Abstract
Histamine and hypocretin neurons are localized to the hypothalamus, a brain area critical to autonomic function and sleep. Narcolepsy type 1, also known as narcolepsy with cataplexy, is a neurological disorder characterized by excessive daytime sleepiness, impaired night-time sleep, cataplexy, sleep paralysis and short latency to rapid eye movement (REM) sleep after sleep onset. In narcolepsy, 90% of hypocretin neurons are lost; in addition, two groups reported in 2014 that the number of histamine neurons is increased by 64% or more in human patients with narcolepsy, suggesting involvement of histamine in the aetiology of this disorder. Here, we review the role of the histamine and hypocretin systems in sleep–wake modulation. Furthermore, we summarize the neuropathological changes to these two systems in narcolepsy and discuss the possibility that narcolepsy-associated histamine abnormalities could mediate or result from the same processes that cause the hypocretin cell loss. We also review the changes in the hypocretin and histamine systems, and the associated sleep disruptions, in Parkinson disease, Alzheimer disease, Huntington disease and Tourette syndrome. Finally, we discuss novel therapeutic approaches for manipulation of the histamine system.
Introduction
The hypothalamus is a brain area with a critical role in the control of autonomic function and sleep. The involvement of the posterior hypothalamus in the maintenance of wakefulness was first recognized during an encephalitis lethargica epidemic (1918–1926) by the neurologist von Economo, who described extreme sleepiness associated with damage to this area.1 The posterior hypothalamus is a heterogeneous structure that includes neurons containing histamine, glutamate, γ-aminobutyric acid (GABA), hypocretin (also known as orexin) and melanin-concentrating hormone (MCH).
In normal individuals, hypocretin neurons are present throughout the hypothalamus. More than a decade ago, two groups2,3 identified loss of hypocretin neurons as the cause of human narcolepsy with cataplexy. The discovery of this hypocretin deficiency led to a change in the International Classification of Sleep Disorders, with the ‘narcolepsy’ category being divided into ‘narcolepsy type 1’ and ‘narcolepsy type 2’.
Narcolepsy is diagnosed in patients who have a daily irrepressible need for sleep. In narcolepsy type 1 (also called hypocretin deficiency syndrome or narcolepsy with cataplexy), patients demonstrate one or both of the following: cataplexy plus mean sleep latencies of ≤8 min and at least two sleep-onset rapid eye movement (REM) periods on a multiple sleep latency test, and/or low levels of hypocretin peptide 1 (≤110 pg/ml) in the cerebrospinal fluid (CSF). By contrast, patients with narcolepsy type 2 (initially called narcolepsy without cataplexy) also demonstrate mean sleep latencies of ≤8 min and at least two sleep-onset REM periods, but do not experience cataplexy, have normal CSF levels of hypocretin peptide 1 (if tested), and their hypersomnolence and/or sleep latency findings cannot be better described by another cause.4 Recently, two groups reported a massive increase in the number of histamine neurons in narcolepsy type 1.5,6
Both histamine and hypocretin have been implicated in the modulation of brain functions, such as sleep–wake regulation, energy and endocrine homeostasis, reward-motivated behaviours and motor functions.7–12 This Review focuses on the contribution of histamine and hypocretin to the control of the sleep–wake cycle. We review recent anatomical and physiological evidence for the role of histamine and hypocretin in normal sleep–wake behaviours, as well as recent findings concerning their involvement in a variety of pathologies, including narcolepsy, Alzheimer disease (AD), Parkinson disease (PD), Huntington disease (HD) and Tourette syndrome (TS). Moreover, we pay special attention to recently published results on over-the-counter antihistamines, and histamine H3 receptor (H3R) antagonists and inverse agonists in treating sleep–wake abnormalities in these diseases.
Neurobiology
In the human brain, both histamine and hypocretin neurons are found in the medial, lateral and posterior hypothalamus, where they are intermixed with other types of neurons (Figure 1).7,8
Figure 1 |.
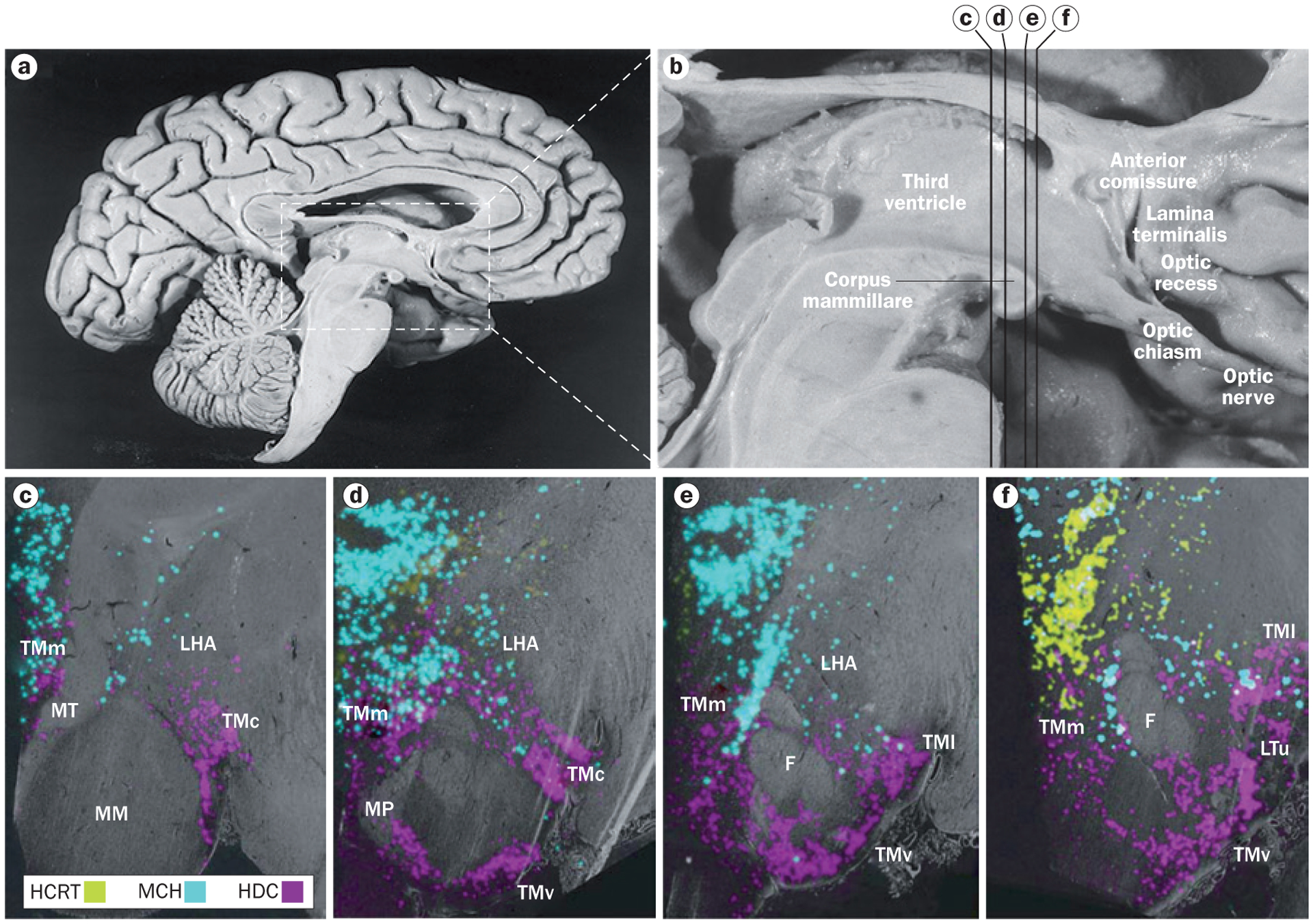
Neurons containing histamine, hypocretin and melanin-concentrating hormone in the human hypothalamus. a | Overview of the medial surface of the human brain, and b | detail of the hypothalamus. The distribution of neurons containing histamine, hypocretin and melanin-concentrating hormone are shown with in situ hybridization in coronal sections of hypothalamus at the c | posterior level through the medial mammillary nucleus, d | rostromammillary level at the principal mammillary fasciculus, e | level of the caudal end of the fornix and f | premamillary level. In panels c–f, the third ventricle is on the left side. Abbreviations: F, fornix; HCRT, hypocretin neurons; HDC, histidine-decarboxylase-positive (histamine) neurons; LHA, lateral hypothalamic area; LTu, lateral segment of the lateral tuberal nucleus; MCH, melanin-concentrating hormone neurons; MM, medial mammillary nucleus; MP, mammillary peduncle; MT, mammillothalamic tract; TMc, caudal tuberomammillary nucleus; TMl; lateral tuberomammillary nucleus; TMm, medial tuberomammillary nucleus; TMv, ventral tuberomammillary nucleus. Panels a and b reprinted with permission from Elsevier © Handbook of Clinical Neurology Vol. 79. Swaab, D. F. The human hypothalamus: basic and clinical aspects. Part 1: Nuclei of the human hypothalamus 3–38 (2003). Parts c–f adapted with permission from John Wiley & Sons © Krolewski, D. M. et al. J. Comp. Neurol. 518, 4591–4611 (2010).
Histamine neurons
Histamine metabolism and histamine receptors
Histamine neurons express l-histidine decarboxylase (HDC), which is the rate-limiting enzyme for neuronal histamine synthesis. In ex vivo studies, histamine neurons can be detected by immunological staining of HDC.13,14
Brain histamine is reduced to an inactivated form, tele-methylhistamine (t-MeHA), by histamine N-methyltransferase.7,8 All four types of histamine receptors (H1R–H4R) in the brain belong to the G-protein-coupled receptor superfamily. H1R has a primarily excitatory effect on neurons via G-protein-coupled depolarization.7 H3R can act as either an autoreceptor or heteroreceptor, and suppresses the release of histamine and other neurotransmitters, including acetylcholine, dopamine, GABA and serotonin.7,8 The functional similarities and differences, as well as the details of the localization of histamine receptors in the brain have been reviewed elsewhere.7,8
Co-transmitters and control of sleep–wake cycle
GABAergic signalling on histamine neurons alone does not seem to have a major role in controlling the sleep–wake cycle,15 because genetic ablation of GABA receptors on histamine neurons in mice does not affect their sleep–wake cycle.16 This result suggests that other transmitters, such as galanin or glutamate,17 contribute to the control of the sleep–wake cycle via interactions with histamine. Relayed input from the suprachiasmatic or intracellular circadian regulation mechanisms could also be involved; indeed, a 2014 mouse study demonstrated that deletion of Arntl (also known as Bmal1) from histamine neurons resulted in prolonged wakefulness and fragmented sleep.18
Hypocretin neurons
A few hypocretin neurons are found in the anterior hypothalamus at the level where the fornix crosses the paraventricular nucleus (Figure 1).19 At more caudal levels, the fornix projects to the mammillary bodies while passing through the perifornical nucleus, an area with a large number of hypocretin neurons.19 There are also many hypocretin neurons at the junction of the fornix and the mammillary bodies.20
Hypocretin peptides are produced from the precursor molecule preprohypocretin. The longer hypocretin peptide, hypocretin-1, contains 33 amino acids, and the shorter, hypocretin-2, contains 28 amino acids.21 Hypocretin receptors are 7-transmembrane G-protein coupled receptors. Hypocretin receptor type 1 (hypocretin 1R) is abundantly expressed in the locus coeruleus and dorsal raphe nucleus, and has a preferential affinity for hypocretin-1, whereas hypocretin receptor type 2 (hypocretin 2R) binds both forms of hypocretin with similar affinity, and is expressed in several brain areas.21
Co-transmitters
Hypocretin neurons also secrete dynorphin,22,23 glutamate24 and neuronal-activity-regulated pentraxin (NARP).23,25,26 NARP secretion by hypocretin neurons is of particular interest, because it exhibits a circadian rhythm27,28 and is a key regulator for diurnal plasticity of hypocretin neurons.27,28
Dynorphin has been reported to be packed in the same synaptic vesicles as hypocretin.29 Interestingly, dynorphin can mediate depressive-like states in mice,29 and has an opposite action to hypocretin on rewarding behaviours.29 Individuals with narcolepsy have increased incidence of depression compared with individuals with comparably debilitating diseases,30 and hypocretin release in humans is increased during pleasure and inhibited during pain.9 The link between narcolepsy and depression might be explained by a disturbed dynorphin–hypocretin balance.29
Reciprocal connections
An in situ hybridization study has demonstrated that hypocretin and histamine neurons are often found adjacent to each other in the posterior part of the human hypothalamus (Figure 1).19 Their projection areas also largely overlap, as extensively mapped by the Allen Brain Atlas Mouse Connectivity project (http://connectivity.brain-map.org/; Figure 2).32 Moreover, immunohistochemistry and electron microscopy studies in the rodent brain have shown hypocretin fibres to be extensively distributed in the tuberomammillary nucleus (TMN) area,33–36 and an immunochemical study found histamine fibres to project heavily to hypocretin neurons.37 The axonal terminals of hypocretin neurons in the TMN contain both hypocretin and glutamate vesicles;24 both of these transmitters excite histamine neurons.37,38
Figure 2 |.
