Abstract
Aiming at exploring vascular components in multiple sclerosis (MS) with brain outflow disturbance, we combined transcriptome analysis in MS internal jugular vein (IJV) wall with WES in MS families with vertical transmission of disease. Main results were the differential expression in IJV wall of 16 MS-GWAS genes and of seven genes (GRIN2A, GRIN2B, IL20RB, IL26, PER3, PITX2, and PPARGC1A) not previously indicated by GWAS but encoding for proteins functionally interacting with MS candidate gene products. Strikingly, 22/23 genes have been previously associated with vascular or neuronal traits/diseases, nine encoded for transcriptional factors/regulators and six (CAMK2G, GRIN2A, GRIN2B, N1RD1, PER3, PPARGC1A) for circadian entrainment/rhythm components. Among the WES low-frequency (MAF ≤ 0.04) SNPs (n = 7) filtered in the 16 genes, the NR1D1 rs17616365 showed significantly different MAF in the Network for Italian Genomes affected cohort than in the 1000 Genome Project Tuscany samples. This pattern was also detected in five nonintronic variants (GRIN2B rs1805482, PER3 rs2640909, PPARGC1A rs2970847, rs8192678, and rs3755863) in genes coding for functional partners. Overall, the study proposes specific markers and low-frequency variants that might help (i) to understand perturbed biological processes in vascular tissues contributing to MS disease, and (ii) to characterize MS susceptibility genes for functional association with disease-pathways.
Keywords: multiple sclerosis, vascular components, GWAS, transcriptomics, WES, rare variants, circadian entrainment, circadian rhythm
1. Introduction
Several studies have provided evidence for vascular components participating in the complex pathogenetic network in multiple sclerosis (MS). Blood–brain barrier disruption and vascular changes, leading to cerebral hypoperfusion and tissue hypoxia, interact in a vicious cycle favoring the altered immune trafficking and the inflammatory events [1,2,3]. Most of the MS lesions have a perivascular development around small cerebral veins, a feature called “central vein sign” [4,5], which spreads across all MS clinical phenotypes. The elevation in venous pressure [6] might be a vascular feature related to the underlying pathophysiology of MS and a component of the compliance changes found in MS [7].
The cerebrovascular disease and the neurodegeneration process appear to be reciprocally linked [8,9,10], supporting alterations in neurovascular coupling mechanisms. Moreover, the occurrence of cardiovascular comorbidities [11,12,13,14] and the overlapping between genetic associations for MS and cardiovascular disease [15,16] suggest common pathological mechanisms affecting vasculature [12].
Dysfunction in the circadian clock system might contribute to neurovascular pathological conditions by affecting protein homeostasis, immune, and inflammatory functions [17,18,19,20,21,22]. Indeed, recent evidence suggests the role of molecular clocks in regulating blood–brain barrier [23], in dictating the response of immune pathways, and in modulating innate and adaptive immune crosstalk in autoimmune diseases [24]. Data support the relationships between the circadian rhythm and function of the vascular system [25], association of circadian disruption with immune activation–neurodegeneration [26], and increased incidence of MS [24].
Among hundreds of MS susceptibility genes from genome-wide association studies (GWAS) contributing to the polygenic pathways network of MS [27], many are still to be characterized in terms of functional association with MS onset/progression pathways, particularly with respect to the vascular compartment.
This study was aimed at exploring vascular components in MS by combining (i) transcriptome analysis of the internal jugular vein (IJV) walls from MS patients suffering from disturbed brain outflow [28] and (ii) whole exome sequencing (WES) in MS families [29] characterized by disturbed brain outflow and vertical transmission of MS.
2. Results
2.1. The Transcriptomic Approach: Expression Profile of MS-GWAS Genes in Vascular Tissues
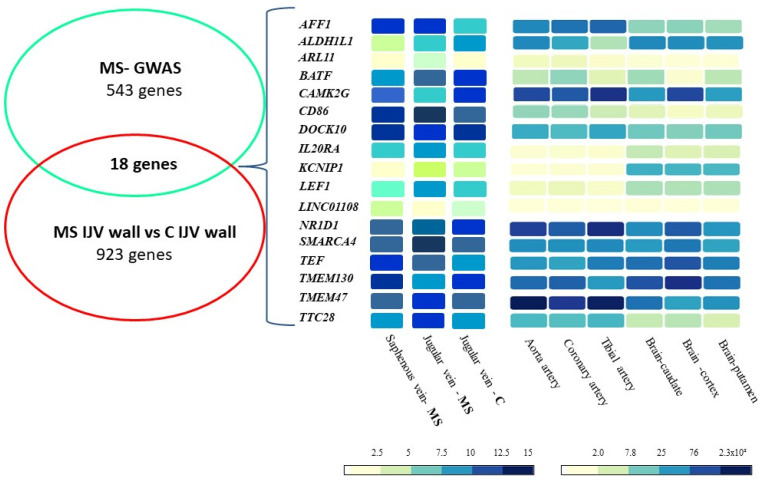
The reference list of MS genes in the GWAS catalogue (n = 543) and the transcripts differentially expressed between MS and control IJV walls (n = 923) included eighteen shared records (2 ncRNA and 16 coding genes) (Table 1). Regarding the 16 coding genes (Table 2), the GO enrichment analysis by STRING (v11.0) revealed significantly (FDR ≤ 0.05) enriched terms related to immune system/response, driven by transcripts for CD86, a receptor of the immune/inflammatory response that showed the highest increase in fold change in the MS jugular wall. Transcription (biological processes) and signal transduction (reactome pathways) were also included among the significantly enriched terms.
Table 1.
MS-GWAS genes differentially expressed between MS and control IJV walls.
| Gene Symbol | Gene Name | Regulation | MS-IJV | C-IJV | FC | p * |
|---|---|---|---|---|---|---|
| AFF1 | AF4/FMR2 family member 1 | up | 9.95 ± 0.30 | 8.84 ± 0.29 | 2.17 | 0.005 |
| ALDH1L1 | Aldehyde dehydrogenase 1 family member L1 | down | 6.03 ± 0.22 | 7.23 ± 0.36 | 2.29 | 0.004 |
| ARL11 | ADP ribosylation factor-like GTPase 11 | up | 3.11 ± 0.42 | 2.07 ± 0.32 | 2.06 | 0.010 |
| BATF | Basic leucine zipper ATF-like transcription factor | up | 10.52 ± 1.39 | 8.94 ± 0.46 | 2.99 | 0.049 |
| CAMK2G | Calcium/calmodulin dependent protein kinase II gamma | down | 6.80 ± 0.57 | 8.01 ± 0.48 | 2.31 | 0.019 |
| CD86 | CD86 molecule | up | 14.38 ± 0.89 | 11.30 ± 0.38 | 8.48 | 0.002 |
| DOCK10 | Dedicator of cytokinesis 10 | down | 8.71 ± 0.98 | 10.01 ± 0.44 | 2.47 | 0.038 |
| IL20RA | Interleukin 20 receptor subunit alpha | up | 7.57 ± 1.23 | 5.97 ± 0.39 | 3.00 | 0.034 |
| KCNIP1 | Potassium voltage-gated channel interacting protein 1 | up | 3.84 ± 0.66 | 2.65 ± 0.70 | 2.28 | 0.042 |
| LEF1 | Lymphoid enhancer binding factor 1 | up | 7.64 ± 0.44 | 6.29 ± 0.96 | 2.55 | 0.042 |
| LINC01108 | ncRNA | down | 2.09 ± 0.54 | 3.17 ± 0.60 | 2.12 | 0.034 |
| LOC100130476 | ncRNA | up | 3.33 ± 0.15 | 2.16 ± 0.35 | 2.24 | 0.047 |
| NR1D1 | Nuclear receptor subfamily 1 group D member 1 | up | 8.31 ± 0.97 | 6.45 ± 1.16 | 3.63 | 0.042 |
| SMARCA4 | SWI/SNF related, matrix associated, actin dependent regulator of chromatin, A4 | up | 14.07 ± 0.86 | 12.12 ± 0.33 | 3.86 | 0.007 |
| TEF | Thyrotroph embryonic factor | up | 9.02 ± 0.47 | 7.76 ± 0.91 | 2.40 | 0.045 |
| TMEM130 | Transmembrane protein 130 | down | 6.73 ± 0.31 | 7.94 ± 0.60 | 2.32 | 0.016 |
| TMEM47 | Transmembrane protein 47 | down | 9.60 ± 0.82 | 10.70 ± 0.54 | 2.21 | 0.043 |
| TTC28 | Tetratricopeptide repeat domain 28 | up | 8.72 ± 0.54 | 7.52 ± 0.33 | 2.30 | 0.011 |
MS-IJV and C-IJV columns, mean ± SD of the log-transformed (log2) expression values. The fold change (FC) is presented as an absolute value. * by moderate t-test, followed by the application of Benjamini–Hochberg multiple testing correction.
Table 2.
