medicinal herbs. However, some studies have been conducted to investigate the suppression of coronavirus replication in plants.63,64 One of the most important compounds in this regard is glycyrrhizin (the chief bioactive constituent of liquorice root), which has various antiviral activities including strong suppression of SARS-CoV replication.35,66,67 Furthermore, N sativa, Anthemis hyalina, and Citrus sinensis all have significant effects on Transient receptor potential (TRP) gene expression and virus load, with A hyalina DC being the most potent one in this regard. 33
Host-Directed Therapies
The first phase of COVID-19 includes infection of the upper respiratory tract, followed by subsequent involvement of the lower respiratory tract and other organs. 68 Pulmonary infection is accompanied by alveolar damage and hypoxia. 69 In many cases, COVID-19 infection is associated with inflammation in other organs. Gastrointestinal symptoms and myalgia are common manifestations of the disease.70,71 The neurotropism of SARS-CoV-2, and the resultant inflammation, edema, and axonal damage, may lead to olfactory/gustatory dysfunction and more severe neurologic complications including acute cerebrovascular events, encephalitis, and Guillain-Barré syndrome. 72 Likewise, major mechanisms of cardiovascular complications, including acute cardiac injury, heart failure, and arrhythmias include direct myocardial injury and systemic inflammation. 73 In addition to decreasing viral load, the following targets can help ameliorate symptoms.
Angiotensin converting enzyme 2 Inhibitors
Several manifestations of COVID-19 are associated with the virus’ affinity to ACE2 receptors. These receptors are widely distributed in body organs including endothelial and neuronal tissues. 74 Debatably, the main route of SARS-CoV-2 entry into neurons is via the ACE2 expressed in the heart and nervous system.71,73 In the lungs, spike-like electron-dense projections along with SARS-CoV-2 antigen have been recognized in the ACE2-positive bronchiolar epithelium. 75 Thus, targeting this receptor can be an effective method in relieving COVID-19 symptoms. Medicinal herbs that act on ACE2 have been discussed in the previous sections.
Anti-Inflammatory and Immunoregulatory Agents
Infection of the conducting airways with SARS-CoV-2 leads to an innate cytokine response. 76 Within a week of upper respiratory tract infection, immunoglobulin M (IgM) and immunoglobulin (IgG) response is usually seen in patients. 77 COVID-19 is also associated with cell-mediated immune responses. Immune response to COVID-19 develops either in the direction of a T-cell-mediated protective immune response or an exacerbated inflammatory response. 77 In the former, CD4 and B cells produce neutralizing antibodies, which is critical for control of SARS-CoV-2 infections. 78 T cell counts are significantly reduced in COVID-19 patients, and also, decreased functionality and exhaustion of these cells exacerbate conditions. 79 Cytotoxic CD8 T-cells play an important role in clearing RNA viruses from the body while sparing damage to the host.69,80 The exacerbated response, is associated with the inability to inhibit viral replication and remove infected cells, and may ultimately lead to a cytokine storm. 77 Profiles of several immune cells in COVID-19 have been recognized. Proinflammatory cytokines like interferon-gamma (IFN-γ), interleukin-1 (IL-1), IL-2, IL-6, tumor necrosis factor-alpha (TNF-α), leptin, and chemokines are among those exhibiting an enhancing tendency. 81 Conversely, reduced NK cells, cytotoxic and helper T cells, monocytes/macrophages, and regulatory T (Treg) cells have been mentioned. 81
Medicinal plants can regulate the immune responses of the host. Ginsenoside Rg1, a phytochemical constituent of Panax ginseng C.A.Mey. can enhance the immune activity of CD4( + ) T cells. 37 Also, a recent study has demonstrated that fermented ginseng extracts improve protection against influenza viruses and survival rates in conditions where adaptive immune components (CD4, CD8, B cell, Major histocompatibility complex(MHCII)) are deficient. 82
An ethanol extract of Lophatherum gracile Brongn. has been found to have antiviral activity against respiratory syncytial virus (RSV) infection in rats. The proposed mechanism mediated slight enhancement of CD4 + /CD8 + T cell ratio and also, inhibition of IL-1β, TNF-α, and IFN-γ expression. 36
Plants can also aid the migration and tissue distribution of immunocompetent cells. In this regard, Viscum album L. (mistletoe) can alter the migratory behavior of human peripheral CD4+ T lymphocytes, and significantly enhance the mean velocity and time locomoting of these cells in collagen lattices. 39 Furthermore, the active aqueous extract of this plant has antiviral activity as shown in an in-vitro study on the human parainfluenza virus. 39
