Abstract
Dural arteriovenous fistulas (DAVFs) are high-flow acquired shunts that can carry high risk of intracranial hemorrhage. Because DAVFs can often be managed by endovascular means, early and accurate diagnosis can markedly improve patient morbidity. Time-of-flight and arterial spin-labeling MRA have increased the diagnostic utility of MRI for DAVF by showing hemodynamic rather than anatomic evidence of shunting. The purpose of this article is to describe the cases of seven patients who had colocalization of arterial spin-labeling signal intensity and time-of-flight flow-related enhancement in the left skull base, resulting in a misdiagnosis of DAVF and a recommendation for catheter angiography by the interpreting radiologist. Benign jugular venous reflux is identified as a common mechanism in each case, and the physiology behind this imaging pitfall is described. An algorithmic diagnostic approach to differentiating physiologic venous reflux from true posterior skull base DAVFs is presented.
Keywords: arterial spin labeling, arteriovenous fistula, cerebral angiography, jugular vein, reflux, MRA
Dural arteriovenous fistulas (DAVFs) are an acquired cerebral vascular disorder characterized by abnormal connections between high-pressure arteries and low-pressure veins without an intervening nidus or normal tissue capillaries [1]. DAVFs have varying presentations, ranging from clinically silent to catastrophic cerebral hemorrhage, and are notoriously challenging to diagnose with conventional noninvasive imaging. Digital subtraction angiography remains the reference standard for diagnosis. However, newer MRI techniques of assessing brain perfusion and vasculature have improved noninvasive assessment of patients with suspected DAVF. Specifically, the use of time-of-flight (TOF) and arterial spin-labeling (ASL) perfusion imaging techniques facilitates detection of small DAVFs that would otherwise be occult on MR images obtained with conventional sequences [2, 3]. TOF technique saturates stationary spins with repetitive radiofrequency pulses, allowing inflowing unsaturated spins to generate flow-related signal. ASL relies on magnetic labeling of inflowing arterial blood in the neck [4]. Both of these techniques rely on identification of what appears to be arterial signal intensity in venous structures to suggest the presence of a DAVF.
Despite improved sensitivity, areas of high signal intensity on both ASL and TOF MRA may be indistinguishable from DAVFs, even for experienced neuroradiologists [5] (Fig. 1). Misinterpretation of the findings creates risk that the interpreting radiologist will refer the patient for catheter angiography. This is one of several imaging pitfalls that have been uncovered in retrospect owing to the increased use of these MRI techniques in combination with subsequent digital subtraction angiography [6]. To explore this pitfall further, we reviewed MRI reports from a single tertiary academic center between October 2014 through October 2019 and identified seven cases in which the impression statement recommended neurointerventional consultation based on artifactual MRI findings suggestive of a posterior skull base DAVF on both ASL and TOF MRA. We present these cases, discuss the likely physiologic mechanism responsible for the artifact, and provide an algorithmic approach for radiologists to use in differentiating artifact from true posterior skull base DAVFs. We suggest an approach to interpretation that can limit misdiagnosis and avert unneeded angiography.
Fig. 1—
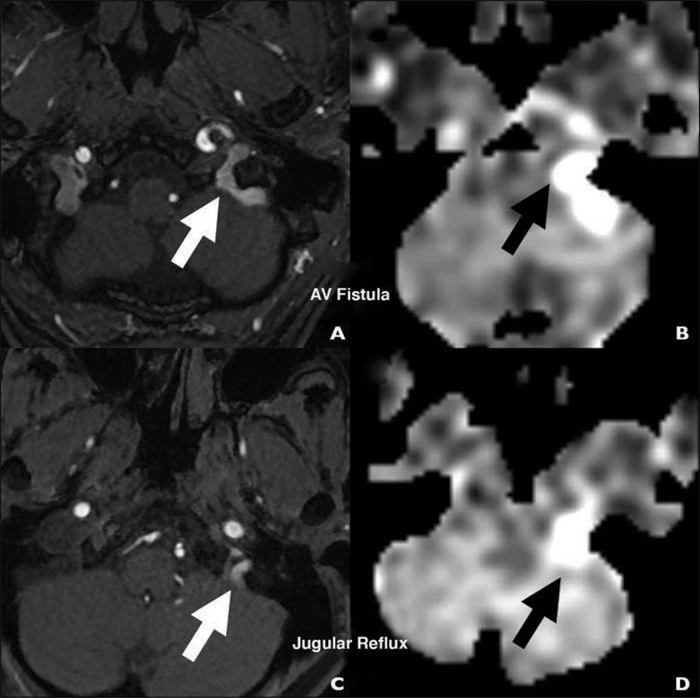
74-year-old woman with new nocturnal pulsatile tinnitus (case 7).
A and B, Colocalized arterial spin-labeling (A) and time-of-flight (B) MRA images show that signal intensity and flow-related enhancement (arrows) can be helpful for recognizing dural arteriovenous fistulas.
