Abstract
Different 2,4-thiazolidinedione-tethered coumarins 5a–b, 10a–n and 11a–d were synthesised and evaluated for their inhibitory action against the cancer-associated hCAs IX and XII, as well as the physiologically dominant hCAs I and II to explore their selectivity. Un-substituted phenyl-bearing coumarins 10a, 10 h, and 2-thienyl/furyl-bearing coumarins 11a–c exhibited the best hCA IX (KIs between 0.48 and 0.93 µM) and hCA XII (KIs between 0.44 and 1.1 µM) inhibitory actions. Interestingly, none of the coumarins had any inhibitory effect on the off-target hCA I and II isoforms. The sub-micromolar compounds from the biochemical assay, coumarins 10a, 10 h and 11a–c, were assessed in an in vitro antiproliferative assay, and then the most potent antiproliferative agent 11a was tested to explore its impact on the cell cycle phases and apoptosis in MCF-7 breast cancer cells to provide more insights into the anticancer activity of these compounds.
Keywords: Carbonic anhydrase inhibitors, 3-acetylcoumarin, apoptosis induction, anticancer agents, hypoxic tumours
1. Introduction
Carbonic anhydrases (CAs, EC 4.2.1.1) are ubiquitous metalloenzymes found in all living organisms and are responsible for the catalysis of the biologically crucial reversible hydration of carbon dioxide to bicarbonate and proton. This is a simple but pivotal physiological reaction which is essential for normal and pathological processes such as CO2 and pH homeostasis, respiration, gluconeogenesis, calcification, bone resorption, fluid secretion and tumorigenesis [1,2]. CAs are grouped into different families, amongst them α-CAs which are present in all vertebrates and are further sub-classified into fifteen isoforms (herein referred to as human CAs or hCAs) that differ by molecular features, expression levels, kinetic properties and cellular distribution in the different tissues [3]. Of note, only twelve hCAs are catalytically active (I–IV, VA, VB, VI, VII, IX, XII–XIV) with an active site containing three histidine residues in a triple coordination with a zinc ion [4]. Regarding the subcellular distribution of the catalytically active hCAs, they can be categorised into different subsets: cytosolic (I, II, III, VII and XIII), trans-membrane (IV, IX, XII, and XIV), mitochondrial (VA and VB), while VI is secreted in saliva and milk [5]. The over-expressed levels and/or dysfunctions of hCAs can lead to many disorders, hence CA inhibitors (CAIs) are utilised for the treatment of glaucoma (targeting hCA II, IV and XII), edoema (targeting hCA II, IV and XIV), mental disorders (targeting hCA II, VII and XIV) and obesity (targeting hCA VA and VB) [4,6,7].
It should be stressed that the trans-membranous hCA IX and XII are hypoxia-induced tumours-associated isozymes and overexpressed in most cancer cells compared to the normal ones [8]. While the overexpressed hCA IX isozyme is mainly linked to cancer poor prognosis and limited to hypoxic tumours, hCA XII can be found in some normal tissues like kidney and colon alongside the hypoxic tumours [9,10]. Interestingly, the tumour growth, angiogenesis, proliferation and metastasis are attributed to the overexpressed levels of hCA IX and XII suggesting a strategy for targeting of such enzymes as a new approach in cancer chemotherapy [8,11]. In this context, selective inhibition of the tumour-associated hCA IX and XII isozymes over the other isoforms, particularly the most prevalent cytosolic hCA I and II is highly desirable and will result in cancer treatment with fewer side effects [12].
In view of this, intensive efforts are being conducted for the development of hCA IX/XII selective inhibitors as a validated approach for cancer treatment [13–15]. hCA IX and XII can be inhibited by different strategies such as coordination to the zinc ion situated in the catalytic active site. Molecules in this class are exemplified by sulfonamide-derived hCAIs and their bioisosters. In addition, the occlusion of the catalytic active cleft is explored and this approach has been explored using coumarins as a newly discovered hCAIs class [16,17].
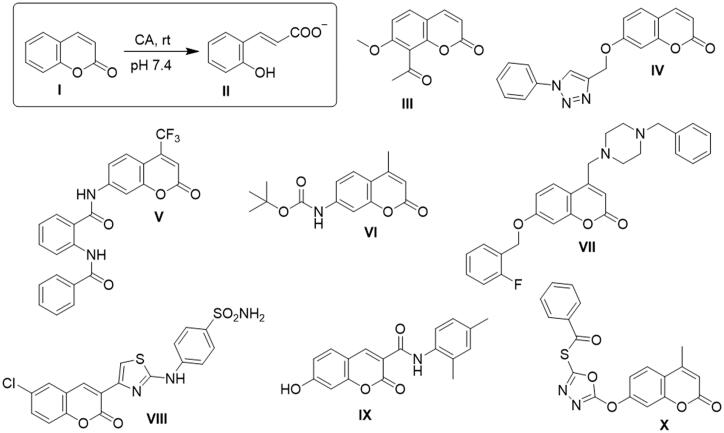
Coumarin I is a naturally-derived, privileged heterocyclic scaffold and molecules containing it show numerous biological properties such as inhibition of CK2, EGFR and PI3K-AKT-mTOR signalling. Moreover, coumarins are known to have anticoagulation, monoamine oxidase inhibition, anti-infective, antioxidant, anti-inflammatory and anticancer activities [14,18–20]. Coumarins are recently discovered as a novel class of hCAIs with inhibitory mechanism different from the sulfonamide-based inhibitors. Coumarinacts as prodrug undergoing hydrolysis by the esterase activity of CA to yield 2-hydroxycinnamic acid derivative II which can bind to the active site cleft occluding its entrance, Figure 1 [15,21]. Since coumarins binding sites are the most heterologous region of the active site between all CAs isoforms, it not surprising that these chemotypes displaying very high selectivity for specific CAs isoforms. Furthermore, the chemical simplicity of coumarins permits the facile incorporation of diverse substituents, leading to generation of a large number of derivatives with interesting biological profiles [22]. Consequently, many ongoing efforts have focussed on developing novel coumarin derivatives as selective hCAs IX/XII inhibitors that could be used for cancer therapy. For instance, diverse coumarins III-X have been reported as selective hCAs IX/XII inhibitors with nanomolar Ki, Figure 1 [8,10,13,17,21–24].
Figure 1.
Some reported coumarins acting as selective CA IX/XII inhibitors.
Inspired by these findings, we prepared a series of 2,4-thiazolidinedione-tethered coumarins, compounds 5a–b, 10a–n and 11a–d, and evaluated their inhibitory action against the cancer-associated hCAs IX and XII, and selectivity over inhibition of the physiologically dominant hCAs I and II to explore their selectivity in order to the cancer-related isoforms. Moreover, the efficient hCAs IX/XII inhibitors 10a, 10h and 11a–c were subjected to in vitro antiproliferative assay under hypoxic conditions and most potent antiproliferative agent 11a was tested to explore its impact on the cell cycle phases and apoptosis in MCF-7 breast cancer cells furnishing more insights on the anticancer activity of such compounds.
2. Experimental
2.1. Chemistry
2.1.1. General
The NMR spectra were recorded by Bruker 400 MHz spectrometer. 1H and 13 C spectra were run at 400 and 100 MHz, respectively, in deuterated dimethylsulphoxide (DMSO-d6) or deuterated triflouroacetic acid. All coupling constant (J) values are given in hertz. IR spectra were recorded with a Bruker FT-IR spectrophotometer. Reaction courses and product mixtures were routinely monitored by thin layer chromatography (TLC) on silica gel precoated F254 Merck plates. Unless otherwise mentioned, all reagents and solvents are commercially available and have been used without further purification. Compounds 2 and 3 are previously reported [25,26].
2.1.2. General procedures for synthesis of 3–(2-oxo-2–(2-oxo-2H-chromen-3-yl)ethyl)thiazolidine-2,4-dione derivatives (5a–b)
To a stirred solution of 3–(2-bromoacetyl)-2H-chromen-2-one 3a (0.27 g, 1.0 mmol) or 6-bromo-3–(2-bromoacetyl)-2H-chromen-2-one 3b (0.34 g, 1.0 mmol) in DMF (7 ml), thiazolidine-2,4-dione 4 (0.12 g, 1.0 mmol), anhydrous K2CO3 (0.28 g, 2.0 mmol) and KI (cat.) were added. The reaction mixture was heated on a water bath for 8h. then was poured over crushed ice. The precipitate was filtered, dried, and crystalsized from hot ethanol to give the corresponding key intermediates 5a-b, respectively.
