Abstract
Introduction
Treatment failures for lupus nephritis (LN) are high with 10%–30% of patients progressing to end-stage renal disease (ESRD) within 10 years. Interstitial fibrosis/tubular atrophy (IFTA) is a predictor of progression to ESRD. Prior studies suggest that tubulointerstitial injury secondary to proteinuria in LN is mediated by complement activation in the tubules, specifically through the membrane attack complex (MAC). This study aimed to investigate the associations between tubular MAC deposition with IFTA and proteinuria.
Methods
In this cross-sectional study, LN kidney biopsies were assessed for MAC deposition by staining for Complement C9, a component of the MAC. Chromogenic immunohistochemistry was performed on paraffin-embedded human renal biopsy sections using unconjugated, murine anti-human Complement C9 (Hycult Biotech, clone X197). Tubular C9 staining intensity was analysed as present versus absent. IFTA was defined as minimal (<10%), mild (10%–24%), moderate (25%–50%) and severe (>50%).
Results
Renal biopsies from 30 patients with LN were studied. There were 24 (80%) female sex, mean age (SD) was 33 (12) years old and 23 (77%) had pure/mixed proliferative LN. Tubular C9 staining was present in 7 (23%) biopsies. 27 patients had minimal-to-mild IFTA and 3 patients had moderate IFTA. Among the C9 + patients, 3 (43%) had moderate IFTA as compared with none in the C9- group, p=0.009. C9 + patients had higher median (IQR) proteinuria as compared with C9- patients: 6.2 g (3.3–13.1) vs 2.4 g (1.3–4.6), p=0.001 at the time of biopsy. There was no difference in estimated glomerular filtration rate (eGFR) between the C9 + and C9- groups.
Conclusion
This study demonstrated that tubular MAC deposition is associated with higher degree of IFTA and proteinuria, which are predictors of progression to ESRD. These results suggest that tubular MAC deposition may be useful in classification of LN. Understanding the role of complement in tubulointerstitial injury will also identify new avenues for LN treatment.
Keywords: lupus nephritis, autoimmunity, lupus erythematosus, systemic, inflammation
Key messages.
What is already known about this subject?
Animal studies showed that complement-sufficient rats developed interstitial fibrosis/tubular atrophy (IFTA) and had evidence of membrane attack complex (MAC) deposition in the renal tubules as compared with C6-deficient rats, suggesting a role of the MAC in tubulointerstitial injury.
What does this study add?
Tubular MAC deposition in human kidney tissue was associated with both a higher percentage of IFTA and proteinuria, which are predictors of progression to chronic kidney disease (CKD).
Tubular MAC deposition adds new information about tubulointerstitial injury beyond existing serum complement markers C3 and C4. We found that low serum complements C3 and/or C4 were not associated with IFTA.
How might this impact on clinical practice or future developments?
There are currently complement targeting therapies available and multiple under development, but none are approved for treatment of lupus nephritis (LN).
Elucidating the relationship between complement and tubulointerstitial fibrosis will shed light on mechanism and aid in the identification of potential biomarkers and treatment targets in LN.
Current ISN/RPS LN classification relies on light microscopic findings in combination with immunofluorescence findings in the glomerulus without accounting for the tubules.
If tubular MAC deposition is confirmed as a marker of tubulointerstitial injury and CKD progression, it will justify its staining in routine care and inclusion in the LN classification.
Introduction
Lupus nephritis (LN) is one of the most severe manifestations of SLE with high morbidity and mortality.1 Treatment failures in LN are high, with only 24%–50% of patients responding to standard treatment at 6 months and 10%–30% progressing to end-stage renal disease (ESRD) within 10 years.2 3 Most studies in LN focus primarily on mechanisms of glomerular disease.4 However, glomerular-based measures of disease activity do not consistently identify patients with LN at highest risk of progression to chronic kidney disease (CKD) and ESRD.5 There is increasing evidence that tubulointerstitial injury plays an important role in renal damage. Specifically, interstitial fibrosis and tubular atrophy (IFTA) strongly associates with poor renal outcomes independent of the extent of glomerular damage and is a reliable predictor of progression to ESRD.6–8 Furthermore, the revised 2018 International Society of Nephrology/Renal Pathology Society (ISN/RPS) classification for LN recommends classifying tubulointerstitial lesions because of their high prognostic values.5
The complement system has an important and under-recognised role in mediating tubulointerstitial injury due to proteinuria in LN.9 Previous studies suggest that proteinuria leads to activation of the terminal complement pathway in the tubules, resulting in the formation of the membrane attack complex (MAC).10–12 The MAC causes kidney injury through cell lysis and release of proinflammatory cytokines, which can lead to progressive tubulointerstitial fibrosis.13 14 To demonstrate the role of the MAC in tubulointerstitial injury in animal models, equivalent levels of proteinuria were induced in normocomplementemic and C6-deficient rats. Complement-sufficient rats developed severe IFTA and had evidence of MAC deposition in the proximal tubules as compared with C6-deficient rats.10 11
Few studies have evaluated tubular MAC deposition in humans. These studies demonstrated MAC deposition in the tubular basement membranes of LN kidney biopsies with limited sample sizes.15–17 Only one group studied the association of tubular MAC deposition with IFTA in patients with LN,18 but their findings had not been reproduced in other LN populations. In addition, none of these studies investigated the association of tubular MAC deposition with proteinuria to understand the relationship between complement activation, proteinuria and tubulointerstitial injury in LN.
