Abstract
This article describes several recent examples of miRNA governing the regulation of the gene expression involved in bone matrix construction. We present the impact of miRNA on the subsequent steps in the formation of collagen type I. Collagen type I is a main factor of mechanical bone stiffness because it constitutes 90–95% of the organic components of the bone. Therefore, the precise epigenetic regulation of collagen formation may have a significant influence on bone structure. We also describe miRNA involvement in the expression of genes, the protein products of which participate in collagen maturation in various tissues and cancer cells. We show how non-collagenous proteins in the extracellular matrix are epigenetically regulated by miRNA in bone and other tissues. We also delineate collagen mineralisation in bones by factors that depend on miRNA molecules. This review reveals the tissue variability of miRNA regulation at different levels of collagen maturation and mineralisation. The functionality of collagen mRNA regulation by miRNA, as proven in other tissues, has not yet been shown in osteoblasts. Several collagen-regulating miRNAs are co-expressed with collagen in bone. We suggest that collagen mRNA regulation by miRNA could also be potentially important in bone metabolism.
Keywords: miRNA, extracellular matrix, collagen type I, bone turnover, non-collagenous proteins
1. Introduction
Collagen fibres, particularly type I, which is the dominant type of bone collagen, are responsible for the strength and elasticity of bone tissues [1,2]. The process of collagen formation requires intracellular and extracellular and enzymatic and non-enzymatic stages, which have been well described in bone tissue (Table 1). The well-known enzyme-dependent steps of collagen formation are regulated by tissue-specific miRNA, targeting COL1A1 mRNA (collagen type I gene) and several other mRNA encoding factors relevant to collagen formation. mRNA profiles in tissues are not the main determinants of cell phenotype, since only 19.3% of the total mRNA is tissue specific [3]. Cava et al. found that 39.7% of miRNAs are tissue specific, proving miRNA critical role as regulators of tissue phenotype [3].
Table 1.
The enzymes involved in the crucial stages of collagen formation.
| Intracellular Stage of Collagen Formation |
Enzyme | Gene | miRNA | References | |
|---|---|---|---|---|---|
| 1. | COL1A1 gene expression | − | COL1A1 | miR-625-5p, miR-92a-1-5p, miR-129-5p, miR-133b, miR-133a-3p, miR-196b-5p, miR-196a, miR-98, miR-29a, miR-513b-5p |
[12,13,15,16,17,18,23,24,25,26,27,28,29] |
| 2. | Hydroxylation of proline residues | Prolyl,4- hydroxylase |
P4HA1, P4HA2, P4HA3, P4HB |
miR-124-3p, miR-122, miR-30e, − miR-210 |
[30,31,32] [33,34] |
| 3. | Hydroxylation of lysine residues | Lysyl hydroxylase |
PLOD1, PLOD2, PLOD3 |
miR-34c, miR-124, miR-26, miR-663a |
[35,36,37,38] |
| 4. | Glycosylation of some hydroxylysine residues | Glycosyltransferase |
GLT25D1
GLT25D2 |
− | [39,40,41] |
| 5. | Formation of intra-chain and inter-chain disulphide bonds in terminal peptides | Protein disulphide isomerase | P4HB | miR-210 | [33,34] |
| 6. | Procollagen triple helix formation | Hsp-47 protein | SERPINH1 | miR-29b, miR-29a |
[42,43,44] |
| Extracellular stage of collagen formation | Enzyme | Gene | miRNA | Sources | |
| 7. | Cleavage of amino propeptides | Specific N-propeptidases | ADAM2,3 and 14 | miR-29b | [45] |
| 8. | Cleavage of carboxy propeptides | Specific C- propeptidase | BMP1 | miR-194, miR-29c, miR-29b |
[45,46,47] |
| 9. | Aggregation of collagen fibres | Self-assembly | − | − | [48] |
| 10. | Crosslinking Oxidative deamination of epsilon-amino groups of lysyl and hydroxylysine residues located in telopeptide domains to aldehydes |
Lysyl oxidase | LOX | miR-27, miR29b, miR29a, miR-30a, miR-30b, miR-30b-2-3p, miR-142-3p |
[43,49,50,51,52,53,54,55] |
MicroRNAs (miRNAs) are small endogenous RNAs with a length of 19–25 nucleotides that regulate gene expression post-transcriptionally, inhibiting their translation or enhancing their degradation. The repression mode that dominates in humans acts without slicing the mRNA and does not require extensive pairing to the miRNA. The deadenylases shorten the poly(A) tail, which in most systems causes mRNA destabilisation through decapping and exonucleolytic decay [4]. miRNAs are involved in essential processes for the development and functioning of the cell, such as cell division, differentiation and programmed death. They regulate the expression of genes at the post-transcriptional level, ultimately affecting the number of individual proteins in the body and ensuring the proper course of processes in the cell. Malfunctions or mutations in the genes encoding miRNAs can lead to serious disorders [4].
Therefore, in this review, we aimed to show how miRNAs regulate collagen and non-collagenous genes in various tissues. Understanding the miRNA-dependent systems that regulate collagen in multiple tissues could be significant in studying similar miRNA–mRNA regulatory mechanisms in bone. The role of miRNA in bone metabolism, development, strength and disease has been described in recent, prominent reviews [5,6,7]. We present here several examples of regulatory relationships encompassing miRNA and its target mRNA. Most of them are involved in collagen maturation in skeletal and non-skeletal tissues. We suggest that the same regulatory relationship as in non-skeletal cells may also occur in bone cells.
2. Collagen Formation
2.1. Collagen Expression
2.1.1. COL1A1 Expression Regulation by miRNAs in Skeletal Tissues
Several types of miRNA regulate COL1A1 expression in skeletal tissues. The mechanism of collagen gene expression was described in detail in a review by Karsenty and Park [8]. Type I collagen is a heterotrimer molecule consisting of two α 1 chains and one α 2 chain encoded by genes placed on the 17 and 7 chromosomes. Two types of mRNA and polypeptide chains are synthesised in a 2:1 ratio. Sp1 (Specificity protein 1), Dlx5 (distal-less homeobox 5) and Runx2 (Runt-related transcription factor 2) transcription factors regulate the expression of Col1a1 [9,10,11].
Activation of the toll-like receptor 4/nuclear factor-kappa B (TLR4/NF-κB) pathway in the intervertebral disc induces pro-inflammatory cytokines, which stimulate the expression of miR-625-5p [12]. An increase in miR-625-5p targeting COL1A1 mRNA results in a decrease in the COL1A1 transcript and eventually contributes to the pathological process of intervertebral disc degeneration [12].
2.1.2. Regulation of COL1A1 Expression by miRNAs in Non-Skeletal Tissues
The miR-196a level is lowered in keloid tissues, and type I collagen is highly deposited in keloid tissues [13]. miR-196a could thus be a new therapeutic target for keloid lesions [13]. Keloids spread beyond the borders of the initial injury and do not regress, while hypertrophic scars are contained within the site of injury and may regress over time [14]. Hypertrophic scarring is a severe disease that results from unusual wound healing. Collagen type I could promote hypertrophic scar formation, and the expression of COL1A1 in scar tissue was markedly higher than that in normal tissue. By targeting COL1A1, increased miR-98 represses the proliferation of hypertrophic scarring [15]. Another study demonstrated upregulated sponging RNA H19 expression in keloid tissue fibroblasts and downregulated miR-29a expression [16]. miR-29a represses type I collagen by interacting with the 3’-untranslated region (UTR) of COL1A1 mRNA [16]. Consequently, miR-29a decreases fibroblast proliferation and allows for the development of keloid-targeted treatments [16]. Collagen production stimulated by transforming growth factor β (TGF-β) is activated in hepatic stellate cells involved in liver fibrosis. miR-29a exhibits an anti-fibrotic effect that directly suppresses the expression of COL1A1 and induces apoptosis [17].
COL1A1 expression diminished by miR-513b-5p affects the viability of vascular smooth muscle cells. This process might involve the formation and rupture of the intracranial Sirtuin 6 (silencing information regulatory protein 6, SIRT6), a member of the sirtuin family proteins [18], which regulates collagen metabolism and is an established anti-ageing protein. miR-128 inhibits osteoblast differentiation in osteoporosis by downregulating SIRT6 expression, thus accelerating the development of osteoporosis [19]. miR-378b represses the mRNA expression levels of COL1A1 via interference with SIRT6 in human dermal fibroblasts [20,21].
Several examples of miRNA described here as COL1A1 targeting, e.g., miR-29b or miR-133, are also involved in bone homeostasis but target other genes [6]. Since the regulation of collagen type I by miR-29b or miR-133 was described in other tissues, and these miRNAs are co-expressed in bone, collagen may possibly be regulated in bone by the same miRNAs.
2.1.3. COL1A1 and miRNAs in the Development of Neoplasms
Bone is the most common target organ for high-grade metastatic prostate cancer [22]. Tumour growth in bone is the result of crosstalk between tumour and bone cells. Cancer-derived exosomes participate in osteoclast differentiation and increase osteolysis [23]. miRNAs are delivered by these exosomes and play a key role in bone homeostasis [23]. Among these delivered miRNAs, miR-92a-1-5p downregulates COL1A1 expression, which promotes osteoclast differentiation and inhibits osteoblastogenesis [23].
Increased COL1A1 expression has been observed in cancer cells, and collagen type I contributes to the differentiation and metastatic abilities of human cancer cells [56,57]. Several miRNAs that interact with COL1A1 mRNA could be classified as suppressor miRNAs. miR-129-5p suppresses gastric cancer cell proliferation, migration and invasion by selectively inhibiting COL1A1 [24]. The study of miRNA provides new therapeutic targets for the clinical treatment of gastric cancer [24]. COL1A1 is also a target gene of miR-133b. An increase in miR-133b inhibits the migration of gastric cancer cells [25]. The assessment of miR-133b expression may have a significant impact on the prognosis of gastric cancer patients. An overexpression of closely related miR-133a-3p (difference with miR-133b only in 3′-terminal A → G) suppresses the proliferation, invasion and mitosis of oral and oesophageal squamous cell carcinoma [26,27]. miR-133, shown to also target RUNX2 mRNA, with a consequent reduction in RUNX2 mRNA or its translation, determines the inhibition of osteogenesis in vitro and leads to bone loss in vivo by inducing osteoclastogenesis [7]. miR-133 upregulation results in oestrogen-deficiency-induced osteoporosis and increases the numbers of monocytes in osteoporotic post-menopausal women [58].
