Abstract
The coronavirus 19 (COVID-19) pandemic has affected hundreds of millions of people worldwide: in most of cases children and young people developed asymptomatic or pauci-symptomatic clinical pictures. However authors have showed that there are some categories of childhood more vulnerable to COVID-19 infection such as newborns or children with comorbidities. We report for the first time to the best of our knowledge about microvascular dysfunction in three pediatric clinical cases who developed COVID-19 infections with need of pediatric critical care. We found that sublingual microcirculation is altered in children with severe COVID-19 infection.
Our findings confirmed most of data already observed by other authors in adult population affected by severe COVID-19 infection, but with distinct characteristics than microcirculation alterations previous observed in a clinical case of MIS-C.
However we cannot establish direct correlation between microcirculation analysis and clinical or laboratory parameters in our series, by our experience we have found that sublingual microcirculation analysis allow clinicians to report directly about microcirculation dysfunction in COVID-19 patients and it could be a valuable bedside technique to monitor thrombosis complication in this population.
Keywords: COVID-19, Sublingual microcirculation analysis, Children, Newborns, Pediatric critical care, Coagulation
1. Introduction
The coronavirus 19 (COVID-19) pandemic has affected hundreds of millions of people worldwide so far and caused over 3 million deaths (Ludvigsson, 2020). The existing evidence suggests that children generally have a milder disease course and better prognosis than adults, and deaths are extremely rare among the pediatric population (Ludvigsson, 2020). Even if elevated inflammatory markers are less common in children with mild COVID-19 disease, those pediatric patients who develop severe COVID-19 show not only a great increase in pro-inflammatory cytokines but also markers of endothelial dysfunction (Diorio et al., 2020a). COVID-19-related endothelitiis has been recently described in several organs by some authors (Varga et al., 2020), suggesting an impaired microvascular function in different vascular beds as a consequence of viral involvement and host inflammatory response (Varga et al., 2020). Furthermore sublingual microcirculation alterations have been described in a young girl affected by multisystem inflammatory syndrome COVID-19 correlated (MIS-C): the sublingual microcirculation analysis (SMA) evidenced low values of Mean Flow Index (MFI), high heterogeneity index (HI) (Bottari et al., 2021).
SMA has allowed the researchers to report directly about microcirculation dysfunction in COVID-19 patients (Favaron et al., 2021; Kanoore Edul et al., 2021; Damiani et al., 2020; Carsetti et al., 2020) showing some correlations between microvascular variables and clinical or laboratory parameters in this population.
We present the first clinical report about sublingual microcirculation analysis in three children with severe COVID-19 infection who required admission to the pediatric Intensive Care Unit (PICU). Five videos per patients were recorded within a week from PICU admission with a single time-point assessment using handheld vital microscope based on incident dark field microscopy imaging (Braedius Medical, Huizen, The Netherlands). Three videos of the best quality were selected (Massey et al., 2013). Videos were analysed offline with dedicated software (Analysis Manager V2) (Braedius Medical, Huizen, The Netherlands) (Carsetti et al., 2017) by two independent operators (GB and VC) (Ince et al., 2018; De Backer et al., 2007). The following parameters were calculated: De Backer score (De Backer et al., 2007), total small vessel density (TVD); proportion of perfused vessels (PPV); perfused microvascular density (PVD). Semi-quantitative analysis of the microcirculatory flow was performed as previously described by Boerma et al. (2005). Each image was divided into four equal quadrants and for each one a quantification of flow was scored (no flow: 0; intermittent flow: 1; sluggish flow: 2; continuous flow: 3). MFI was determined by eye on the basis of the predominant type of flow in each quadrant and averaged over the values obtained in each one. We also calculated the heterogeneity index (HI), following the method of Trzeciak et al. (Trzeciak et al., 2007 based on MFI and PPV values.
