Abstract
Early detection, characterization and monitoring of cancer are possible by using extracellular vesicles (EVs) isolated from non-invasively obtained liquid biopsy samples. They play a role in intercellular communication contributing to cell growth, differentiation and survival, thereby affecting the formation of tumor microenvironments and causing metastases. EVs were discovered more than seventy years ago. They have been tested recently as tools of drug delivery to treat cancer. Here we give a brief review on extracellular vesicles, exosomes, microvesicles and apoptotic bodies. Exosomes play an important role by carrying extracellular nucleic acids (DNA, RNA) in cell-to-cell communication causing tumor and metastasis development. We discuss the role of extracellular vesicles in the pathogenesis of cancer and their practical application in the early diagnosis, follow up, and next-generation treatment of cancer patients.
Keywords: liquid biopsy, exosomes, biomarkers, cell-free nucleic acids, cancer
1. Introduction
Extracellular vesicles (EVs) were discovered in 1946 as procoagulant platelet-derived particles in ultracentrifuged pellets from blood plasma [1]; however, the term “extracellular vesicle” was first used later in independent observations [2,3]. We now know that EVs are nano- to micron-sized vesicles covered by phospholipid bilayers. They are classified into three groups: apoptotic bodies (ABs), ectosomes/microparticles (MPs)/microvesicles (MVs), and exosomes (Exs) [4]. Their subtypes include large dense core vesicles, membrane blebs, oncosomes, outer membrane vesicles (OMVs), and prostasomes (exosome-like vesicles) [5,6,7]. According to current nomenclature, MVB-derived EVs are called exosomes, while plasma membrane-derived EVs are microvesicles [8,9]. The contents, membrane composition and size of EVs are highly heterogenous and depend on cellular location and environment. Extracellular vesicles are released by different tissues and cell types. They are found in body fluids, including amniotic fluid, ascites, bile, breast milk, nasal and bronchial lavage fluid, blood plasma, saliva, semen, synovial fluid, and urine, allowing extraction of EVs from various liquid biopsies [6,7]. EVs contain proteins, lipids, DNA, RNA, and microRNA serving as mediators of cell-to-cell communication [10,11,12]. Membranes protect their contents from nuclease and protease degradation and micro-environment changes (e.g., osmolarity and fluctuations in pH) [12]. Apoptotic bodies are released by blebbing of plasma membrane during apoptosis. The second main group of EVs includes vesicles of different size and components. The last main group, exosomes, belongs to the class of intraluminal vesicles (ILVs) contained in multi-vesicular bodies (MVBs) and are released to the extracellular environment by MVBs fusing with the cell membrane [7,13].
EVs are potent vehicles of intercellular communication providing protection and maintenance for cells and regulating cellular functions [6]. They are involved in cell-to-cell communication, immune response, angiogenesis, and signal transduction [14]. Many cancer cells release EVs, thereby affecting tumor microenvironments and suppressing or, surprisingly, stimulating immune responses, leading to a delicate balance of immune modulation [15]. Tumor-derived EVs were shown to avoid perforin-mediated elimination by CD8+ lymphocytes, a function in which their adenosine content seems to play a pivotal role [16].
Several techniques are available for the isolation of EVs, including density-gradient centrifugation (sucrose and iodixanol gradients), filtration, precipitation, size-exclusion chromatography, and ultracentrifugation; however, subgroups of EVs are not easy to distinguish due to an absence of specific markers. Identification of contents may help (e.g., by antibody-coupled bead flow cytometry analysis, electron microscopy supplemented with immunogold staining method and immunoblotting). Some tetraspanins (CD9, CD63 and CD81) were identified as specific markers for exosomes, but were later noted in the other groups [17,18]. Some other molecules were also determined as markers for EVs (14-3-3 proteins, MHC molecules, stress proteins (HSP), tumor susceptibility gene 101 (TSG101) and ESCRT-3 binding protein ALIX [19]), but CD63 and TSG101 were observed in all EV groups in a comprehensive cancer study showing different distributions based on their appearance and origin [20]. The solution leading to a reliable classification system may be to analyze the glycol-pattern of EVs (shown to be altered from that of the parent cell membrane [21]) as glycosylation of glycans is different between exosomes and apoptotic bodies [22].
In this review, we discuss the current understanding of exosomes’ role in cancer, and as markers of disease progression, making them valuable assets in tumor diagnosis and treatment.
2. Liquid Biopsy
Liquid biopsy has many advantages over conventional, invasive methods: it is less invasive, easily obtainable and repeatable. Liquid biopsies may serve as sources of many important biomarkers including cancer cells, extracellular vesicles (apoptotic bodies, microvesicles and exosomes), tumor-educated platelets, metabolites, proteins, and cell-free nucleic acids (cf-DNA, cf-RNA). Cf-DNAs are used in prenatal testing and characterization of the mutation profile of tumor cells. The number of publications on liquid biopsy-derived exosomes used for cancer detection and monitoring have skyrocketed recently, even with the problems of classification and standardizing extraction methods for different biofluids [23].
3. Extracellular Vesicles
3.1. Apoptotic Bodies
Apoptotic bodies (ABs) were discovered in 1972 by Kerr et al. [24]. They are formed from cells undergoing chromatin condensation, followed by membrane blebbing and fragmentation of cellular components during apoptosis. Finally, ABs are cleared when their translocated phosphatidyl serine membrane components bind to the Annexin V receptor of phagocytes [25], and the C3b complement or thrombospondin is recognized by and bound to phagocytes [26]. The apoptotic death of a cell breaks it up into a variable number of ABs, which are the largest vesicles among EVs (ranging from 1000 nm to 5000 nm) [27]. Apoptotic bodies contain large amounts of RNA both short and long [28], but other macromolecules (DNA, lipids, and proteins) were also observed in them [18,29]. Phosphatidylserine is a useful marker of ABs [30] (Table 1).
Table 1.
Characterization of extracellular vesicles from different aspects.
| Extracellular Vesicle Type | Exosome | Microvesicle | Apoptotic Body |
|---|---|---|---|
| Size | 40–100 nm | 50–1000 nm | 1000–5000 nm |
| Plasma/Serum Concentration | 5.3 particle/mL × 106 | 5–50 g/mL | Much lower compared to MVs and EXs |
| Origin | Inward budding of endosomal membranes forming MVBs and then released by exocytosis | Outward budding/blebbing of plasma membrane | Programmed cell death or apoptosis |
| Mode of extracellular release | Constitutive and regulated | Regulated | Regulated |
| Content | Proteins, lipids, DNA (gDNA, mtDNA, ncDNA), mRNA, miRNA, lncRNA, circRNA | Proteins, lipids, mRNA, miRNA, ncRNAs | Nuclear fractions, cell organelles, proteins, mRNA, ncRNA, DNA |
| Markers | ALIX, TSG101, tetraspanins (CD81, CD63, CD9, CD51), HSP70, flotillin, LAMP-1, MHC-I, -II | Phosphatidylserin, Integrins, selectins, CD40, flotillin-2, metalloproteinases, tissue and cell-specific factors | Annexin V, histones, phosphatidylserin |
| Function | Intracellular communication | Intracellular communication | Facilitation of phagocytosis |
| Morphology | Cup-shape | Cup-shape | Heterogeneous |
| Isolation methods | Ultracentrifugation, size exclusion chromatography, chemical precipitation, peptide affinity method | Centrifugation | No standard method (Centrifugation) |
| Detection | Flow cytometry with capture beads, electron microscopy, Western blot | Flow cytometry, electron microscopy, capture-based assays | Flow cytometry, electron microscopy |
| Reference | [12,55,56,57,68] | [12,55,57,69] | [12,57,70] |
3.2. Microvesicles
Microvesicles (MVs) are another class of EVs, first described in 1967 as “dust” from platelets [31]. Their importance became obvious in the past decades. Microvesicles range from 50–200 nm up to 1 µm in diameter [18,32,33] and contain a portion of the plasma membrane separated by blebbing outward and released into the extracellular space [12]. MVs are found in the blood [34] and in other body fluids, e.g., urine [35], cerebrospinal fluid [36], tears [37], saliva [38] and nasal secretions [39], ascites [40], and semen [41]. They are involved in intercellular communication, signaling pathways and promotion of cell invasion by cell-independent matrix proteolysis. Ectosomes, microparticles, oncosomes, shedding bodies, and shedding vesicles are all referred to as microvesicles [42,43].
Tumor-derived microvesicles (TMVs) and oncosomes originate from cancer cells [44] and an altered release of MVs is associated with cancer progression. Higher numbers of MVs indicate a more severe disease, and a high amount of proteolytic content in MVs correlates with the quick spreading of breast cancer and fibrosarcoma [45]. TMVs transfer bioactive molecules (nucleic acids, lipids, and proteins) to recipient cells causing disease, promoting cancer and providing diagnostic markers. In TMVs, the membrane-type 1 matrix metalloprotease (MT1-MMP) promotes cell invasion of the extracellular matrix (ECM) [46].
Some specific markers might be identified as MV markers, e.g., adenosine diphosphate ribosylation factor 6, CD40 ligand, various integrins and selectins [32,47,48,49]. ARF6 is involved in tumor formation, growth, and metastasis [47] (Table 1).
3.3. Exosomes
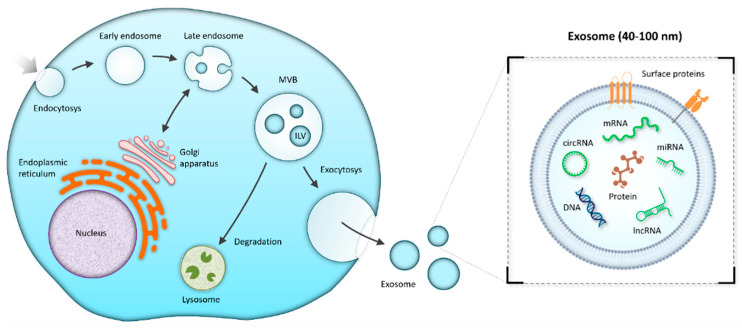
Exosomes were first reported as a “type of small vesicles” in Pan and Johnstone’s sheep reticulocyte-related experiment in 1983 [50], and the name “exosome” was first used (and visually described using transmission microscopy) by Johnstone et al. in 1989 [51]. Among the groups of EVs, exosomes are the smallest (40–100 nm) [52]. They may be verified based on their cup-shaped morphology by negative-staining transmission microscopy and the presence of markers (CD9, CD63, and CD81) [53,54,55]. Besides tetraspanins, other molecules are also found on the surface of exosomes, e.g., ALIX, TSG101, HSP70, flotillin, LAMP-1, MHC-I, -II [8,12,55,56,57]. Exosomes originate as intraluminal vesicles by inward budding of endosomal membranes forming multivesicular bodies (MVBs) from internal multivesicular compartments of the endocytic pathway. Following the secretory pathway, exosomes merge with the plasma membrane and are released into the extracellular milieu or fused with lysosomes for degradation [8,9] (Figure 1).
Figure 1.
Exosome biogenesis. The process of endocytosis results in the formation of an early endosome, followed by a maturation to a late endosome that may bidirectionally exchange vesicles with the Golgi apparatus and the endoplasmic reticulum. Invagination of late endosomal membranes forms the intraluminal vesicles (ILVs) contained in the multivesicular body (MVB). The MVB may fuse with the plasma membrane and release ILVs to the extracellular space as exosomes. On the other hand, the MVB may also be transported to a lysosome for subsequent degradation of its content.
Some studies in the 2000s proved that exosomes may deliver RNA such as mRNA and miRNA involved in cell-to-cell communication [11,54]. During their biogenesis, exosomes are packed with various cargo such as nucleic acids (e.g., DNA, mRNA, miRNA, lncRNA, circRNA) [11,58,59], proteins [60,61], lipids [62], metabolites [63,64] (Figure 1). When each component is released, they affect intercellular communication through direct cell-to-cell interaction contributing to tumorigenesis [11,58,59,60,61,62,63] and tumor-derived exosomes filled with RNAs and proteins may transfer oncogenic activity to recipient non-tumor cells [58,59,60,64] (Figure 1, Table 1).
