Abstract
During the years 1994 to 1998, 10 strains of Salmonella enterica serovar Enteritidis phage type 11 (PT11) and 6 PT9a strains were isolated from Danish hedgehogs, together with 7 strains that did not yield phage susceptibility patterns conforming with any known phage type (routine dilution no conformity [RDNC]). From 1995 to 1998, five Danish patients were reported infected with serovar Enteritidis PT11 and two with PT9a. All serovar Enteritidis PT11, PT9a, and RDNC isolates from hedgehogs and humans were analyzed by pulsed-field gel electrophoresis (PFGE), plasmid profiling, and restriction fragment length polymorphism (RFLP) of plasmids. By use of S1 nuclease and HindIII, the PT11 and PT9a isolates had identical plasmid profiles and RFLP patterns, which differed from the RDNC profiles. The PFGE profiles were identical for all serovar Enteritidis PT11 and PT9a strains from hedgehogs, four of five human strains of serovar Enteritidis PT11, and two human strains of serovar Enteritidis PT9a, irrespective of restriction enzyme, whereas the last human strain deviated slightly when NotI was used but not when XbaI or SpeI was used. The results indicate that serovar Enteritidis PT9a and PT11 are closely related and that PT11 and PT9a from Danish hedgehogs and humans belong to the same clonal lineage.
The distribution of salmonellosis among humans in Denmark has been monitored intensively over the last couple of decades. Salmonella enterica serovar Enteritidis and Salmonella enterica serovar Typhimurium are the most common salmonella types reported to infect humans in Denmark (5). Since 1984, serovar Enteritidis has been the predominant serovar, and phage types 4, 6, and 8 (PT4, PT6, and PT8) have been the most important (5). The main sources of these infections have been meat and table eggs (6). Nevertheless, many other serovar Enteritidis phage types also caused salmonellosis in humans, but with a smaller number of patients. Serovar Enteritidis PT11 and PT9a were some of the uncommon phage types isolated from salmonella-infected humans.
Wildlife has been incriminated as a possible source for salmonella, in particular some of the less common salmonella serovars. Since 1994, the Danish Veterinary Laboratory has systematically analyzed various wildlife species from all areas of Denmark for the presence of salmonella. From these data it was evident that serovar Enteritidis PT11 and PT9a strains were isolated only from hedgehogs (together with some routine dilution no conformity [RDNC] strains).
Serotyping and subsequent phage typing are two of the most commonly used typing methods for primary differentiation of salmonella (10, 11, 18). Serovar Enteritidis can be subdivided into 60 phage types by using the method described by Ward et al. (21). However, by these methods, it is not possible to draw conclusions about the degree of relatedness at the DNA level of strains belonging to the same serotype and phage type. Further verification of possible clonality within specific serovar Enteritidis phage types is often done by using pulsed-field gel electrophoresis (PFGE) analysis together with plasmid profiling and plasmid restriction fragment length polymorphism (RFLP) analysis. These methods have proven efficient for genotypic characterization of many serovar Enteritidis strains (2, 8, 12, 15, 16).
In this study, a comparison between strains of serovar Enteritidis PT11 and PT9a isolated from humans and hedgehogs in Denmark together with a number of RDNC strains from hedgehogs was performed by using PFGE, plasmid profiling, and plasmid RFLP analysis.
MATERIALS AND METHODS
Bacterial strains and phage typing.
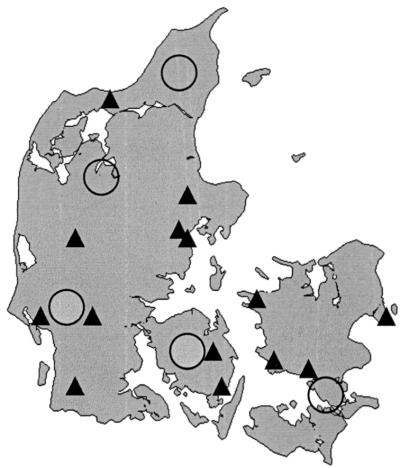
Twenty-three salmonella strains from Danish hedgehogs were collected between 1994 and 1998. The strains were isolated from animals submitted to the laboratory during this period. Most of the animals were submitted from hedgehog nurseries or were road casualties. Five serovar Enteritidis PT11 strains and two serovar Enteritidis PT9a strains of human origin were collected from Danish patients between 1995 and 1998. These seven strains were the total number of strains belonging to these phage types, verified from human infections in Denmark during this period, and were kindly provided by the Statens Serum Institute, Copenhagen, Denmark. Since only about 25% of the human isolates of serovar Enteritidis were routinely phage typed, the true number of cases may have been considerably higher. The geographic distribution of salmonella-infected hedgehogs and human patients is shown in Fig. 1. It may be observed that the isolates originated from the entire country. All isolates were serotyped according to the Kauffmann-White scheme (13) and phage typed in accordance with the scheme of Ward et al. (21).