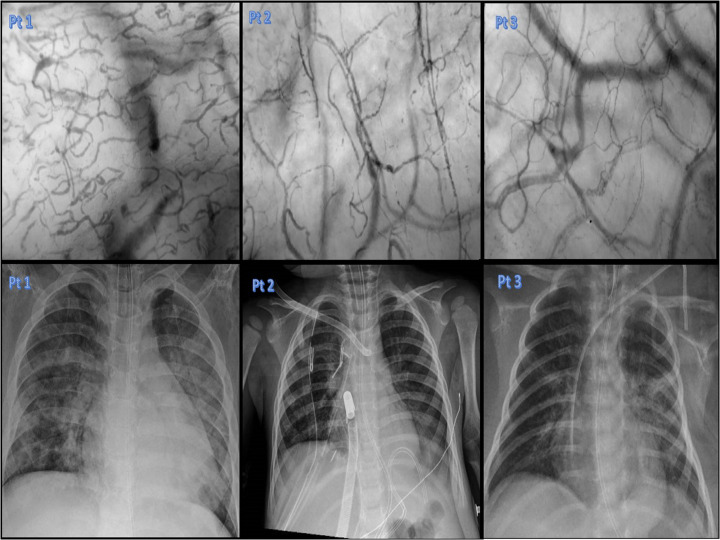
Patient number 1 (Pt1), a 14 year-old boy, with a relapsed bone lymphoblastic B lymphoma. He showed already in the previous weeks a blood cytopenia because of chemiotherapy treatments. He was admitted to the PICU due to a COVID-19 infection inducing hypoxic respiratory failure with a clinical picture of acute respiratory distress syndrome (ARDS) and need for oro-tracheal intubation and invasive mechanical ventilation. SARS-CoV2 was confirmed before and after PICU-admission by means of real-time polymerase chain reaction (RT-PCR) on nasopharyngeal swab and bronco-alveolar lavage. At day 1 blood chemistry evidenced White Blood Cells (WBC) 0.22 103/uL, C-Reactive Protein (CRP) 10.52 mg/dL, Ferritin 4879 ng/mL, Procalcitonin (PCT) 1.05 ng/mL, D-dimers 2.65 μg/mL. He was treated with a protective ventilation strategy, prono-supine position, dexamethasone (0.15 mg/kg/ day) and low molecular weight heparin (100 U/kg bpd). In Table 1 we show the microvascular parameters, as well as ventilatory and hemodynamic variables and the main biomarkers of inflammation measured at the same time of sublingual microcirculation analysis (day 7). As showed in Table 1 he developed a severe ARDS (PEEP > 5 PaO2/FiO2 < 100, Oxygen Index 23.9) with bilateral opacities at the chest X Ray. Fig. 1 reports sublingual microcirculation and chest X-ray performed at the same time point. Microbiological work-up did not evidence other positive microbiological agents responsible for concomitant infections except for polymerase chain reaction test (blood sample) positive for Candida krusei (day 3), which was not confirmed by standard blood culture and become negative by day 7. During the PICU-stay the patient was generally hemodynamically stable and required only low dose of Noradrenaline (0.03–0.06 μg/kg/min). The patient developed a massive bilateral pneumothorax at day 7 with progressive refractory hypoxemia in the following days and despite the medical and pharmacological therapies he did not improve and died at day 14.
Table 1.
Patients' characteristics, respiratory, hemodynamic, laboratory and microcirculatory parameters. MAP = Mean arterial pressure; WBC=White Blood Cells; RBC = Red Blood Cells; CRP=C-Reactive Protein; PT = prothrombin time ratio, LDH = lactose dehydrogenase; TVD = total small vessel density; PVD = Perfused Vessels Density; PPV = Proportion of Perfused Vessels; HI = heterogeneity index.
| Patient's characteristics | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Age | 14 ys | 1 years 6 month | 15 days |
| Weight | 47 | 9,5 | 3,4 |
| Comorbidity | Lymphoma | Cystic fibrosis | |
| Ventilation setting | |||
| PIP | 32 | 20 | 18 |
| Peep | 15 | 7 | 6 |
| PaO2/FiO2 | 96 | 220 | 250 |
| FiO2 (%) | 80 | 40 | 35 |
| ECMO VA setting | |||
| LPM (l/min) | 0.58 | ||
| RPM | 3250 | ||
| FiO2 (%) | 75% | ||
| Hemodynamic variables | |||
| MAP (mmHg) | 70 | 85 | 65 |
| Noradrenaline (μg/kg/min) | 0.03 | 0.04 | – |
| Lactate (mmol/L) | 1.5 | 0.6 | 1.3 |
| Laboratory parameters | |||
| WBC (103/μL) | 0.26 | 25.24 | 5.56 |
| RBC (106/μL) | 3.54 | 3.61 | 4.08 |
| Platelets (103/μL) | 26.000 | 139.000 | 183.000 |
| Hemoglobin (gr/dL) | |||
| Ferritin (ng/mL) | 4879 | 501 | 531 |
| CRP (mg/dL) | 10.5 | 1.55 | 0.36 |
| D-dimer (μg/mL) | 2.65 | 1.46 | 2.45 |
| PT-ratio (%) | 1.34 | 1.89 | 1.09 |
| LDH (U/L) | 239 | 339 | 285 |
| Microcirculation parameters | |||
| TVD (mm/mm2) | 18.5 | 16.36 | 12.7 |
| PVD (mm/mm2) | 9.31 | 14.9 | 12.4 |
| PPV (%) | 79.3 | 89.7 | 98.7 |
| MFI (AU) | 1.3 | 2.6 | 2.6 |
| HI | 2.25 | 0.28 | 0.18 |
| Outcome | |||
| Survival PICU discharge | No | Yes | Yes |
| Survival at 28 days | No | Yes | Yes |
Fig. 1.