Moreover, such tumor-derived exosomes may also serve as biomarkers of prognosis and response to therapy [65,66,67].
4. Exosomes in Tumors
Some studies described the connection between exosomes and the development and progression of different cancer types. Exosomes may serve as multicomponent biomarkers in tumor diagnostics [71,72].
4.1. Exosomes in Tumor Progression
Under physiological conditions, exosomes contribute to normal cellular function, while their altered function may cause cancer. Exosomes induce tumor development by changing the landscape of tumor microenvironments, and immune system activation by changing vascularity and cell polarity. They are also responsible for epithelial–mesenchymal transition (EMT) and an interconversion to mesenchymal–epithelial transition (MET) in several human malignancies [73]. Exosomes are involved in promoting tumorigenesis and metastasis and are associated with gaining chemoresistance, but exosomes derived from dendritic cells may be engineered to trigger antitumor immune responses (“dexosomes”) [74,75].
Exosomes have different ways to participate in tumor development. They may alter gene expression. Post-translational modifications such as ubiquitination frequently occur on EVs. Some studies showed that a dysregulation of ubiquitination and deubiquitination may lead to various diseases such as cancer, and they are part of the regulation of metabolic reprogramming in cancer cells [76]. Exosomes rich in Wnt5b have been associated with head and neck squamous cell carcinomas, invasive breast cancer, and lung and pancreatic cancer [77].
In addition to their effect on gene expression, they may be involved in posttranslational modifications as explained below. EVs from ovarian carcinoma were found to be enriched in mannose and sialic acid residues [22], i.e., glycosylation is modified.
Phosphorylation is another example: the role of Src-phosphorylation in the angiogenesis of myeloid leukemia is promoted by exosomes. This type of phosphorylation may be therapeutically targeted [78].
4.2. Exosomes in Cancer Immunology
Exosomes containing nucleic acids may control the innate and adaptive immune responses [74]. Secreted exosomes may stimulate the anticancer activity of effector CD4+ T cells [4] and promote their proliferation by indirectly activating naïve T cells and B cells by interacting with antigen-presenting cells [79]. Exosomes derived from various cells may release molecules which evoke immune responses in tumor formation. The role of Th17 cells is well-known in ovarian cancer: they may secrete pro- and anticancer factors, and promote angiogenesis [80,81]. Ye et al. (2014) reported that exosomes from nasopharyngeal carcinoma (NPC) cells hindered the proliferation of T cells and the differentiation of Th1 and Th17 cells by reducing the level of interleukin-2 (IL-2), interferon gamma (IFN-γ), and interleukin-17 (IL-17). However, the same exosomes activated Treg cells as a consequence of increasing the levels of interleukin-1b (IL-1b), interleukin-6 (IL-6), and interleukin-10 (IL-10) released from T cells [82]. The Treg/Th17 ratio was elevated in primary and metastatic tumors of patients with epithelial ovarian cancer compared to benign tumors and peritoneum. Inequality of the Treg/Th17 ratio is caused by an exosome-mediated transfer of miR-29a-3p and miR-21-5p from macrophages to T helper (CD4+) cells; the process is suppressed by signal transducer and activator of transcription 3 (STAT3) signaling [83]. Macrophage-derived exosomes of liver cancer cells induced the secretion of IL-6, MCP-1, IL-10, and TNF-α via STAT3 signaling [84]. Transfer of miRNA let-7d via exosomes—as analyzed in TME cells—reduced Th1 proliferation and secretion of IFN-γ [85].
4.3. Exosomes in Immunosuppression
Exosomes carry programmed cell death receptor ligand 1 (PD-L1), which may directly prevent the anticancer function of CD8+ T cells in vivo [65,86], so evasion of immune surveillance may be possible by exosomes. Some authors are optimistic about the future of anti-PD-L1 therapy of cancer [87,88]. PD-L1 on the surface of extracellular vesicles is associated with immunosuppression, disease progression of tumor patients and altered response to immunotherapy [65,86].
4.4. Exosomes in Angiogenesis and Lymphangiogenesis
Cellular microenvironments have a major impact on cancer progression. Exosomes released by cancer cells precondition tissue environments for local spreading and distant metastasis by delivering inflammatory and other factors [89,90,91]. Exosomes from breast cancer promote adhesion of cells to extracellular matrix proteins. Exosome-induced metastases were reported by several authors. Tumor-derived exosomes may affect factors related to EMT and escaping from immune surveillance (β-catenin, caveolin-1, transforming growth factor beta (TGFβ), etc.) [91].
The growth and spread of tumors are associated with the formation of new blood and lymph vessels. Cells located in a tumor and its microenvironment secrete angiogenic factors leading to tumor angiogenesis and growth factors contributing to lymphangiogenesis. Tumor cell-derived exosomes loaded with non-coding RNAs are involved in the same processes [92,93]. Several recent studies proved that cancer cell-derived exosomal miRNAs promote angiogenesis and/or lymphangiogenesis using different signaling pathways affecting endothelial cells [94,95,96,97] and non-endothelial cells [98]. Tumor-derived exosomes containing miR-21 or let-7a (under hypoxic stress) increase M2 polarization of macrophages which may stimulate tumor-associated angiogenesis and lymphangiogenesis [99,100]. Exosomal long non-coding RNAs are also reported to be involved in the process [101,102,103]. For example, lncRNA H19 and HOTAIR stimulate angiogenesis by synthesis and secretion of vascular endothelial growth factors [104,105] and other lncRNAs are involved by sponging microRNAs [106,107]. Exosomal circRNA-100338 may regulate angiogenesis to promote metastasis in hepatocellular carcinoma [108].
Other factors delivered by exosomes may also play key roles in promoting angiogenesis. Some studies have shown that exosomes from breast cancer transfer Annexin II (a tumorigenic factor) both in vivo and in vitro [109]. New vessel formation was detected predominantly in hypoxic regions of tumors with a low level of chemoresponsiveness [110].
4.5. Exosomes in the Therapy Phase
Radiotherapy promotes the secretion of exosomes, and their content plays a significant role in cancer survival. Irradiation may unexpectedly increase survival of cancer cells by triggering the release of exosomes carrying survivin (an apoptosis inhibitor) [110]. It may also affect the ratio of migratory factors (such as Insulin Like Growth Factor Binding Protein 2 (IGFBP2) and exosome connective tissue growth factor (CTGF) in exosomal cargo [111]. Various forms of stress (e.g., heat and oxidative stress) and some chemotherapeutic drugs (such as proteasome inhibitors like Bortezomib and alkylating agents like melphalan) may induce and then increase exosome release from cancer cells [112,113,114].
5. Exosomes in Cancer Detection
Exosomes contain nucleic acids, proteins, lipids, and carbohydrates. They are suitable for the detection of different types of cancer non-invasively from liquid biopsies.
5.1. Nucleic Acids
Nucleic acids found in exosomes show the mutations present in the cells from which they are derived. Types include genomic/nuclear DNA (gDNA, nDNA), mitochondrial DNA (mtDNA), messenger RNA (mRNA), small non-coding RNAs such as microRNA (miRNA), PIWI-interacting RNAs, YRNAs, and long non-coding RNAs (lncRNA) including circular RNAs (circRNA). Many of them are promising biomarker candidates in the diagnosis and prognosis of cancer and monitoring of patients in early- and late-stage disease, therapy selection and follow-up [115,116,117,118,119,120,121,122,123,124,125,126,127]. Other RNAs were also detected in exosomes, such as transfer RNAs (tRNAs) and viral RNAs [128].
5.1.1. DNA
Genomic/Nuclear DNA
In cancer patients, more gDNA content in exosomes is derived from cancer cells than from normal cells due to apoptosis or necrosis. Exosomes with nuclear content are secreted by tumor cells in high quantities, allowing for their application as cancer biomarkers. Recent studies revealed the connection between micronuclei and exosomes with nuclear content [129].
DNAs are shuffled into MVBs by tetraspanins and DNA-binding proteins interacting with CD63 [129]. Exosomes are released from MVBs containing molecules derived from them and then release cell-free DNA into plasma [130]. Circulating tumor DNA is partially derived from exosomes of tumor cells, which may serve as biomarkers in cancer diagnosis [131].
Circulating cell-free DNA encapsulated in exosomes in the plasma of gastrointestinal tumor patients may transform normal gastrointestinal cells into tumor cells, in a process known as genometastasis [132,133,134].
Mitochondrial DNA
Apart from genomic DNA, mtDNA is also present in exosomes and may show relevant copy number differences between cancer patients and healthy controls, as shown by our group in ovarian carcinoma [117]. Release of mtDNA in exosomes was also characteristic for cancer-associated fibroblasts in a breast cancer model [89].
5.1.2. RNA
MicroRNAs
The role of miRNA is highlighted as a marker in diagnosis and monitoring of the progression of many types of cancers [135]. It has been shown that miR-21 is overexpressed in exosomes obtained from patients with esophageal squamous cell carcinoma and glioblastoma [136]. Fabbri and colleagues found that exosomal miR-21 and miR-29a may reduce the overall survival of lung cancer [137]. Anfossi et al. measured the level of miR-21 and found that it is a valuable diagnostic biomarker for breast cancer [138].
Long Non-Coding RNAs
Exosome-derived lncRNAs are emerging as useful cancer biomarkers, and peripheral blood is not the only liquid biopsy from which they are obtained—e.g., urine exosomes carry markers for urothelial bladder cancer, while cervicovaginal lavage yields exosomes containing lncRNA relevant for the diagnosis of cervical cancer [139]. A combination of 2 exosomal mRNAs and an exosomal lncRNA—breast cancer anti-estrogen resistance 4 (BCAR4)—was reported as a robust diagnostic marker set for the screening of colorectal cancer [121]. Plasma lncRNA long intergenic non-protein-coding RNA 152 (LINC00152) is thought to be present exclusively in exosomes. In the diagnosis of gastric cancer, LINC00152 was reported to be more sensitive than established markers [122]. Exosomal Metastasis-associated lung adenocarcinoma transcript 1 (MALAT-1) is overexpressed in non-small cell lung cancer and is thought to increase proliferation and migration capabilities of tumor cells [140].
Circular RNAs
Interestingly, exosomal circRNAs are globally downregulated in most cancer types, but individual tumor biomarkers are usually still present [123]. Their tertiary structure makes them stable and suitable for clinical use. Exosomal circ-0051443 has been reported to be underexpressed in hepatocellular carcinoma [141], while hsa_circ_0065149, circ-KIAA, and hsa_circ_0000419 were downregulated in plasma samples of gastric cancer patients [142,143,144].
5.2. Proteins
5.2.1. Tetraspanins
Tetraspanins such as CD9, CD63, CD81 and CD151 are scaffolding membrane proteins highly enriched in exosomes [145,146]. Logozzi et al. (2009) demonstrated that plasma CD63+ exosomes are significantly increased in melanoma patients [147]. Yoshioka et al. found CD63 to be present in higher levels in exosomes produced by malignant cells, providing evidence that exosomal CD63 could be a protein marker for cancer [20]. CD81 plays a critical role in hepatitis C attachment and cell entry [148].
5.2.2. SNARE Proteins
The main function of SNARE proteins composed of multiple proteins is promoting the fusion of vesicle membranes and plasma membranes [149]. The vesicle associated membrane protein 7 (VAMP7), a member of the SNARE family, is an important component of exosomes involved in their secretion to the extracellular environment [150]. The abnormal lncRNA LINC00511 induces formation of invadopodia by regulating the colocalization of VAMP7 and synaptosome associated protein 23 (SNAP23) and is thus involved in tumor progression as shown in hepatocellular carcinoma (HCC) cells [151].
