Abstract
Objective
Evaluate the impact of pregnancy physiology and medication non-adherence on serum hydroxychloroquine (HCQ) pharmacokinetics (PK) and exposure-response in SLE.
Methods
We conducted a PK analysis using data from two observational pregnancy registries. We enrolled pregnant women with SLE taking HCQ at least 3 months prior to, and throughout pregnancy, and excluded those with multiple gestations. Using the PK model, we conducted dosing simulations and imputed 0%/20%/40%/60% non-adherence to evaluate the impact of adherence versus physiological changes on HCQ concentrations. We compared the effect of pregnancy-average non-adherent concentrations (≤100 ng/mL vs >100 ng/mL) on preterm birth using adjusted logistic regression.
Results
We enrolled 56 women who had 61 pregnancies. By the third trimester, mean apparent HCQ clearance increased by 59.6%. At a dosage of 400 mg/day, fully adherent patients are expected to have HCQ concentrations ≤100 ng/mL only 0.3% of the time, compared with 24.2% when 60% of doses are missed. Persistently low HCQ concentrations throughout pregnancy were associated with a significantly higher odds of preterm birth, controlling for lupus nephritis and race (OR 11.2; 95% CI 2.3 to 54.2; p=0.003).
Conclusions
We observed significant changes in HCQ PK during pregnancy, resulting in a shortening in the drug’s half-life by 10 days; however, medication non-adherence had a more pronounced effect on HCQ exposure compared with physiological changes alone. Moreover, pregnant women with non-adherent HCQ concentrations had significantly higher rates of preterm birth. Accordingly, optimising adherence in pregnancy may be more clinically meaningful than adjusting HCQ dosage to account for physiological changes. PK modelling indicates that serum HCQ concentrations ≤100 ng/mL are suggestive of non-adherence regardless of trimester and may help identify pregnancies at risk for poor outcomes.
Keywords: pharmacokinetics, systemic lupus erythematosus, antirheumatic agents
Key messages.
What is already known about this subject?
Physiological changes can alter hydroxychloroquine (HCQ) pharmacokinetics (PK) during pregnancy; however, the relationship between HCQ PK, medication adherence and pregnancy outcomes is unknown.
What does this study add?
We observed significant changes in HCQ PK during pregnancy, resulting in a shortening in the drug’s half-life by 10 days.
Medication non-adherence had a more pronounced effect on HCQ exposure compared with physiological changes alone, and pregnancies with persistently low HCQ concentrations had significantly higher rates of preterm birth.
How might this impact on clinical practice or future developments?
Serum HCQ concentrations ≤100 ng/mL are suggestive of non-adherence regardless of trimester.
Therapeutic drug monitoring may help identify pregnancies at risk for poor outcomes due to medication non-adherence.
Introduction
Pregnancies in women with SLE are often complicated by fetal loss, preterm birth and in severe cases, maternal death.1 2 Additionally, many women with SLE who are undertreated experience flares of their disease during pregnancy,3 which is independently associated with worse pregnancy outcomes.4–7 Accordingly, it is crucial to optimise drug therapy in lupus pregnancies to promote disease control and improve outcomes.1 Hydroxychloroquine (HCQ) is considered by the American College of Rheumatology and European League Against Rheumatism to be the gold-standard immunomodulatory drug in pregnancies complicated by SLE, due to its safety, prevention of disease flares and improvement in pregnancy and neonatal outcomes.3 8–11
Despite the importance of maintaining therapeutic drug exposure during pregnancy, few studies have evaluated the pharmacokinetics (PK) of HCQ in pregnancy. Several physiological changes in pregnancy may alter HCQ PK, including increases in the apparent volume of distribution, due to increases in fat mass and total body water; and increases in apparent HCQ clearance (CL), due to increases in the glomerular filtration rate and induction of hepatic drug metabolising enzymes.12–14 As a result of these PK changes, women with SLE may be at risk for subtherapeutic HCQ exposure as pregnancy progresses. In addition to changes in PK, subtherapeutic HCQ exposure can also occur in the setting of drug non-adherence. Adherence is difficult to accurately measure in an outpatient setting and varies widely depending on the measure and definition used, but electronic pill monitoring and pharmacy refill data suggest up to three-quarters of patients with lupus are non-adherent.15–18 Pregnancy may further exacerbate drug non-adherence due to frequent nausea and vomiting, perceived concerns about fetal harm from medications, mood changes and life stressors. Accordingly, drug exposure in pregnancy must be interpreted in the context of both physiological changes, disease effects and adherence.
In a cohort of pregnant women with autoimmune diseases, we found that there was an increase in HCQ apparent volume of distribution during pregnancy.14 However, due to the opportunistic nature of data collection, our prior PK study was not powered to detect a change in HCQ CL. Additionally, we found that pregnant women with SLE who had the lowest serum HCQ concentrations (≤100 ng/mL) had higher disease activity and rates of preterm birth, but it was unclear whether this was due to subtherapeutic exposure to HCQ itself, or generalised non-adherence.4 Accordingly, we continued to prospectively enrol women with SLE into a population PK and exposure-response study with the goals of evaluating the: (1) impact of pregnancy on HCQ PK and (2) complex relationship between drug adherence, HCQ drug concentrations and pregnancy outcomes.
Notably, most studies evaluating medication adherence and HCQ concentrations have used whole blood instead of serum.15 16 19 However, in this analysis, we measured serum concentrations because serum is readily available from existing biorepositories and retrospective studies, has commercially available assays that are used for clinical monitoring and avoids potential confounding from pregnancy-induced anaemia.14 20
Methods
Study design
We conducted a population PK and exposure-response analysis using data from two prospective, observational registries of pregnant women with rheumatic diseases at Duke University.