2.1.2.1. 3–(2-Oxo-2–(2-oxo-2H-chromen-3-yl)ethyl)thiazolidine-2,4-dione 5a
Yellow crystals (yield, 80%); m. p. = 180–181 °C (reported: 161–163 °C [27]); 1H NMR (400 MHz, Trifluoroacetic acid-d1): δ 8.79 (s, 1H, H-4 coumarin moiety), 8.01 (d, J = 8.0 Hz, 1H, H-5 coumarin moiety), 7.81 (t, J = 7.2 Hz, 1H, H-7 coumarin moiety), 7.51 (d, J = 8.0 Hz, 1H, H-8 coumarin moiety), 7.46 (t, J = 7.6 Hz, 1H, H-6 coumarin moiety), 5.13 (s, 2H, CH2, –N–CH2–CO–), 4.10 (s, 2H, CH2, –S–CH2–CO–); 13 C NMR (101 MHz, Trifluoroacetic acid-d1) δ 189.47, 172.32, 159.16, 155.25, 149.25, 135.73, 131.67, 125.63, 122.60, 118.53, 116.75, 50.43 (–N–CH2–), 34.54 (–S–CH2–); Anal. Calcd. for C14H9NO5S (303.29): C, 55.44; H, 2.99; N, 4.62; Found: C, 55.68; H, 3.01; N, 4.56.
2.1.2.2. 3–(2-(6-Bromo-2-oxo-2H-chromen-3-yl)-2-oxoethyl)thiazolidine-2,4-dione 5b
Yellow crystals (yield, 75%); m. p.= 239–241 °C (reported: 171–173 °C [27]); 1H NMR (400 MHz, DMSO-d6) δ 8.59 (s, 1H, H-4 coumarin), 8.20 (s, 1H, H-5 coumarin), 7.88 (d, J = 8.8 Hz, 1H, H-7 coumarin), 7.44 (d, J = 8.8 Hz, 1H, H-8 coumarin), 5.14 (s, 2H, CH2), 4.11 (s, 2H, CH2); 13 C NMR (101 MHz, TFA-deuterated) δ 195.41, 158.46, 154.08, 146.09, 137.04, 132.10, 125.85, 120.52, 118.86, 116.82, 60.22 (–N–CH2–), 30.49 (–S–CH2–); Anal. Calcd. for C14H8BrNO5S (382.18): C, 44.00; H, 2.11; N, 3.66; Found: C, 43.85; H, 2.12; N, 3.69.
2.1.3. General procedures for preparation of the intermediates 8a–g and 9a–b
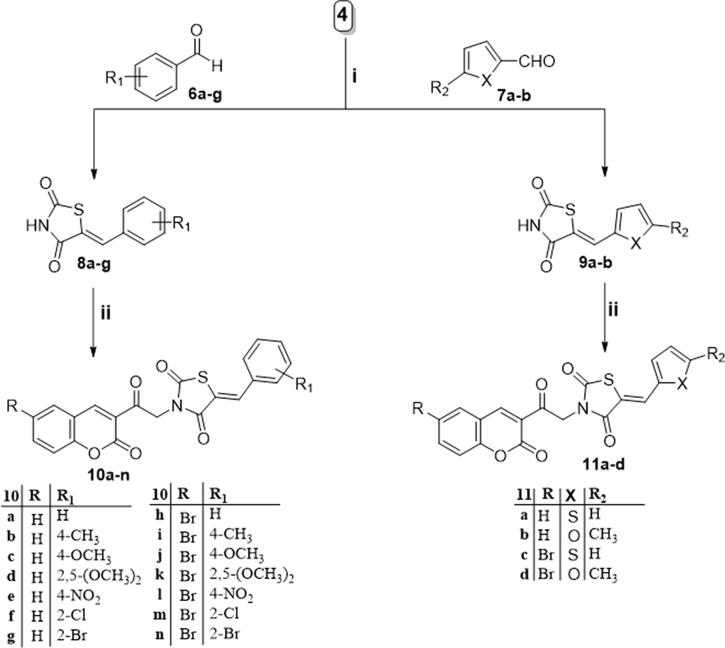
To a solution of thiazolidine-2,4-dione 4 (0.12 g, 1 mmol) in glacial acetic acid (5 ml), anhydrous sodium acetate (0.08 g, 1 mmol) and the appropriate aldehyde derivative (6a–g and 7a–b) were added. The resulting reaction mixture was allowed to stir under reflux for 3h. The precipitated solid was collected by filtration while hot, washed with cold ethanol and water, and dried to afford intermediates 8a–g and 9a–b.
2.1.4. General procedures for preparation of coumarins 10a–n and 11a–d
The appropriate benzylidine derivative 8a–g (2 mmol) was added to a hot stirred mixture of 3-(bromoacetyl)coumarin derivatives 3a–b (2 mmol), K2CO3 (0.55 g, 4 mmol), KI (2 mmol) in DMF (8 ml), then the resulting mixture was stirred under reflux for 8h. The formed precipitates were collected by filtration, washed with water, dried and recrystalslized from DMF/water to yield the final target coumarin-based CAIs 10a–n and 11a–d.
2.1.4.1. 5-Benzylidene-3–(2-oxo-2–(2-oxo-2H-chromen-3-yl)ethyl)thiazolidine-2,4-dione 10a
Grey crystals (yield, 70%); m. p. = 249–251 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.17 (s, 1H, ArH), 8.27 (s, 1H, –CH=), 8.01–7.97 (m, 2H, ArH), 7.70–7.61 (m, 7H, ArH), 5.64 (s, 2H, CH2, –N–CH2–CO–); 13 C NMR (101 MHz, TFA-deuterated) δ 173.27, 172.54, 168.52, 168.28, 153.27, 139.52, 139.52, 137.19, 132.00, 131.79, 131.19, 130.47, 129.01, 126.36, 118.57, 116.70, 115.75, 54.32 (–N–CH2–); Anal. Calcd. for C21H13NO5S (391.40): C, 64.44; H, 3.35; N, 3.58; Found: C, 64.71; H, 3.37; N, 3.52 [28].
2.1.4.2. 5–(4-Methylbenzylidene)-3–(2-oxo-2–(2-oxo-2H-chromen-3-yl)ethyl)thiazolidine-2,4-dione 10b
Yellow crystals (yield, 85%); m. p. = 227–229 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.61 (s, 1H, ArH), 8.04 (d, J = 8.0 Hz, 1H, ArH), 7.79 (t, J = 7.6 Hz, 1H, H-7 ArH), 7.72 (s, 1H, –CH=), 7.51 (d, J = 8.0 Hz, 1H, ArH), 7.47 (d, J = 8.0 Hz, 2H, ArH), 7.48 (t, J = 7.6 Hz, 1H, ArH), 7.33 (d, J = 8.0 Hz, 2H, ArH), 5.13 (s, 2H, CH2, –N–CH2–CO–), 2.41 (s, 3H, CH3); Anal. Calcd. for C22H15NO5S (405.42): C, 65.18; H, 3.73; N, 3.45; Found: C, 64.95; H, 3.76; N, 3.51.
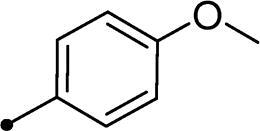
2.1.4.3. 5–(4-Methoxybenzylidene)-3–(2-oxo-2–(2-oxo-2H-chromen-3-yl)ethyl)thiazolidine-2,4-dione 10c
Yellow crystals (yield, 85%); m. p. = 257–259 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.16 (s, 1H, ArH), 8.24 (s, 1H, –CH=), 8.00–7.96 (m, 2H, ArH), 7.71 (d, J = 8.8 Hz, 2H, ArH), 7.65–7.61 (m, 2H, ArH), 7.23 (d, J = 8.8 Hz, 2H, ArH), 5.63 (s, 2H, CH2), 4.10 (s, 3H, OCH3); 13 C NMR (101 MHz, TFA-deuterated) δ 172.54, 168.38, 162.43, 162.00, 161.56, 161.13, 153.19, 139.03, 137.03, 132.98, 131.07, 126.39, 126.00, 118.52, 118.05, 116.05, 115.71, 112.90, 110.08, 54.90 (OCH3), 51.47 (–N–CH2–); Anal. Calcd. for C22H15NO6S (421.42): C, 62.70; H, 3.59; N, 3.32; Found: 62.88; H, 3.62; N, 3.26 [28].
