Abstract
Rapid differentiation of fermentative gram-negative bacilli (fermenters) from nonfermentative gram-negative bacilli (nonfermenters) in positive blood cultures may help physicians to narrow the choice of appropriate antibiotics for empiric treatment. An impedance method for direct differentiation of fermenters from nonfermenters was investigated. The bacterial suspensions (or positive culture broths containing gram-negative bacteria) were inoculated into the module wells of a Bactometer (bioMérieux, Inc., Hazelwood, Mo.) containing 1 ml of Muller-Hinton broth. The inoculated modules were incubated at 35°C, and the change in impedance in each well was continuously monitored. The amount of time required to cause a series of significant deviations from baseline impedance values was defined as the detection time (DT). The percent change of impedance was defined as the change of impedance at the time interval from DT to DT plus 1 h. After testing 857 strains of pure cultures (586 strains of fermenters and 271 strains of nonfermenters), a breakpoint (2.98%) of impedance change was obtained by discriminant analysis. Strains displaying impedance changes of greater than 2.98% were classified as fermenters; the others were classified as nonfermenters. By using this breakpoint, 98.6% (340 of 345) of positive blood cultures containing fermenters and 98% (98 of 100) of positive blood cultures containing nonfermenters were correctly classified. The impedance method was simple, and the results were normally available within 2 to 4 h after direct inoculation of positive blood culture broths.
Nonfastidious aerobic gram-negative bacteria are common pathogens of humans. Conventionally, these microorganisms were subdivided into two major groups: fermentative gram-negative bacteria (fermenters) and nonfermentative gram-negative bacteria (nonfermenters). The dividing line between these types of bacteria is based more on convention than on well-defined genetic or phenotypic characteristics. It is important that an unknown organism be classified by its mode of glucose utilization to select the correct set of biochemical tests for species identification.
Although gram-positive bacteria are the more common causes of bloodstream infections (28), gram-negative bacteremia carries higher risks of severe sepsis, septic shock, and death. When a positive blood culture is reported from the clinical laboratory, the physician normally will start empiric treatment based on the basic information about the organism (gram positive or gram negative) causing bacteremia, as revealed by Gram staining. Rapid institution of an appropriate antimicrobial therapy is important for a good outcome of bacteremia (27, 29). Several of the clinically important nonfermenters are multiresistant organisms (5, 13, 25), and treatments for infections caused by nonfermenters are somewhat different from those for infections caused by fermenters. It is generally recognized that narrow- and expanded-spectrum cephalosporins are minimally active against nonfermenters (2, 4). However, amyloglycoside- or quinolone-resistant strains of Enterobacteriaceae are relatively rare (7, 21, 28). Only a few of the broad-spectrum cephalosporins (e.g., ceftazidime and ceftriaxone) (2, 14, 16, 17, 19) are effective for the clinically important nonfermenters. Other antibiotics useful for nonfermenters are monobactams (1, 2), quinolones (1, 4), imipenem (5), and piperacillin (6). However, this is only a general rule, and some resistant strains of Enterobacteriaceae (e.g., Serratia) may be encountered (10).
The metabolic activities of microorganisms can cause electrical changes (capacitance, impedance, or conductance) in the culture media. The measurement of electrical properties in a culture broth is basically not influenced by the color or turbidity of the clinical specimens (31). The purpose of this study was to evaluate an impedance method for direct differentiation of fermenters from nonfermenters present in positive blood bottles.
MATERIALS AND METHODS
Bacterial strains, media, and culture conditions.
The pure cultures of bacteria used in this study are listed in Table 1. A total of 857 isolates of gram-negative bacilli, including 586 strains of fermenters and 271 strains of nonfermenters, were used. Most strains were isolated at the National Cheng Kung University Hospital (Tainan, Taiwan), but Escherichia coli CCRC 15481 and 11509, Klebsiella pneumoniae CCRC 11546, Pseudomonas aeruginosa CCRC 10944, Acinetobacter baumannii CCRC 15885, and Escherichia vulneris (three strains) were obtained from the Culture Collection and Research Center, Hsinchu, Taiwan. All bacteria were subcultured on sheep blood agar, incubated at 35°C for 18 to 24 h, and then used for testing. Mueller-Hinton broth (MHB), tryptic soy broth (TSB), brain heart infusion broth (BHI), and OF basal medium were obtained from BBL, Becton Dickinson Microbiology Systems (Cockeysville, Md.). OF glucose medium was prepared by supplementing the basal medium with 1% glucose. Some fastidious and unusual fermentative or nonfermentative gram-negative bacilli were not included in this study.
TABLE 1.
