Abstract
Background
During the coronavirus disease 2019 (COVID-19) pandemic, antibody screening is a critical tool to assess anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) immunity. We examined variation in antibody titers associated with age and sex among patients with confirmed COVID-19.
Methods
Blood IgG levels were tested in 1081 patients with positive SARS-CoV-2 quantitative reverse transcription polymerase chain reaction (RT-qPCR) tests between 1 September and 31 December 2020. Patients who did not experience reinfection were identified. Serum IgG levels were measured by immunofluorescence assay. Antibody positivity and antibody titers were analyzed according to time since infection, sex, and age.
Results
The mean (standard deviation) age was 41.2 (14.2) years and 41.2% of patients were women. The lowest antibody positivity rate between the first and ninth month post-infection was detected in the sixth month. The lowest antibody titers among patients aged 20 to 80 years occurred in those aged 30 to 39 years. The IgG titer was positively correlated with age in years (r = 0.125) and decades (r = 0.126).
Conclusions
Six months after infection, anti-SARS-CoV-2 antibody titers increased. Anti-SARS-CoV-2 antibody titers also increased with age. Immunity and pathogenicity should be investigated in addition to antibody positivity rates and antibody titers.
Keywords: Antibody, fluorescent antibody technique, coronavirus disease 2019, severe acute respiratory syndrome coronavirus-2, serology, immunity
Introduction
Coronavirus disease 2019 (COVID-19) was initially identified as an outbreak of pneumonia of unknown origin in Wuhan, China, in December 2019. 1 On 11 March 2020, the World Health Organization declared SARS-CoV-2 pneumonia to be a pandemic, and the first case in Turkey was reported around the same time. 2 As of the end of 2020, 83 million people worldwide had been infected by the causative virus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and 1.8 million individuals had died. COVID-19 can cause symptoms ranging from a mild, self-limiting upper respiratory tract infection to respiratory failure requiring mechanical ventilation and even death. The gold standard diagnostic method for COVID-19 is quantitative reverse transcription polymerase chain reaction (RT-qPCR) testing, which detects viral RNA in respiratory tract samples. 3 Only acute cases may be identified by RT-qPCR testing, and the test provides no information on patient immunity or other characteristics. Levels of inflammatory markers, especially C-reactive protein and blood cells, are altered during SARS-CoV-2-induced inflammation. Antibodies such as IgG, IgM and IgA are the most sensitive and are early serological markers of infection, with levels beginning to rise as early as the second week after onset of symptoms. 4
Although serum IgM and IgG can be positive with low titers as early as the fourth day after symptom onset, higher levels appear during the second and third weeks of the disease. 3 Asymptomatic individuals and others with suspected infection and negative nucleic acid tests can be diagnosed using serological antibody assays.5,6 Experience with other human coronaviruses demonstrated an average duration of immunity of 1 to 2 years. 7 SARS-CoV-2 appears to elicit similar antibody responses compared with other human coronaviruses based on early clinical studies. Antibody responses have been detected against the nucleocapsid and spike proteins. 8 Previous experiences during outbreaks of SARS-CoV, Middle East Respiratory Syndrome (MERS)-CoV, and other seasonal human coronaviruses suggest that, depending on the severity of disease, protection against reinfection may deteriorate within a short period. 9
It is crucial to understand how the immune response to COVID-19 changes with age, disease duration, and disease severity, as well as the duration of post-infection protection afforded by the antibody response. Concerns have been raised regarding the short duration of immunity afforded by vaccines and antibodies elicited by natural infection. This study's goal was to examine differences in antibody titers associated with age and sex in patients with COVID-19 with previous positive RT-qPCR tests.
Methods
Study design and participants
This was a longitudinal observational study. Patients presenting at Biruni University Faculty of Medicine's outpatient clinics between 18 March and 31 August 2020 who had positive SARS-CoV-2 RT-qPCR tests and whose blood IgG levels were tested between 1 September and 31 December 2020 were included in the study. Patients without reinfection were selected among antibody-positive patients if they had previously tested positive by RT-qPCR.