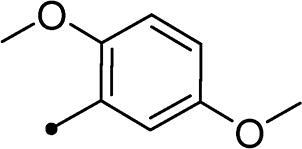
2.1.4.4. 5–(2,5-Dimethoxybenzylidene)-3–(2-oxo-2–(2-oxo-2H-chromen-3-yl)ethyl)thiazolidine-2,4-dione 10d
Grey crystals (yield, 82%); m. p. = 238–240 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.87 (s, 1H, ArH), 8.28 (s, 1H, –CH=), 7.76–7.73 (m, 2H, ArH), 7.42–7.39 (m, 2H, ArH), 7.08–6.93 (m, 3H, ArH), 5.36 (s, 2H, CH2), 3.84 (s, 3H, OCH3), 3.82 (s, 3H, OCH3); 13 C NMR (101 MHz, DMSO) δ 206.65, 189.23, 167.42, 165.61, 159.17, 153.66, 153.59, 152.95, 149.34, 135.63, 131.61, 129.04, 122.59, 122.52, 122.23, 121.89, 117.80, 116.65, 113.64, 56.31 (OCH3), 55.73 (OCH3), 50.67 (–N–CH2–); Anal. Calcd. for C23H17NO7S (451.45): C, 61.19; H, 3.80; N, 3.10; Found: 60.94; H, 3.83; N, 3.14.
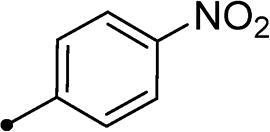
2.1.4.5. 5–(4-Nitrobenzylidene)-3–(2-oxo-2–(2-oxo-2H-chromen-3-yl)ethyl)thiazolidine-2,4-dione 10e
Yellow crystals (yield, 77%); m. p. = 263–265 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.84 (s, 1H, ArH), 8.36 (d, J = 8.8 Hz, 2H, ArH), 8.13 (s, 1H, –CH=), 8.00 (d, J = 8.4 Hz, 1H, ArH), 7.93 (d, J = 8.4 Hz, 2H, ArH), 7.86–7.79 (m, 2H, ArH), 7.44 (t, J = 8.0 Hz, 1H, ArH), 5.24 (s, 2H, CH2, –N–CH2–CO–); 13 C NMR (101 MHz, DMSO) δ 189.33, 165.32, 159.25, 155.31, 149.42, 148.23, 139.55, 135.84, 131.73, 131.65, 131.27, 128.47, 125.66, 124.72, 122.55, 118.57, 116.79, 50.99 (–N–CH2–); Anal. Calcd. for C21H12N2O7S (436.39): C, 57.80; H, 2.77; N, 6.42; Found: C, 58.03; H, 2.75; N, 6.49.
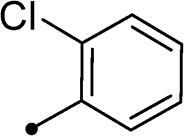
2.1.4.6. 5–(2-Chlorobenzylidene)-3–(2-oxo-2–(2-oxo-2H-chromen-3-yl)ethyl)thiazolidine-2,4-dione 10f
Yellow crystals (yield, 77%); m. p. = 208–211 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.84 (s, 1H, ArH), 8.06 (s, 1H, –CH=), 8.01 (d, J = 8.0 Hz, 1H, ArH), 7.84 (d, J = 8.0 Hz, 1H, ArH), 7.79 (d, J = 8.4 Hz, 1H, ArH), 7.71 (d, J = 8.0 Hz, 1H, ArH), 7.66–7.59 (m, 2H, ArH), 7.51 (d, J = 8.4 Hz, 1H, ArH), 7.44 (t, J = 8.4 Hz, 1H, ArH), 5.23 (s, 2H, CH2, –N–CH2–CO–); 13 C NMR (101 MHz, DMSO) δ 189.39, 167.21, 165.20, 159.24, 149.43, 135.84, 134.17, 133.07, 132.88, 132.04, 131.74, 129.69, 129.24, 125.79, 125.67, 125.19, 122.57, 118.56, 116.79, 55.39 (–N–CH2–); Anal. Calcd. for C21H12ClNO5S (425.84): C, 59.23; H, 2.84; N, 3.29; Found: C, 59.43; H, 2.82; N, 3.25.
2.1.4.7. 5–(2-Bromobenzylidene)-3–(2-oxo-2–(2-oxo-2H-chromen-3-yl)ethyl)thiazolidine-2,4-dione 10g
Yellow crystals (yield, 82%); m. p. = 230–232 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.15 (s, 1H, ArH), 8.64 (s, 1H, –CH=), 8.00–7.97 (m, 2H, ArH), 7.74–7.72 (m, 1H, ArH), 7.66–7.63 (m, 3H, ArH), 7.53–7.56 (m, 2H, ArH), 5.65 (s, 2H, CH2, –N–CH2–CO–); 13 C NMR (101 MHz, TFA-deuterated) δ 190.49, 172.12, 167.88, 163.26, 162.48, 162.04, 161.61, 161.17, 155.14, 136.60, 130.43, 121.61, 119.96, 118.49, 118.05, 115.68, 112.86, 110.05, 50.72 (–N–CH2–); Anal. Calcd. for C21H12BrNO5S (470.29): C, 53.63; H, 2.57; N, 2.98; Found: C, 53.81; H, 2.58; N, 3.01
2.1.4.8. 5-Benzylidene-3–(2-(6-bromo-2-oxo-2H-chromen-3-yl)-2-oxoethyl)thiazolidine-2,4-dione 10h
Yellow crystals (yield, 78%); m. p. = 252–254 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.60 (s, 1H, ArH), 8.21 (d, J = 2.4 Hz, 1H, ArH), 7.87 (d, J = 7.6 Hz, 1H, ArH), 7.78 (s, 1H, –CH=), 7.43–7.61 (m, 6H, ArH), 5.14 (s, 2H, CH2, –N–CH2–CO–); 13 C NMR (101 MHz, DMSO) δ 168.61, 168.30, 146.11, 137.06, 134.29, 133.60, 133.42, 133.01, 131.94, 130.81, 130.45, 129.78, 125.88, 124.43, 120.54, 118.88, 117.04, 116.83, 50.83(–N–CH2–); Anal. Calcd. for C21H12BrNO5S (470.29): C, 53.63; H, 2.57; N, 2.98; Found: C, 53.78; H, 2.56; N, 3.00.
2.1.4.9. 3–(2-(6-Bromo-2-oxo-2H-chromen-3-yl)-2-oxoethyl)-5–(4-methylbenzylidene)thiazolidine-2,4-dione 10i
Yellow crystals (yield, 86%); m. p. = 224–225 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.60 (s, 1H, ArH), 8.20 (s, 1H, ArH), 7.86 (d, J = 8.8 Hz, 1H, ArH), 7.78 (s, 1H, –CH=), 7.47 (d, J = 8.0 Hz, 2H, ArH), 7.42 (d, J = 8.8 Hz, 1H, ArH), 7.33 (d, J = 8.0 Hz, 2H, ArH), 5.13 (s, 2H, –N–CH2–CO–), 2.35 (s, 3H, CH3); Anal. Calcd. for C22H14BrNO5S (484.32): C, 54.56; H, 2.91; N, 2.89; Found: C, 54.75; H, 2.89; N, 2.90.
2.1.4.10. 3–(2-(6-Bromo-2-oxo-2H-chromen-3-yl)-2-oxoethyl)-5–(4-methoxybenzylidene)thiazolidine-2,4-dione 10j
Grey crystals (yield, 72%); m. p. = 245–247 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.60 (s, 1H, ArH), 8.21 (d, J = 2.4 Hz, 1H, ArH), 7.87 (d, J = 8.0 Hz, 1H, ArH), 7.73 (s, 1H, –CH=), 7.54 (d, J = 8.8 Hz, 2H, ArH), 7.43 (d, J = 8.0 Hz, 1H, ArH), 7.08 (d, J = 8.8 Hz, 2H, ArH), 5.20 (s, 2H, –N–CH2–CO–), 3.82 (s, 3H, OCH3); Anal. Calcd. for C22H14BrNO6S (500.32): C, 52.81; H, 2.82; N, 2.80; Found: C, 53.04; H, 2.83; N, 2.80.
2.1.4.11. 3–(2-(6-Bromo-2-oxo-2H-chromen-3-yl)-2-oxoethyl)-5–(2,5 -dimethoxybenzylidene) thiazolidine-2,4-dione 10k
Yellow crystals (yield, 78%); m. p. = 204–206 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.59 (s, 1H, ArH), 8.21 (d, J = 2.4 Hz, 1H, ArH), 7.90 (s, 1H, –CH=), 7.87 (d, J = 8.0 Hz, 1H, ArH), 7.42 (d, J = 8.0 Hz, 1H, ArH), 7.08–7.07 (m, 2H, ArH), 6.91 (d, J = 8.0 Hz, 1H, ArH), 5.19 (s, 2H, –N–CH2–CO–), 3.83 (s, 3H, OCH3), 3.75 (s, 3H, OCH3); 13 C NMR (101 MHz, TFA-deuterated) δ 162.46, 162.02, 161.74, 161.59, 161.15, 154.65, 152.12, 134.08, 122.06, 120.32, 118.49, 115.74, 115.68, 113.18, 112.86, 110.05, 56.32, 55.38. Anal. Calcd. for C23H16BrNO7S (530.35): C, 52.09; H, 3.04; N, 2.64; Found: C, 51.89; H, 3.07; N, 2.63.