Names and function of proteins encoded by MS-GWAS genes differentially expressed between MS and control IJV walls.
| Gene Symbol | Protein Name (Short Name) | Protein Function |
|---|---|---|
| AFF1 | AF4/FMR2 family member 1 (AF4) | Transcription factor, elongation complex of RNA pol II (https://www.ncbi.nlm.nih.gov/gene/4299) |
| ALDH1L1 | Cytosolic 10-formyltetrahydrofolate dehydrogenase (ALDH1L1, FDH) | Enzyme of folate metabolism (https://www.ncbi.nlm.nih.gov/gene/10840) |
| ARL11 | ADP ribosylation factor-like protein 11 (ARLTS1) | GTP-binding protein (https://www.ncbi.nlm.nih.gov/gene/115761) |
| BATF | Basic leucine zipper transcriptional factor ATF-like (B-ATF) | Transcription factor (https://www.ncbi.nlm.nih.gov/gene/10538) |
| CAMK2G | Calcium/calmodulin-dependent protein kinase type II subunit gamma (CAMK-II gamma) | Enzyme, serine/threonine protein kinase, calcium signaling (https://www.ncbi.nlm.nih.gov/gene/818) |
| CD86 | T-lymphocyte activation antigen CD86 (CD86) | Receptor, type 1 membrane protein https://www.ncbi.nlm.nih.gov/gene/942 |
| DOCK10 | Dedicator of cytokinesis 10 (DOC-10) | Guanosine nucleotide exchange factor (https://www.ncbi.nlm.nih.gov/gene/55619) |
| IL20RA | Interleukin-20 receptor subunit alpha (IL-20R-alpha) |
Receptor of IL20, membrane protein (https://www.ncbi.nlm.nih.gov/gene/53832) |
| KCNIP1 | Kv channel-interacting protein 1(KChIP1) | Neuronal membrane excitability (https://www.ncbi.nlm.nih.gov/gene/30820) |
| LEF1 | Lymphoid enhancer-binding factor 1 (LEF1) | Transcription factor, WNT-signaling (https://www.ncbi.nlm.nih.gov/gene/51176) |
| NR1D1 | Nuclear receptor subfamily 1 group D member 1 (EAR-1) Rev-erbA-alpha |
Heme-dependent transcriptional repressor (https://www.ncbi.nlm.nih.gov/gene/9572) |
| SMARCA4 | Transcription activator BRG1 (BAF190A) | Enzyme, helicase and ATPase activities, regulator of transcription (https://www.ncbi.nlm.nih.gov/gene/6597) |
| TEF | Thyrotroph embryonic factor | Transcription factor that binds to and transactivates the TSHB promoter (https://www.ncbi.nlm.nih.gov/gene/7008) |
| TMEM130 | Transmembrane protein 130 | Membrane-bound signaling protein (https://www.ncbi.nlm.nih.gov/gene/222865) |
| TMEM47 | Transmembrane protein 47 | Adherens junction, cell–cell adhesion (https://www.ncbi.nlm.nih.gov/gene/83604) |
| TTC28 | Tetratricopeptide repeat protein 28 | Scaffold-adaptor protein (https://www.ncbi.nlm.nih.gov/gene/23331) |
The gene symbols are reported according to NCBI Gene database (https://www.ncbi.nlm.nih.gov; accessed on 20 December 2021). The recommended protein names and the reported short names (parenthesis) are in accordance with the UniProt Knowledgebase. The molecular function of the proteins is reported according to the Report of the corresponding gene in NCBI Gene database. Noticeably, one third of transcripts upregulated in MS jugular walls encoded for transcription factors (AFF1, BATF, LEF1, NR1D1, and TEF) or for a transcription regulator, SMARCA4, an ATP-dependent chromatin remodeling protein (Table 2).
In order to explore the expression at the mRNA level in other vascular tissues of the 18 selected genes, data from transcriptome analysis of saphenous vein (SV) wall, previously obtained by microarray in vascular specimens from MS patients [30], and data extracted from GTEx portal, only available for arterial vessels, were considered. In addition, the GTEx portal analysis was extended to brain, in particular the caudate, cortex, and putamen regions, of main interest for MS. The relative expression levels concerning two veins, IJV (MS patients and controls) and SV (MS patients), three arteries (aorta, coronary, and tibial), and the selected brain regions are compared in Figure 1. CAMKG2, N1RD1, SMARCA4, TEF, TMEM130, and TMEM47 were characterized by high expression levels in veins, arteries, and brain regions. BATF, CD86, IL20RA, and LEF1 were highly expressed in the veins and modestly in the arteries and brain regions. Differently, ARL11, KCNIP1, and LINC01108 were expressed at very low levels in both veins and arteries, and ARL11 and LINC01108 also displayed low levels in brain regions.
Figure 1.
Schematic representation of the mRNA expression levels of MS-GWAS genes in vascular and brain tissues. Data from venous tissues were obtained by transcriptome analysis of IJV and SV from MS patients and from IJV of controls. Data from arteries and brain tissues were retrieved from GTEx portal. The expression levels are reported as log2 expression values (Table 1) for IJV and saphenous vein, or as transcript per million (TPM) for arteries and brain tissues, according to the color scales reported below the figure. For the ncRNA LOC00130476, no expression data in vascular or brain tissues are reported in GTEx portal.
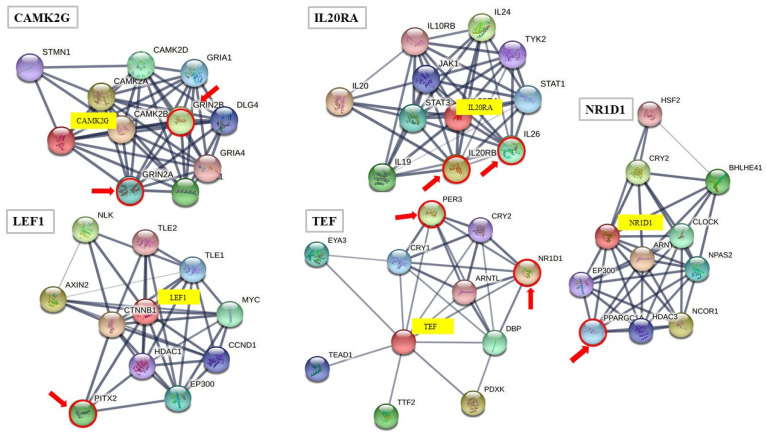
Functional associations of the proteins encoded by the 16 genes were explored in the STRING database, and the top 10 functional partners were considered for each gene. Five proteins (CAMKG2, IL20RA, LEF1, N1RD1, and TEF, Figure 2) were found to be functional partners (scores 0.69–0.99) of seven proteins (GRIN2A, GRIN2B, IL20RB, IL26, PITX2, PPARGC1A, and PER3) encoded by genes differentially expressed in the IJV transcriptome [28]. Among functional partners, transcriptional regulators were well represented (PITX2, PPARGC1A, PER3). Potential interactions between NR1D1 and TEF and between LEF1 and SMARCA4 (not shown in Figure 2) were also reported in the STRING database.
Figure 2.
Functional partners of CAMK2G, IL20RA, LEF1, NR1D1, and TEF predicted by STRING database (v11.0). In the STRING setting, the protein nodes are reported with the corresponding gene symbol. The top ten interactors of CAMK2G, IL20RA, LEF1, NR1D1, and TEF are reported as colored nodes filled with some known or predicted 3D structure. The edges (solid lines) indicate both functional and physical protein associations. The line thickness indicates the strength of data support that ranged from 0.69 to 0.76 (high, TEF), and from 0.92 to 0.99 (highest, CAMK2G, IL20RA, LEF1, NR1D1). The genes differentially expressed between MS and C IJV walls are marked by red arrows and circles.
The DAVID (v6.8) analysis of the 12 genes, differentially expressed in the IJV transcriptome of MS patients, revealed that the terms “circadian entrainment” (KEGG pathway) and “circadian regulation of gene expression” (biological process) were significantly enriched (Benjamini-corrected p-value 0.011 and 0.016, respectively).
2.2. The Genomic–Transcriptomic Approach: WES in MS Families—Expression Profile of MS-GWAS Genes
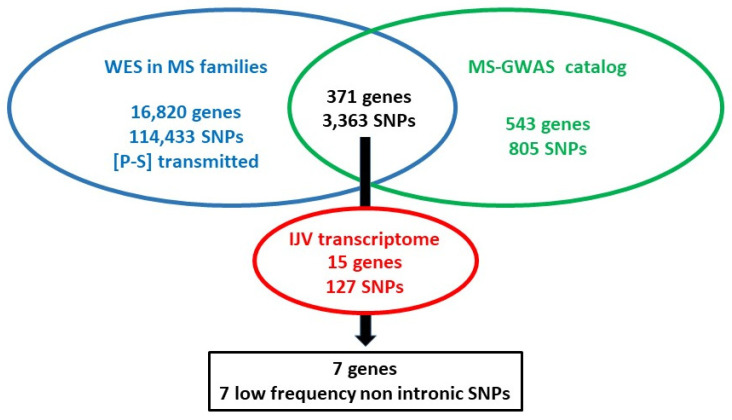
Data from the targeted WES-based pilot analysis in MS families were examined to identify variants showing vertical transmission of disease within families. A total of 114,433 single-nucleotide polymorphisms (SNPs) were observed in 16,820 genes (Figure 3). To prioritize SNPs combining genomic and transcriptomic approaches, data from WES output in families were filtered with both MS-GWAS and transcriptomic sets as schematized in Figure 3, leading to the selection of 127 variants in 15 genes (Figure 3) among the 18 selected by transcriptomic GWAS approach (Table 1). We focused on low-frequency (MAF ≤ 0.04) and nonintronic variants (Figure 3, Table 3 and Table S1, MS-GWAS genes), and extended the investigation of disease association by using data from i) the Network for Italian Genomes “NIG-IT” that includes individuals affected by different diseases, among which includes MS, and ii) controls from gnomAD 3.1.1 (nfe) and from Tuscany in the 1000 Genome Project. Among the seven prioritized SNPs, the DOCK1 rs113265459, the NR1D1 rs17616365, and TMEM130 rs199556348 showed significantly higher MAF in the NIG-IT affected cohort than in the 1000G TSI samples (Table 3). MAF of NR1D1 and TMEM130 variants in the NIG-IT Italian sample was significantly different from that in the gnomAD 3.1.1.
Figure 3.
Schematic representation of the filtering approaches to prioritize SNPs. Variants with vertical transmission of disease within the MS families (WES output, blue) were selected and then filtered using both MS-GWAS (green) and transcriptomic (red) datasets.
Table 3.