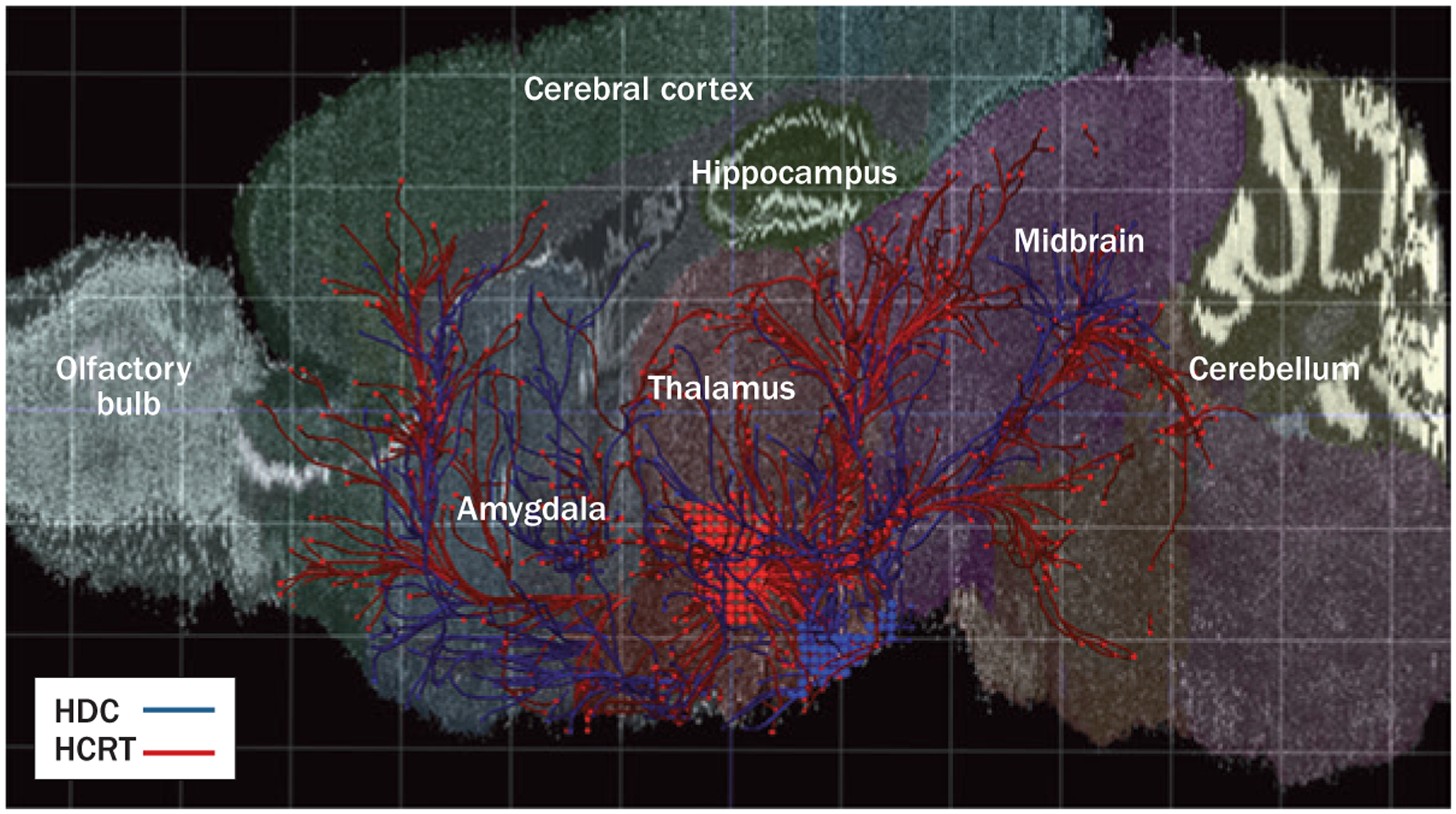
The hypocretin and histamine systems in the mouse brain—origin and general projections. Red dots indicate the location and projections of hypocretin neurons. The blue dots and lines represent the location and innervations of histamine neurons. Mouse brain connectivity was mapped using hypocretin–Cre and histidine-decarboxylase–Cre mouse lines. Adeno-associated viral vectors expressing Cre-dependent enhanced green fluorescent protein was injected to trace axonal projections from both hypocretin (red) and histamine (blue) neurons. Abbreviations: HCRT, hypocretin neurons; HDC, histidine-decarboxylase-positive (histamine) neurons. Reprinted with permission. © 2014 Allen Institute for Brain Science. Allen Mouse Brain Connectivity Atlas [online]. Available from: http://connectivity.brain-map.org/.
The histamine and hypocretin systems have been shown to regulate each other bidirectionally in zebrafish: translational inhibition of HDC reduced the level of hypocretin mRNA and the number of hypocretin neurons, and this reduction was rescued by overexpression of HDC mRNA39 in an H1R-dependent manner.39 The comprehensive interactions between histamine and hypocretin during development and the functional roles of this interaction have been discussed in recent reviews.40,41 It remains, however, unclear whether histamine neurons form synaptic connections with hypocretin neurons. In transgenic mice that retrogradely transfer green fluorescent protein (GFP) to terminals that project to hypocretin neurons, no evidence was found of synaptic connections from the TMN to hypocretin neurons.42 A classical retrograde tracing study also did not find evidence for such connections.43
Regulation of the sleep–wake cycle
Histamine involvement
The histamine neurons of the TMN are active during wakefulness, have a very low level of neuronal activity during non-REM sleep, and reach their minimum activity level in REM sleep.44,45 Knockout studies have implicated both histamine, H1R, and H3R in sleep–wake regulation. Specifically, Hdc knockout mice showed impaired wakefulness and increased REM sleep.46 Mice lacking H1R exhibited much fewer brief waking episodes than did wild-type mice.47,48 Both Hdc and H1R knockout mice demonstrate an increased ratio of delta–theta oscillations, an indicator of sleepiness, during the dark phase of the daily light–dark cycle (when they would normally be active), indicating that the H1R could largely exert the function of histamine in the maintenance of wakefulness.46,48 The H3R knockout mice have been reported to show fragmented sleep and reduced wakefulness during the dark period.49
Hypocretin involvement
Hypocretin neurons can induce wakefulness via excitation of histamine neurons. Optogenetic studies in rodent brain slices have demonstrated fast glutamate transmission from hypocretin neurons to histamine neurons via the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors.38 Histamine neurons in the TMN can also be activated directly through hypocretin 2R.34 In dog models of narcolepsy, a mutation that inactivates the hypocretin 2R decreased histamine levels in the CSF50 despite normal numbers of histamine neurons.
Co-regulation of sleep by hypocretin and histamine
The diurnal activity pattern of hypocretin neurons parallels that of the histamine neurons, with increased c-fos staining, a neuronal activity marker, as well as increased extracellular hypocretin-1 levels reported in rats during the night phase.10,49,51 However, hypocretin and histamine have distinct roles in maintaining wakefulness. Hcrt knockout mice show increased REM sleep duration during the dark phase, whereas Hdc knockout mice show increases in REM sleep duration only during the light phase.52 Most importantly, Hcrt knockout animals exhibit narcolepsy with cataplexy, which is absent in Hdc knockout mice.52
In line with the findings from mouse studies, human brain histamine and hypocretin-1 levels both show a diurnal pattern, being low at wake onset, upregulated in the late waking stage and downregulated during sleep. The expression of HDC mRNA in the human TMN, measured by radioactive quantitative in situ hybridization in the postmortem brain, reaches its highest level between 0800 h and 2000 h.53 A similar profile is also seen in the CSF histamine level in squirrel monkeys54 as well as in histamine metabolites (t-MeHA) in the human CSF.55 A slightly increased hypocretin-1 level is also observed in the late afternoon (1100 h–1800 h) relative to that at night (1800 h–1100 h) in the CSF acquired via lumbar puncture from healthy humans participants.56 It should be noted that there are several steps between brain histamine levels and the levels of metabolites in the CSF, including transcription, translation and degradation. Information from both transcript levels and CSF levels of histamine and histamine metabolites (t-MeHA) can provide additional information on the activity of the brain histamine system.
More evidence for the concerted action of the histamine and hypocretin systems in sleep regulation comes from the findings that intracerebroventricular infusion of pyrilamine, an H1R antagonist, inhibits hypocretin-induced arousal in rats,34 and that mice lacking the H1R do not show hypocretin-induced wakefulness.35 Moreover, H1R knockout mice show reduced hypocretin levels, suggesting that H1R activates hypocretin neurons in situ.57
Histamine and hypocretin in CNS disease
Narcolepsy
Narcolepsy was first described by Westphal (in 1877) and Gelineau (in 1880). Narcolepsy is a chronic sleep disorder characterized by excessive daytime sleepiness, cataplexy (sudden loss of muscle tone in response to certain strong emotions), REM sleep abnormalities (such as hypnagogic hallucinations at the transitions between wakefulness and sleep, sleep-onset REM periods during daytime naps) and sleep paralysis. Individuals with narcolepsy often have disturbed night-time sleep and tend to be obese.58,59
Loss of hypocretin neurons in narcolepsy
The number of hypocretin neurons in patients with type 1 narcolepsy is greatly reduced,2,3 with an average depletion of 90% (Figure 3).3 This specific and subtotal degeneration of hypocretin neurons has been confirmed by quantitative analyses that have shown a similar loss of neurons containing the hypocretin co-transmitters dynorphin and NARP.3,23,26 The loss of hypocretin neurons does not seem to be accompanied by a generalized degeneration of neurons in the hypothalamus, because the number of MCH neurons, which are intermixed with the hypocretin neurons, is unchanged in narcolepsy.3
Figure 3 |.