C and D, Colocalized arterial spin-labeling (C) and time-of-flight (D) MRA images show signal intensity and flow-related enhancement (arrows) also occur in physiologic internal jugular vein reflux.
MRI-MRA Technique
At our institution, MRI-MRA protocols that include ASL are performed exclusively at 3 T (Discovery MR750 or Signa Premier system, both GE Healthcare). We use a modified version of the International Society for Magnetic Resonance in Medicine best practices in ASL recommendations [7]. Images are acquired by means of pseudocontinuous labeling technique with TR/TE, 4931/10.5; FOV, 24 × 24 cm; slice thickness, 4 cm; number of signals acquired, 3; postlabeling delay, 2025 milliseconds. Mean tagged and untagged images are postprocessed to generate perfusion maps according to the methods of Buxton et al. [8]. Three-dimensional unenhanced TOF MRA is performed in a plane orthogonal to the skull base with TR/TE, minimum/1; FOV, 22 × 17.8 cm (frequency × phase direction); slice thickness, 1 cm; chemical fat saturation; flip angle, 15°; matrix, 640 × 480 (frequency × phase direction).
Cases
The seven cases are summarized in Table 1. Strategies used to differentiate DAVF from internal jugular reflux are summarized in Table 2.
TABLE 1:
Summary of Case Series of Patients With Colocalized High Signal Intensity on Both Arterial Spin Labeling and Time-of-Flight MRA in Left Jugular Foramen Without Dural Arteriovenous Fistulas
| Case | Indication for Initial Imaging Study | Side | Diagnostic Confirmation |
|---|---|---|---|
| 1 | Workup of new seizures | Left (nondominant) | MRA (TRICKS), DSA |
| 2 | Workup of acute stroke | Left (nondominant) | CTA, DSA |
| 3 | Incidental finding | Left (nondominant) | Resolution on follow-up MRI-MRA |
| 4 | Pulsatile tinnitus | Left (nondominant) | DSA |
| 5 | Pulsatile tinnitus | Left (nondominant) | DSA |
| 6 | Workup of acute stroke | Left (nondominant) | DSA |
| 7 | Nonpulsatile tinnitus | Left (nondominant) | Stable on follow-up MRI-MRA; resolution of symptoms |
Note—TRICKS = time-resolved imaging of contrast kinetics, DSA = digital subtraction angiography.
TABLE 2:
Summary of Strategies to Differentiate Dural Arteriovenous Fistula From Internal Jugular Reflux
| Strategy | MRI-MRA Sequences | Dural Arteriovenous Fistula | Physiologic Internal Jugular Vein Reflux |
|---|---|---|---|
| Structural | |||
| Assess arterial system for abnormality | Time-of-flight MRA | Enlarged external carotid artery branches; particular attention to ascending pharyngeal-neuromeningeal trunk) | Normal-caliber external carotid artery branches |
| Assess venous system for abnormality | Contrast-enhanced T1-weighted, susceptibility-weighted imaging | Evidence of venous sinus thrombosis | Patent venous sinuses |
| Abnormal oxygenated blood in veins | Normal deoxygenated blood in veins (T2*) | ||
| Functional | |||
| Assess for physiologic evidence of shunting | TRICKS, T2-weighted FLAIR | Early (arterial phase) opacification of veins with contrast material | Venous opacification normal or delayed |
| Susceptibility-weighted imaging | Venous congestive pattern of cerebral edema | No evidence of parenchymal congestion or edema |
Note—TRICKS = time-resolved imaging of contrast kinetics.
Case 1
A 38-year-old woman with a history of Hodgkin lymphoma treated previously with mediastinal radiation was admitted for workup of new seizures. MRI-MRA performed to exclude a vascular cause showed colocalized high signal intensity in the left jugular foramen on ASL and TOF MRA images (Figs. 2A and 2B). Time-resolved contrast-enhanced MRA showed immediate reflux of contrast medium from left antecubital injection with retrograde flow into the sigmoid sinus, inferior petrosal sinus, and pterygoid plexus before arterial filling (Figs. 2C and 2D). Subsequent cerebral angiography showed no early venous filling or enlarged branches of the external carotid artery (ECA) (Fig. 2E).
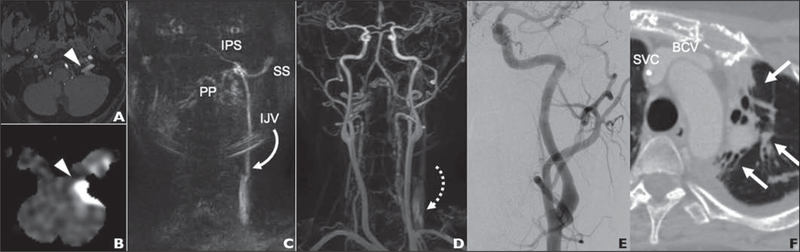
Fig. 2—
38-year-old woman with history of Hodgkin lymphoma treated previously with mediastinal radiation admitted for workup of new seizures (case 1). Video S1, also from this case, can be viewed in the AJR electronic supplement to this article, available at 10.2214/AJR.20.24012.