Population
We included 28 pregnancies in women from November 2013 to December 2016 with SLE who were taking HCQ throughout pregnancy as previously described.4 14 To this dataset, we enrolled an additional cohort of 33 pregnancies in women from January 2017 to January 2020 with a diagnosis of SLE as recorded in the study registry who were: (1) taking HCQ sulfate at least 3 months prior to their first pregnancy visit; (2) continued HCQ throughout pregnancy and (3) had at least two blood samples. We retained one patient initially thought to have SLE, but who was re-classified as mixed connective tissue disease. We excluded women with multiple gestations (eg, twins), due to potential confounding between drug PK, preterm birth and other outcomes of interest.
Data collection
Registry visits occurred at the same time as standard of care clinical visits, which typically occur 2–4 times per participant during pregnancy or post partum. At each visit, serum was obtained and frozen (−20°C to −80°C) for laboratory analysis. In addition, we collected information on concomitant medication use and obtained clinical information including SLE disease activity measures and neonatal outcomes as characterised below.
From 2013 to 2015, we did not routinely collect the time of medication administration. For these patients, HCQ dosage and dosing interval at each visit were extracted from the electronic medical record. From 2016 to 2020, we began recording the date and time the participants reported taking their most recent HCQ dosage and confirmed patient-reported dosage and dosage interval. In 2017, we began measuring patient-reported adherence using the Medication Adherence Self-Reported Inventory (MASRI).15 21 The MASRI is a self-reported measure consisting of multiple questions that prompt the respondent to consider their medication use, followed by a visual analogue scale that is used to quantify their perceived adherence (0%–100%).21 Only the visual analogue scale is used to classify a respondent as adherent or non-adherent, as noted further below.
Neonatal outcomes
We collected neonatal outcomes including neonatal loss, preterm birth and gestational age at birth. Non-live births were excluded from analyses of preterm birth or gestational age. We compared the effect of pregnancy average HCQ concentrations (≤100 ng/mL vs >100 ng/mL) on preterm birth using logistic regression models, controlling for a history of lupus nephritis within the last 3 years and race based on the directed acyclic graph (DAG) (online supplemental figure 1). Generalised estimating equations with an unstructured covariance matrix corrected for multiple pregnancies per patient. There were not enough observations to control by MASRI adherence. In a sensitivity analysis, we also adjusted the logistic regression model for concomitant prednisone and azathioprine use, although these were not considered true confounders based on the DAG.
lupus-2021-000602supp001.pdf (970.4KB, pdf)
All statistical analyses except logistic regression were conducted in R V.4.1.1 (R Foundation for Statistical Computing, Vienna, Austria) and RStudio V.1.4.1717 (RStudio, Boston, Massachusetts, USA), using the following packages: rmcorr, missMethods, ggplot2, tidyverse and dplyr. Logistic regression modelling was conducted using SAS software V.9.4 (SAS Institute, Cary, North Carolina, USA). All hypothesis testing was conducted using a significance level of 0.05.
Bioanalytical method used for HCQ concentration determination
We measured HCQ concentrations in serum using a validated high-performance liquid chromatography/tandem mass spectrometry assay at NMS Labs (Willow Grove, Pennsylvania, USA) as previously described.14 Briefly, we confirmed that HCQ was stable in frozen serum for at least 12 months at our storage temperatures; the precision between and within runs had coefficients of variation between 1.49% and 13.6%. The accuracy at the assay’s lower limit of quantitation (10 ng/mL) was 85.9%–98.2%.
Medication non-adherence
Consistent with prior publications, we defined adherence as a MASRI score of ≥80 on the visual analogue scale.15 21 We compared the differences in median HCQ concentration and adherence categories using the Mann-Whitney U test, stratified by all doses and by those taking the same total daily dose of 400 mg/day. Due to HCQ’s long half-life and low within-day variation of concentrations,22 23 we pooled all samples, irrespective of the timing of HCQ administration.
Population PK model development and evaluation
We developed a population PK model using non-linear mixed effects modelling with Phoenix NLME (Certara, Princeton, New Jersey, USA, V.8.3) software using the first-order conditional estimation with extended least squares method. We limited the PK analysis to subjects with known HCQ administration dates and times. Similar to a prior publication, we also excluded visits with HCQ concentration ≤100 ng/mL, due to concerns of medication non-adherence.14 To determine the impact of these assumptions on PK model parameters, we conducted a sensitivity analysis where we evaluated model performance using data from all subjects (eg, regardless of HCQ concentration and imputing missing administration times to 08:00 on the day of registry visit). Full methods on population PK model development and evaluation are noted in the online supplemental materials.
Dosing simulations and post hoc estimates of apparent clearance and volume of distribution during each trimester
We generated a virtual population of 1000 pregnant women (250 from each trimester and post partum). To ensure the virtual population was representative of real-world pregnant patients with SLE, we used the ‘rnorm’ function in R to randomly sample total body weights from the distribution (average, SD) of total body weight that we observed in our cohort of 55 pregnant women with SLE.
Using this virtual population and the final PK model, we determined the empirical Bayesian estimates (EBEs) for apparent volume of distribution and apparent CL. We conducted 100 virtual simulations and summarised the average (SD) EBE for apparent volume, apparent CL and HCQ half-life. Next, we used the EBEs and between-subject variability for CL to conduct dosing simulations and determine the expected steady-state concentration for 400 mg of HCQ taken once daily for at least 3 months. To explore the impact of medication non-adherence on serum HCQ concentrations throughout pregnancy, we also conducted dosing simulations and imputed 20%, 40% or 60% missing doses completely at random.