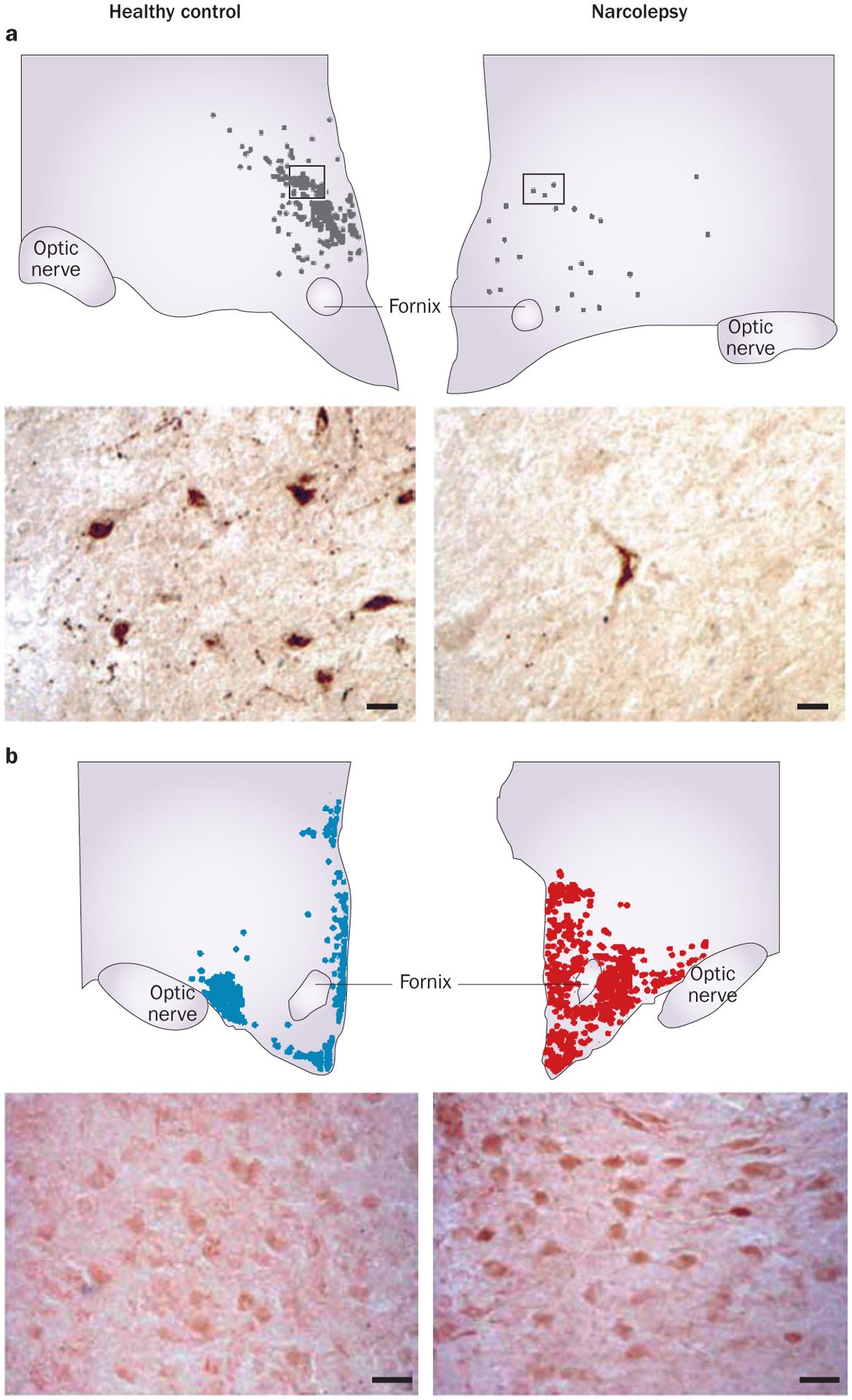
Loss of hypocretin neurons and increase in histamine neurons in narcolepsy. a | Number and distribution of hypocretin neurons, and a representative cell staining of brain slices from a healthy control (left) and an individual with narcolepsy (right). Scale bars = 25 μm. b | Number and distribution of histamine neurons, and a representative cell staining from a healthy control (left) and an individual with narcolepsy (right). Scale bars = 50 μm. Part a reprinted with permission from Elsevier © Thannickal, T. C. et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron 27, 469–474 (2000). Part b adapted with permission from John Wiley & Sons © John, J. et al. Ann. Neurol. 74, 786–793 (2013).
Hypocretin-1 levels in the CSF of patients with narcolepsy type 1 are typically low or undetectable.60–62 Although 10–30% of patients with narcolepsy without cataplexy also show undetectable levels of CSF hypocretin-1, most of them exhibit normal levels and thus have narcolepsy type 2.61,62 A study of the brains of two patients with narcolepsy type 2 showed partial loss of hypocretin neurons,63 suggesting that the extent of hypocretin loss determines the presence of cataplexy in narcolepsy.
Increased histamine neurons in narcolepsy type 1
In two dog strains with narcolepsy resulting from spontaneous loss-of-function mutations in the hypocretin 2R gene,64 histamine content in the cortex and thalamus is decreased.50 These dog strains were the first animal models of narcolepsy, and enabled investigation into the CNS mechanisms of cataplexy. In these dogs, cataplexy is linked to an abrupt cessation of action potentials in the locus coeruleus,66 increased firing of a cluster of medial medullary inhibitory neurons,67 and a maintained or increased firing of histamine cells.68 These findings suggest that activity of histamine neurons is tightly coupled to the waking state that, by definition, persists in cataplexy, whereas locus coeruleus activity is linked to the maintenance of waking muscle tone, which is lost in cataplexy.69
A robust increase (64–94%) in the number of histamine neurons in the TMN has been reported by two independent groups (Figure 3).5,6 However, we did not observe this increase of histamine neurons in a series of narcoleptic animal models we tested (hypocretin 2R mutant dogs, Hcrt knock-out mice, ataxin-3-hypocretin mice, and doxycycline-controlled diphtheria toxin A–hypocretin mice).5 Of these animal models, the postnatal hypocretin cell loss and adult-onset symptoms seen in the doxycycline-controlled diphtheria toxin A–hypocretin mouse resembles the clinical characteristics human narcolepsy most closely.70 Valko et al. reported only a small increase in the number of histamine cells in the Hcrt knockout mice, but not in the ataxin-3-hypocretin mouse.6 These data lead us to conclude that the large increase in histamine neurons in human patients with narcolepsy is not a compensation for the loss of hypocretin neurons. Rather, it might result from or mediate the immune-based loss of hypocretin neurons in these patients (Box 1). As described below, the number of histamine neurons has not been reported to increase in other human neurodegenerative diseases in which hypocretin reduction occurs. In contrast with humans, the hypocretin abnormalities in animal models of narcolepsy are caused by genetic alterations, rather than the presumed immune-mediated neuronal loss in human narcolepsy (Box 1).5
Box 1 |. Hypotheses on increase in histamine neurons in narcolepsy.
Adverse effect of narcolepsy medication
Virtually all patients with narcolepsy take medication to alleviate their symptoms. Pharmacotherapy has been proposed as the cause of the increased number of histamine neurons in human narcolepsy; consistent with this hypothesis, we have not detected an increased number of histamine neurons in any of the four (drug-free) animal models that we have examined.5 The effect of antidepressants and antipsychotics on CSF levels of histamine remains controversial. In one study, a significant reduction in CSF histamine was demonstrated in unmedicated, but not medicated patients with hypocretin deficiency.158 By contrast, another study found no association between treatment status and levels of CSF histamineor tele-methylhistamine,71 and HDC mRNA levels have been reported to be stable in patients with depression who were being treated with antidepressants or antipsychotics.159
However, we are not aware of any drug that has been shown to alter the number of histamine neurons. The consistency and very large magnitude (64–94%5,6) of the increase in the number of histamine neurons in individuals with narcolepsy, independent of the category of the drug taken, makes the drug mediation hypothesis implausible.
‘Neurotransmitter respecification’
We have hypothesized5 that increase in the number of histamine neurons could result from ‘neurotransmitter respecification’,160 in which neurons that do not normally have detectable levels of a particular protein or the enzyme required for its synthesis—in this case the HDC enzyme necessary for histamine synthesis—develop this capacity.
Neurogenesis
We have also suggested5 that postnatal neurogenesis161,162 of histamine neurons is a potential mechanism explaining our observations.
Autoimmune response
Autoimmune insult might trigger T cells, B cells, macrophages, microglia and mast cells to secrete factors that increase the number of histamine neurons (Figure 4).
Abbreviations: CSF, cerebrospinal fluid; HDC, histidine decarboxylase.
Despite the greatly increased number of histamine neurons in human narcolepsy, decreased65 or unchanged histamine levels71 are seen in the CSF of these patients. Normal CSF levels of histamine and t-MeHA were seen in a large population of patients with narcolepsy type 1, as well as in patients with other non-hypocretin-1-deficient central hypersomnias and in neurological controls, with no significant between-group differences and no associations with sleepiness or frequency of cataplexy.71 CSF histamine and t-MeHA are, therefore, not useful biomarkers for assessing the aetiology or severity of centrally mediated hypersomnia.71
Autoimmunity, histamine and hypocretin neuron loss
The overwhelming majority of patients with narcolepsy type 1 express the human leukocyte antigen (HLA) subtype DQB1*06:02, which is present in only 20–30% of the general population.31 Narcolepsy is also linked to T-cell receptor α polymorphisms,72 adding to the evidence for an autoimmune-mediated mechanism of hypocretin cell destruction. As in other presumed autoimmune disorders, the development of narcolepsy sometimes seems to be triggered by environmental factors, such as upper airway infection, H1N1 influenza or H1N1 vaccination with squalene-based adjuvant (Figure 4a);73–75 however, in most cases, such triggers cannot be identified. To date, there is no consistent evidence that specific autoantibodies cause hypocretin neuron degeneration.76,77
Figure 4 |.
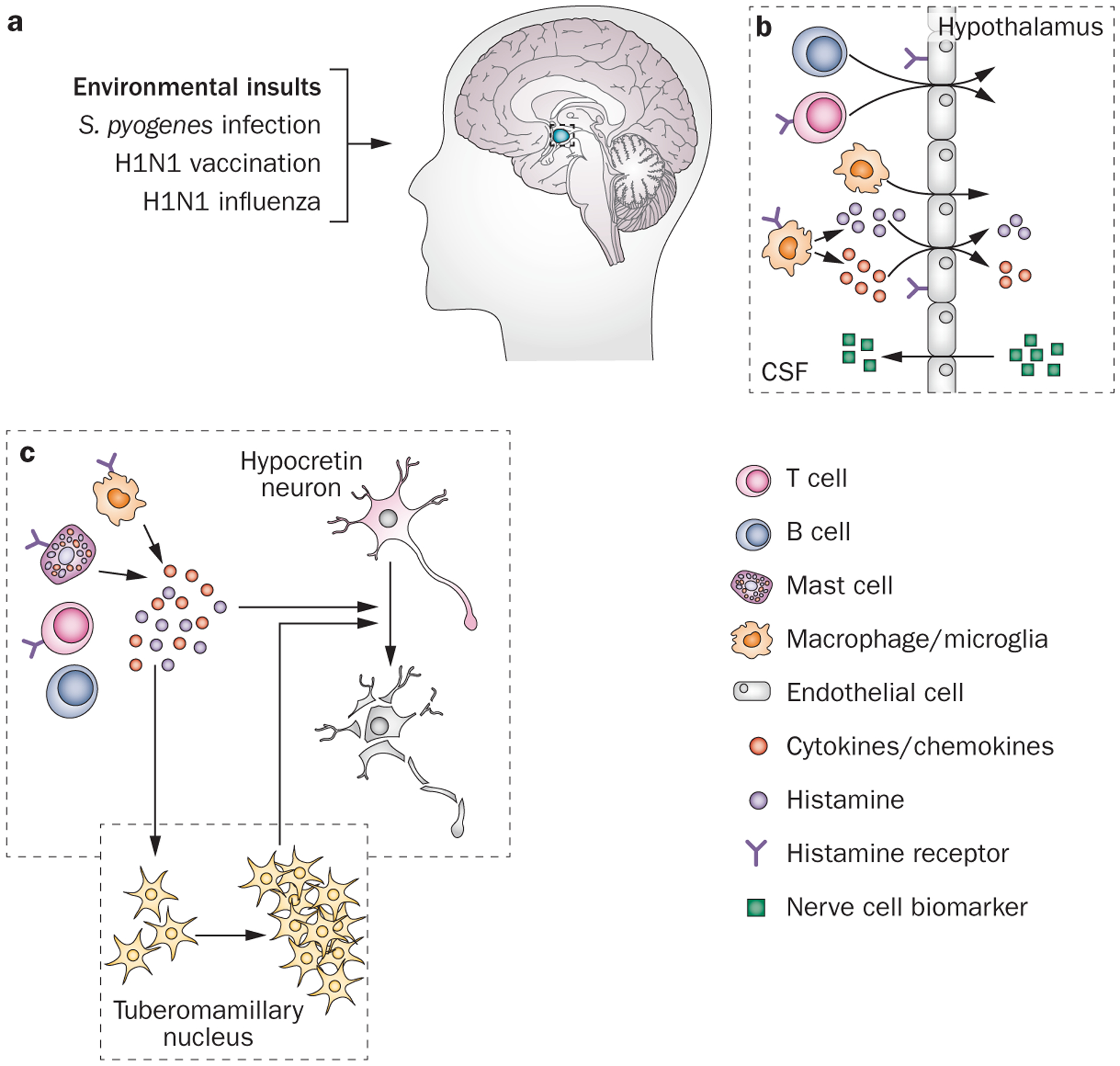
A schematic model of autoimmune-triggered histaminergic involvement in hypocretin neuron degeneration. a | The development of narcolepsy can sometimes be triggered by environmental factors, such as Streptococcus pyogenes infection, upper airway infection, H1N1 influenza or H1N1 vaccination with squalene-based adjuvant. Histaminergic signalling has a crucial role in several autoimmune diseases of the CNS. b | For example, binding of histamine to endothelial cell histamine receptors increases the permeability of the blood–brain barrier, (as indicated by increased levels of neuron-specific markers Aβ, total tau protein, phosphorylated tau, and neuro-specific enolase in the CSF), thereby facilitating T cell entry to the CNS.81–87 Moreover, histamine signalling enhances the activity of type 1 T helper cells through binding to histamine type 1 receptors located on these cells.88 c | T cells, B cells, macrophages, microglia and mast cells secrete histamine and other cytokines and chemokines,78,89,90,163 triggering a local inflammatory response that can damage the sensitive hypocretin neurons. Abbreviations: Aβ, amyloid-β; CSF, cerebrospinal fluid.