Therefore, we set out to investigate the association of tubular MAC deposition with IFTA and proteinuria in LN. We hypothesise that complement activation resulting in MAC deposition in renal tubules is associated with higher degree of IFTA and proteinuria independent of glomerular damage in LN. There is currently no treatment focused on tubulointerstitial injury in LN. With multiple novel complement targeting therapies in development,19 20 it is timely and important to understand the role of the complement system in tubulointerstitial injury as this will identify new avenues for LN treatment.
Methods
This cross-sectional study used deidentified clinically indicated kidney biopsy samples collected at New York Langone Medical Center between July 2014 and July 2016. Informed consent was obtained for using biopsy samples collected for clinical use. A renal pathologist (MW) selected 30 unstained paraffin embedded renal biopsies from specimens previously stored in pathology. Inclusion criteria were age ≥18 years old, confirmation of LN diagnosis on biopsy, lupus nephritis ISN/RPS class II, III, IV, V, III+V or IV+V and at least 10 glomeruli present in the renal biopsy. Patients with advance sclerosing LN Class VI (over 90% of global sclerosis) were excluded because of advance end-stage damage.
We assessed MAC deposition in the renal tubules by using a previously developed immunohistochemistry (IHC) protocol.21 We performed immunohistochemical staining for Complement C9, which is a part of the MAC and a proxy marker for MAC deposition.22
In brief, chromogenic IHC was performed on formalin-fixed, paraffin-embedded, 4 µm human renal biopsy sections using the Ventana Medical Systems Discovery XT instrument and unconjugated, murine anti-human Complement C9 (Hycult Biotech, clone X197). The IHC protocol was optimised using C3 glomerulopathy as positive control and normal renal tissue as negative control.21 C9 staining was assessed in different locations: the tubular basement membrane, glomerulus and arterioles. Tubular C9 stain intensity was reviewed independently by three renal pathologists (MW, JP, DS) and scored on a standard semiquantitative scale of none (0), mild (1+), moderate (2+) and severe (3+) to add internal validity. Percentage of tubules with C9 staining was also analysed. Any discrepancy was resolved with re-evaluation of slides.
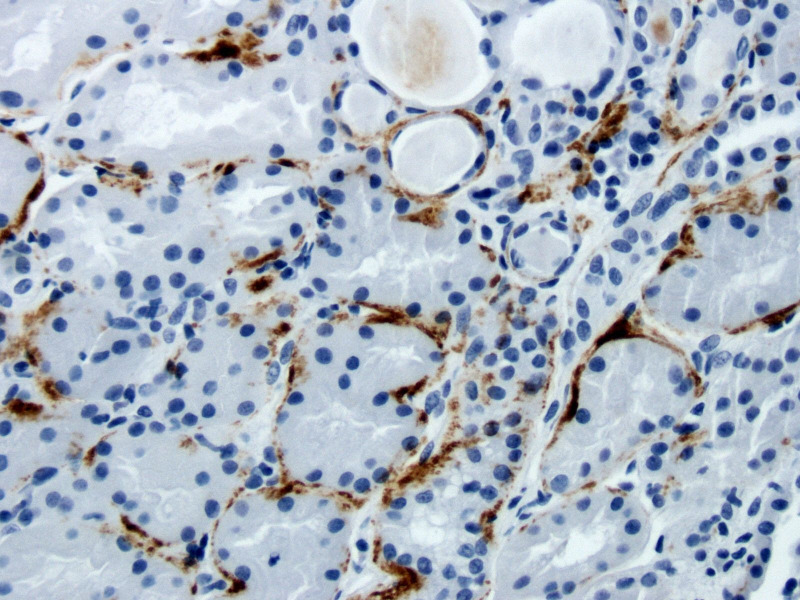
Tubular C9 staining was defined as positive if any staining was present (intensity score of 1 to 3) and negative if staining was absent (intensity score of 0). Figure 1 shows one of the patients with LN with positive C9 staining along the tubular basement membrane. To define IFTA, the main outcome of interest, the percentage of total tubules with fibrosis and/or atrophy was evaluated and categorised based on the NIH criteria as minimal (<10%), mild (10%–24%), moderate (25%–50%) and severe (>50%).6 7 For the main analysis, minimal and mild IFTA were combined. Additional analysis was done for all IFTA categories. The secondary outcome was proteinuria, which was analysed as a continuous variable. We recorded the 24-hour urine protein at the time of biopsy, and if not available, spot urine protein to creatine ratio was recorded. Nephrotic range proteinuria was defined as proteinuria >3.5 g/24 hours. IFTA and proteinuria were compared between C9 positive (+) and C9 negative (-) groups.
