Abstract
Fibrosis is driven by a misdirected cell response causing the overproduction of extracellular matrix and tissue dysfunction. Numerous pharmacological strategies have attempted to prevent fibrosis but have attained limited efficacy with some detrimental side effects. While stem cell treatments have provided more encouraging results, they have exhibited high variability and have not always improved tissue function. To enhance stem cell efficacy, we evaluated whether mechanical memory could direct cell response. We hypothesized that mechanically pre-conditioning on a soft matrix (soft priming) will delay adipose-derived stem cell (ASC) transition to a pro-fibrotic phenotype, expanding their regenerative potential and improving healing in a complex tissue environment. Primary ASCs isolated from rat and human subcutaneous fat exhibited mechanical memory, demonstrated by a delayed cell response to stiffness following two weeks of soft priming including decreased cell area, actin coherency, and extracellular matrix production compared to cells on stiff substrates. Soft primed ASCs injected into our rat model of post-traumatic elbow contracture decreased histological evidence of anterior capsule fibrosis and increased elbow range-of-motion when evaluated by joint mechanics. These findings suggest that exploiting mechanical memory by strategically controlling the culture environment during cell expansion may improve the efficacy of stem cell-based therapies targeting fibrosis.
Keywords: Anterior Capsule, Post-traumatic Joint Contracture, Range-of-motion, Mechanical Priming, Pre-conditioning
Introduction
Fibrosis is the excessive accumulation of extracellular matrix (ECM) proteins which causes soft tissue pathology and dysfunction (1). The negative functional consequences of these ECM changes are readily apparent in advanced organ diseases of the lung, liver and kidney, where regulatory mechanisms and therapeutics have been extensively studied, but fibrosis also has dramatic effects on musculoskeletal tissues which can significantly affect physical function (2–4). Notably, fibrosis is believed to drive post-traumatic joint contracture which affects approximately 50% of patients after injury and permanently limits joint function (5–8). Currently, there are no treatment strategies which have consistently prevented joint contracture (9–11).
Across conditions, fibrosis is thought to derive from a dysregulated wound healing response. Following injury, fibroblasts proliferate and deposit/contract ECM to mechanically stabilize the damaged tissue (12). However, aberrant biochemical and mechanical signaling at the wound site can prevent termination of this cellular response leading to the overproduction of ECM and pathological stiffening of the tissue (12–15). Because of the delicate balance between the physiological and pathological response to injury, treating or preventing fibrosis is difficult and presents a critical clinical challenge. Numerous pharmacological strategies have attempted to inhibit fibrotic signaling (e.g., transforming growth factor β1 (TGFβ1) inhibition), prevent collagen synthesis (e.g., procollagen 1 inhibition), or increase ECM break down (e.g., matrix metalloproteinases (MMPs)) (2–4, 16). While these treatments have been shown to slow the progression of fibrosis, they lack the ability to prevent organ dysfunction (4). Similarly, pharmacological strategies to minimize fibrosis in orthopaedic tissues have exhibited decreased fibroblast proliferation and collagen deposition, but these changes have often not led to a corresponding improvement in function (17–20). Safety concerns also arise because these drugs have pleiotropic effects, which could also alter angiogenesis, immunomodulation and cell proliferation in non-fibrotic tissues (3, 16).
Stem cells are a promising alternative to prevent fibrosis because these cells have the potential to modify the fibrotic microenvironment and promote regeneration through cell-cell signaling (17, 21). Unlike pharmacological strategies, stem cells have the capacity to not only send but also receive signals from resident cell populations which can harness adaptive cell-cell communication to further enhance wound healing (22). These bioactive signals secreted by stem cells are believed to be paracrine factors which contribute to immunomodulation and regeneration (17, 23–25). Thus, numerous studies have attempted to treat fibrosis with stem cells in various organs. However, results have been mixed - ranging from limited benefits to augmented pathology (26–28). Similarly, in orthopaedic applications, while many animal studies that injected either bone-marrow derived stem cells (BD-MSCs) or adipose-derived stem cells (ASCs) showed histological improvements, few studies have reported a persistent improvement in function; typically, only a transient improvement in tissue mechanics or gait has been observed (29–31). Orthopaedic clinical trials have also found mixed efficacy of BD-MSC and ASC treatments. The majority of trials have targeted osteoarthritis. While most of those studies have found improvements in tissue structure by histology or MRI, function was either not improved above a clinically meaningful threshold or not evaluated (32–37). Following tibial osteotomy, addition of BD-MSCs to a platelet gel application failed to improve osseointegration or patient reported function over gel alone (38). Similarly in the shoulder, ASC treatment after rotator cuff repair showed no improvement in structure or function over repair alone (39). The variability in outcomes across stem cell-based therapies highlights a need to improve the efficacy of these cells in a wound environment.
One strategy for enhanced treatment is to harness stem cell mechanical memory. Mechanical memory is the concept that cells retain behavioral features of their previous mechanical environment. Pre-conditioning or priming cells in culture exploits mechanical memory, which delays cell response to a subsequent environment with different properties. Balestrini and colleagues first provided evidence of mechanical memory by priming rodent lung fibroblasts on either soft (~5 kPa; i.e., healthy tissue) or stiff (~100 kPa; i.e., fibrotic tissue) substrates for two weeks, then transferred these cells to stiff or soft substrates, respectively, for an additional two weeks (40). Soft primed cells exhibited decreased evidence of fibrotic conversion, evidenced by reduced cytoskeletal contractility, α smooth muscle actin (αSMA) expression, and ECM production, compared to cells only cultured on stiff substrates or tissue culture plastic (TCP, ~3 GPa). Conversely, stiff priming increased fibrotic conversion compared to cells only cultured on soft substrates. Hence, cells can be directed solely by mechanical cues against or toward a pro-fibrotic phenotype. More recently, both rodent and human BD-MSCs have similarly been shown to exhibit mechanical memory (41, 42). In previous therapeutic applications, isolated stem cells were typically expanded in culture on TCP prior to cell injection. Given our current understanding of mechanical memory, we postulated that TCP expansion could direct cells toward fibrotic conversion causing them to contribute to, rather than mitigate, fibrosis.
Conversely, injecting stem cells expanded on soft substrates may ameliorate the fibrotic response in vivo. Treatment with soft primed BD-MSCs decreased fibrosis during healing in a rodent dermal wound model (41). In another study, conditioned media from soft primed BD-MSCs was also able to enhance dermal wound closure and re-epithelialization demonstrating that the soft primed cell secretome alone could improve healing (43). While these studies suggest that cell mechanical memory can alter fibrotic healing, it is unclear whether soft primed stem cells can prevent fibrosis in a more complex biological environment (e.g., joint injury) and improve function (e.g., increased range-of-motion (ROM)). Furthermore, while BD-MSCs remain a clinically viable cell source, many studies are now evaluating the regenerative potential of ASCs, but mechanical memory in ASCs has yet to be evaluated.
The objective of this study was to (1) determine if ASCs exhibit mechanical memory and (2) investigate if soft primed ASCs could beneficially remodel a complex orthopaedic wound environment and ultimately improve function after injury. First, we provide evidence that ASCs, a more readily available source of autologous stem cells than BD-MSCs (21, 44), exhibit mechanical memory in vitro. Second, we demonstrate that soft primed ASCs mitigate the fibrotic response in our rat model of post-traumatic elbow contracture, improving biomechanical as well as histological outcomes. This evidence supports our hypothesis that soft priming delays ASC transition to a pro-fibrotic phenotype, expanding their regenerative potential and improving healing in complex tissue environments.
