Abstract
Recent developments in genome editing and delivery systems have opened new possibilities for B cell gene therapy. CRISPR/Cas9 nucleases have been used to introduce transgenes into B cell genomes for subsequent secretion of exogenous therapeutic proteins from plasma cells, and to program novel B cell antigen-receptor specificities, allowing for the generation of desirable antibody responses that cannot normally be elicited in animal models. Genome modification of B cells or their progenitor, hematopoietic stem cells (HSCs), could potentially substitute antibody or protein replacement therapies that often require multiple lifelong injections. To date, B cell editing utilizing CRISPR/Cas9 has been solely employed in preclinical studies wherein cells are edited ex vivo. In this review, we discuss current B cell engineering efforts and strategies for the eventual safe and economical adoption of modified B cells into the clinic, including in vivo viral delivery of editing reagents to B cells.
B Cells
B cells, or B lymphocytes, are key effectors of the adaptive immune response. One of their main roles is to produce antibodies (Ab), also called immunoglobulins (Igs). These are secreted proteins made up of covalently linked heavy (H) and kappa (κ) or lambda (λ) light chains with functionally distinct antigen-specific (variable), and immune effector (constant) regions. B cell ontogeny begins in the bone marrow and involves somatic recombination of Ig heavy (IgH) and light (Igκ/Igλ) chain loci. These events result in the cell-surface expression of immunoglobulins in the form of B cell antigen-receptors (BCRs). This process, also called V(D)J-recombination, generates unique BCR’s in each B cell, with antigen specificity encoded by the complementarity-determining regions (CDRs) of the variable heavy (VH) and variable light (VL) regions. Cells with self-reactive BCR’s are counter-selected in the marrow before they egress into the periphery to become circulating naïve B cells (1). When naïve B cells receive activation signals from specific T helper (Th) cells and foreign antigen binding to the BCR, they undergo clonal expansion and affinity maturation in the germinal centers (GCs) of secondary lymphoid organs. In a process called class or isotype-switching, genomic recombination occurs in the IgH constant region, allowing for immunoglobulins to be expressed with alternative (IgG, IgA, IgE) effector, heavy chain constant genes. Mutations are also generated in VH and VL regions that alter BCR antigen affinity (2). Variants with improved BCR-affinities are preferentially selected for survival while those with lower affinity or recognizing self-antigens are deleted or maintained in an anergic state by peripheral tolerance (3). Affinity-matured cells differentiate into long-lived memory or plasma cells. Plasma cells, secrete large quantities of antibodies and can survive for decades in the bone marrow and gut-associated lymphoid tissues (GALT). Memory B cells also exhibit extended lifespans and can generate a rapid, high-affinity antibody response upon subsequent antigen-exposure (4). In addition to antibody production, B cells also express high levels of MHC class-II and act as antigen presenting cells (APC’s), presenting antigens to CD4+ T cells. As professional APC’s B cells can either activate or tolerize T cell responses to antigen (5). This brief review describes recent efforts towards harnessing these various B cell effector functions in genetic medicine.
Gene Therapy
Broadly, gene therapy is the introduction of genetic material or gene-editing nucleases into a patient’s cells for the treatment of disease. Gene therapy encompasses gene replacement, gene alteration, increasing or decreasing the transcribed levels of a gene product, and the introduction of completely novel transgenes. Decades of research have begun to pay off clinically, with 22 gene therapy drugs approved globally in 2019 (6). The main challenge for gene therapy is to achieve safe, specific, and long-term therapeutic changes in the targeted cells or tissues while minimizing off-target modifications. As highlighted with the tragic death of Jesse Gelsinger in 1999, immune tolerance of genetic medicines is also of paramount importance (7). Ideally, a patient would be dosed once with the genetic drug, supplanting the need for multiple injections in the case of enzyme replacement therapies.
Precision Genome Engineering Using DNA Nucleases
Prior to CRISPR/cas, integration-competent lentiviral (LV) transduction was commonly used to deliver transgenes to cultured B cells or HSCs for random insertion into the genome. For example, Lu et al transduced human CD34+ cord blood cells with LVs containing HIV broadly neutralizing antibody (bnAb) IgG expression cassettes. These cells were then subjected to a B cell differentiation culture system leading to maturation of plasma-like cells that could secrete high levels of the bnAb ex vivo (8). In Fusil et al., similar expression cassettes were developed that could be alternatively spliced to generate either cell-surface BCRs in non-secretory B cell lines, or soluble antibody secreted from plasma cells after delivery using integration-competent lentiviral vectors (9). With the adoption of CRISPR/cas, more recent efforts employ precision genome editing strategies to knock-out or deliver transgenes to specific locations in the B cell genome by homology directed repair (HDR).
The advent of CRISPR/Cas genome editing has profoundly impacted emerging cell therapies and has been demonstrated in B cells for a variety of applications including the reprogramming of B cell antigen-receptor specificities and the secretion of therapeutic proteins from plasma cells, as further discussed in this review. CRISPR/Cas has largely displaced traditional DNA-nucleases used for genome modification, such as zinc fingers or TALENs, because this enzyme can be more easily adapted for cutting of different target sites in the genome (10). CRISPR/Cas machinery utilizes protein nucleases known as CRISPR associated proteins, or Cas. These ancient nucleases derived from archaeal and bacterial adaptive immune systems typically use a single guide RNA (gRNA) to locate a DNA target and produce a double stranded break (DSB) (11). The DSBs are then resolved by one of two mechanisms in diploid cells: (i) Nonhomologous end-joining (NHEJ) or (ii) homology-directed repair (HDR). The majority of DSBs will be repaired by NHEJ - a highly efficient, error-prone process that tends to introduce nucleotide insertions or deletions known as indels at the DNA break-site. Indels in a coding region can cause frameshift mutations that will result in gene knockout. In HDR-mediated DNA break-repair, template DNA (usually an exogenous “donor”) is integrated at the site of the DSB using donor homology to sequences on either side of the break-site. This mechanism allows for seamless incorporation of exogenous genes or nucleotides in a controlled manner that does not generate random indels (12). Importantly, HDR occurs primarily during S/G2 phase, and is therefore restricted to cells that are actively dividing. The introduction of a DSB into the genome is inherently a dangerous process, leading to concerns about CRISPR/Cas editing causing undesirable genomic alterations (13). To improve safety, a variety of gRNA design algorithms have been developed to generate gRNAs with high on-target and minimal off-target DNA cutting activity (14). Another strategy utilizes Cas9 nickases that initiate HDR from a nick (single strand DNA break), instead of a DSB, in order to minimize indels (15). Ex vivo editing ensures modifications are restricted to the target cell population, which can be selected post editing for re-engraftment. Technologies being developed for specific in vivo delivery will be described in further sections.
B Cell Genome Editing Strategies
Editing at Safe Harbor Loci
Because transgenes inserted into random genomic locations can interact with the genome in unpredictable ways, recent gene delivery approaches target genomic safe harbor loci that have been evaluated for predictable transgene expression while minimizing unwanted interactions (16). As a proof-of-concept, Johnson et al inserted a splice acceptor and fluorescent reporter gene into a CRISPR/cas cut site in the AAVS1 for expression using cell endogenous promoters (17). Hung et al inserted transgenes of therapeutic proteins under control of the MND (myeloproliferative sarcoma virus enhancer, negative control region deleted, dl587rev primer-binding site substituted) promoter into the CCR5 safe harbor locus as it is not transcriptionally active in human B cells, it is not required for plasma cell differentiation, and null mutations are innocuous to humans (18). In a unique BCR editing approach, Pesch et al modified murine B cells for the expression of a novel chimeric B cell receptor (CBCR), at the Rosa26 safe harbor locus. A single-chain variable fragment (scFv) acting as the extracellular antigen-binding domain was joined to the CD28 transmembrane domain and the cytoplasmic domain of the endogenous murine BCR fused to the CD79β intracellular signaling domain via a spacer encoding a Strep tag. CBCRs could potentially offer a way to activate engineered B cells in an antigen-controllable manner (by vaccination), independent of the endogenous BCR (19). Luo et al. targeted an anti-PD-1 antibody cassette to the GAPDH (Glyceraldehyde-3-phosphate dehydrogenase) locus in mouse primary B cells for co-expression of the transgene along with the GAPDH enzyme (20).