Bacterial strains used in this study
| Microorganism | No. of strains tested |
|---|---|
| Fermenters | |
| Aeromonas hydrophila | 5 |
| Aeromonas spp. | 10 |
| Citrobacter freundii | 30 |
| Citrobacter koseri | 11 |
| Escherichia coli | 222 |
| Escherichia vulneris | 3 |
| Enterobacter aerogenes | 28 |
| Enterobacter cloacae | 31 |
| Enterobacter sakazakii | 4 |
| Klebsiella pneumoniae | 84 |
| Klebsiella oxytoca | 5 |
| Morganella morganii | 34 |
| Pasteurella multocida | 1 |
| Proteus mirabilis | 43 |
| Proteus vulgaris | 6 |
| Providencia stuartii | 2 |
| Salmonella spp. | 22 |
| Serratia marcescens | 32 |
| Shigella sonnei | 10 |
| Vibrio vulnificus | 3 |
| Nonfermenters | |
| Acinetobacter baumannii | 104 |
| Acinetobacter lwoffii | 8 |
| Alcaligenes xylosoxidans | 6 |
| Burkholderia cepacia | 3 |
| Comamonas acidovorans | 2 |
| Chryseobacterium indologenes | 6 |
| Chryseobacterium menigosepticum | 7 |
| Pseudomonas aeruginosa | 61 |
| Pseudomonas fluorescence | 6 |
| Pseudomonas stutzeri | 2 |
| Sphingomonas paucimobilis | 2 |
| Stenotrophomonas maltophilia | 59 |
| Othera | 5 |
Includes one strain of each of the following species: Acinetobacter sp., Flavobacterium odoratum, Flavobacterium sp., Brevundimonas vesicularis, and Agrobacterium tumefaciens.
Selection of electrical signal and medium.
The measurement of electrical change caused by bacterial metabolism in a culture medium was conducted with a Bactometer M-128 (bioMérieux Vitek, Hazelwood, Mo.). Signals of capacitance, total impedance (the term impedance will be used hereafter), and conductance were available in the instrument. Total impedance is a function of both conductance and capacitance. To determine which signal was better for differentiating fermenters from nonfermenters, a panel of four strains (E. coli CCRC 15481, K. pneumoniae CCRC 11546, P. aeruginosa CCRC 10944, and A. baumannii CCRC 15885) was tested. Each module well (bioMérieux Vitek) containing 1 ml of MHB was inoculated with 5 μl of a bacterial suspension having a turbidity of a 0.5 McFarland standard to reach a final inoculum of about 106 CFU/ml. The inoculated modules were incubated at 35°C, and the changes of impedance, conductance, and capacitance in the module wells were continuously monitored by the Bactometer at 6-min intervals for 24 h. The bacterial growth curves were graphically displayed as percent changes of the three electrical signals versus incubation time. In addition to MHB, three other media (TSB, BHI, and OF glucose) were used to test E. coli CCRC 11509 and P. aeruginosa 516 (a clinical isolate) to compare the abilities of different media to distinguish fermenters from nonfermenters.
The amount of time required to cause a series of significant deviations from baseline electrical values was defined as the detection time (DT) (23) and was automatically determined by the Bactometer. The percent change (relative to the initial value) of each electrical signal was defined as the change of that signal at the time interval from DT to DT plus 1 h (DT+1 h). This amount of change reflected the slope of the change at the initial phase of exponential growth of bacteria.
Direct distinguishing of fermenters from nonfermenters in positive blood cultures.
Blood specimens were collected at the National Cheng Kung University Hospital. The BACTEC Aerobic and Aerobic Plus bottles (Becton Dickinson Microbiology Systems, Sparks, Md.) were normally inoculated with 3 to 10 ml of blood from the patients, inserted into BACTEC NR-9240 instruments (Becton Dickinson Microbiology Systems), and incubated at 37°C. Positive bottles showing growth of gram-negative bacteria, as revealed by Gram staining were used for direct inoculation into the Bactometer. Smears showing mixed cultures of gram-negative and gram-positive bacteria or showing two morphologically quite different gram-negative bacteria were not used for study. Ten microliters of the positive culture broths was inoculated into each module well containing 1 ml of MHB, and the impedance change in each well was monitored. A total of 466 positive blood cultures containing gram-negative bacteria were analyzed. All blood isolates obtained on subculture plates were identified by conventional microbiological procedures.
Statistical analysis.
For each species of the pure cultures (Table 1), multiple strains were analyzed and a mean value for the percent change of impedance at the interval from DT to DT+1 h was obtained. Based on these values, discriminant analysis (15) was performed to obtain a linear discriminant function from which a breakpoint was derived to separate strains of fermenters from nonfermenters.
RESULTS
Comparison of electrical signals.
