Abstract
Background
It is uncertain whether awake prone positioning can prevent intubation for invasive ventilation in spontaneous breathing critically ill patients with acute hypoxemic respiratory failure. Awake prone positioning could benefit these patients for various reasons, including a reduction in direct harm to lung tissue, and prevention of tracheal intubation-related complications.
Design and methods
The PRONELIFE study is an investigator-initiated, international, multicenter, randomized clinical trial in patients who may need invasive ventilation because of acute hypoxemic respiratory failure. Consecutive patients admitted to participating ICUs are randomly assigned to standard care with awake prone positioning, versus standard care without awake prone positioning. The primary endpoint is a composite of tracheal intubation and all-cause mortality in the first 14 days after enrolment. Secondary endpoints include time to tracheal intubation and effects of awake prone positioning on oxygenation parameters, dyspnea sensation, and complications. Other endpoints are the number of days free from ventilation and alive at 28 days, total duration of use of noninvasive respiratory support, total duration of invasive ventilation, length of stay in ICU and hospital, and mortality in ICU and hospital, and at 28, 60, and 90 days. We will also collect data regarding the tolerance of prone positioning.
Discussion
The PRONELIFE study is among the first randomized clinical trials investigating the effect of awake prone positioning on intubation rate in ICU patients with acute hypoxemic failure from any cause. The PRONELIFE study is sufficiently sized to determine the effect of awake prone positioning on intubation for invasive ventilation—patients are eligible in case of acute hypoxemic respiratory failure without restrictions regarding etiology. The PRONELIFE study is a pragmatic trial in which blinding is impossible—however, as around 35 ICUs worldwide will participate in this study, its findings will be highly generalizable. The findings of the PRONELIFE study have the potential to change clinical management of patients who may need invasive ventilation because of acute hypoxemic respiratory failure.
Trial registration
ISRCTN ISRCTN11536318. Registered on 17 September 2021. The PRONELIFE study is registered at clinicaltrials.gov with reference number NCT04142736 (October, 2019).
Keywords: ICU, Acute respiratory failure, Hypoxemia, Prone position, Awake prone positioning, Invasive ventilation, Intubation, Invasive ventilation, Randomized controlled trial
Background
Acute hypoxic respiratory failure represents one of the most common reasons for intensive care unit (ICU) admission [1]. Initial management of hypoxemic patients should involve immediate administration of simple supplemental oxygen via a nasal prong or a non-rebreather mask, or more complex forms of respiratory support like high-flow nasal oxygen (HFNO) oxygen or noninvasive ventilation (NIV), depending on severity of hypoxic respiratory failure and the underlying cause, but also patient characteristics and the availability of oxygen interfaces. Unfortunately, patients with acute hypoxemic respiratory failure often need to proceed with invasive ventilation. While life-saving, this intervention comes with disadvantages, including ventilator-induced lung injury and respiratory muscle waist, but also intubation-related side-effects. In addition, intubated patients often need sedation, which has side-effects of its own.
In intubated invasively ventilated ICU patients, prone positioning can be used to improve oxygenation, and this intervention has been shown to improve outcome in patients with severe ARDS. This survival benefit could be mediated, at least in part through a reduction in direct harm to lung tissue, as regional differences in lung aeration, compliance, and shear strain are minimized by this intervention [2, 3]. In theory, nonintubated patients could also benefit from prone positioning [4, 5], a strategy named “awake prone positioning” [6, 7]. Some evidence for benefit of awake prone positioning comes from a handful of studies, mostly case reports and single-center observation case series [5, 8, 9]. The findings thus far show that awake prone positioning can indeed improve oxygenation and also reduce dyspnea sensation. Besides that, awake prone positioning seems to be well-accepted and easy to perform, and to have relatively few side-effects [10].
In the absence of sufficient randomized clinical trial evidence, we designed the PRONELIFE study, a pragmatic study that compares standard care with awake prone positioning versus standard care without awake prone positioning in patients with acute hypoxemic respiratory failure from any cause. We hypothesize that awake prone positioning reduces the need for intubation.
Methods
Design
The “PRone positioN in patients with spontanEous ventiLation and acute hypoxemic respIratory FailurE” (PRONELIFE) study is a pragmatic, investigator-initiated, international and multicenter, two-arm, superiority randomized clinical trial in patients with acute hypoxemic respiratory failure from any cause. The study will be conducted according to the Declaration of Helsinki principles as stated in the current version of Fortaleza, Brazil, 2013 [11]. The Institutional Review Board of the Sagrat Cor University Hospital, Barcelona, Spain, approved the study protocol (reference number 2019/68-UCI-HUSC). The study is registered at www.clinicaltrials.gov (NCT04142736, October 2019). The PRONELIFE study is planned to be performed in the 35 ICUs worldwide. Patients will be provisionally included under a strategy of deferred consent, for reasons as explained below.
Consort diagram
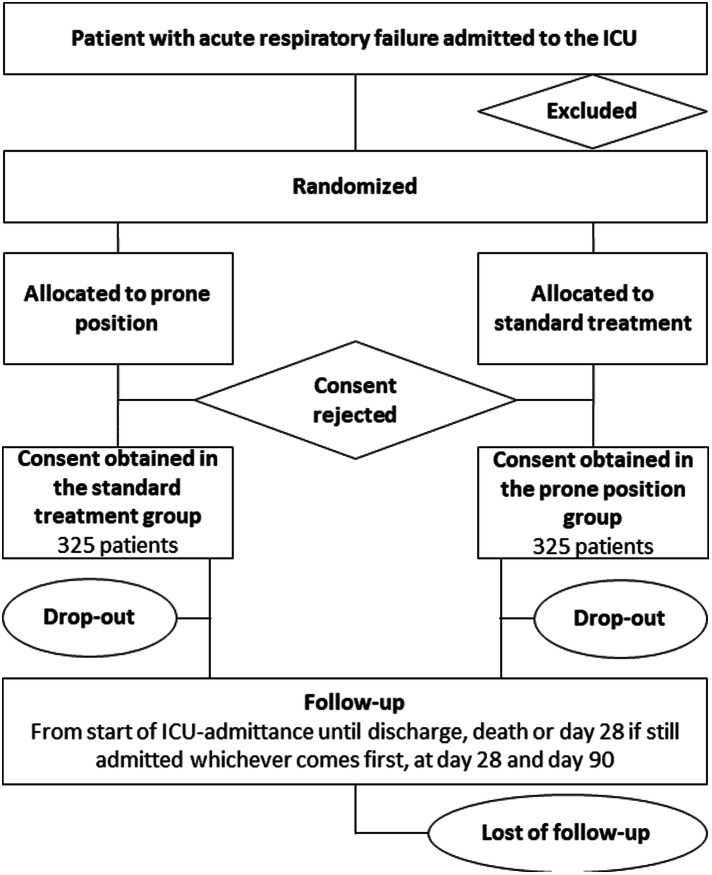
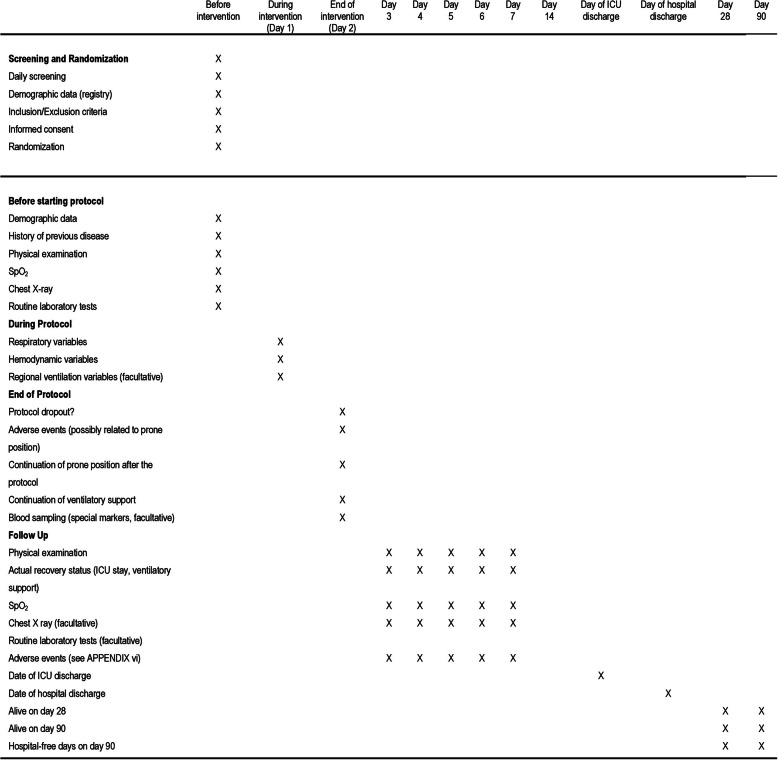
The Consolidated Standards of Reporting Trials (CONSORT) [12] diagram of the PRONELIFE study is presented in Fig. 1. Consecutive patients admitted to the participating ICUs are screened for eligibility. Demographic data are registered regardless of meeting enrollment criteria. If excluded from participation, the reason(s) for exclusion will be reported. The study overview is presented in Fig. 2.
Fig. 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram
Fig. 2.
Study overview
Inclusion and exclusion criteria
Patients admitted to the ICU with acute hypoxemic respiratory failure from any cause will be eligible for participation, unless prompt intubation is irrepressible (Table 1). Acute respiratory failure is defined as the need for supplementary oxygen with an FiO2 of at least 40% by Venturi facemask, HFNO, NIV, or CPAP needed to achieve and maintain an SpO2 of between 88 and 92%.
Table 1.
Contraindication for awake prone position
|
• Suspected increased intracranial pressure (e.g., severe brain injury) • Hemoptysis • Vomiting • Recent abdominal wound (less than 15 days) • Tracheal surgery or sternotomy during the previous 15 days • Facial trauma or facial surgery during the previous 15 days • Deep venous thrombosis treated for less than 2 days • Cardiac pacemaker inserted in the last 2 days • Unstable spine, femur, or pelvic fractures • Hemodynamic instability (defined by a systolic blood pressure below 90 mmHg, mean blood pressure below 65 mmHg, or requirement for vasopressor) • Pregnant women • Presence of chest tube |
The PRONELIFE study has the following exclusion criteria: age < 18 years, having any contraindication for prone positioning (Table 2), previous participation in this trial, and participation in other interventional trials with the same primary endpoint. We also exclude pregnant patients, patients who refuse intubation, and those only receiving comfort care. As patients will be provisionally included under a strategy of deferred consent, there is no need for written informed consent at start of the study—however, if consent is not obtained within 48 h, a patient drops out of the study and will be replaced.
Table 2.
Borg dyspnea scale
| 0 | Nothing at all |
|---|---|
| 0.5 | Very, very slight (just noticeable) |
| 1 | Very slight |
| 2 | Slight breathlessness |
| 3 | Moderate |
| 4 | Somewhat severe |
| 5 | Severe breathlessness |
| 6 | |
| 7 | Very severe breathlessness |
| 8 | |
| 9 | Very, very severe (almost maximal) |
| 10 | Maximal |
Study endpoints
The primary endpoint is the composite of tracheal intubation and all-cause mortality within 14 days after enrollment.
Secondary endpoints are the effects of awake proning on the following clinical outcome variables:
Time to tracheal intubation (only in patients who need invasive ventilation);
Oxygenation parameters;
Dyspnea sensation (while awake in prone versus supine position);
The number of days free from invasive ventilation and alive at day 28;
Duration of use of noninvasive ventilatory support, including the use of a non-rebreather mask, CPAP, high-flow nasal oxygen (HFNO) oxygen, or noninvasive ventilation (NIV);
Duration of invasive ventilation;
Ventilation-free days (VFD) at 28 days from ICU admission, defined as the number of days alive and free from IMV during the first 28 days from start of IMV;
Length of stay in ICU and hospital;
Mortality in ICU and hospital, and at 28, 60, and 90 days; and
Tolerance of prone positioning (only in patients in the prone positioning group).
