Abstract
The aromatase cytochrome P450 (P450arom) enzyme, or estrogen synthase, which is coded by the CYP19A1 gene, is widely expressed in a subpopulation of excitatory and inhibitory neurons, astrocytes, and other cell types in the human brain. Experimental studies in laboratory animals indicate a prominent role of brain aromatization of androgens to estrogens in regulating different brain functions. However, the consequences of aromatase expression in the human brain remain poorly understood. Here, we summarize the current knowledge about aromatase expression in the human brain, abundant in the thalamus, amygdala, hypothalamus, cortex, and hippocampus and discuss its role in the regulation of sensory integration, body homeostasis, social behavior, cognition, language, and integrative functions. Since brain aromatase is affected by neurodegenerative conditions and may participate in sex-specific manifestations of autism spectrum disorders, major depressive disorder, multiple sclerosis, stroke, and Alzheimer's disease, we discuss future avenues for research and potential clinical and therapeutic implications of the expression of aromatase in the human brain.
Keywords: aging, amygdala, cerebral cortex, hippocampus, hypothalamus, thalamus
Introduction
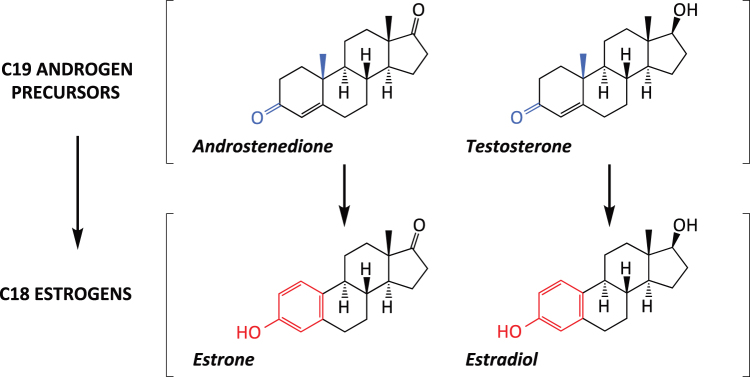
Part of the actions of androgens in the body are exerted after their conversion to estrogens by the enzyme aromatase cytochrome P450 (P450arom) or estrogen synthase. The enzyme converts androst-4-ene-3,17-dione (androstenedione) and testosterone into estrone and estradiol, respectively (Fig. 1). Half a century ago, Naftolin and collaborators reported the existence of aromatase activity in samples of human brain tissue isolated from male fetuses.1,2 The implication of this finding was that aromatizable androgens can regulate human brain function not only through androgen receptor (AR) but also by the activation of estrogen receptors (ERs) after their conversion to estrogenic metabolites. In addition, although aromatase activity in the brain probably does not affect the overall concentration of androgens, it is plausible that by decreasing the levels of androgens at very specific intracellular domains, aromatase activity may also contribute toward modulate AR signaling in the human brain.
FIG. 1.
Aromatase cytochrome P450 (P450arom) or estrogen synthase converts androgen c19 precursors in C18 estrogens. For instance, the enzyme converts androst-4-ene-3,17-dione (androstenedione) in estrone and testosterone into estradiol.
Studies in different vertebrate species, from fish to mammals, have shown that aromatase is expressed in the developing and adult brain and the spinal cord of both males and females. Central aromatase activity participates in a variety of functions that are not restricted to the control of the neuroendocrine axis and the regulation of reproduction or sex differences, but this includes the processing of sensory information, the coordination of sensory inputs with motor outputs, the expression of affective behavior, and the modulation of learning and memory.3–10 To exert these actions, estradiol generated by brain aromatase regulates cellular signaling, gene expression, synaptic transmission, and synaptic plasticity,11–14 and it is part of the endogenous neuroprotective and anti-inflammatory response activated in neural tissue after injury.15,16
Not all of these roles of the enzyme described in animal studies have been ascertained in humans. However, human studies have significantly advanced in recent years and we have considerable new information on the anatomical and cellular distribution of the enzyme in the human central nervous system (CNS), together with new hints on its physiological implication in the neural processing of sensory integration, the modulation of social behavior and cognition, and the central control of body homeostasis. Available data also indicate that brain aromatase expression is altered with aging and under neurodegenerative conditions. Further, genetic and neuropathological findings suggest that the enzyme may participate in the manifestation of brain diseases, including major depressive disorder, autism spectrum disorders (ASDs), and neurodegenerative diseases. Here, we review the available information on the distribution and function of aromatase in the human brain, discussing future avenues for research and potential clinical and therapeutic implications.
Aromatase Expression and Distribution in the Human Brain
The expression of CYP19A1, the aromatase gene, has been detected in the brain of men and women, in structures associated with cognition and memory (frontal cortex, temporal cortex, hippocampus), endocrine and autonomic regulation (hypothalamus), sensorimotor information processing (thalamus, cerebellum), or affective behavior (amygdala).17–21 Aromatase protein distribution in these brain regions has been ascertained by immunohistochemical analysis22 and positron emission tomography (PET) imaging.23 Aromatase messenger RNA (mRNA) and protein expression has been also detected in the human fetal brain, with relative higher levels at the end of the gestational period.24,25 In addition, studies performed in the human temporal lobe with different techniques have allowed to demonstrate the coincidence of CYP19A1 mRNA,19,26 aromatase protein,19 and aromatase enzymatic activity27,28 in the same brain structure. These studies have also shown that men and women have a similar expression, distribution, and activity of aromatase in the brain. Only a small global sex difference, with higher levels in men, has been reported.29
The human CYP19A1 gene contains 9 coding exons that are regulated by at least 10 tissue-specific alternate splicing variants of exon I. Several exon I splicing variants have been identified in the brain of men and women and in human brain cells.17,19,21,30,31 These different promoters could be potentially targeted by a large variety of molecules,17,19,21,32 allowing a complex regulation of aromatase expression in the human brain. In addition, some of these CYP19A1exon I variants have a broad anatomical distribution in the brain, whereas others seem to be restricted to specific brain regions. In agreement with this, aromatase mRNA levels show regional differences, with higher levels in some specific brain structures (thalamus, hypothalamus) compared with others.17
Regional differences in brain aromatase levels have been confirmed and expanded by PET studies, which analyze the distribution of binding sites of a labeled aromatase inhibitor and provide a useful comparative estimation of the expression levels of the enzyme in different brain structures. These studies revealed higher expression in the thalamus followed by the amygdala and the hypothalamus/preoptic area and then by the inferior olivary nucleus, accumbens, pons, occipital and temporal cortex, putamen, cerebellum, and the cerebral white matter.29,33 The possible function of aromatase in these different regions of the human brain will be discussed later in more detail.
Cellular Localization
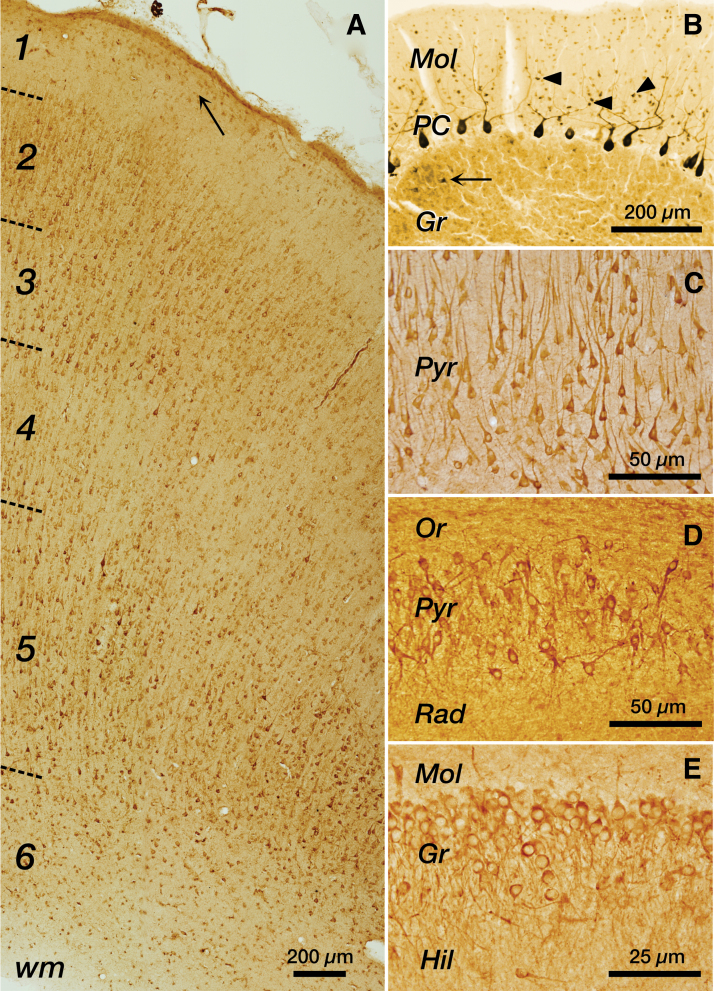
Neurons are the most abundant cell type expressing aromatase in the human brain (Fig. 2). In human neurons, the enzyme is localized in the perikaryon and in dendrites and axons.19,22 The localization of aromatase in these different neuronal compartments suggests that the local production of estradiol by the enzyme may influence a variety of cellular processes, such as signal transduction, gene expression, or synaptic physiology. Aromatase immunoreactivity has been also localized in synaptic terminals of human neurons, including the synaptic vesicles,34 in agreement with the proposal that aromatase may generate estradiol in presynaptic terminals that may target ERs in postsynaptic structures.35
FIG. 2.