A and B, Axial time-of-flight (A) and arterial spin-labeling (B) MRA images show hyperintensity (arrowheads) in left jugular foramen.
C, Coronal time-resolved contrast subtraction MRA image of neck shows early retrograde flow into left internal jugular vein (IJV) extending to jugular foramen, pterygoid plexus (PP), interior petrosal sinus (IPS), and sigmoid sinus (SS).
D, MRA image acquired in later phase than C shows normal opacification of arterial system with no shunting or hypertrophied external carotid artery branches and lagging contrast at level of left IJV valve (arrow).
E, Angiogram obtained after selective injection of left common carotid artery shows no dural arteriovenous fistula.
F, Chest CT image obtained 14 days before E shows patent brachiocephalic vein (BCV) and superior vena cava (SVC) with evidence of substantial postradiation change in adjacent lung parenchyma (arrows).
Case 2
A 91-year-old woman with a history of atrial fibrillation was found unresponsive. Her NIH Stroke Scale score on arrival at the emergency department was 23, and CT angiography showed basilar thrombosis. The patient was treated with IV tissue plasminogen activator and mechanical thrombectomy. On postoperative day 1, MRI-MRA showed scattered infarcts in the thalamus and cerebellum with high signal intensity in the left jugular foramen on ASL images. There were no enlarged ECA branches, dilated venous channels, early venous opacification on CTA, or other imaging features of DAVF. Retrospective review of CTA images showed florid reflux of IV contrast material into the left jugular vein and into the sigmoid and transverse sinuses (Figs. 3A and 3B). This reversal of flow in the left internal jugular vein likely caused the abnormality on ASL images at subsequent MRI (Fig. 3C).
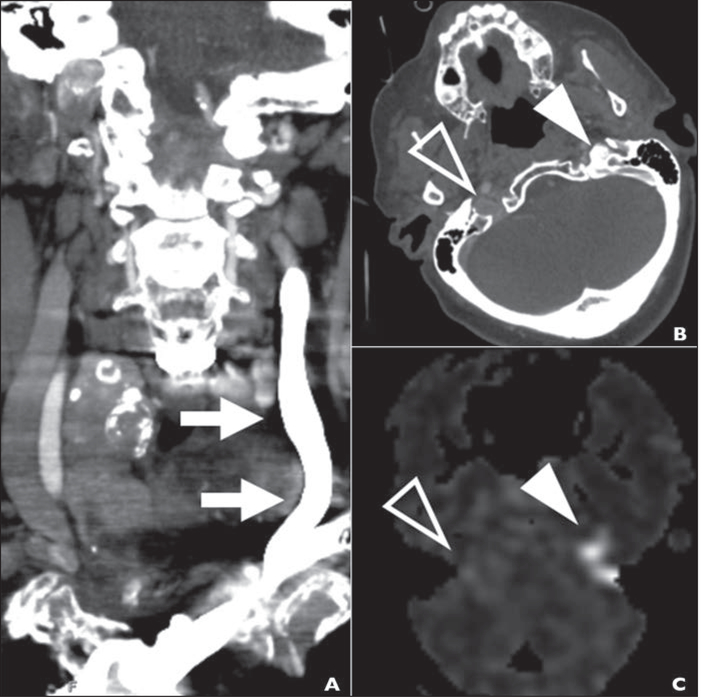
Fig. 3—
91-year-old woman with history of atrial fibrillation who was found unresponsive. NIH Stroke Scale score on arrival at emergency department was 23, and CT angiography showed basilar thrombosis (case 2).
A, CTA image obtained with left antecubital injection site to evaluate acute stroke shows florid retrograde flow of IV contrast material in internal jugular vein (arrows).
B, Axial arterial phase CTA image at skull base shows reflux of contrast material into jugular foramen (solid arrowhead) without reflux on right (open arrowhead).
C, Arterial spin-labeling MRA image obtained 24 hours after B shows pattern of hyperintensity (arrowheads) matching that in B.
Case 3
A 64-year-old woman with a WHO grade IV astrocytoma of the left frontal lobe treated with surgery and adjuvant chemotherapy 5 years earlier presented for routine tumor imaging with the addition of vascular imaging (TOF and time-resolved imaging of contrast kinetics [TRICKS] sequences) because new headaches developed after the patient started bevacizumab therapy. MRI-MRA of the brain showed colocalized high signal intensity in the left jugular vein and sigmoid sinus on ASL and TOF images (Fig. 4A). There were no enlarged ECA branches, dilated venous channels, or other imaging features of DAVF. TRICKS images showed delayed opacification of the left internal jugular vein (IJV), a finding incompatible with fistula. Because the patient had no new neurologic signs or symptoms and no imaging findings suggesting DAVF at numerous prior brain MRI examinations, the finding was suspected to be artifactual. Imaging findings were reviewed by the multidisciplinary tumor board, and 4-week follow-up MRI-MRA was recommended, which showed complete resolution of the finding on both ASL and TOF images (the TRICKS sequence was not repeated).
