Abstract
While there is some evidence that migration to Western countries increases metabolic syndrome (MetS) risk, there is a lack of data pertaining to migration to the Middle East. This study aimed to investigate the relationship between migration and MetS incidence following 24-months of residency in Qatar and identify possible MetS determinants. Migrants to Qatar employed at Hamad Medical Corporation (the national health service) aged 18–65 years were invited to participate. Baseline and follow-up screening for MetS included HbA1c, triglycerides, HDL-cholesterol, blood pressure, and waist circumference. MetS-free migrants were rescreened 24-months post-migration, and the World Health Organization STEPwise questionnaire was administered, assessing changes in lifestyle from baseline. Of 1095 migrants contacted, 472 consented to participate, 205 of whom had normal metabolic parameters at baseline; 160 completed follow-up screening. Most participants were males (74.6%, n = 153) and Asian (81.0%, n = 166/205), and two thirds (66.3%, n = 136/205) were nurses. The incidence of new-onset MetS was 17.0% (n = 27/160, 95%CI; 11.0–23.0%), with 81.0% (n = 129/160, 95%CI; 73.8–86.0%) having at least one MetS element 24-months post-residency in Qatar. Male gender was a risk factor for MetS (adjusted odds ratio (AOR) = 3, p = 0.116), as was consuming medication that could induce MetS (AOR = 6.3, p < 0.001). There is merit in further research targeting these groups.
Keywords: metabolic syndrome, migration, incidence, determinants, Qatar
1. Introduction
The American Heart Association considers metabolic syndrome (MetS) a global epidemic [1,2], affecting around one-quarter of people worldwide and being a significant cause of morbidity and mortality [3,4]. While the syndrome consists of commonly occurring chronic diseases including diabetes mellitus (DM), hypertension (HTN), dyslipidemia, and obesity, it is underdiagnosed by physicians [2,5].
In 2009, the International Diabetes Federation (IDF) task force published the Consensus Worldwide Definition of MetS. Any three of the following five elements are sufficient to diagnose MetS; HTN, DM, elevated triglycerides (TG), low high-density lipoprotein cholesterol (HDL-C), or central obesity (Table 1) [6]. In the past decade, the IDF MetS criteria have been the most widely quoted in the literature [7,8,9,10,11,12], and consequently were adopted throughout this research.
Table 1.
Updated criteria for clinical diagnosis of Metabolic Syndrome [6].
| Measure | Categorical Cut Points |
|---|---|
| Patients were diagnosed as having MetS if they had any three out of the following five elements: | |
| Elevated TG | ≥1.7 mmol/L, or drug treatment for elevated triglycerides |
| Reduced HDL-C | <1.0 mmol/L in males, <1.3 mmol/L in females, or drug treatment for reduced HDL-C |
| Elevated BP | Systolic ≥130 and/or diastolic ≥85 mmHg, or on antihypertensive drug treatment |
| Elevated FBG | ≥5.5 mmol/L, or drug treatment for high glucose |
| Elevated WC | Country/ethnic group waist circumference (a measure of central obesity) [3] |
| Europids: Male ≥ 94 cm, Female ≥ 80 cm | |
| South Asians: Male ≥ 90 cm, Female ≥ 80 cm | |
| Chinese: Male ≥ 90 cm, Female ≥ 80 cm | |
| Japanese: Male ≥ 90 cm, Female ≥ 85 cm | |
| Ethnic South and Central Americans: Use South Asian recommendations until more specific data are available. | |
| Sub-Saharan Africans: Use European data until more specific data are available. | |
| Eastern Mediterranean and Middle East (Arab) populations: Use European data until more specific data are available. | |
TG; triglycerides; HDL-C, high-density lipoprotein cholesterol, BP; blood pressure; FBG, fasting blood glucose; WC, waist circumference.
In addition to modifiable clinical elements, other factors have been linked to an increased risk of MetS development, including psychological factors of anger, stress, anxiety, and sleep deprivation. [13,14]. Moreover, accumulating evidence has linked migration to MetS and its elements of DM, HTN, dyslipidemia, and central obesity [15,16,17,18,19,20].
The process by which migration increase the risk of MetS is multifactorial, with precipitating factors including urbanisation [21,22], Westernisation [23,24,25,26,27,28], reduced leisure-time physical activity [29], acculturation and acculturation stress [30,31,32,33,34,35], and the migration period. The time spent by migrants away from their original home country before developing MetS or any of its core elements is uncertain. Ranges reported in the literature are between 1 and more than 15 years [15,17,30,36,37,38,39,40].
MetS is particularly relevant to the Middle East, with evidence of a high rate of non-communicable diseases amongst migrants to the region. A recent cross-sectional study reported a high prevalence of MetS amongst migrants to Qatar of 48.8% [41]. Moreover, a recent scoping review gave a prevalence of HTN of 30.5%, DM of 9.0–16.0%, and pre-DM of 30.5% amongst South Asian migrants (India, Bangladesh, and Pakistan) [42]. While three studies have assessed MetS prevalence amongst the native Qatar population [41,43,44], only one included migrants, but it did not focus on potential determinants [41].
The aim of this study was to investigate the relationship between migration and the incidence of MetS following 24-month residency in Qatar and to identify possible MetS determinants.
2. Materials and Methods
2.1. Setting
This study was conducted within the Hamad Medical Corporation (the HMC), the national health service provider in Qatar, which comprises 20 facilities. More than 25,000 employees of considerable ethnic diversity work in the HMC, with employees having migrated from more than 90 countries [45].
2.2. Study Design
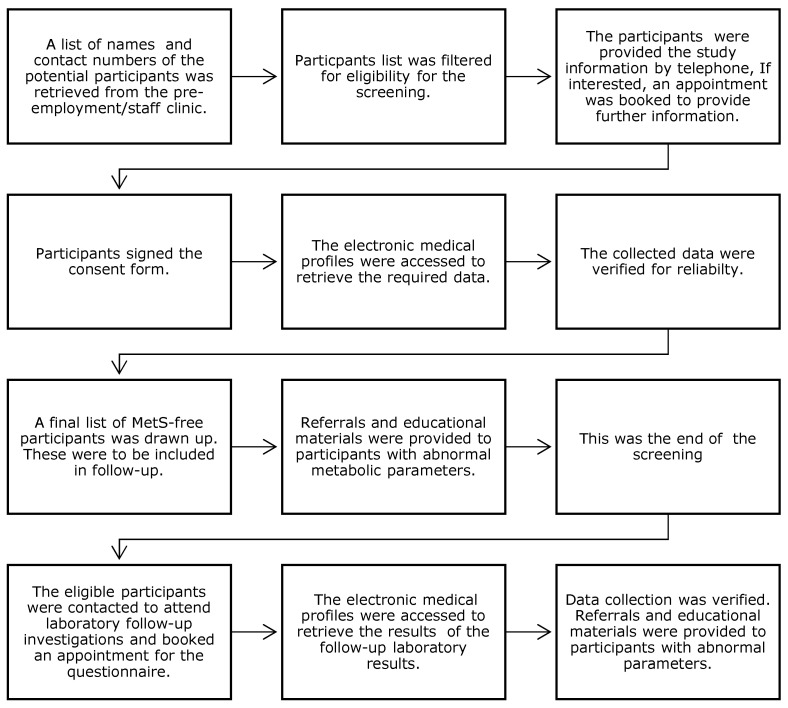
This study had a prospective, longitudinal, observational design with a nested cross-sectional survey. It was conducted in two phases: the first involved baseline screening of migrants within three months of arrival to Qatar (1 July to 31 December 2017). The second phase was a prospective follow-up of those with normal metabolic parameters at baseline at 24-months post-migration. A summary of the recruitment and research processes is shown in Figure 1.
