Abstract
Depression and anxiety disorders are two of the most common and growing mental health concerns in adolescents. Consequently, antidepressant medication (AD) use has increased widely during the last decades. Several classes of antidepressants are used mainly to treat depression, anxiety, and obsessive-compulsive disorders by targeting relevant brain neurochemical pathways. Almost all randomized clinical trials of antidepressants examined patients with no concomitant medications or drugs. This does not address the expected course of therapy and outcome in cannabis users. Cannabis is the most commonly used illicit substance globally. Substantial changes in its regulation are recently taking place. Many countries and US states are becoming more permissive towards its medical and recreational use. The psychological and physiological effects of cannabis (mainly of its major components, tetrahydrocannabinol (THC) and cannabidiol (CBD)) have been extensively characterized. Cannabis use can be a risk factor for depressive and anxiety symptoms, but some constituents or mixtures may have antidepressant and/or anxiolytic potential. The aim of this literature review is to explore whether simultaneous use of AD and cannabis in adolescence can affect AD treatment outcomes. Based on the current literature, it is reasonable to assume that antidepressants are less effective for adolescents with depression/anxiety who frequently use cannabis. The mechanisms of action of antidepressants and cannabis point to several similarities and conjunctions that merit future investigation regarding the potential effectiveness of antidepressants among adolescents who consume cannabis regularly.
Keywords: antidepressants, depression, anxiety, cannabis, tetrahydrocannabinol (THC), cannabidiol (CBD)
1. Introduction
Major depression disorder (MDD) is the prominent cause of disability-adjusted life years lost in 10–19-year-olds, with a global prevalence of 4–25% [1,2,3]. Since COVID-19 was declared an international public health emergency, youth around the world have experienced dramatic disruptions to their everyday lives, which has increased this prevalence and the associated disability [4]. This matter has special importance because it is considered a ‘gateway’ disorder, increasing the likelihood of adult depression and other psychiatric disorders later in life, with associated social, medical, and economic sequelae [5,6,7,8].
Whereas MDD manifests as an episodic but often recurrent illness with a mean duration of 16 weeks, often comorbid with other mental disorders, particularly anxiety [9], the clinical presentation of MDD in adolescents is quite different from the adult one. In fact, it is characterized by heterogeneous and changing symptoms, sometime hidden from somatic complaints and complicated by the high comorbidity rates with anxiety disorders, substance abuse, disruptive behavior disorders, personality disorders, and medical illnesses [10,11,12,13]. A main concern for this is the risk for suicide. In adolescents as well as in adults, although suicide is a complex phenomenon which has many causes, 85% to 95% of those dying by suicide have a psychiatric illness (particularly MDD) [14], and there is an over-representation of individuals with substance-use disorders [15,16,17,18,19]. Furthermore, there are significant psychosocial and educational consequences if such an episode remains undetected [20]. Therefore, in these individuals it is difficult to recognize depressive symptoms and make a correct diagnosis, as well as to establish an adequate therapeutic strategy [21].
Evidence-based treatment for depression and anxiety consists of psychological therapies, e.g., cognitive behavioral therapy, and antidepressant medication (AD) [22]. However, because of insufficient resources, antidepressants are used more frequently than psychological interventions [23] In particular, the rate of its prescription among adolescents has increased over time [24]. Thus, AD has long been the basis of medical treatment for depression and a suitable replacement for benzodiazepines in long-term treatment of anxiety disorders [22]. Although clinical guidelines recommend AD for minimum 6 months following response in MDD and anxiety disorders, medication adherence rates in adolescents are poor [25]. There has been continuous debate about AD efficacy [23,26,27,28], though they remain an effective, yet not fully satisfactory, treatment option for many patients with depression and anxiety [29].
Less is known about AD interaction with cannabis. For instance, whereas ADs are associated with reduction of suicidal behavior [30], recreational cannabis use is often associated with increased suicidality, at least in some populations [31]. It is critical to recognize the joint effect of ADs when consumed together with medical or non-medical cannabis.
Cannabis, commonly known as marijuana, contains Δ-9-tetrahydrocannabinol (THC) and cannabidiol (CBD), the main constituents, and a plethora of phytocannabinoids alongside a vast array of flavonoids and terpenes [32]. It is the most widely used illicit substance worldwide [33]. Over the past 50 years, not only has cannabis been increasingly adopted by adolescents and young adults for recreational use, mainly in social settings to increase sociability and produce euphoric and tranquilizing effects [34], but the age of use initiation is shifting back with younger children and adolescents reporting daily cannabis use [35]. Although cannabis remains illegal in most countries, there have been significant changes in its use as a therapeutic medicine [36,37,38]. Accumulating evidence suggests that some cannabinoids, particularly CBD, may be an effective and safe anxiolytic and potentially also antidepressant agents [32,39,40,41,42,43,44].
A core problem is that use amongst depressed teens has increased more rapidly over the past 15 years compared to their peers [45], yet, almost all clinical studies of ADs examined ‘clean’ patients; with no concomitant use of psychoactive substances. This does not represent the course of therapy in cannabis users.
Hence, the aim of the current literature review is to obtain a deeper understanding regarding the possible effects of ADs efficiency for adolescents who regularly use cannabis.
We hypothesized that due to some similarities between the mechanisms of action of antidepressants and cannabis, the outcomes of AD treatment are limited.
2. Method
Search Strategy and Paper Selection
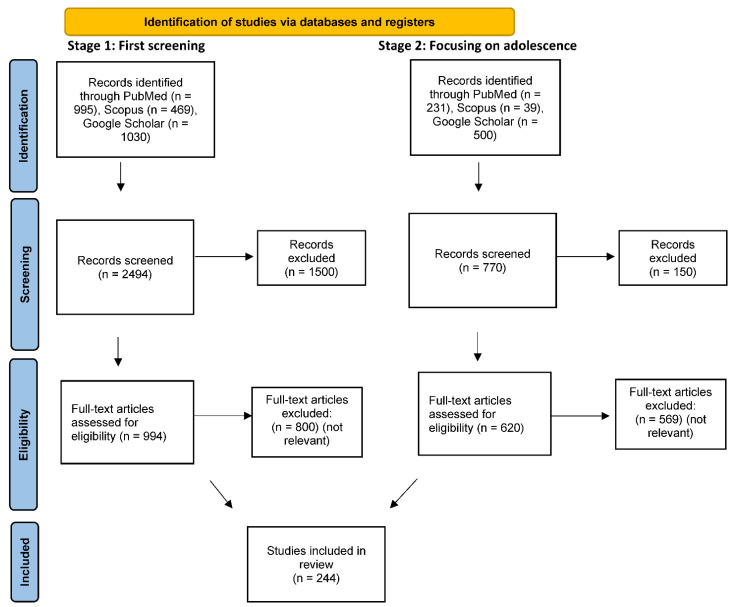
A comprehensive, two-stage, literature search was performed for all papers published up to 30 October 2021, using PubMed, SCOPUS, and Google Scholar electronic databases. In the first stage, we used the search terms: “Depression”, or “Antidepressants” or “Anxiety”, together with “Cannabis”, or “Cannabidiol”, or “THC”, or “Cannabinoids”. We included all original research papers, review articles, non-research letters, communications, commentaries meeting search criteria, but excluded case reports, small case series, and non-English language articles. One independent author (D.H.) screened the titles and abstracts for potential articles. In the second stage, papers with these combined terms on “Adolescents” or “Adolescence” were prioritized. The full texts of remaining articles were evaluated by applying the inclusion and exclusion criteria. Even though this is a narrative review, we have attempted to present our search strategy including approximate numbers of papers at each stage in a PRISMA type flowchart (see Figure 1).
Figure 1.
PRISMA 2020 flow diagram for retrospectively estimated numbers of papers at each stage of screening.
3. The Efficacy of Antidepressants: The Importance of Adherence
ADs are the frontline intervention for treatment of anxiety and depressive disorders and obsessive-compulsive disorder (OCD), in both adults and youngsters, with and without co-morbidity [46,47,48,49,50,51]. Clinicians have a wide choice of drugs [23]. While the prevalence of anxiety and MDD remained stable over the past decades [52], use of antidepressants is increasing worldwide [53,54]. This began in the mid-1950s with the introduction of tricyclic antidepressants (TCAs) (e.g., imipramine) and monoamine oxidase inhibitors (MAOIs) (e.g., iproniazid). Both classes of agents produced a dramatic improvement in depression symptoms but had poor tolerability and safety profiles [55]. Subsequently, along with recognizing the importance of the serotonergic system in pathophysiology and treatment of depression [56], second-generation medications were developed: selective serotonin reuptake inhibitors (SSRIs) (e.g., fluoxetine), selective norepinephrine (e.g., reboxetine), dual serotonin and norepinephrine reuptake inhibitors (SNRIs) (e.g., venlafaxine), norepinephrine/dopamine reuptake inhibitor (NDRIs) (e.g., bupropion), and noradrenergic and specific serotonergic antidepressants (NaSSAs) (e.g., mirtazapine) (Table 1).
Table 1.
Antidepressant classes in clinical use among patients diagnosed with MDD, anxiety, and OCD.
| Drug Name | Active Principle | Main Targets |
Mechanisms of Action | Main Side Effects |
|---|---|---|---|---|
| Tricyclic antidepressants (TCAs) | The chemical structure of a TCA consists of a three-ringed structure with an attached secondary or tertiary amine | Serotonin, norepinephrine, and acetyl choline | Act on approximately five different neurotransmitter pathways to achieve their effects: block the reuptake of Serotonin and Norepinephrine in presynaptic terminals which leads to increased concentration of these neurotransmitters in the synaptic cleft. act as competitive antagonists on post-synaptic alpha cholinergic (alpha1 and alpha2), muscarinic, and histaminergic receptors (H1). |
Constipation, dizziness, blurred vision, confusion, urinary retention, and tachycardia |
| Monoamine oxidase inhibitors (MAOIs) | Blocking monoamine oxidase | Norepinephrine and serotonin | Breaks down different types of neurotransmitters from the brain: norepinephrine, serotonin, dopamine, and tyramine. MAOIs inhibit the breakdown of these neurotransmitters thus, increasing their levels. | Dry mouth, nausea, diarrhea, constipation, drowsiness, insomnia, dizziness, and/or lightheadedness |
| Selective Serotonin Reuptake inhibitors (SSRIs) | Inhibit the reuptake of serotonin | Serotonin | Block the reuptake of serotonin into the presynaptic nerve terminal via the serotonin uptake site, thus increasing the synaptic concentration of serotonin. | Flatulence, somnolence, memory impairment, decreased concentration, yawning, fatigue, dry mouth, weight gain, light headedness, adverse sexual effects, and sweating |
| Selective Norepinephrine | Inhibit reuptake of norepinephrine | Norepinephrine | Block the reuptake of norepinephrine into the presynaptic nerve terminal via somatodendritic 2a-adrenoceptors, thus increasing the synaptic concentration of norepinephrine | Dry mouth, constipation, insomnia, increased sweating, tachycardia, vertigo, urinary hesitancy and/or retention, and impotence |
| Dual Serotonin and Norepinephrine Reuptake Inhibitors (SNRIs) | Inhibit the uptake of serotonin and norepinephrine | Norepinephrine and serotonin | Bind to serotonin and norepinephrine transporters to selectively inhibit the reuptake of these neurotransmitters from the synaptic cleft | Nausea, hypertension, somnolence, dizziness, and dry mouth |
| Norepinephrine/Dopamine Reuptake Inhibitor (NDRIs) | Inhibit the uptake of dopamine and norepinephrine | Norepinephrine and dopamine | Block the reuptake of norepinephrine and dopamine into the presynaptic nerve terminal thus increasing the synaptic concentration of norepinephrine and dopamine | Fatigue, sleepiness, and somnolence |
| Noradrenergic and Specific Serotonergic antidepressants (NaSSAs) | Enhance serotonergic and noradrenergic neurotransmission | Norepinephrine and serotonin | Potent antagonism of central α2-adrenergic autoreceptors and heteroreceptors and antagonism of both 5-HT2 and 5-HT, receptors with low affinity for muscarinic, cholinergic, and dopaminergic receptors | Somnolence, increased appetite, weight gain, dry mouth, constipation, and dizziness |
In spite of the contemporary excessive use of these second-generation drugs, there is a long-lasting debate and concern about their efficacy and effectiveness. Treatment often takes several weeks or months to reach their full therapeutic effects [56], and 30–50% of the patients do not respond to treatment with ADs [57]. In fact, no AD or class of ADs offers a faster onset of action [58] and no single AD treatment is uniformly effective [59,60]. Although international practice guidelines recommend that pharmacotherapy for adolescents should be initiated with fluoxetine, with sertraline or citalopram used in case of non-response to fluoxetine [61], after unsuccessful treatment for depression with an SSRI, it is unclear whether switching to a particular antidepressant is more effective than switching to another [62].