Upper section: Chest x-rays performed during the COVID-19 respiratory infection in our series. Lower section: sublingual microcirculation pictures of our series. The video captions of sublingual microcirculation analysis has been performed the same day of the chest x-rays reported in the upper section.
Patient 2 (pt 2): a 20-month old boy with cystic fibrosis was admitted to the PICU in November 2020 for respiratory distress. At admission, RT-PCR on nasopharyngeal swabs showed positivity to Sars-COV-2 and Rhinovirus. After 24 h the patient developed respiratory failure and refractory hypercapnia (Oxygen Index 28; PaO2/FiO2 68; pCO2 > 70 mmHg) requiring Venous-Arterial (VA) Extra Corporeal Membrane Oxygenation (ECMO). At day 1 blood chemistry evidenced WBC 8.46 103/uL CRP 0.07 mg/dL, Ferritin 97 ng/mL, PCT 0.07 μg/mL, D-dimers 0.59 μg/mL. Thorax chest-X ray evidenced a basal pneumonia associated to a pleural effusion and diffuse thickening of the peri-broncovascular interstitium. He received dexamethasone (0.15 mg/kg/ day) and low molecular weight heparin 100 U/kg/bpd. Table 1 shows microvascular parameters together with ventilatory and hemodynamic variables and inflammatory biomarkers (measured at day 7). Fig. 1 reports the sublingual microcirculation and chest X-ray performed at the same time point. Microbiological workup did not evidence other positive microbiological agents responsible for infection. During the PICU-stay the patient was hemodynamically stable and received only low dose of Noradrenaline 0.03–0.06 μg/kg/min. He was weaned from VA-ECMO at day 10 and was discharged from the PICU at day 16. He survived to hospital discharge.
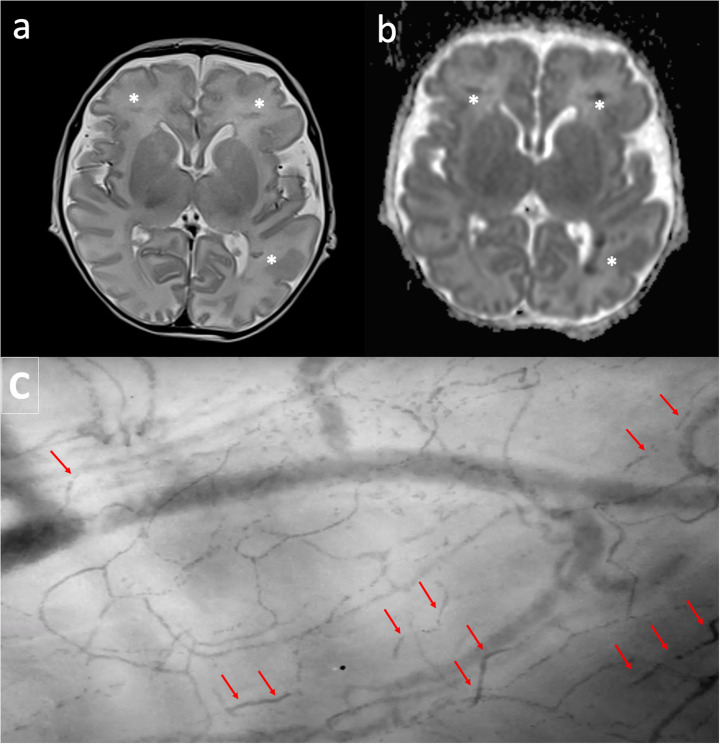
Patient 3 (pt3): a 15 day-old newborn was admitted to the PICU for gastro-intestinal bleeding. He had been admitted to the hospital for fever in the previous week and resulted positive to Sars-Cov2. Need for surgery had been excluded, but 48 h after he showed a progressive respiratory failure with progressive hypercapnia not responsive to non-invasive mechanical ventilation, therefore he required oro-tracheal intubation (PaCO2 > 65 mmHg). At day 1 blood chemistry evidenced WBC 7.84 103/uL, CRP 0.74 mg/dL, D-dimers 1.77 μg/mL. Thorax chest-X ray diffuse thickening of the peri-broncovascular interstitium. He received hyper-immune plasma 15 mL/kg for three days, dexamethasone 0.15 mg/kg/ day, remdesivir 2.5–5 mg /kg/die and low molecular weight heparin 100 U/kg bpd. Table 1 shows microvascular, ventilatory and hemodynamic parameters and the most important biomarkers of inflammation (measured at day 6). Fig. 1 reports the sublingual microcirculation and chest X-ray performed at the same time point. A first attempt of weaning from mechanical ventilation was made on day 7, but it failed for progressive respiratory distress and the baby required re-intubation. We performed MRI for an hypo-reactive baby during the mechanical ventilation weaning trial: MRI evidenced thrombosis of deep medullary veins with ischemic lesions in the related distribution areas. In Fig. 2 we report the MRI images reporting thrombosis of deep medullary veins in association to a second report of the sublingual microcirculation where we have observed obstructed or sluggish flow. On day 18 he was successfully extubated and he was discharged from the PICU on day 22. He survived to hospital discharge.