Methods and Materials
Cell isolation.
Subcutaneous adipose tissue was dissected from male Long-Evans rats (250-350 g, 8-10 weeks old; Charles River Laboratories International, Wilmington, MA, USA) under sterile conditions immediately following sacrifice by CO2 inhalation overdose. The dissected adipose tissue was minced and put into 37°C pre-warmed digestive solution (0.1% collagenase and 1% penicillin/streptomycin in low-glucose Dulbecco’s Modified Eagle’s Medium (DMEM)) for one hour with gentle inversion every 10 minutes. The remaining solid tissue was mechanically broken-down using forceps and the shredded tissue solution was then incubated for 30 minutes at 37°C and 5% CO2. Following incubation, the shredded tissue was further dissociated by gentle pipetting. The cell suspension was then passed through a 70-μm sterile filter and centrifuged at 1300 rpm for 10 minutes at 4°C. The cell pellet was resuspended in standard growth media (10% fetal bovine serum (FBS) and 1% penicillin/streptomycin in low-glucose DMEM) and expanded in culture to passage four, where cultures exhibit >90% homogeneity for MSC markers (e.g., CD13, CD29, CD44, CD73, CD90) (44, 45). Cells were banked in cryostorage prior to experimentation.
Human subcutaneous ASCs were acquired as part of a study approved by the Human Research Protection Office at the Washington University School of Medicine. Briefly, a ~100 mg biopsy of subcutaneous adipose tissue in the calf was acquired during below-the-knee amputation surgery. The biopsy was processed as described above for rat subcutaneous adipose tissue, except that human ASCs were used at passage two. All cell culture experiments were conducted in standard growth media at 37°C and 5% CO2 (44).
Polyacrylamide gels.
The preparation of polyacrylamide hydrogels was adapted from a previously described protocol (46). Briefly, polyacrylamide stock solutions were made to achieve gels with elastic moduli of ~1 kPa (3% acrylamide and 0.1% bis-acrylamide in deionized H2O) and ~120 kPa (15% acrylamide, 1.2% bis-acrylamide in deionized H2O). Stock solutions were combined with a crosslinker and catalyst (10% ammonium persulfate and 0.11% tetramethylethylenediamine (TEMED), respectively) and sandwiched between a 25 mm glass coverslip and a glass microscope slide. Prior to use, coverslips were functionalized in a methacrylate solution (0.5% 3-(trimethyloxysilyl)propyl methacrylate and 0.3% acetic acid in ethanol) to enable covalent attachment of the hydrogel, while microscope slides were treated with dichlorodimethylsiloxane (DCDMS) to repel the hydrogel surface. After one hour, polymerization was complete, and the gels were moved into a six-well culture plate. Gels were rinsed with sterile phosphate buffered saline (1X PBS) to remove any residual unpolymerized acrylamide. To allow for protein coating and cell adhesion, gels were then incubated twice with sulfo-SANPAH (0.2 mg/mL in 50 mM HEPES, pH 8.5), activated with 365 nm ultraviolet light, and then rinsed thoroughly with sterile 1X PBS. Gels were incubated with 10 μg/mL fibronectin overnight at 37°C and 5% CO2.
ASCs were seeded on the polyacrylamide gels at low density to isolate the mechanical effects of substrate stiffness from cell-cell interactions. The number of cells seeded was optimized so that the cell density at the end of the study was approximately the same on each stiffness. Approximately 1000-1500 and 75-150 cells/well were seeded onto 1 and 120 kPa gels, respectively. Cells cultured on either 1 or 120 kPa gels for two weeks were used to demonstrate that ASCs were mechanosensitive and are referred to as Soft and Stiff, respectively. Mechanical memory was evaluated in ASCs by priming cells on 1 kPa for two weeks, then enzymatically transferring the cells with 0.25% Trypsin-EDTA to new 120 kPa gels for either one or two weeks (Soft-to-Stiff (1) or Soft-to-Stiff (2), respectively). As a control, ASCs were cultured on 120 kPa for two weeks, then enzymatically transferred to new 120 kPa gels for either one or two weeks (Stiff-to-Stiff (1) or Stiff-to-Stiff (2), respectively). Three-dimensional printed polylactic acid (PLA) cylinders were used to harvest cells only from the middle of the gels to avoid uneven edge effects (harvested cells from approximately 50% gel total area).
The same protocol was used to make 1 kPa polyacrylamide gels to culture cells for in vivo injection, but 3” x 3.5” rectangular coverslips and 3.25” x 4” microscope slides (Ted Pella, Redding, CA, USA) were used to scale up cell numbers. The number of cells seeded on these large gels was similarly optimized to limit cell-cell interaction through the culture period, so mechanical stimulus was driven by the substrate surface. Hence, approximately 12,500 cells/gel were seeded. ASCs were cultured for two weeks on these large gels before they were harvested with 0.25% Trypsin-EDTA for injection.
Immunocytochemistry.
ASCs were fixed with 4% paraformaldehyde for 15 minutes and then permeabilized with 1% triton-X for 15 minutes. For each six-well plate, five gels were labeled with primary antibodies for either αSMA (1:200; AB5694, Abcam, Cambridge, UK) or yes associated protein (YAP; 1:50; SC-101199, Santa Cruz, Dallas, TX, USA) while one gel served as the isotype control labeled with IgG (1:200; Poly29108, Biolegend, San Diego, CA, USA) or IgG2aκ (1:50; 401501, Biolegend), respectively. The antibodies and isotype controls were diluted in 1.5% buffer serum (1.5% goat serum and 1% bovine serum albumin (BSA) in 1X PBS) and incubated on the gels for 18 hours at room temperature. Alexa Fluor 488 goat anti-rabbit or Alexa Fluor 568 goat anti-mouse (1:400; A1108 or A1104, respectively, Life Technologies, Carlsbad, CA, USA) were diluted in 1.5% buffer serum and incubated on the gels for one hour at room temperature. Gels labeled for YAP were also co-labelled for F-actin (Conjugated Alexa Fluor 488 Phalloidin; 1:200; A12379, ThermoFisher, Waltham, MA, USA) following the same protocol as the secondary antibodies. Cell nuclei were labeled with DAPI (1:1000; Hoechst 33342, H3570, Life Technologies).
Five replicates (gels), 10 cells per replicate, were imaged per group via confocal microscope (DM6B Leica, Wetzlar, Germany). Cells were selected for imaging on the DAPI channel to eliminate experimental bias from viewing αSMA or YAP intensity or cell morphology. Quantification of cell morphology was performed on images with a custom macro in ImageJ (National Institutes of Health, Bethesda, MD, USA). YAP and F-actin labeled cells were used to evaluate cell area, actin coherency (i.e., alignment), and YAP nuclear/cytoplasmic ratio. αSMA labeled cells were used to quantify the number of αSMA positive cells, which is a pro-fibrotic (myofibroblast) marker (14, 40, 47). Imaging, processing, and analysis settings were standardized across all images.
