Abstract
Postoperative fevers are common in hospitalised patients and warrant workup beyond the early post-op period. A 50-year-old man was admitted after sustaining a tibial plateau fracture. Fevers began 3 days after external fixation and persisted through a second surgery despite initial negative workup. Careful review of medications revealed enoxaparin as the instigating agent of a febrile drug reaction, and the fevers resolved after discontinuing the drug. On further questioning, it was discovered the patient had an allergy to pork, from which the main components of enoxaparin are typically derived. To our knowledge, this is the first reported enoxaparin-induced fever in the setting of a pork allergy. Enoxaparin-induced fevers should be considered in patients with unexplained post-op fever. Our case demonstrates the importance of analysing newly administered medications. Simple detailed history may significantly reduce patient morbidity and help to broaden differentials during investigation.
Keywords: haematology (incl blood transfusion), general practice / family medicine, infectious diseases, medical education
Background
Fever following major surgery is a relatively common finding in hospitalised patients and can often be a diagnostic challenge. Traditionally, the cut-off for fever is 38°C or 100.3 °F, although this may vary depending on the institution.1 The timing of fever following surgery is one of the most useful factors in prioritising the differential. Fevers can be classified as immediately post-op (within hours), early post-op (days 0–3), late post-op (days 4–30) or delayed (more than 30 days).
Recent literature suggests that workup for fever prior to post-op day 3 (POD3) without any localising symptoms is unwarranted due to the high incidence of ‘physiological fever’. Many immediate to early post-op fevers are secondary to surgical trauma, inflammation due to tissue damage and exposure to foreign materials.2–5 Generally, these fevers resolve with time or simple antipyretics and do not require further workup. However, fevers that persist beyond this early post-op timeframe should raise suspicion for an underlying pathological cause.
In the early post-op period, the differential may include pulmonary pathology such as atelectasis or pneumonia, urinary tract infections, early surgical site infections, myocardial infarction or continued inflammation due to trauma, burns or surgery. Non-infectious causes of fever should also be ruled out, including venous thromboembolism or pulmonary embolism, febrile drug reactions or gout.6 7 A careful history, physical examination and medication review can be revealing and minimise the amount of testing and imaging performed, as evidenced in our case. Here we present a man with persistent fevers beginning in the early post-op period after surgical intervention for a tibial plateau fracture.
Case presentation
The patient is a 50-year-old man with a medical history of hypertension, paroxysmal atrial fibrillation not on anticoagulation and obstructive sleep apnea who presented to the emergency department after falling off a motorcycle and sustaining a left tibial plateau fracture. He underwent two operations: an external fixation followed 4 days later by an open reduction with internal fixation (ORIF) of both medial and lateral condyles. He initially fevered to 38.9°C 3 days after the external fixation, which resolved with acetaminophen. After ORIF, the patient continued to have persistent fevers ranging between 38°C and 38.9°C despite scheduled acetaminophen 925 mg four times per day.
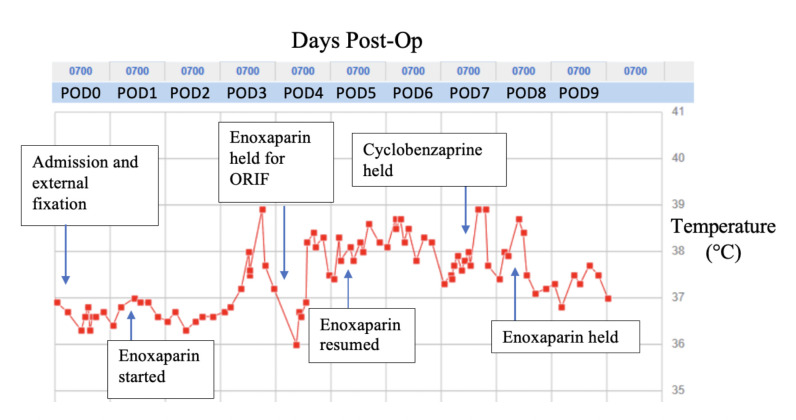
When evaluated on POD5 following the external fixation, review of systems was positive for diaphoresis, occasional productive cough of clear sputum and urinary frequency. Notably, the patient had no shortness of breath, chest pain, constipation, diarrhoea, dysuria or calf pain. Physical examination was unremarkable with the exception of the recent, well-healing surgical incision on the left lower extremity. Outside of fevers, other vital signs were stable throughout admission. Since the patient first fevered 3 days after the external fixation, further investigation was warranted for a precipitating aetiology. Beyond this point, events listed as ‘post-op’ refer to days after the external fixation procedure. Fevers were logged throughout the admission as represented in figure 1.
