Abstract
Background:
The diagnosis of pulmonary embolism (PE) because of nonspecific clinical presentation remains as a challenge for emergency physicians. Arterial to end-tidal partial pressure of carbon dioxide (P(a-Et) CO2) gradient may be useful in the evaluation of PE. This aimed to define the diagnostic role of P(a-Et)CO2 gradient by sidestream capnography, as a noninvasive method, and D-dimer in patients with PE.
Materials and Methods:
Two hundred and three patients with chest pain or dyspnea who attend the hospital emergency ward were enrolled over a study period at a single academic center. PE was confirmed by multidetector computed tomography (MDCT) scans. PaCO2, EtCO2, and D-dimer were measured within 24 h of MDCT by capnograph.
Results:
The combination of P(a-Et)CO2 gradient (cutoff >9.2 ng/ml) and D-dimer (cutoff >3011 ng/ml) with sensitivity and specificity of 30.2% and 87.2% showed a significant diagnostic value in detecting PE (area under the curve = 0.577, P = 0.045) but not alone (P > 0.05).
Conclusion:
As the results show, the combination of P(a-Et)CO2 gradient and D-dimer can show an acceptable diagnostic value in detecting PE, although it suggests further research on evaluating the diagnostic value of P(a-Et)CO2 gradient and combining it with other diagnostic criteria to achieve a definite and generalizable result.
Keywords: Carbon Dioxide Partial Pressure Determination, Capnography, D-dimer, pulmonary embolism
Introduction
Acute pulmonary embolism (APE), which is defined as a sudden occlusion of a pulmonary artery, due to its high risk of mortality and morbidity is known as one of the most important challenges in clinical emergencies.[1] APE is mainly caused by a thrombus-derived embolus that develops in the deep veins of the lower extremities venous system, although it may be caused by thrombosis in the pelvic veins, kidneys, or upper extremities venous.[2] Pulmonary embolism (PE) is globally the third cause of death from cardiovascular diseases.[3] The annual incidence rates for PE range from 39 to 115 per 100,000 population.[4]
The diagnosis of PE is difficult and because of the high variability in clinical presentation and nonspecific symptoms remains a challenge.[5] The diagnosis of PE should be based on a clinical prediction rule, D-dimer, and multidetector computed tomography pulmonary angiography (CTPA).[3,6] CTPA as the gold standard for APE diagnosis is quick and readily available in most institutions, but its unavoidable side effects such as radiation exposure, allergic reactions, acute kidney injury, low patients’ compliance, and imposing costs on the patient and health-care system, along with significant rising in the number of an unnecessary CTPA to exclude a PE, reduce its feasibility to perform, especially in emergency conditions.[1,7,8,9]
D-dimer, as a screening test, is a frequently performed test to excludes PE, the test is highly sensitive that can rule out PE in patients with low or intermediate pretest probability.[10] D-dimer has low specificity in the elderly and false-positive cases of the test can found in the presence of liver disease, high rheumatoid factor, inflammation, malignancy, trauma, pregnancy, and recent surgery. Furthermore, false-negative of D-dimer testing can happen when the test is taken shortly after thrombosis or several days after clot formation, and also, after the use of anticoagulant agents.[11,12]
Arterial to end-tidal partial pressure of carbon dioxide (P (a-Et) CO2) gradient, which is used as one of the standard monitoring methods in patients during anesthesia in recovery and the intensive care unit, recently mentioned as a noninvasive diagnostic test in patients with suspected PE, although the limited diagnostic value on air-blood gas analysis in these patients is a common conception.[13] In patients with PE, the alveolar dead space would be increased because of ventilation-perfusion mismatch and significant disorders of blood flow.[14] This increase impairs the efficient elimination of carbon dioxide and leads to an increase in PaCO2.[15] Hence, it is expected that in patients who experience PE, EtCO2 decreased compared to PaCO2 and showed that P(a-Et)CO2 gradient might be useful for diagnose of embolism.[16,17] Some limited studies evaluated the level of P(a-Et)CO2 gradient as a diagnostic test in patients with PE. These studies show that P(a-Et)CO2 gradient can be used as a diagnostic test, but with low specificity, it cannot be used to diagnose PE, alone. On the other hand, these studies reported that in conjunction with a positive D-dimer test, it has been found to increase diagnostic accuracy.[18,19,20,21,22,23,24,25,26]
This study aimed to investigate the accuracy of P(a-Et)CO2 gradient, measured by sidestream capnography as a noninvasive method with low risk and cost, and D-dimer in the diagnosis of PE.
