Abstract
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease, with poor prognosis and no cure. Substantial evidence implicates inflammation and associated oxidative stress as a potential mechanism for ALS, especially in patients carrying the SOD1 mutation and, therefore, lacking anti-oxidant defense. The brain is particularly vulnerable to oxidation due to the abundance of polyunsaturated fatty acids, such as docosahexaenoic acid (DHA), which can give rise to several oxidized metabolites. Accumulation of a DHA peroxidation product, CarboxyEthylPyrrole (CEP) is dependent on activated inflammatory cells and myeloperoxidase (MPO), and thus marks areas of inflammation-associated oxidative stress. At the same time, generation of an alternative inactive DHA peroxidation product, ethylpyrrole, does not require cell activation and MPO activity. While absent in normal brain tissues, CEP is accumulated in the central nervous system (CNS) of ALS patients, reaching particularly high levels in individuals carrying a SOD1 mutation. ALS brains are characterized by high levels of MPO and lowered anti-oxidant activity (due to the SOD1 mutation), thereby aiding CEP generation and accumulation. Due to DHA oxidation within the membranes, CEP marks cells with the highest oxidative damage. In all ALS cases CEP is present in nearly all astrocytes and microglia, however, only in individuals carrying a SOD1 mutation CEP marks >90% of neurons, thereby emphasizing an importance of CEP accumulation as a potential hallmark of oxidative damage in neurodegenerative diseases.
Keywords: amyotrophic lateral sclerosis, oxidative stress, lipid peroxidation, carboxyethylpyrrole, biomarker
Graphical Abstract
![]()
Introduction
The pathophysiology of many neurodegenerative diseases, ranging from Alzheimer’s disease to amyotrophic lateral sclerosis (ALS), has been associated with chronic inflammation and sustained oxidative stress [1]. ALS is a progressive neurodegenerative disease characterized by the degeneration of upper motor neurons in the motor cortex of the cerebrum and lower motor neurons in the spinal cord [2]. The median overall survival for ALS patients is reported to be as short as 29.8 months from symptoms onset, 15.8 months from diagnosis, and 14.3 months from the first clinic visit [3]. Progressive muscle weakness and atrophy caused by motor neuron degeneration substantially reduces patients’ quality of life, and most of the patients die from respiratory failure resulted from weakening of respiratory muscles.
Although the cause of disease is unknown in the majority of ALS cases, oxidative stress and inflammation have been considered relevant mechanisms in the pathogenesis of ALS as multiple studies have reported increased levels of soluble markers for oxidative stress and inflammation in the central nervous system tissues, spinal fluid, and serum of ALS patients, including 8-hydroxy-2’-deoxyguanosine, 4-hydroxynonenal and ascorbate free radical (reviewed by [4]). While most ALS cases are sporadic and the pathologic processes underlying ALS are multi-factorial and poorly understood, inheritable germline superoxide dismutase-1 (SOD1) mutations that increase the oxidative stress burden in individuals are known to potentiate ALS [2]. Mutations in the SOD1 gene encoding a major antioxidant protein result in the imbalance between generation and removal of reactive oxygen species (ROS), leading to an increase in sustained oxidative stress in the CNS [2]. C9orf72 gene mutation is another frequent cause of ALS. The repeated expansion of C9orf72 gene causes loss of function of the C9orf72 protein that is primarily expressed in myeloid cells or toxic gain of function from repeated RNA, which are both implicated in C9orf72-type ALS (reviewed by [5]).
Lipid peroxidation is one of the major outcomes of oxidative stress that affects cellular membranes and proteins. The brain is particularly vulnerable to such stress due to the high abundance of polyunsaturated fatty acids (PUFAs) [6]. The oxidation of PUFAs such as docosahexaenoic acid (DHA) gives rise to a family of carboxyalkylpyrrole protein adducts (CAPs) with carboxyethylpyrrole (CEP) being one of the most studied CAPs. The clinical significance of CEP is supported by studies reporting its role in inflammatory diseases, including macular degeneration, and tumor progression [7, 8]. CEP is primarily generated during inflammation and oxidative damage as a result of the respiratory burst in neutrophils in inflamed tissues and is absent in healthy tissue [9]. It can be recognized, scavenged, and metabolized by macrophages [10]. CEP is recognized by the pattern recognition Toll-like receptor (TLR) 2, TLR 6, TLR1 and TLR9 [10–12] leading to activation of TLR signaling. CEP was found at high levels in the retina [13] and blood [14] of age-related macular degeneration patients characterized by significant oxidative stress and, therefore, may aid in diagnosis, susceptibility prediction, and therapeutic monitoring in age-related macular degeneration [15, 16]. In addition, CEP has a broad spectrum of effects, ranging from activating platelets [17] and recruiting macrophages [9, 18], to promoting angiogenesis [8, 11]. A previous study described CEP deposition around calcified brain lesions and proposed that CEP accumulation might serve as an indication of neural dysfunction associated with oxidative stress [19]. Yet, in neurodegenerative disorders CEP accumulation might serve both as a readout of oxidative stress and an active pro-inflammatory component contributing to disease progression. In the present study, we first demonstrate that CEP is generated in the presence of myeloid cells from transmembrane PUFAs (e.g., DHA) as well as from supplemented DHA. We show that the production of CEP but not alternative DHA product ethylpyrrole (EP), is augmented by activation of myeloid cells in a myeloperoxidase-dependent manner. Deposits of CEP were found in ALS patients’ brains, with significantly higher levels of CEP in individuals carrying an SOD1 mutation. Finally, we demonstrate that astrocytes and microglia accumulate CEP and most importantly, CEP was observed at high levels in neurons of patients carrying the SOD1 mutation but not in neurons of other ALS patients or control brains. These results show that indeed, accumulation of CEP is a hallmark of oxidative stress and CEP might serve as a potential readout for oxidative damage in ALS and other neurodegenerative disorders.
Material and Methods
Human brains samples
All human ALS tissue samples in this study were acquired from the Health Insurance Portability and Accountability Act-compliant tissue registry which was approved by the institutional review board and written informed consent was obtained from all patients. ALS was diagnosed by the neurologist (with 26 years of experience in clinical neurology) according to the revised EI Escorial criterial [20]. Two of the five human non-ALS tissue samples were obtained from the National Disease Research Interchange (NDRI) and are de-identified. The other three human non-ALS tissue samples were collected as approved by the local institutional review board. Tissues were collected from individuals without microscopic or macroscopic indications of neurological disease according to a neuropathologist’s review of records of individuals who had complete diagnostic autopsies performed with consent from the next of kin.
Isolation of resident and monocyte-derived peritoneal macrophages
Resident macrophages were washed out from the peritoneal cavity of C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) using 5 ml of cold PBS. To obtain monocyte-derived inflammatory macrophages, mice were intraperitoneally injected with 1 ml of 3% thioglycollate (TG), 3 days later, peritoneal cells were harvested with 5 ml of sterile PBS by lavage of the peritoneal cavity.
Macrophage immunostaining with anti-CEP and anti-EP polyclonal antibodies
Isolated macrophages were plated on coverslip in 24-well plate with or without 20 μM DHA for 24 hours. After incubation, cells were fixed with 4% paraformaldehyde and incubated at 4 °C overnight with the primary antibody, rabbit polyclonal anti-CEP (or rabbit polyclonal anti-EP) and rat anti-mouse CD68 (FA-11; Serotec). After washing several times with PBS, the sections were incubated with Alexa Fluor 488-conjugated donkey anti-rabbit IgG and Alexa Fluor 568-conjugated donkey anti-rat for 1 hour at room temperature. Coverslips were mounted onto the slides using VECTASHIELD Mounting Medium with DAPI (4’,6-diamidino-2-phenylindole; Vector Laboratories). Slides were examined with a DM2500 upright fluorescent microscope (Leica Microsystems, GmbH, Wetzlar, Germany). The images were analyzed using Image-Pro software (Media Cybernetics, Rockville, MD).
