Abstract
Patient: Male, 48-year-old
Final Diagnosis: Acute respiratory distress syndrome (ARDS) • parainfluenza virus infection
Symptoms: Dyspnea
Medication: —
Clinical Procedure: —
Specialty: Infectious Diseases • Pulmonology
Objective:
Rare disease
Background:
Human parainfluenza viruses (PIVs) belong to the Paramyxoviridae family. PIVs cause lower respiratory tract infections in children and the elderly. In addition, severe pneumonia due to PIVs has been reported in immunocompromised adults. However, no reports have described PIV infections leading to acute respiratory distress syndrome (ARDS) in immunocompetent hosts.
Case Report:
A 48-year-old otherwise healthy man was transported to our hospital due to worsening dyspnea. On arrival, strong effortful breathing was observed and results of arterial blood gas analysis revealed severe hypoxia. On the basis of the clinical presentation, we intubated the patient for mechanical ventilation. However, mechanical ventilation provided inadequate oxygenation. Finally, veno-venous extracorporeal membrane oxygenation was initiated. Pneumonia was considered to be a cause of the ARDS, based on the patient’s history and blood examination. Repeated reverse transcription-polymerase chain reaction tests for the novel coronavirus were performed, and endotracheal aspirate specimens were cultured for bacteria and fungus; however, the results were all negative. On day 2, the PIV-3-specific antibody titer was elevated. Two weeks later, the PIV-3-specific antibody titer had increased 4-fold. On the basis of these results, we diagnosed pneumonia induced by PIV-3 infection.
Conclusions:
ARDS can occur because of severe pneumonia induced by PIV-3. In cases of unexplained severe pneumonia or ARDS, PIV infection should be included in the differential diagnosis.
Keywords: Extracorporeal Membrane Oxygenation; Paramyxoviridae; Pneumonia, Viral
Background
Acute respiratory distress syndrome (ARDS) is a life-threatening condition defined by the association of bilateral infiltrates and hypoxemia due to a variety of pulmonary and non-pulmonary insults [1]. Pneumonia is the leading cause of ARDS; thus, accurate identification of the pathogen responsible for infection is necessary [2].
Human parainfluenza viruses (PIVs) belong to the Paramyxoviridae family. PIVs are enveloped RNA viruses [3]. PIVs cause upper respiratory tract infections in children and adults and lower respiratory tract infections in children younger than 5 years and elderly or immunocompromised adults. PIVs infections exhibit a distinctly bimodal pattern of age distribution [4]. Severe pneumonia in immunocompromised adults has been reported, particularly in patients with hematologic malignancies and hematopoietic stem cell transplant recipients [5,6]. However, no reports have described PIV infections leading to ARDS in immunocompetent hosts. Here, we report a case of ARDS induced by PIV that was successfully resuscitated using extracorporeal membrane oxygenation (ECMO) in a healthy young adult.
Case Report
A 48-year-old healthy man with no previous medical history (body mass index, 21.1) had been experiencing cold symptoms for 2 weeks and was treated by a nearby physician. The patient was transported to our hospital due to worsening dyspnea.
On arrival, his initial vital signs were as follows: Glasgow Coma Scale score, 15 (E4V5M6); respiratory rate, 32/min; percutaneous oxygen saturation, 85% under assisted ventilation with bag valve mask; heart rate, 120/min; blood pressure, 108/60 mmHg; and body temperature, 38°C. Labored breathing was observed, and arterial blood gas analysis revealed severe hypoxia (Table 1). On the basis of the clinical presentation, we intubated the patient for mechanical ventilation. However, mechanical ventilation provided inadequate oxygenation. The PaO2/FiO2 ratio was 81.8 and did not improve. Finally, venovenous ECMO was initiated. A chest computed tomography (CT) showed bilateral mixed ground-glass opacity (GGO) and consolidation (Figures 1, 2).
Table 1.