Data for enrolled patients were obtained from clinical records. The time elapsed between the positive SARS-CoV-2 RT-qPCR test and the measurement of blood IgG levels was calculated. Patient age, sex, comorbidities, and intensive care requirements were noted. The study excluded patients under the age of 18 years, patients with SARS-CoV-2 reinfection, patients using immunosuppressive medications, and immunocompromised patients.
Serum immunoglobulin G (IgG) levels against SARS-CoV-2 were measured at the time admission to the hospital. The number of times that individuals with the clinical symptoms of COVID-19 underwent RT-qPCR nucleic acid testing and had positive results was recorded. Demographic characteristics and comorbidities of all patients were obtained from hospital records.
The study protocol was reviewed and approved by the Biruni University Faculty of Medicine Ethics Committee and the Ministry of Health (approval number 2021/47-42). The study was conducted according to the principles laid out in the Declaration of Helsinki. All patients were given full information regarding the study procedures before providing written consent. The reporting of this study conformed with the STROBE criteria. 10
Study procedures
SARS-CoV-2 RT-qPCR results were obtained from hospital records. All patients included in the study had oropharyngeal and nasal swab samples taken for COVID-19 RT-qPCR nucleic acid testing. Both mouth and nose swab samples were placed in viral nucleic acid buffer (VNAB). VNAB containing samples was added to the prepared RT-qPCR mix and amplified using a Biorad CFX96 Realtime PCR device. For RT-qPCR testing, 200 relative fluorescence units was used as a threshold value in each channel. The N gene channel (HEX) was used as an internal control. In the N gene channel, sigmoidal curves with cycle threshold (Ct) values ≤32 were considered positive. In the ORF1ab gene channel (FAM), sigmoidal curves with Ct values ≤38 were considered positive. Samples with Ct values >38 in the FAM channel or with no peaks were considered negative provided that internal controls were included in the assay.
For the serological assay, we collected venous blood (2 mL) from each participant between 1 December 2020 and 13 January 2021 (prior to the roll-out of public vaccination in Turkey). Blood samples were tested within 4 hours of collection and were stored at room temperature. We used an immunofluorescence assay (IFA) (IF2084 for Getein 1600, Getein Biotech, Inc. Nanjing, China) to evaluate the presence of serum IgG against SARS-CoV-2 in accordance with the manufacturer's instructions. The test uses mixtures of recombinant SARS-CoV-2 nucleocapsid (N) protein and spike (S) protein. Briefly, each Getein 1600 cartridge contains a specific radio-frequency identification card that is automatically calibrated. The sample diluent is placed at the correct position in the Getein 1600 cartridge, then the samples are placed in the designated area of the sample holder. After inserting the holder and selecting the right test item, the Getein 1600 will perform the test and print the result automatically. The test result is displayed numerically in terms of a cut-off index (COI) value. Test result is negative if the COI is <1.0 and positive if the COI is ≥1.0. The COIs for anti-SARS-CoV-2 IgM/IgG antibody were determined and validated using 500 samples confirmed as negative and 88 samples confirmed as positive. The test had a sensitivity for IgM of 56.7% (17/30) (95% confidence interval [CI], 39.2% to 72.6%) and IgM specificity of 98.8% (79/80). IgM was not used in this study to evaluate immune responses because of its low sensitivity and because it is associated with acute infection. The test had a sensitivity for IgG of 73.3% (22/30) (95% CI, 55.6% to 85.8%) and IgG specificity of 100% (80/80) (95% CI, 95.4% to 100%). 11
Statistical analysis
This was a retrospective cross-sectional study. Differences between normally distributed data were assessed using one-sample Kolmogorov–Smirnov tests. Continuous variables were presented as means ± standard deviations. Categorical variables were presented as counts and percentages. Differences between continuous variables were assessed using two-sided Student's t-tests. Differences between categorical variables were assessed using the Chi-square test or Fisher’s exact test for small samples. Analysis of variance was used to compare the means of multiple groups with post-hoc correction using the Bonferroni method. Pearson’s correlation analysis was applied to numerical and nominal data. Values of p < 0.05 were considered statistically significant. All statistical analyses were performed using SPSS 20.0 software (SPSS, Armonk, NY: IBM Corp, USA).
