Abstract
Most trials on mRNA vaccines against SARS-CoV-2 did not include patients with chronic kidney disease (CKD), hemodialysis (HD) patients, or kidney transplant recipients (KTR). However, those patients have a higher risk for a severe course of COVID-19 disease and mortality. Available literature has demonstrated a reduced efficacy of mRNA vaccines in HD patients and KTR, while data on CKD patients is scarce. Additionally, factors associated with non-response are poorly understood and not well characterized. We assessed antibody (AB) response (n = 582, 160 CKD patients, 206 patients on HD, 216 KTR) after the administration of two doses of a mRNA-vaccine with either BNT162b2 or mRNA-1273. AB measurements were carried out after a median of 91 days after first vaccinations, demonstrating non-response in 12.5% of CKD patients, 12.1% of HD patients, and 50% of KTR. AB titers were significantly higher in CKD patients than in HD patients or KTR. Factors associated with non-response were treated with rituximab in CKD patients, the use of calcineurin inhibitors in HD patients and older age, and the use of BNT162b2, mycophenolic acid, or glucocorticoids and lower hemoglobin levels in KTR. This study contributes to the understanding of the extent and conditions that predispose for non-response in patients with impaired kidney function.
Keywords: SARS-CoV-2, COVID-19, mRNA vaccines, kidney transplantation, hemodialysis, chronic kidney disease
1. Introduction
To combat the ongoing COVID-19 pandemic, significant effort has been undertaken to develop highly effective and safe vaccines against SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2). Two of the most promising vaccines are BNT162b2 by Pfizer and mRNA-1273 by Moderna. However, the respective authorization trials as well as most follow-up trials on these mRNA vaccines did not include patients with chronic kidney disease (CKD), on hemodialysis (HD), or after kidney transplantation (KT). Moreover, immunosuppressive treatment was an exclusion criterion in the original registration studies [1,2]. Several publications have already indicated a reduced humoral immune response in HD [3,4,5] and kidney transplant recipients (KTR) [6,7,8], while data in CKD patients with or without immunosuppressive treatment is scarce. Factors/conditions discussed to predispose non-response and a severe COVID-19 course in these patients are advanced age, comorbidities, the uremic environment in HD, and immunosuppressive medication in KTR. Factors that are associated with non-response in the existing literature include age [9,10], diabetes [11], lower hemoglobin [9], lower eGFR (per mL/min/1.73 m2) [12], longer HD vintage [13], BNT162b2 instead of mRNA-1273 [14,15,16,17], treatment with antimetabolites [18] or belatacept [19,20], treatment with rituximab [21] or high dose cortisone in the last year [9], and triple immunosuppression (calcineurin inhibitor plus antimetabolite plus steroid) [9]. The extent and impact of the abovementioned factors are poorly understood, and possible mitigation strategies are lacking.
Since mortality in SARS-CoV-2-infected kidney transplant recipients [22], hemodialysis patients [23], and CKD patients [24] is high, further understanding of the efficacy of the SARS-CoV-2 vaccination and risk factors for non-response in this highly vulnerable population is urgently needed. Safe and effective vaccination strategies remain especially important due to the fact that, as of date, effective treatments for severe COVID-19 disease are rarely available. Additionally, most of these patients have difficulties to socially distance due to either needing regular hemodialysis, medical appointments, or care through relatives or nursing personnel.
2. Materials and Methods
Between February and April 2021, CKD patients, HD patients, and KT recipients received two doses of mRNA-vaccination based on the recommendations of the Austrian National Advisory Committee on immunization practices. Patients were treated in Tyrolean dialysis centers or by the Department of Internal Medicine IV at the University Hospital Innsbuck. Data were analyzed retrospectively; inclusion in this study did not play any role in immunization practices. Routine laboratory parameters and clinical data were assessed in May and June 2021 through electronic patient records.
Antibody (AB) titers were measured by Abbott SARS-CoV-2 IgG II Quant Assay in 40% of each group and by Liaison® SARS-CoV-2 S1/S2 IgG in 60% of each group based on availability in our laboratory between January and August 2021. Limits for non-response were <7 BAU/mL and <13 AU/mL, respectively, as defined by the manufacturer. Measurements with the Liaison assay were carried out and analyzed before the conversion of the assay to BAU/mL, hence the unit AU/mL is used (the now defined cut-off of 33.8 BAU/mL is equivalent to 13 AU/mL, conversion factor 2.6).
Inclusion criteria comprised either CKD (predominantly CKD stage IV and V), HD or living with a functioning kidney graft as underlying condition, completed prime-booster vaccination with a mRNA vaccine approximately 4 weeks apart, and an available measurement of AB titers with at least one of the above-mentioned assays 60 to 120 days after first vaccine dose. Exclusion criteria included age under 18 years at the time of first vaccination and a history of PCR-proven SARS-CoV-2 infection or the detection of nucleocapsid AB.
BNT162b2 was used in 58% of CKD patients, in 96% of HD patients, and in 49% of KTR; the remainder received mRNA-1273. Both doses were administered approximately 4 weeks apart (median 28 days, 25% and 75% percentiles were 21 and 29 days). AB measurement was carried out after a median of 91 days after the first vaccination.