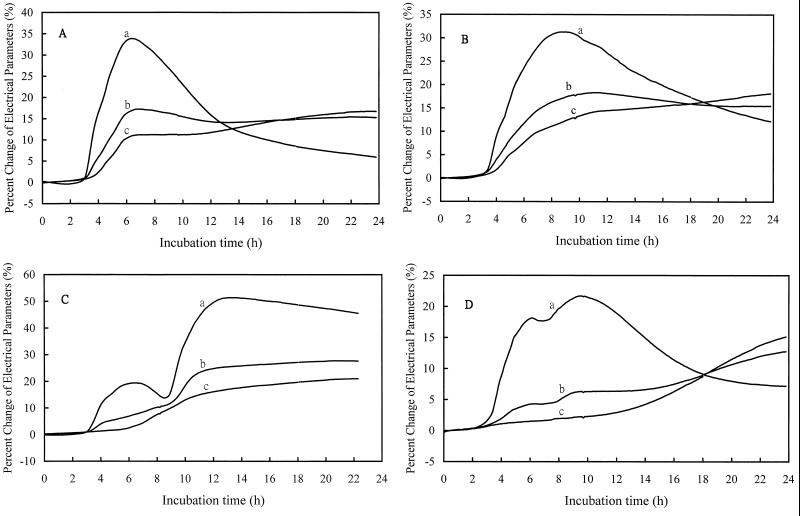
The growth curves of E. coli CCRC 15481, K. pneumoniae CCRC 11546, P. aeruginosa CCRC 10944, and A. baumannii CCRC 15885 in MHB are shown in Fig. 1A, B, C, and D, respectively. These curves were obtained by monitoring the changes of capacitance (curves a), impedance (curves b), and conductance (curves c). The capacitance change in the culture broth at the initial phase of exponential growth was most prominent, followed by the changes in impedance and conductance. Table 2 summarizes the DTs and the percent change of each signal. By using the signal of capacitance, the DTs ranged from 2.6 h (E. coli CCRC 15481) to 3.2 h (P. aeruginosa CCRC 10944) at an inoculum concentration of approximately 106 CFU/ml. The DTs obtained by monitoring the signal of impedance were slightly longer than those obtained by monitoring capacitance.
FIG. 1.
Growth curves of E. coli CCRC 15481 (A), K. pneumoniae CCRC 11546 (B), P. aeruginosa CCRC 10944 (C), and A. baumannii CCRC 15885 (D). Bacterial growth was monitored by the signals of capacitance (curves a), impedance (curves b), and conductance (curves c), respectively.
TABLE 2.
DTs and percent changes in electrical signals in the interval from DT to DT+1 h for E. coli, K. pneumoniae, P. aeruginosa, and A. baumannii
| Microorganism | Capacitance
|
Impedance
|
Conductance
|
|||
|---|---|---|---|---|---|---|
| DT | % Change | DT | % Change | DT | % Change | |
| E. coli CCRC 15481 | 2.6 | 8.6 | 2.9 | 4.5 | 3.5 | 2.7 |
| K. pneumoniae CCRC 11546 | 3.0 | 7.2 | 3.2 | 3.4 | 3.7 | 2.7 |
| P. aeruginosa CCRC 10944 | 3.2 | 9.2 | 3.4 | 2.6 | 6.2 | 1.1 |
| A. baumannii CCRC 15885 | 2.8 | 5.1 | 3.5 | 1.8 | 12.2 | 0.5 |
Drastic changes of capacitance were observed for E. coli (8.6%), K. pneumoniae (7.2%), and P. aeruginosa (9.2%) at the interval from DT to DT+1 h, although the change (5.1%) produced by A. baumannii was relatively small (Table 2). It seemed that the measurement of capacitance was unable to differentiate fermenters from nonfermenters at the initial phase of exponential growth. The signals of impedance and conductance were discriminative (Table 2 and Fig. 1, curves b and curves c); fermenters produced relatively greater changes of impedance and conductance than those produced by nonfermenters. However, the DTs of P. aeruginosa CCRC 10944 (6.2 h) and A. baumannii CCRC 15885 (12.2 h) obtained by the measurement of conductance were much longer than those obtained by measurement of the other two signals (Table 2). The changes of conductance were very small at the initial phase of exponential growth of both nonfermenters (Fig. 1C and D, curves c). Therefore, the signal of impedance was a better choice and was used in the following experiments.
Comparison of media.
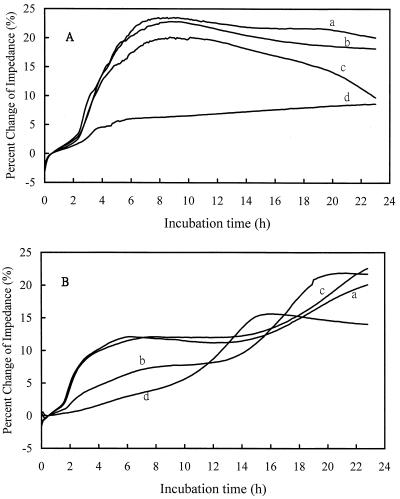
Four media (MHB, TSB, BHI, and OF glucose) were used to test E. coli CCRC 11509 and P. aeruginosa 516 to compare the effect of the medium on the differentiation of fermenters from nonfermenters (Fig. 2). Both E. coli CCRC 11509 (Fig. 2A) and P. aeruginosa 516 (Fig. 2B) caused drastic changes in impedance at the initial phase of exponential growth when TSB (curves a) and BHI (curves c) were used. However, the changes in impedance were very small when both organisms were grown in OF glucose (Fig. 2, curves d). It seemed that MHB (Fig. 2, curves b) was most discriminative for fermenters and nonfermenters. From the above results, MHB and the signal of impedance were used in the following experiments.