Figure 1.
Postoperative fever timeline.
Differential diagnosis and investigations
During the initial evaluation of post-op fever the differential remained broad. The patient had an intermittent, mild leukocytosis with a normal differential ranging between 9.0 and 12.4×103/uL in the post-op period; eosinophilia was notably absent. Preoperative and repeat COVID-19 testing on POD5 were negative. POD6 lactate was mildly elevated at 2.2 mmol/L but down-trended the following day. C reactive protein collected post-op was elevated at 130.48 mg/L and procalcitonin level was within normal limits. Blood and urine cultures were collected before the patient was started on empiric ceftriaxone and vancomycin. With adherence to hourly incentive spirometer use, the patient did not have evidence of atelectasis or pneumonia on chest X-ray. Urinalysis with culture was negative for acute urinary tract infection. D-dimer was mildly elevated at 2.13 ng/mL, with a follow-up bilateral lower extremity ultrasound negative for deep vein thrombosis. The orthopaedic team closely monitored the surgical incision during daily dressing changes, and there was no evidence of erythema, induration or exudate from the wound. Blood and urine cultures were persistently negative after 48 hours and antibiotics were discontinued, but the patient continued to have fever.
After the aforementioned infectious workup was negative, the team began investigating potential causes of drug-induced fevers. Acetaminophen was discontinued on POD6 to monitor for fever improvement. Careful review of the medications revealed fever onset after the initial administration of two drugs: cyclobenzaprine and prophylactic enoxaparin. Cyclobenzaprine was discontinued on POD7 and vital signs were monitored for 24 hours; the patient continued to be febrile. On POD8, prophylactic enoxaparin was held for 24 hours while the patient was monitored for fevers. His temperature decreased throughout that afternoon and overnight and he was finally diagnosed with drug-induced fever secondary to enoxaparin use. On further questioning, it was discovered the patient had a mild food allergy to pork products with diarrhoea, likely a contributing cause of the acute onset fevers.
Outcome and follow-up
After the resolution of fevers, the patient was deemed medically and surgically stable for discharge with close outpatient follow-up. The patient was educated about the porcine-derived composition of enoxaparin and to avoid future consumption of all porcine derivatives with concern for additional allergy-related complications. Since the patient’s CHA2DS2-VASc score was 1 and the patient was in normal sinus rhythm throughout the admission, he was discharged on oral aspirin 325 mg as directed by the primary orthopaedic team. At his 2-week follow-up appointment, he reported no additional fevers, and his wounds continued to heal appropriately.
Discussion
Fever is commonly encountered in the post-op course. A planned systematic approach to evaluate post-op fever is essential not only for diagnosis and treatment, but also for patient safety and resource stewardship. Workup prior to POD3 is often unwarranted and a misuse of hospital resources.2 Both timing and clinical presentation are essential to consider in the initial evaluation to explore aetiologies including but not limited to injury, infection and medications. Of all potential causes, surgical site infections are the most prevalent.6 Pneumonia, urinary tract infection and thrombosis are other common aetiologies that must be ruled out before considering drug-induced fever. Additional causes are listed in table 1.
Table 1.