Isolation of leukocyte subsets from the blood of human donors
The blood after erythrocytes lysis was incubated with ant-CD14 (FITC), anti-CD49d (PE) and anti-CD16 (APC) to identify the different subsets of leukocytes. Monocytes were detected as CD14-positive cells. Eosinophils were distinguished as a high sideward scatter cells that expressed CD49dhigh and CD16low. Neutrophils were detected as CD16high cells. Cell population was sorted based on these parameters by BD FACSAria II flow cytometer (Beckton Dickinson, San Diego, CA). Isolated cells were incubated in 96 well plate (5×104/well) in 100 μl HANKS for 24 hours. In the separate experiment, cells were incubated in a range of pH from 6.8 to 8.0. The pH of HANKS was adjusted by 1 M HCl or 1 M NaOH before the experiment. In a different setup, isolated monocytes were incubated with 20 μM DHA with LPS or with MPO inhibitor 1 μM 4-ABH. Notably, 1 μM 4-ABH does not affect catalase or glutathione peroxidase activities, and displays no effect on the production of superoxide by neutrophils. After incubation, cell media was collected, and EP and CEP concentrations were detected using competitive ELISA.
Detection of CEP and EP in cell culture media by ELISA
Cell supernatants were mixed with a rabbit polyclonal anti-CEP antibody and incubated at 37 °C for 1 hour with gentle shaking. ELISA plates were coated with 221 nM CEP- BSA and blocked with 2% BSA. After 1 hour, the plates were washed three times, filled with the supernatant/antibody mixtures, and incubated at room temperature (RT) for 1 hour. Then, the plates were washed three times, filled with a HRP-conjugated anti-rabbit secondary antibody (Invitrogen), and incubated at RT for 1 hour. For ELISA standard, we used serial dilutions of CEP-BSA starting from 1–5 μM as maximum and plotted a standard curve. CEP concentrations were measured by reading absorbance at 450 nm by using spectroscopy after the plates were washed and incubated with HRP substrates (ABTS, Invitrogen). The same procedure was performed for EP measurements.
Immunostaining of human brains tissues
Postmortem patient brains tissue sections were immersed into preheated antigen retriever solution (Sigma, C9999; citrate buffer, pH 6.0) for 15 min at 85oC followed by rinse with PBS. They were incubated with 1.5% H2O2 + 0.1 % Triton-X 100 in PBS for 15 min at room temperature after that and rinsed three times with PBS followed by blocking with 3% goat serum and 10 % human serum in PBS for 60 min at room temperature. Then, sections were incubated with primary antibodies diluted in blocking solution overnight at room temperature followed by rinse with PBS on the second day. Sections were then rinsed three times with PBS on a rocker and stained with secondary antibodies in PBS at room temperature for one hour. Nuclei were stained by trihydrochloride, trihydrate (Hoechst 33342; 1: 1000 dilution) in PBS for 15 min at room temperature. After rinsed three times with PBS, sections were mounted by mounting medium (ProLong Gold antifade reagent, P36930), and coverslipped. Fluorescent images were taken with a Leica TCS SP5 confocal microscope. The primary antibodies used for staining include anti-myeloperoxidase (Santa Cruz Biotechnology, sc-390109), anti-GFAP (Sigma-Aldrich, G3893), and anti-TMEM119 (Biolegend, 853302), and anti-NeuN (Sigma-Aldrich, MAB377).
Confocal Microscopy
All confocal images were obtained using a Leica TCS SP5 confocal microscope in the Department of Neuroscience at the Lerner Research Institute with either a 40× (1.15 NA) or 63× (1.30 NA) oil immersion lens. Samples were imaged in 2-μm step sizes and spanned the length of tissue samples (20 μm), with at least three regions per sample being imaged. All fluorescence measurement analyses were performed with the measure tool in FIJI (NIH).
Ethical guidelines for animal use
All animal experiments and procedures were performed in accordance with the NIH guidelines on animal care. All protocols were approved by the Cleveland Clinic Animal Care Committee in accordance with Institutional Biosafety Committee guidelines.
Statistical analysis
All statistical tests were performed in GraphPad Prism 8.1.1 using Student’s t test or a one- or two-way analysis of variance (ANOVA) with Tukey’s post hoc test, depending on the number of conditions compared. All experiments represent at least three samples per conditions in cell culture studies and five samples when comparing animal studies unless otherwise indicated. Results are represented as mean ± s.e.m.
Results
Macrophages generate CEP from endogenous and exogenous DHA promoted by inflammatory stimulation
Previous studies have suggested a connection between CEP protein adducts and macrophage infiltration and accumulation in the peritoneal cavity during inflammation. These studies concluded that inflammation augments CEP production [9]. However, little is known about the source of CEP generation. CEP is the end product of PUFAs (e.g., DHA, which is an integral component of the cell membrane) oxidation. Therefore, we tested for CEP generation with and without extra supplementary DHA by resident peritoneal macrophages, as well as by thioglycollate-elicited macrophages to mimic an inflammation scenario.
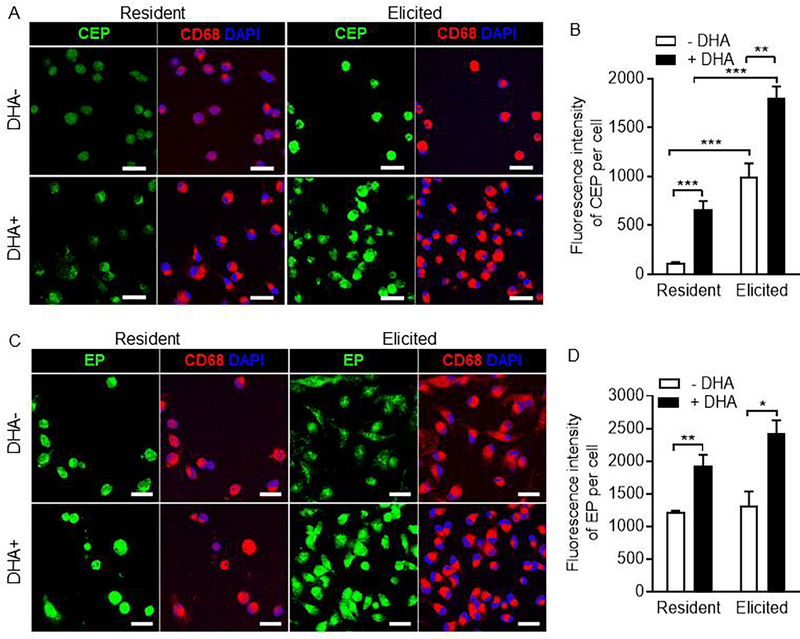
We found that CEP was detected in both resident and elicited peritoneal macrophages with a 10-fold increase in the latter (Fig. 1A upper panels, fig. 1B). Increased CEP production was detected within the cell which indicates intracellular oxidative stress and reactive oxygen species generation upon cell activation. The addition of DHA significantly boosted CEP production (~6-fold increase in resident and ~2-fold increase in elicited peritoneal macrophages compared with no DHA addition) (Fig. 1A lower panel, fig. 1B). We also measured levels of EP, a structural analog to CEP and alternative inactive DHA peroxidation product (Supplementary fig. 1), in peritoneal macrophages. Similar to CEP production, both the resident and the elicited peritoneal macrophages generated high levels of EP which was augmented by the addition of DHA. However, inflammatory stimulation did not significantly increase EP level (Fig. 1C, 1D). These data indicate that macrophages generate CEP from DHA and that CEP generation can be promoted by inflammation. These data also suggest that while EP is generated as a “default” product of PUFA peroxidation, CEP is a specific inflammation-induced end product of PUFA oxidation.