This heterogeneity in the effectiveness in AD treatment is also manifested differently by gender as some studies indicated that men respond better to TCAs than to SSRIs whereas women tend to show the opposite pattern of response [63]. Numerous studies attempt to explain these discrepancies [64,65,66] yet the reason for this remains unclear [67]. Surprisingly, little is known about these differences among adolescents. Another important dimension is period of life, as effectiveness of AD treatment varies across ages [68,69,70,71]. For example, young adults had a lower response to noradrenergic antidepressants than they did to serotonergic antidepressants, whereas there was no differential response in the older age group [70]. The reason for these differences is poorly understood [68].
The primary goal of AD treatment maintenance is to prevent a subsequent episode of depression (recurrence), anxiety, or OCD symptoms and development of chronicity [72,73,74]. However, this goal is not achieved in many cases. While a remitted patient with depression or anxiety is symptom-free [74,75], the probability of achieving and sustaining symptomatic remission for adolescence with MDD with first-line pharmacotherapy is as low as approximately 30–40% [76]. This probability is similar for adolescents with anxiety. For example, in patients with generalized anxiety disorder (GAD), sertraline has demonstrated remission rates that are 34% to 46% higher than placebo [77]. Regarding OCD, studies report 25% to 47% symptomatic remission rates [78]. In fact, long-term AD use may increase, in some cases, biochemical vulnerability to develop depressive episodes and worsen long-term outcome and symptomatic expression of MDD, decreasing likelihood of subsequent response to pharmacologic treatment and duration of symptom-free period [59].
ADs have very broad mechanisms of action which lead to a variety of adverse effects related to their potent activity on cholinergic, adrenergic, and histaminergic receptors [55,79,80]. This is reflected in numerous side effects such as sexual dysfunction [81,82], headaches [83], somnolence [83] and weight gain [81,83,84]. Additionally, these side effects may consequently lead to decreased adherence to AD [85], which is another key issue regarding currently available AD compounds. According to a growing body of research, the effectiveness of AD treatment is highly influenced by adherence rates [86,87,88], since using AD continuously is a key to successful treatment outcomes [89] and reduced likelihood of relapse or recurrence of depression [90]. For AD to be efficacious, patients must remain adherent until symptoms remit, up to 12 weeks [91,92]. To decrease the risk of relapse, the American Academy of Child and Adolescent Psychiatry (AACAP) Practice Parameters recommend continuation of treatment for 6–12 months “for all patients who have responded to the acute treatment” [93]. Moreover, adherence to AD is associated with lower risk of premature mortality [94] in various AD-using populations [51,95,96,97]. However, low adherence rate is commonly reported [95,96,97,98,99,100,101]; about 60% of patients discontinue antidepressants within 3 months [102,103], with both genders following a similar pattern of AD adherence across age decades [104]. It is still unclear whether the limited efficacy of AD is due to low adherence rates or maybe the causal relationship is vice versa. Such putative bi-directional influence may lead to a positive feedback loop or “a vicious cycle” with mounting negative effect.
In the last decade, many studies suggest that cannabinoids be explored as potential novel ADs.
4. Cannabinoids for Treatment of Depression and Anxiety: Changing Perceptions throughout the Years
Over the last 25 years the endocannabinoid system (ECS) has emerged as an important neuromodulatory system [105], which includes ligands, enzymes, and endogenous cannabinoid receptors, widespread throughout the brain and parts of the body [106]. Although this system is activated during consumption of illegal drugs containing exocannabinoids such as marijuana and synthetic cannabinoids [107], research suggests it is important for regulation of many basic physiological functions such as cognition, learning, memory, perception, sleep, pain, appetite, motor control, and regulation of cardiovascular and immune responses [108,109,110,111,112,113,114].
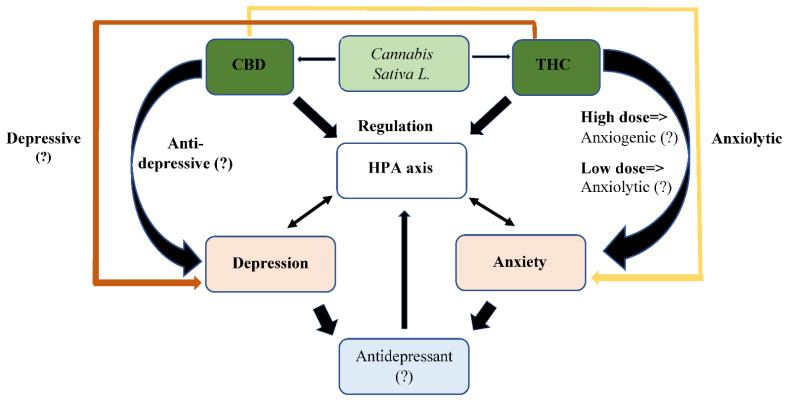
A fundamental element in the discovery of the ECS is Cannabis sativa L., which has a long history as a medicinal plant [115]. Since the discovery of its main psychomimetic constituent, Δ9-Tetrahydrocannabinol (Δ9-THC), about half a century ago [116], studies have shown that it produces many additional compounds, including various phytocannabinoids [117]. The concentration of these compounds depends on tissue type, age, variety, growth conditions (nutrition, humidity, light level), harvest time, and storage conditions [118], leading to a wide range of pharmaceutical effects [119]. To date, out of the ±150 cannabinoids that have been identified, the most studied and most active are THC and CBD [32]. CBD, unlike THC, is devoid of psychotomimetic effects [120]. In general, THC and CBD seem to have opposite effects [121,122,123,124,125] (Figure 2).
Figure 2.
Putative effect of cannabinoids on depression and anxiety. Some of the data presented above is speculative and needs further clarification in future study. In addition, the putative role of additional cannabinoids contained in cannabis has not been well studied and is not represented in this figure.
It is important to distinguish between the multi-purpose applications of cannabis in the context of medicinal and social purposes. It is considered a controversial plant due to its recreational use [126], highlighted by the ‘social high’ induced by marijuana (usually predominantly comprised of THC [127]) [128,129]. Since the recreational use of cannabis was first reported [130], it has spread globally, first to high-income countries, then to low- and middle-income countries [131,132]. Likewise, the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) longitudinal epidemiological study found that, between 2001–2002 and a decade later (2012–2013), marijuana use and Diagnostic and Statistical Manual of Mental Disorders Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV marijuana disorder prevalence doubled [133].
Among the most prominent concerns regarding cannabis use is the connection to mental disorders [134]. Numerous studies support ECS involvement in modulation of the hypothalamic–pituitary–adrenal (HPA) axis; regulation of mood and reward, anxiety, and extinction of fear learning [135,136,137,138,139]. This is expressed both in psychotic and non- psychotic disorders when a large intake of cannabis appears to trigger acute psychotic episodes and worsen outcomes in existing psychosis [140,141,142,143]. In parallel, a growing body of evidence supports an association between cannabis use and depression and anxiety [144,145,146], including in young adults. Cannabis consumption during adolescence is associated with increased risk of developing MDD and suicidality, especially suicidal ideation in young adulthood [147] which is manifested differently across gender [148]. Moreover, it places them at greater risk for maintaining higher levels of anxiety over time [149].
On the other hand, cannabis psychoactive preparations have been used for over 4000 years for medical purposes [150] and a great portion of its medical efficacy is attributed to CBD’s neuroprotection properties [151] which include antioxidant and anti-inflammatory activities [152,153].
From a psychiatric perspective, CBD is a novel promising therapeutic agent. It attenuates the psychotic-like effects of cannabis over time in recreational users [154] and it was repeatedly shown to induce anxiolytic activity in preclinical and clinical studies [41,155,156,157] in addition to its anti-compulsive effects [158,159,160,161]. Furthermore, it exhibits anti-depressive-like abilities in several animal models [42,43,44], yet, to the best of our knowledge, there is no published controlled clinical study that has investigated whether CBD can decrease depressive symptoms in patients. A laboratory study reported that CBD attenuated the transient “amotivational” effects of THC-based cannabis [162] and a small-scale clinical trial focusing on different outcomes reported that depression was an intervening variable in the effects of Sativex (which has a high ratio of CBD:THC) on the main topic of interest [163]. A potential treatment for borderline personality disorder (BPD), based on ratio of high level of CBD to low level of THC has been suggested [32] and CBD-based compounds have been found to be potent in the relief of anxious and depressive symptoms [164,165,166,167].
Unfortunately, medical use of cannabis has been shown to be significantly associated with non-medical use of cannabis [168]. This overlap presents new difficulties when psychiatric patients regularly use cannabis.
5. Antidepressant Treatment Combined with Cannabis Use: A Gap in Knowledge
The knowledge about interactions between cannabis use and long-term AD treatment is vague. HPA axis dysregulation plays a role in vulnerability to stress-related disorders, such as anxiety and depression. AD agents normalize its hyperactivity [169,170,171,172]. The ECS can also regulate the HPA axis activity [173,174,175,176]. Accordingly, deficits in ECS signaling may result in depressive and anxiogenic behavioral responses, while pharmacological augmentation of endocannabinoid signaling can produce both anti-depressive and anxiolytic behavioral responses [177].
This duplicity may possibly be attributed, at least in part, to the bidirectional effects of cannabinoids on anxiety, with low doses having anxiolytic and high doses anxiogenic effects, as well as to the individual’s history and the environmental context [178]. Thus, CBD’s effect on depressive-like behavior in mice has an inverted U-shaped dose-response curve [44], whereas the association between MDD and cannabis use in humans is more complex. Unfortunately, many longitudinal studies exploring the association between cannabis use and MDD employed unclear categories for defining frequency of cannabis use or did not record the frequency of cannabis use [179], while greater exposure to cannabis is expected to lead to a greater incidence of MDD at follow-up [180].
The meaning of this is that combined cannabis and AD use interaction may be dose-dependent. In general, low doses of cannabis are stimulatory as they were found to be anxiolytic, whereas high doses are inhibitory and anxiogenic [178]. This means that any effect of this interaction should be explored carefully, taking into account dose-use patterns of cannabis users. Due to the pivotal role of the ECS in the regulation of emotional states, it is most likely that a patient who uses AD will be affected differently by this combined use of cannabis whether he/she consumes stimulating low doses or inhibitory high doses. In addition, one must take into consideration the relatively unpredictable nature of the response of humans to cannabis consumption, which is derived from multiple factors.
One possible explanation for these biphasic effects of cannabinoids is that distinct receptors with differential sensitivity to cannabinoids are implicated in their inhibitory/anxiogenic and stimulatory/anxiolytic effects [178]. Precise short-term and long-term dose-related effects of cannabinoids in humans remain to be studied carefully [181].
Another aspect that requires examinations is the possible drug–drug interactions resulting from the pharmaco-metabolic processes of ADs when combined with cannabinoids. This issue has not been explored fully; yet, a few similarities between these compounds’ mechanisms of action can point to a competitive effect. Although there are limited studies of direct SSRI–cannabinoid interactions, accumulating data suggest the potential for interactions [182].
Longstanding evidence suggests that changes in activity of 5HT1A serotonergic inhibitory auto-receptors, mediating cortisol, and ACTH secretion, as well as the regulation of serotonergic neuronal firing [183], mediate symptom improvement during antidepressant therapy [184]. Specifically, SSRIs modulate the serotonergic (5HT) system and the HPA axis by affecting sensitivity of 5HT1A auto-receptors [184,185,186], an effect thought to be a necessary prerequisite for clinical response [187,188,189]. Similarly, anxiolytic effects produced by cannabinoids are modulated by 5-HT1A receptors [190,191]. This was also confirmed for CBD, as shown in many animal studies [44,192,193,194].
Additionally, changes in neuronal plasticity and BDNF signaling have been implicated in the etiology of depression and in AD drug action [193]. Specifically, ADs increase the synthesis of BDNF [195,196,197,198,199]. Similarly, cannabinoids also affect BNDF levels. In preclinical studies, THC has been shown to alter BDNF expression [200,201,202,203] and CBD increases BDNF signaling in models of neurodegeneration [204,205,206]. However, this effect may differ by the individual’s cannabis consumption habits; intravenous administration of THC increased serum BDNF levels in healthy controls but not in occasional cannabis users and that the latter have lower basal BDNF levels [207].