Materials and Methods
This cross-sectional diagnostic study included all patients suspicious of PE, presented at the Emergency Department of Al-Zahra Hospital from January 2018 to July 2019.
Of the population, according to studies[26] and sensitivity of PE diagnosis by the combination of EtCO2/O2 and D-dimer which is 84.6%, a confidence level of 95%, the test power of 80%, 0.08% error, and the likelihood of PE incidence of 0.34, we calculated a sample size of 230. The sample was selected using convenience sampling.
All patients with chest pain or dyspnea who attend the hospital emergency ward were screened by an emergency physician for eligibility and those were suspected of having a PE were enrolled. Inclusion criteria were age >18 years, clinical suspicion of acute PE, defined as acute chest pain, new-onset or worsening dyspnea without other obvious causes, and ability to consent. Clinical suspicion of PE was specified according to the clinical signs and symptoms as well as the Wells (Ws) criteria and the positive pulmonary embolism rule-out criteria. Exclusion criteria included: an obvious etiology to the acute presentation other than PE (acute coronary syndrome or pneumothorax), pregnancy, mechanical ventilation, known hypercarbic respiratory failure, neuromuscular disorders, and hemostatic instability.
In this study, 27 cases were excluded due to critical conditions, hypercarbic respiratory failure, and hemostatic instability. Thus, the sample size was reduced to 203.
The proposal was approved by the ethics committee at Isfahan University of Medical Sciences (IR.MUI.MED.REC.1397.202) and written informed consent was obtained from patients. Then, the symptoms and criteria related to the clinical suspicion of PE were evaluated.
Enrolled patients underwent diagnostic multidetector computed tomography (MDCT) scans to evaluate the presence of PE. MDCT was done by an independent radiologist on 64-slice multidetector equipment with <2.5 mm collimation. Images were interpreted by two independent radiologists and were classified as negative PE, APE, chronic PE, other findings, or indeterminate. In the present study, scan results with positive APE were taken as the gold standard.
Within 24 h of MDCT completion, breath and blood collection were done to measure EtCO2, PaCo2, and D-dimer as the main endpoint of the study. Carbon dioxide was measured by a trained tester, who was blinded to the results of MDCT, using sidestream capnography, calibrated in an air room containing 3% Co2. The mask or nasal cannula tubing was placed in the patient's mouth, allowing tidal breathing, while the ETCO2 was measured. Patients were instructed to breathe normally for 10 s. This was repeated three times and an average of values was recorded. Furthermore, blood samples were analyzed to measure D-dimer. Hence, the PaCO2–EtCO2 gradient was calculated and used in the statistical analyses.
MedCalc software version 10.2.0.0 was used to performed statistical analysis. The results are reported as mean ± standard deviation or number (%) as appropriate. Independent sample t-test and Chi-square test were used to compare the continuous and ordinal variables between patients with or without PE, respectively. A receiver operating characteristic (ROC) curve analysis was used to evaluate the areas under the ROC curve which established the best cutoff values for EtCo2 and PaCo2 difference value and D-dimer value in the detection of PE. Sensitivity, specificity, positive and negative predictive values, and positive and negative likelihood ratios were then calculated. A significance level of <0.05 was considered in all analyses.