Figure 1. Myeloid leukocytes generate CEP from endogenous and exogenous DHA during pro-inflammatory stimulation.
A. Representative confocal images of mouse resident and thioglycollate-induced peritoneal macrophages co-cultured with or without DHA immunostained for CEP and CD68. Scale bar is 20μm B. Quantification of CEP fluorescence intensity from images in A. C. Representative confocal images of mouse resident and thioglycollate-induced peritoneal macrophages co-cultured with or without DHA immunostained for EP and CD68. Scale bar is 20μm D. Bar graph showing quantification of EP fluorescent intensity from images in C. All data are mean ± s.e.m. Unpaired two-tailed t-test were used to determine statistical significance. * p < 0.05, ** p < 0.01, and *** p < 0.001.
Activated inflammatory cells generate CEP in a MPO-dependent manner
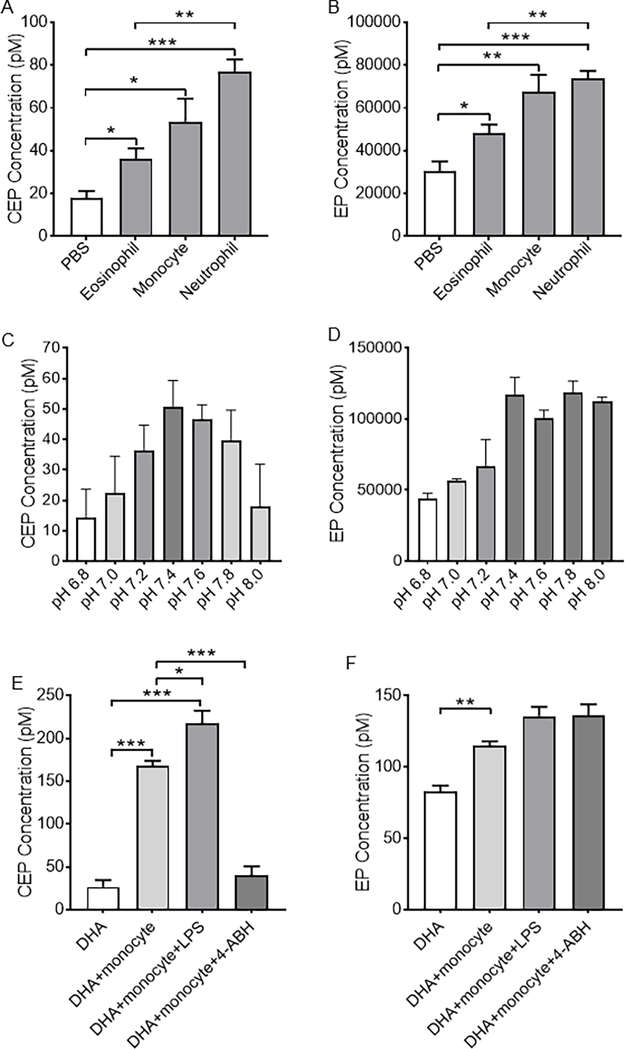
Next, we expanded the cell spectrum from macrophages to human blood leukocytes and assessed CEP and EP generation in FACS-fractioned human blood leukocytes (Fig. 2, supplementary fig. 2). CEP and EP can be generated by human blood leukocytes, especially neutrophils and monocytes (Fig. 2A, 2B). Generation of CEP and EP by neutrophils is pH-dependent with the highest amount achieved at normal physiological pH (7.4) (Fig. 2C, 2D). Similar to macrophages, monocytes can generate CEP with supplementary DHA (Fig. 2E). Based upon our result that inflammatory activation promotes CEP production by macrophages, we next activated human monocytes with lipopolysaccharide (LPS) and observed a significant increase in CEP production (Fig. 2E). Because CEP derives from oxidation of PUFAs and MPO is the major peroxidase secreted by monocytes upon their activation, we tested the role of MPO in CEP production by monocytes using 4-aminobenzoic acid hydrazide (4-ABH), a potent irreversible MPO inhibitor [21]. Strikingly, 4-ABH treatment abolished CEP production by monocytes implying that CEP generation is dependent on MPO, consistent with previous in vitro studies [9]. Likewise, monocytes can generate EP from supplementary DHA. However, this process was not aided by MPO because 4-AHB treatment failed to reduce EP generation by monocytes (Fig. 2F). Collectively, these data confirm that inflammatory cells produce CEP in a MPO-dependent manner and their activation increases CEP production.
Figure 2. CEP, but not EP, is generated by pro-inflammatory myeloid cells in a MPO-dependent manner.
A and B. Bar graphs showing production of CEP (A) and EP (B) by different human blood leukocytes. C and D. CEP (C) and EP (D) production by human neutrophils in different pH values. Note the highest amount of CEP and EP production is achieved at the normal physiological pH = 7.4. E and F. Bar graphs showing CEP (E) and EP (F) production from DHA by human monocytes with or without LPS stimulation or 4-ABH inhibition. LPS, lipopolysaccharide. 4-AHB, 4-aminobenzoic acid hydrazide. All data are mean ± s.e.m. Unpaired two-tailed t-test were used to determine statistical significance. * p < 0.05, ** p < 0.01, and *** p < 0.001.
CEP is accumulated at high level and co-localized to MPO-expressing cells in ALS patients’ brains
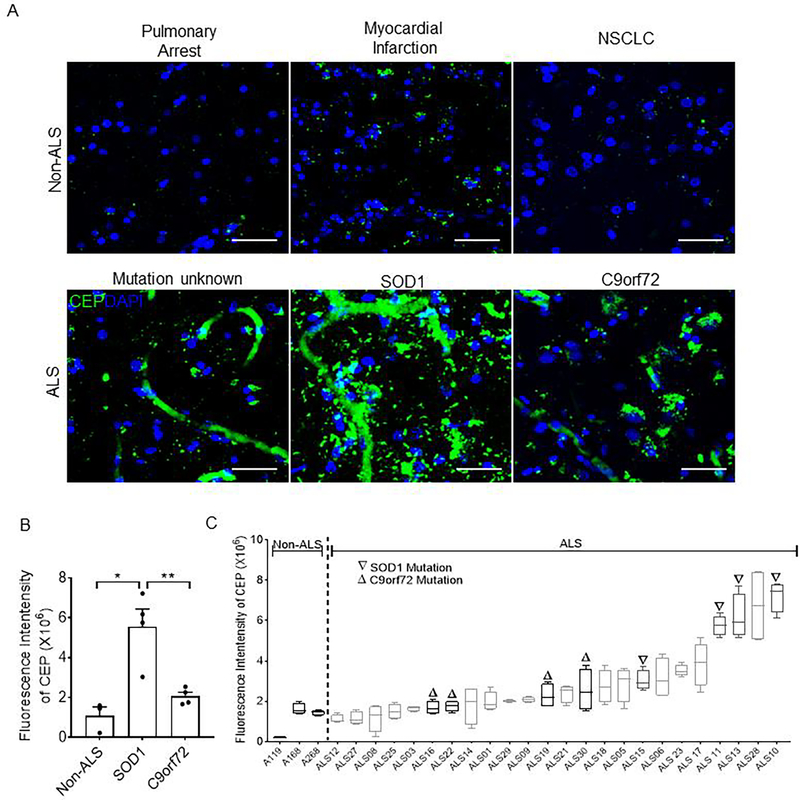
CEP is reported to accumulate at high level in inflamed tissues with excessive oxidative stress [9, 11, 22], and ALS is characterized by extensive oxidative stress [23]. We hypothesized that patients with ALS have high levels of CEP in the brain. To address this question, we determined whether there is an abnormal deposition of CEP in ALS patients’ brains. Postmortem tissue samples collected from the primary motor cortex of human ALS subjects were sectioned and immunostained for CEP to assess CEP levels. While CEP was nearly absent in non-ALS patients’ brains, it accumulated at high level in ALS patients’ brains (Fig. 3A). We further examined CEP levels in SOD1-type and C9orf72-type ALS which are featured by high oxidative stress levels [24, 25] and lowered anti-oxidant activity (SOD1-type) [2]. CEP deposition was remarkably higher in SOD1-type (~5-fold) ALS patients compared with that of C9orf72-type ALS patients and non-ALS patients. These data further implicate the role of oxidative stress-mediated CEP generation as the higher oxidative stress level in SOD1-type ALS was also associated with higher CEP level (Fig. 3B, 3C), which establishes a tight link between CEP deposition and ALS.