Statistical analysis was performed with IBM SPSS Statistics. Non-parametric tests were used for the comparison of continuous data, and logistic regression served for assessment of risk factors for non-response. All factors showing a univariate association with a p-value < 0.100 were entered in the final multivariate model.
This analysis was approved by the Institutional Review Board of the Medical University Innsbruck (ECS 1280/2021).
3. Results
The initial cohort consisted of 871 patients with either CKD, treatment with HD or after kidney transplantation. Sixty-two patients were excluded due to a history of SARS-CoV-2 infection before the first or the second dose, and 17 patients were excluded due to nucleocapsid AB detection before the first or second dose without a clinically apparent history of infection. Nineteen patients had no second dose of vaccination. From the remaining 773 patients, in 191 patients, antibodies were not taken between 60 to 120 days after the first vaccination. Hence, in the final cohort, 582 patients were included: 160 patients with CKD, 206 patients on HD, and 216 patients after kidney transplantation. Table 1 displays baseline characteristics of the final cohort.
Table 1.
Data is displayed as median, 25%, and 75% percentiles. Numeric data is displayed as number of participants (n) and percentage (%) where appropriate (sums do not add up to 100% due to rounding).
| CKD (n = 160) | HD (n = 206) | KTR (n = 216) | |
|---|---|---|---|
| Age (years) | 63.1 (53.5–74.5) | 69.5 (57.5–78.6) | 59.9 (50.7–68.5) |
| Female (n, %) | 66 (41) | 66 (32) | 69 (32) |
| BMI (kg/m2) | 25.6 (23.3–29.3) | 24.9 (22.2–28.4) | 24.8 (21.7–27.8) |
| Primary renal disease (n, %) | |||
| Diabetic nephropathy | 14 (9) | 45 (22) | 28 (13) |
| Glomerulonephritis | 64 (40) | 35 (17) | 71 (33) |
| Other | 49 (31) | 55 (27) | 77 (36) |
| Unknown | 3 (2) | 23 (11) | 11 (5) |
| Vascular nephropathy | 30 (19) | 48 (23) | 29 (13) |
| Comorbidities (n) | |||
| Cardiovascular disease | 45 | 98 | 92 |
| Cerebrovascular disease | 16 | 33 | 34 |
| Active or former malignancy | 13 | 35 | 53 |
| Diabetes mellitus | 36 | 77 | 79 |
| Comedication (n, %) | |||
| Treatment with RAAS inhibitors | 83 (52) | 62 (30) | 93 (43) |
| High-dose glucocorticoid treatment during last year (≥1 mg/kg) | 6 (4) | 4 (2) | 16 (7) |
| Tacrolimus | - | 7 (3) | 152 (70) |
| Cyclosporine A | - | 1 (0.5) | 38 (18) |
| Azathioprine | - | 0 | 33 (15) |
| Mycophenolic acid | - | 2 (1) | 148 (69) |
| Belatacept | - | 0 | 14 (6) |
| Glucocorticoids | - | 8 (4) | 178 (82) |
| mTor inhibitors | - | 0 | 11 (5) |
| Rituximab | 30 (19) | - | - |
| Time between 1st vaccination and rituximab (days) | 242 (127–324) | 2 (1–4) | 2 (1–3) |
| Classes of antihypertensive drugs (n) | 2 (1–3) | ||
| Laboratory values | |||
| Albumin (g/dL) | 4.0 (3.7–4.3) | 3.8 (3.4–4.1) | |
| Hemoglobin (g/dL) | 132 (117–141) | 111 (105–120) | 4.1 (3.9–4.4) |
| C-reactive protein (mg/dL) | 0.20 (0.09–0.46) | 0.33 (0.16–0.95) | 134 (122–146) |
| eGFR (mL/min/1.73 m2) | 29.9 (19.8–49.9) | - | 0.19 (0.08–0.40) |
The median age was 63.1, 69.5, and 59.9 years, respectively, in CKD patients, HD patients, and KTR. The median eGFR was 29.9 mL/min/1.73 m2 in CKD patients and 49.2 mL/min/1.73 m2 in KTR. Details on immunosuppressive treatment and comorbidities are also given in Table 1. The median time between the first vaccination and AB measurement was 91 days in all groups. Table 2 shows the type of vaccine used and the types and results of antibody measurement. Rates of non-response were 12.5%, 12.1%, and 50.0% in CKD patients, HD patients, and KTR.
Table 2.