Furthermore, gut microbiota have been implicated in regulation of pathophysiology of several mental disorders, including anxiety and depression [208,209,210]. Drugs belonging to new classes of ADs have antimicrobial effects [211]. For instance, the gut microbiota–brain axis at least partially mediates the antidepressant actions of (R)-ketamine [212] and vagus nerve dependent gut-brain signaling contributes to the effects of oral SSRI [213]. Interestingly, cannabinoids and the ECS are involved in regulating the gut microbiome [214,215,216,217], with some recent evidence supporting the effects of cannabis consumption on the microbiota–gut–brain axis [218,219].
One more consideration in the overlap between AD treatment and cannabis use is in their rich cross-talk with the immune system. While accumulating evidence indicates immunomodulatory and anti-inflammatory effects of cannabinoids [220,221,222,223,224], the connection of stress, depression, and anxiety with the immune system is well established [225,226,227,228,229,230,231,232]. The meaning of this interaction is that depressed and anxious patients may already have immune disruption [225,226,228,229,230,231,232], and that use of AD normalizes it [232]. Due to the fact that the precise mechanisms whereby antidepressants cause these changes are uncertain, it is unclear whether additional cannabis use, which affects the immune system as well [220,221,222,223,224], may affect AD treatment’s efficiency.
Another important issue to be considered is that cannabis consumption while on AD treatment can potentially cause the patient to use AD improperly. Cannabis is generally used to elevate mood [233]. It enables and increases the subjective sense of well-being [234]. Furthermore, this combination of cannabis and AD can affect the stability of the patients’ mental states, making it hard for them to distinguish between the physical and psychological effects derived from the cannabis use and their AD treatment. A situation like this may lead to mistaken conclusions. Thus, it is reasonable to believe that cannabis use may affect adherence to AD: patients might use their AD medication less than prescribed or even chose to use more cannabis or other drugs instead of the prescribed AD. Indeed, cannabis use is strongly related to use of other drugs [235] which can lead to a greater complication.
In summary, the examination of AD and cannabis mechanisms of action points at several similarities between these two, which can lead to modulatory effects of cannabis on AD’s effectiveness. Still, this issue merits in-depth investigation before drawing conclusions. At present, it is unclear whether ADs are effective for depressive/anxious patients who use cannabis frequently.
6. Summary: What Is Known and What Needs to Be Studied about Antidepressants and Cannabinoids?
The rate of cannabis use while on AD medication is an important issue that became more relevant in the last decades due to the sharp increases in both AD prescription [53] and cannabis use [236]. This is accompanied with a worldwide trend toward liberalizing cannabis policy and commercializing its sale [129]. The upsetting reality is that cannabis use was more than twice as common and increased more rapidly from 2004 to 2016 among youth with depression compared to youth without depression [45].
This dearth of knowledge merits future basic and clinical studies on this important issue. Whereas a thoughtful examination of this is vital to clarify the possible effects, there are a few significant limitations regarding future research. First, an accurate measurement of cannabis use is challenging. There is a connection between mixing different types of cannabis; recreational use, which is considered as non-medical, i.e., in social settings [34], and the use of cannabinoids in various medical conditions [237,238,239]. However, these two uses are significantly associated [168]. Furthermore, it is especially important to consider the level of cannabis use. Some studies grouped cannabis abuse and dependence into ‘use disorders’ [240], in line with DSM-5 guidelines [241], which correlate with diagnosis of depression/anxiety and AD treatment, while other studies have used different controversial levels of use for ‘heavy’ and ‘light’ cannabinoid use [242,243,244]. In addition, most research has focused on THC, CBD, and their dissimilarities regarding depression and anxiety. However, ‘cannabis’ is not a single compound product [32]. Although it is known that both non-medical and medical marijuana use could contribute differentially to clinical outcomes and potentially lead to barriers to mental health care in this population, it is problematic to achieve a valid measurement by estimating the influence of each compound separately. Considering that cannabis inflorescences accumulate hundreds of milligrams of terpenes alongside cannabinoids [32], the ratio of THC:CBD:other chemicals/terpenes in cannabis is frequently unknown, making it difficult to assess the exact ratio of chemicals and their effects on AD treatment. Consequently, it is crucial to specify unambiguous definitions for the level of use and ratio of compounds in the cannabis before exploring its effect on AD treatment.
Secondly, much of the research on CBD and depression/anxiety is preclinical. To the best of our knowledge, there is no published controlled clinical study that has explored the effect of CBD on depression, along with little evidence regarding its effect on anxiety in humans. Perhaps a significant amount of human data may provide new insights for a better understanding of the innovative combinations of it with AD.
7. Conclusions
There is relatively scarce information about the pharmacological interactions between these two groups of drugs and the clinical efficacy of AD when prescribed to a cannabis-using patient is unknown. Hence, the answer as to whether ADs are effective for patients with depression/anxiety who use cannabis remains open.
Based on current literature and current patterns of AD use in adolescence, we assume that cannabis use while combined with ADs can affect ADs’ pharmaco-metabolic processes and lead to adverse long-term effects. Given the similarities between cannabis compounds and AD mechanisms of action, there are some expected effects which may likely diminish the positive outcome and intensify the side effects of AD treatment.
To our knowledge, only one study has examined this possible interaction of marijuana and AD use among adolescents [182]. Nevertheless, this matter warrants intensive research before a definitive conclusion can be drawn.
Acknowledgments
Research in A.W.’s lab is supported by the Israel Science Foundation (grant #1781/16) and the Israel Ministry of Science and Technology (grant #3-15689). The funding sources had no influence on the content of this review.
Author Contributions
Conceptualization, G.S. and D.H.-S.; writing—original draft preparation, D.H.-S.; writing—review and editing, A.W. (Aron Weller), G.S. and A.W. (Abraham Weizman). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Smithson S., Pignone M.P. Screening Adults for Depression in Primary Care. Med. Clin. N. Am. 2017;101:807–821. doi: 10.1016/j.mcna.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Bernaras E., Jaureguizar J., Garaigordobil M. Child and adolescent depression: A review of theories, evaluation instruments, prevention programs, and treatments. Front. Psychol. 2019;10:543. doi: 10.3389/fpsyg.2019.00543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . Global Diffusion of eHealth: Making Universal Health Coverage Achievable: Report of the Third Global Survey on eHealth. World Health Organization; Geneva, Switzerland: 2017. [Google Scholar]
- 4.Lee J. Mental health effects of school closures during COVID-19. Lancet Child Adolesc. Health. 2020;4:421. doi: 10.1016/S2352-4642(20)30109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berndt E.R., Koran L.M., Finkelstein S.N., Gelenberg A.J., Kornstein S.G., Miller I.M., Keller M.B. Lost human capital from early-onset chronic depression. Am. J. Psychiatry. 2000;157:940–947. doi: 10.1176/appi.ajp.157.6.940. [DOI] [PubMed] [Google Scholar]
- 6.Voelker R. Researchers probe depression in children. JAMA. 2003;289:3078–3079. doi: 10.1001/jama.289.23.3078. [DOI] [PubMed] [Google Scholar]
- 7.Weissman M.M., Wolk S., Goldstein R.B., Moreau D., Adams P., Greenwald S., Wickramaratne P. Depressed adolescents grown up. JAMA. 1994;281:1707–1713. doi: 10.1001/jama.281.18.1707. [DOI] [PubMed] [Google Scholar]
- 8.Weissman M.M., Wolk S., Wickramaratne P., Goldstein R.B., Adams P., Greenwald S., Steinberg D. Children with prepubertal-onset major depressive disorder and anxiety grown up. Arch. Gen. Psychiatry. 1999;56:794–801. doi: 10.1001/archpsyc.56.9.794. [DOI] [PubMed] [Google Scholar]
- 9.Paulus M.P., Stein M.B. Interoception in anxiety and depression. Brain Struct. Funct. 2010;214:451–463. doi: 10.1007/s00429-010-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angold A., Costello E.J., Erkanli A. Comorbidity. J. Child Psychol. Psychiatry Allied Discip. 1999;40:57–87. doi: 10.1111/1469-7610.00424. [DOI] [PubMed] [Google Scholar]
- 11.Angold A., Erkanli A., Farmer E.M., Fairbank J.A., Burns B.J., Keeler G., Costello E.J. Psychiatric disorder, impairment, and service use in rural African American and white youth. Arch. Gen. Psychiatry. 2002;59:893–901. doi: 10.1001/archpsyc.59.10.893. [DOI] [PubMed] [Google Scholar]
- 12.Paris J. Recent research in personality disorders. Preface. Psychiatr. Clin. N. Am. 2008;31:xi–xii. doi: 10.1016/j.psc.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Renouf A.G., Kovacs M., Mukerji P. Relationship of depressive, conduct, and comorbid disorders and social functioning in childhood. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36:998–1004. doi: 10.1097/00004583-199707000-00023. [DOI] [PubMed] [Google Scholar]
- 14.Oquendo M.A., Mann J.J. Suicidal behavior: A developmental perspective. Psychiatr. Clin. N. Am. 2008;31:xiii–xvi. doi: 10.1016/j.psc.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sher L., Sperling D., Stanley B.H., Carballo J.J., Shoval G., Zalsman G., Burke A.K., Mann J.J., Oquendo M.A. Triggers for suicidal behavior in depressed older adolescents and young adults: Do alcohol use disorders make a difference? Int. J. Adolesc. Med. Health. 2007;19:91–98. doi: 10.1515/IJAMH.2007.19.1.91. [DOI] [PubMed] [Google Scholar]
- 16.Shoval G., Sever J., Sher L., Diller R., Apter A., Weizman A., Zalsman G. Substance use, suicidality, and adolescent-onset schizophrenia: An Israeli 10-year retrospective study. J. Child Adolesc. Psychopharmacol. 2006;16:767–775. doi: 10.1089/cap.2006.16.767. [DOI] [PubMed] [Google Scholar]
- 17.Shoval G., Shmulewitz D., Wall M.M., Aharonovich E., Spivak B., Weizman A., Hasin D. Alcohol dependence and suicide-related ideation/behaviors in an Israeli household sample, with and without major depression. Alcohol. Clin. Exp. Res. 2014;38:820–825. doi: 10.1111/acer.12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shoval G., Zalsman G., Apter A., Diller R., Sher L., Weizman A. A 10-year retrospective study of inpatient adolescents with schizophrenia/schizoaffective disorder and substance use. Compr. Psychiatry. 2007;48:1–7. doi: 10.1016/j.comppsych.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Zalsman G., Misgav S., Sommerfeld E., Kohn Y., Brunstein-Klomek A., Diller R., Sher L., Schwartz J., Shoval G., Ben-Dor D.H., et al. Children’s Depression Inventory (CDI) and the Children’s Depression Rating Scale-Revised (CDRS-R): Reliability of the Hebrew version. Int. J. Adolesc. Med. Health. 2005;17:255–257. doi: 10.1515/IJAMH.2005.17.3.255. [DOI] [PubMed] [Google Scholar]
