ABSTRACT
Background and Aim
Knowledge of long-term consequences of severe COVID-19 pneumonitis is of outmost importance. Our aim was, therefore, to assess the long-term impact on quality of life and lung function in adults hospitalized with severe COVID-19.
Methods
All patients hospitalized with COVID-19 pneumonitis at Copenhagen University Hospital-Hvidovre, Denmark, were invited to participate in the study 4–5 months after discharge. Of the 160 invited 128 responded positively (80%). Medical history and symptoms were assessed, and patients rated impact on quality of life and functional status with EuroQol-5D-5L and Post Covid Functional Scale. Lung function was assessed by dynamic spirometry and measurement of diffusing capacity.
Results
Fatigue, dyspnea, cough and cognitive dysfunction were the most common symptoms. Of 128 patients, 85% had at least one symptom, and 51% reported two or more symptoms. Self-rated Quality of life was impaired assessed by EuroQol 5D-5L, with dimensions ‘Pain or discomfort’ (61%) and ‘Usual activities’ (54%) mostly affected. Functional status was significantly worse than before COVID-19 assessed by Post-COVID Functional Scale. Among lung function parameters, diffusing capacity was most affected, with 45% having diffusing capacity < 80% of predicted.
Conclusion
Fatigue, respiratory symptoms and cognitive symptoms are highly common months after hospitalization for severe COVID-19. Compared to pre-COVID-19, functional status and usual activities continued to be impaired. In line with this, almost half of the patients were found to have impaired diffusing capacity.
KEYWORDS: COVID-19, sequelae, long term follow up, pulmonary function, health-related quality of life
Introduction
A growing body of data suggests that infection with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) may have long-term health consequences. Furthermore, although symptoms from the upper and lower airways are the primary manifestations of COVID-19, it is recognized as a multi-organ disease with a broad spectrum of manifestations. Many patients report fatigue, dyspnea, cognitive dysfunction, loss of smell and taste, and functional impairment for several months after infection with SARS-CoV-2 [1–3]. The duration of symptoms and functional impairment remains to be elucidated, and the burden of COVID-19 sequelae on health-care resources and its socioeconomic impact has yet to be established.
In line with previous coronavirus infections such as the SARS epidemic and the Middle Eastern Respiratory Syndrome (MERS) outbreak, patients were found to have a similar picture of persistent symptoms [3–6] emphasizing the importance of obtaining knowledge of the long-term health consequences of this new coronavirus disease.
Our aim was, therefore, to explore health-related quality of life, functional impairment and level of lung function in patients previously hospitalized for COVID-19 pneumonitis.
Methods
Patients
The study was conducted at Copenhagen University Hospital-Hvidovre (catchment area of approximately 500,000 citizens). Patients were eligible for inclusion in the study, if they fulfilled the following criteria: 1) SARS-CoV-2 infection verified by Reverse Transcription Polymerase Chain Reaction (PCR), 2) Primary diagnosis of COVID-19, 3) Admitted with COVID-19 between March 1st and November 1st, 2020, and 4) Signs of COVID-19 pneumonitis on chest X-ray, computed tomography scan and/or requiring supplemental oxygen.
Patients were excluded if they fulfilled one of the following criteria: 1) Age < 18 years, 2) Not able to attend the follow-up visit due to severe disability or chronic cognitive deficits, or 3) Deceased prior to the intended time of follow-up.
Baseline demographics, comorbidities and pharmacological history were obtained from the medical records.
Study design
Patients were invited by letter to participate in the study, and responders were given a scheduled appointment at the respiratory outpatient clinic.
Health-related quality of life was assessed by the EuroQol 5D-5L (EQ-5D-5L) and EuroQol Visual Analogue Scale (EQ VAS). Functional status over time was assessed by the Post Covid Functional Scale (PCFS).
EQ-5D-5L is a questionnaire that defines health in five dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression), with five response categories ranging from no problems to extreme problems. The visual analogue EQ VAS scale ranging from 0 to 100 rate the patient’s overall health on the day of the visit, with higher scores representing better subjective health [7].