Some medicinal herbs have been found to help alleviate the cytokine storm. For instance, Scutellaria baicalensis inhibits the expression of IL-1beta, IL-2, IL-6, IL-12, and TNF-α, thereby exhibiting strong anti-inflammatory activity. 40 Evidence of antiviral properties has previously been reported for this plant. 83 Although studies are limited in this regard, evidence of herbal compounds including polyphenols, triterpenoids, and flavonoids, protecting against cytokine storm during severe influenza exists. 84 Another example is parthenolide, the main biologically active constituent of Tanacetum parthenium (L.) Sch.Bip. This sesquiterpene lactone significantly inhibits IL-1, IL-2, IL-6, IL-8, and TNF-α expression. Considering the noticeable contribution of IL-6 in adverse clinical outcomes and fatality, parthenolide may be a potential herbal candidate for clinical evaluation. 38
Overexpression of RAC/CDC42 (P21)-activated kinase 1 (PAK1) in response to SARS-CoV-2 infection in the lung, is a critical mediator of the cytokine storm that frequently increases mortality in hospitalized patients. Propolis-derived compounds decrease PAK1 activation, pro-inflammatory NK cells, cytokine overproduction, improve NF-KB and monocyte/macrophage immunomodulation, and enhance the production of antibodies against SARS-CoV-2. 41
Anti-Complement Agents
Excessive cytokine release causes dysregulated complement activity, which aggravates lung damage, and ARDS in COVID-19 patients. 85 Anti-complement activity has been shown for some medicinal plants like crude polyphenols extracted from blossoms of C aurantium L. var. amara Engl. 43 Herbs with anti-complementary activity can ameliorate pulmonary edema, and improve the oxidant–antioxidant imbalance. 86 Moreover, there are growing reports of COVID-19 causing dermatological disorders, including maculopapular rashes, urticaria, vesicles, petechiae, and purpura. 87 One of the etiologies proposed for these manifestations includes complement-associated vasculitis, 88 in which case herbs acting as anti-complement agents may be helpful.
Conclusion
Natural resources and medicinal herbs are a valuable source of new drug discovery in managing COVID-19. Similar to processes of semisynthetic and synthetic drug design and production, a rational strategy should be undertaken. Natural products can provide both preventive and therapeutic aid in overcoming the pandemic. The present review attempted to highlight the potential of herbal drug discovery according to various aspects of COVID-19 prevention and treatment. Preventive mechanisms that may be considered include boosting the immune response and hand hygiene in the preexposure phase; and blocking of virus binding and entry ACE2, TMPRSS2, and CatL inhibition in the postexposure phase. Potential therapeutic target mechanisms include virus-directed therapies via inhibition of virus replication and 3C-like proteases; and host-directed therapies via ACE2 inhibition, anti-inflammation, immunoregulation, and use of anti-complement agents. Researchers can employ these strategies in the demanding conditions for drug discovery against COVID-19. This opportunity can help prevention and management of the COVID-19 pandemic. Indeed, numerous studies on natural products have been conducted during the pandemic, although many are in silico. Future studies are recommended to both verify these medicinal herbs and also explore new ones.
Abbreviations
- ACE2
angiotensin-converting enzyme 2
- ARDS
acute respiratory distress syndrome
- CatL
cathepsin L protease
- CDC
Centers for Disease Control and Prevention
- COVID-19
coronavirus disease 2019
- 3CLpro
3-chymotrypsin-like protease
- E protein
envelope
- HAT
human airway trypsin-like protease
- HE protein
hemagglutinin esterase
- IFN
interferon
- IL
interleukins
- IRF-3
Interferon regulatory factor 3
- LPS
lipopolysaccharide
- M protein
membrane
- MERS-CoV
Middle East respiratory syndrome-related coronavirus
- MHC
major histocompatibility complex
- MM-PBSA
molecular mechanics/Poisson–Boltzmann or generalized Born and surface area
- N protein
nucleocapsid
- NF-κB
nuclear factor kappa B
- NK
natural killer
- nsp
non-structural proteins
- PAK1
RAC/CDC42-activated kinase 1
- PHEIC
public health emergency of international concern
- PLpro
papain-like protease
- RSV
respiratory syncytial virus
- SARS-CoV
Severe acute respiratory syndrome-related coronavirus
- STAT
signal transducer and activator of transcription
- TNF-α
tumor necrosis factor-alpha
- TMPRSS2
transmembrane protease serine 2
- WHO
World Health Organization.