Figure 1.
C9 deposition in the tubular basement membrane of a patient with lupus nephritis Class V and moderate interstitial fibrosis/tubular atrophy.
As part of additional analyses, we evaluated tubular versus glomerular C9 staining to determine whether complement’s roles in the tubules and glomerulus are independent of one another. In addition, we compared tubular C9 staining to existing complement markers in assessing tubulointerstitial injury.23 Specifically, we assessed the association of low serum C3 and C4 with IFTA.
Clinical, demographic, laboratory and histopathological parameters were compared between C9 + and C9- groups. Medical records and pathology reports were reviewed by a rheumatologist (SW) who was blinded to C9 staining results to minimise bias. The following demographic and clinical data were collected at the time of biopsy: age, sex, self-reported race and ethnicity, systolic and diastolic blood pressure, and average disease activity as measured by the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K).24 Medications including hydroxychloroquine, prednisone and immunosuppression (use of mycophenolate mofetil, cyclophosphamide, tacrolimus, azathioprine, rituximab and belimumab) were recorded at the time of biopsy. Laboratory data included urinalysis, serum complement levels, dsDNA antibodies, creatinine and estimated glomerular filtration rate (eGFR). Low serum complement was categorised as serum C3 <70 mg/dL and/or serum C4 <14 mg/dL. eGFR was estimated using the Modification of Diet in Renal Disease (MDRD) equation in adults.25
As in the pathology reports, each case was classified based on the 2003 ISN/RPS LN classification.5 The NIH Activity Index and Chronicity Indices were used to characterise glomerular disease activity and chronic interstitial damage, respectively. Activity Index was categorised as mild (0 to 5) moderate (6 to 14) and high (≥15). Chronicity Index was categorised as mild (0 to 3), moderate (score 4 to 7) and high (≥8).5 Global glomerulosclerosis was categorised as ≤10% vs >10% of glomeruli with sclerosis. Number of crescents was counted. Routine immunofluorescence (IF) staining of C3, C4, C1q in the renal biopsy was recorded. The presence of concomitant C3, C4 and C1q deposition in renal biopsy was considered evidence for classical complement pathway activation.
Statistical analyses were performed using STATA (V.16). Fisher’s exact test for categorical data and Student’s t-test for continuous measurements were performed for comparison between tubular C9 + and C9- groups. The Wilcoxon Mann-Whitney test was used when the normality assumption was not met. A p value <0.05 was considered statistically significant and ≤0.20 was considered suggestive of possible association.
Patient and public involvement statement
Patients and the public were not directly involved in the design and conduct of this study. The research questions and outcomes were developed to better understand the role of complement-mediated tubulointerstitial injury in LN. The information learnt from this project will affect care of patients with LN including prognostication and treatment, to improve their survival and quality of life. Dissemination of study results will be done through online patient websites, lupus foundations and annual rheumatology meetings.
Results
Renal biopsies from 30 patients with LN were studied. Of the 30 renal biopsies, tubular C9 staining was present in 7 (23%) biopsies. The brown peroxidase immunohistochemical staining was primarily localised along the tubular basement membranes in a granular pattern. There were no missing data for outcome and variable of interest.
Patient characteristics and comparison between C9+ and C9- groups
Table 1 summarises the overall demographic, clinical and laboratory parameters and compares them between C9 + and C9- groups at the time of biopsy. Mean age was 33 (SD) (12) years old. Among the 30 patients, 24 (80%) were female sex, 9 (30%) self-identified as black or African–American; 11 (37%) self-identified as Hispanic or Latino patients. The median SLEDAI-2K score (IQR) was 11 (8–12). Thirteen out of 30 patients (43%) had systolic blood pressure ≥120 mm Hg and/or diastolic blood pressure ≥80 mm Hg. Twenty-five patients (83%) had eGFR ≥60 mL/min/1.73 m2 and 20 patients (66%) had low serum C3 and C4 levels. Mean albumin level (SD) was 3.0 (0.7) g/dL and median (IQR) proteinuria at the time of biopsy was 2.9 g (1.7–5.6).
Table 1.