The Post Covid Functional scale (PCFS) is an ordinal outcome measure with six steps ranging from 0 (no symptoms) to 5 (death) designed to cover the overall functional limitations after SARS-CoV-2 infection. The patients are also asked to provide a pre-COVID-19 status on the PCFS-scale, which allows for assessing a possible change in status [8].
Spirometry with measurements of the forced vital capacity (FVC) and the forced expiratory volume in 1. second (FEV1) and calculation of the ratio of FEV1/FVCs was done according to standard procedure [9].
Diffusing capacity for carbon monoxide (DLCO), diffusion constant (DL/VA) and total lung capacity (TLC) were measured using the single-breath method [10].
All measurements were performed using EasyOnePro (Medizintecknik AG, Zürich, Switzerland).
A 6 min walking test (6MWT) was performed according to ERS guidelines with baseline SpO2 measured by pulse oximetry on the index finger [11].
Statistics and ethics
As this was a standard of care non-interventional study, formal approval from the ethics committee was not required. However, the Ethics Committee in the Capital Region of Denmark was informed about the study (jr.nr. H-20040749)
Access to the medical records was granted by the hospital data protection manager.
Data were collected and registered in Research Electronic Data Capture (REDCap) (REDCap Consortium, Vanderbilt University Medical Centre, Nashville, US) hosted by the Capital Region of Denmark.
Demographics were described with prevalence, median values and interquartile ranges. Lung function parameters were described with mean and standard deviation. PCFS before and after COVID-19 was tested for significant differences with Wilcoxon Signed Rank Test.
Results
A total of 251 patients were discharged during the study period with a diagnosis of COVID-19 (positive PCR for SARS-CoV-2). Of these 46 did not meet the criteria for COVID-19 pneumonitis (need of oxygen and/or radiological findings), 17 were discharged but died before follow-up, 21 were excluded due to cognitive/physical impairment, two were under 18 years of age and five did not have COVID-19 as primary diagnosis. The remaining 160 were invited to participate in the study, of whom 128 attended the examinations (Figure 1).
Figure 1.
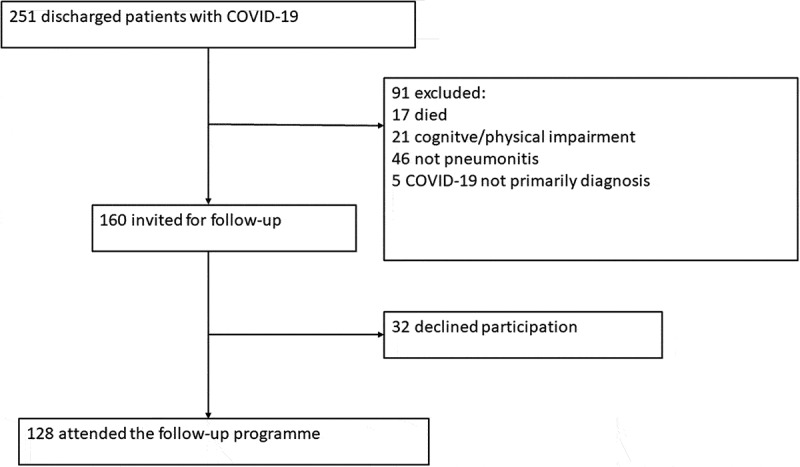
Trial profile.
Table 1 shows the baseline demographics of the final cohort (n = 128). The median age was 64.5 years (IQR: 51.0–75.0) and 42% were women. The median time between discharge and follow-up was 140 days (IQR: 119–157 days). The most common comorbidity was hypertension (48%), followed by diabetes (28%) and heart failure (12%). The majority of patients were never-smokers (54%). Data on medication at admission is given in Table 1.
Table 1.
