Abstract
miR-149 is an miRNA with essential roles in carcinogenesis. This miRNA is encoded by the MIR149 gene on 2q37.3. The miR-149 hairpin produces miR-149-5p and miR-149-3p, which are the “guide” and the sister “passenger” strands, respectively. Deep sequencing experiments have shown higher prevalence of miR-149-5p compared with miR-149-3p. Notably, both oncogenic and tumor suppressive roles have been reported for miR-149-5p. In this review, we summarize the impact of miR-149-5p in the tumorigenesis and elaborate mechanisms of its involvement in this process in a variety of neoplastic conditions based on three lines of evidence, i.e., in vitro, in vivo and clinical settings.
Keywords: miR-149-5p, cancer, biomarker, expression, ncRNAs
1. Introduction
MicroRNAs (miRNAs) are a group of small-sized regulatory noncoding RNAs which bind to the 3′-UTR of target mRNAs in a specific manner to inhibit their translation [1]. miRNAs can affect diverse aspects of carcinogenesis, tumor evolution, metastatic and angiogenic processes, and resistance to chemoradiotherapeutics [2].
miR-149 is an miRNA with essential roles in carcinogenesis. This miRNA is encoded by MIR149 gene on 2q37.3. The miR-149 hairpin produces miR-149-5p and miR-149-3p which are the “guide” and the sister “passenger” strands, respectively [3]. These two miRNAs have completely dissimilar sequences, suggesting their distinct roles in biological processes [3]. Deep sequencing experiments have shown a higher prevalence of miR-149-5p compared with miR-149-3p.
Physiological functions of miR-149-5p have been evaluated by researchers. For instance, it has been shown to be up-regulated in bovine adipocytes in certain times during their differentiation. The functional role of miR-149-5p in this process is exerted through targeting CRTC1 and CRTC2 [4]. Moreover, the expression of miR-149-5p has been found to be decreased in PDGF-BB-induced vascular smooth muscle cells. Up-regulation of miR-149-5p could suppress the proliferation, invasion and migration of vascular smooth muscle cells, whereas its silencing has the opposite effect. Moreover, histone deacetylase 4 (HDAC4) is the miR-149-5p target, through which this function of miR-149-5p is accomplished [5].
Notably, both oncogenic and tumor suppressive roles have been reported for miR-149-5p. In this review, we summarize the impact of miR-149-5p in tumorigenesis and elaborate mechanisms of its involvement in this process in a variety of neoplastic conditions based on three lines of evidence, i.e., in vitro, in vivo and clinical settings.
2. Cell Line Studies
2.1. Gastrointestinal Tumors
A high throughput sequencing experiment in gastric cancer has shown down-regulation of the circular RNA (circRNA) circNRIP1. This circRNA has been shown to act as a sponge for miR-149-5p. This miRNA regulates metabolic pathways through AKT1/mTOR axis. miR-149-5p suppression has been shown to exert similar effects with up-regulation of circNRIP1 in gastric cancer cells [6]. Another study in gastric cancer has shown down-regulation of miR-149-5p parallel with the up-regulation of the long non-coding RNA (lncRNA) BLACAT1 and KIF2A. miR-149-5p has been shown to be sponged by BLACAT1, leading to the up-regulation of KIF2A [7]. Moreover, miR-149-5p has been demonstrated to be sponged by circNHSL1. In fact, the effects of circNHSL1 on cancer cell migration, invasiveness and glutaminolysis is mediated through sponging miR-149-5p. Experiments in gastric cancer cells have led to identification of YWHAZ as the target of miR-149-5p [8].
Two independent studies have shown the sponging effects of LINC00460 on miR-149-5p in the context of colorectal cancer. LINC00460/miR-149-5p has important functions in the regulation of expression of p53 [9] and BGN [10] in this type of cancer.
In addition, miR-149-5p has been found to be sponged by circCTNNA1, a circRNA with pro-proliferative and pro-migratory effects in colorectal cancer cells. miR-149-5p has been shown to decrease the expression of FOXM1. Thus, the circCTNNA1/miR-149-5p/FOXM1 axis has been suggested as a target for therapeutic interventions in colorectal cancer [11]. miR-149-5p has also been shown to be sponged by other oncogenic non-coding RNAs such as PCAT1 [12], DLGAP1-AS1 [13] and circ5615 [14] in colorectal cancer cells.
2.2. Thyroid Cancer
miR-149-5p has a tumor suppressor role in medullary thyroid carcinoma through directly targeting GIT1. miR-149-5p up-regulation could inhibit the proliferation and invasive properties of medullary thyroid carcinoma cells [15]. In papillary thyroid cancer, miR-149-5p has been shown to bind with circ-FLNA, a circRNA whose expression is regulated by TR4. Binding of circ-FLNA with this miRNA releases MMP9 from the inhibitory effects of miR-149-5p. Thus, the TR4/circ-FLNA/miR-149-5p/MMP9 axis has been identified as an important player in the pathogenesis of papillary thyroid cancer [16].
2.3. Oral Cancer
Expression of miR-149-5p has been shown to be reduced in oral squamous cell carcinoma cells, particularly in cisplatin resistant cells. Up-regulation of miR-149-5p could enhance the cytotoxic effects of this drug in both resistant cells and parental cells. miR-149-5p could also decrease the proliferation, migratory aptitude and invasiveness of both cell lines, and promote their apoptosis through decreasing expression of TGFβ2 [17]. In this kind of cancer, miR-149-5p has been shown to be sponged by DLEU1 lncRNA. Since miR-149-5p could decrease the expression of CDK6, down-regulation of miR-149-5p by this lncRNA leads to up-regulation of CDK6 and a subsequent increase in cell proliferation and cell cycle progression [18].
2.4. Ovarian Cancer
In ovarian cancer, two different studies have shown contradictory results about the role of miR-149-5p. Xu et al. have shown that miR-149-5p silencing increases the sensitivity of ovarian cancer cells to cisplatin. miR-149-5p has been found to target the most important kinase elements of the Hippo signaling pathway, i.e., MST1 and SAV1, leading to the inactivation of TEAD expression [19]. Conversely, Li et al. have shown a tumor suppressor role for miR-149-5p in ovarian cancer cells through targeting FOXM1 [20].
2.5. Osteosarcoma
Expression of miR-149-5p has been found to be decreased in osteosarcoma cells. Forced up-regulation of miR-149-5p has inhibited the growth of these cells through targeting TNFRSF12A. The anti-proliferative impacts of miR-149-5p are exerted through modulation of the PI3K/AKT pathway [21].
2.6. Breast Cancer
Expression of miR-149-5p has been found to be down-regulated in breast cancer cells, parallel with the up-regulation of circ_0072995 and SHMT2 levels. This miRNA has a role in the regulation of anaerobic glycolysis through the suppression of SHMT2 expression [22].
Another experiment in breast cancer cells has shown that the anesthetic agent propofol can alter resistance to trastuzumab via modulation of the IL-6/miR-149-5p molecular axis. Authors have reported production of high levels of IL-6 and IL-8 cytokines, were released by resistant cells, induction of the stemness phenotype mammospheres and enhancement of epithelial-mesenchymal transition (EMT) in trastuzumab resistant cells. Notably, propofol could inhibit all of these processes through the up-regulation of miR-149-5p and subsequent down-regulation of IL-6 expression [23]. Another study has shown the effects of ursolic acid in the attenuation of the paclitaxel resistance phenotype in breast cancer cells through influencing the miR-149-5p/myd88 axis [24].
2.7. Urogenital Cancers
In prostate cancer cells, hsa-miR-149-5p has been found to suppress tumorigenic processes through inhibiting expression of RGS17 [25].
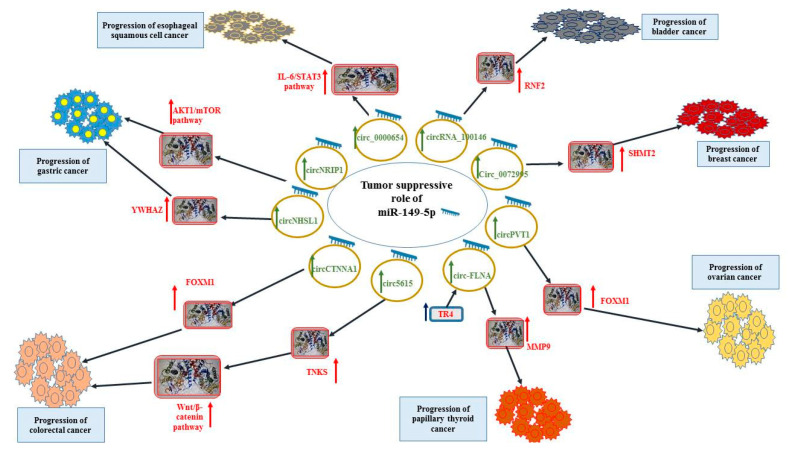
In bladder cancer cells, the anti-cancer effect of miR-149-5p is exerted through the inhibition of expression of RNF2. Notably, miR-149-5p has been shown to be sponged by the oncogenic circRNA_100146 in these cells [26]. Figure 1 shows the tumor-suppressive role of miR-149-5p in esophageal cancer, bladder cancer, breast cancer, ovarian cancer, thyroid cancer and colorectal cancer.