FIG. 1.
Distribution of Danish hedgehogs and humans from whom serovar Enteritidis PT11 and PT9a strains were isolated. Solid triangles, locations of serovar Enteritidis-infected hedgehogs. Open circles, locations of humans reported to be infected with serovar Enteritidis PT11 or PT9a. A symbol may represent more than one isolate from the same area.
Plasmid profile analysis.
All strains were grown overnight in Luria-Bertani (LB) broth at 37°C to an optical density at 620 nm (OD620) of 0.5 to 0.8, after which 2 to 3 ml of the cultures was used for plasmid isolation as described by Olsen (9) with the following modifications. The pH of the lysis buffer was 12.51, the precipitation of DNA with isopropanol was done at −20°C for 15 min, and finally the phenol-chloroform treatment was omitted. Isolated plasmids were digested for 45 min at 37°C with 12 U of S1 nuclease (Amersham) or for 2 h at 37°C with 12 U of HindIII (GIBCO BRL, Life Technologies). The S1 nuclease-digested plasmids were separated in a 1% agarose gel (Pulsed Field Certified Agarose, Bio-Rad) in 0.5× Tris-borate-EDTA (TBE) buffer by using a contour-clamped homogeneous electric field (CHEF)-DR III system (Bio-Rad). The electrophoresis conditions were 6 V/cm at 12°C for 18 h. The ramping time was 0.5 to 15 s for 18 h. The molecular weight markers were a 1-kb DNA extension ladder (GIBCO BRL, Life Technologies) and a Lambda Ladder PFG Marker (New England Biolabs). The HindIII-digested plasmids were separated in a 0.8% agarose gel at 100 V for 20 min followed by 150 V for 2 h and 40 min in a 1× TAE (40 mM Tris, 5 mM sodium acetate, 1 mM EDTA [pH 8.0]) buffer.
PFGE.
Strains of serovar Enteritidis were grown overnight in LB broth at 37°C until an OD620 of ∼0.5 was reached, after which ∼1.5 ml of culture was used for DNA preparation as described by Cameron et al. (3) with the following exceptions. All centrifugation steps were performed in Eppendorf tubes, the lysis step was done for 2 h, and the overnight treatment with proteinase K was carried out at 56°C. Slices of agarose-embedded DNA were digested with 20 U of XbaI, NotI, or SpeI (New England Biolabs) as described by Cameron et al. (3) but with a reduced amount of buffer. The restricted fragments were separated in a 1% agarose gel (Pulsed Field Certified Agarose; Bio-Rad) in 0.5× TBE buffer by using a CHEF-DR III system. The electrophoresis conditions were 6 V/cm at 12°C for 20 h. The ramping times were 7 to 12 s for 10 h, followed by 20 to 40 s for another 10 h. Following electrophoresis, the gel was stained in aqueous ethidium bromide (Bio-Rad), 2 μg/ml, for 15 min, destained in water for 15 min, and photographed under 254-nm UV light.
RESULTS
During the years 1994 to 1998, a total of 176 hedgehogs were analyzed for salmonella at our laboratory. The number of infected animals, with serotypes and phage types, can be seen in Table 1. The distribution of phage types among the salmonella-positive hedgehogs was 10 strains of PT11, 6 strains of PT9a, and 7 RDNC strains (Table 1). During the years 1995 to 1998, seven humans were reported infected with serovar Enteritidis PT11 or PT9a. Five persons were infected with PT11 and two with PT9a (Table 1). Persons infected with serovar Enteritidis RDNC were not included in this investigation.