Nonintronic variants with significant differences in allele frequency between affected and control Italian cohorts.
| Gene |
Chr: Position GRCh38
dbSNP |
Ref. Allele |
mRNA
Molecular Consequences |
mRNA Level Affected by SNP eQTL |
CADD (°)
(Ensembl) |
NIG-IT
MAF |
1000G
TSI MAF |
gnomAD 3.1.1 (nfe)
MAF |
p-Value ($)
NIG-IT vs. 1000G TSI |
p-Value ($)
NIG-IT vs. gnomAD 3.1.1 |
| MS-GWAS genes | ||||||||||
| DOCK10 | chr2:224795011:G:A/C rs113265459 |
A | caC/caT p.His1674His |
ns | A:5.937 | 0.0224 | 0 | 0.0165 | 6.87 × 10−7 | 0.1296 |
| NR1D1 | chr17:40100148:G:A rs17616365 |
A | c.-54C > T 5′-UTR |
CASC3, WIPF2 | A:16.11 | 0.0119 | 0.00485 | 0.0283 | 0.00083 | 0.0011 |
| TMEM130 | chr7:98863351:C:T rs199556348 |
T | gcG/gcA p.Ala45Ala | ns | T:1.532 | 0.00046 | 0.00002 | 0.00003 | 0.0013 | 0.0094 |
| Functional partners | ||||||||||
| GRIN2B | chr12:13611840:G:A rs1805482 |
A | agC/agT p.Ser555Ser | ns | A:0.955 | 0.3215 | 0.3786 | 0.3377 | 0.00011 | 0.2569 |
| GRIN2B | chr12:13865843:G:C/T rs7301328 |
C | ccC/ccG p.Pro122Pro | ns | C:7.840 | 0.3646 | 0.4126 | 0.3892 | 0.00131 | 0.0958 |
| PER3 | chr1:7829881:C:T rs2640908 |
T | acC/acT (+) p.Thr978Thr |
VAMP3, UTS2 | T:0.757 | 0.2044 | 0.2379 | 0.1918 | 0.0093 | 0.2884 |
| PER3 | chr1:7830057:T:C rs2640909 |
C | aTg/aCg (+) p.Met1037Thr |
VAMP3 | C:0.161 | 0.2500 | 0.3204 | 0.2872 | 7.27 × 10−7 | 0.0067 |
| PPARGC1A | chr4:23814301:T:C/A rs2970847 |
C | acA/acG p.Thr394Thr |
ns | C:1.605 | 0.1763 | 0.1408 | 0.1923 | 0.00077 | 0.1791 |
| PPARGC1A | chr4:23814039:C:T rs8192678 |
T | Ggt/Agt p.Gly482Ser |
ns | T:16.24 | 0.3572 | 0.4272 | 0.3450 | 3.33 × 10−6 | 0.3964 |
| PPARGC1A | chr4:23813899:C:T/A/G rs3755863 |
T | acG/acA p.Thr528Thr | ns | T:9.102 | 0.4380 | 0.5097 | 0.4054 | 2.42 × 10−6 | 0.0287 |
Genomic coordinates from NCBI Build GRCh38.p13 (hg38) and dbSNP refSNP (rs) identifiers from build 155 are provided. mRNA levels significantly affected by SNPs in vascular and/or brain tissues (reported in GTEx portal as expression quantitative traits, eQTL) are provided. ns, no significant eQTL was found. CASC3, exon junction complex subunit GJD3, involved into spliceosomes at the exon–exon junction; WIPF2, WAS/WASL interacting protein family member 2, involved in the WASP-mediated organization of the actin cytoskeleton. VAMP3, a protein involved in docking/fusion of synaptic vesicles; UTS2, urotensin 2. Minor allele frequency (MAF) from WES of affected subjects collected by the Network for Italian Genomes (NIG-IT); controls from 1000 Genome Tuscany (TSI) and gnomAD 3.1.1 non-Finnish European (nfe) databases are given. ($) Bonferroni’s multiple test correction was set to p < 0.002: in red, SNPs significant after Bonferroni correction; in blue, SNPs at borderline significance after Bonferroni correction; in black, nominally significant variants. (°) Estimated effect on protein function was assessed with the Combined Annotation-Dependent Depletion (CADD) phred-scale scores v1.4 (for convenience, scores above 30 are regarded as “likely deleterious” and scores below as “likely benign”).
The expression quantitative trait loci (eQTL) analysis (GTEx portal), focused on vascular and brain tissues, supported the functional impact of eight SNPs among the MS-GWAS low-frequency nonintronic variants (n = 2) and among nonintronic variants (n = 6) in functional partner genes (Table S1).
The NR1D1 rs17616365 (Table 3 and Table S1, MS-GWAS genes) affects the mRNAs of CASC3, a protein of the exon–junction complex, and of WIPF2, a protein involved in the organization of cytoskeleton. Further, the 5′UTR NR1D1 rs17616365 has been associated with sex hormone-binding globulin levels. The CD86 rs11575853 (Table S1) affects the expression of mRNA encoding NPHP5, a nephrocystin protein that interacts with calmodulin and the retinitis pigmentosa GTPase regulator protein. The stop codon gains of ARL11 rs34301344 and the amino acid substitution (Val145Leu) of LEF1 rs141850161 could also mark functional variants (Table S1).
The detection of variants by WES, extended to the genes (GRIN2A, GRIN2B, IL20RB, IL26, PITX2, PPARGC1A, and PER3) encoding the predicted functional partners (Figure 2) revealed 57 SNPs, 39 intronic and 18 nonintronic. The analysis of the nonintronic SNPs (Table 3, functional partners) highlighted five variants (GRIN2B rs1805482, PER3 rs2640909, PPARGC1A rs2970847, rs8192678, and rs3755863) differing significantly in frequency (p < 0.0008) between the NIG-IT cohort and the 1000G TSI. For two polymorphisms, PER3 rs2640908 and GRIN2B rs7301328, a borderline significance was observed. When compared with gnomAD controls, the PER3 rs2640909 and PPARGC1A rs3755863 showed a borderline or nominal significant p-value, respectively.
The eQTL analysis for the nonintronic SNPs in functional partners (Table 3 and Table S1, functional partners) showed that three PER3 variants (the synonymous rs228669 and two missense rs228696 and rs228697) affect the expression of the PER3 mRNA. Moreover, four PER3 variants (two synonymous rs2640908, rs2859387 and two missense rs2640909, rs228696) affect the expression of VAMP3 mRNA, a protein of the complex involved in docking/fusion of synaptic vesicles. The PER3 rs2859387 also affects the splicing (sQTL) of VAMP3 mRNA and the PER3 rs2640908 the mRNA levels of UTS2, a vasoconstrictor peptide (Table 3 and Table S1, functional partners).
2.3. Associations with Vascular/Neuronal Traits/Diseases
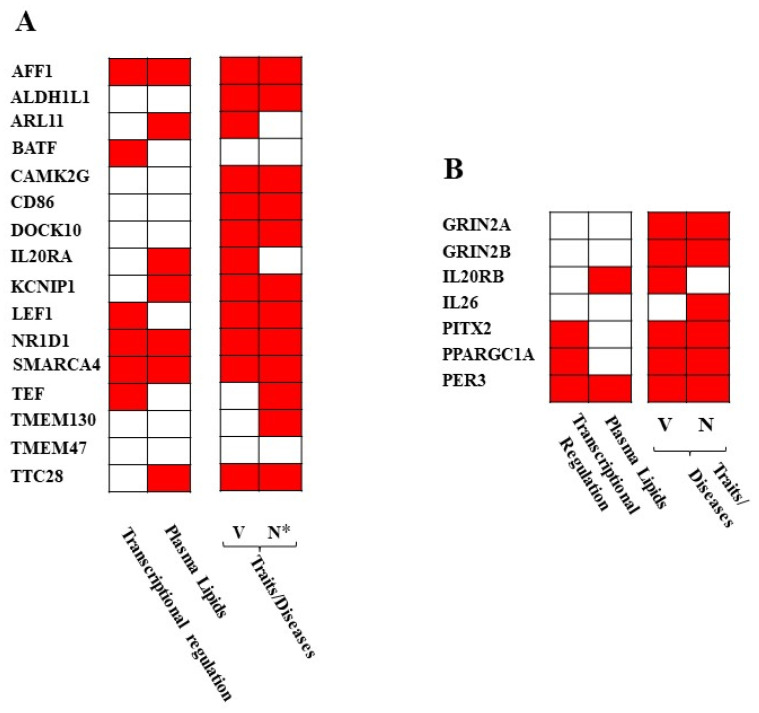
To provide insights into association of selected genes/proteins with diseases, particularly with vascular and neuronal aspects, the associations of the 16 GWAS coding genes and of the seven functional partners were investigated by bioinformatics (DAVID v6.8). Although the terms “pharmacogenomics”, “immune”, and “hematological” were listed in DAVID, none reached significance after Benjamini correction. In literature mining, for five out of the 16 genes (BATF, CD86, IL20RA, KCNIP1, LEF1), association with MS was detected also in studies conducted at RNA or protein levels (Table 4). Both vascular and neuronal associations, additional to MS, were detected for 11 out of 16 genes, and eight were also associated with plasma lipid levels (Figure 4A). Interestingly, several of these genes were immune-related and one of them (LEF1) has been previously related to the blood–brain barrier (Table 4).
Table 4.