Demographics, comorbidities, and medicine at admission
| Admitted with COVID-19 (n = 128) | Median (25–75% percentile) or percentage |
|---|---|
| Age (years) | 64.5 (51.0–75.0) |
| Women/Men | 42%/58% |
| BMI (kg/m2) | 27.2 (24.0–30.4) |
| Smoking status (never/former/current) | 54.3%/43.3%/2.4% |
| Pack years (former and current smokers) | 16.0 (3.5–31.5) |
| Length of Stay (days) | 5 (3–8) |
| Respiratory Support (Oxygen/CPAP/MV) | 86.7%/8.6%/3.9% |
| Comorbidities | Percentage of patients |
| Diabetes | 28% |
| Hypertension | 48% |
| Heart Failure | 12% |
| Atrial fibrillation | 8% |
| COPD | 7% |
| Asthma | 9% |
| Medical treatment at admission | Percentage of patients |
| Beta blockers | 15% |
| Calcium channel blockers | 24% |
| Diuretics | 30% |
| Angiotensin converting enzyme inhibitors | 11% |
| Angiotensin II receptor antagonists | 24% |
| Direct oral anticoagulants | 8% |
| Vitamin K antagonists | 4% |
| Platelet inhibitors | 15% |
| Oral antidiabetics | 24% |
| Insulin and insulin analogs | 8% |
| Non-steroidal anti-inflammatory drugs | 12% |
| Prednisolone and/or immunosuppressants | 5% |
| Long-acting muscarinic antagonists | 3% |
| Long-acting beta-2 agonists | 9% |
| Inhaled corticosteroids | 13% |
BMI, body mass index; CPAP, continuous positive airway pressure; MV, mechanical ventilation; COPD, chronic obstructive pulmonary disease.
All patients included in the cohort required supplemental oxygen during admission either through nasal cannula or high flow nasal cannula (HFNC). In the cohort, 8.6% and 3.9%, respectively, required Continuous Positive Airway Pressure (CPAP) and invasive mechanical ventilation. The overall median length of stay was 6 days (IQR: 3–10 days).
Symptoms
Figure 2 shows the prevalence of the most common symptoms at follow-up. At least one symptom was reported by 85% of the patients, and 51% reported two or more symptoms. The most frequent symptoms were fatigue (69%), dyspnea (48%) and cough (35%). Approximately one-fourth of the cohort reported cognitive impairment and pain/discomfort. Smell and/or taste abnormalities were reported by 16%.
Figure 2.
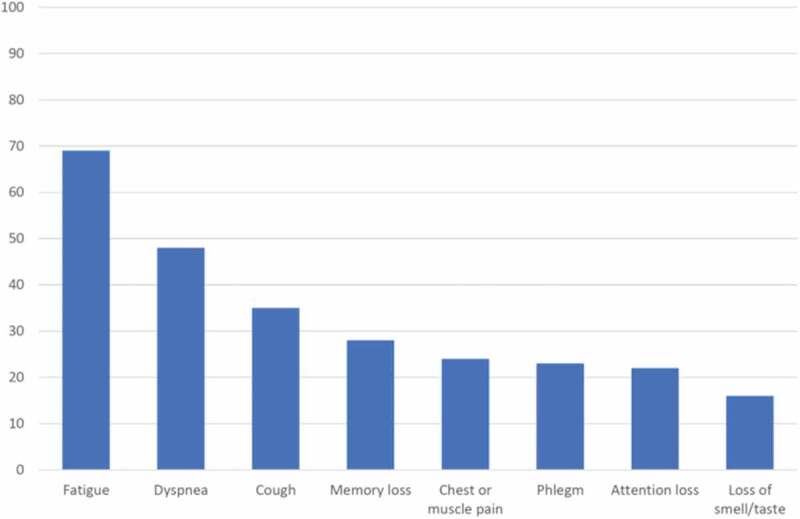
Prevalence of major symptoms at follow-up.
Health related quality of life
Overall, the self-reported health at follow-up was moderately affected (Figure 3). The dimensions most affected in the EQ-5D-5L was pain and discomfort (61% affected), usual activities (54% affected) and mobility (51% affected). Mental health was affected but less common (40% affected). Self-rated health on a 0 to 100 scale with EQ VAS revealed a median score of 75 (IQR: 50–90).
Figure 3.