Results
The demographics and comorbidities of the 1081 patients enrolled in the study are shown in Table 1. The mean (standard deviation) patient age was 41. 2 (14.2) years, and 41. 2% of patients were women. There was no age difference between men and women participating in the study. The frequencies of most comorbidities were similar by sex although men had a higher prevalence of hypertension (p < 0.001). Antibody positivity rate was determined to be 98.4% among all patients; 17 patients were antibody negative. The antibody positivity rate was similar between male and female patients.
Table 1.
Demographic characteristics and comorbidities of the study population.
| All patients (n = 1081) | Women (n = 445) | Men (n = 636) | p-value | |
|---|---|---|---|---|
| Age (years), mean±SD | 41.3 ± 14.2 | 40.5 ± 14.8 | 41.8 ± 13.8 | 0.120 |
| Time* from positive SARS-CoV-2 RT-qPCR to first serological test (months), median (range) | 2.5 (1, 6) | 3 (1, 6) | 2.5 (1, 6) | 0.912 |
| Comorbidities | ||||
| Hypertension, n (%) | 368 (34) | 120 (26.9) | 248 (38.9) | <0.001 |
| Diabetes mellitus, n (%) | 163 (15.1) | 62 (13.9) | 101 (15.9) | 0.389 |
| COPD/asthma, n (%) | 114 (10.5) | 45 (10.1) | 69 (10.8) | 0.763 |
| Coronary artery disease, n (%) | 95 (8.8) | 31 (6.9) | 64 (10.1) | 0.081 |
| Hyperlipidemia, n (%) | 80 (7.4) | 29 (6.5) | 51 (8) | 0.409 |
SD, standard deviation; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; RT-qPCR, quantitative reverse transcription polymerase chain reaction; COPD, chronic obstructive pulmonary disease.
Table 2 shows antibody positivity rates and antibody taters of patients by month. Antibody positivity rates by month did not differ between sexes. The lowest antibody positivity rate was observed in the sixth month of the 9-month study duration (p < 0.001). The mean antibody titer of all patients was 39.8 ± 20.2 COI. The antibody titers of all patients were significantly lower in the sixth month of the 9-month study duration (p < 0.001).
Table 2.
Monthly antibody titers in the study population.
| n | All patients (n = 1081) | Women (n = 445) | Men (n = 636) | p-value | |
|---|---|---|---|---|---|
| 1st month | 787 | 39.8 ± 19.7 | 40.7 ± 18.6 | 39.1 ± 20.5 | 0.275 |
| 2nd month | 146 | 44.9 ± 19.1 | 43.9 ± 21.3 | 45.5 ± 17.6 | 0.651 |
| 3rd month | 52 | 42.9 ± 19.8 | 34.2 ± 20.4 | 47.9 ± 17.9 | 0.014 |
| 4th month | 19 | 31.7 ± 23.8 | 30.4 ± 16.8 | 31.9 ± 25.4 | 0.925 |
| 5th month | 23 | 23.2 ± 19.8 | 12.6 ± 11.4 | 25.4 ± 20.7 | 0.250 |
| 6th month | 13 | 16.8 ± 16.4 | 15.1 ± 18.6 | 18.3 ± 15.6 | 0.739 |
| 7th month | 19 | 38.9 ± 23.9 | 38.8 ± 31.8 | 38.8 ± 21.1 | 0.997 |
| 8th month | 17 | 38.4 ± 27.9 | 29.4 ± 47.4 | 40.3 ± 24.3 | 0.555 |
| 9th month | 5 | 36.9 ± 22.6 | 35.6 ± 43.8 | 37.9 ± 7.9 | 0.927 |
Note: all titers are expressed as mean ± standard deviation cut-off index.