Figure 3. ALS patients’ brains, especially SOD1-type, is marked by high concentration of CEP deposition.
A. Representative confocal micrographs of non-ALS and ALS patients’ brain tissues immunostained for CEP. Scale bar is 50 μm B. Bar graph representing quantification of CEP fluorescence intensity from images in A. N = 3, 4, and 4 patients for non-ALS, C9orf72-type ALS, and SOD1-type ALS respectively. All data are mean ± s.e.m. Comparisons between groups were made using one-way analysis of variance (ANOVA) test followed by Tukey’s post hoc test. NSCLC, non-small cell lung cancer. * p < 0.05, ** p < 0.01. C. Box plot showing CEP fluorescence intensity in brains tissues of all patients.
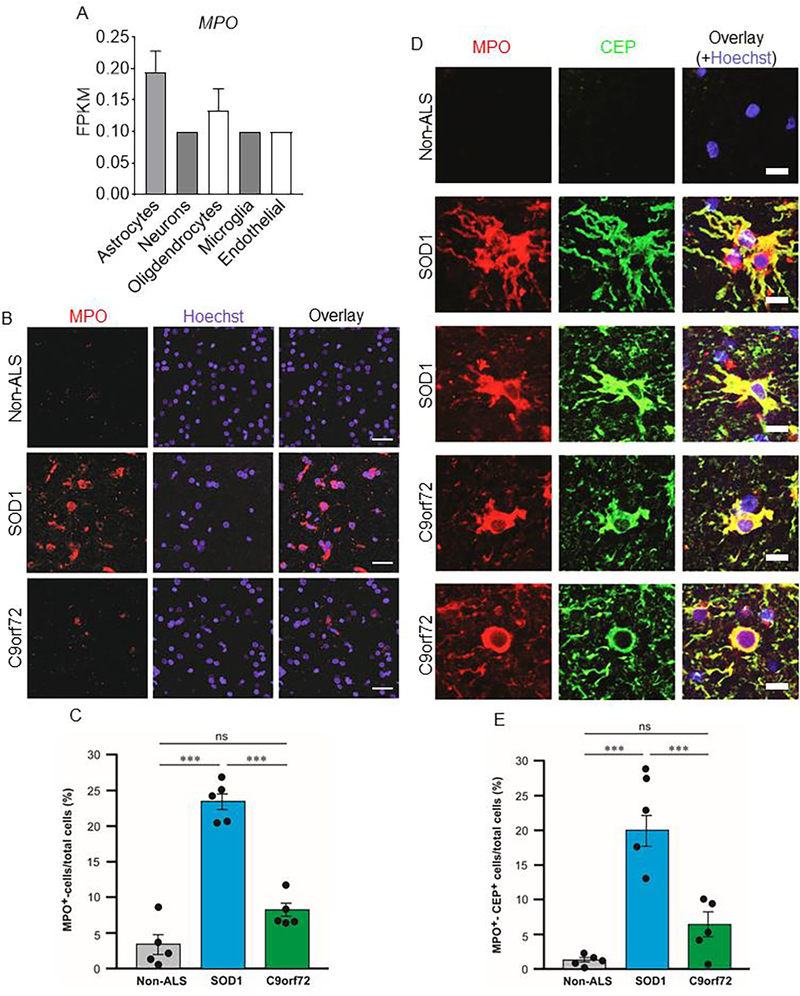
Considering the causative relationship between MPO and CEP generation and differential expression of the MPO gene in different brain cells (Fig. 4A) [26], we examined MPO in ALS patients’ brains and found substantially more MPO expressing cells in SOD1-type (~8-fold) and C9orf72-type (~2-fold) ALS patients’ brains compared with those of non-ALS patients (Fig. 4B, 4C). Furthermore, CEP deposition was co-localized to MPO-expressing cells in ALS’s patients’ brains (Fig. 4D, 4E), which is consistent with our data that inflammatory cells generate CEP in a MPO-dependent manner. Thus, ALS patients’ brains are marked by high level of CEP deposition derived from MPO-expressing cells.
Figure 4. MPO-expressing cells are detected in primary motor cortex of ALS patients and co-localized with CEP deposition.
A. Bar graph showing expression of MPO gene in different human brain cells in published brain single cell RNA sequencing database (https://www.brainrnaseq.org). B. Representative confocal images of brain tissues from non-ALS patients, SOD1-type ALS patients, and C9orf72-type ALS patients immunostained for MPO. Scale bar is 28 μm C. Quantification of fractions of MPO+ cells showing significantly more MPO+ cells in SOD1-type ALS patients’ primary motor cortex. D. Representative confocal images showing brain tissues from non-ALS patients, SOD1-type ALS patients, and C9orf72-type ALS patients immunostained for MPO and CEP. Scale bar is 14 μm. E. Quantification of fractions of MPO+CEP+ cells revealing significantly more MPO+CEP+ cells in SOD1-type ALS patients’ primary motor cortex. All data are mean ± s.e.m. Comparisons between groups were made using one-way ANOVA followed by Tukey’s post hoc test. * p < 0.05, ** p < 0.01, and *** p < 0.001. MPO, myeloperoxidase. ns, not significant.