The blood examination results. Blood gas analysis was conducted under assisted ventilation with a bag valve mask (using 15 L/min of oxygen).
| Biochemistry | ||
| Total protein | 5.7 | g/dl |
| Albumin | 1.7 | g/dl |
| Aspartate transaminase | 28 | IU/l |
| Alanine transaminase | 27 | IU/l |
| Lactate dehydrogenase | 247 | IU/l |
| Blood urea nitrogen | 13 | mg/dl |
| Creatinine | 0.74 | mg/dl |
| Sodium | 132 | mEq/l |
| Potassium | 4.0 | mEq/l |
| Chloride | 98 | mEq/l |
| C-reactive protein | 22.35 | mg/dl |
| Coagulation | ||
| Prothrombin time-INR | 1.36 | |
| Activated partial thromboplastin time | 33.0 | sec |
| Fibrinogen | 746 | mg/dL |
| D-dimer | 16.1 | µg/mL |
| Hematology | ||
| White blood cells | 24000 | /μl |
| Red blood cells | 499 | ×104/μl |
| Hemoglobin | 13.6 | g/dl |
| Hematocrit | 41.1 | % |
| Platelet | 50.6 | ×104/μl |
| Differential leukocyte count | ||
| Neutrophils | 89.1 | % |
| Lymphocytes | 5.0 | % |
| Monocytes | 5.6 | % |
| Eosinophils | 0.1 | % |
| Basophils | 0.2 | % |
| Arterial blood gas analysis | ||
| pH | 7.513 | |
| Partial oxygen pressure | 62.8 | mmHg |
| Partial carbon dioxide pressure | 29.7 | mmHg |
| Bicarbonate ion | 26.0 | mmol/l |
| Base excess | 0.9 | mmol/l |
| Lactate | 1.3 | mmol/l |
INR – international normalized ratio.
Figure 1.

Chest X-ray on arrival showing peripheral bilateral ground-glass opacities.
Pneumonia was considered to be the cause of the ARDS, based on the patient’s history and blood tests. Administration of broad-spectrum antimicrobial agents was initiated while a search for the etiology of pathogens was conducted. At this time, the novel coronavirus (severe acute respiratory syndrome coronavirus 2; SARS-CoV-2) was beginning to spread in Japan, and a few ARDS cases due to the novel coronavirus had occurred. Therefore, pneumonia caused by the novel coronavirus was suspected. Reverse transcription-polymerase chain reaction (RT-PCR) test was repeated 3 times; however, the RT-PCR tests using a nasopharyngeal swab, sputum, and bronchoalveolar lavage fluid were all negative. Blood, endotracheal aspirate, and bronchoalveolar lavage fluid specimen cultures were negative for bacteria and fungus. Tests for bacterial and viral antigens that cause pneumonia were also negative (Table 2).
Table 2.
The results of microbiological tests.
| Bacterial tests | |
| Cultures | |
| Sputum | (–) |
| BALF | (–) |
| Mycoplasma antigen | (–) |
| Chlamydophila pneumoniae IgG | (–) |
| Chlamydophila pneumoniae IgM | (–) |
| Fungal tests | |
| Cultures | |
| Sputum | (–) |
| BALF | (–) |
| Aspergillus antigen | (–) |
| Candida antigen | (–) |
| Beta-D-glucan | (–) |
| Viral tests | |
| HIV-1/HIV-2 antibody | (–) |
| HTLV-1 antibody | (–) |
| CMV IgM | (–) |
| HSV IgM | (–) |
| VZV IgM | (–) |
| EBV IgM | (–) |
| Influenza virus antigen | (–) |
| Adenovirus antigen | (–) |
| RSV antigen | (–) |
| hMPV antigen | (–) |
| SARS-CoV-2 PCR | (–) |
BALF – bronchoalveolar lavage fluid; IgG – immunoglobulin G; IgM – immunoglobulin M; HIV – human immunodeficiency virus; HTLV – human T-lymphotropic virus; CMV – cytomegalovirus; HSV – herpes simplex virus; VZV – varicella-zoster virus; EBV – Epstein-Barr virus; RSV – respiratory syncytial virus; hMPV – human metapneumovirus; SARS-CoV-2 – severe acute respiratory syndrome coronavirus 2; PCR – polymerase chain reaction.
On day 2, the PIV antibody titer in the serum specimen was measured, and the PIV-3-specific antibody titer was elevated. Two weeks later, the PIV-3-specific antibody titer had increased 4-fold (Table 3). On the basis of the elevated antibody titers in paired serum specimens, we diagnosed pneumonia induced by PIV-3 infection.
Table 3.