Results
Of 203 studied patients with symptoms and risk factors of PE, 39 (19.2%) patients had PE. Patients with PE were significantly older than those without PE (60.7 ± 18.1 vs. 53.8 ± 18.6 years respectively, P = 0.037). Most of the patients in both the groups of with and without PE were male. The levels of PaCO2 and D-dimer were similar between those patients with and without PE. The mean levels of PaCo2 and D-dimer were not significantly different between patients with and without PE (P > 0.05). However, EtCo2 in patients with PE with a mean of 27.3 ± 10.9 was significantly lower than patients without PE with a mean of 31.0. 9.4 (P = 0.032). Besides, the P(a-Et)CO2 gradient in patients with PE with a mean of 15.6 ± 13.6 was significantly higher than patients without PE with a mean of 11.4 ± 10.2 (P = 0.030) [Table 1].
Table 1.
Characteristics, end-tidal carbon dioxide, partial pressure of carbon dioxide, and D-dimer in studied patients based on multidetector computed tomography findings
| Variables | Presence of pulmonary embolism | P | |
|---|---|---|---|
|
| |||
| Positive (n=39), n (%) | Negative (n=164), n (%) | ||
| Age (year) | 60.7±18.1 | 53.8±18.6 | 0.037 |
| Sex | |||
| Male | 24 (64.1) | 103 (62.8) | 0.880 |
| Female | 14 (35.9) | 61 (37.2) | |
| EtCo2 | 27.3±10.9 | 31.0±9.4 | 0.032 |
| PaCo2 | 41.2±9.6 | 40.6±11.0 | 0.756 |
| P (a-Et) CO2 gradient | 15.6±13.6 | 11.4±10.2 | 0.030 |
| D-dimer | 2741.5±2455.6 | 2709.5±2710.1 | 0.946 |
| 1615.0 (873.0-4019.0) | 1413.50 (861.5-33.47.2) | ||
Data are mean±SD or median (IQR), or n (%). P value calculated using independent sample t-test or Chi-square test. EtCo2: End-tidal carbon dioxide, PaCo2: Partial pressure of carbon dioxide, SD: Standard deviation, P (a-Et) CO2: End-tidal partial pressure of carbon dioxide, IQR: Interquartile range
On the other hand, evaluation of the diagnostic value of P(a-Et)CO2 gradient and D-dimer in the diagnosis of PE indicated that the D-dimer had no significant value in the diagnosis of PE (cutoff point >3011 ng/ml, area under the curve [AUC] =0.514, P = 0.791). In addition, the P(a-Et)CO2 gradient criterion had no significant value in detecting PE with a cutoff point of 9.2 (AUC = 0.636, P = 0.016). In combination of two diagnostic criteria, D-dimer (cutoff point = 3011 ng/ml) and P(a-Et)CO2 gradient (cutoff point = 9.2) with a sensitivity of 30.2 and a specificity of 87.2 were suitable for identifying PE (AUC = 0.577, P = 0.045) [Table 2 and Figure 1].
Table 2.
The diagnostic value of end-tidal partial pressure of carbon dioxide gradient and D-dimer and both test in the detection of pulmonary embolism
| Parameters of ROC analysis | P (a-Et) CO2 gradient | D-dimer | Combination P (a-Et) CO2 with D-dimer* |
|---|---|---|---|
| ARC (95% CI) | 0.589 (0.518-0.658) | 0.514 (0.442-0.585) | 0.577 (0.506-0.646) |
| P | 0.089 | 0.791 | 0.045 |
| Cutoff point | >9.2 | >3011 | - |
| Sensitivity, % (95% CI) | 64.1 (47.2-78.8) | 38.5 (23.4-55.4) | 30.2 (15.0-44.9) |
| Specificity, % (95% CI) | 54.3 (46.3-62.1) | 80.0 (63.4-77.8) | 87.2 (81.1-91.9) |
| PPV, % (95% CI) | 25.0 (16.9-34.7) | 24.2 (14.3-36.9) | 34.4 (18.6-53.2) |
| NPV, % (95% CI) | 86.4 (78.2-92.4) | 82.7 (75.4-88.6) | 83.6 (77.2-88.8) |
| PLR | 1.4 (1.1-1.8) | 1.3 (0.9-2.0) | 2.2 (1.2-4.2) |
| NLR | 0.7 (0.4-0.1) | 0.9 (0.1-1.2) | 0.82 (0.7-1.0) |
*Combination P(a-Et)CO2 with D-dimer defined P(a-Et)CO2 >9.2 and D-dimer >3011 ng/ml. CI: Confidence interval, PPV: Positive predictive value, NPV: Negative predictive value, ROC: Receiver operating characteristic, ARC: Area under the ROC curve, PLR: Positive likelihood ratio, NLR: Negative likelihood ratio, P(a-Et)CO2: End-tidal partial pressure of carbon dioxide
Figure 1.