- 20.Sepede G., Farano M., Santacroce R., Santoro R., Marini S., Mangifesta R., Salerno R.M. Depressive symptoms in adolescence: The role of gender and personality traits. Res. Adv. Psychiatry. 2015;2:9–16. [Google Scholar]
- 21.Nardi B., Francesconi G., Catena-Dell’osso M., Bellantuono C. Adolescent depression: Clinical features and therapeutic strategies. Eur. Rev. Med. Pharmacol. Sci. 2013;17:1546–1551. [PubMed] [Google Scholar]
- 22.Hollingworth S.A., Burgess P.M., Whiteford H.A. Affective and anxiety disorders: Prevalence, treatment and antidepressant medication use. Aust. N. Z. J. Psychiatry. 2010;44:513–519. doi: 10.3109/00048670903555138. [DOI] [PubMed] [Google Scholar]
- 23.Cipriani A., Furukawa T.A., Salanti G., Chaimani A., Atkinson L.Z., Ogawa Y., Egger M. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. Lancet. 2018;16:420–429. doi: 10.1016/S0140-673632802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bachmann C.J., Aagaard L., Burcu M., Glaeske G., Kalverdijk L.J., Petersen I., Hoffmann F. Trends and patterns of antidepressant use in children and adolescents from five western countries, 2005–2012. Eur. Neuropsychopharmacol. 2016;26:411–419. doi: 10.1016/j.euroneuro.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Hamrin V., Iennaco J.D. Evaluation of motivational interviewing to improve psychotropic medication adherence in adolescents. J. Child Adolesc. Psychopharmacol. 2017;27:148–159. doi: 10.1089/cap.2015.0187. [DOI] [PubMed] [Google Scholar]
- 26.Cipriani A., Zhou X., Del Giovane C., Hetrick S.E., Qin B., Whittington C., Cuijpers P. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: A network meta-analysis. Lancet. 2016;388:881–890. doi: 10.1016/S0140-6736(16)30385-3. [DOI] [PubMed] [Google Scholar]
- 27.Geoffroy P.A., Schroder C.M., Reynaud E., Bourgin P. Efficacy of light therapy versus antidepressant drugs, and of the combination versus monotherapy, in major depressive episodes: A systematic review and meta-analysis. Sleep Med Rev. 2019;48:101213. doi: 10.1016/j.smrv.2019.101213. [DOI] [PubMed] [Google Scholar]
- 28.Kirsch I., Deacon B.J., Huedo-Medina T.B., Scoboria A., Moore T.J., Johnson B.T. Initial severity and antidepressant benefits: A meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 2008;5:e45. doi: 10.1371/journal.pmed.0050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cipriani A., Furukawa T.A., Salanti G., Geddes J.R., Higgins J.P., Churchill R., Tansella M. Comparative efficacy and acceptability of 12 new-generation antidepressants: A multiple-treatments meta-analysis. Lancet. 2009;373:746–758. doi: 10.1016/S0140-6736(09)60046-5. [DOI] [PubMed] [Google Scholar]
- 30.Mulder R.T., Joyce P.R., Frampton C.M., Luty S.E. Antidepressant treatment is associated with a reduction in suicidal ideation and suicide attempts. Acta Psychiatr. Scand. 2008;118:116–122. doi: 10.1111/j.1600-0447.2008.01179.x. [DOI] [PubMed] [Google Scholar]
- 31.Price C., Hemmingsson T., Lewis G., Zammit S., Allebeck P. Cannabis and suicide: Longitudinal study. Br. J. Psychiatry. 2009;195:492–497. doi: 10.1192/bjp.bp.109.065227. [DOI] [PubMed] [Google Scholar]
- 32.Ferber S.G., Namdar D., Hen-Shoval D., Eger G., Koltai H., Shoval G., Weller A. The “entourage effect”: Terpenes coupled with cannabinoids for the treatment of mood disorders and anxiety disorders. Curr. Neuropharmacol. 2020;18:87–96. doi: 10.2174/1570159X17666190903103923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hurst T. The Encyclopedia of Women and Crime. Wiley on line; New York, NY, USA: 2019. World drug report; pp. 1–2. [Google Scholar]
- 34.Hall W., Degenhardt L. Adverse health effects of non-medical cannabis use. Lancet. 2010;374:1383–1391. doi: 10.1016/S0140-6736(09)61037-0. [DOI] [PubMed] [Google Scholar]
- 35.Camchong J., Lim K.O., Kumra S. Adverse effects of cannabis on adolescent brain development: A longitudinal study. Cereb. Cortex. 2017;27:1922–1930. doi: 10.1093/cercor/bhw015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonomo Y., Souza J.D.S., Jackson A., Crippa J.A.S., Solowij N. Clinical issues in cannabis use. Br. J. Clin. Pharmacol. 2018;84:2495–2498. doi: 10.1111/bcp.13703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krediet E., Janssen D.G., Heerdink E.R., Egberts T.C., Vermetten E. Experiences with medical cannabis in the treatment of veterans with PTSD: Results from a focus group discussion. Eur. Neuropsychopharmacol. 2020;36:244–254. doi: 10.1016/j.euroneuro.2020.04.009. [DOI] [PubMed] [Google Scholar]
- 38.Lintzeris N., Mills L., Suraev A., Bravo M., Arkell T., Arnold J.C., McGregor I.S. Medical cannabis use in the Australian community following introduction of legal access: The 2018–2019 Online Cross-Sectional Cannabis as Medicine Survey (CAMS-18) Harm. Reduct. J. 2020;17:37. doi: 10.1186/s12954-020-00377-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krishnan V., Nestler E.J. Linking molecules to mood: New insight into the biology of depression. Am. J. Psychiatry. 2010;167:1305–1320. doi: 10.1176/appi.ajp.2009.10030434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Mello Schier A.R., de Oliveira Ribeiro N.P., Coutinho D.S., Machado S., Arias-Carrión O., Crippa J.A., Silva A.C. Antidepressant-like and anxiolytic-like effects of cannabidiol: A chemical compound of Cannabis sativa. CNS Neurol. Disord. Drug Targets. 2014;13:953–960. doi: 10.2174/1871527313666140612114838. [DOI] [PubMed] [Google Scholar]
- 41.Resstel L.B., Tavares R.F., Lisboa S.F., Joca S.R., Correa F., Guimarães F.S. 5-HT1A receptors are involved in the cannabidiol-induced attenuation of behavioural and cardiovascular responses to acute restraint stress in rats. Br. J. Pharmacol. 2009;156:181–188. doi: 10.1111/j.1476-5381.2008.00046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shbiro L., Hen-Shoval D., Hazut N., Rapps K., Dar S., Zalsman G., Shoval G. Effects of cannabidiol in males and females in two different rat models of depression. Physiol. Behav. 2019;201:59–63. doi: 10.1016/j.physbeh.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 43.Shoval G., Shbiro L., Hershkovitz L., Hazut N., Zalsman G., Mechoulam R., Weller A. Pro-hedonic effect of cannabidiol in a rat model for depression. Neuropsychobiology. 2016;73:123–129. doi: 10.1159/000443890. [DOI] [PubMed] [Google Scholar]
- 44.Zanelati T.V., Biojone C., Moreira F.A., Guimaraes F.S., Joca S.R.L. Antidepressant-like effects of cannabidiol in mice: Possible involvement of 5-HT1A receptors. Br. J. Pharmacol. 2010;159:122–128. doi: 10.1111/j.1476-5381.2009.00521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weinberger A.H., Zhu J., Lee J., Anastasiou E., Copeland J., Goodwin R.D. Cannabis use among youth in the United States, 2004–2016: Faster rate of increase among youth with depression. Drug Alcohol Depend. 2020;209:107894. doi: 10.1016/j.drugalcdep.2020.107894. [DOI] [PubMed] [Google Scholar]
- 46.Shoval G., Zalsman G., Sher L., Apter A., Weizman A. Clinical characteristics of inpatient adolescents with severe obsessive-compulsive disorder. Depress. Anxiety. 2006;23:62–70. doi: 10.1002/da.20135. [DOI] [PubMed] [Google Scholar]
- 47.Fournier J.C., DeRubeis R.J., Hollon S.D., Dimidjian S., Amsterdam J.D., Shelton R.C., Fawcett J. Antidepressant drug effects and depression severity: A patient-level meta-analysis. JAMA. 2010;303:47–53. doi: 10.1001/jama.2009.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huijbregts K.M., Hoogendoorn A., Slottje P., van Balkom A.J., Batelaan N.M. Long-term and short-term antidepressant use in general practice: Data from a large cohort in the Netherlands. Psychother. Psychosom. 2017;86:362–369. doi: 10.1159/000480456. [DOI] [PubMed] [Google Scholar]
- 49.Kellner M. Drug treatment of obsessive-compulsive disorder. Dialogues Clin. Neurosci. 2010;12:187. doi: 10.31887/DCNS.2010.12.2/mkellner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krivoy A., Stubbs B., Balicer R.D., Weizman S., Feldman B., Hoshen M., Shoval G. Low adherence to antidepressants is associated with increased mortality following stroke: A large nationally representative cohort study. Eur. Neuropsychopharmacol. 2017;27:970–976. doi: 10.1016/j.euroneuro.2017.08.428. [DOI] [PubMed] [Google Scholar]
- 51.Rubio G., Jiménez-Arriero M.A., Martínez-Gras I., Manzanares J., Palomo T. The effects of topiramate adjunctive treatment added to antidepressants in patients with resistant obsessive-compulsive disorder. J. Clin. Psychopharmacol. 2006;26:341–344. doi: 10.1097/01.jcp.0000220524.44905.9f. [DOI] [PubMed] [Google Scholar]
- 52.De Graaf R., Ten Have M., Van Gool C., Van Dorsselaer S. Prevalence of mental disorders, and trends from 1996 to 2009. Results from NEMESIS-2. Tijdschr. Psychiatr. 2012;54:27. [PubMed] [Google Scholar]
- 53.Öztürk G., Yetkiner H., Özden E. Macroeconomic determinants of antidepressant use. J. Policy. Model. 2020;42:1394–1407. doi: 10.1016/j.jpolmod.2020.06.001. [DOI] [Google Scholar]
- 54.Noordam R., Aarts N., Verhamme K.M., Sturkenboom M.C., Stricker B.H., Visser L.E. Prescription and indication trends of antidepressant drugs in the Netherlands between 1996 and 2012: A dynamic population-based study. Eur. J. Clin. Pharmacol. 2015;71:369–375. doi: 10.1007/s00228-014-1803-x. [DOI] [PubMed] [Google Scholar]
- 55.Pacher P., Kecskemeti V. Trends in the development of new antidepressants. Is there a light at the end of the tunnel? Curr. Med. Chem. 2004;11:925–943. doi: 10.2174/0929867043455594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Insel T.R., Wang P.S. The STAR* D trial: Revealing the need for better treatments. Psychiatr. Serv. 2009;60:1466–1467. doi: 10.1176/ps.2009.60.11.1466. [DOI] [PubMed] [Google Scholar]
- 57.Bschor T., Ising M., Erbe S., Winkelmann P., Ritter D., Uhr M., Lewitzka U. Impact of citalopram on the HPA system. A study of the combined DEX/CRH test in 30 unipolar depressed patients. J. Psychiatr. Res. 2012;46:111–117. doi: 10.1016/j.jpsychires.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 58.McIntyre R.S. When should you move beyond first-line therapy for depression? J. Clin. Psychiatry. 2010;71((Suppl. 1)):16–20. doi: 10.4088/JCP.9104su1c.03. [DOI] [PubMed] [Google Scholar]
- 59.Fava M., McGrath P.J., Sheu W.P., Reboxetine Study Group Switching to reboxetine: An efficacy and safety study in patients with major depressive disorder unresponsive to fluoxetine. J. Clin. Psychopharmacol. 2003;23:365–369. doi: 10.1097/01.jcp.0000085409.08426.4c. [DOI] [PubMed] [Google Scholar]
- 60.De Vries Y.A., de Jonge P., Kalverdijk L., Bos J.H., Schuiling-Veninga C.C., Hak E. Poor guideline adherence in the initiation of antidepressant treatment in children and adolescents in the Netherlands: Choice of antidepressant and dose. Eur. Child Adolesc. Psychiatry. 2016;25:1161–1170. doi: 10.1007/s00787-016-0836-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rush A.J., Fava M., Wisniewski S.R., Lavori P.W., Trivedi M.H., Sackeim H.A., Kupfer D.J. Sequenced treatment alternatives to relieve depression (STAR* D): Rationale and design. Control. Clin. Trials. 2004;25:119–142. doi: 10.1016/S0197-2456(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 62.Rush A.J., Trivedi M.H., Wisniewski S.R., Stewart J.W., Nierenberg A.A., Thase M.E., Shores-Wilson K. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N. Engl. J. Med. 2006;354:1231–1242. doi: 10.1056/NEJMoa052963. [DOI] [PubMed] [Google Scholar]