Patient characteristics at the time of biopsy overall and stratified by C9 staining groups
| Overall sample n=30 |
Tubular C9 positive (stain intensity 1–3) n=7 |
Tubular C9 negative (stain intensity 0) n=23 |
P value | |
| Demographics/clinical parameters | ||||
| Age, years | 35 (23–41) | 36 (31–56) | 27 (21–39) | 0.20 |
| Female sex, n (%) | 24 (80%) | 5 (71%) | 19 (83%) | 0.60 |
| Black, n (%) | 9 (30%) | 9 (30%) | 21 (70%) | 0.30 |
| Hispanic, n (%) | 11 (37%) | 4 (57%) | 7 (30%) | 0.37 |
| Hydroxychloroquine, n (%) | 24 (80%) | 4 (57%) | 20 (87%) | 0.12 |
| Prednisone, n (%) | 19 (63%) | 2 (29%) | 17 (74%) | 0.07 |
| Immunotherapy*, n (%) | 16 (53%) | 4 (57%) | 12 (52%) | >0.99 |
| SLEDAI-2K† | 11 (8–12) | 12 (10–12) | 10 (6–12) | 0.83 |
| Systolic BP, mm Hg | 120 (112–133) | 133 (113–150) | 120 (110–130) | 0.18 |
| Diastolic BP, mm Hg | 75 (67–86) | 80 (70–100) | 70 (65–84) | 0.24 |
| Laboratory parameters | ||||
| eGFR, mL/min/1.73 m2 | 111.2 (74.7–132.0) | 57.9 (16.1–139.6) | 118.2 (95.2–132.0) | 0.17 |
| Creatinine, mg/dL | 0.7 (0.6–0.9) | 1.6 (0.5–3) | 0.7 (0.6–0.8) | 0.23 |
| Proteinuria‡, g/24 hours | 2.9 (1.7–5.6) | 6.2 (3.3–13.1) | 2.4 (1.3–4.6) | 0.001 |
| Nephrotic range proteinuria§, n (%) | 11 (37%) | 5 (71%) | 6 (26%) | 0.07 |
| Albumin, mean±SD, g/dL | 3.0±0.7 | 2.5±0.6 | 3.1±0.7 | 0.05 |
| Hematuria¶, n (%) | 12 (40%) | 4 (57%) | 8 (35%) | 0.39 |
| Pyuria¶**, n (%) | 13 (43%) | 2 (29%) | 11 (48%) | 0.43 |
| Serum C3 <70 mg/dL, n (%) | 20 (67%) | 4 (57%) | 16 (70%) | 0.66 |
| Serum C4 <14 mg/dL, n (%) | 20 (67%) | 3 (43%) | 17 (74%) | 0.18 |
| dsDNA ≤75 IU/mL, n (%) | 13 (43%) | 3 (43%) | 10 (44%) | >0.99 |
All continuous variables presented as median (IQR) except for albumin.
*Mycophenolate mofetil, cyclophosphamide, tacrolimus, azathioprine, rituximab or belimumab.
†SLEDAI-2K= Systemic Lupus Erythematosus. Disease Activity Index 2000.
‡24-hour urine protein collection and, if not available, spot urine protein to creatinine ratio.
§Proteinuria>3.5 g/24 hours.
¶Presence of 5 or more red blood cells per high-power field.
**Presence of 5 or more white blood cells per high-power field.
BP, blood pressure; eGFR, estimated glomerular filtration rate.
Compared with C9- patients, C9 + patients had higher median (IQR) age (36 (31–56) vs 27 (21-–39) years, p=0.20) and median (IQR) systolic blood pressure (133 (113–150) vs 120 (110–130) mm Hg, p=0.18). Hydroxychloroquine and prednisone use at the time of biopsy were less prevalent among those patients with tubular C9 deposition (table 1). There were no differences among the groups with regard to sex, immunotherapy use and median SLEDAI-2K.
The median (IQR) proteinuria was significantly higher for C9 + patients compared with C9- patients: 6.2 g (3.3–13.1) vs 2.4 g (1.3–4.6) g/24 hours, p=0.001. Five (71%) patients with tubular C9 staining had nephrotic range proteinuria as compared with 6 (26%) patients without C9 staining, p=0.07. Compared with C9- patients, C9 + patients had clinically meaningful lower median (IQR) eGFR (57.9 (16.1–139.6) vs 118.2 (95.2–132.0) mL/min/1.73 m2, p=0.17) and lower mean (SD) serum albumin (2.5 (0.6) vs 3.1 (0.7) g/dL, p=0.05), but did not reach statistical significance. There was no difference in prevalence of low serum C3 and dsDNA between the groups.
Histopathological characteristics and comparison between C9+ and C9- groups
Table 2 summarises the overall histopathological findings and compares them between C9+ and C9- groups. Twenty-seven patients had minimal-to-mild IFTA, three patients had moderate IFTA and none had severe IFTA. Twenty-three out of 30 (77%) patients had pure/mixed proliferative LN. Twelve (40%) and five (17%) of the patients had a moderate NIH Activity and Chronicity indices, respectively. None of the patients had a high NIH Activity and/or Chronicity indices. In addition, 13 out of 30 (43%) biopsies had positive glomerular C9 staining. C9 arterioles/arteries staining were present in 27 (90%) of the biopsies.
Table 2.