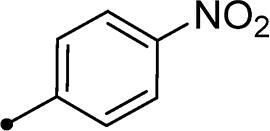
2.1.4.12. 3–(2-(6-Bromo-2-oxo-2H-chromen-3-yl)-2-oxoethyl)-5–(4- nitrobenzylidene)thiazolidine-2,4-dione 10 l
Yellow crystals (yield, 82%); m. p. = 229–231 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.77 (s, 1H, ArH), 8.38 (s, 1H, ArH), 8.32 (d, J = 8.8 Hz, 2H, ArH), 7.93 (dd, J = 8.8, 2.0 Hz, 1H, ArH), 7.88 (s, 1H, –CH=), 7.84 (d, J = 8.8 Hz, 2H, ArH), 7.49 (d, J = 8.8 Hz, 1H, ArH), 5.13 (s, 2H, –N–CH2–CO–); 13 C NMR (101 MHz, DMSO) δ 189.24, 168.05, 167.94, 166.89, 165.28, 158.81, 154.31, 148.23, 147.88, 139.95, 139.52, 131.65, 131.35, 129.28, 128.90, 125.63, 124.73, 123.59, 120.40, 117.06, 50.98. Anal. Calcd. for C21H11BrN2O7S (515.29): C, 48.95; H, 2.15; N, 5.44; Found: C, 49.11; H, 2.14; N, 5.48.
2.1.4.13. 3–(2-(6-Bromo-2-oxo-2H-chromen-3-yl)-2-oxoethyl)-5–(2-chlorobenzylidene)thiazolidine-2,4-dione 10m
Yellow crystals (yield, 77%); m. p. = 192–194 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.65 (s, 1H, ArH), 8.12 (s, 1H, ArH), 8.05 (d, J = 8.8 Hz, 1H, ArH), 7.75–7.51 (m, 6H, ArH), 5.64 (s, 2H, –N–CH2–CO–); 13 C NMR (101 MHz, TFA-deuterated) δ 162.52, 162.09, 161.66, 161.22, 151.67, 139.52, 138.48, 135.27, 133.00, 132.66, 132.42, 130.36, 128.68, 127.03, 119.33, 118.51, 115.69, 112.88, 110.07. Anal. Calcd. for C21H11BrClNO5S (504.74): C, 49.97; H, 2.20; N, 2.78; Found: C, 50.13; H, 2.21; N, 2.80.
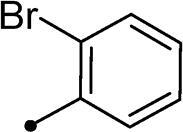
2.1.4.14. 3–(2-(6-Bromo-2-oxo-2H-chromen-3-yl)-2-oxoethyl)-5–(2-bromobenzylidene)thiazolidine-2,4-dione 10n
Yellow crystals (yield, 72%); m. p. = 208–210 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.61 (s, 1H, ArH), 8.22 (s, 1H, ArH), 7.87 (d, J = 8.8 Hz, 1H, ArH), 7.79 (s, 1H, –CH=), 7.51–7.59 (m, 3H, ArH), 7.43 (d, J = 8.8 Hz, 1H, ArH), 7.34 (t, J = 8.0 Hz, 1H, ArH), 5.14 (s, 2H, –N–CH2–CO–); 13 C NMR (101 MHz, DMSO) δ 207.46, 158.94, 158.47, 154.43, 154.09, 146.11, 137.06, 134.21, 134.16, 133.92, 133.01, 131.75, 129.88, 129.61, 129.39, 129.23, 128.93, 120.54, 118.88, 116.83, 30.50. Anal. Calcd. for C21H11Br2NO5S (549.19): C, 45.93; H, 2.02; N, 2.55; Found: C, 46.09; H, 2.03; N, 2.53.
2.1.5. General procedures for preparation of target coumarins 11a–d
5-(Thiophen-2-ylmethylene)thiazolidine-2,4-dione 7a (15 mmol) and/or 5-((5-methylfuran-2-yl)methylene)thiazolidine-2,4-dione 7b (15 mmol) was added to a hot stirred solution of 3-(bromoacetyl) coumarin derivatives 3a,b (15 mmol) in DMF (10 ml), K2CO3 (15 mmol), KI (15 mmol), then the resulting mixture was stirred under reflux for 8h. The precipitates were collected by filtration, washed with water, dried and recrystalslized from hexane/ethanol to yield the final target compounds 11a-d.
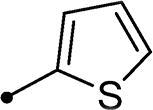
2.1.5.1. 3–(2-Oxo-2–(2-oxo-2H-chromen-3-yl)ethyl)-5-(thiophen-2-ylmethylene)thiazolidine-2,4-dione 11a
Yellow crystals (yield, 83%); m. p. = 250–251 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.13 (s, 1H, ArH), 8.44 (s, 1H, –CH=), 7.97–7.91 (m, 3H, ArH), 7.68–7.61 (m, 3H, ArH), 7.33 (t, J = 4.8 Hz, 1H, ArH), 5.62 (s, 2H, CH2, –N–CH2–CO–); 13 C NMR (101 MHz, TFA-deuterated) δ 190.74, 162.45, 162.02, 161.58, 161.14, 153.33, 137.00, 136.28, 134.41, 131.79, 131.08, 128.63, 126.38, 119.93, 118.48, 115.67, 112.85, 110.04, 50.74. Anal. Calcd. for C19H11NO5S2 (397.42): C, 57.42; H, 2.79; N, 3.52; Found: C, 57.26; H, 2.78; N, 3.55.
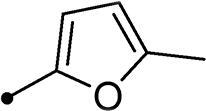
2.1.5.2. 5-((5-Methylfuran-2-yl)methylene)-3–(2-oxo-2–(2-oxo-2H- chromen-3-yl)ethyl)thiazolidine-2,4-dione 11b
Yellow crystals (yield, 70%); m. p. = 227–229 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.12 (s, 1H, ArH), 7.96–7.90 (m, 3H, –CH = and ArH), 7.62–7.60 (m, 2H, ArH), 7.09 (d, J = 4.0 Hz, 1H, ArH), 6.40 (d, J = 4.0 Hz, 1H, ArH), 5.60 (s, 2H, CH2, –N–CH2–CO–), 2.54 (s, 3H, CH3); 13 C NMR (101 MHz, TFA-deuterated) δ 162.45, 162.02, 161.57, 161.14, 153.32, 147.87, 136.96, 131.05, 126.35, 124.21, 118.47, 118.02, 116.58, 115.66, 112.84, 110.55, 110.03, 110.03, 50.50, 12.05; Anal. Calcd. for C20H13NO6S (395.39): C, 60.76; H, 3.31; N, 3.54; Found: C, 60.92; H, 3.34; N, 3.51.
2.1.5.3. 3–(2-(6-Bromo-2-oxo-2H-chromen-3-yl)-2-oxoethyl)-5-(thiophen-2-ylmethylene)thiazolidine-2,4-dione 11c
Yellow crystals (yield, 76%); m. p. = 255–257 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.60 (s, 1H, ArH), 8.27 (d, J = 2.4 Hz, 1H, ArH), 8.04 (s, 1H, –CH=), 7.99 (d, J = 5.2 Hz, 1H, ArH), 7.93 (dd, J = 8.8, 2.4 Hz, 1H, ArH), 7.66 (d, J = 8.0 Hz, 1H, ArH), 7.48 (d, J = 3.6 Hz, 1H, ArH), 7.27 (t, J = 3.6 Hz, 1H, ArH), 5.20 (s, 2H, –N–CH2–CO–); 13 C NMR (101 MHz, DMSO) δ 189.40, 167.97, 167.84, 165.35, 158.79, 147.90, 146.11, 137.84, 137.41, 136.00, 134.92, 133.42, 129.39, 127.63, 125.40, 121.86, 120.42, 117.04, 50.93. Anal. Calcd. for C19H10BrNO5S2 (476.32): C, 47.91; H, 2.12; N, 2.94; Found: C, 47.79; H, 2.13; N, 2.96.
2.1.5.4. 3–(2-(6-Bromo-2-oxo-2H-chromen-3-yl)-2-oxoethyl)-5-((5-methylfuran-2-yl)methylene)thiazolidine-2,4-dione 11d
Yellow crystals (yield, 79%); m. p. = 236–238 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.60 (s, 1H, ArH), 8.22 (d, J = 2.4 Hz, 1H, ArH), 7.99 (dd, J = 8.8, 2.4 Hz, 1H, ArH), 7.74 (s, 1H, –CH=), 7.43 (d, J = 8.8 Hz, 1H, ArH), 6.99 (d, J = 4.0 Hz, 1H, ArH), 6.39 (d, J = 4.0 Hz, 1H, ArH), 5.17 (s, 2H, –N–CH2–CO–), 2.38 (s, 3H, CH3); 13 C NMR (101 MHz, DMSO) δ 189.51, 169.37, 167.80, 157.74, 148.41, 147.87, 146.11, 137.80, 137.06, 133.40, 133.01, 122.00, 120.69, 119.25, 119.05, 118.80, 111.03, 110.72, 50.58, 14.22. Anal. Calcd. for C20H12BrNO6S (474.28): C, 50.65; H, 2.55; N, 2.95; Found: C, 50.52; H, 2.57; N, 2.98.