Footnotes
Author Contributions: SS and AN contributed to data gathering, analysis of data, drafting the manuscript, and approving the final version of the manuscript to be submitted; MK and AZ contributed to making the idea, supervise the project, analyze data and revise the draft and approve the final version of the manuscript to be submitted; RM, GK, and NE contributed to data gathering, revising the draft of manuscript, and approving the final version of the manuscript to be submitted.
Ethical Approval: It is a review and done according to the Committee on Publication Ethics guidelines.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Arman Zargaran https://orcid.org/0000-0003-4351-3861
References
- 1.Lipsitch M, Swerdlow DL, Finelli L. Defining the epidemiology of COVID-19—studies needed. N Engl J Med. 2020;382(13):1194-1196. [DOI] [PubMed] [Google Scholar]
- 2.Vivek-Ananth R Rana A Rajan N, et al. In silico identification of potential natural product inhibitors of human proteases key to SARS-CoV-2 infection. Molecules 2020;25(17):3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int/ (accessed November 20, 2020).
- 4.Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109:102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi Y, Wang Y, Shao C, et al. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27:1451-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Z, Xiao X, Wei X, et al. Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS-CoV-2. J Med Virol. 2020;92(6):595-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrovska BB. Historical review of medicinal plants’ usage. Pharmacogn Rev. 2012;6(11):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan S-Y, Zhou S-F, Gao S-H, et al. New perspectives on how to discover drugs from herbal medicines: CAM’s outstanding contribution to modern therapeutics. Evid Based Complementary Altern Med. 2013;2013:1-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaman W, Saqib S, Ullah F, . et al. COVID-19: phylogenetic approaches may help in finding resources for natural cure. Phytother Res. 2020;34(11):2783-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosales-Mendoza S. Will plant-made biopharmaceuticals play a role in the fight against COVID-19? Expert Opin Biol Ther. 2020;20(6):545-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan S, Ali A, Shi H, . et al. COVID-19: clinical aspects and therapeutics responses. Saudi Pharm J. 2020;28(8):1004-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jalali A, Dabaghian F, Akbrialiabad H, Foroughinia F, Zarshenas MM. A pharmacology-based comprehensive review on medicinal plants and phytoactive constituents possibly effective in the management of COVID-19. Phytother Res. 2021;35(4):1925-1938. [DOI] [PubMed] [Google Scholar]
- 14.Benarba B, Pandiella A. Medicinal plants as sources of active molecules against COVID-19. Front Pharmacol. 2020;11:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adhikari B, Marasini BP, Rayamajhee B, et al. Potential roles of medicinal plants for the treatment of viral diseases focusing on COVID-19: a review. Phytother Res. 2021;35(3):1298-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mrityunjaya M, Pavithra V, Neelam R, et al. Immune-boosting, antioxidant and anti-inflammatory food supplements targeting pathogenesis of COVID-19. Front Immunol. 2020;11:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arshad MS, Khan U, Sadiq A, et al. Coronavirus disease (COVID-19) and immunity booster green foods: a mini review. Food Sci Nutr. 2020;8(8):3971-3976. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Zahedipour F, Hosseini SA, Sathyapalan T, et al. Potential effects of curcumin in the treatment of COVID-19 infection. Phytother Res. 2020; 34(11):2911-2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bae G-S, Kim M-S, Jung W-S, et al. Inhibition of lipopolysaccharide-induced inflammatory responses by piperine. Eur J Pharmacol. 2010;642(1–3):154-162. [DOI] [PubMed] [Google Scholar]
- 20.Donma MM, Donma O. The effects of Allium Sativum on immunity within the scope of COVID-19 infection. Med Hypotheses. 2020;144:109934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arreola R, Quintero-Fabián S, López-Roa RI, et al. Immunomodulation and anti-inflammatory effects of garlic compounds. J Immunol Res. 2015;2015:401630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mirabeau TY, Samson ES. Effect of Allium cepa and Allium sativum on some immunological cells in rats. Afr J Tradit Complement Altern Med. 2012;9(3):374-379. [PMC free article] [PubMed] [Google Scholar]