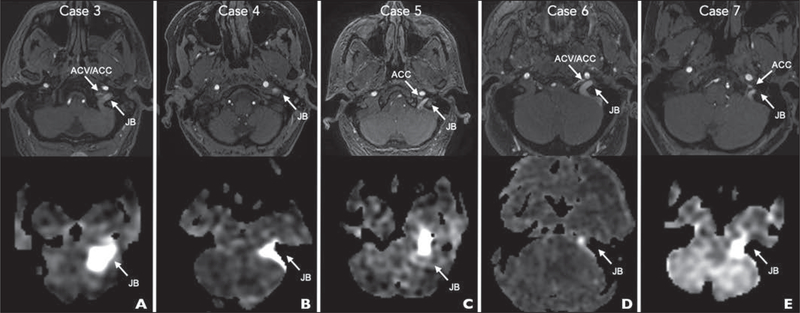
Fig. 4—
Time-of-flight (top row) and arterial spin-labeling (bottom row) MRA images show positions of jugular bulb (JB), anterior condylar vein (ACV), and anterior condylar confluence (ACC).
A, 64-year-old woman with WHO grade IV astrocytoma of left frontal lobe treated with surgery and adjuvant chemotherapy 5 years earlier presenting for routine tumor imaging (case 3).
B, 69-year-old woman with 1-year history of right-sided whooshing pulsatile tinnitus without headache (case 4).
C, 63-year-old man referred to pulsatile tinnitus clinic with episodic vertigo, nausea, headaches, and low-frequency whooshing pulsatile tinnitus (case 5).
D, 48-year-old man with vomiting, left arm clumsiness, and global weakness for 1 week and history of aortic valve replacement (case 6).
E, 74-year-old woman with new nocturnal pulsatile tinnitus (case 7).
Case 4
A 69-year-old woman presented with a 1-year history of right-sided whooshing pulsatile tinnitus without a headache. The pulsatile tinnitus was worse at night, and there were no clear provoking or alleviating factors. MRI-MRA showed abnormal ASL and TOF signal intensity in the region of the left jugular foramen (Fig. 4B). There were no enlarged ECA branches, dilated venous channels, early venous opacification on TRICKS images, or other imaging features of DAVF. Catheter angiography confirmed no DAVF but showed mild outflow stenosis of the upper cervical left internal jugular vein.
Case 5
A 63-year-old man was referred to the pulsatile tinnitus clinic with episodic vertigo, nausea, headaches, and low-frequency whooshing pulsatile tinnitus. He had a history of remote left jugular and sigmoid sinus thrombosis. The neurologic examination findings were normal. MRI-MRA showed focal high ASL signal intensity and subtle flow-related enhancement in the left jugular foramen on TOF images (Fig. 4C). There were no enlarged ECA branches, dilated venous channels, early venous opacification on TRICKS images, or other imaging features of DAVF. Imaging also revealed features of intracranial hypotension (bilateral subdural collections, sagging pontomedullary junction, and diffuse pachymeningeal enhancement). Subsequent catheter angiography excluded DAVF but showed nonocclusive thrombus of the left transverse sinus without evidence of flow restriction.
Case 6
A 48-year-old man with a history of aortic valve replacement presented after experiencing vomiting, left arm clumsiness, and global weakness for 1 week. MRI showed late acute infarctions of the posterior inferior cerebellar artery territory due to dissection of the left vertebral artery. CTA confirmed the clinical diagnosis of lateral medullary syndrome. MRI-MRA showed abnormal high signal intensity on ASL images and flow-related enhancement in the left jugular foramen, sigmoid sinus, and transverse sinus on TOF images (Fig. 4D). There were no enlarged ECA branches, dilated venous channels, early venous opacification on TRICKS, or other imaging features of DAVF. A catheter angiogram confirmed vertebral artery dissection but showed no fistula.
Case 7
A 74-year-old woman presented with new nocturnal pulsatile tinnitus. She had no headache, ataxia, dizziness, or other focal neurologic deficits. MRI showed colocalization of ASL signal intensity and abnormal flow-related TOF enhancement in the left internal jugular vein and sigmoid sinus (Fig. 4E). There were no enlarged ECA branches, dilated venous channels, early venous opacification on TRICKS images, or other imaging features of DAVF. The patient was evaluated in a pulsatile tinnitus clinic, where DAVF was considered unlikely owing to absence of bruit, exacerbation of symptoms in the supine position, low pitch, and lack of a whooshing character to the tinnitus. Therefore, digital subtraction angiography was deferred. Follow-up MRI-MRA performed 7 months later showed findings identical to those of the initial examination. At a follow-up neurologic evaluation, the pulsatile tinnitus symptoms had abated, and the patient was relieved that no invasive procedures were indicated.