FIG. 2.
Impedance growth curves of E. coli CCRC 11509 (A) and P. aeruginosa 516 (a clinical isolate) (B) in TSB (curves a), MHB (curves b), BHI (curves c), and OF glucose medium (curves d).
Effect of inoculum concentration on DT.
The DT of E. coli CCRC 11509 at an inoculum concentration of 107 CFU/ml was only 1 h, and it was 8.5 h when the inoculum density was 101 CFU/ml. With a decrease of inoculum concentration of 1 order of magnitude, the DT increased by 1 to 1.2 h (data not shown). The DT was inversely proportional to the bacterial concentration at the time of inoculation. The patterns of impedance growth curves were not affected by the inoculum concentrations.
Testing of pure cultures.
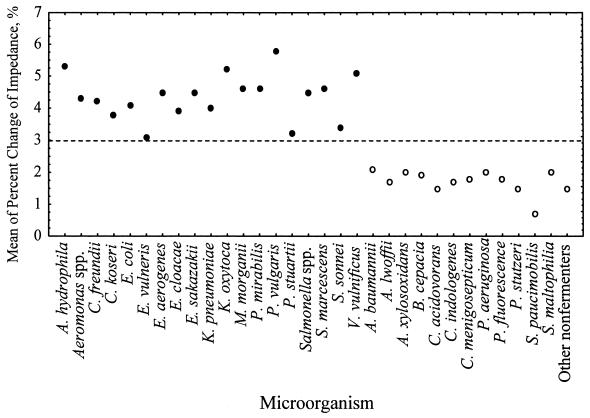
A total of 857 strains, including 586 strains of fermenters and 271 strains of nonfermenters (Table 1), were analyzed. For multiple strains of each species tested, a mean value of the percent change of impedance at the interval from DT to DT+1 h was obtained. Discriminant analysis (15) was used to analyze these values, and a linear discriminant function (7.44χ > 22.18%) was obtained. A breakpoint (χ = 2.98%) was derived from this function. The breakpoint divided all strains into two groups: fermenters and nonfermenters (Fig. 3). If a strain produced an impedance change that was greater than 2.98%, then it was classified as a fermenter; otherwise, it was classified as a nonfermenter.
FIG. 3.
Discriminant analysis of pure cultures of fermenters and nonfermenters. Each closed circle (fermenters) or open circle (nonfermenters) represents the mean of the percent change of impedance in the interval from DT to DT+1 h. A breakpoint (2.98%) (dashed line) was obtained by discriminant analysis. The breakpoint divided strains into two groups: the fermenters and nonfermenters.
Among the 586 strains of fermenters, 581 strains (99.1%) produced values of impedance change of greater than 2.98%. The five misclassified strains were E. coli (two strains), K. pneumoniae (one strain), Serratia marcescens (one strain), and Shigella sonnei (one strain). Among the 271 strains of nonfermenters, 268 (98.9%) produced values of impedance change of less than 2.98%. The three misclassified strains were A. baumannii (one strain), P. aeruginosa (one strain), and Stenotrophomonas maltophilia (one strain). The overall misclassification rate for pure cultures was 0.93% (8 of 857). The eight misclassified strains were subcultured and retested by the impedance method; however, similar results were obtained. In addition, no abnormal biochemical reactions were found for these strains.
At an inoculum concentration of 106 CFU/ml, the average DT of fermenters was 2.1 h (range, 0.6 to 2.7 h), whereas the average DT of nonfermenters was 3.8 h (range, 1.9 to 6.2 h). Generally speaking, the DTs of nonfermenters were longer than those of fermenters.
Direct differentiation of fermenters from nonfermenters in positive blood cultures.
Ten microliters of positive blood culture broth showing growth of gram-negative bacilli was directly inoculated into the wells of Bactometer. The results are shown in Table 3. Among the 466 positive blood bottles containing gram-negative bacteria, there were 345 (74.1%) and 100 (21.5%) bottles containing single strains of fermenters and nonfermenters, respectively. The remaining 18 bottles (3.9%) were mixed cultures, and 3 bottles (0.6%) were Bacteroides spp. Among the 345 strains of fermenters, 340 strains (98.6%) produced impedance changes greater than the breakpoint (2.98%) at the interval from DT to DT+1 h (Table 3). Of the 100 strains of nonfermenters, 98 strains (98%) produced impedance changes smaller than the breakpoint. The overall misclassification rate for blood cultures was 1.6% (7 of 445). The dominating species of nonfermenters isolated from blood cultures were P. aeruginosa, A. baumannii, and S. maltophilia (Table 3).