Histopathological characteristics overall and stratified by C9 staining groups
| Overall sample n=30 |
Tubular C9 positive (stain intensity 1–3) n=7 |
Tubular C9 negative (stain intensity) n=23 |
P value | |
| Light microscopy | ||||
| IFTA*, n (%) | 0.009 | |||
| Minimal to mild (<25%) | 27 (90%) | 4 (57%) | 23 (100%) | |
| Moderate (25%–50%) | 3 (10%) | 3 (43%) | 0 (0%) | |
| Moderate NIH Chronicity Index*, n (%) | 5 (17%) | 3 (43%) | 2 (9%) | 0.07 |
| Pure/Mixed Proliferative LN class (3, 4, 3+5, 4+5), n (%) | 23 (77%) | 6 (86%) | 17 (74%) | >0.99 |
| Moderate NIH Activity Index†, n (%) | 12 (40%) | 2 (29%) | 10 (44%) | 0.67 |
| >10% glomeruli with sclerosis, n (%) | 10 (33%) | 5 (71%) | 5 (22%) | 0.03 |
| At least one crescent, n (%) | 14 (47%) | 4 (57%) | 10 (43%) | 0.68 |
| Complement glomerular IF microscopy | ||||
| C3 (score >1), n (%) | 29 (97%) | 7 (100%) | 22 (96%) | >0.99 |
| C4 (score >1), n (%) | 18 (60%) | 6 (86%) | 12 (52%) | 0.19 |
| C1q (score >1), n (%) | 30 (100%) | 7 (100%) | 23 (100%) | >0.99 |
| Classical complement pathway activation (C3+, C4+, C1q+), n (%) | 18 (60%) | 6 (86%) | 12 (52%) | 0.19 |
| C9 deposition, non-tubular | ||||
| Glomerular (score >0), n (%) | 13 (43%) | 3 (43%) | 10 (44%) | >0.99 |
| Arterioles/arteries (score >0), n (%) | 27 (90%) | 7 (100%) | 20 (87%) | >0.99 |
*Score 4–7.
†Score 6–14.
IF, immunofluorescence; IFTA, interstitial fibrosis/tubular atrophy.
IFTA was associated with tubular C9 deposition: 3 out of 7 (43%) C9 + patients had moderate IFTA versus 0 out of 23 (0%) C9- patients, p=0.009. When IFTA was analysed as minimal versus mild versus moderate, we obtained the same significant association between IFTA and tubular C9 deposition (p=0.007). There was a greater proportion of patients with moderate Chronicity Index among the C9 + group versus C9- group (43% vs 9%, p=0.07), which is clinically meaningful but did not reach statistical significance. In addition, 5 out of 7 (71%) C9 + patients had >10% of glomeruli with sclerosis versus 5 out of 23 (22%) C9- patients, p=0.03. There was no significant difference in glomerular measures of disease activity: LN class, NIH Activity Index and presence of crescents.
There was no association found between tubular C9 staining with glomerular C9 staining by IHC. Of the 7 patients with tubular C9 staining, only 3 patients had concurrent glomerular C9 staining.
In addition, there was no association between tubular C9 immunohistochemical staining with glomerular C3 or C1q deposition seen by IF. Compared with C9- patients, a higher proportion of C9 + patients had concomitant C3, C4 and C1q staining, which would suggest classical complement pathway activation (86% vs 52%, p=0.19). Analysing the data by percentage of tubules with C9 staining yielded similar results (data not shown).
Lack of association between low serum complement with outcomes
Low serum complements C3 and C4 were not associated with higher percentage of IFTA and NIH Chronicity Index. In addition, there was no association found between low serum complements with proteinuria at the time of biopsy (table 3). On the other hand, low serum C3 and C4 were significantly associated with glomerular measures of disease activity: pure/mixed proliferative LN class and NIH Activity index.
Table 3.
Comparison of low vs normal serum complements on laboratory and histopathology
| Low serum C3 (<70 mg/dL) n=20 |
Normal serum C3 (≥70 mg/dL) n=10 |
P value | Low serum C4 (<14 mg/dL) n=20 |
Normal serum C4 (≥14 mg/dL) n=10 |
P value | |
| Laboratory parameters | ||||||
| eGFR, mL/min/1.73 m2 | 108.6 (64.4–123.5) | 129.1 (111.0–139.6) | 0.08 | 109.8 (64.4–125.0) | 127.0 (96.0–129.6) | 0.16 |
| Urine protein, g/24 hours | 3.2 (1.6–5.7) | 2.7 (1.7–3.1) | 0.62 | 2.8 (1.4–5.3) | 3.9 (2.2–5.7) | 0.47 |
| Histopathological parameters | ||||||
| Pure/mixed proliferative LN class (3, 4, 3+5, 4+5), n (%) | 18 (90%) | 5 (50%) | 0.03 | 19 (95%) | 4 (40%) | 0.002 |
| Moderate NIH Activity Index*, n (%) |
10 (50%) | 2 (20%) | 0.24 | 11 (55%) | 1 (10%) | 0.02 |
| Moderate IFTA†, n (%) | 2 (10%) | 1 (10%) | >0.99 | 2 (10%) | 1 (10%) | 0.61 |
| Moderate NIH Chronicity Index‡, n (%) | 4 (20%) | 1 (10%) | 0.64 | 4 (20%) | 1 (10%) | 0.64 |
| >10% glomeruli with sclerosis, n (%) |
5 (25%) | 5 (50%) | 0.23 | 4 (20%) | 6 (60%) | 0.05 |
All continuous variable presented as medium (IQR).