Figure 1.
Flow chart summarising the research process in chronological order.
2.3. Sample Size
Literature MetS rates amongst migrants range from 17.0–39.0%, with the majority of studies reporting 20.0–30.0% [46,47,48,49,50]. Using an estimated rate of 25.0 ± 5%, the study sample size was calculated to be 289 participants, with a precision of 5.0% and 95.0% CIs [51].
2.4. Study Participants and Recruitment
The study population comprised migrants employed at HMC, where all new employees are subjected to a pre-employment medical examination. Participants were included if they were new migrants and had joined HMC during the 6-month recruitment period (July–December 2017) and were aged 16–65 years. Participants were excluded if they were pregnant females or former citizens in Qatar or the Gulf region, due to cultural and economic similarities between the Gulf countries.
Phase 1 (screening): Following ethical approval, the pre-employment staff clinic provided contact details for those who joined HMC from 1 July to 31 December 2017. Participants who fulfilled the inclusion criteria were contacted via telephone and provided with brief study information. If interested, an appointment was booked at which further information was provided and written consent obtained.
Phase 2 (follow-up): Study participants were those identified as MetS-free at Phase 1. Those identified as having MetS or any MetS elements at Phases 1 or 2 were referred for appropriate management and follow-up.
2.5. Data Collection Tools and Data Collection
A data collection tool was developed to address the research objectives, and was piloted among 10 participants to ensure accuracy and comprehensiveness. The pilot data was excluded from the main study. The following data were extracted from the electronic medical records at baseline and follow-up: socio-demographic information, physical measurements, and biochemical measurements of blood glucose and lipid profiles (TG and HDL-C). Data collection was repeated in a 50% random sample by a second researcher to confirm reliability.
At follow-up, participants were contacted to remind them to attend any HMC laboratory, and to book an appointment to complete the WHO STEPwise questionnaire. Laboratory results were extracted from electronic medical profiles, and reliability was again confirmed. The follow-up laboratory tests were fasting TG, fasting blood glucose (FBG), HDL-C, and HbA1c.
The WHO STEPwise characterised any changes in lifestyle as part of the migration process. This validated questionnaire was developed to aid the surveillance of risk factor trends within and between countries [52] and comprises eleven core domains. Participants were requested to answer each question relating to lifestyle habits at baseline and 24-months post-migration.
2.6. Data Handling and Analysis
Data were entered into Microsoft Excel® (Microsoft Corporation, 2018) then exported to SPSS® Version 26 (IBM Corp. Released 2015. IBM statistical analysis for Windows Version 26. Armonk, NY: USA IBM Corp). The Kolmogorov–Smirnov test determined the normality of continuous data. The incidence of new MetS-development and each element were determined by Incidence rate = (Number of new MetS cases or elements)/Number of participants who completed the follow-up) × 100 [53]. Differences in means of normally distributed data from baseline to follow-up were tested using the paired sample t-test. The Wilcoxon Signed Rank test was used for non-normally distributed data. Associations between the incidence of MetS or the elements (1 or 2 elements) with the categorical parameters and the non-normally distributed continuous parameters were tested using the Pearson Chi-square test. Associations with the normally distributed parameters were tested using one-way ANOVA. Significant variables from the univariate analysis were utilised to construct the multiple univariate logistic regression model to determine any association with MetS incidence. All p-values presented were two-tailed, and p-values < 0.05 were considered statistically significant.
2.7. Research Ethics
The study was approved by the School of Pharmacy and Life Sciences ethics committee at Robert Gordon University (RGU) and the Institutional Review Board (IRB) of the Medical Research Center at the HMC in Qatar [RGU S97; HMC-IRB Registration: SCH-HMC-020-2015]. Written consent was obtained from all participants.
3. Results
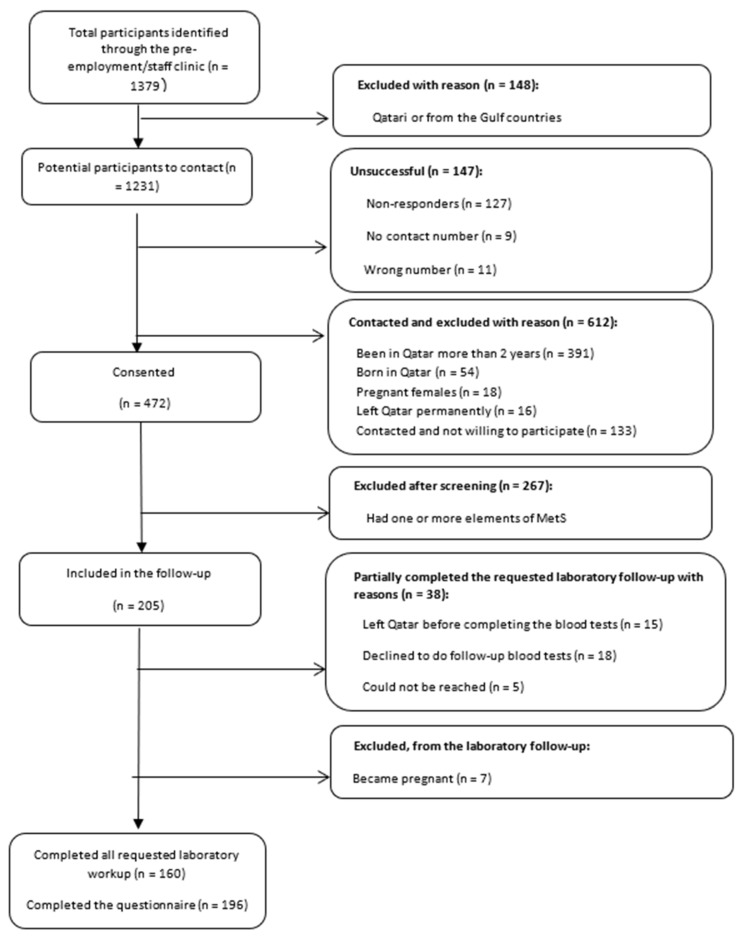
During the study period, 1379 employees joined the HMC, of whom 1084 were contacted (Figure 2). Following access to individual electronic medical profiles, 205 participants were identified as having no element of MetS and were included in the follow-up. Seven participants became pregnant and were excluded from follow-up, and 38 only partially completed the laboratory follow-up (Figure 2).
Figure 2.
Participant flow at screening follow-up.
Of the 205 MetS-free participants at baseline, 153 (74.6%) were males, and 166 (81.0%) came from Asia. The majority were healthcare providers, predominantly nurses (n= 136, 66.3%; Table 2).
Table 2.
Characteristics of the MetS-free participants (n = 205) at baseline within 3 months of arrival to Qatar in 2017.
| Characteristic | Values % (n) |
|---|---|
| Age | 31.2 ± 5.2 years ¥ |
| Gender | |
| Male | 74.6% (153) |
| Female | 25.4% (52) |
| Ethnic origin | |
| Arabs | 8.8% (18) |
| Asian | 81.0% (166) |
| Africans | 2.4% (5) |
| Europeans | 3.4% (7) |
| Others * | 4.4% (9) |
| Marital status | |
| Married | 48.8% (100) |
| Single | 50.2% (103) |
| Divorcee | 1.0% (2) |
| Education | |
| Below bachelor’s degree | 1.5% (3) |
| Graduate (Bachelor & Diploma) | 77.0% (158) |
| Postgraduate (Master and above) | 21.5% (44) |
| Occupation | |
| Doctors | 13.7% (28) |
| Nurses | 66.3% (136) |
| Allied healthcare | 15.1% (31) |
| Others ** | 4.9% (10) |
WHO, World Health Organisation. ¥ ± represents standard deviation (SD) of the mean. * Others included: North Americans, South Americans, Australians–Oceanians. ** Other occupations included: clerk, engineer, IT and aides. Median and IQ range reported due to data skewness.