Cut-offs for HCQ concentration and medication non-adherence in pregnancy
To determine the serum HCQ concentration cut-off that differentiates between medication adherence and pregnancy-induced PK changes, we also conducted dosing simulations using the final PK model (inclusive of intra-individual variability, between-subject variability and residual error) and compared the number of expected trough concentrations after at least 3 months of HCQ administration that were below several proposed cut-offs (eg, ≤50, ≤100, ≤150, ≤200). We conducted the dosing simulations using the same virtual population (100 replicates of 1000 pregnant women) and imputed full adherence, or 20%/40%/60% non-adherence.
Patient and public involvement
Patients or members of the public were not involved in the design, conduct or reporting of the research.
Results
Population
We enrolled a total of 56 women who had a total of 61 pregnancies and 202 visits during the study period. Baseline demographics and clinical characteristics during each pregnancy are noted in table 1. From the 61 pregnancies, we obtained a total of 202 serum samples. Twenty samples (9.9%) were below the quantifiable limit. Samples were obtained during each pregnancy period as follows: first trimester, n=40 (19.8%); second trimester, n=80 (39.6%); third trimester, n=42 (20.8%) and post partum, n=40 (19.8%). The median (range) postpartum visit occurred at 6 (2–38) weeks.
Table 1.
Demographics and clinical characteristics
| All patients 56 women 61 pregnancies 202 visits |
Subgroup for PK model 22 women 22 pregnancies 55 visits |
|
| N (%) or median (IQR) | N (%) or median (IQR) | |
| Age (years) | 31 (27.5–34.3) | 32 (28.9–34.6) |
| Race | ||
| White | 29 (47.5%) | 9 (40.9%) |
| Black | 30 (49.2%) | 12 (54.5%) |
| Other | 2 (3.3%) | 1 (4.5%) |
| Weight (kg), visit* | 79.4 (66.7–96.3) | 81.1 (65.7–100) |
| Missing weight, visit | 5 (2.5%) | 2 (3.6%) |
| Active LN in last 3 years | 9 (14.8%) | 2 (9.1%) |
| Serum creatinine (mg/dL), visit* | 0.6 (0.5–0.8) | 0.7 (0.6–0.8) |
| Missing serum creatinine, visit* | 24 (11.9%) | 7 (12.7%) |
| Disease activity (PGA) | 0.33 (0–0.75) | 0.25 (0.05–0.67) |
| Disease activity (PGA), visit* | 0.39 (0–0.63) | 0.18 (0–0.62) |
| Prednisone use, visit* | 68 (33.7%) | 14 (25.5%) |
| Prednisone dosage (mg), visit* | 10 (5–20) | 7.5 (5–13.4) |
| Azathioprine use, visit* | 74 (36.6%) | 17 (30.9%) |
| HCQ daily dosage (mg), visit* | ||
| 400 | 157 (77.7%) | 41 (74.5%) |
| 200 | 18 (8.9%) | 5 (9.1%) |
| Other | 27 (13.4%) | 9 (16.4%) |
| HCQ dosing interval (hour), visit* | ||
| 24 | 141 (69.8%) | 39 (70.9%) |
| 12 | 61 (30.2%) | 16 (29.1%) |
*'Visit’ represents the value across all study visits; whereas the data otherwise represent values averaged across all time points for each unique pregnancy. Weight and creatinine represent observed value before imputing missing data.
HCQ, hydroxychloroquine; LN, lupus nephritis; PGA, Physician Global Assessment; PK, pharmacokinetic.
Baseline demographics and clinical characteristics for the subgroup of patients used to develop the PK model are also listed in table 1. For the PK subgroup, 7 (12.7%) samples were obtained during the first trimester; 23 (41.8%) from the second trimester; 11 (20%) from the third trimester and 14 (25.5%) post partum.
MASRI and serum HCQ concentration during pregnancy and post partum
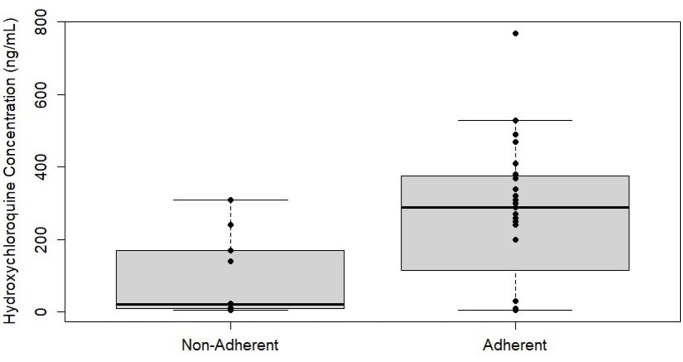
The MASRI score was available for 37/202 visits (18.3%), representing 18 patients with 18 pregnancies. The mean (median) HCQ concentration was higher for those with adherent MASRI scores, 266.6 (290) vs 93.7 (22.5) ng/mL (figure 1). When restricted to patients taking the same total daily dosage of 400 mg, the mean (median) HCQ concentration was 267.8 (295) ng/mL in those as categorised adherent, compared with 103 (24) ng/mL in those believed to be non-adherent based on the MASRI score. Based on the mean HCQ concentrations, we used a serum cut-off of ≤100 ng/mL as a surrogate of medication non-adherence during PK model development. Using this cut-off, non-adherence to HCQ was observed at 51 (25.2%) of pregnancy or postpartum visits for the entire cohort. In addition, 12 pregnancies (19.7%) had average HCQ concentrations throughout pregnancy and post partum that were ≤100 ng/mL, suggesting chronic non-adherence.