Representative examples of aromatase immunoreactive cells in the human brain. (A) Panoramic view of a section of the temporal cortex. Anti-aromatase immunoreactivity is observed in all cortical layers (1–6) and in the wm, but it is particularly intense in pyramidal cells of neocortical layers 2/3 and 5. In layer 1, aromatase immunoreactive cells, most of them with astrocyte morphology, are abundant in proximity of the pial surface (arrow). Female, 63 years old. (B) Cerebellar cortex. Intense immunoreactivity is observed in Purkinje neuronal perikarya in the PC and in their dendrites in the Mol, whereas granule cells are not immunoreactive. Immunoreactivity is also observed in interneurons in the molecular layer (arrowheads) and in a few neurons in the Gr (arrow), which may correspond to Golgi neurons. Male, 49 years old. (C) Pyramidal neurons in the Pyr of the hippocampal Ammon's horn CA1 region showing aromatase immunoreactivity in the perikaryon and in the basal and apical dendrites. Male, 65 years old. (D) Pyramidal neurons in the Pyr of the hippocampal Ammon's horn CA3 region showing aromatase immunoreactivity in the perikaryon and dendrites. Female, 43 years old. (E) Hippocampal dentate gyrus showing aromatase immunoreactivity in granule cell neurons in the Gr and a few interneurons in the Hil. Mol, molecular layer of the dentate gyrus. Female, 35 years old. All panels are from immunoperoxidase-stained sections using hydrogen peroxide as substrate and 3,3′-diaminobenzidine tetrahydrochloride as chromogen. In (B), the reaction was intensified by adding a small amount of nickel chloride. Gr, granular layer; Hil, hilus; Mol, molecular layer; Or, Stratum oriens; PC, Purkinje cell layer; Pyr, pyramidal layer; Rad, Stratum radiatum; Wm, white matter.
Although aromatase immunoreactive neurons have been observed in all the regions of the adult human brain examined so far, not all neurons in a given region are immunoreactive for the enzyme (Fig. 2). For instance, in the temporal cortex a high proportion of pyramidal neurons and a small proportion of calbindin and parvalbumin interneurons are immunoreactive for aromatase.19 In the hippocampus, pyramidal neurons from CA1 to CA3 are the main neuronal population expressing aromatase, together with some granule cells of the dentate gyrus and specific hilar interneurons that co-express the calcium-binding proteins calbindin, calretinin, or parvalbumin.20 In the cerebellum, aromatase immunoreactivity is detected in Purkinje cells, molecular layer interneurons, and a few neurons in the granular layer (Fig. 2).22 Aromatase immunoreactive neurons have been also observed in other regions of the human brain, including magnocellular neurons of the supraoptic nucleus of the hypothalamus, the medial mamillary nucleus, the infundibular nucleus, or the basal nucleus of Meynert.36 The presence of aromatase in excitatory and inhibitory neurons suggests that estrogen synthesis may play a role in maintaining a balanced interplay between excitatory and inhibitory neuronal activity required for correct brain function.
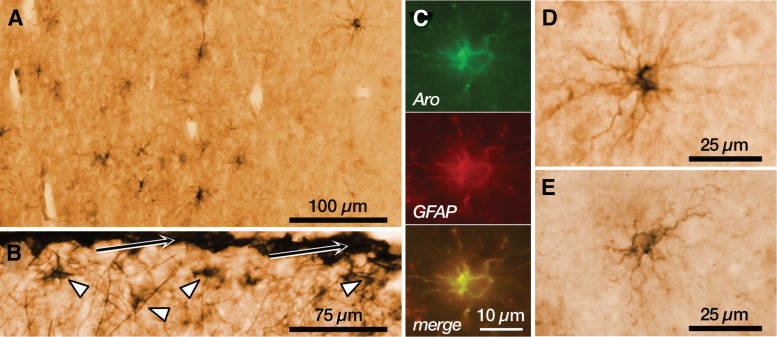
In addition to neurons, a subpopulation of other cells of different types, including fibrous and protoplasmic astrocytes (Fig. 3), oligodendrocytes, some ependymal cells, and choroid plexus cells, show immunoreactivity for aromatase in different human brain regions.19,20,36–38
FIG. 3.
Aromatase immunoreactivity in astrocytes. (A) Panoramic view of the cortical white matter in the temporal lobe, showing immunoreactivity in cells with the morphology of fibrous astrocytes. (B) Detail of the layer 1 of the temporal cortex showing numerous immunoreactive astrocytes (arrowheads) in the proximity of the pial surface (arrows). (C) Immunofluorescence labeling of aromatase (green), the astrocyte cell marker GFAP (red), and the colocalization signal (yellow) in a cortical astrocyte. (D) Representative example of a fibrous astrocyte immunoreactive for aromatase in the cortical white matter. (E) Representative example of a protoplasmic astrocyte immunoreactive for aromatase in the cortical gray matter. All panels are from a 23-year-old male. GFAP, glial fibrillary acidic protein.
Physiological and Pathophysiological Function of Brain Aromatase
Thalamus: sensory integration
Animal research has shown that aromatase is expressed in sensory systems and that local estradiol production by the enzyme modulates the transmission of sensory information,39–41 including pain.42–45 Aromatase activity is involved in the modulation of sensory function also in humans, because cancer patients treated with aromatase inhibitors often suffer from arthralgia and myalgia46–48 and these molecules have been reported to affect the function of sensory systems, such as the retina.49 This role of aromatase activity is further supported by the localization of the enzyme by PET29 and immunohistochemistry22 in the human thalamus, because this highly complex structure is the main sensory information computing brain center. In addition, the thalamus integrates sensorimotor function with high-level cognitive processes. Thus, the thalamus is involved in perception, attention, emotions, memory, language, behavioral flexibility, and the generation of mental representations.50–53 Interestingly, aromatase PET signal in the thalamus shows a significant negative correlation with cooperativeness scores in both men and women,33 suggesting that aromatase expression in this brain region may be involved in the regulation of estrogen-sensitive thalamo-cortical circuits interpreting sensory information to control social abilities.
Amygdala and hypothalamus: body homeostasis, social behavior, and major depressive disorder
The amygdala is involved in emotional learning, social behavior, motivation, aggression, and fear and in the processing and representation of emotions. Aromatase levels in the amygdala have been found to be associated with different personality traits. In women, higher aromatase levels in the left amygdala are associated with higher aggression, whereas higher aromatase levels in the right amygdala are negatively associated with novelty seeking and persistence and positively correlated with cooperativeness and self-transcendence.33 In contrast, higher aromatase levels in the amygdala are associated with lower verbal and nonverbal cognitive performance in men, but not in women.54 Aromatase regulation of androgen/estrogen balance together with sex-specific actions of gonadal hormones in the amygdala may be involved in these functional differences.
In addition, aromatase availability in the amygdala has other functional implications that are similar in both sexes. For instance, aromatase in the amygdala is negatively associated with body mass index in men and women,55 in agreement with the association of obesity with negative emotional states and the association of amygdala activity with body mass index.56 This function of the amygdala is mediated by its reciprocal connections with the hypothalamus, the main control center of body homeostasis.
The hypothalamus integrates body signals and controls body organs through the autonomic nervous system and by the release of hormones in the neurohypophysis and hormonal-releasing factors in the adenohypophysis. One of the well-characterized roles of the hypothalamus is the regulation of the secretion of gonadotrophins. Studies in female monkeys have shown that aromatase-mediated local estradiol synthesis and action in the hypothalamus is involved in the ovarian positive feedback for the induction of luteinizing hormone surges.57 Probably, a similar function of hypothalamic aromatase is operating in women, but this has not yet been studied.