TABLE 1.
Number of hedgehogs examined and salmonella-infected hedgehogs aligned with the number of humans infected with same phage types
| Yr | No. of hedgehogs examined | No. of hedgehogs infected with serovar Enteritidis
|
No. of humans infected with serovar Enteritidis PT11 or PT9a
|
|||
|---|---|---|---|---|---|---|
| PT11 | PT9a | RDNC | PT11 | PT9a | ||
| 1994 | 22 | 1 | 1 | 1 | ||
| 1995 | 30 | 1 | 1 | 1 | ||
| 1996 | 40 | 5 | 1 | 1 | 1 | |
| 1997 | 63 | 3 | 2 | 4 | 1 | 1 |
| 1998 | 21 | 2 | 1 | 2 | ||
The geographic distribution of infected hedgehogs and humans reported infected with serovar Enteritidis PT11 and PT9a can be seen from Fig. 1. Hedgehogs infected with serovar Enteritidis were found in all parts of Denmark. Phage types PT11 and PT9a occurred in all parts of the country, whereas the RDNC type was detected only in Jutland. The three types of strains were represented during several years and from different areas.
All strains were characterized by using PFGE, plasmid profiling, and RFLP of plasmids using HindIII as the restriction enzyme. For PFGE analysis, three rare-cutting enzymes, XbaI, NotI, and SpeI, were used. The results for the hedgehogs and humans are listed in Table 2. Strains were divided into two distinct groups, irrespective of the restriction enzyme. One PFGE pattern group included the PT11 and PT9a strains, whereas the other group consisted of the RDNC strains. The differences in banding patterns between the two groups were as follows: eight bands for XbaI, six bands for NotI, and five bands for SpeI (Fig. 2). The two human serovar Enteritidis PT9a isolates and four of the five human PT11 isolates had profiles identical to those of the corresponding strains from hedgehogs, irrespective of the restriction enzyme. The last human PT11 strain was identical to the other four when restriction enzymes XbaI and SpeI were used, whereas NotI produced a single-band difference.
TABLE 2.
Description of the 23 serovar Enteritidis strains found in Danish hedgehogs during the period 1994 to 1998 and 7 serovar Enteritidis PT11 and PT9a strains isolated from humans during the same period
| Straina | Source | Geographic area | Age (yr) | Phage type | PFGE pattern
|
Plasmid profile
|
|||
|---|---|---|---|---|---|---|---|---|---|
| XbaI | NotI | SpeI | HindIII RFLP patternb | S1 nuclease | |||||
| 94-1 | Hedgehog | Dragør | 11 | XI | NI | SI | I | ∼90 kb | |
| 95-1 | Hedgehog | Dragør | 11 | XI | NI | SI | I | ∼90 kb | |
| 96-1 | Hedgehog | Dragør | 11 | XI | NI | SI | I | ∼90 kb | |
| 96-2 | Hedgehog | Skælskør | 11 | XI | NI | SI | I | ∼90 kb | |
| 96-3 | Hedgehog | Næstved | 11 | XI | NI | SI | I | ∼90 kb | |
| 96-4 | Hedgehog | Løgumkloster | 11 | XI | NI | SI | I | ∼90 kb | |
| 96-5 | Hedgehog | Herning | 11 | XI | NI | SI | I | ∼90 kb | |
| 97-1 | Hedgehog | Løgumkloster | 11 | XI | NI | SI | I | ∼90 kb | |
| 97-2 | Hedgehog | Kalundborg | 11 | XI | NI | SI | I | ∼90 kb | |
| 97-3 | Hedgehog | Svendborg | 11 | XI | NI | SI | I | ∼90 kb | |
| 94-2 | Hedgehog | Dragør | 9a | XI | NI | SI | I | ∼90 kb | |
