Abstract
The aim of the study is to determine the trajectories of physical activity and depressive symptoms and their reciprocal relationship among community-dwelling older adults in the COVID-19 pandemic era. The study population consisted of a cohort of 511 participants aged 60 years and over, who were recruited from eight community health centers in Ya'an, China. The Physical Activity Scale for the Elderly and the Patient Health Questionnaire were respectively used to measure physical activity and depressive symptoms at three time points: before the COVID-19 outbreak (T0), during the outbreak period (T1), and after the subsidence of COVID-19 (T2). The results revealed that physical activity and depressive symptoms fluctuated substantially across T0, T1, and T2. In addition, more severe depressive symptoms at T0 and T1 were significantly associated with lower levels of physical activity at T1 and T2, but the obverse direction of physical activity being associated with subsequent depressive symptoms was not observed in the current study. These findings highlight the importance of supporting old people to remain physically active and combat mental distress early in a pandemic, and prevention and management of depressive symptoms may also be beneficial to promote physical activity.
Keywords: Physical activity, Depressive symptoms, Older adults, COVID-19, Cross-lagged panel model, Longitudinal design
1. Introduction
Physical activity confers a broad spectrum of health benefits and is a crucial component of a healthy lifestyle and disease prevention. Physically active older adults (≥60 years) are at a reduced risk of chronic diseases, functional disabilities, cognitive declines, and role limitations [1,2]. They also experience healthier ageing trajectories and improved quality of life [2]. Conversely, adopting a physically inactive lifestyle is associated with an unfavorable biomarker profile [3] and ranked the fourth leading cause of death [4]. The worldwide economic burden incurred by physical inactivity has been estimated at $67.5 billion annually for healthcare systems [5]. Physical activity guidelines for older people have recommended a minimum of 150–300 min per week of moderate-intensity aerobic physical activity or its equivalent to achieve health benefits [1,6,7]. Despite strong evidence and clear recommendations, physical activity uptake and adherence in older people remain relatively low even during “normal” times [8].
In the context of Coronavirus disease 2019 (COVID-19) pandemic, older adults are disproportionately affected by higher incidence of complications and case fatality rates, and widely regarded as the most vulnerable population group for the infection [9,10]. Public health authorities have hence notably focused on targeting this population and multiplied their efforts to convince the elderly to comply with stringent lockdowns and social distancing protocols [11]. While these confinement policies are useful in terms of infection prevention and control for the pandemic of COVID-19, restrictive practices would inevitably aggregate the pandemic of physical inactivity [12].
Moreover, social isolation and loneliness as consequences of limited interactions could possibly undermine the emotional or psychological well-being of individuals affected [13]. With a rapidly expanding geriatric population worldwide, depressive symptoms in late-life has been increasingly considered as a major health concern that is linked with great suffering and impaired functioning in daily life [14]. The overall prevalence of depressive symptoms has reached 23.6% in the Chinese elderly [15]. Living in the midst of the COVID-19 crisis, older people have to confront an overwhelming sense of uncertainty and heightened concerns of getting infected, which could intensify existing psychological distress and exacerbate the risk of mental health problems [16].
Evidence from cross-sectional investigations has found that higher levels of physical activity play a critical role in the prevention and management of depression [17,18]. Several longitudinal studies have further demonstrated that regular physical activity at baseline is related to lower risk for subsequent depression in the elderly [[19], [20], [21]]. Yet, the lagged effect of physical activity on depressive symptoms failed to gain unanimous support from earlier research. For instance, Rothon et al. [22] has found that physical activity does not protect depression longitudinally, although a cross-sectional effect was identified. On the other side, depressive symptoms have been found to inhibit subsequent participation in physical activity owing to associated feelings of insufficient energy, loss of interest or pleasure, and lack of motivation [23]. A systematic review also confirmed that baseline depression was negatively associated with ensuing levels of activity or adherence to the physical exercise regimens recommended by physicians [24]. Thus far, previous evidence does not provide a consistent picture regarding the nature of the relationship between physical activity and depressive symptoms. Additionally, COVID-19 marks an extraordinary global public health crisis of modern times that has triggered tremendous health, social, economic, and political changes around the world, which could exert profound influences on old people's physical activity and depression patterns, and subsequently induce alterations in the interplay between physical activity and depression.
Therefore, the present study aimed to determine the trajectories of physical activity and depressive symptoms and their longitudinal relationship among community-dwelling older adults in the COVID-19 pandemic era. Specific research questions were: (1) how physical activity and depressive symptoms among community-dwelling older adults fluctuated dynamically with the different stages of COVID-19 pandemic; and (2) whether changes in physical activity precede, follow, or co-occur with changes in depressive symptoms in this population.