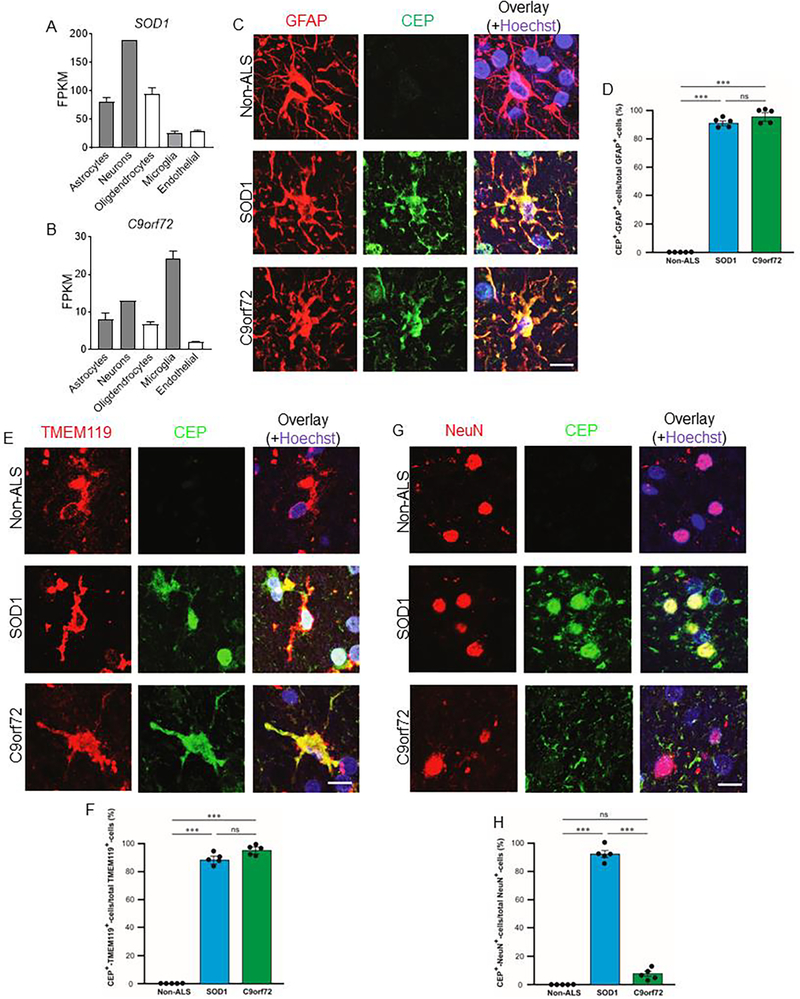
CEP is produced by astrocytes, microglia, and neurons in ALS patients’ brains
The SOD1 gene is preferentially expressed by neurons in the brain, while the C9orf72 gene is enriched in microglia (Fig. 5A, 5B) [26], therefore, the major cell types affected by gene mutations in SOD1-type ALS and C9orf72-type ALS may be different. We scrutinized different kinds of cells in nervous tissue extensively involved in ALS for CEP deposition and production. Astrocytes and microglia are known as the major orchestrator of the brain’s inflammatory response, and are activated during neurodegenerative diseases including ALS, therefore, we first evaluated CEP deposition in astrocytes and microglia. Considerable CEP deposition was found in activated astrocytes and microglia in ALS patients’ brains compared with healthy and resting astrocytes and microglia (Fig. 5C–5F), indicative of elevated intracellular oxidative stress. ALS is characterized by degeneration of both upper motor neurons and lower motor neurons, therefore, we next measured CEP deposition in NeuN+ neurons in ALS patients’ brains. CEP was heavily deposited in neurons in SOD1-type ALS patients’ brains, while little to no CEP was detected in those of C9orf72-type ALS or non-ALS patients’ brains (Fig. 5G, 5H). Together, these data indicate that CEP is produced by major resident cell types involved in ALS. We propose that CEP serves as a potential marker for oxidative damage in ALS, especially in individuals with SOD1-type ALS.
Figure 5. CEP is produced by astrocytes, microglia, and neurons in ALS patients’ brains.
A and B. Bar graphs showing expressions of SOD1 gene (A) and C9orf72 gene (B) in different human brain cells in published brain single cell RNA sequencing database (https://www.brainrnaseq.org). C, E, and G. Representative confocal images of brains tissues from non-ALS patients, SOD1-type ALS patients, and C9orf72-type ALS patients immunostained for GFAP (C, astrocytes), TMEM119 (E, microglia), NeuN (G, neuron) and CEP. Scale bar is 14 μm D, F, and H. Quantification of fractions of CEP+GFAP+ cells (D), CEP+TMEM119+ cells (F), and CEP+NeuN+ cells (H) in primary motor cortex of different patients. All data are mean ± s.e.m. Comparisons between groups were made using one-way ANOVA followed by Tukey’s post hoc test. * p < 0.05, ** p < 0.01, and *** p < 0.001. ns, not significant.
Discussion
We sought to demonstrate that CEP accumulation serves as a readout of oxidative stress in the brain. The main findings from the study are: (1) inflammatory cells generate CEP from PUFA peroxidation as activation-dependent and MPO-mediated process. (2) CEP accumulates in ALS patients’ brains at high level, particularly in the SOD1-type ALS cases, and is co-localized with inflammatory and MPO-expressing cells. (3) CEP is present in major cell types which contribute to ALS pathogenesis, including astrocytes and microglia. However, only in SOD1-type ALS cases CEP is accumulated in neurons.
CEP was reported to be involved in inflammation-associated pathologies, including atherosclerosis, hyperlipidemia, thrombosis, macular degeneration and tumor progression [7]. More mechanistic studies revealed that CEP promotes macrophage adhesion to the extracellular matrix and their migration to the inflamed site [9, 18], polarization toward the pro-inflammatory M1 phenotype [27, 28], and expression of pro-inflammatory cytokines [28]. The level of CEP in pathological processes is well-regulated. For example, the fluctuation of CEP levels adopts a bell-shaped pattern during wound healing. At the early stage of wound healing, CEP is generated rapidly and heavily deposited in wound tissue. CEP level returns to normal when the wound is healed [11]. CEP can be recognized, scavenged, and cleared by macrophages, preferentially and more effectively by M2 macrophages [10]. Collectively, these data indicate that endogenous levels of CEP is tightly coupled with and regulated by inflammation.
The intracellular increase in CEP production upon macrophage activation is a sign of cellular ROS production resulting from inflammatory stimuli. Inflammatory cells produce more CEP upon activation, therefore, CEP marks area of inflammation-related oxidative stress. We showed that addition of exogenously provided PUFAs (e.g., DHA) dramatically augmented CEP production by macrophages. Macrophages can generate CEP even without addition of supplementary PUFAs, by oxidizing PUFAs present within their own lipid membranes. Upon oxidation, DHA can generate several alternative products, including CEP and EP. However, compared with EP which is generated at baseline as the “default” lipid peroxidation end product, the generation of CEP is more fine-tuned and occurs only in an inflammatory environment. Resident macrophages do not generate meaningful amount of CEP without inflammatory stimuli. In contrast, EP was generated at a much higher level than CEP by resident macrophages at baseline with inflammatory stimuli appearing to have no effect on its production. Our result is consistent with a previous study reporting that CEP is absent in healthy tissue and accumulates at the site of inflammation while EP is presents in both healthy and inflammatory tissue at equal amounts [9]. While CEP is recognized by TLR2 and CD36 and activates downstream signaling, EP is not recognized by these receptors due to the lack of a carboxyl group [10]. Inflammation is always accompanied by pH change and microenvironment pH controls inflammatory response in macrophages [28]. The lack of function and the fact that pH change does not impact EP generation as much as CEP generation confirms EP as a “default product”, underscoring CEP as a characteristic of inflammation-associated oxidative stress.
DHA is particularly abundant in the mammalian brain [29] and stands out as the most oxidizable PUFA in human tissues due to its high content of double bonds [6], making it a perfect source of CEP generation in brains with excessive oxidative stress. Our result showed that normal non-ALS brains rarely expresses MPO, but high amounts of MPO-expressing cells are found in ALS brains, consistent with previous reports showing upregulation of MPO in neurodegenerative diseases [30–32]. With DHA as the lipid substrate and MPO as the source of ROS, CEP is therefore continuously generated as a result and would be indicative of sustained oxidative stress in ALS. While much more CEP is found within cells compared with outside of the cells, this is not unexpected since a) endogenous DHA serves as a primary substrate for oxidation and b) myeloid cells are able to uptake and metabolize CEP produced by other cells via phagocytosis [10].
Myeloid cells including microglia express the highest level of the C9orf72 gene in the brain and loss of C9orf72 expression leads to defects in maturation of phagosomes to lysosomes in microglia [33] which is crucial for CEP metabolism [10]. A recent study incorporating comprehensive bioinformatics analyses with robust in vitro and in vivo assays revealed and confirmed distinct pro-inflammatory and anti-inflammatory/phagocytic phenotypes within microglia in neurodegenerative diseases [34]. The imbalance between the two activation states in progression of neurodegenerative diseases and mutation of the C9orf72 gene could cause defective phagocytosis in microglia [33, 34], leading to deficient clearance of CEP by phagocytosis. In addition, CEP modification of existing extracellular matrix proteins transforms them into stronger adhesive ligands for macrophage migration [9, 18], leading to recruitment and aggregation of macrophages/microglia to the ALS lesions, which might exacerbate the neuroinflammation in ALS brains. Because astrocytes expresses TLR2, TLR6 [35] and CD36 [36], which are the major receptors involved in phagocytosis of CEP, it is tempting to speculate that in ALS brains, activated astrocytes may be another scavenger for excessive biologically active CEP apart from microglia. While clearance of CEP is mediated by TLR2, TLR6 and CD36, the signaling of CEP is mainly transduced via TLR1/2 [11, 37]. These receptors are not only found to be expressed on astrocytes, but also involved in their activation [35, 36], creating a possibility of astrocyte activation by CEP in ALS.