MS- GWAS genes differentially expressed between MS and control IJV walls: association with neuro/vascular processes, traits, and diseases.
| Gene | Protein Function | Gene/SNPs-Associated Processes/Traits/Diseases | |
|---|---|---|---|
| Vascular | Neuronal | ||
| AFF1 | Transcription factor; elongation complex RNA pol II | TG, HDL-LDL cholesterol levels, CAD [31] | Cognitive decline in AD, glioblastoma [32] |
| ALDH1L1 | Enzyme; folate metabolism | Aortic stenosis, ischemic stroke [33] | Neural tube defects [34] |
| ARL11 | GTP- binding protein, tumor suppressor gene | Total cholesterol levels [35] | nr |
| BATF | Transcription factor | Blood pressure [15] | MS* [36] |
| CAMK2G | Enzyme; calcium signaling | VSMC proliferation and vascular remodeling [37] | AD, intellectual developmental disorder [38] |
| CD86 | Receptor, immune-inflammatory response | HDL-cholesterol, atherogenic process, stroke, vascular rejection [15,39] | MS* [40,41], autoimmune peripheral neuropathy [42] |
| DOCK10 | Guanyl–nucleotide exchange factor activity | Circulating thrombin activatable fibrinolysis inhibitor [43], platelet count | Dendritic spine morphogenesis, innate immunity and neuroinflammation in EAE, autism [44,45] |
| IL20RA | Receptor of IL20, membrane protein | HDL-LDL cholesterol levels, plasma trimethyllysine # [46,47] | MS* [48], neuroinflammation in EAE [49] |
| KCNIP1 | Ion transmembrane transport | TG levels; atrial fibrillation, hypertension, heart rate in heart failure [50,51] | MS* [52], neuronal calcium sensor, neuronal membrane excitability, ALS, schizophrenia [53] |
| LEF1 | Transcription factor; WNT-signaling | Maturation of the blood–brain barrier, vascular remodeling, and angiogenesis [54,55] | MS* [56], schizophrenia, sporadic ALS [57] |
| NR1D1 | Transcription factor (REV-ERBα); transcriptional repressor, circadian clock component | Lipoproteins levels, anti-inflammatory and atheroprotective action, negative regulation of PAI-1 [58,59] | Neuroinflammation -neurodegeneration, autism [26,60] |
| SMARCA4 | Enzyme, helicase and ATPase activities, regulator of transcription | LDL cholesterol levels, CAD, MI, stroke, carotid atherosclerosis, PAD [31,61] | Growth retardation and neuronal degeneration, ALS [62,63] |
| TEF | Transcription factor | nr | Depressive disorders, PD [64] |
| TMEM130 | Membrane-bound signaling protein | nr | Autism [65] |
| TMEM47 | Adherens junction, cell–cell adhesion | nr | nr |
| TTC28 | scaffold- adaptor protein | TG levels, systolic blood pressure [31] | Cerebrospinal T-tau levels [66] |
MS*, association with MS detected also in RNA/protein studies; #, plasma trimethyllysine are associated with progression of atherosclerosis and myocardial infarction (MI) risk; AD, Alzheimer’s disease; ALS, amyotrophic lateral sclerosis; EAE, experimental autoimmune encephalomyelitis; PAD, peripheral artery disease; PD, Parkinson’s disease; TG, triglycerides; VSMC, vascular smooth muscle cell; nr, not reported in literature.
Figure 4.
Association of GWAS (A) and interactor (B) genes and their encoded mRNA/proteins with transcription, and with vascular (V)/neuronal (N) traits or diseases according to literature and databases. N*, neuronal diseases additional to MS. Associations of ncRNAs (LINC01108 and LOC00130476) with diseases were not found in literature and databases.
Virtually all the functional partners showed associations with vascular traits/diseases and with neuronal traits/diseases, including MS (Figure 4B, Table S2). To note, GRIN2A, GRIN2B, IL20RB, IL26, PITX2, PPARGC1A, and PER3 are not present in the MS-GWAS catalog.
3. Discussion
We propose candidate vascular components in MS by combining the transcriptional pattern in the wall of IJV, the main cerebral venous outflow pathway [67], with MS-GWAS and analysis of vertically transmitted disease variants within three multi-incidence MS families with disturbed brain outflow. Within GWAS genes with differential expression, we focused on low-frequency variants, which are not individually detectable at genome-wide thresholds but are potentially endowed with functional impact [15,68]. Noticeably, the analysis, extended to the non-MS-GWAS functional partners of transcriptionally dysregulated genes, highlighted numerous associations with vascular or neuronal traits/diseases, including MS.
3.1. Transcription Factor Expression—Vascular Function Alteration in MS-Cardiovascular Risk Traits
Genomic and transcriptomic approaches pointed out a noticeable proportion of genes (9/23), MS-GWAS, and functional interactors encoding for transcription factors or regulators, which could bridge alteration of vascular function with MS disease. Moreover, finding virtually all selected genes (22/23) associated with vascular or neuronal traits/diseases strongly supports the presence of pleiotropic loci between MS and cardiovascular comorbidities.
LEF1 and SMARCA4 belong to the WNT/beta-catenin pathway, upregulated in demyelinating events characterizing neurodegeneration [69] and modulating the immune response in MS. LEF1, a brain endothelium-specific transcription factor, has been associated [54] with vascular remodeling and post-stroke angiogenesis [55], and SMARCA4 [70] with cardio–cerebral–peripheral vascular diseases in GWAS [61].
SMARCA4, AFF1, and NR1D1/Rev-Erbα provide a link between immunity-lipid metabolism–vascular function. SMARCA4 and AFF1 contain several SNPs associated with triglyceride and cholesterol plasma levels [61,71], suggesting the genetic overlap between blood lipids and immune-mediated diseases [72]. NR1D1/Rev-Erbα may exert atheroprotective action through inhibition of (i) the human APOC3 promoter gene activity [58] and (ii) PAI1 expression [59], a major negative regulator of fibrinolysis.
CAMKG2/CaMKIIγ, a component of the Ca2+-dependent signaling pathways that influences proliferation of vascular smooth muscle cells and vascular remodeling [37], is shared between two major neurological diseases: ischemic stroke and MS [73].
CD86, driving the atherogenic process [74] in the adaptive immune system, and BATF relate MS disease with cardiovascular disease risk factors, high-density lipoprotein cholesterol (CD86), and systolic blood pressure (BATF) [15]. To note, the 5′UTR CD86 rs11575853 was highlighted in the MS families under study and has been found associated with lower expression in several arterial tissues and brain regions of the MS-GWAS gene IQCB1, which encodes for the nephrocystin NPHP5. An intriguing hypothesis is that the altered expression of NPHP5, involved in amaurosis and retinopathies [75], represents a potential link between retinal diseases and neurodegeneration [76].
3.2. NR1D1–PPARGC1A–PER3 Expression: Vascular and Circadian Clock Components in MS
Several lines of evidence support the involvement of circadian rhythm in the pathogenesis of many neurodegenerative diseases, including MS [20,22]. We highlight differential expression in the wall of IJV, which drains blood from the brain, of several circadian entrainment/rhythm components (Figure 5). These further support relationships between the complex circadian network and MS disease. Of interest, NR1D1, highlighted both by the MS GWAS/IJV transcriptome and GWAS/MS family transmission analyses, provides another piece of the complex neurovascular network in MS, represented by the circadian clock. Indeed, NR1D1/Rev-Erbα negatively regulates the expression of circadian clock proteins. Evidence has been provided for relationships between the circadian rhythm and function of the vascular system [25], and for association of circadian disruption with immune activation–neurodegeneration [26] and increased incidence of MS [24]. The NR1D1 5′UTR rs17616365 is associated in brain and arterial tissues with the mRNA levels of CASC3, a protein found to participate to a regulatory network in MS [77].
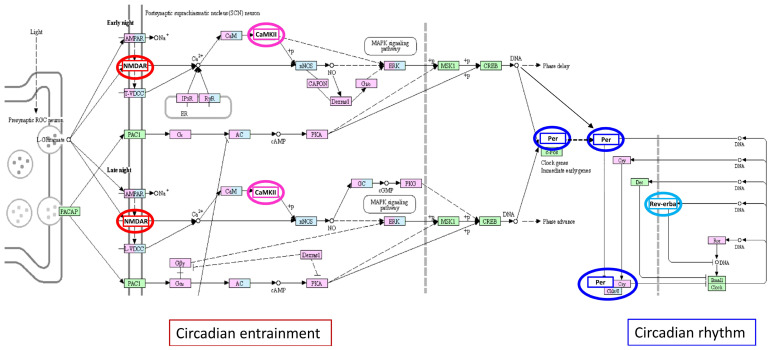
Figure 5.
The “circadian entrainment” and “circadian rhythm” KEGG pathway members encoded by genes differentially expressed in the wall of IJV (CAMK2G, GRIN2A, GRIN2B, PER3, NR1D1). In the KEGG graph, the proteins encoded by these genes are marked as colored circles: the red circle indicates the glutamate ionotropic receptor NMDA that contains subunit 2A and 2B coded by GRIN2A, GRIN2B; the pink circle indicates the CaMKIIγ protein kinase coded by CAMK2G; the blue circle indicates the PER proteins among which the period circadian clock 3 is coded by PER3; the light blue circle indicates the Rev-Erbα protein coded by NR1D1. In accordance with KEGG color codes, pink rectangles indicate disease genes; light blue rectangles indicate drug target; pink/light blue rectangles indicate disease gene and drug target elements; green rectangles indicate specific signaling pathway elements.
Noticeably, two other genes, PPARGC1A and PER3, not reported in the MS-GWAS catalog and involved in the circadian clock, both functional partners of NR1D1, were found differentially expressed in MS vs. control jugular wall and, in families with disturbed brain blood outflow, harbored SNPs with intriguing links to vascular aspect in MS.
PPARGC1A, a transcriptional coactivator, represents a key component of the circadian oscillator that integrates the mammalian clock and energy metabolism [78]. The PPARGC1A rs8192678 (Gly482Ser) was very recently found to confer an increased risk for the occurrence of MS and lipid/metabolic disorders [79]. The finding of lower mRNA levels in MS than in control IJV walls is compatible with the reduced mRNA expression in peripheral blood mononuclear cells of RR-MS [80] and with reduced expression of PPARGC1A and neuronal loss in MS cortex [81].
PER3, a transcriptional repressor, has been associated with homeostatic regulation of sleep, circadian clock phenotypes [82], and immune response [83].
The PER3 rs2640909 (Met1037Thr) and rs2640908 (synonymous), not in linkage disequilibrium, were found to predict in arterial tissues the expression levels of the adjacent VAMP3, involved in synaptic vesicles biology and potential target for preventing disturbed flow-dependent thrombus formation [84]. The relationship of PER3 variations/circadian clock/MS is supported by a PER3 VNTR polymorphism, found to impact the sleep disturbances in MS [85].
As the disruption of circadian rhythms can lead to dysregulation of cellular and molecular mechanisms of the immune cells, and therefore contribute to aberrant immune response [17,18], finding signals in genes belonging to this complex circadian network in MS is of great interest.