Discussion
We describe a series of seven patients who had findings on TOF and ASL MRA that led to an imaging misdiagnosis of DAVF. This prompted referral to neurointerventional radiologists in all cases and further investigation with digital subtraction angiography in four (57.1%). Retrospective critical review of these cases revealed that these venous signal-intensity abnormalities reflected IJV reflux rather than arteriovenous shunting in posterior skull base DAVFs.
Although a spectrum of artifacts are well characterized for both ASL [6, 9] and TOF [10] MRA, colocalization of ASL signal intensity and TOF enhancement at the jugular foramen has only recently been recognized as a source of misdiagnosis [5]. Our review extends previous work showing TOF artifact related to reflux of venous blood, which leads to increased signal intensity in the cavernous and dural venous sinuses [11]. The possibility that jugular reflux can cause ASL artifact was first proposed by Amukotuwa et al. [6], who described it in patients who had surgical brachial arteriovenous fistulas for hemodialysis, although the phenomenon was deemed “likely quite rare.” In this series, we selected only cases that led to neurointerventional referral; the true incidence of the finding may be much higher than in this series. This finding therefore represents an important neuroimaging pitfall that should be recognized by interpreting radiologists.
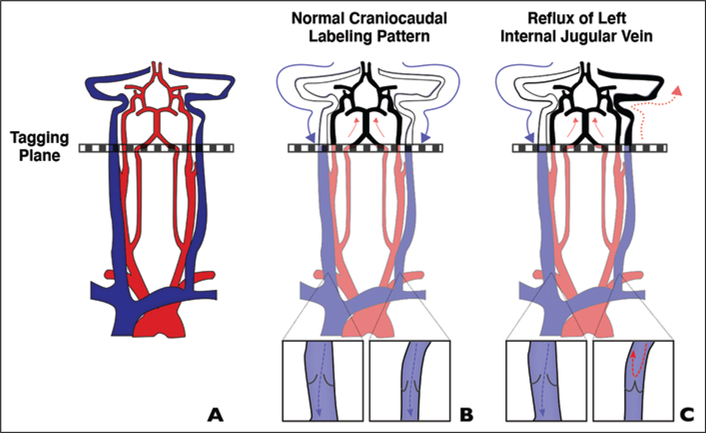
The pattern of abnormal signal intensity described, which consistently localizes to the left IJV and sigmoid sinus, suggests that IJV reflux is a common underlying causative factor. In our series, the left IJV was found to be nondominant in every case, which supports the mechanism of reflux and is consistent with the observation by Jang et al. [12]. The left IJV is nondominant in most people and tends to return a much lower fraction of cerebral blood than the larger right IJV [13]. In addition, the IJV-subclavian valve tends to be closed more often on the left than the right, which predisposes to stasis and retrograde flow of blood in the left IJV, particularly in the supine position as a patient is undergoing MRI [14, 15]. This caudal to cranial flow is likely responsible for the increased signal in the left IJV and sigmoid sinus in patients who do not have a posterior fossa DAVF (Fig. 5), as evidenced by delayed contrast filling on time-resolved MRA images in cases 3–7.
Fig. 5—
Schematic shows proposed mechanism for false localization of signal intensity to left jugular foramen on arterial spin-labeling (ASL) images.
A, In typical ASL protocols, spin-labeling is performed in cross-sectional slab (tagging plane) above carotid bifurcation.
B, With conventional flow, tagged arterial blood travels cranially to brain (red arrows) and tagged venous blood flows caudally (blue arrows), remaining below imaging plane. Inset shows valve configuration.
C, In certain patients, spin-labeled jugular blood may flow in retrograde manner to level of jugular foramen (solid red arrows) or dural sinuses (dotted red arrow), resulting in misleading appearance of arterial signal intensity. Our findings suggest that physiologic asymmetry in internal jugular vein (IJV; more frequently closed on left) contributes to reversal of flow either through increased resistance (closed valve) or from retrograde flow across incompetent left IJV valve (cases 1 and 2). Blue arrow indicates normal caudal venous blood flow. Inset shows valve configuration.
A second mechanism of reflux likely caused the abnormal signal intensity in cases 1 and 2 (Figs. 2 and 3). In these cases, contrast injection in the left arm regurgitated via an incompetent jugular valve, likely owing to increased back pressure from the rapid injection of contrast material necessary to achieve adequate bolus geometry [16]. Both mechanisms (valve closure, valve incompetence) result in retrograde venous flow to the left skull base, mimicking dural fistula. Conversely, because right-sided reflux is unusual, its presence in the right jugular foramen on ASL or TOF MRA should raise suspicion of shunting (Fig. 6). Although several reports [17–20] have linked dysfunctional venous outflow and IJV reflux with various neurologic diseases, the clinical effect of incidentally diagnosed left IJV reflux remains contentious; some degree of reflux is likely within physiologic normal limits. The importance is in recognizing the physiologic state and not misunderstanding it as a potentially dangerous DAVF.