Figure 1.
Hydroxychloroquine concentration and adherence based on the Medication Adherence Self-Reported Inventory score at each visit.
Of visits with low HCQ concentrations (≤100 ng/mL), 7/13 (53.8%) were classified as adherent based on the MASRI score. Conversely, 20/24 visits (83.3%) of visits with HCQ concentrations >100 ng/mL were classified as adherent based on the MASRI score.
Population PK model development and evaluation
The original dataset consisted of 61 pregnancies with 202 visits. In total, for the PK analysis, we excluded 39 pregnancies and 147 visits due to either: (1) HCQ concentrations being ≤100 ng/mL or (2) missing date and time of the most recent HCQ administration. Accordingly, the evaluable PK population included 22 pregnancies with 55 samples (table 1). The base model that best characterised the PK data was a one-compartment model with between-subject variability estimated for CL/F. Residual error was best described using a proportional error model. The results of the final population PK model are noted in online supplemental table 1.
Results of the stepwise covariate searches are noted in online supplemental table 2. During stepwise covariate testing, weight was found to be a significant covariate on the apparent volume of distribution (decrease in Objective Function Value (OFV) by 9.2, p<0.01), and trimester was also found to be a significant covariate on apparent CL. Once body weight was added as a covariate in the model, a power relationship between trimester and CL was statistically superior and retained in the final model (decrease in OFV by −5.4, p<0.05). Including an effect of trimester on CL decreased the between-subject variability by 0.9%. Compared with the base model, weight and trimester as covariates reduced the unexplained residual error by 2.9%; the precision of the population estimate for the apparent volume of distribution improved from 111% to 43.4%, but the precision for the population estimate of apparent CL worsened slightly from 9% to 19.5%. The structural final PK model was:
Where tv is the typical population value of a parameter; Ka is the absorption rate constant; Tlag is the lag time after oral administration in hours; V/F is apparent volume of distribution at steady state in litres; WT is the subject’s actual weight in kilograms normalised to a 70 kg individual; θ1 is the exponential, allometric scaling of weight on apparent volume; CL/F is the apparent CL at steady state in litres per hour; θ2 is the exponent characterising the effects of each successive trimester on CL/F compared with post partum; ηCL/F is the deviation from the average population value for apparent CL.
Overall, the model evaluation showed a good fit between observed and predicted concentrations (online supplemental figure 2), and the visual predictive check demonstrated that the majority of concentrations fell in the 90% prediction interval (online supplemental figure 3). Convergence was achieved for all bootstrap replicates. All parameter estimates were within the 95% CIs for the bootstrap and there was no evidence of bias in the estimates for V/F or the exponent of trimester on CL/F.
Post hoc estimates of apparent clearance and volume of distribution during each trimester
Post hoc estimates from a virtual population of 1000 pregnant women with SLE demonstrated a progressive increase in HCQ CL throughout pregnancy (table 2). Overall, mean CL increased by 59.6% between post partum and the third trimester. In addition, because weight generally increased throughout pregnancy, mean HCQ volume of distribution increased by 31.2% in the third trimester compared with the first trimester. Due to these PK changes, the half-life of HCQ decreased from 32.4 days post partum to 22.1 days in the third trimester.
Table 2.
Predicted HCQ pharmacokinetics throughout pregnancy and post partum*
| Trimester | Half-life (days) | Apparent clearance (L/hour) | Apparent volume of distribution (L) |
| First | 21.4 (16.1) | 42.1 (15.3) | 27 589 (17 248) |
| Second | 24.1 (16.7) | 48.8 (17.6) | 35 935 (19 645) |
| Third | 22.1 (12.7) | 53.0 (18.9) | 36 201 (15 627) |
| Post partum | 32.4 (20.2) | 33.2 (11.8) | 33 164 (16 478) |
*Data presented as mean (SD) of the empirical Bayesian estimates.
HCQ, hydroxychloroquine.
Dosing simulations
We observed a progressive decline in median HCQ concentration during each trimester compared with post partum (figure 2). Assuming no missed doses, there was a median decrease in HCQ concentration of approximately 138 ng/mL throughout pregnancy. As expected, there was also a notable decrease in median HCQ concentrations with medication non-adherence. For example, compared with full adherence, the median postpartum concentration declined from approximately 440 ng/mL to, 354, 264 and 173 for 20%, 40% and 60% non-adherence, respectively.
Figure 2.
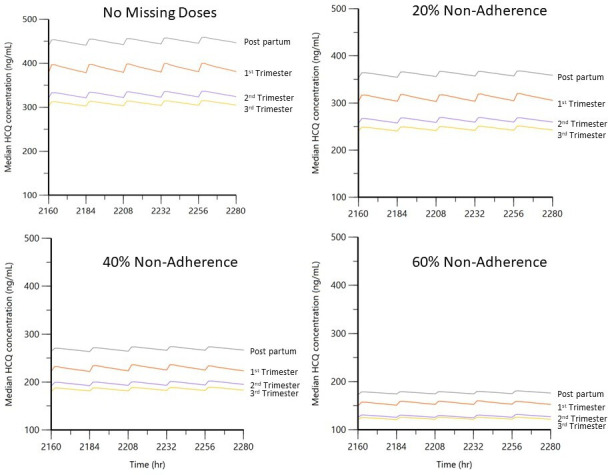
Serum HCQ concentrations throughout pregnancy and post partum. Median predicted serum HCQ concentrations throughout pregnancy and post partum, 400 mg/day. Simulations were conducted using the empirical Bayesian estimates from the final pharmacokinetic model and between-subject variability for clearance. HCQ, hydroxychloroquine.