Histamine and histamine receptors have a crucial role in autoimmune diseases of the CNS.78–80 For example, binding of histamine to histamine receptors expressed in endothelial cells along the ventricles increases blood–brain barrier permeability, and thereby facilitates T cell entry to the CNS (Figure 4b).82–87 Histamine signalling also directly enhances the activity of type 1 T helper cells by binding to the H1R expressed on these cells.88 Histamine activation triggers T cells, B cells, macrophages, microglia and mast cells to secrete histamine and cytokines and chemokines, such as IFN-γ, tumour necrosis factor, quinolinic acid, glutamate and radical oxygen intermediates (Figure 4b),78,89,90 initiating a local inflammatory response (Figure 4c).
In multiple sclerosis, aberrant local inflammation results in myelin sheath destruction78—a similar process might underlie hypocretin neuron degeneration in narcolepsy. Hypocretin neurons are particularly sensitive to autoinflammatory insult, which could explain why the damage is specific to hypocretin neurons (Figure 4c). For example, histamine induction can cause microglia to release nitric oxide,91 which might mediate the selective degeneration via increased endoplasmic reticulum stress in hypocretin but not MCH neurons.92 Similarly, histamine activation causes microglia and macrophages to secrete quinolinic acid,93 which can lead to pathological activation of N-methyl-d-aspartate receptors and subsequent selective death of vulnerable hypocretin neurons, but not adjacent MCH neurons.94
Hypothetically, the above described processes might also affect TMN neurons: hitherto unknown factors (secreted by T cells, B cells, macrophages, microglia and mast cells) might increase the number of histamine neurons as a response to autoimmune insult.5,6 Elevated extracellular histamine might accelerate the degeneration of adjacent hypocretin neurons and inhibit histamine production and release in TMN neurons via the H3R autoreceptor (Figure 4).5,6
The role of increased histamine in narcolepsy pathogenesis might be similar to its role in PD, in which increased histamine levels in the substantia nigra contribute to an accelerated degeneration of dopaminergic neurons. Human postmortem studies have reported an increased density of histaminergic fibres and augmented local histamine levels in the substantia nigra of patients with PD,95–97 and in a classic rat model of PD (unilateral 6-hydroxydopamine lesions of the substantia nigra), an injection of HDC inhibitor α-fluoromethylhistidine at an early stage of degeneration greatly reduces the loss of dopaminergic neurons.98,99 Whether inhibition of neuronal histamine could prevent hypocretin neuron loss in human narcolepsy is currently unknown.
Alzheimer disease
Sleep impairment and neuropathology
The signs of AD include impairment of episodic memory, and deficits in language, semantic memory, executive functioning and visuospatial abilities.100 In addition, patients with AD have more-frequent and prolonged awakenings at night, a decreased amount of sleep, and REM sleep dysregulation.101,102 In fact, the sleep disruption in AD is often the main reason for their institutionalization.101,102 Progressive accumulation of extracellular amyloid-β (Aβ) plaques and intracellular neurofibrillary tangles of tau protein are the two main neuropathological markers for AD.103,104
Hypocretin system involvement
A correlation between CSF hypocretin-1 and total tau protein levels has been demonstrated in patients with AD.105 Hypocretin levels also seem to correlate with Aβ pathology in mice, and a hypocretin antagonist can reduce Aβ levels in the interstitial fluid.106 Another 2014 study confirmed the correlation between hypocretin-1 and Aβ42 levels in human patients with advanced AD.81 The correlation between hypocretin and Aβ pathology is seen neither in other dementias (frontotemporal lobar degeneration, dementia with Lewy bodies) nor in narcolepsy type 1.81
The level of Aβ in the brain interstitial fluid is high during periods of wakefulness, indicating that increased wakefulness might promote the development of AD by increasing the accumulation of extracellular Aβ.106 Because narcolepsy typically develops in the teens or twenties, one would expect it to protect from the subsequent development of AD; however, there is no evidence for a reduced prevalence of AD in patients with narcolepsy,107 and coexistence of narcolepsy and AD has been observed. Moreover, in a postmortem study, the number of hypocretin neurons and levels of CSF hypocretin were reduced in patients with advanced AD.108
Histamine system involvement
Neurofibrillary tangles have been reported to accumulate in the TMN in the early stage of AD.103 In the late stages of AD, the number of histamine neurons in the TMN is substantially decreased,109,110 though the total HDC mRNA expression level is not significantly reduced,110 which is in line with the finding that CSF t-MeHA is not significantly lowered in patients with AD.111 These findings imply that the remaining TMN neurons are activated to maintain histamine levels. A similar apparent compensation effect has been observed in the locus coeruleus of patients with AD, where remaining noradrenergic neurons are capable of maintaining normal noradrenaline levels.112,113 Both diminished and increased levels of histamine and t-MeHA levels have been reported in different brain regions associated with AD;95 studies with larger sample sizes are necessary to resolve the discrepancy regarding histamine alterations in AD.
Challenges in translational studies
Antagonists and inverse agonists of the H3R stimulate the release of various neurotransmitters, including acetylcholine, which might partly explain the beneficial effects of H3R antagonists on cognition seen in animal studies, including animal models of AD.114 However, H3R antagonists have failed to improve cognition in patients with mild to moderate AD in several controlled trials.115–117 Further trials using H3R antagonists in AD are required to understand the effect of increasing brain histamine on noncognitive symptoms, such as excessive sleepiness during daytime.
Parkinson disease
PD is the second-most prevalent age-related neurodegenerative disorder, affecting approximately 1% of the population above the age of 65 years.118 The main clinical features of PD are resting tremor, rigidity, slowness of voluntary movement and postural instability.118 Most of these motor symptoms are linked to the extensive loss (about 80%) of dopaminergic neurons in substantia nigra, which results in reduced dopamine levels in the striatum.118 Lewy neurites and Lewy bodies, the neuropathological hallmarks of PD, have been detected in various brain regions outside the substantia nigra—including the hypothalamus119—and could underlie the nonmotor symptoms in PD, such as autonomic dysfunction, and disorders of mood and sleep.120
Disturbed sleep patterns
About 15–50% of patients with PD show excessive daytime sleepiness with frequent napping and sleep attacks.121 This symptom frequently precedes the motor symptoms of PD.122 The combination of excessive daytime sleepiness, hypnagogic hallucinations, REM-sleep behaviour disorder and daytime sleep-onset REM periods are similar to symptoms seen in narcolepsy.123 Excessive daytime sleepiness in PD is caused by several factors, some being disease-related, whereas others are driven by medication, reduced activity in daily living, pain, altered BMI, depression, and night-time sleep problems, such as apnoea.
It is noteworthy that daytime sleepiness in PD has rarely been objectively assessed using the multiple sleep latency test, which is the gold-standard neurophysiological evaluation of sleepiness.124 In the largest study using this method, abnormally short sleep latencies were found in only 13% of 134 consecutive patients with PD, and none of the patients displayed two or more sleep-onset REM periods.125
Hypocretin system involvement
Hypocretin neurons are progressively lost over the course of PD; unlike in narcolepsy, MCH neurons are also lost in PD (Figure 5).126,127 The higher number of spared hypocretin neurons (40–50%) in PD compared with narcolepsy with cataplexy (10%) might explain the lack of cataplexy in PD.126,127 The levels of hypocretin-1 in the cortex and ventricular CSF of patients with PD are significantly reduced,127,128 but levels are mostly normal in CSF drawn from lumber puncture, suggesting regional differences in hypocretin release in PD; it is also possible that hypocretin is released in spinal regions.60,129,130
Figure 5 |.
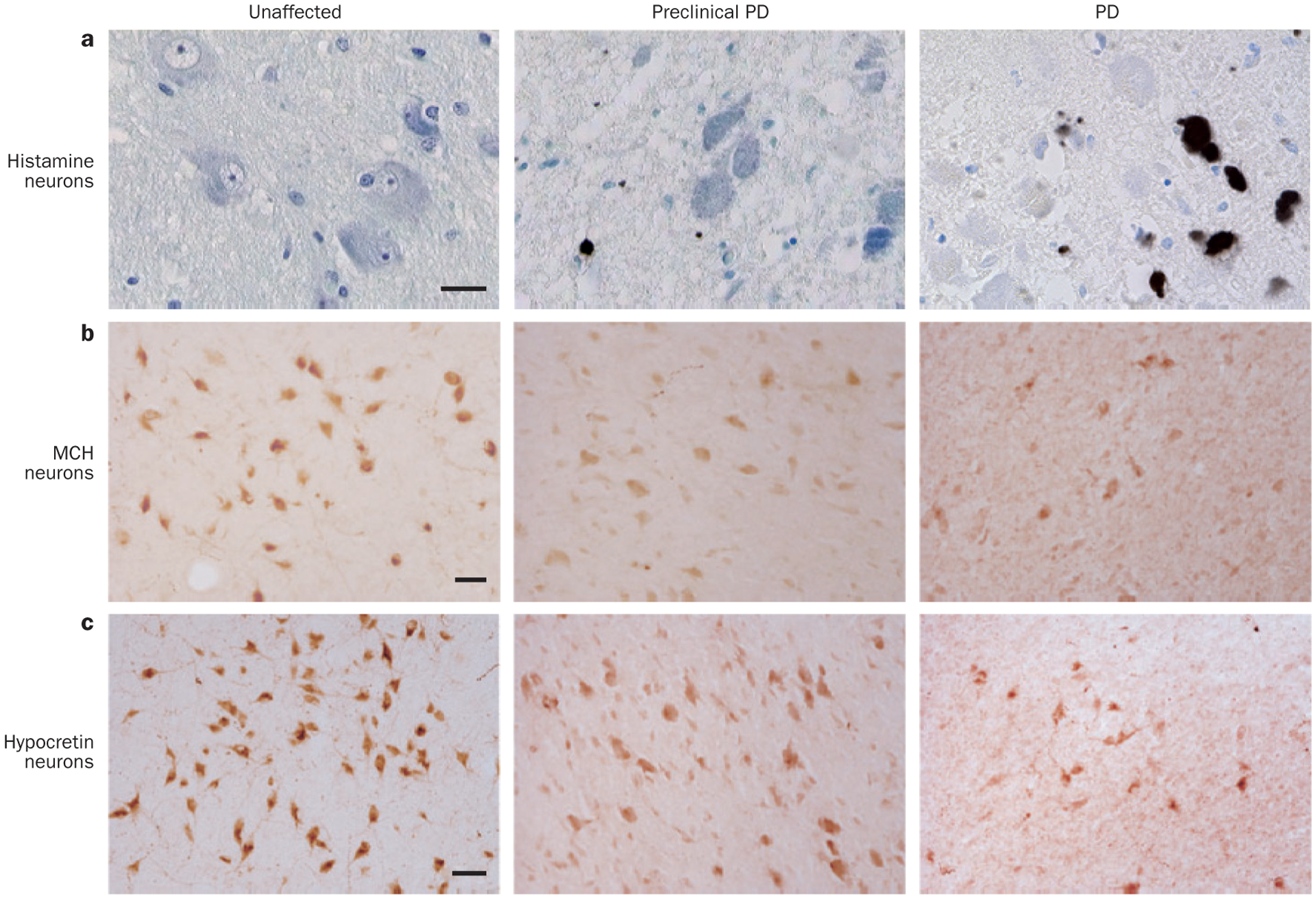
Specific degeneration of histamine, MCH and hypocretin neurons in PD. The course of PD can be divided into several stages,119 including unaffected, preclinical PD, and PD (from left to right). a | The number of histamine neurons in the TMN remains stable despite the accumulation of Lewy bodies and Lewy neurites. TMN neurons were stained for α-synuclein (brown) and counterstained by thionin (blue). Scale bar = 250 μm. The numbers of b | MCH neurons and c | hypocretin neurons decrease in line with increasing disease severity. MCH and hypocretin neurons were immunostained by their peptide-specific antibodies, followed by the diaminobenzidine colouring (reddish brown). Scale bars = 50 μm. Abbreviations: MCH, melanin-concentrating hormone; PD, Parkinson disease; TMN, tuberomamillary nucleus. Part a reprinted with permission from Elsevier Ltd © Shan, L. et al. Neuronal histamine production remains unaltered in Parkinson’s disease despite the accumulation of Lewy bodies and Lewy neurites in the tuberomamillary nucleus. Neurobiol. Aging 33, 1343–1344 (2012). Parts b and c adapted with permission from Oxford University Press © Thannickal, T. C. et al. Hypocretin (orexin) cell loss in Parkinson’s disease. Brain 130, 1586–1595 (2007).