Editing the Immunoglobulin Loci
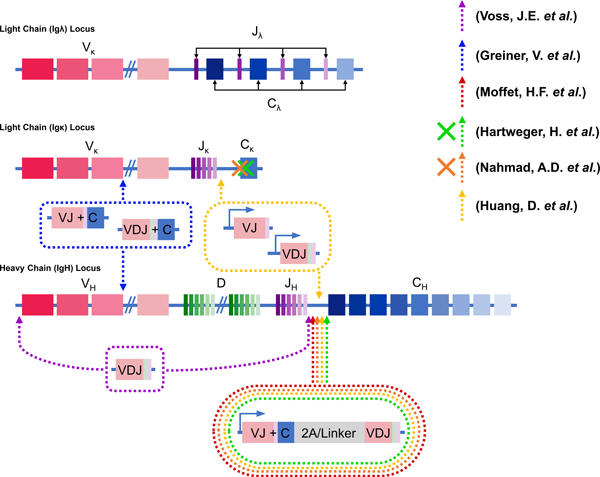
Engineering antibody transgenes into the immunoglobulin loci where they can be expressed using B cell endogenous heavy chain constant gene exons, allows them to function as both B cell antigen-receptors and subsequently as secreted therapeutic antibodies from long-lived plasma cells (Figure 1). Voss et al replaced the entire VH locus between distant (2MB) CRISPR/cas9 cut-sites with a homology repair template containing the VDJ sequence from a heavy chain-encoded HIV broadly neutralizing antibody in human B cells. The engineered heavy chain could be expressed using cell endogenous V-gene promoters, HC constant gene exons, and light chains. These antibodies could broadly recognize HIV and engineered cell lines could be affinity matured in vitro, however low engineering efficiencies in primary cells are limiting for clinical applications (21). Greiner et al. demonstrated that homology repair templates could replace or be inserted into commonly recombined VDJ and VJ regions in mature primary human B cells. Transgenes were expressed in primary human B cells using these endogenous V-gene promoters (22). Several groups have converged on a high efficiency engineering solution that introduces an antibody homology repair template encoding an IgH V-gene promoter, the light chain (including constant gene), heavy chain variable (VDJ) region and a splice donor site, into the heavy chain locus between the 3’-most J-gene and the downstream V-gene enhancer and constant gene region. In this way the antibody light and heavy chains are expressed from a single transcript spliced to cell endogenous HC-constant genes. A flexible linker or P2A self-cleaving peptide are placed between light and heavy chains to allow them to pair together for secretion as functional BCR or antibody (Figure 1). Hartweger et al. included a splice acceptor, stop codon and terminator sequence in front of the donor DNA V-gene promoter to stop transcription of upstream endogenous rearranged VDJ sequences, and a P2A self-cleaving peptide between light and heavy chains. They used CRISPR/cas9 protein complexed with guide RNA (ribonucleoprotein or RNPs) to target indels into the endogenous light chain kappa constant gene to minimize mispairing with endogenous light chains. They showed that ex vivo engineered mouse B cells could secrete class-switched 3BNC60 HIV bnAb for several days after adoptive transfer into congenic immunocompetent mice (23). Moffett et al used a 54-amino acid linker to join light and heavy chains in order to prevent mispairing with endogenous light chains. They could achieve high efficiency insertion with up to 60% or 24% of targeted cells expressing the engineered BCR in ex vivo activated primary human and mouse B cells respectively. Two antibody donor DNAs could be targeted to both HC alleles in up to 6% of double targeted B cells. They also showed that protective levels of pathogen specific RSV (Respiratory Syncytial Virus) antibody could be secreted from engineered cells for several days post adoptive-transfer into congenic immunocompetent mice, or for several weeks as bone marrow engrafted plasma cells in immunocompromised mice (24). Nahmad et al showed that primary mouse B cells engineered to express the 3BNC117 HIV bnAb as antigen receptor could be matured in germinal centers after adoptive transfer and vaccination in congenic immunocompetent mice, resulting in a class-switched and somatically mutated memory and plasma cell response (25). Huang et al showed similar results in mice using HIV bnAb VRC01 engineered B cells, and that such responses could be durable and boosted multiple times. They also showed that ex vivo activated mouse primary cells go on to express memory markers and remain subject to peripheral tolerance mechanisms that delete autoreactive cells after adoptive transfer into immunocompetent animals. In addition, they developed an alternative engineering strategy where heavy and kappa chain variable regions in separate donor DNAs were targeted to their endogenous loci for expression using cell native constant genes. Cells engineered in this way however did not respond to immunization after adoptive transfer in to immunocompetent mice (26).
Figure 1: CRISPR/Cas9 BCR Editing.
Schematic representation of the targeting strategies used to engineer the endogenous BCR of primary B cells. The human κ and λ light chain loci lie on chromosomes 2 and 22 respectively, while both mouse light chain loci lie on chromosome 6. The light chain loci are comprised of variable (V), joining (J) and constant (C) regions. The heavy chain locus lies on the human chromosome 14 and the mouse chromosome 12. The heavy chain locus is comprised of variable (V), diversity (D), joining (J), and constant (C) regions. Cutting sites used by different groups for transgene insertion are indicated by dashed arrows and cutting sites for light chain ablation are indicated by x’s. Depictions of the expression cassettes for each group are depicted in circles of dashed lines (purple (25), blue (29), red (26), green (27), orange (28), and yellow (30)).
Applications of Engineered B Cells
B cells have several immune effector functions that make them intriguing targets for genetic modification, mainly: (i) their ability to clonally expand and differentiate into long-lived memory and plasma cells capable of secreting large quantities of highly specific antibodies, (ii) their ability to function as antigen presenting cells, sustaining effector T cell and T follicular helper responses and iii) their production of inflammatory or immune suppressive cytokines (27). These characteristics make them promising cells for the treatment of genetic diseases (18, 19, 28), infectious diseases (20–24, 26, 29, 30), cancers (20, 22, 31, 32), and autoimmune disorders (22).
Secretion of therapeutic proteins from plasma cells
B cells are the natural secretory cells for antibodies and cytokines, making them an ideal gene therapy target cell choice for secreted therapeutic proteins with clinical relevance for monogenic disorders (18, 24, 33, 34). A major challenge for protein replacement therapy is the ability to sustain effective serum concentrations of the desired exogenous protein long-term without eliciting anti-drug immune responses that could reduce or neutralize the therapeutic effect (35). As plasma cells can persist for an entire life span, one injection of engineered plasma cells secreting high doses of therapeutic proteins might act as a life-long cure. Alternatively, engineered B cells expressing both a secreted therapeutic protein and an antigen-specific BCR could be regulated in vivo to express the transgene through vaccination as demonstrated in Takacs et al., using adoptive transfer and immunization of Ig knock-in mouse B cells expressing therapeutic transgenes (33). Furthermore, antigen-presentation by B cells often leads to T cell tolerance (36–38), raising the possibility that transgene expression from B cells may generate reduced neutralizing anti-drug immune responses as compared with injected protein or gene therapies that result in exogenous protein secretion from other tissues such as the liver or muscle (39). Most immunocompetent mouse models used to study in vivo engineered B cells to date have made use of the black 6 (C57BL/6 or B6) strain because this inbred model is most reliable for congenic adoptive cell transfer experiments where donor cells are tolerated by the recipient animals immune system. This strain has also been shown to tolerate foreign transgene-encoded proteins however, such as human factor IX (FIX), α−1 trypsin and human Erythropoietin (hEPO) (40–42) and therefore may not be the ideal choice for experiments designed to study the tolerogenic properties of B cells. Humanized mouse models have often been used to assess cell-based therapies, wherein immunodeficient mice are injected with human hematopoietic stem cells capable of recapitulating the human immune system. While stem cells are capable of differentiating into mostly transitional or immature B cells in these models, antigen-driven B cell maturation with memory and plasma cell differentiation remains difficult (43). Adoptive transfer of CD19+ human B cells, modified ex vivo for transgene expression, along with autologous CD4+ human T cells into immune-deficient NSG (NOD scid gamma) mice, could allow engineered B cells to home to the spleen and bone marrow, generating plasma cells in 4–5 weeks. The homing was believed to be due to expression of the crucial homing receptors CD62L, CXCR4 and LFA1 on select transduced plasma cells. Lévy et al, used this model to transduce B cells to secrete human factor IX (FIX), a clotting factor that is deficient in Hemophilia B patients, at therapeutic levels (28). Hung et al showed that B cell activating factor (BAFF) could be delivered to human primary B cells and these cells showed improved engraftment and secretion of antibody in NSG mice after ex vivo differentiation to plasma cells (18).