Magnocellular neurons in the human supraoptic and paraventricular hypothalamic nuclei, which produce oxytocin and vasopressin, are immunoreactive for aromatase36,58 and this enzyme mediates the effect of testosterone on vasopressin expression by human neuroblastoma cells,59 suggesting that locally synthesized aromatized androgen metabolites may be involved in the regulation of magnocellular neurons in the human hypothalamus. The best studied function of vasopressin is blood pressure regulation. However, there is not a clear effect of aromatase inhibitors on blood pressure levels in postmenopausal breast cancer patients.48 Still, estradiol generated by aromatase activity in magnocellular neurons may impact other physiological processes regulated by oxytocin and vasopressin, such as reproductive physiology, social bonding, social behavior, anxiety, fear conditioning, and fear extinction.60 In this regard, it should be mentioned that a study has found that aromatase levels in the supraoptic nucleus are negatively associated with novelty seeking, reward dependence, and persistence and positively associated with harm avoidance, cooperativeness, and self-transcendence in women.33
Aromatase is highly expressed in the hypothalamic paraventricular nucleus of healthy men and women. However, aromatase immunoreactivity is significantly decreased in this hypothalamic region in major depressive disorder patients,58 suggesting a possible link of aromatase in the hypothalamus and depression. In this regard, it is relevant to mention that some single-nucleotide CYP19A1 polymorphisms increase the risk of late-life depression in women without a previous history of major depression, whereas other variants of the gene, associated with higher estradiol levels, decrease the risk of late-life depression in women with a history of major depression.61
Cerebral neocortex and hippocampus: cognition, language, and integrative functions
Aromatase expression in regions of the human brain associated with cognitive function, such as the hippocampus, the prefrontal cortex, and the temporal cortex (Fig. 2),19–21,27,37,38 suggest that local conversion of androgens to estrogens participates in the regulation of learning and memory in humans, as it has been demonstrated in animal experimentation studies.6 Learning and memory rely on specific patterns of neuronal activity that induce synaptic plasticity. Interestingly, neuronal activity also regulates aromatase activity and estrogen synthesis in the CNS of birds and rodents.62,63 However, the role of androgens64,65 and estrogens66–68 in human cognition is still controversial. Nevertheless, some single-nucleotide CYP19A1 polymorphisms are associated with decreased performance in memory tests in women69 and aromatase inhibitors used for cancer treatment have a negative impact on cognition in some patients,70 with subtle effects on hippocampal-dependent verbal and visual memory.71 In contrast, treatment with the aromatase inhibitor letrozole does not impair the development of cognitive performance in boys.72
Aromatase activity may influence many other cortical functions, in addition to memory and learning. For instance, local production of estradiol by aromatase in the temporal cortex19,27 may be involved in visual and auditory integration and language. In this regard, the discovery of single-nucleotide polymorphisms within CYP19A1 associated with dyslexia categorical traits and with phonological phenotypes is of high relevance.73 Interestingly, cyp19A1 is associated with the control of vocalization in songbirds and teleost fish74,75 and CYP19A1 expression in the human brain correlates with the expression of other dyslexia-associated genes, such as ROBO1 and DYX1C1.73
Aromatase activity in associative regions of the occipital, parietal, and frontal lobes may also participate in complex integrative functions, as suggested by the finding that aromatase availability in these cerebral cortex regions is associated with different personality traits in men and women.33 These associations have been identified in specific regions of the occipital lobe (lingual gyrus), the parietal lobe (inferior parietal gyrus), and the frontal lobe (superior and inferior frontal gyrus, anterior cingulate gyrus). Thus, for instance, persistence shows a negative association with aromatase in the anterior cingulate gyrus, supramarginal gyrus, and lingual gyrus in men and a positive association with aromatase in the lingual gyrus in women; however, cooperativeness shows a positive association with aromatase in the anterior cingulate gyrus in men and a negative association with aromatase in the superior and inferior frontal gyrus in both sexes. These findings suggest that aromatase activity in some cortical structures may influence specific personality traits in humans, with sex-differentiated characteristics.
Alterations of Brain Aromatase with Aging
There is limited information on the effects of aging on aromatase in the human brain. PET studies suggest a small age-associated decrease in aromatase expression in most brain regions.29 However, aromatase PET signal increases in the cortical white matter at older ages.29 Further, an immunohistochemical study detected a significant increase in aromatase immunoreactivity in all hippocampal regions of older (58–90 years old) compared with younger (34–43 years old) premenopausal women.37 The same study revealed an upregulation of ERα in the same hippocampal regions of older women, suggesting that the drop in ovarian estrogen production with menopause is compensated by an increase in estrogen production and signaling in the hippocampus.37 It is unknown whether a similar increase in aromatase and ERs occurs in other specific brain regions of postmenopausal women or in older men. However, in both men and women, brain levels of estradiol remain unaltered with aging, even if in men aging is associated with a decline in androgen levels in the brain.76 It is, therefore, plausible that brain aromatase activity, at least in some regions such as the hippocampus, could contribute toward maintaining local estrogen levels in older men and women. The preservation of the local estradiol levels in the aged brain may represent an endogenous protective response to maintain brain health, given that estradiol activates neuroprotective mechanisms in neurons and glial cells.77 However, further studies are still necessary to confirm this interpretation.
Brain Aromatase Under Pathological Conditions
Aromatase in ASDs
There is some speculation on the possible involvement of aromatase activity in ASDs. Single nucleotide polymorphisms within CYP19A1 are associated with autistic traits and Asperger syndrome.78 In addition, a significant decrease in aromatase immunoreactivity was detected in a quantitative immunofluorescence analysis on postmortem samples from the frontal cortex (area 9 of Brodmann) of 12 autistic individuals, including males and females.79 Another study has reported a 38% reduction in CYP19A1 mRNA levels in the middle frontal gyrus of 13 male ASD patients relative to controls, together with a 35% decrease in ERβ mRNA expression in the same region of the frontal cortex. Both changes in aromatase and ERβ expression were confirmed at protein level.80
CYP19A1 mRNA expression in the frontal cortex is positively correlated with ERα and ERβ mRNA levels and with the mRNA levels of different ER cofactors,80 suggesting that ERs may be involved in the regulation of aromatase in this brain region. In this regard, it is important to consider that ERβ is not only a receptor for estradiol but also has affinity for the dihydrotestosterone (DHT) reduced metabolite 5α-androstane-3β,17β-diol.81 On the other hand, the expression of aromatase in the frontal cortex of ASD patients positively correlates with the expression of retinoic acid-related orphan receptor-α (RORA), which is also decreased in the brain of autistic patients.79,82 Interestingly, RORA is upregulated by estradiol through ERα and is downregulated by DHT through AR in human neuroblastoma cells. In turn, RORA transcriptionally regulates aromatase expression.79 Thus, it is tempting to speculate that the feedback mechanisms involved in hormonal regulation of RORA and aromatase are impaired in autistic patients. In addition, given the different effect of androgens and estradiol on RORA expression, it would be interesting to determine whether this molecule is involved in sex differences in ASDs, which affect predominantly males.
An implication of aromatase activity in autism would have important implications, considering that the enzyme is a target for endocrine disruptors and other environmental contaminants that may interact with ASD-associated genes during brain development.83 Indeed, as previously mentioned, aromatase is expressed in the developing human brain. Thus, by gestational week 17, aromatase immunoreactive cells are located at the proximity of the growing neuroepithelium of the ventricular and subventricular zones of the human cortex. Then, by gestational weeks 20–24, groups of immunoreactive aromatase cells are detected first in the cortical subplate and then in the parietal cortical plate. Later on, at perinatal and early postnatal periods, aromatase is expressed in glial fibrillary acidic protein immunoreactive glial cells in the cerebral cortex.25 Thus, aromatase activity could be potentially involved in neurodevelopmental disorders by the generation of estradiol, which may regulate specification, proliferation, or migration of neuronal and glial cells. Indeed, sex-specific deficits in cortical migration have been detected in aromatase knockout mice84 and estradiol reduces the impact of ASD-related mutations on cortical neurogenesis,85 suggesting that at least in animal models alterations in aromatase expression or activity during cortical development may cause permanent deficits in cortical function.