Figure 1.
Tumor-suppressive role of miR-149-5p in esophageal cancer, bladder cancer, breast cancer, ovarian cancer, thyroid cancer and colorectal cancer.
2.8. Lung Cancer
In lung cancer cells, miR-149-5p has been found to down-regulate the expression of B3GNT3, an oncogene that influences lung cancer cell proliferation and invasiveness [27]. Moreover, expression of this miRNA has been found to be decreased by HOTAIR [28], HNF1A-AS1 [29] and MIAT [30] lncRNAs in these cells. In fact, HOTAIR has been shown to induce cisplatin resistance and increase the proliferation, migratory potential and invasiveness of cisplatin-resistant lung cancer cells through targeting miR-149-5p [31].
Another study on lung cancer cells has shown the role of miR-149-5p in the enhancement of the response to gefitinib. The oncogenic lncRNA LINC00460 has been found to promote resistance to EGFR-TKI through sequestering miR-149-5p, thus increasing IL-6 levels and facilitating EMT process [32]. Conversely, comparison of miRNA profiles in gefitinib-resistant human lung cancer cells and the parental cells has shown up-regulation of miR-149-5p in resistant cells. miR-149-5p mimics could reduce the motility of lung cancer cells. Up-regulation of miR-149-5p could efficiently evaluate the half maximal inhibitory concentrations of the cell following treatment with gefitinib. Besides, expressions of miR-149-5p in both cell lines have been inversely correlated with caspase-3 levels. Taken together, miR-149-5p expression is increased in the gefitinib-resistant human lung cancer cells contributing in the acquired resistance to gefitinib [33].
Moreover, over-expression of miR-149-5p in cancer-derived exosomes could enhance growth of tumor cells and inhibit their apoptosis through suppression of AMOTL2 levels [34].
2.9. Hepatocellular Carcinoma
In hepatocellular carcinoma, M2 macrophages have been shown to increase the invasiveness and migratory potential of cancer cells through decreasing miR-149-5p levels and subsequent activation of MMP9 signaling [35]. In this kind of cancer, miR-149-5p can enhance response to sorafenib through decreasing AKT1 levels [36]. Moreover, it can exert anti-cancer effects through decreasing MAP2K1 expression [37].
miR-149-5p has also been shown to target 3’-UTR of the MTHFR transcript. Expression of miR-149-5p has been shown to be increased in both normal hepatocytes and hepatocellular carcinoma cells in response to folic acid deficiency. However, this condition has resulted in different responses in the expression of MTHFR in these two cell lines. In fact, MTHFR levels are reduced in cancerous cells but remained constant in normal hepatocytes in response to folic acid deficiency. Thus, miR-149-5p exerts distinct post-transcriptional influences on this gene upon folic acid deficiency in normal hepatocytes and hepatocellular carcinoma cells. Moreover, this miRNA may have an anti-cancer effect in longstanding folic acid deficiency conditions [38].
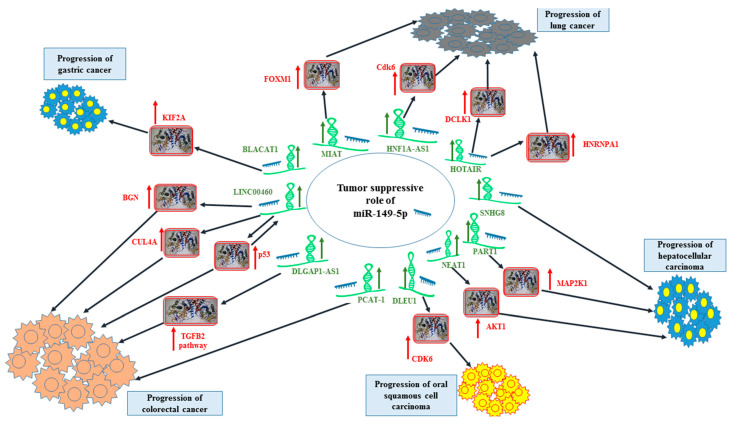
In esophageal squamous cell carcinoma, miR-149-5p exerts anti-cancer effects through the modulation of IL-6/STAT3 axis [39]. Figure 2 shows the tumor-suppressive role of miR-149-5p in gastric cancer, lung cancer, colorectal cancer, hepatocellular carcinoma and oral squamous cell carcinoma.
Figure 2.
Tumor-suppressive role of miR-149-5p in gastric cancer, lung cancer, colorectal cancer, hepatocellular carcinoma and oral squamous cell carcinoma.
Table 1 summarizes the results of studies which assessed the role of miR-149-5p or miR-149-5p-interacting genes in carcinogenesis in cell lines.
Table 1.
Role of miR-149-5p or miR-149-5p-interacting genes in carcinogenesis based on cell line studies (∆: knock-down or deletion, ↑: up-regulation or enhancement, ↓: down-regulation, DDP and CDDP: cisplatin, UA: ursolic acid, PTX: Paclitaxel).
| Tumor Type | Targets/Regulators and Signaling Pathways that Interact with miR-149-5p | Function | Reference |
|---|---|---|---|
| Gastric cancer | circNRIP1, AKT1/mTOR pathway | ∆ circNRIP1: 44001proliferation, ↓ invasion, ↓ migration | [6] |
| BLACAT1, KIF2A | ↑ miR-149-5p: ↓ proliferation, ↓ invasion, ↓ migration | [7] | |
| circNHSL1, YWHAZ | ∆ circNRIP1: ↓ invasion, ↓ migration, ↓ glutaminolysis | [8] | |
| Colorectal cancer | LINC00460, p53, miR-150-5p | ∆ p53 or LINC00460: ↓ oxaliplatin resistance | [9] |
| LINC00460, BGN | ∆ LINC00460: ↓ invasion, ↓ migration | [10] | |
| circCTNNA1, FOXM1 | ↑ circCTNNA1: ↑ proliferation, ↑ invasion, ↑ migration, ↑ viability, ↑ colony-forming, ↑ DNA synthesis ∆ circCTNNA1: ↓ proliferation, ↓ viability, ↓ colony-forming, ↑ G1 arrest |
[11] | |
| PCAT-1 | ∆ PCAT-1: ↓ proliferation, ↓ invasion, ↓ migration, ↑ apoptosis | [12] | |
| DLGAP1-AS1, TGFB2 signaling pathway | ∆ DLGAP1-AS1: ↓ proliferation, ↓ invasion, ↓ migration, ↑ apoptosis, ↑ sensitivity to 5-FU ∆ miR-149-5p: ↑ proliferation, ↓ apoptosis |
[13] | |
| circ5615, TNKS, Wnt/β-catenin pathway | ∆ DLGAP1-AS1: ↓ proliferation, ↓ invasion ↑ DLGAP1-AS1: ↑ proliferation, ↑ transition from G1/S to G2/M phase |
[14] | |
| LINC00460, CUL4A | ∆ DLGAP1-AS1: ↓ proliferation, ↑ apoptosis, ↑ G0/G1 phase arrest | [40] | |
| Medullary thyroid carcinoma | GIT1 | ∆ miR-149-5p: ↑ proliferation, ↑ invasion ↑ miR-149-5p: ↓ proliferation, ↓ invasion |
[15] |
| Papillary thyroid cancer | circ-FLNA, TR4, MMP9 | ∆ TR4: ↓ proliferation, ↓ invasion, ↓ migration | [16] |
| Oral squamous cell carcinoma | TGFβ2 | ↑ miR-149-5p: ↓ proliferation, ↓ invasion, ↓ migration, ↓ CDDP resistance, ↑ apoptosis | [17] |
| DLEU1, CDK6 | ∆ DLEU1: ↓ proliferation, ↑ G1 cell cycle arrest | [18] | |
| Ovarian cancer | Hippo signaling pathway, MST1, SAV1 | ↑ miR-149-5p: ↓ apoptosis, ↑ resistance to CDDP ∆ miR-149-5p: ↑ apoptosis, ↓ resistance to CDDP |
[19] |
| circPVT1, FOXM1 | ∆ circPVT1: ↓ viability, ↓ invasion, ↓ migration | [20] | |
| Osteosarcoma | TNFRSF12A, TWEAK/EGFR pathway | ∆ miR-149-5p: ↑ proliferation, ↑ colony formation | [21] |
| Breast cancer | Circ_0072995, SHMT2 | ∆ Circ_0072995: ↓ proliferation, ↓ invasion, ↓ migration, ↓ anaerobic glycolysis↑ apoptosis ∆ SHMT2: ↓ proliferation, ↓ invasion, ↓ migration, ↓ anaerobic glycolysis↑ apoptosis |
[22] |
| IL-6 | ∆ miR-149-5p: ↓ propofol effects (↓ sensitization to trastuzumab) | [23] | |
| MyD88 | UA treatment: ↑ miR-149-5p = ↓ PTX resistance | [24] | |
| Lung cancer | B3GNT3 | ∆ B3GNT3: ↓ proliferation, ↓ invasion, ↓ colony formation ↑ miR-149-5p: ↓ proliferation, ↓ tumorigenesis |