- 63.Berlanga C., Flores-Ramos M. Different gender response to serotonergic and noradrenergic antidepressants. A comparative study of the efficacy of citalopram and reboxetine. J. Affect. Disord. 2006;95:119–123. doi: 10.1016/j.jad.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 64.Bigos K.L., Pollock B.G., Stankevich B.A., Bies R.R. Sex differences in the pharmacokinetics and pharmacodynamics of antidepressants: An updated review. Gend. Med. 2009;6:522–543. doi: 10.1016/j.genm.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 65.Dalla C., Pitychoutis P.M., Kokras N., Papadopoulou-Daifoti Z. Sex differences in animal models of depression and antidepressant response. Basic Clin. Pharmacol. Toxicol. 2010;106:226–233. doi: 10.1111/j.1742-7843.2009.00516.x. [DOI] [PubMed] [Google Scholar]
- 66.Fernandez-Guasti A., Fiedler J.L., Herrera L., Handa R.J. Sex, stress, and mood disorders: At the intersection of adrenal and gonadal hormones. Horm. Metab. Res. 2012;44:607. doi: 10.1055/s-0032-1312592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.LeGates T.A., Kvarta M.D., Thompson S.M. Sex differences in antidepressant efficacy. Neuropsychopharmacology. 2019;44:140–154. doi: 10.1038/s41386-018-0156-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bylund D.B., Reed A.L. Childhood and adolescent depression: Why do children and adults respond differently to antidepressant drugs? Neurochem. Int. 2007;51:246–253. doi: 10.1016/j.neuint.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Joyce P.R., Mulder R.T., Luty S.E., McKenzie J.M., Miller A.L., Rogers G.R., Kennedy M.A. Age-dependent antidepressant pharmacogenomics: Polymorphisms of the serotonin transporter and G protein β3 subunit as predictors of response to fluoxetine and nortriptyline. Int. J. Neuropsychopharmacol. 2003;6:339–346. doi: 10.1017/S1461145703003663. [DOI] [PubMed] [Google Scholar]
- 70.Mulder R.T., Watkins W.G., Joyce P.R., Luty S.E. Age may affect response to antidepressants with serotonergic and noradrenergic actions. J. Affect. Disord. 2003;76:143–149. doi: 10.1016/S0165-0327(02)00083-6. [DOI] [PubMed] [Google Scholar]
- 71.Tedeschini E., Levkovitz Y., Iovieno N., Ameral V.E., Nelson J.C., Papakostas G.I. Efficacy of antidepressants for late-life depression: A meta-analysis and meta-regression of placebo-controlled randomized trials. J. Clin. Psychiatry. 2011;72:1660–1668. doi: 10.4088/JCP.10r06531. [DOI] [PubMed] [Google Scholar]
- 72.Bauer M., Severus E., Koehler S., Whybrow P.C., Angst J., Moeller H.J., Wfsbp Task Force on Treatment Guidelines for Unipolar Depressive Disorders World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders. part 2: Maintenance treatment of major depressive disorder-update 2015. World J. Biol. Psychiatry. 2015;16:76–95. doi: 10.3109/15622975.2014.1001786. [DOI] [PubMed] [Google Scholar]
- 73.Burchi E., Hollander E., Pallanti S. From treatment response to recovery: A realistic goal in OCD. Int. J. Neuropsychopharmacol. 2018;21:1007–1013. doi: 10.1093/ijnp/pyy079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kjernisted K.D., Bleau P. Long-term goals in the management of acute and chronic anxiety disorders. Can. J. Psychiatry. 2004;49:51–63. [PubMed] [Google Scholar]
- 75.Lam R.W., Kennedy S.H. Evidence-based strategies for achieving and sustaining full remission in depression: Focus on metaanalyses. Can. J. Psychiatry. 2004;49:17–26. [PubMed] [Google Scholar]
- 76.Cheung A.H., Emslie G.J., Mayes T.L. Review of the efficacy and safety of antidepressants in youth depression. J. Child Psychol. Psychiatry. 2005;46:735–754. doi: 10.1111/j.1469-7610.2005.01467.x. [DOI] [PubMed] [Google Scholar]
- 77.Ginsburg G.S., Kendall P.C., Sakolsky D., Compton S.N., Piacentini J., Albano A.M., March J. Remission after acute treatment in children and adolescents with anxiety disorders: Findings from the CAMS. J. Consult. Clin. Psychol. 2011;79:806. doi: 10.1037/a0025933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wagner K.D., Cook E.H., Chung H., Messig M. Remission status after long-term sertraline treatment of pediatric obsessive-compulsive disorder. J. Child Adolesc. Psychopharmacol. 2003;13((Suppl. 1)):53–60. doi: 10.1089/104454603322126340. [DOI] [PubMed] [Google Scholar]
- 79.Whiskey E., Taylor D. A review of the adverse effects and safety of noradrenergic antidepressants. J. Psychopharmacol. 2013;27:732–739. doi: 10.1177/0269881113492027. [DOI] [PubMed] [Google Scholar]
- 80.Nahshoni E., Spitzer S., Berant M., Shoval G., Zalsman G., Weizman A. QT interval and dispersion in very young children treated with antipsychotic drugs: A retrospective chart review. J. Child Adolesc. Psychopharmacol. 2007;17:187–194. doi: 10.1089/cap.2007.0061. [DOI] [PubMed] [Google Scholar]
- 81.Hu X.H., Bull S.A., Hunkeler E.M., Ming E., Lee J.Y., Fireman B., Markson L.E. Incidence and duration of side effects and those rated as bothersome with selective serotonin reuptake inhibitor treatment for depression: Patient report versus physician estimate. J. Clin. Psychiatry. 2004;65:959–965. doi: 10.4088/JCP.v65n0712. [DOI] [PubMed] [Google Scholar]
- 82.Kostev K., Rex J., Eith T., Heilmaier C. Which adverse effects influence the dropout rate in selective serotonin reuptake inhibitor (SSRI) treatment? Results for 50,824 patients. Ger. Med. Sci. 2014;12:Doc15. doi: 10.3205/000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Burra T.A., Chen E., McIntyre R.S., Grace S.L., Blackmore E.R., Stewart D.E. Predictors of self-reported antidepressant adherence. Behav. Med. 2007;32:127–134. doi: 10.3200/BMED.32.4.127-134. [DOI] [PubMed] [Google Scholar]
- 84.De las Cuevas C., Peñate W., Sanz E.J. Risk factors for non-adherence to antidepressant treatment in patients with mood disorders. Eur. J. Clin. Pharmacol. 2014;70:89–98. doi: 10.1007/s00228-013-1582-9. [DOI] [PubMed] [Google Scholar]
- 85.Milan R., Vasiliadis H.M. The association between side effects and adherence to antidepressants among primary care community-dwelling older adults. Aging Ment. Health. 2020;24:1229–1236. doi: 10.1080/13607863.2019.1594165. [DOI] [PubMed] [Google Scholar]
- 86.Aljumah K., Hassali A.A., AlQhatani S. Examining the relationship between adherence and satisfaction with antidepressant treatment. Neuropsychiatr. Dis. Treat. 2014;10:1433–1438. doi: 10.2147/NDT.S67008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Geddes J.R., Carney S.M., Davies C., Furukawa T.A., Kupfer D.J., Frank E., Goodwin G.M. Relapse prevention with antidepressant drug treatment in depressive disorders: A systematic review. Lancet. 2003;361:653–661. doi: 10.1016/S0140-6736(03)12599-8. [DOI] [PubMed] [Google Scholar]
- 88.Sawada N., Uchida H., Suzuki T., Watanabe K., Kikuchi T., Handa T., Kashima H. Persistence and compliance to antidepressant treatment in patients with depression: A chart review. BMC Psychiatry. 2009;9:38. doi: 10.1186/1471-244X-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu C.H., Erickson S.R., Piette J.D., Balkrishnan R. The association of race, comorbid anxiety, and antidepressant adherence among Medicaid enrollees with major depressive disorder. Res. Soc. Adm. Pharm. 2012;8:193–205. doi: 10.1016/j.sapharm.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 90.Sood N., Treglia M., Obenchain R.L., Dulisse B., Melfi C.A., Croghan T.W. Determinants of antidepressant treatment outcome. Am. J. Manag. Care. 2000;6:1327–1339. [PubMed] [Google Scholar]
- 91.Posternak M.A., Baer L., Nierenberg A.A., Fava M. Response rates to fluoxetine in subjects who initially show no improvement. J. Clin. Psychiatry. 2011;72:949–954. doi: 10.4088/JCP.10m06098. [DOI] [PubMed] [Google Scholar]
- 92.Thase M.E. Evaluating antidepressant therapies: Remission as the optimal outcome. J. Clin. Psychiatry. 2003;64((Suppl. 13)):18–25. [PubMed] [Google Scholar]
- 93.Birmaher B., Brent D., AACAP Work Group on Quality Issues Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2007;46:1503–1526. doi: 10.1097/chi.0b013e318145ae1c. [DOI] [PubMed] [Google Scholar]
- 94.Krivoy A., Balicer R.D., Feldman B., Hoshen M., Zalsman G., Weizman A., Shoval G. Adherence to antidepressants is associated with lower mortality: A 4-year population-based cohort study. J. Clin. Psychiatry. 2016;77:566–572. doi: 10.4088/JCP.14m09531. [DOI] [PubMed] [Google Scholar]
- 95.Krivoy A., Balicer R.D., Feldman B., Hoshen M., Zalsman G., Weizman A., Shoval G. Adherence to antidepressant therapy and mortality rates in ischaemic heart disease: Cohort study. Br. J. Psychiatry. 2015;206:297–301. doi: 10.1192/bjp.bp.114.155820. [DOI] [PubMed] [Google Scholar]
- 96.Shoval G., Stubbs B., Balicer R.D., Feldman B., Hoshen M., Zalsman G., Krivoy A. Low adherence to antidepressants is associated with increased mortality in Parkinson disease patients. Parkinsonism Relat. Disord. 2017;43:92–96. doi: 10.1016/j.parkreldis.2017.07.032. [DOI] [PubMed] [Google Scholar]
- 97.Shoval G., Balicer R.D., Feldman B., Hoshen M., Eger G., Weizman A., Krivoy A. Adherence to antidepressant medications is associated with reduced premature mortality in patients with cancer: A nationwide cohort study. Depress. Anxiety. 2019;36:921–929. doi: 10.1002/da.22938. [DOI] [PubMed] [Google Scholar]
- 98.Bambauer K.Z., Adams A.S., Zhang F., Minkoff N., Grande A., Weisblatt R., Ross-Degnan D. Physician alerts to increase antidepressant adherence: Fax or fiction? Arch. Intern. Med. 2006;166:498–504. doi: 10.1001/archinte.166.5.498. [DOI] [PubMed] [Google Scholar]
- 99.Hunot V.M., Horne R., Leese M.N., Churchill R.C. A cohort study of adherence to antidepressants in primary care: The influence of antidepressant concerns and treatment preferences. Prim. Care Companion. J. Clin. Psychiatry. 2007;9:91. doi: 10.4088/pcc.v09n0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Olfson M., Marcus S.C. National patterns in antidepressant medication treatment. Arch. Gen. Psychiatry. 2009;66:848–856. doi: 10.1001/archgenpsychiatry.2009.81. [DOI] [PubMed] [Google Scholar]
- 101.Adhikari K., Patten S.B., Lee S., Metcalfe A. Adherence to and persistence with antidepressant medication during pregnancy: Does it differ by the class of antidepressant medication prescribed? Can J. Psychiatry. 2019;64:199–208. doi: 10.1177/0706743718802809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fontanella C.A., Bridge J.A., Marcus S.C., Campo J.V. Factors associated with antidepressant adherence for Medicaid-enrolled children and adolescents. Ann. Pharmacother. 2011;45:898–909. doi: 10.1345/aph.1Q020. [DOI] [PubMed] [Google Scholar]
- 103.Rossom R.C., Shortreed S., Coleman K.J., Beck A., Waitzfelder B.E., Stewart C., Simon G.E. Antidepressant adherence across diverse populations and healthcare settings. Depress. Anxiety. 2016;33:765–774. doi: 10.1002/da.22532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Krivoy A., Balicer R.D., Feldman B., Hoshen M., Zalsman G., Weizman A., Shoval G. The impact of age and gender on adherence to antidepressants: A 4-year population-based cohort study. Psychopharmacology. 2015;232:3385–3390. doi: 10.1007/s00213-015-3988-9. [DOI] [PubMed] [Google Scholar]
- 105.Lu H.C., Mackie K. Review of the Endocannabinoid System. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2021;6:607–615. doi: 10.1016/j.bpsc.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Di Marzo V., Melck D., Bisogno T., De Petrocellis L. Endocannabinoids: Endogenous cannabinoid receptor ligands with neuromodulatory action. Trends Neurosci. 1998;21:521–528. doi: 10.1016/S0166-2236(98)01283-1. [DOI] [PubMed] [Google Scholar]