Cut-offs for HCQ concentration and medication non-adherence in pregnancy
We conducted dosing simulations (100 replications of 1000 pregnant women across all trimesters and post partum) to determine the percentage of expected samples with an HCQ trough concentration below one of several cut-offs (≤50 ng/mL, ≤100 ng/mL, ≤150 ng/mL and ≤200 ng/mL, table 3). In addition, to differentiate the effects of pregnancy physiology alone from that of pregnancy physiology plus drug non-adherence, we imputed various degrees of adherence. As expected, drug non-adherence increased the number of samples below each cut-off compared with full adherence. In patients taking the most common dosage of 400 mg/day, very few samples (0.3%) were expected to be ≤100 ng/mL based on physiology alone. However, the percentage of expected trough samples ≤100 ng/mL rose to 1.2%, 5.3% and 24.2% when assuming 20%, 40% and 60% non-adherence, respectively. These findings underscore that low HCQ concentrations are increasingly common with progressive non-adherence. Furthermore, ≤100 ng/mL appeared to be a reasonable cut-off to differentiate pregnancy physiology alone versus pregnancy physiology and severe medication non-adherence, regardless of dosage.
Table 3.
Expected per cent of trough concentrations in pregnancy and post partum with medication non-adherence*
| HCQ cut-off (ng/mL) | 100% adherence | 80% adherence† | 60% adherence† | 40% adherence† | |
| 200 mg/day | 400 mg/day | 400 mg/day | 400 mg/day | 400 mg/day | |
| ≤50 | 0.1% | 0.1% | 0.1% | 0.3% | 2.2% |
| ≤100 | 0.6% | 0.3% | 1.2% | 5.3% | 24.2% |
| ≤150 | 27.0% | 2.5% | 7.3% | 22.4% | 57.9% |
| ≤200 | 74.4% | 9.0% | 21.3% | 46.5% | 80.4% |
Values in bold correspond to the optimal HCQ cutoff of </= 100 ng/mL.
*Example: patients taking 400 mg/day of HCQ are expected to have a trough HCQ concentration ≤100 ng/mL only 0.3% of the time if fully adherent and 24.2% of the time if taking 40% of doses.
†Corresponds to 20%, 40% and 60% non-adherence, respectively.
HCQ, hydroxychloroquine.
PK model sensitivity analysis
Compared with using the entire dataset (61 pregnancies, 202 visits), a sensitivity analysis showed better PK model performance by restricting the PK analysis to a population with HCQ concentrations >100 ng/mL, and with known administration dates and times. Specifically, there was a reduction in residual error from approximately 60% to 23.3%, improved plausibility of parameter estimates and the individual predicted HCQ concentrations more closely aligned with observed concentrations.
Neonatal outcomes
Neonatal outcomes were available for 56 pregnancies with 190 clinic visits. There were two neonatal losses, which were excluded from further analysis, yielding 54 pregnancies with live births. Overall, the median (IQR) gestational age at birth was 37 weeks (36–39) and 17 (31.5%) pregnancies delivered preterm. Of the 54 total pregnancies, 10/15 (66.7%) with pregnancy average HCQ concentrations ≤100 ng/mL delivered preterm; 2/30 (6.7%) with average concentrations 101–500 ng/mL delivered preterm and 5/9 (55.6%) with average HCQ concentrations ≥500 ng/mL delivered preterm.
In logistic regression modelling, average HCQ concentrations ≤100 ng/mL were associated with a higher odds of preterm birth, controlling for lupus nephritis and race (OR 11.2; 95% CI 2.3 to 54.2; p=0.003). In a sensitivity analysis, low HCQ concentrations were still significantly associated with preterm birth, controlling for concomitant azathioprine and prednisone use and controlling for lupus nephritis and race (p=0.004). The median Physician Global Assessment (PGA) of maternal disease activity during pregnancy was higher in the ≤100 ng/mL group compared with the >100 ng/mL group (median 0.75 vs 0.24).
Discussion
We observed significant changes to HCQ PK during pregnancy, including an increase in the apparent clearance by approximately 60% throughout pregnancy, and an increase in the apparent volume of distribution by 31%. As a result of these changes, the half-life of HCQ decreased by approximately 10 days during pregnancy compared with post partum. Collectively, the PK changes result in a decrease in median serum HCQ concentration by 138 ng/mL throughout pregnancy at a standard dose of 400 mg/day. From a pharmacological perspective, and assuming linear first-order kinetics, a 60% increase in clearance would require a 60% increase in dose (eg, 200 mg/day prepregnancy to 320 mg/day during the third trimester) to maintain the same average steady-state exposure (assuming no change in bioavailability). Despite these important observations, the need for HCQ dosage adjustments during pregnancy is not straightforward for several reasons. First, the physiological changes that impact HCQ PK (eg, weight gain, increases in the glomerular filtration rate, induction of hepatic metabolising enzymes) occur progressively throughout pregnancy. Second, HCQ concentrations change relatively slowly due to the long half-life and sequestration in blood cells and tissues.24 Third, the impact of pregnancy on HCQ bioavailability is unknown. Lastly, the target concentration for HCQ in lupus pregnancies requires further clarification. Accordingly, although it is possible that some women with lupus do not receive optimal HCQ dosing in pregnancy, a better understanding of the exposure-response relationship is needed before specific dosing adjustments can be recommended.