Randomization and blinding
Randomization will be performed using a dedicated password-protected website and will be balanced per center. Central randomization with the use of a permutated-block randomization list (with block sizes of 4 to 8) will be used. Participants will be allocated to the prone positioning or standard care on a 1:1 ratio. By the nature of the intervention, it will not be possible to blind clinicians to whether a participant has been randomized to awake proning or standard care. Interventions will be blinded to data analysts and outcome assessors.
Prone positioning
The study intervention will last for at least 48 h and is divided in 4 blocks of 6 h each: patients will be placed in the prone position for up to 2 h, which can be prolonged if the patient feels comfortable, but could also be interrupted if a patient meets any of the discontinuation criteria which are any of the following:
Developing a contraindication (Table 1);
Worsening of dyspnea (at any time, according to predefined criteria, as described in Table 2);
A further and sustained drop in SpO2 refractory to an increase in FiO2; nausea or vomiting; and
Increasing hemodynamic instability that is unrelated to sedatives (if given) and cannot be corrected by vasopressor or inotrope infusion (as described in Table 3)
Table 3.
Hemodynamic instability definition
| Defined as any of the following not responding to fluid resuscitation: | |
|---|---|
|
• Systolic arterial pressure < 90 mmHg, or • Mean arterial pressure < 65 mmHg, or • Increased needs of vasopressor agents, or • ECG evidence of ischemia or significant uncontrolled ventricular arrhythmia |
During the change in position, from supine to prone or from prone to supine, FiO2 will be increased by 25% above baseline. Each change in position is guided by two healthcare workers and an attending physician, but more healthcare workers could be needed. While the patient remains in a prone position, skin protection will be used to avoid pressure sores. Also, the application of cushions will enhance patient tolerance. Arms can be at the side, in a swimmer’s position, and can be moved to increase comfort.
Food and comfort breaks are planned while patients are in supine. If the patient is receiving enteral or oral feeding, this is interrupted from 1 h before prone until a patient is in a supine position.
The best-fitting and most-tolerated oxygen interface will be used in the prone position—this could be different from patient to patient, and different from what is used in the supine position, and could differ between patients but also institutions (i.e., depending on the availability of masks with or without a reservoir bag and with or without the Venturi system, HFNO, CPAP or NIV).
Standard of care
In all patients, whether receiving prone positioning or not, the best standard of care is provided, according to the standard care by the local teams.
When in a supine position, the patient will be placed in 30–45° semi-recumbent position but this can be changed for the comfort of the patient to supine, semi-sitting, sitting, or a lateral decubitus position.
Vitals parameters, including SpO2 and the SpO2/FiO2, are continuously monitored. The oxygenation target ranges for SpO2 are 88 to 92%; this is 7 to 8 kPa for PaO2. For patients in whom the risk of potentially dangerous hypoxemia could become unacceptable during the study (e.g., in patients who develop cardiac ischemia due to cardiac infarction or failed revascularization, or severe untreatable anemia such as with Jehovah’s Witnesses), oxygenation target ranges can be higher, 94 to 96% for SpO2 and 9 to 11.5 kPa for PaO2 [13–15].
Opiates and benzodiazepines are allowed at low dosages to improve comfort.
Intubation criteria
The decision to continue with invasive mechanical ventilation is based on clinical judgment rather than isolated gasometrical criteria. Any of the following criteria should be considered for proceeding to endotracheal intubation:
Respiratory or cardiac arrest;
Respiratory pauses;
Altered level of consciousness such as uncontrolled agitation not responding to medical treatment, or a drop in the Glasgow Coma Score;
Evidence of exhaustion such as an unacceptable increase in use of accessory muscles or thoracoabdominal paradox;
Inability to clear secretions from the airway in patients with abundant sputum production, or evidence of aspiration; or
Hemodynamic instability as defined in Table 3
In addition, the presence of 2 of the following criteria within 1 h of start noninvasive ventilatory support:
Respiratory rate > 35 breaths/min, or increased respiratory rate from the baseline;
Not improving or increased dyspnea;
pH < 7.30 or less from its baseline, or PaCO2 > 20% from the baseline value; or
SpO2 < 88%
Weaning from the noninvasive oxygen delivery system
Weaning from the oxygen therapy delivery system will be done according to local protocols and preferences. Table 4 provides guidance for oxygen requirements coupled with the reduction in gas flow rates of the HFNO.
Table 4.
High-flow nasal cannula scheme for oxygen requirements coupled with gas flow rates
| FiO2 | 21–30% | 30–40% | 40–60% | 60–100% |
|---|---|---|---|---|
| Flow | 30 L/min | 30–40 L/min | 40–50 L/min | 50–70 L/min |
In case the patient is using NIV, sessions could be interrupted when the respiratory rate is < 25 breaths/min and the FiO2 < 40% (for a SpO2 88%)
Invasive ventilation
If patient continues with invasive ventilation, settings are chosen in line with the local guidelines for invasive ventilation. The use of lung-protective ventilation with a low tidal volume and low pressures is advocated. Also, sufficient levels of PEEP should be used, and prone positioning is to be applied if a patient develops severe hypoxemia, defined as PaO2/FiO2 < 150 mmHg at a minimum FiO2 of 60% and 5 cmH2O.
Fluid regimens
We advise to use a restricted fluid strategy, i.e., targeting a neutral cumulative fluid balance as soon as a patient can be weaned of vasopressors. Crystalloid infusions are preferred over colloid infusions.
Sedation
In patients under invasive ventilation, the local guideline for sedation is to be followed, and preferably consists of combinations of use of analgo-sedation over hypno-sedation, use of bolus over continuous infusion of sedating agents, and the use of sedation scores (e.g., 3 times per day, and using a Richmond Agitation Sedation Scale) [16, 17]. Also, the level of pain is to be determined, e.g., by using the Numeric Rating Scale, the Visual Analog Scale (VAS), the Critical Care Pain Observation Tool (CCPOT) or Behavioral Pain Scale (BPS).
Weaning from invasive ventilation
Weaning from invasive ventilation follows local protocols and preferences. In all patients, it should be tested whether the patient accepts assist ventilation at least two times a day; this should also be tried when the patient shows respiratory muscle activity during assist ventilation. The attending physician decides when to extubate a patient, based on general extubation criteria (i.e., responsive and cooperative, adequate cough reflex, adequate oxygenation with FiO2 ≤ 0.4, hemodynamically stable, no uncontrolled arrhythmia and a rectal temperature > 36 °C, and after successfully passing a spontaneous breathing trial (SBT) with a T-piece or ventilation with minimal support (pressure support level < 10 cmH2O) and FiO2 ≤ 0.4). In case SBTs are used, an SBT is judged as successful when the following criteria are met for at least 30 min, and the attending physician takes the final decision for extubation:
Respiratory rate < 35 breath/min;
Peripheral oxygen saturation > 90%;
Increase < 20% of heart rate and blood pressure; and
No signs of anxiety and diaphoresis.
Data collection
Data will be entered via an electronic case report from (eCRF). On ICU admission and within 48 h, demographic and baseline data and data on disease severity will be collected. Data collection includes gender, age, height, weight, date of hospital admission, date of ICU admission, cause of respiratory failure, chest X-ray, the Sequential Organ Failure Assessment (SOFA) score, the Acute Physiology and Chronic Health Evaluation II (APACHE II) score, and the Simplified Acute Physiology Score II (SAPS II).
Data on the standard of care (described below) will be collected daily for the first 48 h and every 6 h, up to day 28. The total number of hours spent in prone position in a day (cumulative), the number of prone sessions, and their duration is recorded. After the protocol, every day until day 14, clinical outcome variables are discharge of the ICU or death, whichever comes first. Data on duration of noninvasive respiratory support, length of stay in ICU and hospital, location of the patient (in ICU, hospital, another facility, or home), and life status (alive or deceased) will be assessed on 28 and 90 days (Fig. 3).
Fig. 3.
Schedule of events
One hour before each position change (supine to prone and prone to supine), the following variables will be collected for the duration of the protocol: respiratory variables (respiratory rate SpO2, SpO2/FiO2, ROX index; SpO2/FiO2: RR, dyspnea defined according to the Borg dyspnea scale); noninvasive mean arterial pressure; heart rate; noninvasive support device parameters: Venturi facemask, HFNO, CPAP, NIV; adverse effects possibly related to prone position (hypotension, bradycardia hypoxemia, pressure ulcers, vomiting, displacements of the respiratory support device, peripheral or venous central line, orogastric or nasogastric tube, or urinary catheter), if any are recorded in the eCRF.
In case prone positioning is not tolerated, all reasons for a premature change in body position are reported.
The following parameters of the respiratory support devices will be collected within 1 h before and 1 h after randomization, and every day at a fixed time point until cessation of the respiratory support: Venturi facemask: flow (liters/min), FiO2; HFNO: flow (liters/min), FiO2; CPAP: PEEP, flow (liters/min), tidal volume, FiO2; NIV: PEEP, pressure support over PEEP, flow (liters/min), tidal volume, FiO2.
If the patient meets the criteria for intubation, the reasons for intubation are documented in the eCRF.
Data on the administration on the administration of steroids, Remdesivir, Tocilizumab, Heparin/low molecular weight heparin, and antihypertensive medications are recorded. Fluid balance is collected from the nursing chart.
Power calculation
We will include a total of 650 patients. The required sample size is calculated using data from two multicenter randomized controlled trials reporting intubation rates in patients with acute hypoxemic respiratory failure [18, 19]. It was conservatively assumed that the rate of tracheal intubation will be 45% in the standard treatment group. Accordingly, 650 patients (n = 325 per group) would provide 80% power to detect a relative risk of 0.75 for the primary endpoint at a 2-sided α level of 0.05, assuming a dropout rate of 10%. After enrollment of 70% of the patients, an unblinded sample size reestimation will be performed, and sample size could be increased to a maximum of 1000 patients if needed.
Deferred informed consent
For this study, we will include patients using a strategy using deferred informed consent because we explicitly want to randomize and start ventilation according to randomization within 1 h after ICU admission. Nevertheless, the legal representative’s written informed consent will be requested as soon as possible thereafter and never later than 48 h after randomization. If informed consent is not obtained within those 48 h, or if a legal representative denies participation within this time frame, the patient is excluded, and data will no longer be used, nor will this patient be counted for the sample size of 325 inclusions in each group (that is the provisionally included patient for whom informed consent is not obtained within the time frame of 48 h is “replaced” by a new patient until the total number of 325 patients in each arm is definitively included). On the consent form, participants will be asked if they agree to use their data should they choose to withdraw from the trial. Participants will also be asked for permission for the research team to share relevant data with people from the Hospitals taking part in the research or from regulatory authorities. This trial does involve collecting biological specimens.
Statistical analysis
Statistical analysis will be based on the intention-to-treat principle. We will also perform a per-protocol analysis, comparing patients who received awake prone positioning and patients who received standard of care.
Continuous normally distributed variables will be expressed by their mean and standard deviation or when not normally distributed as medians and their interquartile ranges. Categorical variables will be expressed as n (%). Where appropriate, statistical uncertainty will be expressed by the 95% confidence levels.
The primary outcome, the number of days free of ventilation at day 14, will be analyzed using Cox’s regression. The possible imbalance between groups will be modeled in the Cox model. To further compare groups, Student’s t test will be used. If continuous data is not normally distributed, the Mann-Whitney U test will be used. Categorical variables will be compared with the chi-square test or Fisher’s exact tests. Time-dependent data will be analyzed using a proportional hazard model adjusted for possible imbalances of patients’ baseline characteristics. No interim analysis will be conducted.
The level and pattern of missing data in the baseline variables and outcomes will be established by forming appropriate tables and the likely causes of any missing data will be investigated. If necessary, multiple imputation or Bayesian methods for missing data will be used.
A P value < 0.05 is considered statistical significance. The analysis will be performed with R statistics version 3.0.2 (R Foundation, Vienna, Austria).