| 95-2 | Hedgehog | Esbjerg | 9a | XI | NI | SI | I | ∼90 kb | |
| 97-4 | Hedgehog | Årslev | 9a | XI | NI | SI | I | ∼90 kb | |
| 97-5 | Hedgehog | Fjerritslev | 9a | XI | NI | SI | I | ∼90 kb | |
| 98-1 | Hedgehog | Svendborg | 9a | XI | NI | SI | I | ∼90 kb | |
| 98-2 | Hedgehog | Vejen | 9a | XI | NI | SI | I | ∼90 kb | |
| 94-3 | Hedgehog | Mundelstrup | RDNC | XII | NII | SII | II | ∼90 kb | |
| 96-6 | Hedgehog | Randers | RDNC | XII | NII | SII | II | ∼90 kb | |
| 97-6 | Hedgehog | Randers | RDNC | XII | NII | SII | II | ∼90 kb | |
| 97-7 | Hedgehog | Randers | RDNC | XII | NII | SII | II | ∼90 kb | |
| 97-8 | Hedgehog | Fjerritslev | RDNC | XII | NII | SII | II | ∼90 kb | |
| 97-9 | Hedgehog | Århus | RDNC | XII | NII | SII | ∼90 kb | ||
| 98-3 | Hedgehog | Vejen | RDNC | XII | NII | SII | II | ∼90 kb | |
| 95-3 | Human | Storstrøms amt | 5 | 11 | XI | NI | SI | I | ∼90 kb |
| 96-7 | Human | Storstrøms amt | 58 | 11 | XI | NIvar | SI | I | ∼90 kb |
| 97-10 | Human | Fyns amt | 45 | 11 | XI | NI | SI | I | ∼90 kb |
| 98-4 | Human | Storstrøms amt | 2 | 11 | XI | NI | SI | I | ∼90 kb |
| 98-5 | Human | Viborg amt | 2 | 11 | XI | NI | SI | I | ∼90 kb |
| 96-8 | Human | Nordjyllands amt | 0 | 9a | XI | NI | SI | I | ∼90 kb |
| 97-11 | Human | Ribe amt | 58 | 9a | XI | NI | SI | I | ∼90 kb |
The first two numerals indicate the year in which the strain was found.
Fragment sizes for pattern I, approximately 30.2, 15, 11, 10, 9, 7.3, 4, and 3.5 kb; fragment sizes for pattern II, approximately 23, 19, 18, 14, 10, 3.5, and 2.5 kb.
FIG. 2.
PFGE patterns obtained from XbaI analysis of serovar Enteritidis strains from humans and hedgehogs in Denmark. First and last lanes, molecular size markers (from the bottom, 48.5, 97.0, 145.5, 194.0, 242.5, 291.0, 339.5, 388.0, 436.5, and 485.0 kb [some of which are indicated by arrows]); second lane, strain 95-1 (PT11; from a hedgehog); third lane, strain 98-2 (PT9a; from a hedgehog); fourth lane, strain 98-3 (RDNC; from a hedgehog); fifth lane, strain 98-4 (PT11; from a human); sixth lane, strain 96-8 (PT9a; from a human).
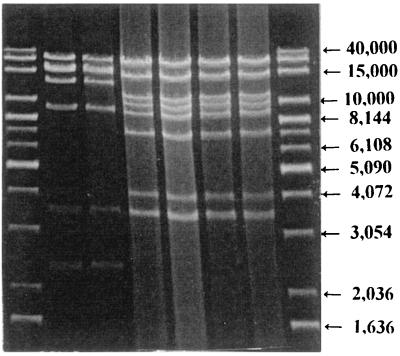
All strains contained a single plasmid of approximately 90 kb (Table 2). Furthermore, all PT11 and PT9a strains had identical HindIII restriction patterns, but these were distinct from the HindIII profiles of the RDNC strains, which also were identical within the group (Fig. 3).
FIG. 3.
HindIII RFLP plasmid analysis of different serovar Enteritidis PT11, PT9a, and RDNC strains from humans and hedgehogs. First and last lanes, molecular size markers (from the top, 40,000, 20,000, 15,000, 10,000, 8,144, 7,126, 6,108, 5,090, 4,072, 3,054, 2,036, and 1,636 bp [some of which are indicated by arrows]); second lane, strain 96-6 (RDNC; from a hedgehog); third lane, strain 98-3 (RDNC; from a hedgehog); fourth lane, strain 95-1 (PT11; from a hedgehog); fifth lane, strain 97-4 (PT9a; from a hedgehog); sixth lane, strain 98-4 (PT11; from a human); seventh lane, strain 96-8 (PT9a; from a human).