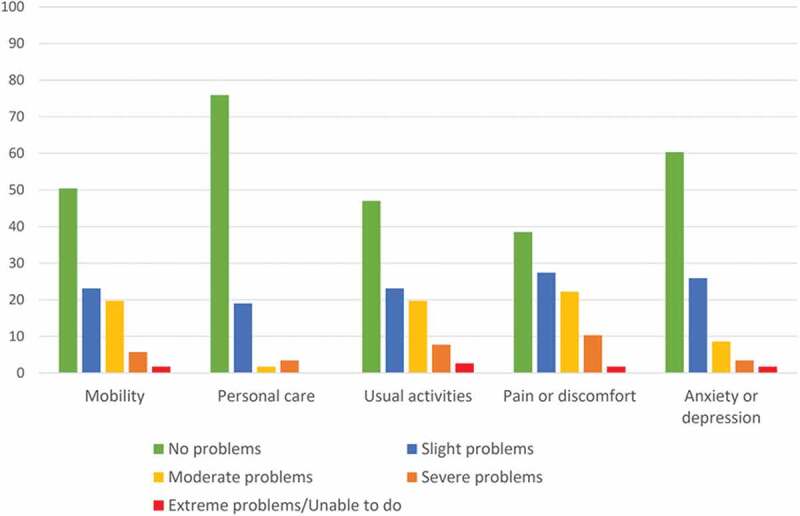
EuroQol 5D-5L at follow-up.
The PCFS questionnaire showed an impairment in functional status compared to before the COVID-19 infection (Figure 4). Mild to severe impairment was reported by 59% at follow-up compared to 32% before COVID-19 (p < 0.001). Of 75 patients reporting PCFS before as well as after COVID-19, 4% reported improvement, 60% reported unchanged status and 36% reported worse functional status.
Figure 4.
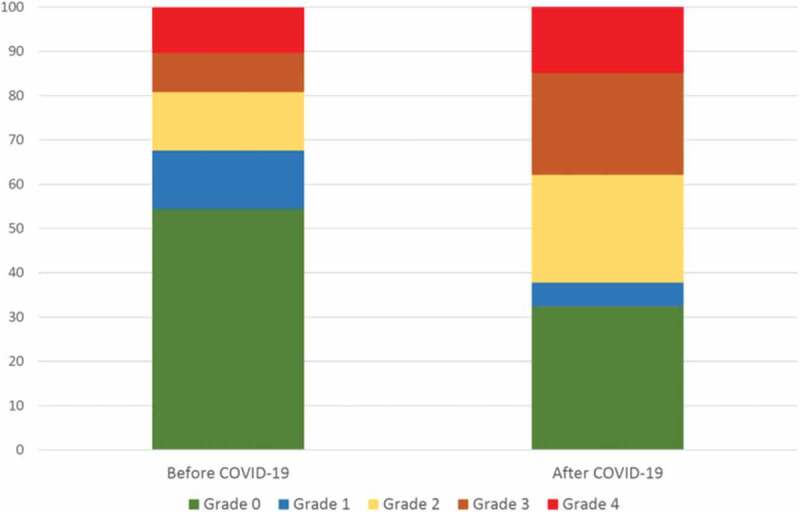
Post COVID Functional Scale before and after COVID-19. Grade 0: No functional limitations; Grade 1: Negligible functional limitations; Grade 2 Slight functional limitations; Grade 3: Moderate functional limitations; Grade 4: Severe functional limitations.
Lung function and 6-minutes walking test
The results of the pulmonary tests are shown in Table 2. The group means for the 123 patients completing the dynamic spirometry was within normal limits; however, FEV1 and FVC were below 80% in 25.2% and 19.5%, respectively, of the patients. An FEV1/FVC ratio < 0.70 and TLC < 80% of predicted, respectively, was found in 13.8% and 27.9% of the patients. Values for DLCO and DL/VA were below normal in 45.0% and 27.9% of patients, respectively. The median 6-min walking distance was 463 m (IQR: 357–540).
Table 2.