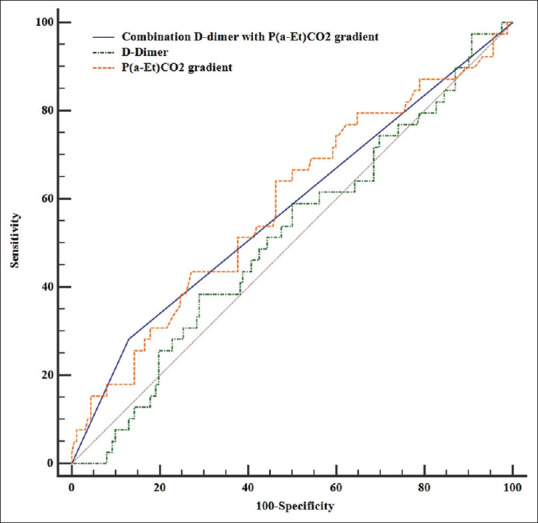
Receiver operating characteristic curves to compare the diagnostic value of D-dimer, P(a-Et)CO2 gradient, and combination P(a-Et)CO2 with D-dimer
Discussion
In the emergency departments, the diagnosis of PE due to its nonspecific clinical presentation is difficult which raises concerns for the potential overuse of diagnostic tests such as CTPA.[11,22] In the present study, we investigated the diagnostic role of P(a-Et)CO2 gradient measured by sidestream capnography, as a noninvasive method, and plasma D-dimer in patients with PE. We found that P(a-Et)CO2 gradient was significantly increased in the presence of PE (P = 0.030). P(a-Et)CO2 gradient in the detection of PE had low sensitivity and specificity. In addition, D-dimer in our study had low sensitivity (38.5%) and specificity (80.0%). Hence, P(a-Et)CO2 gradient and D-dimer could not use as a diagnostic test alone in the detection of PE.
The incidence of PE in the population in the present study was 19.2%, which was similar to other studies that reported the prevalence of PE between 15% and 40%.[12,23,24,25] In our study, the average ETCO2 in patients with a positive PE (27.3 mmHg) was significantly lower than in patients without PE (31 mmHg). Similarly, in Riaz and Jacob's study, the mean ETCO2 in patients with a positive PE was scientifically lower than patients without PE (25.1 vs. 33.1 mmHg, respectively).[20] Another study by Ozdemir et al. revealed a significantly lower ETCO2 level in the PE group.[7] In addition, Yüksel et al. reported that the ETCO2 level in patients with PE was significantly lower than patients without PE.[21] In contrast, Hemnes et al. reported that the mean ETCO2 values were similar in patients with (36.3 mmHg) or without (35.5 mmHg) PE.[16] The differences among these studies can be due to discrepancies in the characteristics of the patient population and measurement methods.