TABLE 3.
Direct differentiation of fermenters from nonfermenters in positive blood cultures by measurement of impedance change in MHB
| Microorganism | No. of strains tested | Impedance change (%)a, mean ± SD | No. (%) of strains with impedance change > breakpoint |
|---|---|---|---|
| Fermenters | |||
| Aeromonas hydrophila | 11 | 5.7 ± 1.2 | 11 (100) |
| Aeromonas spp. | 7 | 4.9 ± 1.7 | 7 (100) |
| Citrobacter freundii | 7 | 4.4 ± 0.5 | 7 (100) |
| Citrobacter koseri | 2 | 5.2 ± 0.2 | 2 (100) |
| Citrobacter spp. | 7 | 4.4 ± 0.5 | 7 (100) |
| Escherichia coli | 156 | 4.7 ± 0.8 | 154 (98.7) |
| Enterobacter cloacae | 22 | 4.0 ± 0.7 | 21 (95.5) |
| Enterobacter spp. | 4 | 4.7 ± 1.2 | 4 (100) |
| Klebsiella pneumoniae | 85 | 4.3 ± 1.0 | 84 (98.8) |
| Morganella morganii | 6 | 4.3 ± 1.1 | 6 (100) |
| Pasteurella multocida | 1 | 3.2 | 1 (100) |
| Proteus mirabilis | 10 | 4.8 ± 0.8 | 10 (100) |
| Salmonella spp. | 16 | 4.7 ± 1.2 | 15 (93.8) |
| Serratia marcescens | 3 | 4.1 ± 1.0 | 3 (100) |
| Vibrio vulnificus | 6 | 5.0 ± 1.2 | 6 (100) |
| Vibrio cholerae, non-O1 | 3 | 5.0 ± 1.4 | 3 (100) |
| Nonfermenters | |||
| Acinetobacter baumannii | 17 | 2.0 ± 0.6 | 1 (5.9) |
| Acinetobacter lwoffii | 8 | 1.7 ± 0.8 | 0 (0) |
| Acinetobacter calcoaceticus | 8 | 2.5 ± 0.4 | 0 (0) |
| Acinetobacter spp. | 3 | 1.9 ± 0.6 | 0 (0) |
| Burkholderia pickettii | 5 | 2.0 ± 0.4 | 0 (0) |
| Flavobacterium spp. | 5 | 1.8 ± 0.7 | 0 (0) |
| Pseudomonas aeruginosa | 37 | 1.9 ± 0.5 | 1 (2.7) |
| Pseudomonas spp. | 8 | 1.6 ± 0.6 | 0 (0) |
| Stenotrophomonas maltophilia | 9 | 1.9 ± 0.7 | 0 (0) |
Percent change of impedance in the interval from DT to DT+1 h.
The average DT of fermenters was 1.3 h after direct inoculation, whereas the average DT of nonfermenters was 2.1 h. This indicated that the cell densities were higher than 106 CFU/ml after direct inoculation of positive culture broth into MHB. Since an additional 1 h was required to read the impedance change at DT+1 h, approximately 3 h was required to distinguish fermenters from nonfermenters in positive blood cultures. From the results of the 18 mixed cultures, it was found that a mixed culture containing a fermenter and a nonfermenter tended to mimic a fermenter, and a mixed culture encompassing two or more fermenters normally displayed an impedance growth curve like that of a fermenter. A small percentage (0.3%) of the blood isolates were Bacteroids spp. that were unable to grow in MHB in 24 h.
DISCUSSION
In this study, a completely new and rapid method based on the measurement of electrical properties in the medium was proposed to distinguish fermenters from nonfermenters in positive blood cultures (Table 3). The average time needed for this purpose was about 3 h after direct inoculation. Obviously, the method was also effective for testing pure cultures (Fig. 3). The impedance growth curves were quite similar for each bacterial strain, irrespective of the source of the inocula (from a pure culture or from a positive blood culture bottle). This indicated that the presence of blood cells did not interfere with the measurement of impedance.
As shown in Fig. 1, the changes in conductance also could be used to differentiate fermenters from nonfermenters. Changes in conductance are primarily due to the ionic metabolites produced by the growing bacteria (24). The relatively small changes of conductance produced by nonfermenters (Fig. 1C and D, curves c) revealed that very small amounts of charged molecules were produced by these bacteria. The low production of charged metabolites resulted in longer DTs, and this rendered the signal of conductance not suitable for differentiating fermenters from nonfermenters.