Pulmonary function at follow up
| Pulmonary function | Mean ± SD | Percentage with < 80% predicted |
|---|---|---|
| FEV1% predicted (n = 123) | 90.1 ± 19.4 | 25.2% |
| FVC % predicted (n = 123) | 93.3 ± 18.1 | 19.5% |
| FEV1/FVC (n = 123) | 0.77 ± 0.09 | (Value below 0.7) 13.8% |
| DLCO % predicted (n = 111) | 79.1 ± 20.4 | 45.0% |
| DLCO/VA % predicted (n = 111) | 92.1 ± 20.4 | 27.9% |
| TLC % predicted (n = 111) | 87.8 ± 14.6 | 27.0% |
FEV1, forced vital capacity in the first second; FVC, forced vital capacity, DLco, diffusion capacity of the lungs for carbon monoxide; VA, alveolar volume; TLC, total lung capacity.
Length of stay, symptoms and lung function
Using length of stay (LOS) as a proxy for the severity of the acute illness, patients were categorized as having short LOS (≤ 5 days) or long LOS (> 5 days). LOS was short for 47.7% of patients and long for 52.3% of patients. There was no significant correlation between LOS and age, body mass index or number of symptoms at follow-up. Women had on average shorter LOS than men (59.3% versus 39.2% with short LOS, p = 0.025). Lung function was significantly lower in patients with long LOS. FEV1% predicted was on average 95% for patients with short LOS and 86% for patients with long LOS (p = 0.008), FVC % predicted was on average 99% for patients with short LOS and 88% for patients with long LOS (p = 0.001), and DLCO % predicted was 84% for patients with short LOS and 75% for patients with long LOS (p = 0.01).
Discussion
Symptoms and self-rated health
This study shows that at follow-up 4 to 5 months after discharge there are several persistent symptoms in survivors of COVID-19 pneumonitis. Only 15% of the patients were asymptomatic at follow-up and approximately half of the patients reported at least two symptoms.
Fatigue (69%) along with respiratory symptoms such as dyspnea (48%), cough (35%) and phlegm (22%) was the most reported symptoms. This is in line with symptoms reported from other out-patient clinics in Europe at 2–3 months follow-up [12,13]. In an Italian study with 143 patients of whom 72.7% had signs of interstitial pneumonia, 53.1% complained of fatigue and 43.4% had a sensation of dyspnea two months after symptom onset [1].
In a larger Spanish study including 277 patients, the numbers were a bit lower with 34.8% of the patients reporting fatigue and 34.4% reporting dyspnea. However, only 65% of the patients in this study needed hospitalization [14].
Neurological complaints such as memory loss and attention loss were also frequently reported [3]. The term ‘Brain fog’ has been used to describe this cognitive disorder and is suggested to evolve from mechanism such as deconditioning or Posttraumatic Stress Disorder [15]. Although psychological sequelae are not reported in our study, a large Chinese study reported anxiety, depression and sleep disorder in up to one-quarter of the 1,733 patients who had been hospitalized with COVID-19 at 6-month follow-up [2].
This relatively high number of symptoms persisting at least four months after discharge is also reflected in the health-related quality of life scores reported in our study. The dimensions of pain and discomfort, usual activities and mobility being mostly affected and a median EuroQol Visual analogue scale of 75 suggestive of a large impact on quality of life several months after discharge. The EuroQol Visual analogue scale at 6 months in the large Chinese study by Huang was 80 [2], almost similar to the median score in our study.
The patients were only asked to report new symptoms and the nature and prevalence of the symptoms in comparison to the reported comorbidities, makes it less likely that the symptoms reported after COVID-19 would be a result of aggravation in premorbid conditions.
Most studies report self-rated health at a point in time after COVID-19, and therefore lack information on the burden of symptoms before the illness. The PCFS has been suggested to serve as a proxy that summarizes and monitors burden of symptoms over time, and our study points to a substantial effect of COVID-19 disease on the functional status 4–5 months after the acute infection. A limitation in this comparison is the risk of recall bias, as patients had to assess their functional status before onset of the acute illness several months earlier.