2.2. Biological evaluation
The experimental procedures for CA stopped-flow [29–31], MTT cell viability [32,33], cell cycle [34] and Annexin V-FITC/PI [35] assays are included in the Supporting Information.
3. Results and discussion
3.1. Chemistry
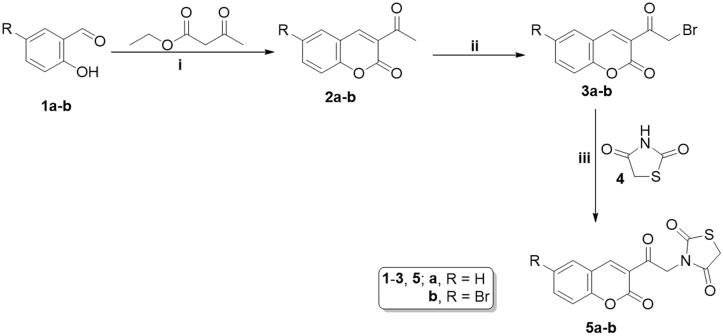
The proposed synthetic routes to obtain the target coumarins are depicted in Scheme 1 and Scheme 2. First, condensation of 2-hydroxybenzaldehydes 1a–b with ethyl 3-oxobutanoate in refluxing absolute ethanol in the presence of a few drops of piperidine yielded 3-acetylcoumarins 2a–b. These were subjected to bromination via reaction with Br2 in glacial acetic acid to yield the key 3-(bromoacetyl)coumarin intermediates 3a–b, which were subsequently treated with thiazolidine-2,4-dione 4 in refluxing DMF using anhydrous K2CO3 as base and KI as a nucleophilic catalyst to afford 3–(2-oxo-2–(2-oxo-2H-chromen-3-yl)ethyl)thiazolidine-2,4-dione derivatives 5a–b (Scheme 1).
Scheme 1.
Reagents and conditions: (i) Abs. Ethanol, piperidine, reflux, 2 h.; (ii) bromine 99%, glacial acetic acid, r.t., 6 h.; (iii) anhydrous DMF, potassium carbonate, potassium iodide, heating on a water bath, 8 h.
Scheme 2.
Reagents and conditions: (i) glacial acetic acid, reflux 3 h.; (ii) DMF, potassium carbonate, potassium iodide, reflux 8 h.
Synthesis of compounds 8a–g and 9a–b (Scheme 2) was achieved via refluxing of thiazolidine-2,4-dione 4 with benzaldehyde derivatives 6a–g and 7a–b in glacial acetic acid and anhydrous sodium acetate. Treatment of 8a–g and 9a–b with the key intermediates 3a–b in refluxing DMF using anhydrous K2CO3 and KI furnished the corresponding final targets 5-benzylidene-3–(2-oxo-2–(2-oxo-2H-chromen-3-yl)ethyl)thiazolidine-2,4-dione 10a–n and 11a–d, respectively. Proposed structures for the synthesised coumarins were in agreement with their various spectroscopic and analytical data.
3.2. Carbonic anhydrase inhibition
The inhibitory influence of all the synthesised coumarins 5a–b, 10a–n and 11a–d was investigated against hCA I, II IX and XII isoforms using a stopped flow CO2 hydrase assay and a well-known hCAI, acetazolamide (AAZ) as control [36]. From the resulting inhibition constants (KI) shown in Table 1, certain structure activity relationship (SAR) can be inferred.
Table 1.
Inhibition data for hCA I, II, IX and XII isoforms with 2,4-thiazolidinedione-tethered coumarins (5a–b, 10a–n and 11a–d) and AAZ. 
| Cmpd | R1 | R2 |
KI (μM)a,b |
|||
|---|---|---|---|---|---|---|
| CA I | CA II | CA IX | CA XII | |||
| 5a | H | – | >100 | >100 | 0.12 | 0.15 |
| 5b | Br | – | >100 | >100 | 0.24 | 0.31 |
| 10a | H |
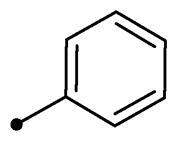
|
>100 | >100 | 0.82 | 0.75 |
| 10b | H |
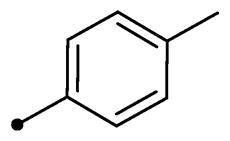
|
>100 | >100 | 4.3 | 4.0 |
| 10c | H |
|
>100 | >100 | 5.8 | 4.5 |
| 10d | H |
|
>100 | >100 | 8.4 | 6.2 |
| 10e | H |
|
>100 | >100 | 12.3 | 8.0 |
| 10f | H |
|
>100 | >100 | 2.2 | 3.8 |
| 10g | H |

|
>100 | >100 | 2.3 | 4.1 |
| 10h | Br |
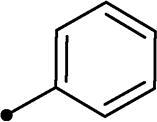
|
>100 | >100 | 0.93 | 0.87 |
| 10i | Br |
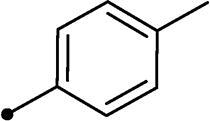
|
>100 | >100 | 6.2 | 2.3 |
| 10j | Br |
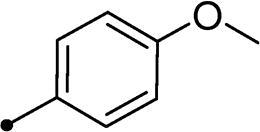
|
>100 | >100 | 8.9 | 4.9 |
| 10k | Br |
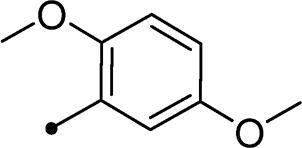
|
>100 | >100 | 16.4 | 6.6 |
| 10l | Br |
|
>100 | >100 | 18.2 | 10.4 |
| 10m | Br |

|
>100 | >100 | 2.9 | 3.2 |
| 10n | Br |
|
>100 | >100 | 3.4 | 2.8 |
| 11a | H |
|
>100 | >100 | 0.48 | 0.83 |
| 11b | H |
|
>100 | >100 | 0.79 | 1.1 |
| 11c | Br |
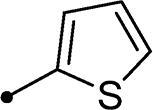
|
>100 | >100 | 0.59 | 0.44 |
| 11d | Br |
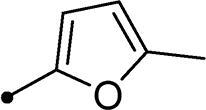
|
>100 | >100 | 0.91 | 0.82 |
| AAZ | – | – | 250 | 12.5 | 25.0 | 5.7 |
aMean from 3 different assays, by a stopped flow technique (errors were in the range of ± 5–10% of the reported values); bincubation time of 6 h.
Coumarins 5a–b, 10a–n and 11a–d are devoid of significant inhibition towards the off-target ubiquitous hCA I and the physiologically dominant hCA II (KIs > 100 µM) isoforms, Table 1. In contrast, these coumarins inhibited the cancer-related hCA IX with inhibition constants spanning a range between 0.12 and 18.2 µM, (Table 1). Regarding the unsubstituted thiazolidinedione-bearing coumarins 5a–b, the absence of substitution at 6-position of coumarin scaffold furnished the most effective hCA IX inhibitor in this work displaying KI of 0.12 µM, whereas, the 6-bromination decreased the hCA IX inhibitory power 2-folds (KI = 0.24 µM).
In the context of hCA IX inhibition constants of the benzylidene counterparts 10a–n, it was found that 6-unsubsituted coumarins 10a–g showed effective inhibition (KIs ranged from 0.82 to 12.3 µM) compared to the 6-bromo analogues 10h–n (KIs spanned between 0.93 and 18.2 µM). In the term of coumarins with the benzylidene moiety, 10a–g, it is worth stressing that appending an unsubstituted aryl ring to the thiazolidinedione moiety 10a provided the most effective hCA IX inhibitor within this series (KI = 0.82 µM). Indeed, the incorporation of ortho-chloro or ortho-bromophenyl (10f and 10 g, respectively) decreased the inhibition constants to low micromolar values (KIs = 2.2 and 2.3 µM, respectively). Regrettably, the remaining phenyl substitution pattern were similarly poorer hCA IX inhibitors with KIs equalling 4.3 µM (p-methyl, 10b), 5.8 µM (p-methoxy, 10c), 8.4 µM (2,5-dimethoxy, 10d), 12.3 µM (p-nitro, 10e).