Among the three signals tested, the changes in capacitance were most prominent (Fig. 1). Changes in capacitance were primarily due to increases in the amount of charge stored at the electrode-medium interface and/or changes associated with passivation of the surface layer (due to pH) (22, 23). The amount of charge stored at the electrode-medium interface was negligible due to the high ionic strengths of the media used in this study (22, 23). Therefore, changes in capacitance were mostly due to changes in the oxide layer at the surfaces of the metal electrodes. Since the capacitance signal did not discriminate between fermenters and nonfermenters, it was concluded that changes in the oxide layer were not significant enough to be detected among these two categories of bacteria. Total impedance is a function of both conductance and capacitance. In this study, the impedance signal was found to be most appropriate for distinguishing fermenters from nonfermenters, based on the criteria of the magnitude of signal change and the discriminative ability (Fig. 1).
The Bactometer is not standard equipment in clinical microbiology laboratories. We think a simpler device that could measure impedance changes in culture media would be enough for differentiating fermenters from nonfermenters in blood cultures. There are two purposes for this procedure: for microbiologists to select a correct set of biochemical tests for species identification and for physicians to narrow the selection of antibiotics.
The fact that a microorganism can grow in an aerobic environment does not necessarily mean that oxygen is metabolically used. We found that the impedance growth curves of some fermenters (e.g., E. coli and K. pneumoniae) grown under aerobic or anaerobic conditions were quite similar (data not shown). This means that these organisms use the Embden-Meyerhof-Parnas pathway for carbohydrate degradation even under atmospheric oxygen (18). However, nonfermenters either are nonsaccharolytic or utilize the Entner-Doudoroff pathway to produce pyruvate, which is then oxidized via the Krebs cycle to form water. The acids that are formed in the Entner-Doudoroff pathway (glucuronic acid and its derivatives) and those produced in the Krebs cycle (citric acid and its derivatives) are extremely weak compared with the acids (lactic acid or so-called mixed acids) produced in the Embden-Meyerhof-Parnas pathway (18). This fact might explain the difference in impedance (and conductance) change caused by fermenters and nonfermenters at the initial phase of exponential growth.
The present method was based on the measurement of impedance changes caused by microorganisms cultivated in MHB. Fermenters produced a relatively high change (>2.98%) of impedance in the interval from DT to DT+1 h, whereas the impedance change was relatively small (<2.98%) for nonfermenters in the same time period. The measurement was reproducible and was not influenced by the inoculum concentration, which affects only DT. For pure cultures tested at an inoculum of 106 CFU/ml, the average DTs of fermenters and nonfermenters were 2.1 and 3.8 h, respectively (data not shown). In addition to the bacteria listed in Table 1, two strains of Haemophilus influenzae were further tested. However, both strains were unable to grow in MHB that did not contain the growth factors (hemin and NAD) for H. influenzae. It seems that fastidious microorganisms are not suitable for testing by the present method.
In a recent survey conducted on 2,124 patients with gram-negative bacteremia, Leibovici et al. (20) found that 670 (31.5%) were given inappropriate empiric antibiotic treatment, and the mortality rate of this group of patients was 31.5%. However, the mortality rate was only 18% for the remaining 1,454 patients who were given appropriate empiric antibiotic treatment (P = 0.0001). Other studies also showed that inappropriate antimicrobial treatment was an independent factor in poor outcomes of bacteremia (3, 9, 11, 12, 26, 29). Other factors that may be associated with a poor prognosis of bacteremia are pneumonia, underlying disease, the source of bacteremia, and malignancy (3, 30). Among these factors, only antibiotic treatment is amenable to medical intervention, and rapid diagnoses may have clinical impact (7, 8).
The most common nonfermenters (P. aeruginosa, A. baumannii, and S. maltophilia) isolated from bacteremia are usually multiresistant bacteria (5, 10, 13). P. aeruginosa bacteremia represented about 5.7% of the total number of bacteremias (29) and may be as many as 25% of nosocomial gram-negative bacteremias (3, 29). It is generally recognized that narrow- and expanded-spectrum cephalosporins are minimally active against nonfermenters (2, 4). With rare exceptions, the nonfermenters are resistant to benzylpenicillin, oxacillin, lincomycin, ampicillin, and cephaloridine. However, S. maltophilia is inherently susceptible to trimethoprim-sulfamethoxazole (16, 18). Therefore, earlier information on the grouping of a gram-negative bacteremia may help physicians narrow the selection of antibiotics for empiric treatment of bacteremia.
In view of the high rates of isolation of nonfastidious aerobic gram-negative bacilli from bacteremic episodes, a rapid method to differentiate fermenters from nonfermenters in positive blood cultures may have clinical importance. The signal detection in the Bactometer is a continuous and real-time process, with results being available within a few hours after direct incubation.
ACKNOWLEDGMENTS
This project was supported by a grant (NSC 89-232-B006-049) from the National Science Council, Taiwan, Republic of China.
We thank W. C. Ko for critical reading of the manuscript and P. Y. Wu for technical assistance.