However, CEP deposition in neurons in ALS brains differs between ALS subtypes. CEP is only detected in neurons of the SOD1-type ALS, and neither C9orf72-type ALS nor non-ALS brains have positive CEP staining. The SOD1 gene encodes the superoxide dismutase, which is a major antioxidant within the cell as well as a transcription factor for genes involved in resistance to oxidative stress and restoration of oxidative damage in neurons [38, 39]. SOD1 mutations can cause neurotoxicity in ALS as a result of elevated oxidative stress and mitochondrial dysfunction [3]. Together, the increased level of oxidative stress [4] combined with an inability to counteract as a result of the SOD1 gene mutation, lead to the overload of neurons with oxidative stress in ALS. Thus, in contrast to microglia and astrocytes where cell activation and upregulation of MPO is the major contributor to excessive oxidative stress, oxidative stress in neurons in SOD1-type ALS is mainly caused by the failure of anti-oxidative mechanism because neurons express significantly less MPO [30]. Therefore, neurons in the SOD1-type ALS brain experience substantially higher oxidative stress than those in the C9orf72-type ALS brain. Since neurons represent the majority of cells in the brain, the elevated CEP level in neurons could lead to higher overall CEP levels in the brain of SOD1-type ALS patients compared with C9orf72-type ALS patients. In addition, our supplementary data and previous studies [26, 33, 40] both show that SOD1 gene expression is highest in neurons in the brain, while C9orf72 gene expression is highest in microglia. The differential enrichment of SOD1 gene and C9orf72 gene expressions could lead to the observed heterogeneity of CEP accumulation in ALS patients carrying SOD1 or C9orf72 gene mutations. Together, excessive ROS primarily affects neurons of SOD1-type ALS brains and microglia of C9orf72-type ALS brains.
CEP was also previously demonstrated to be a novel ligand for TLR9 [12] and neurons were identified as the main cell type expressing TLR9 in the brains [41]. Stimulation of TLR9 in neurons resulted in neuronal apoptosis [42], degeneration [43], and excitotoxic death [44], therefore, CEP could contribute to neuronal death in ALS through TLR9 as well. The expression of TLR9 in neurons also increases with age [41] and CEP accumulates at a high level in aging tissue [11], which would imply a potential role of CEP in neuronal survival in age-related neurodegenerative diseases (e.g., Alzheimer’s disease).
ALS is not considered to be a neuron autonomous disease [45]. Mice harboring mutated SOD1 (mSOD1) only in neurons have reduced clinical disease compared to mice that globally express mSOD1 [46]. Cell specific deletion of mSOD1 in astrocytes or microglia slow clinical disease onset and extend lifespan, supporting the concept that glial cells play essential roles in ALS disease progression [47, 48]. Reactive astrocytes are a prominent feature of postmortem ALS tissues [49]. Positron emission tomography imaging revealed widespread cerebral microglial activation in individuals with ALS [50] and bioinformatics analysis of postmortem ALS brains detected elevated levels of microglial transcripts [51]. CEP accumulation in astrocytes and microglia identifies a mechanism by which astrocytes and microglia amplify oxidative damage and neurodegeneration in ALS.
It remains unknown whether CEP plays a causative role in ALS development or is merely symptomatic in its progression. Future studies may focus on CEP in early ALS brain tissues to delineate its effects on different cell types involved in ALS before massive death of neurons and glial cells. To utilize clinically CEP as a biomarker of ALS progression, it is important to quantify CEP levels in the brains and the serum. To this end, the development of liquid chromatography-mass spectrometry (LC-MS) method to measure CEP levels is required. To date, no enrichment methods are available for CEP adducts. Without an efficient enrichment method, the detection of CEP adducts in tissues using LC-MS would be difficult. There are no methods available for non-invasively monitoring CEP levels in neurons and glia in ALS brains. Therefore, to estimate the accumulation of CEP in neurons and glia in the brains at different stages of ALS progression, patient plasma CEP level may be used alternatively. It has been shown that in patients with the age-related macular degeneration, CEP levels in the retina are well correlated with those in serum [15, 16]. Future studies can focus the relationship between plasma CEP levels and ALS progression.
The present study demonstrates the generation of oxidative metabolite CEP via DHA peroxidation in an MPO-dependent manner, elaborates the enrichment of CEP in astrocytes, microglia, and neurons in ALS brains, and proposes CEP as a potential biomarker for oxidative damage in ALS.
Supplementary Material
Supplementary figure 1. Generation of CEP and EP from DHA.
The oxidative fragmentation of docosahexaenoic acyl (DHA) chain at the sn-2 position of 1-palmitoyl-2-docosahexaenoyl-sn-glycero-3-phosphocholine (PDPC) generates a series of oxidized lipids, including the short chain (a) 4-hydroxy-2-hexenal (HHE) and (b) 4-Hydroxy-7-oxo-5-heptenoic Acid (HOHA) which is released from the phospholipid backbone after hydrolysis catalyzed by phospholipase A2 (PLA2). The γ-hydroxyalkenal group of (a) HHE and (b) HOHA react with amino group (e.g. ε-amino group of lysine residues in proteins) to form a Schiff base adduct, which is converted to γ-hydroxy enamine and then to γ-keto-enamine through two consecutive steps of tautomerization. The intramolecular reaction of the enamine group with the keto group generates a five-membered ring, and the subsequent dehydration reaction leads to the formation of a pyrrole ring: ethylpyrrole (EP) for HHE (a) and carboxyetheylpyrrole (CEP) for HOHA (b). “R” in the figure represents the “Rest of the molecule”.
Supplementary figure 2. Isolation of different leukocyte subsets from human blood by FACS.
Table 1. Abbreviations and full names
Highlights:
Macrophages generate CEP from DHA promoted by inflammation in a MPO-dependent manner
CEP accumulates in and co-localize with MPO-expressing cells in ALS brains
Astrocytes, microglia, and neurons produce high level of CEP in ALS
CEP deposition is a hallmark of oxidative damage in neurodegenerative disease
Acknowledgements
We thank the patients who contributed their brain tissue to the NDRI and the Health Insurance Portability and Accountability Act-compliant tissue registry, which made this study possible.
Funding
This work was supported by the National Institutes of Health [R01 HL145536 and HL142772].
Footnotes
Declaration of Interest: none.