[27] |
| HOTAIR, HNRNPA1 | ↑ miR-149-5p: ↓ proliferation, ↓ invasion, ↓ migration, ↑ G0/G1 phase arrest, did not affect apoptosis | [28] | |
| HNF1A-AS1, Cdk6 | ∆ HNF1A-AS1: ↓ proliferation, ↓ invasion, ↓ migration | [29] | |
| MIAT, FOXM1 | ∆ MIAT: ↓ proliferation, ↓ invasion, ↓ migration, ↓ colony formation, ↑ G1 phase arrest | [30] | |
| _ | ∆ miR-149-5p: ↓ gefitinib resistance, ↑ apoptosis ↑ miR-149-5p: ↑ proliferation, ↑ gefitinib resistance |
[33] | |
| HOTAIR, DCLK1 | ∆ HOTAIR: ↓ proliferation, ↓ invasion, ↓ migration, ↓ DDP resistance | [31] | |
| AMTOL2 | ↑ miR-149-5p: ↑ proliferation, ↓ apoptosis ∆ miR-149-5p: ↓ proliferation, ↑ apoptosis |
[34] | |
| LINC00460, IL-6 | ∆ LINC00460: ↓ EMT process, ↓ migration, ↓ viability following treatment with various EGFR-TKIs, ↑ apoptosis following treatment with various EGFR-TKIs | [32] | |
| Hepatocellular carcinoma | MMP9 | co-culture: PMA + IL-4 and IL-13: ↑ invasion, ↑ migration | [35] |
| NEAT1, AKT1 | ∆ NEAT1: ↑ sensitivity to sorafenib, ↑ apoptosis, ↓ viability | [36] | |
| PART1, MAP2K1 | ∆ PART1: ↓ proliferation, ↓ invasion, ↓ migration ↑ miR-149-5p: ↓ proliferation, ↓ invasion, ↓ migration |
[37] | |
| SNHG8 | ∆ SNHG8: ↓ proliferation, ↓ invasion, ↓ migration, ↓ viability, ↓ colony formation | [41] | |
| MTHFR, TP53INP1, PDCD4 | FA deficiency: ↑ miR-149-5p | [38] | |
| Prostate carcinoma | RGS17 | ↑ miR-149-5p: ↓ proliferation, ↓ migration, ↓ viability | [25] |
| Esophageal squamous cell cancer | circ_0000654, IL-6/STAT3 signaling pathway | ∆ circ_0000654: ↓ proliferation, ↓ invasion, ↓ migration, ↑ apoptosis ↑ circ_0000654: ↑ proliferation, ↑ invasion, ↑ migration, ↓ apoptosis ↑ miR-149-5p: ↓ proliferation, ↓ invasion, ↓ migration, ↑ apoptosis |
[39] |
| Bladder cancer | circRNA_100146, RNF2 | ∆ circRNA_100146: ↓ proliferation, ↓ invasion, ↓ migration, ↑ apoptosis | [26] |
| Renal cell carcinoma | _ | ↑ miR-149-5p: ↓ proliferation, ↓ migration, ↑ apoptosis | [42] |
| FOXM1 | ↑ miR-149-5p: ↓ proliferation, ↓ invasion, ↓ migration | [43] | |
| Cervical cancer | circ_0075341, AURKA | ∆ circ_0075341: ↓ proliferation, ↓ invasion | [44] |
| circ_0011385, SOX4 | ∆ circ_0011385: ↓ multiplication, ↓ invasion, ↓ migration | [45] | |
| Melanoma | LRIG2 | ↑ miR-149-5p: ↓ colony formation, ↑ apoptosis | [46] |
| Acute myeloid leukemia | FASLG, p-FADD, caspases | ∆ miR-149-5p: ↑ apoptosis | [47] |
| Acute lymphocytic leukemia | circADD2, AKT2 | ↑ circADD2: ↓ proliferation, ↓ miR-149-5p | [48] |
3. Animal Studies
In gastric cancer, in vivo studies have indirectly confirmed the tumor suppressor role of miR-149-5p. In fact, the silencing of circNRIP1 [6], BLACAT1 [7] or circNHSL1 [8] which sequester this miRNA has led to a reduction of tumor size in animal models. Similarly, silencing of LINC00460 [9], circCTNNA1 [11], DLGAP1-AS1 [13] or circ5615 [14] has attenuated tumor growth in animal models of colorectal cancer.
In vivo studies have shown the impact of miR-149-5p in the facilitation of the inhibitory function of propofol against lung metastases in a breast cancer xenograft model [23]. On the other hand, miR-149-5p has been shown to exert oncogenic effects in ovarian cancer, since its silencing has decreased tumor volume and resistance to cisplatin [19]. Table 2 shows the role of miR-149-5p or miR-149-5p-intercating genes in carcinogenesis based on animal models.
Table 2.
Role of miR-149-5p or miR-149-5p-intercating genes in the carcinogenesis based on animal models (∆: knock-down or deletion, ↑: up-regulation or enhancement, ↓: down-regulation, CDDP: cisplatin, UA: ursolic acid, PTX: Paclitaxel).
| Tumor Type | Animal Models | Results | Reference |
|---|---|---|---|
| Gastric cancer | BALB/c nude mice | ∆ circNRIP1 (which sponges miR-149-5p): ↓ tumor volume, ↓ tumor weight | [6] |
| female BALB/c nude mice | ∆ BLACAT1 (which sponges miR-149-5p): ↓ tumor volume, ↓ tumor weight | [7] | |
| male BALB/C nude mice | ∆ circNHSL1 (which sponges miR-149-5p): ↓ tumor volume, ↓ tumor weight | [8] | |
| Colorectal cancer | BALB/c nude mice | ∆ LINC00460 (which sponges miR-149-5p): ↓ tumor volume, ↓ tumor weight | [9] |
| female BALB/c nude mice | ↑ circCTNNA1 (which sponges miR-149-5p): ↑tumor volume, ↑tumor weight | [11] | |
| female nude mice | ∆ DLGAP1-AS1 (which sponges miR-149-5p): ↓tumor growth, ↑5-FU sensitivity | [13] | |
| male BALB/C nude mice | ∆ circ5615 (which sponges miR-149-5p): ↓ tumor growth | [14] | |
| athymic male mice | ∆ LINC00460 (which sponges miR-149-5p): ↓tumor volume, ↓tumor weight | [40] | |
| Papillary thyroid cancer | male nude mice | ∆ circ-FLNA (which sponges miR-149-5p): ↓tumor growth, ↓tumor volume, ↓ metastasis | [16] |
| Ovarian cancer | BALB/c-nu mice | ∆ miR-149-5p: ↓ tumor volume, ↓ tumor weight, ↓ resistance to CDDP | [19] |
| Breast cancer | female BALB/c nude mice | ∆ circ_0072995: ↓ tumor volume, ↓ tumor weight | [22] |
| female athymic mice | ∆ miR-149-5p: ↓ ability of propofol to inhibit lung metastasis | [23] | |
| female athymic nude mice | UA and PTX treatment: ↓tumor volume, ↓ tumor weight | [24] | |
| Lung cancer | BALB/c nude mice | ∆ B3GNT3: ↓ tumor volume, ↓ tumor weight | [27] |
| female BALB/c nude mice | ∆ HNF1A- AS1: ↓ tumor volume, ↓ tumor weight | [29] | |
| female BALB/c nude mice | ∆ MIAT: ↓ tumor volume, ↓ tumor weight | [30] | |
| athymic BALB/c mice | ∆ HOTAIR: ↓ tumor volume, ↓ tumor weight, ↓ DDP resistance | [31] | |
| Hepatocellular carcinoma | nude mice | ↑ macrophages (THP-1): ↑ metastasis miR-149-5p: ↓ metastasis |
[35] |
| nude mice | ∆ SNHG8: ↓ tumor volume, ↓ tumor weight, ↓ metastasis | [41] | |
| Esophageal squamous cell cancer | male BALB/c athymic nude mice | ∆ circ_0000654: ↓ tumor volume, ↓ tumor weight, ↓ metastasis | [39] |
| Acute lymphocytic leukemia | BALB/c nude mice | ↑ circADD2: ↓ tumor volume, ↓ tumor weight | [48] |
4. Clinical Studies
Independent studies in gastric, liver, colorectal, medullary/papillary thyroid, breast, prostate, esophageal, renal, cervical and oral squamous cell cancers as well as osteosarcoma have shown down-regulation of miR-149-5p or its sequestering lncRNAs/circRNAs, thus providing evidence for tumor suppressor role of miR-149-5p (Table 3). In gastric cancer, up-regulation of circNRIP1 (which sponges miR-149-5p) has been associated with shorter overall survival and disease-free survival (DFS) times [6]. In colorectal cancer, up-regulation of LINC00460 (which sponges miR-149-5p) has been associated with poorer DFS [10].