Another developmental factor associated with increased ASD risk that may alter aromatase expression is inflammation. Indeed, aromatase expression positively correlates with the expression of inflammatory markers in postmortem cerebellar samples of children who died between 1 and 9 years of age from pathologies associated with inflammatory conditions.86 This finding is relevant for the possible implication of local estradiol production in the brain in ASD, because the hormone is known to participate in the regulation of the development of cerebellar Purkinje neurons87 and because cerebellar alterations during infancy have been implicated in autism.88,89
Aromatase and epilepsy
Changes in serum estradiol/progesterone ratio during the menstrual cycle or exogenous administration of estradiol are known to increase seizure susceptibility in women with epilepsy.90,91 In men, testosterone has mixed effects on epileptic seizures, because the reduced testosterone metabolite 3α-androstanediol decreases the frequency of seizures, whereas the aromatization of testosterone to estradiol increases seizures.92 This later effect may be mediated by the facilitating effect of estradiol on excitatory synaptic transmission and the suppression of inhibitory neurotransmission involved in seizures.93,94 Therefore, aromatase inhibitors, by decreasing estradiol levels, reduce seizures in men with epilepsy.95
Although estradiol is epileptogenic, it may also exert protective effects in epilepsy. Thus, the hormone decreases the latency to initiate seizures but reduces the duration of late-stage seizures in female rats treated with the epileptogenic drug kainic acid.96 In addition, estradiol protects gamma aminobutyric acid metabolism and prevents spine synaptic loss in hippocampal slices in which epileptogenic activity is induced with bicuculine.97 Further, aromatase inhibitors reduce seizures,98 but they increase hippocampal neuronal damage15 induced by kainic acid in rodents. These paradoxical effects of estradiol, which increases the frequency of epileptic seizures but at the same time decreases seizure severity and protects neurons from epileptic-induced excitotoxicity, could be explained by the rapid synaptic actions of the hormone, which potentiate excitatory transmission and suppress inhibition in neurons,99,100 in combination with the hormonal long-term transcriptional effects, which decrease excitotoxicity and oxidative stress, inhibit apoptosis, and promote synaptogenesis by acting on both glial and neuronal cells.77
Given these neuroprotective actions of estradiol, it would be important to assess the potential neurological long-term effects of aromatase inhibitors when considering their potential clinical use for the treatment of epilepsy. This information will be relevant, because histological studies have shown that most surviving parvalbumin inhibitory interneurons in the hippocampus of long-term epileptic patients express aromatase,20 suggesting that local estradiol formation in the epileptic hippocampus may still be able to regulate inhibition.
Aromatase and stroke
Some CYP19A1 polymorphisms are associated with an increased risk of stroke in both men and women, whereas other gene variants are associated with a decreased risk.101 The effects of these CYP19A1 polymorphisms may be related with differences in systemic estradiol levels, because the hormone regulates numerous cardiovascular and metabolic parameters that affect the risk of stroke. However, central aromatase activity may also have some implications in the outcome of stroke.
Studies in rodents have shown an increased expression of the enzyme in reactive astrocytes in the regions of the neural tissue affected by secondary neurodegeneration after middle cerebral artery occlusion.15,102 This, together with the demonstration that estradiol generated by astrocytes after ischemic brain injury is neuroprotective,103 suggests a direct participation of aromatase activity in the outcome of stroke.
A similar increase in aromatase expression in reactive astrocytes has been detected after traumatic brain injury, where aromatase is induced by inflammatory signals and is neuroprotective by the local generation of estradiol.15,104 This suggests that central aromatase induction may be a general neuroprotective mechanism activated after an acute brain injury. Unfortunately, there is no information on whether aromatase expression is modified in the human brain after traumatic injury or stroke. Nevertheless, a recent study has reported an increase in serum aromatase levels 24 h after stroke in postmenopausal women.105 The functional significance of this finding is, however, unclear. Although aromatase concentration in serum has been shown to represent a good biomarker for some types of cancer,106 it is unknown whether it may be affected by modifications in aromatase expression in the brain.
Aromatase and chronic neurodegenerative diseases
As observed for the risk of stroke, some aromatase gene variants also modify the risk of suffering chronic neurodegenerative diseases, such as Alzheimer's disease (AD). Thus, it has been reported that some CYP19A1 polymorphisms, either per se107–112 or in combination with polymorphisms for other genes,113,114 are associated with increased AD susceptibility, whereas other aromatase gene polymorphisms may decrease AD risk.115
A direct evidence of changes in brain aromatase expression under neurodegenerative conditions in humans has been obtained in postmortem studies of multiple sclerosis lesions, where the enzyme is upregulated in astrocytes in male patients.116 Aromatase immunoreactivity is also increased in the hippocampus21 and the prefrontal cortex38 of male and female AD patients, compared with sex- and aged-matched controls. Further, aromatase immunoreactivity in the basal nucleus of Meynert, which sends cholinergic projections to the cerebral cortex and is important to maintain cortical function, is also increased in AD patients.36 In contrast, other brain regions of AD patients, including the hypothalamus, show decreased aromatase immunoreactivity compared with controls.36
The causes and significance of the changes in aromatase expression under chronic neurodegenerative conditions are not yet determined. As previously mentioned, animal studies suggest that increased aromatase expression after acute brain injury is neuroprotective by the local generation of estradiol. If these results are translatable to humans and to chronic neurodegenerative diseases, the enhanced expression of aromatase in multiple sclerosis lesions and in the hippocampus, the frontal cortex, and the basal nucleus of AD patients could represent an endogenous mechanism to protect the nervous system. On the other hand, aromatase upregulation in multiple sclerosis lesions and in the prefrontal cortex of AD patients is observed predominantly in astrocytes,38,117 which are known to actively participate in the neuroprotective actions of the enzyme by the generation of estradiol in animal experimental models.16,103,117,118
Aromatase and sex differences in neurodegenerative diseases
The similarity in the expression of aromatase in the brain of men and women is intriguing, considering that robust sex differences are presented by the enzyme in the brain of other species, including laboratory rodents. This may be so because aromatase plays different functions in the sexual differentiation of the brain in rodents and primates. Indeed, although estradiol generated by brain aromatase is essential for rodent brain masculinization, it does not appear to play a major role in this process in humans and non-human primates.119–121 However, estradiol is important for male physiology122,123 and both testosterone and estradiol are involved in men libido.124,125 Further, the analysis of ERs and AR polymorphisms in transsexual populations suggests an interaction of AR and ERβ in the development of transsexuality in androphilic male-to-female transsexuals.126 However, in addition to estradiol, reduced metabolites of DHT are also ligands of ERβ127 and, therefore, the role of aromatase activity in the generation of sexual identity in men is still an unsolved question.
Aromatase activity could also be potentially involved in the generation of sex differences in neurodegenerative diseases. This is suggested by the studies showing that it is only in women that the plasmatic levels of the enzyme increase after stroke,105 whereas it is only in men that aromatase expression is increased in multiple sclerosis lesions.116 In addition, the association of CYP19A1 gene polymorphisms with AD risk and mild cognitive impairment is more frequent in women than in men,114,128 further suggesting that aromatase activity may have a different effect on the neurodegenerative alterations of males and females.
Although not obvious sex differences in aromatase immunoreactivity have been detected in the brain of AD patients, studies in rodents suggest that aromatase activity may protect the female brain from AD pathology by increasing local estradiol levels but may have a negative impact on the male brain by decreasing local levels of testosterone.129,130 Whether this is the case in the human brain is unknown. Nevertheless, it is interesting to note that, compared with control individuals, estradiol levels decrease in the brain of women with AD, whereas testosterone but not estradiol levels decrease in the brain of men with AD.76
Conclusions and Perspectives
The studies reviewed here indicate that aromatase is expressed in most, if not all, regions of the human brain, but in specific cellular populations within each region. Although some functional consequences of aromatase activity in the human brain have been explored, there are still numerous gaps in our knowledge of the role of the enzyme in the human CNS. For instance, although neurons are the main cell type expressing aromatase in the human brain, the enzyme is also expressed in astrocytes, oligodendrocytes, ependymal cells, and choroid plexus cells. These cells have numerous homeostatic, metabolic, and trophic functions in the brain; regulate cerebrospinal fluid formation and composition; generate myelin; control the blood–brain barrier; and participate in synaptic transmission and plasticity. However, we have very limited information on the role of local estradiol generated by aromatase in these cells, which are also involved in a variety of neurological alterations.
To our knowledge, nothing has been published about the local distribution and function of aromatase in the human spinal cord. In this regard, it would be important to confirm in humans the results of animal studies showing that aromatase is expressed by dorsal root ganglion neurons41 and that estradiol locally produced in the spinal cord and the thalamus by the enzyme is involved in pain transmission.43–45
Another CNS region where aromatase function needs to be better explored is the retina, where local estradiol synthesis may have a protective role.49,131 The role of aromatase activity also remains to be clarified in numerous brain regions; for instance, in the lateral habenula, where local estradiol synthesis by axonal afferent may participate in the modulation of neuronal circuits linking body homeostasis and motivation,132 or in the striatum/nucleus accumbens region,29 where testosterone and estradiol may influence motivation, anxiety-associated symptoms, reward cue processing, and drug abuse. It will be also important to clarify the role of aromatase activity in the vestibular nuclei, the cerebellum, and the olivo-cerebellar system,22,29,133 where estradiol locally produced by the enzyme may potentially be involved in the coordination or integration of sensory inputs with motor and cognitive processes.134 A better understanding of the role of aromatase activity in the different regions of the human brain will contribute toward clarifying the controversial cognitive effects of aromatase inhibitors used as breast cancer therapies135,136 and may also help to develop new treatments for specific brain pathological alterations based on drugs that modulate aromatase enzymatic activity or expression.
