Abstract
G protein-coupled receptors (GPCRs), key regulators of a variety of critical biological processes, are attractive targets for insecticide development. Given the importance of these receptors in many organisms, including humans, it is critical that novel pesticides directed against GPCRs are designed to be species-specific. Here, we present characterization of an interfering RNA pesticide (IRP) targeting the mosquito GPCR-encoding dopamine 1 receptor (dop1) genes. A small interfering RNA corresponding to dop1 was identified in a screen for IRPs that kill Aedes aegypti during both the adult and larval stages. The 25 bp sequence targeted by this IRP is conserved in the dop1 genes of multiple mosquito species, but not in non-target organisms, indicating that it could function as a biorational mosquito insecticide. Aedes aegypti adults treated through microinjection or attractive toxic sugar bait delivery of small interfering RNA corresponding to the target site exhibited severe neural and behavioral defects and high levels of adult mortality. Likewise, A. aegypti larval consumption of dried inactivated yeast tablets prepared from a Saccharomyces cerevisiae strain engineered to express short hairpin RNA corresponding to the dop1 target site resulted in severe neural defects and larval mortality. Aedes albopictus and Anopheles gambiae adult and larval mortality was also observed following treatment with dop1 IRPs, which were not toxic to non-target arthropods. The results of this investigation indicate that dop1 IRPs can be used for species-specific targeting of dop1 GPCRs and may represent a new biorational strategy for control of both adult and larval mosquitoes.
Keywords: Aedes, Anopheles, vector, insecticide, RNAi, G protein-coupled receptor
1. Introduction
Mosquito control is the primary means of preventing the spread of existing and the emergence of new mosquito-borne illnesses. However, resistance to every class of chemical insecticides has been documented in many mosquito species globally (Airs and Bartholomay, 2017), and the potential impacts of pesticides on non-target organisms are of ongoing concern (EPA, 2019). Discovery of new insecticides and mosquito control strategies that pose no risk to human health and which do not harm the environment is of critical importance (WHO, 2009). GPCRs, which comprise a large family of membrane bound receptors that regulate numerous intracellular signaling cascades and mediate a variety of critical biological processes, are attractive yet underexploited pesticide targets (Hill et al., 2016; Meyer et al., 2012; Ngai and McDowell, 2017; Nowling et al., 2013). Recent advances in mosquito genome sequencing and reference genome assemblies (Arensburger et al., 2010; Holt et al., 2002; Matthews et al., 2018; Neafsey et al., 2015; Nene et al., 2007) have revealed a mosquito GPCR superfamily consisting of hundreds of new putative insecticide targets in Aedes (dengue, Zika, chikungunya, and yellow fever vectors), Anopheles (malaria vectors), and Culex (lymphatic filariasis and West Nile virus vectors) mosquitoes (Hill et al., 2016; Hill et al., 2018; Ngai and McDowell, 2017; Nowling et al., 2013). Roughly one third of human pharmaceutical drugs impact GPCRs (Sriram and Insel, 2018; Wise et al., 2002), indicating that these receptors can be readily targeted, but highlighting the importance of identifying novel pesticides that are specific to mosquito GPCRs yet do not impact these receptors in humans or other non-target species (Hill et al., 2016).
RNAi, an endogenous regulatory pathway in eukaryotic cells that silences gene expression through the production of small interfering RNAs (siRNAs), has traditionally been applied for functional characterization of mosquito genes in the laboratory (Airs and Bartholomay, 2017), but has attracted the interest of the insect agricultural pest control community (Zhang et al., 2017). We recently initiated a large-scale effort to assess the potential use of RNAi technology for mosquito control. These studies began with high-throughput screens that led to the identification of siRNAs which function as mosquito larvicides (Hapairai et al., 2017; Mysore et al., 2017), several of which recognize conserved target sites in multiple mosquito species and function as broad-based mosquito pesticides (Mysore et al., 2019a,b). A proportion of the siRNA larvicides identified in these screens target genes with orthologs that are known to be required for both larval and adult viability in Drosophila melanogaster, a well-characterized genetic model insect. Here, through the development and evaluation of IRPs corresponding to a conserved target site in mosquito dop1 genes, we examine the hypothesis that IRPs can function as insecticides that target both developing and adult mosquitoes.
Previous characterization of A. aegypti dop1 demonstrated that this gene (the subject of this investigation) and dop2, a second GPCR family member in A. aegypti, encode D1-like dopaminergic GPCRs that respond to dopamine in a dose-dependent manner (Meyer et al., 2012). A screen for lead chemistries active at Dop2 identified amitriptyline and doxepin as Dop2-selective compounds that induce high levels of A. aegypti larval mortality (Meyer et al., 2012). Dop2 antagonists also induce high rates of mortality in Culex quinquefasciatus (Nuss et al., 2015) and Anopheles gambiae larvae (Hill et al., 2016). Likewise, manipulation of dopamine levels in D. melanogaster larvae through inhibition of tyrosine hydroxylase activity results in larval death (Neckameyer, 1996). RNAi-mediated targeting of dop1R2, the D. melanogaster ortholog of dop2, as well as treatments with the D1-like receptor antagonist flupenthixol resulted in late larval/prepupal defects and developmental arrest in developing fruit flies (Regna et al., 2016), and silencing a dopamine-2 like receptor (D2R) in Tribolium castaneum caused larval death (Bai et al., 2011). These findings suggest that silencing A. aegypti dop1 could also result in larval mortality. Furthermore, neural expression of dsRNA targeting dop1R1 in D. melanogaster adults results in a flightless phenotype and adult lethality (Dietzl et al., 2007). Given that the A. aegypti dop1 gene is expressed in developing and adult mosquitoes (Akbari et al., 2013), and that dopamine signaling is required for viability at multiple stages of the fruit fly life cycle (Dietzl et al., 2007; Mummery-Widmer et al., 2009; Neckameyer, 1996; Regna et al., 2016), it was hypothesized that silencing dop1 in A. aegypti would induce mortality at multiple life cycle stages.
In this study, we describe a scalable attractive toxic sugar bait- (ATSB-) based delivery system that was used for effective delivery of siRNAs targeting dop1 to adult A. aegypti mosquitoes under simulated deployment conditions. We also discuss the development and characterization of a yeast-based system for delivery of dop1 IRPs to A. aegypti larvae. We demonstrate that silencing dop1 in A. aegypti results in high levels of mortality in adults and larvae and show that IRPs which silence dop1 can be used for selective, biorational targeting of multiple species of disease vector mosquitoes.
2. Materials and Methods
2.1. Mosquito rearing
A. aegypti Liverpool-IB12 (LVP-IB12) strain mosquitoes, A. albopictus (obtained from BEI Resources, NIAID, NIH: A. albopictus, Strain Gainesville, MRA-804, contributed by Sandra A. Allan), and A. gambiae G3 strain mosquitoes (obtained through BEI Resources, NIAID, NIH: A. gambiae, Strain G3, Eggs, MRA-112, contributed by Mark Q. Benedict) were reared as described (Clemons et al., 2010a). Adult females of each species were provided with blood meals through use of a Hemotek artificial membrane feeding system (Hemotek Limited, Blackburn, UK) that was used to deliver sheep blood that had been purchased from HemoStat Laboratories (Dixon, CA). The mosquitoes were cultured in an insectary that is maintained at 26° C, ~80% relative humidity, and which has a 12 hr light/12 hr dark cycle with 1hr crepuscular periods at the beginning and end of each cycle.
2.2. Discovery of siRNA #462:
siRNA #462, which was not described previously, was identified in larval soaking (Hapairai et al., 2017; Mysore et al., 2017) and adult microinjection (see below) screens for genes essential for mosquito viability. A subset of the siRNAs tested in these screens, including siRNA #462, uncover target sites conserved in orthologous genes of multiple mosquito species, but not humans. These genes were screened in A. aegypti larvae and adults if the D. melanogaster orthologs were known to be required for larval viability (Thurmond et al., 2019), when the A. aegypti orthologs were known to be expressed throughout larval development (Akbari et al., 2013), if the genes are required for adult D. melanogaster survival (Thurmond et al., 2019), and if the target sites were not known to reside in the genomes of organisms other than mosquitoes [per blastn searches (Giraldo-Calderon et al., 2015; Johnson et al., 2008)]. Larval soaking screen experiments were performed as described (Singh et al., 2013) using first instar (L1) larvae and the following siRNAs purchased from Integrated DNA Technologies (Coralville, Iowa): #462: 5’- AUAUCAUCGCCGCGUUCUGCAAGAC −3’ in dop1 (AAEL019437) and a control sequence that has not been identified in mosquito or other genomes (Tomchaney et al., 2014): 5’-GAAGAGCACUGAUAGAUGUUAGCGU-3’. The larval soaking screen was performed in duplicate experiments in which 20 L1 larvae were placed in 20 ul of 0.5 μg/μl siRNA for four hours, then placed in sterile distilled water, reared, and assessed as described in the larvicide testing guidelines of the World Health Organization (WHO, 2005), with the experiment concluding when mosquitoes had either died or emerged as adults. Larval screen data were evaluated using the Fisher’s exact test.
The adulticidal capacity of siRNA #462 (hereafter referred to as dop1.462 siRNA) was assessed in a microinjection screen conducted in A. aegypti. For the microinjection experiments, adult female mosquitoes were injected using an embryo microinjection protocol (Clemons et al., 2010b) that was adapted for adults. In summary, non-blood fed three day old females were anesthetized with carbon dioxide and injected with a dose of 2.25 μg siRNA (250 nl of 9 μg/μl dop1.462 or control siRNA) vertical to the body axis in the thoracic region. After injection, the mosquitoes were placed in a cage where they recovered and were observed for behavioral defects and death over the course of the next week. 20 adult females/treatment were injected in each of three replicate experiments, and data were evaluated with the Fisher’s exact test. The susceptibility of A. albopictus and A. gambiae adult females to dop1.462 siRNA was evaluated in the same manner, except that the siRNA dose was reduced to 150 nl of 6 μg/μl siRNA per mosquito for A. gambiae adult females, which are smaller than A. aegypti and A. albopictus adult females.
2.3. ATSB simulated field trials:
ATSB feedings were performed using a protocol modified from Coy et al. (2012). To create the bait station, the pointed end of a 0.2 ml plastic tube (Eppendorf, Hauppauge, NY) was cut off using a razor blade, creating a 1 mm opening through which a small piece of cotton (~4 mg) that served as a wick was placed. 64 μl of 10% sucrose in sterile DEPC-treated water with 0.5% of blue tracer dye (McCormick) alone, or containing 2.5 μg/μl of dop1.462 or control siRNA, was added to the tube. The tube was then capped and hung with the wick facing down at the top of a 3.75 L cage (Berry Global, Evansville, IN). 25 non-blood fed 4–5 day old adult females that had been sugar-starved for 48 hrs were permitted to feed from the wick for four hrs beginning at dawn. Females that were semi-engorged or that had failed to feed were discarded, while fully engorged females were collected as individuals and placed in fruit fly rearing vials. After 24 hrs, the mosquitoes were fed with 10% sucrose solution, which was subsequently provided every two days over the next six days, at which time behavioral phenotypes and mortality were assessed. The G-test of independence was used to compare feeding rates among treatments in each of three biological replicate experiments, while the log-rank test was used for comparison of survival rates among the control or experimental sugar bait treatments.