Type of vaccine and antibody measurement in CKD and HD patients, and KTR. Continuous data is displayed as median, 25%, and 75% percentiles. Numeric data is displayed as number of participants (n) and percentage (%) where appropriate (sums do not add up to 100% due to rounding).
| CKD (n = 160) | HD (n = 206) | KTR (n = 216) | |
|---|---|---|---|
| Type of antibody measurement (n, %) | |||
| Abbott SARS-CoV-2 IgG II Quant Assay | 65 (40.6) | 84 (40.8) | 88 (40.7) |
| Liaison® SARS-CoV-2 S1/S2 IgG | 95 (59.4) | 122 (59.2) | 128 (59.3) |
| Vaccine (n, %) | |||
| BNT162b2 | 93 (58.1) | 198 (96.1) | 106 (49.1) |
| mRNA-1273 | 67 (41.9) | 8 (3.9) | 110 (50.9) |
| Results of antibody measurement | |||
| Abbott SARS-CoV-2 IgG II Quant Assay titer (BAU/mL) |
230.3 (48.3–497.6) | 151.6 (47.7–458.4) | 4.75 (3.0–30.2) |
| Liaison® SARS-CoV-2 S1/S2 IgG titer (AU/mL) | 602.0 (252.5–800.0) | 121.5 (32.0–293.0) | 10.3 (1.9–74.3) |
| Non-response (n, %) | 20 (12.5) | 25 (12.1) | 108 (50.0) |
| Time between 1st vaccination and AB measurement (days) | 91 (90–96) | 91 (88–94) | 91 (90–95) |
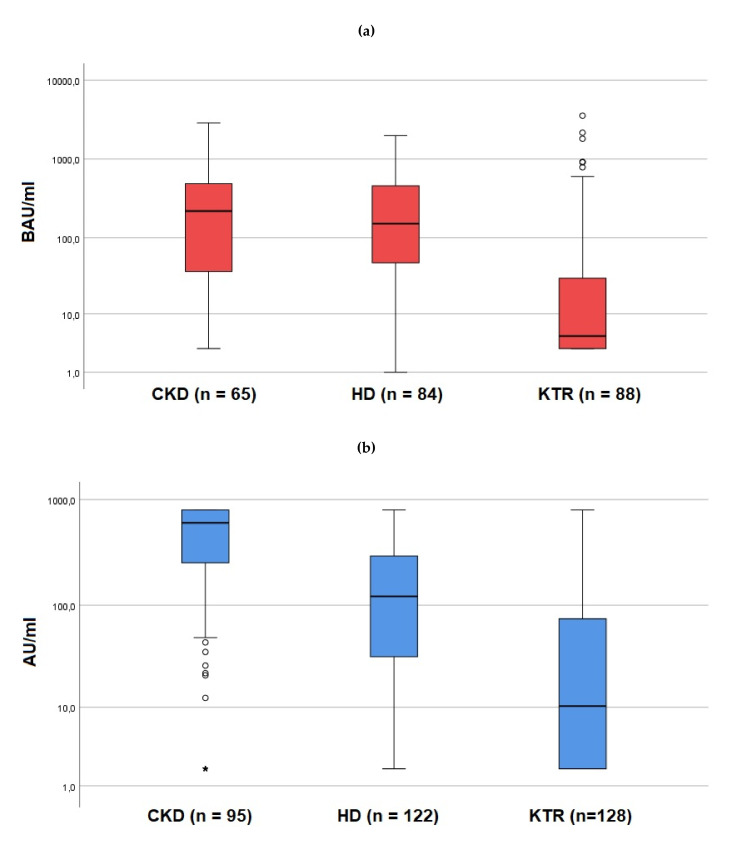
Levels of antibody titers were significantly higher in CKD patients (Abbott: 230.3 BAU/mL [48.3–497.6]; Liaison: 602.0 AU/mL [252.5–800.0]) than in HD patients (Abbott: 151.6 BAU/mL [47.7–458.4]; Liaison: 121.5 AU/mL [32.0–293.0]); (p < 0.001) and in KTR (Abbott: 4.75 BAU/mL [3.0–30.2]; Liaison: 10.3 AU/mL [1.9–74.3]); (all p < 0.001, Figure 1).
Figure 1.
(a,b): Levels of antibody titers in CKD patients, HD patients, and KTR. (a) shows the results of the Abbott SARS-CoV-2 IgG II Quant Assay test (median, 25, and 75% percentile for CKD: 230.3 (48.3–497.6), for HD: 151.6 (47.7–458.4), for KTR: 4.8 (3.0–30.2); (b) shows the results of the Liaison® SARS-CoV-2 S1/S2 IgG test (median, 25, and 75% percentile for CKD: 602.0 (252.5–800.0), for HD: 121.5 (32.0–293.0), for KTR: 10.3 (1.9–74.3). Logarithmic scale. Circles represent statistical outliers between 1.5 to 3.0 times of the interquartile range (IQR), asterisks represent statistical outliers more than 3 times of the IQR.
In a multivariate analysis, risk factors for non-response were examined. In CKD, a significant risk factor for non-response was treatment with rituximab (OR 27.2, 95% CI 5.12–144.63, p < 0.001) before vaccination (Table 3). The rate of non-response was 53.3% in CKD patients treated with rituximab (n = 30) versus 3.1% in those without rituximab treatment (n = 130). Longer temporal distance of rituximab application to first vaccination significantly reduced the risk for non-response (OR 0.98, 0.96–1.00, p = 0.020 per day).
Table 3.