DISCUSSION
During the years 1994 to 1998, many species of wildlife animals (n = 1,287) from different Danish geographic areas were analyzed for salmonella at our laboratory. However, serovar Enteritidis was found only among hedgehogs, except for one fox analyzed in 1998, which was infected with a PT1 strain (Danish Veterinary Laboratory, unpublished results). Serovar Enteritidis PT11 was the dominant phage type (50% of strains), whereas PT9a and RDNC strains together accounted for the other 50%. The incidence of serovar Enteritidis in hedgehogs varied from 6.7% (1995) to 25% (1996), depending on the year. The hedgehogs examined were not collected randomly; they were predominantly sick animals from hedgehog nurseries that were submitted for laboratory investigation. Therefore, the recorded incidence of infection may not reflect the true infection level among free-living hedgehogs.
Canadian hedgehogs kept as pets have been reported infected with Salmonella enterica serovar Tilene or serovar Typhimurium (22). Furthermore, serovar Tilene was isolated from African pygmy hedgehogs in the State of Washington (4). In Britain, 14 of 74 hedgehogs were reported infected with salmonella. One animal was infected with serovar Typhimurium definitive type 104, whereas the rest were infected with serovar Enteritidis PT11 (7). According to the literature, serovar Enteritidis PT11 was isolated sporadically from dogs and pigs in Germany (19) and from poultry, meat products, and table eggs in The Netherlands (20). Similar information about PT9a is very limited. The occurrence of RDNC strains in hedgehogs was about 33%. However, these strains were not analyzed further, as a comparison with human RDNC strains would not necessarily be relevant.
Since 1995, 25% (or at least 25 per month) of human serovar Enteritidis strains isolated in Denmark have been phage typed (5). This implies that probably four times as many humans suffered recorded cases of infection with serovar Enteritidis PT11 or PT9a (about 28 people in 4 years). The age distribution among the human patients (Table 2) indicated a rather high incidence in children, as four out of seven patients were between 0 and 5 years old. This is in agreement with the observations from Britain, where two-thirds of patients suffering from a serovar Enteritidis PT11 infection were children under 5 years of age (http://www.healthnet.org/programs/promed-hma/9811/msg00056.html). Whether this is due to a higher susceptibility of children to this phage type or merely a higher exposure to infection, e.g., contact with hedgehogs or their habitats, is not known, but the latter seems more likely. Both human and hedgehog isolates were distributed over the entire country, indicating that these phage types are widely distributed among hedgehogs. Likewise, the very low prevalence of these phage types among humans and wildlife other than hedgehogs possibly indicates a certain level of host adaptation to hedgehogs that needs to be further investigated.
Analysis of the PFGE pattern divided the isolates from hedgehogs into two distinct groups, irrespective of which restriction enzyme was used. All serovar Enteritidis PT11 and PT9a isolates had identical PFGE patterns, but these were different from the RDNC strain PFGE patterns, which also were identical within the group (Table 2 and Fig. 2). The differences between the two PFGE pattern groups varied from five to eight bands depending on the restriction enzyme; hence the strains should be regarded as unrelated when XbaI is used and as possibly related when NotI or SpeI is used (17). Serovar Enteritidis RDNC strains were found only in Jutland. However, this may be a coincidence due to the small number of isolates; it warrants further investigation. Identification of both RDNC strains and a PT9a strain in the area around Fjerritslev, North Jutland, showed that hedgehogs from the same area might carry different strains of serovar Enteritidis. However, it was only the RDNC strains that were limited in their distribution, as PT11 and PT9a strains could be isolated from hedgehogs all over the country (Table 2). The PFGE patterns for the human salmonella strains were slightly more complex, as one of the five serovar Enteritidis PT11 strains deviated from the other four PT11 strains and the two PT9a strains in a single band in the NotI profile.