*Score 6–14.
†25%–50% of total tubules with fibrosis and/or atrophy.
‡Score 4–7.
eGFR, estimated glomerular filtration rate; IFTA, interstitial fibrosis/tubular atrophy.
Discussion
The role of the complement system in LN is an understudied but increasingly important area of research. In this cross-sectional study, we aimed to investigate tubular MAC deposition in LN biopsies to highlight the complement-mediated tubulointerstitial injury in LN. We found that tubular MAC deposition was present in 23% of renal biopsies and is associated with both a higher percentage of IFTA and proteinuria at time of biopsy. The association of tubular MAC deposition with IFTA in LN suggests that the terminal complement pathway plays an important role in tubulointerstitial injury.
This study is one of the first in humans to support the postulated link between proteinuria and complement-mediated tubulointerstitial injury in LN. We showed an association that has biological plausibility based on prior studies. Based on Hostetter and colleague’s proposed mechanism from animal studies,26 27 filtered protein through the damaged glomerular capillary walls is broken down in the proximal tubules to ammonia, which has been shown to activate the complement cascade leading to assembly of the MAC in the proximal renal tubules. In the absence of proteinuria, the MAC did not mediate tubulointerstitial disease in three distinct mouse models of non-proteinuric CKD.12
In this study, we found that tubular MAC deposition was associated with increased chronicity including higher degree of IFTA, glomerular sclerosis and NIH chronicity score while our previous study showed that glomerular MAC deposition was associated with hypertension and worse treatment response.21 Current ISN/RPS LN classification relies on light microscopic findings in combination with IF findings in the glomerulus without accounting for the tubules. Interestingly, the NIH Chronicity Index weights equally glomerular and tubulointerstitial findings with two (eg, sclerosis and crescents) and two (eg, fibrosis and atrophy) components for each. Our findings emphasise the importance of tubulointerstitial injury and suggest it may be useful to consider tubular C9 deposition in classification and prognostication of LN. This aligns with the revised 2018 ISN/RPS’s recommendations to assess tubulointerstitial lesions for prognostic significance in LN.
Tubular MAC deposition adds new information about tubulointerstitial injury beyond existing serum complement markers C3 and C4. We found that low serum complements C3 and/or C4 were not associated with IFTA, which is consistent with prior studies.7 A possible explanation for our findings is that the MAC is a more sensitive marker of complement activation because it is amplified compared with earlier complement components C1 to C4, and its presence implies it has surpassed the upstream regulatory complement proteins.28
Deposition of the MAC in renal tubules was found to be associated with IFTA in other diseased human kidneys such as IgA and diabetic nephropathy but less is known in LN.29 30 Only one other group (Wilson et al 201918) showed tubular MAC immunohistochemical staining was associated with IFTA in lupus using 57 biopsies with class III, IV and V LN obtained at a tertiary medical centre from England.18 Using different antibodies, IHC staining protocol and patient population (different demographics and medication use), we showed similar results that tubular MAC deposition was associated with higher degree of IFTA. To take a step further, we demonstrated an association between tubular MAC deposition and proteinuria, indicating a mechanism for complement-mediated tubulointerstitial injury. The reproducibility of the findings by these two groups strengthens and validates this important association between the MAC and indices of tubulointerstitial injury in LN.
This pilot study has several limitations related to its retrospective design and relatively small sample size. First, this study may be underpowered to detect small but important clinical differences between C9 + and C9- groups, and therefore p values should be interpreted with caution recognising the possibility of type II error. Because of relatively small number of patients with LN with moderate IFTA and tubular C9 staining, multivariable analysis could not be performed to adjust for possible confounders. Our findings demonstrated an association between tubular C9 deposition with IFTA and proteinuria, but not causation. Our results do not differentiate whether tubular C9 deposition seen in LN with proteinuria is due to passive filtration of complement proteins or complement activation in the tubules.
Second, our study did not include patients with severe (>50%) IFTA, therefore our results apply to patients with mild to moderate IFTA. However, the difference in tubular C9 deposition between minimal-to-mild versus moderate IFTA is meaningful because patients with moderate IFTA have a window of opportunity for potential treatment. In comparison, patients with severe IFTA are on a trajectory towards ESRD even without ongoing immune-mediated injury related to the inexorable progression that accompanies hyperperfusion of remaining functioning kidney.
Strengths of this study include using LN biopsies from a well-defined urban lupus population at a large tertiary medical centre. To minimise bias and ensure internal validity, medical records were reviewed by the first author (SW) who was blinded to C9 staining results. In addition, tubular C9 staining was interpreted by three different pathologists independently. An additional strength is that many of our findings are consistent with prior studies,6 7 adding to the external validity. Similar to prior studies that showed factors associated with IFTA, we found patients with tubular MAC staining were older, less likely to be on lupus medications (hydroxychloroquine and prednisone), had higher systolic blood pressure and lower eGFR at time of biopsy.