2.4. Yeast engineering and culturing
Custom DNA oligonucleotides (Invitrogen Life Technologies, Carlsbad, CA) encoding a short hairpin RNA (shRNA) expression cassette corresponding to dop1 target sequence 5’-AUAUCAUCGCCGCGUUCUGCA-3’ were used to produce stably transformed S. cerevisiae as previously described (Hapairai et al., 2017). In short, the dop1.462 shRNA-encoding nucleotide was ligated downstream of the galactose-inducible Gal1 promoter (Bassel and Mortimer, 1971) and upstream of the cyc1 terminator. The resulting construct was inserted into the multiple cloning sites of the pRS404 and pRS406 integrating shuttle vectors (Sikorski and Hieter, 1989), which are marked by TRP1 and URA3, respectively. The resulting plasmids facilitated chromosomal integration and selection of recombinant S. cerevisiae CEN.PK strain yeast [genotype MATα ura3–52 trp1–289 leu2–3_112 his3Δ1 MAL2–8C SUC2 (van Dijken et al., 2000)] which were able to grow on synthetic complete media lacking tryptophan and uracil. PCR and sequencing were used to verify integration of the dop1.462 shRNA expression cassettes at both loci. This strain, which is hereafter referred to as dop1.462 yeast IRP, as well as a previously described control shRNA expression strain (Hapairai et al., 2017), were cultured as described (Hapairai et al., 2017). The yeast cultures were used in the preparation of 40 mg dried inactivated yeast interfering RNA larvicide tablets as detailed in a methods protocol (Mysore et al., 2019c).
2.5. Larvicide trials
Lab assays:
Laboratory larvicide trials which conformed to the WHO larvicide testing guidelines (WHO, 2005) were performed as described previously (Mysore et al., 2017). In short, in each of three biological replicate experiments, 20 first instar (L1) larvae were placed in 50 ml of distilled water in each of three 500 ml replicate containers per condition (control or dop1.462 treatment). In each replicate container, the 20 larvae were fed a single 40 mg control or dop1.462 yeast tablet at the beginning of the trial, which permitted ad libitum larval feeding throughout the experimental trial period. Fourth instar (L4) larvae were given a dietary supplement, which consisted of 150 μl of 6% w/v liver powder (MP Biomedicals) mixed in distilled water as described (Mysore et al., 2019a). Larval mortality was assessed, and the percentages of larval mortality were arcsine transformed prior to analyzing data from replicate experiments using Student’s t-test.
For generation of dose-response curves, which were produced as described (Hapairai et al., 2017), three biological replicate experiments, each with three larvicide-treated replicate containers per dose, were performed. To generate the different doses of dop1.462 IRP yeast, various proportions of the larvicidal yeast culture were mixed with the control shRNA yeast. Replicate data were pooled for analysis, and LD50 values with 95% confidence intervals were determined using SPSS 25 software (IBM, Armonk, NY) and log dosage-probit mortality regression as previously described (Hapairai et al., 2017; Mysore et al., 2017). The data were also assessed separately using linear regression analysis that was performed using Microsoft Excel (Microsoft Corp, Seattle, WA).
2.6. Semi-field larvicide trials:
Larvicide trials were also conducted outdoors in a rooftop laboratory in Notre Dame, IN during May and June 2019. These semi-field trials, which were completed in accordance with the WHO larvicide testing guidelines (WHO, 2005), were conducted as previously described (Mysore et al., 2019a,b) on LVP-IB12 strain A. aegypti mosquitoes. To prepare each replicate container, 20 L1 larvae, 3.7 L of distilled water (water height of 10 cm), and one dop1.462 or control yeast larvicide tablet were placed in a 10 L plastic container (height = 25 cm, diameter = 23 cm). To prevent mosquito escape and the entrance of macrobiota into the test site, the containers were covered with mesh and placed in a screened (472 openings per centimeter) SansBug 1-Person Free-Standing Pop-Up Mosquito-Net tent (Hakuna Matata Tents, Ontario, Canada) located underneath an overhang. A total of 14 replicate containers/condition were evaluated over the course of three biological replicate trials. At the conclusion of the trials, the percentages of larval mortality were arcsine transformed, and Student’s t-test was used to evaluate data from multiple replicate experiments. During the trial period, humidity levels averaged 75±15%, while temperatures ranged from 9° C to 35° C, with mean daytime temperatures of 23.5±5° C and mean nighttime temperatures of 19±4° C.
2.7. Evaluation of dop1 transcript levels
A riboprobe corresponding to bases 272–708 of the A. aegypti dop1 gene (AAEL019437) was prepared as described (Patel, 1996) and used in in situ hybridization experiments that were conducted as described (Haugen et al., 2010) and used to assess transcript levels in adult and L4 larval brains. For analysis of adult transcripts, brains from 20 adult females that had been microinjected with dop1.462 or control siRNAs were evaluated in each of three biological replicate experiments. For analysis of larval transcripts, the brains of 20 larvae that had been fed with either dop1.462 or control yeast tablets were assessed in each of three biological replicate experiments. Following imaging of the processed brains using a Zeiss Axioimager (Carl Zeiss Microscopy, LLC, Thornwood, NY) that is equipped with a Spot Flex camera (Diagnostic Instruments, Inc. Sterling Heights, MI), FIJI ImageJ software (Schindelin, 2019) was used to calculate mean gray values (average signal intensity over the selected area). This permitted quantification of digoxigenin-labeled dop1 transcript signals in the brains of control or dop1.462-treated L4 or adult mosquitoes as described (Mysore et al., 2015). Data from the three larval or adult biological replicate experiments were combined and statistically evaluated with Student’s t-test.
2.8. Immunohistochemical analysis of mosquito brains:
Three biological replicate immunohistochemical staining experiments were performed on the brains of control or dop1.462 IRP-treated adult or L4 mosquitoes as described (Clemons et al., 2010c; Mysore et al., 2011). The following reagents were used in these studies: anti-Bruchpilot mAb nc82 antibody (Wagh et al., 2006) (Developmental Studies Hybridoma Bank, Iowa City, Iowa, Product nc82, which was deposited by E. Buchner) and TO-PRO-3 iodide (Molecular Probes, Eugene, OR). Three biological replicate experiments were performed on the brains of 20 L4 larvae or adults per control or experimental treatment in each biological replicate experiment. Processed brain tissues were mounted and imaged using a Zeiss 710 confocal microscope and Zen software, and the images were analyzed with Adobe Photoshop CC 2018 and FIJI ImageJ (Schindelin, 2019) software. This permitted quantification of mean gray values, which were calculated and statistically analyzed using Student’s t-test as described (Mysore et al., 2015).
2.9. Evaluation of dop1.462 pesticide in non-target species:
Tribolium castaneum:
Adult Tribolium castaneum were obtained from Carolina Biologicals (Burlington, NC), and the beetles were reared according to the provider’s instructions. For the toxicity assays, in each of two biological replicate experiments, 20 newly hatched T. castaneum larvae were reared in a plastic tube (provided by Carolina Biologicals) on 10 g of an 8:8:1:1 mixture of white flour, brown flour, nutritional yeast (provided by Carolina Biologicals), and control or dop1.462 yeast. The tubes were stored in an incubator maintained at 32° C, and beetle survival was monitored throughout development. The number of adults that eclosed per replicate tube was observed and recorded, and combined data from two biological replicate experiments were analyzed with the Fisher’s exact text.
Daphnia:
Daphnia pulex and Daphnia magna were acquired from Carolina Biologicals (Burlington, NC) and evaluated as previously described (Mysore et al., 2019c) at 22° C, under ambient laboratory illumination (12 hr light/12 hr dark), and in COMBO medium containing 0.0001% sodium selenium (Kilham SS, 1998) and control or dop1.462 yeast. In each of three biological replicate trials, a 40 mg dop1.462 or control yeast tablet was dissolved in 50 mL of distilled water, and this solution was fed to 20 Daphnia over a five day period, with 10 ml of solution provided each day. A Fischer’s exact test was used to analyze combined survival data from each of three 10 day biological replicate trials.
D. melanogaster:
Survival of Oregon R (Thurmond et al., 2019) D. melanogaster larvae that fed on control or dop1.462 yeast was assessed in assays that were performed at 22° C under ambient laboratory illumination (12 hr light/12 hr dark). In summary, for each assay performed as previously described (Mysore et al., 2019b), a tablet of control or dop1.462 yeast was resuspended in 200 μl of distilled water and 10 μl of red food dye (McCormick’s) and mixed with 10 ml of standard fly media. In each replicate trial, 20 L1 larvae were placed in a vial of food containing control or larvicidal yeast, and yeast consumption was verified through observation of red food dye in the larval guts. The number of adults that emerged from each of seven biologial replicate trials was recorded as a measurement of survival, and data were evaluated using the Fischer’s exact test.
For analysis of adults, 20 females were placed in a cage with a 100×150mm petri dish with 32 μl of 10% sucrose solution containing 0.5% of blue tracer dye (McCormick) alone or with 2.5 μg/μl of control or dop1.462 siRNA that was divided into four 8 μl droplets. The adult flies were permitted to feed overnight before being transferred into plastic vials containing standard rearing media. These assays were performed under ambient laboratory illumination (12 hr light/12 hr dark) at 22° C. Mortality was recorded daily for six days, and data from two biological replicate experiments were combined and analyzed using a G-test of independence.
3. Results
3.1. Discovery of dop1.462 siRNA, a dual-action adulticidal/larvicidal mosquito IRP:
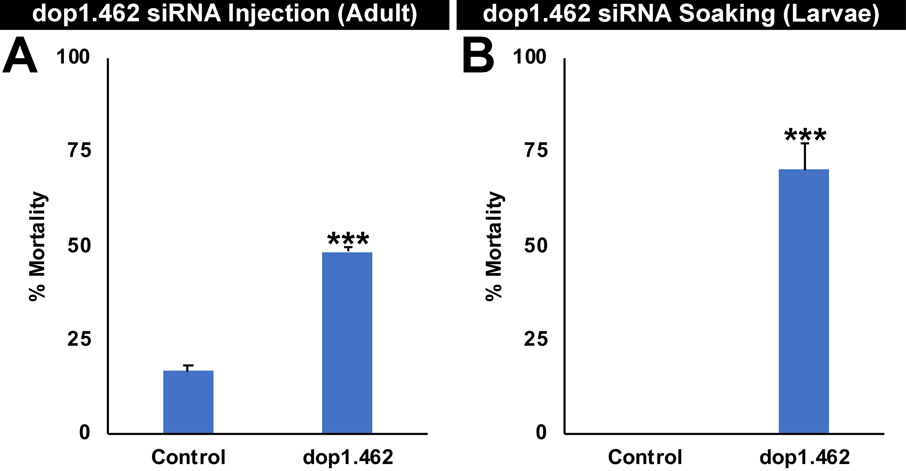
siRNA #462, hereafter referred to as dop1.462 siRNA, was prioritized for testing in screens for genes required at multiple stages of the mosquito life cycle (see methods for detail). dop1.462 siRNA corresponds to a target sequence located in exon six of A. aegypti dop1 (Meyer et al., 2012) that is conserved in multiple Aedes and Anopheles mosquito species (Giraldo-Calderon et al., 2015), but has not yet been identified outside of mosquitoes (Supplementary files 1, 2). Injection of A. aegypti adult females with 9 μg/μl of dop1.462 siRNA induced 48±1% mortality in A. aegypti adult females (Fig. 1A; P=0.00015253 vs. control siRNA treatment). Soaking L1 A. aegypti larvae in 0.5 μg/μl dop1.462 siRNA resulted in 70±7% larval mortality (Fig. 1B; P=9.2733×10−5 vs. control siRNA treatment). The results of these screening studies support the hypothesis that dop1.462 siRNA has both adulticidal and larvicidal activity in A. aegypti.
Fig. 1. Adulticidal and larvicidal activity of dop1.462 in A. aegypti.
siRNA dop1.462 was discovered in screens for mosquito adult lethal (A) and larval lethal (B) genes. Significant adult mortality was observed following microinjection of adult females with 250 nl of 9 μg/μl dop1.462 siRNA (A; data compiled from three biological replicate experiments, each with 20 adults/treatment are shown; mortality levels were recorded after six days of observation). Significant larval mortality was observed after four hour L1 soaking treatments with 0.5 μg/μl dop1.462 siRNA (B; the soaking screen was performed in duplicate with 20 larvae/treatment, with the experiment concluding when all mosquitoes had either died or emerged as adults). Data from dop1.462 vs. control siRNA-treated A. aegypti, represented here as mean percentage mortality, were statistically analyzed using the Fischer’s exact test; error bars denote standard errors of the mean (SEM), and *** denotes P<0.001 vs. control.