Of the 205 eligible participants, 196 (95.6%) completed the STEPwise questionnaire 24-months post-residency in Qatar. Most were non-smokers (n = 181, 92.3%) and met the WHO criteria for physical activity (n = 183, 93.4%). The lifestyle parameters at baseline and 24-months post-migration are given in Table 3. The mean times spent in vigorous and moderate activities at follow-up dropped by 50% compared to baseline (p = 0.001 for both, Wilcoxon Signed Rank test). The reduced moderate and vigorous physical activity levels at follow-up were not associated with MetS incidence (p = 0.792, p = 0.680 respectively, Pearson Chi-Square test).
Table 3.
Lifestyle parameters at baseline and 24-months post-migration (n = 196).
| Lifestyle Parameters | Baseline % (n) | 24-Months Post-Migration % (n) |
|---|---|---|
| Smokers | ||
| Yes | 2.1% (4) | 11.2% (22) |
| No | 92.3% (181) | 88.8% (174) |
| Missing | 5.6% (11) | 0 |
| Alcohol consumers | ||
| Yes | 41.8% (82) | |
| No | NA | 58.2% (114) |
| Mean (SD) number of days fruit consumed in a week (days/week) | 3.8 ± 2.3 days/week ¥ | 4.4 ± 2.3 days/week ¥ |
| Mean (SD) number of servings of fruit consumed on average per day | 1.32 ± 0.7 serves/day ¥ | 1.25 ± 0.6 serves/day ¥ |
| Mean (SD) number of days vegetables consumed in a week (days/week) | 4.7 ± 2.2 days/week ¥ | 4 ± 2.3 days/week ¥ |
| Mean (SD) number of servings of vegetables consumed on average per day | 1.6 ± 0.7 serves/day ¥ | 1.35 ± 0.6 serves/day ¥ |
| Median (IQR) number of mins of moderate activities per week | 1800 (540–2850) min ¶ | 1410 (418–2400) min ¶ |
| Median (IQR) number of mins of vigorous activities week | 120 (0–300) min ¶ | 60 (0–240) min |
| Met WHO criteria for moderate activity | ||
| Yes | 89.3% (175) | 84.7% (166) |
| No | 9.2% (18) | 15.3% (30) |
| Missing | 1.5% (3) | 0 |
| Met WHO criteria for vigorous activity | ||
| Yes | 54.6% (107) | 43.9% (86) |
| No | 42.3% (83) | 51.0% (100) |
| Missing | 3.1% (6) | 5.1% (10) |
| Met the WHO criteria for physical activity (vigorous or moderate) | ||
| Yes | 93.4% (183) | 91.3% (179) |
| No | 6.6% (13) | 8.7% (17) |
| Met WHO recommendations for diet baseline | ||
| Yes | 5.1% (10) | 2.5% (5) |
| No | 94.9% (186) | 97.5% (191) |
¥ ± represents standard deviation (SD) of the mean. ¶ Median and IQ range reported due to data skewness.
At follow-up, 160 of 205 participants completed all the requested laboratory workup (the five elements of MetS). The incidence of new-onset MetS during the 24 months of residing in Qatar was 17.0% (n = 27/160, 95% CI; 11.0–23.0%); 81.0% (n = 129/160) of participants developed at least one element of MetS (Table 4). Only 19.0% (31/160) of participants were still MetS free 24-months post-migration.
Table 4.
Incidence of MetS and MetS elements 24-months post-migration.
| Metabolic/Parameters | Values % (n) | 95% CI |
|---|---|---|
| Zero elements of MetS (n = 160) ¥ | 19.0% (31) | 14.0–26.0% |
| One element of MetS (n = 160) ¥ | 33.0% (52) | 25.0–40.0% |
| Two elements of MetS (n = 160) ¥ | 31.0% (50) | 24.0–39.0% |
| Three or more elements of MetS (n = 160) ¥ | 17.0% (27) | 11.0–23.0% |
| HTN | ||
| SBP ≥ 130 mmHg (n = 197) ¥ | 19.3% (38) | 14.0–26.0% |
| DBP ≥ 85 mmHg (n = 197) ¥ | 13.7% (27) | 9.0–19.0% |
| SBP ≥ 130 mmHg or DBP ≥ 85 mmHg or on antihypertensive medications (n = 197) ¥ | 33.0% (65) | 27.0–40.0% |
| DM, pre-DM | ||
| FBG > 5.5 mmol/L or on medications (n = 169) ¥ | 26.6% (45) | 20.0–34.0% |
| FBG > 5.5 mmol/L or HbA1c > 5.6% or on medications (n = 167) ¥ | 33.5% (56) | 26.0–41.0% |
| FBG > 5.5 mmol/L or HbA1c > 6.5% or on medications (n = 167) ¥ | 3.6% (6) | 1.0–8.0% |
| Dyslipidaemia | ||
| TG ≥ 1.7 mmol/L (n = 166) ¥ | 18.7% (31) | 13.0–26.0% |
| HDL-C < 1.03 mmol/L for males or <1.29 mmol/L for females (n = 165) ¥ | 9.7% (16) | 6.0–15.0% |
| HDL-C < 1.03 mmol/L for males or <1.29 mmol/L for females or TG ≥ 1.7 mmol/L or on medications (n = 166) ¥ | 30.7% (51) | 22.0–37.0% |
| Obesity | ||
| WC ≥ 90 cm for male and ≥80 cm for females * (n = 191) ¥ | 56.5% (108) | 49.0–64.0% |
| WC above the range * or on anti-obesity medications (n = 192) ¥ | 56.8% (109) | 49.0–64.0% |
MetS, metabolic syndrome; HTN, hypertension; SBP, systolic blood pressure; DBP, diastolic blood pressure; DM, diabetes mellitus; FBG, fasting blood glucose; HbA1c, glycated haemoglobin; TG; triglycerides; HDL-C, high-density lipoprotein cholesterol; WC, waist circumference. * WC ≥ 90 cm for males and ≥80 cm for females, except for Europid males ≥ 95 cm and Japanese females ≥ 85 cm—please refer to Table 1. ¥ The number of the participants vary between the parameters, due to missing data. Thirty-eight partially completed the laboratory workup, while 160 completed it all.
As shown in Table 4, at follow-up more than half of the participants developed central obesity (56.5%, n = 108/191, 95% CI; 49.0–64.0%), 33.5% (n = 56/167, 95% CI; 26.0–41.0%) developed elevated glycaemic parameters (either abnormal FBG, HbA1c or on DM medications), while 33.0% (n = 65/197, 95% CI; 27.0–40.0%) developed HTN (elevated SBP, and DBP, or on antihypertensive medications), and 18.7% (n = 31/166, CI; 13.0–26.0%) had new-onset hypertriglyceridemia.
All metabolic parameters were statistically significantly raised at follow-up compared to baseline, with the exception of SBP (Table 5). FBG values were not compared because these were not available for all participants at baseline.
Table 5.
Comparison of metabolic parameters at baseline and follow-up.
| Metabolic Parameter | Baseline (2017) ¥ | Follow-Up (2019) ¥ | t-Value ¶ | df | p-Value * |
|---|---|---|---|---|---|
| SBP mmHg (n = 196) | 119 ± 9.3 | 120 ± 12.0 | −1.73 | 195 | 0.085 |
| DBP mmHg (n = 197) | 71 ± 9.0 | 75 ± 10.0 | −4.90 | 196 | <0.001 |
| HbA1c % (n = 166) | 5.1 ± 0.3 | 5.4 ± 0.3 | −13.90 | 165 | <0.001 |
| TG mg/dL (n = 166) | 1 ± 0.4 | 1.2 ± 0.7 | −4.56 | 165 | <0.001 |
| HDL-C mg/dL (n = 165) | 1.29 ± 0.3 | 1.34 ± 0.3 | −3.15 | 164 | 0.002 |
| WC cm (n = 192) | 84.2 ± 5.5 | 88.4 ± 6.9 | −12.05 | 191 | <0.001 |
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, glycated haemoglobin; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol. ¥ ± represents standard deviation (SD) of the mean. ¶ Paired Sample t-test test-value. * Paired Sample t-test.