- 107.Shalit N., Barzilay R., Shoval G., Shlosberg D., Mor N., Zweigenhaft N., Krivoy A. Characteristics of synthetic cannabinoid and cannabis users admitted to a psychiatric hospital: A comparative study. J. Clin. Psychiatry. 2016;77:989–995. doi: 10.4088/JCP.15m09938. [DOI] [PubMed] [Google Scholar]
- 108.Acharya N., Penukonda S., Shcheglova T., Hagymasi A.T., Basu S., Srivastava P.K. Endocannabinoid system acts as a regulator of immune homeostasis in the gut. Proc. Natl. Acad. Sci. USA. 2017;114:5005–5010. doi: 10.1073/pnas.1612177114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Achterberg E.M., van Swieten M.M., Driel N.V., Trezza V., Vanderschuren L.J. Dissociating the role of endocannabinoids in the pleasurable and motivational properties of social play behaviour in rats. Pharmacol. Res. 2016;110:151–158. doi: 10.1016/j.phrs.2016.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hanlon E.C., Tasali E., Leproult R., Stuhr K.L., Doncheck E., De Wit H., Van Cauter E. Sleep restriction enhances the daily rhythm of circulating levels of endocannabinoid 2-arachidonoylglycerol. Sleep. 2016;39:653–664. doi: 10.5665/sleep.5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Morera-Herreras T., Miguelez C., Aristieta A., Torrecilla M., Ruiz-Ortega J.A., Ugedo L. Cannabinoids and motor control of the basal ganglia: Therapeutic potential in movement disorders. In: Meccariello R., Chianese R., editors. Cannabinoids in Health and Disease. InTech; Rijeka, Croatia: 2016. pp. 59–92. [Google Scholar]
- 112.Kruk-Slomka M., Dzik A., Budzynska B., Biala G. Endocannabinoid system: The direct and indirect involvement in the memory and learning processes—A short review. Mol. Neurobiol. 2017;54:8332–8347. doi: 10.1007/s12035-016-0313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sierra S., Luquin N., Navarro-Otano J. The endocannabinoid system in cardiovascular function: Novel insights and clinical implications. Clin. Auton. Res. 2018;28:35–52. doi: 10.1007/s10286-017-0488-5. [DOI] [PubMed] [Google Scholar]
- 114.Soria-Gómez E., Bellocchio L., Reguero L., Lepousez G., Martin C., Bendahmane M., Wiesner T. The endocannabinoid system controls food intake via olfactory processes. Nat. Neurosci. 2014;17:407–415. doi: 10.1038/nn.3647. [DOI] [PubMed] [Google Scholar]
- 115.Gertsch J., Pertwee R.G., Di Marzo V. Phytocannabinoids beyond the Cannabis plant–do they exist? Br. J. Pharmacol. 2010;160:523–529. doi: 10.1111/j.1476-5381.2010.00745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gaoni Y., Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish. J. Am. Chem. Soc. 1964;86:1646–1647. doi: 10.1021/ja01062a046. [DOI] [Google Scholar]
- 117.Hanuš L.O., Meyer S.M., Muñoz E., Taglialatela-Scafati O., Appendino G. Phytocannabinoids: A unified critical inventory. Nat. Prod. Rep. 2016;33:1357–1392. doi: 10.1039/C6NP00074F. [DOI] [PubMed] [Google Scholar]
- 118.Khan B.A., Warner P., Wang H. Antibacterial properties of hemp and other natural fibre plants: A review. BioResources. 2014;9:3642–3659. doi: 10.15376/biores.9.2.3642-3659. [DOI] [Google Scholar]
- 119.Andre C.M., Hausman J.F., Guerriero G. Cannabis sativa: The plant of the thousand and one molecules. Front. Plant Sci. 2016;7:19. doi: 10.3389/fpls.2016.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ligresti A., De Petrocellis L., Di Marzo V. From phytocannabinoids to cannabinoid receptors and endocannabinoids: Pleiotropic physiological and pathological roles through complex pharmacology. Physiol. Rev. 2016;96:1593–1659. doi: 10.1152/physrev.00002.2016. [DOI] [PubMed] [Google Scholar]
- 121.Batalla A., Crippa J.A., Busatto G.F., Guimaraes F.S., Zuardi A.W., Valverde O., Martin-Santos R. Neuroimaging studies of acute effects of THC and CBD in humans and animals: A systematic review. Curr. Pharm. Des. 2014;20:2168–2185. doi: 10.2174/13816128113199990432. [DOI] [PubMed] [Google Scholar]
- 122.Bhattacharyya S., Morrison P.D., Fusar-Poli P., Martin-Santos R., Borgwardt S., Winton-Brown T., Mehta M.A. Opposite effects of Δ-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35:764–774. doi: 10.1038/npp.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Colizzi M., Bhattacharyya S. Does cannabis composition matter? Differential effects of delta-9-tetrahydrocannabinol and cannabidiol on human cognition. Curr. Addict. Rep. 2017;4:62–74. doi: 10.1007/s40429-017-0142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dewey W.L. Cannabinoid pharmacology. Pharmacol. Rev. 1986;38:151–178. doi: 10.1016/0378-8741(87)90061-4. [DOI] [PubMed] [Google Scholar]
- 125.Guimarães F.S., Chiaretti T.M., Graeff F.G., Zuardi A.W. Antianxiety effect of cannabidiol in the elevated plus-maze. Psychopharmacology. 1990;100:558–559. doi: 10.1007/BF02244012. [DOI] [PubMed] [Google Scholar]
- 126.Luginbuhl A.M. Industrial hemp (Cannabis sativa L.): The geography of a controversial plant. Calif. Geogr. 2001;41:1–14. [Google Scholar]
- 127.Mead A. The legal status of cannabis (marijuana) and cannabidiol (CBD) under US law. Epilepsy Behav. 2017;70:288–291. doi: 10.1016/j.yebeh.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 128.Jones R.T. Tetrahydrocannabinol and the marijuana-induced social “high,” or the effects of the mind on marijuana. Ann. N. Y. Acad. Sci. 1971;191:155–165. doi: 10.1111/j.1749-6632.1971.tb13995.x. [DOI] [Google Scholar]
- 129.Murray R.M., Hall W. Will legalization and commercialization of cannabis use increase the incidence and prevalence of psychosis? JAMA Psychiatry. 2020;77:777–778. doi: 10.1001/jamapsychiatry.2020.0339. [DOI] [PubMed] [Google Scholar]
- 130.DeFleur L.B., Garrett G.R. Dimensions of marijuana usage in a land-grant university. J. Couns. Psychol. 1970;17:468. doi: 10.1037/h0029858. [DOI] [Google Scholar]
- 131.Hall W. What has research over the past two decades revealed about the adverse health effects of recreational cannabis use? Addiction. 2015;110:19–35. doi: 10.1111/add.12703. [DOI] [PubMed] [Google Scholar]
- 132.Hall W., Degenhardt L. Prevalence and correlates of cannabis use in developed and developing countries. Curr. Opin. Psychiatry. 2007;20:393–397. doi: 10.1097/YCO.0b013e32812144cc. [DOI] [PubMed] [Google Scholar]
- 133.Woodruff S.I., Shillington A.M. Sociodemographic and drug use severity differences between medical marijuana users and non-medical users visiting the emergency department. Am. J. Addict. 2016;25:385–391. doi: 10.1111/ajad.12401. [DOI] [PubMed] [Google Scholar]
- 134.Patton G.C., Coffey C., Carlin J.B., Degenhardt L., Lynskey M., Hall W. Cannabis use and mental health in young people: Cohort study. BMJ. 2002;325:1195–1198. doi: 10.1136/bmj.325.7374.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Abush H., Akirav I. Cannabinoids ameliorate impairments induced by chronic stress to synaptic plasticity and short-term memory. Neuropsychopharmacology. 2013;38:1521–1534. doi: 10.1038/npp.2013.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fattore L., Fadda P., Spano M.S., Pistis M., Fratta W. Neurobiological mechanisms of cannabinoid addiction. Mol. Cell. Endocrinol. 2008;286:S97–S107. doi: 10.1016/j.mce.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 137.Ganon-Elazar E., Akirav I. Cannabinoids prevent the development of behavioral and endocrine alterations in a rat model of intense stress. Neuropsychopharmacology. 2012;37:456–466. doi: 10.1038/npp.2011.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Marsicano G., Wotjak C.T., Azad S.C., Bisogno T., Rammes G., Cascio M.G., Di Marzo V. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- 139.Steiner M.A., Wotjak C.T. Role of the endocannabinoid system in regulation of the hypothalamic-pituitary-adrenocortical axis. Prog. Brain Res. 2008;170:397–432. doi: 10.1016/S0079-612300433-0. [DOI] [PubMed] [Google Scholar]
- 140.Hall W. Cannabis use and psychosis. Drug. Alcohol Rev. 1998;17:433–444. doi: 10.1080/09595239800187271. [DOI] [PubMed] [Google Scholar]
- 141.Linszen D.H., Dingemans P.M., Lenior M.E. Cannabis abuse and the course of recent-onset schizophrenic disorders. Arch. Gen. Psychiatry. 1994;51:273–279. doi: 10.1001/archpsyc.1994.03950040017002. [DOI] [PubMed] [Google Scholar]
- 142.Machielsen M., van der Sluis S., de Haan L. Cannabis use in patients with a first psychotic episode and subjects at ultra high risk of psychosis: Impact on psychotic-and pre-psychotic symptoms. Aust. N. Z. J. Psychiatry. 2010;44:721–728. doi: 10.3109/00048671003689710. [DOI] [PubMed] [Google Scholar]
- 143.Schoeler T., Petros N., Di Forti M., Pingault J.B., Klamerus E., Foglia E., Bhattacharyya S. Association between continued cannabis use and risk of relapse in first-episode psychosis: A quasi-experimental investigation within an observational study. JAMA Psychiatry. 2016;73:1173–1179. doi: 10.1001/jamapsychiatry.2016.2427. [DOI] [PubMed] [Google Scholar]
- 144.Buckner J.D., Crosby R.D., Wonderlich S.A., Schmidt N.B. Social anxiety and cannabis use: An analysis from ecological momentary assessment. J. Anxiety Disord. 2012;26:297–304. doi: 10.1016/j.janxdis.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cheung J.T., Mann R.E., Ialomiteanu A., Stoduto G., Chan V., Ala-Leppilampi K., Rehm J. Anxiety and mood disorders and cannabis use. Am. J. Drug Alcohol Abuse. 2010;36:118–122. doi: 10.3109/00952991003713784. [DOI] [PubMed] [Google Scholar]
- 146.Kedzior K.K., Laeber L.T. A positive association between anxiety disorders and cannabis use or cannabis use disorders in the general population-a meta-analysis of 31 studies. BMC Psychiatry. 2014;14:136. doi: 10.1186/1471-244X-14-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gobbi G., Atkin T., Zytynski T., Wang S., Askari S., Boruff J., Mayo N. Association of cannabis use in adolescence and risk of depression, anxiety, and suicidality in young adulthood: A systematic review and meta-analysis. JAMA Psychiatry. 2019;76:426–434. doi: 10.1001/jamapsychiatry.2018.4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shalit N., Shoval G., Shlosberg D., Feingold D., Lev-Ran S. The association between cannabis use and suicidality among men and women: A population-based longitudinal study. J. Affect. Disord. 2016;205:216–224. doi: 10.1016/j.jad.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 149.Duperrouzel J., Hawes S.W., Lopez-Quintero C., Pacheco-Colón I., Comer J., Gonzalez R. The association between adolescent cannabis use and anxiety: A parallel process analysis. Addict. Behav. 2018;78:107–113. doi: 10.1016/j.addbeh.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Abel E.L. Marihuana: The First Twelve Thousand Years. Springer Science & Business Media; Berlin/Heidelberg, Germany: 2013. [Google Scholar]
- 151.Larsen C., Shahinas J. Dosage, efficacy and safety of cannabidiol administration in adults: A systematic review of human trials. J. Clin. Med. Res. 2020;12:129. doi: 10.14740/jocmr4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mannucci C., Navarra M., Calapai F., Spagnolo E.V., Busardò F.P., Cas R.D., Calapai G. Neurological aspects of medical use of cannabidiol. CNS Neurol. Disord. Drug Targets. 2017;16:541–553. doi: 10.2174/1871527316666170413114210. [DOI] [PubMed] [Google Scholar]
- 153.Vuolo F., Petronilho F., Sonai B., Ritter C., Hallak J.E., Zuardi A.W., Dal-Pizzol F. Evaluation of serum cytokines levels and the role of cannabidiol treatment in animal model of asthma. Mediat. Inflamm. 2015;2015:538670. doi: 10.1155/2015/538670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Morgan C.J.A., Gardener C., Schafer G., Swan S., Demarchi C., Freeman T.P., Wingham G. Sub-chronic impact of cannabinoids in street cannabis on cognition, psychotic-like symptoms and psychological well-being. Psychol. Med. 2012;42:391. doi: 10.1017/S0033291711001322. [DOI] [PubMed] [Google Scholar]