Declaration of Interests
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Rekatsina M, Paladini A, Piroli A, Zis P, Pergolizzi JV, Varrassi G, Pathophysiology and Therapeutic Perspectives of Oxidative Stress and Neurodegenerative Diseases: A Narrative Review, Advances in therapy 37(1) (2020) 113–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hardiman O, Al-Chalabi A, Chio A, Corr EM, Logroscino G, Robberecht W, Shaw PJ, Simmons Z, van den Berg LH, Amyotrophic lateral sclerosis, Nature reviews. Disease primers 3 (2017) 17071. [DOI] [PubMed] [Google Scholar]
- [3].Traxinger K, Kelly C, Johnson BA, Lyles RH, Glass JD, Prognosis and epidemiology of amyotrophic lateral sclerosis: Analysis of a clinic population, 1997–2011, Neurology. Clinical practice 3(4) (2013) 313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Barber SC, Shaw PJ, Oxidative stress in ALS: key role in motor neuron injury and therapeutic target, Free radical biology & medicine 48(5) (2010) 629–41. [DOI] [PubMed] [Google Scholar]
- [5].Balendra R, Isaacs AM, C9orf72-mediated ALS and FTD: multiple pathways to disease, Nature reviews. Neurology 14(9) (2018) 544–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fliesler SJ, Anderson RE, Chemistry and metabolism of lipids in the vertebrate retina, Progress in lipid research 22(2) (1983) 79–131. [DOI] [PubMed] [Google Scholar]
- [7].Yakubenko VP, Byzova TV, Biological and pathophysiological roles of end-products of DHA oxidation, Biochimica et biophysica acta. Molecular and cell biology of lipids 1862(4) (2017) 407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].McCoy MG, Nascimento DW, Veleeparambil M, Murtazina R, Gao D, Tkachenko S, Podrez E, Byzova TV, Endothelial TLR2 promotes proangiogenic immune cell recruitment and tumor angiogenesis, Sci Signal 14(666) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yakubenko VP, Cui K, Ardell CL, Brown KE, West XZ, Gao D, Stefl S, Salomon RG, Podrez EA, Byzova TV, Oxidative modifications of extracellular matrix promote the second wave of inflammation via β(2) integrins, Blood 132(1) (2018) 78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kim YW, Yakubenko VP, West XZ, Gugiu GB, Renganathan K, Biswas S, Gao D, Crabb JW, Salomon RG, Podrez EA, Byzova TV, Receptor-Mediated Mechanism Controlling Tissue Levels of Bioactive Lipid Oxidation Products, Circulation research 117(4) (2015) 321–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].West XZ, Malinin NL, Merkulova AA, Tischenko M, Kerr BA, Borden EC, Podrez EA, Salomon RG, Byzova TV, Oxidative stress induces angiogenesis by activating TLR2 with novel endogenous ligands, Nature 467(7318) (2010) 972–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Panigrahi S, Ma Y, Hong L, Gao D, West XZ, Salomon RG, Byzova TV, Podrez EA, Engagement of platelet toll-like receptor 9 by novel endogenous ligands promotes platelet hyperreactivity and thrombosis, Circulation research 112(1) (2013) 103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Crabb JW, Miyagi M, Gu X, Shadrach K, West KA, Sakaguchi H, Kamei M, Hasan A, Yan L, Rayborn ME, Salomon RG, Hollyfield JG, Drusen proteome analysis: an approach to the etiology of age-related macular degeneration, Proceedings of the National Academy of Sciences of the United States of America 99(23) (2002) 14682–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang H, Guo J, West XZ, Bid HK, Lu L, Hong L, Jang GF, Zhang L, Crabb JW, Linetsky M, Salomon RG, Detection and biological activities of carboxyethylpyrrole ethanolamine phospholipids (CEP-EPs), Chemical research in toxicology 27(12) (2014) 2015–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gu X, Meer SG, Miyagi M, Rayborn ME, Hollyfield JG, Crabb JW, Salomon RG, Carboxyethylpyrrole protein adducts and autoantibodies, biomarkers for age-related macular degeneration, The Journal of biological chemistry 278(43) (2003) 42027–35. [DOI] [PubMed] [Google Scholar]
- [16].Renganathan K, Gu J, Rayborn ME, Crabb JS, Salomon RG, Collier RJ, Kapin MA, Romano C, Hollyfield JG, Crabb JW, CEP biomarkers as potential tools for monitoring therapeutics, PloS one 8(10) (2013) e76325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Biswas S, Xin L, Panigrahi S, Zimman A, Wang H, Yakubenko VP, Byzova TV, Salomon RG, Podrez EA, Novel phosphatidylethanolamine derivatives accumulate in circulation in hyperlipidemic ApoE−/− mice and activate platelets via TLR2, Blood 127(21) (2016) 2618–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cui K, Podolnikova NP, Bailey W, Szmuc E, Podrez EA, Byzova TV, Yakubenko VP, Inhibition of integrin α(D)β(2)-mediated macrophage adhesion to end product of docosahexaenoic acid (DHA) oxidation prevents macrophage accumulation during inflammation, The Journal of biological chemistry 294(39) (2019) 14370–14382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zarb Y, Weber-Stadlbauer U, Kirschenbaum D, Kindler DR, Richetto J, Keller D, Rademakers R, Dickson DW, Pasch A, Byzova T, Nahar K, Voigt FF, Helmchen F, Boss A, Aguzzi A, Klohs J, Keller A, Ossified blood vessels in primary familial brain calcification elicit a neurotoxic astrocyte response, Brain : a journal of neurology 142(4) (2019) 885–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Brooks BR, Miller RG, Swash M, Munsat TL, El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis, Amyotrophic lateral sclerosis and other motor neuron disorders : official publication of the World Federation of Neurology, Research Group on Motor Neuron Diseases 1(5) (2000) 293–9. [DOI] [PubMed] [Google Scholar]
- [21].Kettle AJ, Gedye CA, Winterbourn CC, Mechanism of inactivation of myeloperoxidase by 4-aminobenzoic acid hydrazide, The Biochemical journal 321 ( Pt 2)(Pt 2) (1997) 503–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mulfaul K, Ozaki E, Fernando N, Brennan K, Chirco KR, Connolly E, Greene C, Maminishkis A, Salomon RG, Linetsky M, Natoli R, Mullins RF, Campbell M, Doyle SL, Toll-like Receptor 2 Facilitates Oxidative Damage-Induced Retinal Degeneration, Cell reports 30(7) (2020) 2209–2224.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ferraiuolo L, Kirby J, Grierson AJ, Sendtner M, Shaw PJ, Molecular pathways of motor neuron injury in amyotrophic lateral sclerosis, Nature reviews. Neurology 7(11) (2011) 616–30. [DOI] [PubMed] [Google Scholar]
- [24].Masrori P, Van Damme P, Amyotrophic lateral sclerosis: a clinical review, European journal of neurology 27(10) (2020) 1918–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lopez-Gonzalez R, Lu Y, Gendron TF, Karydas A, Tran H, Yang D, Petrucelli L, Miller BL, Almeida S, Gao FB, Poly(GR) in C9ORF72-Related ALS/FTD Compromises Mitochondrial Function and Increases Oxidative Stress and DNA Damage in iPSC-Derived Motor Neurons, Neuron 92(2) (2016) 383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhang Y, Sloan SA, Clarke LE, Caneda C, Plaza CA, Blumenthal PD, Vogel H, Steinberg GK, Edwards MS, Li G, Duncan JA 3rd, Cheshier SH, Shuer LM, Chang EF, Grant GA, Gephart MG, Barres BA, Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse, Neuron 89(1) (2016) 37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cruz-Guilloty F, Saeed AM, Echegaray JJ, Duffort S, Ballmick A, Tan Y, Betancourt M, Viteri E, Ramkhellawan GC, Ewald E, Feuer W, Huang D, Wen R, Hong L, Wang H, Laird JM, Sene A, Apte RS, Salomon RG, Hollyfield JG, Perez VL, Infiltration of proinflammatory m1 macrophages into the outer retina precedes damage in a mouse model of age-related macular degeneration, International journal of inflammation 2013 (2013) 503725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhou X, Pope SD, Medzhitov R, Microenvironment controls inflammatory response in macrophages, The Journal of Immunology 206(1 Supplement) (2021) 111.03. [Google Scholar]