Histamine as a potential modulator of PD pathology
In PD, Lewy bodies and Lewy neurites are found in the TMN (Figure 5),131 but the number of histamine neurons remains stable despite the reduced number of hypocretin neurons.126,127 This finding is consistent with stable HDC mRNA expression in TMN neurons,132 stable HDC enzymatic activity,133 and stable CSF levels of t-MeHA isomer134 in patients with PD.
Postmortem human and animal data collectively show that increased levels of histamine could accelerate degeneration of dopaminergic neurons in the substantia nigra of patients with PD. Inhibition of endogenous histamine production in a rat model of PD prevented the loss of dopamine neurons in the substantia nigra,95 a finding supporting our hypothesis that the increased number of histamine neurons, which we reported in patients with narcolepsy,5 could have caused the loss of hypocretin neurons in narcolepsy.
Trials assessing the benefits of H3R antagonists, such as pitolisant, in PD are currently ongoing, with the primary objective of alleviation of excessive daytime sleepiness.114 H3R antagonists promote histamine release in the brain and might be of interest in treating overt sleepiness in PD because histamine neurons are spared in this disorder. However, the large variability in the phenotype, severity and pathogenesis of daytime sleepiness in PD could prevent the global effectiveness of any psychostimulant in this condition. Moreover, the possibility of degeneration caused by an increased local release of histamine in the substantia nigra of patients with PD95 should remind us to administer these compounds with caution.
Huntington disease
HD is an autosomal dominant neurodegenerative disorder caused by a CAG repeat expansion in the gene encoding huntingtin.135 This CAG repeat in the N-terminal of the huntingtin gene (HTT) translates into an abnormally long polyglutamine tract. The disease occurs when the critical threshold of about 37 glutamine units is exceeded.136 Patients develop characteristic motor, cognitive and behavioural deficits over the course of the disease,135 with chorea as a core feature.135
Hypocretin system involvement
Among all the hypothalamic nuclei, the TMN and lateral hypothalamic area contain the highest frequency of the cytoplasmatic inclusions of mutant HTT.137 The number of hypocretin neurons is reduced by about 30% in patients with HD,137–139 and hypocretin neurons are also markedly reduced (by 70%) in an HD mouse model, R6/2,139 which expresses exon 1 of the human mutant HTT with 150 CAG repeats, and displays several clinical symptoms of HD.140
The loss of hypocretin neurons in HD is relatively specific owing to their relative sensitivity: the number and size of MCH neurons adjacent to the hypocretin neurons in patients with HD did not differ from that in matched controls,138 and several studies have shown that the level of hypocretin-1 peptide in the CSF of patients with HD is similar to that of healthy controls.138,141 This result indicates that the levels of hypocretin-1 peptide in the CSF does not closely track the 30% loss of hypocretin cells seen in these patients.
Histamine system involvement
A postmortem assessment of brains of patients with HD has demonstrated a substantial increase in HDC mRNA expression without a change in the number of histamine neurons;142 moreover, histamine N-methyltransferase mRNA was upregulated in the inferior frontal gyrus of patients with HD, indicating increased histamine degradation.142 Although this study did not evaluate protein and enzymatic activity of HDC, the reported augmentation of the histamine system in HD is consistent with a previous study that described increased levels of histamine metabolites in the CSF.134
An in vitro electrophysiological study showed that the response of histamine neurons to hypocretin is unchanged in HD model mice, suggesting that the histamine neurons remain functionally intact despite a markedly reduced input from hypocretin fibres;143 however, neither the tuberomammillary complex nor the neuronal histamine system has been directly studied in vivo.
Alterations in both hypothalamic hypocretin and histamine levels in HD might explain, at least partly, the disintegration of circadian rhythm and sleep disorders in patients with HD.144,145 However, despite the 30% loss of hypocretin neurons, these patients do not display REM sleep at sleep onset, cataplexy, hypnagogic hallucinations or sleep paralysis146—these symptoms probably occur only with more-extensive loss of hypocretin neurons, such as seen in narcolepsy type 1.
Tourette syndrome
TS is a childhood-onset neurological disorder that is characterized by motor and phonic tics. In a rare two-generation pedigree, an autosomal dominant mutation (Trp317X) of the HDC gene, which truncated full-length HDC protein from 662 amino acids to 316 amino acids, was reported to deplete the enzymatic activity of HDC.147 This mutation occurred in one of the HDC alleles and caused haploinsufficiency in histamine production.147 The association of TS with HDC was also supported by a larger sample of 520 European families.148
A 2014 study reported Hdc+/− knockout mice to have intermediate levels of brain histamine.149 These mice exhibited tic-like behaviours and might serve as an animal model for TS. As we previously discussed, homozygous Hdc knockout mice show reduced wakefulness at lights-off, and signs of somnolence.46 The sleep–wake pattern of the heterozygous Hdc knockout mice could mimic the sleep disorders described in TS.150 One patient affected with TS plus hypersomnia had a dramatic decrease in daytime sleepiness after being treated with pitolisant, whereas tic scores remained constant.151 A clinical investigation of H3R antagonists in the treatment of TS symptoms is, therefore, warranted.
Therapeutic prospects
H1R antagonists
H1R antagonists are well known to cross the blood–brain barrier and cause drowsiness.152 Several drugs with H1R antagonist activity—such as diphenhydramine, chlorpheniramine, doxylamine and brompheniramine—have been prescribed to treat allergies, cold symptoms, itching, nausea, but also insomnia, and are available over-the-counter.153 However, despite being widely used, very few data exist on the effectiveness of these drugs for the treatment of insomnia. Some antidepressants or antipsychotics reported to have beneficial effects on insomnia, such as doxepin, amitriptyline, olanzapine and risperidone, are also H1R blockers.153 However, most of the antidepressants and antipsychotics with H1R antagonist activity also substantially alter the sleep–wake cycle via mechanisms not related to histamine, with main actions on cholinergic, dopaminergic, serotonergic, and adrenergic receptors. These various actions could also explain the adverse events (constipation, nausea, urinary retention, fatigue and weight gain) that are commonly reported in association with these compounds.
Recently, a well designed placebo-controlled trial showed that a low dose (1–6 mg) of doxepin, a relatively selective H1R antagonist, alleviated the symptoms of primary insomnia.154 The improvement was observed mainly as prolonged sleep during the latter part of the night (that is, prevention of early morning awakening) rather than as decreased sleep-onset latency, as occurs with the use of most of the GABAergic sleep-promoting agents (such as the benzodiazepines).154 Neither sedation nor impairment was found upon awakening in patients taking doxepin compared with those taking placebo.154 Altogether, these results suggest a stronger efficacy of doxepin in the second part of the night, in accordance with the circadian histamine level being higher toward the end of the night.
H3R agonists and antagonists
Drugs that inhibit histamine release through H3R agonism could also promote sleep, as previous studies114,155 have shown that increasing brain histamine levels promotes wakefulness. Thus, H3R has been targeted for the treatment of daytime sleepiness in several neurological pathologies associated with hypersomnia.114
Pitolisant is one of the first H3R antagonists found to have a good preclinical and clinical benefit–risk ratio; it has been reported to enhance the activity of histamine, acetylcholine, dopamine and noradrenaline neurons.155 In Hcrt knockout mice (an animal model for narcolepsy) pitolisant significantly improved symptoms of narcolepsy, such as sleepiness, and decreased abnormally short REM sleep latency at sleep onset.155 Two small pilot trials indicated that pitolisant decreased excessive daytime sleepiness in adults and children with narcolepsy.155,156 In a double-blind randomized trial comparing pitolisant with placebo and modafinil, an approved pharmacological treatment for narcolepsy, pitolisant was found to ameliorate excessive daytime sleepiness in patients with narcolepsy at a level similar to that seen with modafinil (Figure 6), moreover, pitolisant use was linked to reduced number of cataplexy episodes.157 Pitolisant at doses up to 40 mg was well tolerated in patients with narcolepsy, and no withdrawal symptoms, dependence or abuse were detected, which is in accordance with in vitro results that demonstrated no change in dopamine release in the striatal complex.157
Figure 6 |.