- 155.Izzo A.A., Borrelli F., Capasso R., Di Marzo V., Mechoulam R. Non-psychotropic plant cannabinoids: New therapeutic opportunities from an ancient herb. Trends Pharmacol. Sci. 2009;30:515–527. doi: 10.1016/j.tips.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 156.Pertwee R.G. Pharmacological and therapeutic targets for Δ 9 tetrahydrocannabinol and cannabidiol. Euphytica. 2014;140:73–82. doi: 10.1007/s10681-004-4756-9. [DOI] [Google Scholar]
- 157.Zuardi A.W. Cannabidiol: From an inactive cannabinoid to a drug with wide spectrum of action. Braz. J. Psychiatry. 2008;30:271–280. doi: 10.1590/S1516-44462008000300015. [DOI] [PubMed] [Google Scholar]
- 158.Casarotto P.C., Gomes F.V., Resstel L.B., Guimarães F.S. Cannabidiol inhibitory effect on marble-burying behaviour: Involvement of CB1 receptors. Behav. Pharmacol. 2010;21:353–358. doi: 10.1097/FBP.0b013e32833b33c5. [DOI] [PubMed] [Google Scholar]
- 159.Deiana S., Watanabe A., Yamasaki Y., Amada N., Arthur M., Fleming S., Platt B. Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Δ 9-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive–compulsive behaviour. Psychopharmacology. 2012;219:859–873. doi: 10.1007/s00213-011-2415-0. [DOI] [PubMed] [Google Scholar]
- 160.Nardo M., Casarotto P.C., Gomes F.V., Guimaraes F.S. Cannabidiol reverses the mCPP-induced increase in marble-burying behavior. Fundam. Clin. Pharmacol. 2014;28:544–550. doi: 10.1111/fcp.12051. [DOI] [PubMed] [Google Scholar]
- 161.Thomas A., Burant A., Bui N., Graham D., Yuva-Paylor L.A., Paylor R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology. 2009;204:361–373. doi: 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lawn W., Freeman T.P., Pope R.A., Joye A., Harvey L., Hindocha C., Das R.K. Acute and chronic effects of cannabinoids on effort-related decision-making and reward learning: An evaluation of the cannabis ‘amotivational’ hypotheses. Psychopharmacology. 2016;233:3537–3552. doi: 10.1007/s00213-016-4383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Selvarajah D., Gandhi R., Emery C.J., Tesfaye S. Randomized placebo-controlled double-blind clinical trial of cannabis-based medicinal product (Sativex) in painful diabetic neuropathy: Depression is a major confounding factor. Diabetes Care. 2010;33:128–130. doi: 10.2337/dc09-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.De Gregorio D., McLaughlin R.J., Posa L., Ochoa-Sanchez R., Enns J., Lopez-Canul M., Gobbi G. Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety-like behavior in a model of neuropathic pain. Pain. 2019;160:136. doi: 10.1097/j.pain.0000000000001386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hen-Shoval D., Amar S., Shbiro L., Smoum R., Haj C.G., Mechoulam R., Shoval G. Acute oral cannabidiolic acid methyl ester reduces depression-like behavior in two genetic animal models of depression. Behav. Brain Res. 2018;351:1–3. doi: 10.1016/j.bbr.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 166.Pertwee R.G., Rock E.M., Guenther K., Limebeer C.L., Stevenson L.A., Haj C., Mechoulam R. Cannabidiolic acid methyl ester, a stable synthetic analogue of cannabidiolic acid, can produce 5-HT1A receptor-mediated suppression of nausea and anxiety in rats. Br. J. Pharmacol. 2018;175:100–112. doi: 10.1111/bph.14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rock E.M., Limebeer C.L., Petrie G.N., Williams L.A., Mechoulam R., Parker L.A. Effect of prior foot shock stress and Δ 9-tetrahydrocannabinol, cannabidiolic acid, and cannabidiol on anxiety-like responding in the light-dark emergence test in rats. Psychopharmacology. 2017;234:2207–2217. doi: 10.1007/s00213-017-4626-5. [DOI] [PubMed] [Google Scholar]
- 168.Ware M.A., Adams H., Guy G.W. The medicinal use of cannabis in the UK: Results of a nationwide survey. Int. J. Clin. Pract. 2005;59:291–295. doi: 10.1111/j.1742-1241.2004.00271.x. [DOI] [PubMed] [Google Scholar]
- 169.Binder E.B., Künzel H.E., Nickel T., Kern N., Pfennig A., Majer M., Holsboer F. HPA-axis regulation at in-patient admission is associated with antidepressant therapy outcome in male but not in female depressed patients. Psychoneuroendocrinology. 2009;34:99–109. doi: 10.1016/j.psyneuen.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 170.De Kloet E.R., DeRijk R.H., Meijer O.C. Therapy insight: Is there an imbalanced response of mineralocorticoid and glucocorticoid receptors in depression? Nat. Clin. Pract. Endocrinol. Metab. 2007;3:168–179. doi: 10.1038/ncpendmet0403. [DOI] [PubMed] [Google Scholar]
- 171.Lam V.Y., Raineki C., Wang L.Y., Chiu M., Lee G., Ellis L., Weinberg J. Role of corticosterone in anxiety-and depressive-like behavior and HPA regulation following prenatal alcohol exposure. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2019;90:1–15. doi: 10.1016/j.pnpbp.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Morilak D.A., Frazer A. Antidepressants and brain monoaminergic systems: A dimensional approach to understanding their behavioural effects in depression and anxiety disorders. Int. J. Neuropsychopharmacol. 2004;7:193–218. doi: 10.1017/S1461145704004080. [DOI] [PubMed] [Google Scholar]
- 173.Cota D. The role of the endocannabinoid system in the regulation of hypothalamic-pituitary-adrenal axis activity. J. Neuroendocrinol. 2008;20:35–38. doi: 10.1111/j.1365-2826.2008.01673.x. [DOI] [PubMed] [Google Scholar]
- 174.Gobbi G., Bambico F.R., Mangieri R., Bortolato M., Campolongo P., Solinas M., Tontini A. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc. Natl. Acad. Sci. USA. 2005;102:18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hill M.N., Gorzalka B.B. Pharmacological enhancement of cannabinoid CB1 receptor activity elicits an antidepressant-like response in the rat forced swim test. Eur. Neuropsychopharmacol. 2005;15:593–599. doi: 10.1016/j.euroneuro.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 176.Witkin J.M., Tzavara E.T., Nomikos G.G. A role for cannabinoid CB1 receptors in mood and anxiety disorders. Behav. Pharmacol. 2005;16:315–331. doi: 10.1097/00008877-200509000-00005. [DOI] [PubMed] [Google Scholar]
- 177.Hill M.N., Gorzalka B.B. The endocannabinoid system and the treatment of mood and anxiety disorders. CNS Neurol. Disord. Drug Targets. 2009;8:451–458. doi: 10.2174/187152709789824624. [DOI] [PubMed] [Google Scholar]
- 178.Viveros M.P., Marco E.M., File S.E. Endocannabinoid system and stress and anxiety responses. Pharmacol. Biochem. Behav. 2005;81:331–342. doi: 10.1016/j.pbb.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 179.Lev-Ran S., Roerecke M., Le Foll B., George T.P., McKenzie K., Rehm J. The association between cannabis use and depression: A systematic review and meta-analysis of longitudinal studies. Psychol. Med. 2014;44:797. doi: 10.1017/S0033291713001438. [DOI] [PubMed] [Google Scholar]
- 180.Feingold D., Weiser M., Rehm J., Lev-Ran S. The association between cannabis use and mood disorders: A longitudinal study. J. Affect. Disord. 2015;172:211–218. doi: 10.1016/j.jad.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 181.Ranganathan M., Braley G., Pittman B., Cooper T., Perry E., Krystal J., D’Souza D.C. The effects of cannabinoids on serum cortisol and prolactin in humans. Psychopharmacology. 2009;203:737. doi: 10.1007/s00213-008-1422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Vaughn S.E., Strawn J.R., Poweleit E.A., Sarangdhar M., Ramsey L.B. The impact of marijuana on antidepressant treatment in adolescents: Clinical and pharmacologic considerations. J. Pers. Med. 2021;11:615. doi: 10.3390/jpm11070615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Dinan T.G. Serotonin and the regulation of hypothalamic-pituitary-adrenal axis function. Life Sci. 1996;58:1683–1694. doi: 10.1016/0024-3205(96)00066-5. [DOI] [PubMed] [Google Scholar]
- 184.Stahl S.M. Mechanism of action of serotonin selective reuptake inhibitors: Serotonin receptors and pathways mediate therapeutic effects and side effects. J. Affect. Disord. 1998;51:215–235. doi: 10.1016/S0165-0327(98)00221-3. [DOI] [PubMed] [Google Scholar]
- 185.Leonard B.E. The HPA and immune axes in stress: The involvement of the serotonergic system. Eur. Psychiatry. 2005;20:S302–S306. doi: 10.1016/S0924-9338(05)80180-4. [DOI] [PubMed] [Google Scholar]
- 186.Stahl S. 5HT1A receptors and pharmacotherapy. Is serotonin receptor down-regulation linked to the mechanism of action of antidepressant drugs? Psychopharmacol. Bull. 1994;30:39–43. [PubMed] [Google Scholar]
- 187.Blier P. Pharmacology of rapid-onset antidepressant treatment strategies. J. Clin. Psychiatry. 2001;62:12. [PubMed] [Google Scholar]
- 188.Cryan J.F., Leonard B.E. 5-HT1A and beyond: The role of serotonin and its receptors in depression and the antidepressant response. Hum. Psychopharmacol. 2000;15:113–135. doi: 10.1002/(SICI)1099-1077(200003)15:2<113::AID-HUP150>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 189.Papakostas G.I., Chuzi S.E., Sousa J.L., Fava M. 5HT1A-mediated stimulation of cortisol release in major depression: Use of non-invasive cortisol measurements to predict clinical response. Eur. Arch. Psychiatry Clin. Neurosci. 2010;260:175–180. doi: 10.1007/s00406-009-0035-z. [DOI] [PubMed] [Google Scholar]
- 190.Braida D., Limonta V., Malabarba L., Zani A., Sala M. 5-HT1A receptors are involved in the anxiolytic effect of Δ9-tetrahydrocannabinol and AM 404, the anandamide transport inhibitor, in Sprague–Dawley rats. Eur. J. Pharmacol. 2007;555:156–163. doi: 10.1016/j.ejphar.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 191.Malone D.T., Taylor D.A. Involvement of somatodendritic 5-HT1A receptors in Δ9-tetrahydrocannabinol-induced hypothermia in the rat. Pharmacol. Biochem. Behav. 2001;69:595–601. doi: 10.1016/S0091-3057(01)00567-6. [DOI] [PubMed] [Google Scholar]
- 192.Campos A.C., Guimarães F.S. Involvement of 5HT1A receptors in the anxiolytic-like effects of cannabidiol injected into the dorsolateral periaqueductal gray of rats. Psychopharmacology. 2008;199:223. doi: 10.1007/s00213-008-1168-x. [DOI] [PubMed] [Google Scholar]
- 193.Hayakawa K., Mishima K., Nozako M., Ogata A., Hazekawa M., Liu A.X., Iwasaki K. Repeated treatment with cannabidiol but not Δ9-tetrahydrocannabinol has a neuroprotective effect without the development of tolerance. Neuropharmacology. 2007;52:1079–1087. doi: 10.1016/j.neuropharm.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 194.Resstel L.B., Joca S.R., Moreira F.A., Corrêa F.M., Guimarães F.S. Effects of cannabidiol and diazepam on behavioral and cardiovascular responses induced by contextual conditioned fear in rats. Behav. Brain Res. 2006;172:294–298. doi: 10.1016/j.bbr.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 195.Castrén E., Rantamäki T. The role of BDNF and its receptors in depression and antidepressant drug action: Reactivation of developmental plasticity. Dev. Neurobiol. 2010;70:289–297. doi: 10.1002/dneu.20758. [DOI] [PubMed] [Google Scholar]
- 196.Duman R.S., Monteggia L.M. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 197.Monteggia L.M., Barrot M., Powell C.M., Berton O., Galanis V., Gemelli T., Nestler E.J. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc. Natl. Acad. Sci. USA. 2004;101:10827–10832. doi: 10.1073/pnas.0402141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Björkholm C., Monteggia L.M. BDNF–a key transducer of antidepressant effects. Neuropharmacology. 2016;102:72–79. doi: 10.1016/j.neuropharm.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Nibuya M., Morinobu S., Duman R.S. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J. Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Butovsky E., Juknat A., Goncharov I., Elbaz J., Eilam R., Zangen A., Vogel Z. In vivo up-regulation of brain-derived neurotrophic factor in specific brain areas by chronic exposure to Δ9-tetrahydrocannabinol. J. Neurochem. 2005;93:802–811. doi: 10.1111/j.1471-4159.2005.03074.x. [DOI] [PubMed] [Google Scholar]