- 23.Daverey A, Dutta K. COVID-19: eco-friendly hand hygiene for human and environmental safety. J Environ Chem Eng. 2020: 9(2);104754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Duan S, Chu C, et al. Melaleuca alternifolia concentrate inhibits in vitro entry of influenza virus into host cells. Molecules. 2013;18(8):9550-9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdelli I, Hassani F, Bekkel Brikci S, Ghalem S. In silico study the inhibition of angiotensin converting enzyme 2 receptor of COVID-19 by Ammoides verticillata components harvested from western Algeria. J Biomol Struct Dyn. 2020;39(9):1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmad S, Abbasi HW, Shahid S, Gul S, Abbasi SW. Molecular docking, simulation and MM-PBSA studies of nigella sativa compounds: a computational quest to identify potential natural antiviral for COVID-19 treatment. J Biomol Struct Dyn. 2020;39(12):4225-4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boozari M, Hosseinzadeh H. Natural products for COVID-19 prevention and treatment regarding to previous coronavirus infections and novel studies. Phytother Res. 2020;35(2):864-876. [DOI] [PubMed] [Google Scholar]
- 28.Güler HI, Tatar G, Yildiz O, Belduz AO, Kolayli S. Investigation of potential inhibitor properties of ethanolic propolis extracts against ACE-II receptors for COVID-19 treatment by molecular docking study. ScienceOpen Preprints. 2020; 203(6):3557-3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang L, Li Y-T, Miao J, et al. Network pharmacology studies on the effect of Chai-Ling decoction in coronavirus disease 2019. J Tradit Complement Med. 2020;5(3):145-159. [Google Scholar]
- 30.Sekiou O Bouziane I Bouslama Z, et al. In-Silico Identification of Potent Inhibitors of COVID-19 Main Protease (Mpro) from Natural Products. Int J Biochem Physiol. 2020; 5(3): 1-12. [Google Scholar]
- 31.Kumar V, Dhanjal JK, Bhargava P, et al. Withanone and withaferin-A are predicted to interact with transmembrane protease serine 2 (TMPRSS2) and block entry of SARS-CoV-2 into cells. J Biomol Struct Dyn. 2020:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chowdhury P. In silico investigation of phytoconstituents from Indian medicinal herb ‘Tinospora cordifolia (giloy)’ against SARS-CoV-2 (COVID-19) by molecular dynamics approach. J Biomol Struct Dyn. 2020;39(17):6792-6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ulasli M, Gurses SA, Bayraktar R, et al. The effects of Nigella sativa (Ns), Anthemis hyalina (Ah) and Citrus sinensis (Cs) extracts on the replication of coronavirus and the expression of TRP genes family. Mol Biol Rep. 2014;41(3):1703-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chikhale RV, Sinha SK, Patil RB, et al. In-silico investigation of phytochemicals from Asparagus racemosus as plausible antiviral agent in COVID-19. J Biomol Struct Dyn. 2020;39(14):5033-5047. [DOI] [PubMed] [Google Scholar]
- 35.Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr HW. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. The Lancet. 2003;361(9374):2045-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen LF, Zhong YL, Luo D, et al. Antiviral activity of ethanol extract of Lophatherum gracile against respiratory syncytial virus infection. J Ethnopharmacol. 2019;242:111575. [DOI] [PubMed] [Google Scholar]
- 37.Lee EJ, Ko E, Lee J, et al. Ginsenoside Rg1 enhances CD4( + ) T-cell activities and modulates Th1/Th2 differentiation. Int Immunopharmacol. 2004;4(2):235-244. [DOI] [PubMed] [Google Scholar]
- 38.Bahrami M, Kamalinejad M, Latifi SA, Seif F, Dadmehr M. Cytokine storm in COVID-19 and parthenolide: preclinical evidence. Phytother Res. 2020;34(10):2429-2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nikolai G, Friedl P, Werner M, Niggemann B, Zänker KS. Effect of a mistletoe extract (Iscador QuFrF) on viability and migratory behavior of human peripheral CD4+and CD8+ T lymphocytes in three-dimensional collagen lattices. In Vitro Cell Dev Biol Animal. 1997;33(9):710-716. [DOI] [PubMed] [Google Scholar]
- 40.Kim EH, Shim B, Kang S, et al. Anti-inflammatory effects of Scutellaria baicalensis extract via suppression of immune modulators and MAP kinase signaling molecules. J Ethnopharmacol. 2009;126(2):320-331. [DOI] [PubMed] [Google Scholar]