In a similar fashion, the appending of unsubstituted aryl ring to the 6-bromocoumarins 10h–n produced the most potent hCA IX inhibitor within this series (10h; KI = 0.93 µM), whereas ortho chlorination or bromination reduced the inhibition constants to low micromolar values (10m and 10n; KIs = 2.9 and 3.4 µM, respectively). Similar to the 6-unsubstituted coumarins 10a-g, it was noted that in the 6-bromocoumarin series, inclusion of other phenyl substituents(compounds10h–n) lowered the hCA IX inhibition constants showing KIs in the range 6.2–18.2 µM. Superiorly, the replacement of six-membered phenyl with five-membered 2-thienyl ring potentially elevated the inhibition constants for both 6-unsubstituted and 6-bromocoumarins (11a and 11c; KIs = 0.48 and 0.59 µM, respectively) compared to their phenyl counterparts (10a and 10h; KIs = 0.82 and 0.93 µM, respectively). Notably, utilising 2-furyl functionality in place of phenyl/thienyl group did not result in significant change in potency (11b and 11d; KIs = 0.79 and 0.91 µM, respectively).
Collectively, the deduced SAR for hCA IX inhibition suggests that applying of unsubstituted thiazolidinedione (5a–b) is more favoured than their substituted analogues (10a–n and 11a–d) affording the most potent hCA IX inhibitors in this study. Furthermore, the lack of substitution at 6-position of coumarin (5a, 10a–g and 11a–b) is more advantageous for such type of activity relative to 6-bromo counterparts (5b, 10h–n and 11c–d). Additionally, appending of unsubstituted phenyl ring (10a, 10h) is the most beneficial pattern within all tested benzylidene counterparts (10a–n), while replacement of phenyl with 2-thienyl moiety gave the most potent hCA IX inhibitors (11a and 11c) within all arylidene derivatives (10a–n and 11a–d).
Finally, the inhibition profiles (Table 1) revealed that the cancer-related hCA XII isoform was inhibited by the coumarins 5a–b, 10a–n and 11a–d displaying a range of inhibition constants from submicromolar level to low micromolar values (KIs ranged from 0.15 to 10.4 µM). The unsubstituted thiazolidinedione-bearing coumarins 5a-b emerged as the most effective hCA XII inhibitors, with submicromolar inhibition constants (5a; KI = 0.15 µM and 5b; KI = 0.31 µM).
It should be pointed out that bromination at 6-position of coumarin 5b led to 2-fold diminished inhibition for hCA XII relative to the unsubstituted analogue 5a, in a similar manner observed in the SAR for hCA IX inhibition, Table 1. Concerning the benzylidene derivatives 10a–n, it was observed that appending a phenyl to thiazolidinedione moiety resulted in the most potent hCA XII inhibitors (at submicromolar level) within this series (10a; KI = 0.75 µM and 10h; KI = 0.87 µM). The incorporation of different substituents to the phenyl group reduced the inhibitory potential affording KIs spanning between 2.3 and 10.4 µM. Furthermore, the absence of substitution at 6-position of coumarin is beneficial for inhibition (10a; KI = 0.75 µM), whereas 6-bromination decreased the activity (10h; KI = 0.87 µM), Table 1. It was noted that replacement of the phenyl group (10a; KI = 0.75 µM and 10h; KI = 0.87 µM) with 2-thienyl or 2-furyl functionalities reduced KIs for the 6-unsubstituted coumarins (11a; KI = 0.83 µM and 11b; KI = 1.1 µM, respectively), while raised KIs for the 6-bromocoumarins (11c; KI = 0.44 µM and 11d; KI = 0.82 µM, respectively). This is unlike the pattern in hCA IX inhibition profile and points to a potential future avenue of exploration towards selectivity of hCA XII over hCA IX.
To summarise, the elicited SAR highlighted that unsubstituted thiazolidinedione derivatives (5a–b) exerted more superior potency relative to their substituted counterparts (10a–n and 11a–d) resulting in the most potent hCA XII inhibitors in this work (5a-b). Moreover, within all benzylidene derivatives 10a–n, the unsubstituted phenyl counterparts exerted the best inhibition profiles (10a and 10h), however the replacement of phenyl with 2-thienyl or 2-furyl along with 6-bromination at coumarin scaffold potentiated the inhibitory impact of compounds (11c and 11d). Overall, the herein reported coumarins emerge as selective inhibitors towards the tumour-related hCA IX and XII over the off-target hCA I and II that suggests their use as promising candidates for the development of more potent, selective hCA IX and XII inhibitors as anticancer agents.
3.3. Anticancer activity
3.3.1. In vitro antiproliferative activity against MCF-7 breast cancer cell line
The antiproliferative action of the most potent and selective hCA IX/XII inhibitors 10a, 10h and 11a–d was assessed against MCF-7 breast cancer cell line, since the overexpression of hCA IX is well-reported to be associated with poor prognosis of breast cancer [37] and the cell line has been previously used as a model in CA medicinal chemistry investigations. The antiproliferative potential was investigated using MTT assay [38] under hypoxic conditions employing staurosporine as a reference anticancer drug. The results are presented in Table 2 as median inhibitory concentration (IC50) which denotes the concentration of the tested drug required to produce 50% growth inhibition of the cancer cell compared to the negative control.
Table 2.
Anti-proliferative activities of 2,4-thiazolidinedione-tethered coumarins 10a, 10 h and 11a–c against MCF-7 cell line.
| Compound | IC50 (μM)a (MCF-7) |
|---|---|
| 10a | 3.13 ± 0.18 |
| 10h | 11.1 ± 0.65 |
| 11a | 0.48 ± 0.03 |
| 11b | 4.14 ± 0.24 |
| 11c | 9.56 ± 0.56 |
| 11d | 1.65 ± 0.1 |
| Staurosporine | 2.44 ± 0.14 |
aIC50 values are the mean ± SD of three experiments.
Investigation of the antiproliferative effects towards MCF-7 breast cancer cell line confirmed that the tested coumarins 10a, 10h and 11a–c exhibited moderate to excellent growth inhibitory influence (IC50 ranged between 0.48 and 11.1 µM). Of special interest, the 2-thienyl-bearing 6-unsubstitued coumarin 11a, that displayed potent hCA IX/XII inhibition at submicromolar level, exerted excellent antiproliferative action at submicromolar value (IC50 equals 0.48 µM). Likewise, the other tested coumarins 10a, 10h, 11b–d demonstrated moderate growth inhibitory action with IC50 values equal 3.13, 11.1, 4.14, 9.56 and 1.65 µM, respectively compared to staurosporine as reference drug (IC50 = 2.44 µM), Table 2.
3.3.2. Cell cycle analysis
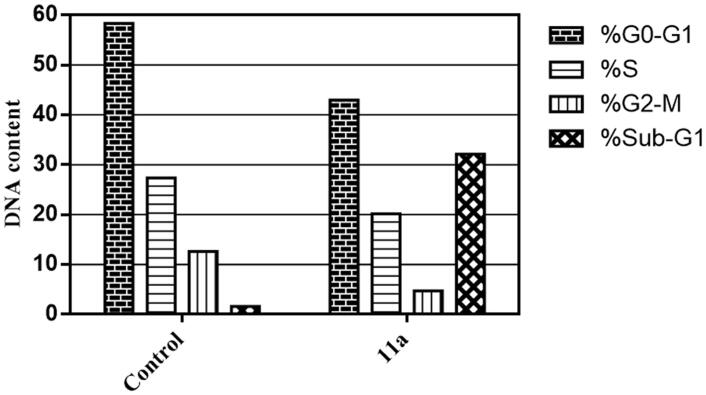
The influence of 2-thienyl-bearing 6-unsubstitued coumarin 11a on the cell cycle progression was investigated by flow cytometric in MCF-7 breast cancer cells, at 24h following treatment at its IC50 value (0.48 ± 0.03 µM), Figure 2.
Figure 2.
Impact of the tested coumarin 11a on the progression of cell cycle of MCF-7 cells.
As illustrated in Figure 2, the flow cytometric results showed that the exposure of MCF-7 breast cancer cells to compound 11a gave rise to a significant rise in the cell populations at Sub-G1, which increased by 19.7 folds with concomitant decrease in G2-M phase by 2.6 folds compared to the control, in addition to decline in cell populations within S and G0-G1 phases. This observation strongly suggests coumarin 11a induces apoptosis in MCF-7 cells.