Infectious Diseases
Vaccines are designed to provide protection from infectious diseases, often through the production of pathogen-specific neutralizing antibodies. However, traditional vaccines have failed to generate broadly neutralizing antibodies (bnAbs) capable of generating universal protection against pathogens with diverse and rapidly evolving immunodominant regions, such as Human Immunodeficiency Virus (HIV), Influenza and Hepatitis C (44, 45). The importance of this problem is emphasized by the frequent spillover of antigenically distinct pathogenic coronaviruses into humans leading to the current COVID-19 pandemic (46), and by antigenic drift of the SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus-2) pandemic strain that has threatened the efficacy of neutralizing antibody responses generated by approved vaccines (47). The ability to elicit broadly-neutralizing antibodies from engineered B cells could revolutionize the development of vaccines against such variable pathogens when traditional approaches fail. Programming engineered Ab isotypes could also be useful. Cheong et al., developed a method to induce isotype switching by mimicking AID cleavages with CRISPR/cas (48). For example IgA was found to contribute to neutralization of SARS-CoV-2 to a greater extent compared to other isotypes and could be programmed into bnAb-engineered cells (49). Infusion of recombinant monoclonal antibodies (mAbs) has been explored for several diseases including COVID-19 (50), but this approach is not ideal for therapy in the case of chronic viral infections, or as a prevention strategy, because the short half-life of mAbs (1–3 weeks) necessitates repeated administration, driving practical and financial challenges (51). Immunogenicity of the therapeutic mAb for some patients (~7%), is also an issue (52, 53). Traditional gene therapy approaches have been utilized for in vivo antibody generation from non-B cell populations. Vectored delivery of antibody genes to muscle cells has been developed as a strategy to generate long-term expression of protective antibodies (39, 54, 55). A drawback to this approach is the fixed quantity of antibody produced, which is unresponsive to a dynamic infection. Additionally, such an approach is unable to stimulate the natural immune response to develop the cellular and humoral memory necessary for successful, long-term vaccination. As clearly demonstrated in one study using rhesus monkeys given a vector encoding anti-HIV bnAbs, there is also the danger of developing antibodies that neutralize the transgenic bnAbs (39). Furthermore, pre-existing immunity to viral vectors can cause low levels of transgene antibody expression (56, 57).
Self-renewing HSCs have also been targeted for anti-pathogen antibody production, as stem cells are capable of B cell and plasma differentiation. This approach has the potential advantage of short-circuiting V(D)J-recombination to prevent generation of endogenous Igs during B cell development and continuous generation of transgene expressing B cells in vivo. Several groups have used lentiviral vectors encoding secreted bnAbs against HIV to edit HSCs (8, 29). This HSC-based delivery of bnAbs was capable of persistent secretion, penetrating relevant viral reservoir tissues, and stimulating a protective immune response in humanized mice (30). However, current HSC gene therapy involves harvesting HSCs from the patient, ex vivo genetic engineering, and re-infusion following myeloablative conditioning. This procedure is largely unsuitable for vaccination purposes due to the associated toxicities and risks of low engraftment.
Until recently, genetic engineering of B cells has been limited by technical challenges including delivery of genome editing reagents, the complexity of native antibody development, and the culture and differentiation of HSCs and primary B cells. CRISPR/cas has recently enabled methods to reprogram novel antigen receptor specificities into B cells, an approach that could help surmount difficulties eliciting protective responses against antigenically variable pathogens (58). BCR editing has been used to introduce antibodies specific for HIV (21, 23–26) and respiratory syncytial virus (RSV) (24), with promising results, indicating that engineered B cells are capable of initiating a protective immune response in animal models. In sum, potential advantages of expressing antibody transgenes from BCR-engineered B cells for infectious disease applications are that: 1) Titers could be increased by vaccination or in response to infection. 2) The engineered antibody response would be able to mature in affinity against rapidly evolving pathogens. 3) Engineered antibody could be expressed as all effector isotypes. 4) The engineered antibody should be tolerated by the immune system and eliminated if self-reactive.
Cancer
While not requiring B cell genome editing, the antigen-presentation function of B cells can potentially be harnessed to initiate T cell responses targeting cancer neoantigens. Indeed, there have been a few early-stage clinical trials for B cell-based cancer vaccines, many utilizing the CD40 pathway to activate B cells for presentation of cancer antigens (59). B cells have been loaded with antigens through BCR-mediated uptake (60), CD19 receptor-targeting (61), or through microfluidic-based loading (62). The isolation and enrichment of B cells with defined anti-tumor specificity from the tumor site or surrounding lymph nodes is another approach that has shown some success (63).
Monoclonal antibodies have also been used to enhance immune responses, such as checkpoint blockade therapy, which uses antibodies targeting PD-1, PD-L1, or CTLA-4 to activate T cell anti-cancer effector functions (64). In addition, chimeric antigen receptor (CAR) T cells edited to target a tumor-associated antigen have achieved great clinical results for the treatment of multiple myeloma (65). CAR-T cells have even been modified to constitutively secrete inhibitory checkpoint antibodies, such as anti-PD-1 (66) and anti-PD-L1 (67). Luo et al edited B cells to express an anti-PD-1 antibody at the GAPDH locus using integrase-defective lentiviral vectors containing either CRISPR/cas9 and gRNA or homology repair template. The engineered B cells demonstrated antitumor activity comparable to existing anti-PD-1 monoclonal antibodies in a tumor xenograft mouse model (20). A drawback to this approach, is that engineered cells expressing antibodies targeting self-antigens will have to bypass deactivation by peripheral tolerance (26) and also persistent secretion of a checkpoint antibody might induce an overly activated immune system and increase T cell autoimmunity against the patient (68). One potential solution would be to edit these cells to express a stable selection marker or suicide gene that could be used to eliminate the engineered cells and avoid adverse side effects. An additional applicati84on of B cell editing is the induction of chromosomal translocations for the development of new models to study B cell malignancies. Johnson et al modeled the t(8;14) translocation characteristic of Burkitt Lymphoma using RNPs targeting c-Myc and the IgH locus (17).
Autoimmune Disorders
Autoimmune disorders develop when self-tolerance mechanisms fail, resulting in the immune system attacking the body’s own cells. B cells are known to play a mediating role in autoimmunity through the production of autoantibodies, presentation of “self” proteins to T cells, and cytokine secretion (69) (70). There have been a few studies editing activated primary B cells ex vivo for the induction of tolerance to self-antigens via retroviral delivery of antigen fusions to the tolerogenic Ig backbone (71). Adoptive transfer of such genetically modified B cells has been shown to induce tolerance in animal models for diseases including diabetes (72), multiple sclerosis (73), rheumatoid arthritis (74), posterior uveitis (75), hemophilia (76), and experimental allergic encephalomyelitis (77). Another potential application for B cell editing to treat autoimmune disorders lies in the secretion of antibodies targeting inflammatory cytokines (e.g., TNF-α and IL6) (22, 77). However, in vivo use of engineered B cells that continuously secrete antibodies specific to self-antigens may be problematic as previously described. It remains challenging to intervene in autoimmune disease without overly compromising the normal immune response.