There was a significant association between male gender and developing MetS (Chi-square = 13.4, df = 4, p = 0.01). Additionally, administration of medications that potentially induce MetS was significantly associated with an increased risk of MetS or its elements (Chi-square = 16.9, df = 4, p = 0.002) compared to those not taking such medications (Table 6). Age was not compared as most participants were of a similar age (mean age 31.2 + 5.2 years). Ethnic groups, marital status, education, occupation, diet, and exercise had no statistically significant effect on incidence of new-onset MetS. Of note, no patients were identified as having five elements of MetS at the end of the 24 months.
Table 6.
Comparison of migrants with one, two, three and four elements of MetS and MetS-free migrants 24 months post-migration to Qatar regarding variables of demographics and the modifiable risk factors (lifestyle variables) (n = 160) α.
| No MetS% (n) | One Element of MetS % (n) | Two Elements of MetS % (n) | Three Elements of MetS % (n) | Four Elements of MetS % (n) | Test Value | df | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Gender | ||||||||
| Male | 64.5% (20) | 73.1% (38) | 86.0% (43) | 100% (18) | 55.6% (5) | |||
| Female | 35.5% (11) | 26.9% (14) | 14.0% (7) | 0 | 44.4% (4) | 13.4 | 4 | 0.01 * |
| Ethnic group | ||||||||
| Arabs | 6.5% (2) | 7.7% (4) | 10.0% (5) | 5.6% (1) | 0 | |||
| Asians | 90.3% (28) | 78.8% (41) | 74.0% (37) | 94.4% (17) | 88.9% (8) | |||
| Africans | 0 | 1.9% (1) | 6.0% (3) | 0 | 0 | |||
| European | 0 | 5.8% (3) | 4.0% (2) | 0 | 0 | |||
| Others † | 3.2% (1) | 5.8% (3) | 6.0% (3) | 0 | 11.1% (1) | 11.3 | 16 | 0.788 * |
| Marital status | ||||||||
| Married | 22.6% (7) | 48.1% (25) | 50.0% (25) | 61.1% (11) | 55.6% (5) | |||
| Single | 71.0% (22) | 50.0% (26) | 50.0% (25) | 38.9% (7) | 44.4% (4) | |||
| Divorced | 6.4% (2) | 1.9% (1) | 0 | 0 | 0 | 10.2 | 8 | 0.254 * |
| Education | ||||||||
| Below Bachelor’s degree | 6.4% (2) | 0 | 0 | 0 | 0 | |||
| Graduate (Bachelor, Diploma) | 83.9% (26) | 80.8% (42) | 72.0% (36) | 77.8% (14) | 66.7% (6) | |||
| Postgraduate (Master and above) | 9.7% (3) | 19.2% (10) | 28.0% (14) | 22.2% (4) | 33.3% (3) | 12.7 | 8 | 0.123 * |
| Occupation | ||||||||
| Doctors | 6.5% (2) | 11.5% (6) | 12.0% (6) | 11.1% (2) | 22.2% (2) | |||
| Nurses | 70.9% (22) | 71.2% (37) | 58.0% (29) | 83.3% (15) | 66.7% (6) | |||
| Allied healthcare providers | 16.1% (5) | 15.4% (8) | 24.0% (12) | 0 | 0 | |||
| Others ‡ | 6.5% (2) | 1.9% (1) | 6.0% (3) | 5.6% (1) | 11.1% (1) | 11.5 | 12 | 0.489 * |
| Medication that might induce MetS | ||||||||
| Yes | 19.4% (6) | 13.5% (7) | 12.0% (6) | 27.8% (5) | 66.7% (6) | |||
| No | 80.6% (25) | 86.5% (45) | 88.0% (44) | 72.2% (13) | 33.3% (3) | 16.9 | 4 | 0.002 * |
| Number of days fruit consumed in a week ¥ | 4.2 ± 2.3 | 4.3 ± 2.3 | 4.3 ± 2.3 | 4.7 ± 2.3 | 3.8 ± 2.6 | 0.26 | 4 | 0.905 € |
| Mean (SD) number of servings of fruit consumed per day ¥ | 1.3 ± 0.8 | 1.3 ± 0.5 | 1.2 ± 0.6 | 1 ± 0.4 | 1.2 ± 0.4 | 0.93 | 4 | 0.448 € |
| Number of days vegetables consumed per week ¥ | 3.6 ± 1.9 | 4 ± 2.2 | 4 ± 2.5 | 4.9 ± 2.4 | 4.1 ± 2.8 | 0.85 | 4 | 0.493 € |
| Number of servings of vegetables consumed per day ¥ | 1.3 ± 0.6 | 1.4 ± 0.6 | 1.3 ± 0.5 | 1.4 ± 0.5 | 1.4 ± 0.5 | 0.39 | 4 | 0.811 € |
| Met WHO recommendation for moderate activity (≥150 min/week) | ||||||||
| Yes | 88.2% (30) | 86.3% (44) | 80.0% (40) | 88.2% (15) | 88.9% (8) | |||
| No | 11.8% (4) | 13.7% (7) | 20.0% (10) | 11.8% (2) | 11.1% (1) | 4.67 | 8 | 0.792 * |
| Met WHO recommendation for vagarious activity (≥75 min/week) | ||||||||
| Yes | 37.5% (12) | 48.0% (24) | 46.9% (23) | 57.9% (11) | 30.0% (3) | 5. | ||
| No | 62.5% (20) | 52.0% (26) | 53.1% (26) | 42.1% (8) | 70.0% (7) | 70 | 8 | 0.680 * |
| Met WHO criteria for moderate or vagarious activity | ||||||||
| Yes | 93.7% (30) | 92.3% (48) | 86.0% (43) | 88.2% (15) | 100% (9) | |||
| No | 6.3% (2) | 7.7% (4) | 14.0% (7) | 11.8% (2) | 0 | 7.81 | 8 | 0.452 * |
αn = 160 since this only includes participants whose full laboratory workup was available. * Pearson Chi-Square test. € One-way ANOVA test. ¥ ± represents the standard deviation (SD). † Others included: North Americans, South Americans, Australians–Oceanians. ‡ Other occupations included: clerk, engineer, IT and aides.
Univariate logistic regression analysis was undertaken to identify the determinants of MetS. Consuming medications that potentially induce MetS (Such as β blocker, steroids and anti-psychotics) was associated with a four-fold higher risk of MetS (unadjusted OR 4.4, 95% CI; 1.74–10.92, p = 0.001). Multiple logistic regression analysis was used to adjust for gender and physical activities (moderate and vigorous, baseline, and follow-up). Consuming medications that potentially induce MetS was found to be associated with a six-fold risk of MetS (AOR 6.3, 95% CI; 2.27–17.73, p < 0.001; Table 7).
Table 7.