Another important question that remains to be elucidated is the role of aromatase activity during brain development. As discussed in previous sections, aromatase is expressed in the human fetal cerebral cortex25 and local production of estradiol by the enzyme is involved in the regulation of brain development in experimental animals. Further studies are necessary to determine its role in the developing human brain and whether its activity or expression during this sensitive period is altered by endocrine disruptor chemicals or by other pathological insults, such as maternal infections or by maternal chronic stress. Aromatase expression in the brain may be also altered by infections or inflammation during the early years of postnatal development, as it has been shown to occur in the cerebellum of male and female children between 1 and 9 years of age, where the enzyme expression is significantly increased in those children who experienced some form of inflammation proximate to death.86 If these changes in aromatase expression have an impact on brain development and early brain function, as animal experimentation studies suggest, they could play a role in the onset of developmental-associated brain disorders and in the generation of permanent functional deficits.
In addition to clarifying the role of aromatase activity in acute and chronic neurodegenerative diseases, affective disorders, and the brain aging process, further research is also needed to explore another very important clinical aspect: the role of the enzyme in brain tumors. Aromatase is expressed by human glioma cells,32,137,138 where it may play a role in tumor evolution by the generation of estradiol. Indeed, it has been found that in human glioblastoma multiforme (GBM) tissue the number of cells immunoreactive to aromatase and ERs decreased as the grade of tumor malignity increased.139 In addition, it has been recently reported that high expression of aromatase and ERα in human GBM is associated with significantly longer survival times of GBM patients, regardless of gender.140 These findings may pave the way for promising therapeutic perspectives for the control of glioma growth.
In conclusion, there are many unsolved questions and gaps in our knowledge on human brain aromatase that remain to be explored by future research. However, the limited available information that has been reviewed here suggests that the aromatization of androgens to estrogens by the human nervous tissue is involved in many more physiological and pathological processes than previously believed.
Abbreviations Used
- AD
Alzheimer's disease
- AR
androgen receptor
- ASD
autism spectrum disorder
- CNS
central nervous system
- DHT
dihydrotestosterone
- ER
estrogen receptor
- GBM
glioblastoma multiforme
- GFAP
glial fibrillary acidic protein
- Gr
granular layer
- Hil
hilus
- Mol
molecular layer
- mRNA
messenger RNA
- Or
Stratum oriens
- PC
Purkinje cell layer
- PET
positron emission tomography
- Pyr
pyramidal layer
- Rad
Stratum radiatum
- RORA
retinoic acid-related orphan receptor-α
- Wm
white matter
Authors' Contributions
All authors participated in the conception and design, as well as the drafting and revising of the article. The article was approved by all authors.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by Agencia Estatal de Investigación, Ministerio de Ciencia e Innovación (BFU2017-82754-R, RYC-2015-18545, and BFU2017-84490-P), Centro de Investigación Biomédica en Red de Fragilidad y Envejecimiento Saludable (CIBERFES), Instituto de Salud Carlos III, and Fondo Europeo de Desarrollo Regional (FEDER).
Cite this article as: Azcoitia I, Mendez P, Garcia-Segura LM (2021) Aromatase in the human brain, Androgens: Clinical Research and Therapeutics 2.1, 189–202, DOI: 10.1089/andro.2021.0007.
References
- 1. Naftolin F, Ryan KJ, Petro Z. Aromatization of androstenedione by the diencephalon. J Clin Endocrinol Metab. 1971;33(2):368–370. [DOI] [PubMed] [Google Scholar]
- 2. Naftolin F, Ryan KJ, Petro Z. Aromatization of androstenedione by limbic system tissue from human foetuses. J Endocrinol. 1971;51(4):795–796. [DOI] [PubMed] [Google Scholar]
- 3. Garcia-Segura LM. Aromatase in the brain: Not just for reproduction anymore. J Neuroendocrinol. 2008;20(6):705–712. [DOI] [PubMed] [Google Scholar]
- 4. Cornil CA. On the role of brain aromatase in females: Why are estrogens produced locally when they are available systemically? J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2018;204(1):31–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Diotel N, Charlier TD, Lefebvre d'Hellencourt C, et al. Steroid transport, local synthesis, and signaling within the brain: Roles in neurogenesis, neuroprotection, and sexual behaviors. Front Neurosci. 2018;12:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rosenfeld CS, Shay DA, Vieira-Potter VJ. Cognitive effects of aromatase and possible role in memory disorders. Front Endocrinol (Lausanne). 2018;9:610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shaw K. Aromatase expression and function in the brain and behavior: A comparison across communication systems in teleosts. J Chem Neuroanat. 2018;94:139–153. [DOI] [PubMed] [Google Scholar]
- 8. Vahaba DM, Remage-Healey L. Neuroestrogens rapidly shape auditory circuits to support communication learning and perception: Evidence from songbirds. Horm Behav. 2018;104:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van der Linden A, Balthazart J. Rapid changes in auditory processing in songbirds following acute aromatase inhibition as assessed by fMRI. Horm Behav. 2018;104:63–76. [DOI] [PubMed] [Google Scholar]
- 10. Brocca ME, Garcia-Segura LM. Non-reproductive functions of aromatase in the central nervous system under physiological and pathological conditions. Cell Mol Neurobiol. 2019;39(4):473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saldanha CJ, Remage-Healey L, Schlinger BA. Synaptocrine signaling: Steroid synthesis and action at the synapse. Endocr Rev. 2011;32(4):532–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fester L, Rune GM. Sexual neurosteroids and synaptic plasticity in the hippocampus. Brain Res. 2015;1621:162–169. [DOI] [PubMed] [Google Scholar]
- 13. Rudolph LM, Cornil CA, Mittelman-Smith MA, et al. Actions of steroids: New neurotransmitters. J Neurosci. 2016;36(45):11449–11458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Azcoitia I, Arevalo MA, Garcia-Segura LM. Neural-derived estradiol regulates brain plasticity. J Chem Neuroanat. 2018;89:53–59. [DOI] [PubMed] [Google Scholar]
- 15. Garcia-Segura LM, Veiga S, Sierra A, Melcangi RC, Azcoitia I. Aromatase: A neuroprotective enzyme. Prog Neurobiol. 2003;71(1):31–41. [DOI] [PubMed] [Google Scholar]
- 16. Duncan KA, Saldanha CJ. Central aromatization: A dramatic and responsive defense against threat and trauma to the vertebrate brain. Front Neuroendocrinol. 2020;56:100816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sasano H, Takashashi K, Satoh F, Nagura H, Harada N. Aromatase in the human central nervous system. Clin Endocrinol (Oxf). 1998;48(3):325–329. [DOI] [PubMed] [Google Scholar]
- 18. Stoffel-Wagner B, Watzka M, Schramm J, Bidlingmaier F, Klingmüller D. Expression of CYP19 (aromatase) mRNA in different areas of the human brain. J Steroid Biochem Mol Biol. 1999;70(4–6):237–241. [DOI] [PubMed] [Google Scholar]
- 19. Yague JG, Muñoz A, de Monasterio-Schrader P, Defelipe J, Garcia-Segura LM, Azcoitia I. Aromatase expression in the human temporal cortex. Neuroscience. 2006;138(2):389–401. [DOI] [PubMed] [Google Scholar]
- 20. Yague JG, Azcoitia I, DeFelipe J, Garcia-Segura LM, Muñoz A. Aromatase expression in the normal and epileptic human hippocampus. Brain Res. 2010;1315:41–52. [DOI] [PubMed] [Google Scholar]
- 21. Prange-Kiel J, Dudzinski DA, Pröls F, Glatzel M, Matschke J, Rune GM. Aromatase expression in the hippocampus of AD patients and 5xFAD mice. Neural Plast. 2016;2016:9802086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Azcoitia I, Yague JG, Garcia-Segura LM. Estradiol synthesis within the human brain. Neuroscience. 2011;191:139–147. [DOI] [PubMed] [Google Scholar]
- 23. Biegon A, Kim SW, Alexoff DL, et al. Unique distribution of aromatase in the human brain: In vivo studies with PET and [N-methyl-11C]vorozole. Synapse. 2010;64(11):801–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pezzi V, Mathis JM, Rainey WE, Carr BR. Profiling transcript levels for steroidogenic enzymes in fetal tissues. J Steroid Biochem Mol Biol. 2003;87(2–3):181–189. [DOI] [PubMed] [Google Scholar]