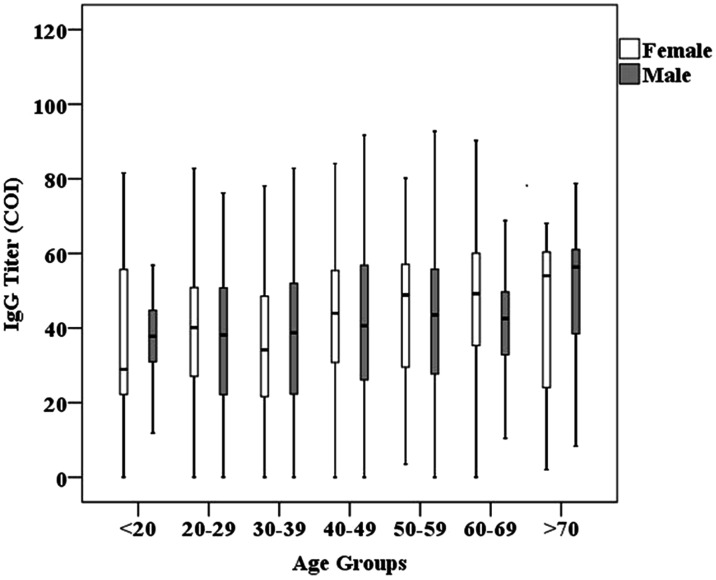
Table 3 shows antibody titers by age in the study population. There were no age-related differences in antibody titers in men or women. The lowest antibody titers among patients aged 20 to 80 years were observed in those between the ages of 30 and 39 years (p = 0.003) (Figure 1). IgG titers were positively correlated with age (r = 0.133, p < 0.001).
Table 3.
Antibody titers by age in the study population.
| Age (years) | n | All patients (n = 1081) | Women (n = 445) | Men (n = 636) | p-value |
|---|---|---|---|---|---|
| <20 | 26 | 36.7 ± 18.1 | 38.1 ± 27.7 | 36.2 ± 14.2 | 0.871 |
| 20–29 | 254 | 37.6 ± 19.5 | 38.8 ± 19.1 | 36.5 ± 19.9 | 0.343 |
| 30–39 | 305 | 36.8 ± 19.3 | 35.1 ± 18.7 | 37.9 ± 19.7 | 0.206 |
| 40–49 | 220 | 42.1 ± 21.4 | 43.1 ± 20.5 | 41.5 ± 21.9 | 0.592 |
| 50–59 | 172 | 42.9 ± 20.6 | 44.3 ± 19.1 | 42.1 ± 21.5 | 0.499 |
| 60–69 | 70 | 43.2 ± 18.6 | 47.2 ± 19.4 | 41.1 ± 18.1 | 0.201 |
| >70 | 34 | 47.7 ± 23.5 | 42.7 ± 25.2 | 51.6 ± 21.9 | 0.277 |
Note: all titers are expressed as mean ± standard deviation cut-off index.
Figure 1.
Distribution of antibody titers by age groups and sex.
Discussion
In this study, we observed that antibodies were elicited against SARS-CoV-2 at similar levels in women and men. The antibody titers among women and all patients reached their lowest levels during the fifth month post-infection. Antibody positivity was observed after the sixth month post-infection and antibody titer was positively associated with age.
COVID-19 remains a significant concern. The disease has an average incubation time of 3 to 5 days, rapid human-to-human transmission, and a global death rate of 2.1%. There are no effective treatments available. Induction of humoral immunity via community immunization campaigns represents a promising strategy. 12 Zhao et al. reported that the average seroconversion periods for IgM and IgG were 11 and 12 days, respectively. 13 Tan et al. observed that different serological kits had comparable performance for 170 patients with COVID-19. The authors concluded that serological testing should be performed after 14 days, and that some patients (three patients, 1.8%) did not have IgG seroconversion even at 40-day follow-up. In the same study, it was observed that antibody titers increased day by day during the 40-day follow-up. Measurement of antibody levels after the 21st day of follow up had a sensitivity of 90.6% (95% CI, 75.8%–96.8%) and an average COI of 44.2 (95% CI, 32–56.4). Similarly, antibody positivity was present in 98.4% of patients over the 9-month follow-up in our study, with antibody levels of 39.8 ± 19.6 COI during the first month. Our study differs from others in this regard because it had a bigger sample size and a longer follow-up period.