5.2.3. Rab Proteins
There are more than 60 different Rab proteins in humans. Rab proteins are small GTPase proteins regulating membrane trafficking, intracellular transport, lipid remodeling, fusion, and exosome release [152,153]. RAB11 was the first protein from the RAB family that was shown to be involved in the secretion of exosomes containing TFR and HSC70 from myelogenous leukemia cell lines [154]. Depending on the cell type, Rab5, Rab7, Rab11, Rab27, and Rab35 are involved in vesicle secretion and thus cancer progression. It was observed that ovarian cancer cells significantly increased their exosome release in hypoxia by upregulating Rab27a and downregulating Rab7, LAMP1/2, and NEU-1 [155].
5.2.4. Annexins
Annexins are a group of calcium- and phospholipid-binding proteins highly expressed in exosomes in cancer. Maji et al. demonstrated their participation in breast cancer pathogenesis [109].
5.2.5. Flotillins
Flotillins are membrane-associated proteins involved in scaffolding, signaling, and endocytosis. They are enriched in exosomes and may be used as exosomal biomarkers. Phuyal et al. showed that they affect the composition of exosomes [156].
5.2.6. Proteins Involved in ESCRT Complex
Programmed cell death 6-interacting protein (PDCD6IP) or ALIX is a cytoplasmic protein involved in apoptosis as a binding protein in endosomal sorting complex required for transport (ESCRT) complexes [157]. Specific components are sorted into ILVs by ESCRT complexes (ESCRT-0, ESCRT-I, ESCRT-II, and ESCRT-III, which contain specific proteins, e.g., VPS4, VTA1, and ALIX) involved in the biogenesis of exosomes [158,159]. The hepatocyte growth factor-regulated tyrosine kinase substrate (HRS), which is part of ESCRT-0, also has a significant role in the exosome biogenesis [159]. HRS recognizes ubiquitinated proteins and interacts with STAM (a part of ESCRT-0) [160]. ALIX (ESCRT-III-related protein) may cooperate with syndecan-syntenin and is also involved in exosome formation [161].
5.2.7. Heat Shock Proteins
Exosomes also contain heat shock proteins (HSPs) which are produced under stressful conditions. HSPs were found to play a role in antigen presentation by loading peptides onto the major histocompatibility complex (MHC). Thus, they have the capacity to stimulate antitumor immune responses. Lv et al. found that exosomes from resistant human hepatocellular carcinoma cells may improve tumor immunogenicity by the induction of HSP-specific natural killer (NK) cell responses [162].
5.3. Lipids
The lipid composition of exosomes varies and is affected by the cell of origin. Skotland et al. used exosomal lipids as biomarkers and reported that engineering lipid composition of exosomes may yield useful drug delivery vehicles [163].
5.4. Glycans
During glycosylation, glycans (a subgroup of saccharides) may be attached to lipids, proteins, and other glycans [164]. They are essential in exosome function [165]. Conserved and unique glycan contents were found in exosomes corresponding to parent cell membranes (e.g., specific sialic acid-containing glycoproteins in exosomes derived from SKOV3 cells) [166,167]. Bisecting GlcNAc-containing N-glycans were found in exosomes derived from SKOV3 and OVM ovarian carcinoma cell lines [167]. These molecules have been associated with a lower occurrence of metastasis in multiple cancer types [168], suggesting that exosomes containing glycans may be used in cancer vaccine development. The glycan properties of cancer cell-derived exosomes make them promising early diagnosis markers such as lectin-conjugated nanoparticles in pancreatic cancer [169].
Nucleic acid, protein, lipid, and carbohydrate contents of exosomes are summarized in Table 2.
Table 2.
A summary of exosome contents.
| Type | Function | Application | Reference | ||
|---|---|---|---|---|---|
| Nucleic acids | DNA | gDNA/nDNA | unknown | prenatal diagnosis, biomarker | [115,116] |
| mtDNA | unknown | biomarker | [117] | ||
| RNA | mRNA | codes for proteins | data | [118] | |
| miRNA | gene regulation | diagnosis | [119,120] | ||
| lncRNA | regulation of gene transcription, epigenetic modification | diagnostic biomarker | [121,122] | ||
| circRNA | gene regulation, cell proliferation, epithelial-mesenchymal transition, metastasis, invasion, chemoresistance | diagnostic biomarker | [123] | ||
| Proteins | Tetraspanins | CD9, CD63, CD81, CD51 | adhesion, proliferation, migration, binding, entrance, motility | biomarker | [145,146] |
| Rab proteins | Rab5, Rab7, Rab11, Rab27 and Rab35 | vesicle secretion | cancer prognosis | [155] | |
| SNARE proteins | e.g., VAMP7 | secretion of exosomes, involved in tumor progression |
monitoring the tumor progression | [150] | |
| Annexins | cell life cycle, exocytosis, apoptosis | cancer pathogenesis | [109] | ||
| Flotillins | scaffolding, signaling, endocytosis | biomarker | [156] | ||
| Heat shock proteins | antigen presentation | improving tumor immunogenicity | [162] | ||
| Lipids | formation of exosomes and releasing of exosomes to the extracellular environment | biomarker | [163] | ||
| Glycans | decrease in metastasis | possible use in cancer vaccine development | [168] |
6. Exosomes as Next-Generation Treatment Options
Recently, exosome research has been focused on developing designed drug or nucleic acid delivery to cancerous cells or tissues to improve the effectiveness of cancer therapy [170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192] (Table 3).
Table 3.
Application of exosomes in cancer therapy.
| Cancer | Application | Reference |
|---|---|---|
| Breast | Effective doxorubicin therapy using targeted iRGD-exosome delivery of doxorubicin | [170] |
| Breast | Exosomes loaded with miR-379 from engineered mesenchymal stem cells may reduce tumor activity | [171] |
| Bladder | Delivery of polo-like kinase-1 (PLK-1) siRNA containing exosomes to cancer cells decreases the PLK-1 mRNA | [172,173] |
| Glioma | Anti-survivin immunotherapy leads to decreased release of CD9+/GFAP+/SVN+ and CD9+/SVN+ exosomes which may be associated with longer progression-free survival | [174] |
| Glioma | miRNA-146b (anti-glioma miRNA) containing exosomes derived from marrow stromal cells may suppress glioma growth in vitro | [175] |
| Glioblastoma | Natural-killer-derived exosomes may stimulate T cell proliferation and promote the maturation of DCs | [176] |
| Hepatocellular carcinoma | Exosomes enriched with miR-335-5p may decrease cancer growth and invasion | [177] |
| Hepatocellular carcinoma | Dendritic cell-derived exosomes (DEXs) promote natural killer cell and T cell activation and proliferation | [178,179] |
| Leukemia | Tumor-derived exosomes (TEXs) carry tumor-associated antigens that trigger tumor antigen-specific immune response | [180] |
| Lymphoma | TNF-alpha-related-apoptosis-inducing-ligand (TRAIL)—armed exosomes may promote apoptosis in cancer cells | [181] |
| Murine Lewis lung carcinoma | Paclitaxel (PTX) loaded exosomes (exoPTX) increased cytotoxicity in cancer cells (drug resistant MDCKMDR1 (Pgp+) cells) | [182] |
| Murine melanoma | Macrophage-derived exosome-encapsulated Trp2 vaccine may induce a stronger antigen-specific cytotoxic T cell response via Th1 response | [183] |
| Nasopharyngeal carcinoma | TEXs loaded with galectin-9 suppress T-cell proliferation, and increase apoptosis in mature Th1 lymphocytes | [184,185] |
| Osteosarcoma | Exosomes filled with miR-101 may suppress lung metastasis in osteosarcoma | [186] |
| Ovarian cancer | Tumor-derived exosomes expressing Fas ligand and TRAIL induce apoptosis of the precursors of DCs and PBMCs | [187] |
| Pancreatic ductal adenocarcinoma | Exosomes transfected with miR-145-5p may suppress pancreatic ductal adenocarcinoma cell proliferation and invasion through TGF-β/Smad3 pathways | [188] |
| Prostate | Tumor exosomes expressing Fas ligand induce apoptosis of CD8 (+) T cells | [189] |
| Prostate | Delivery of paclitaxel from cancer cell-derived exosome increases drug cytotoxicity | [190] |
| Prostate | Presence of ASC-derived exosomal miR-145 initiates apoptosis in prostate cancer | [191] |
| Prostate | Knockdown of ACTN4 gene decreases the invasion and proliferation of prostate cancer | [192] |
Useful properties of exosomes (small size, compatibility with biological processes, long circulatory halflife, compliance to adaptation and modification, enhanced permeability and retention (EPR) effect, prolonged circulation, tumor-targeting capacity) make them promising therapeutic shuttle vesicles [193,194,195,196,197] with low toxicity following administration [198]. They may be used to transport anticancer drugs, biomolecules, nucleic acids, soluble proteins, antibodies, and nanoparticles [199,200,201,202,203,204,205]. Exosome-based immunotherapeutics are under development and testing in animal models and clinical trials. Exosome-based clinical trials involved in cancer immunotherapy are available in the database of privately and publicly funded clinical studies (https://www.clinicaltrials.gov/, accessed on 4 December 2021).
Recent reviews highlighted the possible therapeutic application of exosomes for personalized medicine. Engineered extracellular vesicles loaded with various molecules may find application in EV-based personalized medicine as a new option for tailoring clinical treatment [206,207,208,209].
7. Conclusions
In this review, we summarized the role of extracellular vesicles in cancer progression focusing on exosomes carrying extracellular nucleic acids (DNA, RNA) from cell to cell, causing tumor and metastasis development. We outlined the roles of liquid-biopsy-derived exosomes in tumor pathology and therapy against cancer. They serve as excellent sources of various markers for early non-invasive detection, classification of cancer and follow up. They are suitable for the targeted delivery of drugs to tumor cells, making them promising next-generation treatment vehicles.