A limitation of this study is the low number of investigated patients and families, who, on the other hand, were selected for disturbed brain outflow. Based on these features, the selected genes and markers could be more tightly associated with a subgroup of MS patients.
4. Conclusions
Based on transcriptome and genomic analysis in MS, our study highlights the differential expression in venous tissue, particularly in the wall of the IJV, which drains blood from the brain, of 16 MS-GWAS genes, and of seven genes not previously indicated by GWAS, but functionally interacting with MS candidates. Virtually all genes have been previously associated with vascular or neuronal traits/diseases additional to MS and nine encoded for transcriptional factors/regulators. Eight genes were associated with plasma lipid levels supporting the link between lipids and immune-mediated diseases.
Differential expression and frequency of low-frequency variants were observed for six genes involved in circadian entrainment/rhythm.
Future studies should focus on the functional characterization of selected markers and SNPs (i) “in vitro”, by recombinant expression of SNPs which predict alteration/modulation of the gene/protein expression; (ii) in plasma/WBC of MS patients to evaluate protein/mRNA [86] with predicted alteration/modulation (i.e, plasma lipids), and (iii) in patients undergoing exercise and rehabilitation strategies [87], grouped for specific alleles of the circadian entrainment/rhythm genes.
5. Materials and Methods
The list of genes associated with MS was created by the NHGRI-EBI GWAS catalogue [88]. SNPs listed under “multiple sclerosis” and with p-value ≤ 5 × 10−6 were selected. Based on these selected common variants, the reference gene list for the present study included 805 SNPs attributable to 543 genes.
Microarray-based transcriptome analysis of IJV walls has been already described [28]. The list of differentially expressed genes between MS- and control-IJV walls (fold change ≥2, p ≤ 0.05, multiple testing correction), included 923 genes [28].
Expression data of selected genes in arterial vessels and brain regions were extracted from Genotype-Tissue Expression (GTEx) portal [89].
For selected genes, gene ontology enrichment analysis (FDR ≤ 0.05) for biological processes, molecular functions, and reactome pathways was conducted with STRING [90]. Functional partners (protein–protein interaction, PPI) were explored by STRING database (v11.0, https://version-11-0b.string-db.org/, accessed on 1 July 2021). In the STRING setting, the protein nodes are reported with the corresponding gene symbol and as colored nodes filled with some known or predicted 3D structure. The network edges were reported with the “confidence” mode (solid lines). The line thickness indicates the degree of confidence prediction of the interaction (low 0.150; medium 0.400; high 0.700; highest 0.900). Only the top ten (high to highest) interactors were included in the analysis. The Database for Annotation, Visualization and Integrated Discovery (DAVID, https://david.ncifcrf.gov/home.jsp, accessed on 10 November 2021) v6.8 [91] was used to perform functional enrichment analyses of the differentially expressed functional partner and related genes. Significantly enriched terms were defined as having Benjamini-corrected p-value < 0.05.
Pathways of molecular interactions and relations of selected genes were obtained from Kyoto Encyclopedia of Genes and Genomes (KEGGS) portal [92].
A WES was performed on 11 individuals, seven diagnosed with MS and four unaffected, from three independent families. Sequencing was performed by BGI (Shenzhen, China) using nanoarray-based short-read sequencing-by-ligation technology (cPALTM) as previously described [29].
To identify new exonic and/or potentially functional variants we firstly analyzed data from the targeted WES-based pilot analysis in MS families to identify all variants showing vertical transmission of disease within families. Then, we filtered these SNPs with both MS-GWAS and transcriptomic sets. To prioritize the selected variants, we investigated these candidate SNPs in publicly available datasets of human DNA sequence variation (1000 Genomes Project [93]; Genome Aggregation Database gnomAD [94]).
Based on the higher genetic variability in the Italian population as compared with other European populations [95,96], leading to recommending the use of population-specific databases for a more accurate assessment of variant pathogenicity [97], we compared populations with MAFs obtained from (i) the Network for Italian Genomes “NIG-IT” which include whole-exome sequencing for 1098 unrelated Italian individuals affected by different diseases, MS included; (ii) Controls from 1000 Genome Project which contains allelic frequencies for a sample of Tuscany, Italy (1000G TSI), an optimal reference population for individuals under study. The low prevalence (188 per 100,000 individuals) of MS in Tuscany [98] makes improbable the presence of individuals with MS in the Tuscany control sample. Although the 1000G TSI cohort is the most closely related to our sample, it is too small to produce accurate frequencies for low-frequency variants. Thus, we used the non-Finnish European (nfe) populations from gnomAD 3.1.1 as a further control population.
To test the difference in MAFs between reference and study populations, a two-proportion z-test with a 0.05 two-sided significance level was applied. A threshold of p < 0.002, assuming the Bonferroni correction for multiple testing, was used for significance.
Acknowledgments
We thank the Italian foundation “Fondazione Il Bene Onlus” for its support to multiple sclerosis research.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms23010310/s1.
Author Contributions
Conceptualization, F.B., G.M., C.S. and N.Z.; methodology, C.S., N.Z., B.L., E.M., M.B., F.S., P.Z. and F.M.; software, C.S., N.Z. and B.L.; formal analysis, C.S., N.Z., B.L. and G.M.; data curation, C.S., N.Z., B.L. and G.M.; writing—original draft preparation, C.S., N.Z., B.L. and G.M.; writing—review and editing, C.S., N.Z., B.L., M.B., G.M. and F.B.; supervision, F.B.; funding acquisition, F.B. and P.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the strategic 2010–2012 Research Program of Emilia Romagna Region (Italy), grant number 1786/2012, and by the Italian Ministry of University and Research, grant number 2010XE5L2R_002.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the S. Anna University-Hospital, Ferrara, Italy (protocol number 7-2010, date of approval 10 June 2010).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The “NIG—Network for Italian Genomes” database is freely available at the following link http://www.nig.cineca.it/, accessed on 10 November 2021.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.D’Haeseleer M., Cambron M., Vanopdenbosch L., De Keyser J. Vascular Aspects of Multiple Sclerosis. Lancet Neurol. 2011;10:657–666. doi: 10.1016/S1474-4422(11)70105-3. [DOI] [PubMed] [Google Scholar]
- 2.Spencer J.I., Bell J.S., DeLuca G.C. Vascular Pathology in Multiple Sclerosis: Reframing Pathogenesis around the Blood-Brain Barrier. J. Neurol. Neurosurg. Psychiatry. 2018;89:42–52. doi: 10.1136/jnnp-2017-316011. [DOI] [PubMed] [Google Scholar]
- 3.Balasa R., Barcutean L., Mosora O., Manu D. Reviewing the Significance of Blood-Brain Barrier Disruption in Multiple Sclerosis Pathology and Treatment. Int. J. Mol. Sci. 2021;22:8370. doi: 10.3390/ijms22168370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sati P.J., Oh R.T., Constable N., Evangelou C.R., Guttmann R.G., Henry E.C., Klawiter C., Mainero L., Massacesi H., McFarland F., et al. Reich, and Naims Cooperative. The Central Vein Sign and Its Clinical Evaluation for the Diagnosis of Multiple Sclerosis: A Consensus Statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat. Rev. Neurol. 2016;12:714–722. doi: 10.1038/nrneurol.2016.166. [DOI] [PubMed] [Google Scholar]
- 5.Suh C.H., Kim S.J., Jung S.C., Choi C.G., Kim H.S. The “Central Vein Sign” on T2*-Weighted Images as a Diagnostic Tool in Multiple Sclerosis: A Systematic Review and Meta-Analysis Using Individual Patient Data. Sci. Rep. 2019;9:18188. doi: 10.1038/s41598-019-54583-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zamboni P., Galeotti R., Menegatti E., Malagoni A.M., Tacconi G., Dall’Ara S., Bartolomei I., Salvi F. Chronic Cerebrospinal Venous Insufficiency in Patients with Multiple Sclerosis. J. Neurol. Neurosurg. Psychiatry. 2009;80:392–399. doi: 10.1136/jnnp.2008.157164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bateman G.A., Lechner-Scott J., Carey M.F., Bateman A.R., Lea R.A. Does the Venous Pressure Theory of Multiple Sclerosis Pathophysiology Deserve a Second Chance? Mult. Scler. Relat. Disord. 2021;56:103262. doi: 10.1016/j.msard.2021.103262. [DOI] [PubMed] [Google Scholar]