According to previous studies of SARS-CoV, there is a correlation between antibody titers and protection against disease. Moreover, seroconversion occurs earlier and antibody titers are higher in patients with severe disease requiring intensive care, supplemental oxygen, and corticosteroids. 13 Although naturally occurring neutralizing antibody activity associated with IgG improves clearance of SARS-CoV, antibody does not protect against disease progression. Long et al. reported that the duration of transmission was longer in symptomatic patients, IgG levels were lower in patients with asymptomatic or mild disease, and 40% of asymptomatic patients became seronegative during the early recovery period. 14 While early SARS-CoV studies revealed transient immunity, recent studies have documented the presence of neutralizing antibodies even after 12 to 17 years. 13 Similar to the data for SARS-CoV, Ripperger et al. reported that in their study of 5882 patients, neutralizing antibodies were produced stably for 5 to 7 months. 15 The authors concluded that long-term studies of antibodies following vaccination or infection are required. In a recent study, Lumley et al. reported that 11,182 healthcare workers were anti-spike and anti-nucleocapsid antibody negative, with a 1.08% RT-qPCR positive rate. There were no symptoms in antibody-positive individuals. In the same study, the authors found that as the antibody titer increased, PCR positivity decreased. 16 They reported that 223 of 11,364 seronegative healthcare workers had experienced reinfection, while 2 of 1256 seropositive healthcare workers had experienced reinfection. 16 In our study, we observed no evidence for reinfection. We attributed this to patients' elevated antibody levels. According to Pilz et al., the rate of reinfection among 14,860 individuals was 0.27%, while the overall infection rate in Austria was 2.85%. 17
In our study, we observed that antibody positivity rates among patients admitted to intensive care units remained quite high during the 9-month follow-up after infection. These data support the notion that severe SARS-CoV-2 infection induces a stronger immune response. Orner et al. studied 89 hospitalized patients with RT-qPCR-confirmed SARS-CoV-2 infections and found that median seroconversion among men occurred on the eighty day and median seroconversion among women occurred on the seventh day; there was no significant difference between men and women. The authors stated that seroconversion occurred earlier than the sixth day in those over 65 years of age; there was no disparity between the IgG titers of men and women, and titers were slightly higher in those over 65 years of age. 18 In a study of 30,576 participants, Gudbjartason et al. observed 91% seropositivity among 1215 patients diagnosed using RT-qPCR. Antibody positivity rates were higher in hospitalized patients. Positive correlations were observed between body mass index and antibody titers, and low antibody titers were observed in smokers and patients receiving anti-inflammatory medication. The authors also found that antibody titers were higher in elderly hospitalized patients. 19 Similarly, we found that antibody titers were positively associated with age. Because they have more symptomatic disease, elderly individuals with weaker immune systems are more likely to have more durable and higher antibody titers. Wajnberg et al. reported a 95% seroconversion rate and antibody persistence for 5 months in a study of 2347 RT-qPCR-positive patients. 20 In our study, antibody seropositivity rates were the lowest after the sixth month of the 9-month follow-up period, and the titer among all patients was the lowest during the fifth month. However, that seropositivity extended until the ninth month and antibody titers were sustained after the sixth month. Although there was an absence of symptoms or RT-qPCR positivity in patients, antibody titers and antibody positivity persisted. In our study, antibody titers were lowest among those aged 18 to 20 years and 30 to 39 years, and antibody titers increased with age. These data indicated that increased severity of disease associated with age could induce stronger antibody responses.
We could not conduct any neutralization assays in this study. Previously, Dogan et al. showed that the 50% neutralization titer (NT50) was much higher in hospitalized patients than in outpatients. Levels of all antibodies, including S-receptor binding domain (RBD) IgG (rs = 0.81), N IgG (rs = 0.689), S-RBD IgA (rs = 0.60) and S-RBD IgM (rs = 0.47), were significantly correlated with NT50 values in each participant. 21
The current study had several limitations. First, the results cannot be generalized to the whole population because it was a cross-sectional study conducted at a single center. Only patients admitted to hospital were included and asymptomatic cases and individuals under age 18 years were excluded. Second, low numbers of patients over the age of 70 years and low numbers of individuals tested for antibodies after 6 months were included. Third, the study could not provide information on the relationships between antibody titers or seroconversion rates with RT-qPCR positivity or active disease during follow-up.