2. Materials and methods
2.1. Study design and participants
This study employed a prospective longitudinal design with three-wave repeated data collections. The baseline data were obtained by face-to-face interviews between December 2019 and January 2020 (T0), before the COVID-19 outbreak in mainland China. We followed-up the participants via a telephone survey between March and April 2020 (T1), a period within the outbreak of COVID-19; and then followed-up again approximately seven months later (T2), when the pandemic had almost subsided in China and the social life had returned to normal.
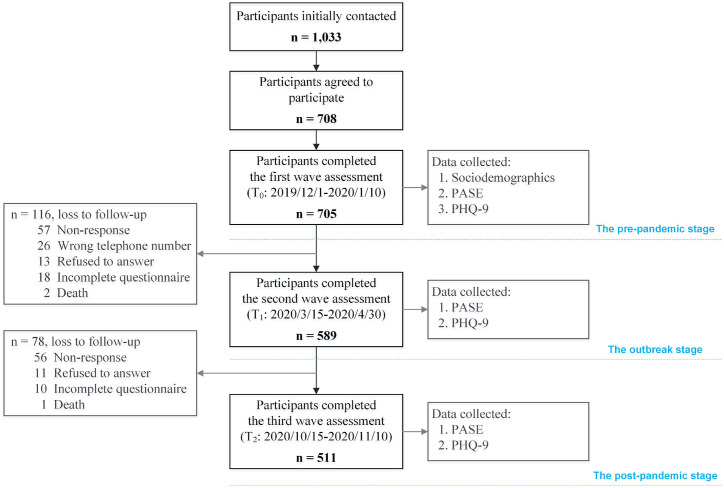
We recruited participants for this study from eight community health centers in Ya'an, a prefecture-level city with a high degree of aging located in the western part of Sichuan province, China. The local authority had rigorously implemented risk prevention and control measures deployed by central government to contain the spread of the COVID-19. Older adults were eligible to participate if they met the following inclusion criteria: aged 60 years and above, lived in the community for at least one year, able to communicate verbally in Chinese, and cognitively intact. Of 1033 participants initially approached, 705 (68.2%) took part in the first wave assessment (T0). For the subsequent two waves, only the original respondents were contacted to ensure that we could depict intrapersonal changes throughout the pandemic and maintain the longitudinal design. Finally, a total of 511 older adults provided complete data for all three measurement waves, resulting in an overall drop-out rate of 27.5% (see Fig. 1 for more details). There were no obvious differences in sociodemographic characteristics between the participants who completed the final survey and those who were lost to follow-up. The study complies with the Declaration of Helsinki, and the locally appointed ethics committee approved the research protocol and written informed consent was obtained from all participants.
Fig. 1.
Flowchart of the study participants over the study period. (PASE, the Physical Activity Scale for the Elderly; PHQ-9, the Patient Health Questionnaire).
2.2. Measures and procedure
2.2.1. Physical activity
The level of physical activity was assessed using the validated Chinese version of the Physical Activity Scale for the Elderly (PASE) questionnaire [25]. The PASE is a widely used measure in epidemiological studies to assess physical activity of older adults [26], and has been verified to be reliable and valid among the Chinese population [25,27]. The instrument is comprised of 12 self-reported items addressing leisure-, household-, and work-related activities over the preceding week. Leisure time-related activities were rated on two ordinal scales: (1) Days of Activity (0 = never to 3 = often) and (2) Hours Per Day of Activity (<1 to >4 h) to determine the frequency value (Hours Per Day) for each activity, whereas household and work-related activities were rated as ‘yes’ or ‘no’. One point was allotted for every ‘yes’ answer for a particular activity, and zero points were given for a ‘no’ response. Scores on the 12 types of activities were weighted by the corresponding strenuousness of the activity and then summed to generate an overall PASE score, which may range from 0 to 400 or more, with a higher score indicating a greater amount of physical activity.
2.2.2. Depressive symptoms
The presence and severity of depressive symptoms were measured by the Chinese version of the Patient Health Questionnaire (PHQ-9) [28]. The PHQ-9 is an internationally used depression-screening tool with robust psychometric properties, reliability and validity in adult community populations. Based on the DSM-IV depression diagnostic criteria, this tool consists of 9-item depressive symptom modules that require respondents to rate the frequency of being bothered by the particular depressive symptom experienced during the prior two weeks [29]. Items are scored on a four-point Likert scale of 0 (not at all) to 3 (nearly every day), with the scores summated to derive a total score ranging between 0 and 27. A cut-off score of 7 or greater on the PHQ-9 was recommended to indicate probable clinical depression in the general Chinese population [30].
2.2.3. Covariates
Potential covariates considered in this study included gender, age, marital status, living arrangements, individual's monthly income, presence of chronic diseases or disorders, and level of education, and were assessed at the first wave interview.