Study organization
The steering committee comprises the principal investigator, the coordinating investigators, and eight international experts in ventilatory support in critically ill patients.
The coordinating investigator is responsible for administrative management and communication with the local investigators and assists at the participating clinical sites in trial management, record keeping, and data management. The coordinating investigators help set up local training in the participating ICUs to ensure the study is conducted according to the ICH-GCP guidelines, guarantee data collection integrity, and ensure timely completion of the case report forms. The local investigators provide structural and scientific leadership. They guarantee the integrity of data collection and ensure timely completion of the case report forms.
An independent monitor will be installed. Remote monitoring utilizing queries on the database will be done by a statistician and analyzed by the monitor to signalize early aberrant patterns, trends, issues with consistency or credibility, and other anomalies. On-site monitoring will comprise controlling the presence and completeness of the research dossier and the informed consent forms, and source data checks will be performed in the files of 25% of the patients.
An independent Data and Safety Monitoring Board (DSMB) watches over the ethics of conducting the study under the Declaration of Helsinki and monitors safety parameters and the overall conduct of the research. The DSMB is composed of three independent individuals (Prof. Arthur Slutsky, Prof. Claude Guerin, and Dr. Tài Pham). The DSMB will meet by conference calls. The first meeting is scheduled after the first 100 patients. After this meeting, the DSMB will meet every 6 months.
All unexpected adverse events will be reported to the DSMB. Any report or advice of the DSMB will be sent to the sponsor of the study, the Institut d'Investigació i Innovació Parc Taulí (I3PT), Sabadell, Spain. Should the sponsor decide not to implement the advice of the DSMB fully, the sponsor will send the recommendation to the reviewing Institutional Review Board, including a note to substantiate why (part of) the advice of the DSMB will not be followed. All substantial amendments will be notified to the Sponsor Ethical Review Board first and the Funder. Then the PI will notify the centers, and a copy of a revised protocol will be sent back to the PI to add to the Investigator Site File. Any deviations in the protocol will be fully documented using a breach report form. Non-substantial amendments (typing errors and administrative changes) will not be notified to the Sponsor Ethical Review Board. The protocol will be updated in the clinical trial registry.
Discussion
PRONELIFE is among the first clinical trials of awake prone positioning in patients with acute hypoxemic respiratory failure that will recruit a sufficient number of patients to test the hypothesis that this intervention prevents tracheal intubation. PRONELIFE will also collect data regarding other patient-centered outcomes, including duration of ventilation if needed, length of stay in ICU and hospital, and mortality. We will also study the tolerability of awake prone positioning and its effects on oxygenation.
Invasive ventilation is a life-support strategy associated with serious complications that may result in significant morbidity and mortality. In addition, endotracheal intubation is a high-risk procedure in critically ill patients, with major complications like severe hypoxemia, cardiovascular instability, and even cardiac arrest [20–22]. The primary endpoint of this study is a composite of the need for invasive ventilation and death within the first 14 days. We decide to use this composite endpoint, hypothesizing that the intervention under study will impact two outcomes that are invariable related. Using a composite outcome will also increase the event rate, improve the statistical efficiency for sample size calculation, and improve the study’s precision. We chose the need for invasive ventilation and death within the first 14 days because we hypothesize that the impact of awake prone positioning will not last beyond the first 2 weeks. Of note, we will report the two components of the composite as two secondary endpoints.
Evidence for the benefit of awake prone positioning in nonintubated ICU patients is scarce. Its first use was described in a case series of patients with hypoxemic respiratory failure, showing improvements in oxygenation [8]. Improvements in oxygenation were also reported in two studies in patients with severe hypoxemia after lung transplantation [5, 23]. A rapidly growing number of mainly small observational studies describe the use of awake prone positioning in patients with spontaneous breathing with acute respiratory failure in whom hypoxemia is refractory to supplementary oxygen. It has been shown that awake prone positioning can improve oxygenation within minutes [24–28], and the effects are maintained for up to 1 h after turning back to supine and disappear mostly after 6 to 12 h [29]. A total of 15 studies, representing 449 patients, were recently used in one meta-analysis [30], assessing the change in oxygenation (i.e., PaO2/FiO2 ratio, PaO2, and SpO2) induced by awake prone positioning. Two recent studies failed to show benefit of awake prone positioning in patients with acute respiratory failure [31, 32]. Of note, one of these studies had an observational design, and it remained uncertain whether the intervention was used as a routine or life-saving therapy [32]. The other study was a randomized clinical trial that was underpowered for sample size estimation [31]. In both studies, patients were only included patients with COVID-19 infection. Evidence suggests that direct versus indirect causes of lung injury possess different pathophysiologic characteristics that might impact both the progression and outcome of ARDS [33–35]. Therefore, the prone position could affect mortality, and its impact may vary according to the etiology of lung injury. Also, in both studies, the intubation criteria were not clearly defined. Our study will include different etiologies of acute respiratory failure, and we have clearly described intubation criteria.
Awake prone positioning is an attractive intervention for several reasons. First, many patients with hypoxemic acute respiratory failure seem to tolerate awake prone positioning relatively well [24, 36, 37]. Awake prone positioning is also associated with few complications. Data are limited, but thus far, no severe adverse events have been described in the literature. Pressure sores, frequently developing in intubated ICU patients [38] and with higher morbidity [39], have only been rarely described in patients who received awake prone positioning [9, 31]. Intolerance to awake prone positioning could be related to musculoskeletal discomfort [24, 26, 37, 40], nausea and vomiting [26], cough [37], and anxiety [40]. Our study will focus on these aspects.
PRONELIFE will also collect other important and patient-centered endpoints such as dyspnea sensation, duration of use of noninvasive ventilatory support, and duration of invasive ventilation and the classical ICU endpoints like the length of ICU and hospital stay, and various mortality rates.
We anticipate the PRONELIFE study to be highly feasible and easy to conduct as the study procedures are straightforward, without difficult or complex interventions making the treatment of patients clear and easy. Furthermore, the PRONELIFE study uses a deferred consent strategy to include patients rapidly in the study and those admitted during evenings or nights when researchers and family members are often not around to ask for informed consent. This will create a study population representative of the average ICU population. Also, PRONELIFE will be performed in the ICUs of different hospitals worldwide, increasing the generalizability of its findings. Furthermore, we include patients with any cause of respiratory failure.
One important limitation of PRONELIFE is that blinding is not possible due to the nature of the intervention tested, which could induce bias. However, the indication for invasive ventilation, which directly influences the primary outcome, is clearly stated, and all analyses will be performed in a blinded fashion. Second, we foresee that another limitation could derive from the time under prone position a patient with spontaneous breathing could tolerate continuously. Knowing from studies that the benefit of prone position in ARDS could depend on its duration [41], the protocol design will count the total time of prone positioning for each patient. Although patients randomized to the intervention will have 2 h under prone position, this strategy could be further prolonged if the patient feels comfortable.
The results of PRONELIFE have the potential to change clinical practice in terms of how we manage patients with acute hypoxemic respiratory failure.
In conclusion, the PRONELIFE study is an international, investigator-initiated randomized clinical trial that is adequately powered to test the hypothesis that awake prone positioning benefits ICU patients with acute hypoxemic respiratory failure.
Trial status
Pending to start recruiting.
Date recruitment will begin in 1 February 2022.
Recruitment will be completed in February 2024.
Protocol Version 5.4, 16 July 2021.
Acknowledgements
Funding source: this study is an investigator-initiated trial, funded by the Institut d'Investigació i Innovació Parc Taulí (I3PT). This study is partially funded and Sponsored by the European Society of Intensive Care Medicine (ESICM) and the Spanish Respiratory Society (SEPAR).
Abbreviations
- APACHE II
Acute physiology and chronic health evaluation II
- ARDS
Acute respiratory distress syndrome
- BPS
Behavioral pain scale
- CCPOT
Critical care pain observation tool
- CONSORT
Consolidated standards of reporting trials
- CPAP
Continuous positive airway pressure
- DSMB
Data and safety monitoring board
- eCRF
Electronic case report form
- FiO2
Fraction of inspired oxygen
- HFNO
High-flow nasal oxygen
- ICH-GCP
International conference on harmonization-good clinical practice
- IMV
Invasive mechanical ventilation
- ICU
Intensive care unit
- NIV
Noninvasive ventilation
- PaCO2
Partial pressure of arterial carbon dioxide
- PaO2
Partial pressure of arterial oxygen
- PEEP
Positive end-expiratory pressure
- ROX
Respiratory OXygenation
- SAPS II
Simplified acute physiology score II
- SBT
Spontaneous breathing trial
- SOFA
Sequential organ failure assessment
- SpO2
Peripheral capillary oxygen saturation
- VAS
Visual analog scale
- VFD
Ventilator-free days
Authors’ contributions
LMQ, MJS, and AA designed the study. LMQ, MJS, ASN, MA, DLG, OR, NJ, LlB, CH, DM, MCR, and AA approved the design of PRONELIFE. ASN performed the power calculations and designed the statistical analysis plan. LMQ prepared the initial draft of this manuscript. LMQ, MJS, ASN, MA, DLG, OR, NJ, LlB, CH, DM, MCR, and AA approved the initially submitted version of this manuscript. The authors read and approved the final manuscript.
Funding
This article has partial funding from the Institut d'Investigació i Innovació Parc Taulí (I3PT).
Availability of data and materials
Morales-Quinteros, Serpa Neto, Artigas, and Schultz had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; the members of the Steering Committee for PRONELIFE Collaborative Group vouch for the accuracy and completeness of the data and for the fidelity of the study to the protocol. All data needed to evaluate the conclusions of the trial will be present and tabulated in the final manuscript. Individual de-identified raw data will be available from the corresponding author on reasonable request during the first year after publication of the primary manuscript arising from this study.