In breast cancer, miR-196b-5p inhibits COL1A1 mRNA by transcript downregulation [28,29]. The closely related miR-196a (difference with miR-196b-5p only in a single nucleotide C → A) directly binds to the 3’-UTR of COL1A1 [13].
2.2. Intracellular Stages of Collagen Formation
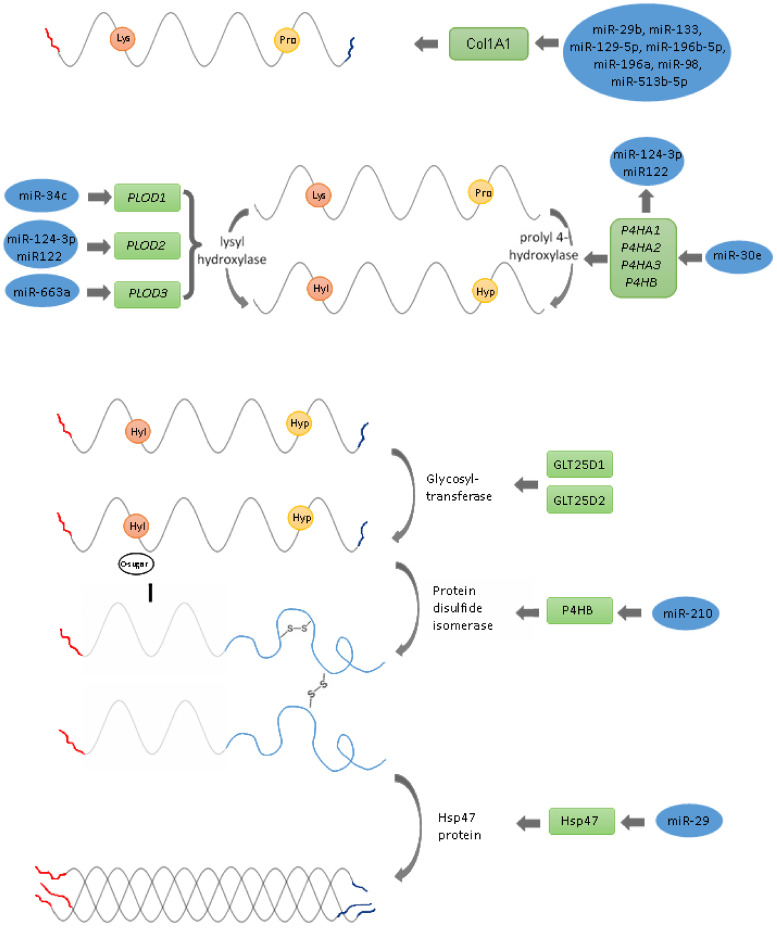
The collagen type I is post-translationally modified in several steps: hydroxylation of proline and lysine residues, glycosylation of some hydroxylysine residues and the formation of intra-chain and inter-chain disulphide bonds in the terminal peptide (Figure 1).
Figure 1.
Schematic presentation of the key steps of collagen synthesis in the cell.
2.2.1. Hydroxylation of Proline and Lysine Residues, Glycosylation of Some Hydroxylysine Residues
Collagen hydroxylation begins in the endoplasmatic reticulum (ER), where prolyl and lysyl hydroxylases convert proline into hydroxyproline or lysine into hydroxylysine, respectively. Three specific enzymes mediate hydroxylation: prolyl 4-hydroxylase, prolyl 3-hydroxylase and the family of lysyl hydroxylases [59].
The hydroxylation of proline residues on collagen, catalysed by collagen prolyl 4-hydroxylase (C-P4H), is essential for the stability of the collagen triple helix. C-P4H is an α2β2 tetramer consisting of three isoenzymes differing in the catalytic α-subunits, which are encoded by P4HA1, P4HA2 and P4HA3 genes and a β-subunit, which is encoded by a single gene, P4HB [60]. The expressions of P4HAs are regulated by multiple cellular factors, including miRNAs [60]. miR-124-3p inhibits the collagen synthesis of vascular smooth muscle cells (VSMC) by directly targeting P4HA1 [32]. miR-122 plays an important role in negatively regulating collagen production in haematopoietic stem cells [61]. miR-122 is significantly upregulated in osteoclasts from osteoporotic patients and showed a significant sensitivity and specificity in distinguishing osteoporotic patients [62].
P4HA2 enhances collagen deposition in the liver in vivo and in vitro, leading to liver fibrosis and liver cancer progression. miR-30e, targeting P4HA2 mRNA, controls collagen production [63].
Proline hydroxylation by procollagen-lysine, 2-oxoglutarate 5-dioxygenase (PLOD) is indispensable for cross-linking and maintaining the mature collagen network and for the formation of the tight triple-helical structure of collagen [64,65]. The reduced form of vitamin C—ascorbic acid—and α-ketoglutarate function as cofactors for these reactions [66]. Three lysyl hydroxylases (LH1, LH2 and LH3) are identified, encoded by PLOD1, PLOD2 and PLOD3 genes. The expression of PLODs is regulated by multiple cytokines, transcription factors and miRNAs [67]. miR-34c inhibits PLOD1 expression in osteosarcoma at both mRNA and protein levels [35]. miR-34c, by targeting and suppressing special AT-rich sequence binding protein 2 (SATB2), has negative effects on bone development [68]. miR-34a was found to suppress the osteoblast differentiation of human MSCs in vitro and reduce the formation of bone following the subcutaneous transfer of hMSC-loaded ceramic beads in SCID mice [69].
miR-124 and miR-26 targets PLOD2 mRNA in regulating the malignant behaviours of laryngeal and renal carcinoma cells and is increased in osteoporotic patients’ blood [36,37,62]. In osteogenesis in vitro miR-26 regulates glycogen synthase kinase 3 β gene GSK3B [70]. PLOD3 is held by miR-663a, modulating collagen IV secretions in physiological conditions and in response to ER stress [38].
2.2.2. Procollagen Glycosylation
Hydroxylated lysyl residues allow galactosyltransferase (GLT25D1 and GLT25D2, also known as COLGALT1 and COLGALT2, and glucosyltransferase to transfer glucose and galactose to the hydroxylysine residues of procollagen [39,40]. The gene encoding the 1-2 glucosyltransferase enzyme remains unknown [40]. miRNA regulates glycosyltransferases, but GLT25D1 and GLT25D2 regulatory miRNAs have not been identified [41]. Glycosylation is engaged in the folding and stability of procollagen and the prevention of inter-chain cross-linking after hydroxylation and glycosylation. C propeptides from two α chains and one β chain are associated with forming intrachain and interchain disulphide bonds [71].
Many patients diagnosed with low bone mineral mass do not experience risky fractures [72]. Enzymatic (glycosylation) and non-enzymatic (glycation) are the two types of post-translational modifications of extracellular matrix proteins and collagen proteins that influence bone quality at the macro-, micro- and nanoscale. Protein in connection with carbohydrates participates in mineralisation, microdamage and the mechanical properties of bone [73].
2.2.3. Formation of Intra-Chain and Inter-Chain Disulphide Bonds in Terminal Peptides
Protein disulphide isomerase (PDI) encoded by P4HB catalyses the formation of intra-chain and inter-chain disulphide bonds in the C-propeptide regions of each collagen peptide [33,74,75]. PDI is the β-subunit of α2β2 heterotetramer of collagen prolyl 4-hydroxylase, and acts as a chaperone, stabilising the functional conformation of collagen [76]. The miR-210 P4HB target is highly downregulated in chemotherapeutic agent temozolomide-resistant glioblastoma multiforme cells [34]. These findings suggest new directions for COL1A1 studies to examine if miR-210 regulates collagen formation in bone.
2.2.4. Procollagen Triple Helix Formation
Hsp-47 (heat shock protein 47, encoded by SERPINH1) is a collagen-specific chaperone essential for correct procollagen triple helix folding in the ER [77,78]. Hsp-47 also prevents the local unfolding of procollagen and aggregation [77,78]. Mutations in the Hsp-47 gene lead to osteogenesis imperfecta [77,78]. In Hsp-47-negative cells, the folding of procollagen is impaired, and due to the malformation of its triple helix, it is more sensitive to proteolysis [77,78]. Hsp47 mRNA contains a binding site for miRNA-29b in the 3′-UTR [43]. The simultaneous downregulation of miR-29a and upregulation of Hsp47 has been reported in cervical squamous cell carcinoma [42]. In breast cancer and glioma cells, the expression of miR-29b suppresses malignant phenotypes by reducing Hsp47 and collagen deposition [43,79]. The downregulation of miR-29b and upregulation of Hsp47 were observed during wound healing [80]. These findings bear translational possibilities, e.g., that targeting miR-29b/Hsp47 might be a strategy to reduce scar formation [80]. Since the miR-29 family plays an important role in regulating osteoclasts, osteoblasts and bone marrow mesenchymal stem cells (BM-MSC), it is likely that Hsp47 is also a target of miR-29 in bone [6]. The triple helix formation is secreted extracellularly via the Golgi apparatus, and probably also the large coat protein complex II (COPII) and TANGO1 [81,82].
2.3. Extracellular Stages of Collagen Formation
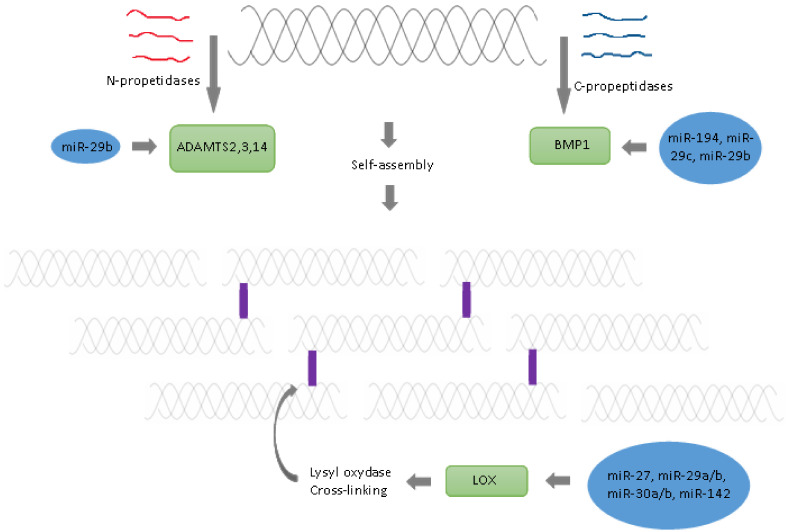
2.3.1. Cleavage of Amino- and Carboxy Propeptides
Extracellularly, procollagen undergoes several additional modifications. Specific N- and C- propeptides remove amino- and carboxy-terminal propeptides, respectively [83]. Procollagens are modified by the amino-terminal propeptides disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS2, -3 and -14), and the carboxy-terminal propeptides bone morphogenetic protein 1 (BMP1)/Tolloid-like families, respectively [84]. BMP1 also cleaves and activates the lysyl oxidase (LOX) precursor, the enzyme catalysing the formation of covalent collagen cross-links, an essential process for fibril stabilisation.