3.3.3. Annexin V-FITC/propidium iodide (AV/PI) apoptosis assay
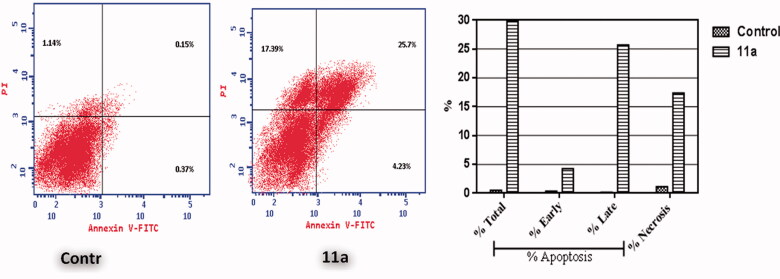
Annexin V-FITC/propidium iodide (AnxV/PI) dual staining assay was employed to confirm the potential apoptotic impact of coumarin 11a on early and late apoptosis percentages in MCF-7 breast cancer cells (Figure 3 and Table 3). This flow cytometric analysis highlighted that compound 11a was able to induce apoptosis in MCF-7 cells as indicated by the significant elevation in the percentage of annexin V-FITC-stained apoptotic cells including early apoptosis (Figure 3, lower right) from 0.37 to 4.23% and late apoptosis, Figure 3, upper right) from 0.15 to 25.7%. This represents 57 folds total increase relative to the control in apoptotic cells.
Figure 3.
Effect of coumarin 11a on the percentage of AV positive staining in breast MCF-7 cells.
Table 3.
Distribution of AV-FITC/PI positive stained apoptotic MCF-7 cells.
| Comp. | Apoptosis |
Necrosis | ||
|---|---|---|---|---|
| Total | Early | Late | ||
| 11a | 29.93 | 4.23 | 25.7 | 17.39 |
| Control | 0.52 | 0.37 | 0.15 | 1.14 |
4. Conclusions
In this study, different 2,4-thiazolidinedione-tethered coumarins 5a–b, 10a–n and 11a–d have been synthesised and evaluated for their inhibitory action against the cancer-associated hCAs IX and XII, in addition to the physiologically dominant hCAs I and II, in order to explore their selectivity. Interestingly, none of the coumarins had any inhibitory effect on off-target hCA I and II isoforms. Unsubstituted phenyl-bearing coumarins 10a, 10h, and 2-thienyl/furyl-bearing coumarins 11a–c exhibited the best hCA IX (KIs between 0.48 and 0.93 µM) and hCA XII (KIs between 0.44 and 1.1 µM) inhibitory actions. Coumarins 10a, 10h and 11a–c were subjected to an in vitro antiproliferative assay, and then the most potent antiproliferative agent 11a was tested to explore its impact on the cell cycle phases and apoptosis in MCF-7 breast cancer cells furnishing more insights on the potential anticancer activity of such compounds.
Supplementary Material
Funding Statement
This work was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University, through the Research Groups Program Grant no [RGP-1440 − 0025](2). The work was also financed by the Italian Ministry for Education and Science (MIUR), grant PRIN: rot. 2017XYBP2R, and by Ente Cassa di Risparmio di Firenze (ECRF), grant [CRF2020.1395] to CTS.
Disclosure statement
No potential conflict of interest was reported by the author(s). CT Supuran is Editor-in-Chief of the Journal of Enzyme Inhibition and Medicinal Chemistry. He was not involved in the assessment, peer review, or decision-making process of this paper. The authors have no relevant affiliations of financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
- 1.(a) Supuran CT. Structure and function of carbonic anhydrases. Biochem J 2016;473:2023–32. [DOI] [PubMed] [Google Scholar]; (b) Supuran CT. Experimental carbonic anhydrase inhibitors for the treatment of hypoxic tumors. J Exp Pharmacol 2020;12:603–17. [DOI] [PubMed] [Google Scholar]
- 2.(a) Supuran CT. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin Drug Discov 2017;12:61–88; [DOI] [PubMed] [Google Scholar]; (b) Supuran CT. Emerging role of carbonic anhydrase inhibitors. Clin Sci 2021;135:1233–49. [DOI] [PubMed] [Google Scholar]
- 3.(a) Supuran CT, How many carbonic anhydrase inhibition mechanisms exist? J Enzyme Inhib Med Chem 2016;31: 345–60. [DOI] [PubMed] [Google Scholar]; (b) Nocentini A, Angeli A, Carta F, et al. Reconsidering anion inhibitors in the general context of drug design studies of modulators of activity of the classical enzyme carbonic anhydrase. J Enzyme Inhib Med Chem 2021;36:561–80. [DOI] [PubMed] [Google Scholar]
- 4.(a) Supuran CT, Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81. [DOI] [PubMed] [Google Scholar]; (b) Neri D, Supuran CT.. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 2011;10:767–77. [DOI] [PubMed] [Google Scholar]
- 5.(a) Supuran CT. Carbonic anhydrases-an overview. Curr Pharm Des 2008;14:603–14. [DOI] [PubMed] [Google Scholar]; (b) Angeli A, Carta F, Nocentini A, et al. Carbonic anhydrase inhibitors targeting metabolism and tumor microenvironment. Metabolites 2020;10:412. [DOI] [PubMed] [Google Scholar]; (c) Pastorekova S, Casini A, Scozzafava A, et al. Carbonic anhydrase inhibitors: the first selective, membrane-impermeant inhibitors targeting the tumor-associated isozyme IX. Bioorg Med Chem Lett 2004;14:869–73. [DOI] [PubMed] [Google Scholar]
- 6.(a) Carta F, Supuran CT.. Diuretics with carbonic anhydrase inhibitory action: a patent and literature review (2005–2013). Expert Opin Ther Pat 2013;23:681–91. [DOI] [PubMed] [Google Scholar]; (b) Supuran CT. Carbonic anhydrase inhibitors and their potential in a range of therapeutic areas. Expert Opin Ther Pat 2018;28:709–12. [DOI] [PubMed] [Google Scholar]
- 7.Mincione F, Nocentini A, Supuran CT.. Advances in the discovery of novel agents for the treatment of glaucoma. Expert Opin Drug Discov 2021;16:1209–25. [DOI] [PubMed] [Google Scholar]
- 8.Narella SG, Shaik MG, Mohammed A, et al. Synthesis and biological evaluation of coumarin-1,3,4-oxadiazole hybrids as selective carbonic anhydrase IX and XII inhibitors. Bioorg Chem 2019;87:765–72. [DOI] [PubMed] [Google Scholar]
- 9.(a) Supuran CT. Carbonic anhydrase inhibitors: an update on experimental agents for the treatment and imaging of hypoxic tumors. Expert Opin Investig Drugs 2022;in press. [DOI] [PubMed] [Google Scholar]; (b) Supuran CT. Carbonic anhydrase inhibitors as emerging agents for the treatment and imaging of hypoxic tumors. Expert Opin Investig Drugs 2018;27:963–70. [DOI] [PubMed] [Google Scholar]
- 10.(a) Thacker PS, Alvala M, Arifuddin M, et al. Design, synthesis and biological evaluation of coumarin-3-carboxamides as selective carbonic anhydrase IX and XII inhibitors. Bioorg Chem 2019;86:386–92. [DOI] [PubMed] [Google Scholar]; (b) Supuran CT. Carbonic anhydrase inhibition and the management of hypoxic tumors. Metabolites 2017;7:48. [DOI] [PubMed] [Google Scholar]
- 11.(a) Nocentini A, Supuran CT.. Carbonic anhydrase inhibitors as antitumor/antimetastatic agents: a patent review (2008-2018). Expert Opin Ther Pat 2018;28:729–40. [DOI] [PubMed] [Google Scholar]; (b) Chafe SC, Vizeacoumar FS, Venkateswaran G, et al. Genome-wide synthetic lethal screen unveils novel CAIX-NFS1/xCT axis as a targetable vulnerability in hypoxic solid tumors. Sci Adv 2021;7:eabj0364. [DOI] [PubMed] [Google Scholar]; (c) McDonald PC, Chia S, Bedard PL, et al. A phase 1 study of SLC-0111, a novel inhibitor of carbonic anhydrase IX, in patients with advanced solid tumors. Am J Clin Oncol 2020;43:484–90. [DOI] [PubMed] [Google Scholar]
- 12.(a)Alterio V, Di Fiore A, D'Ambrosio K, et al. Multiple binding modes of inhibitors to carbonic anhydrases: how to design specific drugs targeting 15 different isoforms? Chem Rev 2012;112:4421–68. [DOI] [PubMed] [Google Scholar]; (b) Supuran CT, Alterio V, Di Fiore A, et al. Inhibition of carbonic anhydrase IX targets primary tumors, metastases, and cancer stem cells: three for the price of one. Med Res Rev 2018;38:1799–836. [DOI] [PubMed] [Google Scholar]
- 13.(a) Eldehna WM, Abdelrahman MA, Nocentini A, et al. Synthesis, biological evaluation and in silico studies with 4-benzylidene-2-phenyl-5(4H)-imidazolone-based benzenesulfonamides as novel selective carbonic anhydrase IX inhibitors endowed with anticancer activity. Bioorg Chem 2019;90:103102. [DOI] [PubMed] [Google Scholar]; (b) Briganti F, Pierattelli R, Scozzafava A, Supuran CT.. Carbonic anhydrase inhibitors. Part 37. Novel classes of carbonic anhydrase inhibitors and their interaction with the native and cobalt-substituted enzyme: kinetic and spectroscopic investigations. Eur J Med Chem 1996;31:1001–10. [DOI] [PubMed] [Google Scholar]