Logistic regression analysis for factors associated with MetS development at 24-months post-migration (n = 160) ¥.
| Univariate Logistic Regression | Multiple Logistic Regression | ||||||
|---|---|---|---|---|---|---|---|
| Unadjusted OR | CI | p-value | Adjusted OR | CI | p-value | ||
| Gender | |||||||
| Male | 17.9% (22) | 1.4 | 3 | ||||
| Female | 13.5% (5) | 1 (reference) | (0.48–3.98) | 0.534 | 1 (reference) | (0.76–11.85) | 0.116 |
| WHO for moderate activities at baseline met (2017) | |||||||
| Yes | 17.5% (25) | 2.9 | 4.4 | ||||
| No | 6.7% (1) | 1 (reference) | (0.37–23.60) | 0.285 | 1 (reference) | (0.37–52.74) | 0.242 |
| WHO for vigorous activities at baseline met (2017) | |||||||
| Yes | 18.7% (17) | 1.5 | 0.9 | ||||
| No | 13.4% (9) | 1(reference) | (0.62–3.56) | 0.379 | 1 (reference) | (0.31–2.87) | 0.939 |
| WHO for vigorous activities at follow-up met (2019) | |||||||
| Yes | 17.3% (23) | 1.5 | (0.40–5.32) | 0.561 | 0.9 | (0.21–4.70) | 0.996 |
| No | 12.5% (3) | 1 (reference) | 1 (reference) | ||||
| WHO for moderate activities at follow-up met (2019) | |||||||
| Yes | 16.4% (12) | 0.9 | (0.42–2.29) | 0.969 | 1.2 | (0.41–3.32) | 0.764 |
| No | 16.7% (14) | 1 (reference) | 1 (reference) | ||||
| Taking medications that induce MetS | |||||||
| Yes | 36.7% (11) | 4.4 | (1.74–10.92) | 0.001 | 6.3 | (2.27–17.73) | <0.001 |
| No | 11.7% (15) | 1 (reference) | 1 (reference) | ||||
¥ The total number of participants included in the analysis equals 160; however, if there were any single missing values in the participants’ data, the whole data set related to that participant was excluded.
4. Discussion
Key study findings were that among migrants with normal metabolic parameters at baseline, 17.0% (95% CI; 11.0–23.0%) developed MetS during their 24-months of residence in Qatar, with around 81.0% (95% CI; 73.8–86.0%) developing at least one element of MetS—most commonly central obesity. Administration of medications that potentially induce MetS increased MetS risk by six-fold.
The incidence of MetS amongst initially MetS-free Qatar migrants rose to 17.0% over the study period. Van der Linden reported similarly high rates of MetS among Ghanaian migrants to Europe, compared to their counterparts in Ghana (ranged from 31.4% to 38.4% vs 8.3%, respectively) [50].
In this study, more than half of the study population developed central obesity during the study, most likely due to reduced physical activity and a diet with fewer fruit and vegetable portions—as highlighted in the analysis of the survey data. In a systematic review of 39 cross-sectional studies, Goulão et al. highlighted the positive association between obesity prevalence and the length-of-stay in a host country [54,55]. Migration was associated with weight gain, which directly increased with the duration of migration. Similar to the findings of the current study, a recent study conducted amongst Qatar migrants in 2020 also highlighted that male migrants were more likely than females to develop MetS [41]. Central and South American male migrants to Washington tended to develop MetS more than female migrants [47]. This variation was attributed to sex hormones that make men more prone to develop central obesity and subsequent insulin resistance [56,57].
Regarding sociodemographic parameters, previous studies have shown the MetS-protective effect of being unmarried (single, divorced or widow), having high education, occupation levels, and consuming diets rich in fruits and vegetables [58,59,60,61,62,63,64,65,66,67]. Other studies have also highlighted the variation of MetS prevalence between ethnic groups [41,68], being most marked in South Asian migrants. Conversely, the current study showed no association between occupation, education, marital status, ethnic origin, and diet with MetS incidence. However, the limited sample size, the extensive medical background (95%, n = 195) with similar work environments, and the lack of ethnic variability (81.0% were Asians) could explain these differences.
Compared to migrants not receiving any medications that alter metabolic parameters, migrants consuming these medications were at increased risk of MetS by six-fold. The most commonly reported medications were anti-psychotics, β blockers, steroids, and diuretics. Several previous studies have confirmed these adverse metabolic effects, with proposed mechanisms including the promotion of weight gain, fat disposition in the visceral area, subsequent impaired glucose tolerance, and insulin resistance [69,70,71,72,73,74,75]. Caution should be exercised when prescribing these medications, with periodic monitoring of metabolic parameters [76].
There are numerous strengths to this study. While all previous studies have focused on MetS prevalence at one time-point, this is the first study to screen migrants for MetS at baseline and determine its incidence 24-months post-migration. Hence, the current study associates MetS incidence to the migration process itself and subsequent lifestyle modifications. In addition, this is the first study to assess MetS and its potential determinants amongst migrants to the Middle East and the Gulf. A validated and well-established questionnaire (WHO STEPwise) was used to address lifestyle modifications [77].
There are limitations to this study; hence, the study findings should be interpreted with caution. These include the relatively low participation rate, resulting in the study size being below the calculated sample estimation. There are potential issues of generalisability beyond the study setting. Notably, most of the population were Asian, and almost all had a medical background. Baseline questionnaire data may also be subject to recall bias, and the follow-up period of two years was relatively short. Evidence indicates that a more extended migration period may be associated with higher MetS prevalence [15].
This study provides evidence on the impact of migration to the Middle East in new-onset MetS incidence. Assessment and follow-up of these migrants in terms of MetS and its elements will generally improve health status, control of risk factors, decrease the likelihood of longer-term consequences and enhance quality of life. This study will guide policymakers within the Ministry of Public Health and the HMC in implementing preventative measures to combat MetS among migrants and develop strategies for early warning systems. Further studies with ethnic and occupational diversity and a more extended follow-up period are warranted to determine MetS incidence and its determinants amongst Middle East migrants.
5. Conclusions
There is limited evidence concerning the incidence of MetS and its elements post-migration, particularly in the Middle East. Migrants to Qatar, particularly males, showed increased MetS incidence 24-months following migration. Consuming medications that potentially induce MetS was a significant determinant. These factors should be the subject of prospective intervention studies.
Acknowledgments
The authors would like to acknowledge Anh Jochebeth, Shilpa Kuttikrishnan, Ann O’Connor, Monica Young, and Martin Steinhoff for their contribution and support. Additionally, we would like to acknowledge Prem Chandra for their statistical analysis-related support.
Abbreviations
| AOR | Adjusted Odds Ratio |
| BP | Blood Pressure |
| DBP | Diastolic Blood Pressure |
| DM | Diabetes Mellitus |
| FBG | Fasting Blood Glucose |
| HbA1c | Glycated Haemoglobin |
| HDL-C | High-Density Lipoprotein Cholesterol |
| HMC | Hamad Medical Corporation |
| HTN | Hypertension |
| IDF | International Diabetes Federation |
| IRB | Institutional Review Board |
| Mets | Metabolic Syndrome |
| RGU | Robert Gordon University |
| SBP | Systolic Blood Pressure |
| SD | Standard Deviation |
| TG | Triglycerides |
| WC | Waist Circumference |
Author Contributions
Conceptualization, H.A., R.M.A.-A., A.P.T., D.S. and K.S.P.; methodology, R.M.A.-A., A.P.T., D.S., H.A. and C.R.; validation, R.M.A.-A., A.P.T., M.I.M.I., M.E., S.U. and D.S.; writing—original draft preparation, R.M.A.-A., A.P.T. and D.S.; writing—review and editing, R.M.A.-A., A.P.T., D.S. and C.R.; supervision, A.P.T. and D.S.; project administration, R.M.A.-A. and K.S.P.; funding acquisition, K.S.P. and R.M.A.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Hamad Medical Corporation, grant number [IRGC-03-IN-17-070].