Table 3.
Role of miR-149-5p or miR-149-5p-interacting genes in carcinogenesis based on clinical studies (OS: Overall survival, DFS: disease-free survival, PFS: progression-free rate TNM: tumor-node-metastasis, ANCTs: adjacent non-cancerous tissues, NSCLC: non-small cell lung cancer, ccRCC: clear cell renal cell carcinoma).
| Tumor Type | Samples | Expression of miR-149-5p or Other Genes (Tumor vs. Normal) |
Kaplan-Meier Analysis (Impact of miR-149-5p Dysregulation or Other Genes Dysregulation) | Association of Expression of miR-149-5p or Expression of Other Genes with Clinicopathologic Characteristics | Reference |
|---|---|---|---|---|---|
| Gastric cancer (GC) | 3 pairs of GC tissues and ANCTs | Up-regulation of circNRIP1 (which sponges miR-149-5p) | shorter OS and DFS | GC tumor size and lymphatic invasion | [6] |
| 40 GC patients and 40 normal controls | Up-regulation of circNRIP1 (which sponges miR-149-5p) | _ | _ | ||
| 52 pairs of GC tissues and ANCTs | Up-regulation of BLACAT1 (which sponges miR-149-5p) | _ | WHO grade and TNM stage | [7] | |
| 20 GC patients and 20 normal controls | Up-regulation of CircNHSL1 (which sponges miR-149-5p) |
_ | tumor size, TNM stages, lymphatic metastasis, and distant metastasis | [8] | |
| GEO databases: (GSE23739, GSE26595, GSE26645, GSE28700, GSE33743, GSE54397, GSE63121, GSE78091, and GSE93415 |
Down-regulation of miR-149-5p | _ | distal stomach | [50] | |
| TCGA database | Down-regulation of miR-149-5p | _ | _ | ||
| Stomach Adenocarcinoma (STAD) | serum samples from 130 STAD patients and 116 healthy controls | Down-regulation of miR-149-5p | _ | _ | [51] |
| serum samples from 30 STAD patients and 24 healthy controls | Down-regulation of miR-149-5p | _ | _ | ||
| Colorectal cancer (CRC) | 21 pairs of CRC tissues and ANCTs | Up-regulation of LINC00460 (which sponges miR-149-5p) |
_ | clinical stage and node status | [9] |
| TCGA database | Up-regulation of LINC00460 (which sponges miR-149-5p) | _ | _ | ||
| 40 pairs of CRC tissues and ANCTs | Up-regulation of LINC00460 (which sponges miR-149-5p) | Poorer DFS | _ | [10] | |
| 60 pairs of colon cancer tissues and ANCTs | Up-regulation of circCTNNA1 (which sponges miR-149-5p), Up-regulation of FOXM1 | _ | advanced TNM stage | [11] | |
| 180 pairs of CRC tissues and ANCTs | Up-regulation of circCTNNA1 (which sponges miR-149-5p) | lower OS | _ | ||
| 55 pairs of CRC tissues and ANCTs | Down-regulation of miR-149-5p | _ | _ | [12] | |
| 42 pairs of CRC tumor tissues and ANCTs | Up-regulation of DLGAP1-AS1 (which sponges miR-149-5p) | lower OS | advanced clinical stage (phase III–IV) | [13] | |
| GSE142837 analysis | Up-regulation of circ5615 (which sponges miR-149-5p) | _ | _ | [14] | |
| 99 pairs of CRC tissues and ANCTs | Up-regulation of circ5615 (which sponges miR-149-5p) | shorter OS | higher T stage | ||
| TCGA and GEO dataset: GSE33113 and GSE41328 |
Up-regulation of LINC00460 (which sponges miR-149-5p) | shorter OS | larger tumor sizes, advanced TNM stages, and lymph node metastasis | [40] | |
| Medullary thyroid carcinoma (MTC) | 36 42 pairs of MTC tumor tissues and ANCTs | Down-regulation of miR-149-5p | shorter OS | distant metastases and TNM stage | [15] |
| Papillary thyroid cancer (PTC) | 20 pairs of primary PTC tumors and the paired lymph node metastatic PTC tumors | Up-regulation of TR4 | shorter OS | nodal metastasis | [16] |
| Oral squamous cell carcinoma (OSCC) | 34 pairs of OSCC tissues and ANCTs | Down-regulation of miR-149-5p | _ | _ | [17] |
| 10 pairs of OSCC tissues and ANCTs | Up-regulation of DLEU1 (which sponges miR-149-5p) | _ | advanced stage | [18] | |
| Ovarian cancer | TCGA datasets | Up-regulation of miR-149-5p in chemoresistant tissues | _ | _ | [19] |
| 20 ovarian cancer tissues and 10 benign ovarian lesion tissues | Up-regulation of miR-149-5p in ovarian cancer tissues than benign ovarian tissues | _ | _ | ||
| GTEx database | Up-regulation of circPVT1 | shorter PFS | stage 3/4 and grade 3 OV | [20] | |
| Osteosarcoma | 191 sarcoma patients and 66 adjacent normal samples | Down-regulation of miR-149-5p | shorter OS | age and tumor size | [21] |
| Breast cancer | 70 pairs of breast cancer tissues and ANCTs | Up-regulation of circ_0072995 | _ | _ | [22] |
| 1104 breast cancer tissues and 113 adjacent normal tissues | Up-regulation of SHMT2 | _ | _ | ||
| Lung cancer | TCGA dataset | Up-regulation of B3GNT3 (a target of miR-149-5p) | lower OS and DFS | advanced TNM stages | [27] |
| 120 pairs of lung cancer tissues and ANCTs | Up-regulation of B3GNT3 (a target of miR-149-5p) | lower OS and DFS | late TNM stages, bigger tumor size, distant metastasis and recurrence | ||
| GEO dataset: GSE19188 (91 NSCLC tissues and 65 ANCTs | Up-regulation of HOTAIR (which sponges miR-149-5p) | unfavorable DFS | _ | [28] | |
| 60 pairs of NSCLC tissues and ANCTs | Up-regulation of HNF1A-AS1 (which sponges miR-149-5p) | shorter OS | advanced TMN stage, big tumor size, and lymph node metastasis | [29] | |
| 80 pairs of NSCLC and ANCTs | Up-regulation of MIAT (which sponges miR-149-5p) | shorter OS | advanced pathological stage | [30] | |
| 35 DDP-resistant NSCLC tumors and 35 DDP-sensitive NSCLC tumors | Up-regulation of HOTAIR in DDP-resistant NSCLC tumors than DDP-sensitive NSCLC tumors | _ | TNM stage, lymph node metastasis and DDP response | [31] | |
| GEO dataset: GSE111803 analysis (5 lung adenocarcinoma patients and 5 healthy controls) | Up-regulation of miR-149-5p | _ | advanced clinical stage | [34] | |
| TCGA dataset | Up-regulation of LINC00460 in lung adenocarcinoma tissues with EGFR-activating mutations than in normal tissues | _ | _ | [32] | |
| 62 patients with EGFR-mutant lung adenocarcinoma treated with EGFR-TKI | Up-regulation of LINC00460 | shorter OS and PFS | _ | ||
| Hepatocellular carcinoma (HCC) | 79 pairs of HCC tissues and ANCTs | Up-regulation of NEAT1 (which sponges miR-149-5p) Down-regulation of miR-149-5p |
shorter OS and DFS | tumor stage, lymphatic metastasis, and sorafenib resistance | [36] |