Delivery Methods
Ex vivo
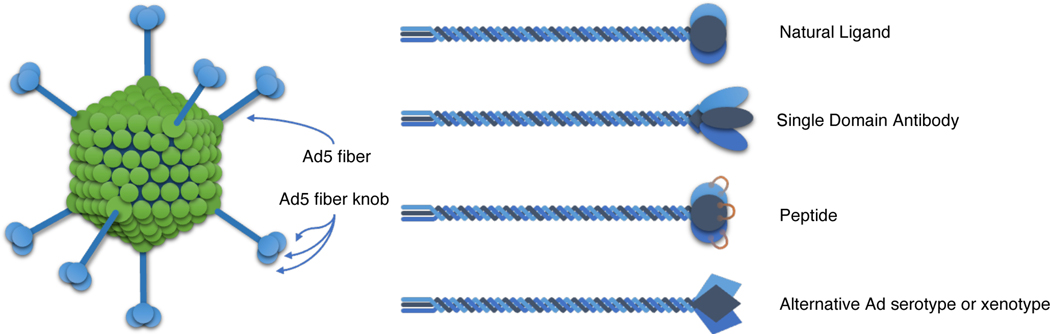
Ex vivo gene delivery to HSCs and primary T cells is now routine in cell therapies used to treat several diseases (78, 79). Ex vivo editing allows for the selection of targeted cell types prior to editing and subsequent enrichment of successfully engineered cells. Almost all published B cell editing studies utilizing CRISPR/Cas found at the time of this review’s writing have described ex vivo engineering methods (Table 1). In these studies, CRISPR/cas9 and corresponding gRNAs are generally delivered as RNPs by electroporation. Electroporation of nuclease in this format is extremely efficient, non-toxic, and ensures minimal off-target cutting compared with mRNA or DNA formats. (17, 18) (19, 22–26). Plasmid DNA is also toxic (dsDNA), and is not as efficiently electroporated into B cells (26). Nonviral methods for delivery of homology repair templates to primary B cells has included electroporation of single stranded DNA (ssDNA) or endotoxin-free plasmid donor DNA (17, 18, 21, 23, 26). Viral vector transduction of donor DNA has also been used with great success. Adeno-associated virus (AAV) vectors delivering homology repair templates in combination with electroporated RNPs is the most widely used method for delivery of HDR genome-editing reagents at this time, and has shown some of the highest reported efficiencies in mouse and human primary B cells, ranging from 10–84% (Table 1). AAVs are commonly used to deliver donor DNA templates because of their stability and low toxicity, however AAV has several limitations, including a small packaging capacity (~5 kb), modest transgene expression levels, and production of low titers (80). AAV serotype-6 is most commonly reported for transduction of human primary B cells while AAV.2 or AAV.DJ has been successfully used in mouse B cells (Table 1). Lentiviral vector transduction of donor DNA templates has not been widely used for CRISPR-based genome editing of B cells however they are becoming increasingly popular because they are capable of transducing both replicating and non-replicating cells, and because they can be psuedotyped with receptor binding proteins that allow for retargeting to different cells types. They are however limited by a small packaging capacity (9 kb), issues with large-scale production (81–83), and risk of insertional mutagenesis (84). LV’s pseudotyped with alternative envelopes such as BaEV, measles virus (H/F glycoproteins), feline endogenous retrovirus (RD114), or gibbon-ape leukemia virus (GaLV) have shown naïve B cell transduction rates up to 20–50% (9, 20, 85). Luo et al. could achieve 20% HDR efficiency in activated human B cells by transduction of two BaEV-pseudotyped integration-deficient LVs that delivered both HDR template and CRISPR/cas9 by transduction (14). Adenovirus (Ad) vectors are another common choice for gene delivery. They are stable, have a large packaging capacity (36 kb), can be grown to high titer, efficiently transduce dividing and non-dividing cells, and pose a low risk for insertional mutagenesis (86). The main limitation for Ad-based ex vivo editing is poor transduction of primary B cells (87). This problem could be addressed with strategies to incorporate novel B-cell targeting moieties into the Ad fiber protein, which mediates cell infection: (i) a B-cell specific natural ligand (88), (ii) a B-cell specific single domain antibody (89), (iii) peptides (90), or (iv) alternative Ad serotype or xenotype (91) (Figure 2). One group showed that an Ad5 vector with a polylysine-containing fiber was able to transduce lipopolysaccharide (LPS)-activated murine B cells and B cell lines (92). Another group found up to 6.5-fold enhancement binding of B cell lines upon insertion of a CD21 binding sequence into the Ad5 fiber (93).
Table 1:
CRISPR/Cas9-mediated HDR in Primary B cells
| Johnson (17) | Hung (18) | Pesch (19) | Luo (20) | Voss (21) | Greiner (22) | Hartweger (23) | Moffett (24) | Nahmad (25) | Huang (26) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Modified Cell Type | Human B cells | Human B cells | Murine B cells | Human B cells | Human B cells | Human B cells | Human and murine B cells | Human and murine B cells | Human and murine B cells | Murine B cells |
| Pre-transduction activation | CD40L and IL4 | CD40L, CpG, IL2/10/15 | IL4, BAFF, CD40L feeder cells | CD40L, IL2/10/15, and CpG | CD40L and IL4 | CellXVivo Human B Cell Expansion Kit | Anti-TLR4 Ab (RP105) | hCD40L, IL2/10/15, and CpG (h) mCD40L + IL4 (m) | Anti-TLR4 Ab (RP105), IL7 (h) LPS (m) | LPS, CpG |
| Delivery Vehicle | AAV6 + RNPs | AAV6 + RNPs | Linear dsDNA/AAVDJ +RNPs | BaEV-pseudtotyped Integrase-deficient LV | plasmid | ssDNA + RNPs | ssDNA + RNPS | AAV6 + RNPs | AAV6 + RNPs (h) AAVDJ + RNPs (m) | Plasmid / AAV2 RNPs |
| Integration Site | AAVS1 locus | CCR5 locus | Rosa26 locus | GAPDH locus | IgH locus | IgH and IgK loci | IgH locus | IgH locus | IgH locus | IgH and IgK loci |
| Cutting efficiency | Not evaluated for AAVS1 locus | 43–84% | Not evaluated | 48.3% | Not evaluated | not quantified for Ig loci | 64% (h) 80% (m) | 72% (h) 71% (m) | 29% (h) 30% (m) | 55–80% |
| Inserted sequences | EGFP | FIX and BAFF | CBCR | αPD1-CD90 | VH | HC/nanobody (into IgH) KC (into IgK) | KC-P2A-VH | KC-linker-VH | KC-linker/P2A-VH | KC-P2A-VH or VH (into IgH) VL (into IgK) |
| HDR efficiency | 25% | 10–40% | 0.3% | 21.6% | 0.21% | Approx. 15–35% | 0.4% (h) 4% (m) | 10–60% (h) 10–24% (m) | 11% (h) 10–84% (m) | 10% (HC) |
| Promoter | endogenous PPP1R12C | donor derived MND | donor derived CMV | endogenous GAPDH | endogenous HC V-gene | endogenous HC V-gene | donor derived HC V-gene | donor derived HC V-gene | HC V-gene promoter or splice acceptor | donor derived HC V-gene |
| Target | N/A | Hemophilia/ B cell engraftment | HEL | PD-1 | HIV | TNF-α | HIV | RSV, HIV, Influenza, and EBV | HIV | HIV |
| Secretion in immunocompetent mice | N/A | N/A | N/A | N/A | N/A | N/A | 7 days | 7–12 days | 33 days | 252 days |
| Secretion in NSG mice | N/A | 21 days | N/A | 150 days | N/A | N/A | N/A | 72 days | N/A | N/A |
Figure 2: Strategies for Ad Transductional Targeting of B Cells.
Adenoviral cell entry involves interactions of the Ad fiber knob with cell surface receptors. B cell targeting using an Ad vector requires ablation of tropism for its native receptor and incorporation of a targeting moiety into the knob that can redirect binding to a B cell surface receptor. This can be accomplished using a fiber-fibritin chimera fused to a novel knob epitope. Strategies to target B cells include modifying the knob to incorporate: (i) a B cell specific natural ligand, (ii) a B cell specific single domain antibody, (iii) a peptide with B cell affinity, and (iv) an alternative Ad serotype or xenotype knob.
In Vivo
Despite the success of ex vivo cell engineering, there are several drawbacks that make it an impractical method for several major applications of genetically altered B cells, particularly vaccines. Briefly, ex vivo editing of primary B cells for adoptive transfer requires (i) cell isolation and sorting (ii) incubation with immune activating agents or differentiation media (HCSs/plasma cells) (iii) transduction with a gene-delivery vehicle, and (iv) characterization of cells before reinfusion into the patient. Furthermore, quiescent B cells require stimulation with cytokines, activating ligands, or other factors to transition from the G0 to G1 phase of the cell cycle to be transduced/modified by HDR. This process is complex, costly and requires significant infrastructure. It would be highly advantageous to simply inject an appropriate vector into a patient and introduce desired changes to the cells in vivo.