Multivariate analysis of risk factors for non-response in CKD patients. OR odds ratio, CI confidence interval.
| OR (95% CI) | p-Value | |
|---|---|---|
| Glomerulonephritis | 1.53 (0.24–9.75) | 0.651 |
| Rituximab treatment | 27.20 (5.12–144.63) | <0.001 |
| Days between 1st vaccination and Rituximab (per day) | 0.98 (0.96–1.00) | 0.020 |
In HD patients with renal transplant in situ (n = 39), the use of calcineurin inhibitors was significantly associated with non-response (OR 14.85, 2.68–82.43, p = 0.002); no other risk factors could be identified in HD patients (Table 4).
Table 4.
Multivariate analysis of risk factors for non-response in HD patients. OR odds ratio, CI confidence interval.
| OR (95% CI) | p-Value | |
|---|---|---|
| Glomerulonephritis | 2.87 (0.95–8.70) | 0.062 |
| High-dose glucocorticoid treatment during last year (≥1 mg/kg) | 6.36 (0.67–60.44) | 0.107 |
| Treatment with tacrolimus or cyclosporine A | 14.85 (2.68–82.43) | 0.002 |
| Glucocorticoids | 1.90 (0.25–14.49) | 0.537 |
| Hemoglobin (per g/dL) | 0.97 (0.95–1.01) | 0.098 |
In KTR, higher age (OR 1.06, 1.03–1.09, p < 0.001 per year) was significantly associated with non-response (Table 5). The use of mycophenolic acid (OR 6.61, 2.31–18.86, p < 0.001) or glucocorticoids (OR 4.95, 1.48–16.57, p = 0.010) was also significantly associated with non-response, whereas vaccination with mRNA-1273 (OR 0.41, 0.20–0.83, p = 0.014) and higher levels of hemoglobin (OR 0.97, 0.95–0.99, p < 0.001 per g/L significantly reduced the risk for non-response. Treatment with belatacept was not significantly associated with non-response, possibly due to a low patient number (n = 14).
Table 5.
Multivariate analysis of risk factors for non-response in KTR. OR odds ratio, CI confidence interval, KT kidney transplantation.
| OR (95% CI) | p-Value | |
|---|---|---|
| Age (per year) | 1.06 (1.03–1.09) | <0.001 |
| Type of vaccination | ||
| BNT162b2 | Ref. | - |
| mRNA-1273 | 0.41 (0.20-0.83) | 0.014 |
| Cerebrovascular disease | 3.11 (0.99–9.76) | 0.052 |
| CMV reactivation during the last 6 months | 1.22 (0.47–3.18) | 0.681 |
| Basis of immunosuppression | ||
| Antimetabolite and/or steroids | Ref. | - |
| Cyclosporine A | 0.89 (0.06–14.23) | 0.934 |
| Tacrolimus | 1.59 (0.11–22.38) | 0.730 |
| mTOR inhibitors | 0.31 (0.01–10.42) | 0.512 |
| Belatacept | 11.01 (0.45–269.68) | 0.142 |
| Antimetabolites | ||
| No antimetabolites | Ref. | - |
| Azathioprine | 0.73 (0.18–2.92) | 0.652 |
| Mycophenolic acid | 6.61 (2.31–18.86) | <0.001 |
| Glucocorticoids | 4.95 (1.48–16.57) | 0.010 |
| Hemoglobin (per g/dL) | 0.97 (0.95–0.99) | <0.001 |
| Time since last KT (per year) | 0.99 (0.94–1.04) | 0.660 |
Presence of diabetes or the underlying primary renal disease (diabetic nephropathy, vascular/hypertensive nephropathy, glomerulonephritis, other, unknown) did not predict non-response in any of the groups. In addition, the presence of cardiovascular or cerebrovascular disease were not associated with non-response. Further non-significant factors were treatment with RAAS inhibitors and the number of classes of antihypertensive drugs.
4. Discussion
This is one of the first studies showing a direct comparison of SARS-CoV-2 antibody titers in CKD patients with and without immunosuppressive treatment, HD patients, and KTR. We found levels of antibody titers to be significantly higher in CKD patients than in HD patients and KTR. Interestingly, primary non-response in CKD and HD patients was approximately the same (12.5% vs. 12.1%). The non-response rate in HD is in line with recent literature [25]. It has been demonstrated that immunosuppressive medication after transplantation impairs immune response to (not only SARS-CoV-2) vaccines [26] and influences immunization practices of listed HD patients [27]. Our data also suggests a possible advantage of vaccination before the initiation of renal replacement therapy (RRT).
The low AB titers in HD patients and KTR might indicate the need for a scheduled booster regimen to enhance immune response in those patients. In particular, the very low titers in KTR are alarming, not only regarding the potential of vaccines to protect against COVID-19 disease, but also the longevity of the supposed protection. Several trials have already reported on immune responses to a third vaccine dose in KTR non-responders [28,29,30] and weak responders [31], as well as HD patients [32,33]. Approximately 50% of KTR developed a positive AB response after a third dose. A fourth dose seems to have a similar efficacy [34]. This raises the question of the efficiency of available vaccines (or vaccination strategies) to grant adequate protection from COVID-19 in KTR. In the case of continuous non-/or very low-response, basic hygiene measures, social distancing, and especially a high vaccination coverage of care providers, relatives, and the general population remain highly important. To the best of our knowledge, no systematic analysis of efficacy of a third vaccine dose in CKD patients exists.