Fig. 2.
Upper section: MRI shows focal linear hypointensity on T2 weighted transverse image (a), mainly located into the deep white matter on both hemispheres (asterisk). Lesions appears hypointense on ADC map of DWI (b) and correlate with focal deep venous thrombosis with restricted diffusion. Typically cortex is spared. Lower section: sublingual microcirculation picture: red arrows show obstructed blood flow in small vessels. Sublingual microcirculation analysis and MRI has been performed the same day. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2. Discussion
Recently other authors (Favaron et al., 2021; Kanoore Edul et al., 2021; Damiani et al., 2020; Carsetti et al., 2020) described microvascular alterations in adult patients affected by COVID-19. Despite the evidence that children with COVID-19 are often asymptomatic or pauci-symptomatic, endothelial damage associated with hyper-cytokinemia due to COVID-19 was recently described also in the pediatric population (Varga et al., 2020; Diorio et al., 2020b). We report for the first time on sublingual microvascular analysis on three children with severe COVID-19 disease. Microvascular alterations consisted in an increased number of small vessels with stopped, intermittent or sluggish flow. However normal values in pediatric populations are still not validated, we have observed TVD values very similar to what reported by other authors (Favaron et al., 2021): increase in TVD in these patients may be correlated with the hypoxia or with a neoangiogenesis that was described in COVID-19 patients (Kanoore Edul et al., 2021). PVD, PPV and MFI values were clearly disturbed than normal in all patients: these findings are consistent with data reported in adult patients with severe COVID-19 infection (Kanoore Edul et al., 2021; Damiani et al., 2020; Carsetti et al., 2020). Some common findings on microcirculation has been observed between our series and the recent case report on microcirculation alteration in MIS-C (Bottari et al., 2021) in particular regarding the alterations in MFI and in HI, on the other hand the most important differences observed are related to the TDV values that were clearly disturbed in the report of the patient affected by MIS-C.
The most severe alteration in blood flow quality, with increased flow heterogeneity (Trzeciak et al., 2007), was observed in patient 1: this could suggest a correlation between microvascular alterations and outcome, similarly to what happens during sepsis (Trzeciak et al., 2007), which merits further investigations in future studies. Furthermore MFI < 2.5 correlated with an adverse outcome. This patient had a pre-existing relevant comorbidity of a methastatic lymphoma with a chemotherapy-induced cytopenia. The observed microvascular alterations were not related to sepsis since the patient did never show signs of septic shock during the clinical course. A previous report (Karvunidis et al., 2012) reported no statistical difference in microvascular parameters between cytopenic patients and healthy control. Therefore we can assume that COVID-19 infection played a potential role in determining microcirculatory dysfunction in this case. The MFI < 2.5 correlated with an adverse outcome. Microcirculatory alterations in COVID-19 patients may be associated with clotting abnormalities and formation of microthrombi. In this case however, despite severe alterations in microcirculatory perfusion, D-dimer levels were never severely increased throughout the PICU stay.
Patient number 2 had an MFI of 2.6 suggesting some degree of impairment in microvascular flow even in absence of significant hemodynamic instability. Previous reports did not show a correlation between microvascular alterations in patients undergoing ECMO and the extracorporeal treatment per se (Carsetti et al., 2020; Erdem et al., 2019). Furthermore this type of alteration did not correlate with other biomarkers of inflammation.