- 25. Montelli S, Peruffo A, Zambenedetti P, et al. Expression of aromatase P450(AROM) in the human fetal and early postnatal cerebral cortex. Brain Res. 2012;1475:11–18. [DOI] [PubMed] [Google Scholar]
- 26. Stoffel-Wagner B, Watzka M, Steckelbroeck S, et al. Expression of CYP19 (aromatase) mRNA in the human temporal lobe. Biochem Biophys Res Commun. 1998;244(3):768–771. [DOI] [PubMed] [Google Scholar]
- 27. Wozniak A, Hutchison RE, Morris CM, Hutchison JB. Neuroblastoma and Alzheimer's disease brain cells contain aromatase activity. Steroids. 1998;63(5–6):263–267. [DOI] [PubMed] [Google Scholar]
- 28. Steckelbroeck S, Heidrich DD, Stoffel-Wagner B, et al. Characterization of aromatase cytochrome P450 activity in the human temporal lobe. J Clin Endocrinol Metab. 1999;84(8):2795–2801. [DOI] [PubMed] [Google Scholar]
- 29. Biegon A. In vivo visualization of aromatase in animals and humans. Front Neuroendocrinol. 2016;40:42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Honda S, Harada N, Takagi Y. Novel exon 1 of the aromatase gene specific for aromatase transcripts in human brain. Biochem Biophys Res Commun. 1994;198(3):1153–1160. [DOI] [PubMed] [Google Scholar]
- 31. Tan W, Zhu Z, Ye L, Leung LK. Methylation dictates PI.f-specific CYP19 transcription in human glial cells. Mol Cell Endocrinol. 2017;452:131–137. [DOI] [PubMed] [Google Scholar]
- 32. Yague JG, Garcia-Segura LM, Azcoitia I. Selective transcriptional regulation of aromatase gene by vitamin D, dexamethasone, and mifepristone in human glioma cells. Endocrine. 2009;35(2):252–261. [DOI] [PubMed] [Google Scholar]
- 33. Takahashi K, Hosoya T, Onoe K, et al. , Association between aromatase in human brains and personality traits. Sci Rep. 2018;8(1):16841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Naftolin F, Horvath TL, Jakab RL, Leranth C, Harada N, Balthazart J. Aromatase immunoreactivity in axon terminals of the vertebrate brain. An immunocytochemical study on quail, rat, monkey and human tissues. Neuroendocrinology. 1996;63(2):149–155. [DOI] [PubMed] [Google Scholar]
- 35. Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29(5):241–249. [DOI] [PubMed] [Google Scholar]
- 36. Ishunina TA, van Beurden D, van der Meulen G, et al. Diminished aromatase immunoreactivity in the hypothalamus, but not in the basal forebrain nuclei in Alzheimer's disease. Neurobiol Aging. 2005;26(2):173–194. [DOI] [PubMed] [Google Scholar]
- 37. Ishunina TA, Fischer DF, Swaab DF. Estrogen receptor alpha and its splice variants in the hippocampus in aging and Alzheimer's disease. Neurobiol Aging. 2007;28(11):1670–1681. [DOI] [PubMed] [Google Scholar]
- 38. Luchetti S, Bossers K, Van de Bilt S, et al. Neurosteroid biosynthetic pathways changes in prefrontal cortex in Alzheimer's disease. Neurobiol Aging. 2011;32(11):1964–1976. [DOI] [PubMed] [Google Scholar]
- 39. Horvath TL, Wikler KC. Aromatase in developing sensory systems of the rat brain. J Neuroendocrinol. 1999;11(2):77–84. [DOI] [PubMed] [Google Scholar]
- 40. Schaeffer V, Meyer L, Patte-Mensah C, Mensah-Nyagan AG. Progress in dorsal root ganglion neurosteroidogenic activity: Basic evidence and pathophysiological correlation. Prog Neurobiol. 2010;92(1):33–41. [DOI] [PubMed] [Google Scholar]
- 41. Bereiter DA, Thompson R, Rahman M. Sex differences in estradiol secretion by trigeminal brainstem neurons. Front Integr Neurosci. 2019;13:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Evrard HC, Balthazart J. Aromatization of androgens into estrogens reduces response latency to a noxious thermal stimulus in male quail. Horm Behav. 2004;45(3):181–189. [DOI] [PubMed] [Google Scholar]
- 43. Ghorbanpoor S, Garcia-Segura LM, Haeri-Rohani A, Khodagholi F, Jorjani M. Aromatase inhibition exacerbates pain and reactive gliosis in the dorsal horn of the spinal cord of female rats caused by spinothalamic tract injury. Endocrinology. 2014;155(11):4341–4355. [DOI] [PubMed] [Google Scholar]
- 44. Robarge JD, Duarte DB, Shariati B, Wang R, Flockhart DA, Vasko MR. Aromatase inhibitors augment nociceptive behaviors in rats and enhance the excitability of sensory neurons. Exp Neurol. 2016;281:53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kramer PR, Rao M, Stinson C, Bellinger LL, Kinchington PR, Yee MB. Aromatase derived estradiol within the thalamus modulates pain induced by varicella zoster virus. Front Integr Neurosci. 2018;12:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fusi C, Materazzi S, Benemei S, et al. Steroidal and non-steroidal third-generation aromatase inhibitors induce pain-like symptoms via TRPA1. Nat Commun. 2014;5:5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tenti S, Correale P, Cheleschi S, Fioravanti A, Pirtoli L. Aromatase inhibitors-induced musculoskeletal disorders: Current knowledge on clinical and molecular aspects. Int J Mol Sci. 2020;21(16):5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhao F, Ren D, Shen G, et al. Toxicity of extended adjuvant endocrine with aromatase inhibitors in patients with postmenopausal breast cancer: A Systemtic review and Meta-analysis. Crit Rev Oncol Hematol. 2020;156:103114. [DOI] [PubMed] [Google Scholar]
- 49. Moschos MM, Chatziralli IP, Sergentanis T, et al. Electroretinographic and optical coherence tomography findings in breast cancer patients using aromatase inhibitors. Cutan Ocul Toxicol. 2016;35(1):13–20. [DOI] [PubMed] [Google Scholar]
- 50. Hwang K, Bertolero MA, Liu WB, D'Esposito M. The human thalamus is an integrative hub for functional brain networks. J Neurosci. 2017;37(23):5594–5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rikhye RV, Wimmer RD, Halassa MM. Toward an integrative theory of thalamic function. Annu Rev Neurosci. 2018;41:163–183. [DOI] [PubMed] [Google Scholar]
- 52. Wolff M, Vann SD. The cognitive thalamus as a gateway to mental representations. J Neurosci. 2019;39(1):3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wolff M, Morceau S, Folkard R, Martin-Cortecero J, Groh A. A thalamic bridge from sensory perception to cognition. Neurosci Biobehav Rev. 2021;120:222–235. [DOI] [PubMed] [Google Scholar]
- 54. Alia-Klein N, Preston-Campbell RN, Kim SW, et al. Human cognitive ability is modulated by aromatase availability in the brain in a sex-specific manner. Front Neurosci. 2020;14:565668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Biegon A, Alia-Klein N, Alexoff DL, et al. Relationship of estrogen synthesis capacity in the brain with obesity and self-control in men and women. Proc Natl Acad Sci U S A. 2020;117(37):22962–22966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Park BY, Hong J, Park H. Neuroimaging biomarkers to associate obesity and negative emotions. Sci Rep. 2017;7(1):7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kenealy BP, Keen KL, Garcia JP, Kohlenberg LK, Terasawa E. Obligatory role of hypothalamic neuroestradiol during the estrogen-induced LH surge in female ovariectomized rhesus monkeys. Proc Natl Acad Sci U S A. 2017;114(52):13804–13809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wu JL, He Y, Hrubý R, et al. Aromatase changes in depression: A postmortem and animal experimental study. Psychoneuroendocrinology. 2017;77:56–62. [DOI] [PubMed] [Google Scholar]
- 59. Grassi D, Bellini MJ, Acaz-Fonseca E, Panzica G, Garcia-Segura LM. Estradiol and testosterone regulate arginine-vasopressin expression in SH-SY5Y human female neuroblastoma cells through estrogen receptors-α and -β. Endocrinology. 2013;154(6):2092–2100. [DOI] [PubMed] [Google Scholar]
- 60. Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: Social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12(9):524–538. [DOI] [PubMed] [Google Scholar]
- 61. Ancelin ML, Norton J, Canonico M, Scarabin PY, Ritchie K, Ryan J. Aromatase (CYP19A1) gene variants, sex steroid levels, and late-life depression. Depress Anxiety. 2020;37(2):146–155. [DOI] [PubMed] [Google Scholar]
- 62. Charlier TD, Cornil CA, Balthazart JJ. Rapid modulation of aromatase activity in the vertebrate brain. Exp Neurosci. 2013;7:31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fester L, Brandt N, Windhorst S, Pröls F, Bläute C, Rune GM. Control of aromatase in hippocampal neurons. J Steroid Biochem Mol Biol. 2016;160:9–14. [DOI] [PubMed] [Google Scholar]