In summary, we found that tubular MAC deposition was associated with a higher percentage of IFTA and proteinuria, which are predictors of progression to CKD and ESRD. Although our study is preliminary in nature, our findings generate an important hypothesis that tubular MAC deposition may be useful in LN classification as a marker of tubulointerstitial injury. If our findings are confirmed in larger studies, it will justify C9 staining as part of routine care and its inclusion in the ISN/RPS LN classification. This will set a new paradigm for LN diagnosis and treatment. In addition, elucidating the role of the MAC in tubulointerstitial injury in LN will also aid in the identification of potential treatment targets for tubulointerstitial disease. This is timely and impactful with multiple complement targeting therapies under development.19 20
Footnotes
Twitter: @WangShudanMD, @BGoilav
AB and HMB contributed equally.
Contributors: Conceptualisation: Shudan Wang, AB, BG, JP, HMB. Methodology: Shudan Wang, AB, LC, BZ, MW, JP, DS, HMB. Formal analysis and investigation: Shudan Wang, AB, MW, JP. Writing—original draft preparation: Shudan Wang. Writing—review and editing: Shudan Wang, Shuwei Wang, BG, CP, ALJ, JP, DS, HMB, AB. Funding acquisition: Shudan Wang, AB. Resources: MW, JP, LC. Supervision: AB, HMB. All authors read and approved the final manuscript. Shudan Wang responsible for the overall content as the guarantor.
Funding: All individuals with direct involvement of this study are listed as authors. This research was supported by NIH/National Center for Advancing Translational Science (NCATS) Einstein – Montefiore CTSA Grant Number KL2 TR002558 (Shudan Wang) and supported by NIH/NIAMS K23 AR068441 (Anna Broder). The NIH provide K grants to support Drs Wang and Broder in their career development and is not involved in study design, data analysis and writing of this manuscript.The NYULH Center for Biospecimen Research and Development, Histology and Immunohistochemistry Laboratory (RRID:SCR_018304), is supported in part by the Laura and Isaac Perlmutter Cancer Center Support Grant; NIH/NCI P30CA016087 (Luis Chiriboga).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. Data are available on reasonable request. Data are from deidentified patients who had their kidney biopsy done either Tisch Hospital (NYU) or Bellevue Hospital.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was approved by the New York University (NYU) Institutional Review Board and was performed in accordance with the ethical standards of the Declaration of Helsinki. The manuscript adheres to the ‘Strengthening the Reporting of Observational Studies in Epidemiology’ (STROBE) guidelines for cross-sectional study.
References
- 1.Mok CC, Kwok RCL, Yip PSF. Effect of renal disease on the standardized mortality ratio and life expectancy of patients with systemic lupus erythematosus. Arthritis Rheum 2013;65:2154–60. 10.1002/art.38006 [DOI] [PubMed] [Google Scholar]
- 2.Hoover PJ, Costenbader KH. Insights into the epidemiology and management of lupus nephritis from the US rheumatologist's perspective. Kidney Int 2016;90:487–92. 10.1016/j.kint.2016.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appel GB, Contreras G, Dooley MA, et al. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol 2009;20:1103–12. 10.1681/ASN.2008101028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lech M, Anders H-J. The pathogenesis of lupus nephritis. J Am Soc Nephrol 2013;24:1357–66. 10.1681/ASN.2013010026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bajema IM, Wilhelmus S, Alpers CE, et al. Revision of the International Society of Nephrology/Renal pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of health activity and chronicity indices. Kidney Int 2018;93:789–96. 10.1016/j.kint.2017.11.023 [DOI] [PubMed] [Google Scholar]
- 6.Broder A, Mowrey WB, Khan HN, et al. Tubulointerstitial damage predicts end stage renal disease in lupus nephritis with preserved to moderately impaired renal function: a retrospective cohort study. Semin Arthritis Rheum 2018;47:545–51. 10.1016/j.semarthrit.2017.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Londoño Jimenez A, Mowrey WB, Putterman C, et al. Brief report: tubulointerstitial damage in lupus nephritis: a comparison of the factors associated with tubulointerstitial inflammation and renal scarring. Arthritis Rheumatol 2018;70:1801–6. 10.1002/art.40575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsieh C, Chang A, Brandt D, et al. Predicting outcomes of lupus nephritis with tubulointerstitial inflammation and scarring. Arthritis Care Res 2011;63:865–74. 10.1002/acr.20441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu SI-H, Couser WG. Chronic progression of tubulointerstitial damage in proteinuric renal disease is mediated by complement activation: a therapeutic role for complement inhibitors? J Am Soc Nephrol 2003;14:S186–91. 10.1097/01.ASN.0000070032.58017.20 [DOI] [PubMed] [Google Scholar]