- 8.Sweeney D.M., Kisler K., Montagne A., Toga A.W., Zlokovic B.V. The Role of Brain Vasculature in Neurodegenerative Disorders. Nat. Neurosci. 2018;21:1318–1331. doi: 10.1038/s41593-018-0234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Presta I., Vismara M., Novellino F., Donato A., Zaffino P., Scali E., Pirrone K.C., Spadea M.F., Malara N., Donato G. Innate Immunity Cells and the Neurovascular Unit. Int. J. Mol. Sci. 2018;19:3856. doi: 10.3390/ijms19123856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lendahl U., Nilsson P., Betsholtz C. Emerging Links between Cerebrovascular and Neurodegenerative Diseases-a Special Role for Pericytes. EMBO Rep. 2019;20:e48070. doi: 10.15252/embr.201948070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marrie A.R., Rudick R., Horwitz R., Cutter G., Tyry T., Campagnolo D., Vollmer T. Vascular Comorbidity Is Associated with More Rapid Disability Progression in Multiple Sclerosis. Neurology. 2010;74:1041–1047. doi: 10.1212/WNL.0b013e3181d6b125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karmon Y., Ramanathan M., Minagar A., Zivadinov R., Weinstock-Guttman B. Arterial, Venous and Other Vascular Risk Factors in Multiple Sclerosis. Neurol. Res. 2012;34:754–760. doi: 10.1179/1743132812Y.0000000077. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan T.B., Berkowitz A.L., Samuels M.A. Cardiovascular Dysfunction in Multiple Sclerosis. Neurologist. 2015;20:108–114. doi: 10.1097/NRL.0000000000000064. [DOI] [PubMed] [Google Scholar]
- 14.Jakimovski D., Gandhi S., Paunkoski I., Bergsland N., Hagemeier J., Ramasamy D.P., Hojnacki D., Kolb C., Benedict R.H.B., Weinstock-Guttman B., et al. Hypertension and Heart Disease Are Associated with Development of Brain Atrophy in Multiple Sclerosis: A 5-Year Longitudinal Study. Eur. J. Neurol. 2019;26:87-e8. doi: 10.1111/ene.13769. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y., Bos S.D., Harbo H.F., Thompson W.K., Schork A.J., Bettella F., Witoelar A., Lie B.A., Li W., McEvoy L.K., et al. Genetic Overlap between Multiple Sclerosis and Several Cardiovascular Disease Risk Factors. Mult. Scler. 2016;22:1783–1793. doi: 10.1177/1352458516635873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tseveleki V., Rubio R., Vamvakas S.S., White J., Taoufik E., Petit E., Quackenbush J. Probert. Comparative Gene Expression Analysis in Mouse Models for Multiple Sclerosis, Alzheimer’s Disease and Stroke for Identifying Commonly Regulated and Disease-Specific Gene Changes. Genomics. 2010;96:82–91. doi: 10.1016/j.ygeno.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Downton P., Early J.O., Gibbs J.E. Circadian Rhythms in Adaptive Immunity. Immunology. 2020;161:268–277. doi: 10.1111/imm.13167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Druzd D., Matveeva O., Ince L., Harrison U., He W., Schmal C., Herzel H., Tsang A.H., Kawakami N., Leliavski A.O., et al. Lymphocyte Circadian Clocks Control Lymph Node Trafficking and Adaptive Immune Responses. Immunity. 2017;46:120–132. doi: 10.1016/j.immuni.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rijo-Ferreira F., Takahashi J.S. Genomics of Circadian Rhythms in Health and Disease. Genome Med. 2019;11:82. doi: 10.1186/s13073-019-0704-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leng Y., Musiek E.S., Hu K., Cappuccio F.P., Yaffe K. Association between Circadian Rhythms and Neurodegenerative Diseases. Lancet Neurol. 2019;18:307–318. doi: 10.1016/S1474-4422(18)30461-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coogan N.A., Baird A.L., Popa-Wagner A., Thome J. Circadian Rhythms and Attention Deficit Hyperactivity Disorder: The What, the When and the Why. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2016;67:74–81. doi: 10.1016/j.pnpbp.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Li D.M., Xin H., Yuan Y., Yang X., Li H., Tian D., Zhang H., Zhang Z., Han T.L., Chen Q., et al. Circadian Clock-Controlled Checkpoints in the Pathogenesis of Complex Disease. Front. Genet. 2021;12:721231. doi: 10.3389/fgene.2021.721231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L.S., Lahens N.F., Yue Z., Arnold D.M., Pakstis P.P., Schwarz J.E., Sehgal A. A Circadian Clock Regulates Efflux by the Blood-Brain Barrier in Mice and Human Cells. Nat. Commun. 2021;12:617. doi: 10.1038/s41467-020-20795-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sutton C.E., Finlay C.M., Raverdeau M., Early J.O., DeCourcey J., Zaslona Z., O’Neill L.A.J., Mills K.H.G., Curtis A.M. Loss of the Molecular Clock in Myeloid Cells Exacerbates T Cell-Mediated Cns Autoimmune Disease. Nat. Commun. 2017;8:1923. doi: 10.1038/s41467-017-02111-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crnko S., Cour M., van Laake L.W., Lecour S. Vasculature on the Clock: Circadian Rhythm and Vascular Dysfunction. Vascul. Pharmacol. 2018;108:1–7. doi: 10.1016/j.vph.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Wang L.X., Li L. Circadian Clock Regulates Inflammation and the Development of Neurodegeneration. Front. Cell. Infect. Microbiol. 2021;11:696554. doi: 10.3389/fcimb.2021.696554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shepard J.C., Cline S.G., Hinds D., Jahanbakhsh S., Prokop J.W. Breakdown of Multiple Sclerosis Genetics to Identify an Integrated Disease Network and Potential Variant Mechanisms. Physiol. Genom. 2019;51:562–577. doi: 10.1152/physiolgenomics.00120.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marchetti G., Ziliotto N., Meneghetti S., Baroni M., Lunghi B., Menegatti E., Pedriali M., Salvi F., Bartolomei I., Straudi S., et al. Changes in Expression Profiles of Internal Jugular Vein Wall and Plasma Protein Levels in Multiple Sclerosis. Mol. Med. 2018;24:42. doi: 10.1186/s10020-018-0043-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ziliotto N., Marchetti G., Scapoli C., Bovolenta M., Meneghetti S., Benazzo A., Lunghi B., Balestra D., Laino L.A., Bozzini N., et al. C6orf10 Low-Frequency and Rare Variants in Italian Multiple Sclerosis Patients. Front. Genet. 2019;10:573. doi: 10.3389/fgene.2019.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ziliotto N., Meneghetti S., Menegatti E., Baroni M., Lunghi B., Salvi F., Ferracin M., Branchini A., Gemmati D., Mascoli F., et al. Expression Profiles of the Internal Jugular and Saphenous Veins: Focus on Hemostasis Genes. Thromb. Res. 2020;191:113–124. doi: 10.1016/j.thromres.2020.04.039. [DOI] [PubMed] [Google Scholar]
- 31.Richardson G.T., Sanderson E., Palmer T.M., Ala-Korpela M., Ference B.A., Smith G.D., Holmes M.V. Evaluating the Relationship between Circulating Lipoprotein Lipids and Apolipoproteins with Risk of Coronary Heart Disease: A Multivariable Mendelian Randomisation Analysis. PLoS Med. 2020;17:e1003062. doi: 10.1371/journal.pmed.1003062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iourov Y.I., Zelenova M.A., Vorsanova S.G., Voinova V.V., Yurov Y.B. 4q21.2q21.3 Duplication: Molecular and Neuropsychological Aspects. Curr. Genom. 2018;19:173–178. doi: 10.2174/1389202918666170717161426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams R.S., Yang Q., Chen F., Liu X., Keene K.L., Jacques P., Chen W.M., Weinstein G., Hsu F.C., Beiser A., et al. Genome-Wide Meta-Analysis of Homocysteine and Methionine Metabolism Identifies Five One Carbon Metabolism Loci and a Novel Association of Aldh1l1 with Ischemic Stroke. PLoS Genet. 2014;10:e1004214. doi: 10.1371/journal.pgen.1004214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Au S.K., Findley T.O., Northrup H. Finding the Genetic Mechanisms of Folate Deficiency and Neural Tube Defects-Leaving No Stone Unturned. Am. J. Med. Genet. A. 2017;173:3042–3057. doi: 10.1002/ajmg.a.38478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klarin D., Damrauer S.M., Cho K., Sun Y.V., Teslovich T.M., Honerlaw J., Gagnon D.R., DuVall S.L., Li J., Peloso G.M., et al. Genetics of Blood Lipids among ~300,000 Multi-Ethnic Participants of the Million Veteran Program. Nat. Genet. 2018;50:1514–1523. doi: 10.1038/s41588-018-0222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akkad A.D., Bellenberg B., Esser S., Weiler F., Epplen J.T., Gold R., Lukas C., Haghikia A. Multiple Sclerosis Risk Loci Correlate with Cervical Cord Atrophy and May Explain the Course of Disability. Neurogenetics. 2015;16:161–168. doi: 10.1007/s10048-015-0438-0. [DOI] [PubMed] [Google Scholar]
- 37.Saddouk Z.F., Sun L.Y., Liu Y.F., Jiang M., Singer D.V., Backs J., van Riper D., Ginnan R., Schwarz J.J., Singer H.A. Ca2+/Calmodulin-Dependent Protein Kinase Ii-Gamma (Camkiigamma) Negatively Regulates Vascular Smooth Muscle Cell Proliferation and Vascular Remodeling. FASEB J. 2016;30:1051–1064. doi: 10.1096/fj.15-279158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Proietti Onori M., Koopal B., Everman D.B., Worthington J.D., Jones J.R., Ploeg M.A., Mientjes E., van Bon B.W., Kleefstra T., Schulman H., et al. The Intellectual Disability-Associated Camk2g P.Arg292pro Mutation Acts as a Pathogenic Gain-of-Function. Hum. Mutat. 2018;39:2008–2024. doi: 10.1002/humu.23647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hosiawa A.K., Wang H., DeVries M.E., Garcia B., Liu W., Zhou D., Akram A., Jiang J., Sun H., Cameron M.J., et al. Cd80/Cd86 Costimulation Regulates Acute Vascular Rejection. J. Immunol. 2005;175:6197–6204. doi: 10.4049/jimmunol.175.9.6197. [DOI] [PubMed] [Google Scholar]