Author Contributions
Conceptualization, B.S., and B.N.; writing—original draft preparation, B.S., B.N.; writing—review and editing, G.B., N.N., M.S., I.B., O.P., T.S.; supervision, B.S. and B.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Operational Programme Integrated Infrastructure for the project ITMS2014+: 313011V446 co-financed by the European Regional Development Fund.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chargaff E., West R. The biological significance of the thromboplastic protein of blood. J. Biol. Chem. 1946;166:189–197. doi: 10.1016/S0021-9258(17)34997-9. [DOI] [PubMed] [Google Scholar]
- 2.Ali S.Y., Sajdera S.W., Anderson H.C. Isolation and Characterization of Calcifying Matrix Vesicles from Epiphyseal Cartilage. Proc. Natl. Acad. Sci. USA. 1970;67:1513–1520. doi: 10.1073/pnas.67.3.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonucci E. Fine structure and histochemistry of “calcifying globules” in epiphyseal cartilage. Z. Zellforsch. Mikrosk. Anat. 1970;103:192–217. doi: 10.1007/BF00337312. [DOI] [PubMed] [Google Scholar]
- 4.Raposo G., Nijman H.W., Stoorvogel W., Liejendekker R., Harding C.V., Melief C.J., Geuze H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stegmayr B., Ronquist G. Promotive effect on human sperm progressive motility by prostasomes. Urol. Res. 1982;10:253–257. doi: 10.1007/BF00255932. [DOI] [PubMed] [Google Scholar]
- 6.Yáñez-Mó M., Siljander P.R.-M., Andreu Z., Zavec A.B., Borràs F.E., Buzas E.I., Buzas K., Casal E., Cappello F., Carvalho J., et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Théry C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., Antoniou A., Arab T., Archer F., Atkin-Smith G.K., et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathieu M., Névo N., Jouve M., Valenzuela J.I., Maurin M., Verweij F.J., Palmulli R., Lankar D., Dingli F., Loew D., et al. Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9. Nat. Commun. 2021;12:4389. doi: 10.1038/s41467-021-24384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Witwer K.W., Théry C. Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. J. Extracell. Vesicles. 2019;8:1648167. doi: 10.1080/20013078.2019.1648167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ratajczak J., Miękus K., Kucia M., Zhang J., Reca R., Dvorak P., Ratajczak M.Z. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: Evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20:847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 11.Valadi H., Ekstrom K., Bossios A., Sjöstrand M., Lee J.J., Lötvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 12.Kim K.M., Abdelmohsen K., Mustapic M., Kapogiannis D., Gorospe M. RNA in extracellular vesicles. Wiley Interdiscip. Rev. RNA. 2017;8:e1413. doi: 10.1002/wrna.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colombo M., Raposo G., Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 14.Gézsi A., Kovács Á., Visnovitz T., Buzás E.I. Systems biology approaches to investigating the roles of extracellular vesicles in human diseases. Exp. Mol. Med. 2019;51:1–11. doi: 10.1038/s12276-019-0226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scholl J.N., Dias C.K., Muller L., Battastini A.M.O., Figueiró F. Extracellular vesicles in cancer progression: Are they part of the problem or part of the solution? Nanomedicine. 2020;15:2625–2641. doi: 10.2217/nnm-2020-0256. [DOI] [PubMed] [Google Scholar]
- 16.Tadokoro H., Hirayama A., Kudo R., Hasebe M., Yoshioka Y., Matsuzaki J., Yamamoto Y., Sugimoto M., Soga T., Ochiya T. Adenosine leakage from perforin-burst extracellular vesicles inhibits perforin secretion by cytotoxic T-lymphocytes. PLoS ONE. 2020;15:e0231430. doi: 10.1371/journal.pone.0231430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tauro B.J., Greening D., Mathias R., Mathivanan S., Ji H., Simpson R.J. Two Distinct Populations of Exosomes Are Released from LIM1863 Colon Carcinoma Cell-derived Organoids. Mol. Cell. Proteom. 2013;12:587–598. doi: 10.1074/mcp.M112.021303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crescitelli R., Lässer C., Szabó T.G., Kittel A., Eldh M., Dianzani I., Buzás E.I., Lötvall J. Distinct RNA profiles in subpopulations of extracellular vesicles: Apoptotic bodies, microvesicles and exosomes. J. Extracell. Vesicles. 2013;2:20677. doi: 10.3402/jev.v2i0.20677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Witwer K.W., Buzás E.I., Bemis L.T., Bora A., Lässer C., Lötvall J., Nolte-’t Hoen E.N., Piper M.G., Sivaraman S., Skog J., et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles. 2013;2:20360. doi: 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshioka Y., Konishi Y., Kosaka N., Katsuda T., Kato T., Ochiya T. Comparative marker analysis of extracellular vesicles in different human cancer types. J. Extracell. Vesicles. 2013;2:20424. doi: 10.3402/jev.v2i0.20424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krishnamoorthy L., Bess J.W., Preston A.B., Nagashima K., Mahal L.K. HIV-1 and microvesicles from T cells share a common glycome, arguing for a common origin. Nat. Chem. Biol. 2009;5:244–250. doi: 10.1038/nchembio.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Escrevente C., Keller S., Altevogt P., Costa J. Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer. 2011;11:108. doi: 10.1186/1471-2407-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou B., Xu K., Zheng X., Chen T., Wang J., Song Y., Shao Y., Zheng S. Application of exosomes as liquid biopsy in clinical diagnosis. Signal Transduct. Target. Ther. 2020;5:144. doi: 10.1038/s41392-020-00258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerr J.F.R., Wyllie A.H., Currie A.R. Apoptosis: A Basic Biological Phenomenon with Wideranging Implications in Tissue Kinetics. Br. J. Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martínez M.C., Freyssinet J.-M. Deciphering the plasma membrane hallmarks of apoptotic cells: Phosphatidylserine transverse redistribution and calcium entry. BMC Cell Biol. 2001;2:20. doi: 10.1186/1471-2121-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erwig L.-P., Henson P.M. Clearance of apoptotic cells by phagocytes. Cell Death Differ. 2008;15:243–250. doi: 10.1038/sj.cdd.4402184. [DOI] [PubMed] [Google Scholar]
- 27.Borges F.T., Reis L.A., Schor N. Extracellular vesicles: Structure, function, and potential clinical uses in renal diseases. Braz. J. Med. Biol. Res. 2013;46:824–830. doi: 10.1590/1414-431X20132964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hardy M.-P., Audemard É., Migneault F., Feghaly A., Brochu S., Gendron P., Boilard É., Major F., Dieudé M., Hébert M.-J., et al. Apoptotic endothelial cells release small extracellular vesicles loaded with immunostimulatory viral-like RNAs. Sci. Rep. 2019;9:7203. doi: 10.1038/s41598-019-43591-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blander J.M. The many ways tissue phagocytes respond to dying cells. Immunol. Rev. 2017;277:158–173. doi: 10.1111/imr.12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atkin-Smith G.K., Tixeira R., Paone S., Mathivanan S., Collins C., Liem M., Goodall K., Ravichandran K., Hulett M., Poon I.K. A novel mechanism of generating extracellular vesicles during apoptosis via a beads-on-a-string membrane structure. Nat. Commun. 2015;6:7439. doi: 10.1038/ncomms8439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolf P. The Nature and Significance of Platelet Products in Human Plasma. Br. J. Haematol. 1967;13:269–288. doi: 10.1111/j.1365-2141.1967.tb08741.x. [DOI] [PubMed] [Google Scholar]
- 32.Théry C., Ostrowski M., Segura E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y., Li G., Liu M.-L. Microvesicles as Emerging Biomarkers and Therapeutic Targets in Cardiometabolic Diseases. Genom. Proteom. Bioinform. 2018;16:50–62. doi: 10.1016/j.gpb.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dignat-George F., Lacroix R. Microparticles: New Protagonists in Pericellular and Intravascular Proteolysis. Semin. Thromb. Hemost. 2013;39:33–39. doi: 10.1055/s-0032-1333310. [DOI] [PubMed] [Google Scholar]
- 35.Smalley D.M., Ley K. Plasma-derived microparticles for biomarker discovery. Clin. Lab. 2008;54:67–80. [PubMed] [Google Scholar]
- 36.Manek R., Moghieb A., Yang Z., Kumar D., Kobessiy F., Sarkis G.A., Raghavan V., Wang K.K.W. Protein Biomarkers and Neuroproteomics Characterization of Microvesicles/Exosomes from Human Cerebrospinal Fluid Following Traumatic Brain Injury. Mol. Neurobiol. 2018;55:6112–6128. doi: 10.1007/s12035-017-0821-y. Erratum in Mol. Neurobiol. 2018, 55, 6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grigor’Eva A., Tamkovich S., Eremina A., Tupikin A., Kabilov M., Chernykh V., Vlassov V., Laktionov P., Ryabchikova E. Characteristics of exosomes andmicroparticles discovered in human tears. Biomeditsinskaya Khimiya. 2016;62:99–106. doi: 10.18097/PBMC20166201099. [DOI] [PubMed] [Google Scholar]
- 38.Sun Y., Huo C., Qiao Z., Shang Z., Uzzaman A., Liu S., Jiang X., Fan L.Y., Ji L., Guan X., et al. Comparative Proteomic Analysis of Exosomes and Microvesicles in Human Saliva for Lung Cancer. J. Proteome Res. 2018;17:1101–1107. doi: 10.1021/acs.jproteome.7b00770. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi T., Kato A., Berdnikovs S., Stevens W.W., Suh L.A., Norton J.E., Carter R.G., Harris K.E., Peters A.T., Hulse K.E., et al. Microparticles in nasal lavage fluids in chronic rhinosinusitis: Potential biomarkers for diagnosis of aspirin-exacerbated respiratory disease. J. Allergy Clin. Immunol. 2017;140:720–729. doi: 10.1016/j.jaci.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi D.-S., Park J.O., Jang S.C., Yoon Y.J., Jung J.W., Choi D.-Y., Kim J.-W., Kang J.S., Park J., Hwang D., et al. Proteomic analysis of microvesicles derived from human colorectal cancer ascites. Proteomics. 2011;11:2745–2751. doi: 10.1002/pmic.201100022. Erratum in Proteomics 2011, 11, 3438. [DOI] [PubMed] [Google Scholar]
- 41.Wang L., Lv J., Guo C., Li H., Xiong C. Recovery of cell-free mRNA and microRNA from human semen based on their physical nature. Biotechnol. Appl. Biochem. 2014;61:342–348. doi: 10.1002/bab.1172. [DOI] [PubMed] [Google Scholar]
- 42.Clancy J., Sedgwick A., Rosse C., Muralidharan-Chari V., Raposo G., Method M., Chavrier P., D’Souza-Schorey C. Regulated delivery of molecular cargo to invasive tumour-derived microvesicles. Nat. Commun. 2015;6:6919. doi: 10.1038/ncomms7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tricarico C., Clancy J., D’Souza-Schorey C. Biology and biogenesis of shed microvesicles. Small GTPases. 2017;8:220–232. doi: 10.1080/21541248.2016.1215283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.D’Souza-Schorey C., Clancy J.W. Tumor-derived microvesicles: Shedding light on novel microenvironment modulators and prospective cancer biomarkers. Genes Dev. 2012;26:1287–1299. doi: 10.1101/gad.192351.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ginestra A., La Placa M.D., Saladino F., Cassarà D., Nagase H., Vittorelli M.L. The amount and proteolytic content of vesicles shed by human cancer cell lines correlates with their in vitro invasiveness. Anticancer Res. 1998;18:3433–3437. [PubMed] [Google Scholar]