2.3. Statistical analysis
Only participants with complete data for all waves were included in our analysis. Descriptive statistics were used for participants’ characteristics and outcome measures. The trajectories of changes in physical activity and depressive symptoms over time were examined by fitting mixed-effects models which could effectively control for statistical dependencies due to repeated measures on the same participant [31]. We applied Bonferroni-corrected pairwise comparisons as post hoc tests when significant main effects on time were observed [32]. In addition, reliable change index (RCI) differentiating a reliable change from change that may have occurred due to random fluctuation in measurement [33] were calculated to determine the proportion of individuals who experienced reliable improvements or deterioration in terms of physical activity and depressive symptoms during the pandemic period.
Pearson correlations were performed to examine the associations between physical activity and depressive symptoms, both cross-sectionally and longitudinally. We then employed three-wave cross-lagged panel models (CLPM) in structural equation modeling framework [34] to explore the relationships between the outcome variables with adjustment for participants' baseline characteristics. The following four competing models were tested and compared: (1) M1, a stability model with only autoregressive paths; (2) M2, a model with autoregressive paths and cross-lagged paths from prior physical activity to later depressive symptoms; (3) M3, a model with autoregressive paths and cross-lagged paths from prior depressive symptoms to later physical activity; and (4) M4, a fully cross-lagged model with both autoregressive paths and cross-lagged paths representing reciprocal effects. The goodness of model fit was evaluated using multiple indicators including χ [2]/df, root mean square error of approximation (RMSEA), comparative fit index (CFI), and standardized root mean square residual (SRMR) [35]. An acceptable model fit is indicated by a nonsignificant χ [2], a χ [2]/df ratio of no greater than 3, a RMSEA value of 0.08 or below, a CFI value of 0.9 or above, and an SRMR value of less than 0.08 [35]. The Satorra-Bentler scaled chi-square difference tests (Δχ [2]) were applied to compare the relative fit of the nested models [36], where a significant Δχ [2] value indicates a difference in model fit and the model with a lower χ [2] value better fits to the data.
Statistical analyses were undertaken with the SPSS Statistics for Windows, version 26 (IBM Corp., Chicago, IL) and Mplus, version 8.0 (Muthen & Muthen, Los Angeles, CA). The alpha level adopted for significance (2-tailed) was set at 0.05 for all analyses.
3. Results
The final sample consisted of 511 community-dwelling adults with a mean age of 72.0 [standard deviation (SD) = 7.4] years and the majority were female (65.6%). More baseline characteristics of our analytical sample are given in Table 1 .
Table 1.
Characteristics of participants at baseline (N = 511).
| Characteristics | n (%) or mean ± SDa |
|---|---|
| Gender, male | 176 (34.4%) |
| Age (years) | 72.0 ± 7.4 |
| 60–64 | 87 (17.0%) |
| 65–69 | 128 (25.0%) |
| 70–75 | 141 (27.6%) |
| ≥76 | 155 (30.3%) |
| Marital statusb | |
| Single | 385 (75.3%) |
| Married | 126 (24.7%) |
| Living arrangement | |
| Living alone | 132 (25.8%) |
| Living with family members | 240 (47.0%) |
| Living with paid caregiver | 113 (22.1%) |
| Living with common-law partner | 26 (5.1%) |
| Monthly income (¥) | 1739.5 ± 873.2 |
| ≤1000 | 135 (26.4%) |
| 1001–1400 | 125 (24.5%) |
| 1401–2000 | 132 (25.8%) |
| >2000 | 119 (23.3%) |
| Having chronic diseases | 274 (46.4%) |
| Educational levelc | |
| Low | 245 (47.9%) |
| Medium | 79 (15.5%) |
| High | 187 (36.6%) |
SD, standard deviation.
Single indicated separated, divorced, widowed, or never married, and married indicated married or partnered.
Low educational level indicated less than lower secondary education, medium educational level indicated upper secondary or vocational training, and high educational level indicated tertiary education.