- 41.Berretta AA, Silveira MAD, Cóndor Capcha JM, De Jong D. Propolis and its potential against SARS-CoV-2 infection mechanisms and COVID-19 disease: running title: propolis against SARS-CoV-2 infection and COVID-19. Biomed Pharmacother. 2020;131:110622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Austarheim I, Pham AT, Nguyen C, et al. Antiplasmodial, anti-complement and anti-inflammatory in vitro effects of Biophytum umbraculum welw. Traditionally used against cerebral malaria in Mali. J Ethnopharmacol. 2016;190:159-164. [DOI] [PubMed] [Google Scholar]
- 43.Shen C-Y, Jiang J-G, Huang C-L, Zhu W, Zheng C-Y. Polyphenols from blossoms of Citrus aurantium L. var. amara engl. Show significant anti-complement and anti-inflammatory effects. J Agric Food Chem. 2017;65(41):9061-9068. [DOI] [PubMed] [Google Scholar]
- 44.Patil A, Kakde M. Medicinal plant as a natural immunity booster for COVID-19—a review. Ind J Integr Med. 2020; 2(2):24-27. [Google Scholar]
- 45.Berardi A, Perinelli DR, Merchant HA, et al. Hand sanitisers amid COVID-19: a critical review of alcohol-based products on the market and formulation approaches to respond to increasing demand. Int J Pharm. 2020; 584:119431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mpiana PT, Tshibangu DS, Kilembe JT, et al. Aloe vera (L.) Burm. F. as a potential anti-COVID-19 plant: a mini-review of Its antiviral activity. Eur J Med Plants. 2020;31(8):86-93. [Google Scholar]
- 47.Boopathi S, Poma AB, Kolandaivel P. Novel 2019 coronavirus structure, mechanism of action, antiviral drug promises and rule out against its treatment. J Biomol Struct Dyn. 2020;39(9): 3409-3418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Babadaei MMN, Hasan A, Bloukh SH, et al. The expression level of angiotensin-converting enzyme 2 determines the severity of COVID-19: lung and heart tissue as targets. J Biomol Struct Dyn. 2020;39(10):3780-3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bekhechi C, Boti JB, Bekkara FA, Abdelouahid DE, Casanova J, Tomi F. Isothymol in Ajowan essential oil. Nat Prod Commun. 2010;5(7):1107-1110. [PubMed] [Google Scholar]
- 50.Koshak DAE, Koshak PEA. Nigella sativa L as a potential phytotherapy for coronavirus disease 2019: a mini review of in silico studies. Curr Ther Res Clin Exp. 2020;93:100602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boozari M, Hosseinzadeh H. Natural products for COVID-19 prevention and treatment regarding to previous coronavirus infections and novel studies. Phytother Res. 2020; 35(2):864-876. [DOI] [PubMed] [Google Scholar]
- 52.Wach A, Biesaga M. Quercetin content in some food and herbal samples. Food Chem. 2007;100(2):699-704. [Google Scholar]
- 53.Williamson G, Kerimi A. Testing of natural products in clinical trials targeting the SARS-CoV-2 (COVID-19) viral spike protein-angiotensin converting enzyme-2 (ACE2) interaction. Biochem Pharmacol. 2020;178:114123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Antonio AdS, Wiedemann LSM, Veiga-Junior VF. Natural products’ role against COVID-19. RSC Adv. 2020;10(39):23379-23393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou N, Pan T, Zhang J, et al. Glycopeptide antibiotics potently inhibit cathepsin L in the late endosome/lysosome and block the entry of Ebola virus, Middle East respiratory syndrome coronavirus (MERS-CoV), and severe acute respiratory syndrome coronavirus (SARS-CoV). J Biol Chem. 2016;291(17):9218-9232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vivek-Ananth RP Rana A Rajan N, et al. In silico identification of potential natural product inhibitors of human proteases key to SARS-CoV-2 infection. Molecules. 2020;25(17):3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leung P-C. The efficacy of Chinese medicine for SARS: a review of Chinese publications after the crisis. Am J Chin Med. 2007;35(4):575-581. [DOI] [PubMed] [Google Scholar]
- 58.Li S-Y, Chen C, Zhang H-Q, et al. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antiviral Res. 2005;67(1):18-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin L-T, Hsu W-C, Lin C-C. Antiviral natural products and herbal medicines. J Tradit Complement Med. 2014;4(1):24-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chojnacka K, Witek-Krowiak A, Skrzypczak D, Mikula K, Młynarz P. Phytochemicals containing biologically active polyphenols as an effective agent against COVID-19-inducing coronavirus. J Funct Foods. 2020;73:104146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 2015;1282:1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tanaka Y, Sato Y, Sasaki T. Suppression of coronavirus replication by cyclophilin inhibitors. Viruses. 