Declarations
Ethics approval and consent to participate
The study was approved by the referral ethics committee Sagrat Cor University Hospital, Barcelona, Spain (reference number 2019/68-UCI-HUSC) (Additional file 1). For inclusion into the study, and due to national emergency regulations, verbal informed consent from the patient’s relatives or legal representative will be provided (Additional file 2). See Additional file 3 for the SPIRIT checklist of the study protocol.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stefan MS, Shieh MS, Pekow PS, Rothberg MB, Steingrub JS, Lagu T, Lindenauer PK. Epidemiology and outcomes of acute respiratory failure in the United States, 2001 to 2009: a national survey. J Hosp Med. 2013;8(2):76–82. doi: 10.1002/jhm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gattinoni L, Taccone P, Carlesso E, Marini JJ. Prone position in acute respiratory distress syndrome. Rationale, indications, and limits. Am J Respir Crit Care Med. 2013;188(11):1286–1293. doi: 10.1164/rccm.201308-1532CI. [DOI] [PubMed] [Google Scholar]
- 3.Scholten EL, Beitler JR, Prisk GK, Malhotra A. Treatment of ARDS with prone positioning. Chest. 2017;151(1):215–224. doi: 10.1016/j.chest.2016.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ding L, Wang L, Ma W, Hangyon H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Crit Care. 2020;24(1):28. doi: 10.1186/s13054-020-2738-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feltracco P, Serra E, Barbieri S, Persona P, Rea F, Loy M, Ori C. Non-invasive ventilation in prone position for refractory hypoxemia after bilateral lung transplantation. Clin Transpl. 2009;23(5):748–750. doi: 10.1111/j.1399-0012.2009.01050.x. [DOI] [PubMed] [Google Scholar]
- 6.Stilma W, Åkerman E, Artigas A, Bentley A, Bos LD, Bosman TJC, de Bruin H, Brummaier T, Buiteman-Kruizinga LA, Carcò F, Chesney G, Chu C, Dark P, Dondorp AM, Gijsbers HJH, Gilder ME, Grieco DL, Inglis R, Laffey JG, Landoni G, Lu W, Maduro LMN, McGready R, McNicholas B, de Mendoza D, Morales-Quinteros L, Nosten F, Papali A, Paternoster G, Paulus F, Pisani L, Prud’homme E, Ricard JD, Roca O, Sartini C, Scaravilli V, Schultz MJ, Sivakorn C, Spronk PE, Sztajnbok J, Trigui Y, Vollman KM, van der Woude MCE. Awake proning as an adjunctive therapy for refractory hypoxemia in non-intubated patients with COVID-19 acute respiratory failure: guidance from an international group of healthcare workers. Am J Trop Med Hyg. 2021;104(5):1676–1686. doi: 10.4269/ajtmh.20-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koeckerling D, Barker J, Mudalige NL, Oyefeso O, Pan D, Pareek M, Thompson JP, Ng GA. Awake prone positioning in COVID-19. Thorax. 2020;75(10):833–834. doi: 10.1136/thoraxjnl-2020-215133. [DOI] [PubMed] [Google Scholar]
- 8.Valter C, Christensen AM, Tollund C, Schønemann NK. Response to the prone position in spontaneously breathing patients with hypoxemic respiratory failure. Acta Anaesthesiol Scand. 2003;47(4):416–418. doi: 10.1034/j.1399-6576.2003.00088.x. [DOI] [PubMed] [Google Scholar]
- 9.Scaravilli V, Grasselli G, Castagna L, Zanella A, Isgrò S, Lucchini A, Patroniti N, Bellani G, Pesenti A. Prone positioning improves oxygenation in spontaneously breathing nonintubated patients with hypoxemic acute respiratory failure: A retrospective study. J Crit Care. 2015;30(6):1390–1394. doi: 10.1016/j.jcrc.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Jayakumar D, Ramachandran Dnb P, Rabindrarajan Dnb E, Vijayaraghavan Md B, et al. Standard care versus awake prone position in adult nonintubated patients with acute hypoxemic respiratory failure secondary to COVID-19 infection-a multicenter feasibility randomized controlled trial. J Intensive Care Med. 2021;36(8):918–924. doi: 10.1177/08850666211014480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet. 2001;357(9263):1191–1194. doi: 10.1016/S0140-6736(00)04337-3. [DOI] [PubMed] [Google Scholar]
- 13.Schjørring OL, Klitgaard TL, Perner A, Wetterslev J, Lange T, Siegemund M, Bäcklund M, Keus F, Laake JH, Morgan M, Thormar KM, Rosborg SA, Bisgaard J, Erntgaard AES, Lynnerup ASH, Pedersen RL, Crescioli E, Gielstrup TC, Behzadi MT, Poulsen LM, Estrup S, Laigaard JP, Andersen C, Mortensen CB, Brand BA, White J, Jarnvig IL, Møller MH, Quist L, Bestle MH, Schønemann-Lund M, Kamper MK, Hindborg M, Hollinger A, Gebhard CE, Zellweger N, Meyhoff CS, Hjort M, Bech LK, Grøfte T, Bundgaard H, Østergaard LHM, Thyø MA, Hildebrandt T, Uslu B, Sølling CG, Møller-Nielsen N, Brøchner AC, Borup M, Okkonen M, Dieperink W, Pedersen UG, Andreasen AS, Buus L, Aslam TN, Winding RR, Schefold JC, Thorup SB, Iversen SA, Engstrøm J, Kjær MBN, Rasmussen BS. Lower or higher oxygenation targets for acute hypoxemic respiratory failure. N Engl J Med. 2021;384(14):1301–1311. doi: 10.1056/NEJMoa2032510. [DOI] [PubMed] [Google Scholar]
- 14.Mackle D, Bellomo R, Bailey M, Beasley R, Deane A, et al. Conservative oxygen therapy during mechanical ventilation in the ICU. N Engl J Med. 2020;382(11):989–998. doi: 10.1056/NEJMoa1903297. [DOI] [PubMed] [Google Scholar]
- 15.Barrot L, Asfar P, Mauny F, Winiszewski H, Montini F, Badie J, Quenot JP, Pili-Floury S, Bouhemad B, Louis G, Souweine B, Collange O, Pottecher J, Levy B, Puyraveau M, Vettoretti L, Constantin JM, Capellier G. Liberal or conservative oxygen therapy for acute respiratory distress syndrome. N Engl J Med. 2020;382(11):999–1008. doi: 10.1056/NEJMoa1916431. [DOI] [PubMed] [Google Scholar]
- 16.Ely EW, Truman B, Shintani A, Thomason JW, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289(22):2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 17.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O'Neal PV, Keane KA, Tesoro EP, Elswick RK. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 18.Frat J-P, Thille AW, Mercat A, Girault C, Ragot S, Perbet S, Prat G, Boulain T, Morawiec E, Cottereau A, Devaquet J, Nseir S, Razazi K, Mira JP, Argaud L, Chakarian JC, Ricard JD, Wittebole X, Chevalier S, Herbland A, Fartoukh M, Constantin JM, Tonnelier JM, Pierrot M, Mathonnet A, Béduneau G, Delétage-Métreau C, Richard JCM, Brochard L, Robert R. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372(23):2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 19.Grieco DL, Menga LS, Cesarano M, Rosà T, Spadaro S, Bitondo MM, Montomoli J, Falò G, Tonetti T, Cutuli SL, Pintaudi G, Tanzarella ES, Piervincenzi E, Bongiovanni F, Dell’Anna AM, Delle Cese L, Berardi C, Carelli S, Bocci MG, Montini L, Bello G, Natalini D, de Pascale G, Velardo M, Volta CA, Ranieri VM, Conti G, Maggiore SM, Antonelli M, COVID-ICU Gemelli Study Group. Anzellotti GM, Cascarano L, Ceccaroni F, de Santis P, di Muro M, Durante M, Filetici N, Gennenzi V, Gullì A, Lombardi G, Maccaglia A, Maviglia R, Mele A, Mercurio G, Michi T, Morena TC, Murdolo M, Pennisi MA, Postorino S, Potalivo A, Pozzana F, Rubino C, Savino M, Scarascia R, Scavone A, Settanni D, Silva S, Torrini F, Vargas J, Zaccone C. Effect of helmet noninvasive ventilation vs high-flow nasal oxygen on days free of respiratory support in patients with COVID-19 and moderate to severe hypoxemic respiratory failure: the HENIVOT Randomized Clinical Trial. JAMA. 2021;325(17):1731–1743. doi: 10.1001/jama.2021.4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaber S, Amraoui J, Lefrant J, Arich C, et al. Clinical practice and risk factors for immediate complications of endotracheal intubation in the intensive care unit: a prospective multicenter study. Crit Care Med. 2006;34(9):2355–2361. doi: 10.1097/01.CCM.0000233879.58720.87. [DOI] [PubMed] [Google Scholar]
- 21.Simpson GD, Ross MJ, McKeown DW, Ray DC. Tracheal intubation in the critically ill: a multi-centre national study of practice and complications. Br J Anaesth. 2012;108(5):792–799. doi: 10.1093/bja/aer504. [DOI] [PubMed] [Google Scholar]