Fig. 6—
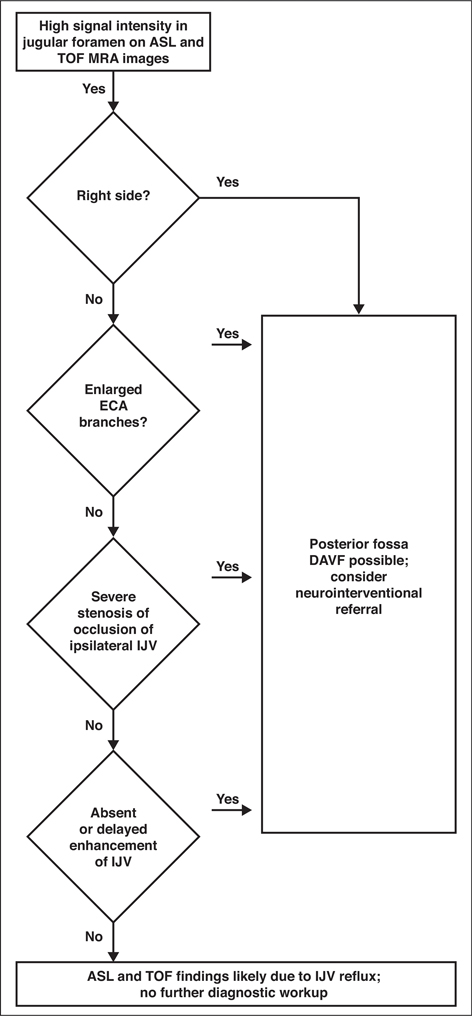
Chart shows proposed diagnostic algorithm for differentiating arterial spin-labeling (ASL) and time-of-flight (TOF) MRA findings likely due to physiologic internal jugular vein (IJV) reflux from similar imaging patterns that can indicate dural arteriovenous fistula (DAVF). ECA = external carotid artery.
Posterior fossa DAVFs, including marginal sinus, condylar vein, and sigmoid variations, should always remain in the differential diagnosis of abnormal blood flow near the skull base [21]. Heterogeneous venous drainage of these fistulas can result in a range of clinical presentations, but many are high risk. Therefore, early diagnosis with noninvasive imaging is crucial in the reduction of their associated morbidity and mortality [22]. Most posterior skull base DAVFs are supplied by branches of the ascending pharyngeal artery, which arises from the ECA. Arterial branches that supply a DAVF become enlarged. We therefore recommend assessing the size of the ipsilateral ascending pharyngeal artery and its tributaries when evaluating for DAVF. If the ipsilateral artery is not enlarged, DAVF is unlikely, and the findings are probably due to physiologic IJV reflux. Posterior fossa DAVFs typically converge with venous drainage into the IJV (Fig. 7) and as such would be expected to show increased signal intensity in the IJV on ASL and TOF MRA. However, this case series shows that this colocalizing abnormal signal intensity can be seen in the absence of a fistula.
Fig. 7—
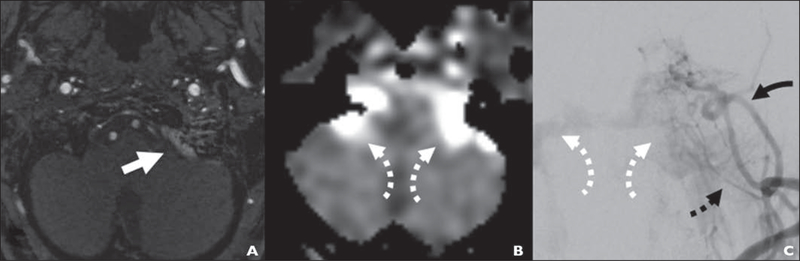
74-year-old woman with new nocturnal pulsatile tinnitus (case 7; same patient as in Fig. 1).
A, Time-of-flight MRA image shows left-sided marginal sinus fistula presenting with focal signal intensity (arrow) in region of left jugular foramen.
B, Arterial spin-labeling perfusion image shows bilateral areas of high signal intensity in jugular foramen extending anteriorly toward inferior petrosal sinuses, which is reflux pathway responsible for pressurizing cavernous sinuses from marginal sinus (arrows).
C, Anteroposterior left external carotid injection digital subtraction angiogram shows extensive marginal sinus fistula supplied by several branches, including stylomastoid artery (solid black arrow) and branches of ascending pharyngeal artery (dashed black arrow). Fistula drainage refluxes via both inferior petrosal sinuses, mirroring appearance (white arrows) in B.