2013;5(5):1250-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen C-J, Michaelis M, Hsu H-K, et al. Toona sinensis roem tender leaf extract inhibits SARS coronavirus replication. J Ethnopharmacol. 2008;120(1):108-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim H-Y, Shin H-S, Park H, et al. In vitro inhibition of coronavirus replications by the traditionally used medicinal herbal extracts, Cimicifuga rhizoma, Meliae cortex, Coptidis rhizoma, and Phellodendron cortex. J Clin Virol. 2008;41(2):122-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim H-Y, Eo E-Y, Park H, et al. Medicinal herbal extracts of Sophorae radix, acanthopanacis cortex, Sanguisorbae radix and Torilis fructus inhibit coronavirus replication in vitro. Antivir Ther. 2010;15(5):697-709. [DOI] [PubMed] [Google Scholar]
- 66.Fiore C, Eisenhut M, Krausse R, et al. Antiviral effects of Glycyrrhiza species. Phytother Res. 2008;22(2):141-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bailly C, Vergoten G. Glycyrrhizin: an alternative drug for the treatment of COVID-19 infection and the associated respiratory syndrome? Pharmacol Therapeut. 2020;214:107618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of COVID-19 in New York City. N Engl J Med. 2020;382(24):2372-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stratton CW, Tang YW, Lu H. Pathogenesis-directed therapy of 2019 novel coronavirus disease. J Med Virol. 2020;93(3):1320-1342. [DOI] [PubMed] [Google Scholar]
- 70.Villapol S. Gastrointestinal symptoms associated with COVID-19: impact on the gut microbiome. Transl Res. 2020;226:57-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paliwal VK, Garg RK, Gupta A, Tejan N. Neuromuscular presentations in patients with COVID-19. J Neurol Sci. 2020;41(11):3039-3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bridwell R, Long B, Gottlieb M. Neurologic complications of COVID-19. Am J Emerg Med. 2020;38(7):1549.e3–.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndr. 2020;14(3):247-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martines RB, Ritter JM, Matkovic E, et al. Pathology and pathogenesis of SARS-CoV-2 associated with fatal coronavirus disease, United States. Emerg Infect Dis. 2020;26(9):2005-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mason RJ. Pathogenesis of COVID-19 from a cell biology perspective. Eur Respiratory Soc. 2020;55(4):2000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.García LF. Immune response, inflammation, and the clinical spectrum of COVID-19. Front Immunol. 2020;11(1441):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Swain SL, McKinstry KK, Strutt TM. Expanding roles for CD4+T cells in immunity to viruses. Nat Rev Immunol. 2012;12(2):136-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Diao B, Wang C, Tan Y, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). Front Immunol. 2020;11(827):1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li G, Fan Y, Lai Y, et al. Coronavirus infections and immune responses. J Med Virol. 2020 ;92(4):424.-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Donma MM, Donma O. The effects of allium sativum on immunity within the scope of COVID-19 infection. Med Hypotheses. 2020;144:109934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang Y, Jung YJ, Kim KH, et al. Antiviral activity of fermented Ginseng extracts against a broad range of influenza viruses. Viruses. 2018;10(9): 471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nagai T, Suzuki Y, Tomimori T, Yamada H. Antiviral activity of plant flavonoid, 5,7,4′-trihydroxy-8-methoxyflavone, from the roots of Scutellaria baicalensis against influenza A (H3N2) and B viruses. Biol Pharm Bull. 1995;18(2):295-299. [DOI] [PubMed] [Google Scholar]
- 84.Liu Q, Zhou Y-h, Yang Z-q. The cytokine storm of severe influenza and development of immunomodulatory therapy. Cell Mol Immunol. 2016;13(1):3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Spanopoulou C, Kassimos D, Cassimos D. Potential role of anti-complement agents in the treatment of COVID-19-related ARDS. Mediterr J Rheumatol. 2020;31(Suppl 2):304-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lu Y, Jiang Y, Ling L, Zhang Y, Li H, Chen D. Beneficial effects of Houttuynia cordata polysaccharides on “two-hit” acute lung injury and endotoxic fever in rats associated with anti-complementary activities. Acta Pharm Sin B. 2018;8(2):218-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gottlieb M, Long B. Dermatologic manifestations and complications of COVID-19. Am J Emerg Med. 2020;38(9):1715-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]