3.1. The trajectories of physical activity and depressive symptoms
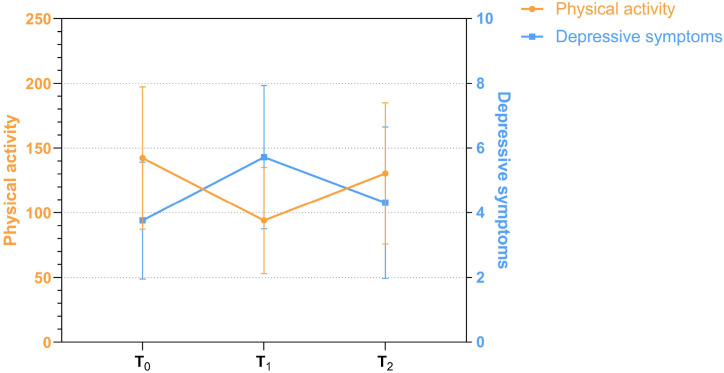
Table 2 presents the descriptive statistics for three-time repeated measurements of physical activity and depressive symptoms, and their longitudinal patterns of change are graphically depicted in Fig. 2 . The mixed-effects models revealed a significant main effect of time for both PASE [F (2, 612) = 582.0, p < 0.001] and PHQ-9 [F (2, 449) = 652.0, p < 0.001] scores. Post hoc pairwise comparisons showed that physical activity decreased substantially from T0 (the pre-pandemic stage) to T1 (the outbreak stage; ΔMPA1−0 = −48.30, p < 0.001), and then began to rebound from T1 to T2 (the post-pandemic stage; ΔMPA2−1 = 36.39, p < 0.001), but still being significantly lower than the pre-pandemic baseline level (ΔMPA2−0 = −11.91, p < 0.001). As shown in Fig. 2, the trajectory of depressive symptoms exhibited an exactly opposite direction against that of the physical activity. The interval between T0 and T1 saw an obvious surge in depression levels (ΔMDS1−0 = 1.96, p < 0.001), then the subsequent interval witnessed a relief from the heightened feelings of depression (ΔMDS2−1 = −1.41, p < 0.001). However, overall PHQ-9 scores at T2 were significantly higher than that of the baseline (ΔMDS2−0 = 0.55, p < 0.001). Further RCI analysis found that 36.4% of participants experienced clinically significant and statistically reliable reductions in physical activity (RCI < −1.96), while 62.4% suffered deterioration in depressive symptoms (RCI>1.96) during the outbreak of the COVID-19 pandemic.
Table 2.
Descriptive statistics and bivariate correlations of study variables (N = 511).
| Characteristics | Mean ± SD | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|
| 1. Physical activity at T0 | 142.3 ± 55.0 | – | |||||
| 2. Physical activity at T1 | 94.0 ± 41.0 | 0.45*** | – | ||||
| 3. Physical activity at T2 | 130.4 ± 54.5 | 0.71*** | 0.56*** | – | |||
| 4. Depressive symptoms at T0 | 3.76 ± 1.8 | −0.39*** | −0.40*** | −0.41*** | – | ||
| 5. Depressive symptoms at T1 | 5.72 ± 2.2 | −0.31*** | −0.35*** | −0.36*** | 0.68*** | – | |
| 6. Depressive symptoms at T2 | 4.31 ± 2.3 | −0.34*** | −0.33*** | −0.39*** | 0.78*** | 0.70*** | – |
*p < 0.05, **p < 0.01, ***p < 0.001.
Fig. 2.
The trajectories of physical activity and depressive symptoms from T0 to T2. (T0 = the pre-pandemic stage, T1 = the outbreak stage, T2 = the post-pandemic stage).
3.2. Longitudinal relationships between physical activity and depressive symptoms
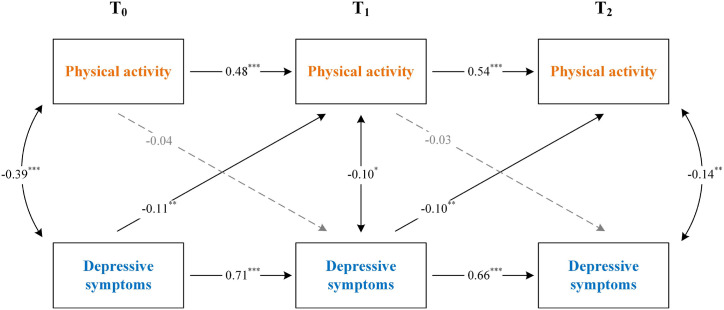
As summarized in Table 2, both cross-sectional and longitudinal associations were observed between the outcome variables, and thus there was merit in proceeding the proposed cross-lagged panel models [34]. We tested and compared four competing models to examine the relationships between physical activity and depressive symptoms. The model-data fit indicators corresponding to each hypothetical model as well as the differences in fit between models are presented in Table 3 . According to the fit indices and Satorra-Bentler chi-square tests, the fully cross-lagged model with autoregressive and cross-lagged paths (M4, see Fig. 3 ) was accepted as the best fit to the study data (χ2/df = 1.64, CFI = 0.921, RMSEA = 0.062, SRMR = 0.072). Statistically significant autoregressive loadings indicate substantial stability in study variables across assessment waves. Moreover, the CLPM unveiled significant cross-lags linking perceived depression prior to the pandemic with lower levels of physical activity during the pandemic outbreak stage (β for T0 to T1 = −0.11, p < 0.01), and also with depression during the pandemic associated with lower physical activity after the pandemic (β for T1 to T2 = −0.10, p < 0.01). However, the obverse direction of physical activity being associated with depressive symptoms was not observed (β for T0 to T1 = −0.04, p = 0.887; β for T1 to T2 = −0.03, p = 0.139). Taken together, these findings demonstrated that depressive symptoms at a preceding time point were predictive of subsequent performance on physical activity but not vice versa.