Extracellular matrix labeling.
Nascent ECM proteins were labeled following methods previously described (48, 49). For each six-well plate, five gels were pulse labeled the last two days of the culture period with L-Azidohomoalanine (AHA; Click Chemistry Tools, Scottsdale, AZ, USA) supplemented growth media (10% FBS, 0.9% sodium pyruvate, 0.1% ascorbic acid, 1% glutamax, 1% penicillin/streptomysin, 0.1% cystine, 0.2% AHA in DMEM without L-methionine (21013024, ThermoFisher)), while one gel served as the control and was given standard growth media. At the end of the culture period, the plasma membrane was labeled with Cell Mask (1:250; C10045, ThermoFisher) for 30 minutes at 37°C and 5% CO2. AHA was then labeled with 30 μM DBCO-488 (Click Chemistry Tools) for 40 minutes at 37°C and 5% CO2. The gels were fixed with 4% paraformaldehyde for 15 minutes at room temperature. Cell nuclei were labeled with DAPI (1:1000).
Five replicates, 10 cells per replicate, were imaged per group via confocal microscope (DM6B Leica). A custom macro in ImageJ was used to quantify the nascent ECM of each cell. The plasma membrane label was used to identify the cell boundary. Twenty radial intensity profiles emanating from the center of the cell were symmetrically mapped and then truncated to include only the region outside of the cell. These 20 intensity profiles were averaged for each cell. Heatmaps were generated by expanding the cell boundary region-of-interest (ROI, determined from the plasma membrane label) outward by 14.5 μm (~20 pixels) and measuring the mean pixel intensity in the area between two ROI, which was then plotted based on a color scale representative of ECM intensity. The ROI expansion was repeated eight times. Any matrix deposited by other nearby cells was manually excluded from all quantification. Imaging, processing, and analysis settings were standardized across all images.
Adipokine Array.
Growth media was collected after 72 hours of culture for detection of adipokines by Proteome Profiler (ARY016, R&D Systems, Minneapolis, MN, USA). Briefly, the array membrane was blocked, incubated with growth media and detection antibody cocktail, and detected with IRDye 800CW Streptavidin (1:10,000; 926-32230, LI-COR, Lincoln, NE, USA). Membranes were then imaged on an Odyssey Scanner (9120, LI-COR). The Image Studio Lite Ver 5.2 (LI-COR) was used to quantify antibody intensities. These images were background subtracted and normalized to the average membrane intensity. Data are presented as a ratio of intensities between groups (e.g., Soft/Stiff and Soft-to-Stiff (1)/Stiff-to-Stiff (1)) and there were six replicates per group.
ELISA.
For six replicates per group, growth media was conditioned for 24 hours of culture and collected for detection of MCP-1 by Quantikine ELISA (DCP00, R&D Systems). Briefly, growth media and standards were incubated on the plate at room temperature for two hours, followed by incubations with conjugate for one hour and substrate solution for 30 minutes. Finally, stop solution was added and plate fluorescence was read at 450 nm (Synergy II, BioTek, Winooski, VT, USA). Cells were trypsinized with 0.25% Trypsin-EDTA and counted via hemocytometer for signal normalization.
Animal model.
In this Institutional Appropriate Care and Animal Use Committee (IACUC) approved study, twelve male Long-Evans rats were used to evaluate whether ASC injection could enhance tissue remodeling and wound healing in vivo. We used a previously developed injury and immobilization protocol, which surgically induced a unilateral injury (anterior capsulotomy with lateral collateral ligament transection) to mimic soft tissue damage that occurs as a result of elbow dislocation (50, 51). Immediately following surgery, the injured limbs were immobilized in a flexed position with an external bandage for 21 days. This period of immobilization was selected because it was previously shown to be the earliest time point at which significant contracture developed (7). Animals were randomized into two groups (n = 6/group): INJ-TCP (injury + immobilization + TCP-expanded ASC injection), and INJ-GEL (injury + immobilization + soft primed ASC injection). Data from control (no injury + no immobilization) and INJ (injury + immobilization) animals were reported in previous studies, but were included here to enable statistical comparison between control, untreated and treated groups (7, 50, 51).
Cell injections.
ASCs were either soft primed for two-weeks on 1 kPa polyacrylamide gels or expanded on TCP for one-week to represent standard culture conditions used in previous stem cell injection studies (26-28, 30-34, 39). ASCs were injected three days after the surgically induced injury to target fibroblasts which in other connective tissues have been reported to proliferate and infiltrate the wound site 3-5 days post-injury and transition to pro-fibrotic myofibroblasts after 8-9 days (12). Prior to injection, rats were anesthetized using isoflurane at 2.5-4% with an oxygen carrier via nasal inhalation using a nose cone. The external immobilization bandage and staples used for skin closure at the original surgery, which induced the injury, were removed. Animals received a dose of a local analgesic (0.5 mL 5 mg/mL bupivacaine, Hospira, Lake Forest, IL, USA) subcutaneously at the original incision site 20 minutes before the cell injection. After 20 minutes, the skin incision was re-opened superficially to provide visualization of the joint for injection. The suture used to close the muscle fascial layer at the original surgery was used as a landmark to guide access to the anterior capsule where the cells were injected. Two million soft primed or TCP expanded ASCs were injected anteriorly in 50 μL sterile saline using a 25-gauge needle (INJ-GEL or INJ-TCP, respectively). Following injection, the skin was closed with suture glue (3M Vetbond Tissue Adhesive, St. Paul, MN, USA) and the injured limb was immediately immobilized with the external bandage using the same protocol developed previously (50). After 21 days of immobilization, animals were sacrificed with CO2 inhalation overdose and stored in −20°C.
Flexion-extension joint mechanics.
Animals were thawed 24 hours prior to dissection and prepared for mechanical testing as described previously (50, 51). Briefly, forelimbs were skinned, disarticulated at the glenohumeral joint, and the paw was removed. The proximal humerus and distal ulna/radius were secured in test fixtures. A custom mechanical test system was used to evaluate elbow ROM in flexion-extension. The system design and post-test analysis protocol were published previously (50, 51). A custom written Matlab code (MathWorks, Natick, MA, USA) converted the force-displacement data to torque-angular position which was analyzed to quantify joint motion. ROM measurements included total ROM, neutral zone length, and neutral zone stiffness. Neutral zone length is a clinically relevant measure defined as the amount of motion possible before increased force is required to move the joint further, while neutral zone stiffness is the slope of the force-displacement curve in the neutral zone.
Joint histology.