3.2. Silencing the Aae dop1 gene results in neural defects in the A. aegypti adult brain
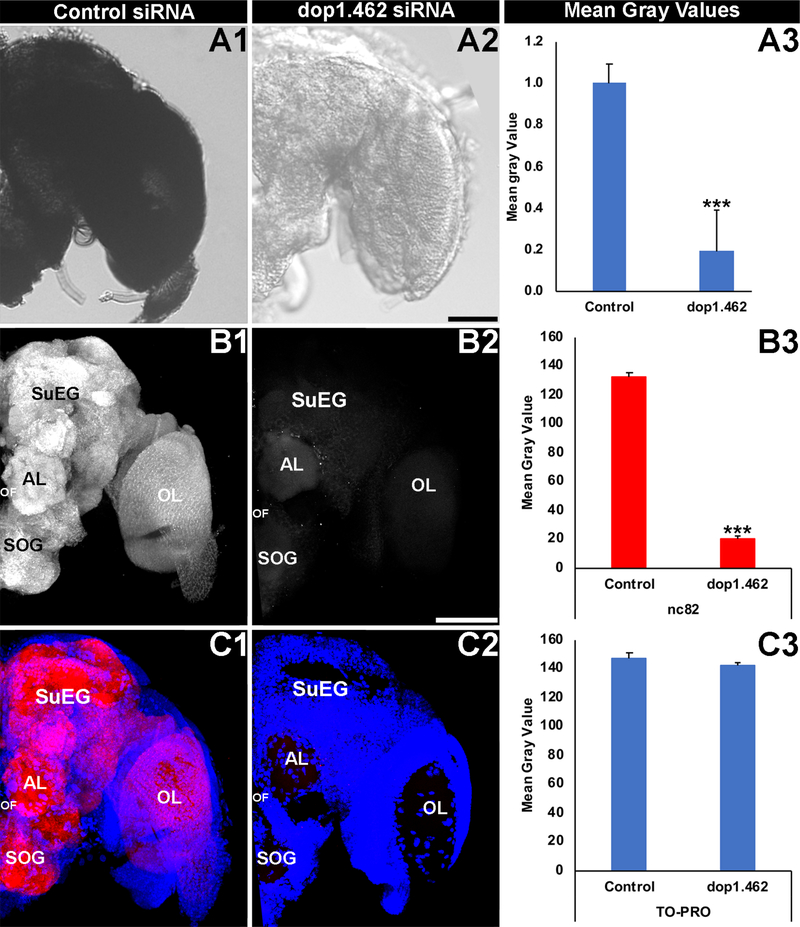
The functions of dop1 were characterized in the A. aegypti adult brain, in which it was hypothesized that silencing of dop1 expression by dop1.462 siRNA would impact neural activity. In wild type and control-injected animals, Aae dop1 is expressed broadly throughout the adult brain (the control-injected animal is shown for reference in Fig. 2A1). A significant reduction in dop1 transcripts was observed in the brains of A. aegypti adult females that were injected with dop1.462 siRNA (Fig. 2A2; 86±1.5% reduction with respect to control siRNA-treated brains, P=1.42X10−103). Although neural density, which was evaluated through quantification of TO-PRO nuclear staining (Fig. 2C3), was not significantly different (P>0.05) in dop1.462 (Fig. 2C2) vs. control-treated brains (Fig. 2C1), levels of Bruchpilot expression, a marker of active neural synapses (Wagh et al., 2006), were reduced by 82±2% (Fig 2B3, P=3.741X10−54) in the brains of adults injected with dop1.462 siRNA (Fig. 2B2,C2; compare to control-treated brains in Fig. 2B1,C1). These experiments confirmed that dop1.462 siRNA effectively silences dop1 and demonstrated that this silencing results in disruption of neural function in the A. aegypti adult brain.
Fig. 2. Neural defects are observed in dop1.462-treated A. aegypti adults.
Broad expression of dop1.462 transcripts detected at high levels throughout the control-injected and wild-type A. aegypti adult female brain (a brain from a control siRNA-microinjected animals is shown in A1) was significantly reduced in the brains of adults injected with dop1.462 siRNA (A2; mean gray value results from three biological replicate experiments are shown in A3; n = 50 control-treated brains, and n = 60 dop1.462-treated brains). Although levels of TO-PRO nuclear staining (blue in C1, C2) were not significantly different (C3) in the brains of adults injected with control (Cl) or dop1.462 (C2) siRNA, levels of Bruchpilot (white in B1, B2; red in C1, C2), a marker of synaptic active zones (labeled by mAb nc82) were significantly reduced (B3) in the synaptic neuropil following microinjection of dop1.462 siRNA (B2, C2; compare to control siRNA treatment in B1, C1). Data were compiled from three biological replicate experiments performed on a total of 43 control-treated brains and 37 dop1.462-treated brains (B3, C3) and are represented as average mean gray values in A3, B3, and C3, in which error bars represent SEM. In all panels, brains were dissected and fixed 24 hours following injection of the mosquitoes with a 2.25 μg dose of siRNA. Student’s t-tests were used for statistical analyses of control vs. dop1.462 siRNA injected animals; ***=P<0.001 vs. control. Representative adult brains are oriented dorsal upward; scale Bar=100 μm. Labels are as follows: AL: antennal lobe; OF: oesophageous foramen; OL: optic lobe; SOG: sub-esophageal ganglion; SuEG: supra-esophageal ganglion.
3.3. ATSB-mediated delivery of dop1.462 IRPs
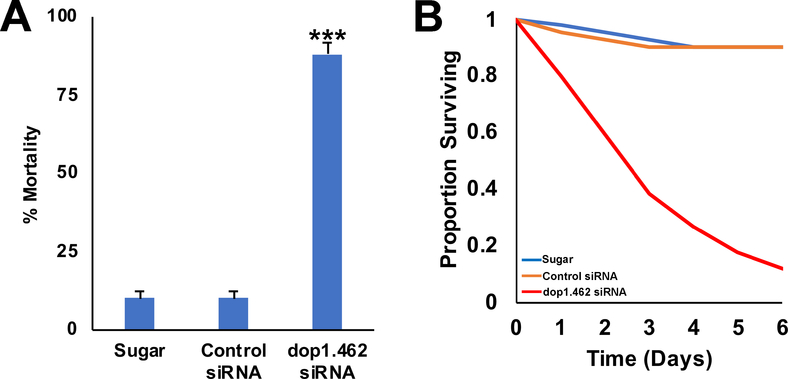
The adulticidal activity of dop1.462 siRNA observed in microinjection assays (Fig. 1A) suggested that it may be useful to identify a mechanism for delivery of dop1.462 that could potentially translate to the field. To this end, a sugar-baited delivery system was evaluated in simulated deployment trials conducted in the laboratory in which adult females were fed 10% sucrose sugar bait marked with blue tracer dye alone, with control siRNA, or with dop1.462 siRNA. From a total of 75 female mosquitoes subjected to each treatment (25/treatment in each of three biological replicate trials), 55± 4% fed on sugar bait, 55± 4% on sugar bait containing control siRNA, and 45±4% on sugar bait with dop1.462 ATSB (resulting in an average dose of ~12 μg siRNA/mosquito); no significant differences were observed in feeding rates among the three treatments (G=1.75, d.f=2, P=0.413). Although negligible mortality was observed in mosquitoes fed with sugar bait or sugar bait containing control siRNA, 88±4% of mosquitoes that fed on dop1.462 ATSB died [Fig. 3A; P<0.001 compared to sugar (χ2=53.21) or sugar with control siRNA (χ2=49.73)] over the course of a six day trial period (see survival curve in Fig. 3B). All dop1.462 ATSB-treated mosquitoes (n=34 individuals combined from three biological replicate experiments), including the 12% of dop1.462-treated mosquitoes that recovered and survived treatment (Fig. 3A), failed to fly and exhibited very limited, uncoordinated walking behavior (Video 1). 100% morbidity, defined here as a failure to respond to stimuli for 10 sec, was observed in mosquitoes that consumed dop1.462 ATSB (n=34). In summary, ATSB-mediated delivery of dop1.462 resulted in severe behavioral deficits and high levels of adult mortality (Fig. 3A,B) that exceeded those observed in adult microinjection experiments (Fig. 1B).
Fig. 3. Delivery of dop1.462 siRNA as an ATSB results in high levels of A. aegypti adult mortality.
A. In simulated field trials, high levels of adult mortality were observed in adult females that fed on ATSB with 2.5 μg/μl dop1.462 siRNA (n=34) vs. sugar bait alone (Sugar; n=41) or sugar bait with 2.5 μg/μl control siRNA (Control siRNA, n=41). The average dose was ~12 μg siRNA per mosquito. The data shown, which were compiled from three biological replicates trials, are represented as mean percentage mortality and were analyzed using the log-rank test; error bars represent SEM; ***=P<0.001 in comparison to sugar alone or control siRNA treatments. B. The survival curves over a six day trial period are shown for adult females that fed on sugar bait, control siRNA sugar bait, or dop1.462 ATSB.
Video 1. Defective motor behavior observed in mosquitoes that consumed dop1.462 ATSB.
Defective locomotor behavior was observed in adult female mosquitoes that consumed dop1.462 ATSB. In the video, individual females that consumed sugar bait (right) or sugar bait with control siRNA (center) display typical locomotor behaviors, including flight and exploration of their local environments. The dop1.462-treated female (left) attempts to perform these activities, but fails (well beyond the recording session). The video can be viewed at this link.
3.4. Targeting dop1 induces neural defects and death in A. aegypti larvae:
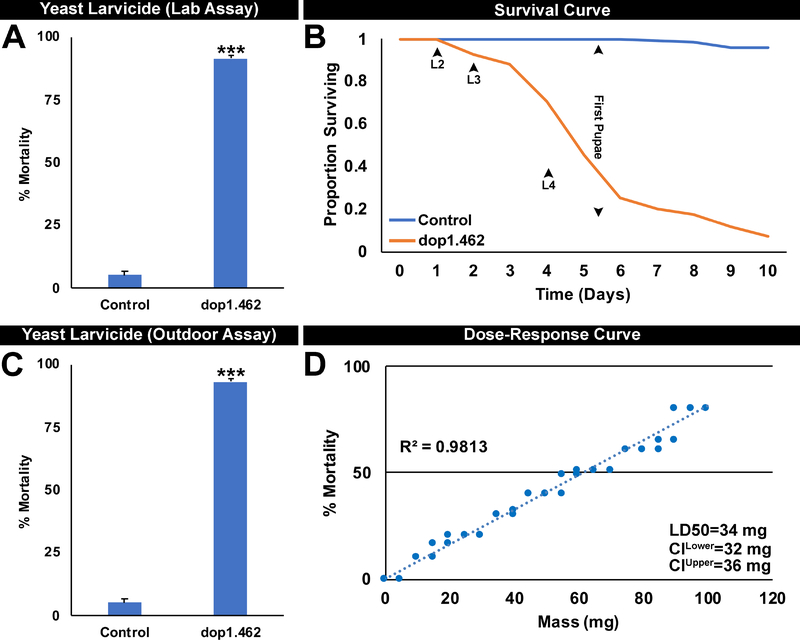
The impact of silencing dop1 in A. aegypti larvae was examined using a yeast delivery system (Duman-Scheel, 2019; Hapairai et al., 2017) for dop1.462 IRP. S. cerevisiae engineered to express shRNA corresponding to dop1.462 siRNA (hereafter referred to as dop1.462 yeast) were generated. Larval consumption of inactivated dop1.462 yeast tablets prepared from this strain resulted in 92±1% A. aegypti larval mortality in indoor laboratory trials (Fig. 4A; P=8.754X10−18 vs. control yeast interfering RNA treatment; LD50=34 mg, Fig. 4D). Likewise, dop1.462 yeast induced 93±1% larval death in semi-field experiments conducted in an outdoor rooftop laboratory in Notre Dame, IN (Fig. 4C; P=9.6608X10−18 vs. control). Larvae treated with dop1.462 yeast (beginning in L1) died as fourth instar (L4) larvae or as early pupae (Fig. 4B). Comparable to adults (Fig 2A1,A2), broad expression of dop1 throughout the L4 larval brain (Supplementary File 3.A1) was reduced by 93±1% in dop1.462 yeast-treated larvae (Supplementary File 3.A2; P=9.0902X10−130) that were harvested in early L4 just prior to the time that treated animals typically die (Fig. 4B). As observed in adults (Fig. 2), silencing of dop1 transcripts correlated with significant loss (78±1.5%) of Bruchpilot expression (P=1.803X10−46) in L4 brains (Supplementary File 3.B2,C2 vs. control in 2.B1,C1), but no significant differences in neural density were detected (Supplementary File 3.C3, P>0.05; compare dop1.462 treated brain in Supplementary File 3.B2,C2 vs. control-treated brain in 2.B1,C1). This loss of neural activity in the L4 nervous system correlated with the timing of larval death (Fig. 4B). These experiments, combined with analyses of adults (Figs. 2, 3), indicated that Dop1 neural activity is required at multiple stages of the A. aegypti life cycle.