Previous evidences suggest that COVID-19 in newborn induces more severe symptoms in comparison to older children (De Rose et al., 2020). In patient 3 we observed respiratory failure with need of mechanical ventilation associated with thrombotic complications. It is relevant to underline that in this patient we found images of obstructed blood flow in small and we have observed also thrombotic complications involving central nervous system (Fig. 2). We can suppose that there is a relationship between these findings and COVID-19 infection and this is also a plausible hypothesis if we consider previously reported data in adults of an inverse relationship between PVD and D-dimers levels (Damiani et al., 2020).
Due to the small number of patients studied, we cannot establish correlations among microvascular parameters and laboratory values or mechanical ventilation variables. Our patients had severe and moderate ARDS, an increase of D-dimers and a mild increase in ferritin levels. Even if they were hemodynamically stable and two of them received only low doses of vasopressors, they showed sublingual microvascular alterations. Other markers of endothelial damage such as Il-8 ore C3a and C5a (Diorio et al., 2020a; Diorio et al., 2020b) could better correlate with our sublingual microcirculation analysis, but unfortunately these were not measured in our patients.
The potential harmful role of viral agents against vascular endothelium has been already reported in scientific literature: Senchenkov and others have showed that persistent CMV infection induces adhesion molecules upregulation finally responsible of leukocyte and platelets recruitment and microvascular dysfunction (Khoretonenko et al., 2014; Senchenkov et al., 2011). However our patients had not evidence of CMV infections, a similiar model could be hypothesized to explain the potential mechanisms of microvascular dysfunction COVID-19 related.
Although we are aware that we cannot draw definitive conclusions, some important observations could be done based on these clinical cases. The three patients had very different severity of clinical conditions and also a different prognosis. Our experience confirms that children with comorbidity, in particular with congenital and acquired immunodeficiencies could show a higher risk of severe COVID-19 infections (Brisca et al., 2021). At present, limited evidences exist on the impact of COVID-19 in newborns, although a higher vulnerability has been described by some authors (De Rose et al., 2020). We confirmed that, in children affected by COVID-19, the sublingual TVD is normal or increased, whereas flow parameters (convective) MFI and PPV are decreased and may be correlated with the outcome. A limitation of this report is that we performed a single time point evaluation: evaluating the evolution of microvascular alterations in these patients would have provided additional important information on the possible relationship between the course of microcirculatory dysfunction and the patient prognosis. Furthermore with multiple time points of sublingual microcirculation evaluation we could better evaluate the impact of the main pathology of each patient than the impact of the COVID-19 infection itself.
3. Conclusions
Children affected by COVID 19 with need of PICU show a wide spectrum of clinical pictures. Pre-existing comorbidities can impact of the clinical course of this infection which is mild in the most healthy children (Brisca et al., 2021). In children with severe COVID-19 microvascular parameters are altered.
CRediT authorship contribution statement
All the authors contributed equally to write the manuscript. GB drafted the manuscript. GB and VC performed the sublingual microcirculation analysis. ED, EC, AD and CC reviewed the manuscript. FS and CG reviewed the neuro-imaging and the manuscript.
Declaration of competing interest
-
•
All authors have participated in (a) conception and design, or analysis and interpretation of the data; (b) drafting the article or revising it critically for important intellectual content; and (c) approval of the final version.
-
•
This manuscript has not been submitted to, nor is under review at, another journal or other publishing venue.
-
•
The authors have no affiliation with any organization with a direct or indirect financial interest in the subject matter discussed in the manuscript.