Postoperative fever aetiologies by timeframe
| Immediate (within hours) | Early (days 1–3) | Late (days 4–30) |
|
|
|
Drug-induced fever is defined as a fever that starts soon after initiation of a drug, diminishes after stopping the drug and cannot be attributed to any other aetiology.8 9 It is an underdiagnosed phenomenon, which can be a diagnostic enigma in the setting of other physiological disorders mimicking signs of sepsis. Antibiotics have been identified as the most common culprit.9 Fever onset may be immediate if the patient is already sensitised to the offending agent, or delayed in the case of a first-time exposure.10 The average onset is 7–10 days, though can range from less than 24 hours to several months.11 12 It most commonly presents in a remittent, intermittent pattern, as with our patient.8 Other associated findings can include ‘relative bradycardia’, cutaneous lesions (18%–29%), leukocytosis with eosinophilia (<20%) and elevated inflammatory markers.8 The fever typically resolves within 48 hours of discontinuing the inciting agent as shown in our case presentation. Re-introduction of the same agent usually results in fever recurrence, and can confirm the diagnosis.13
It is reported that drug-induced fever could be a potential side effect from enoxaparin use 4%–8% of the time, however exact incidence is unknown due to scarcity of reports.13 There is one case report listing enoxaparin as a causative agent of drug fever, though ultimately DRESS syndrome was diagnosed.14 Other cases of fevers secondary to factor Xa inhibitor dalteparin10 and therapeutic heparin15 16 have been reported. Heparin-induced fever can specifically be difficult to diagnose in critically ill or post-op patients who commonly receive the drug for thromboembolic prophylaxis.15 The mechanism responsible for the febrile reaction is likely an idiosyncratic response. Our patient is the first reported case of an enoxaparin-induced fever in the setting of a pork allergy. We suspect there was a contributing component of a hypersensitivity reaction to his fever. Meat allergies are relatively uncommon, although exact prevalence has not been well-established. It is estimated that 5% of adults have food allergies,17 and of that small population, meat allergies may account for 3% of those cases.18 Hypersensitivity reactions to enoxaparin have frequently been reported in literature, though most often manifesting as a delayed skin reaction in the absence of fever.19–22 The components of enoxaparin are typically derived from livestock, primarily bovine and porcine intestines and lungs, lending to its potential for hypersensitivity reaction.13 Chen et al reported ovine-derived heparin products have a similar composition to bovine enoxaparin and should be further evaluated for future use in the setting of a pork allergy.23 For patients with known heparin allergies, appropriate alternatives for anticoagulation include DOACs, intravenous lepirudin or subcutaneous pentosanpolysulfate.21
Enoxaparin-induced fevers should be considered in the setting of any unexplained post-op fever in a patient receiving prophylactic enoxaparin. Our case demonstrates the importance of analysing newly administered medications in addition to a careful review of the patient’s allergy history. While drug-induced fevers are often an underdiagnosed phenomenon, a simple detailed history may significantly reduce patient morbidity and help to broaden differentials during investigation.
Learning points.
Postoperative fevers are a common finding in the hospital and should be evaluated beyond the early postoperative period.
A planned systematic approach is necessary for patient safety, resource stewardship diagnosis and treatment of postoperative fever.
Drug-induced fever due to enoxaparin usage is likely an underreported cause of postoperative fever, even in the absence of allergies.
Enoxaparin is a porcine-derived product, and this case should prompt additional attentiveness to food allergies when prescribing animal-derived medications.
Acknowledgments
To our patient for bearing with us while we figured his illness.
Footnotes
Contributors: HG, AS and DEVA contributed to the design, analysis, literature review and to the writing of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Garibaldi RA, Brodine S, Matsumiya S, et al. Evidence for the non-infectious etiology of early postoperative fever. Infect Control 1985;6:273–7. 10.1017/S0195941700061749 [DOI] [PubMed] [Google Scholar]