After mechanical testing all forelimbs were prepared for histological evaluation. Briefly, elbow joints were fixed for 72 hours in 10% neutral buffered formalin at 90° flexion and then decalcified for 17 days in 14% ethylenediamine tetraacetic acid (EDTA, pH 7.2). The forelimbs were dehydrated in a series of 30%, 50%, and 70% ethanol washes and then embedded in paraffin. For each limb, three 5-μm-thick sagittal sections were cut per stain. Hematoxylin and eosin (H&E) and toluidine blue were used to evaluate biological characteristics of interest including tissue thickness, adhesions, fibrosis, cellularity, inflammation, vascularity, myofibroblasts, and proteoglycans following the same method used previously (7, 50, 51). Blinded sections were scored by a musculoskeletal pathologist to analyze the anterior capsule using the following symbolic scoring method: adhesions were scored as present (+) or absent (−); fibrosis was scored as < 30% (+), 31-60% (+ +), or > 60% (+ + +); cellularity was scored as minimal (+), mild (+ +), moderate (+ + +), or marked (+ + + +); inflammation was scored as none (−), mild (+), moderate (+ +), or marked (+ + +); vascularity was scored as < 6 (+), 6-10, or > 10 blood vessels per high power field at 40x; myofibroblasts were scored as mild (+), moderate (+ +), or marked (+ + +); and matrix proteoglycans were scored as weak to mild (+), moderate (+ +), or strong (+ + +). Capsular thickness was measured on each section and reported semi-quantitatively to account for variation throughout the tissue volume. Numerical thickness values were averaged across each group, normalized by the thickness of the corresponding uninjured contralateral limb capsule and converted to a symbolic score: 0-150 μm (−), 151-300 μm (+), 301-450 μm (+ +), 451-600 μm (+ + +), and > 600 μm (+ + + +).
Statistical analysis.
Quantifications of immunohistochemistry and ECM labeling images, and ELISA data were analyzed with a two-way analysis of variance (ANOVA) for stiffness and priming with post-hoc Bonferroni comparisons between groups at each time point. Adipokine array data were analyzed with a two-way ANOVA for stiffness and priming with post-hoc Bonferroni comparisons corrected with False Discovery Rate using the Benjamini-Hochberg method. Flexion-extension joint mechanics data were analyzed using a one-way ANOVA with post-hoc Bonferroni comparisons between each group. All statistical analysis was performed in GraphPad Prism (GraphPad Software Inc., La Jolla, CA, USA) and statistical significance was defined as p < 0.05.
Results
Mechanical memory was first assessed in primary rat ASCs by examining pro-fibrotic changes in morphology and αSMA expression with two weeks of soft priming followed by transfer to stiff substrates for an additional 1-2 weeks (Figure 1A). Qualitatively, cells cultured on soft substrates appeared smaller with a less developed and organized cytoskeleton compared to cells only cultured on stiff substrates at all time points (Figure 1B). Quantitatively, the Soft group exhibited decreased actin coherency (i.e., alignment) and αSMA positive cells compared to the Stiff group (p < 0.0001 and < 0.0001, respectively); while the decreased cell area exhibited a trend towards significance (p = 0.053; Figures 1C–E). After two weeks of soft priming, ASCs transferred to a stiff substrate for one week (Soft-to-Stiff (1)) retained these differences with decreased cell area, actin coherency, and αSMA positive cells compared to Stiff-to-Stiff (1) (p = 0.004, < 0.0001, and < 0.0001, respectively; Figures 1C–E). However, following an additional week of culture on a stiff substrate, no significant difference in actin coherency or αSMA positive cells was detected between groups (Figures 1D–E). Only cell area for the Soft-to-Stiff (2) group remained decreased compared to Stiff-to-Stiff (2) (p = 0.002; Figure 1C). Quantitation of YAP localization, a putative transcriptional regulator of mechanosensing, showed that the nuclear to cytoplasmic ratio was decreased in the Soft group compared to the Stiff group (p < 0.0001), but there was no difference in either soft primed group compared to their respective stiff controls (Supplemental Figure 1), suggesting that long term retention of mechanical memory was not mediated by YAP in these cells. Overall, these data indicate that soft primed ASCs exhibit mechanical memory, retaining morphological features of a soft environment for at least one-week, thereby postponing ASC transition to a pro-fibrotic phenotype.
Figure 1.
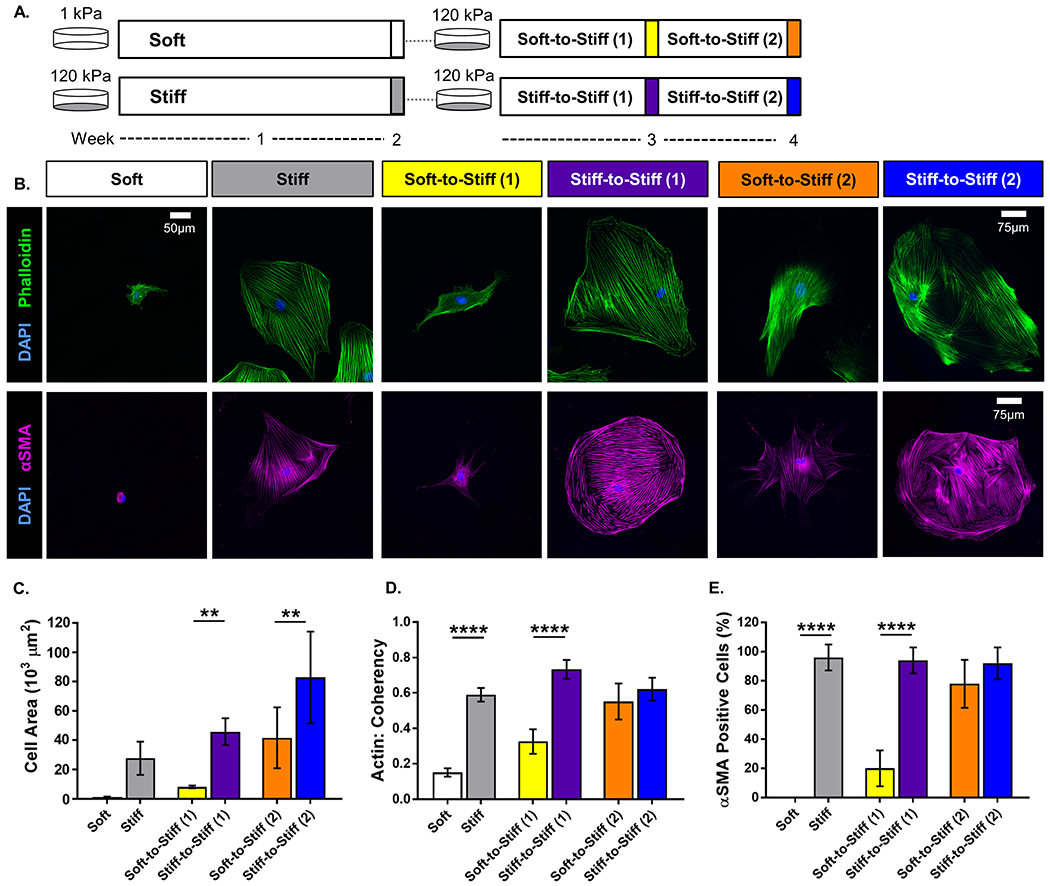
Schematic of the in vitro experimental method timeline. Adipose-derived stem cells (ASCs) were isolated from rat subcutaneous adipose tissue expanded for four passages and frozen down for storage. ASCs were cultured on either 1 or 120 kPa polyacrylamide gels functionalized with 10 μg/mL fibronectin. (· · · = enzymatically transferred to a new gel using 0.25% trypsin-EDTA). (A) Representative images of rat ASCs immunolabeled for (top row) nuclei (blue) and F-actin (green), and (bottom row) nuclei (blue) and α-smooth muscle actin (αSMA, magenta). (scale bar = 50 μm for all groups, except Stiff-to-Stiff (2): scale bar = 75 μm). Quantitative measures computed from fluorescent images: (B) cell area, (C) actin coherency (alignment), and (D) αSMA positive cells. Data are shown as mean ± standard deviation. ** p < 0.01. **** p < 0.0001.