Pulmonary function
Abnormalities in diffusing capacity were the most common impairment in pulmonary function. The high number reported in our study (45%) might reflect that all patients included had signs of pneumonitis. In a small Chinese study, with a 30-day follow-up after discharge, 52.6% had a DLCO less than 80% of predicted and the reduction in DLCO was most pronounced for the severe cases [16]. Zhao et al. reported that in 55 patients, 3 months after discharge 25% had decreased diffusing capacity [17].
These findings are in concordance with the large Chinese study [2], which reported lung function parameters in a sample of 390 patients at six-month follow-up. In this study, DLCO was reduced to less than 80% of predicted in 22% of mild cases and 56% of the most severe cases. Not all patients had signs of COVID-19 pneumonitis and only 68% of patients required oxygen supplementation during admission with acute COVID-19.
In studies of patients with Acute Respiratory Distress Syndrome (ARDS), Middle East Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS) the persistence of low DLCO was the most common abnormality in pulmonary function testing with a decrement directly related to the severity of the illness [4,5,18,19]. We were not able to correlate lung function parameters to the severity of the acute illness, as we had only a few patients treated with CPAP or invasive mechanical ventilation, but not surprisingly, we found length of stay, a surrogate marker of severity, to be correlated to a reduced lung function. This is probably due to length of stay being a marker of more severe lung injury and a subsequent reduction in lung function at follow-up, but another possibility is that a low lung function at admission resulted in a protracted disease course. However, against this speaks that we found no association between LOS and reported lung disease at admission.
Our study has some limitations. This is a single-center study with a limited number of cases. Although all patients had a need of oxygen supplementation, only 3.9% received mechanical ventilation. Thus, our findings mainly apply to hospitalized patients with moderate COVID-19. Furthermore, the study was conducted during the first phase of the COVID-19 pandemic, reflected in a limited number of patients treated with CPAP and also very few receiving remdesivir (7.0%) or dexamethasone (5.5%), which might alter the outcome on short term as well as long term.
Other limitations of the study include the lack of information on symptom history before COVID-19 and lack of grading of symptom severity. Regarding the lung function values and the 6-min walking test, we have no information on values before the onset of the acute illness. However, the limited number of patients with a history of chronic lung disease makes it unlikely that the reduction in lung function parameters, especially DLCO, is due to chronic conditions.
In conclusion, we found that fatigue, respiratory symptoms, and cognitive symptoms are highly prevalent four to five months after hospitalization with severe COVID-19. The most affected lung function parameter was diffusing capacity with impairment in close to half of the patients. Self-reported functional impairment is often light to moderate, but significantly worse compared to status before COVID-19.
Acknowledgments
The authors thank Anne-Kirsten Midjord, Ulla Gundersen, Gerd Martinez, Anne Rasmussen, Fereba Masumi, Lisa Capion and Sofie Otterup Hjulmand at the outpatient clinic at Copenhagen University Hospital-Hvidovre for assistance with the study.
Biographies
Marie Vejen is a Senior Registrar at the Department of Respiratory Medicine at Hvidovre Hospital.
Ejvind Frausing Hansen is a Senior Consultant at the Department of Respiratory Medicine at Hvidovre Hospital. His field of research is Respiratory Failure.
Bakir Nabil Ibrahim Al-Jarah is a Senior Registrar at the Department of Respiratory Medicine at Hvidovre Hospital.
Casper Jensen is a Senior Registrar at the Department of Respiratory Medicine at Hvidovre Hospital.
Klaus Nielsen Jeschke is a Senior Consultant at the Department of Respiratory Medicine at Hvidovre Hospital. His field of research is Respiratory Failure.