- 46.Skog J., Würdinger T., Van Rijn S., Meijer D.H., Gainche L., Curry W.T., Jr., Carter B.S., Krichevsky A.M., Breakefield X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grossmann A.H., Zhao H., Jenkins N., Zhu W., Richards J.R., Yoo J.H., Winter J.M., Rich B., Mleynek T.M., Li D.Y., et al. The small GTPase ARF6 regulates protein trafficking to control cellular function during development and in disease. Small GTPases. 2019;10:1–12. doi: 10.1080/21541248.2016.1259710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mobarrez F., Sjövik C., Soop A., Hållström L., Frostell C., Pisetsky D.S., Wallén H. CD40L expression in plasma of volunteers following LPS administration: A comparison between assay of CD40L on platelet microvesicles and soluble CD40L. Platelets. 2015;26:486–490. doi: 10.3109/09537104.2014.932339. [DOI] [PubMed] [Google Scholar]
- 49.Cocucci E., Racchetti G., Meldolesi J. Shedding microvesicles: Artefacts no more. Trends Cell Biol. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 50.Pan B.-T., Johnstone R.M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell. 1983;33:967–978. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- 51.Johnstone R.M., Bianchini A., Teng K. Reticulocyte maturation and exosome release: Transferrin receptor containing exosomes shows multiple plasma membrane functions. Blood. 1989;74:1844–1851. doi: 10.1182/blood.V74.5.1844.1844. [DOI] [PubMed] [Google Scholar]
- 52.Stoorvogel W., Kleijmeer M.J., Geuze H.J., Raposo G. The Biogenesis and Functions of Exosomes. Traffic. 2002;3:321–330. doi: 10.1034/j.1600-0854.2002.30502.x. [DOI] [PubMed] [Google Scholar]
- 53.Conde-Vancells J., Rodriguez-Suarez E., Embade N., Gil D., Matthiesen R., Valle M., Elortza F., Lu S.C., Mato J.M., Falcon-Perez J.M. Characterization and Comprehensive Proteome Profiling of Exosomes Secreted by Hepatocytes. J. Proteome Res. 2008;7:5157–5166. doi: 10.1021/pr8004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simons M., Raposo G. Exosomes—vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 2009;21:575–581. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 55.Mathivanan S., Ji H., Simpson R.J. Exosomes: Extracellular organelles important in intercellular communication. J. Proteom. 2010;73:1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 56.Lässer C., Eldh M., Lötvall J. Isolation and Characterization of RNA-Containing Exosomes. J. Vis. Exp. 2012;59:e3037. doi: 10.3791/3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pospíchalová V., Svoboda J., Dave Z., Kotrbová A., Kaiser K., Klemova D., Ilkovics L., Hampl A., Crha I., Jandakova E., et al. Simplified protocol for flow cytometry analysis of fluorescently labeled exosomes and microvesicles using dedicated flow cytometer. J. Extracell. Vesicles. 2015;4:25530. doi: 10.3402/jev.v4.25530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kahlert C., Melo S., Protopopov A., Tang J., Seth S., Koch M., Zhang J., Weitz J., Chin L., Futreal A., et al. Identification of Double-stranded Genomic DNA Spanning All Chromosomes with Mutated KRAS and p53 DNA in the Serum Exosomes of Patients with Pancreatic Cancer. J. Biol. Chem. 2014;289:3869–3875. doi: 10.1074/jbc.C113.532267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun Z., Yang S., Zhou Q., Wang G., Song J., Li Z., Zhang Z., Xu J., Xia K., Chang Y., et al. Emerging role of exosome-derived long non-coding RNAs in tumor microenvironment. Mol. Cancer. 2018;17:82. doi: 10.1186/s12943-018-0831-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hurwitz S.N., Rider M.A., Bundy J.L., Liu X., Singh R.K., Meckes D.G., Jr. Proteomic profiling of NCI-60 extracellular vesicles uncovers common protein cargo and cancer type-specific biomarkers. Oncotarget. 2016;7:86999–87015. doi: 10.18632/oncotarget.13569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kowal J., Arras G., Colombo M., Jouve M., Morath J.P., Primdal-Bengtson B., Dingli F., Loew D., Tkach M., Théry C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA. 2016;113:E968–E977. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haraszti R.A., Didiot M.-C., Sapp E., Leszyk J., Shaffer S.A., Rockwell H.E., Gao F., Narain N.R., DiFiglia M., Kiebish M.A., et al. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. J. Extracell. Vesicles. 2016;5:32570. doi: 10.3402/jev.v5.32570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Altadill T., Campoy I., Lanau L., Gill K., Rigau M., Gil-Moreno A., Reventos J., Byers S., Colas E., Cheema A.K. Enabling Metabolomics Based Biomarker Discovery Studies Using Molecular Phenotyping of Exosome-Like Vesicles. PLoS ONE. 2016;11:e0151339. doi: 10.1371/journal.pone.0151339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ludwig N., Gillespie D.G., Reichert T.E., Jackson E.K., Whiteside T.L. Purine Metabolites in Tumor-Derived Exosomes May Facilitate Immune Escape of Head and Neck Squamous Cell Carcinoma. Cancers. 2020;12:1602. doi: 10.3390/cancers12061602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen G., Huang A.C., Zhang W., Zhang G., Wu M., Xu W., Yu Z., Yang J., Wang B., Sun H., et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560:382–386. doi: 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cordonnier M., Nardin C., Chanteloup G., Derangere V., Algros M., Arnould L., Garrido C., Aubin F., Gobbo J. Tracking the evolution of circulating exosomal-PD-L1 to monitor melanoma patients. J. Extracell. Vesicles. 2020;9:1710899. doi: 10.1080/20013078.2019.1710899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Melo S.A., Luecke L.B., Kahlert C., Fernandez A.F., Gammon S.T., Kaye J., LeBleu V.S., Mittendorf E.A., Weitz J., Rahbari N., et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177–182. doi: 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mehdiani A., Maier A., Pinto A., Barth M., Akhyari P., Lichtenberg A. An Innovative Method for Exosome Quantification and Size Measurement. J. Vis. Exp. 2015;95:e50974. doi: 10.3791/50974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jayachandran M., Miller V.M., Heit J.A., Owen W.G. Methodology for isolation, identification and characterization of microvesicles in peripheral blood. J. Immunol. Methods. 2012;375:207–214. doi: 10.1016/j.jim.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lázaro-Ibáñez E., Sanz-Garcia A., Visakorpi T., Escobedo-Lucea C., Siljander P., Ayuso-Sacido Á., Yliperttula M. Different gDNA content in the subpopulations of prostate cancer extracellular vesicles: Apoptotic bodies, microvesicles, and exosomes. Prostate. 2014;74:1379–1390. doi: 10.1002/pros.22853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.LeBleu V.S., Kalluri R. Exosomes as a Multicomponent Biomarker Platform in Cancer. Trends Cancer. 2020;6:767–774. doi: 10.1016/j.trecan.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 72.Kim H., Kim D.W., Cho J.-Y. Exploring the key communicator role of exosomes in cancer microenvironment through proteomics. Proteome Sci. 2019;17:5. doi: 10.1186/s12953-019-0154-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rajagopal C., Harikumar K.B. The Origin and Functions of Exosomes in Cancer. Front. Oncol. 2018;8:66. doi: 10.3389/fonc.2018.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mashouri L., Yousefi H., Aref A.R., Ahadi A.M., Molaei F., Alahari S.K. Exosomes: Composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol. Cancer. 2019;18:75. doi: 10.1186/s12943-019-0991-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun T., Liu Z., Yang Q. The role of ubiquitination and deubiquitination in cancer metabolism. Mol. Cancer. 2020;19:146. doi: 10.1186/s12943-020-01262-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Harada T., Yamamoto H., Kishida S., Kishida M., Awada C., Takao T., Kikuchi A. Wnt5b-associated exosomes promote cancer cell migration and proliferation. Cancer Sci. 2017;108:42–52. doi: 10.1111/cas.13109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mineo M., Garfield S.H., Taverna S., Flugy A., De Leo G., Alessandro R., Kohn E.C. Exosomes released by K562 chronic myeloid leukemia cells promote angiogenesis in a src-dependent fashion. Angiogenesis. 2012;15:33–45. doi: 10.1007/s10456-011-9241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Montecalvo A., Shufesky W.J., Stolz D.B., Sullivan M.G., Wang Z., DiVito S.J., Papworth G.D., Watkins S.C., Robbins P.D., Larregina A.T., et al. Exosomes As a Short-Range Mechanism to Spread Alloantigen between Dendritic Cells during T Cell Allorecognition. J. Immunol. 2008;180:3081–3090. doi: 10.4049/jimmunol.180.5.3081. [DOI] [PubMed] [Google Scholar]
- 80.Bilska M., Pawłowska A., Zakrzewska E., Chudzik A., Suszczyk D., Gogacz M., Wertel I. Th17 Cells and IL-17 As Novel Immune Targets in Ovarian Cancer Therapy. J. Oncol. 2020;2020:8797683. doi: 10.1155/2020/8797683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Winkler I., Pyszniak M., Pogoda K., Semczuk A., Gogacz M., Miotla P., Adamiak-Godlewska A., Darmochwal-Kolarz D., Rechberger T., Tabarkiewicz J. Assessment of Th17 lymphocytes and cytokine IL-17A in epithelial ovarian tumors. Oncol. Rep. 2017;37:3107–3115. doi: 10.3892/or.2017.5559. [DOI] [PubMed] [Google Scholar]
- 82.Ye S.-B., Li Z.-L., Luo D.-H., Huang B.-J., Chen Y.-S., Zhang X.-S., Cui J., Zeng Y.-X., Li J. Tumor-derived exosomes promote tumor progression and T-cell dysfunction through the regulation of enriched exosomal microRNAs in human nasopharyngeal carcinoma. Oncotarget. 2014;5:5439–5452. doi: 10.18632/oncotarget.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou J., Li X., Wu X., Zhang T., Zhu Q., Wang X., Wang H., Wang K., Lin Y., Wang X. Exosomes Released from Tumor-Associated Macrophages Transfer miRNAs That Induce a Treg/Th17 Cell Imbalance in Epithelial Ovarian Cancer. Cancer Immunol. Res. 2018;6:1578–1592. doi: 10.1158/2326-6066.CIR-17-0479. [DOI] [PubMed] [Google Scholar]
- 84.He C., Hua W., Liu J., Fan L., Wang H., Sun G. Exosomes derived from endoplasmic reticulum-stressed liver cancer cells enhance the expression of cytokines in macrophages via the STAT3 signaling pathway. Oncol. Lett. 2020;20:589–600. doi: 10.3892/ol.2020.11609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Okoye I.S., Coomes S.M., Pelly V.S., Czieso S., Papayannopoulos V., Tolmachova T., Seabra M.C., Wilson M.S. MicroRNA-containing Tregulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity. 2014;41:89–103. doi: 10.1016/j.immuni.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Theodoraki M.-N., Yerneni S.S., Hoffmann T.K., Gooding W.E., Whiteside T.L. Clinical Significance of PD-L1+ Exosomes in Plasma of Head and Neck Cancer Patients. Clin. Cancer Res. 2018;24:896–905. doi: 10.1158/1078-0432.CCR-17-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dong H., Strome S.E., Salomao D.R., Tamura H., Hirano F., Flies D.B., Roche P.C., Lu J., Zhu G., Tamada K., et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002;8:793–800. doi: 10.1038/nm730. Erratum in Nat. Med. 2002, 8, 1039. [DOI] [PubMed] [Google Scholar]