Institutional Review Board Statement
All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Hamad Medical Corporation and RGU (protocol code IRGC-03-IN-17-070 and S97, respectively) in 2019.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kereiakes D.J., Willerson J.T. Metabolic syndrome epidemic. Am. Heart Assoc. 2003;108:1552–1553. doi: 10.1161/01.CIR.0000093203.00632.2B. [DOI] [PubMed] [Google Scholar]
- 2.Saklayen M.G. The global epidemic of the metabolic syndrome. Curr. Hypertens. Rep. 2018;20:12. doi: 10.1007/s11906-018-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alberti K., Zimmet P., Shaw J. Metabolic syndrome—A new world-wide definition. A consensus statement from the international diabetes federation. Diabet. Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 4.O’Neill S., O’Driscoll L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015;16:1–12. doi: 10.1111/obr.12229. [DOI] [PubMed] [Google Scholar]
- 5.Fujiyoshi A., Murad M.H., Luna M., Rosario A., Ali S., Paniagua D., Molina J., Lopez M., Jacobs S., Lopez-Jimenez F. Metabolic syndrome and its components are underdiagnosed in cardiology clinics. J. Eval. Clin. Pract. 2011;17:78–83. doi: 10.1111/j.1365-2753.2010.01371.x. [DOI] [PubMed] [Google Scholar]
- 6.Alberti K., Eckel R., Grundy S., Zimmet P., Cleeman J., Donato K. Harmonizing the metabolic syndrome. A joint interim statement of the IDF task force on epidemiology and prevention; NHL and Blood Institute; AHA.; WHF.; IAS; and IA for the study of obesity. Am. Heart Assoc. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 7.Gundogan K., Bayram F., Gedik V., Kaya A., Karaman A., Demir O., Sabuncu T., Kocer D., Coskun R. Metabolic syndrome prevalence according to ATP III and IDF criteria and related factors in Turkish adults. AMS. 2013;9:243. doi: 10.5114/aoms.2013.34560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rębak D., Suliga E., Grabowska U., Głuszek S. The prevelance of metabolic syndrome on the sample of paramedics. Int. J. Occup. Med. Environ. Health. 2018;31:741–751. doi: 10.13075/ijomeh.1896.01212. [DOI] [PubMed] [Google Scholar]
- 9.Athyros V.G., Ganotakis E.S., Tziomalos K., Papageorgiou A.A., Anagnostis P., Griva T., Kargiotis K., Mitsiou E.K., Karagiannis A., Mikhailidis D.P., et al. Comparison of four definitions of the metabolic syndrome in a Greek (Mediterranean) population. Curr. Med. Res. Opin. 2010;26:713–719. doi: 10.1185/03007991003590597. [DOI] [PubMed] [Google Scholar]
- 10.Herningtyas E.H., Ng T.S. Prevalence and distribution of metabolic syndrome and its components among provinces and ethnic groups in Indonesia. BMC Public Health. 2019;19:377. doi: 10.1186/s12889-019-6711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention Metabolic syndrome prevalence by race/ethnicity and sex in the United States, National Health and Nutrition Examination Survey, 1988–2012. Preventing Chronic Disease. [(accessed on 22 March 2016)]; Available online: https://www.cdc.gov/pcd.
- 12.Sigit F.S., Tahapary D.L., Trompet S., Sartono E., van Dijk K.W., Rosendaal F.R., De Mutsert R. The prevalence of metabolic syndrome and its association with body fat distribution in middle-aged individuals from Indonesia and the Netherlands: A cross-sectional analysis of two population-based studies. Diabetol. Metab. Syndr. 2020;12:1–11. doi: 10.1186/s13098-019-0503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gustafson B., Hammarstedt A., Andersson C.X., Smith U. Inflamed adipose tissue: A culprit underlying the metabolic syndrome and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2007;27:2276–2283. doi: 10.1161/ATVBAHA.107.147835. [DOI] [PubMed] [Google Scholar]
- 14.Türkoglu Ç., Duman B.S., Günay D., Çagatay P., Özcan R., Büyükdevrim A.S. Effect of abdominal obesity on insulin resistance and the components of the metabolic syndrome: Evidence supporting obesity as the central feature. Obes. Surg. 2003;13:699–705. doi: 10.1381/096089203322509255. [DOI] [PubMed] [Google Scholar]
- 15.Bharmal N., Kaplan R.M., Shapiro M.F., Mangione C.M., Kagawa-Singer M., Wong M.D., McCarthy W.J. The association of duration of residence in the United States with cardiovascular disease risk factors among South Asian immigrants. J. Immigr. Minor. Health. 2015;17:781–790. doi: 10.1007/s10903-013-9973-7. [DOI] [PubMed] [Google Scholar]
- 16.Liu J., Probst J.C., Harun N., Bennett K.J., Torres M.E. Acculturation, physical activity, and obesity among Hispanic adolescents. Ethn. Health. 2009;14:509–525. doi: 10.1080/13557850902890209. [DOI] [PubMed] [Google Scholar]
- 17.Bursztyn M., Raz I. Blood pressure, glucose, insulin and lipids of young Ethiopian recent immigrants to Israel and in those resident for 2 years. J. Hypertens. 1993;11:455–459. doi: 10.1097/00004872-199304000-00018. [DOI] [PubMed] [Google Scholar]
- 18.Sobngwi E., Mbanya J.C., Unwin N.C., Porcher R., Kengne A.P., Fezeu L., Minkolou E.M., Tournoux C., Gautier J.-F., Aspray T.J., et al. Exposure over the life course to an urban environment and its relation with obesity, diabetes, and hypertension in rural and urban Cameroon. Int. J. Epidemiol. 2004;33:769–776. doi: 10.1093/ije/dyh044. [DOI] [PubMed] [Google Scholar]
- 19.Steyn K., Kazenellenbogen J.M., Lombard C.J., Bourne L.T. Urbanization and the risk for chronic diseases of lifestyle in the black population of the Cape Peninsula, South Africa. J. Cardiovasc. Risk. 1997;4:135–142. doi: 10.1097/00043798-199704000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Singh R.B., Bajaj S., Niaz M.A., Rastogi S.S., Moshiri M. Prevalence of type 2 diabetes mellitus and risk of hypertension and coronary artery disease in rural and urban population with low rates of obesity. Int. J. Cardiol. 1998;66:65–72. doi: 10.1016/S0167-5273(98)00141-7. [DOI] [PubMed] [Google Scholar]
- 21.Bursztyn M., Raz I. Prediction of hypertension by the insulinogenic index in young Ethiopian immigrants. J. Hypertens. 1995;13:57–62. doi: 10.1097/00004872-199501000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Poulter N.R., Khaw K.T., Hopwood B.E., Mugambi M., Peart W.S., Rose G., Sever P.S. The Kenyan Luo migration study: Observations on the initiation of a rise in blood pressure. BMJ. 1990;300:967–972. doi: 10.1136/bmj.300.6730.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Satia-Abouta J. Dietary acculturation: Definition, process, assessment, and implications. Int. J. Hum. Ecol. 2003;4:71–86. [Google Scholar]