Reflux of flow in the IJV causing high signal intensity near the skull base on TOF and ASL images can be discerned from a true DAVF through a systematic approach to MRI interpretation. The angiographic hallmark of DAVF is shunting of contrast medium from the arteries directly into the vein. Therefore, in contrast-enhanced MRA, it would be difficult to identify a DAVF involving a vein that has delayed contrast enhancement unless the vein is thrombosed or otherwise diseased. In our experience, identifying the thrombosis or diseased sinus is fairly straightforward. As such, if the IJV is otherwise normal appearing, without contrast enhancement on arterial phase TRICKS MR or CTA images, DAVF can be excluded. In addition, we suggest that reflux of contrast medium (typically from a closed IJV-subclavian valve) is responsible for delayed contrast opacification on TRICKS or CTA images owing to increased resistance to jugular venous outflow. Because the transverse sinuses, sigmoid sinuses, and jugular veins are a parallel flow circuit, preferential venous egress would occur via the dominant, lower resistance right IJV. We propose an algorithmic approach to diagnosis that summarizes these observations (Fig. 6).
Susceptibility-weighted imaging has been studied for detection of DAVFs [23]. This technique can highlight venous congestion and may reveal shunting. Such shunting is evidenced by lack of T2* effect, which indicates oxygenated (arterial) hemoglobin [24]. This technique is likely less useful in the posterior fossa venous drainage, which is highly variable and in which artifacts are more common [25]. We use susceptibility-weighted imaging as a troubleshooting tool to exclude sinus thrombosis, a finding that should raise suspicion of DAVF. CTA may also be useful when MRI-MRA findings are equivocal for DAVF. In case 2, contrast reflux on CTA was diagnostic of IJV reflux, but this pattern was not observed on the TRICKS images of other patients, suggesting that CTA reflux may be a specific but not sensitive finding. Other authors [26] have described high accuracy with respect to identifying venous drainage patterns in intracranial DAVFs. Because contrast enhancement on MRA differs mechanistically from the flow-related enhancement of TOF technique, we anticipate that ambiguous signal intensity would not be present in skull base veins unless a true DAVF were present.
Our study had several limitations. Given the small sample of patients and specific institutional imaging protocol, it is not known how generalizable this imaging pattern is to other populations or MRI system protocols. In this series, all areas of hyperintensity on ASL and TOF images were located on the left, but this does not imply that this phenomenon is limited to the left side, particularly in patients with codominant or left-dominant venous drainage. Future work should evaluate the overall prevalence of this artifact and its associations with other alterations in physiology.
Conclusion
High signal intensity in the region of the left jugular foramen on ASL and TOF MRA may be caused by physiologic reflux, which can mimic the appearance of DAVFs. Systematic review of additional imaging findings can help differentiate these diagnoses and avoid unnecessary catheter angiography.
Supplementary Material
HIGHLIGHTS.
Reflux in the internal jugular vein can produce similar ASL and time-of-flight MRA; this benign finding should not be misdiagnosed as dural arteriovenous fistula.
Acknowledgments
Supported by National Institute on Deafness and Other Communication Disorders of the National Institutes of Health (no. R21DC016087-01A1) (M. R. Amans) and National Heart, Lung, and Blood Institute of the National Institutes of Health (no. R56HL149124-01) (M. R. Amans).
Footnotes
The authors declare that they have no disclosures relevant to the subject matter of this article.
An electronic supplement is available online at 10.2214/AJR.20.24012.