Table 3.
Summary statistics of the cross-lagged panel models and model comparison.
| Model | χ2 | df | χ2/df | CFI | RMSEA | SRMR |
|---|---|---|---|---|---|---|
| M1. Autoregressive model | 126.61 | 71 | 1.78 | 0.898 | 0.109 | .078 |
| M2. Physical activity → Depressive symptoms | 123.58 | 69 | 1.79 | 0.910 | 0.091 | .077 |
| M3. Depressive symptoms → Physical activity | 112.38 | 69 | 1.63 | 0.919 | 0.061 | .072 |
| M4. Fully cross-lagged model | 109.95 | 67 | 1.64 | 0.921 | 0.062 | .072 |
| Model comparisons | Δχ2 | df | ||||
| M1 vs. M2 | 3.03* | 2 | ||||
| M1 vs. M3 | 14.23*** | 2 | ||||
| M1 vs. M4 | 16.66*** | 4 | ||||
| M2 vs. M4 | 13.63*** | 2 | ||||
| M3 vs. M4 | 2.43* | 2 |
*p < 0.05, **p < 0.01, ***p < 0.001.
Fig. 3.
The fully cross-lagged model showing the relationships between physical activity and depressive symptoms over time. (Single headed arrows represent regression paths and double-headed arrows represent correlations. The path coefficients reflect model estimated standardized beta values. Characteristics of participants were entered in as covariates in this model, but are not depicted for clarity. *p < 0.05, **p < 0.01, ***p < 0.001).
4. Discussion
The prospective longitudinal study with three-wave repeated measures throughout the COVID-19 crisis served to illustrate the patterns of variation in physical activity and depressive symptoms among a cohort of community-dwelling Chinese adults aged 60 and above, and to clarify the nature of the relationship between them. We found the level of physical activity dramatically declined and the severity of depression remarkably worsened during the COVID-19 pandemic, and did not bounce back to their pre-pandemic levels when the crisis had generally subsided. As indicated by the RCI criterion, 36.4% and 62.4% of participants, respectively, experienced reliable and significant changes in levels of physical activity and perceived depression during the pandemic. The CLPM analyses pointed towards a unidirectional relationship whereby initial deterioration in depressive symptoms precedes a decrease in physical activity engagement. In contrast, early activity participation behaviors were not associated with later severity of depression.
The current study, based on prospectively collected longitudinal data, extended evidence from previous cross-sectional and retrospective studies of physical activity declines during the pandemic period [37,38] and reflected a substantial negative impact of COVID-19 on the amount of activity engagement. The imposition of bundles of highly restrictive measures, for instance, social distancing and self-isolation, to counteract the pandemic contagion has fostered an environment that dissuades people from remaining physically active and promotes fear of being infected. Consequently, physical inactivity became prevalent during the period and what's worse, most participants failed to resume previous activity levels several months after the restrictions lifted. This is alarming as previous research suggests that even brief periods of exposure to physical inactivity can be deleterious [39]. Therefore, the impact of lockdown restrictions on overall physical activity, an essential determinant of public health, especially for the old, vulnerable population, should be thoroughly considered in the pandemic era, particularly if prolonged restrictions are required.
We found the depression levels reported by the elderly fluctuated with the development of COVID-19 and displayed the highest level during the outbreak stage. This finding corroborates that of other recent studies suggesting the unprecedented mental distress laid by the pandemic across the whole population and vulnerable groups [16,40]. With the soaring morbidity and mortality related to COVID-19, socio-emotional fallout resulted from the rapidly changing health, social, and economic conditions was not surprising [41]. The elevated level of depression in the old population might represent a psychological response to the heightened levels of fear, perceived health risks, and an overwhelming sense of dread in the midst of a pandemic. Our findings, together with prior studies comparing populations before and after the outbreak of the COVID-19 pandemic [16,40], consolidate the significance of supporting older adults to try to adapt smoothly to new psychological demands of life early in a pandemic and providing timely and appropriately tailored mental health support throughout lockdown and its aftermath.