- 64. Batra A, Marchioni M, Hashmi AZ, et al. Cognition and depression effects of androgen receptor axis-targeted drugs in men with prostate cancer: A systematic review. J Geriatr Oncol. 2021;12(5):687–695. [DOI] [PubMed] [Google Scholar]
- 65. Cai Z, Li H. An updated review: Androgens and cognitive impairment in older men. Front Endocrinol (Lausanne). 2020;11:586909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hogervorst E, Bandelow S. Sex steroids to maintain cognitive function in women after the menopause: A meta-analyses of treatment trials. Maturitas. 2010;66(1):56–71. [DOI] [PubMed] [Google Scholar]
- 67. Maki PM. Critical window hypothesis of hormone therapy and cognition: A scientific update on clinical studies. Menopause. 2013;20(6):695–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Albert K, Hiscox J, Boyd B, et al. Estrogen enhances hippocampal gray-matter volume in young and older postmenopausal women: A prospective dose-response study. Neurobiol Aging. 2017;56:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kravitz HM, Meyer PM, Seeman TE, Greendale GA, Sowers MR. Cognitive functioning and sex steroid hormone gene polymorphisms in women at midlife. Am J Med. 2006;119:S94–S102. [DOI] [PubMed] [Google Scholar]
- 70. Collins B, Mackenzie J, Stewart A, Bielajew C, Verma S. Cognitive effects of hormonal therapy in early stage breast cancer patients: A prospective study. Psychooncology. 2009;18(8):811–821. [DOI] [PubMed] [Google Scholar]
- 71. Bayer J, Rune G, Schultz H, et al. The effect of estrogen synthesis inhibition on hippocampal memory. Psychoneuroendocrinology. 2015;56:213–225. [DOI] [PubMed] [Google Scholar]
- 72. Hero M, Maury S, Luotoniemi E, Service E, Dunkel L. Cognitive effects of aromatase inhibitor therapy in peripubertal boys. Eur J Endocrinol. 2010;163(1):149–155. [DOI] [PubMed] [Google Scholar]
- 73. Anthoni H, Sucheston LE, Lewis BA, et al. The aromatase gene CYP19A1: Several genetic and functional lines of evidence supporting a role in reading, speech and language. Behav Genet. 2012;42(4):509–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Forlano PM, Schlinger BA, Bass AH. Brain aromatase: New lessons from non-mammalian model systems. Front Neuroendocrinol. 2006;27(3):247–274. [DOI] [PubMed] [Google Scholar]
- 75. Remage-Healey L, Coleman MJ, Oyama RK, Schlinger BA. Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proc Natl Acad Sci U S A. 2010;107(8):3852–3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rosario ER, Chang L, Head EH, Stanczyk FZ, Pike CJ. Brain levels of sex steroid hormones in men and women during normal aging and in Alzheimer's disease. Neurobiol Aging. 2011;32(4):604–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Azcoitia I, Barreto GE, Garcia-Segura LM. Molecular mechanisms and cellular events involved in the neuroprotective actions of estradiol. Analysis of sex differences. Front Neuroendocrinol. 2019;55:100787. [DOI] [PubMed] [Google Scholar]
- 78. Chakrabarti B, Dudbridge F, Kent L, et al. Genes related to sex steroids, neural growth, and social-emotional behavior are associated with autistic traits, empathy, and Asperger syndrome. Autism Res. 2009;2(3):157–177. [DOI] [PubMed] [Google Scholar]
- 79. Sarachana T, Hu VW. Differential recruitment of coregulators to the RORA promoter adds another layer of complexity to gene (dys) regulation by sex hormones in autism. Mol Autism. 2013;4(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Crider A, Thakkar R, Ahmed AO, Pillai A. Dysregulation of estrogen receptor beta (ERβ), aromatase (CYP19A1), and ER co-activators in the middle frontal gyrus of autism spectrum disorder subjects. Mol Autism. 2014;5(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Handa RJ, Pak TR, Kudwa AE, Lund TD, Hinds L. An alternate pathway for androgen regulation of brain function: Activation of estrogen receptor beta by the metabolite of dihydrotestosterone, 5alpha-androstane-3beta,17beta-diol. Horm Behav. 2008;53(5):741–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hu VW, Sarachana T, Sherrard RM, Kocher KM. Investigation of sex differences in the expression of RORA and its transcriptional targets in the brain as a potential contributor to the sex bias in autism. Mol Autism. 2015;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cheroni C, Caporale N, Testa G. Autism spectrum disorder at the crossroad between genes and environment: Contributions, convergences, and interactions in ASD developmental pathophysiology. Mol Autism. 2020;11(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sellers KJ, Denley MCS, Saito A, et al. Brain-synthesized oestrogens regulate cortical migration in a sexually divergent manner. Eur J Neurosci. 2020;52(1):2646–2663. [DOI] [PubMed] [Google Scholar]
- 85. Willsey HR, Exner CRT, Xu Y, et al. Parallel in vivo analysis of large-effect autism genes implicates cortical neurogenesis and estrogen in risk and resilience. Neuron. 2021;109(5):788..e8–804.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wright CL, Hoffman JH, McCarthy MM. Evidence that inflammation promotes estradiol synthesis in human cerebellum during early childhood. Transl Psychiatry. 2019;9(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sakamoto H, Mezaki Y, Shikimi H, Ukena K, Tsutsui K. Dendritic growth and spine formation in response to estrogen in the developing Purkinje cell. Endocrinology. 2003;144(10):4466–4477. [DOI] [PubMed] [Google Scholar]
- 88. Wang SS, Kloth AD, Badura A. The cerebellum, sensitive periods, and autism. Neuron. 2014;83(3):518–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hampson DR, Blatt GJ. Autism spectrum disorders and neuropathology of the cerebellum. Front Neurosci. 2015;9:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Herzog AG. Catamenial epilepsy: Definition, prevalence pathophysiology and treatment. Seizure. 2008;17(2):151–159. [DOI] [PubMed] [Google Scholar]
- 91. Herzog AG, Fowler KM, Sperling MR, et al. Variation of seizure frequency with ovulatory status of menstrual cycles. Epilepsia. 2011;52(10):1843–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Reddy DS. Testosterone modulation of seizure susceptibility is mediated by neurosteroids 3alpha-androstanediol and 17beta-estradiol. Neuroscience. 2004;129(1):195–207. [DOI] [PubMed] [Google Scholar]
- 93. Murphy DD, Cole NB, Greenberger V, Segal M. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J Neurosci. 1998;18(7):2550–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW. 17beta-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J Neurophysiol. 1999;81(2):925–929. [DOI] [PubMed] [Google Scholar]
- 95. Harden C, MacLusky NJ. Aromatase inhibitors as add-on treatment for men with epilepsy. Expert Rev Neurother. 2005;5(1):123–127. [DOI] [PubMed] [Google Scholar]
- 96. Ledoux VA, Smejkalova T, May RM, Cooke BM, Woolley CS. Estradiol facilitates the release of neuropeptide Y to suppress hippocampus-dependent seizures. J Neurosci. 2009;4;29(5):1457–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zhou L, Lehan N, Wehrenberg U, et al. Neuroprotection by estradiol: A role of aromatase against spine synapse loss after blockade of GABA(A) receptors. Exp Neurol. 2007;203(1):72–81. [DOI] [PubMed] [Google Scholar]
- 98. Iqbal R, Jain GK, Siraj F, Vohora D. Aromatase inhibition by letrozole attenuates kainic acid-induced seizures but not neurotoxicity in mice. Epilepsy Res. 2018;143:60–69. [DOI] [PubMed] [Google Scholar]
- 99. Smith SS, Woolley CS. Cellular and molecular effects of steroid hormones on CNS excitability. Cleve Clin J Med. 2004;71(Suppl. 2):S4–S10. [DOI] [PubMed] [Google Scholar]
- 100. Huang GZ, Woolley CS. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron. 2012;74(5):801–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Cai Q, Zheng J, Bai M, et al. Genetic variations of CYP19A1 gene and stroke susceptibility: A case-control study in the Chinese Han Population. J Cardiovasc Pharmacol. 2020;75(4):344–350. [DOI] [PubMed] [Google Scholar]
- 102. Carswell HV, Dominiczak AF, Garcia-Segura LM, Harada N, Hutchison JB, Macrae IM. Brain aromatase expression after experimental stroke: Topography and time course. J Steroid Biochem Mol Biol. 2005;96(1):89–91. [DOI] [PubMed] [Google Scholar]