The expression of multiple genes involved in the synthesis and deposition of extracellular matrix in human trabecular meshwork cells is negatively regulated by miR-29b [45]. COL1A1, BMP1 and ADAM12 mRNAs were identified as direct targets of miR-29b. miR-194 expression was found to be strongly negatively associated with metastasis in clinical specimens of non-small cell lung cancer [46]. miR-194 directly targets BMP1 [46]. The resulting downregulation of BMP1 leads to the suppression of TGF-β activity and thus the downregulation of the expression of critical oncogenic genes (matrix metalloproteinases MMP2 and MMP9) [46]. The progression of hepatocellular carcinoma was negatively regulated by miR-29c and promoted by BMP1 [47]. miR-29b overexpression has been implicated in osteoblast differentiation of cell line MC3T3-E1 [85]. Thus, miR-29b is a crucial regulator of the osteoblast phenotype by targeting anti-osteogenic factors and modulating bone extracellular matrix proteins [85]. It remains to be seen if BMP1 is also a target of miR-29b in bones.
2.3.2. Aggregation of Collagen Fibres
Collagen fibres aggregate spontaneously due to a self-assembly mechanism, and the shift between fibres is kept to 1/4 of the length [48]. The mechanical strength of bone is highly dependent on the formation of covalent cross-links within collagen fibrils. The cross-link is initiated by the enzymatic action of LOX [43,84]. LOX catalyses the oxidative deamination of epsilon-amino groups of lysyl and hydroxylysine residues located in telopeptide domains to aldehydes (allysine and hydroxylysine) [2,86,87]. Reactive aldehydes initiate non-enzymatic condensation reactions to form covalent bonds with lysine or hydroxylysine residues, which initiates collagen cross-linking [2]. LOX requires copper and several cofactors: pyridoxal phosphate (vitamin B6) and tyrosyl-lysine quinone [88,89]. Active vitamin D3 (calcitriol) increases the expression of LOX [90]. Thus, a vitamin B6 deficiency, or a copper deficiency lowering LOX activity, impairs enzymatic cross-linking and causes a decrease in bone strength [2,86]. Another possibility resulting from the deficient action of lysyl oxidase causing defects in enzymatic collagen cross-linking is Menkes disease [91]. LOX expression is regulated by miR-27, miR29a/b, miR30a/b and miR-142.
Lysyl oxidase is upregulated and functions as an essential factor during bone morphogenetic protein 4-induced murine mesenchymal stem cells (C3H10T1/2) differentiation to adipocytic cell lineage. miR-27, by targeting Lox mRNA, represses adipogenic lineage differentiation [49]. Additionally, in mesenchymal stem cells, miR-27 determines the inhibition of osteoblastic differentiation by affecting a negative regulator of the Wnt signal [7,92]. miR-27, by targeting SP7 gene (osterix, OSX), suppresses the osteogenic differentiation of maxillary sinus membrane stem cells [93]. miR-27a is one of the most strongly downregulated miRNAs in larger, post-menopausal, osteoporotic women and healthy, pre-menopausal women [92].
miR-29a has been shown to exert a hepatoprotective effect on hepatocellular damage and liver fibrosis [50]. In hepatocellular carcinoma, miR-29a-3p exerts inhibitory activity directly, binding to the 3’-UTR of LOX [50]. A low expression of miR-29a and high expression of LOX indicates poor survival [50]. miR-29a promotes osteogenesis and suppresses histone deacetylase 4 (HDAC4), indicating that decreasing miR-29a may be feasible in the management of osteoporosis [94]. miR-29b is also implicated in the pathogenesis and progression of liver fibrosis by regulating the post-translational processing of extracellular matrix (ECM) and fibril formation [43]. In rat hepatic stellate cells, miR-29b targeting the 3’-UTR sequence in LOX mRNA leads to abnormal collagen structure [43].
Direct regulation of LOX by miR-30a was confirmed in human keratinocytes. miR-30a overexpression strongly impaired epidermal differentiation; a significant increase was also observed in the level of apoptotic cells overexpressing miR-30a in the epidermis [51]. LOX was involved in primary tumour formation, and the establishment of metastases was verified as the downstream target of miR-30c-2-3p gastric cancer [52].
miR-142-3p may be developed and tested with other LOX inhibitors to overcome chemoresistance in triple-negative breast cancer [53]. Chemoresistance is a trait of triple-negative breast cancer, but inhibiting LOX by miR-142-3p reduces collagen cross-linking and fibronectin assembly, increases drug penetration and causes the induction of apoptosis and re-sensitisation to chemotherapy [53].
2.3.3. Crosslinking
Finally, cross-links are formed between and within the chains, which is a significant post-translational modification of collagen, providing the neighbouring collagen molecules with a stable structure and toughness in the bone [2]. Cross-links can be created both enzymatically and non-enzymatically [2,95]. LOX regulate enzymatic cross-link formation [2]. LOX gene expression is regulated by several miRNA families: miR-27, miR29, miR-30 and miR-142 (see Table 1 and Figure 2) [43,49,50,51,52,53,54,55]. The non-enzymatic process is pathological, and it is mainly based on non-enzymatic glycation [2,95,96,97]. During the ageing process or some diseases (e.g., diabetes), the non-enzymatic cross-links, also known as advanced glycation end products, accumulate in collagen and cause reduced plasticity, loss of toughness, lower bone strength and increased fracture risk [2,91,97,98,99].
Figure 2.
Schematic representation of the key steps of collagen synthesis in the extracellular matrix.
3. Collagen Interactions with Non-Collagenous Proteins and Their Impact on Bone Quality
Collagen fibres function as a scaffold for bone cells, and they are the main components providing bone tissue with strength and elasticity. Any disorders during the process of collagen formation affect the mechanical properties of the bone. The bone owes its remarkable mechanical features and resistance to fracture to its hierarchical composite structure. The involvement of miRNA in bone remodelling induced by mechanical forces was recently described [5]. Despite collagen and hydroxyapatite being the most abundant, various non-collagenous matrix components, such as proteoglycans and glycoproteins, play a crucial role in the mechanical properties of bone tissue (Table 2). The matrix of bone comprises collagen type I stabilised by water and non-collagenous proteins (NCPs). NCPs can accumulate Ca2+ ions in their proximity and bind collagen [100]. Interactions between collagens and NCPs significantly impact bone strength and resistance to fracture [101]. NCPs attach cells to the bone matrix, and they are essential for remodelling, which alters the material and whole bone quality [101]. This work focuses on a few significant NCPs and their influence. One of the most important NCPs is osteopontin (OPN), which is produced by osteoblastic and osteoclastic cells at high levels [102].
Table 2.
Regulation of non-collagenous proteins by miRNA.
| Protein | Function | Gene | miRNA | References |
|---|---|---|---|---|
| TGF-β | Regulates the elastic modulus and the hardness of the bone | TGFB1 | See review | [118] |
| Decorin | Models the effects of TGF-β, participates in fibrogenesis of collagen, prevents the mineralisation of collagen [30] | DCN | miR-181b | [122] |
| Biglycan | Inhibits the effects of TGF-β | BGN | miR-330-5p | [120] |
| Osteonectin | Responsible for bone density | SPARC | miRs-29a and -29c | [127] |
| Osteopontin | Crucial for the deformability and resistance of bones to fractures, provides strong adhesion for hydroxyapatite and bone sialoprotein I (BSP-1 or BNSP) | OPN | miRNA-127-5p miR-4262 |
[106, 107] |
| Thrombospondin-2 | Incorporates collagen into the insoluble cross-linked bone matrix | THBS2 | miR-221-3p miR-93-5p miR-203a-3p |
[111,112,113] |
| Alkaline phosphatase | Alters the Pi/PPi ratio in the bone microenvironment to favour bone mineralisation | ALPL | hsa-miR-149, miR-99a-5p MiR-9-5p miR-204-5p |
[128,129,130,131] |
3.1. Osteopontin
OPN is a phosphorylated glycoprotein, and it plays a role in the mineralisation, organisation and deposition of the extracellular matrix in bone [103]. OPN provides the cohesion of collagen fibrils and prevents the collapse of its structures by mineralisation [104]. After an intrafibrillar mineral is formed, OPN may alter the growth habit of extrafibrillar hydroxyapatite crystals through the binding of phosphate and carboxylate groups to minerals [101]. First, the intrafibrillar collagen spaces are mineralised; second, an extrafibrillar mineral coating is formed [104]. Opn knock-out mice had no macroscopic alterations in their bones; however, they showed a high mineral content with larger, more perfect crystals, which reduces fracture properties, such as strength and flexibility [104]. Tests showed a greater decrease in hardness or elastic modulus in younger animals than in adult mice [101]. Hence, OPN promotes the early stages of mineralisation, osteoclast activity and the adherence of osseous cells [102,104,105]. Opn-deprived mice can still mineralise bone; therefore, there must also be other NCPs that play a role in mineralisation [101].
Interestingly, miR-127-5p inhibited the proliferation of chondrocytes through a decrease in OPN expression [106]. miRNA-127-5p mimics suppressed OPN production and the activity of a reporter construct containing the 3’-UTR of human OPN mRNA [106]. The levels of miR-4262 were significantly decreased, and the level of OPN was increased considerably in osteosarcoma specimens compared to the paired adjacent non-tumour tissue [107]. miR-4262 overexpression inhibited OPN-mediated cell invasion, highlighting miR-4262 as an intriguing therapeutic target to prevent osteosarcoma metastases [107]. The role of miR-127-5p and miR-4262, which target the 3’-UTR of OPN in bone, has not yet been reported.