- 24.Park S., Paik H., Skinner J.D., Spindler A.A., Park H. Nutrient intake of Korean-American, Korean, and American adolescents. J. Am. Diet. Assoc. 2004;104:242–245. doi: 10.1016/j.jada.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 25.Lv N., Cason K.L. Dietary pattern change and acculturation of Chinese Americans in Pennsylvania. J. Am. Diet. Assoc. 2004;104:771–778. doi: 10.1016/j.jada.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 26.Holmboe-Ottesen G., Wandel M. Changes in dietary habits after migration and consequences for health: A focus on South Asians in Europe. Food Nutr. Res. 2012;56:18891. doi: 10.3402/fnr.v56i0.18891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kousar R. Ph.D. Thesis. Victoria University; St Albans, Victoria, Australia: Mar, 2010. Metabolic Syndrome: Effect of a Culturally Appropriate Diet and Physical Activity in Female Pakistani Immigrants. [Google Scholar]
- 28.Daryani A., Berglund L., Andersson Å., Kocturk T., Becker W., Vessby B. Risk factors for coronary heart disease among immigrant women from Iran and Turkey, compared to women of Swedish ethnicity. Ethn. Dis. 2005;15:213–220. [PubMed] [Google Scholar]
- 29.Misra K.B., Endemann S.W., Ayer M. Leisure time physical activity and metabolic syndrome in Asian Indian immigrants residing in northern California. Ethn. Groups. 2005;10:19–23. [PubMed] [Google Scholar]
- 30.Jaffe A., Giveon S., Wulffhart L., Oberman B., Freedman L., Ziv A., Kalter-Leibovici O. Diabetes among Ethiopian immigrants to Israel: Exploring the effects of migration and ethnicity on diabetes risk. PLoS ONE. 2016;11:e0157354. doi: 10.1371/journal.pone.0157354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finch B.K., Vega W.A. Acculturation stress, social support, and self-rated health among Latinos in California. J. Immigr. Health. 2003;5:109–117. doi: 10.1023/A:1023987717921. [DOI] [PubMed] [Google Scholar]
- 32.Fang C.Y., Boden G., Siu P.T., Tseng M. Stressful life events are associated with insulin resistance among Chinese immigrant women in the United States. Prev. Med. Rep. 2015;2:563–567. doi: 10.1016/j.pmedr.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Brien M.J., Also V.A., Davey A., Bueno A., Whitaker R.C. Peer reviewed: Acculturation and the prevalence of diabetes in US Latino adults, national health and nutrition examination survey 2007–2010. Prev. Chronic Dis. 2014;11:A638. doi: 10.5888/pcd11.140142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sundquist J., Winkleby M. Country of birth, acculturation status and abdominal obesity in a national sample of Mexican-American women and men. Int. J. Epidemiol. 2000;29:470–477. doi: 10.1093/ije/29.3.470. [DOI] [PubMed] [Google Scholar]
- 35.Daviglus M.L., Talavera G.A., Avilés-Santa M.L., Allison M., Cai J., Criqui M.H., Gellman M., Gaichello A.L., Gouskova N., Kaplan R.C., et al. Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States. JAMA. 2012;308:1775–1784. doi: 10.1001/jama.2012.14517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Modesti P.A., Tamburini C., Hagi M.I., Cecioni I., Migliorini A., Serneri G.G.N. Twenty-four-hour blood pressure changes in young Somalian blacks after migration to Italy. Am. J. Hypertens. 1995;8:201–205. doi: 10.1016/0895-7061(94)00189-I. [DOI] [PubMed] [Google Scholar]
- 37.Goldbourt U., Khoury M., Landau E., Reisin L.H., Rubinstein A. Blood pressure in Ethiopian immigrants: Relationship to age and anthropometric factors, and changes during their first year in Israel. Isr. J. Med. Sci. 1991;27:264–267. [PubMed] [Google Scholar]
- 38.Rosenthal T., Grossman E., Knecht A., Goldbourt U. Levels and correlates of blood pressure in recent and earlier Ethiopian immigrants to Israel. J. Hum. Hypertens. 1990;4:425–430. [PubMed] [Google Scholar]
- 39.Bursztyn M., Raz I. Blood pressure and insulin in Ethiopian immigrants: Longitudinal study. J. Hum. Hypertens. 1995;9:245–248. [PubMed] [Google Scholar]
- 40.Creatore M.I., Moineddin R., Booth G., Manuel D.H., DesMeules M., McDermott S., Glazier R.H. Age- and sex-related prevalence of diabetes mellitus among immigrants to Ontario, Canada. CMAJ. 2010;182:781–789. doi: 10.1503/cmaj.091551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Syed M.A., Al Nuaimi A.S., Latif Zainel A.J.A., Qotba H.A. Prevalence of metabolic syndrome in primary health settings in Qatar: A cross sectional study. BMC Public Health. 2020;20:1–7. doi: 10.1186/s12889-020-08609-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mishra S.R., Ghimire S., Joshi C., Gyawali B., Shrestha A., Neupane D., Sharma S.R., Pokharel Y., Virani S.S. Cardio-metabolic disease risk factors among South Asian labour migrants to the Middle East: A scoping review and policy analysis. Glob. Health. 2019;15:33. doi: 10.1186/s12992-019-0468-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ismail M.F. Metabolic syndrome among obese Qataris attending primary health care centers in Doha, 2010. J. Fam. Community Med. 2012;19:7–11. doi: 10.4103/2230-8229.94004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Musallam M., Bener A., Zirie M., Al-Gaud Y.K., Al-Hamaq A., Othman M., Tewfik I. Metabolic syndrome and its components among Qatari population. Int. J. Food Saf. Nutr. Public Health. 2008;1:88–102. doi: 10.1504/IJFSNPH.2008.018858. [DOI] [Google Scholar]
- 45.HAMAD MEDICAL CORPORATION (HMC) About us. Our People. [(accessed on 18 May 2018)]. [Homepage on the Internet] Available online: https://www.hamad.qa/EN/About-Us/Our%20People/Pages/default.aspx.
- 46.Kim Y.J., Lee Y.H., Lee Y.J., Kim K.J., An J.H., Kim N.H., Kim H.Y., Choi D.S., Kim S.G. Prevalence of metabolic syndrome and its related factors among North Korean refugees in South Korea: A cross-sectional study. BMJ Open. 2016;6:e010849. doi: 10.1136/bmjopen-2015-010849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gill R.M., Khan S.A., Jackson R.T., Duane M. Prevalence of the metabolic syndrome in Central and South American immigrant residents of the Washington, DC, area. J. Nutr. Metab. 2017;2017 doi: 10.1155/2017/9531964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jowitt L.M., Lu L.W., Rush E.C. Migrant Asian Indians in New Zealand; prediction of metabolic syndrome using body weights and measures. Asia Pac. J. Clin. Nutr. 2014;23:385. doi: 10.3316/informit.573625836789552. [DOI] [PubMed] [Google Scholar]
- 49.Ajjan R., Carter A., Somani R., Kain K., Grant P. Ethnic differences in cardiovascular risk factors in healthy Caucasian and South Asian individuals with the metabolic syndrome. J. Thromb. Haemost. 2007;5:754–760. doi: 10.1111/j.1538-7836.2007.02434.x. [DOI] [PubMed] [Google Scholar]
- 50.Van Der Linden E.L., Meeks K., Beune E., de-Graft Aikins A., Addo J., Owusu-Dabo E., Mockenhaupt F.P., Bahendeka S., Danquah I., Schulze M.B., et al. The prevalence of metabolic syndrome among Ghanaian migrants and their homeland counterparts: The Research on obesity and type 2 diabetes among African migrants (RODAM) study. Eur. J. Public Health. 2019;29:906–913. doi: 10.1093/eurpub/ckz051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dean A.G., Sullivan K.M., Soe M.M. OpenEpi. Open Source Epidemiologic Statistics for Public Health, Version. [(accessed on 2 February 2017)]. [homepage on the Internet] Available online: www.OpenEpi.com.