D-dimer level in our study was similar to patients with PE compared with those without PE. In contrast in most of the previous studies, the level of D-dimer was reported being significantly higher in patients with PE compared with those without PE.[16,17,18,26] We found that in patients with PE, P(a-Et)CO2 gradient was significantly higher than patients without PE, but it has low sensitivity and specificity for the detection of PE. In a study by Kline et al., patients with PE had significantly lower EtCO2/O2 values compared with those without PE. However, patients with PE had significantly higher mean D-dimer. They reported that the combination of D-dimer and ETCO2/O2 could produce clinically important improvements as a screening strategy for PE in a moderate-risk population.[26] It has previously been shown that dead space fraction, which has been measured by comparing total exhaled CO2 tension with arterial CO2 tension, to be abnormal in patients with PE.[27] Sanchez et al. show that dead space (measured by capnograph) had a sensitivity of 68.5% and a specificity of 81.5% for the detection of PE.[24] Verschuren et al. reported that the late dead space fraction performed well for diagnosis in PE.[14] Yoon et al. reported that the combined result of a positive D-dimer test and alveolar dead space fraction improved the specificity for diagnosing PE compared with the D-dimer test alone.[28] These findings show the usefulness of studied tests when combined with the D-dimer test and often reported low reliability for tests alone. In similar to previous studies, our finding shows that the combination of P(a-Et)CO2 gradient (with cutoff >9.2) and D-dimer (with cutoff >3011 ng/ml) with sensitivity 30.2 and specificity 87.2 had a significant value in the diagnosis of PE.
This study has several limitations. First, the study had low generalizability of the results because it is conducted at a single center. Second, the D-dimer level in all studied patients was more than 500 ng per ml that used as the threshold as negative or positive (all patients had positive D-dimer).[29,30] Hence, we could not assess the combined use of a D-dimer (with cutoff >500 ng/ml) result and P(a-Et)CO2 gradient as a screening test.
Conclusion
According to the results of the current study, ETCO2 in patients with PE was significantly lower than in patients without PE. In the presence of PE, the P(a-Et)CO2 gradient was significantly higher than patients without PE. P(a-Et)CO2 gradient and D-dimer tests had no significant diagnostic value in the detection of PE. However, the combination of D-dimer and P(a-Et)CO2 gradient could have a significant diagnostic value as a screening test for PE. Hence, P(a-Et)CO2 gradient, by sidestream capnography as a noninvasive method with low cost, can be used as a diagnostic test in combination with D-dimer in the emergency department to detect PE in suspected patients. However, further studies are needed to more clarify the diagnostic role of P(a-Et)CO2 gradient alone and in combination with D-dimer for diagnosis of PE in suspected patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
This study was supported by a grant from Isfahan University of Medical Sciences.
References
- 1.Rubini G, Ferrari C, Cimino A, Fanelli M, Altini C, Gaudiano A, et al. How often suspected pulmonary embolism is diagnosed and its main diagnostic characteristics, in an emergency nuclear medicine service? Four years experience. Hell J Nucl Med. 2019;22:187–93. doi: 10.1967/s002449911054. [DOI] [PubMed] [Google Scholar]