- 2.Ashley B, Spiegel DA, Cahill P, et al. Post-operative fever in orthopaedic surgery: How effective is the 'fever workup?'. J Orthop Surg 2017;25:230949901772795. 10.1177/2309499017727953 [DOI] [PubMed] [Google Scholar]
- 3.Guinn S, Castro FP, Garcia R, et al. Fever following total knee arthroplasty. Am J Knee Surg 1999;12:161-4. [PubMed] [Google Scholar]
- 4.Hobar PC, Masson JA, Herrera R, et al. Fever after craniofacial surgery in the infant under 24 months of age. Plast Reconstr Surg 1998;102:32–6. 10.1097/00006534-199807000-00005 [DOI] [PubMed] [Google Scholar]
- 5.Livelli FD, Johnson RA, McEnany MT, et al. Unexplained in-hospital fever following cardiac surgery. natural history, relationship to postpericardiotomy syndrome, and a prospective study of therapy with indomethacin versus placebo. Circulation 1978;57:968–75. 10.1161/01.CIR.57.5.968 [DOI] [PubMed] [Google Scholar]
- 6.Hyder JA, Wakeam E, Arora V, et al. Investigating the "Rule of W," a mnemonic for teaching on postoperative complications. J Surg Educ 2015;72:430–7. 10.1016/j.jsurg.2014.11.004 [DOI] [PubMed] [Google Scholar]
- 7.Pile JC. Evaluating postoperative fever: a focused approach. Cleve Clin J Med 2006;73 Suppl 1:S62–6. 10.3949/ccjm.73.Suppl_1.S62 [DOI] [PubMed] [Google Scholar]
- 8.Laun J, Laun K, Farooqi A, et al. Heparin-Induced fever: a case report and literature review. J Burn Care Res 2019;40:723–4. 10.1093/jbcr/irz064 [DOI] [PubMed] [Google Scholar]
- 9.Vodovar D, Le Beller C, Lillo-Le-Louet A, et al. [Drug-induced fever: a diagnosis to remember]. Rev Med Interne 2014;35:183–8. 10.1016/j.revmed.2013.02.023 [DOI] [PubMed] [Google Scholar]
- 10.Wackernagel D, Obaya S, Nydert P. Dalteparin-sodium induced drug fever in a neonate. BMJ Case Rep 2016;2016. 10.1136/bcr-2016-217621. [Epub ahead of print: 13 Oct 2016]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackowiak PA, LeMaistre CF. Drug fever: a critical appraisal of conventional concepts. An analysis of 51 episodes in two Dallas hospitals and 97 episodes reported in the English literature. Ann Intern Med 1987;106:728–33. 10.7326/0003-4819-106-5-728 [DOI] [PubMed] [Google Scholar]
- 12.Patel RA, Gallagher JC. Drug fever. Pharmacotherapy 2010;30:57–69. 10.1592/phco.30.1.57 [DOI] [PubMed] [Google Scholar]
- 13.Ng QX, Seng C, Ho CYX, et al. Enoxaparin: a cause of postoperative fever? Med Hypotheses 2018;121:47–8. 10.1016/j.mehy.2018.09.027 [DOI] [PubMed] [Google Scholar]
- 14.Ronceray S, Dinulescu M, Le Gall F, et al. Enoxaparin-Induced dress syndrome. Case Rep Dermatol 2012;4:233–7. 10.1159/000345096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forni AL, Murray HW. Drug fever induced by heparin. Am J Med 1992;92:107. 10.1016/0002-9343(92)90025-7 [DOI] [PubMed] [Google Scholar]
- 16.CLEJ JE. Drug fever. Prog Allergy 1964;8:149–94. [Google Scholar]
- 17.Sicherer SH, Sampson HA. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol 2014;133:291–307. 10.1016/j.jaci.2013.11.020 [DOI] [PubMed] [Google Scholar]
- 18.EMW B. Ige-Mediated food allergies including oral allergy syndrome in 383 patients. Allergologie 1998;21. [Google Scholar]
- 19.Cabañas R, Caballero MT, López-Serrano MC, et al. Delayed hypersensitivity to enoxaparin. J Investig Allergol Clin Immunol 1998;8:383–4. [PubMed] [Google Scholar]
- 20.Gonzalez-Delgado P, Fernandez J. Hypersensitivity reactions to heparins. Curr Opin Allergy Clin Immunol 2016;16:315–22. 10.1097/ACI.0000000000000281 [DOI] [PubMed] [Google Scholar]
- 21.Koch P, Hindi S, Landwehr D. Delayed allergic skin reactions due to subcutaneous heparin-calcium, enoxaparin-sodium, pentosan polysulfate and acute skin lesions from systemic sodium-heparin. Contact Dermatitis 1996;34:156–8. 10.1111/j.1600-0536.1996.tb02162.x [DOI] [PubMed] [Google Scholar]
- 22.Méndez J, Sanchís ME, de la Fuente R, et al. Delayed-Type hypersensitivity to subcutaneous enoxaparin. Allergy 1998;53:999–1003. 10.1111/j.1398-9995.1998.tb03804.x [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Yu Y, Fareed J, et al. Comparison of low-molecular-weight heparins prepared from ovine heparins with enoxaparin. Clin Appl Thromb Hemost 2019;25:107602961984070. 10.1177/1076029619840701 [DOI] [PMC free article] [PubMed] [Google Scholar]