It is interesting that isolates with different phage types showed the same PFGE pattern, i.e., the same genotype. This indicates a close relationship between PT11 and PT9a. It has previously been reported that strains of serovar Enteritidis may alter their phage type due to the introduction of a resistance plasmid (1). Such phage type conversion without change of genotype usually, but not always, followed the evolutionary clonal lineages of serovar Enteritidis (1).
In all serovar Enteritidis strains included in this work, the approximate size of plasmids was 90 kb. It was expected that a plasmid of this size would be found in the serovar Enteritidis PT11 strains, as this has been reported by other authors (2, 15). However, it was reported that some PT11 strains contained a plasmid of 80 kb (1, 14), but whether this is due to differences in clonal lineages or merely differences in methods for calculating molecular weights is at present unknown.
Analysis of the HindIII RFLP patterns divided the salmonella strains into two different groups (Fig. 3). One group consisted of the serovar Enteritidis PT11 and PT9a strains, where only one specific banding pattern was seen (pattern I in Table 2; Fig. 3, fourth through seventh lanes). The other group consisted of the RDNC strains, which also had a specific banding pattern (pattern II in Table 2; Fig. 3, second and third lanes).
This analysis of serovar Enteritidis PT11 and PT9a isolated from Danish hedgehogs and humans strongly indicates a close relatedness of the strains, as they were genetically indistinguishable (except for one strain) when analyzed by plasmid profiling and PFGE. More than half the patients in this investigation were children who probably often play in outdoor areas where hedgehogs live, and it is possible that the primary source of these salmonella infections arises from hedgehogs, e.g., from contaminated feces.
ACKNOWLEDGMENTS
We thank P. Gerner-Smidt, Statens Serum Institute, Copenhagen, Denmark, for providing the human isolates of Salmonella serovar Enteritidis PT11 and PT9a.
The technical assistance of K. Absalonsen, A. Brandstrup, L. Nielsen, and A. Wetter is gratefully appreciated.
REFERENCES
- 1.Brown D J, Baggesen D L, Platt D J, Olsen J E. Phage type conversion in Salmonella enterica serotype Enteritidis caused by the introduction of a resistance plasmid of incompatibility group X (IncX) Epidemiol Infect. 1999;122:19–22. doi: 10.1017/s0950268898001794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown D J, Threlfall E J, Hampton M D, Rowe B. Molecular characterization of plasmids in Salmonella Enteritidis phage types. Epidemiol Infect. 1993;110:209–216. doi: 10.1017/s0950268800068126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cameron D N, Khambaty F M, Wachsmuth I K, Tauxe R V, Barrett T J. Molecular characterization of Vibrio cholerae O1 strains by pulsed-field gel electrophoresis. J Clin Microbiol. 1994;32:1685–1690. doi: 10.1128/jcm.32.7.1685-1690.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. African pygmy hedgehog-associated salmonellosis—Washington, 1994. JAMA. 1995;274:294. [PubMed] [Google Scholar]
- 5.Gerner-Smidt P, Wegener H C. Salmonella enterica serovar Enteritidis in Denmark. In: Saeed A M, Gast R K, Potter M E, Wall P G, editors. Salmonella enterica serovar Enteritidis in humans and animals. Epidemiology, pathogenesis, and control. Ames, Iowa: Iowa State University Press; 1999. pp. 63–69. [Google Scholar]
- 6.Hald T, Wegener H C, Jørgensen B B. Annual report on zoonoses in Denmark 1998. Copenhagen, Denmark: Datagraf Auning; 1999. [Google Scholar]
- 7.Keymer I F, Gibson E A, Reynolds D J. Zoonoses and other findings in hedgehogs (Erinaceus europaeus): a survey of mortality and review of the literature. Vet Rec. 1991;128:245–249. doi: 10.1136/vr.128.11.245. [DOI] [PubMed] [Google Scholar]
- 8.Liebisch B, Schwarz S. Molecular typing of Salmonella enterica subsp. enterica serovar Enteritidis isolates. J Med Microbiol. 1996;44:52–59. doi: 10.1099/00222615-44-1-52. [DOI] [PubMed] [Google Scholar]