3.2. Thrombospondin-2
Thrombospondin-2 (TSP2, encoded by THBS2) belongs to a family of five extracellular proteins, promoting osteoblast lineage progression [108]. Thsp2-knockout mice had reduced matrix collagen levels compared to the wild types [109]. TSP2 incorporates collagen into the insoluble cross-linked bone matrix, although it does not affect total collagen production [110]. TSP2 is an osteogenesis regulator, expressed in bone progenitor cells, such as MC3T3, acting in an autocrine manner and promoting osteoblast differentiation and bone deposition [111]. However, TSP2 is also produced by non-skeletal cells, such as trophoblasts and colon cancer cells, where the THBS2 gene is expressed and regulated by miRNAs.
miR-221-3p negatively regulated the expression of THBS2 in human first-trimester placenta (HTR-8/SVneo) cells [111]. In vitro functional assays have revealed that miR-221-3p promotes trophoblast growth, invasion and migration partly via targeting THBS2 [111]. In terminal osteoblasts, miR-221 attenuates differentiation through Dkk2 mRNA targeting. THBS2 has been predicted and experimentally verified as a direct target of miR-93-5p [112]. The promotion function of miR-93-5p on cervical cancer operates by targeting the THBS2/matrix metalloproteinases signalling pathway. miR-93-5p might be a potential therapeutic target for the treatment of cervical cancer [112].
miR-203a-3p binds to the 3’-untranslated region of THBS2 mRNA, downregulating THBS2 expression and inhibiting colorectal cancer progression and metastasis [113]. The expression of miR-203a-3p, which serves a tumour-suppressive role, was significantly downregulated in colorectal cancer tissues. miR-203a-3p was determined to target THBS2 to inhibit colorectal cancer progression and metastasis; thus, miR-203a-3p may be considered a potentially novel approach to treating colorectal cancer [113].
miR-93, miR-203 and miR-221 are also involved in bone homeostasis by targeting BMP2, Smad9 and RUNX2, respectively [114,115,116]. Since miR-93, miR-203 and miR-221 targeting THBS2 was described in other tissues, THBS2 may possibly be regulated in bone by the same miRNAs.
3.3. Biglycan and Decorin
Signalisation of TGF-β has been identified as responsible for the elasticity and hardness of the bone matrix [117]. The miRNA regulation of the TGF-β pathway has already been reviewed [118]. The secretion of TGF-β by bone cells increases minerals in the bone matrix from about 33–42% [117]. While TGF-β is an essential regulator of osteogenesis, proteoglycans are significant regulators of TGF-β [117]. Small leucine-rich proteoglycans, such as biglycan and decorin, are responsible for binding this growth factor. Biglycan and decorin are necessary to maintain an adequate number of mature osteoblasts by modulating the proliferation and survival of bone marrow stromal cells. Biglycan deficiency in bones impacts the number of collagen fibril anomalies, including changes in both fibril size and fibril shape, as well as peak bone mass, and can result in the development of osteopenia [119].
Biglycan is the target gene of miR-330-5p in bone marrow stem cells [120]. Importantly, biglycan was able to reverse the regulatory effects of miR-330-5p on the BMP/Smad pathway, alkaline phosphatase activity and mineralisation ability in BM-MSCs [120]. miR-330-5p facilitates osteogenesis in bone marrow stromal cells through the biglycan-induced BMP/Smad pathway, thus alleviating the progression of osteoporosis. Osteoporosis mice with in vivo knockdown of miR-330-5p presented higher bone mineral density and BV/TV than controls [120].
Osteoblastic differentiation is regulated by TGF-β signalling molecules, such as TGF-β type I receptor (TβR-I/Alk5). miR-181a promotes osteoblastic differentiation by repressing TGF-β type I receptors [121]. Furthermore, blocking miR-181b reversed TGF-β1-induced decorin downregulation [122]. On the other hand, by blocking miR-181b, it was proven that decorin is an miR-181b target gene in dermal fibroblasts [122]. The deletion of miR-181 in mice revealed a potential role for this miRNA in controlling growth plate and bone development, as well as the enhancement of osteogenesis in vitro [123].
3.4. Osteonectin
Osteonectin, also called ‘secreted protein acidic and rich in cysteine’ (encoded by SPARC, BM-40), is produced by osteoblasts. Osteonectin is responsible for bone density and trabecular volume, with effects on microarchitecture, the most abundant non-collagenous matrix protein in bone. The loss of osteonectin in the bone results in decreased trabecular bone [124]. Osteonectin modifies the balance between bone formation and bone resorption, affecting both sides of the remodelling process. Furthermore, it initiates active mineralisation, and complexes of osteonectin–collagen bind apatite crystals and free calcium ions [125]. The proximal region of the mouse osteonectin 3′-UTR contains a well-conserved, dominant regulatory motif that interacts with miRs-29a and miR-29c. Wnt signalling, which is increased during osteoblastic differentiation, induces the expression of miR-29 [126].
4. Interactions between Collagen and the Mineral Phase in Bone
Here, we will focus on the organisation of mineralised collagen fibrils and the interactions of mineral compounds with collagen. Phenomena that occur on the nanoscale level are fundamental to understanding the structure of bone. Mineral compounds in bone belong to the calcium phosphate family. Precisely, it is a type of apatite [Ca5(PO4)3(F, Cl, OH)], the form of which resembles hydroxyapatite [Ca10(PO4)6(OH)2] but varies in its contents [100]. The presence of substantial carbonate CO32− together with some cations (H+, Na+, Mg2+, K+) and anions (F−, Cl−) proves the specificity of the apatite [100]. The mineral occurs in bone in the form of flake-like nanosized platelets, whose length ranges from 15–200 nm, thickness from 1–7 nm and width from 10–80 nm [100,132,133]. According to classical models, the apatite crystals lie between collagen fibrils [134,135]. It has been postulated that interactions between collagen and the mineral phase provide bone with appropriate mechanical properties.
When implemented into the fibril, the mineral phase causes a decrease in length and induces internal stress. On the other hand, the presence of the mineral phase improves the stiffness, strength, extensibility and toughness of the fibril.
Alkaline phosphatase (gene ALPL) activity alters the Pi/PPi ratio in the bone microenvironment to favour bone mineralisation [136] and is an agent in mineralisation regulated by miRNA. Skeletal ALPL is anchored to membrane inositol-phosphate on the outer surface of osteoblasts [137]. ALPL-mediated hydrolysis of PPi has two implications. First, it reduces the amount of PPi in the bone microenvironment. Second, it increases the amount of mineralisation promoting Pi ions by liberating them from PPi [136]. Lower protein expression levels were observed using Western blotting, confirming that ALPL is directly targeted by hsa-miR-149 [128]. Inhibition of miR-99a-5p in MC3T3 pre-osteoblastic cells promoted osteogenic differentiation, whereas its overexpression suppressed the levels of osteogenic-specific ALPL expression [129]. miR-9-5p could bind directly to the 3’-UTR of ALPL and inhibit the expression of ALPL. Circular RNA circSIPA1L1 upregulates ALPL by targeting miR-204-5p and promoting the osteogenic differentiation of stem cells from the apical papilla [131].
There are many lines of evidence indicating that mechanical stress regulates bone metabolism and promotes bone growth. BMP, Wnt, ERK1/2 and OPG/RANKL are the main molecules that regulate the effects of mechanical loading on bone formation. Recently, miRNAs were found to be involved in mechanical stress-mediated bone metabolism [138].
However, Wolff’s law of bone remodelling states that bone adapts to its loads [139]. Some concepts hold that miRNAs may be critical for exercise-induced bone remodelling [140].
5. Conclusions
The quantity and spatial organisation of collagen, non-collagenous proteins, polysaccharides and minerals determine the mechanical properties of bone. This review discusses the epigenetic regulation of collagen and non-collagenous protein by miRNA in numerous tissues and diseases. Several collagen-regulating miRNAs are co-expressed with collagen in bone. The functionality of miRNA–collagen relationships has not yet been proven in osteoblasts, osteocytes and osteoclasts. We conclude that the regulatory mechanisms of these collagen and target miRNAs could also regulate bone metabolism, which is a matter for future research.
Author Contributions
Conceptualisation, T.P.L.; writing—original draft preparation, U.G., K.K., M.M., A.U., A.C. and T.P.L.; writing—review and editing, P.P.J.; supervision, M.S., P.K., A.P. and M.G. All authors have read and agreed to the published version of the manuscript.