Footnotes
Acknowledgements: We would like thank all the medical and nursing PICU staff of the Bambino Gesù Childrens Hospital who continue to care for patients and parents.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Boerma E.C., Mathura K.R., Van Der Voort P.H.J., et al. Quantifying bedside-derived imaging of microcirculatory abnormalities in septic patients: a prospective validation study. Crit. Care. 2005;9 doi: 10.1186/cc3809. R601-R606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottari G., Confalone V., Cotugno N., et al. Efficacy of CytoSorb in a pediatric case of severe multisystem inflammatory syndrome (MIS-C): a clinical case report. Front. Pediatr. 2021;11(9) doi: 10.3389/fped.2021.676298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisca G., Mariani M., Rotulo A.G., et al. Clinical course of COVID-19 in children with pre-existing medical conditions. Acta Paediatr. 2021;110:1291–1292. doi: 10.1111/apa.15730. [DOI] [PubMed] [Google Scholar]
- Carsetti A., Hollmann A., Pierantazzi S., et al. Ability and efficient of an automatic analysis software to measure microvascular parameters. J. Clin. Monit. Comput. 2017;31:669–676. doi: 10.1007/s10877-016-9928-3. [DOI] [PubMed] [Google Scholar]
- Carsetti A., Damiani E., Casarotta E. Sublingual microcirculation in patients with SARS-CoV-2 undergoing veno-venous extracorporeal membrane oxigenation. Microvasc. Res. 2020;132 doi: 10.1016/j.mvr.2020.104064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiani E., Carsetti A., Casarotta E., et al. Microvascular alterations in patients with SARS-COV_2 severe pneumonia. Ann. Intensive Care. 2020;10:60. doi: 10.1186/s13613-020-00680-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Backer D., Hollenberg S., Boerma C., et al. How to evaluate the microcirculation: report of a round table conference. Crit. Care. 2007;11(5) doi: 10.1186/cc6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rose D., Piersigilli F., Ronchetti M.P., et al. Novel Coronavirus disease (COVID-19) in newborns and infants: what we know so far. Ital. J. Pediatr. 2020;46:56. doi: 10.1186/s13052-020-0820-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diorio C., Henrickson S.E., Vella L., et al. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS-CoV-2. J. Clin. Invest. 2020;130(11):5967–5975. doi: 10.1172/JCI140970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diorio C., McNerney K.O., Lambert M., et al. Evidence of thrombotic microangiopathy in children with SARS-CoV-2 across the spectrum presentations. Blood Adv. 2020;4:6051–6063. doi: 10.1182/bloodadvances.2020003471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdem O., Kuiper J.W., Van Rosmalen J., et al. The sublingual microcirculation throughout neonatal and pediatric extracorporeal membrane oxygenation treatment: it is altered bu systemic extracorporeal support? Front. Pediatr. 2019;7:272. doi: 10.3389/fped.2019.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaron E., Ince C., Hilthy M.P., et al. Capillary leukocytes, microaggregates, and the response to hypoxemia in the microcirculation of coronavirus disease 2019 patients. Crit. Care Med. 2021;49:661–670. doi: 10.1097/CCM.0000000000004862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ince C., Boerma E., Cecconi M., et al. Second consensus on the assessment of sublingual microcirculation in critically ill patients: results from a task force of the european Society of Intensive Care Medicine. Intensive Care Med. 2018;44:281–299. doi: 10.1007/s00134-018-5070-7. [DOI] [PubMed] [Google Scholar]
- Kanoore Edul V.S., Caminos Eguillor J.F., Ferrara G., et al. Microcirculation alterations in severe COVID-19 pneumonia. J. Crit. Care. 2021;61:73–75. doi: 10.1016/j.jcrc.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karvunidis T., Chvojka J., Sykora R., et al. Septic shock and chemotherapy-induced cytopenia: effects on microcirculation. Intensive Care Med. 2012;38(8):1336–1344. doi: 10.1007/s00134-012-2582-4. [DOI] [PubMed] [Google Scholar]
- Khoretonenko M.V., Brunson J., Senchenkov E., et al. Platelets, acting in part via P-selectin, mediate cytomegalovirus-induced microvascular dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2014;307:H1745–H1753. doi: 10.1152/ajpheart.00201.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludvigsson J.F. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;00:1–8. doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey M., LaRochelle E., Najarro G., et al. The microcirculation image quality score: development and preliminary evaluation of a proposed approach to grading quality of image acquisition for bedside videomicroscopy. J. Crit. Care. 2013;28:913–917. doi: 10.1016/j.jcrc.2013.06.015. [DOI] [PubMed] [Google Scholar]
- Senchenkov E., Khoretonenko M.V., Leskov I.L., et al. P-Selectin mediates the microvascular dysfunction associated with persistent cytomegalovirus infection in normocholesterolemic and hypercholesterolemic mice. Microcirculation. 2011;18(6):452–462. doi: 10.1111/j.1549-8719.2011.00106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzeciak S., Dellinger R.P., Parrillo J.E., et al. Early microcirculation perfusion derangements in patients with severe sepsis and septic shock: relationship to hemodynamic, oxygen transport, and survival. Ann. Emerg. Med. 2007;49:9888–9896. doi: 10.1016/j.annemergmed.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;17:1–2. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]