In the final accepted model, initial depressive symptoms were negatively related to ensuing physical activity, such that higher levels of depressive symptoms were predictive of less physical activity in older adults in the context of the pandemic. The findings confirm those of previous cross-sectional studies [17,42] and further point out the direction of the relationship between physical activity and depression. Similarly, the 6-year Longitudinal Aging Study Amsterdam (LASA) [43] that has investigated the relationship between changes in depressive symptoms and unhealthy lifestyles found that an emerging depression was associated with becoming sedentary, irrespective of a person's disease status at baseline, and was associated with a decrease in minutes of physical activity. The distressing symptoms of depression such as fatigue, lack of motivation, low self-esteem, having difficulty with problem-solving, and feelings of helplessness might explain why depressed individuals are reluctant to perform potentially rewarding health behaviors and inclined to become physically inactive. Apart from that, the behavioral model of depression in later life [44] has proposed self-critical cognitions as a mediating factor that plays a role in curtailing a depressed person's engagement in activities. The model suggested that activities are often followed by self-critical cognitions in depressed individuals, which would elicit a punishing effect on the person's efforts, and then resulting in further declines in physical activity engagement [43].
Conversely, we did not discern any lagged effects of physical activity on depressive symptoms neither at pre-pandemic stage nor at the outbreak stage, which is inconsistent with certain studies, such as studies of Lindwall et al. [20] and Ku et al. [21] The divergence between prior studies and the current one might be explained, on the one hand, by the different instruments used for assessing outcome variables; on the other, by the different study subjects we focused on. More importantly, the disruption of daily routines induced by the COVID-19 pandemic has immense deconditioning effects on the geriatric group, thereby altering the magnitude and direction of the relationship between physical activity and depressive symptoms.
4.1. Strengths and limitations
The data collection process of the present study coincided with different evolution phases of the COVID-19 pandemic, which allowed us to amass pre-pandemic baseline data and enabled the longitudinal tracking of participants’ physical activity and depressive symptoms before, during, and after the pandemic. The prospective design with repeated measures over time made it possible to scrutinize the patterns of changes, and to our knowledge, this study is the first attempt to explore the reciprocal relationship between physical activity and depressive symptoms over the pandemic period in a sample of community-dwelling older adults.
However, several limitations should be acknowledged. First, the precision and reliability of outcome assessment might be compromised due to inherent biases underpinning self-report methods and the use of telephone surveys for the second and third wave of participant followed-up. Second, while we have attempted to obtain a random, representative sample of community-dwelling older adults, females were slightly over-represented (65.6%), and importantly, caution is warranted in generalizing our findings to a larger population or different geographic settings where the COVID-19 pandemic and related public health measures might operate in a unique way. Additionally, three waves of assessments were conducted over different seasons, so the contribution of seasonality to findings remains to be examined; nevertheless, the results presented here indicate effects exceeding usual fluctuations [45]. Finally, the study was also limited by the long intervals between follow-ups, which deterred us from sketching a more specific and precise changing pattern of study variables.
5. Conclusions
While pandemic mitigation measures are essential in protecting public health, the study indicates that they affect old people's physical activity and emotional status in a way that compromises health. The study also indicates that depressive symptoms predict diminished physical activity over time, rather than the other way around among community-dwelling older adults in the COVID-19 pandemic era. The findings highlight the importance of supporting old people to remain physically active and combat mental distress early in a pandemic, and noteworthily, prevention and management of depressive symptoms may also be beneficial to postpone or diminish physical inactivity, though more research is needed to elucidate the underlying mechanisms.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors want to thank all participants for their good collaboration and nursing students from Ya'an Polytechnic College for their efforts to collect the longitudinal data.
References
- 1.World Health Organization . 2010. Global Recommendations on Physical Activity for Health.https://www.who.int/dietphysicalactivity/global-PA-recs-2010.pdf accessed 10 December 2021. [PubMed] [Google Scholar]