REFERENCES
- 1.Appelbaum P C, Spangler S K, Tamarree T. Susceptibility of 310 nonfermentative gram-negative bacteria to azthreonam, carmonam, ciprofloxacin, ofloxacin and fleroxacin. Chemotherapy. 1988;34:40–45. doi: 10.1159/000238546. [DOI] [PubMed] [Google Scholar]
- 2.Appelbaum P C, Tamin J, Pankuch G A, Aber R C. Susceptibility of 324 nonfermentative gram-negative rods to 6 cephalosporins and azthreonam. Chemotherapy. 1983;29:337–344. doi: 10.1159/000238217. [DOI] [PubMed] [Google Scholar]
- 3.Bisbe J, Gatell J M, Puig J, Mallolas J, Martinez J A, Jimenez de Anta M T, Soriano E. Pseudomonas aeruginosa bacteremia: univariate and multivariate analyses of factors influencing the prognosis in 133 episodes. Rev Infect Dis. 1988;10:629–635. doi: 10.1093/clinids/10.3.629. [DOI] [PubMed] [Google Scholar]
- 4.Chang S C, Chen Y C, Luh K T, Hsieh W C. In vitro activities of antimicrobial agents, alone and in combination, against Acinetobacter baumannii isolated from blood. Diagn Microbiol Infect Dis. 1995;23:105–110. doi: 10.1016/0732-8893(95)00170-0. [DOI] [PubMed] [Google Scholar]
- 5.Cisneros J M, Reyes M J, Pachon J, Becerril B, Caballero F J, Garcia-Garmendia J L, Ortiz C, Cobacho A R. Bacteremia due to Acinetobacter baumannii: epidemiology, clinical findings, and prognostic features. Clin Infect Dis. 1996;22:1026–1032. doi: 10.1093/clinids/22.6.1026. [DOI] [PubMed] [Google Scholar]
- 6.Daschner F, Langmaack H, Grehn M, Steffens A, Just M. Combination effect of piperacillin with four aminoglycosides on nonfermenting gram-negative bacteria. Chemotherapy. 1981;27:39–43. doi: 10.1159/000237953. [DOI] [PubMed] [Google Scholar]
- 7.Doern G V, Scott D R, Rashad A L. Clinical impact of rapid antimicrobial susceptibility testing of blood culture isolates. Antimicrob Agents Chemother. 1982;21:1023–1024. doi: 10.1128/aac.21.6.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doern G V, Vautour R, Gaudet M, Levy B. Clinical impact of rapid in vitro susceptibility testing and bacterial identification. J Clin Microbiol. 1994;32:1757–1762. doi: 10.1128/jcm.32.7.1757-1762.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edgeworth J D, Treacher D F, Eykyn S J. A 25-year study of nosocomial bacteremia in an adult intensive care unit. Crit Care Med. 1999;27:1421–1428. doi: 10.1097/00003246-199908000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Farmer J J., III . Enterobacteriaceae: introduction and identification. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 7th ed. Washington, D.C.: American Society for Microbiology; 1999. pp. 442–458. [Google Scholar]
- 11.Gatell J M, Trilla A, Latorre X, Almela M, Mensa J, Moreno A, Miro J M, Martinez J A, Jimenez de Anta M T, Soriano E. Nosocomial bacteremia in a large Spanish teaching hospital: analysis of factors influencing prognosis. Rev Infect Dis. 1988;10:203–210. doi: 10.1093/clinids/10.1.203. [DOI] [PubMed] [Google Scholar]
- 12.Gomez J, Simarro E, Banos V, Requena L, Ruiz J, Garcia F, Canteras M, Valdes M. Six-year prospective study of risk and prognostic factors in patients with nosocomial sepsis caused by Acinetobacter baumannii. Eur J Clin Microbiol Infect Dis. 1999;18:358–361. doi: 10.1007/pl00015019. [DOI] [PubMed] [Google Scholar]
- 13.Hancock R E. Resistance mechanisms in Pseudomonas aeruginosa and other nonfermentative gram-negative bacteria. Clin Infect Dis. 1998;27(Suppl. 1):S93–S99. doi: 10.1086/514909. [DOI] [PubMed] [Google Scholar]
- 14.Jang T N, Kuo B I, Shen S H, Fung C P, Lee S H, Yang T L, Huang C S. Nosocomial gram-negative bacteremia in critically ill patients: epidemiologic characteristics and prognostic factors in 147 episodes. J Formos Med Assoc. 1999;98:465–473. [PubMed] [Google Scholar]
- 15.Johnson R A, Wichern D W. Applied multivariate statistical analysis. Upper Saddle River, N.J: Prentice-Hall International, Inc.; 1998. pp. 629–725. [Google Scholar]