Recent publications demonstrated a 100% percent development of AB after vaccination in CKD patients [35,36]. Our finding of a response rate of 87.5% can be partially explained by the fact that, in contrast to these studies, we included CKD patients with immunosuppressive medication. In our cohort, 19% of CKD patients received rituximab treatment, while six patients received a high dose glucocorticoid treatment (>1 mg/kg body weight) during the last year.
A limitation of our study is the fact that we did not assess cellular immune responses. While it is reasonable to assume that immunosuppressive medication impairs cellular immune response to a similar extent than humoral response (with the exception of rituximab), some studies exist that demonstrated a significant T-cell immunity in cases of humoral non-response [37,38]. The extent to which S1-AB titer correlates with protection against infection and severe COVID-19 courses is incompletely understood. An interesting point was raised in recent publications: while the development of positive AB titers seems to correlate reasonably well with neutralizing antibodies [39] and neutralizing capacity of patient plasma [40], 10% of seropositive KTRs did not develop neutralizing capacity [39]. Comparison and interpretation of cellular assays is difficult due to the heterogeneity of employed analyses. While the analysis of S1-AB assays might not be able to inform on the whole immunologic picture, an inexpensive tool, easy to deploy and compare, is needed to combat the ongoing pandemic and inform on sufficient or inadequate protection. The lack of follow-up is a limitation of our study; “real life” data on break-through infections and COVID-19 infection courses of vaccinated immunosuppressed patients are urgently needed.
Furthermore, specific immunosuppressive agents (such as rituximab in CKD patients, calcineurin inhibitors in HD patients with transplant in situ, and mycophenolic acid and glucocorticoids in KTR) might influence immune response, suggesting a possible need for a change in immunosuppressive therapy ahead of vaccination. While changes to immunosuppression in KTR should be handled with caution, in HD patients temporarily pausing immunosuppressive medication prescribed to preserve residual kidney function seems feasible, though it is unclear if pausing immunosuppression is sufficient to restore an adequate vaccine response. Our data also suggests aiming for a temporal distance of vaccination to the last rituximab administration in CKD patients, which is in line with current literature [41]. While induction treatment with rituximab, for example in ANCA-associated vasculitis, usually cannot be delayed, postponing COVID-19 vaccination until the reconstitution of CD19+ B-cells, a transient switch to other immunosuppressive treatments (such as azathioprine) or a delay of rituximab treatment in stable patients may be warranted [42]. In any case, a careful benefit-risk analysis by the providing physician is needed in such instances.
In line with the available literature, our data favours the usage of mRNA-1273 over BNT162b2 in KTR [16,17] as well as HD [14,15] to ensure optimal response. As of to date, no such recommendation has found entry in international, national or scientific guidelines on vaccination strategies.
The data of our study were collected at a time where SARS-CoV-2 variants of concern (Delta, Omicron) did not play a major epidemiological role in Austria.
In general, the vaccination of household members and other close contacts is essential and has been shown to reduce the transmission rate [43].
In summary, our study expands the knowledge of AB response to mRNA-based SARS-CoV-2 vaccinations in different groups of patients with impaired kidney function. Patients with CKD had a significantly better immune response than patients on HD or KTR, while still over 10% of CKD patients did not mount a detectable immune response. Clinical studies on antibody levels as well as cellular immune responses, duration of protection, and break through infections in those patients are clearly needed to inform on future immunization strategies and optimal treatment.
Acknowledgments
We want to thank all participating centers in Tyrol, especially Hermann Kathrein, Matthias Post, Florian Reinstaller, Regina Schlacher, Angelika Senn, Karin Erhart, Eva Tamerl, and Christine Wohlgemuth.
Author Contributions
Conceptualization, L.B. and J.K.; methodology, J.K.; software, C.A.S., J.M. and J.K.; validation, C.A.S. and J.K.; formal analysis, J.K.; investigation, L.B., M.P., M.R. and J.K.; resources, M.P., M.R. and G.M.; data curation, C.A.S., J.M. and J.K.; writing—original draft preparation, L.B. and J.K.; writing—review and editing, L.B., C.A.S., J.M., M.P., M.R., G.M. and J.K.; supervision, G.M.; project administration, L.B. and J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of the Medical University Innsbruck (ECS 1280/2021, 31 August 2021).