Fig. 4. Oral consumption of dop1.462 yeast by A. aegypti larvae results in high levels of mortality.
Larval consumption of inactivated dried dop1.462 yeast tablets resulted in significant larval mortality in laboratory (A) and outdoor semi-field larvicide trials conducted in Indiana (C). Data shown in panels A (n=260 total larvae/treatment) and C (n=280 total larvae/treatment), were compiled from three biological replicate experiments and are represented here as mean percentage mortality; error bars represent SEM. Mortality data from dop1.462 vs. control yeast IRP-treated larvae were arcsine transformed and analyzed using Student’s t-test (***=P<0.001 vs. control). Consumption of inactivated dried dop1.462 yeast larvicide tablets beginning in L1 resulted in death beginning in L3 or during the L4 or early pupal stages (B; compare to larvae fed with control yeast IRP that survived). In A-C, a single 40 mg yeast tablet was provided to 20 larvae in each replicate container at the onset of each experiment. D. A dose-response curve shows the mass of dop1.462 yeast vs. the resulting percentage of A. aegypti mortality following treatment; LD50 = 34 mg. Regression analysis (D) indicated that these dose-response data are linearly correlated (R2=0.9813).
3.5. Dop1.462 IRPs function as broad-range mosquito insecticides and are not toxic to a selection of non-target arthropods:
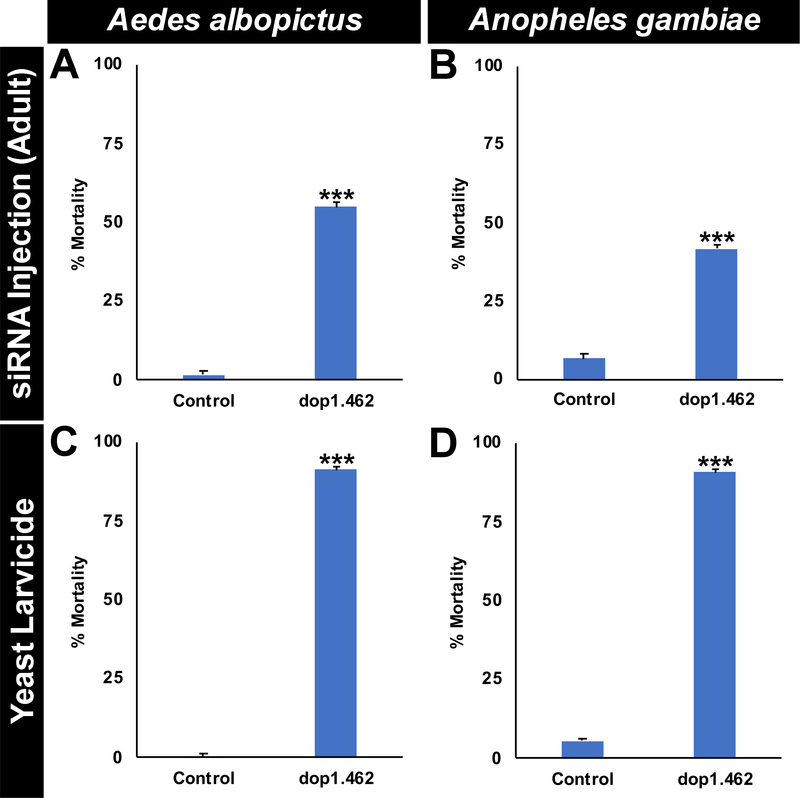
The target site of dop1.462 IRPs is conserved in A. albopictus and multiple species of Anopheles malaria vector mosquitoes, but not in the sequenced genomes of other arthropods, humans, or other non-target organisms (Supplementary file 1). Given the conservation of this target site in multiple mosquito species, it was hypothesized that dop1.462 yeast would function as a biorational broad-range mosquito IRP. In support of this hypothesis, as observed in A. aegypti adult microinjection experiments (Fig. 1A), in adult female mosquitoes, microinjection of dop1.462 siRNA resulted in 55±1% adult female A. albopictus mortality (Fig. 5A; P=1.8246X10−12 vs. control siRNA treatment) and 42±1% adult female A. gambiae mortality (Fig. 5B; P=3.6901X10−7 vs. control siRNA treatment). Likewise, dop1.462 yeast larvicide treatments induced 91±1% mortality in A. albopictus (Fig. 5C; P=1.607X10−23 vs. control yeast treatment) and 91±1% mortality in A. gambiae (Fig. 5D; P=3.277X10−18 vs. control yeast treatment). This adulticidal and larvicidal activity of dop1.462 appears to be restricted to mosquitoes, as no significant mortality (P>0.05) was observed in Daphnia pulex (Fig. 6A) and Daphnia magna adults (Fig. 6B), Tribolium castaneum larvae (Fig. 6C), or D. melanogaster larvae (Fig. 6D) or adults (Fig. 6E), all of which have known dop1 orthologs (Kriventseva, 2019) that lack the dop1.462 target site (Supplementary file 1) and survived treatment with dop1.462 IRPs. Combined, these results support the hypothesis that dop1.462 IRPs function as a biorational broad-range mosquito IRP.
Fig. 5. Broad-range activity of dop1.462 IRP in mosquitoes.
Microinjection of dop1.462 siRNA induces mortality in A. albopictus (A; n=60/treatment; dose = 250 nl of 9 μg/μl siRNA per mosquito) and A. gambiae (B; n=60/treatment; dose = 150 nl of 6 μg/μl siRNA per mosquito) adult females (compare to control siRNA injections of the same doses). For the experiments in A and B, adult mortality was evaluated for six days following injection, after which time the final results were recorded. Likewise, larval consumption of inactivated dop1.462 yeast tablets (C, D) induces high levels of mortality in A. albopictus (C; n=240/treatment) and A. gambiae (D; n=240/treatment). In the experiments shown in A and B, one 40 mg dop1.462 or control yeast tablet was provided to 20 larvae in each replicate container trial; the data shown were compiled from a total of three biological replicate experiments, with the experiment concluding when all animals had either died (typically during L4) or emerged as adults. Data were arcsine transformed and analyzed with a Fisher’s exact test (A, B) or Student’s t-test (C, D); ***=P<0.001 vs. control.
Fig. 6. Non-target arthropods survive treatments with dop1.462 IRPs.
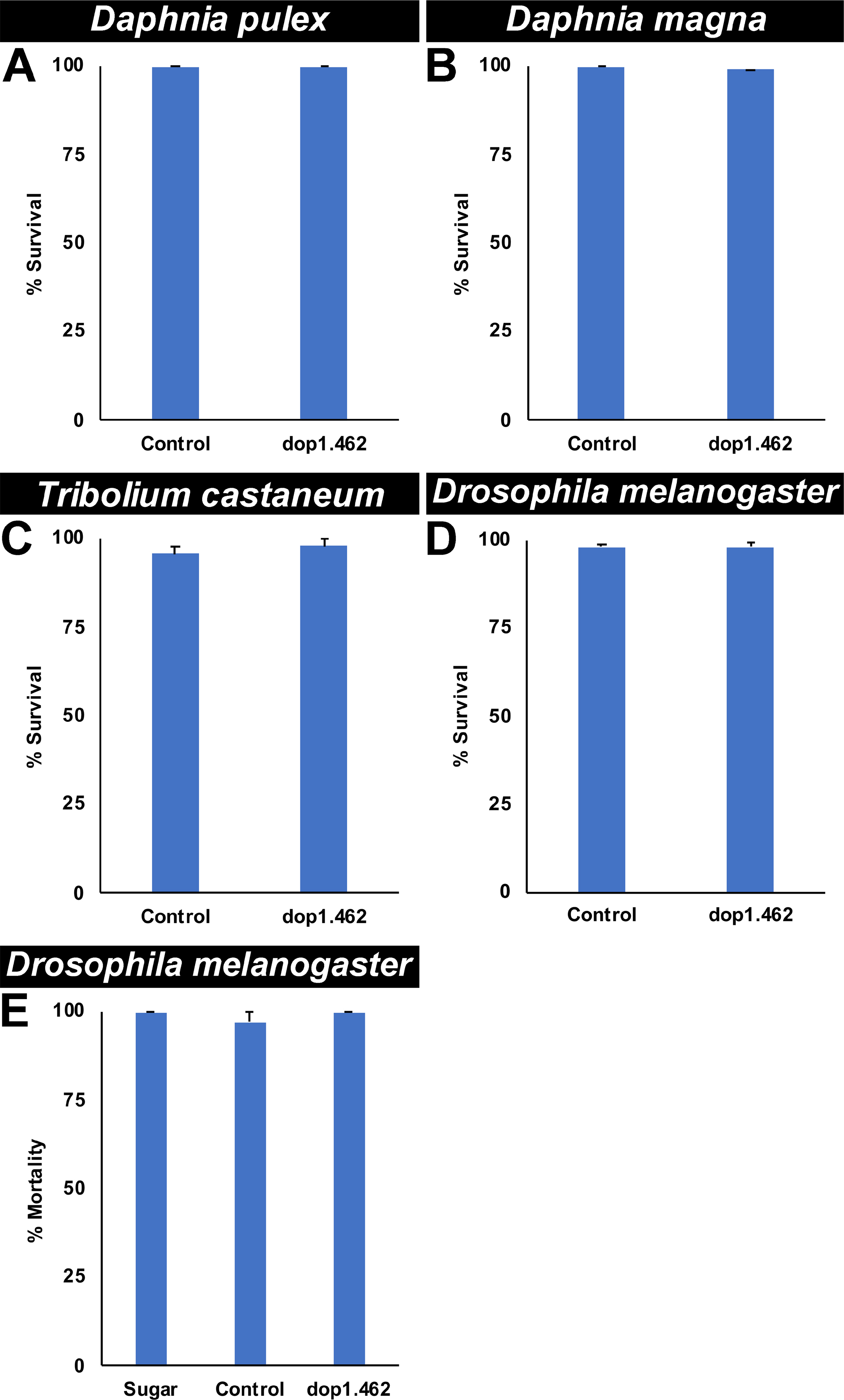
Consumption of dop1.462 yeast by D. pulex (A; n=60/treatment), D. magna adults (B; n=60/treatment), Tribolium castaneum larvae (C; n=120/treatment), or D. melanogaster larvae (D; n=140/treatment) had no significant impact on survival (P>0.05 with respect to arthropods treated with control IRPs). In all replicate trials conducted on these larvae (A-D), 20 individuals were fed 40 mg of yeast at the onset of the trial, which concluded when all insects had emerged as adults. Likewise, no significant differences in mortality were observed in adult D. melanogaster fed with dop1.462 ATSB, sugar bait alone, or sugar bait with control siRNA, (E; n=40/treatment; 20 adults were fed 32 μl of 2.5 μg/μl siRNA at the onset of the trial, with mortality assessed six days following treatment). Data in A-E are displayed as mean percentages of survival with error bars representing SEM. The survival data shown in A-D were compiled from multiple biological replicate trials (three in A and B, six in C, seven in D) and analyzed using the Fisher’s exact test, while survival data shown in E were compiled from two replicate experiments and analyzed using the G-test.