- 40.Li Z., Liu Y., Jia A., Cui Y., Feng J. Cerebrospinal Fluid Cells Immune Landscape in Multiple Sclerosis. J. Transl. Med. 2021;19:125. doi: 10.1186/s12967-021-02804-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brynedal B., Khademi M., Wallstrom E., Hillert J., Olsson T., Duvefelt K. Gene Expression Profiling in Multiple Sclerosis: A Disease of the Central Nervous System, but with Relapses Triggered in the Periphery? Neurobiol. Dis. 2010;37:613–621. doi: 10.1016/j.nbd.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 42.Yang M., Shi X.Q., Peyret C., Oladiran O., Wu S., Chambon J., Fournier S., Zhang J. Effector/Memory Cd8(+) T Cells Synergize with Co-Stimulation Competent Macrophages to Trigger Autoimmune Peripheral Neuropathy. Brain Behav. Immun. 2018;71:142–157. doi: 10.1016/j.bbi.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 43.Stanne M.T., Olsson M., Lorentzen E., Pedersen A., Gummesson A., Gils A., Jood K., Engstrom G., Melander O., Declerck P.J., et al. A Genome-Wide Study of Common and Rare Genetic Variants Associated with Circulating Thrombin Activatable Fibrinolysis Inhibitor. Thromb. Haemost. 2018;118:298–308. doi: 10.1160/TH17-04-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jaudon F., Raynaud F., Wehrle R., Bellanger J.M., Doulazmi M., Vodjdani G., Gasman S., Fagni L., Dusart I., Debant A., et al. The Rhogef Dock10 Is Essential for Dendritic Spine Morphogenesis. Mol. Biol. Cell. 2015;26:2112–2127. doi: 10.1091/mbc.E14-08-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Namekata K., Guo X., Kimura A., Azuchi Y., Kitamura Y., Harada C., Harada T. Roles of the Dock-D Family Proteins in a Mouse Model of Neuroinflammation. J. Biol. Chem. 2020;295:6710–6720. doi: 10.1074/jbc.RA119.010438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kathiresan S.A.K., Manning S., Demissie R.B., D’Agostino A., Surti C., Guiducci L., Gianniny N.P., Burtt O., Melander M., Orho-Melander D.K., et al. A Genome-Wide Association Study for Blood Lipid Phenotypes in the Framingham Heart Study. BMC Med. Genet. 2007;8:S17. doi: 10.1186/1471-2350-8-S1-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li S.X., Wang Z., Cajka T., Buffa J.A., Nemet I., Hurd A.G., Gu X., Skye S.M., Roberts A.B., Wu Y., et al. Untargeted Metabolomics Identifies Trimethyllysine, a Tmao-Producing Nutrient Precursor, as a Predictor of Incident Cardiovascular Disease Risk. JCI Insight. 2018;3:e99096. doi: 10.1172/jci.insight.99096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin P., McGovern A., Massey J., Schoenfelder S., Duffus K., Yarwood A., Barton A., Worthington J., Fraser P., Eyre S., et al. Identifying Causal Genes at the Multiple Sclerosis Associated Region 6q23 Using Capture Hi-C. PLoS ONE. 2016;11:e0166923. doi: 10.1371/journal.pone.0166923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dayton J.R., Yuan Y., Pacumio L.P., Dorflinger B.G., Yoo S.C., Olson M.J., Hernandez-Suarez S.I., McMahon M.M., Cruz-Orengo L. Expression of Il-20 Receptor Subunit Beta Is Linked to Eae Neuropathology and Cns Neuroinflammation. Front. Cell. Neurosci. 2021;15:683687. doi: 10.3389/fncel.2021.683687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Varga T.V., Kurbasic A., Aine M., Eriksson P., Ali A., Hindy G., Gustafsson S., Luan J., Shungin D., Chen Y., et al. Novel Genetic Loci Associated with Long-Term Deterioration in Blood Lipid Concentrations and Coronary Artery Disease in European Adults. Int. J. Epidemiol. 2017;46:1211–1222. doi: 10.1093/ije/dyw245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsai T.C., Hsieh C.S., Chang S.N., Chuang E.Y., Ueng K.C., Tsai C.F., Lin T.H., Wu C.K., Lee J.K., Lin L.Y., et al. Genome-Wide Screening Identifies a Kcnip1 Copy Number Variant as a Genetic Predictor for Atrial Fibrillation. Nat. Commun. 2016;7:10190. doi: 10.1038/ncomms10190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fransen L.N., Crusius J.B.A., Smolders J., Mizee M.R., van Eden C.G., Luchetti S., Remmerswaal E.B.M., Hamann J., Mason M.R.J., Huitinga I. Post-Mortem Multiple Sclerosis Lesion Pathology Is Influenced by Single Nucleotide Polymorphisms. Brain Pathol. 2020;30:106–119. doi: 10.1111/bpa.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chattopadhyay D., Sengupta S. First Evidence of Pathogenicity of V234i Mutation of Hvapb Found in Amyotrophic Lateral Sclerosis. Biochem. Biophys. Res. Commun. 2014;448:108–113. doi: 10.1016/j.bbrc.2014.04.102. [DOI] [PubMed] [Google Scholar]
- 54.Hupe M., Li M.X., Kneitz S., Davydova D., Yokota C., Kele J., Hot B., Stenman J.M., Gessler M. Gene Expression Profiles of Brain Endothelial Cells During Embryonic Development at Bulk and Single-Cell Levels. Sci. Signal. 2017;10:487. doi: 10.1126/scisignal.aag2476. [DOI] [PubMed] [Google Scholar]
- 55.Buga M.A., Margaritescu C., Scholz C.J., Radu E., Zelenak C., Popa-Wagner A. Transcriptomics of Post-Stroke Angiogenesis in the Aged Brain. Front. Aging Neurosci. 2014;6:44. doi: 10.3389/fnagi.2014.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sferruzza G., Clarelli F., Mascia E., Ferre L., Ottoboni L., Sorosina M., Santoro S., Moiola L., Martinelli V., Comi G., et al. Transcriptomic Analysis of Peripheral Monocytes Upon Fingolimod Treatment in Relapsing Remitting Multiple Sclerosis Patients. Mol. Neurobiol. 2021;58:4816–4827. doi: 10.1007/s12035-021-02465-z. [DOI] [PubMed] [Google Scholar]
- 57.Wisniewska M.B. Physiological Role of Beta-Catenin/Tcf Signaling in Neurons of the Adult Brain. Neurochem. Res. 2013;38:1144–1155. doi: 10.1007/s11064-013-0980-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Coste H., Rodriguez J.C. Orphan Nuclear Hormone Receptor Rev-Erbalpha Regulates the Human Apolipoprotein Ciii Promoter. J. Biol. Chem. 2002;277:27120–27129. doi: 10.1074/jbc.M203421200. [DOI] [PubMed] [Google Scholar]
- 59.Wang J., Yin L., Lazar M.A. The Orphan Nuclear Receptor Rev-Erb Alpha Regulates Circadian Expression of Plasminogen Activator Inhibitor Type 1. J. Biol. Chem. 2006;281:33842–33848. doi: 10.1074/jbc.M607873200. [DOI] [PubMed] [Google Scholar]
- 60.Goto M., Mizuno M., Matsumoto A., Yang Z., Jimbo E.F., Tabata H., Yamagata T., Nagata K.I. Role of a Circadian-Relevant Gene Nr1d1 in Brain Development: Possible Involvement in the Pathophysiology of Autism Spectrum Disorders. Sci. Rep. 2017;7:43945. doi: 10.1038/srep43945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Myocardial Infarction Genetics, Consortium. Kathiresan S., Voight B.F., Purcell S., Musunuru K., Ardissino D., Mannucci P.M., Anand S., Engert J.C., Samani N.J., et al. Genome-Wide Association of Early-Onset Myocardial Infarction with Single Nucleotide Polymorphisms and Copy Number Variants. Nat. Genet. 2009;41:334–341. doi: 10.1038/ng.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deng L., Li G., Rao B., Li H. Central Nervous System-Specific Knockout of Brg1 Causes Growth Retardation and Neuronal Degeneration. Brain Res. 2015;1622:186–195. doi: 10.1016/j.brainres.2015.06.027. [DOI] [PubMed] [Google Scholar]
- 63.Tibshirani M., Zhao B., Gentil B.J., Minotti S., Marques C., Keith J., Rogaeva E., Zinman L., Rouaux C., Robertson J., et al. Dysregulation of Chromatin Remodelling Complexes in Amyotrophic Lateral Sclerosis. Hum. Mol. Genet. 2017;26:4142–4152. doi: 10.1093/hmg/ddx301. [DOI] [PubMed] [Google Scholar]
- 64.Rao S., Leung C.S., Lam M.H., Wing Y.K., Waye M.M., Tsui S.K. Resequencing Three Candidate Genes Discovers Seven Potentially Deleterious Variants Susceptibility to Major Depressive Disorder and Suicide Attempts in Chinese. Gene. 2017;603:34–41. doi: 10.1016/j.gene.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 65.Vincent B.J., Choufani S., Horike S., Stachowiak B., Li M., Dill F.J., Marshall C., Hrynchak M., Pewsey E., Ukadike K.C., et al. A Translocation T(6;7)(P11-P12;Q22) Associated with Autism and Mental Retardation: Localization and Identification of Candidate Genes at the Breakpoints. Psychiatr. Genet. 2008;18:101–109. doi: 10.1097/YPG.0b013e3282f97df7. [DOI] [PubMed] [Google Scholar]
- 66.Li S.Q., Parrado A.R., Samtani M.N., Narayan V.A., Alzheimer’s Disease Neuroimaging Initiative Variations in the Fra10ac1 Fragile Site and 15q21 Are Associated with Cerebrospinal Fluid Abeta1-42 Level. PLoS ONE. 2015;10:e0134000. doi: 10.1371/journal.pone.0134000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zivadinov R., Chung C.P. Potential Involvement of the Extracranial Venous System in Central Nervous System Disorders and Aging. BMC Med. 2013;11:260. doi: 10.1186/1741-7015-11-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ramagopalan S.V., Dyment D.A., Cader M.Z., Morrison K.M., Disanto G., Morahan J.M., Berlanga-Taylor A.J., Handel A., De Luca G.C., Sadovnick A.D., et al. Rare Variants in the Cyp27b1 Gene Are Associated with Multiple Sclerosis. Ann. Neurol. 2011;70:881–886. doi: 10.1002/ana.22678. [DOI] [PubMed] [Google Scholar]