To date, there is a single report of successful in vivo HDR-based editing of B cells using CRISPR/cas and homology repair templates in the mouse model. Here, saCas9/gRNA (transcriptionally targeted for expression in only B cells), and HIV bnAb donor DNA, were packaged in separate AAV.DJ vectors (due to packaging size restrictions). Systemic co-delivery of the vectors in mice could edit enough B cells to allow subsequent elicitation of transgene derived bnAb serum responses through vaccination with HIV envelope immunogens. (94). This success highlights that despite significant reduction in engineering efficiency that occurs when nuclease and donor DNA must be packaged separately, relatively few engineered cells are required for generation of a therapeutic effect. Vaccination of low numbers of engineered cells can drive 100x clonal expansion and affinity maturation in vivo after boosting (26). While B cell targeting in vivo can be fairly efficient, it is so far non-specific. One group used high doses of an exosome-associated AAV8 vector in vivo that transduced 3–9.5% of the primary B cells in the blood, spleen, and lymph nodes and 40% of the B cells in the liver, with significant off-target cell expression (95). Several strategies exist for improving in vivo targeting of B cells using AAVs however. ‘Designed ankyrin repeat proteins’ (DARPins) (96) or nanobodies (97) can be embedded into the viral capsid to enhance B cell specific targeting for example. Recent work engineering B cells ex vivo and new vector developments highlight other possibilities for achieving safe and effective in vivo B cell therapies. LV vectors are commonly used for in vivo editing and exhibit low levels of immunogenicity (98) and as mentioned, pseudotyped LVs can specifically target B cells. The packaging capacity is again problematic for LVs when both nuclease and HDR template DNA must be delivered to the same cell in vivo. Some recent reports have described novel IDLVs capable of packaging RNP into the same vector as donor DNA, which should dramatically improve efficiencies for in vivo applications (99, 100). Ad vectors also have a well-established safety profile and have recently been able to deliver CRISPR/Cas machinery for long-term gene expression at therapeutic levels (101). Ads are extremely immunogenic (102). Approaches for dealing with Ad immunogenicity for in vivo gene delivery include: vector genome modifications to dampen immune responses; chemical shielding to reduce unwanted surface interactions (i.e. polyethylene glycol) (103); capsid modifications and the use of non-human serotypes; and the use of immunosuppressive drugs during vector administration for transient dampening of anti-vector responses (104). The hepatotoxicity of first-generation Ad vectors has also been addressed using a chimeric capsid strategy for liver de-targeting (105). One study showed in vivo delivery of antigen to B cells in mice using an Ad5 vector modified with an Ad serotype 35 fiber. The treatment was capable of suppressing tumor growth and eliciting antigen-specific T cell responses (106). Although it will likely require significant development before clinical adaptation as compared with ex vivo methods, in vivo delivery to B cells holds more potential as feasible one-shot, universal B cell gene therapy.
Conclusions
Genetic engineering of B cells is a novel but rapidly growing field, driven by recent technological advances in genome editing and delivery technologies, and by the numerous potential therapeutic applications in disease treatment and prevention. B cells have several important immune effector functions that could be harnessed for genetic medicine. For example, they could be used to durably express therapeutic proteins or antibodies from long-lived plasma cell transgenes. Desirable antibodies can be elicited from B cell receptor engineered cells using vaccine immunogens, and these cells have been shown to be capable of affinity maturation in germinal centers in immunocompetent animal models. However promising, further characterization of in vivo function, safety, and therapeutic efficacy of engineered B cells in relevant animal models is required before adapting the most promising applications for clinical testing. Current B cell engineering methods target ex vivo activated cells which would require autologous re-engraftment into the patient. Ex vivo engineering offers many important advantages including the ability to specifically target and select correctly engineered B cells. However, if genome modified B cells are to become a cost-effective and widely available therapeutic option, methods for safe, specific, and efficient in vivo editing should be developed. Adenoviral vectors (Ads), integration-deficient lentiviral vectors (IDLVs) and adeno-associated viral (AAV) vectors are all promising options for in vivo delivery of genome editing payloads to B cells. Each of these options have unique advantages and disadvantages that should be explored as this fledging cell therapy matures towards the clinic.
Acknowledgments
This work was supported by the Bill and Melinda Gates Foundation (OPP1183956 to J.E.V. and by the National Institutes of Health (R01AI165143 and R01AI167003 to JEV, and R01 CA211096 and UG3 TR002851 to DTC).
Abbreviations used in this article:
- AAV
adeno-associated virus
- Ab
antibody
- Ad
adenovirus
- APC
antigen presenting cells
- BaEV
baboon retrovirus envelope
- BAFF
b cell activating factor
- BCR
b cell receptor
- bnAb
broadly neutralizing antibody
- C
constant
- CAR
chimeric antigen receptor
- Cas
crispr associated proteins
- CBCR
chimeric b cell receptor
- CDR
complementarity-determining region
- CPG
5’-cytosine-phosphodiester bond-guanine-3’
- CRISPR
clustered regularly interspaced short palindromic repeats
- D
diversity
- DSB
double stranded break
- EBV
epstein-barr virus
- FIX
factor IX
- GALT
gut-associated lymphoid tissues
- GaLV
gibbon-ape leukemia virus
- GC
germinal center
- gRNA
guide ribonucleic acid
- H
heavy
- HC
heavy chain
- HDR
homology-directed repair
- HEL
hen egg lysozyme
- HIV
human immunodeficiency virus
- HSC
hematopoietic stem cell
- Ig
immunoglobulin
- IgA
immunoglobulin isotype A
- IgE
immunoglobulin isotype E
- IgG
immunoglobulin isotype G
- IgH
immunoglobulin heavy
- Ig κ
immunoglobulin kappa light
- Ig λ
immunoglobulin lambda light
- Indel
insertion or deletion
- IRES
internal ribosome entry site
- J
joining
- LC
light chain
- LPS
lipopolysaccharide
- LV
lentivirus
- mAb
monoclonal antibody
- NHEJ
nonhomologous end-joining
- NSG
non-obese diabetic severe combined immunodeficiency gamma
- RNP
ribonucleoprotein
- RSV
respiratory syncytial virus
- scFv
single-chain variable fragment
- SLO
secondary lymphoid organ
- TALEN
transcription activator-like effector nuclease
- Th
t helper
- TNF-α
tumor necrosis factor alpha
- V
variable
- VH
variable heavy
- VL
variable light
- κ
kappa
- λ
lambda
Footnotes
Disclosures
David T. Curiel serves as a Scientific Advisor for Walking Fish Therapeutics and is founder and CSO of Precision Virologics. The other authors have no financial conflicts of interest to declare.