4. Discussion:
4.1. RNAi-based approaches for mosquito-specific GPCR targeting:
GPCRs, which have been successfully targeted for human drug development (Wise et al., 2002), have been described as underexploited pesticide targets (Hill et al., 2016; Ngai and McDowell, 2017). As discussed by (Hill et al., 2016), new insecticides that target GPCRs will ideally be mosquito-selective, yet effective against multiple species of disease vector mosquitoes. Genome sequencing efforts (Arensburger et al., 2010; Holt et al., 2002; Matthews et al., 2018; Neafsey et al., 2015; Nene et al., 2007) have facilitated the design of RNAi-based pesticides that recognize conserved targets in multiple mosquito species, but which are not conserved in non-target species (Supplementary file 1), such as honey bees and other pollinators, suggesting that RNAi pesticides could represent a “green” approach for mosquito-specific GPCR targeting. The results of this investigation demonstrate that RNAi-mediated silencing of dop1 causes mosquito mortality at both the adult (Figs. 1, 3) and larval (Figs. 1, 4) stages of the mosquito life cycle. Severe defects in synaptic activity in the mosquito adult (Fig. 2) and larval (Supplementary file 3) brain correlate, and are likely primary contributors to the high levels of mortality induced by dop1.462 IRPs. The adult behavioral defects observed are comparable to those resulting from neural silencing of dop1R1 in D. melanogaster, in which a flightless phenotype and adult lethality were also reported (Dietzl et al., 2007). The results of this investigation also demonstrate that the requirement for dop1 function is conserved between A. aegypti, A. albopictus, and A. gambiae mosquitoes (Fig. 5). Conservation of the dop1 target site in multiple other Anopheles spp. (Supplementary file 1) suggests that dop1.462 IRPs will have activity in multiple species of malaria vector mosquitoes, indicating that IRPs targeting mosquito dop1 genes could one day be used in integrated control programs targeting multiple malaria vectors. Although the dop1.462 target site is not identically conserved in the C. quinquefasciatus dop1 ortholog (Kriventseva, 2019), which was consequently not included in this investigation, the design of siRNAs or shRNAs to match the C. quinquefasciatus dop1 gene, as well as the genes of other Anopheles species with known dop1 orthologs (Kriventseva, 2019), would likely permit successful targeting of these species as well.
In silico analyses failed to detect the dop1.462 IRP target sites outside of mosquitoes (Supplementary files 1, 2). It is difficult, however, to rule out the potential for non-target impacts solely on the basis of a lack of sequence similarity (Jackson and Linsley, 2010). To begin to address this, dop1.462 activity was evaluated in several non-target arthropods that lack dop1.462 target sites (Supplementary file 1, Fig. 6). D. melanogaster (Fig. 6D,E), a dipteran insect relative of vector mosquitoes, as well as Tribolium castaneum (Fig. 6C), a more distantly related insect, were not impacted by consumption of dop1.462 IRPs. T. castaneum, in which a variety of tissue types are known to respond to extracellular dsRNA (Miller et al., 2008), is sensitive to oral RNAi pesticides (Alshukri et al., 2019). T. casteneum is known to have a stronger systemic RNAi response than D. melanoagaster, in which most tissues do not take up extracellular dsRNA (Miller et al., 2008), but neither insect died following dop1.462 treatment (Fig. 6C). Likewise, dop1.462 yeast consumption was not toxic to two species of Daphnia (Fig. 6A). Daphnia were selected for these non-target assays because these distantly related aquatic arthropods are often used in U.S. Environmental Protection Agency (EPA) toxicity assays (EPA, 2002) and are known to be sensitive to RNAi (Hiruta et al., 2013; Guo et al., 2016; Schumpert et al., 2015). The results of these initial toxicity assays suggest that dop1.462 may have little, if any activity in non-target organisms. These preliminary results suggest that RNAi-based insecticides could help overcome challenges associated with discovery of vector selective chemistries targeting GPCRs, perhaps helping to eliminate a need for timed pesticide applications that would avoid pollinator activities (Hill et al., 2018). If dop1.462 insecticides were ever to be commercialized, it will of course still be important to pursue additional requisite toxicity assays with commercial-ready dop1.462 formulations in additional species, including vertebrate organisms. However, the present results indicate that dop1.462 IRPs, which appear to have a desirable safety profile with respect to current chemical pesticides, may represent a new tool for the biorational control of multiple species of disease vector mosquitoes during both the adult and larval stages of the mosquito life cycle.
4.2. ATSB-mediated delivery of dop1.462:
In recent years, we have worked to develop a yeast-based IRP production and delivery system (Duman-Scheel, 2019; Hapairai et al., 2017) that was used to successfully characterize the larvicidal capacity of dop1.462 activity in this investigation (Fig. 4). This yeast-based system, one of the most effective methods for larval gene silencing evaluated in our laboratory to date (Hapairai et al., 2017), has many advantages. For example, interfering RNA is produced through relatively inexpensive yeast culturing, significantly reducing RNA production costs. Yeast, a non-toxic natural product that is used for food and beverage preparation and sold as a dietary supplement, has been cultivated worldwide for thousands of years, and yeast interfering RNA technology, which could be implemented in the field, could be readily scaled for use in global larvicide programs (Duman-Scheel, 2019). In this investigation, microinjection experiments demonstrated that dop1.462 siRNA can also function as an adulticide (Fig. 1A), suggesting that identification of a field-appropriate method for delivery of IRPs to adult mosquitoes would be beneficial. Unfortunately, effective methods for topical application of IRPs to insects have not yet been developed. We therefore turned to sugar-baited delivery, a system that enabled successful delivery of dop1.462 to A. aegypti in the laboratory (Fig. 3) and which could one day translate to the field.
ATSBs, an emerging new paradigm for vector control (Fiorenzano et al., 2017), exploit the sugar feeding behavior of female and male mosquitoes, which are attracted to feed on sugar sources containing toxins. ATSBs, which are delivered through sprays or at bait stations, can be used both outdoors and indoors for mosquito control (Fiorenzano et al., 2017; Müller, 2016). Significant reductions in disease vector mosquitoes were observed in ATSB field trials (Fiorenzano et al., 2017; Müller, 2016; Sissoko et al., 2019), suggesting that this technology will significantly advance integrated mosquito control programs. Although sugar baits facilitate more targeted pesticide delivery, pesticide resistance is still of concern (Faraji and Unlu, 2016), and efforts to develop IRPs as a new class of pesticides which are compatible with ATSB technology may therefore be beneficial. Furthermore, many of the IRPs that are currently in use, for example garlic oil and boric acid insecticides, are not specific to mosquitoes and could impact non-target organisms (Fiorenzano et al., 2017). Advancements such as the addition of protective barriers to ATSB bait stations and limiting ATSB application to non-flowering vegetation (Fiorenzano et al., 2017; Müller, 2016) have improved the specificity of this application. The added specificity of IRPs, which appear to have a highly desirable safety profile (MonSanto, 2014) (Supplementary file 1, Fig. 6), could further enhance ATSB technology. At this time, dop1.462 ATSBs have only been tested on A. aegypti, but it would be interesting to further develop this technology for use in A. albopictus and A. gambiae, both of which are also sensitive to dop1.462 microinjection treatment (Fig. 6A,B). Given the increased insecticidal capacity of dop1.462 observed when it was delivered as an orally consumed ATSB (Fig. 3) rather than through injection (Fig. 1A), it is anticipated that ATSB-mediated delivery of dop1.462 to A. albopictus and A. gambiae would result in high levels of mortality in these species, as well. The higher levels of mortality observed following ATSB delivery likely result from the increased dose of siRNA delivered in an average sugar meal (~12 μg siRNA per mosquito in an average ATSB treatment, opposed to 4.5 μg siRNA per mosquito through microinjection). Moreover, given that the small percentage of mosquitoes which recover and survive dop1.462 treatment in the laboratory have severe locomotor and flight defects (Video 1) that would likely prohibit their survival in the wild, it is anticipated that field mortality rates could approach 100%.
4.3. Prospects and considerations for future field implementation of mosquito IRPs:
In addition to demonstrating the potential for using IRP-ATSBs to control adult mosquitoes, the results of this investigation support continued efforts to develop yeast IRPs for control of mosquito larvae. Activity of dop1.462 yeast, like that of several other IRP yeast larvicides recently developed in our laboratory (Mysore et al., 2019a,b) was retained when the yeast was used outdoors, where it was exposed to temperatures that reached 35° C (Fig. 4C). These results, combined with previous studies in which it was demonstrated that yeast IRP larvicide activity levels were retained in different types of water, in different sizes of containers that held 50 ml to 26 L of water, in both laboratory and field strains of mosquitoes, and when A. aegypti larval numbers and densities were varied (Hapairai et al., 2017; Mysore et al., 2019a,b), suggest that these larvicides could become useful components of integrated mosquito control programs. However, yeast IRP technology would be further enhanced by the development of robust formulations that are resistant to extreme conditions (i.e. high heat and humidity) that may be encountered during shipment to and long-term storage in the tropics. Moreover, the development of long-lasting formulations with residual activities that extend beyond two weeks in water, the residual activity of the present formulation (Hapairai et al., 2017), would also be beneficial. We are presently working to address these concerns and are also pursuing efforts to scale yeast production to commercially-appropriate levels (Duman-Scheel, 2019). Likewise, the use of ATSB systems for delivery of insecticidal RNAs would also require scaled IRP production, as well as the development of shelf-stable robust formulations with residual activities that are sufficient for deployments in bait stations or as foliar sprays in the tropics. San Miguel and Scott ( 2016) demonstrated that foliar application of dsRNA targeting actin in the Colorado potato beetle was active for at least a month and was not easily removed after it had dried on plant leaves. Furthermore, in field trials, Hunter et al. (2010) successfully delivered dsRNA in sugar solutions to honey bees, which were subsequently protected from Israeli Acute Paralysis Virus infections. These investigations suggest that ATSB-mediated IRP delivery systems could be effectively deployed for mosquito control.
Following development of commercial-ready dop1.462 yeast and ATSB formulations, field testing in the United States must be conducted to support future EPA registry applications. Moreover, in addition to the United States, the use of RNAi-based pesticides will need to be approved in each country of intended use. Approvals for use of yeast-based IRPs, which are genetically modified organisms, may be challenging, particularly given that some nations lack a regulatory body equivalent to the United States EPA and have no mechanism to review requests to use IRP technology. The demonstrated ability to use heat-killed yeast for RNAi-based applications (Hapairai et al., 2017), as was the case in the present study, may help to overcome these challenges. Moreover, the pursuit of further toxicology testing, field testing in the United States, and EPA registry approval for both larvicidal and adulticidal RNAi-based pesticides would likely help to facilitate approval for these technologies elsewhere.