The results of parainfluenza virus antibody titer for the serum specimens.
| Day 2 | Day 16 | |
|---|---|---|
| PIV-1 | <10 | <10 |
| PIV-2 | 20 | 40 |
| PIV-3 | 40 | 160 |
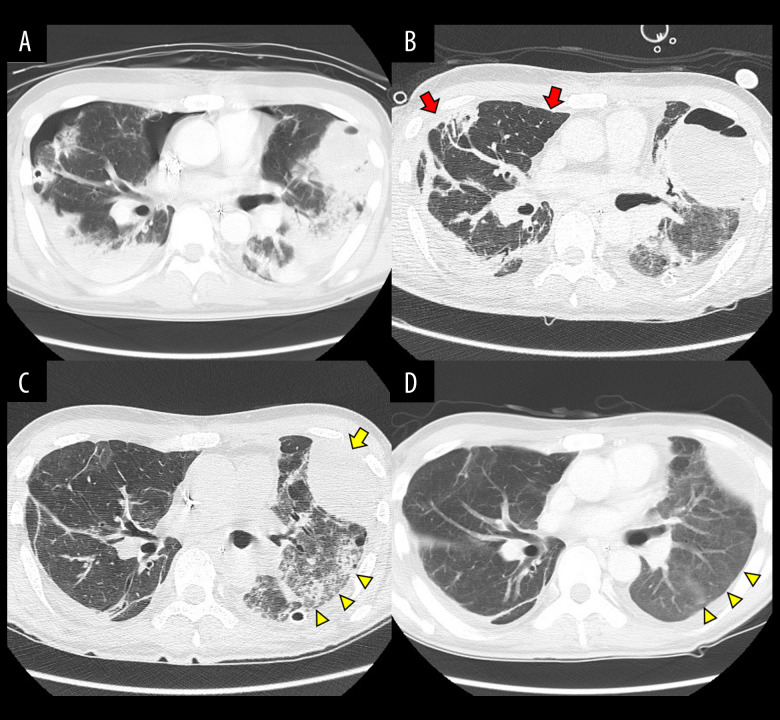
On day 9, the patient went into cardiac arrest when we were changing the ECMO circuit. He underwent chest compressions and developed bilateral hemopneumothorax; therefore, thoracic drainage and a partial lung resection were performed. He had secondary refractory pneumothorax, which resulted in prolonged ECMO treatment. The patient’s respiratory condition gradually improved, and the GGO and consolidation on chest CT disappeared (Figure 3). Finally, the patient was taken off of ECMO on day 52 and transferred to another hospital for continued rehabilitation on day 67, without neurological deficits.
Figure 3.
Chest computed tomography. (A) On day 9, the patient had hemorrhagic shock due to bilateral hemopneumothorax. Thoracic drainage and a partial lung resection were performed. (B) On day 37, the secondary refractory pneumothorax disappeared (red arrows). (C) On day 52, the left lung hematoma shrank (yellow arrow) and the ground-glass opacity improved (arrow heads). (D) On day 65, the ground-glass opacity almost disappeared (arrow heads).
Discussion
This is the first report of severe pneumonia progressing to ARDS caused by PIV-3 in an otherwise healthy adult. ARDS caused by pneumonia may be reversible; therefore, microbiological examinations for a wide range of pathogens are important for the initial assessment of patients with ARDS [4]. PIVs cause 22–29% of community-acquired pneumonia, with influenza viruses and rhinoviruses being the most common [7–9]. PIV infections account for 1–15% of respiratory illnesses in adults, with infrequent reports of pneumonia in young adults and a higher risk for severe disease in frail older adults [10]. There are 4 types (1 through 4) and 2 subtypes (4a and 4b) of PIVs. The prevalence of each serotype attributed to infection varies with time, but most studies show that PIV-3 is the most common cause of clinically significant infection [11]. Acquisition of PIV-3 usually occurs early in life, with 50% and 92% of children infected by 1 and 3 years of age, respectively. PIV-3 is isolated predominantly from patients under 5 years of age [4]. Severe pneumonia due to PIV-3 also occurs in hematopoietic stem cell transplant or lung transplant recipients [5,6,12]. However, no reports have described severe pneumonia caused by PIV-3 in immunocompetent hosts. In the present case, there was no co-infection with any virus other than PIV-3. We did not measure the levels of cytokines and could not assess the immune response. However, 50% hemolytic complement activity (CH50) was activated, and we considered that the immune response was normal. Our case suggests that PIV-3 can be the cause of severe pneumonia and ARDS of unknown origin.
The clinical presentation of PIV infection in adults, including cough, rhinorrhea, and sore throat, is indistinguishable from other respiratory illnesses [4]. Typical chest CT findings for PIV pneumonia are multifocal patchy consolidation with GGO and centrilobular nodules with bronchial wall thickening [13]. In this case, the patient already had severe respiratory failure upon arrival at the hospital, and chest CT showed widespread GGO and consolidation. Thus, the cause of ARDS was difficult to diagnose.