| 48 pairs of HCC tissues and ANCTs | Up-regulation of PART1 (which sponges miR-149-5p) | _ | _ | [37] | |
| 23 pairs of HCC tissues and ANCTs | Up-regulation of SNHG8 (which sponges miR-149-5p) | higher recurrence rates | _ | [41] | |
| Prostate carcinoma (PCa) | GEO DataSets: GSE17317 and GSE34932 |
Down-regulation of miR-149-5p | _ | _ | [25] |
| 30 pairs of PCa tissues and ANCTs | Down-regulation of miR-149-5p | _ | _ | ||
| Esophageal squamous cell cancer (ESCC) | 55 pairs of ESCC tissues and ANCTs | Up-regulation of circ_0000654 (which sponges miR-149-5p) |
_ | higher T stage and local lymph node metastasis | [39] |
| Bladder cancer | 68 pairs of Bladder cancer tissues and ANCTs | Up-regulation of circRNA_100146 (which sponges miR-149-5p) Down-regulation of miR-149-5p |
_ | tumor stage, LN status, histological grade, and tumor size | [26] |
| Bladder cancer | 10 Bladder cancer patients and 10 healthy controls | Up-regulation of miR-149-5p | _ | _ | [49] |
| TCGA database | Up-regulation of miR-149-5p | poor OS | _ | ||
| urine of 70 Bladder cancer patients and 90 healthy controls | Up-regulation of miR-149-5p | _ | _ | ||
| Renal cell carcinoma (RCC) |
32 pairs of RCC tissues and ANCTs | Down-regulation of miR-149-5p | _ | _ | [42] |
| TCGA database (237 pairs of ccRCC tissues and ANCTs) | Down-regulation of miR-149-5p | shorter OS | _ | [52] | |
| 16 pairs of ccRCC tissues and ANCTs | Down-regulation of miR-149-5p | _ | _ | [43] | |
| Cervical cancer (CC) | 37 pairs of CC tissues and ANCTs | Up-regulation of circ_0075341 (which sponges miR-149-5p) | _ | larger tumor size, advanced FIGO stage, and lymph-node metastasis | [44] |
| GEO database: GSE102686 | Up-regulation of circ_0011385 (which sponges miR-149-5p) | _ | _ | [45] | |
| 50 pairs of CC tissues and ANCTs | Up-regulation of circ_0011385 (which sponges miR-149-5p) | _ | higher FIGO stage | ||
| Melanoma | _ melanoma tissue and ANCTs | Up-regulation of LRIG2 (a target of miR-149-5p) | _ | _ | [46] |
| Acute myeloid leukemia (AML) | 45 AML, T cell ALL, and CML patients and 20 healthy controls | Up-regulation of miR-149-5p | _ | _ | [47] |
| Acute lymphocytic leukemia (ALL) | GSE166579 | Dow-regulation of circADD2 (which sponges miR-149-5p) | _ | _ | [48] |
| 30 ALL patients and 30 controls | Dow-regulation of circADD2 (which sponges miR-149-5p) | _ | _ | ||
| Pancreatic ductal adenocarcinoma (PDAC) | 27 PDAC patients and 3 healthy controls | Up-regulation of miR-149-5p | _ | _ | [53] |
In bladder cancer, both tumor suppressor [26] and oncogenic [49] effects have been reported for mR-149-5p. In ovarian cancer, miR-149-5p has been shown to be up-regulated in cancerous tissues, particularly chemoresistant ones compared with controls [19]. However, another study has shown up-regulation of circPVT1, a miR-149-5p-sequestering circRNA in this type of cancer [20]. In lung cancer, while most studies have indicated a tumor suppressor role for miR-149-5p (Table 3), assessment of a GEO dataset has shown up-regulation of this miRNA in cancer patients compared with controls [34].
5. Discussion
miR-149-5p has been shown to be sponged by a number of circRNAs and lncRNAs such as circNRIP1, circNHSL1, circCTNNA1, circ5615, circ-FLNA, circPVT1, circ_0072995, circ_0000654, circ_0075341, circRNA_100146, circ_0011385, circADD2, BLACAT1, LINC00460, PCAT1, DLEU1, DLGAP1-AS1, HOTAIR, MIAT, HNF1A-AS1, NEAT1 and SNHG8. Thus, the most appreciated mechanism of miR-149-5p dysregulation in cancer cells is the sequestering effects of these lncRNAs and circRNAs on it. Identification of the complex network between lncRNAs/circRNAs and miR-149-5p is a prerequisite for the development of targeted therapies for modulation of expression of this miRNA.
TGF-β2, Wnt/β-catenin, Hippo, TWEAK/EGFR and IL-6/STAT3 pathways are the main signaling pathways being regulated by miR-149-5p. Based on the results of Target Scan online tool (www.targetscan.org/cgi-bin/targetscan/vert_71/targetscan.cgi?species=Human&gid=&mir_c=miR-149-5p&mir_sc=&mir_nc=&sortType=cs&allTxs=&incl_nc=100, accessed on 28 November 2021), the top predicted targets of miR-149-5p are summarized in Table 4. Thus, miR-149-5p can regulate several independent cellular processes linked with immune function, gene expression and cell motility.
Table 4.
Top predicted targets of miR-149-5p.
| Rget Gene | Representative Transcript | Gene Name | Number of 3P-seq Tags Supporting UTR + 5 | Conserved Sites | Poorly Conserved Sites | 6mer Sites | Cumulative Weighted Context++ Score | Total Context++ Score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | 8mer | 7mer-m8 | 7mer-A1 | Total | 8mer | 7mer-m8 | 7mer-A1 | |||||||
| LRIG2 | ENST00000361127.5 | leucine-rich repeats and immunoglobulin-like domains 2 | 348 | 1 | 1 | 0 | 0 | 3 | 1 | 2 | 0 | 1 | −0.96 | −1.08 |
| STRADB | ENST00000392249.2 | STE20-related kinase adaptor beta | 12 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | −0.87 | −0.87 |
| ZBTB37 | ENST00000367701.5 | zinc finger and BTB domain containing 37 | 213 | 0 | 0 | 0 | 0 | 5 | 0 | 1 | 4 | 4 | −0.83 | −0.92 |
| TTLL9 | ENST00000375921.2 | tubulin tyrosine ligase-like family, member 9 | 5 | 0 | 0 | 0 | 0 | 3 | 2 | 0 | 1 | 1 | −0.82 | −0.82 |
| FRMD7 | ENST00000370879.1 | FERM domain containing 7 | 5 | 0 | 0 | 0 | 0 | 2 | 1 | 1 | 0 | 0 | −0.81 | −0.81 |
| TSPAN31 | ENST00000547992.1 | tetraspanin 31 | 72 | 0 | 0 | 0 | 0 | 2 | 1 | 1 | 0 | 2 | −0.78 | −1.10 |
| CTRC | ENST00000375943.2 | chymotrypsin C (caldecrin) | 5 | 0 | 0 | 0 | 0 | 3 | 1 | 1 | 1 | 2 | −0.77 | −0.77 |
| STRA6 | ENST00000395105.4 | stimulated by retinoic acid 6 | 42 | 0 | 0 | 0 | 0 | 4 | 1 | 3 | 0 | 1 | −0.77 | −0.77 |
| GUCD1 | ENST00000447813.2 | guanylyl cyclase domain containing 1 | 2990 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 1 | −0.77 | −0.77 |
| KCNJ5 | ENST00000529694.1 | potassium inwardly-rectifying channel, subfamily J, member 5 | 12 | 0 | 0 | 0 | 0 | 4 | 0 | 4 | 0 | 2 | −0.75 | |
Then, we assessed the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways of miR-149-5p and its associated genes (Table 5).
Table 5.