In advance of further development of RNAi-based pesticides for mosquito control, concerns for the potential to develop resistance to RNAi-based pesticides have also been raised. With the addition of dop1.462 to the growing arsenal of IRPs, we continue to develop new pesticides that could effectively replace any specific IRP to which mosquitoes become resistant. Furthermore, given that RNAi machinery regulates endogenous cellular mechanisms, one could argue that resistance to RNAi as a whole might not be likely to develop. However, Khajuria et al. (2018) recently reported on the development and characterization of a dsRNA-resistant Diabrotica virgifera virgifera (western corn rootworm) population. This raised the question of whether the ~5–10% of larvae in some containers that survive treatment with dop1.462 (Fig. 4) or other larvicidal yeast strains (Hapairai et al., 2017; Mysore et al., 2019a,b) are resistant to dsRNA. This does not appear to be the case, as all larvae die when reared as individual larvae following yeast IRP treatments (Mysore et al., 2019a,b), suggesting that survivors are likely eating dead larvae (which are rarely observed in the containers) rather than yeast. Likewise, detection of severe behavioral phenotypes or death in all mosquitoes that consumed dop1.462 ATSB (Fig. 3, Video 1) suggests that adults are not resistant to this insecticide. Finally, in ongoing studies with two different yeast IRPs, no evidence of resistance has been observed following 10 generations of selection (MDS, unpublished). Given that the evolution of insecticide resistance must be considered with every new class of pesticides under consideration (Khajuria et al., 2018), further research on this topic is nevertheless warranted.
It should also be noted that although siRNAs and shRNAs 21–25 bp in length appear to work well in A. aegypti, A. albopictus, A. gambiae, and C. quinquefasciatus (this study and Mysore et al., 2019a,b), short interfering RNAs do not perform as well in some other insects. For example, siRNAs are not effectively taken up by D. melanogaster S2 cells (Saleh et al., 2006). Likewise, oral RNAi studies in Diabrotica virgifera virgifera demonstrated that a 21 bp siRNA targeting the DvSnf7 gene did not appear to be taken up in midgut cells. By contrast, midgut uptake of a longer 240 bp dsRNA was noted in Diabrotica virgifera virgifera and supported a size-activity relationship in bioassays (Bolognesi et al., 2012). Thus, mosquitoes appear to have more efficient uptake of siRNAs than the western corn rootworm. The endocytic pathway mediates dsRNA entry in D. melanogaster (Saleh et al., 2006), and T. castaneum processes ingested dsRNA via clathrin-dependent endocytosis (Xiao et al., 2015). It is presumed that mosquito siRNA and microbe/shRNA uptake is through endocytosis, but the mechanism will need to be further investigated in mosquitoes. Likewise, the overall mechanisms leading to spreading of the RNAi response in tissues beyond the mosquito midgut, for example the larval or adult brain in which dop1 transcript levels were significantly reduced (Fig 2A2, Supplemental file 3A2), will need to be further investigated in mosquitoes and other insects (Cooper et al., 2018). Evaluation of the mechanisms of siRNA and yeast/shRNA uptake and systemic spreading of the RNA response in mosquitoes could help to explain the differences in RNAi efficiency noted between mosquitoes and other insects. Such research could facilitate the enhancement of RNAi methodology in insects that are less amenable to RNAi (Cooper et al., 2018). The mosquito RNAi research agenda should therefore include elucidation of the cellular machinery that permits siRNA and microbe-shRNA uptake, how these species are processed and degraded, whether the mechanisms differ between various delivery methods, and evaluation of the potential for developing RNAi resistance when utilizing various interfering RNA delivery strategies.
4.4. Conclusions and Future Directions:
In conclusion, these studies demonstrated that dop1.462, a dual-action adulticidal and larvicidal IRP with target sites conserved in the orthologous genes of multiple species of mosquitoes (Supplementary file 1), may represent a new method of controlling Aedes and Anopheles mosquitoes (Figs. 1, 3, 4, 5) at multiple stages of the mosquito life cycle. Characterization of dop1.462 indicated that the mode of action for this IRP is through silencing of the dop1 gene, resulting in disruption of neural function in adults (Fig. 2) and larvae (Supplementary file 3). In silico data which indicate that the dop1.462 target site is only present in mosquitoes (Supplementary file 1), combined with the lack of dop1.462 toxicity observed following treatments of non-target organisms (Fig. 6), indicate that this IRP may offer a method for specifically targeting mosquito dop1 GPCRs that will not impact GPCR activity in non-target species. Moreover, the results of simulated field trials demonstrated that adulticidal dop1.462 siRNA can be delivered to adult mosquitoes in the form of an ATSB (Fig. 3), suggesting that use of IRPs could promote the development of ATSBs with increased species-specificity. Efforts to generate and characterize the dop1.462 yeast IRP (Figs. 4, 5) in this investigation have resulted in the addition of an additional larvicide to the growing arsenal of yeast larvicidal IRPs (Hapairai et al., 2017; Mysore et al., 2017, 2019a,b). Future studies will aim to further build upon this arsenal of larvicides and to develop a comparable arsenal of adulticidal IRPs.
Supplementary Material
Supplementary file 1. Evaluation of dop1.462 target site conservation. The 21 and 25 bp sequences targeted by dop1.462 IRPs were used as a query sequence against all mosquito genomes in Vectorbase (Giraldo-Calderon et al., 2015). Mosquito species in which a perfectly conserved target sequence was identified in the indicated genes (when known) or scaffold (s) locations are indicated. The 21 and 25 bp target sequences were also used to conduct blastn searches against all sequences in the NCBI nucleotide collection (Johnson et al., 2008). As of January 2020, other than disease vector mosquitoes, these searches failed to uncover any 2½1 or 25/25 identical matches in any genome, including the indicated taxonomic groups (NCBI TaxIDs are listed, including Diptera, Coleoptera, and Crustacea, from which representative species were assessed in toxicity assays).
Supplementary file 2. Results of blastn searches for dop1.462 target sites in non-target organisms. Outside of the disease vector mosquito identities detailed in supplementary file 1, as of January 2020, blastn searches did not uncover 21/21 or 25/25 nucleotide sequence identities for the 21 bp (A) or 25 bp (B) dop1.462 IRP target sites in any sequences found in the NCBI nucleotide collection (Johnson et al., 2008). The ratios of identity, bit scores (max score), E values, and accession numbers for the top 20 blast results (outside of mosquitoes) are shown. Several 19/21 and 22/25 match identities were uncovered, but perfect matches covering the full 21 bp (A) or 25 bp (B) query sequences were only identified in mosquitoes.
Supplementary file 3. A. aegypti larval consumption of dop1.462 yeast results in severe neural defects. High levels of dop1 expression detected in the A. aegypti L4 larval brain (A1, which shows a brain from a control yeast-treated larva that is comparable to wild-type larva expression) are significantly reduced (A3) in the brains of L4 larvae that consumed dop1.462 yeast (A2). In A3, mean gray value results that were compiled from three biological replicate trials are shown (n=53 brains from dop1.462-treated larvae, and n=53 brains from control-treated larvae). Larval brains were labeled with mAbnc82 (white in B1, B2; red in C1, C2) and counter-stained with TO-PRO nuclear label (blue in C1, C2). Although nc82 levels were significantly reduced (B3) in the synaptic neuropil of larvae fed with dop1.462 yeast (B2, C2), no significant differences were observed in TO-PRO levels (C3), which were similar in dop1.462 (C2) and control-treated (C1) larvae. Data shown in B3 and C3 were compiled from three biological replicate experiments performed on a total of 37 dop1.462-treated brains and 43 control-treated brains and are represented here as average mean gray values; error bars correspond to SEM. Data were analyzed with Student’s t-test; ***=P<0.001 vs. control. Representative adult brains are oriented dorsal upward and labeled as follows: AL: antennal lobe; OF: oesophageous foramen; OL: optic lobe; SOG: sub-esophageal ganglion; SuEG: supra-esophageal ganglion. The scale bar corresponds to 100 μm.
Acknowledgements
We thank Scott Emrich for assistance in identifying conserved sequences in the A. gambiae and A. aegypti and genomes and are grateful for the technical assistance of Jacob Realey, Joi Misenti, and Joe Roethele. We also thank the Innovative Vector Control Consortium for useful discussions about this project.
Funding
This work was supported through the Indiana University Showalter Scholar program (to MDS), the National Institutes of Health/National Institute of Allergy and Infectious Disease (1 R21 AI128116–01 to MDS, NW, and DWS), the United States Agency for International Development (AID-OAA-F-16–00097 to MDS), and the U.S. Department of Defense Deployed Warfighter Protection Program (W911QY-17–1-0002 to MDS). The sponsors did not play a role in study design, in the collection, analysis and interpretation of data, in the writing of the report, nor the decision to submit the article for publication.
Abbreviations:
- ATSB
attractive toxic sugar bait
- dop1
dopamine 1 receptor
- EPA
Environmental Protection Agency
- L1
first instar
- GPCR
G protein-coupled receptor
- IRP
interfering RNA pesticide
- LVP-IB12
Liverpool-IB12
- shRNA
short hairpin RNA
- siRNA
small interfering RNA
- WHO
World Health Organization
Footnotes
Declaration of interests: MDS, DWS and NW are inventors on pending Patent Application 62/361,704. This application did not impact interpretation of the data in this study and will not impact the authors’ adherence to journal policies on sharing materials and data. LKH, KM, LS, PL, CWW, NDS, AML, MPS, and JI declare that they have no competing interests.
References
- Airs PM, Bartholomay LC, 2017. RNA interference for mosquito and mosquito-borne disease control. Insects 8(1), pii: E4. doi: 10.3390/insects8010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari OS, Antoshechkin I, Amrhein H, Williams B, Diloreto R, Sandler J, Hay BA, 2013. The developmental transcriptome of the mosquito Aedes aegypti, an invasive species and major arbovirus vector. G3 (Bethesda) 3, 1493–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshukri B, Astarita F, Al-Esawy M, Al-Esawy M, Pennacchio F, Gatehouse AMR, Edwards MG, 2019. Targeting the potassium ion channel genes SK and SH as a novel approach for control of insect pests: efficacy and biosafety. Pest Manag Sci 75, 2505–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arensburger P, Megy K, Waterhouse RM, Abrudan J, Amedeo P, Antelo B, Bartholomay L, Bidwell S, Caler E, Camara F, Campbell CL, Campbell KS, Casola C, Castro MT, Chandramouliswaran I, Chapman SB, Christley S, Costas J, Eisenstadt E, Feschotte C, Fraser-Liggett C, Guigo R, Haas B, Hammond M, Hansson BS, Hemingway J, Hill SR, Howarth C, Ignell R, Kennedy RC, Kodira CD, Lobo NF, Mao C, Mayhew G, Michel K, Mori A, Liu N, Naveira H, Nene V, Nguyen N, Pearson MD, Pritham EJ, Puiu D, Qi Y, Ranson H, Ribeiro JM, Roberston HM, Severson DW, Shumway M, Stanke M, Strausberg RL, Sun C, Sutton G, Tu ZJ, Tubio JM, Unger MF, Vanlandingham DL, Vilella AJ, White O, White JR, Wondji CS, Wortman J, Zdobnov EM, Birren B, Christensen BM, Collins FH, Cornel A, Dimopoulos G, Hannick LI, Higgs S, Lanzaro GC, Lawson D, Lee NH, Muskavitch MA, Raikhel AS, Atkinson PW, 2010. Sequencing of Culex quinquefasciatus establishes a platform for mosquito comparative genomics. Science 330, 86–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai H, Zhu F, Shah K, Palli SR, 2011. Large-scale RNAi screen of G protein-coupled receptors involved in larval growth, molting and metamorphosis in the red flour beetle. BMC Genomics 12, 388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassel J, Mortimer R, 1971. Genetic order of the galactose structural genes in Saccharomyces cerevisiae. Journal of bacteriology 108, 179–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemons A, Mori A, Haugen M, Severson DW, Duman-Scheel M, 2010a. Culturing and egg collection of Aedes aegypti. Cold Spring Harb Protoc 2010, pdb prot5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemons A, Haugen M, Severson D, Duman-Scheel M, 2010b. Functional analysis of genes in Aedes aegypti embryos. Cold Spring Harb Protoc 2010, pdb prot5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemons A, Flannery E, Kast K, Severson D, Duman-Scheel M, 2010c. Immunohistochemical analysis of protein expression during Aedes aegypti development. Cold Spring Harb Protoc 2010, pdb prot5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AMW, Silver K, Zhang J, Park Y, and Zhu KY 2019. Molecular mechanisms influencing efficiency of RNA interference in insects. Pest Manag Sci 75, 18–28. [DOI] [PubMed] [Google Scholar]
- Coy MR, S.N.D., Chalaire KC, Inberg A, Maayan I, Glick E, Paldi N, Becnel JJ, 2012. Gene silencing in adult Aedes aegypti mosquitoes through oral delivery of double-stranded RNA. J. Appl. Entomol 2012, 741–748. [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, Couto A, Marra V, Keleman K, Dickson BJ, 2007. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448, 151–156. [DOI] [PubMed] [Google Scholar]
- Duman-Scheel M, 2019. Saccharomyces cerevisiae (baker’s yeast) as an interfering RNA expression and delivery system. Curr Drug Targets 20, 942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA, 2002. Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms. United States Environmental Protection Agency, Washington D.C. [Google Scholar]
- EPA, 2019. Pesticides. https://www.epa.gov/pesticides. Accessed March 2019.