Informed Consent Statement
Patient consent was waived due to the retrospective design of the study collecting data on routine measurements.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to data protection.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yanay N.B., Freiman S., Shapira M., Wishahi S., Hamze M., Elhaj M., Zaher M., Armaly Z. Experience with SARS-CoV-2 BNT162b2 mRNA vaccine in dialysis patients. Kidney Int. 2021;99:1496–1498. doi: 10.1016/j.kint.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grupper A., Sharon N., Finn T., Cohen R., Israel M., Agbaria A., Rechavi Y., Schwartz I.F., Schwartz D., Lellouch Y., et al. Humoral Response to the Pfizer BNT162b2 Vaccine in Patients Undergoing Maintenance Hemodialysis. Clin. J. Am. Soc. Nephrol. 2021;16:1037–1042. doi: 10.2215/CJN.03500321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Billany R.E., Selvaskandan H., Adenwalla S.F., Hull K.L., March D.S., Burton J.O., Bishop N.C., Carr E.J., Beale R., Tang J.W., et al. Seroprevalence of antibody to S1 spike protein following vaccination against COVID-19 in patients receiving hemodialysis: A call to arms. Kidney Int. 2021;99:1492–1494. doi: 10.1016/j.kint.2021.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyarsky B.J., Werbel W.A., Avery R.K., Tobian A.A.R., Massie A.B., Segev D.L., Garonzik-Wang J.M. Antibody Response to 2-Dose SARS-CoV-2 mRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA. 2021;325:2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benotmane I., Gautier-Vargas G., Cognard N., Olagne J., Heibel F., Braun-Parvez L., Martzloff J., Perrin P., Moulin B., Fafi-Kremer S., et al. Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int. 2021;99:1498–1500. doi: 10.1016/j.kint.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korth J., Jahn M., Dorsch O., Anastasiou O.E., Sorge-Hädicke B., Eisenberger U., Gaeckler A., Dittmer U., Witzke O., Wilde B., et al. Impaired Humoral Response in Renal Transplant Recipients to SARS-CoV-2 Vaccination with BNT162b2 (Pfizer-BioNTech) Viruses. 2021;13:756. doi: 10.3390/v13050756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grupper A., Rabinowich L., Schwartz D., Schwartz I.F., Ben-Yehoyada M., Shashar M., Katchman E., Halperin T., Turner D., Goykhman Y., et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am. J. Transplant. 2021;21:2719–2726. doi: 10.1111/ajt.16615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Attias P., Sakhi H., Rieu P., Soorkia A., Assayag D., Bouhroum S., Nizard P., El Karoui K. Antibody response to the BNT162b2 vaccine in maintenance hemodialysis patients. Kidney Int. 2021;99:1490–1492. doi: 10.1016/j.kint.2021.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cucchiari D., Egri N., Bodro M., Herrera S., Del Risco-Zevallos J., Casals-Urquiza J., Cofan F., Moreno A., Rovira J., Banon-Maneus E., et al. Cellular and humoral response after MRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am. J. Transplant. 2021;21:2727–2739. doi: 10.1111/ajt.16701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rozen-Zvi B., Yahav D., Agur T., Zingerman B., Ben-Zvi H., Atamna A., Tau N., Mashraki T., Nesher E., Rahamimov R. Antibody response to SARS-CoV-2 mRNA vaccine among kidney transplant recipients: A prospective cohort study. Clin. Microbiol. Infect. 2021;27:1173.e1–1173.e4. doi: 10.1016/j.cmi.2021.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anand S., Montez-Rath M.E., Han J., Garcia P., Cadden L., Hunsader P., Kerschmann R., Beyer P., Dittrich M., Block G.A., et al. Antibody Response to COVID-19 Vaccination in Patients Receiving Dialysis. J. Am. Soc. Nephrol. 2021;32:2435–2438. doi: 10.1681/ASN.2021050611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaiser R.A., Haller M.C., Apfalter P., Kerschner H., Cejka D. Comparison of BNT162b2 (Pfizer–BioNtech) and mRNA-1273 (Moderna) SARS-CoV-2 mRNA vaccine immunogenicity in dialysis patients. Kidney Int. 2021;100:697–698. doi: 10.1016/j.kint.2021.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Praet J., Reynders M., de Bacquer D., Viaene L., Schoutteten M., Caluwé R., Doubel P., Heylen L., De Bel A.V., Van Vlem B., et al. Predictors and Dynamics of the Humoral and Cellular Immune Response to SARS-CoV-2 mRNA Vaccines in Hemodialysis Patients: A Multicenter Observational Study. J. Am. Soc. Nephrol. 