- 14.Shaldam M, Eldehna WM, Nocentini A, et al. Development of novel benzofuran-based SLC-0111 analogs as selective cancer-associated carbonic anhydrase isoform IX inhibitors. Eur J Med Chem 2021;216:113283. [DOI] [PubMed] [Google Scholar]
- 15.Abdelrahman MA, Eldehna WM, Nocentini A, et al. Novel benzofuran-based sulphonamides as selective carbonic anhydrases IX and XII inhibitors: synthesis and in vitro biological evaluation. J Enzyme Inhib Med Chem 2020;35:298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdelrahman MA, Ibrahim HS, Nocentini A, et al. Novel 3-substituted coumarins as selective human carbonic anhydrase IX and XII inhibitors: synthesis, biological and molecular dynamics analysis. Eur J Med Chem 2021;209:112897. [DOI] [PubMed] [Google Scholar]
- 17.Chandak N, Ceruso M, Supuran CT, Sharma PK.. Novel sulfonamide bearing coumarin scaffolds as selective inhibitors of tumor associated carbonic anhydrase isoforms IX and XII. Bioorganic Med Chem 2016;24:2882–6. [DOI] [PubMed] [Google Scholar]
- 18.(a) Supuran CT. Coumarin carbonic anhydrase inhibitors from natural sources. J Enzyme Inhib Med Chem 2020;35:1462–70. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Supuran CT. Multitargeting approaches involving carbonic anhydrase inhibitors: hybrid drugs against a variety of disorders. J Enzyme Inhib Med Chem 2021;36:1702–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Warhi T, Sabt A, Elkaeed EB, Eldehna WM.. Recent advancements of coumarin-based anticancer agents: an up-to-date review. Bioorg Chem 2020;103:104163. [DOI] [PubMed] [Google Scholar]
- 20.Garg SS, Gupta J, Sharma S, Sahu D.. An insight into the therapeutic applications of coumarin compounds and their mechanisms of action. Eur J Pharm Sci 2020;152:105424. [DOI] [PubMed] [Google Scholar]
- 21.(a) Maresca A, Scozzafava A, Supuran CT.. 7, 8-Disubstituted-but not 6, 7-disubstituted coumarins selectively inhibit the transmembrane, tumor-associated carbonic anhydrase isoforms IX and XII over the cytosolic ones I and II in the low nanomolar/subnanomolar range. Bioorg Med Chem Lett 2010;20:7255–8. [DOI] [PubMed] [Google Scholar]; (b) Mishra CB, Tiwari M, Supuran CT.. Progress in the development of human carbonic anhydrase inhibitors and their pharmacological applications: where are we today? Med Res Rev 2020;40:2485–565. [DOI] [PubMed] [Google Scholar]
- 22.Nocentini A, Carta F, Ceruso M, et al. Click-tailed coumarins with potent and selective inhibitory action against the tumor-associated carbonic anhydrases IX and XII. Bioorg Med Chem 2015;23:6955–66. [DOI] [PubMed] [Google Scholar]
- 23.(a) Bozdag M, Alafeefy AM, Altamimi AM, et al. Coumarins and other fused bicyclic heterocycles with selective tumor-associated carbonic anhydrase isoforms inhibitory activity. Bioorg Med Chem 2017;25:677–83. [DOI] [PubMed] [Google Scholar]; (b) Krasavin M, Kalinin S, Sharonova T, Supuran CT.. Inhibitory activity against carbonic anhydrase IX and XII as a candidate selection criterion in the development of new anticancer agents. J Enzyme Inhib Med Chem 2020;35:1555–61. [DOI] [PubMed] [Google Scholar]; (c) Innocenti A, Gülçin I, Scozzafava A, Supuran CT.. Carbonic anhydrase inhibitors. Antioxidant polyphenols effectively inhibit mammalian isoforms I-XV. Bioorg Med Chem Lett 2010;20:5050–3. [DOI] [PubMed] [Google Scholar]
- 24.(a) Carta F, Maresca A, Scozzafava A, Supuran CT.. Novel coumarins and 2-thioxo-coumarins as inhibitors of the tumor-associated carbonic anhydrases IX and XII. Bioorg Med Chem 2012;20:2266–73. [DOI] [PubMed] [Google Scholar]; (b) Dar'in D, Kantin G, Kalinin S, et al. Investigation of 3-sulfamoyl coumarins against cancer-related IX and XII isoforms of human carbonic anhydrase as well as cancer cells leads to the discovery of 2-oxo-2H-benzo[h]chromene-3-sulfonamide – a new caspase-activating proapoptotic agent. Eur J Med Chem 2021;222:113589. [DOI] [PubMed] [Google Scholar]
- 25.Koelsch CF. Bromination of 3-acetocoumarin. J Am Chem Soc 1950;72:2993–5. [Google Scholar]
- 26.Ben Mohamed S, Rachedi Y, Hamdi M, et al. An efficient synthetic access to substituted thiazolyl‐pyrazolyl‐chromene‐2‐ones from dehydroacetic acid and coumarin derivatives by a multicomponent approach. Eur J Org Chem 2016;2016:2628–36. [Google Scholar]
- 27.Valadbeigi E, Ghodsi S.. Synthesis and characterization of some new thiazolidinedione derivatives containing a coumarin moiety for their antibacterial and antifungal activities. Med Chem 2017;07:178–85. [PMC free article] [PubMed] [Google Scholar]
- 28.Mishra G, Sachan N, Chawla P.. Synthesis and evaluation of thiazolidinedione-coumarin adducts as antidiabetic, anti-inflammatory and antioxidant agents. Lett Org Chem 2015;12:429–55. [Google Scholar]
- 29.Eldehna WM, Abo-Ashour MF, Nocentini A, et al. Enhancement of the tail hydrophobic interactions within the carbonic anhydrase IX active site via structural extension: design and synthesis of novel N-substituted isatins-SLC-0111 hybrids as carbonic anhydrase inhibitors and antitumor agents. Eur J Med Chem 2019;162:147–60. [DOI] [PubMed] [Google Scholar]
- 30.Eldehna WM, Nocentini A, Elsayed ZM, et al. Benzofuran-based carboxylic acids as carbonic anhydrase inhibitors and antiproliferative agents against breast cancer. ACS Med Chem Lett 2020;11:1022–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elbadawi MM, Eldehna WM, Nocentini A, et al. Identification of N-phenyl-2-(phenylsulfonyl)acetamides/propanamides as new SLC-0111 analogues: synthesis and evaluation of the carbonic anhydrase inhibitory activities . Eur J Med Chem 2021;218:113360. [DOI] [PubMed] [Google Scholar]
- 32.Sabt A, Eldehna WM, Al-Warhi T, et al. Discovery of 3,6-disubstituted pyridazines as a novel class of anticancer agents targeting cyclin-dependent kinase 2: synthesis, biological evaluation and in silico insights. J Enzyme Inhib Med Chem 2020;35:1616–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Rashood ST, Hamed AR, Hassan GS, et al. Antitumor properties of certain spirooxindoles towards hepatocellular carcinoma endowed with antioxidant activity. J Enzyme Inhib Med Chem 2020;35:831–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eissa IH, El-Helby AG, Mahdy HA, et al. Discovery of new quinazolin-4(3H)-ones as VEGFR-2 inhibitors: design, synthesis, and anti-proliferative evaluation . Bioorg Chem 2020;105:104380. [DOI] [PubMed] [Google Scholar]
- 35.El-Helby AG, Sakr H, Ayyad RR, et al. Design, synthesis, molecular modeling, in vivo studies and anticancer activity evaluation of new phthalazine derivatives as potential DNA intercalators and topoisomerase II inhibitors. Bioorg Chem 2020;103:104233. [DOI] [PubMed] [Google Scholar]
- 36.Khalifah RG. The carbon dioxide hydration activity of carbonic anhydrase: I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem 1971;246:2561–73. [PubMed] [Google Scholar]
- 37.(a) Hedlund EE, McDonald PC, Nemirovsky O, et al. Harnessing induced essentiality: targeting carbonic anhydrase IX and angiogenesis reduces lung metastasis of triple negative breast cancer xenografts. Cancers 2019;11:1002. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Gieling RG, Babur M, Mamnani L, et al. Antimetastatic effect of sulfamate carbonic anhydrase IX inhibitors in breast carcinoma xenografts. J Med Chem 2012;55:5591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65:55–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.