In this study, we found that SARS-CoV-2 antibodies were produced at the same rate in both men and women. Decreased antibody titers were observed during the fifth month of follow-up and the antibody positivity rate was reached its minimum during the sixth month of follow-up. However, the antibody positivity rate and antibody titers improved again after the sixth month. We also found that antibody titers increased with age. Although serological tests are useful during the pandemic period, further studies are needed to evaluate vaccine efficacy, to assess duration of natural immunity, and to assess protection against reinfection.
Footnotes
Availability of data and material: The anonymized data are available and can be provided upon request.
Author contributions: BBU: Conceptualization, data curation, writing – original draft preparation, SY: writing – original draft preparation; MSI: data curation, writing – original draft preparation; MC: methodology, writing – reviewing.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Mehmet Sami Islamoglu https://orcid.org/0000-0003-3426-6950
References
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Coronavirus disease 2019 (COVID-19) outbreak. https://www.who.int (2020, accessed 14 December 2021).
- 3.Sethuraman N Jeremiah SS andRyo A.. Interpreting diagnostic tests for SARS-CoV-2. JAMA 2020; 323: 2249–2251. [DOI] [PubMed] [Google Scholar]
- 4.Lou B, Li TD, Zheng SF, et al. Serology characteristics of SARS-CoV-2 infection after exposure and post-symptom onset. Eur Respir J 2020; 56: 2000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Z, Yi Y, Luo X, et al. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J Med Virol 2020; 92: 1518–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen Y, Ba Y, Hu Y, et al. Relationship between the dynamic changes of serum 2019-nCoV IgM/IgG and patient immunity after 6 month hospital discharge. Inflamm Res 2021; 70: 241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Post N, Eddy D, Huntley C, et al. Antibody response to SARS-CoV-2 infection in humans: A systematic review. PLoS One 2020; 15: e0244126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang AT, Garcia-Carreras B, Hitchings MDT, et al . A systematic review of antibody mediated immunity to coronaviruses: Antibody kinetics, correlates of protection, and association of antibody responses with severity of disease. medRxiv 2020; 2020.04.14.20065771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kellam P andBarclay W.. The dynamics of humoral immune responses following SARS-CoV-2 infection and the potential for reinfection. J Gen Virol 2020; 101: 791–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 11.GP Getein Biotech, Inc. Serology test evaluation report for “One Step Test for Novel Coronavirus (2019-nCoV) IgM/IgG antibody (Colloidal Gold).” https://www.accessdata.fda.gov/cdrh_docs/presentations/maf/maf3270-a001.pdf (2020, accessed 14 December 2021).
- 12.Iwasaki A andYang Y.. The potential danger of suboptimal antibody responses in COVID-19. Nat Rev Immunol 2020; 20: 339–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao J, Yuan Q, Wang H, et al. Antibody Responses to SARS-CoV-2 in Patients With Novel Coronavirus Disease 2019. Clin Infect Dis 2020; 71: 2027–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Long QX, Tang XJ, Shi QL, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med 2020; 26: 1200–1204. [DOI] [PubMed] [Google Scholar]
- 15.Ripperger TJ, Uhrlaub JL, Watanabe M, et al. Orthogonal SARS-CoV-2 serological assays enable surveillance of low-prevalence communities and reveal durable humoral immunity. Immunity 2020; 53: 925–933.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lumley SF, O'Donnell D, Stoesser NE, et al. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med 2021; 384: 533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pilz S, Chakeri A, Ioannidis JP, et al . SARS-CoV-2 re-infection risk in Austria. Eur J Clin Invest 2021; 51: e13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orner EP, Rodgers MA, Hock K, et al. Comparison of SARS-CoV-2 IgM and IgG seroconversion profiles among hospitalized patients in two US cities. Diagn Microbiol Infect Dis 2021; 99: 115300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gudbjartsson DF, Norddahl GL, Melsted P, et al . Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med 2020; 383: 1724–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wajnberg A, Amanat F, Firpo A, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science 2020; 370: 1227–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dogan M, Kozhaya L, Placek L, et al. Novel SARS-CoV-2 specific antibody and neutralization assays reveal wide range of humoral immune response during COVID-19. medRxiv 2020; 2020.07.07.20148106. [DOI] [PMC free article] [PubMed] [Google Scholar]