References
- 1.Nemazee D. 2017. Mechanisms of central tolerance for B cells. Nat Rev Immunol 17: 281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chi X, Li Y, and Qiu X. 2020. V(D)J recombination, somatic hypermutation and class switch recombination of immunoglobulins: mechanism and regulation. Immunology 160: 233–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooks JF, Murphy PR, Barber JEM, Wells JW, and Steptoe RJ. 2020. Peripheral Tolerance Checkpoints Imposed by Ubiquitous Antigen Expression Limit Antigen-Specific B Cell Responses under Strongly Immunogenic Conditions. J Immunol 205: 1239–1247. [DOI] [PubMed] [Google Scholar]
- 4.Suan D, Sundling C, and Brink R. 2017. Plasma cell and memory B cell differentiation from the germinal center. Curr Opin Immunol 45: 97–102. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, and Jensen PE. 2008. The role of B lymphocytes as antigen-presenting cells. Arch Immunol Ther Exp (Warsz) 56: 77–83. [DOI] [PubMed] [Google Scholar]
- 6.Ma CC, Wang ZL, Xu T, He ZY, and Wei YQ. 2020. The approved gene therapy drugs worldwide: from 1998 to 2019. Biotechnol Adv 40: 107502. [DOI] [PubMed] [Google Scholar]
- 7.Branca MA 2005. Gene therapy: cursed or inching towards credibility? Nat Biotechnol 23: 519–521. [DOI] [PubMed] [Google Scholar]
- 8.Luo XM, Maarschalk E, O’Connell RM, Wang P, Yang L, and Baltimore D. 2009. Engineering human hematopoietic stem/progenitor cells to produce a broadly neutralizing anti-HIV antibody after in vitro maturation to human B lymphocytes. Blood 113: 1422–1431. [DOI] [PubMed] [Google Scholar]
- 9.Fusil F, Calattini S, Amirache F, Mancip J, Costa C, Robbins JB, Douam F, Lavillette D, Law M, Defrance T, Verhoeyen E, and Cosset FL. 2015. A Lentiviral Vector Allowing Physiologically Regulated Membrane-anchored and Secreted Antibody Expression Depending on B-cell Maturation Status. Mol Ther 23: 1734–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khalil AM 2020. The genome editing revolution: review. J Genet Eng Biotechnol 18: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lieber MR 2010. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu Rev Biochem 79: 181–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeh CD, Richardson CD, and Corn JE. 2019. Advances in genome editing through control of DNA repair pathways. Nat Cell Biol 21: 1468–1478. [DOI] [PubMed] [Google Scholar]
- 13.Han HA, Pang JKS, and Soh BS. 2020. Mitigating off-target effects in CRISPR/Cas9-mediated in vivo gene editing. J Mol Med (Berl) 98: 615–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu G, Zhang Y, and Zhang T. 2020. Computational approaches for effective CRISPR guide RNA design and evaluation. Comput Struct Biotechnol J 18: 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rees HA, Yeh WH, and Liu DR. 2019. Development of hRad51-Cas9 nickase fusions that mediate HDR without double-stranded breaks. Nat Commun 10: 2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papapetrou EP, and Schambach A. 2016. Gene Insertion Into Genomic Safe Harbors for Human Gene Therapy. Mol Ther 24: 678–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson MJ, Laoharawee K, Lahr WS, Webber BR, and Moriarity BS. 2018. Engineering of Primary Human B cells with CRISPR/Cas9 Targeted Nuclease. Sci Rep 8: 12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hung KL, Meitlis I, Hale M, Chen CY, Singh S, Jackson SW, Miao CH, Khan IF, Rawlings DJ, and James RG. 2018. Engineering Protein-Secreting Plasma Cells by Homology-Directed Repair in Primary Human B Cells. Mol Ther 26: 456–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pesch T, Bonati L, Kelton W, Parola C, Ehling RA, Csepregi L, Kitamura D, and Reddy ST. 2019. Molecular Design, Optimization, and Genomic Integration of Chimeric B Cell Receptors in Murine B Cells. Front Immunol 10: 2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo B, Zhan Y, Luo M, Dong H, Liu J, Lin Y, Zhang J, Wang G, Verhoeyen E, Zhang Y, and Zhang H. 2020. Engineering of alpha-PD-1 antibody-expressing long-lived plasma cells by CRISPR/Cas9-mediated targeted gene integration. Cell Death Dis 11: 973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voss JE, Gonzalez-Martin A, Andrabi R, Fuller RP, Murrell B, McCoy LE, Porter K, Huang D, Li W, Sok D, Le K, Briney B, Chateau M, Rogers G, Hangartner L, Feeney AJ, Nemazee D, Cannon P, and Burton DR. 2019. Reprogramming the antigen specificity of B cells using genome-editing technologies. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greiner V, Bou Puerto R, Liu S, Herbel C, Carmona EM, and Goldberg MS. 2019. CRISPR-Mediated Editing of the B Cell Receptor in Primary Human B Cells. iScience 12: 369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartweger H, McGuire AT, Horning M, Taylor JJ, Dosenovic P, Yost D, Gazumyan A, Seaman MS, Stamatatos L, Jankovic M, and Nussenzweig MC. 2019. HIV-specific humoral immune responses by CRISPR/Cas9-edited B cells. J Exp Med 216: 1301–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moffett HF, Harms CK, Fitzpatrick KS, Tooley MR, Boonyaratanakornkit J, and Taylor JJ. 2019. B cells engineered to express pathogen-specific antibodies protect against infection. Sci Immunol 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nahmad AD, Raviv Y, Horovitz-Fried M, Sofer I, Akriv T, Nataf D, Dotan I, Carmi Y, Burstein D, Wine Y, Benhar I, and Barzel A. 2020. Engineered B cells expressing an anti-HIV antibody enable memory retention, isotype switching and clonal expansion. Nat Commun 11: 5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang D, Tran JT, Olson A, Vollbrecht T, Tenuta M, Guryleva MV, Fuller RP, Schiffner T, Abadejos JR, Couvrette L, Blane TR, Saye K, Li W, Landais E, Gonzalez-Martin A, Schief W, Murrell B, Burton DR, Nemazee D, and Voss JE. 2020. Vaccine elicitation of HIV broadly neutralizing antibodies from engineered B cells. Nat Commun 11: 5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leon B, Ballesteros-Tato A, Misra RS, Wojciechowski W, and Lund FE. 2012. Unraveling effector functions of B cells during infection: the hidden world beyond antibody production. Infect Disord Drug Targets 12: 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levy C, Fusil F, Amirache F, Costa C, Girard-Gagnepain A, Negre D, Bernadin O, Garaulet G, Rodriguez A, Nair N, Vandendriessche T, Chuah M, Cosset FL, and Verhoeyen E. 2016. Baboon envelope pseudotyped lentiviral vectors efficiently transduce human B cells and allow active factor IX B cell secretion in vivo in NOD/SCIDgammac(−/−) mice. J Thromb Haemost 14: 2478–2492. [DOI] [PubMed] [Google Scholar]
- 29.Joseph A, Zheng JH, Chen K, Dutta M, Chen C, Stiegler G, Kunert R, Follenzi A, and Goldstein H. 2010. Inhibition of in vivo HIV infection in humanized mice by gene therapy of human hematopoietic stem cells with a lentiviral vector encoding a broadly neutralizing anti-HIV antibody. J Virol 84: 6645–6653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuhlmann AS, Haworth KG, Barber-Axthelm IM, Ironside C, Giese MA, Peterson CW, and Kiem HP. 2019. Long-Term Persistence of Anti-HIV Broadly Neutralizing Antibody-Secreting Hematopoietic Cells in Humanized Mice. Mol Ther 27: 164–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Page A, Fusil F, and Cosset FL. 2020. Towards Physiologically and Tightly Regulated Vectored Antibody Therapies. Cancers (Basel) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tian M, Cheng HL, Kimble MT, McGovern K, Waddicor P, Chen Y, Cantor E, Qiu M, Tuchel ME, Dao M, and Alt FW. 2021. An in vivo method for diversifying the functions of therapeutic antibodies. Proc Natl Acad Sci U S A 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takacs K, Du Roure C, Nabarro S, Dillon N, McVey JH, Webster Z, Macneil A, Bartok I, Higgins C, Gray D, Merkenschlager M, and Fisher AG. 2004. The regulated long-term delivery of therapeutic proteins by using antigen-specific B lymphocytes. Proc Natl Acad Sci U S A 101: 16298–16303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karasuyama H, and Melchers F. 1988. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. Eur J Immunol 18: 97–104. [DOI] [PubMed] [Google Scholar]
- 35.Singh SK 2011. Impact of product-related factors on immunogenicity of biotherapeutics. J Pharm Sci 100: 354–387. [DOI] [PubMed] [Google Scholar]
- 36.Fuchs EJ, and Matzinger P. 1992. B cells turn off virgin but not memory T cells. Science 258: 1156–1159. [DOI] [PubMed] [Google Scholar]