- 22.Russotto V, Myatra SN, Laffey JG, Tassistro E, Antolini L, Bauer P, Lascarrou JB, Szuldrzynski K, Camporota L, Pelosi P, Sorbello M, Higgs A, Greif R, Putensen C, Agvald-Öhman C, Chalkias A, Bokums K, Brewster D, Rossi E, Fumagalli R, Pesenti A, Foti G, Bellani G, INTUBE Study Investigators. Abdelkarem Ahmed H, Adhikari NKJ, Agrawal K, Agrawal N, Aguirre-Bermeo H, Agvald-Öhman C, Ahmad M, Ajami S, Akhtar SN, Alghamdi A, Alhadi A, Ali SM, Ali MN, Alias A, Almekhlafi G, Alonso J, Alvarez Montenegro D, Aman R, Anstey M, Aragão I, Arnaoutoglou E, Azoulay E, Baccari L, Baliga N, Ballekatte Manjunath R, Bamane S, Bandert A, Bartholdy R, Basto M, Baturova V, Bauer PR, Bellissima A, Belsky V, Bendre P, Benini A, Besset S, Bhattacharyya M, Bielanski P, Bigatello L, Boissier F, Bokums K, Boni E, Bonney I, Bowen D, Boyer A, Brazzi L, Brewster D, Broman L, Browne A, Bruel C, Brunin Y, Bugedo G, Calamai I, Campos P, Canavosio FG, Cappellini I, Cascella M, Catorze N, Chalkias A, Champigneulle B, Chandwani J, Chao A, Chaurasia S, Chawla R, Chawla A, Cheetham O, Chemouni F, Chew Kiok L, Chien JY, Chimunda T, Chiu CT, Chiumiento F, Chou NK, Chudeau N, Colica S, Colin G, Constantin JM, Contou D, Cortegiani A, Costa PF, Costa V, Costamagna A, Cotoia A, Cracchiolo AN, Crone P, Cunha RP, Curic Radivojevic R, Das A, Dash S, de Pascale G, de Rosa S, del Sorbo L, Della Torre V, di Caprio B, di Fenza R, di Giacinto I, Dimitropoulou A, Dudda M, Edmunds C, Ehrentraut SF, el-Fellah N, Elhadi M, Elhadi A, Escudero-Acha P, Espinoza M, Esposito C, Fabretti F, Fein DG, Ferluga M, Fernandes M, Ferre A, Ferrier J, Flaksa M, Flores F, Flores Gonzalez J, Fonseca Fuentes XE, Francis R, Franco DG, Franczyk P, Frat JP, Furman M, Fusari M, Galkin P, Gallo de Moraes A, Gammaldi R, García Aguilera MF, Garofalo E, Gaszynski T, Gatward J, Ghula M, Giacomucci A, Giovannini I, Gnanadurai K, Godet T, Goffi A, Goma Fernandez G, Gonzalez M, González D, González-Castro A, Gopalakrishna KN, Gottesman E, Gros A, Guervilly C, Guitton C, Gupta M, Gupta K, Hamid T, Hamzaoui O, Hannesdottir K, Hasan S, Hossain M, Hossein S, Hraiech S, Huang CK, Hypes C, Imhmed Alkhumsi S, Islam M, Ismail MA, Ivancan V, Jacquier S, Jagiasi B, Jain N, Jamaluddin MFH, Jankowski M, Jeswani D, Jeswani D, Jha S, Jones L, Jones B, Jozwiak M, Jumic A, Kamp O, Karametos I, Karelov A, Katsoulis P, Kaufman DA, Kaushik S, Kaye CT, Kesavarapu SR, Khaled A, Khalidah H, Khan A, Khunteta S, Kindgen-Milles D, Korula SV, Kothekar A, Koul SS, Krog D, Ku SC, Kuellmar M, Kuo LC, Kuragayala SD, Kyparissi A, Labarca G, Laffey JG, Lalwani J, Landaverde A, Lascarrou JB, Laserna A, Lee CC, Legriel S, Lehr A, Leonor T, Li Y, Licciardi AL, Litton E, Lomivorotov V, Longhini F, Lopez Nava CL, Loza Gallardo LR, Lungu R, Luzi A, Ma W, Magomedov M, Makris A, Mallapura Maheshwarappa H, Maraffi T, Marcelli ME, Mariano K, Marin N, Marova N, Martin M, Martinez Gonzalez M, Maseda E, Mastroianni F, Matas M, Matos D, Maugeri JG, Mazlan MZ, Meersch M, Meher R, Mehesry TH, Meirik M, Mekontso Dessap A, Mensah K, Mercier E, Michalek P, Midya A, Mihaljevic S, Mirouse A, Mishra P, Mistry R, Moguš M, Mohd Nordin N, Mohd Samat N, Montini L, Montrucchio G, Moro V, Morocho Tutillo D, Mosier J, Mrinal S, Mudyna W, Muller G, Munta K, Munusamy S, Musso S, Muttini S, Nahla Irtiza I, Nakou E, Narkhede A, Nates J, Nespoli MR, Nespoli F, Nikitenko A, Nogueira C, O'Grady R, Odeyemi YE, Ohlsson A, Orsello A, Palaniswamy V, Palma DM, Palmese S, Pantoja Leal JN, Papandreou E, Papanikolaou M, Parotto M, Patel M, Pavlek M, Pedrotti N, Pei Hwa N, Pelagalli L, Pérez Ruiz M, Persson E, Petsiou A, Pezzi A, Philip S, Philippard F, Piegat M, Pili-Floury S, Pinciroli R, Pinto M, Piton G, Plantefeve G, Pouplet C, Pouriki S, Pradella A, Prashant K, Putensen C, Quayle A, Rahmani L, Randall I, Ray B, Regli A, Reza ST, Ricard JD, Riva I, Roca O, Rona R, Rosell J, Rowley R, Ruan SY, Rumschuessel K, Rundo A, Russo P, Russotto V, Sahu S, Sales G, Salmon-Gandonnière C, San Juan Roman N, Sánchez-Hurtado L, Sandefur BJ, Santafe M, Santoro L, Sanyal R, Saravanabavan L, Shah B, Shah M, Shin MH, Silva M, Simpson S, Sinah A, Singh AK, Singh DK, Singh N, Singh L, Skowronski L, Sosa MA, Spadaro S, Spangfors M, Sperber J, Spina R, Srivastava A, Steel A, Suarez de la Rica A, Sujeet Kumar S, Sundrani O, Sunil N, Suparna B, Surath MR, Syed Y, Szakmany T, Sztrymf B, Tabah A, Tarantino S, Tileli M, Tirape-Castro H, Toledo-Salinas O, Tramarin J, Tsiftsis D, Tucic I, Tutillo León JA, Tutino L, Tyagi VN, Vagdatli K, Varkey S, Vera MM, von Seth M, Wahlstrom C, Wan Hassan WMN, Wan Ismail WN, Wang KC, Winiszewski H, Wu J, Wu L, Yeh YC, Young P, Zani G, Zarka J, Zhao D, Zlotnik D. Intubation practices and adverse peri-intubation events in critically ill patients from 29 countries. JAMA. 2021;325(12):11164–11172. doi: 10.1001/jama.2021.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feltracco P, Serra E, Barbieri S, Milevoj M, Michieletto E, Carollo C, Rea F, Zanus G, Boetto R, Ori C. Noninvasive high-frequency percussive ventilation in the prone position after lung transplantation. Transpl Proc. 2012;44(7):2016–2021. doi: 10.1016/j.transproceed.2012.05.062. [DOI] [PubMed] [Google Scholar]
- 24.Elharrar X, Trigui Y, Dols AM, Touchon F, Martinez S, Prud’homme E, Papazian L. Use of prone positioning in nonintubated patients with COVID-19 and hypoxemic acute respiratory failure. JAMA. 2020;323(22):2336–2338. doi: 10.1001/jama.2020.8255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Despres C, Brunin Y, Berthier F, Pili-Floury S, et al. Prone positioning combined with high-flow nasal or conventional oxygen therapy in severe Covid-19 patients. Crit Care. 2020;24:256. doi: 10.1186/s13054-020-03001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ng Z, Tay WC, Ho CH. Awake prone positioning for non-intubated oxygen dependent COVID-19 pneumonia patients. Eur Respir J. 2020;56(1):2001198. doi: 10.1183/13993003.01198-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic. Acad Emerg Med. 2020;27(5):375–378. doi: 10.1111/acem.13994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Damarla M, Zaeh S, Niedermeyer S, Merck S, Niranjan-Azadi A, Broderick B, Punjabi N. Prone positioning of nonintubated patients with COVID-19. Am J Respir Crit Care Med. 2020;202(4):604–606. doi: 10.1164/rccm.202004-1331LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hallifax RJ, Porter BM, Elder PJ, Evans SB, et al. Successful awake proning is associated with improved clinical outcomes in patients with COVID-19: single-centre high-dependency unit experience. BMJ Open Respir Res. 2020;7(1):e000678. doi: 10.1136/bmjresp-2020-000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reddy MP, Subramaniam A, Lim ZJ, Zubarej A, et al. Prone positioning of non-intubated patients with COVID-19 - a systematic review and meta-analysis. Crit Care Med. 2021;49(10):e1001–e1014. doi: 10.1097/CCM.0000000000005086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosén J, von Oelreich E, Fors D, Jonsson Fagerlund M, Taxbro K, Skorup P, Eby L, Campoccia Jalde F, Johansson N, Bergström G, Frykholm P, the PROFLO Study Group. Gradin A, Ali M, Lennborn U, Bogdanovic D, Roos A, Modie M, Giesecke J. Awake prone positioning in patients with hypoxemic respiratory failure due to COVID-19: the PROFLO multicenter randomized clinical trial. Crit Care. 2021;25(1):209. doi: 10.1186/s13054-021-03602-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrando C, Mellado-Artigas R, Gea A, Arruti E, Aldecoa C, Adalia R, Ramasco F, Monedero P, Maseda E, Tamayo G, Hernández-Sanz ML, Mercadal J, Martín-Grande A, Kacmarek RM, Villar J, Suárez-Sipmann F, for the COVID-19 Spanish ICU Network. Vendrell M, Sánchez-Etayo G, Alcón A, Belda I, Agustí M, Carramiñana A, Gracia I, Panzeri M, León I, Balust J, Navarro R, Arguís MJ, Carretero MJ, Ibáñez C, Perdomo J, López A, López-Baamonde M, Cuñat T, Ubré M, Ojeda A, Calvo A, Rivas E, Hurtado P, Pujol R, Martín N, Tercero J, Sanahuja P, Magaldi M, Coca M, del Rio E, Martínez-Ocon J, Masgoret P, Caballero A, Risco R, Gómez L, de Riva N, Ruiz A, Tena B, Tio M, Jaramillo S, Balibrea JM, de Lacy FB, Otero A, Ibarzabal A, Bravo R, Carreras A, Martín-Barreda D, Alias AJ, Balaguer M, Aliaga J, Almuedo A, Alonso JR, Andrea R, Angelès GS, Arias M, Aziz F, Badía JR, Barbeta E, Torres T, Batiste G, Benet P, Borrat X, Borrell M, Bragulat E, Carmona I, Castellà M, Castro P, Ceravalls J, Comino O, Cucciniello C, de Deray C, de Diego O, de la Matta P, Farrero M, Fernández J, Fernández S, Fernández A, Ferrer M, Fervienza A, Forga MT, Forné D, Galán C, Gómez A, Guasch E, Hernández- Tejero M, Jacas A, Jiménez B, Leyes P, López T, Martínez JA, Martínez-Pallí G, Mercadal J, Muñoz G, Muñoz J, Nicolás JM, Ortiz JT, Peiró A, Pérez M, Poch E, Pujol M, Quintana E, Ramis B, Reverter E, Rovira I, Ruiz P, Sandoval E, Schneider S, Sibila O, Solé C, Soriano A, Soy D, Suárez M, Téllez A, Toapanta ND, Torres A, Urra X, Aldecoa C, Bordell A, Martín S, Andrés J, Ruiz AM, Medel GT, Villasante IB, Clavero FI, Álvarez CP, Herrera Díez JT, Trancho AG, Mandiola IS, Suarez CR, Bocos AR, Izagirre EU, de Urbina Fernández PO, López NA, Molano LP, Martínez EG, Hidalgo IV, de Orte Sancho K, Paniagua CG, Labrador GO, Larrañaga MP, Miguelez ML, Andrés EB, Laureano EU, Boedo MJM, Rodríguez BE, Camiruaga AE, Aguirre DL, Maeztu AZ, Gala AG, Korro IC, Campo AÁ, Viana AC, Enríquez AA, Rementeria XO, Campos AS, Rico RG, Damborenea PB, Momeñe MG, Ruiz BC, Rico AL, Polo AR, Grijelmo CG, Reta MC, Arroyo EM, Aparicio LA, Aspiazu II, Basabe AI, Julian IM, Rico ID, Martínez MP, Adalia R, Zattera L, Hernández IA, Altuna LL, Castells AS, Garcia AV, Núñez M, Román L, Calvo FJR, González RV, González VB, Faba P, Montenegro O, Ramírez NB, Contreras SM, Rodríguez AG, Vázquez SR, Pérez CG, Miguelez EH, Blanco IP, Rivera DG, de la Fuente AM, Pardo M, Rodriguez V, Bengoetxea U, Ramasco F, Bernal SOS, Hernando ASC, Roca AP, Yusta CF, Villabona EG, Lantero CV, Rodriguez EP, Toledo AE, Méndez DA, Rodriguez MO, Hernández RM, Alonso JN, Artazcoz II, Carazo SE, Guerrero CR, Rodríguez ER, González RM, Santos JH, Pérez JT, Mena