Funding
The project was funded by the Polish National Science Centre (NCN), a Polish governmental agency, under project number 2016/21/B/NZ7/02748 (OPUS 11 competition). No commercial resources were used.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Buckwalter J.A., Glimcher M.J., Cooper R.R., Recker R. Bone biology. I: Structure, blood supply, cells, matrix, and mineralization. Instr. Course Lect. 1996;45:371–386. [PubMed] [Google Scholar]
- 2.Saito M., Marumo K. Effects of Collagen Crosslinking on Bone Material Properties in Health and Disease. Calcif. Tissue Int. 2015;97:242–261. doi: 10.1007/s00223-015-9985-5. [DOI] [PubMed] [Google Scholar]
- 3.Cava C., Bertoli G., Castiglioni I. Portrait of Tissue-Specific Coexpression Networks of Noncoding RNAs (miRNA and lncRNA) and mRNAs in Normal Tissues. Comput. Math. Methods Med. 2019;2019:9029351. doi: 10.1155/2019/9029351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartel D.P. Metazoan MicroRNAs. Cell. 2018;173:20–51. doi: 10.1016/j.cell.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y., Jia L., Zheng Y., Li W. Bone remodeling induced by mechanical forces is regulated by miRNAs. Biosci. Rep. 2018;38:38. doi: 10.1042/BSR20180448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao Y., Patil S., Qian A. The Role of MicroRNAs in Bone Metabolism and Disease. Int. J. Mol. Sci. 2020;21:6081. doi: 10.3390/ijms21176081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellavia D., De Luca A., Carina V., Costa V., Raimondi L., Salamanna F., Alessandro R., Fini M., Giavaresi G. Deregulated miRNAs in bone health: Epigenetic roles in osteoporosis. Bone. 2019;122:52–75. doi: 10.1016/j.bone.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Karsenty G., Park R.W. Regulation of type I collagen genes expression. Int. Rev. Immunol. 1995;12:177–185. doi: 10.3109/08830189509056711. [DOI] [PubMed] [Google Scholar]
- 9.Moradifard S., Hoseinbeyki M., Emam M.M., Parchiniparchin F., Ebrahimi-Rad M. Association of the Sp1 binding site and -1997 promoter variations in COL1A1 with osteoporosis risk: The application of meta-analysis and bioinformatics approaches offers a new perspective for future research. Mutat. Res. Rev. Mutat. Res. 2020;786:108339. doi: 10.1016/j.mrrev.2020.108339. [DOI] [PubMed] [Google Scholar]
- 10.Tadić T., Erceg I., Stover M.L., Rowe D.W., Lichtler A.C. Dlx5 induces expression of COL1A1 promoter contained in a retrovirus vector. Croat. Med. J. 2001;42:436–439. [PubMed] [Google Scholar]
- 11.Komori T. Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell Tissue Res. 2010;339:189–195. doi: 10.1007/s00441-009-0832-8. [DOI] [PubMed] [Google Scholar]
- 12.Shen L., Xiao Y., Wu Q., Liu L., Zhang C., Pan X. TLR4/NF-ÎşB axis signaling pathway-dependent up-regulation of miR-625–5p contributes to human intervertebral disc degeneration by targeting COL1A1. Am. J. Transl Res. 2018;11:1374–1388. [PMC free article] [PubMed] [Google Scholar]
- 13.Kashiyama K., Mitsutake N., Matsuse M., Ogi T., Saenko V.A., Ujifuku K., Utani A., Hirano A., Yamashita S. miR-196a downregulation increases the expression of type I and III collagens in keloid fibroblasts. J. Investig. Dermatol. 2012;132:1597–1604. doi: 10.1038/jid.2012.22. [DOI] [PubMed] [Google Scholar]
- 14.Gauglitz G.G., Korting H.C., Pavicic T., Ruzicka T., Jeschke M.G. Hypertrophic scarring and keloids: Pathomechanisms and current and emerging treatment strategies. Mol. Med. 2011;17:113–125. doi: 10.2119/molmed.2009.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bi S., Chai L., Yuan X., Cao C., Li S. MicroRNA-98 inhibits the cell proliferation of human hypertrophic scar fibroblasts via targeting Col1A1. Biol. Res. 2017;50:22. doi: 10.1186/s40659-017-0127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z., Feng C., Song K., Qi Z., Huang W., Wang Y. lncRNA-H19/miR-29a axis affected the viability and apoptosis of keloid fibroblasts through acting upon COL1A1 signaling. J. Cell Biochem. 2020;121:4364–4376. doi: 10.1002/jcb.29649. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto Y., Itami S., Kuroda M., Yoshizato K., Kawada N., Murakami Y. MiR-29a Assists in Preventing the Activation of Human Stellate Cells and Promotes Recovery from Liver Fibrosis in Mice. Mol. Ther. 2016;24:1848–1859. doi: 10.1038/mt.2016.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng Z., Chen Y., Wang Y., Li Y., Cheng Q. MicroRNA-513b-5p targets COL1A1 and COL1A2 associated with the formation and rupture of intracranial aneurysm. Sci. Rep. 2021;11:14897. doi: 10.1038/s41598-021-94116-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao J., Liu S., Zhang W., Ni L., Hu Z., Sheng Z., Yin B. MiR-128 inhibits the osteogenic differentiation in osteoporosis by down-regulating SIRT6 expression. Biosci. Rep. 2019;39 doi: 10.1042/BSR20191405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joo D., An S., Choi B.G., Kim K., Choi Y.M., Ahn K.J., An I.S., Cha H.J. MicroRNA-378b regulates α-1-type 1 collagen expression via sirtuin 6 interference. Mol. Med. Rep. 2017;16:8520–8524. doi: 10.3892/mmr.2017.7657. [DOI] [PubMed] [Google Scholar]
- 21.Kim K., An S., Choi B.G., Joo D., Choi Y.M., Ahn K.J., An I.S., Cha H.J. Arctiin regulates collagen type 1α chain 1 mRNA expression in human dermal fibroblasts via the miR-378b-SIRT6 axis. Mol. Med. Rep. 2017;16:9120–9124. doi: 10.3892/mmr.2017.7679. [DOI] [PubMed] [Google Scholar]
- 22.Probert C., Dottorini T., Speakman A., Hunt S., Nafee T., Fazeli A., Wood S., Brown J.E., James V. Communication of prostate cancer cells with bone cells via extracellular vesicle RNA; a potential mechanism of metastasis. Oncogene. 2019;38:1751–1763. doi: 10.1038/s41388-018-0540-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu L., Sui B., Fan W., Lei L., Zhou L., Yang L., Diao Y., Zhang Y., Li Z., Liu J., et al. Exosomes derived from osteogenic tumor activate osteoclast differentiation and concurrently inhibit osteogenesis by transferring COL1A1-targeting miRNA-92a-1–5p. J. Extracell Vesicles. 2021;10:e12056. doi: 10.1002/jev2.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Q., Yu J. MiR-129-5p suppresses gastric cancer cell invasion and proliferation by inhibiting COL1A1. Biochem. Cell Biol. 2018;96:19–25. doi: 10.1139/bcb-2016-0254. [DOI] [PubMed] [Google Scholar]
- 25.Guo Y., Lu G., Mao H., Zhou S., Tong X., Wu J., Sun Q., Xu H., Fang F. miR-133b Suppresses Invasion and Migration of Gastric Cancer Cells via the COL1A1/TGF-Î Axis. OncoTargets Ther. 2020;13:7985–7995. doi: 10.2147/OTT.S249667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He B., Lin X., Tian F., Yu W., Qiao B. MiR-133a-3p Inhibits Oral Squamous Cell Carcinoma (OSCC) Proliferation and Invasion by Suppressing COL1A1. J. Cell Biochem. 2018;119:338–346. doi: 10.1002/jcb.26182. [DOI] [PubMed] [Google Scholar]
- 27.Yin Y., Du L., Li X., Zhang X., Gao Y. miR-133a-3p suppresses cell proliferation, migration, and invasion and promotes apoptosis in esophageal squamous cell carcinoma. J. Cell Physiol. 2019;234:12757–12770. doi: 10.1002/jcp.27896. [DOI] [PubMed] [Google Scholar]
- 28.Zhu X., Rao X., Yao W., Zou X. Downregulation of MiR-196b-5p impedes cell proliferation and metastasis in breast cancer through regulating COL1A1. Am. J. Transl. Res. 2018;10:3122–3132. [PMC free article] [PubMed] [Google Scholar]
- 29.Lu Q., Guo Z., Xie W., Jin W., Zhu D., Chen S., Ren T. The lncRNA H19 Mediates Pulmonary Fibrosis by Regulating the miR-196a/COL1A1 Axis. Inflammation. 2018;41:896–903. doi: 10.1007/s10753-018-0744-4. [DOI] [PubMed] [Google Scholar]
- 30.Ito S., Nagata K. Mutants of collagen-specific molecular chaperone Hsp47 causing osteogenesis imperfecta are structurally unstable with weak binding affinity to collagen. Biochem. Biophys. Res. Commun. 2016;469:437–442. doi: 10.1016/j.bbrc.2015.12.028. [DOI] [PubMed] [Google Scholar]
- 31.Yamauchi M., Noyes C., Kuboki Y., Mechanic G.L. Collagen structural microheterogeneity and a possible role for glycosylated hydroxylysine in type I collagen. Proc. Natl. Acad. Sci. USA. 1982;79:7684–7688. doi: 10.1073/pnas.79.24.7684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen W., Yu F., Di M., Li M., Chen Y., Zhang Y., Liu X., Huang X., Zhang M. MicroRNA-124-3p inhibits collagen synthesis in atherosclerotic plaques by targeting prolyl 4-hydroxylase subunit alpha-1 (P4HA1) in vascular smooth muscle cells. Atherosclerosis. 2018;277:98–107. doi: 10.1016/j.atherosclerosis.2018.08.034. [DOI] [PubMed] [Google Scholar]
- 33.Wilson R., Lees J.F., Bulleid N.J. Protein disulfide isomerase acts as a molecular chaperone during the assembly of procollagen. J. Biol. Chem. 1998;273:9637–9643. doi: 10.1074/jbc.273.16.9637. [DOI] [PubMed] [Google Scholar]
- 34.Lee D., Sun S., Zhang X.Q., Zhang P.D., Ho A.S., Kiang K.M., Fung C.F., Lui W.M., Leung G.K. MicroRNA-210 and Endoplasmic Reticulum Chaperones in the Regulation of Chemoresistance in Glioblastoma. J. Cancer. 2015;6:227–232. doi: 10.7150/jca.10765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu X., Xiang H., Cong W., Yang H., Zhang G., Wang Y., Guo Z., Shen Y., Chen B. PLOD1, a target of miR-34c, contributes to cell growth and metastasis via repressing LATS1 phosphorylation and inactivating Hippo pathway in osteosarcoma. Biochem. Biophys. Res. Commun. 2020;527:29–36. doi: 10.1016/j.bbrc.2020.04.052. [DOI] [PubMed] [Google Scholar]