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways analyses of miR-149-5p and its associated genes were extracted from the EVmiRNA: the extracellular vesicles miRNA database.
| KEGG ID | KEGG Description | Genes | p Value |
|---|---|---|---|
| ko04962 | Vasopressin-regulated water reabsorption | VAMP2, RAB5C, ADCY9, DCTN2, DCTN4, DCTN5 | 2.45 × 10−52 |
| ko04380 | Osteoclast differentiation | ITGB3, SOCS1, SOCS3, FOS, IFNG, JUN, GAB2, CSF1, IFNAR2, SYK, GRB2, IFNGR2, MITF, IL1A, IL1B, TGFB2 | 1.47 × 10−118 |
| ko05218 | Melanoma | PDGFRB, PDGFRA, TP53, RB1, CDK6, MITF, FGFR1 | 4.09× 10−58 |
| ko05210 | Colorectal cancer | TCF7, TP53, AXIN1, FOS, BIRC5, JUN, TGFB2, MSH6, LEF1 | 1.58× 10−70 |
| ko05332 | Graft-versus-host disease | IFNG, IL6, IL1B, PRF1, IL1A | 3.12× 10−28 |
| ko05202 | Transcriptional misregulation in cancers | MEN1, TAF15, NCOR1, FLT1, CEBPA, SPINT1, ZBTB16, SIX4, GOLPH3, AFF1, KLF3, JMJD1C, TRAF1, SP1, PLAU, PBX1, TP53, CCND2, IL6, ELK4, MMP9, BCL6, EWSR1 | 4.43× 10−160 |
| ko05142 | Chagas disease (American trypanosomiasis) | TGFB2, IRAK1, ADCY1, FOS, IFNG, CALR, JUN, NOS2, IL6, IFNGR2, IL1B, MYD88, CD3E, IRAK4, GNAQ | 3.41× 10−103 |
| ko05140 | Leishmaniasis | MARCKSL1, ITGB1, TGFB2, MYD88, FOS, IFNG, JUN, NOS2, IFNGR2, IL1A, IL1B, IRAK1, IRAK4 | 8.32× 10−71 |
| ko05412 | Arrhythmogenic right ventricular cardiomyopathy (ARVC) | ITGB1, ITGA9, ITGB3, SGCD, GJA1, CACNB2, CACNA1C, CACNA1D, TCF7, LEF1, CACNG4 | 5.27× 10−89 |
| ko04540 | Gap junction | GJA1, PDGFRA, GNAQ, PDGFRB, DRD2, ADCY9, SRC, GRB2, ADCY1 | 7.36× 10−78 |
| ko03015 | mRNA surveillance pathway | SMG5, PABPN1, SMG6, RNPS1, SYMPK, CSTF2, SAP18, UPF2, ACIN1, RNMT | 8.17× 10−83 |
| ko04930 | Type II diabetes mellitus | SOCS4, CACNA1C, SOCS1, SOCS3, SLC2A4, CACNA1D | 1.12× 10−50 |
Moreover, we retrieved genes and gene products associated with GO terms of miR-149-5p (Table 6).
Table 6.
Genes and gene products associated with GO terms of miR-149-5p extracted from AmiGO 2. ARUK-UCL: The Alzheimer’s Research UK University College London, BHF-UCL: British Heart Foundation UK University College London.
| GO Class | Contributor | Reference |
|---|---|---|
| negative regulation of tumor necrosis factor production | ARUK-UCL | PMID:23595570 |
| negative regulation of cell migration involved in sprouting angiogenesis | BHF-UCL | PMID:24463821 |
| negative regulation of blood vessel endothelial cell proliferation involved in sprouting angiogenesis | BHF-UCL | PMID:24463821 |
| negative regulation of endothelial cell chemotaxis to fibroblast growth factor | BHF-UCL | PMID:24463821 |
| mRNA binding involved in posttranscriptional gene silencing | ARUK-UCL | PMID:23595570 |
| negative regulation of epithelial to mesenchymal transition | ARUK-UCL | PMID:25916550 |
| negative regulation of cell migration | ARUK-UCL | PMID:26498692 |
| negative regulation of interleukin-6 production | ARUK-UCL | PMID:24299952 |
| positive regulation of transforming growth factor beta3 production | ARUK-UCL | GO_REF:0000024 |
| positive regulation of collagen biosynthetic process | ARUK-UCL | GO_REF:0000024 |
| gene silencing by miRNA | ARUK-UCL | PMID:23595570 |
| negative regulation of fibroblast growth factor receptor signaling pathway | BHF-UCL | PMID:24463821 |
| cellular response to fibroblast growth factor stimulus | BHF-UCL | PMID:24463821 |
| negative regulation of inflammatory response | ARUK-UCL | GO_REF:0000024 |
| negative regulation of stress fiber assembly | ARUK-UCL | PMID:26498692 |
| negative regulation of NIK/NF-kappaB signaling | ARUK-UCL | GO_REF:0000024 |
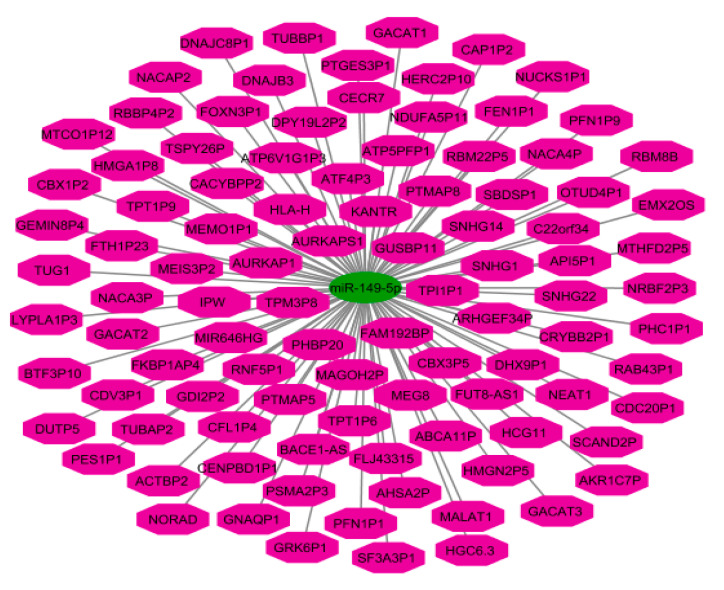
Finally, we depicted an interaction network between miR-149-5p and lncRNAs (Figure 3).
Figure 3.
An interaction network between miR-149-5p and long non-coding RNAs (lncRNAs). The pink octagon and green ellipse indicate lncRNAs and miR-149-5p, respectively. The graph visualization was constructed by Cytoscape version 3.6.1.
The impact of miR-149-5p in carcinogenesis has been appraised by a number of in vitro and in vivo studies which have silenced this miRNA or the related lncRNAs/circRNAs. This miRNA can affect the responses of cancer cells to oxaliplatin, cisplatin, 5-fluouracil, sorafenib, gefitinib and trastuzumab.
Clinical studies have suggested a tumor suppressor role for mR-149-5p in gastric, liver, colorectal, medullary/papillary thyroid, breast, prostate, esophageal, renal, cervical and oral squamous cell cancers as well as osteosarcoma. In bladder and ovarian cancers, both tumor-suppressor and oncogenic effects have been reported for mR-149-5p. In lung cancer, while most studies have indicated a tumor-suppressor role for miR-149-5p, a single study has shown up-regulation of this miRNA in cancer patients compared with controls. Thus, most conducted studies are in favor of a tumor-suppressor role for mR-149-5p. However, a context-dependent role might be considered for this miRNA. Consistent with the tumor-suppressor role for this miRNA, several studies have shown correlation between the down-regulation of this miRNA and shorter survival of patients, indicating a role for miR-149-5p as a prognostic predictor.
Cumulatively, miR-149-5p partakes in a complex functional network which is constructed by several cancer-related lncRNAs and circRNAs. This network efficiently controls the activity of several cancer-related signaling pathways. Modulation of expression of miR-149-5p can be regarded as a therapeutic modality for the attenuation of cancer cells’ growth and the induction of chemosensitivity in these cells.
In conclusion, miR-149-5p is an example of miRNAs with important physiological roles whose expression has been dysregulated in various types of cancer. Future studies should focus on the design of targeted therapies for the amendment of dysregulation of this miRNA.
Author Contributions
S.G.-F. wrote the draft and revised it. M.T. designed and supervised the study. A.T., T.K., S.K. and B.M.H. collected the data and designed the figures and tables. All authors have read and agreed to the published version of the manuscript.