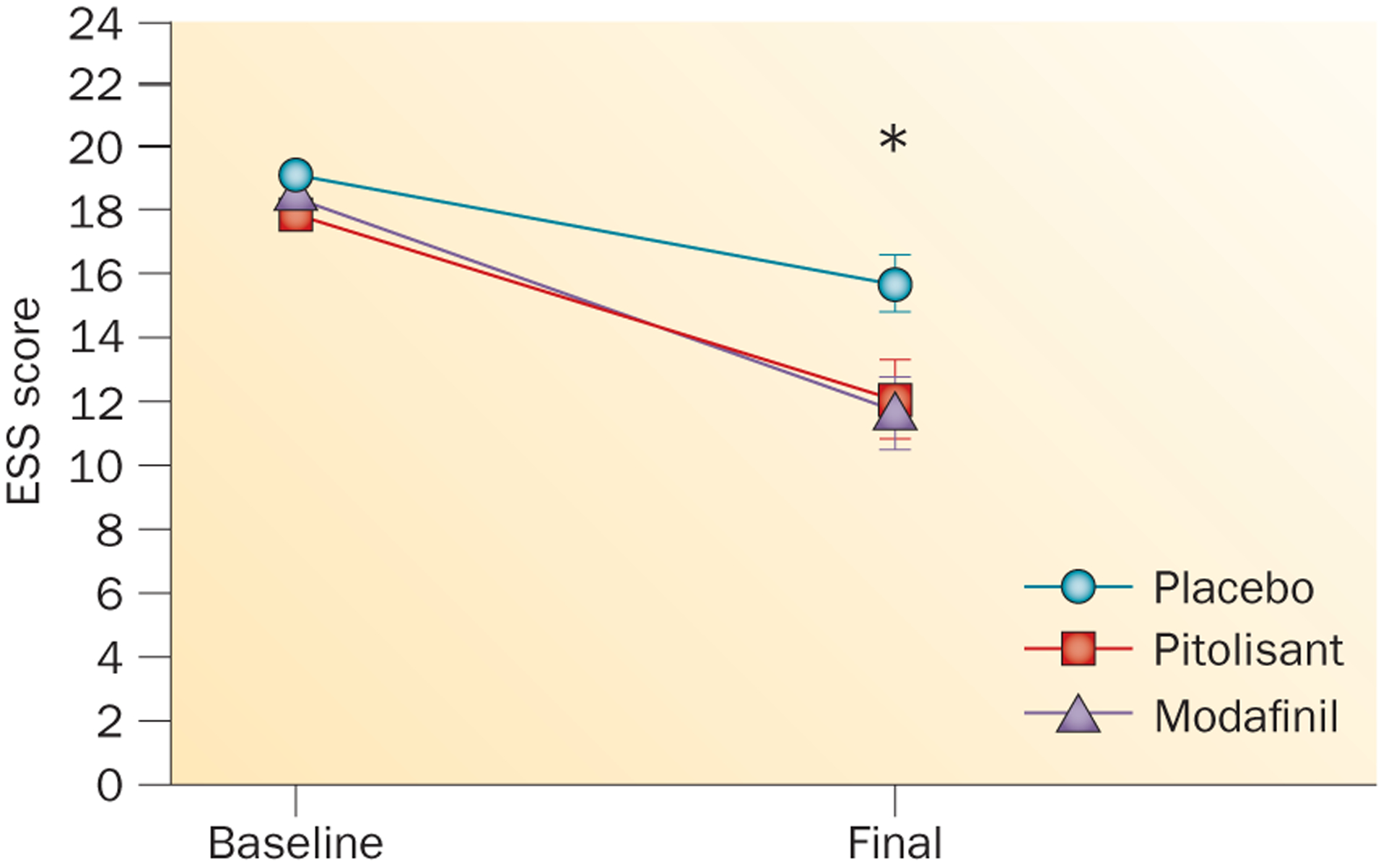
Effects of pitolisant, modafinil and placebo on patients with narcolepsy. Changes in Epworth sleepiness scale (ESS) score from the beginning of a double-blind, randomized phase III study (‘Baseline’) to the end of the 9-week treatment period (‘Final’).157 Data points indicate the mean ESS score; error bars indicate SEM. *A significant reduction in ESS was found for both modafinil and pitolisant versus placebo. No significant difference in ESS was found between pitolisant and modafinil.157
The recent findings of a robust increase in the number of histamine neurons5,6 and unchanged histamine levels71 in patients with narcolepsy highlights the need for further study into the exact pharmacological effects of pitolisant. Taken together, the results suggest pitolisant as a promising treatment for narcolepsy. Other pitolisant trials—using lower doses, monitored on a long-term open basis, and assessing the anticataplectic activity in drug-free patients with narcolepsy—are currently ongoing. According to the NIH Clinical Trials Database (ClinicalTrials.gov), other H3R antagonist are also being evaluated.
The studies discussed above will provide more insight into the role of central histamine levels in the regulation of daytime sleepiness associated with narcolepsy, with PD, AD and TS, and with residual excessive sleepiness associated with obstructive sleep apnoea syndrome despite continuous positive airway pressure therapy.
Conclusions
The neurodegenerative processes in AD, PD and HD can lead to a slow degeneration of hypocretin neurons over the course of the disease. By contrast, in narcolepsy type 1, loss of hypocretin neurons and peptide manifests close to the time of symptom onset.
Functionally, the substantial loss of hypocretin neurons in neurodegenerative diseases (40% in AD, 60% in PD, 30% in HD and 90% in narcolepsy) could contribute to the excessive daytime sleepiness and other sleep abnormalities seen in these disorders.
In addition, neuropathological findings by us5 and others6 demonstrate an increased number of histamine neurons in type 1 narcolepsy, in contrast with other neurological conditions involving loss of hypocretin neurons, namely AD, PD and HD. Recent results showed that increasing histamine release might alleviate narcolepsy, as indicated by positive results on a phase III clinical trial using an H3R inverse agonist.155,157 The increased number of histamine neurons in human narcolepsy deserves further investigation to determine the clinical effect of this increase, and to elucidate the possible link to hypocretin cell loss.
Key points.
Over-the-counter histamine H1 receptor antagonists (antihistamines) block the effects of histamine, have sedating properties and are commonly used to treat insomnia
A large increase in the number of histamine neurons is seen in type 1 narcolepsy (narcolepsy with cataplexy), but not in animal models of narcolepsy
Low levels of cerebrospinal fluid (CSF) hypocretin are characteristic of type 1 narcolepsy; changes in CSF histamine levels are small and variable in this disorder
In several neurological disorders including Parkinson and Alzheimer diseases, hypocretin-containing cells degenerate while the number of histamine cells and histamine levels are in the normal range
Histamine H3 receptor antagonists and inverse agonists promote histamine release and are a promising class of drugs for the promotion of wakefulness, as has been shown in patients with narcolepsy
Acknowledgements
We thank the National Institutes of Health (NS14610, MH064109, DA034748) and the Medical Research Service of the US Department of Veterans Affairs for support. L.S. is supported by a 2014 NARSAD Young Investigator Grant from the Brain & Behaviour Research Foundation. The authors want to thank Dr Ronald McGregor for drawing Figure 4.
Competing interests
Y.D. has received funds for speaking and board engagements from Bioprojet, Jazz and UCB Pharma. L.S. and J.M.S. declare no competing interests.
References
- 1.von Economo C Sleep as a problem of localization. J. Nerv. Ment. Dis 71, 249–259 (1930). [Google Scholar]
- 2.Peyron C et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat. Med 6, 991–997 (2000). [DOI] [PubMed] [Google Scholar]
- 3.Thannickal TC et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron 27, 469–474 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Academy of Sleep Medicine. International Classification of Sleep Disorders: Diagnostic and Coding Manual 3rd edn (AASM, 2014). [Google Scholar]
- 5.John J et al. Greatly increased numbers of histamine cells in human narcolepsy with cataplexy. Ann. Neurol 74, 786–793 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valko PO et al. Increase of histaminergic tuberomammillary neurons in narcolepsy. Ann. Neurol 74, 794–804 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Haas HL, Sergeeva OA & Selbach O Histamine in the nervous system. Physiol. Rev 88, 1183–1241 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Panula P & Nuutinen S The histaminergic network in the brain: basic organization and role in disease. Nat. Rev. Neurosci 14, 472–487 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Blouin AM et al. Human hypocretin and melanin-concentrating hormone levels are linked to emotion and social interaction. Nat. Commun 4, 1547 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiyashchenko LI et al. Release of hypocretin (orexin) during waking and sleep states. J. Neurosci 22, 5282–5286 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGregor R, Wu MF, Barber G, Ramanathan L & Siegel JM Highly specific role of hypocretin (orexin) neurons: differential activation as a function of diurnal phase, operant reinforcement versus operant avoidance and light level. J. Neurosci 31, 15455–15467 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mileykovskiy BY, Kiyashchenko LI & Siegel JM Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron 46, 787–798 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trottier S et al. Co-localization of histamine with GABA but not with galanin in the human tuberomamillary nucleus. Brain Res. 939, 52–64 (2002). [DOI] [PubMed] [Google Scholar]
- 14.Ericson H, Watanabe T & Kohler C Morphological analysis of the tuberomammillary nucleus in the rat brain: delineation of subgroups with antibody against l-histidine decarboxylase as a marker. J. Comp. Neurol 263, 1–24 (1987). [DOI] [PubMed] [Google Scholar]
- 15.Sherin JE, Elmquist JK, Torrealba F & Saper CB Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J. Neurosci 18, 4705–4721 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zecharia AY et al. GABAergic inhibition of histaminergic neurons regulates active waking but not the sleep-wake switch or propofol-induced loss of consciousness. J. Neurosci 32, 13062–13075 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.John J, Kodama T & Siegel JM Caffeine promotes glutamate and histamine release in the posterior hypothalamus. Am. J. Physiol. Regul. Integr. Comp. Physiol 307, R704–R710 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu X et al. Circadian factor BMAL1 in histaminergic neurons regulates sleep architecture. Curr. Biol 24, 2838–2844 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krolewski DM et al. Expression patterns of corticotropin-releasing factor, arginine vasopressin, histidine decarboxylase, melanin-concentrating hormone, and orexin genes in the human hypothalamus. J. Comp. Neurol 518, 4591–4611 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fronczek R, Lammers GJ, Balesar R, Unmehopa UA & Swaab DF The number of hypothalamic hypocretin (orexin) neurons is not affected in Prader–Willi syndrome. J. Clin. Endocrinol. Metab 90, 5466–5470 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Sakurai T et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92, 573–585 (1998). [DOI] [PubMed] [Google Scholar]
- 22.Chou TC et al. Orexin (hypocretin) neurons contain dynorphin. J. Neurosci 21, RC168 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crocker A et al. Concomitant loss of dynorphin, NARP, and orexin in narcolepsy. Neurology 65, 1184–1188 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torrealba F, Yanagisawa M & Saper CB Colocalization of orexin a and glutamate immunoreactivity in axon terminals in the tuberomammillary nucleus in rats. Neuroscience 119, 1033–1044 (2003). [DOI] [PubMed] [Google Scholar]
- 25.Reti IM, Reddy R, Worley PF & Baraban JM Selective expression of Narp, a secreted neuronal pentraxin, in orexin neurons. J. Neurochem 82, 1561–1565 (2002). [DOI] [PubMed] [Google Scholar]
- 26.Blouin AM et al. Narp immunostaining of human hypocretin (orexin) neurons: loss in narcolepsy. Neurology 65, 1189–1192 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Appelbaum L et al. Circadian and homeostatic regulation of structural synaptic plasticity in hypocretin neurons. Neuron 68, 87–98 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maret S et al. Homer1a is a core brain molecular correlate of sleep loss. Proc. Natl Acad. Sci. USA 104, 20090–20095 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muschamp JW et al. Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proc. Natl Acad. Sci. USA 111, E1648–E1655 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vourdas A et al. Narcolepsy and psychopathology: is there an association? Sleep Med. 3, 353–360 (2002). [DOI] [PubMed] [Google Scholar]
- 31.Mignot E, Hayduk R, Black J, Grumet FC & Guilleminault C HLA DQB1*0602 is associated with cataplexy in 509 narcoleptic patients. Sleep 20, 1012–1020 (1997). [PubMed] [Google Scholar]
- 32.Oh SW et al. A mesoscale connectome of the mouse brain. Nature 508, 207–214 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peyron C et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci 18, 9996–10015 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamanaka A et al. Orexins activate histaminergic neurons via the orexin 2 receptor. Biochem. Biophys. Res. Commun 290, 1237–1245 (2002). [DOI] [PubMed] [Google Scholar]
- 35.Huang ZL et al. Arousal effect of orexin A depends on activation of the histaminergic system. Proc. Natl Acad. Sci. USA 98, 9965–9970 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chemelli RM et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell 98, 437–451 (1999). [DOI] [PubMed] [Google Scholar]
- 37.Eriksson KS, Sergeeva O, Brown RE & Haas HL Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. J. Neurosci 21, 9273–9279 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schone C et al. Optogenetic probing of fast glutamatergic transmission from hypocretin/orexin to histamine neurons in situ. J. Neurosci 32, 12437–12443 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sundvik M et al. The histaminergic system regulates wakefulness and orexin/hypocretin neuron development via histamine receptor H1 in zebrafish. FASEB J. 25, 4338–4347 (2011). [DOI] [PubMed] [Google Scholar]
- 40.Panula P, Sundvik M & Karlstedt K Developmental roles of brain histamine. Trends Neurosci. 37, 159–168 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Sundvik M & Panula P Interactions of the orexin/hypocretin neurones and the histaminergic system. Acta Physiol. (Oxf.) 213, 321–333 (2015). [DOI] [PubMed] [Google Scholar]
- 42.Sakurai T et al. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron 46, 297–308 (2005). [DOI] [PubMed] [Google Scholar]