EM, Martínez MJM, Muñoz EA, Serrano PM, Muñoz LC, Mjertan A, Martínez DG, García CR, Viejo OA, Pereira JA, Bonet AC, López DP, de Dios Tomas E, Celemin RM, Paz MLM, Gutiérrez LQ, Velasco ND, Hernández GM, del Corral FG, Arias GH, Cuesta DR, Rice AG, Sevillano EM, Molpeceres NO, Domínguez B, Lima AV, Candela Á, Acevedo Bambaren IA, Blanco MIA, Montoiro PA, Utrera FÁ, Esteruelas JA, López AA, Balvis AJB, Bardi T, Martín MB, Haserfaty JB, Camacho AB, Weimer LB, del Mar Carbonell Soto M, Seral CC, Zaballos CC, Llamas EC, Orduna PC, Cortes Forero IP, Aliseda PAC, de Pablo Pajares MA, Remesal YD, Díaz TD, Blasco NE, Martín MEE, Triviño JF, López NF, Martín CF, Pozuelo NF, Martín LG, Santos CG, Mayo DG, Rojo MG, Cibrián CG, López EH, Olmedillo BH, Gallego BI, Khonsari S, Ruiz MNM, Arroyo MM, Ortega AMM, Burcio SM, del Carmen Martín González M, Grande AM, López JJM, Rabes CM, Borja MM, Castro NM, Pérez AM, Matcan S, Viñas CM, Herrera LM, Betancur AM, Carbajo MM, Moradas JM, Pérez LM, Murias MN, González EO, Recio ÓO, Rodriguez MÁP, Roux DP, Torres LP, Lagunas DP, Corraliza JMP, Rodrigo MP, Diaz-Regaño IR, Esteban DR, Pernia VR, Saiz ÁR, Villarino BS, Palero NS, Pérez GS, Pérez JS, Romero ABS, López JT, García CT, de la Torre Concostrina M, Mesa EMU, Olarte EV, Martínez JV, Palacios RV, García GV, de Medeiros CV, Ovejero SG, Orgaz MV, Herradon PL, Gómez CC, Sarmiento-Trujillo T, Medina NG, García MM, Ramírez CE, Rivero NM, Gil JAB, Martín S, Moral MV, Galán J, Paniagua P, Pérez S, Bainac A, Arias A, Ramil E, Escudero J, Monedero P, Cara C, Lara A, Martínez EM, Mendoza J, Baines ÍR, Trull CS, López PM, Gea A, Montero A, Ibañez RA, Pitarch JVL, Alcóver FR, Herreros CÁ, Martín CS, Olmos LLO, Moruno MN, Montoto FG, Rodriguez M, Coco LF, Gamito CH, Orejudo AB, Vielma LGS, Marín YG, de Borja Amador Penco F, Domínguez MD, Ramírez SE, Carbonell JA, López BM, Martínez-Castro S, Aguilar G, Gestal M, Casas P, Rosato AO, Pan AN, Portela MA, Romar AG, Rodríguez EM, Seijo DR, Maceiras PR, Castro-Ceoane F, López EM, Gil S, Antón JG, Tirado PGC, Calvo AC, Lisbona LF, Romero MC, Gil BA, Jarne LP, Lozano MS, López DL, Abarca AP, Serrano J, Pérez-Asenjo J, Díez-Domínguez Á, Zubizarreta I, Ramos J, Fernández I, Maseda E, de la Rica AS, Veganzones J, Insausti I, Sagra J, Carrasco SD, Feijoo AM, Yagüe J, Garutti I, Parga EB, Garcia CD, Rosa EP, Navarro AT, Gabernet RF, Bernat MJ, Valls MS, Garcia-Bernalt CC, Anton JF, Sierra AA, Gomez LG, Vaqueiro OG, Marin SH, Pinzon LP, Aranda SG, Orejuela CB, Sanchez EC, Fernandez AR, Sanjosé EF, Garsabal PI, Lopez GI, Vicol A, Malagon SE, Loewe MS, Torradeflo LG, Alcaide LB, Sanclemente GB, Pujol PS, Mendoza GC, Konarska M, Almenara FB, Golska A, Blesa AC, Serra AM, Melchor JR, Moreno AN, Novo KC, López SG, Moreno EN, Tonal BG, de Pablo EL, Yañez BA, Rivero BV, Mateo BN, de Retes M, Escoda NA, Mayo CG, González RS, Escobar AR, López MLP, Peña MV, Dueñas DS, Motos AA, Abad-Gurumeta A, Errazquin AT, Ruiz ES, Pérez NG, de Borja Bau González F, Serra CM, Higueras JS, Vicente R, Ferrandis R, Martín SP, Moncho AP, Puigdollers IM, Cortés JPA, Calvo AM, Peña AP, Fernández MC, Varela M, Parada PD, Carlín RR, Aragunde SB, Chiva MIF, Agulló AJ, Ferrer AP, Galiana M, Margarit A, del Rio VM, Muxella EH, Vidal A. Awake prone positioning does not reduce the risk of intubation in COVID-19 treated with high-flow nasal oxygen therapy: a multicenter, adjusted cohort study. Crit Care. 2020;24(1):597. doi: 10.1186/s13054-020-03314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dicker RA, Morabito DJ, Pittet JF, Campbell AR, Mackersie RC. Acute respiratory distress syndrome criteria in trauma patients: why the definitions do not work. J Trauma. 2004;57(3):522–526. doi: 10.1097/01.TA.0000135749.64867.06. [DOI] [PubMed] [Google Scholar]
- 34.Calfee CS, Janz DR, Bernard GR, May AK, Kangelaris KN, Matthay MA, Ware LB. Distinct molecular phenotypes of direct vs indirect ARDS in single-center and multicenter studies. Chest. 2015;147(6):1539–1548. doi: 10.1378/chest.14-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo L, Shaver CM, Zhao Z, Koyama T, Calfee CS, Bastarache JA, Ware LB. Clinical predictors of hospital mortality differ direct and indirect acute respiratory distress syndrome. Chest. 2017;151(4):755–763. doi: 10.1016/j.chest.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sartini C, Tresoldi M, Scarpellini P, Tettamanti A, Carcò F, Landoni G, Zangrillo A. Respiratory parameters in patients with COVID-19 after using noninvasive ventilation in the prone position outside the intensive care unit. JAMA. 2020;323(22):2338–2340. doi: 10.1001/jama.2020.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coppo A, Bellani G, Winterton D, Di Pierro M, et al. Feasibility and physiological effects of prone positioning in non-intubated patients with acute respiratory failure due to COVID-19 (PRON-COVID): a prospective cohort study. Lancet Respir Med. 2020;8(8):765–774. doi: 10.1016/S2213-2600(20)30268-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Girard R, Baboi L, Ayzac L, Richard J-C, et al. The impact of patient positioning on pressure ulcers in patients with severe ARDS: results from a multicentre randomised controlled trial on prone positioning. Intensive Care Med. 2014;40(3):397–403. doi: 10.1007/s00134-013-3188-1. [DOI] [PubMed] [Google Scholar]
- 39.Labeau SO, Afonso E, Benbenishty J, Blackwood B, Boulanger C, Brett SJ, Calvino-Gunther S, Chaboyer W, Coyer F, Deschepper M, François G, Honore PM, Jankovic R, Khanna AK, Llaurado-Serra M, Lin F, Rose L, Rubulotta F, Saager L, Williams G, Blot SI, on behalf of the DecubICUs Study Team. the European Society of Intensive Care Medicine (ESICM) Trials Group Collaborators. Muzha D, Ribas AM, Lipovesty F, Loudet C, Coyer F, Eller P, Mostafa N, Honoré PM, Telleria VM, Smajic J, Nogueira PC, Nafees KMK, Hentchoya R, Rose L, Soledad J, Lin F, Cardenas Y, Reyes AG, Sustic A, Mpouzika M, Vymazal T, Jensen HI, Aguirre-Bermeo H, Maddison L, Valta M, Calvino-Gunther S, Bloos F, Adipa FE, Koulouras V, Enamorado J, Ágoston Z, Birgisdóttir H, Gupta A, Gurjar M, Kilapong B, Hashemian SM, Martin-Loeches I, Benbenishty J, Cortegiani A, Fletcher K, Hayashi Y, Waweru-Siika W, Abidi K, Lee SM, Hadri B, Dolgusevs M, Abillama FF, Jovaisa T, Thix C, Elhadi M, Nor BM, Ratnam S, Mazlan MZ, Maiyalagan S, Sánchez-Hurtado L, Belii A, Naranpurev M, Gautam P, de lange D, Parke R, Ilesanmi RE, Shosholcheva M, Petosic A, Lind R, Ffarcsi MH, Bogarin J, Hernandez AM, Mikaszewska-Sokolewicz M, Sousa B, Tomescu D, Sandesc D, Twagirumugabe T, Gusarov V, Ebaid M, Jankovic R, Slobodianiuk G, Martonova A, Knafelj R, Mer M, Maseda E, Panka B, Schefold JC, Joelsson-Alm E, Trongtrakul K, Merritt-Charles L, Besbes LO, Dikmen Y, Zgrzheblovska L, Fielding M, Rubulotta F, Khanna AK, Saager L, von der Osten I, Muzha D, Greca A, Cani A, Xhindi N, Hyska G, Ribas AM, Pinto S, Alves P, Esposito R, Valgolio E, Minope JTS, Abdala A, Ayala M, Bravo S, Bantar A, Delgado P, Badariotti G, Lipovestky F, Diaz A, Saul P, Setten M, Aucapina A, Acosta Y, Gonzalez V, Camputaro L, Baccaro F, Villa R, Diaz A, Mastantuono M, Dean E, Rostello OF, Brizuela P, Bartoli JR, Guereschi M, Quiroga C, Putruele S, Villegas P, Curilen V, Fernandez R, Nocheretti MG, Escalante RG, Loudet CI, Fernandez S, Gonzalez AL, Alvarez GA, Iglesias F, Chaparro S, Zakalik G, Pagella G, Baini M, Campos PA, Sabbag I, Schmukler A, Fonseca IP, Alvarez GM, Ramirez M, Tapia F, Bascary CA, del Valle Gimenez G, Bertoletti FP, Milioto E, Bonsignore PJM, Fernandez MA, Smith J, Chimunda T, Thompson L, Maguire T, Lin F, Chaboyer W, Coyer F, Watts S, Mitchell M, Powell M, Lye I, Parsons L, Baker N, Reynolds C, Thompson A, Masters K, Sosnowski K, Morrison L, Leslie GD, Lakshmanan R, Tabah A, Brown W, McDowell-Skaines S, McLucas A, Smith C, Tallot M, Jones S, Barakat-Johnson M, Leong T, Butcher R, Martin K, Douschan P, von Lewinski D, Eller P, Schmutz R, Kolussi U, Salman F, Ateya Z, Mostafa N, de Decker K, van Regenmortel N, Jans A, Wijnands P, Coremans S, Honore PM, de Bels D, Depuydt T, Paillet C, Jacquet LM, Swinnen W, Hannes F, Mergeay M, van de Velde S, Allaert S, Hoste P, Borin C, Balon S, Fraipont V, Biston P, de Schryver N, Dugernier T, van Cotthem I, Telleria VM, Smajic J, de Almeida AO, Jorge SA, Becker D, Schmidt RC, Oliveira E, Ramalho A, Mazocoli E, Fioretti A, Barros E, Serpa L, Bianchini S, Campanili T, Pantaleao T, Garcia PC, Ronchini ALV, Santos R, Nafees KMK, Manap NBA, Hentchoya R, Bagshaw S, Carney D, Bagshaw S, Davidow J, Bagshaw S, Rokosh E, Bagshaw S, Laizner AM, Smith S, McQuirter M, Kampayana BS, Favre R, Sills M, Laizner AM, Dallaire J, Laizner AM, Becker C, Microys S, Bowes B, Lajeunesse J, Ghosh R, Baptiste-Savoie J, Raizman R, Bagshaw S, Suen G, Taghavi N, Smith O, Fielding C, Rose L, Canales J, Molina P, Chaparro J, Sepulveda MI, Zamorano MJF, Rocha P, Villanueva X, Araya P, Dayan M, Avalos F, Li X, Liu Y, Li X, Chen X, Jiang Z, Yang J, Chen J, Yang L, Wang K, Gao J, Fang