- 52.Riley L., Guthold R., Cowan M., Savin S., Bhatti L., Armstrong T., Bonita R. The World Health Organization STEPwise approach to noncommunicable disease risk-factor surveillance: Methods, challenges, and opportunities. Am. J. Public Health. 2016;106:74–78. doi: 10.2105/AJPH.2015.302962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Centers for Disease Control and Prevention (CDC) Centers for Disease Control and Prevention; [(accessed on 22 March 2018)]. Principles of Epidemiology | Lesson 3—Section 2. [homepage on the Internet] Available online: https://www.cdc.gov/csels/dsepd/ss1978/lesson3/section2.html. [Google Scholar]
- 54.Goulão B., Santos O., Carmo I. The impact of migration on body weight: A review. Cad. Saúde Pública. 2015;31:229–245. doi: 10.1590/0102-311X00211913. [DOI] [PubMed] [Google Scholar]
- 55.Higgins V., Nazroo J., Brown M. Pathways to ethnic differences in obesity: The role of migration, culture and socio-economic position in the UK. SSM Popul. Health. 2019;7:100394. doi: 10.1016/j.ssmph.2019.100394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Link J.C., Reue K. Genetic basis for sex differences in obesity and lipid metabolism. Annu. Rev. Nutr. 2017;37:225–245. doi: 10.1146/annurev-nutr-071816-064827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zore T., Palafox M., Reue K. Sex differences in obesity, lipid metabolism, and inflammation—A role for the sex chromosomes? Mol. Metab. 2018;15:35–44. doi: 10.1016/j.molmet.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cho D.Y., Koo J. Differences in metabolic syndrome prevalence by employment type and sex. Int. J. Environ. Res. Public Health. 2018;15:1798. doi: 10.3390/ijerph15091798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Al-Daghri N.M., Alkharfy K.M., Al-Attas O.S., Khan N., Alfawaz H.A., Alghanim S.A., Al-Yousef M.A., Al-Ajlan A.S.M., Alokail M.S. Gender-dependent associations between socioeconomic status and metabolic syndrome: A cross-sectional study in the adult Saudi population. BMC Cardiovasc. Disord. 2014;14:51. doi: 10.1186/1471-2261-14-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Al-Thani M.H., Al-Thani A.A., Cheema S., Sheikh J., Mamtani R., Lowenfels A.B., Al-Chetachi W.F., Almalki B.A., Khalifa S.A.H., Bakri A.O.H., et al. Prevalence and determinants of metabolic syndrome in Qatar: Results from a National health survey. BMJ Open. 2016;6:e009514. doi: 10.1136/bmjopen-2015-009514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Santos A.C., Ebrahim S., Barros H. Gender, socio-economic status and metabolic syndrome in middle-aged and old adults. BMC Public Health. 2008;8:62. doi: 10.1186/1471-2458-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cho K.I., Kim B.H., Je H.G., Jang J.S., Park Y.H. Gender-specific associations between socioeconomic status and psychological factors and metabolic syndrome in the Korean population: Findings from the 2013 Korean national health and nutrition examination survey. BioMed Res. Int. 2016;2016:3973197. doi: 10.1155/2016/3973197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khunti K., Taub N., Tringham J., Jarvis J., Farooqi A., Skinner T.C., Davies M.J. Screening for the metabolic syndrome using simple anthropometric measurements in south Asian and white Europeans: A population-based screening study. The Leicester ethnic atherosclerosis and diabetes risk (LEADER) study. Prim. Care Diabetes. 2010;4:25–32. doi: 10.1016/j.pcd.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 64.Unwin N., Bhopal R., Hayes L., White M., Patel S., Ragoobirsingh D., Alberti G. A comparison of the new international diabetes federation definition of metabolic syndrome to WHO and NCEP definitions in Chinese, European and South Asian origin adults. Ethn. Dis. 2007;17:522–528. [PubMed] [Google Scholar]
- 65.Tillin T., Forouhi N., Johnston D., McKeigue P., Chaturvedi N., Godsland I. Metabolic syndrome and coronary heart disease in South Asians, African-Caribbeans and white Europeans: A UK population-based cross-sectional study. Diabetologia. 2005;48:649–656. doi: 10.1007/s00125-005-1689-3. [DOI] [PubMed] [Google Scholar]
- 66.Lim M., Kim J. Association between fruit and vegetable consumption and risk of metabolic syndrome determined using the Korean Genome and Epidemiology Study (KoGES) Eur. J. Nutr. 2019;7:1–12. doi: 10.1007/s00394-019-02021-5. [DOI] [PubMed] [Google Scholar]
- 67.Hosseinpour-Niazi S., Mirmiran P., Mirzaei S., Azizi F. Cereal, fruit and vegetable fibre intake and the risk of the metabolic syndrome: A prospective study in the tehran lipid and glucose study. J. Hum. Nutr. Diet. 2015;28:236–245. doi: 10.1111/jhn.12242. [DOI] [PubMed] [Google Scholar]
- 68.Liu J., Hanley A., Young T., Harris S., Zinman B. Characteristics and prevalence of the metabolic syndrome among three ethnic groups in Canada. Int. J. Obes. 2006;30:669–676. doi: 10.1038/sj.ijo.0803179. [DOI] [PubMed] [Google Scholar]
- 69.Bergman R.N., Kim S.P., Hsu I.R., Catalano K.J., Chiu J.D., Kabir M., Richey J.M., Ader M. Abdominal obesity: Role in the pathophysiology of metabolic disease and cardiovascular risk. Am. J. Med. 2007;120:S3–S8. doi: 10.1016/j.amjmed.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 70.Freyberg Z., Aslanoglou D., Shah R., Ballon J.S. Intrinsic and antipsychotic drug-induced metabolic dysfunction in schizophrenia. Front. Neurosci. 2017;11:432. doi: 10.3389/fnins.2017.00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carli M., Kolachalam S., Longoni B., Pintaudi A., Baldini M., Aringhieri S., Fasciani I., Annibale P., Maggio R., Scarselli M. Atypical antipsychotics and metabolic syndrome: From molecular mechanisms to clinical differences. Pharmaceuticals. 2021;14:238. doi: 10.3390/ph14030238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oguz A., Mesci B., Sagun G., Kilic D.C., Yetkin D.O., Akalin A. Secondary metabolic syndrome: The frequency of factors which may underlie the parameters of metabolic syndrome. Ann. Saudi Med. 2013;33:566–571. doi: 10.5144/0256-4947.2013.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rizza R.A., Mandarino L.J., Gerich J.E. Cortisol-induced insulin resistance in man: Impaired suppression of glucose production and stimulation of glucose utilization due to a postreceptor defect of insulin action. J. Clin. Endocrinol. Metab. 1982;54:131–138. doi: 10.1210/jcem-54-1-131. [DOI] [PubMed] [Google Scholar]
- 74.Dimitriadis G., Leighton B., Parry-Billings M., Sasson S., Young M., Krause U., Bevan S., Piva T., Wegener G., Newsholme E.A. Effects of glucocorticoid excess on the sensitivity of gluscose transport and metabolism to insulin in rat skeletal muscle. Biochem. J. 1997;321:707–712. doi: 10.1042/bj3210707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dimitriadis G., Leighton B., Parry-Billings M., Tounas C., Raptis S., Newsholme E.A. Fuosemide decreases the sensitivity of glucose transport to insulin in skeletal muscle in vitro. EJE. 1998;139:118–122. doi: 10.1530/eje.0.1390118. [DOI] [PubMed] [Google Scholar]
- 76.Keepers G.A., Fochtmann L.J., Anzia J.M., Benjamin S., Lyness J.M., Mojtabai R., Servis M., Walaszek A., Buckley P., Lenzenweger M.F., et al. The American Psychiatric Association practice guideline for the treatment of patients with schizophrenia. Am. J. Psychiatry. 2020;177:868–872. doi: 10.1176/appi.ajp.2020.177901. [DOI] [PubMed] [Google Scholar]
- 77.World Health Organization The WHO STEPwise Approach to Surveillance of Noncommunicable Diseases (STEPS). STEPS Instruments for NCD Risk Factors (Core and Expanded Version 1.4) [(accessed on 10 September 2020)]. Available online: http://www.who.int/chp/steps/en.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.