- 43.Yoshida K, McCormack S, Espana RA, Crocker A & Scammell TE Afferents to the orexin neurons of the rat brain. J. Comp. Neurol 494, 845–861 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steininger TL, Alam MN, Gong H, Szymusiak R & McGinty D Sleep–waking discharge of neurons in the posterior lateral hypothalamus of the albino rat. Brain Res. 840, 138–147 (1999). [DOI] [PubMed] [Google Scholar]
- 45.Lin JS Brain structures and mechanisms involved in the control of cortical activation and wakefulness, with emphasis on the posterior hypothalamus and histaminergic neurons. Sleep Med. Rev 4, 471–503 (2000). [DOI] [PubMed] [Google Scholar]
- 46.Parmentier R et al. Anatomical, physiological, and pharmacological characteristics of histidine decarboxylase knock-out mice: evidence for the role of brain histamine in behavioral and sleep-wake control. J. Neurosci 22, 7695–7711 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang ZL et al. Altered sleep-wake characteristics and lack of arousal response to H3 receptor antagonist in histamine H1 receptor knockout mice. Proc. Natl Acad. Sci. USA 103, 4687–4692 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishiguro T et al. Impaired ventilation and metabolism response to hypoxia in histamine H1 receptor-knockout mice. Respir. Physiol. Neurobiol 154, 331–341 (2006). [DOI] [PubMed] [Google Scholar]
- 49.Gondard E et al. Enhanced histaminergic neurotransmission and sleep-wake alterations, a study in histamine H3-receptor knock-out mice. Neuropsychopharmacology 38, 1015–1031 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nishino S et al. Decreased brain histamine content in hypocretin/orexin receptor-2 mutated narcoleptic dogs. Neurosci. Lett 313, 125–128 (2001). [DOI] [PubMed] [Google Scholar]
- 51.Yoshida Y et al. Fluctuation of extracellular hypocretin-1 (orexin A) levels in the rat in relation to the light-dark cycle and sleep–wake activities. Eur. J. Neurosci 14, 1075–1081 (2001). [DOI] [PubMed] [Google Scholar]
- 52.Anaclet C et al. Orexin/hypocretin and histamine: distinct roles in the control of wakefulness demonstrated using knock-out mouse models. J. Neurosci 29, 14423–14438 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shan L et al. Diurnal fluctuation in histidine decarboxylase expression, the rate limiting enzyme for histamine production, and its disorder in neurodegenerative diseases. Sleep 35, 713–715 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zeitzer JM et al. Time-course of cerebrospinal fluid histamine in the wake-consolidated squirrel monkey. J. Sleep Res 21, 189–194 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kiviranta T, Tuomisto L & Airaksinen EM Diurnal and age-related changes in cerebrospinal fluid tele-methylhistamine levels during infancy and childhood. Pharmacol. Biochem. Behav 49, 997–1000 (1994). [DOI] [PubMed] [Google Scholar]
- 56.Salomon RM et al. Diurnal variation of cerebrospinal fluid hypocretin-1 (Orexin-A) levels in control and depressed subjects. Biol. Psychiatry 54, 96–104 (2003). [DOI] [PubMed] [Google Scholar]
- 57.Lin L et al. Measurement of hypocretin/orexin content in the mouse brain using an enzyme immunoassay: the effect of circadian time, age and genetic background. Peptides 23, 2203–2211 (2002). [DOI] [PubMed] [Google Scholar]
- 58.Dauvilliers Y, Siegel JM, Lopez R, Torontali ZA & Peever JH Cataplexy—clinical aspects, pathophysiology and management strategy. Nat. Rev. Neurol 10, 386–395 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dauvilliers Y, Arnulf I & Mignot E Narcolepsy with cataplexy. Lancet 369, 499–511 (2007). [DOI] [PubMed] [Google Scholar]
- 60.Ripley B et al. CSF hypocretin/orexin levels in narcolepsy and other neurological conditions. Neurology 57, 2253–2258 (2001). [DOI] [PubMed] [Google Scholar]
- 61.Mignot E et al. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch. Neurol 59, 1553–1562 (2002). [DOI] [PubMed] [Google Scholar]
- 62.Nishino S, Ripley B, Overeem S, Lammers GJ & Mignot E Hypocretin (orexin) deficiency in human narcolepsy. Lancet 355, 39–40 (2000). [DOI] [PubMed] [Google Scholar]
- 63.Thannickal TC, Nienhuis R & Siegel JM Localized loss of hypocretin (orexin) cells in narcolepsy without cataplexy. Sleep 32, 993–998 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scammell TE, Willie JT, Guilleminault C, Siegel JM & International Working Group on Rodent Models of, N. A consensus definition of cataplexy in mouse models of narcolepsy. Sleep 32, 111–116 (2009). [PMC free article] [PubMed] [Google Scholar]
- 65.Nishino S et al. Decreased CSF histamine in narcolepsy with and without low CSF hypocretin-1 in comparison to healthy controls. Sleep 32, 175–180 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu MF et al. Locus coeruleus neurons: cessation of activity during cataplexy. Neuroscience 91, 1389–1399 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Siegel JM et al. Neuronal activity in narcolepsy: identification of cataplexy-related cells in the medial medulla. Science 252, 1315–1318 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.John J, Wu MF, Boehmer LN & Siegel JM Cataplexy-active neurons in the hypothalamus: implications for the role of histamine in sleep and waking behavior. Neuron 42, 619–634 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Siegel JM in Principles and Practice of Sleep Medicine 5th edn (eds Kryger MH et al.) 92–111(Elsevier Saunders, 2011). [Google Scholar]
- 70.Tabuchi S et al. Conditional ablation of orexin/ hypocretin neurons: a new mouse model for the study of narcolepsy and orexin system function. J. Neurosci 34, 6495–6509 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dauvilliers Y et al. Normal cerebrospinal fluid histamine and tele-methylhistamine levels in hypersomnia conditions. Sleep 35, 1359–1366 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fontana A et al. Narcolepsy: autoimmunity, effector T cell activation due to infection, or T cell independent, major histocompatibility complex class II induced neuronal loss? Brain 133, 1300–1311 (2010). [DOI] [PubMed] [Google Scholar]
- 73.Aran A et al. Elevated anti-streptococcal antibodies in patients with recent narcolepsy onset. Sleep 32, 979–983 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dauvilliers Y et al. Increased risk of narcolepsy in children and adults after pandemic H1N1 vaccination in France. Brain 136, 2486–2496 (2013). [DOI] [PubMed] [Google Scholar]
- 75.Partinen M et al. Narcolepsy as an autoimmune disease: the role of H1N1 infection and vaccination. Lancet Neurol. 13, 600–613 (2014). [DOI] [PubMed] [Google Scholar]
- 76.Bergman P et al. Narcolepsy patients have antibodies that stain distinct cell populations in rat brain and influence sleep patterns. Proc. Natl Acad. Sci. USA 111, E3735–E3744 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cvetkovic-Lopes V et al. Elevated Tribbles homolog 2-specific antibody levels in narcolepsy patients. J. Clin. Invest 120, 713–719 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pedotti R, De Voss JJ, Steinman L & Galli SJ Involvement of both ‘allergic’ and ‘autoimmune’ mechanisms in EAE, MS and other autoimmune diseases. Trends Immunol. 24, 479–484 (2003). [DOI] [PubMed] [Google Scholar]
- 79.Alonso A, Jick SS & Hernan MA Allergy, histamine 1 receptor blockers, and the risk of multiple sclerosis. Neurology 66, 572–575 (2006). [DOI] [PubMed] [Google Scholar]
- 80.Lock C et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat. Med 8, 500–508 (2002). [DOI] [PubMed] [Google Scholar]
- 81.Dauvilliers YA, Lehmann S, Jaussent I & Gabelle A Hypocretin and brain beta-amyloid peptide interactions in cognitive disorders and narcolepsy. Front. Aging Neurosci 6, 119 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Steinman L Optic neuritis, a new variant of experimental encephalomyelitis, a durable model for all seasons, now in its seventieth year. J. Exp. Med 197, 1065–1071 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Steinman L Multiple sclerosis: a two-stage disease. Nat. Immunol 2, 762–764 (2001). [DOI] [PubMed] [Google Scholar]
- 84.Jutel M, Watanabe T, Akdis M, Blaser K & Akdis CA Immune regulation by histamine. Curr. Opin. Immunol 14, 735–740 (2002). [DOI] [PubMed] [Google Scholar]
- 85.Teuscher C et al. Central histamine H3 receptor signaling negatively regulates susceptibility to autoimmune inflammatory disease of the CNS. Proc. Natl Acad. Sci. USA 104, 10146–10151 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Magistretti PJ Regulation of glycogenolysis by neurotransmitters in the central nervous system. Diabete. Metab 14, 237–246 (1988). [PubMed] [Google Scholar]
- 87.Heier MS, Skinningsrud A, Paus E & Gautvik KM Increased cerebrospinal fluid levels of nerve cell biomarkers in narcolepsy with cataplexy. Sleep Med. 15, 614–618 (2014). [DOI] [PubMed] [Google Scholar]
- 88.Jutel M et al. Histamine regulates T-cell and antibody responses by differential expression of H1 and H2 receptors. Nature 413, 420–425 (2001). [DOI] [PubMed] [Google Scholar]
- 89.Silver R, Silverman AJ, Vitković L & Lederhendler II Mast cells in the brain: evidence and functional significance. Trends Neurosci. 19, 25–31 (1996). [DOI] [PubMed] [Google Scholar]
- 90.Chikahisa S et al. Histamine from brain resident MAST cells promotes wakefulness and modulates behavioral states. PLoS ONE 8, e78434 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rocha SM, Pires J, Esteves M, Graca B & Bernardino L Histamine: a new immunomodulatory player in the neuron-glia crosstalk. Front. Cell. Neurosci 8, 120 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Obukuro K et al. Nitric oxide mediates selective degeneration of hypothalamic orexin neurons through dysfunction of protein disulfide isomerase. J. Neurosci 33, 12557–12568 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stone TW Kynurenines in the CNS: from endogenous obscurity to therapeutic importance. Prog. Neurobiol 64, 185–218 (2001). [DOI] [PubMed] [Google Scholar]
- 94.Katsuki H & Akaike A Excitotoxic degeneration of hypothalamic orexin neurons in slice culture. Neurobiol. Dis 15, 61–69 (2004). [DOI] [PubMed] [Google Scholar]
- 95.Shan L, Bao AM & Swaab DF The human histaminergic system in neuropsychiatric disorders. Trends Neurosci. 38, 167–177 (2015). [DOI] [PubMed] [Google Scholar]
- 96.Anichtchik OV, Rinne JO, Kalimo H & Panula P An altered histaminergic innervation of the substantia nigra in Parkinson’s disease. Exp. Neurol 163, 20–30 (2000). [DOI] [PubMed] [Google Scholar]
- 97.Rinne JO et al. Increased brain histamine levels in Parkinson’s disease but not in multiple system atrophy. J. Neurochem 81, 954–960 (2002). [DOI] [PubMed] [Google Scholar]
- 98.Liu CQ, Chen Z, Liu FX, Hu DN & Luo JH Involvement of brain endogenous histamine in the degeneration of dopaminergic neurons in 6-hydroxydopamine-lesioned rats. Neuropharmacology 53, 832–841 (2007). [DOI] [PubMed] [Google Scholar]
- 99.Liu CQ, Hu DN, Liu FX, Chen Z & Luo JH Apomorphine-induced turning behavior in 6-hydroxydopamine lesioned rats is increased by histidine and decreased by histidine decarboxylase, histamine H1 and H2 receptor antagonists, and an H3 receptor agonist. Pharmacol. Biochem. Behav 90, 325–330 (2008). [DOI] [PubMed] [Google Scholar]
- 100.Defina PA, Moser RS, Glenn M, Lichtenstein JD & Fellus J Alzheimer’s disease clinical and research update for health care practitioners. J. Aging Res 2013, 207178 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Van Someren EJ Circadian and sleep disturbances in the elderly. Exp. Gerontol 35, 1229–1237 (2000). [DOI] [PubMed] [Google Scholar]