A particular strength of our analysis is the use of population PK modelling to differentiate between physiological pregnancy changes and medication non-adherence on HCQ drug exposure. We demonstrated that serum HCQ concentrations ≤100 ng/mL are consistent with non-adherence in pregnancy through several complementary observations: (1) a small subset of patients with MASRI scores <80 at each visit and average HCQ concentrations 93.7–103 ng/mL; (2) improved PK model performance by excluding visits with very low HCQ concentrations and (3) dosing simulations predicting that <1% of samples from fully adherent pregnant women will have serum trough HCQ concentrations ≤100 ng/mL from physiological changes alone. Conversely, approximately 24% of samples in patients taking 400 mg/day of HCQ are expected to be ≤100 ng/mL when they miss 60% of their doses. These results align with observations from a large prospective cohort study of patients with SLE, which suggested that it requires ‘severe’ non-adherence to reduce HCQ concentrations.15 Moreover, our results are similar to a study in non-pregnant adults where a threshold of 106 ng/mL in serum corresponded to low whole blood concentrations.16 Although we chose to define a single HCQ cut-off in serum to define non-adherence in pregnancy and post partum for practical reasons, our modelling and simulation can also be used to refine this cut-off by trimester and total daily HCQ dosage.
Determining an appropriate cut-off for drug concentrations to identify medication non-adherence during pregnancy is especially relevant because patients with SLE who do not routinely take their medication are more likely to need emergency room care17 and have worse pregnancy outcomes.4 Conversely, HCQ use can prevent recurrent congenital heart block in at-risk pregnancies by >50%.25 Moreover, studies suggest that monitoring whole blood HCQ concentrations can increase the proportion of patients achieving target adherence levels.19 Notably, HCQ concentrations vary widely between individuals even at the same dosage,4 therefore, no cut-off will be perfectly sensitive or specific for identifying medication non-adherence. Nevertheless, HCQ concentrations may be superior to patient-reported adherence, as 7/13 (53.8%) of visits with low HCQ concentrations in our study were classified as adherent using the MASRI, suggesting questionnaires may underestimate medication non-adherence. In addition, drug levels and questionnaires may capture different types of medication non-adherence (ie, severe vs less severe non-adherence).15
Additionally, we sought to evaluate the relationship between HCQ concentrations and neonatal outcomes. Our data suggest a significantly higher odds of preterm birth with persistently non-adherent HCQ concentrations, when controlling for the confounders of lupus nephritis and race. Furthermore, this association was maintained even when controlling for concomitant medication use. However, because of the small sample sizes in our study, the CIs for the effect of low HCQ concentrations on preterm birth were wide, and this finding requires confirmation in other cohorts. We also found that these low concentrations occur most often in the setting of severe non-adherence to HCQ (eg, missing 60% or more of doses); and therefore, it is possible that the higher rate of preterm birth is due to general non-adherence to other medications and prenatal care, especially if pregnancies were unplanned and had limited preconception counselling.26 Alternatively, because PGA was higher in pregnancies with low HCQ concentrations, the higher preterm birth rate could at least partially be due to maternal disease activity. Altogether, it is likely that preterm birth in women with low HCQ concentrations is multifactorial.4
HCQ partitions into multiple different blood cells,24 27 28 so it is important to highlight that HCQ concentrations in serum cannot be directly compared with other matrices, particularly whole blood.4 14 16 29 Historically, measuring HCQ concentrations in whole blood is preferred to serum, due to higher precision that can result when centrifugation causes haemolysis of red blood cells and alters serum concentrations.16 However, similar performance of whole blood and serum assays can occur with rigorous standardisation of sample handling and centrifugation, and there remains a very strong correlation between serum and whole-blood HCQ concentrations (r=0.8, p<0.0001).16 Furthermore, a particular strength of our PK modelling is that we were able to quantify the residual variability in HCQ concentrations due to assay performance, the handling of serum samples, unmeasured covariates and errors in data collection. The relatively low residual variability (20.4%), precision of PK parameters and simulation-based diagnostic plots suggests that we were able to precisely characterise HCQ disposition in pregnancy and post partum, despite using a serum matrix. Our PK model performance is further supported by the low coefficient of variation (14%) found for serum during assay development.29 Reassuringly, and assuming a whole blood to serum ratio of 1.47–2.63,16 the predicted HCQ concentrations for the adherent patients in our cohort are similar to whole blood concentrations reported by Izmirly et al in a population of highly motivated pregnant women.25 Additionally, it has been appropriately argued that certain SLE disease states (eg, acute haemolytic anaemia) can alter serum concentrations.16 Conversely, we argue that other SLE disease states (eg, anaemia of chronic disease) and normal pregnancy physiology (eg, anaemia of pregnancy), may disproportionately alter whole blood concentrations, making serum preferable.4 For example, it is known that measuring whole blood tacrolimus concentrations (which like HCQ also partitions into red blood cells) during pregnancy may lead to erroneous dosage adjustments.4 20 Accordingly, we maintain that serum is an acceptable matrix to measure HCQ concentrations during pregnancy, but highlight the need for clinicians to understand the relative pros and cons of both serum and whole blood measurements, and interpret results in light of patient-specific confounders.