- 37.Gilbert KM, and Weigle WO. 1994. Tolerogenicity of resting and activated B cells. J Exp Med 179: 249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bennett SR, Carbone FR, Toy T, Miller JF, and Heath WR. 1998. B cells directly tolerize CD8(+) T cells. J Exp Med 188: 1977–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez-Navio JM, Fuchs SP, Pantry SN, Lauer WA, Duggan NN, Keele BF, Rakasz EG, Gao G, Lifson JD, and Desrosiers RC. 2019. Adeno-Associated Virus Delivery of Anti-HIV Monoclonal Antibodies Can Drive Long-Term Virologic Suppression. Immunity 50: 567–575 e565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barr D, Tubb J, Ferguson D, Scaria A, Lieber A, Wilson C, Perkins J, and Kay MA. 1995. Strain related variations in adenovirally mediated transgene expression from mouse hepatocytes in vivo: comparisons between immunocompetent and immunodeficient inbred strains. Gene Ther 2: 151–155. [PubMed] [Google Scholar]
- 41.Tripathy SK, Black HB, Goldwasser E, and Leiden JM. 1996. Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirus vectors. Nat Med 2: 545–550. [DOI] [PubMed] [Google Scholar]
- 42.Snyder RO, Miao CH, Patijn GA, Spratt SK, Danos O, Nagy D, Gown AM, Winther B, Meuse L, Cohen LK, Thompson AR, and Kay MA. 1997. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat Genet 16: 270–276. [DOI] [PubMed] [Google Scholar]
- 43.Brendel C, Rio P, and Verhoeyen E. 2020. Humanized mice are precious tools for evaluation of hematopoietic gene therapies and preclinical modeling to move towards a clinical trial. Biochem Pharmacol 174: 113711. [DOI] [PubMed] [Google Scholar]
- 44.Walker LM, and Burton DR. 2018. Passive immunotherapy of viral infections: ‘super-antibodies’ enter the fray. Nat Rev Immunol 18: 297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pollard AJ, and Bijker EM. 2021. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol 21: 83–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cui J, Li F, and Shi ZL. 2019. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol 17: 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garcia-Beltran WF, Lam EC, St Denis K, Nitido AD, Garcia ZH, Hauser BM, Feldman J, Pavlovic MN, Gregory DJ, Poznansky MC, Sigal A, Schmidt AG, Iafrate AJ, Naranbhai V, and Balazs AB. 2021. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell 184: 2372–2383 e2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheong TC, Compagno M, and Chiarle R. 2016. Editing of mouse and human immunoglobulin genes by CRISPR-Cas9 system. Nat Commun 7: 10934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sterlin D, Mathian A, Miyara M, Mohr A, Anna F, Claer L, Quentric P, Fadlallah J, Devilliers H, Ghillani P, Gunn C, Hockett R, Mudumba S, Guihot A, Luyt CE, Mayaux J, Beurton A, Fourati S, Bruel T, Schwartz O, Lacorte JM, Yssel H, Parizot C, Dorgham K, Charneau P, Amoura Z, and Gorochov G. 2021. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci Transl Med 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pecetta S, Finco O, and Seubert A. 2020. Quantum leap of monoclonal antibody (mAb) discovery and development in the COVID-19 era. Semin Immunol 50: 101427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Both L, Banyard AC, van Dolleweerd C, Wright E, Ma JK, and Fooks AR. 2013. Monoclonal antibodies for prophylactic and therapeutic use against viral infections. Vaccine 31: 1553–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jackisch C, Kim SB, Semiglazov V, Melichar B, Pivot X, Hillenbach C, Stroyakovskiy D, Lum BL, Elliott R, Weber HA, and Ismael G. 2015. Subcutaneous versus intravenous formulation of trastuzumab for HER2-positive early breast cancer: updated results from the phase III HannaH study. Ann Oncol 26: 320–325. [DOI] [PubMed] [Google Scholar]
- 53.Doevendans E, and Schellekens H. 2019. Immunogenicity of Innovative and Biosimilar Monoclonal Antibodies. Antibodies (Basel) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skaricic D, Traube C, De B, Joh J, Boyer J, Crystal RG, and Worgall S. 2008. Genetic delivery of an anti-RSV antibody to protect against pulmonary infection with RSV. Virology 378: 79–85. [DOI] [PubMed] [Google Scholar]
- 55.Schnepp BC, and Johnson PR. 2014. Adeno-associated virus delivery of broadly neutralizing antibodies. Curr Opin HIV AIDS 9: 250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Priddy FH, Lewis DJM, Gelderblom HC, Hassanin H, Streatfield C, LaBranche C, Hare J, Cox JH, Dally L, Bendel D, Montefiori D, Sayeed E, Ackland J, Gilmour J, Schnepp BC, Wright JF, and Johnson P. 2019. Adeno-associated virus vectored immunoprophylaxis to prevent HIV in healthy adults: a phase 1 randomised controlled trial. Lancet HIV 6: e230–e239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Verdera HC, Kuranda K, and Mingozzi F. 2020. AAV Vector Immunogenicity in Humans: A Long Journey to Successful Gene Transfer. Mol Ther 28: 723–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kepler TB, and Wiehe K. 2017. Genetic and structural analyses of affinity maturation in the humoral response to HIV-1. Immunol Rev 275: 129–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wennhold K, Shimabukuro-Vornhagen A, and von Bergwelt-Baildon M. 2019. B Cell-Based Cancer Immunotherapy. Transfus Med Hemother 46: 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rodriguez-Pinto D, and Moreno J. 2005. B cells can prime naive CD4+ T cells in vivo in the absence of other professional antigen-presenting cells in a CD154-CD40-dependent manner. Eur J Immunol 35: 1097–1105. [DOI] [PubMed] [Google Scholar]
- 61.Ma Y, Xiang D, Sun J, Ding C, Liu M, Hu X, Li G, Kloecker G, Zhang HG, and Yan J. 2013. Targeting of antigens to B lymphocytes via CD19 as a means for tumor vaccine development. J Immunol 190: 5588–5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Szeto GL, Van Egeren D, Worku H, Sharei A, Alejandro B, Park C, Frew K, Brefo M, Mao S, Heimann M, Langer R, Jensen K, and Irvine DJ. 2015. Microfluidic squeezing for intracellular antigen loading in polyclonal B-cells as cellular vaccines. Sci Rep 5: 10276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wennhold K, Thelen M, Schlosser HA, Haustein N, Reuter S, Garcia-Marquez M, Lechner A, Kobold S, Rataj F, Utermohlen O, Chakupurakal G, Theurich S, Hallek M, Abken H, Shimabukuro-Vornhagen A, and von Bergwelt-Baildon M. 2017. Using Antigen-Specific B Cells to Combine Antibody and T Cell-Based Cancer Immunotherapy. Cancer Immunol Res 5: 730–743. [DOI] [PubMed] [Google Scholar]
- 64.Hargadon KM, Johnson CE, and Williams CJ. 2018. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol 62: 29–39. [DOI] [PubMed] [Google Scholar]
- 65.Lin Q, Zhao J, Song Y, and Liu D. 2019. Recent updates on CAR T clinical trials for multiple myeloma. Mol Cancer 18: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li S, Siriwon N, Zhang X, Yang S, Jin T, He F, Kim YJ, Mac J, Lu Z, Wang S, Han X, and Wang P. 2017. Enhanced Cancer Immunotherapy by Chimeric Antigen Receptor-Modified T Cells Engineered to Secrete Checkpoint Inhibitors. Clin Cancer Res 23: 6982–6992. [DOI] [PubMed] [Google Scholar]
- 67.Suarez ER, Chang de K, Sun J, Sui J, Freeman GJ, Signoretti S, Zhu Q, and Marasco WA. 2016. Chimeric antigen receptor T cells secreting anti-PD-L1 antibodies more effectively regress renal cell carcinoma in a humanized mouse model. Oncotarget 7: 34341–34355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, Shabafrouz K, Ribi C, Cairoli A, Guex-Crosier Y, Kuntzer T, Michielin O, Peters S, Coukos G, Spertini F, Thompson JA, and Obeid M. 2019. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol 16: 563–580. [DOI] [PubMed] [Google Scholar]
- 69.Eynon EE, and Parker DC. 1992. Small B cells as antigen-presenting cells in the induction of tolerance to soluble protein antigens. J Exp Med 175: 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tobon GJ, Izquierdo JH, and Canas CA. 2013. B lymphocytes: development, tolerance, and their role in autoimmunity-focus on systemic lupus erythematosus. Autoimmune Dis 2013: 827254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Borel Y. 1980. Haptens bound to self IgG induce immunologic tolerance, while when coupled to syngeneic spleen cells they induce immune suppression. Immunol Rev 50: 71–104. [DOI] [PubMed] [Google Scholar]
- 72.Song L, Wang J, Wang R, Yu M, Sun Y, Han G, Li Y, Qian J, Scott DW, Kang Y, Soukhareva N, and Shen B. 2004. Retroviral delivery of GAD-IgG fusion construct induces tolerance and modulates diabetes: a role for CD4+ regulatory T cells and TGF-beta? Gene Ther 11: 1487–1496. [DOI] [PubMed] [Google Scholar]