- 102.Bianchetti A et al. Predictors of mortality and institutionalization in Alzheimer disease patients 1 year after discharge from an Alzheimer dementia unit. Dementia 6, 108–112 (1995). [DOI] [PubMed] [Google Scholar]
- 103.Braak H, Braak E & Bohl J Staging of Alzheimer-related cortical destruction. Eur. Neurol 33, 403–408 (1993). [DOI] [PubMed] [Google Scholar]
- 104.Shan L, Swaab DF & Bao AM Neuronal histaminergic system in aging and age-related neurodegenerative disorders. Exp. Gerontol 48, 603–607 (2013). [DOI] [PubMed] [Google Scholar]
- 105.Liguori C et al. Orexinergic system dysregulation, sleep impairment, and cognitive decline in Alzheimer disease. JAMA Neurol. 71, 1498–1505 (2014). [DOI] [PubMed] [Google Scholar]
- 106.Kang JE et al. Amyloid-β dynamics are regulated by orexin and the sleep-wake cycle. Science 326, 1005–1007 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Scammell TE, Matheson JK, Honda M, Thannickal TC & Siegel JM Coexistence of narcolepsy and Alzheimer’s disease. Neurobiol. Aging 33, 1318–1319 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fronczek R et al. Hypocretin (orexin) loss in Alzheimer’s disease. Neurobiol. Aging 33, 1642–1650 (2012). [DOI] [PubMed] [Google Scholar]
- 109.Airaksinen MS et al. Histamine neurons in human hypothalamus: anatomy in normal and Alzheimer diseased brains. Neuroscience 44, 465–481 (1991). [DOI] [PubMed] [Google Scholar]
- 110.Shan L, Bossers K, Unmehopa U, Bao AM & Swaab DF Alterations in the histaminergic system in Alzheimer’s disease: a postmortem study. Neurobiol. Aging 33, 2585–2598 (2012). [DOI] [PubMed] [Google Scholar]
- 111.Motawaj M, Peoc’h K, Callebert J & Arrang JM CSF levels of the histamine metabolite tele-methylhistamine are only slightly decreased in Alzheimer’s disease. J. Alzheimers Dis 22, 861–871 (2010). [DOI] [PubMed] [Google Scholar]
- 112.Hoogendijk WJ et al. Increased activity of surviving locus ceruleus neurons in Alzheimer’s disease. Ann. Neurol 45, 82–91 (1999). [DOI] [PubMed] [Google Scholar]
- 113.Szot P et al. Compensatory changes in the noradrenergic nervous system in the locus ceruleus and hippocampus of postmortem subjects with Alzheimer’s disease and dementia with Lewy bodies. J. Neurosci 26, 467–478 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Passani MB & Blandina P Histamine receptors in the CNS as targets for therapeutic intervention. Trends Pharmacol. Sci 32, 242–249 (2011). [DOI] [PubMed] [Google Scholar]
- 115.Egan M et al. Pilot randomized controlled study of a histamine receptor inverse agonist in the symptomatic treatment of AD. Curr. Alzheimer Res 9, 481–490 (2012). [DOI] [PubMed] [Google Scholar]
- 116.Haig GM et al. A randomized study of H3 antagonist ABT-288 in mild-to-moderate Alzheimer’s dementia. J. Alzheimers Dis 42, 959–971 (2014). [DOI] [PubMed] [Google Scholar]
- 117.Grove RA et al. A randomized, double-blind, placebo-controlled, 16-week study of the H3 receptor antagonist, GSK239512 as a monotherapy in subjects with mild-to-moderate Alzheimer’s disease. Curr. Alzheimer Res 11, 47–58 (2014). [DOI] [PubMed] [Google Scholar]
- 118.Sulzer D Multiple hit hypotheses for dopamine neuron loss in Parkinson’s disease. Trends Neurosci. 30, 244–250 (2007). [DOI] [PubMed] [Google Scholar]
- 119.Braak H et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 24, 197–211 (2003). [DOI] [PubMed] [Google Scholar]
- 120.De Cock VC, Vidailhet M & Arnulf I Sleep disturbances in patients with parkinsonism. Nat. Clin. Pract. Neurol 4, 254–266 (2008). [DOI] [PubMed] [Google Scholar]
- 121.Hobson DE et al. Excessive daytime sleepiness and sudden-onset sleep in Parkinson disease: a survey by the Canadian Movement Disorders Group. JAMA 287, 455–463 (2002). [DOI] [PubMed] [Google Scholar]
- 122.Abbott RD et al. Excessive daytime sleepiness and subsequent development of Parkinson disease. Neurology 65, 1442–1446 (2005). [DOI] [PubMed] [Google Scholar]
- 123.Arnulf I Excessive daytime sleepiness in parkinsonism. Sleep Med. Rev 9, 185–200 (2005). [DOI] [PubMed] [Google Scholar]
- 124.Arnulf I et al. Parkinson’s disease and sleepiness: an integral part of PD. Neurology 58, 1019–1024 (2002). [DOI] [PubMed] [Google Scholar]
- 125.Cochen De Cock V et al. Daytime sleepiness in Parkinson’s disease: a reappraisal. PLoS ONE 9, e107278 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Thannickal TC, Lai YY & Siegel JM Hypocretin (orexin) and melanin concentrating hormone loss and the symptoms of Parkinson’s disease. Brain 131, e87 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fronczek R et al. Hypocretin (orexin) loss in Parkinson’s disease. Brain 130, 1577–1585 (2007). [DOI] [PubMed] [Google Scholar]
- 128.Drouot X et al. Low levels of ventricular CSF orexin/hypocretin in advanced PD. Neurology 61, 540–543 (2003). [DOI] [PubMed] [Google Scholar]
- 129.Overeem S et al. Normal hypocretin-1 levels in Parkinson’s disease patients with excessive daytime sleepiness. Neurology 58, 498–499 (2002). [DOI] [PubMed] [Google Scholar]
- 130.Compta Y et al. Cerebrospinal hypocretin, daytime sleepiness and sleep architecture in Parkinson’s disease dementia. Brain 132, 3308–3317 (2009). [DOI] [PubMed] [Google Scholar]
- 131.Shan L et al. Neuronal histamine production remains unaltered in Parkinson’s disease despite the accumulation of Lewy bodies and Lewy neurites in the tuberomamillary nucleus. Neurobiol. Aging 33, 1343–1344 (2012). [DOI] [PubMed] [Google Scholar]
- 132.Nakamura S et al. Large neurons in the tuberomammillary nucleus in patients with Parkinson’s disease and multiple system atrophy. Neurology 46, 1693–1696 (1996). [DOI] [PubMed] [Google Scholar]
- 133.Garbarg M, Javoy-Agid F, Schwartz JC & Agid Y Brain histidine decarboxylase activity in Parkinson’s disease. Lancet 1, 74–75 (1983). [DOI] [PubMed] [Google Scholar]
- 134.Prell GD & Green JP Histamine metabolites and pros-methylimidazoleacetic acid in human cerebrospinal fluid. Agents Actions Suppl. 33, 343–363 (1991). [DOI] [PubMed] [Google Scholar]
- 135.Phillips W, Shannon KM & Barker RA The current clinical management of Huntington’s disease. Mov. Disord 23, 1491–1504 (2008). [DOI] [PubMed] [Google Scholar]
- 136.Kremer B et al. A worldwide study of the Huntington’s disease mutation. The sensitivity and specificity of measuring CAG repeats. N. Engl. J. Med 330, 1401–1406 (1994). [DOI] [PubMed] [Google Scholar]
- 137.Aziz A et al. Hypocretin and melanin-concentrating hormone in patients with Huntington disease. Brain Pathol. 18, 474–483 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Aziz NA, Swaab DF, Pijl H & Roos RA Hypothalamic dysfunction and neuroendocrine and metabolic alterations in Huntington’s disease: clinical consequences and therapeutic implications. Rev. Neurosci 18, 223–251 (2007). [DOI] [PubMed] [Google Scholar]
- 139.Petersen A et al. Orexin loss in Huntington’s disease. Hum. Mol. Genet 14, 39–47 (2005). [DOI] [PubMed] [Google Scholar]
- 140.Mangiarini L et al. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell 87, 493–506 (1996). [DOI] [PubMed] [Google Scholar]
- 141.Baumann CR, Hersberger M & Bassetti CL Hypocretin-1 (orexin A) levels are normal in Huntington’s disease. J. Neurol 253, 1232–1233 (2006). [DOI] [PubMed] [Google Scholar]
- 142.van Wamelen DJ et al. Functional increase of brain histaminergic signaling in Huntington’s disease. Brain Pathol. 21, 419–427 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Williams RH, Morton AJ & Burdakov D Paradoxical function of orexin/hypocretin circuits in a mouse model of Huntington’s disease. Neurobiol. Dis 42, 438–445 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Morton AJ et al. Disintegration of the sleep-wake cycle and circadian timing in Huntington’s disease. J. Neurosci 25, 157–163 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Morton AJ Circadian and sleep disorder in Huntington’s disease. Exp. Neurol 243, 34–44 (2013). [DOI] [PubMed] [Google Scholar]
- 146.Arnulf I et al. Rapid eye movement sleep disturbances in Huntington disease. Arch. Neurol 65, 482–488 (2008). [DOI] [PubMed] [Google Scholar]
- 147.Ercan-Sencicek AG et al. L-histidine decarboxylase and Tourette’s syndrome. N. Engl. J. Med 362, 1901–1908 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Karagiannidis I et al. Support of the histaminergic hypothesis in Tourette syndrome: association of the histamine decarboxylase gene in a large sample of families. J. Med. Genet 50, 760–764 (2013). [DOI] [PubMed] [Google Scholar]
- 149.Castellan Baldan L et al. Histidine decarboxylase deficiency causes tourette syndrome: parallel findings in humans and mice. Neuron 81, 77–90 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ghosh D et al. Sleep disorders in children with Tourette syndrome. Pediatr. Neurol 51, 31–35 (2014). [DOI] [PubMed] [Google Scholar]
- 151.Hartmann A, Worbe Y & Arnulf I Increasing histamine neurotransmission in Gilles de la Tourette syndrome. J. Neurol 259, 375–376 (2012). [DOI] [PubMed] [Google Scholar]
- 152.Lieberman P Histamine, antihistamines, and the central nervous system. Allergy Asthma Proc. 30, 482–486 (2009). [DOI] [PubMed] [Google Scholar]
- 153.Krystal AD, Richelson E & Roth T Review of the histamine system and the clinical effects of H1 antagonists: basis for a new model for understanding the effects of insomnia medications. Sleep Med. Rev 17, 263–272 (2013). [DOI] [PubMed] [Google Scholar]
- 154.Roth T et al. Efficacy and safety of doxepin 1 mg, 3 mg, and 6 mg in adults with primary insomnia. Sleep 30, 1555–1561 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lin JS et al. An inverse agonist of the histamine H(3) receptor improves wakefulness in narcolepsy: studies in orexin−/− mice and patients. Neurobiol. Dis 30, 74–83 (2008). [DOI] [PubMed] [Google Scholar]
- 156.Inocente C et al. Pitolisant, an inverse agonist of the histamine H3 receptor: an alternative stimulant for narcolepsy-cataplexy in teenagers with refractory sleepiness. Clin. Neuropharmacol 35, 55–60 (2012). [DOI] [PubMed] [Google Scholar]
- 157.Dauvilliers Y et al. Pitolisant versus placebo or modafinil in patients with narcolepsy: a double-blind, randomised trial. Lancet Neurol. 12, 1068–1075 (2013). [DOI] [PubMed] [Google Scholar]
- 158.Kanbayashi T et al. CSF histamine contents in narcolepsy, idiopathic hypersomnia and obstructive sleep apnea syndrome. Sleep 32, 181–187 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shan L Qi XR, Balesar R, Swaab DF & Bao AM Unaltered histaminergic system in depression: a postmortem study. J. Affect. Disord 146, 220–223 (2013). [DOI] [PubMed] [Google Scholar]
- 160.Spitzer NC Activity-dependent neurotransmitter respecification. Nat. Rev. Neurosci 13, 94–106 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lee DA & Blackshaw S Functional implications of hypothalamic neurogenesis in the adult mammalian brain. Int. J. Dev. Neurosci 30, 615–621 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Migaud M et al. Emerging new sites for adult neurogenesis in the mammalian brain: a comparative study between the hypothalamus and the classical neurogenic zones. Eur. J. Neurosci 32, 2042–2052 (2010). [DOI] [PubMed] [Google Scholar]
- 163.Lecendreux M et al. Impact of cytokine in type 1 narcolepsy: role of pandemic H1N1 vaccination? J. Autoimmun 60, 20–31 (2015). [DOI] [PubMed] [Google Scholar]