Charlotte Suppli Ulrik is professor in pulmonary medicine and Head of the Respiratory Research Unit and Severe Asthma Clinic at Hvidovre Hospital.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- [1].Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group . Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–7. PMID: 32644129; PMCID: PMC7349096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021. Jan 16;397(10270):220–232. Epub 2021 Jan 8. PMID: 33428867; PMCID: PMC7833295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021. Apr;27(4):601–615. Epub 2021 Mar 22. PMID: 33753937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ahmed H, Patel K, Greenwood DC, et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: a systematic review and meta-analysis. J Rehabil Med. 2020. May 31;52(5):jrm00063. PMID: 32449782. [DOI] [PubMed] [Google Scholar]
- [5].Ong KC, Ng AW, Lee LS, et al. Pulmonary function and exercise capacity in survivors of severe acute respiratory syndrome. Eur Respir J. 2004. Sep;24(3):436–442. PMID: 15358703. [DOI] [PubMed] [Google Scholar]
- [6].Ngai JC, Ko FW, Ng SS, et al. The long-term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology. 2010. Apr;15(3):543–550. Epub 2010 Mar 19. PMID: 20337995; PMCID: PMC7192220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rabin R, de Charro F.. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001. Jul;33(5):337–343. PMID: 11491192. [DOI] [PubMed] [Google Scholar]
- [8].Klok FA, Boon GJAM, Barco S, et al. The post-COVID-19 functional status scale: a tool to measure functional status over time after COVID-19. Eur Respir J. 2020. Jul 2;56(1):2001494. PMID: 32398306; PMCID: PMC7236834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Graham BL, Steenbruggen I, Miller MR, et al. Standardization of spirometry 2019 update. An official American thoracic society and European respiratory society technical statement. Am J Respir Crit Care Med. 2019. Oct 15;200(8):e70–e88. PMID: 31613151; PMCID: PMC6794117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Graham BL, Brusasco V, Burgos F, et al. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J. 2017. Jan 3;49(1):1600016. Erratum in: Eur Respir J. 2018 Nov 22;52(5): PMID: 28049168. [DOI] [PubMed] [Google Scholar]
- [11].Puente-Maestu L, Palange P, Casaburi R, et al. Use of exercise testing in the evaluation of interventional efficacy: an official ERS statement. Eur Respir J. 2016. Feb;47(2):429–460. Epub 2016 Jan 21. PMID: 26797036. [DOI] [PubMed] [Google Scholar]
- [12].Arnold DT, Hamilton FW, Milne A, et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax. 2021. Apr;76(4):399–401. Epub 2020 Dec 3. PMID: 33273026; PMCID: PMC7716340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Garrigues E, Janvier P, Kherabi Y, et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020. Dec;81(6):e4–e6. Epub 2020 Aug 25. PMID: 32853602; PMCID: PMC7445491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Moreno-Pérez O, Merino E, Leon-Ramirez JM, et al. Post-acute COVID-19 syndrome. Incidence and risk factors: a Mediterranean cohort study. J Infect. 2021. Mar;82(3):378–383. Epub 2021 Jan 12. PMID: 33450302; PMCID: PMC7802523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kaseda ET, Levine AJ. Post-traumatic stress disorder: a differential diagnostic consideration for COVID-19 survivors. Clin Neuropsychol. 2020. Oct-Nov;34(7–8):1498–1514. Epub 2020 Aug 26. PMID: 32847484. [DOI] [PubMed] [Google Scholar]
- [16].Huang Y, Tan C, Wu J, et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir Res. 2020. Jun 29;21(1):163. PMID: 32600344; PMCID: PMC7323373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhao YM, Shang YM, Song WB, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020. Aug;25:100463. Epub 2020 Jul 15. PMID: 32838236; PMCID: PMC7361108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Luhr O, Aardal S, Nathorst-Westfelt U, et al. Pulmonary function in adult survivors of severe acute lung injury treated with inhaled nitric oxide. Acta Anaesthesiol Scand. 1998. Apr;42(4):391–398. PMID: 9563856. [DOI] [PubMed] [Google Scholar]
- [19].Hsieh MJ, Lee WC, Cho HY, et al. Recovery of pulmonary functions, exercise capacity, and quality of life after pulmonary rehabilitation in survivors of ARDS due to severe influenza A (H1N1) pneumonitis. Influenza Other Respir Viruses. 2018. Sep;12(5):643–648. Epub 2018 Jun 12. PMID: 29676537; PMCID: PMC6086854. [DOI] [PMC free article] [PubMed] [Google Scholar]