References
- 1.Lasjaunias P, Chiu M, ter Brugge K, Tolia A, Hurth M, Bernstein M. Neurological manifestations of intracranial dural arteriovenous malformations. J Neurosurg 1986; 64:724–730 [DOI] [PubMed] [Google Scholar]
- 2.Le TT, Fischbein NJ, André JB, Wijman C, Rosenberg J, Zaharchuk G. Identification of venous signal on arterial spin labeling improves diagnosis of dural arteriovenous fistulas and small arteriovenous malformations. AJNR 2012; 33:61–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noguchi K, Melhem ER, Kanazawa T, Kubo M, Kuwayama N, Seto H. Intracranial dural arteriovenous fistulas: evaluation with combined 3D time-of-flight MR angiography and MR digital subtraction angiography. AJR 2004; 182:183–190 [DOI] [PubMed] [Google Scholar]
- 4.Al-Kwifi O, Emery DJ, Wilman AH. Vessel contrast at three Tesla in time-of-flight magnetic resonance angiography of the intracranial and carotid arteries. Magn Reson Imaging 2002; 20:181–187 [DOI] [PubMed] [Google Scholar]
- 5.Toledano-Massiah S, Badat N, Ghorra C, et al. Jugular venous reflux may mimic type I dural arterio-venous fistula on arterial spin labeling magnetic resonance images. Neuroradiology 2020; 62:447–454 [DOI] [PubMed] [Google Scholar]
- 6.Amukotuwa SA, Yu C, Zaharchuk G. 3D pseudocontinuous arterial spin labeling in routine clinical practice: a review of clinically significant artifacts. J Magn Reson Imaging 2016; 43:11–27 [DOI] [PubMed] [Google Scholar]
- 7.Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 2015; 73:102–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buxton RB, Frank LR, Wong EC, Siewert B, Warach S, Edelman RR. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med 1998; 40:383–396 [DOI] [PubMed] [Google Scholar]
- 9.Deibler AR, Pollock JM, Kraft RA, Tan H, Burdette JH, Maldjian JA. Arterial spin-labeling in routine clinical practice. Part 3. Hyperperfusion patterns. AJNR 2008; 29:1428–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsuruda J, Saloner D, Norman D. Artifacts associated with MR neuroangiography. AJNR 1992; 13:1411–1422 [PMC free article] [PubMed] [Google Scholar]
- 11.Kim E, Kim JH, Choi BS, Jung C, Lee DH. MRI and MR angiography findings to differentiate jugular venous reflux from cavernous dural arteriovenous fistula. AJR 2014; 202:839–846 [DOI] [PubMed] [Google Scholar]
- 12.Jang J, Kim BS, Kim BY, et al. Reflux venous flow in dural sinus and internal jugular vein on 3D time-of-flight MR angiography. Neuroradiology 2013; 55:1205–1211 [DOI] [PubMed] [Google Scholar]
- 13.Lichtenstein D, Saïfi R, Augarde R, et al. The internal jugular veins are asymmetric. Usefulness of ultrasound before catheterization. Intensive Care Med 2001; 27:301–305 [DOI] [PubMed] [Google Scholar]
- 14.San Millán Ruíz D, Gailloud P, Rüfenacht DA, Delavelle J, Henry F, Fasel JH. The craniocervical venous system in relation to cerebral venous drainage. AJNR 2002; 23:1500–1508 [PMC free article] [PubMed] [Google Scholar]
- 15.Akkawi NM, Agosti C, Borroni B, et al. Jugular valve incompetence: a study using air contrast ultrasonography on a general population. J Ultrasound Med 2002; 21:747–751 [DOI] [PubMed] [Google Scholar]
- 16.Carroll TJ, Korosec FR, Swan JS, Hany TF, Grist TM, Mistretta CA. The effect of injection rate on time-resolved contrast-enhanced peripheral MRA. J Magn Reson Imaging 2001; 14:401–410 [DOI] [PubMed] [Google Scholar]
- 17.Modabbernia A, Taslimi S, Ashrafi M, Modabbernia MJ, Hu HH. Internal jugular vein reflux in patients with transient global amnesia: a meta-analysis of case-control studies. Acta Neurol Belg 2012; 112:237–244 [DOI] [PubMed] [Google Scholar]
- 18.Beggs C, Chung CP, Bergsland N, et al. Jugular venous reflux and brain parenchyma volumes in elderly patients with mild cognitive impairment and Alzheimer’s disease. BMC Neurol 2013; 13:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zamboni P, Galeotti R, Menegatti E, et al. Chronic cerebrospinal venous insufficiency in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 2009; 80:392–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Traboulsee AL, Knox KB, Machan L, et al. Prevalence of extracranial venous narrowing on catheter venography in people with multiple sclerosis, their siblings, and unrelated healthy controls: a blinded, case-control study. Lancet 2014; 383:138–145 [DOI] [PubMed] [Google Scholar]
- 21.McDougall CG, Halbach VV, Dowd CF, Higashida RT, Larsen DW, Hieshima GB. Dural arteriovenous fistulas of the marginal sinus. AJNR 1997; 18:1565–1572 [PMC free article] [PubMed] [Google Scholar]
- 22.Turner RD, Gonugunta V, Kelly ME, Masaryk TJ, Fiorella DJ. Marginal sinus arteriovenous fistulas mimicking carotid cavernous fistulas: diagnostic and therapeutic considerations. AJNR 2007; 28:1915–1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Letourneau-Guillon L, Krings T. Simultaneous arteriovenous shunting and venous congestion identification in dural arteriovenous fistulas using susceptibility-weighted imaging: initial experience. AJNR 2012; 33:301–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jagadeesan BD, Delgado Almandoz JE, Moran CJ, Benzinger TL. Accuracy of susceptibility-weighted imaging for the detection of arteriovenous shunting in vascular malformations of the brain. Stroke 2011; 42:87–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haacke EM, Mittal S, Wu Z, Neelavalli J, Cheng YC. Susceptibility-weighted imaging: technical aspects and clinical applications. Part 1. AJNR 2009; 30:19–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishimura S, Hirai T, Sasao A, et al. Evaluation of dural arteriovenous fistulas with 4D contrast-enhanced MR angiography at 3T. AJNR 2010; 31:80–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.