X, Zhao R, Xia X, Liu H, Li J, Wang H, Meng G, di Y, Wang D, Zhao RH, Hu LP, Fang X, Peipei X, Jiao QF, Wang HY, Xia CJ, Liu Y, Ye M, Wan Y, Wang W, Ding Y, Ren A, Gao Y, Li Q, du G, Yang J, Shen Y, Ding Y, Li N, Yuan C, Li J, Tan L, Lin Q, Guo H, Yan H, Xu X, Zhang W, Liang J, Zhang L, Tian E, Zhao Q, InSu L, Dong J, Gu Y, Liu Y, Zhao L, Wang W, Qiao H, Tuo L, Lv M, Zhu JY, Zhu JF, Wei Y, Liu M, He Y, Cheng J, Liu J, Jia N, Wei D, Li Q, Wu X, Duan H, Lin D, Liang Q, Luo X, Xiong Y, Huang RF, Fu J, Zan T, Ye M, Shi Z, Long Y, Lei Y, Liu X, Yumei C, Wang LL, Zhang Y, Xu Y, Cheng, Zhijuan W, Sun C, Song JH, Wang Y, Liu XM, Liu Y, Yuan Y, Huang Q, Yang F, Wu Y, Luo X, Bai X, Zheng H, Song M, Sun Y, Li ZS, Luo F, Liu M, Li LC, Li X, Zhang G, Xiao L, Yu T, Gao G, Wei W, Wang F, Han T, Li T, Zeng Q, Zeng JM, Long Y, Pan F, Wang J, He G, Chen H, Zhang F, Chao Y, Chunhua G, Yao X, Bai D, Liu L, Xu X, Wang Y, Liang X, Zhang N, Li X, Zhang A, Chen X, Hu XC, Zhang H, Wang RX, Tak PS, Ho SW, Jiang QX, Ding X, Hong L, Miao L, Feng Z, Huang LP, Wu J, Wang Y, Guo J, Zhang B, Ma C, Han Y, Liu C, Ding M, Luan L, Zheng J, Lv S, Jiang S, Cao W, Xue X, Li J, Liu G, Wang J, Wei X, Zhang W, Jiang Y, Yao Z, Gao L, Li J, Zhao W, Jiang M, Hao J, Zhang J, Song C, Chen F, Wang S, Hu L, Cao D, Liu Y, Wan J, Wang X, Shao H, Zhang Z, Cui X, Liu J, Zhao L, Li X, Fan L, Zhang L, Yu M, Li B, Li C, Liu L, Liu XL, Chen W, Li Y, Zhigang Z, Yuchen W, Mu C, Zhu G, Yang F, Bo Q, Li L, Chen M, Jiang JH, Yin H, Pang X, Gong YY, Yang S, Yan X, Zheng X, Lei DH, Lei L, Guo Y, Liu L, Yu J, Sun W, Bi AP, Li W, Wu Y, Li J, Ni D, Li X, Liu Y, Wu Z, Song B, Chen J, Fei Q, Xiaoyan Y, Ran Q, Xixi L, Jiao X, Ji H, Zhiping S, Hong M, Jianhong M, Hao Y, Yin L, Wang Y, Hui C, Ju W, Xia X, Huo Y, Wang Y, Chen L, Yan Y, Zhao Q, Chen H, Bao G, Cao Y, Hong L, Zhang H, Zhang Y, Xu L, Guixiang J, Li Y, Zhao HM, Huang X, Dai Z, Jian Y, Zhang H, Tian Z, Cao ZQ, Li M, Liu Y, Ouyang F, Ma F, Jin W, Ge L, Wu SF, Li J, Yuan W, Chen T, Shi G, Chen Z, Liu K, Lin X, Yuemen L, Lijuan S, Tian XF, Wang S, Feng ZX, Liu XZ, Dong Y, Zhang J, Bocui N, Jiang ZX, Yang J, Wang GX, Zhao Y, Wu X, Yang Q, Hu RL, Li XQ, Yu ZJ, Yao Y, Deng X, Xiao Y, Xie Y, Yang Y, Yang H, Zhou Y, Li Z, Xiao M, Yang YX, Tian Y, Gama LMS, Hernandez JS, Cardenas Y, Caicedo N, Marin J, Ochoa ME, Gomez M, Rojas-Suarez J, Gonzalez J, Reyes AJG, Chapeta E, Orozco E, Filipović-Grčić I, Vuković A, Pečenković S, Šuput A, Sustic A, Zivanovic-Posilovic G, Bozena A, Udiljak N, Milic M, Radivojevic RC, Mihaljevic S, Matas M, Tonkovic D, Čuljak H, Herceg I, Pavlisa G, Dobric M, Beker T, Adam VN, Goranovic T, Markoulias C, Mathaios M, Mylordou M, Achilleos E, Kleanthous P, Kotanidi V, Foka M, Charalabous I, Alexandrou A, Georgiou M, Patsalos A, Zepoy S, Constantinou C, Piza P, Vymazal T, Wiborg E, Bruhn L, Kaasby K, Pedersen KR, Mikkelsen S, Collet M, Langvad A, Andresen H, Fischer S, Kjærgård IE, Jepsen B, Husted B, Bestle M, Kodal AM, Hansen TCB, Pedersen ASB, Thomsen TD, Hoegenhaven A, From M, Frandsen TM, Henning G, Hansen A, Jensen HI, Bliksted IA, Tamayo LM, Mogrovejo P, Aguirre-Bermeo H, Palaez C, Tutillo DRM, Hurtado CV, García MF, Alvarez D, Guerrero F, Vasquez A, Kütimets M, Tamme K, Maddison L, Anvelt E, Dlamini-Sserumaga L, Löfqvist C, Lusenius V, Kauppi O, Sakki JK, Tervo-Heikkinen T, Kesti U, Merilainen M, Karjula E, Peltomaa M, Palmu A, Ahtiala M, Valta MA, Mentec H, Plantefève G, Besch G, Pili-Floury S, Ledochowski S, Déserts MD, Giacardi C, Daubin C, Massard A, le Guen Y, Blanc A, Mandaroux S, Günther SC, Avogadro P, Radavidson A, Turc J, Jochmans S, Quintard H, Boyer L, Bruel C, Philippart F, Montravers P, Atchade E, Flessel N, Chinardet B, Soulisse L, Pillard C, Ngo D, Bongiorno B, Heitzler N, Souppart V, Gautheret N, Timsit JF, Essardy F, Fartoukh M, Mehay D, Etourneau F, Farkas JC, Beuret P, Preda G, de Montmollin E, Castelain V, Jaschinski U, Rothenfusser M, Kindgen-Milles D, Dimski T, Fiedler C, Heinicke T, Meybohm P, Schulze T, Bota M, Pelz S, Odenthal T, Christ M, Bloos F, Bösl K, Chovas A, Stehr S, Simon P, Grotheer S, Schüppel S, Schaller S, Albrecht L, Stübner A, Graeser S, Kolbe N, Lausch M, Diers A, Guenther U, Riessen R, Roller M, Osei IP, Kusi-Appiah AC, Yakubu YH, Guadi-Gosh B, Dragoumanis C, Christofis C, Kazakos N, Bastani S, Martinos C, Bekos V, Papanikolaou M, Papavasilopoulou T, Efthymiou A, Chantziara V, Kyriakoudi A, Kakaras N, Diakaki C, Flevari A, Nikolaou C, Katerina K, Avramopoulou L, Tsikritsaki K, Gkiokas G, Pantiora E, Katsenos C, Patsiou EC, Alexandropoulou P, Koutsodimitropoulos I, Farmakis E, Nestora K, Chatzis M, Kondili E, Soundoulounaki S, Mousafiri O, Lepida D, Liarmakopoulou A, Koulouras V, Papathanakos G, Oikonomou M, Ioannides P, Papadopoulos D, Staikos I, Stafylaraki M, Raitsiou B, Mandis K, Ravani I, Kourelea S, Efthimiou A, Thoma G, Bakas A, Psarulis K, Anisoglou S, Papageorgiou E, Michailidou E, Tholioti T, Lavrentieva A, Sourla E, Spyropoulou A, Pantelas N, Stalika KMM, Georgakas I, Karathanou A, Tsikriki S, Dimoula A, Kanakaki S, Vakalos A, Pagioulas K, Enamorado JE, Nardai G, Hawchar F, Blondal A, Rygvadottir B, Jonasdottir RJ, Birgisdottir H, Shah B, Kaushik S, Tripathy S, Singh M, Agarwal S, Gupta M, Ahmad M, Mangal K, Bhargava V, Kushare V, Jha S, Bhakhtiani L, Gupta A, Kamal M, Gurjar M, Baronia A, Kilapong B, Susanti A, Lestari MI, Zulkifli Z, Baskoro W, Zand F, Zarei F, Mahmoodpoor A, Heidari F, Jafaraghaee F, O’Shea A, O’Shea F, O’Donnell C, Craig G, Fitzpatrick G, Dunne L, Hastings J, Marsh B, Cody C, Campbell E, Doyle D, Pacturanan M, Sheehan C, Carey A, Carter C, Mulvey R, Finn DO’CR, Motherway C, Walsh A, Kehoe J, Delossantos S, Lalor J, O’Nuallain S, Behan H, McPherson S, Corcoran A, Gordon P, Rooney G, Levy D, Azencot M, Benbenishty J, Gurevich V, Lavy A, Bendelari V, Marconi R, Barone A, Gatti C, Giampaoletti A, Borgognoni C, Ghioldi DM, Raimondo A, Castiglione G, Bruno AV, Rubulotta G, Mo A, Corso A, Girianni S, Bruni A, Garofalo E, Maggiore SM, di Risio A, Calamai I, Spina R, Spadaro S, Volta CA, Cotoia A, Mirabella L, Maulicino L, Abregal G, Donvito M, D’Ambrosio P, Binda F, Adamina I, Galazzi A, Negro A, Vaschetto R, Capuzzi F, Boschetto M, Stivanello L, Bonaccorso L, Megna C, Cortegiani A, Iozzo P, Rizzo A, Scire G, Taibi MR, Tranello FP, Manzo A, Traina L, Pastore B, Quaini A, Giusti GD, Montaldi G, Piergentili F, Mancini F, Casaioli S, Uccelli F, Guarracino F, Onelli A, di Gravio V, Cossu M, Matrona O, Rocco M, Alampi D, Dellafiore F, Ranalli F, Bossolasco M, Brizio E, Migliorino P, Cortellazi P, Rosati M, D’Ambrosio F, Quagliotto C, Roman-Pognuz E, Peratoner A, de Rosa S, Martin MA, de Sanctis F, Ciorba P, Fletcher K, Toppin P, Harding-Goldson H, Taito S, Shime N, Yamamoto R, Kanda F, Hirao A, Egi M, Noguchi A, Hashimoto S, Aya U, Sakuramoto H, Ohuchi A, Kataoka J, Maruyama K, Nakayama I, Nishime Y, Fujimoto K, Takahashi K, Tsujimoto M, Shimizu M, Waweru-Siika W, Tole E, Correia MC, Kim JH, Park S, Kim KC, Baek J, Bae JM, Park SY, Park TS, Lee HB, Park SY, Park J, Yeon-Joo L, Young-Jae C, Lee SM, Jeon K, Kim SC, Lee J, Chee HK, Huh JW, Sim YS, Kim J, Chang Y, Ahn JJ, Kang BJ, Lee WY, Lee SJ, Hadri B, Baftiu N, Krastins I, Dolgusevs M, Abillama FF, Stiban S, Feghaly ME, Gharios E, Merheb M, Benlamin M, Khaled A, Belkhair WA, Tabib M, Ashour F, Elhadi A, Tababa OWE, Khaled T, Alkhumsi SIR, Alshrif AI, Aboufray AA, Alabuzidi A, Triki AR, Elgammudi M, Zahra HB, Soula E, al-Alawi MMS, Ahmed H, Ghula MAA, Vosylius S, Mouton L, Rastegar T, Sertznig C, Martin G, Thix C, Theisen C, Ferretti C, Gils F, Gallion M, Zainudin A, Bahrin LKK, Deva SR, Rahim AHA, Wahab S, Mazlan MZ, Hassan WNW, Ismail WNW, Ali MN, Khoo TM, Samat NM, Tong JMG, Adib NAN, Nor MBM, Ratnam S, Ismail N, Ratnam S, Sulaiman SR, Foong KW, Alias A, Hua NP, Maiyalagan S, Maiyalagan S, Zermeno JM, Blanco D, Duran K, Nava CLL, Nandyelly SJR, Sanchez-Hurtado LA, Tejeda-Huezo B, del Moral Armengol M, Nava LPA, Herrera JG, de Anda GFV, Gallegos-Perez H, Hernandez-Sanchez N, Hernandez-Ponce L, Gorordo-Delsol L, Hernandez-Romero M, Gomez S, Molinar F, Ñamendys-Silva SA, Romero-Gonzalez JP, Gonzalez D, Landaverde A, Sosa MÁ, Navarro B, de Molina Serrano JIR, Iburrigarro SR, Ibarra A, Aguirre J, Martinez-Gonzalez M, Padilla NRC, Pineda AAV, Villafuerte MVE, Herrera MOG, Belii A, Naranpurev M, Baasanjav B, Hachimi A, Elkhayari M, Abidi K, Dendane T, Subedi NB, Pathak SD, Gautam P, Manandhar M, van Gulik L, van den Brink M, van Vliet P, Gerretsen B, van den Berg L, de Haan M, Tuinstra B, Kuijpers P, Reijntjens J, Vermeijden JW, Rinket M, Vanroest M, Reidinga A, Loef B, Dieperink W, Onrust M, Dormans T, Bormans L, Koopmans M, Gerritsen RT, van den Elst A, Evers M, Oiting O, Wilting R, Ramaker B, van der Kuil M, Fijen JW, Haas L, de Lange D, Haringman J, Newby L, Parke R, Gilder E, Hacking D, Dagooc R, Song R, Waibel H, Dawn F, Rapley J, Chadwick L, Chapman C, Crone P, Albrett J, Marko P, Goodson J, Browne T, Whitticase R, Davidson