- 16.Jones R N, Pfaller M A, Marshall S A, Hollis R J, Wilke W W. Antimicrobial activity of 12 broad-spectrum agents against 270 nosocomial blood stream infection isolates caused by non-enteric gram-negative bacilli: occurrence of resistance, molecular epidemiology, and screening of metallo-enzymes. Diagn Microbiol Infect Dis. 1997;29:187–192. doi: 10.1016/s0732-8893(97)81808-1. [DOI] [PubMed] [Google Scholar]
- 17.Just H M, Beckert A, Bassler M, Daschner F D. Combination effect of cefriazone with four aminoglycosides on nonfermenting gram-negative bacteria. Chemotherapy. 1982;28:397–401. doi: 10.1159/000238128. [DOI] [PubMed] [Google Scholar]
- 18.Koneman E W, Allen S D, Janda W M, Schreckenberger P C, Winn W C., Jr . Color atlas and textbook of diagnostic microbiology. 5th ed. New York, N.Y: Lippincott; 1997. pp. 253–320. [Google Scholar]
- 19.Lai S W, Ng K C, Yu W L, Liu C S, Lai M M, Lin C C. Acinetobacter baumannii bloodstream infection: clinical features and antimicrobial susceptibilities of isolates. Kaoshiung J Med Sci. 1999;17:406–413. [PubMed] [Google Scholar]
- 20.Leibovici L, Paul M, Poznanski O, Drucker M, Samra Z, Konigsberger H, Pitlik S D. Monotherapy versus beta-lactam–aminoglycoside combination treatment for gram-negative bacteremia: a prospective, observational study. Antimicrob Agents Chemother. 1997;41:1127–1133. doi: 10.1128/aac.41.5.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mezer E, Gelfand Y A, Lotan R, Tamir A, Miller B. Bacteriological profile of ophthalmic infections in an Israeli hospital. Eur J Ophthalmol. 1999;9:120–124. doi: 10.1177/112067219900900208. [DOI] [PubMed] [Google Scholar]
- 22.Noble P A. Hypothetical model for monitoring microbial growth by using capacitance measurements—a minireview. J Microbiol Methods. 1999;37:45–49. doi: 10.1016/s0167-7012(99)00041-x. [DOI] [PubMed] [Google Scholar]
- 23.Noble P A, Dziuba M, Harrison D J, Albritton W L. Factors influencing capacitance-based monitoring of microbial growth. J Microbiol Methods. 1999;37:51–64. doi: 10.1016/s0167-7012(99)00040-8. [DOI] [PubMed] [Google Scholar]
- 24.Owen J D. Formulation of culture media for conductimetric assays: theoretical considerations. J Gen Microbiol. 1985;131:3055–3076. [Google Scholar]
- 25.Seifert H, Strate A, Pulverer G. Nosocomial bacteremia due to Acinetobacter baumannii. Medicine. 1995;74:340–349. doi: 10.1097/00005792-199511000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Siau H, Yuen K Y, Ho P L, Wong S S, Woo P C. Acinetobacter bacteremia in Hong Kong: prospective study and review. Clin Infect Dis. 1999;28:26–30. doi: 10.1086/515068. [DOI] [PubMed] [Google Scholar]
- 27.Spanik S, Trupl J, Ilavska I, Helpianska L, Drgona L, Demitrovicova A, Kukuckova E, Studena M, Pichna P, Oravcova E, Rusnakova V, Koren P, Lacka J, Krcmery V., Jr Bacteria and fungemia occurring during antimicrobial prophylaxis with ofloxacin in cancer patients: risk factors, etiology and outcome. J Chemother. 1996;8:387–393. doi: 10.1179/joc.1996.8.5.387. [DOI] [PubMed] [Google Scholar]
- 28.Trupl J, Kunova A, Oravcova E, Pichna P, Kukuckova E, Grausova S, Grey E, Spanik S, Demitrovicova A, Kralovicova K, Lacka J, Krupova I, Svec J, Koren P, Krcmery V., Jr Resistance pattern of 2816 isolates isolated from 17631 blood cultures and etiology of bacteremia and fungemia in a single cancer institution. Acta Oncol. 1997;36:643–649. doi: 10.3109/02841869709001329. [DOI] [PubMed] [Google Scholar]
- 29.Vidal F, Mensa J, Almela M, Martinez J A, Marco F, Casals C, Gatell J M, Soriano E, Jimenez de Anta M T. Epidemiology and outcome of Pseudomonas aeruginosa bacteremia, with special emphasis on the influence of antibiotic treatment. Analysis of 189 episodes. Arch Intern Med. 1996;156:2121–2126. [PubMed] [Google Scholar]
- 30.Weinstein M P, Towns M L, Quartey S M, Mirrett S, Reimer L G, Parmigiani G, Reller L B. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis. 1997;24:584–602. doi: 10.1093/clind/24.4.584. [DOI] [PubMed] [Google Scholar]
- 31.Wu J J, Huang A H, Dai J H, Chang T C. Rapid detection of oxacillin-resistant Staphylococcus aureus in blood cultures by an impedance method. J Clin Microbiol. 1997;35:1460–1464. doi: 10.1128/jcm.35.6.1460-1464.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]