There are important limitations in our analysis. First, we obtained data and samples from a real-world registry, which can be prone to disease misclassification, missing data and other challenges to data quality. For example, we encountered several discrepancies between the dose that patients reported taking and the dose prescribed in the medical record, requiring adjudication. To overcome these limitations, we made several assumptions as discussed in the ‘Methods’ section. Second, we used a slightly less stringent cut-off value for covariate selection (p=0.05) during backwards elimination, due to our small sample size; therefore, it is theoretically possible that the relationship between trimester and clearance was a false positive, but a false positive covariate effect is very unlikely because HCQ is known to undergo renal clearance, and the glomerular filtration rate increases by up to 40% in pregnancy.12 Lastly, it is possible that by excluding visits with very low HCQ concentrations during PK model development (eg, by erroneously imputing non-adherence), we could have underestimated renal clearance.14 Despite this potential limitation, we showed in a sensitivity analysis that the PK model was much more precise when excluding these low concentrations and, overall, our analysis provides strong support that low concentrations are best explained by medication non-adherence.
Summary
We observed significant changes in HCQ PK during pregnancy, resulting in a shortening of the drug’s half-life by approximately 10 days, yet medication non-adherence had a more pronounced effect on overall HCQ exposure compared with physiological changes alone. Moreover, pregnant women with non-adherent HCQ concentrations had significantly higher rates of preterm birth. Accordingly, optimising adherence to HCQ may be more clinically important than adjusting dosage to account for physiological changes. Our PK modelling indicates that serum HCQ concentrations ≤100 ng/mL are suggestive of non-adherence regardless of trimester and may help identify pregnancies at risk for poor outcomes.
Acknowledgments
The authors would like to thank Kim Brouwer, PharmD, PhD, and Klarissa D Jackson, PhD, for analytical guidance and manuscript review. The authors would also like to thank Erin Campbell, MS, for manuscript review.
Footnotes
Contributors: All authors have contributed to the analysis design and oversight, manuscript conception and drafting, statistical analysis and/or editorial review. Our paper is not under consideration elsewhere. All authors have also reviewed and approved the manuscript. Potential conflicts of interest are reported in the manuscript for all authors. SJB is responsible for the overall content as guarantor.
Funding: Support for the project was provided by the Rheumatology Research Foundation’s Scientist Development Award, Thrasher Research Fund, the Derfner Foundation, a Duke Health ENABLE grant and the National Institutes of Health.
Competing interests: SB receives support from the National Institutes of Health (NIH), US Food and Drug Administration, Patient Centered Outcomes Research Institute, the Rheumatology Research Foundation’s Scientist Development Award, the Childhood Arthritis and Rheumatology Research Alliance and consulting for UCB. DW is an Independent Director for Simulations Plus. MEBC receives funding from GSK and is a consultant for GSK and UCB. AE receives support from the NIH, Pfizer and Exagen. ARM receives research support from the non-profit organisation, Thrasher Research Fund. MC-W receives support for research from the NIH, National Institute of Allergy and Infectious Diseases, NICHD, US Food and Drug Administration and industry for drug development in adults and children. DG receives salary support for research from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and from Nabriva Therapeutics through a contract with the University of North Carolina at Chapel Hill. In addition, DG serves as a consultant for Tellus Therapeutics, focusing on neonatal drug development.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The Duke Institutional Review Board approved the study protocol and registries, and written informed consent was obtained from all study participants (IRB #Pro00000756, #Pro00084014, #Pro00000775).
References
- 1.Moyer A, Chakravarty EF. Management of pregnancy in lupus. Rheum Dis Clin North Am 2021;47:441–55. 10.1016/j.rdc.2021.04.008 [DOI] [PubMed] [Google Scholar]
- 2.Mehta B, Luo Y, Xu J, et al. Trends in maternal and fetal outcomes among pregnant women with systemic lupus erythematosus in the United States: a cross-sectional analysis. Ann Intern Med 2019;171:164–71. 10.7326/M19-0120 [DOI] [PubMed] [Google Scholar]
- 3.Eudy AM, Siega-Riz AM, Engel SM, et al. E ffect of pregnancy on disease flares in patients with systemic lupus erythematosus. Ann Rheum Dis 2018;38:annrheumdis-2017-212535–60. 10.1136/annrheumdis-2017-212535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balevic SJ, Cohen-Wolkowiez M, Eudy AM, et al. Hydroxychloroquine levels throughout pregnancies complicated by rheumatic disease: implications for maternal and neonatal outcomes. J Rheumatol 2019;46:57–63. 10.3899/jrheum.180158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim J-W, Jung J-Y, Kim H-A, et al. Lupus low disease activity state achievement is important for reducing adverse outcomes in pregnant patients with systemic lupus erythematosus. J Rheumatol 2021;48:707–16. 10.3899/jrheum.200802 [DOI] [PubMed] [Google Scholar]
- 6.Buyon JP, Kim MY, Guerra MM, et al. Predictors of pregnancy outcomes in patients with lupus: a cohort study. Ann Intern Med 2015;163:153–63. 10.7326/M14-2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skorpen CG, Lydersen S, Gilboe I-M, et al. Influence of disease activity and medications on offspring birth weight, pre-eclampsia and preterm birth in systemic lupus erythematosus: a population-based study. Ann Rheum Dis 2018;77:264–9. 10.1136/annrheumdis-2017-211641 [DOI] [PubMed] [Google Scholar]