- 73.Xu B, and Scott DW. 2004. A novel retroviral gene therapy approach to inhibit specific antibody production and suppress experimental autoimmune encephalomyelitis induced by MOG and MBP. Clin Immunol 111: 47–52. [DOI] [PubMed] [Google Scholar]
- 74.Satpute SR, Soukhareva N, Scott DW, and Moudgil KD. 2007. Mycobacterial Hsp65-IgG-expressing tolerogenic B cells confer protection against adjuvant-induced arthritis in Lewis rats. Arthritis Rheum 56: 1490–1496. [DOI] [PubMed] [Google Scholar]
- 75.Liang W, Karabekian Z, Mattapallil M, Xu Q, Viley AM, Caspi R, and Scott DW. 2006. B-cell delivered gene transfer of human S-Ag-Ig fusion protein protects from experimental autoimmune uveitis. Clin Immunol 118: 35–41. [DOI] [PubMed] [Google Scholar]
- 76.Lei TC, and Scott DW. 2005. Induction of tolerance to factor VIII inhibitors by gene therapy with immunodominant A2 and C2 domains presented by B cells as Ig fusion proteins. Blood 105: 4865–4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Melo ME, Qian J, El-Amine M, Agarwal RK, Soukhareva N, Kang Y, and Scott DW. 2002. Gene transfer of Ig-fusion proteins into B cells prevents and treats autoimmune diseases. J Immunol 168: 4788–4795. [DOI] [PubMed] [Google Scholar]
- 78.Morgan RA, Gray D, Lomova A, and Kohn DB. 2017. Hematopoietic Stem Cell Gene Therapy: Progress and Lessons Learned. Cell Stem Cell 21: 574–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.June CH, O’Connor RS, Kawalekar OU, Ghassemi S, and Milone MC. 2018. CAR T cell immunotherapy for human cancer. Science 359: 1361–1365. [DOI] [PubMed] [Google Scholar]
- 80.Naso MF, Tomkowicz B, Perry WL 3rd, and Strohl WR. 2017. Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs 31: 317–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Munis AM 2020. Gene Therapy Applications of Non-Human Lentiviral Vectors. Viruses 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang D, and Gao G. 2014. State-of-the-art human gene therapy: part I. Gene delivery technologies. Discov Med 18: 67–77. [PMC free article] [PubMed] [Google Scholar]
- 83.Milone MC, and O’Doherty U. 2018. Clinical use of lentiviral vectors. Leukemia 32: 1529–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E, Clappier E, Caccavelli L, Delabesse E, Beldjord K, Asnafi V, MacIntyre E, Dal Cortivo L, Radford I, Brousse N, Sigaux F, Moshous D, Hauer J, Borkhardt A, Belohradsky BH, Wintergerst U, Velez MC, Leiva L, Sorensen R, Wulffraat N, Blanche S, Bushman FD, Fischer A, and Cavazzana-Calvo M. 2008. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest 118: 3132–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gutierrez-Guerrero A, Cosset FL, and Verhoeyen E. 2020. Lentiviral Vector Pseudotypes: Precious Tools to Improve Gene Modification of Hematopoietic Cells for Research and Gene Therapy. Viruses 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee CS, Bishop ES, Zhang R, Yu X, Farina EM, Yan S, Zhao C, Zheng Z, Shu Y, Wu X, Lei J, Li Y, Zhang W, Yang C, Wu K, Wu Y, Ho S, Athiviraham A, Lee MJ, Wolf JM, Reid RR, and He TC. 2017. Adenovirus-Mediated Gene Delivery: Potential Applications for Gene and Cell-Based Therapies in the New Era of Personalized Medicine. Genes Dis 4: 43–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Richardson C, Brennan P, Powell M, Prince S, Chen YH, Spiller OB, and Rowe M. 2005. Susceptibility of B lymphocytes to adenovirus type 5 infection is dependent upon both coxsackie-adenovirus receptor and alphavbeta5 integrin expression. J Gen Virol 86: 1669–1679. [DOI] [PubMed] [Google Scholar]
- 88.Belousova N, Korokhov N, Krendelshchikova V, Simonenko V, Mikheeva G, Triozzi PL, Aldrich WA, Banerjee PT, Gillies SD, Curiel DT, and Krasnykh V. 2003. Genetically targeted adenovirus vector directed to CD40-expressing cells. J Virol 77: 11367–11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sharma PK, Dmitriev IP, Kashentseva EA, Raes G, Li L, Kim SW, Lu ZH, Arbeit JM, Fleming TP, Kaliberov SA, Goedegebuure SP, Curiel DT, and Gillanders WE. 2018. Development of an adenovirus vector vaccine platform for targeting dendritic cells. Cancer Gene Ther 25: 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Belousova N, Krendelchtchikova V, Curiel DT, and Krasnykh V. 2002. Modulation of adenovirus vector tropism via incorporation of polypeptide ligands into the fiber protein. J Virol 76: 8621–8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mahsoub HM, Yuan L, and Pierson FW. 2020. Turkey adenovirus 3, a siadenovirus, uses sialic acid on N-linked glycoproteins as a cellular receptor. J Gen Virol 101: 760–771. [DOI] [PubMed] [Google Scholar]
- 92.Li L, Wickham TJ, and Keegan AD. 2001. Efficient transduction of murine B lymphocytes and B lymphoma lines by modified adenoviral vectors: enhancement via targeting to FcR and heparan-containing proteins. Gene Ther 8: 938–945. [DOI] [PubMed] [Google Scholar]
- 93.Mailly L, Renaut L, Rogee S, Grellier E, D’Halluin JC, and Colin M. 2006. Improved gene delivery to B lymphocytes using a modified adenovirus vector targeting CD21. Mol Ther 14: 293–304. [DOI] [PubMed] [Google Scholar]
- 94.Nahmad AD, Lazzarotto CR, Zelikson N, Kustin T, Tenuta M, Huang D, Reuveni M, Horovitz-Fried M, Dotan I, Rosin-Arbesfeld R, Nemazee D, Voss JE, Stern A, Tsai SQ, and Barzel A. 2021. In vivo engineered B cells retain memory and secrete high titers of anti-HIV antibodies in mice. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Breuer CB, Hanlon KS, Natasan JS, Volak A, Meliani A, Mingozzi F, Kleinstiver BP, Moon JJ, and Maguire CA. 2020. In vivo engineering of lymphocytes after systemic exosome-associated AAV delivery. Sci Rep 10: 4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Munch RC, Janicki H, Volker I, Rasbach A, Hallek M, Buning H, and Buchholz CJ. 2013. Displaying high-affinity ligands on adeno-associated viral vectors enables tumor cell-specific and safe gene transfer. Mol Ther 21: 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Eichhoff AM, Borner K, Albrecht B, Schafer W, Baum N, Haag F, Korbelin J, Trepel M, Braren I, Grimm D, Adriouch S, and Koch-Nolte F. 2019. Nanobody-Enhanced Targeting of AAV Gene Therapy Vectors. Mol Ther Methods Clin Dev 15: 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bulcha JT, Wang Y, Ma H, Tai PWL, and Gao G. 2021. Viral vector platforms within the gene therapy landscape. Signal Transduct Target Ther 6: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Uchida N, Drysdale CM, Nassehi T, Gamer J, Yapundich M, DiNicola J, Shibata Y, Hinds M, Gudmundsdottir B, Haro-Mora JJ, Demirci S, and Tisdale JF. 2021. Cas9 protein delivery non-integrating lentiviral vectors for gene correction in sickle cell disease. Mol Ther Methods Clin Dev 21: 121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lyu P, Javidi-Parsijani P, Atala A, and Lu B. 2019. Delivering Cas9/sgRNA ribonucleoprotein (RNP) by lentiviral capsid-based bionanoparticles for efficient ‘hit-and-run’ genome editing. Nucleic Acids Res 47: e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Boucher P, Cui X, and Curiel DT. 2020. Adenoviral vectors for in vivo delivery of CRISPR-Cas gene editors. J Control Release 327: 788–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ahi YS, Bangari DS, and Mittal SK. 2011. Adenoviral vector immunity: its implications and circumvention strategies. Curr Gene Ther 11: 307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kreppel F, and Hagedorn C. 2021. Capsid and Genome Modification Strategies to Reduce the Immunogenicity of Adenoviral Vectors. Int J Mol Sci 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thacker EE, Timares L, and Matthews QL. 2009. Strategies to overcome host immunity to adenovirus vectors in vaccine development. Expert Rev Vaccines 8: 761–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Short JJ, Rivera AA, Wu H, Walter MR, Yamamoto M, Mathis JM, and Curiel DT. 2010. Substitution of adenovirus serotype 3 hexon onto a serotype 5 oncolytic adenovirus reduces factor X binding, decreases liver tropism, and improves antitumor efficacy. Mol Cancer Ther 9: 2536–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim EK, Seo HS, Chae MJ, Jeon IS, Song BY, Park YJ, Ahn HM, Yun CO, and Kang CY. 2014. Enhanced antitumor immunotherapeutic effect of B-cell-based vaccine transduced with modified adenoviral vector containing type 35 fiber structures. Gene Ther 21: 106–114. [DOI] [PubMed] [Google Scholar]