- 2.Cunningham C O’ Sullivan R., Caserotti P., Tully M.A. Consequences of physical inactivity in older adults: a systematic review of reviews and meta-analyses. Scand. J. Med. Sci. Sports. 2020;30(5):816–827. doi: 10.1111/sms.13616. [DOI] [PubMed] [Google Scholar]
- 3.Wirth K., Klenk J., Brefka S., et al. Biomarkers associated with sedentary behaviour in older adults: a systematic review. Ageing Res. Rev. 2017;35:87–111. doi: 10.1016/j.arr.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization . 2018. Global Action Plan on Physical Activity 2018-2030: More Active People for a Healthier World.https://apps.who.int/iris/bitstream/handle/10665/272722/9789241514187-eng.pdf accessed 10 December 2021. [Google Scholar]
- 5.Ding D., Lawson K.D., Kolbe-Alexander T.L., et al. The economic burden of physical inactivity: a global analysis of major non-communicable diseases. Lancet. 2016;388(10051):1311–1324. doi: 10.1016/S0140-6736(16)30383-X. [DOI] [PubMed] [Google Scholar]
- 6.U.S. Department of Health and Human Services . second ed. U.S. Department of Health and Human Services; Washington, DC: 2018. Physical Activity Guidelines for Americans. [Google Scholar]
- 7.Australian Government Department of Health . 2021. Recommendations on Physical Activity for Health for Older Australians.https://www.health.gov.au/health-topics/physical-activity-and-exercise/physical-activity-and-exercise-guidelines-for-all-australians/for-older-australians-65-years-and-over accessed 10 December 2021. [Google Scholar]
- 8.Sun F., Norman I.J., While A.E. Physical activity in older people: a systematic review. BMC Publ. Health. 2013;13:449. doi: 10.1186/1471-2458-13-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jordan R.E., Adab P., Cheng K.K. Covid-19: risk factors for severe disease and death. BMJ. 2020;368:m1198. doi: 10.1136/bmj.m1198. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization . 2020. Health Care Considerations for Older People during COVID-19 Pandemic.https://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/publications-and-technical-guidance/vulnerable-populations/health-care-considerations-for-older-people-during-covid-19-pandemic accessed 10 December 2021. [Google Scholar]
- 12.Kohl H.W., 3rd, Craig C.L., Lambert E.V., et al. The pandemic of physical inactivity: global action for public health. Lancet. 2012;380(9838):294–305. doi: 10.1016/S0140-6736(12)60898-8. [DOI] [PubMed] [Google Scholar]
- 13.Armitage R., Nellums L.B. COVID-19 and the consequences of isolating the elderly. Lancet Public Health. 2020;5(5):e256. doi: 10.1016/S2468-2667(20)30061-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization . 2017. Mental Health of Older Adults.https://www.who.int/news-room/fact-sheets/detail/mental-health-of-older-adults accessed 10 December 2021. [Google Scholar]
- 15.Li D., Zhang D.J., Shao J.J., Qi X.D., Tian L. A meta-analysis of the prevalence of depressive symptoms in Chinese older adults. Arch. Gerontol. Geriatr. 2014;58(1):1–9. doi: 10.1016/j.archger.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 16.Fancourt D., Steptoe A., Bu F. Trajectories of anxiety and depressive symptoms during enforced isolation due to COVID-19 in England: a longitudinal observational study. Lancet Psychiatr. 2021;8(2):141–149. doi: 10.1016/S2215-0366(20)30482-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacob L., Tully M.A., Barnett Y., et al. The relationship between physical activity and mental health in a sample of the UK public: a cross-sectional study during the implementation of COVID-19 social distancing measures. Ment. Health. Phys. Act. 2020;19:100345. doi: 10.1016/j.mhpa.2020.100345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiménez-Pavón D., Carbonell-Baeza A., Lavie C.J. Physical exercise as therapy to fight against the mental and physical consequences of COVID-19 quarantine: special focus in older people. Prog. Cardiovasc. Dis. 2020;63(3):386–388. doi: 10.1016/j.pcad.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Callow D.D., Arnold-Nedimala N.A., Jordan L.S., et al. The mental health benefits of physical activity in older adults survive the COVID-19 pandemic. Am. J. Geriatr. Psychiatr. 2020;28(10):1046–1057. doi: 10.1016/j.jagp.2020.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindwall M., Larsman P., Hagger M.S. The reciprocal relationship between physical activity and depression in older European adults: a prospective cross-lagged panel design using SHARE data. Health Psychol. 2011;30(4):453–462. doi: 10.1037/a0023268. [DOI] [PubMed] [Google Scholar]
- 21.Ku P.W., Fox K.R., Chen L.J., Chou P. Physical activity and depressive symptoms in older adults: 11-year follow-up. Am. J. Prev. Med. 2012;42(4):355–362. doi: 10.1016/j.amepre.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Rothon C., Edwards P., Bhui K., Viner R.M., Taylor S., Stansfeld S.A. Physical activity and depressive symptoms in adolescents: a prospective study. BMC Med. 2010;8:32. doi: 10.1186/1741-7015-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharifian N., Gu Y., Manly J.J., et al. Linking depressive symptoms and cognitive functioning: the mediating role of leisure activity. Neuropsychology. 2020;34(1):107–115. doi: 10.1037/neu0000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roshanaei-Moghaddam B., Katon W.J., Russo J. The longitudinal effects of depression on physical activity. Gen. Hosp. Psychiatr. 2009;31(4):306–315. doi: 10.1016/j.genhosppsych.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Ngai S.P., Cheung R.T., Lam P.L., Chiu J.K., Fung E.Y. Validation and reliability of the physical activity scale for the elderly in Chinese population. J. Rehabil. Med. 2012;44(5):462–465. doi: 10.2340/16501977-0953. [DOI] [PubMed] [Google Scholar]