- 8.Levy RA, Vilela VS, Cataldo MJ, et al. Hydroxychloroquine (HCQ) in lupus pregnancy: double-blind and placebo-controlled study. Lupus 2001;10:401–4. 10.1191/096120301678646137 [DOI] [PubMed] [Google Scholar]
- 9.Duan J, Ma D, Wen X, et al. Hydroxychloroquine prophylaxis for preeclampsia, hypertension and prematurity in pregnant patients with systemic lupus erythematosus: a meta-analysis. Lupus 2021;30:1163–74. 10.1177/09612033211007199 [DOI] [PubMed] [Google Scholar]
- 10.Sammaritano LR, Bermas BL, Chakravarty EE, et al. 2020 American College of rheumatology guideline for the management of reproductive health in rheumatic and musculoskeletal diseases. Arthritis Rheumatol 2020;72:529–56. 10.1002/art.41191 [DOI] [PubMed] [Google Scholar]
- 11.Andreoli L, Bertsias GK, Agmon-Levin N, et al. EULAR recommendations for women's health and the management of family planning, assisted reproduction, pregnancy and menopause in patients with systemic lupus erythematosus and/or antiphospholipid syndrome. Ann Rheum Dis 2017;76:476–85. 10.1136/annrheumdis-2016-209770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ke AB, Rostami-Hodjegan A, Zhao P, et al. Pharmacometrics in pregnancy: an unmet need. Annu Rev Pharmacol Toxicol 2014;54:53–69. 10.1146/annurev-pharmtox-011613-140009 [DOI] [PubMed] [Google Scholar]
- 13.Abduljalil K, Furness P, Johnson TN, et al. Anatomical, physiological and metabolic changes with gestational age during normal pregnancy: a database for parameters required in physiologically based pharmacokinetic modelling. Clin Pharmacokinet 2012;51:365–96. 10.2165/11597440-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 14.Balevic SJ, Green TP, Clowse MEB, et al. Pharmacokinetics of hydroxychloroquine in pregnancies with rheumatic diseases. Clin Pharmacokinet 2019;58:525–33. 10.1007/s40262-018-0712-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costedoat-Chalumeau N, Houssiau F, Izmirly P, et al. A prospective international study on adherence to treatment in 305 patients with flaring SLE: assessment by drug levels and self-administered questionnaires. Clin Pharmacol Ther 2019;106:374–82. 10.1002/cpt.1194 [DOI] [PubMed] [Google Scholar]
- 16.Blanchet B, Jallouli M, Allard M, et al. Hydroxychloroquine levels in patients with systemic lupus erythematosus: whole blood is preferable but serum levels also detect non-adherence. Arthritis Res Ther 2020;22:223. 10.1186/s13075-020-02291-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feldman CH, Yazdany J, Guan H, et al. Medication nonadherence is associated with increased subsequent acute care utilization among Medicaid beneficiaries with systemic lupus erythematosus. Arthritis Care Res 2015;67:1712–21. 10.1002/acr.22636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marengo MF, Waimann CA, de Achaval S, et al. Measuring therapeutic adherence in systemic lupus erythematosus with electronic monitoring. Lupus 2012;21:1158–65. 10.1177/0961203312447868 [DOI] [PubMed] [Google Scholar]
- 19.Durcan L, Clarke WA, Magder LS, et al. Hydroxychloroquine blood levels in systemic lupus erythematosus: Clarifying dosing controversies and improving adherence. J Rheumatol 2015;42:2092–7. 10.3899/jrheum.150379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hebert MF, Zheng S, Hays K, et al. Interpreting tacrolimus concentrations during pregnancy and postpartum. Transplantation 2013;95:908–15. 10.1097/TP.0b013e318278d367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koneru S, Shishov M, Ware A, et al. Effectively measuring adherence to medications for systemic lupus erythematosus in a clinical setting. Arthritis Rheum 2007;57:1000–6. 10.1002/art.22898 [DOI] [PubMed] [Google Scholar]
- 22.Rainsford KD, Parke AL, Clifford-Rashotte M, et al. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology 2015;23:231–69. 10.1007/s10787-015-0239-y [DOI] [PubMed] [Google Scholar]
- 23.Tett SE, Day RO, Cutler DJ. Concentration-effect relationship of hydroxychloroquine in rheumatoid arthritis--a cross sectional study. J Rheumatol 1993;20:1874–9. [PubMed] [Google Scholar]
- 24.Browning DJ. Pharmacology of chloroquine and hydroxychloroquine. hydroxychloroquine and chloroquine retinopathy, 2014: 35–63. [Google Scholar]
- 25.Izmirly P, Kim M, Friedman DM, et al. Hydroxychloroquine to prevent recurrent congenital heart block in fetuses of anti-SSA/Ro-positive mothers. J Am Coll Cardiol 2020;76:292–302. 10.1016/j.jacc.2020.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajendran A, Eudy AM, Balevic SJ, et al. The importance of pregnancy planning in lupus pregnancies. Lupus 2021;30:741–51. 10.1177/0961203321989803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jallouli M, Galicier L, Zahr N, et al. Determinants of hydroxychloroquine blood concentration variations in systemic lupus erythematosus. Arthritis Rheumatol 2015;67:2176–84. 10.1002/art.39194 [DOI] [PubMed] [Google Scholar]
- 28.Yeon Lee J, Lee J, Ki Kwok S, et al. Factors related to blood hydroxychloroquine concentration in patients with systemic lupus erythematosus. Arthritis Care Res 2017;69:536–42. 10.1002/acr.22962 [DOI] [PubMed] [Google Scholar]
- 29.Carlsson H, Hjorton K, Abujrais S, et al. Measurement of hydroxychloroquine in blood from SLE patients using LC-HRMS-evaluation of whole blood, plasma, and serum as sample matrices. Arthritis Res Ther 2020;22:125. 10.1186/s13075-020-02211-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
lupus-2021-000602supp001.pdf (970.4KB, pdf)
Data Availability Statement
No data are available.