- 36.Huang J., Zhou X.H., Huang S., Li S., Wu T.T. Involvement of microRNA-124 in biological behaviors of laryngeal carcinoma via PLOD2 signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2020;24:4855–4862. doi: 10.26355/eurrev_202005_21174. [DOI] [PubMed] [Google Scholar]
- 37.Kurozumi A., Kato M., Goto Y., Matsushita R., Nishikawa R., Okato A., Fukumoto I., Ichikawa T., Seki N. Regulation of the collagen cross-linking enzymes LOXL2 and PLOD2 by tumor-suppressive microRNA-26a/b in renal cell carcinoma. Int. J. Oncol. 2016;48:1837–1846. doi: 10.3892/ijo.2016.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amodio G., Sasso E., D’Ambrosio C., Scaloni A., Moltedo O., Franceschelli S., Zambrano N., Remondelli P. Identification of a microRNA (miR-663a) induced by ER stress and its target gene PLOD3 by a combined microRNome and proteome approach. Cell Biol. Toxicol. 2016;32:285–303. doi: 10.1007/s10565-016-9335-z. [DOI] [PubMed] [Google Scholar]
- 39.Schegg B., Hülsmeier A.J., Rutschmann C., Maag C., Hennet T. Core glycosylation of collagen is initiated by two beta(1-O)galactosyltransferases. Mol. Cell Biol. 2009;29:943–952. doi: 10.1128/MCB.02085-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hennet T. Collagen glycosylation. Curr. Opin. Struct. Biol. 2019;56:131–138. doi: 10.1016/j.sbi.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 41.Dall’Olio F., Trinchera M. Epigenetic Bases of Aberrant Glycosylation in Cancer. Int. J. Mol. Sci. 2017;18:998. doi: 10.3390/ijms18050998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamamoto N., Kinoshita T., Nohata N., Yoshino H., Itesako T., Fujimura L., Mitsuhashi A., Usui H., Enokida H., Nakagawa M., et al. Tumor-suppressive microRNA-29a inhibits cancer cell migration and invasion via targeting HSP47 in cervical squamous cell carcinoma. Int. J. Oncol. 2013;43:1855–1863. doi: 10.3892/ijo.2013.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y., Ghazwani M., Li J., Sun M., Stolz D.B., He F., Fan J., Xie W., Li S. MiR-29b inhibits collagen maturation in hepatic stellate cells through down-regulating the expression of HSP47 and lysyl oxidase. Biochem. Biophys. Res. Commun. 2014;446:940–944. doi: 10.1016/j.bbrc.2014.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamada Y., Sugawara S., Arai T., Kojima S., Kato M., Okato A., Yamazaki K., Naya Y., Ichikawa T., Seki N. Molecular pathogenesis of renal cell carcinoma: Impact of the anti-tumor miR-29 family on gene regulation. Int. J. Urol. 2018;25:953–965. doi: 10.1111/iju.13783. [DOI] [PubMed] [Google Scholar]
- 45.Luna C., Li G., Qiu J., Epstein D.L., Gonzalez P. Role of miR-29b on the regulation of the extracellular matrix in human trabecular meshwork cells under chronic oxidative stress. Mol. Vis. 2009;15:2488–2497. [PMC free article] [PubMed] [Google Scholar]
- 46.Wu X., Liu T., Fang O., Leach L.J., Hu X., Luo Z. miR-194 suppresses metastasis of non-small cell lung cancer through regulating expression of BMP1 and p27(kip1) Oncogene. 2014;33:1506–1514. doi: 10.1038/onc.2013.108. [DOI] [PubMed] [Google Scholar]
- 47.Tong Y., Wang M., Dai Y., Bao D., Zhang J., Pan H. LncRNA HOXA-AS3 Sponges miR-29c to Facilitate Cell Proliferation, Metastasis, and EMT Process and Activate the MEK/ERK Signaling Pathway in Hepatocellular Carcinoma. Hum. Gene Ther. Clin. Dev. 2019;30:129–141. doi: 10.1089/humc.2018.266. [DOI] [PubMed] [Google Scholar]
- 48.Ushiki T. Collagen fibers, reticular fibers and elastic fibers. A comprehensive understanding from a morphological viewpoint. Arch. Histol. Cytol. 2002;65:109–126. doi: 10.1679/aohc.65.109. [DOI] [PubMed] [Google Scholar]
- 49.Chen S.Z., Xu X., Ning L.F., Jiang W.Y., Xing C., Tang Q.Q., Huang H.Y. miR-27 impairs the adipogenic lineage commitment via targeting lysyl oxidase. Obesity. 2015;23:2445–2453. doi: 10.1002/oby.21319. [DOI] [PubMed] [Google Scholar]
- 50.Yang Y.L., Tsai M.C., Chang Y.H., Wang C.C., Chu P.Y., Lin H.Y., Huang Y.H. MIR29A Impedes Metastatic Behaviors in Hepatocellular Carcinoma via Targeting LOX, LOXL2, and VEGFA. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22116001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muther C., Jobeili L., Garion M., Heraud S., Thepot A., Damour O., Lamartine J. An expression screen for aged-dependent microRNAs identifies miR-30a as a key regulator of aging features in human epidermis. Aging. 2017;9:2376–2396. doi: 10.18632/aging.101326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Z., Shi L., Li X., Wang X., Wang H., Liu Y. RNF144A-AS1, a TGF-Î1- and hypoxia-inducible gene that promotes tumor metastasis and proliferation via targeting the miR-30c-2-3p/LOX axis in gastric cancer. Cell Biosci. 2021;11:177. doi: 10.1186/s13578-021-00689-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saatci O., Kaymak A., Raza U., Ersan P.G., Akbulut O., Banister C.E., Sikirzhytski V., Tokat U.M., Aykut G., Ansari S.A., et al. Targeting lysyl oxidase (LOX) overcomes chemotherapy resistance in triple negative breast cancer. Nat. Commun. 2020;11:2416. doi: 10.1038/s41467-020-16199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duan Z., Li L., Li Y. Involvement of miR-30b in kynurenine-mediated lysyl oxidase expression. J. Physiol Biochem. 2019;75:135–142. doi: 10.1007/s13105-019-00686-4. [DOI] [PubMed] [Google Scholar]
- 55.Yu Y., Shi E., Gu T., Tang R., Gao S., Wang Y., Liu H. Overexpression of microRNA-30a contributes to the development of aortic dissection by targeting lysyl oxidase. J. Thorac. Cardiovasc. Surg. 2017;154:1862–1869. doi: 10.1016/j.jtcvs.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 56.Liu J., Shen J.X., Wu H.T., Li X.L., Wen X.F., Du C.W., Zhang G.J. Collagen 1A1 (COL1A1) promotes metastasis of breast cancer and is a potential therapeutic target. Discov. Med. 2018;25:211–223. [PubMed] [Google Scholar]
- 57.Hou L., Lin T., Wang Y., Liu B., Wang M. Collagen type 1 alpha 1 chain is a novel predictive biomarker of poor progression-free survival and chemoresistance in metastatic lung cancer. J. Cancer. 2021;12:5723–5731. doi: 10.7150/jca.59723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lv H., Sun Y., Zhang Y. MiR-133 is Involved in Estrogen Deficiency-Induced Osteoporosis through Modulating Osteogenic Differentiation of Mesenchymal Stem Cells. Med. Sci. Monit. 2015;21:1527–1534. doi: 10.12659/MSM.894323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Viguet-Carrin S., Garnero P., Delmas P.D. The role of collagen in bone strength. Osteoporos Int. 2006;17:319–336. doi: 10.1007/s00198-005-2035-9. [DOI] [PubMed] [Google Scholar]
- 60.Shi R., Gao S., Zhang J., Xu J., Graham L.M., Yang X., Li C. Collagen prolyl 4-hydroxylases modify tumor progression. Acta Biochim. Biophys. Sin. 2021;53:805–814. doi: 10.1093/abbs/gmab065. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Li J., Ghazwani M., Zhang Y., Lu J., Li J., Fan J., Gandhi C.R., Li S. miR-122 regulates collagen production via targeting hepatic stellate cells and suppressing P4HA1 expression. J. Hepatol. 2013;58:522–528. doi: 10.1016/j.jhep.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seeliger C., Karpinski K., Haug A.T., Vester H., Schmitt A., Bauer J.S., Van Griensven M. Five freely circulating miRNAs and bone tissue miRNAs are associated with osteoporotic fractures. J. Bone Miner. Res. 2014;29:1718–1728. doi: 10.1002/jbmr.2175. [DOI] [PubMed] [Google Scholar]
- 63.Feng G.X., Li J., Yang Z., Zhang S.Q., Liu Y.X., Zhang W.Y., Ye L.H., Zhang X.D. Hepatitis B virus X protein promotes the development of liver fibrosis and hepatoma through downregulation of miR-30e targeting P4HA2 mRNA. Oncogene. 2017;36:6895–6905. doi: 10.1038/onc.2017.291. [DOI] [PubMed] [Google Scholar]
- 64.Aghajanian P., Hall S., Wongworawat M.D., Mohan S. The Roles and Mechanisms of Actions of Vitamin C in Bone: New Developments. J. Bone Miner. Res. 2015;30:1945–1955. doi: 10.1002/jbmr.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nagata K., Saga S., Yamada K.M. A major collagen-binding protein of chick embryo fibroblasts is a novel heat shock protein. J. Cell Biol. 1986;103:223–229. doi: 10.1083/jcb.103.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trackman P.C. Enzymatic and non-enzymatic functions of the lysyl oxidase family in bone. Matrix Biol. 2015;52:7–18. doi: 10.1016/j.matbio.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qi Y., Xu R. Roles of PLODs in Collagen Synthesis and Cancer Progression. Front. Cell Dev. Biol. 2018;6:66. doi: 10.3389/fcell.2018.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wei J., Shi Y., Zheng L., Zhou B., Inose H., Wang J., Guo X.E., Grosschedl R., Karsenty G. miR-34s inhibit osteoblast proliferation and differentiation in the mouse by targeting SATB2. J. Cell Biol. 2012;197:509–521. doi: 10.1083/jcb.201201057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen L., Holmstrøm K., Qiu W., Ditzel N., Shi K., Hokland L., Kassem M. MicroRNA-34a inhibits osteoblast differentiation and in vivo bone formation of human stromal stem cells. Stem. Cells. 2014;32:902–912. doi: 10.1002/stem.1615. [DOI] [PubMed] [Google Scholar]
- 70.Hu H., Zhao C., Zhang P., Liu Y., Jiang Y., Wu E., Xue H., Liu C., Li Z. miR-26b modulates OA induced BMSC osteogenesis through regulating GSK3beta-catenin pathway. Exp. Mol. Pathol. 2019;107:158–164. doi: 10.1016/j.yexmp.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 71.Gelse K., Pöschl E., Aigner T. Collagens-structure, function, and biosynthesis. Adv. Drug. Deliv. Rev. 2003;55:1531–1546. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 72.Sornay-Rendu E., Boutroy S., Munoz F., Delmas P.D. Alterations of cortical and trabecular architecture are associated with fractures in postmenopausal women, partially independent of decreased BMD measured by DXA: The OFELY study. J. Bone Miner. Res. 2007;22:425–433. doi: 10.1359/jbmr.061206. [DOI] [PubMed] [Google Scholar]