- 201.Derkinderen P., Valjent E., Toutant M., Corvol J.C., Enslen H., Ledent C., Girault J.A. Regulation of extracellular signal-regulated kinase by cannabinoids in hippocampus. J. Neurosci. 2003;23:2371–2382. doi: 10.1523/JNEUROSCI.23-06-02371.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Maj P.F., Collu M., Fadda P., Cattaneo A., Racagni G., Riva M.A. Long-term reduction of brain-derived neurotrophic factor levels and signaling impairment following prenatal treatment with the cannabinoid receptor 1 receptor agonist (R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinyl-methyl) pyrrolo [1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone. Eur. J. Neurosci. 2007;25:3305–3311. doi: 10.1111/j.1460-9568.2007.05565.x. [DOI] [PubMed] [Google Scholar]
- 203.Rubino T., Viganò D., Premoli F., Castiglioni C., Bianchessi S., Zippel R., Parolaro D. Changes in the expression of G protein-coupled receptor kinases and β-arrestins in mouse brain during cannabinoid tolerance. Mol. Neurobiol. 2006;33:199–213. doi: 10.1385/MN:33:3:199. [DOI] [PubMed] [Google Scholar]
- 204.Giacoppo S., Pollastro F., Grassi G., Bramanti P., Mazzon E. Target regulation of PI3K/Akt/mTOR pathway by cannabidiol in treatment of experimental multiple sclerosis. Fitoterapia. 2017;116:77–84. doi: 10.1016/j.fitote.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 205.Mori M.A., Meyer E., Soares L.M., Milani H., Guimarães F.S., de Oliveira R.M.W. Cannabidiol reduces neuroinflammation and promotes neuroplasticity and functional recovery after brain ischemia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2017;75:94–105. doi: 10.1016/j.pnpbp.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 206.Sales A.J., Fogaça M.V., Sartim A.G., Pereira V.S., Wegener G., Guimarães F.S., Joca S.R. Cannabidiol induces rapid and sustained antidepressant-like effects through increased BDNF signaling and synaptogenesis in the prefrontal cortex. Mol. Neurobiol. 2019;56:1070–1081. doi: 10.1007/s12035-018-1143-4. [DOI] [PubMed] [Google Scholar]
- 207.D’Souza D.C., Pittman B., Perry E., Simen A. Preliminary evidence of cannabinoid effects on brain-derived neurotrophic factor (BDNF) levels in humans. Psychopharmacology. 2009;202:569. doi: 10.1007/s00213-008-1333-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Foster J.A., Neufeld K.A.M. Gut–brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 209.Luna R.A., Foster J.A. Gut brain axis: Diet microbiota interactions and implications for modulation of anxiety and depression. Curr. Opin. Biotechnol. 2015;32:35–41. doi: 10.1016/j.copbio.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 210.Molina-Torres G., Rodriguez-Arrastia M., Roman P., Sanchez-Labraca N., Cardona D. Stress and the gut microbiota-brain axis. Behav. Pharmacol. 2019;30:187–200. doi: 10.1097/FBP.0000000000000478. [DOI] [PubMed] [Google Scholar]
- 211.Macedo D., Chaves Filho A.J.M., de Sousa C.N.S., Quevedo J., Barichello T., Júnior H.V.N., de Lucena D.F. Antidepressants, antimicrobials or both? Gut microbiota dysbiosis in depression and possible implications of the antimicrobial effects of antidepressant drugs for antidepressant effectiveness. J. Affect. Disord. 2017;208:22–32. doi: 10.1016/j.jad.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 212.Yang C., Qu Y., Fujita Y., Ren Q., Ma M., Dong C., Hashimoto K. Possible role of the gut microbiota–brain axis in the antidepressant effects of (R)-ketamine in a social defeat stress model. Transl. Psychiatry. 2017;7:1294. doi: 10.1038/s41398-017-0031-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Neufeld K.A.M., Bienenstock J., Bharwani A., Champagne-Jorgensen K., Mao Y., West C., Forsythe P. Oral selective serotonin reuptake inhibitors activate vagus nerve dependent gut-brain signalling. Sci. Rep. 2019;9:14290. doi: 10.1038/s41598-019-50807-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Cani P.D., Plovier H., Van Hul M., Geurts L., Delzenne N.M., Druart C., Everard A. Endocannabinoids—at the crossroads between the gut microbiota and host metabolism. Nat. Rev. Endocrinol. 2016;12:133. doi: 10.1038/nrendo.2015.211. [DOI] [PubMed] [Google Scholar]
- 215.Muccioli G.G., Naslain D., Bäckhed F., Reigstad C.S., Lambert D.M., Delzenne N.M., Cani P.D. The endocannabinoid system links gut microbiota to adipogenesis. Mol. Syst. Biol. 2010;6:392. doi: 10.1038/msb.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sharkey K.A., Wiley J.W. The role of the endocannabinoid system in the brain–gut axis. Gastroenterology. 2016;151:252–266. doi: 10.1053/j.gastro.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zoppi S., Madrigal J.L., Pérez-Nievas B.G., Marín-Jiménez I., Caso J.R., Alou L., Menchén L. Endogenous cannabinoid system regulates intestinal barrier function in vivo through cannabinoid type 1 receptor activation. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;302:G565–G571. doi: 10.1152/ajpgi.00158.2011. [DOI] [PubMed] [Google Scholar]
- 218.Sherwin E., Dinan T.G., Cryan J.F. Recent developments in understanding the role of the gut microbiota in brain health and disease. Ann. N. Y. Acad. Sci. 2017;1420:5–25. doi: 10.1111/nyas.13416. [DOI] [PubMed] [Google Scholar]
- 219.Karoly H.C., Mueller R.L., Bidwell L.C., Hutchison K.E. Cannabinoids and the Microbiota–Gut–Brain Axis: Emerging Effects of Cannabidiol and Potential Applications to Alcohol Use Disorders. Alcohol Clin. Exp. Res. 2020;44:340–353. doi: 10.1111/acer.14256. [DOI] [PubMed] [Google Scholar]
- 220.Cabral G.A., Pettit D.A.D. Drugs and immunity: Cannabinoids and their role in decreased resistance to infectious disease. J. Neuroimmunol. 1998;83:116–123. doi: 10.1016/S0165-5728(97)00227-0. [DOI] [PubMed] [Google Scholar]
- 221.Croxford J.L., Yamamura T. Cannabinoids and the immune system: Potential for the treatment of inflammatory diseases? J. Neuroimmunol. 2005;166:3–18. doi: 10.1016/j.jneuroim.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 222.Klein T.W., Friedman H., Specter S. Marijuana, immunity and infection. J. Neuroimmunol. 1998;83:102–115. doi: 10.1016/S0165-5728(97)00226-9. [DOI] [PubMed] [Google Scholar]
- 223.Massi P., Vaccani A., Parolaro D. Cannabinoids, immune system and cytokine network. Curr. Pharm. Des. 2006;12:3135–3146. doi: 10.2174/138161206777947425. [DOI] [PubMed] [Google Scholar]
- 224.Roth M.D., Baldwin G.C., Tashkin D.P. Effects of delta-9-tetrahydrocannabinol on human immune function and host defense. Chem. Phys. Lipids. 2002;1211:229–239. doi: 10.1016/S0009-3084(02)00159-7. [DOI] [PubMed] [Google Scholar]
- 225.Eyre H., Baune B.T. Neuroplastic changes in depression: A role for the immune system. Psychoneuroendocrinology. 2012;37:1397–1416. doi: 10.1016/j.psyneuen.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 226.Leonard B. Stress, depression and the activation of the immune system. World J. Biol. Psychiatry. 2000;1:17–25. doi: 10.3109/15622970009150562. [DOI] [PubMed] [Google Scholar]
- 227.Leonard B.E. The immune system, depression and the action of antidepressants. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2001;25:767–780. doi: 10.1016/S0278-5846(01)00155-5. [DOI] [PubMed] [Google Scholar]
- 228.Leonard B.E., Song C. Stress and the immune system in the etiology of anxiety and depression. Pharmacol. Biochem. Behav. 1996;54:299–303. doi: 10.1016/0091-3057(95)02158-2. [DOI] [PubMed] [Google Scholar]
- 229.Linn B.S., Linn M.W., Jensen J. Anxiety and immune responsiveness. Psychol. Rep. 1981;49:969–970. doi: 10.2466/pr0.1981.49.3.969. [DOI] [PubMed] [Google Scholar]
- 230.Nautiyal K.M., Ribeiro A.C., Pfaff D.W., Silver R. Brain mast cells link the immune system to anxiety-like behavior. Proc. Natl. Acad. Sci. USA. 2008;105:18053–18057. doi: 10.1073/pnas.0809479105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Miller A.H. Neuroendocrine and immune system interactions in stress and depression. Psychiatr. Clin. N. Am. 1998;21:443–463. doi: 10.1016/S0193-953X(05)70015-0. [DOI] [PubMed] [Google Scholar]
- 232.Stein M., Keller S.E., Schleifer S.J. Immune system: Relationship to anxiety disorders. Psychiatr. Clin. N. Am. 1988;11:349–360. doi: 10.1016/S0193-953X(18)30502-1. [DOI] [PubMed] [Google Scholar]
- 233.Fischer J.A., Clavarino A.M., Plotnikova M., Najman J.M. Cannabis use and quality of life of adolescents and young adults: Findings from an Australian birth cohort. J. Psychoact. Drugs. 2015;47:107–116. doi: 10.1080/02791072.2015.1014121. [DOI] [PubMed] [Google Scholar]
- 234.Volkow N.D., Hampson A.J., Baler R.D. Don’t worry, be happy: Endocannabinoids and cannabis at the intersection of stress and reward. Annu. Rev. Pharmacol. Toxicol. 2017;57:285–308. doi: 10.1146/annurev-pharmtox-010716-104615. [DOI] [PubMed] [Google Scholar]
- 235.Degenhardt L., Hall W., Lynskey M. Alcohol, cannabis and tobacco use among Australians: A comparison of their associations with other drug use and use disorders, affective and anxiety disorders, and psychosis. Addiction. 2001;96:1603–1614. doi: 10.1046/j.1360-0443.2001.961116037.x. [DOI] [PubMed] [Google Scholar]
- 236.United Nations Office on Drugs and Crime (UNODC) World Drug Report, United Nations Office on Drugs and Crime. 2015. [(accessed on 21 October 2020)]. Available online: https://www.unodc.org/documents/wdr2015/World_Drug_Report_2015.pdf.
- 237.Leung L. Cannabis and its derivatives: Review of medical use. J. Am. Board Fam. Med. 2011;24:452–462. doi: 10.3122/jabfm.2011.04.100280. [DOI] [PubMed] [Google Scholar]
- 238.Lotan I., Treves T.A., Roditi Y., Djaldetti R. Cannabis (medical marijuana) treatment for motor and non–motor symptoms of Parkinson disease: An open-label observational study. Clin. Neuropharmacol. 2014;37:41–44. doi: 10.1097/WNF.0000000000000016. [DOI] [PubMed] [Google Scholar]
- 239.Woolridge E., Barton S., Samuel J., Osorio J., Dougherty A., Holdcroft A. Cannabis use in HIV for pain and other medical symptoms. J. Pain Symptom Manag. 2005;29:358–367. doi: 10.1016/j.jpainsymman.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 240.Anthony J.C., Helzer J.E. Syndromes of Drug Abuse and Dependence. Psychiatric Disorders in America: The Epidemiologic Catchment Area Study. The Free Press; New York, NY, USA: 1991. pp. 116–154. [Google Scholar]
- 241.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-5®) American Psychiatric Pub.; Washington, DC, USA: 2013. [Google Scholar]
- 242.Hollister L.E., Kanter S.L. Laboratory verification of “heavy” and “light” users of cannabis. Drug Alcohol Depend. 1980;5:151–152. doi: 10.1016/0376-8716(80)90192-1. [DOI] [PubMed] [Google Scholar]
- 243.Tait R.J., Mackinnon A., Christensen H. Cannabis use and cognitive function: 8-year trajectory in a young adult cohort. Addiction. 2011;106:2195–2203. doi: 10.1111/j.1360-0443.2011.03574.x. [DOI] [PubMed] [Google Scholar]
- 244.Taylor M., Lees R., Henderson G., Lingford-Hughes A., Macleod J., Sullivan J., Hickman M. Comparison of cannabinoids in hair with self-reported cannabis consumption in heavy, light and non-cannabis users. Drug Alcohol Rev. 2017;36:220–226. doi: 10.1111/dar.12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.