- 2.Pelletier-Galarneau M, Zannier E, Zuckier LS, Le Gal G. Referral patterns and diagnostic yield of lung scintigraphy in the diagnosis of acute pulmonary embolism. Thrombosis. 2017;2017:1–6. doi: 10.1155/2017/1623868. doi: 10.1155/2017/1623868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galiè N, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35:3033. [Google Scholar]
- 4.Wendelboe AM, Raskob GE. Global burden of thrombosis: Epidemiologic aspects. Circ Res. 2016;118:1340–7. doi: 10.1161/CIRCRESAHA.115.306841. [DOI] [PubMed] [Google Scholar]
- 5.Pollack CV, Schreiber D, Goldhaber SZ, Slattery D, Fanikos J, O’Neil BJ, et al. Clinical characteristics, management, and outcomes of patients diagnosed with acute pulmonary embolism in the emergency department: Initial report of EMPEROR (Multicenter Emergency Medicine Pulmonary Embolism in the Real World Registry) J Am Coll Cardiol. 2011;57:700–6. doi: 10.1016/j.jacc.2010.05.071. [DOI] [PubMed] [Google Scholar]
- 6.Raja AS, Greenberg JO, Qaseem A, Denberg TD, Fitterman N, Schuur JD, et al. Evaluation of patients with suspected acute pulmonary embolism: Best practice advice from the clinical guidelines committee of the American College of Physicians. Ann Intern Med. 2015;163:701–11. doi: 10.7326/M14-1772. [DOI] [PubMed] [Google Scholar]
- 7.Remy-Jardin M. Are we overdiagnosing pulmonary embolism? “No”. J Thoracic Imaging. 2018;33:348–9. doi: 10.1097/RTI.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 8.Bozorgmehr R, Pishgahi M, Mohaghegh P, Bayat M, Khodadadi P, Ghafori A. Relationship between thrombosis risk factors, clinical symptoms, and laboratory findings with pulmonary embolism diagnosis; A cross-sectional study. Arch Acad Emerg Med. 2019;7:41. [PMC free article] [PubMed] [Google Scholar]
- 9.Kruger PC, Eikelboom JW, Douketis JD, Hankey GJ. Pulmonary embolism: Update on diagnosis and management. Med J Aust. 2019;210:516–24. doi: 10.5694/mja2.50201. [DOI] [PubMed] [Google Scholar]
- 10.Bass AR, Fields KG, Goto R, Turissini G, Dey S, Russell LA. Clinical decision rules for pulmonary embolism in hospitalized patients: A systematic literature review and meta-analysis. Thromb Haemost. 2017;117:2176–85. doi: 10.1160/TH17-06-0395. [DOI] [PubMed] [Google Scholar]
- 11.Adam SS, Key NS, Greenberg CS. D-dimer antigen: Current concepts and future prospects. Blood. 2009;113:2878–87. doi: 10.1182/blood-2008-06-165845. [DOI] [PubMed] [Google Scholar]
- 12.Koch V, Biener M, Müller-Hennessen M, Vafaie M, Staudacher I, Katus HA, et al. Diagnostic performance of D-dimer in predicting venous thromboembolism and acute aortic dissection? Eur Heart J Acute Cardiovasc Care. 2020:1–9. doi: 10.1177/2048872620907322. doi: 10.1177/2048872620907322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takano Y, Sakamoto O, Kiyofuji C, Ito K. A comparison of the end-tidal CO2 measured by portable capnometer and the arterial P CO2 in spontaneously breathing patients. Respir Med. 2003;97:476–81. doi: 10.1053/rmed.2002.1468. [DOI] [PubMed] [Google Scholar]
- 14.Verschuren F, Liistro G, Coffeng R, Thys F, Roeseler J, Zech F, et al. Volumetric capnography as a screening test for pulmonary embolism in the emergency department. Chest. 2004;125:841–50. doi: 10.1378/chest.125.3.841. [DOI] [PubMed] [Google Scholar]
- 15.Ishaaya E, Tapson VF. Advances in the diagnosis of acute pulmonary embolism. F1000Res. 2020;9 doi: 10.12688/f1000research.21347.1. F1000 Faculty Rev. doi: 10.12688/f1000research. 21347.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hemnes AR, Newman AL, Rosenbaum B, Barrett TW, Zhou C, Rice TW, et al. Bedside end-tidal CO2 tension as a screening tool to exclude pulmonary embolism. Eur Respir J. 2010;35:735–41. doi: 10.1183/09031936.00084709. [DOI] [PubMed] [Google Scholar]