- 73.Sroga G.E., Vashishth D. Effects of bone matrix proteins on fracture and fragility in osteoporosis. Curr. Osteoporos Rep. 2012;10:141–150. doi: 10.1007/s11914-012-0103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bottomley M.J., Batten M.R., Lumb R.A., Bulleid N.J. Quality control in the endoplasmic reticulum: PDI mediates the ER retention of unassembled procollagen C-propeptides. Curr. Biol. 2001;11:1114–1118. doi: 10.1016/S0960-9822(01)00317-7. [DOI] [PubMed] [Google Scholar]
- 75.Sharma U., Carrique L., Vadon-Le Goff S., Mariano N., Georges R.N., Delolme F., Koivunen P., Myllyharju J., Moali C., Aghajari N., et al. Structural basis of homo- and heterotrimerization of collagen I. Nat. Commun. 2017;8:14671. doi: 10.1038/ncomms14671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koski M.K., Anantharajan J., Kursula P., Dhavala P., Murthy A.V., Bergmann U., Myllyharju J., Wierenga R.K. Assembly of the elongated collagen prolyl 4-hydroxylase α2β2 heterotetramer around a central α2 dimer. Biochem. J. 2017;474:751–769. doi: 10.1042/BCJ20161000. [DOI] [PubMed] [Google Scholar]
- 77.Ito S., Nagata K. Biology of Hsp47 (Serpin H1), a collagen-specific molecular chaperone. Semin. Cell Dev. Biol. 2017;62:142–151. doi: 10.1016/j.semcdb.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 78.Ito S., Nagata K. Roles of the endoplasmic reticulum-resident, collagen-specific molecular chaperone Hsp47 in vertebrate cells and human disease. J. Biol. Chem. 2019;294:2133–2141. doi: 10.1074/jbc.TM118.002812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhu J., Xiong G., Fu H., Evers B.M., Zhou B.P., Xu R. Chaperone Hsp47 Drives Malignant Growth and Invasion by Modulating an ECM Gene Network. Cancer Res. 2015;75:1580–1591. doi: 10.1158/0008-5472.CAN-14-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhu Y., Li Z., Wang Y., Li L., Wang D., Zhang W., Liu L., Jiang H., Yang J., Cheng J. Overexpression of miR-29b reduces collagen biosynthesis by inhibiting heat shock protein 47 during skin wound healing. Transl. Res. 2016;178:38–53. doi: 10.1016/j.trsl.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 81.Gorur A., Yuan L., Kenny S.J., Baba S., Xu K., Schekman R. COPII-coated membranes function as transport carriers of intracellular procollagen I. J. Cell Biol. 2017;216:1745–1759. doi: 10.1083/jcb.201702135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Malhotra V., Erlmann P. The pathway of collagen secretion. Annu. Rev. Cell Dev. Biol. 2015;31:109–124. doi: 10.1146/annurev-cellbio-100913-013002. [DOI] [PubMed] [Google Scholar]
- 83.Hulmes D.J.S. Roles of the procollagen C-propeptides in health and disease. Essays Biochem. 2019;63:313–323. doi: 10.1042/EBC20180049. [DOI] [PubMed] [Google Scholar]
- 84.Rosell-Garcia T., Paradela A., Bravo G., Dupont L., Bekhouche M., Colige A., Rodriguez-Pascual F. Differential cleavage of lysyl oxidase by the metalloproteinases BMP1 and ADAMTS2/14 regulates collagen binding through a tyrosine sulfate domain. J. Biol. Chem. 2019;294:11087–11100. doi: 10.1074/jbc.RA119.007806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li Z., Hassan M.Q., Jafferji M., Aqeilan R.I., Garzon R., Croce C.M., Van Wijnen A.J., Stein J.L., Stein G.S., Lian J.B. Biological functions of miR-29b contribute to positive regulation of osteoblast differentiation. J. Biol. Chem. 2009;284:15676–15684. doi: 10.1074/jbc.M809787200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McNerny E.M.B., Gardinier J.D., Kohn D.H. Exercise increases pyridinoline cross-linking and counters the mechanical effects of concurrent lathyrogenic treatment. Bone. 2015;81:327–337. doi: 10.1016/j.bone.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sasaki T., Stoop R., Sakai T., Hess A., Deutzmann R., Schlotzer-Schrehardt U., Chu M.L., Von der Mark K. Loss of fibulin-4 results in abnormal collagen fibril assembly in bone, caused by impaired lysyl oxidase processing and collagen cross-linking. Matrix Biol. 2016;50:53–66. doi: 10.1016/j.matbio.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 88.Bird T.A., Levene C.I. Lysyl oxidase: Evidence that pyridoxal phosphate is a cofactor. Biochem. Biophys. Res. Commun. 1982;108:1172–1180. doi: 10.1016/0006-291X(82)92124-6. [DOI] [PubMed] [Google Scholar]
- 89.Wang S.X., Mure M., Medzihradszky K.F., Burlingame A.L., Brown D.E., Dooley D.M., Smith A.J., Kagan H.M., Klinman J.P. A crosslinked cofactor in lysyl oxidase: Redox function for amino acid side chains. Science. 1996;273:1078–1084. doi: 10.1126/science.273.5278.1078. [DOI] [PubMed] [Google Scholar]
- 90.Nagaoka H., Mochida Y., Atsawasuwan P., Kaku M., Kondoh T., Yamauchi M. 1,25(OH)2D3 regulates collagen quality in an osteoblastic cell culture system. Biochem. Biophys. Res. Commun. 2008;377:674–678. doi: 10.1016/j.bbrc.2008.10.036. [DOI] [PubMed] [Google Scholar]
- 91.Depalle B., Duarte A.G., Fiedler I.A.K., Pujo-Menjouet L., Buehler M.J., Berteau J.P. The different distribution of enzymatic collagen cross-links found in adult and children bone result in different mechanical behavior of collagen. Bone. 2018;110:107–114. doi: 10.1016/j.bone.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 92.You L., Pan L., Chen L., Gu W., Chen J. MiR-27a is Essential for the Shift from Osteogenic Differentiation to Adipogenic Differentiation of Mesenchymal Stem Cells in Postmenopausal Osteoporosis. Cell Physiol. Biochem. 2016;39:253–265. doi: 10.1159/000445621. [DOI] [PubMed] [Google Scholar]
- 93.Peng W., Zhu S., Li X., Weng J., Chen S. miR-27b-3p Suppressed Osteogenic Differentiation of Maxillary Sinus Membrane Stem Cells by Targeting Sp7. Implant. Dent. 2017;26:492–499. doi: 10.1097/ID.0000000000000637. [DOI] [PubMed] [Google Scholar]
- 94.Tan K., Peng Y.T., Guo P. MiR-29a promotes osteogenic differentiation of mesenchymal stem cells via targeting HDAC4. Eur. Rev. Med. Pharmacol. Sci. 2018;22:3318–3326. doi: 10.26355/eurrev_201806_15151. [DOI] [PubMed] [Google Scholar]
- 95.Gauthier R., Follet H., Langer M., Gineyts E., Rongieras F., Peyrin F., Mitton D. Relationships between human cortical bone toughness and collagen cross-links on paired anatomical locations. Bone. 2018;112:202–211. doi: 10.1016/j.bone.2018.04.024. [DOI] [PubMed] [Google Scholar]
- 96.Collier T.A., Nash A., Birch H.L., De Leeuw N.H. Intra-molecular lysine-arginine derived advanced glycation end-product cross-linking in Type I collagen: A molecular dynamics simulation study. Biophys. Chem. 2016;218:42–46. doi: 10.1016/j.bpc.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Poundarik A.A., Wu P.C., Evis Z., Sroga G.E., Ural A., Rubin M., Vashishth D. A direct role of collagen glycation in bone fracture. J. Mech. Behav. Biomed. Mater. 2015;52:120–130. doi: 10.1016/j.jmbbm.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Depalle B., Qin Z., Shefelbine S.J., Buehler M.J. Influence of cross-link structure, density and mechanical properties in the mesoscale deformation mechanisms of collagen fibrils. J. Mech. Behav. Biomed. Mater. 2015;52:1–13. doi: 10.1016/j.jmbbm.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schmidt F.N., Zimmermann E.A., Campbell G.M., Sroga G.E., Puschel K., Amling M., Tang S.Y., Vashishth D., Busse B. Assessment of collagen quality associated with non-enzymatic cross-links in human bone using Fourier-transform infrared imaging. Bone. 2017;97:243–251. doi: 10.1016/j.bone.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stock S.R. The Mineral-Collagen Interface in Bone. Calcif. Tissue Int. 2015;97:262–280. doi: 10.1007/s00223-015-9984-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Morgan S., Poundarik A.A., Vashishth D. Do Non-collagenous Proteins Affect Skeletal Mechanical Properties? Calcif. Tissue Int. 2015;97:281–291. doi: 10.1007/s00223-015-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Icer M.A., Gezmen-Karadag M. The multiple functions and mechanisms of osteopontin. Clin. Biochem. 2018;59:17–24. doi: 10.1016/j.clinbiochem.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 103.Bailey S., Karsenty G., Gundberg C., Vashishth D. Osteocalcin and osteopontin influence bone morphology and mechanical properties. Ann. N. Y. Acad. Sci. 2017;1409:79–84. doi: 10.1111/nyas.13470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rodriguez D.E., Thula-Mata T., Toro E.J., Yeh Y.W., Holt C., Holliday L.S., Gower L.B. Multifunctional role of osteopontin in directing intrafibrillar mineralization of collagen and activation of osteoclasts. Acta Biomater. 2014;10:494–507. doi: 10.1016/j.actbio.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Singh A., Gill G., Kaur H., Amhmed M., Jakhu H. Role of osteopontin in bone remodeling and orthodontic tooth movement: A review. Prog. Orthod. 2018;19:18. doi: 10.1186/s40510-018-0216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tu M., Li Y., Zeng C., Deng Z., Gao S., Xiao W., Luo W., Jiang W., Li L., Lei G. MicroRNA-127-5p regulates osteopontin expression and osteopontin-mediated proliferation of human chondrocytes. Sci. Rep. 2016;6:25032. doi: 10.1038/srep25032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Song K., Liu N., Yang Y., Qiu X. Regulation of osteosarcoma cell invasion through osteopontin modification by miR-4262. Tumour. Biol. 2016;37:6493–6499. doi: 10.1007/s13277-015-4530-8. [DOI] [PubMed] [Google Scholar]