Infection with PIVs can be confirmed by: 1) direct detection of the viral genome using PCR, 2) direct detection of viral antigens in respiratory secretions (collected within 1 week of symptom onset) using immunofluorescence or enzyme immunoassays, 3) isolation and identification of the virus in cell culture, or 4) demonstration of a significant rise in PIV-specific IgG antibodies between appropriately collected paired serum specimens or specific IgM antibodies in a single serum specimen [14]. When this case occurred, a worldwide pandemic of the novel coronavirus infection had started and was beginning to spread in Japan. Almost all of the PCR assays were used to test for novel coronavirus infections and were not available for the diagnosis of PIVs. Thus, we used the serologic diagnosis to show a significant rise in IgG antibodies between paired serum specimens. If the PCR test had been used to diagnose PIV, the diagnosis could have been made more promptly.
Conclusions
ARDS can occur because of severe pneumonia induced by PIV-3 without viral co-infection. Our experience suggests that PIV infection should be included in the differential diagnosis in cases of unexplained severe pneumonia or ARDS in immunocompetent hosts.
Figure 2.

Computed tomography on arrival showing patchy bilateral ground-glass opacities (arrow heads) and consolidations (arrows).
Footnotes
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Fan E, Brodie D, Slutsky AS. Acute respiratory distress syndrome: Advances in diagnosis and treatment. JAMA. 2018;319:698–710. doi: 10.1001/jama.2017.21907. [DOI] [PubMed] [Google Scholar]
- 2.Papazian L, Calfee CS, Chiumello D, et al. Diagnostic workup for ARDS patients. Intensive Care Med. 2016;42:674–85. doi: 10.1007/s00134-016-4324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henrickson KJ. Parainfluenza viruses. Clin Microbiol Rev. 2003;16:242–64. doi: 10.1128/CMR.16.2.242-264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Branche AR, Falsey AR. Parainfluenza virus infection. Semin Respir Crit Care Med. 2016;37:538–54. doi: 10.1055/s-0036-1584798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chalkias S, Mackenzie MR, Gay C, et al. DAS181 treatment of hematopoietic stem cell transplant patients with parainfluenza virus lung disease requiring mechanical ventilation. Transpl Infect Dis. 2014;16:141–44. doi: 10.1111/tid.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shima T, Yoshimoto G, Nonami A, et al. Successful treatment of parainfluenza virus 3 pneumonia with oral ribavirin and methylprednisolone in a bone marrow transplant recipient. Int J Hematol. 2008;88:336–40. doi: 10.1007/s12185-008-0148-6. [DOI] [PubMed] [Google Scholar]
- 7.Kurai D, Sasaki Y, Saraya T, et al. Pathogen profiles and molecular epidemiology of respiratory viruses in Japanese inpatients with community-acquired pneumonia. Respir Investig. 2016;54:255–63. doi: 10.1016/j.resinv.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alimi Y, Lim WS, Lansbury L, et al. Systematic review of respiratory viral pathogens identified in adults with community-acquired pneumonia in Europe. J Clin Virol. 2017;95:26–35. doi: 10.1016/j.jcv.2017.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radovanovic D, Sotgiu G, Jankovic M, et al. An international perspective on hospitalized patients with viral community-acquired pneumonia. Eur J Intern Med. 2019;60:54–70. doi: 10.1016/j.ejim.2018.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falsey AR, Walsh EE. Viral pneumonia in older adults. Clin Infect Dis. 2006;42:518–24. doi: 10.1086/499955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russell E, Ison MG. Parainfluenza virus in the hospitalized adult. Clin Infect Dis. 2017;65:1570–76. doi: 10.1093/cid/cix528. [DOI] [PubMed] [Google Scholar]
- 12.Drozd DR, Limaye AP, Moss RB, et al. DAS181 treatment of severe parainfluenza type 3 pneumonia in a lung transplant recipient. Transpl Infect Dis. 2013;15:E28–32. doi: 10.1111/tid.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koo HJ, Lim S, Choe J, Choi SH, et al. Radiographic and CT features of viral pneumonia. RadioGraphics. 2018;38:719–39. doi: 10.1148/rg.2018170048. [DOI] [PubMed] [Google Scholar]
- 14.CDC (Centers for Disease Control and Prevention) Human parainfluenza viruses (HPIVs), for healthcare professionals, laboratory diagnosis. 2009. Available at: https://www.cdc.gov/parainfluenza/hcp/lab.html [accessed July 24 2021]