- Faraji A, Unlu I, 2016. The eye of the tiger, the thrill of the fight: Effective larval and adult control measures against the Asian tiger mosquito, Aedes albopictus (Diptera: Culicidae), in North America. Journal of medical entomology 53, 1029–1047. [DOI] [PubMed] [Google Scholar]
- Fiorenzano JM, Koehler PG, Xue RD, 2017. Attractive toxic sugar Bait (ATSB) for control of mosquitoes and its impact on non-target organisms: a review. Int J Environ Res Public Health 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldo-Calderon GI, Emrich SJ, MacCallum RM, Maslen G, Dialynas E, Topalis P, Ho N, Gesing S, VectorBase C, Madey G, Collins FH, Lawson D, 2015. VectorBase: an updated bioinformatics resource for invertebrate vectors and other organisms related with human diseases. Nucleic Acids Res 43, D707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo CY, Chen P, Zhang MM, Ning JJ, Wang CL, Wang DL, Zhao YL 2016. Expression analysis of a transformer gene in Daphnia pulex after RNAi. Mol Biol 50: 847–854. [DOI] [PubMed] [Google Scholar]
- Hapairai LK, Mysore K, Chen Y, Harper EI, Scheel MP, Lesnik AM, Sun L, Severson DW, Wei N, Duman-Scheel M, 2017. Lure-and-kill yeast interfering RNA larvicides targeting neural genes in the human disease vector mosquito Aedes aegypti. Sci Rep 7, 13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen M, Tomchaney M, Kast K, Flannery E, Clemons A, Jacowski C, Simanton Holland W, Le C, Severson D, Duman-Scheel M, 2010. Whole-mount in situ hybridization for analysis of gene expression during Aedes aegypti development. Cold Spring Harb Protoc 2010, pdb prot5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CA, Doyle T, Nuss AB, Ejendal KF, Meyer JM, Watts VJ, 2016. Comparative pharmacological characterization of D1-like dopamine receptors from Anopheles gambiae, Aedes aegypti and Culex quinquefasciatus suggests pleiotropic signaling in mosquito vector lineages. Parasit Vectors 9, 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CA, Sharan S, Watts VJ, 2018. Genomics, GPCRs and new targets for the control of insect pests and vectors. Curr Opin Insect Sci 30, 99–106. [DOI] [PubMed] [Google Scholar]
- Hiruta C, Toyota K, Miyakawa H, Ogino Y, Miyagawa S, Tatarazako N, Shaw JR, Iguchi T 2013. Development of a microinjection system for RNA interference in the water flea Daphnia pulex. BMC Biotechnol 13, 96, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt RA, Subramanian GM, Halpern A, Sutton GG, Charlab R, Nusskern DR, Wincker P, Clark AG, Ribeiro JM, Wides R, Salzberg SL, Loftus B, Yandell M, Majoros WH, Rusch DB, Lai Z, Kraft CL, Abril JF, Anthouard V, Arensburger P, Atkinson PW, Baden H, de Berardinis V, Baldwin D, Benes V, Biedler J, Blass C, Bolanos R, Boscus D, Barnstead M, Cai S, Center A, Chaturverdi K, Christophides GK, Chrystal MA, Clamp M, Cravchik A, Curwen V, Dana A, Delcher A, Dew I, Evans CA, Flanigan M, Grundschober-Freimoser A, Friedli L, Gu Z, Guan P, Guigo R, Hillenmeyer ME, Hladun SL, Hogan JR, Hong YS, Hoover J, Jaillon O, Ke Z, Kodira C, Kokoza E, Koutsos A, Letunic I, Levitsky A, Liang Y, Lin JJ, Lobo NF, Lopez JR, Malek JA, McIntosh TC, Meister S, Miller J, Mobarry C, Mongin E, Murphy SD, O’Brochta DA, Pfannkoch C, Qi R, Regier MA, Remington K, Shao H, Sharakhova MV, Sitter CD, Shetty J, Smith TJ, Strong R, Sun J, Thomasova D, Ton LQ, Topalis P, Tu Z, Unger MF, Walenz B, Wang A, Wang J, Wang M, Wang X, Woodford KJ, Wortman JR, Wu M, Yao A, Zdobnov EM, Zhang H, Zhao Q, Zhao S, Zhu SC, Zhimulev I, Coluzzi M, della Torre A, Roth CW, Louis C, Kalush F, Mural RJ, Myers EW, Adams MD, Smith HO, Broder S, Gardner MJ, Fraser CM, Birney E, Bork P, Brey PT, Venter JC, Weissenbach J, Kafatos FC, Collins FH, Hoffman SL, 2002. The genome sequence of the malaria mosquito Anopheles gambiae. Science 298, 129–149. [DOI] [PubMed] [Google Scholar]
- Hunter W, Ellis J, Vanengelsdorp D, Hayes J, Westervelt D, Glick E, Williams M, Sela I, Maori E, Pettis J, Cox-Foster D, Paldi N, 2010. Large-scale field application of RNAi technology reducing Israeli acute paralysis virus disease in honey bees (Apis mellifera, Hymenoptera: Apidae). PLoS Pathog 6, e1001160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AL, Linsley PS, 2010. Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat Rev Drug Discov 9, 57–67. [DOI] [PubMed] [Google Scholar]
- Johnson M, Zaretskaya I, Raytselis Y, Merezhuk Y, McGinnis S, Madden TL, 2008. NCBI BLAST: a better web interface. Nucleic Acids Res 36, W5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khajuria C, Ivashuta S, Wiggins E, Flagel L, Moar W, Pleau M, Miller K, Zhang Y, Ramaseshadri P, Jiang C, Hodge T, Jensen P, Chen M, Gowda A, McNulty B, Vazquez C, Bolognesi R, Haas J, Head G, Clark T, 2018. Development and characterization of the first dsRNA-resistant insect population from western corn rootworm, Diabrotica virgifera virgifera LeConte. PloS one 13, e0197059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilham SS, K.D., Lynn SG, Goulden CE, Herrera L, 1998. COMBO: a defined freshwater culture medium for algae and zooplankton. Hydrobiologia 377, 147–159. [Google Scholar]
- Matthews BJ, Dudchenko O, Kingan SB, Koren S, Antoshechkin I, Crawford JE, Glassford WJ, Herre M, Redmond SN, Rose NH, Weedall GD, Wu Y, Batra SS, Brito-Sierra CA, Buckingham SD, Campbell CL, Chan S, Cox E, Evans BR, Fansiri T, Filipovic I, Fontaine A, Gloria-Soria A, Hall R, Joardar VS, Jones AK, Kay RGG, Kodali VK, Lee J, Lycett GJ, Mitchell SN, Muehling J, Murphy MR, Omer AD, Partridge FA, Peluso P, Aiden AP, Ramasamy V, Rasic G, Roy S, Saavedra-Rodriguez K, Sharan S, Sharma A, Smith ML, Turner J, Weakley AM, Zhao Z, Akbari OS, Black W.C.t., Cao H, Darby AC, Hill CA, Johnston JS, Murphy TD, Raikhel AS, Sattelle DB, Sharakhov IV, White BJ, Zhao L, Aiden EL, Mann RS, Lambrechts L, Powell JR, Sharakhova MV, Tu Z, Robertson HM, McBride CS, Hastie AR, Korlach J, Neafsey DE, Phillippy AM, Vosshall LB, 2018. Improved reference genome of Aedes aegypti informs arbovirus vector control. Nature 563, 501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriventseva EV, Kuznetsov D, Tegenfeldt F, Manni M, Dias R, Simão FA, Zdobnov EM, 2019. OrthoDB v10: sampling the diversity of animal, plant, fungal, protist, bacterial and viral genomes for evolutionary and functional annotations of orthologs. Nucleic Acids Res 47, D807–D811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JM, Ejendal KF, Avramova LV, Garland-Kuntz EE, Giraldo-Calderon GI, Brust TF, Watts VJ, Hill CA, 2012. A “genome-to-lead” approach for insecticide discovery: pharmacological characterization and screening of Aedes aegypti D(1)-like dopamine receptors. PLoS Negl Trop Dis 6, e1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SC, Brown SJ, and Tomoyasu Y, 2008. Larval RNAi in Drosophila? Dev Genes Evol 218, 505–510. [DOI] [PubMed] [Google Scholar]
- MonSanto, 2014. Docket ID: EPA-HQ-OPP-2013–0485. https://www.apsnet.org/members/outreach/ppb/Documents/Monsanto%20posted%20written%20comment%20Jan%202014.pdf, Accessed June 2016.