- 17.Ozdemir M, Sonmez BM, Yilmaz F, Yilmaz A, Duyan M, Komut S. Is bedside end-tidal CO2 measurement a screening tool to exclude pulmonary embolism in emergency department? J Clin Med Res. 2019;11:696–702. doi: 10.14740/jocmr3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozsu S, Abul Y, Yilmaz I, Ozsu A, Oztuna F, Bulbul Y, et al. Prognostic significance of PaO2/PaCO2 ratio in normotensive patients with pulmonary embolism. Clin Respir J. 2012;6:104–11. doi: 10.1111/j.1752-699X.2011.00253.x. [DOI] [PubMed] [Google Scholar]
- 19.Aminiahidashti H, Shafiee S, Zamani Kiasari A, Sazgar M. Applications of end-tidal carbon dioxide (ETCO2) monitoring in emergency department; A narrative review. Emerg (Tehran) 2018;6:e5. [PMC free article] [PubMed] [Google Scholar]
- 20.Riaz I, Jacob B. Pulmonary embolism in Bradford, UK: Role of end-tidal CO2 as a screening tool. Clin Med (Lond) 2014;14:128–33. doi: 10.7861/clinmedicine.14-2-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yüksel M, Pekdemir M, Yilmaz S, Yaka E, Kartal AG. Diagnostic accuracy of noninvasive end-tidal carbon dioxide measurement in emergency department patients with suspected pulmonary embolism84-90. Turk J Med Sci. 2016;46:84–90. doi: 10.3906/sag-1404-108. [DOI] [PubMed] [Google Scholar]
- 22.Righini M, Van Es J, Den Exter PL, Roy PM, Verschuren F, Ghuysen A, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: The ADJUST-PE study. JAMA. 2014;311:1117–24. doi: 10.1001/jama.2014.2135. [DOI] [PubMed] [Google Scholar]
- 23.Manara A, D’hoore W, Thys F. Capnography as a diagnostic tool for pulmonary embolism: A meta-analysis. Ann Emerg Med. 2013;62:584–91. doi: 10.1016/j.annemergmed.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez O, Wermert D, Faisy C, Revel MP, Diehl JL, Sors H, et al. Clinical probability and alveolar dead space measurement for suspected pulmonary embolism in patients with an abnormal D-dimer test result. J Thromb Haemost. 2006;4:1517–22. doi: 10.1111/j.1538-7836.2006.02021.x. [DOI] [PubMed] [Google Scholar]
- 25.Rodger MA, Jones G, Rasuli P, Raymond F, Djunaedi H, Bredeson CN, et al. Steady-state end-tidal alveolar dead space fraction and D-dimer: Bedside tests to exclude pulmonary embolism. Chest. 2001;120:115–9. doi: 10.1378/chest.120.1.115. [DOI] [PubMed] [Google Scholar]
- 26.Kline JA, Hogg MM, Courtney DM, Miller CD, Jones AE, Smithline HA, et al. D-dimer and exhaled CO2/O2 to detect segmental pulmonary embolism in moderate-risk patients. Am J Respir Crit Care Med. 2010;182:669–75. doi: 10.1164/rccm.201001-0129OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robert-Ebadi H, Glauser F, Planquette B, Moumneh T, Le Gal G, Righini M. Safety of multidetector computed tomography pulmonary angiography to exclude pulmonary embolism in patients with a likely pretest clinical probability. J Thromb Haemost. 2017;15:1584–90. doi: 10.1111/jth.13746. [DOI] [PubMed] [Google Scholar]
- 28.Yoon YH, Lee SW, Jung DM, Moon SW, Horn JK, Hong YS. The additional use of end-tidal alveolar dead space fraction following D-dimer test to improve diagnostic accuracy for pulmonary embolism in the emergency department. Emerg Med J. 2010;27:663–7. doi: 10.1136/emj.2008.071118. [DOI] [PubMed] [Google Scholar]
- 29.Lim W, Le Gal G, Bates SM, Righini M, Haramati LB, Lang E, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: Diagnosis of venous thromboembolism. Blood Adv. 2018;2:3226–56. doi: 10.1182/bloodadvances.2018024828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Es N, van der Hulle T, van Es J, den Exter PL, Douma RA, Goekoop RJ, et al. Wells rule and d-dimer testing to rule out pulmonary embolism: A systematic review and individual-patient data meta-analysis. Ann Intern Med. 2016;165:253–61. doi: 10.7326/M16-0031. [DOI] [PubMed] [Google Scholar]