- 103. Wang J, Sareddy GR, Lu Y, et al. Astrocyte-derived estrogen regulates reactive astrogliosis and is neuroprotective following Ischemic Brain Injury. J Neurosci. 2020;40(50):9751–9771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Saldanha CJ. Estrogen as a Neuroprotectant in both sexes: Stories from the bird brain. Front Neurol. 2020;11:497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Manwani B, Fall P, Zhu L, et al. Increased P450 aromatase levels in post-menopausal women after acute ischemic stroke. Biol Sex Differ. 2021;12(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Guszcz T, Szymańska B, Kozlowski R, Lukaszewski Z, Laskowski P, Gorodkiewicz E. Plasma aromatase as a sensitive and selective potential biomarker of bladder cancer and its role in tumorigenesis. Oncol Lett. 2020;19(1):562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Huang R, Poduslo SE. CYP19 haplotypes increase risk for Alzheimer's disease. J Med Genet. 2006;43(8):e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Iivonen S, Corder E, Lehtovirta M, et al. Polymorphisms in the CYP19 gene confer increased risk for Alzheimer disease. Neurology. 2004;62(7):1170–1176. [DOI] [PubMed] [Google Scholar]
- 109. Chace C, Pang D, Weng C, et al. Variants in CYP17 and CYP19 cytochrome P450 genes are associated with onset of Alzheimer's disease in women with down syndrome. J Alzheimers Dis. 2012;28(3):601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Janicki SC, Park N, Cheng R, Schupf N, Clark LN, Lee JH. Aromatase variants modify risk for Alzheimer's disease in a multiethnic female cohort. Dement Geriatr Cogn Disord. 2013;35(5–6):340–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Zheng J, Yan H, Shi L, et al. The CYP19A1 rs3751592 variant confers susceptibility to Alzheimer disease in the Chinese Han population. Medicine (Baltimore). 2016;95(35):e4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Song Y, Lu Y, Liang Z, Yang Y, Liu X. Association between rs10046, rs1143704, rs767199, rs727479, rs1065778, rs1062033, rs1008805, and rs700519 polymorphisms in aromatase (CYP19A1) gene and Alzheimer's disease risk: A systematic review and meta-analysis involving 11,051 subjects. Neurol Sci. 2019;40(12):2515–2527. [DOI] [PubMed] [Google Scholar]
- 113. Combarros O, Riancho JA, Infante J, et al. Interaction between CYP19 aromatase and butyrylcholinesterase genes increases Alzheimer's disease risk. Dement Geriatr Cogn Disord. 2005;20(2–3):153–157. [DOI] [PubMed] [Google Scholar]
- 114. Medway C, Combarros O, Cortina-Borja M, et al. The sex-specific associations of the aromatase gene with Alzheimer's disease and its interaction with IL10 in the Epistasis Project. Eur J Hum Genet. 2014;22(2):216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Combarros O, Sánchez-Juan P, Riancho JA, et al. Aromatase and interleukin-10 genetic variants interactively modulate Alzheimer's disease risk. J Neural Transm (Vienna). 2008;115(6):863–867. [DOI] [PubMed] [Google Scholar]
- 116. Luchetti S, van Eden CG, Schuurman K, van Strien ME, Swaab DF, Huitinga I. Gender differences in multiple sclerosis: Induction of estrogen signaling in male and progesterone signaling in female lesions. J Neuropathol Exp Neurol. 2014;73(2):123–135. [DOI] [PubMed] [Google Scholar]
- 117. Azcoitia I, Sierra A, Veiga S, Garcia-Segura LM. Aromatase expression by reactive astroglia is neuroprotective. Ann N Y Acad Sci. 2003;1007:298–305. [DOI] [PubMed] [Google Scholar]
- 118. Lu Y, Sareddy GR, Wang J, et al. Neuron-derived estrogen is critical for astrocyte activation and neuroprotection of the ischemic brain. J Neurosci. 2020;40(38):7355–7374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Wallen K. Hormonal influences on sexually differentiated behavior in nonhuman primates. Front Neuroendocrinol. 2005;26(1):7–26. [DOI] [PubMed] [Google Scholar]
- 120. Bao AM, Swaab DF. Sexual differentiation of the human brain: Relation to gender identity, sexual orientation and neuropsychiatric disorders. Front Neuroendocrinol. 2011;32(2):214–226. [DOI] [PubMed] [Google Scholar]
- 121. Kelava I, Chiaradia I, Pellegrini L, Kalinka AT, Lancaster MA. Male sex hormones increase excitatory neuron production in developing human neocortex. bioRxiv 2020.10.24.353359.
- 122. Cooke PS, Nanjappa MK, Ko C, Prins GS, Hess RA. Estrogens in male physiology. Physiol Rev. 2017;97(3):995–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Russell N, Grossmann M. Estradiol as a male hormone. Eur J Endocrinol. 2019;181(1):R23–R43. [DOI] [PubMed] [Google Scholar]
- 124. Schulster M, Bernie AM, Ramasamy R. The role of estradiol in male reproductive function. Asian J Androl. 2016;18(3):435–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Finkelstein JS, Lee, H, Burnett-Bowie SA, et al. 2013. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 2013;369: 1011–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Fernández R, Guillamon A, Cortés-Cortés J, et al. Molecular basis of Gender Dysphoria: Androgen and estrogen receptor interaction. Psychoneuroendocrinology. 2018;98:161–167. [DOI] [PubMed] [Google Scholar]
- 127. Lund TD, Hinds LR, Handa RJ. The androgen 5alpha-dihydrotestosterone and its metabolite 5alpha-androstan-3beta, 17beta-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor beta-expressing neurons in the hypothalamus. J Neurosci. 2006;26(5):1448–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Butler HT, Warden DR, Hogervorst E, Ragoussis J, Smith AD, Lehmann DJ. Association of the aromatase gene with Alzheimer's disease in women. Neurosci Lett. 2010;468(3):202–206. [DOI] [PubMed] [Google Scholar]
- 129. McAllister C, Long J, Bowers A, et al. Genetic targeting aromatase in male amyloid precursor protein transgenic mice down-regulates beta-secretase (BACE1) and prevents Alzheimer-like pathology and cognitive impairment. J Neurosci. 2010;30(21):7326–7334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Overk CR, Lu PY, Wang YT, et al. Effects of aromatase inhibition versus gonadectomy on hippocampal complex amyloid pathology in triple transgenic mice. Neurobiol Dis. 2012;45(1):479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Cascio C, Deidda I, Russo D, Guarneri P. The estrogenic retina: The potential contribution to healthy aging and age-related neurodegenerative diseases of the retina. Steroids. 2015;103:31–41. [DOI] [PubMed] [Google Scholar]
- 132. Zhang L, Hernández VS, Swinny JD, et al. A GABAergic cell type in the lateral habenula links hypothalamic homeostatic and midbrain motivation circuits with sex steroid signaling. Transl Psychiatry. 2018;8(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Scarduzio M, Panichi R, Pettorossi VE, Grassi S. Synaptic long-term potentiation and depression in the rat medial vestibular nuclei depend on neural activation of estrogenic and androgenic signals. PLoS One. 2013;8(11):e80792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Dieni CV, Contemori S, Biscarini A, Panichi R. De novo synthesized estradiol: A role in modulating the cerebellar function. Int J Mol Sci. 2020;21(9):3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Gervais NJ, Remage-Healey L, Starrett JR, Pollak DJ, Mong JA, Lacreuse A. Adverse effects of aromatase inhibition on the brain and behavior in a nonhuman primate. J Neurosci. 2019;39(5):918–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Branigan GL, Soto M, Neumayer L, Rodgers K, Brinton RD. Association between hormone-modulating breast cancer therapies and incidence of neurodegenerative outcomes for women with breast cancer. JAMA Netw Open. 2020;3(3):e201541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Yague JG, Lavaque E, Carretero J, Azcoitia I, Garcia-Segura LM. Aromatase, the enzyme responsible for estrogen biosynthesis, is expressed by human and rat glioblastomas. Neurosci Lett. 2004;368(3):279–284. [DOI] [PubMed] [Google Scholar]
- 138. Tan W, Wong TY, Wang Y, Huang J, Leung LK. CYP19 expression is induced by 2,3,7,8-tetrachloro-dibenzo-para-dioxin in human glioma cells. Mol Cell Endocrinol. 2013;375(1–2):106–112. [DOI] [PubMed] [Google Scholar]
- 139. Dueñas Jiménez JM, Candanedo Arellano A, Santerre A, et al. Aromatase and estrogen receptor alpha mRNA expression as prognostic biomarkers in patients with astrocytomas. J Neurooncol. 2014;119(2):275–284. [DOI] [PubMed] [Google Scholar]
- 140. Hönikl LS, Lämmer F, Gempt J, Meyer B, Schlegel J, Delbridge C. High expression of estrogen receptor alpha and aromatase in glial tumor cells is associated with gender-independent survival benefits in glioblastoma patients. J Neurooncol. 2020;147(3):567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]