Funding
Not applicable.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Macfarlane L.-A., Murphy P.R. MicroRNA: Biogenesis, Function and Role in Cancer. Curr. Genom. 2010;11:537–561. doi: 10.2174/138920210793175895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hussen B.M., Hidayat H.J., Salihi A., Sabir D.K., Taheri M., Ghafouri-Fard S. MicroRNA: A signature for cancer progression. Biomed. Pharmacother. 2021;138:111528. doi: 10.1016/j.biopha.2021.111528. [DOI] [PubMed] [Google Scholar]
- 3.He Y., Yu D., Zhu L., Zhong S., Zhao J., Tang J. miR-149 in Human Cancer: A Systemic Review. J. Cancer. 2018;9:375–388. doi: 10.7150/jca.21044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan R., Raza S.H.A., Junjvlieke Z., Wang X., Wang H., Cheng G., Mei C., Elnour I.E., Zan L. Bta-miR-149-5p inhibits proliferation and differentiation of bovine adipocytes through targeting CRTCs at both transcriptional and posttranscriptional levels. J. Cell. Physiol. 2020;235:5796–5810. doi: 10.1002/jcp.29513. [DOI] [PubMed] [Google Scholar]
- 5.Zhang B., Dong Y., Liu M., Yang L., Zhao Z. miR-149-5p Inhibits Vascular Smooth Muscle Cells Proliferation, Invasion, and Migration by Targeting Histone Deacetylase 4 (HDAC4) Med. Sci. Monit. 2019;25:7581–7590. doi: 10.12659/MSM.916522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X., Wang S., Wang H., Cao J., Huang X., Chen Z., Xu P., Sun G., Xu J., Lv J., et al. Circular RNA circNRIP1 acts as a microRNA-149-5p sponge to promote gastric cancer progression via the AKT1/mTOR pathway. Mol. Cancer. 2019;18:1–24. doi: 10.1186/s12943-018-0935-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Z., Liu X., Liu X., Niu D. Long Non-Coding RNA BLACAT1 Promotes the Tumorigenesis of Gastric Cancer by Sponging microRNA-149-5p and Targeting KIF2A. Cancer Manag. Res. 2020;12:6629–6640. doi: 10.2147/CMAR.S258178. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Hui C., Tian L., He X. Circular RNA circNHSL1 Contributes to Gastric Cancer Progression Through the miR-149-5p/YWHAZ Axis. Cancer Manag. Res. 2020;12:7117–7130. doi: 10.2147/CMAR.S253152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meng X., Sun W., Yu J., Zhou Y., Gu Y., Han J., Zhou L., Jiang X., Wang C. LINC00460-miR-149-5p/miR-150-5p-Mutant p53 Feedback Loop Promotes Oxaliplatin Resistance in Colorectal Cancer. Mol. Ther. Nucleic Acids. 2020;22:1004–1015. doi: 10.1016/j.omtn.2020.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruan T., Lu S., Xu J., Zhou J.-Y. lncRNA LINC00460 Functions as a Competing Endogenous RNA and Regulates Expression of BGN by Sponging miR-149-5p in Colorectal Cancer. Technol. Cancer Res. Treat. 2021;20:1533033820964238. doi: 10.1177/1533033820964238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen P., Yao Y., Yang N., Gong L., Kong Y., Wu A. Circular RNA circCTNNA1 promotes colorectal cancer progression by sponging miR-149-5p and regulating FOXM1 expression. Cell Death Dis. 2020;11:1–12. doi: 10.1038/s41419-020-02757-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang A.-H., Fan W.-J., Fu L., Wang X.-T. LncRNA PCAT-1 regulated cell proliferation, invasion, migration and apoptosis in colorectal cancer through targeting miR-149-5p. Eur. Rev. Med. Pharmacol. Sci. 2019;23:8310–8320. doi: 10.26355/eurrev_201910_19142. [DOI] [PubMed] [Google Scholar]
- 13.Qu L., Chen Y., Zhang F., He L. The lncRNA DLGAP1-AS1/miR-149-5p/TGFB2 axis contributes to colorectal cancer progression and 5-FU resistance by regulating smad2 pathway. Mol. Ther. Oncol. 2021;20:607–624. doi: 10.1016/j.omto.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma Z., Han C., Xia W., Wang S., Li X., Fang P., Yin R., Xu L., Yang L. circ5615 functions as a ceRNA to promote colorectal cancer progression by upregulating TNKS. Cell Death Dis. 2020;11:1–14. doi: 10.1038/s41419-020-2514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye X., Chen X. miR-149-5p inhibits cell proliferation and invasion through targeting GIT1 in medullary thyroid carcinoma. Oncol. Lett. 2018;17:372–378. doi: 10.3892/ol.2018.9628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ouyang X., Feng L., Yao L., Xiao Y., Hu X., Zhang G., Liu G., Wang Z. Testicular orphan receptor 4 (TR4) promotes papillary thyroid cancer invasion via activating circ-FNLA/miR-149-5p/MMP9 signaling. Mol. Ther. Nucleic Acids. 2021;24:755–767. doi: 10.1016/j.omtn.2021.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo K., He J., Yu D., Açil Y. MiR-149-5p regulates cisplatin chemosensitivity, cell growth, and metastasis of oral squamous cell carcinoma cells by targeting TGFβ2. Int. J. Clin. Exp. Pathol. 2019;12:3728–3739. [PMC free article] [PubMed] [Google Scholar]
- 18.Lv T., Liu H., Wu Y., Huang W. Knockdown of lncRNA DLEU1 inhibits the tumorigenesis of oral squamous cell carcinoma via regulation of miR-149-5p/CDK6 axis. Mol. Med. Rep. 2021;23:1–11. doi: 10.3892/mmr.2021.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu M., Xiao J., Chen M., Yuan L., Li J., Shen H., Yao S. miR-149-5p promotes chemotherapeutic resistance in ovarian cancer via the inactivation of the Hippo signaling pathway. Int. J. Oncol. 2018;52:815–827. doi: 10.3892/ijo.2018.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li M., Chi C., Zhou L., Chen Y., Tang X. Circular PVT1 regulates cell proliferation and invasion via miR-149-5p/FOXM1 axis in ovarian cancer. J. Cancer. 2021;12:611–621. doi: 10.7150/jca.52234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu R.D., Feng F., Yu X.-S., Liu Z.-D., Lao L.-F. miR-149-5p inhibits cell growth by regulating TWEAK/Fn14/PI3K/AKT pathway and predicts favorable survival in human osteosarcoma. Int. J. Immunopathol. Pharmacol. 2018;32:2058738418786656. doi: 10.1177/2058738418786656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qi C., Qin X., Zhou Z., Wang Y., Yang Q., Liao T. Circ_0072995 promotes cell carcinogenesis via up-regulating miR-149-5p-mediated SHMT2 in breast cancer. Cancer Manag. Res. 2020;12:11169–11181. doi: 10.2147/CMAR.S272274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tian D., Tian M., Ma Z.-m., Zhang L.-l., Cui Y.-f., Li J.-l. Anesthetic propofol epigenetically regulates breast cancer trastuzumab resistance through IL-6/miR-149-5p axis. Sci. Rep. 2020;10:1–8. doi: 10.1038/s41598-020-65649-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiang F., Fan Y., Ni Z., Liu Q., Zhu Z., Chen Z., Hao W., Yue H., Wu R., Kang X. Ursolic Acid Reverses the Chemoresistance of Breast Cancer Cells to Paclitaxel by Targeting MiRNA-149-5p/MyD88. Front. Oncol. 2019;9:501. doi: 10.3389/fonc.2019.00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma J., Wei H., Li X., Qu X. Hsa-miR-149-5p Suppresses Prostate Carcinoma Malignancy by Suppressing RGS17. Cancer Manag. Res. 2021;13:2773–2783. doi: 10.2147/CMAR.S281968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H., Niu X., Mao F., Liu X., Zhong B., Jiang H., Fu G. Hsa_circRNA_100146 Acts as a Sponge of miR-149-5p in Promoting Bladder Cancer Progression via Regulating RNF2. OncoTargets Ther. 2020;13:11007–11017. doi: 10.2147/OTT.S273622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun Y., Liu T., Xian L., Liu W., Liu J., Zhou H. B3GNT3, a Direct Target of miR-149-5p, Promotes Lung Cancer Development and Indicates Poor Prognosis of Lung Cancer. Cancer Manag. Res. 2020;12:2381–2391. doi: 10.2147/CMAR.S236565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H., Cui Z., Lv X., Li J., Gao M., Yang Z., Bi Y., Zhang Z., Wang S., Li S., et al. Long Non-coding RNA HOTAIR Function as a Competing Endogenous RNA for miR-149-5p to Promote the Cell Growth, Migration, and Invasion in Non-small Cell Lung Cancer. Front. Oncol. 2020;10 doi: 10.3389/fonc.2020.528520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu L., Chen Y., Li Q., Duan P. lncRNA HNF1A-AS1 modulates non–small cell lung cancer progression by targeting miR-149-5p/Cdk6. J. Cell. Biochem. 2019;120:18736–18750. doi: 10.1002/jcb.29186. [DOI] [PubMed] [Google Scholar]