To determine whether morphologic features of ASC mechanical memory translated to changes in cellular function, ECM secretion was quantified on different substrate stiffnesses and following transfer. Qualitatively, ASCs on soft substrates generated less ECM which appeared globular and only close to the cell membrane, while those on stiff produced a larger ECM network throughout which individual fibers could be observed (Figure 2A, top row). ECM secretion was not symmetric around ASCs in any culture condition. Heatmaps of signal intensity surrounding each cell body illustrated that ASCs on stiff substrates had a higher density of surrounding ECM compared to those on soft (Figure 2A, bottom row). Quantitation of the intensity profile for the Soft group illustrated that ECM production was not only lower immediately next to the cell membrane, but also decreased at a faster rate moving radially away from the membrane compared to the Stiff group (Figure 2B). The Soft-to-Stiff (1) ECM intensity profile exhibited a similar shape as Stiff-to-Stiff (1), but with a downward shift (Figure 2C). Quantification of the area under the curve for these ECM intensity profiles showed that ECM production was decreased in the Soft group compared with Stiff and in the Soft-to-Stiff (1) group compared with Stiff-to-Stiff (1) (p = 0.0001 and 0.003, respectively; Figure 2D). Thus, soft priming ASCs not only delayed the development of pro-fibrotic morphology but also fibrotic activity, demonstrating that mechanical memory can be used to control cell behavior.
Figure 2.
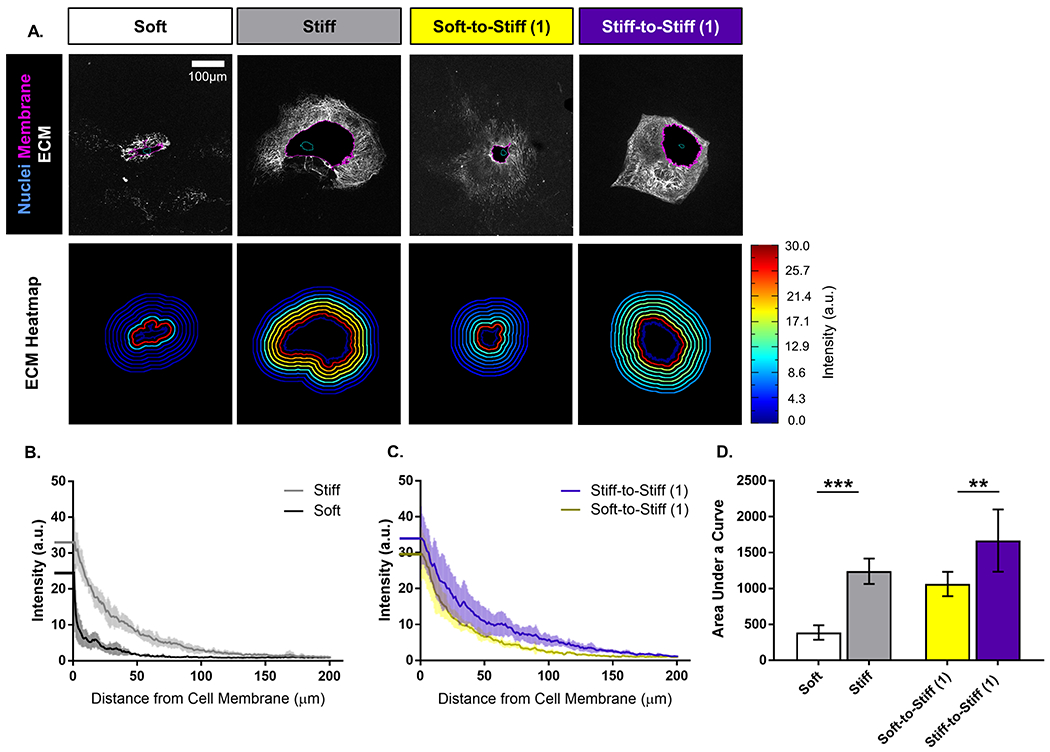
(A) Representative images of rat adipose-derived stem cells (ASCs) immunolabeled for (top row) nuclei (blue outline), cell membrane (magenta outline), and extracellular matrix (white), and (bottom row) intensity heatmap of extracellular matrix deposition. (scale bar = 100 μm). Quantitative measures computed from fluorescent images for extracellular matrix intensity profile for (B) Soft and Stiff groups, and (C) Soft-to-Stiff (1) and Stiff-to-Stiff (1) groups. (dark line = mean, and light lines = standard deviation). (D) Area under the curve for the extracellular matrix intensity profiles for each group. Data are shown as mean ± standard deviation. ** p < 0.01. *** p < 0.001.
To evaluate the effect of mechanical memory in an in vivo injury environment, soft primed ASCs were injected into the anterior capsule of our rat elbow contracture model (Figure 3A). ASCs expanded on TCP were injected as a control to represent culture conditions in previous stem cell injection studies (26–28, 30–34, 39). Limbs harvested 18 days after injection (21 days after injury) were evaluated histologically to identify the effect on the anterior capsule. Qualitatively, both cell injection groups (INJ-TCP and INJ-GEL) displayed an altered biological response as shown by the overall joint morphology and appearance of the anterior capsule compared to injury alone (INJ) and control (Figure 3B). Blinded histological scoring of the anterior capsule semi-quantitatively reported these differences (Table 1). INJ scores slightly varied with previous publications because these sections were blinded and rescored with the data collected in this study; importantly, these subtle differences do not alter the interpretation of the results (7). While most histological parameters for INJ, INJ-TCP and INJ-GEL were increased compared to control, indicative of a wound healing response, there were a few notable differences. While INJ-TCP and INJ-GEL capsule thickness values were not different compared to control, both groups exhibited increased myofibroblasts and inflammation. Compared with INJ and INJ-TCP, INJ-GEL capsules exhibited decreased fibrosis and increased cellularity suggestive of regenerative remodeling of the wound environment.
Figure 3.