- Müller GCA, Galili A, 2016. Attractive Toxic Sugar Baits (ATSB): from Basic Science to Product to a New Paradigm for Vector Control. Westham Innovations. https://endmalaria.org/sites/default/files/7_Gunter%20Mueller.pdf, Accessed June 2016. [Google Scholar]
- Mummery-Widmer JL, Yamazaki M, Stoeger T, Novatchkova M, Bhalerao S, Chen D, Dietzl G, Dickson BJ, Knoblich JA, 2009. Genome-wide analysis of Notch signalling in Drosophila by transgenic RNAi. Nature 458, 987–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mysore K, Flister S, Muller P, Rodrigues V, Reichert H, 2011. Brain development in the yellow fever mosquito Aedes aegypti: a comparative immunocytochemical analysis using cross-reacting antibodies from Drosophila melanogaster. Dev Genes Evol 221, 281–296. [DOI] [PubMed] [Google Scholar]
- Mysore K, Hapairai LK, Sun L, Harper EI, Chen Y, Eggleson KK, Realey JS, Scheel ND, Severson DW, Wei N, Duman-Scheel M, 2017. Yeast interfering RNA larvicides targeting neural genes induce high rates of Anopheles larval mortality. Malar J 16, 461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mysore K, Li P, Wang CW, Hapairai LK, Scheel ND, Realey JS, Sun L, Roethele JB, Severson DW, Wei N, Duman-Scheel M, 2019a. Characterization of a yeast interfering RNA larvicide with a target site conserved in the synaptotagmin gene of multiple disease vector mosquitoes. PLoS Negl Trop Dis 13, e0007422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mysore K, Li P, Wang CW, Hapairai LK, Scheel ND, Realey JS, Sun L, Severson DW, Wei N, Duman-Scheel M, 2019b. Characterization of a broad-based mosquito yeast interfering RNA larvicide with a conserved target site in mosquito semaphorin-1a genes. Parasit Vectors 12, 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mysore K, Hapairai LK, Wei N, Realey JS, Scheel ND, Severson DW, Duman-Scheel M, 2019c. Preparation and use of a yeast shRNA delivery system for gene silencing in mosquito larvae. Methods Mol Biol 1858, 213–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mysore K, Sun L, Tomchaney M, Sullivan G, Adams H, Piscoya AS, Severson DW, Syed Z, Duman-Scheel M, 2015. siRNA-Mediated Silencing of doublesex during female development of the dengue vector mosquito Aedes aegypti. PLoS Negl Trop Dis 9, e0004213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neafsey DE, Waterhouse RM, Abai MR, Aganezov SS, Alekseyev MA, Allen JE, Amon J, Arca B, Arensburger P, Artemov G, Assour LA, Basseri H, Berlin A, Birren BW, Blandin SA, Brockman AI, Burkot TR, Burt A, Chan CS, Chauve C, Chiu JC, Christensen M, Costantini C, Davidson VL, Deligianni E, Dottorini T, Dritsou V, Gabriel SB, Guelbeogo WM, Hall AB, Han MV, Hlaing T, Hughes DS, Jenkins AM, Jiang X, Jungreis I, Kakani EG, Kamali M, Kemppainen P, Kennedy RC, Kirmitzoglou IK, Koekemoer LL, Laban N, Langridge N, Lawniczak MK, Lirakis M, Lobo NF, Lowy E, MacCallum RM, Mao C, Maslen G, Mbogo C, McCarthy J, Michel K, Mitchell SN, Moore W, Murphy KA, Naumenko AN, Nolan T, Novoa EM, O’Loughlin S, Oringanje C, Oshaghi MA, Pakpour N, Papathanos PA, Peery AN, Povelones M, Prakash A, Price DP, Rajaraman A, Reimer LJ, Rinker DC, Rokas A, Russell TL, Sagnon N, Sharakhova MV, Shea T, Simao FA, Simard F, Slotman MA, Somboon P, Stegniy V, Struchiner CJ, Thomas GW, Tojo M, Topalis P, Tubio JM, Unger MF, Vontas J, Walton C, Wilding CS, Willis JH, Wu YC, Yan G, Zdobnov EM, Zhou X, Catteruccia F, Christophides GK, Collins FH, Cornman RS, Crisanti A, Donnelly MJ, Emrich SJ, Fontaine MC, Gelbart W, Hahn MW, Hansen IA, Howell PI, Kafatos FC, Kellis M, Lawson D, Louis C, Luckhart S, Muskavitch MA, Ribeiro JM, Riehle MA, Sharakhov IV, Tu Z, Zwiebel LJ, Besansky NJ, 2015. Mosquito genomics. Highly evolvable malaria vectors: the genomes of 16 Anopheles mosquitoes. Science 347, 1258522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckameyer WS, 1996. Multiple roles for dopamine in Drosophila development. Dev Biol 176, 209–219. [DOI] [PubMed] [Google Scholar]
- Nene V, Wortman JR, Lawson D, Haas B, Kodira C, Tu ZJ, Loftus B, Xi Z, Megy K, Grabherr M, Ren Q, Zdobnov EM, Lobo NF, Campbell KS, Brown SE, Bonaldo MF, Zhu J, Sinkins SP, Hogenkamp DG, Amedeo P, Arensburger P, Atkinson PW, Bidwell S, Biedler J, Birney E, Bruggner RV, Costas J, Coy MR, Crabtree J, Crawford M, Debruyn B, Decaprio D, Eiglmeier K, Eisenstadt E, El-Dorry H, Gelbart WM, Gomes SL, Hammond M, Hannick LI, Hogan JR, Holmes MH, Jaffe D, Johnston JS, Kennedy RC, Koo H, Kravitz S, Kriventseva EV, Kulp D, Labutti K, Lee E, Li S, Lovin DD, Mao C, Mauceli E, Menck CF, Miller JR, Montgomery P, Mori A, Nascimento AL, Naveira HF, Nusbaum C, O’Leary S, Orvis J, Pertea M, Quesneville H, Reidenbach KR, Rogers YH, Roth CW, Schneider JR, Schatz M, Shumway M, Stanke M, Stinson EO, Tubio JM, Vanzee JP, Verjovski-Almeida S, Werner D, White O, Wyder S, Zeng Q, Zhao Q, Zhao Y, Hill CA, Raikhel AS, Soares MB, Knudson DL, Lee NH, Galagan J, Salzberg SL, Paulsen IT, Dimopoulos G, Collins FH, Birren B, Fraser-Liggett CM, Severson DW, 2007. Genome sequence of Aedes aegypti, a major arbovirus vector. Science 316, 1718–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngai M, McDowell MA, 2017. The search for novel insecticide targets in the post-genomics era, with a specific focus on G-protein coupled receptors. Mem Inst Oswaldo Cruz 112, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowling RJ, Abrudan JL, Shoue DA, Abdul-Wahid B, Wadsworth M, Stayback G, Collins FH, McDowell MA, Izaguirre JA, 2013. Identification of novel arthropod vector G protein-coupled receptors. Parasit Vectors 6, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuss AB, Ejendal KF, Doyle TB, Meyer JM, Lang EG, Watts VJ, Hill CA, 2015. Dopamine receptor antagonists as new mode-of-action insecticide leads for control of Aedes and Culex mosquito vectors. PLoS Negl Trop Dis 9, e0003515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel NH, 1996. In situ hybridization to whole mount Drosophila embryos, in: Krieg PA (Ed.), A Laboratory Guide to RNA: Isolation, Analysis, and Synthesis. Wiley-Liss, New York, pp. 357–370. [Google Scholar]
- Regna K, Kurshan PT, Harwood BN, Jenkins AM, Lai CQ, Muskavitch MA, Kopin AS, Draper I, 2016. A critical role for the Drosophila dopamine D1-like receptor Dop1R2 at the onset of metamorphosis. BMC Dev Biol 16, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh MC, van Rij RP, Hekele A, Gillis A, Foley E, O’Farrell PH, Andino R 2006. The endocytic pathway mediates cell entry of dsRNA to induce RNAi silencing. Nat Cell Biol 8, 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Miguel K, S.J., 2016. The next generation of insecticides: dsRNA is stable as a foliar-applied insecticide. Pest Manag Sci 72, 801–809. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A, 2019. Fiji: an open-source platform for biological-image analysis. Nature Methods 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P, 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AD, Wong S, Ryan CP, Whyard S, 2013. Oral delivery of double-stranded RNA in larvae of the yellow fever mosquito, Aedes aegypti: implications for pest mosquito control. J Insect Sci 13, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumpert CA, Dudycha JL, Patel RC 2015. Development of an efficient RNA interference method by feeding for the microcrustacean Daphnia. BMC Biotechnol 15, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sissoko F, Junnila A, Traore MM, Traore SF, Doumbia S, Dembele SM, Schlein Y, Traore AS, Gergely P, Xue RD, Arheart KL, Revay EE, Kravchenko VD, Beier JC, Muller GC, 2019. Frequent sugar feeding behavior by Aedes aegypti in Bamako, Mali makes them ideal candidates for control with attractive toxic sugar baits (ATSB). PloS one 14, e0214170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram K, Insel PA, 2018. G protein-coupled receptors as targets for approved drugs: how many targets and how many drugs? Mol Pharmacol 93, 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurmond J, Goodman JL, Strelets VB, Attrill H, Gramates LS, Marygold SJ, Matthews BB, Millburn G, Antonazzo G, Trovisco V, Kaufman TC, Calvi BR, FlyBase C, 2019. FlyBase 2.0: the next generation. Nucleic Acids Res 47, D759–D765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomchaney M, Mysore K, Sun L, Li P, Emrich SJ, Severson DW, Duman-Scheel M, 2014. Examination of the genetic basis for sexual dimorphism in the Aedes aegypti (dengue vector mosquito) pupal brain. Biol Sex Differ 5, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijken JP, Bauer J, Brambilla L, Duboc P, Francois JM, Gancedo C, Giuseppin ML, Heijnen JJ, Hoare M, Lange HC, Madden EA, Niederberger P, Nielsen J, Parrou JL, Petit T, Porro D, Reuss M, van Riel N, Rizzi M, Steensma HY, Verrips CT, Vindelov J, Pronk JT, 2000. An interlaboratory comparison of physiological and genetic properties of four Saccharomyces cerevisiae strains. Enzyme and microbial technology 26, 706–714. [DOI] [PubMed] [Google Scholar]
- Wagh DA, Rasse TM, Asan E, Hofbauer A, Schwenkert I, Durrbeck H, Buchner S, Dabauvalle MC, Schmidt M, Qin G, Wichmann C, Kittel R, Sigrist SJ, Buchner E, 2006. Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron 49, 833–844. [DOI] [PubMed] [Google Scholar]
- WHO, 2005. Guidelines for laboratory and field testing of mosquito larvicides. World Health Organization, Geneva. [Google Scholar]
- WHO, 2009. Dengue guidelines for diagnosis, treatment, prevention and control: new edition. World Health Organization, Geneva. [PubMed] [Google Scholar]
- Wise A, Gearing K, Rees S, 2002. Target validation of G-protein coupled receptors. Drug Discov Today 7, 235–246. [DOI] [PubMed] [Google Scholar]
- Xiao D, Gao X, Xu J, Liang X, Li Q, Yao J, Zhu KY Clathrin-dependent endocytosis plays a predominant role in cellular uptake of double-stranded RNA in the red flour beetle. Insect Biochem Mol Biol 60, 68–77. [DOI] [PubMed] [Google Scholar]
- Zhang J, Khan SA, Heckel DG, Bock R, 2017. Next-generation insect-resistant plants: RNAi-Mediated Crop Protection. Trends Biotechnol. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file 1. Evaluation of dop1.462 target site conservation. The 21 and 25 bp sequences targeted by dop1.462 IRPs were used as a query sequence against all mosquito genomes in Vectorbase (Giraldo-Calderon et al., 2015). Mosquito species in which a perfectly conserved target sequence was identified in the indicated genes (when known) or scaffold (s) locations are indicated. The 21 and 25 bp target sequences were also used to conduct blastn searches against all sequences in the NCBI nucleotide collection (Johnson et al., 2008). As of January 2020, other than disease vector mosquitoes, these searches failed to uncover any 2½1 or 25/25 identical matches in any genome, including the indicated taxonomic groups (NCBI TaxIDs are listed, including Diptera, Coleoptera, and Crustacea, from which representative species were assessed in toxicity assays).
Supplementary file 2. Results of blastn searches for dop1.462 target sites in non-target organisms. Outside of the disease vector mosquito identities detailed in supplementary file 1, as of January 2020, blastn searches did not uncover 21/21 or 25/25 nucleotide sequence identities for the 21 bp (A) or 25 bp (B) dop1.462 IRP target sites in any sequences found in the NCBI nucleotide collection (Johnson et al., 2008). The ratios of identity, bit scores (max score), E values, and accession numbers for the top 20 blast results (outside of mosquitoes) are shown. Several 19/21 and 22/25 match identities were uncovered, but perfect matches covering the full 21 bp (A) or 25 bp (B) query sequences were only identified in mosquitoes.
Supplementary file 3. A. aegypti larval consumption of dop1.462 yeast results in severe neural defects. High levels of dop1 expression detected in the A. aegypti L4 larval brain (A1, which shows a brain from a control yeast-treated larva that is comparable to wild-type larva expression) are significantly reduced (A3) in the brains of L4 larvae that consumed dop1.462 yeast (A2). In A3, mean gray value results that were compiled from three biological replicate trials are shown (n=53 brains from dop1.462-treated larvae, and n=53 brains from control-treated larvae). Larval brains were labeled with mAbnc82 (white in B1, B2; red in C1, C2) and counter-stained with TO-PRO nuclear label (blue in C1, C2). Although nc82 levels were significantly reduced (B3) in the synaptic neuropil of larvae fed with dop1.462 yeast (B2, C2), no significant differences were observed in TO-PRO levels (C3), which were similar in dop1.462 (C2) and control-treated (C1) larvae. Data shown in B3 and C3 were compiled from three biological replicate experiments performed on a total of 37 dop1.462-treated brains and 43 control-treated brains and are represented here as average mean gray values; error bars correspond to SEM. Data were analyzed with Student’s t-test; ***=P<0.001 vs. control. Representative adult brains are oriented dorsal upward and labeled as follows: AL: antennal lobe; OF: oesophageous foramen; OL: optic lobe; SOG: sub-esophageal ganglion; SuEG: supra-esophageal ganglion. The scale bar corresponds to 100 μm.