- [29].Neuringer M, Anderson GJ, Connor WE, The essentiality of n-3 fatty acids for the development and function of the retina and brain, Annual review of nutrition 8 (1988) 517–41. [DOI] [PubMed] [Google Scholar]
- [30].Green PS, Mendez AJ, Jacob JS, Crowley JR, Growdon W, Hyman BT, Heinecke JW, Neuronal expression of myeloperoxidase is increased in Alzheimer’s disease, Journal of neurochemistry 90(3) (2004) 724–33. [DOI] [PubMed] [Google Scholar]
- [31].Gray E, Thomas TL, Betmouni S, Scolding N, Love S, Elevated myeloperoxidase activity in white matter in multiple sclerosis, Neuroscience letters 444(2) (2008) 195–8. [DOI] [PubMed] [Google Scholar]
- [32].Maki RA, Tyurin VA, Lyon RC, Hamilton RL, DeKosky ST, Kagan VE, Reynolds WF, Aberrant expression of myeloperoxidase in astrocytes promotes phospholipid oxidation and memory deficits in a mouse model of Alzheimer disease, The Journal of biological chemistry 284(5) (2009) 3158–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].O’Rourke JG, Bogdanik L, Yáñez A, Lall D, Wolf AJ, Muhammad AK, Ho R, Carmona S, Vit JP, Zarrow J, Kim KJ, Bell S, Harms MB, Miller TM, Dangler CA, Underhill DM, Goodridge HS, Lutz CM, Baloh RH, C9orf72 is required for proper macrophage and microglial function in mice, Science (New York, N.Y.) 351(6279) (2016) 1324–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rangaraju S, Dammer EB, Raza SA, Rathakrishnan P, Xiao H, Gao T, Duong DM, Pennington MW, Lah JJ, Seyfried NT, Levey AI, Identification and therapeutic modulation of a pro-inflammatory subset of disease-associated-microglia in Alzheimer’s disease, Molecular neurodegeneration 13(1) (2018) 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Carpentier PA, Begolka WS, Olson JK, Elhofy A, Karpus WJ, Miller SD, Differential activation of astrocytes by innate and adaptive immune stimuli, Glia 49(3) (2005) 360–74. [DOI] [PubMed] [Google Scholar]
- [36].Bao Y, Qin L, Kim E, Bhosle S, Guo H, Febbraio M, Haskew-Layton RE, Ratan R, Cho S, CD36 is involved in astrocyte activation and astroglial scar formation, Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 32(8) (2012) 1567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Saeed AM, Duffort S, Ivanov D, Wang H, Laird JM, Salomon RG, Cruz-Guilloty F, Perez VL, The oxidative stress product carboxyethylpyrrole potentiates TLR2/TLR1 inflammatory signaling in macrophages, PloS one 9(9) (2014) e106421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kirby J, Halligan E, Baptista MJ, Allen S, Heath PR, Holden H, Barber SC, Loynes CA, Wood-Allum CA, Lunec J, Shaw PJ, Mutant SOD1 alters the motor neuronal transcriptome: implications for familial ALS, Brain : a journal of neurology 128(Pt 7) (2005) 1686–706. [DOI] [PubMed] [Google Scholar]
- [39].Tsang CK, Liu Y, Thomas J, Zhang Y, Zheng XF, Superoxide dismutase 1 acts as a nuclear transcription factor to regulate oxidative stress resistance, Nature communications 5 (2014) 3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Rizzu P, Blauwendraat C, Heetveld S, Lynes EM, Castillo-Lizardo M, Dhingra A, Pyz E, Hobert M, Synofzik M, Simón-Sánchez J, Francescatto M, Heutink P, C9orf72 is differentially expressed in the central nervous system and myeloid cells and consistently reduced in C9orf72, MAPT and GRN mutation carriers, Acta neuropathologica communications 4(1) (2016) 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kaul D, Habbel P, Derkow K, Krüger C, Franzoni E, Wulczyn FG, Bereswill S, Nitsch R, Schott E, Veh R, Naumann T, Lehnardt S, Expression of Toll-like receptors in the developing brain, PloS one 7(5) (2012) e37767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mukherjee P, Winkler CW, Taylor KG, Woods TA, Nair V, Khan BA, Peterson KE, SARM1, Not MyD88, Mediates TLR7/TLR9-Induced Apoptosis in Neurons, Journal of immunology (Baltimore, Md. : 1950) 195(10) (2015) 4913–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Maatouk L, Compagnion AC, Sauvage MC, Bemelmans AP, Leclere-Turbant S, Cirotteau V, Tohme M, Beke A, Trichet M, Bazin V, Trawick BN, Ransohoff RM, Tronche F, Manoury B, Vyas S, TLR9 activation via microglial glucocorticoid receptors contributes to degeneration of midbrain dopamine neurons, Nature communications 9(1) (2018) 2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Acioglu C, Mirabelli E, Baykal AT, Ni L, Ratnayake A, Heary RF, Elkabes S, Toll like receptor 9 antagonism modulates spinal cord neuronal function and survival: Direct versus astrocyte-mediated mechanisms, Brain, behavior, and immunity 56 (2016) 310–24. [DOI] [PubMed] [Google Scholar]
- [45].Ilieva H, Polymenidou M, Cleveland DW, Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond, The Journal of cell biology 187(6) (2009) 761–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Pramatarova A, Laganière J, Roussel J, Brisebois K, Rouleau GA, Neuron-specific expression of mutant superoxide dismutase 1 in transgenic mice does not lead to motor impairment, The Journal of neuroscience : the official journal of the Society for Neuroscience 21(10) (2001) 3369–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Boillée S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW, Onset and progression in inherited ALS determined by motor neurons and microglia, Science (New York, N.Y.) 312(5778) (2006) 1389–92. [DOI] [PubMed] [Google Scholar]
- [48].Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, Takahashi R, Misawa H, Cleveland DW, Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis, Nature neuroscience 11(3) (2008) 251–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, Barres BA, Neurotoxic reactive astrocytes are induced by activated microglia, Nature 541(7638) (2017) 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Corcia P, Tauber C, Vercoullie J, Arlicot N, Prunier C, Praline J, Nicolas G, Venel Y, Hommet C, Baulieu JL, Cottier JP, Roussel C, Kassiou M, Guilloteau D, Ribeiro MJ, Molecular imaging of microglial activation in amyotrophic lateral sclerosis, PloS one 7(12) (2012) e52941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Dols-Icardo O, Montal V, Sirisi S, López-Pernas G, Cervera-Carles L, Querol-Vilaseca M, Muñoz L, Belbin O, Alcolea D, Molina-Porcel L, Pegueroles J, Turón-Sans J, Blesa R, Lleó A, Fortea J, Rojas-García R, Clarimón J, Motor cortex transcriptome reveals microglial key events in amyotrophic lateral sclerosis, Neurology(R) neuroimmunology & neuroinflammation 7(5) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1. Generation of CEP and EP from DHA.
The oxidative fragmentation of docosahexaenoic acyl (DHA) chain at the sn-2 position of 1-palmitoyl-2-docosahexaenoyl-sn-glycero-3-phosphocholine (PDPC) generates a series of oxidized lipids, including the short chain (a) 4-hydroxy-2-hexenal (HHE) and (b) 4-Hydroxy-7-oxo-5-heptenoic Acid (HOHA) which is released from the phospholipid backbone after hydrolysis catalyzed by phospholipase A2 (PLA2). The γ-hydroxyalkenal group of (a) HHE and (b) HOHA react with amino group (e.g. ε-amino group of lysine residues in proteins) to form a Schiff base adduct, which is converted to γ-hydroxy enamine and then to γ-keto-enamine through two consecutive steps of tautomerization. The intramolecular reaction of the enamine group with the keto group generates a five-membered ring, and the subsequent dehydration reaction leads to the formation of a pyrrole ring: ethylpyrrole (EP) for HHE (a) and carboxyetheylpyrrole (CEP) for HOHA (b). “R” in the figure represents the “Rest of the molecule”.
Supplementary figure 2. Isolation of different leukocyte subsets from human blood by FACS.
Table 1. Abbreviations and full names