- 10.Nangaku M, Pippin J, Couser WG. C6 mediates chronic progression of tubulointerstitial damage in rats with remnant kidneys. J Am Soc Nephrol 2002;13:928–36. 10.1681/ASN.V134928 [DOI] [PubMed] [Google Scholar]
- 11.Nangaku M, Pippin J, Couser WG. Complement membrane attack complex (C5b-9) mediates interstitial disease in experimental nephrotic syndrome. J Am Soc Nephrol 1999;10:2323–31. 10.1681/ASN.V10112323 [DOI] [PubMed] [Google Scholar]
- 12.Rangan GK, Pippin JW, Coombes JD, et al. C5B-9 does not mediate chronic tubulointerstitial disease in the absence of proteinuria. Kidney Int 2005;67:492–503. 10.1111/j.1523-1755.2005.67106.x [DOI] [PubMed] [Google Scholar]
- 13.Pippin JW, Durvasula R, Petermann A, et al. Dna damage is a novel response to sublytic complement C5b-9-induced injury in podocytes. J Clin Invest 2003;111:877–85. 10.1172/JCI200315645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu VW, Esser AF, Podack ER, et al. The membrane attack mechanism of complement: photolabeling reveals insertion of terminal proteins into target membrane. J Immunol 1981;127:380–6. [PubMed] [Google Scholar]
- 15.Ootaka T, Suzuki M, Sudo K, et al. Histologic localization of terminal complement complexes in renal diseases. An immunohistochemical study. Am J Clin Pathol 1989;91:144–51. 10.1093/ajcp/91.2.144 [DOI] [PubMed] [Google Scholar]
- 16.Hinglais N, Kazatchkine MD, Bhakdi S, et al. Immunohistochemical study of the C5b-9 complex of complement in human kidneys. Kidney Int 1986;30:399–410. 10.1038/ki.1986.198 [DOI] [PubMed] [Google Scholar]
- 17.Biesecker G, Katz S, Koffler D. Renal localization of the membrane attack complex in systemic lupus erythematosus nephritis. J Exp Med 1981;154:1779–94. 10.1084/jem.154.6.1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson HR, Medjeral-Thomas NR, Gilmore AC, et al. Glomerular membrane attack complex is not a reliable marker of ongoing C5 activation in lupus nephritis. Kidney Int 2019;95:655–65. 10.1016/j.kint.2018.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zelek WM, Xie L, Morgan BP, et al. Compendium of current complement therapeutics. Mol Immunol 2019;114:341–52. 10.1016/j.molimm.2019.07.030 [DOI] [PubMed] [Google Scholar]
- 20.Barilla-Labarca M-L, Toder K, Furie R. Targeting the complement system in systemic lupus erythematosus and other diseases. Clin Immunol 2013;148:313–21. 10.1016/j.clim.2013.02.014 [DOI] [PubMed] [Google Scholar]
- 21.Wang S, Wu M, Chiriboga L, et al. Membrane attack complex (MAC) deposition in lupus nephritis is associated with hypertension and poor clinical response to treatment. Semin Arthritis Rheum 2018;48:256–62. 10.1016/j.semarthrit.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 22.Hatanaka M, Seya T, Yoden A, et al. Analysis of C5b-8 binding sites in the C9 molecule using monoclonal antibodies: participation of two separate epitopes of C9 in C5b-8 binding. Mol Immunol 1992;29:911–6. 10.1016/0161-5890(92)90129-l [DOI] [PubMed] [Google Scholar]
- 23.Birmingham DJ, Irshaid F, Nagaraja HN, et al. The complex nature of serum C3 and C4 as biomarkers of lupus renal flare. Lupus 2010;19:1272–80. 10.1177/0961203310371154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gladman DD, Ibañez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol 2002;29:288–91. [PubMed] [Google Scholar]
- 25.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006;145:247–54. 10.7326/0003-4819-145-4-200608150-00004 [DOI] [PubMed] [Google Scholar]
- 26.Clark EC, Nath KA, Hostetter MK, et al. Role of ammonia in tubulointerstitial injury. Miner Electrolyte Metab 1990;16:315–21. [PubMed] [Google Scholar]
- 27.Nath KA, Hostetter MK, Hostetter TH. Pathophysiology of chronic tubulo-interstitial disease in rats. interactions of dietary acid load, ammonia, and complement component C3. J Clin Invest 1985;76:667–75. 10.1172/JCI112020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serna M, Giles JL, Morgan BP, et al. Structural basis of complement membrane attack complex formation. Nat Commun 2016;7:10587. 10.1038/ncomms10587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alexopoulos E, Papaghianni A, Papadimitriou M. The pathogenetic significance of C5b-9 in IgA nephropathy. Nephrol Dial Transplant 1995;10:1166–72. 10.1093/ndt/10.7.1166 [DOI] [PubMed] [Google Scholar]
- 30.Zheng J-M, Ren X-G, Jiang Z-H, et al. Lectin-Induced renal local complement activation is involved in tubular interstitial injury in diabetic nephropathy. Clin Chim Acta 2018;482:65–73. 10.1016/j.cca.2018.03.033 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request. Data are available on reasonable request. Data are from deidentified patients who had their kidney biopsy done either Tisch Hospital (NYU) or Bellevue Hospital.