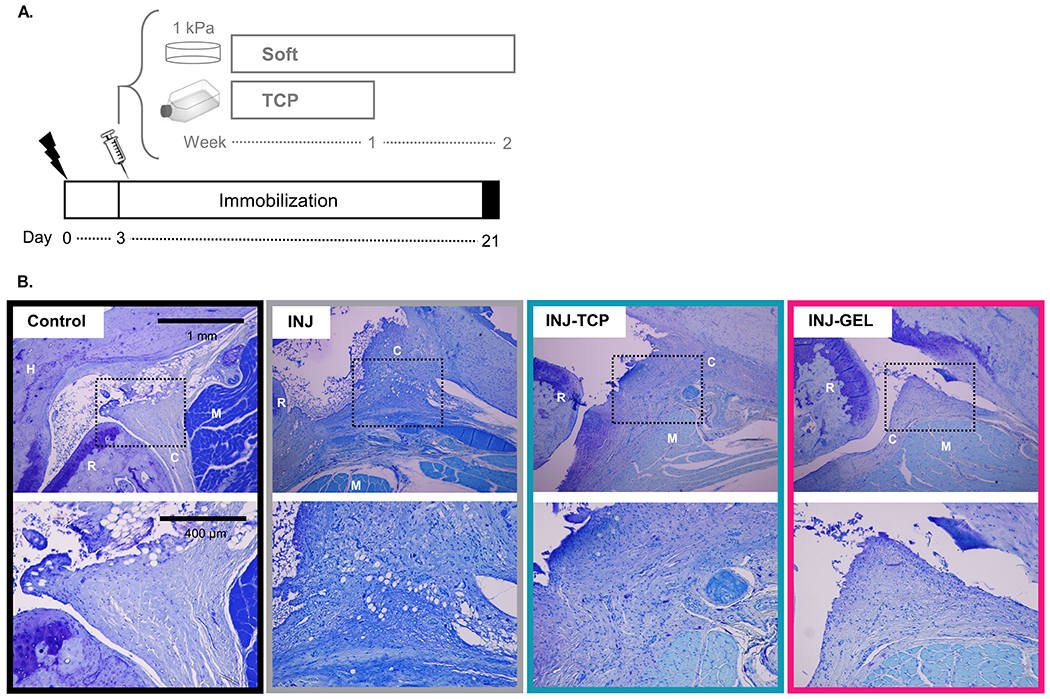
Schematic of the in vivo experimental method timeline. Adipose-derived stem cells (ASCs) were isolated from rat subcutaneous adipose tissue then cultured on either fibronectin-coated 1 kPa polyacrylamide gels for two weeks or tissue culture plastic (TCP) for one week before injection. (lightning bolt = surgery, syringe = injection). Representative sagittal histology (toluidine blue) illustrate joint anatomy (top row scale bar = 1 mm) and general characteristics of the anterior capsule (bottom row scale bar = 400 μm) after 21 days of immobilization. (dotted square = region of interest shown at higher magnification in the bottom row, C = capsule, H = humerus, R = radius, M = muscle, INJ-TCP = injured and injected with cells cultured on tissue culture plastic (TCP), INJ-GEL = injured and injected with cells cultured on fibronectin-coated 1 kPa polyacrylamide gels).
Table 1.
Histological evaluation of the anterior capsule.
| Name | Adhesion | Fibrosis | Cell. | Inflamm. | Vascularity | Myofibro. | Proteoglycan | Thickness |
|---|---|---|---|---|---|---|---|---|
| Control | − | − | + | − | + | + | + | − |
| INJ | + | + + | + | − | + | + | + + | + |
| INJ-TCP | + | + + | + + | + | + | + + | + + | − |
| INJ-GEL | + | + | + + + | + | + | + + | + + | − |
Thickness grading scheme: 0-150 μm (−), 151-300 μm (+), 301-450 μm (+ +), 451-600 μm (+ + +), and > 601 μm (+ + + +). Data for control and INJ were scored and published previously (7). (Cell. = Cellularity, Inflamm. = Inflammation, Myofibro. = Myofibroblasts).
These limbs were also biomechanically tested to determine the effect of stem cell treatment on joint ROM and stiffness. As we previously reported, elbow injury and immobilization (i.e., INJ) resulted in significantly decreased total ROM and neutral zone length, and increased neutral zone stiffness (7). Treatment of injured joints with TCP-expanded ASCs (INJ-TCP) elicited no significant biomechanical changes compared to injury alone (INJ; p > 0.999, > 0.999, and 0.815, respectively; Figure 4A–C) and hence remained significantly altered compared to control (p < 0.0001, 0.0002, and 0.0004, respectively; Figure 4A–C). However, treatment with soft primed ASCs (INJ-GEL) significantly increased total ROM compared to INJ (p = 0.026; Figure 4A). Furthermore, unlike in the INJ and INJ-TCP groups, total ROM, neutral zone length, and neutral zone stiffness in the INJ-GEL group were not significantly different from control (p = 0.087, 0.123, and > 0.999, respectively; Figure 4A– C). Taken together, these results indicate that injection of soft primed ASCs improved joint function after injury compared to untreated animals and was not significantly different from control.
Figure 4.
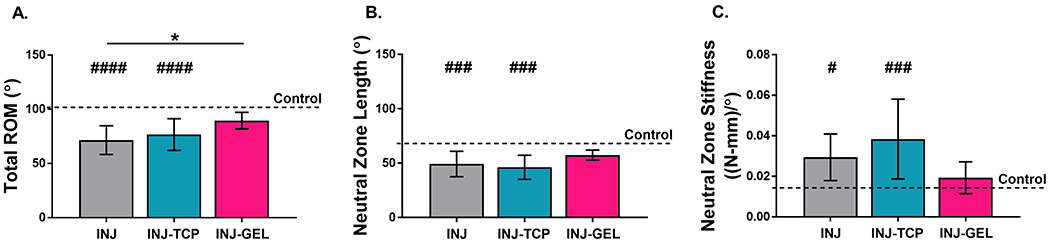
Quantitative results are shown for flexion-extension: (A) total range-of-motion (ROM), (B) neutral zone length and (C) neutral zone stiffness. Limbs were evaluated after 21 days of immobilization. Data are shown as mean ± standard deviation. # p < 0.05, ### p < 0.001, and #### p < 0.0001 different from control. * p < 0.05. Data for control and INJ were published previously (7). (INJ-TCP = injured and injected with cells cultured on tissue culture plastic (TCP), INJ-GEL = injured and injected with cells cultured on fibronectin-coated 1 kPa polyacrylamide gels).
To further explore the mechanism underlying the biological and functional benefits of soft primed ASCs in vivo, we next sought to determine whether soft priming altered the cell secretome. Out of 30 cytokines evaluated via an adipokine array, only three exhibited increased (> 1) intensity ratios on soft compared to stiff substrates for both ratios, with at least one significant difference (i.e., significantly increased expression on soft compared to stiff substrates): Endocan, insulin-like growth factor-binding protein-6 (IGFBP-6), and monocyte chemoattractant protein-1 (MCP-1; Figure 5, Table 2, Supplemental Table 1). However, out of these three cytokines, only MCP-1 retained significantly increased secretion following transfer (Soft-to-Stiff (1) to Stiff-to-Stiff (1) ratio). These data indicate that soft priming can alter the ASC secretome and that MCP-1 could play a role in the modified healing response following injection of these cells in vivo.
Figure 5.
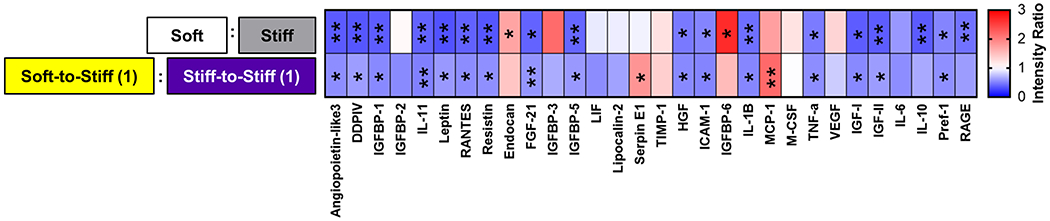
Adipokine array results: Heatmap of normalized intensity ratio for Soft/Stiff and Soft-to-Stiff (1)/Stiff-to-Stiff (1) with statistical results from two-way analysis of variance (ANOVA) for stiffness and priming with post-hoc Bonferroni corrected with False Discovery Rate using the Benjamini-Hochberg method. * p < 0.05. ** p < 0.01.