2021;32:3208–3220. doi: 10.1681/ASN.2021070908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stumpf J., Siepmann T., Lindner T., Karger C., Schwöbel J., Anders L., Faulhaber-Walter R., Schewe J., Martin H., Schirutschke H., et al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: A prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg. Health Eur. 2021;9:100178. doi: 10.1016/j.lanepe.2021.100178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dębska-Ślizień A., Ślizień Z., Muchlado M., Kubanek A., Piotrowska M., Dąbrowska M., Tarasewicz A., Chamienia A., Biedunkiewicz B., Renke M., et al. Predictors of Humoral Response to mRNA COVID19 Vaccines in Kidney Transplant Recipients: A Longitudinal Study-The COViNEPH Project. Vaccines. 2021;9:1165. doi: 10.3390/vaccines9101165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kantauskaite M., Müller L., Kolb T., Fischer S., Hillebrandt J., Ivens K., Andree M., Luedde T., Orth H.M., Adams O., et al. Intensity of mycophenolate mofetil treatment is associated with an impaired immune response to SARS-CoV-2 vaccination in kidney transplant recipients. Am. J. Transplant. 2021:1–6. doi: 10.1111/ajt.16851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chavarot N., Ouedrani A., Marion O., Leruez-Ville M., Vilain E., Baaziz M., Del Bello A., Burger C., Sberro-Soussan R., Martinez F., et al. Poor Anti-SARS-CoV-2 Humoral and T-cell Responses After 2 Injections of mRNA Vaccine in Kidney Transplant Recipients Treated with Belatacept. Transplantation. 2021;105:e94–e95. doi: 10.1097/TP.0000000000003784. [DOI] [PubMed] [Google Scholar]
- 20.Terrec F., Jouve T., Malvezzi P., Janbon B., Naciri Bennani H., Rostaing L., Noble J. Belatacept Use after Kidney Transplantation and Its Effects on Risk of Infection and COVID-19 Vaccine Response. J. Clin. Med. 2021;10:5159. doi: 10.3390/jcm10215159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moor M.B., Suter-Riniker F., Horn M.P., Aeberli D., Amsler J., Möller B., Njue L.M., Medri C., Angelillo-Scherrer A., Borradori L., et al. Humoral and cellular responses to mRNA vaccines against SARS-CoV-2 in patients with a history of CD20 B-cell-depleting therapy (RituxiVac): An investigator-initiated, single-centre, open-label study. Lancet Rheumatol. 2021;3:e789–e797. doi: 10.1016/S2665-9913(21)00251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jager K.J., Kramer A., Chesnaye N.C., Couchoud C., Sánchez-Álvarez J.E., Garneata L., Collart F., Hemmelder M.H., Ambuehl P., Kerschbaum J., et al. Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020;98:1540–1548. doi: 10.1016/j.kint.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., Curtis H.J., Mehrkar A., Evans D., Inglesby P., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gansevoort R.T., Hilbrands L.B. CKD is a key risk factor for COVID-19 mortality. Nat. Rev. Nephrol. 2020;16:705–706. doi: 10.1038/s41581-020-00349-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J.-J., Lee T.H., Tian Y.-C., Lee C.-C., Fan P.-C., Chang C.-H. Immunogenicity Rates after SARS-CoV-2 Vaccination in People with End-stage Kidney Disease: A Systematic Review and Meta-analysis. JAMA Netw. Open. 2021;4:e2131749. doi: 10.1001/jamanetworkopen.2021.31749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grupper A., Katchman E., Ben-Yehoyada M., Rabinowich L., Schwartz D., Schwartz I.F., Shashar M., Halperin T., Turner D., Goykhman Y., et al. Kidney transplant recipients vaccinated before transplantation maintain superior humoral response to SARS-CoV-2 vaccine. Clin. Transplant. 2021;35:e14478. doi: 10.1111/ctr.14478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blanchard-Rohner G., Enriquez N., Lemaître B., Cadau G., Combescure C., Giostra E., Hadaya K., Meyer P., Gasche-Soccal P.M., Berney T., et al. Usefulness of a systematic approach at listing for vaccine prevention in solid organ transplant candidates. Am. J. Transplant. 2019;19:512–521. doi: 10.1111/ajt.15097. [DOI] [PubMed] [Google Scholar]
- 28.Kamar N., Abravanel F., Marion O., Couat C., Izopet J., Del Bello A. Three Doses of an mRNA Covid-19 Vaccine in Solid-Organ Transplant Recipients. N. Engl. J. Med. 2021;385:661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Westhoff T.H., Seibert F.S., Anft M., Blazquez-Navarro A., Skrzypczyk S., Zgoura P., Meister T.L., Pfaender S., Stumpf J., Hugo C., et al. A third vaccine dose substantially improves humoral and cellular SARS-CoV-2 immunity in renal transplant recipients with primary humoral nonresponse. Kidney Int. 2021;100:1135–1136. doi: 10.1016/j.kint.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benotmane I., Gautier G., Perrin P., Olagne J., Cognard N., Fafi-Kremer S., Caillard S. Antibody Response After a Third Dose of the mRNA-1273 SARS-CoV-2 Vaccine in Kidney Transplant Recipients with Minimal Serologic Response to 2 Doses. JAMA. 2021;326:1063–1065. doi: 10.1001/jama.2021.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masset C., Kerleau C., Garandeau C., Ville S., Cantarovich D., Hourmant M., Kervella D., Houzet A., Guillot-Gueguen C., Guihard I., et al. A third injection of the BNT162b2 mRNA COVID-19 vaccine in kidney transplant recipients improves the humoral immune response. Kidney Int. 2021;100:1132–1135. doi: 10.1016/j.kint.2021.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bensouna I., Caudwell V., Kubab S., Acquaviva S., Pardon A., Vittoz N., Dogan-Firat B., Hanafi L., Faucon A.L., Housset P. SARS-CoV-2 Antibody Response After a Third Dose of the BNT162b2 Vaccine in Patients Receiving Maintenance Hemodialysis or Peritoneal Dialysis. Am. J. Kidney Dis. 2021. in press . [DOI] [PMC free article] [PubMed]