- 26.Washburn R.A., Smith K.W., Jette A.M., Janney C.A. The physical activity scale for the elderly (PASE): development and evaluation. J. Clin. Epidemiol. 1993;46(2):153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 27.Vaughan K., Miller W.C. Validity and reliability of the Chinese translation of the physical activity scale for the elderly (PASE) Disabil. Rehabil. 2013;35(3):191–197. doi: 10.3109/09638288.2012.690498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen S., Fang Y., Chiu H., Fan H., Jin T., Conwell Y. Validation of the nine-item Patient Health Questionnaire to screen for major depression in a Chinese primary care population. Asia Pac. Psychiatr. 2013;5(2):61–68. doi: 10.1111/appy.12063. [DOI] [PubMed] [Google Scholar]
- 29.Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang W., Bian Q., Zhao Y., et al. Reliability and validity of the Chinese version of the Patient Health Questionnaire (PHQ-9) in the general population. Gen. Hosp. Psychiatr. 2014;36(5):539–544. doi: 10.1016/j.genhosppsych.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 31.Hayat M.J., Hedlin H. Modern statistical modeling approaches for analyzing repeated-measures data. Nurs. Res. 2012;61(3):188–194. doi: 10.1097/NNR.0b013e31824f5f58. [DOI] [PubMed] [Google Scholar]
- 32.Cao J., Zhang S. Multiple comparison procedures. JAMA. 2014;312(5):543–544. doi: 10.1001/jama.2014.9440. [DOI] [PubMed] [Google Scholar]
- 33.Jacobson N.S., Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J. Consult. Clin. Psychol. 1991;59(1):12–19. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- 34.Selig J.P., Little T.D. In: Handbook of Developmental Research Methods. Laursen B., Little T.D., Card N.A., editors. The Guilford Press; New York: 2011. Autoregressive and cross-lagged panel analysis for longitudinal data; pp. 265–278. [Google Scholar]
- 35.Cohen J., Cohen P., West S.G., Aiken L.S. third ed. Routledge; New York: 2002. Applied Multiple regression/Correlation Analysis for the Behavioral Sciences. [Google Scholar]
- 36.Satorra A., Bentler P.M. A scaled difference chi-square test statistic for moment structure analysis. Psychometrika. 2001;66(4):507–514. doi: 10.1007/BF02296192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ammar A., Brach M., Trabelsi K., et al. Effects of COVID-19 home confinement on eating behaviour and physical activity: results of the ECLB-COVID19 international online survey. Nutrients. 2020;12(6):1583. doi: 10.3390/nu12061583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rahman M.E., Islam M.S., Bishwas M.S., Moonajilin M.S., Gozal D. Physical inactivity and sedentary behaviors in the Bangladeshi population during the COVID-19 pandemic: an online cross-sectional survey. Heliyon. 2020;6(10) doi: 10.1016/j.heliyon.2020.e05392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bowden Davies K.A., Sprung V.S., Norman J.A., et al. Short-term decreased physical activity with increased sedentary behaviour causes metabolic derangements and altered body composition: effects in individuals with and without a first-degree relative with type 2 diabetes. Diabetologia. 2018;61(6):1282–1294. doi: 10.1007/s00125-018-4603-5. [DOI] [PubMed] [Google Scholar]
- 40.Sepúlveda-Loyola W., Rodríguez-Sánchez I., Pérez-Rodríguez P., Ganz F., Torralba R., Oliveira D.V., Rodríguez-Mañas L. Impact of social isolation due to COVID-19 on health in older people: mental and physical effects and recommendations. J. Nutr. Health Aging. 2020;24(9):938–947. doi: 10.1007/s12603-020-1469-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fitzpatrick K.M., Harris C., Drawve G. Living in the midst of fear: depressive symptomatology among US adults during the COVID-19 pandemic. Depress. Anxiety. 2020;37(10):957–964. doi: 10.1002/da.23080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brailovskaia J., Cosci F., Mansueto G., et al. The association between depression symptoms, psychological burden caused by Covid-19 and physical activity: an investigation in Germany, Italy, Russia, and Spain. Psychiatr. Res. 2021;295:113596. doi: 10.1016/j.psychres.2020.113596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Gool C.H., Kempen G.I., Penninx B.W., Deeg D.J., Beekman A.T., van Eijk J.T. Relationship between changes in depressive symptoms and unhealthy lifestyles in late middle aged and older persons: results from the Longitudinal Aging Study Amsterdam. Age Ageing. 2003;32(1):81–87. doi: 10.1093/ageing/32.1.81. [DOI] [PubMed] [Google Scholar]
- 44.Fiske A., Wetherell J.L., Gatz M. Depression in older adults. Annu. Rev. Clin. Psychol. 2009;5:363–389. doi: 10.1146/annurev.clinpsy.032408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harmatz M.G., Well A.D., Overtree C.E., Kawamura K.Y., Rosal M., Ockene I.S. Seasonal variation of depression and other moods: a longitudinal approach. J. Biol. Rhythm. 2000;15(4):344–350. doi: 10.1177/074873000129001350. [DOI] [PubMed] [Google Scholar]