- 69.Vallee A., Vallee J.N., Guillevin R., Lecarpentier Y. Interactions between the Canonical Wnt/Beta-Catenin Pathway and Ppar Gamma on Neuroinflammation, Demyelination, and Remyelination in Multiple Sclerosis. Cell. Mol. Neurobiol. 2018;38:783–795. doi: 10.1007/s10571-017-0550-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Griffin C.T., Curtis C.D., Davis R.B., Muthukumar V., Magnuson T. The Chromatin-Remodeling Enzyme Brg1 Modulates Vascular Wnt Signaling at Two Levels. Proc. Natl. Acad. Sci. USA. 2011;108:2282–2287. doi: 10.1073/pnas.1013751108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Waterworth M.D., Ricketts S.L., Song K., Chen L., Zhao J.H., Ripatti S., Aulchenko Y.S., Zhang W., Yuan X., Lim N., et al. Genetic Variants Influencing Circulating Lipid Levels and Risk of Coronary Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2010;30:2264–2276. doi: 10.1161/ATVBAHA.109.201020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Andreassen A.O., Desikan R.S., Wang Y., Thompson W.K., Schork A.J., Zuber V., Doncheva N.T., Ellinghaus E., Albrecht M., Mattingsdal M., et al. Abundant Genetic Overlap between Blood Lipids and Immune-Mediated Diseases Indicates Shared Molecular Genetic Mechanisms. PLoS ONE. 2015;10:e0123057. doi: 10.1371/journal.pone.0123057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tian Z., Song Y., Yao Y., Guo J., Gong Z., Wang Z. Genetic Etiology Shared by Multiple Sclerosis and Ischemic Stroke. Front. Genet. 2020;11:646. doi: 10.3389/fgene.2020.00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gerdes N., Zirlik A. Co-Stimulatory Molecules in and Beyond Co-Stimulation—Tipping the Balance in Atherosclerosis? Thromb. Haemost. 2011;106:804–813. doi: 10.1160/TH11-09-0605. [DOI] [PubMed] [Google Scholar]
- 75.Otto A.E., Loeys B., Khanna H., Hellemans J., Sudbrak R., Fan S., Muerb U., O’Toole J.F., Helou J., Attanasio M., et al. Nephrocystin-5, a Ciliary Iq Domain Protein, Is Mutated in Senior-Loken Syndrome and Interacts with Rpgr and Calmodulin. Nat. Genet. 2005;37:282–288. doi: 10.1038/ng1520. [DOI] [PubMed] [Google Scholar]
- 76.Choi S., Guo L., Cordeiro M.F. Retinal and Brain Microglia in Multiple Sclerosis and Neurodegeneration. Cells. 2021;10:1507. doi: 10.3390/cells10061507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gnanakkumaar P., Murugesan R., Ahmed S. Gene Regulatory Networks in Peripheral Mononuclear Cells Reveals Critical Regulatory Modules and Regulators of Multiple Sclerosis. Sci. Rep. 2019;9:12732. doi: 10.1038/s41598-019-49124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu C., Li S., Liu T., Borjigin J., Lin J.D. Transcriptional Coactivator Pgc-1alpha Integrates the Mammalian Clock and Energy Metabolism. Nature. 2007;447:477–481. doi: 10.1038/nature05767. [DOI] [PubMed] [Google Scholar]
- 79.Kolic I., Stojkovic L., Stankovic A., Stefanovic M., Dincic E., Zivkovic M. Association Study of Rs7799039, Rs1137101 and Rs8192678 Gene Variants with Disease Susceptibility/Severity and Corresponding Lep, Lepr and Pgc1a Gene Expression in Multiple Sclerosis. Gene. 2021;774:145422. doi: 10.1016/j.gene.2021.145422. [DOI] [PubMed] [Google Scholar]
- 80.Kolic I., Stojkovic L., Dincic E., Jovanovic I., Stankovic A., Zivkovic M. Expression of Lep, Lepr and Pgc1a Genes Is Altered in Peripheral Blood Mononuclear Cells of Patients with Relapsing-Remitting Multiple Sclerosis. J. Neuroimmunol. 2020;338:577090. doi: 10.1016/j.jneuroim.2019.577090. [DOI] [PubMed] [Google Scholar]
- 81.Witte E.M., Nijland P.G., Drexhage J.A., Gerritsen W., Geerts D., Hof B.v., Reijerkerk A., de Vries H.E., van der Valk P., van Horssen J. Reduced Expression of Pgc-1alpha Partly Underlies Mitochondrial Changes and Correlates with Neuronal Loss in Multiple Sclerosis Cortex. Acta Neuropathol. 2013;125:231–243. doi: 10.1007/s00401-012-1052-y. [DOI] [PubMed] [Google Scholar]
- 82.Dijk D.J., Archer S.N. Period3, Circadian Phenotypes, and Sleep Homeostasis. Sleep Med. Rev. 2010;14:151–160. doi: 10.1016/j.smrv.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 83.Mazzoccoli G., Palmieri O., Corritore G., Latiano T., Bossa F., Scimeca D., Biscaglia G., Valvano M.R., D’Inca R., Cucchiara S., et al. Association Study of a Polymorphism in Clock Gene Period3 and Risk of Inflammatory Bowel Disease. Chronobiol. Int. 2012;29:994–1003. doi: 10.3109/07420528.2012.705935. [DOI] [PubMed] [Google Scholar]
- 84.Zhu J.J., Jiang Z.T., Liu C., Xi Y.F., Wang J., Yang F.F., Yao W.J., Pang W., Han L.L., Zhang Y.H., et al. Vamp3 and Snap23 as Potential Targets for Preventing the Disturbed Flow-Accelerated Thrombus Formation. Front. Cell. Dev. Biol. 2020;8:576826. doi: 10.3389/fcell.2020.576826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Golalipour M., Maleki Z., Farazmandfar T., Shahbazi M. Per3 Vntr Polymorphism in Multiple Sclerosis: A New Insight to Impact of Sleep Disturbances in Ms. Mult. Scler. Relat. Disord. 2017;17:84–86. doi: 10.1016/j.msard.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 86.Ziliotto N., Lamberti N., Manfredini F., Straudi S., Tisato V., Carantoni M., Melloni E., Secchiero P., Basaglia N., Bernardi F., et al. Baseline and Overtime Variations of Soluble Adhesion Molecule Plasma Concentrations Are Associated with Mobility Recovery after Rehabilitation in Multiple Sclerosis Patients. J. Neuroimmunol. 2021;352:577473. doi: 10.1016/j.jneuroim.2020.577473. [DOI] [PubMed] [Google Scholar]
- 87.Ziliotto N., Lamberti N., Manfredini F., Straudi S., Baroni M., Tisato V., Carantoni M., Secchiero P., Basaglia N., Marchetti G., et al. Functional Recovery in Multiple Sclerosis Patients Undergoing Rehabilitation Programs Is Associated with Plasma Levels of Hemostasis Inhibitors. Mult. Scler. Relat. Disord. 2020;44:102319. doi: 10.1016/j.msard.2020.102319. [DOI] [PubMed] [Google Scholar]
- 88.Buniello A., MacArthur J.A.L., Cerezo M., Harris L.W., Hayhurst J., Malangone C., McMahon A., Morales J., Mountjoy E., Sollis E., et al. The Nhgri-Ebi Gwas Catalog of Published Genome-Wide Association Studies, Targeted Arrays and Summary Statistics 2019. Nucleic Acids Res. 2019;47:D1005–D1012. doi: 10.1093/nar/gky1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Battle A., Brown C.D., Engelhardt B.E., Montgomery S.B. Genetic Effects on Gene Expression across Human Tissues. Nature. 2017;550:204–213. doi: 10.1038/nature24277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Szklarczyk D., Gable A.L., Lyon D., Junge A., Wyder S., Huerta-Cepas J., Simonovic M., Doncheva N.T., Morris J.H., Bork P. String V11: Protein-Protein Association Networks with Increased Coverage, Supporting Functional Discovery in Genome-Wide Experimental Datasets. Nucleic Acids Res. 2019;47:D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang D.W., Sherman B.T., Lempicki R.A. Systematic and Integrative Analysis of Large Gene Lists Using David Bioinformatics Resources. Nat. Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 92.Kanehisa M., Sato Y., Kawashima M. Kegg Mapping Tools for Uncovering Hidden Features in Biological Data. Protein Sci. 2021 doi: 10.1002/pro.4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Genomes Project Consortium. Auton A., Brooks L.D., Durbin R.M., Garrison E.P., Kang H.M., Korbel J.O., Marchini J.L., McCarthy S., McVean G.A., et al. A Global Reference for Human Genetic Variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Karczewski K.J., Francioli L.C., Tiao G., Cummings B.B., Alfoldi J., Wang Q., Collins R.L., Laricchia K.M., Ganna A., Birnbaum D.P. The Mutational Constraint Spectrum Quantified from Variation in 141,456 Humans. Nature. 2020;581:434–443. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fiorito G., di Gaetano C., Guarrera S., Rosa F., Feldman M.W., Piazza A., Matullo G. The Italian Genome Reflects the History of Europe and the Mediterranean Basin. Eur. J. Hum. Genet. 2016;24:1056–1062. doi: 10.1038/ejhg.2015.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Raveane A., Aneli S., Montinaro F., Athanasiadis G., Barlera S., Birolo G., Boncoraglio G., di Blasio A.M., di Gaetano C., Pagani L.S., et al. Population Structure of Modern-Day Italians Reveals Patterns of Ancient and Archaic Ancestries in Southern Europe. Sci. Adv. 2019;5:eaaw3492. doi: 10.1126/sciadv.aaw3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Birolo G., Aneli S., di Gaetano C., Cugliari G., Russo A., Allione A., Casalone E., Giorgio E., Paraboschi E.M., Ardissino D.S. Functional and Clinical Implications of Genetic Structure in 1686 Italian Exomes. Hum. Mutat. 2021;42:272–289. doi: 10.1002/humu.24156. [DOI] [PubMed] [Google Scholar]
- 98.Bezzini D., Policardo L., Meucci G., Ulivelli M., Bartalini S., Profili F., Battaglia M.A., Francesconi P. Prevalence of Multiple Sclerosis in Tuscany (Central Italy): A Study Based on Validated Administrative Data. Neuroepidemiology. 2016;46:37–42. doi: 10.1159/000441567. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The “NIG—Network for Italian Genomes” database is freely available at the following link http://www.nig.cineca.it/, accessed on 10 November 2021.