Table 2.
Cytokines which exhibited increased intensity ratios in both soft culture conditions (> 1), where at least one ratio was significantly increased (i.e., significantly increased expression on soft compared to stiff substrates).
| Soft : Stiff |
Soft-to-Stiff (1) : Stiff-to-Stiff (1) |
|||
|---|---|---|---|---|
| Cytokine | Intensity Ratio | p value | Intensity Ratio | p value |
| Endocan | 1.7 ± 0.5 | 0.0124* | 1.5 ± 0.3 | 0.0994 |
| IGFBP-6 | 2.7 ± 1.6 | 0.0148* | 1.5 ± 0.6 | 0.8053 |
| MCP-1 | 1.7 ± 0.2 | 0.0597 | 2.2 ± 0.7 | 0.0090** |
Data are shown as mean ± standard deviation.
p < 0.05.
p < 0.01.
(IGFBP-6 = Insulin-like growth factor-binding protein-6, MCP-1 = Monocyte chemoattractant protein-1).
To probe the translational potential of ASC mechanical memory, we also assessed the effects of mechanical priming in human ASCs. Similar to rat ASCs, human ASCs primed on soft substrates appeared qualitatively smaller compared to those on stiff in all groups (Figure 6A). Quantitation of cell area demonstrated that Soft, Soft-to-Stiff (1), and Soft-to-Stiff (2) exhibited decreased cell area compared to their respective controls (p < 0.0001, 0.0003, and < 0.0001, respectively; Figure 6B). Actin coherency was decreased on the Soft group compared with Stiff and the Soft-to-Stiff (2) group compared with Stiff-to-Stiff (2) (p < 0.0001 and < 0.0001, respectively; Figure 6C). Upregulated MCP-1 secretion in soft primed ASCs was also similarly conserved between both species; specifically, it was increased in the Soft group compared with Stiff and in the Soft-to-Stiff (1) group compared with Stiff-to-Stiff (1) (p = 0.0002 and 0.0218, respectively; Figure 6D). Quantitation of YAP localization showed that the nuclear to cytoplasmic ratio was decreased on Soft compared to Stiff (p < 0.0001), but there was no difference in either soft primed group compared to their respective stiff controls, similar to rat ASCs (Supplemental Figure 2). Overall, human ASCs not only exhibited mechanical memory, but their memory was retained longer than in rat ASCs. Thus, soft priming human ASCs has the potential to expand their regenerative capacity in clinical settings by postponing their transition to a pro-fibrotic phenotype.
Figure 6.
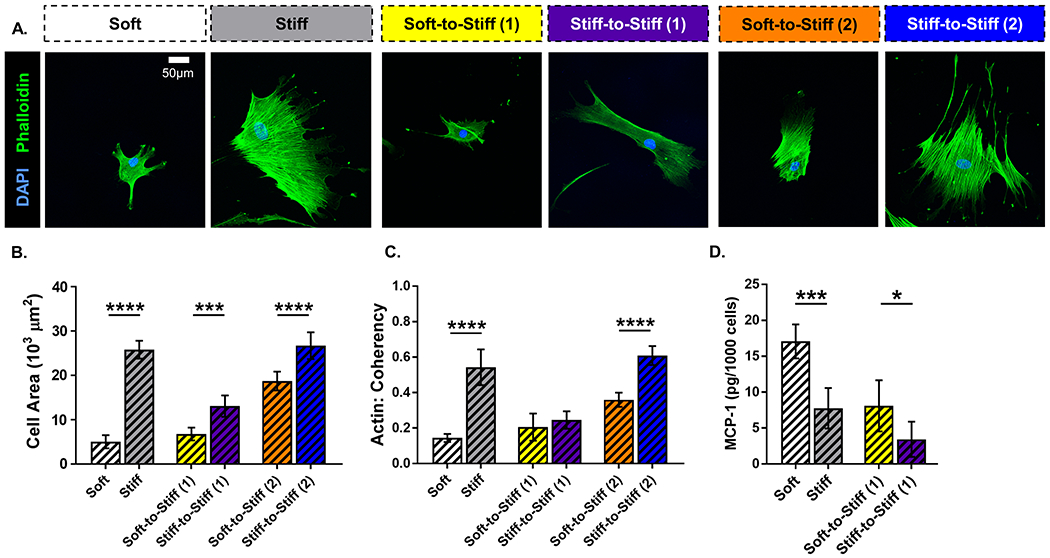
(A) Representative images of human adipose-derived stem cells (ASCs) immunolabeled for nuclei (blue) and F-actin (green). (scale bar = 50 μm). Quantitative measures computed from fluorescent images: (B) cell area and (C) actin coherency (alignment). (D) Monocyte chemoattractant protein-1 (MCP-1) expression in human ASC conditioned media. Data are shown as mean ± standard deviation. * p < 0.05. *** p < 0.001. **** p < 0.0001.
Discussion
In this work, we demonstrated that ASCs from both rat and human sources exhibited mechanical memory in vitro, retaining morphological and functional features of a soft environment following transfer to a stiff environment. These features suggested resistance to a pro-fibrotic phenotype characterized by increased cell size, cytoskeletal alignment, expression of αSMA and secretion of ECM. In line with these in vitro results, we found that injection of soft primed rat ASCs into an injured in vivo environment reduced histological evidence of fibrosis and joint contracture. Together, these findings suggest that exploiting mechanical memory is a promising tool to direct ASCs to improve healing in complex wound environments.
The mechanosensitivity of ASCs has been previously demonstrated by altered morphology and stiffness-driven differentiation (52, 53). We additionally report that ECM and cytokine secretion are altered by matrix stiffness (Figures 2 and 5, Table 2, Supplemental Table 1). Furthermore, we show that ASCs exhibit mechanical memory. ASCs soft primed for two weeks and subsequently cultured on stiff substrates for one week still exhibited decreased cell area, actin coherency, and αSMA positive cells compared to cells cultured only on stiff substrates (Figure 1). While most of these differences were lost after soft primed ASCs were cultured an additional week on stiff substrates, the capability of ASCs to retain memory of their previous culture environment for at least one week may be sufficient to increase their regenerative potential in vivo. Histological and functional improvements following ASC injection in our rat model of elbow contracture support this notion.
Mechanical memory is not unique to ASCs. Previous work in lung fibroblasts and BD-MSCs have demonstrated mechanical memory using pro-fibrotic cell markers like αSMA (40, 41). Rat lung fibroblasts also exhibited decreased contraction (i.e., gel wrinkles) and collagen production with soft priming (40). Additionally, investigations evaluating mechanical memory in breast cancer cell lines (e.g., MDA-MD-231, MCF-10A) showed that soft priming decreased proliferation rate and migration speed compared to cells cultured only on stiff substrates (54, 55). Thus, some cellular functions exhibit mechanical memory. In the current study, the effect of mechanical memory on ASC activity was evaluated by the quantification of individual cell ECM production and cytokine secretion. Soft primed ASCs, even after one week of culture on stiff substrates, produced less ECM than cells cultured only on stiff substrates and maintained an altered cytokine secretion profile (Figures 2 and 5, Table 2, Supplemental Table 1). Thus, soft priming not only delayed changes in morphology but also in function.