- 33.Dekervel M., Henry N., Torreggiani M., Pouteau L.-M., Imiela J.-P., Mellaza C., Garnier A.S., Dujardin A., Asfar M., Ducancelle A., et al. Humoral response to a third injection of BNT162b2 vaccine in patients on maintenance haemodialysis. Clin. Kidney J. 2021;14:2349–2355. doi: 10.1093/ckj/sfab152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alejo J.L., Mitchell J., Chiang T.P.-Y., Abedon A.T., Boyarsky B.J., Avery R.K., Tobian A.A.R., Levan M.L., Massie A.B., Garonzik-Wang J.M., et al. Antibody Response to a Fourth Dose of a SARS-CoV-2 Vaccine in Solid Organ Transplant Recipients: A Case Series. Transplantation. 2021;105:e280. doi: 10.1097/TP.0000000000003934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanders J.-S.F., Bemelman F.J., Messchendorp A.L., Baan C.C., van Baarle D., van Binnendijk R., Diavatopoulos D.A., Froelke S.C., Geers D., GeurtsvanKessel C.H., et al. The RECOVAC Immune-response Study: The Immunogenicity, Tolerability, and Safety of COVID-19 Vaccination in Patients with Chronic Kidney Disease, on Dialysis, or Living with a Kidney Transplant. Transplantation. 2021. in press . [DOI] [PMC free article] [PubMed]
- 36.Quiroga B., Soler M.J., Ortiz A., Vaquera S.M., Mantecón C.J.J., Useche G., Sánchez Márquez M.G., Carnerero M., Jaldo Rodríguez M.-T., Ramos P.M., et al. Safety and immediate humoral response of COVID-19 vaccines in chronic kidney disease patients: The SENCOVAC study. Nephrol. Dial. Transplant. 2021. in press . [DOI] [PMC free article] [PubMed]
- 37.Thieme C.J., Blazquez-Navarro A., Safi L., Kaliszczyk S., Paniskaki K., Neumann I.E., Schmidt K., Stockhausen M., Hoerstrup J., Cinkilic O., et al. Impaired Humoral but Substantial Cellular Immune Response to Variants of Concern B1.1.7 and B.1.351 in Hemodialysis Patients after Vaccination with BNT162b2. J. Am. Soc. Nephrol. 2021;32:2725–2727. doi: 10.1681/ASN.2021050672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hall V.G., Ferreira V.H., Ierullo M., Ku T., Marinelli T., Majchrzak-Kita B., Yousuf A., Kulasingam V., Humar A., Kumar D. Humoral and cellular immune response and safety of two-dose SARS-CoV-2 mRNA-1273 vaccine in solid organ transplant recipients. Am. J. Transplant. 2021;12:3980–3989. doi: 10.1111/ajt.16766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pedersen R.M., Bang L.L., Tornby D.S., Kierkegaard H., Nilsson A.C., Johansen I.S., Bistrup C., Jensen T.G., Justesen U.S., Andersen T.E. The SARS-CoV-2-neutralizing capacity of kidney transplant recipients 4 weeks after receiving a second dose of the BNT162b2 vaccine. Kidney Int. 2021;100:1129–1131. doi: 10.1016/j.kint.2021.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hod T., Ben-David A., Olmer L., Levy I., Ghinea R., Mor E., Lustig Y., Rahav G. Humoral Response of Renal Transplant Recipients to the BNT162b2 SARS-CoV-2 mRNA Vaccine Using Both RBD IgG and Neutralizing Antibodies. Transplantation. 2021;105:e234–e243. doi: 10.1097/TP.0000000000003889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Connolly C., Koenig D., Ravi S., Azar A., Kant S., Dalal M., Duchen J., Seo P., Antiochos B., Paik J.P., et al. Correspondence on “SARS-CoV-2 vaccination in rituximab-treated patients: Evidence for impaired humoral but inducible cellular immune response” by Bonelli et al. Ann. Rheum. Dis. 2021;80:e164. doi: 10.1136/annrheumdis-2021-220972. [DOI] [PubMed] [Google Scholar]
- 42.Kronbichler A., Geetha D., Smith R.M., Egan A.C., Bajema I.M., Schönemarck U., Mahr A., Anders H.J., Bruchfeld A., Cid M.C., et al. The COIVD-19 pandemic and ANCA-associated vasculitis—Reports from the EUVAS meeting and EUVAS education forum. Autoimmun. Rev. 2021;20:102986. doi: 10.1016/j.autrev.2021.102986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harris R.J., Hall J.A., Zaidi A., Andrews N.J., Dunbar J.K., Dabrera G. Effect of vaccination on household transmission of SARS-CoV-2 in England. N. Engl. J. Med. 2021;385:759–760. doi: 10.1056/NEJMc2107717. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to data protection.