- 88.Chen L., Han X. Anti–PD-1/PD-L1 therapy of human cancer: Past, present, and future. J. Clin. Investig. 2015;125:3384–3391. doi: 10.1172/JCI80011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wortzel I., Dror S., Kenific C.M., Lyden D. Exosome-Mediated Metastasis: Communication from a Distance. Dev. Cell. 2019;49:347–360. doi: 10.1016/j.devcel.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 90.Jabbari N., Akbariazar E., Feqhhi M., Rahbarghazi R., Rezaie J. Breast cancer-derived exosomes: Tumor progression and therapeutic agents. J. Cell. Physiol. 2020;235:6345–6356. doi: 10.1002/jcp.29668. [DOI] [PubMed] [Google Scholar]
- 91.Wu M., Wang G., Hu W., Yao Y., Yu X.-F. Emerging roles and therapeutic value of exosomes in cancer metastasis. Mol. Cancer. 2019;18:53. doi: 10.1186/s12943-019-0964-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lin X.-J., Fang J.-H., Yang X.-J., Zhang C., Yuan Y., Zheng L., Zhuang S. Hepatocellular carcinoma cell-secreted exosomal microRNA-210 promotes angiogenesis in vitro and in vivo. Mol. Ther. Nucleic Acids. 2018;11:243–252. doi: 10.1016/j.omtn.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao W., Liu Y., Zhang C., Duan C. Multiple Roles of Exosomal Long Noncoding RNAs in Cancers. BioMed Res. Int. 2019;2019:1460572. doi: 10.1155/2019/1460572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Duan B., Shi S., Yue H., You B., Shan Y., Zhu Z., Bao L., You Y. Exosomal miR-17-5p promotes angiogenesis in nasopharyngeal carcinoma via targeting BAMBI. J. Cancer. 2019;10:6681–6692. doi: 10.7150/jca.30757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim D.H., Park S., Kim H., Choi Y.J., Kim S.Y., Sung K.J., Sung Y.H., Choi C.-M., Yun M., Yi Y.-S., et al. Tumor-derived exosomal miR-619-5p promotes tumor angiogenesis and metastasis through the inhibition of RCAN1.4. Cancer Lett. 2020;475:2–13. doi: 10.1016/j.canlet.2020.01.023. [DOI] [PubMed] [Google Scholar]
- 96.Masoumi-Dehghi S., Babashah S., Sadeghizadeh M. microRNA-141-3p-containing small extracellular vesicles derived from epithelial ovarian cancer cells promote endothelial cell angiogenesis through activating the JAK/STAT3 and NF-κB signaling pathways. J. Cell Commun. Signal. 2020;14:233–244. doi: 10.1007/s12079-020-00548-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li J., Yuan H., Xu H., Zhao H., Xiong N. Hypoxic Cancer-Secreted Exosomal miR-182-5p Promotes Glioblastoma Angiogenesis by Targeting Kruppel-like Factor 2 and 4. Mol. Cancer Res. 2020;18:1218–1231. doi: 10.1158/1541-7786.MCR-19-0725. [DOI] [PubMed] [Google Scholar]
- 98.Fan J., Xu G., Chang Z., Zhu L., Yao J. miR-210 transferred by lung cancer cell-derived exosomes may act as proangiogenic factor in cancer-associated fibroblasts by modulating JAK2/STAT3 pathway. Clin. Sci. 2020;134:807–825. doi: 10.1042/CS20200039. Erratum in Clin. Sci. 2020, 134, 1801–1804. [DOI] [PubMed] [Google Scholar]
- 99.Hsieh C.-H., Tai S.-K., Yang M.-H. Snail-overexpressing Cancer Cells Promote M2-Like Polarization of Tumor-Associated Macrophages by Delivering MiR-21-Abundant Exosomes. Neoplasia. 2018;20:775–788. doi: 10.1016/j.neo.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Park J.E., Dutta B., Tse S.W., Gupta N., Tan C.F., Low J.K., Yeoh K.W., Kon O.L., Tam J.P., Sze S.K. Hypoxia-induced tumor exosomes promote M2-like macrophage polarization of infiltrating myeloid cells and microRNA-mediated metabolic shift. Oncogene. 2019;38:5158–5173. doi: 10.1038/s41388-019-0782-x. [DOI] [PubMed] [Google Scholar]
- 101.Yu B., Wang S. Angio-LncRs: LncRNAs that regulate angiogenesis and vascular disease. Theranostics. 2018;8:3654–3675. doi: 10.7150/thno.26024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Arcucci V., Stacker S., Achen M. Control of Gene Expression by Exosome-Derived Non-Coding RNAs in Cancer Angiogenesis and Lymphangiogenesis. Biomolecules. 2021;11:249. doi: 10.3390/biom11020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Balandeh E., Mohammadshafie K., Mahmoudi Y., Hossein Pourhanifeh M., Rajabi A., Bahabadi Z.R., Mohammadi A.H., Rahimian N., Hamblin M.R., Mirzaei H. Roles of Non-coding RNAs and Angiogenesis in Glioblastoma. Front Cell Dev. Biol. 2021;9:716462. doi: 10.3389/fcell.2021.716462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Conigliaro A., Costa V., Dico A.L., Saieva L., Buccheri S., Dieli F., Manno M., Raccosta S., Mancone C., Tripodi M., et al. CD90+ liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Mol. Cancer. 2015;14:155. doi: 10.1186/s12943-015-0426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ma X., Li Z., Li T., Zhu L., Li Z., Tian N. Long non-coding RNA HOTAIR enhances angiogenesis by induction of VEGFA expression in glioma cells and transmission to endothelial cells via glioma cell derived-extracellular vesicles. Am. J. Transl. Res. 2017;9:5012–5021. [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang C., Luo Y., Cao J., Wang X., Miao Z., Shao G. Exosomal lncRNA FAM225A accelerates esophageal squamous cell carcinoma progression and angiogenesis via sponging miR-206 to upregulate NETO2 and FOXP1 expression. Cancer Med. 2020;9:8600–8611. doi: 10.1002/cam4.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cheng C., Zhang Z., Cheng F., Shao Z. Exosomal lncRNA RAMP2-AS1 Derived from Chondrosarcoma Cells Promotes Angiogenesis Through miR-2355-5p/VEGFR2 Axis. OncoTargets Ther. 2020;13:3291–3301. doi: 10.2147/OTT.S244652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huang X.-Y., Huang Z.-L., Huang J., Xu B., Huang X.-Y., Xu Y.-H., Zhou J., Tang Z.-Y. Exosomal circRNA-100338 promotes hepatocellular carcinoma metastasis via enhancing invasiveness and angiogenesis. J. Exp. Clin. Cancer Res. 2020;39:20. doi: 10.1186/s13046-020-1529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Maji S., Chaudhary P., Akopova I., Nguyen P.M., Hare R.J., Gryczynski I., Vishwanatha J. Exosomal Annexin II Promotes Angiogenesis and Breast Cancer Metastasis. Mol. Cancer Res. 2017;15:93–105. doi: 10.1158/1541-7786.MCR-16-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Suchorska W., Lach M. The role of exosomes in tumor progression and metastasis (Review) Oncol. Rep. 2016;35:1237–1244. doi: 10.3892/or.2015.4507. [DOI] [PubMed] [Google Scholar]
- 111.Ni J., Bucci J., Malouf D., Knox M., Graham P., Li Y. Exosomes in Cancer Radioresistance. Front. Oncol. 2019;9:869. doi: 10.3389/fonc.2019.00869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hedlund M., Nagaeva O., Kargl D., Baranov V., Mincheva-Nilsson L. Thermal- and Oxidative Stress Causes Enhanced Release of NKG2D Ligand-Bearing Immunosuppressive Exosomes in Leukemia/Lymphoma T and B Cells. PLoS ONE. 2011;6:e16899. doi: 10.1371/journal.pone.0016899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lehmann B., Paine M.S., Brooks A.M., McCubrey J., Renegar R.H., Wang R., Terrian D.M. Senescence-Associated Exosome Release from Human Prostate Cancer Cells. Cancer Res. 2008;68:7864–7871. doi: 10.1158/0008-5472.CAN-07-6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yu X., Harris S.L., Levine A.J. The Regulation of Exosome Secretion: A Novel Function of the p53 Protein. Cancer Res. 2006;66:4795–4801. doi: 10.1158/0008-5472.CAN-05-4579. [DOI] [PubMed] [Google Scholar]
- 115.Pös O., Budis J., Szemes T. Recent trends in prenatal genetic screening and testing. F1000Research. 2019;8:764. doi: 10.12688/f1000research.16837.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Thakur B.K., Zhang H., Becker A., Matei I., Huang Y., Costa-Silva B., Zheng Y., Hoshino A., Brazier H., Xiang J., et al. Double-stranded DNA in exosomes: A novel biomarker in cancer detection. Cell Res. 2014;24:766–769. doi: 10.1038/cr.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Keserű J.S., Soltész B., Lukács J., Márton É., Szilágyi-Bónizs M., Penyige A., Póka R., Nagy B. Detection of cell-free, exosomal and whole blood mitochondrial DNA copy number in plasma or whole blood of patients with serous epithelial ovarian cancer. J. Biotechnol. 2019;298:76–81. doi: 10.1016/j.jbiotec.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 118.De Souza M.F., Kuasne H., Barros-Filho M.D.C., Cilião H.L., Marchi F.A., Fuganti P.E., Paschoal A.R., Rogatto S.R., Cólus I.M.D.S. Circulating mRNAs and miRNAs as candidate markers for the diagnosis and prognosis of prostate cancer. PLoS ONE. 2017;12:e0184094. doi: 10.1371/journal.pone.0184094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Armand-Labit V., Pradines A. Circulating cell-free microRNAs as clinical cancer biomarkers. Biomol. Concepts. 2017;8:61–81. doi: 10.1515/bmc-2017-0002. [DOI] [PubMed] [Google Scholar]
- 120.Montani F., Bianchi F. Circulating Cancer Biomarkers: The Macro-revolution of the Micro-RNA. EBioMedicine. 2016;5:4–6. doi: 10.1016/j.ebiom.2016.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dong L., Lin W., Weiqi S., Xu M.-D., Wu X., Ni S., Huang D., Weng W.-W., Tan C., Sheng W., et al. Circulating Long RNAs in Serum Extracellular Vesicles: Their Characterization and Potential Application as Biomarkers for Diagnosis of Colorectal Cancer. Cancer Epidemiol. Biomark. Prev. 2016;25:1158–1166. doi: 10.1158/1055-9965.EPI-16-0006. [DOI] [PubMed] [Google Scholar]
- 122.Lijun Q., Shao Y., Zhang X., Zheng T., Miao M., Qin L., Wang B., Ye G., Xiao B., Guo J. Plasma long noncoding RNA protected by exosomes as a potential stable biomarker for gastric cancer. Tumor Biol. 2015;36:2007–2012. doi: 10.1007/s13277-014-2807-y. [DOI] [PubMed] [Google Scholar]
- 123.Seimiya T., Otsuka M., Iwata T., Shibata C., Tanaka E., Suzuki T., Koike K. Emerging Roles of Exosomal Circular RNAs in Cancer. Front. Cell Dev. Biol. 2020;8:568366. doi: 10.3389/fcell.2020.568366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ramalingam N., Jeffrey S. Future of Liquid Biopsies with Growing Technological and Bioinformatics Studies: Opportunities and Challenges in Discovering Tumor Heterogeneity with Single-Cell Level Analysis. Cancer J. 2018;24:104–108. doi: 10.1097/PPO.0000000000000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Heitzer E., Haque I.S., Roberts C.E.S., Speicher M.R. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat. Rev. Genet. 2019;20:71–88. doi: 10.1038/s41576-018-0071-5. [DOI] [PubMed] [Google Scholar]
- 126.Szilágyi M., Pös O., Márton É., Buglyó G., Soltész B., Keserű J., Penyige A., Szemes T., Nagy B. Circulating Cell-Free Nucleic Acids: Main Characteristics and Clinical Application. Int. J. Mol. Sci. 2020;21:6827. doi: 10.3390/ijms21186827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ignatiadis M., Sledge G.W., Jeffrey S.S. Liquid biopsy enters the clinic—Implementation issues and future challenges. Nat. Rev. Clin. Oncol. 2021;18:297–312. doi: 10.1038/s41571-020-00457-x. [DOI] [PubMed] [Google Scholar]