- 108.Calabro N.E., Kristofik N.J., Kyriakides T.R. Thrombospondin-2 and extracellular matrix assembly. Biochim. Biophys. Acta. 2014;1840:2396–2402. doi: 10.1016/j.bbagen.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Alford A.I., Golicz A.Z., Cathey A.L., Reddy A.B. Thrombospondin-2 facilitates assembly of a type-I collagen-rich matrix in marrow stromal cells undergoing osteoblastic differentiation. Connect. Tissue Res. 2013;54:275–282. doi: 10.3109/03008207.2013.811236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Manley E., Jr., Perosky J.E., Khoury B.M., Reddy A.B., Kozloff K.M., Alford A.I. Thrombospondin-2 deficiency in growing mice alters bone collagen ultrastructure and leads to a brittle bone phenotype. J. Appl. Physiol. 2015;119:872–881. doi: 10.1152/japplphysiol.00340.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yang Y., Li H., Ma Y., Zhu X., Zhang S., Li J. MiR-221-3p is down-regulated in preeclampsia and affects trophoblast growth, invasion and migration partly via targeting thrombospondin 2. Biomed. Pharmacother. 2019;109:127–134. doi: 10.1016/j.biopha.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 112.Sun X.Y., Han X.M., Zhao X.L., Cheng X.M., Zhang Y. MiR-93-5p promotes cervical cancer progression by targeting THBS2/MMPS signal pathway. Eur Rev. Med. Pharmacol. Sci. 2019;23:5113–5121. doi: 10.26355/eurrev_201906_18175. [DOI] [PubMed] [Google Scholar]
- 113.Qian Z., Gong L., Mou Y., Han Y., Zheng S. MicroRNA-203a-3p is a candidate tumor suppressor that targets thrombospondin 2 in colorectal carcinoma. Oncol. Rep. 2019;42:1825–1832. doi: 10.3892/or.2019.7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang Y., Wei Q.S., Ding W.B., Zhang L.L., Wang H.C., Zhu Y.J., He W., Chai Y.N., Liu Y.W. Increased microRNA-93-5p inhibits osteogenic differentiation by targeting bone morphogenetic protein-2. PLoS ONE. 2017;12:e0182678. doi: 10.1371/journal.pone.0182678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fan F.Y., Deng R., Qiu L., Wen Q., Zeng Y., Gao L., Zhang C., Kong P., Zhong J., Zeng N., et al. miR-203a-3p.1 is involved in the regulation of osteogenic differentiation by directly targeting Smad9 in MM-MSCs. Oncol Lett. 2019;18:6339–6346. doi: 10.3892/ol.2019.10994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang Y., Gao Y., Cai L., Li F., Lou Y., Xu N., Kang Y., Yang H. MicroRNA-221 is involved in the regulation of osteoporosis through regulates RUNX2 protein expression and osteoblast differentiation. Am. J. Transl. Res. 2017;9:126–135. [PMC free article] [PubMed] [Google Scholar]
- 117.Balooch G., Balooch M., Nalla R.K., Schilling S., Filvaroff E.H., Marshall G.W., Marshall S.J., Ritchie R.O., Derynck R., Alliston T. TGF-beta regulates the mechanical properties and composition of bone matrix. Proc. Natl. Acad. Sci. USA. 2005;102:18813–18818. doi: 10.1073/pnas.0507417102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Garcia J., Delany A.M. MicroRNAs regulating TGFÎ and BMP signaling in the osteoblast lineage. Bone. 2021;143:115791. doi: 10.1016/j.bone.2020.115791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Corsi A., Xu T., Chen X.D., Boyde A., Liang J., Mankani M., Sommer B., Iozzo R.V., Eichstetter I., Robey P.G., et al. Phenotypic effects of biglycan deficiency are linked to collagen fibril abnormalities, are synergized by decorin deficiency, and mimic Ehlers-Danlos-like changes in bone and other connective tissues. J. Bone Miner. Res. 2002;17:1180–1189. doi: 10.1359/jbmr.2002.17.7.1180. [DOI] [PubMed] [Google Scholar]
- 120.Jin S.L., Bai Y.M., Zhao B.Y., Wang Q.H., Zhang H.S. Silencing of miR-330-5p stimulates osteogenesis in bone marrow mesenchymal stem cells and inhibits bone loss in osteoporosis by activating Bgn-mediated BMP/Smad pathway. Eur. Rev. Med. Pharmacol. Sci. 2020;24:4095–4102. doi: 10.26355/eurrev_202004_20987. [DOI] [PubMed] [Google Scholar]
- 121.Bhushan R., Grünhagen J., Becker J., Robinson P.N., Ott C.E., Knaus P. miR-181a promotes osteoblastic differentiation through repression of TGF-Î signaling molecules. Int. J. Biochem Cell Biol. 2013;45:696–705. doi: 10.1016/j.biocel.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 122.Kwan P., Ding J., Tredget E.E. MicroRNA 181b regulates decorin production by dermal fibroblasts and may be a potential therapy for hypertrophic scar. PLoS ONE. 2015;10:e0123054. doi: 10.1371/journal.pone.0123054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hensley A.P., McAlinden A. The role of microRNAs in bone development. Bone. 2021;143:115760. doi: 10.1016/j.bone.2020.115760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Machado do Reis L., Kessler C.B., Adams D.J., Lorenzo J., Jorgetti V., Delany A.M. Accentuated osteoclastic response to parathyroid hormone undermines bone mass acquisition in osteonectin-null mice. Bone. 2008;43:264–273. doi: 10.1016/j.bone.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Thurner P.J., Chen C.G., Ionova-Martin S., Sun L., Harman A., Porter A., Ager J.W. , III; Ritchie, R.O.; Alliston, T. Osteopontin deficiency increases bone fragility but preserves bone mass. Bone. 2010;46:1564–1573. doi: 10.1016/j.bone.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kapinas K., Kessler C.B., Delany A.M. miR-29 suppression of osteonectin in osteoblasts: Regulation during differentiation and by canonical Wnt signaling. J. Cell Biochem. 2009;108:216–224. doi: 10.1002/jcb.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dole N.S., Kapinas K., Kessler C.B., Yee S.P., Adams D.J., Pereira R.C., Delany A.M. A single nucleotide polymorphism in osteonectin 3′ untranslated region regulates bone volume and is targeted by miR-433. J. Bone Miner. Res. 2015;30:723–732. doi: 10.1002/jbmr.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wei J., Chen H., Fu Y., Zhang B., Zhang L., Tao S., Lin F. Experimental study of expression profile and specific role of human microRNAs in regulating atrophic bone nonunion. Medicine. 2020;99:e21653. doi: 10.1097/MD.0000000000021653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Moura S.R., Bras J.P., Freitas J., Osório H., Barbosa M.A., Santos S.G., Almeida M.I. miR-99a in bone homeostasis: Regulating osteogenic lineage commitment and osteoclast differentiation. Bone. 2020;134:115303. doi: 10.1016/j.bone.2020.115303. [DOI] [PubMed] [Google Scholar]
- 130.Zheng C., Bai C., Sun Q., Zhang F., Yu Q., Zhao X., Kang S., Li J., Jia Y. Long noncoding RNA XIST regulates osteogenic differentiation of human bone marrow mesenchymal stem cells by targeting miR-9-5p. Mech. Dev. 2020;162:103612. doi: 10.1016/j.mod.2020.103612. [DOI] [PubMed] [Google Scholar]
- 131.Li Y., Bian M., Zhou Z., Wu X., Ge X., Xiao T., Yu J. Circular RNA SIPA1L1 regulates osteoblastic differentiation of stem cells from apical papilla via miR-204-5p/ALPL pathway. Stem. Cell Res. Ther. 2020;11:461. doi: 10.1186/s13287-020-01970-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bini F., Pica A., Marinozzi A., Marinozzi F. A 3D Model of the Effect of Tortuosity and Constrictivity on the Diffusion in Mineralized Collagen Fibril. Sci. Rep. 2019;9:2658. doi: 10.1038/s41598-019-39297-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Depalle B., Qin Z., Shefelbine S.J., Buehler M.J. Large Deformation Mechanisms, Plasticity, and Failure of an Individual Collagen Fibril with Different Mineral Content. J. Bone Miner. Res. 2016;31:380–390. doi: 10.1002/jbmr.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jager I., Fratzl P. Mineralized collagen fibrils: A mechanical model with a staggered arrangement of mineral particles. Biophys. J. 2000;79:1737–1746. doi: 10.1016/S0006-3495(00)76426-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Landis W.J., Song M.J., Leith A., McEwen L., McEwen B.F. Mineral and organic matrix interaction in normally calcifying tendon visualized in three dimensions by high-voltage electron microscopic tomography and graphic image reconstruction. J. Struct. Biol. 1993;110:39–54. doi: 10.1006/jsbi.1993.1003. [DOI] [PubMed] [Google Scholar]
- 136.Murshed M. Mechanism of Bone Mineralization. Cold Spring Harb. Perspect. Med. 2020;8 doi: 10.1101/cshperspect.a031229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Anh D.J., Dimai H.P., Hall S.L., Farley J.R. Skeletal alkaline phosphatase activity is primarily released from human osteoblasts in an insoluble form, and the net release is inhibited by calcium and skeletal growth factors. Calcif. Tissue Int. 1998;62:332–340. doi: 10.1007/s002239900441. [DOI] [PubMed] [Google Scholar]
- 138.Yuan Y., Zhang L., Tong X., Zhang M., Zhao Y., Guo J., Lei L., Chen X., Tickner J., Xu J., et al. Mechanical Stress Regulates Bone Metabolism Through MicroRNAs. J. Cell Physiol. 2017;232:1239–1245. doi: 10.1002/jcp.25688. [DOI] [PubMed] [Google Scholar]
- 139.Robling A.G., Turner C.H. Mechanical signaling for bone modeling and remodeling. Crit. Rev. Eukaryot Gene Expr. 2009;19:319–338. doi: 10.1615/CritRevEukarGeneExpr.v19.i4.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Qi Z., Liu W., Lu J. The mechanisms underlying the beneficial effects of exercise on bone remodeling: Roles of bone-derived cytokines and microRNAs. Prog. Biophys. Mol. Biol. 2016;122:131–139. doi: 10.1016/j.pbiomolbio.2016.05.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.