- 9.Olsen J E. An improved method for rapid isolation of plasmid DNA from wild-type gram-negative bacteria for plasmid restriction profile analysis. Lett Appl Microbiol. 1990;10:209–212. doi: 10.1111/j.1472-765x.1990.tb01335.x. [DOI] [PubMed] [Google Scholar]
- 10.Olsen J E, Baggesen D L, Christensen H, Platt D J. A typing concept for Salmonella. In: Aspán A, Mulder R W A W, editors. COST Action 97. Pathogenic micro-organisms in poultry and eggs. 4. Development of monitoring procedures, rapid detection methods and techniques. Molecular epidemiology of campylobacter and salmonella. Brussels, Belgium: CEC; 1998. pp. 97–103. [Google Scholar]
- 11.Olsen J E, Brown D J, Skov M N, Christensen J P. Bacterial typing methods suitable for epidemiological analysis. Applications in investigations of salmonellosis among livestock. Vet Q. 1993;15:125–135. doi: 10.1080/01652176.1993.9694390. [DOI] [PubMed] [Google Scholar]
- 12.Olsen J E, Skov M N, Threlfall E J, Brown D J. Clonal lines of Salmonella enterica serotype Enteritidis documented by IS200-, ribo-, pulsed-field gel electrophoresis and RFLP typing. J Med Microbiol. 1994;40:15–22. doi: 10.1099/00222615-40-1-15. [DOI] [PubMed] [Google Scholar]
- 13.Popoff M Y, Le Minor L. Antigenic formulas of the Salmonella serovars, 7th revision. WHO Collaborating Centre for Reference and Research on Salmonella. Paris, France: Institut Pasteur; 1999. [Google Scholar]
- 14.Rankin S, Platt D J. Phage conversion in Salmonella enterica serotype Enteritidis: implications for epidemiology. Epidemiol Infect. 1995;114:227–236. doi: 10.1017/s0950268800057897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ridley A M, Threlfall E J, Rowe B. Genotypic characterization of Salmonella Enteritidis phage types by plasmid analysis, ribotyping, and pulsed-field gel electrophoresis. J Clin Microbiol. 1998;36:2314–2321. doi: 10.1128/jcm.36.8.2314-2321.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki Y, Ishihara M, Matsumoto M, Arakawa S, Saito M, Ishikawa N, Yokochi T. Molecular epidemiology of Salmonella Enteritidis. An outbreak and sporadic cases studied by means of pulsed-field gel electrophoresis. J Infect. 1995;31:211–217. doi: 10.1016/s0163-4453(95)80029-8. [DOI] [PubMed] [Google Scholar]
- 17.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Threlfall E J, Ridley A M. Application of molecular methods to Salmonella epidemiology. In: Aspán A, Mulder R W A W, editors. COST Action 97. Pathogenic micro-organisms in poultry and eggs. 4. Development of monitoring procedures, rapid detection methods and techniques. Molecular epidemiology of campylobacter and salmonella. Brussels, Belgium: CEC; 1998. pp. 45–51. [Google Scholar]
- 19.Tschäpe H, Liesegang A, Gericke B, Prager R, Rabsch W, Helmuth R. Ups and downs of Salmonella enterica serovar Enteritidis in Germany. In: Saeed A M, Gast R K, Potter M E, Wall P G, editors. Salmonella enterica serovar Enteritidis in humans and animals. Epidemiology, pathogenesis, and control. Ames, Iowa: Iowa State University Press; 1999. pp. 51–61. [Google Scholar]
- 20.van de Giessen A W, van Leeuwen W J, van Pelt W. Salmonella enterica serovar Enteritidis in the Netherlands: epidemiology, prevention, and control. In: Saeed A M, Gast R K, Potter M E, Wall P G, editors. Salmonella enterica serovar Enteritidis in humans and animals. Epidemiology, pathogenesis, and control. Ames, Iowa: Iowa State University Press; 1999. pp. 71–80. [Google Scholar]
- 21.Ward L R, de Sa J D, Rowe B. A phage-typing scheme for Salmonella enteritidis. Epidemiol Infect. 1987;99:291–294. doi: 10.1017/s0950268800067765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woodward D L, Khakhria R, Johnson W M. Human salmonellosis associated with exotic pets. J Clin Microbiol. 1997;35:2786–2790. doi: 10.1128/jcm.35.11.2786-2790.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]