- 128.Dreux M., Garaigorta U., Boyd B., Décembre E., Chung J., Whitten-Bauer C., Wieland S., Chisari F.V. Short-Range Exosomal Transfer of Viral RNA from Infected Cells to Plasmacytoid Dendritic Cells Triggers Innate Immunity. Cell Host Microbe. 2012;12:558–570. doi: 10.1016/j.chom.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yokoi A., Villar-Prados A., Oliphint P.A., Zhang J., Song X., De Hoff P., Morey R., Liu J., Roszik J., Clise-Dwyer K., et al. Mechanisms of nuclear content loading to exosomes. Sci. Adv. 2019;5:eaax8849. doi: 10.1126/sciadv.aax8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fernando M.R., Jiang C., Krzyzanowski G.D., Ryan W.L. New evidence that a large proportion of human blood plasma cell-free DNA is localized in exosomes. PLoS ONE. 2017;12:e0183915. doi: 10.1371/journal.pone.0183915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vagner T., Spinelli C., Minciacchi V.R., Balaj L., Zandian M., Conley A., Zijlstra A., Freeman M.R., Demichelis F., De S., et al. Large extracellular vesicles carry most of the tumour DNA circulating in prostate cancer patient plasma. J. Extracell. Vesicles. 2018;7:1505403. doi: 10.1080/20013078.2018.1505403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.García-Olmo D.C., Domínguez C., García-Arranz M., Anker P., Stroun M., García-Verdugo J.M., García-Olmo D. Cell-Free Nucleic Acids Circulating in the Plasma of Colorectal Cancer Patients Induce the Oncogenic Transformation of Susceptible Cultured Cells. Cancer Res. 2010;70:560–567. doi: 10.1158/0008-5472.CAN-09-3513. [DOI] [PubMed] [Google Scholar]
- 133.Peters D.L., Pretorius P.J. Continuous adaptation through genetic communication—A putative role for cell-free DNA. Expert Opin. Biol. Ther. 2012;12((Suppl. 1)):S127–S132. doi: 10.1517/14712598.2012.668518. [DOI] [PubMed] [Google Scholar]
- 134.Thierry A.R., El Messaoudi S., Gahan P.B., Anker P., Stroun M. Origins, structures, and functions of circulating DNA in oncology. Cancer Metastasis Rev. 2016;35:347–376. doi: 10.1007/s10555-016-9629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Filipów S., Laczmanski L. Blood Circulating miRNAs as Cancer Biomarkers for Diagnosis and Surgical Treatment Response. Front. Genet. 2019;10:169. doi: 10.3389/fgene.2019.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Birkó Z., Nagy B., Klekner Á., Virga J. Novel Molecular Markers in Glioblastoma—Benefits of Liquid Biopsy. Int. J. Mol. Sci. 2020;21:7522. doi: 10.3390/ijms21207522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fabbri M., Paone A., Calore F., Galli R., Gaudio E., Santhanam R., Lovat F., Fadda P., Mao C., Nuovo G.J., et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc. Natl. Acad. Sci. USA. 2012;109:E2110–E2116. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Anfossi S., Giordano A., Gao H., Cohen E., Tin S., Wu Q., Garza R.J., Debeb B.G., Alvarez R.H., Valero V., et al. High Serum miR-19a Levels Are Associated with Inflammatory Breast Cancer and Are Predictive of Favorable Clinical Outcome in Patients with Metastatic HER2+ Inflammatory Breast Cancer. PLoS ONE. 2014;9:e83113. doi: 10.1371/journal.pone.0083113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dragomir M., Chen B., Calin G.A. Exosomal lncRNAs as new players in cell-to-cell communication. Transl. Cancer Res. 2018;7((Suppl. 2)):S243–S252. doi: 10.21037/tcr.2017.10.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang R., Xia Y., Wang Z., Zheng J., Chen Y., Li X., Wang Y., Ming H. Serum long non coding RNA MALAT-1 protected by exosomes is up-regulated and promotes cell proliferation and migration in non-small cell lung cancer. Biochem. Biophys. Res. Commun. 2017;490:406–414. doi: 10.1016/j.bbrc.2017.06.055. [DOI] [PubMed] [Google Scholar]
- 141.Chen W., Quan Y., Fan S., Wang H., Liang J., Huang L., Chen L., Liu Q., He P., Ye Y. Exosome-transmitted circular RNA hsa_circ_0051443 suppresses hepatocellular carcinoma progression. Cancer Lett. 2020;475:119–128. doi: 10.1016/j.canlet.2020.01.022. [DOI] [PubMed] [Google Scholar]
- 142.Tang W., Fu K., Sun H., Rong D., Wang H., Cao H. CircRNA microarray profiling identifies a novel circulating biomarker for detection of gastric cancer. Mol. Cancer. 2018;17:137. doi: 10.1186/s12943-018-0888-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shao Y., Tao X., Lu R., Zhang H., Ge J., Xiao B., Ye G., Guo J. Hsa_circ_0065149 is an Indicator for Early Gastric Cancer Screening and Prognosis Prediction. Pathol. Oncol. Res. 2020;26:1475–1482. doi: 10.1007/s12253-019-00716-y. [DOI] [PubMed] [Google Scholar]
- 144.Tao X., Shao Y., Lu R., Ye Q., Xiao B., Ye G., Guo J. Clinical significance of hsa_circ_0000419 in gastric cancer screening and prognosis estimation. Pathol.-Res. Pract. 2020;216:152763. doi: 10.1016/j.prp.2019.152763. [DOI] [PubMed] [Google Scholar]
- 145.Zou F., Wang X., Han X., Rothschild G., Zheng S.G., Basu U., Sun J. Expression and Function of Tetraspanins and Their Interacting Partners in B Cells. Front. Immunol. 2018;9:1606. doi: 10.3389/fimmu.2018.01606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lin J., Li J., Huang B., Liu J., Chen X., Chen X.-M., Xu Y.-M., Huang L.-F., Wang X.-Z. Exosomes: Novel Biomarkers for Clinical Diagnosis. Sci. World J. 2015;2015:657086. doi: 10.1155/2015/657086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Logozzi M., De Milito A., Lugini L., Borghi M., Calabro’ L., Spada M., Perdicchio M., Marino M.L., Federici C., Iessi E., et al. High Levels of Exosomes Expressing CD63 and Caveolin-1 in Plasma of Melanoma Patients. PLoS ONE. 2009;4:e5219. doi: 10.1371/journal.pone.0005219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Malik M.A., Mirza J.I.A., Umar M., Manzoor S. CD81+ Exosomes Play a Pivotal Role in the Establishment of Hepatitis C Persistent Infection and Contribute Toward the Progression of Hepatocellular Carcinoma. Viral Immunol. 2019;32:453–462. doi: 10.1089/vim.2019.0077. [DOI] [PubMed] [Google Scholar]
- 149.Zylbersztejn K., Galli T. Vesicular traffic in cell navigation. FEBS J. 2011;278:4497–4505. doi: 10.1111/j.1742-4658.2011.08168.x. [DOI] [PubMed] [Google Scholar]
- 150.Proux-Gillardeaux V., Raposo G., Irinopoulou T., Galli T. Expression of the Longin domain of TI-VAMP impairs lysosomal secretion and epithelial cell migration. Biol. Cell. 2007;99:261–271. doi: 10.1042/BC20060097. [DOI] [PubMed] [Google Scholar]
- 151.Peng X., Li X., Yang S., Huang M., Wei S., Ma Y., Li Y., Wu B., Jin H., Li B., et al. LINC00511 drives invasive behavior in hepatocellular carcinoma by regulating exosome secretion and invadopodia formation. J. Exp. Clin. Cancer Res. 2021;40:183. doi: 10.1186/s13046-021-01990-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tzeng H.-T., Wang Y.-C. Rab-mediated vesicle trafficking in cancer. J. Biomed. Sci. 2016;23:70. doi: 10.1186/s12929-016-0287-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Le N.-Q., Ho Q.-T., Ou Y.-Y. Classifying the molecular functions of Rab GTPases in membrane trafficking using deep convolutional neural networks. Anal. Biochem. 2018;555:33–41. doi: 10.1016/j.ab.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 154.Savina A., Fader C.M., Damiani M.T., Colombo M.I. Rab11 Promotes Docking and Fusion of Multivesicular Bodies in a Calcium-Dependent Manner. Traffic. 2005;6:131–143. doi: 10.1111/j.1600-0854.2004.00257.x. [DOI] [PubMed] [Google Scholar]
- 155.Dorayappan K.D.P., Wanner R., Wallbillich J.J., Saini U., Zingarelli R., Suarez A.A., Cohn D.E., Selvendiran K. Hypoxia-induced exosomes contribute to a more aggressive and chemoresistant ovarian cancer phenotype: A novel mechanism linking STAT3/Rab proteins. Oncogene. 2018;37:3806–3821. doi: 10.1038/s41388-018-0189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Phuyal S., Hessvik N.P., Skotland T., Sandvig K., Llorente A. Regulation of exosome release by glycosphingolipids and flotillins. FEBS J. 2014;281:2214–2227. doi: 10.1111/febs.12775. [DOI] [PubMed] [Google Scholar]
- 157.Hoshino A., Kim H.S., Bojmar L., Gyan K.E., Cioffi M., Hernandez J., Zambirinis C.P., Rodrigues G., Molina H., Heissel S., et al. Extracellular Vesicle and Particle Biomarkers Define Multiple Human Cancers. Cell. 2020;182:1044–1061.e18. doi: 10.1016/j.cell.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hanson P.I., Cashikar A. Multivesicular Body Morphogenesis. Annu. Rev. Cell Dev. Biol. 2012;28:337–362. doi: 10.1146/annurev-cellbio-092910-154152. [DOI] [PubMed] [Google Scholar]
- 159.Colombo M., Moita C.F., van Niel G., Kowal J., Vigneron J., Benaroch P., Manel N., Moita L.F., Théry C., Raposo G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J. Cell Sci. 2013;126:5553–5565. doi: 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- 160.Sahu R., Kaushik S., Clement C.C., Cannizzo E.S., Scharf B., Follenzi A., Potolicchio I., Nieves E., Cuervo A.M., Santambrogio L. Microautophagy of Cytosolic Proteins by Late Endosomes. Dev. Cell. 2011;20:131–139. doi: 10.1016/j.devcel.2010.12.003. Erratum in Dev. Cell 2011, 20, 405–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Baietti M.F., Zhang Z., Mortier E., Melchior A., DeGeest G., Geeraerts A., Ivarsson Y., Depoortere F., Coomans C., Vermeiren E., et al. Syndecan–syntenin–ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012;14:677–685. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- 162.Lv L.-H., Wan Y.-L., Lin Y., Zhang W., Yang M., Li G.-L., Lin H.-M., Shang C.-Z., Chen Y.-J., Min J. Anticancer Drugs Cause Release of Exosomes with Heat Shock Proteins from Human Hepatocellular Carcinoma Cells That Elicit Effective Natural Killer Cell Antitumor Responses in Vitro. J. Biol. Chem. 2012;287:15874–15885. doi: 10.1074/jbc.M112.340588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Skotland T., Sandvig K., Llorente A. Lipids in exosomes: Current knowledge and the way forward. Prog. Lipid Res. 2017;66:30–41. doi: 10.1016/j.plipres.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 164.Varki A. Biological roles of glycans. Glycobiology. 2017;27:3–49. doi: 10.1093/glycob/cww086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Williams C., Royo F., Aizpurua-Olaizola O., Pazos R., Boons G.-J., Reichardt N.-C., Falcon-Perez J.M. Glycosylation of extracellular vesicles: Current knowledge, tools and clinical perspectives. J. Extracell. Vesicles. 2018;7:1442985. doi: 10.1080/20013078.2018.1442985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Batista B.S., Eng W.S., Pilobello K.T., Hendricks-Muñoz K.D., Mahal L.K. Identification of a Conserved Glycan Signature for Microvesicles. J. Proteome Res. 2011;10:4624–4633. doi: 10.1021/pr200434y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Escrevente C., Grammel N., Kandzia S., Zeiser J., Tranfield E.M., Conradt H.S., Costa J. Sialoglycoproteins and N-Glycans from Secreted Exosomes of Ovarian Carcinoma Cells. PLoS ONE. 2013;8:e78631. doi: 10.1371/journal.pone.0078631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gu J., Sato Y., Kariya Y., Isaji T., Taniguchi N., Fukuda T. A Mutual Regulation between Cell−Cell Adhesion and N-Glycosylation: Implication of the Bisecting GlcNAc for Biological Functions. J. Proteome Res. 2009;8:431–435. doi: 10.1021/pr800674g. [DOI] [PubMed] [Google Scholar]
- 169.Choi Y., Park U., Koo H.-J., Park J.-S., Lee D.H., Kim K., Choi J. Exosome-mediated diagnosis of pancreatic cancer using lectin-conjugated nanoparticles bound to selective glycans. Biosens. Bioelectron. 2021;177:112980. doi: 10.1016/j.bios.2021.112980. [DOI] [PubMed] [Google Scholar]