- 30.Zhou Z., Zhang S., Xiong Y. Long noncoding RNA MIAT promotes non-small cell lung cancer progression by sponging miR-149-5p and regulating FOXM1 expression. Cancer Cell Int. 2020;20:1–10. doi: 10.1186/s12935-020-01432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhan Y., Abuduwaili K., Wang X., Shen Y., Nuerlan S., Liu C. Knockdown of Long Non-Coding RNA HOTAIR Suppresses Cisplatin Resistance, Cell Proliferation, Migration and Invasion of DDP-Resistant NSCLC Cells by Targeting miR-149-5p/Doublecortin-Like Kinase 1 Axis. Cancer Manag. Res. 2020;12:7725–7737. doi: 10.2147/CMAR.S246299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakano Y., Isobe K., Kobayashi H., Kaburaki K., Isshiki T., Sakamoto S., Takai Y., Tochigi N., Mikami T., Iyoda A., et al. Clinical importance of long non-coding RNA LINC00460 expression in EGFR-mutant lung adenocarcinoma. Int. J. Oncol. 2019;56:243–257. doi: 10.3892/ijo.2019.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu Y., Qin X., Yan D., Cao H., Zhou L., Fan F., Zang J., Ni J., Xu X., Sha H., et al. Genome-wide profiling of micro-RNA expression in gefitinib-resistant human lung adenocarcinoma using microarray for the identification of miR-149-5p modulation. Tumor Biol. 2017;39:1010428317691659. doi: 10.1177/1010428317691659. [DOI] [PubMed] [Google Scholar]
- 34.Tian W., Yang H., Zhou B. Integrative analysis of exosomal microRNA-149-5p in lung adenocarcinoma. Aging. 2021;13:7382–7396. doi: 10.18632/aging.202596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu G., Yin L., Ouyang X., Zeng K., Xiao Y., Li Y. M2 Macrophages Promote HCC Cells Invasion and Migration via miR-149-5p/MMP9 Signaling. J. Cancer. 2020;11:1277–1287. doi: 10.7150/jca.35444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu X., Niu Y., Tang G., Wu C. LncRNA NEAT1 modulates sorafenib resistance in hepatocellular carcinoma through regulating the miR-149-5p/AKT1 axis. Saudi J. Gastroenterol. 2020;26:194–203. doi: 10.4103/sjg.SJG_4_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou C., Wang P., Tu M., Huang Y., Xiong F., Wu Y. Long Non-Coding RNA PART1 Promotes Proliferation, Migration and Invasion of Hepatocellular Carcinoma Cells via miR-149-5p/MAP2K1 Axis. Cancer Manag. Res. 2020;12:3771–3782. doi: 10.2147/CMAR.S246311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li C., Ni J., Liu Y.-X., Wang H., Liang Z.-Q., Wang X. Response of MiRNA-22-3p and MiRNA-149-5p to Folate Deficiency and the Differential Regulation of MTHFR Expression in Normal and Cancerous Human Hepatocytes. PLoS ONE. 2017;12:e0168049. doi: 10.1371/journal.pone.0168049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Z., Tie X., Li N., Yi Z., Shen F., Zhang Y. Circular RNA hsa_circ_0000654 promotes esophageal squamous cell carcinoma progression by regulating the miR-149-5p/IL-6/STAT3 pathway. IUBMB Life. 2020;72:426–439. doi: 10.1002/iub.2202. [DOI] [PubMed] [Google Scholar]
- 40.Lian Y., Yan C., Xu H., Yang J., Yu Y., Zhou J., Shi Y., Ren J., Ji G., Wang K. A Novel lncRNA, LINC00460, Affects Cell Proliferation and Apoptosis by Regulating KLF2 and CUL4A Expression in Colorectal Cancer. Mol. Ther. Nucleic Acids. 2018;12:684–697. doi: 10.1016/j.omtn.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dong J., Teng F., Guo W., Yang J., Ding G., Fu Z. lncRNA SNHG8 Promotes the Tumorigenesis and Metastasis by Sponging miR-149-5p and Predicts Tumor Recurrence in Hepatocellular Carcinoma. Cell. Physiol. Biochem. 2018;51:2262–2274. doi: 10.1159/000495871. [DOI] [PubMed] [Google Scholar]
- 42.Jin L., Li Y., Liu J., Yang S., Gui Y., Mao X., Nie G., Lai Y. Tumor suppressor miR-149-5p is associated with cellular migration, proliferation and apoptosis in renal cell carcinoma. Mol. Med. Rep. 2016;13:5386–5392. doi: 10.3892/mmr.2016.5205. [DOI] [PubMed] [Google Scholar]
- 43.Okato A., Arai T., Yamada Y., Sugawara S., Koshizuka K., Fujimura L., Kurozumi A., Kato M., Kojima S., Naya Y. Dual strands of pre-miR-149 inhibit cancer cell migration and invasion through targeting FOXM1 in renal cell carcinoma. Int. J. Mol. Sci. 2017;18:1969. doi: 10.3390/ijms18091969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shao S., Wang C., Wang S., Zhang H., Zhang Y. Hsa_circ_0075341 is up-regulated and exerts oncogenic properties by sponging miR-149-5p in cervical cancer. Biomed. Pharmacother. 2020;121:109582. doi: 10.1016/j.biopha.2019.109582. [DOI] [PubMed] [Google Scholar]
- 45.Xu A.L., Wang W.-S., Zhao M.-Y., Sun J.-N., Chen X.-R., Hou J.-Q. Circular RNA circ_0011385 promotes cervical cancer progression through competitively binding to miR-149-5p and up-regulating SOX4 expression. Kaohsiung J. Med. Sci. 2021 doi: 10.1002/kjm2.12432. [DOI] [PubMed] [Google Scholar]
- 46.Chen W., Zhang J., Xu H., Dai J., Zhang X. The negative regulation of miR-149-5p in melanoma cell survival and apoptosis by targeting LRIG2. Am. J. Transl. Res. 2017;9:4331–4340. [PMC free article] [PubMed] [Google Scholar]
- 47.Tian P., Yan L. Inhibition of MicroRNA-149-5p Induces Apoptosis of Acute Myeloid Leukemia Cell Line THP-1 by Targeting Fas Ligand (FASLG) Med. Sci. Monit. 2016;22:5116–5123. doi: 10.12659/MSM.899114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu Y., Ma X., Zhang H., Wu Y., Kang M., Fang Y., Xue Y. Mechanism of circADD2 as ceRNA in Childhood Acute Lymphoblastic Leukemia. Front. Cell Dev. Biol. 2021:9. doi: 10.3389/fcell.2021.639910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin J.-T., Tsai K.-W. Circulating miRNAs Act as Diagnostic Biomarkers for Bladder Cancer in Urine. Int. J. Mol. Sci. 2021;22:4278. doi: 10.3390/ijms22084278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu X., Pu K., Wang Y., Chen Y., Zhou Y. Gastric cancer-associated microRNA expression signatures: Integrated bioinformatics analysis, validation, and clinical significance. Ann. Transl. Med. 2021;9:797. doi: 10.21037/atm-21-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen X., Li X., Peng X., Zhang C., Liu K., Huang G., Lai Y. Use of a Four-miRNA Panel as a Biomarker for the Diagnosis of Stomach Adenocarcinoma. Dis. Markers. 2020;2020 doi: 10.1155/2020/8880937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xie M., Lv Y., Liu Z., Zhang J., Liang C., Liao X., Liang R., Lin Y., Li Y. Identification and validation of a four-miRNA(miRNA-21-5p, miRNA-9-5p, miR-149-5p, and miRNA-30b-5p) prognosis signature in clear cell renal cell carcinoma. Cancer Manag. Res. 2018;10:5759–5766. doi: 10.2147/CMAR.S187109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang C., Wang J., Cui W., Liu Y., Zhou H., Wang Y., Chen X., Chen X., Wang Z. Serum Exosomal miRNA-1226 as Potential Biomarker of Pancreatic Ductal Adenocarcinoma. OncoTargets Ther. 2021;14:1441–1451. doi: 10.2147/OTT.S296816. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.