C, Judd H, Owens D, Onyeka T, Ugwu I, Ilesanmi R, Adejumo PO, Owojuyigbe A, Adenekan A, Uba S, Chime C, Jibrin D, Sankey BJ, Adekola O, Olanipekun S, Olanipekun S, Adekola O, Shosolcheva M, Gievski V, Kartalov A, Naumovski F, Kuzmanovska B, Trposak A, Bogoevska-Miteva Z, Rosalia R, Olsen BF, Sjobo B, Jensen KD, Sykehus D, Johansen BF, Straede E, Johansen E, Finnstrom IJ, Toellefsen A, Ostenjo H, Bjorgen H, Bratsberg B, Kristoffersen E, Skorstad EM, Hansen S, Vullum S, Lunde GA, Arntsen W, Lund M, Akselsen GR, Monstad KR, Stenset A, Haugom H, Monsen B, Høgvall L, Trudvang S, Galaaen B, Malmin SK, Andersen MH, Hargott RF, Andersen Y, Steffenak E, Nyhus M, Meland B, Hashmi M, Rivas N, Maidana E, de Jesús Ortiz A, Cabral DMB, Simi M, Aponte C, Rivas JC, Gill S, Garcia A, Alvarenga G, Cespedes L, Perez H, Moreira ML, Canete F, Gonzalez R, Monges N, Garcia A, Coman M, Pederzani M, Franco N, Aganon F, Martinez R, Noblezada-Uy D, Ellazar CG, Cerezo FD, Hernandez AM, Palo JE, Aperocho CAJ, Isanan M, Tubacka M, Jasiewicz P, Czuczwar M, Borys M, Gutysz-Wojnicka A, Glinka L, Gawda R, Mikaszewska-Sokolewicz M, Bilawicz J, Cabrita P, Vieira J, Figueiredo MF, Pinheiro CM, Antunes N, Pedro L, Ferreira F, Parente I, Varela M, Fernandes F, Martins C, Viveiros A, Cavaco R, Rita CS, Dias S, Feranandes AM, Silva P, Nunes C, Cabral J, Sousa B, Pires F, Ferreira H, Santos J, Pinto VMV, Bispo BM, Ferreira A, Molinos E, Lafuente E, Gregorio R, Costa H, Lima Â, Ferreira S, Seromenho V, Luis E, Valerio I, Cesar H, Tavares A, Alsheikhly AS, Mahmood S, Guran CT, Moise A, Filipescu DC, Luchian M, Tomescu D, Popescu M, Scutariu MA, Petrisor C, Hagau N, Grigoras I, Patrichi T, Gusarev V, Pivkina A, Kulakov V, Ignatenko O, Kovaleva J, Zhivotneva T, Zhedaeva M, Matiushkov N, Ershova O, Egorova N, Khoronenko V, Baskakov D, Sergeev D, Piradov M, Grishina L, Magomedov M, Zuev E, Gorokhovatsky U, Leonova A, Fadeeva L, Belskiy V, Galishevskiy D, Zubareva N, Tribulev M, Zueva O, Kiselev A, Kamenshchikov N, Tokareva E, Petrushin M, Starchenko I, Twagirumugabe T, Nshimyumuremyi I, Muhizi J, Buregeya E, Nzarora J, Assiri A, Ebaid MS, Almekhlafi G, Mandourah Y, Velickovic J, Veličković D, Jovanovic B, Hadzibegovic A, Stefanovic B, Misic V, Bumbasirevic V, Rajković M, Jankovic R, Stojanovic M, Gavrilovic S, Stanojević M, Martonova A, Yaghi A, Turčan A, Firment P, Slobodianiuk G, Rabarova D, Lančaričová D, Vlaovic J, Groznik M, Lukic M, Perme J, Sostaric M, Umek N, Mirkovic T, Dolenc S, Knafelj R, Fister M, Zorko N, Markota A, Yeni NP, Jali P, Schmollgruber S, Syed MR, Parag N, Wise R, Galiana M, Navarro JA, de Pablo AM, Albert P, Martinez P, Mendiara Y, Garcia B, Llinas AA, Riveiro M, Gallart E, Riera A, Sanz M, Salo S, Lajara MAG, Nieto MV, Garcia R, Pena JMG, Gorgolas MC, Isasi MA, Sierra R, Gordo F, Conejo I, Salvà-Costa V, Garzón-Tovar C, Lospitao S, Gonzalez R, Gutierrez P, Girona M, Adamuz J, Olivares PG, de Ceballos JPG, Tirado C, de Wit I, Polo ABC, del Mar Diaz Salcedo M, Ripolles-Melchor J, Martinez-Hurtado E, Alvarez JD, Arcas MLB, Gonzalez JIT, de la Ventana ABS, Calleja PLA, Alvarez RG, Zamora PS, Guerrero AO, Cosano R, Perez-Vacas J, Campos-Perez M, Barreiro EM, Sanchez LC, Diaz MG, Jimenez R, del Rio Cabajo L, Muriel DS, Alonso HF, Fernández AW, Piñan IS, Albaiceta GM, Fernandez MCI, Abos FJS, Monedero P, Chueca RM, Aguirre LG, Manosa SC, Luque CP, Calpe N, Losilla MR, Fores MT, Farre O, Fernandez O, del Rosario Villar Redondo M, Arteta Arteta DS, Sanchez MAH, Espinosa CP, Reyes LM, Domenech LC, Guillén CV, Alvarez JT, del Cotillo M, Barrueco-Francioni JE, Conde BB, Blanco MPS, Blasco ML, Clement AI, Hurtado C, Sanz LC, Perez-Torres D, Prol-Silva E, Pereira J, González IA, Cano AE, Nuñez CR, Fernadez IL, Fernandez AAM, del Bosque Diez R, Hilario B, Zalba-Etayo B, Pascual-Bielsa A, Panka B, Banwarie P, Nahar D, van Axel A, Boedjawan NN, Jansson EB, Malvemyr AS, Johansson L, Sandberg U, Tingsvik C, Mattsson G, Löf G, Spångfors M, Ringdal M, Geijer S, Orvelius L, Hylen M, Lagerhäll C, Joelsson-Alm E, Åkerman E, Hellkvist VH, Mickelsson U, Åkerman E, Wahlbom E, Larsson IM, Wallin E, Boroli F, Ory S, Schefold JC, Jong ML, Dullenkopf A, Lang M, Fleury Y, Maus M, Ben-Hamouda N, Fishman A, Hsu MY, Chang SC, Trongtratul K, Sawawiboon C, Morakul S, Khwannimit B, Merritt-Charles L, Singh K, Ventour D, Figaro-Barclay D, Sankar-Maharaj S, Mebazaa MS, Kamoun S, Elatrous S, Besbes L, Abroug F, Naija W, Elhechmi YZ, Sellami W, Hajjej Z, Merhabene T, Talik I, Kuscu OO, Dilek O, Zerman A, Dal HC, Turan S, Aydemir S, Yilmaz H, Calili DK, İzdes S, Cengiz M, Gümüş A, Taşdemir B, Kağnıcı A, Ay M, Ay SA, Caliskan G, Akbas T, Balbay AO, Efe S, Inal V, Elay G, Karabacak P, Özserezli B, Şentürk E, Demirkiran O, Bozbay S, Dikmen Y, Erdogan E, Akker M, Peker N, Ozgultekin A, Serin SO, Turan C, Karaoren G, Goksu S, Karakurt S, Arikan H, Gül F, Cinel İ, Kara I, Undar HN, Bayraktar YS, Çelik JB, Tokur ME, Aydin DT, Yildiz İ, Özcan B, Erdivanli B, Erdivanli B, Özcan B, Eroglu A, Akdağ D, Ünlü N, Fielding M, Dungca A, Ali A, Thankamma B, Reyes PE, John S, Rajendran A, Ahmad FKE, Smiley KA, Hojden S, Miller MT, Das Sasidharan Nair V, Antonio MGS, Qawasmeh KA, Shawish SA, Twiggs H, Rosado I, Babych V, Morren F, Young C, Vaughan-Jones N, Harris S, Burns K, Georgiev C, Shayamano R, Kerslake I, Creber P, Vochin A, O’Brien C, Caddell P, Hagan S, Hughes M, Torlinski T, Sherwin J, Kannan S, Markham A, Lebon R, Cupitt J, Cranshaw J, White N, Marriott V, Milner W, Groba CB, Azoia J, Polgarova P, George S, Kapoor R, Lynch C, Fox N, Cranmer K, Fox N, Llewellym T, Matthews K, Maltby L, Ibao J, Boulton K, Jarman R, Baxter K, Raj AS, Moghal A, White J, Barrowcliffe S, Pulletz M, Ganeshalingam V, Baruah R, Boulanger C, Baker H, Woods J, Ei PP, Ogbeide V, Hayden P, Matthews K, Hughes J, Balasubramanian M, Salberg A, Saha R, Holmquist D, Young C, Derbyshire C, Smith N, Stones E, Ademokun J, Popescu M, Legorburo MS, North S, Brett C, Jaundoo H, Craig J, Whiteley S, Howcroft C, Wilby L, Delve P, Shaw D, Williams K, Welters ID, McMullen J, Brett S, Flores L, Trueman-Dawkins T, Rubulotta F, Templeton M, Adams J, Smith C, Prowle J, Byers H, McDonnell A, Rose BO, Reece-Anthony R, Mendes L, Vizcaychipi M, Bull R, Lacaden G, Santiago E, Delgado CC, Farnell-Ward S, Thorpe E, Somerville J, Williams A, Cummings D, Derrick H, Brumwell S, Randell C, McCann N, Aves E, Berry G, Szakmany T, Gunter U, Pulak P, Sarkar N, Wright K, Gomes V, Jones J, Palfrey R, Camsooksai J, Lewis A, Eneas A, Tridente A, Barr L, Jovaisa T, Thomas B, Parkin E, Horner D, Frey C, Bench S, Baumber R, Broadhurst P, Jackson M, Williams L, Clark M, Paddle J, Bean S, Buckley S, Palfreeman C, Liu S, Allison N, Attwood B, Parsons P, Houghton V, Turner SJ, Higgins D, Bielskute E, Horrigan N, Jacob R, Habgood K, Zaki A, Collins A, Lord J, Ramiro C, Kubisz-Pudelko A, Kotze M, Williams H, Iovenko I, Tsarev A, Zgrzheblovska L, Briva A, Mendez G, Napolitano L, Saager L, Teig M, Lee J, Rodriguez GE, Ben-Jacob T, Potestio C, Eng T, Mahanes D, Khanna A, Duggal A, Nananmori M, Lois M, Diaz A, Karamchandani K, Bealer C, Barefield C, Terry D, Fivecoat P, Idowu O, Cata J, Clesi T, Peterson J, Hatton K, Dhaliwal J, Mueller D, Tao J, Eltorai AS, Pastores SM, Remor N, Salazar J, Bogarin J, Barkas D, Joffe A, Barnes C, Barnes C, Sona C, Schallom M, Short J, Lorenzo J, von der Osten I, Borkowska M, Llaurado-Serra M, Demarré L, Pleitinckx V, Lin F, Xing C, Li J, Debue AS, Goller S, Cortegiani A, Megna C, Afonso E, Gusarov V, Larina E, Llaurado-Serra M, Trongtrakul K, Dikmen Y, Erdogan E. Prevalence, associated factors and outcomes of pressure injuries in adult intensive care unit patients: the DecubICUs study. Intensive Care Med. 2021;47(2):160–169. doi: 10.1007/s00134-020-06234-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu Q, Wang T, Qin X, Jie Y, Zha L, Lu W. Early awake prone position combined with high-flow nasal oxygen therapy in severe COVID-19: a case series. Crit Care. 2020;24(1):250. doi: 10.1186/s13054-020-02991-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guérin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, Mercier E, Badet M, Mercat A, Baudin O, Clavel M, Chatellier D, Jaber S, Rosselli S, Mancebo J, Sirodot M, Hilbert G, Bengler C, Richecoeur J, Gainnier M, Bayle F, Bourdin G, Leray V, Girard R, Baboi L, Ayzac L. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Morales-Quinteros, Serpa Neto, Artigas, and Schultz had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; the members of the Steering Committee for PRONELIFE Collaborative Group vouch for the accuracy and completeness of the data and for the fidelity of the study to the protocol. All data needed to evaluate the conclusions of the trial will be present and tabulated in the final manuscript. Individual de-identified raw data will be available from the corresponding author on reasonable request during the first year after publication of the primary manuscript arising from this study.