- 170.Tian Y., Li S., Song J., Ji T., Zhu M., Anderson G.J., Wei J., Nie G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35:2383–2390. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- 171.O’Brien K.P., Khan S., Gilligan K., Zafar H., Lalor P., Glynn C., O’Flatharta C., Ingoldsby H., Dockery P., De Bhulbh A., et al. Employing mesenchymal stem cells to support tumor-targeted delivery of extracellular vesicle (EV)-encapsulated microRNA-379. Oncogene. 2018;37:2137–2149. doi: 10.1038/s41388-017-0116-9. [DOI] [PubMed] [Google Scholar]
- 172.Greco K.A., Franzen C.A., Foreman K.E., Flanigan R.C., Kuo P.C., Gupta G. PLK-1 Silencing in Bladder Cancer by siRNA Delivered With Exosomes. Urology. 2016;91:241.e1–241.e7. doi: 10.1016/j.urology.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 173.Franzen C., Blackwell R.H., Foreman K.E., Kuo P.C., Flanigan R.C., Gupta G.N. Urinary Exosomes: The Potential for Biomarker Utility, Intercellular Signaling and Therapeutics in Urological Malignancy. J. Urol. 2016;195:1331–1339. doi: 10.1016/j.juro.2015.08.115. [DOI] [PubMed] [Google Scholar]
- 174.Galbo P., Ciesielski M.J., Figel S., Maguire O., Qiu J., Wiltsie L., Minderman H., Fenstermaker R.A. Circulating CD9+/GFAP+/survivin+ exosomes in malignant glioma patients following survivin vaccination. Oncotarget. 2017;8:114722–114735. doi: 10.18632/oncotarget.21773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Katakowski M., Buller B., Zheng X., Lu Y., Rogers T., Osobamiro O., Shu W., Jiang F., Chopp M. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013;335:201–204. doi: 10.1016/j.canlet.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Federici C., Shahaj E., Cecchetti S., Camerini S., Casella M., Iessi E., Camisaschi C., Paolino G., Calvieri S., Ferro S., et al. Natural-killer-derived extracellular vesicles: Immune sensors and interactors. Front. Immunol. 2020;11:262. doi: 10.3389/fimmu.2020.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wang F., Li L., Piontek K., Sakaguchi M., Selaru F.M. Exosome miR-335 as a novel therapeutic strategy in hepatocellular carcinoma. Hepatology. 2018;67:940–954. doi: 10.1002/hep.29586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Viaud S., Terme M., Flament C., Taieb J., Andre F., Novault S., Escudier B., Robert C., Caillat-Zucman S., Tursz T., et al. Dendritic Cell-Derived Exosomes Promote Natural Killer Cell Activation and Proliferation: A Role for NKG2D Ligands and IL-15Rα. PLoS ONE. 2009;4:e4942. doi: 10.1371/journal.pone.0004942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Li J., Huang S., Zhou Z., Lin W., Chen S., Chen M., Ye Y. Exosomes derived from rAAV/AFP-transfected dendritic cells elicit specific T cell-mediated immune responses against hepatocellular carcinoma. Cancer Manag. Res. 2018;10:4945–4957. doi: 10.2147/CMAR.S178326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Huang F., Wan J., Hu W., Hao S. Enhancement of Anti-Leukemia Immunity by Leukemia–Derived Exosomes Via Downregulation of TGF-β1 Expression. Cell. Physiol. Biochem. 2017;44:240–254. doi: 10.1159/000484677. [DOI] [PubMed] [Google Scholar]
- 181.Rivoltini L., Chiodoni C., Squarcina P., Tortoreto M., Villa A., Vergani B., Bürdek M., Botti L., Arioli I., Cova A., et al. TNF-Related Apoptosis-Inducing Ligand (TRAIL)–Armed Exosomes Deliver Proapoptotic Signals to Tumor Site. Clin. Cancer Res. 2016;22:3499–3512. doi: 10.1158/1078-0432.CCR-15-2170. [DOI] [PubMed] [Google Scholar]
- 182.Kim M.S., Haney M.J., Zhao Y., Mahajan V., Deygen I., Klyachko N.L., Inskoe E., Piroyan A., Sokolsky M., Okolie O., et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine. 2016;12:655–664. doi: 10.1016/j.nano.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Cheng L., Wang Y., Huang L. Exosomes from M1-Polarized Macrophages Potentiate the Cancer Vaccine by Creating a Pro-inflammatory Microenvironment in the Lymph Node. Mol. Ther. 2017;25:1665–1675. doi: 10.1016/j.ymthe.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Keryer-Bibens C., Pioche-Durieu C., Villemant C., Souquère S., Nishi N., Hirashima M., Middeldorp J.M., Busson P. Exosomes released by EBV-infected nasopharyngeal carcinoma cells convey the viral latent membrane protein 1 and the immunomodulatory protein galectin 9. BMC Cancer. 2006;6:283. doi: 10.1186/1471-2407-6-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Klibi J., Niki T., Adhikary D., Mautner J., Busson P., Riedel A., Pioche-Durieu C., Souquere S., Rubinstein E., Le Moulec S., et al. Blood diffusion and Th1-suppressive effects of galectin-9–containing exosomes released by Epstein-Barr virus–infected nasopharyngeal carcinoma cells. Blood. 2009;113:1957–1966. doi: 10.1182/blood-2008-02-142596. Erratum in Blood 2009, 114, 3974. [DOI] [PubMed] [Google Scholar]
- 186.Zhang K., Dong C., Chen M., Yang T., Wang X., Gao Y., Wang L., Wen Y., Chen G., Wang X., et al. Extracellular vesicle-mediated delivery of miR-101 inhibits lung metastasis in osteosarcoma. Theranostics. 2020;10:411–425. doi: 10.7150/thno.33482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Peng P., Yan Y., Keng S. Exosomes in the ascites of ovarian cancer patients: Origin and effects on anti-tumor immunity. Oncol. Rep. 2011;25:749–762. doi: 10.3892/or.2010.1119. [DOI] [PubMed] [Google Scholar]
- 188.Ding Y., Cao F., Sun H., Wang Y., Liu S., Wu Y., Cui Q., Mei W., Li F. Exosomes derived from human umbilical cord mesenchymal stromal cells deliver exogenous miR-145-5p to inhibit pancreatic ductal adenocarcinoma progression. Cancer Lett. 2019;442:351–361. doi: 10.1016/j.canlet.2018.10.039. [DOI] [PubMed] [Google Scholar]
- 189.Abusamra A.J., Zhong Z., Zheng X., Li M., Ichim T.E., Chin J.L., Min W.-P. Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis. Blood Cells Mol. Dis. 2005;35:169–173. doi: 10.1016/j.bcmd.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 190.Saari H.O., Lázaro-Ibáñez E., Viitala T., Vuorimaa-Laukkanen E., Siljander P., Yliperttula M. Microvesicle- and exosome-mediated drug delivery enhances the cytotoxicity of Paclitaxel in autologous prostate cancer cells. J. Control. Release. 2015;220 Pt B:727–737. doi: 10.1016/j.jconrel.2015.09.031. [DOI] [PubMed] [Google Scholar]
- 191.Pan J., Ding M., Xu K., Yang C., Mao L.-J. Exosomes in diagnosis and therapy of prostate cancer. Oncotarget. 2017;8:97693–97700. doi: 10.18632/oncotarget.18532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ishizuya Y., Uemura M., Narumi R., Tomiyama E., Koh Y., Matsushita M., Nakano K., Hayashi Y., Wang C., Kato T., et al. The role of actinin-4 (ACTN4) in exosomes as a potential novel therapeutic target in castration-resistant prostate cancer. Biochem. Biophys. Res. Commun. 2020;523:588–594. doi: 10.1016/j.bbrc.2019.12.084. [DOI] [PubMed] [Google Scholar]
- 193.Syn N., Wang L., Chow E., Lim C.T., Goh B.-C. Exosomes in Cancer Nanomedicine and Immunotherapy: Prospects and Challenges. Trends Biotechnol. 2017;35:665–676. doi: 10.1016/j.tibtech.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 194.Bell B.M., Kirk I.D., Hiltbrunner S., Gabrielsson S., Bultema J.J. Designer exosomes as next-generation cancer immunotherapy. Nanomed. Nanotechnol. Biol. Med. 2016;12:163–169. doi: 10.1016/j.nano.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 195.Kibria G., Ramos E.K., Wan Y., Gius D.R., Liu H. Exosomes as a Drug Delivery System in Cancer Therapy: Potential and Challenges. Mol. Pharm. 2018;15:3625–3633. doi: 10.1021/acs.molpharmaceut.8b00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yong T., Zhang X., Bie N., Zhang H., Zhang X., Li F., Hakeem A., Hu J., Gan L., Santos H.A., et al. Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat. Commun. 2019;10:3838. doi: 10.1038/s41467-019-11718-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Xunian Z., Kalluri R. Biology and therapeutic potential of mesenchymal stem cell-derived exosomes. Cancer Sci. 2020;111:3100–3110. doi: 10.1111/cas.14563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Xu Z., Zeng S., Gong Z., Yan Y. Exosome-based immunotherapy: A promising approach for cancer treatment. Mol. Cancer. 2020;19:160. doi: 10.1186/s12943-020-01278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gong C., Tian J., Wang Z., Gao Y., Wu X., Ding X., Qiang L., Li G., Han Z., Yuan Y., et al. Functional exosome-mediated co-delivery of doxorubicin and hydrophobically modified microRNA 159 for triple-negative breast cancer therapy. J. Nanobiotechnology. 2019;17:93. doi: 10.1186/s12951-019-0526-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yim N., Ryu S.-W., Choi K., Lee K.R., Lee S., Choi H., Kim J., Shaker M.R., Sun M.R.S.W., Park J.-H., et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein–protein interaction module. Nat. Commun. 2016;7:12277. doi: 10.1038/ncomms12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhao L., Gu C., Gana Y., Shaoa L., Chenc H., Zhua H. Exosome-mediated siRNA delivery to suppress postoperative breast cancer metastasis. J. Control. Release. 2020;318:1–15. doi: 10.1016/j.jconrel.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 202.Kooijmans S.A.A., Aleza C.G., Roffler S.R., Van Solinge W., Vader P., Schiffelers R.M. Display of GPI-anchored anti-EGFR nanobodies on extracellular vesicles promotes tumour cell targeting. J. Extracell. Vesicles. 2016;5:31053. doi: 10.3402/jev.v5.31053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Shi X., Cheng Q., Hou T., Han M., Smbatyan G., Lang J., Epstein A.L., Lenz H.-J., Zhang Y. Genetically Engineered Cell-Derived Nanoparticles for Targeted Breast Cancer Immunotherapy. Mol. Ther. 2020;28:536–547. doi: 10.1016/j.ymthe.2019.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Liang G., Zhu Y., Ali D.J., Tian T., Xu H., Si K., Sun B., Chen B., Xiao Z. Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. J. Nanobiotechnology. 2020;18:10. doi: 10.1186/s12951-019-0563-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Mendt M., Kamerkar S., Sugimoto H., McAndrews K.M., Wu C.-C., Gagea M., Yang S., Blanko E.V.R., Peng Q., Ma X., et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight. 2018;3:e99263. doi: 10.1172/jci.insight.99263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yu X., Odenthal M., Fries J.W.U. Exosomes as miRNA Carriers: Formation–Function–Future. Int. J. Mol. Sci. 2016;17:2028. doi: 10.3390/ijms17122028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Shankar G.M., Balaj L., Stott S.L., Nahed B., Carter B.S. Liquid biopsy for brain tumors. Expert Rev. Mol. Diagn. 2017;17:943–947. doi: 10.1080/14737159.2017.1374854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Contreras-Naranjo J.C., Wu H.-J., Ugaz V.M. Microfluidics for exosome isolation and analysis: Enabling liquid biopsy for personalized medicine. Lab Chip. 2017;17:3558–3577. doi: 10.1039/C7LC00592J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Piffoux M., Nicolás-Boluda A., Mulens-Arias V., Richard S., Rahmi G., Gazeau F., Wilhelm C., Silva A.K. Extracellular vesicles for personalized medicine: The input of physically triggered production, loading and theranostic properties. Adv. Drug Deliv. Rev. 2019;138:247–258. doi: 10.1016/j.addr.2018.12.009. [DOI] [PubMed] [Google Scholar]