A first look into the possible mechanisms driving mechanical memory in rat and human ASCs started with the evaluation of the mechanosensitive protein YAP. Since YAP is reported to localize within the nucleus on stiff substrates and cytoplasm on soft substrates, if YAP contributes to long-term maintenance of cell mechanical memory then it would remain cytoplasmic in soft primed cells following transfer to a stiff substrate (42, 56). However, in vitro studies with soft primed rat and human ASCs showed that YAP was nuclear at both time points following transfer (Supplemental Figures 1 and 2). Consistent with recent reports, YAP does not likely drive long-term mechanical memory (41, 57, 58). Future investigations will focus on miR-21 and epigenetic changes (Figure 7), which have been proposed to affect cell mechanosensitivity (41, 59).
Figure 7.
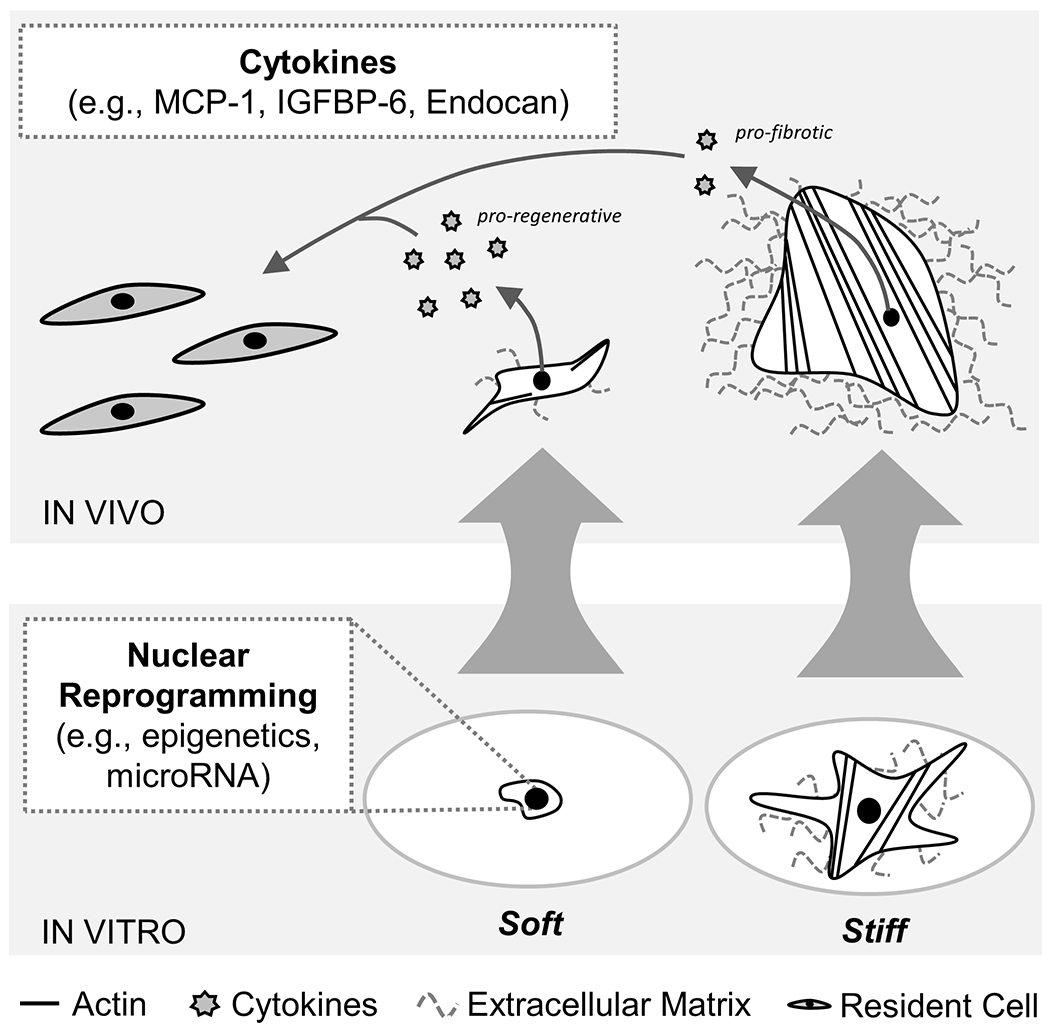
While YAP does not drive long-term mechanical memory in vitro, cell memory may potentially be regulated by nuclear reprogramming (e.g., altered epigenetics and/or microRNA expression) (41, 59). Increased pro-regenerative cytokine secretion (e.g., MCP-1, IGFBP-6, Endocan) after soft priming may signal to the resident cell population in vivo to mitigate the fibrotic wound healing response in our rat elbow contracture model which subsequently improved joint motion.
Maintenance of mechanical memory in vitro suggests the possibility to pre-direct, or prime, cells toward a specific function in vivo. Of course, functional changes in vitro do not always translate to functional changes in vivo. Few groups have investigated the effects of mechanically primed cells in vivo. Notably, Li and colleagues showed that injecting two million soft primed BD-MSCs via fibrin glue onto a dermal wound in a hypertrophic scarring rodent model improved histological parameters at the wound site (e.g., increased cellularity, decreased collagen density) compared to untreated animals (41). In a similar model, Yang et al. showed that conditioned media from soft primed BD-MSCs enhanced dermal wound closure (e.g., re-epithelialization) which provides evidence that indirect signaling from soft primed cells can improve healing (43). However, this previous work only reported indirect changes in tissue mechanics (e.g., wound area/tension). In the current study, intra-articular injection of two million soft primed ASCs into our rat model of elbow contracture not only reduced histological evidence of fibrosis but also improved biomechanical measures of joint contracture. Evaluation of flexion-extension joint mechanics demonstrated that injection of ASCs cultured on TCP (INJ-TCP) did not improve elbow contracture, evidenced by decreased total ROM and neutral zone length with increased neutral zone stiffness compared with control (Figure 4). However, injection of ASCs cultured on soft substrates (INJ-GEL) improved these parameters such that joint mechanics were not statistically different from control (Figure 4).
These improved functional changes were likely driven by an altered biological response in the anterior capsule after cell injection. Histologically, we found evidence of cell- and tissue-level changes including increased inflammation and cellularity as well as decreased fibrosis and thickness in the ASC treated elbows (INJ-TCP and INJ-GEL) compared to untreated (INJ; Figure 3, Table 1). When comparing INJ-TCP to INJ-GEL groups (i.e. evaluating the effect of mechanical priming), we found decreased fibrosis and increased cellularity only. Similar quantities of myofibroblasts between these groups was surprising, but this quantification did not account for myofibroblast activity. It is possible that in the INJ-GEL group, the myofibroblasts were signaled to contribute to ECM remodeling rather than fibrosis (40). Similarly, while the quantity of inflammatory cells was not different between INJ-TCP and INJ-GEL, the characteristics of these cells may be altered by paracrine signals from soft primed ASCs such that they exhibit a pro-regenerative, rather than pro-inflammatory phenotype. Our in vitro results suggest that soft primed ASCs, compared with ASCs expanded on TCP, may also directly limit the fibrotic response in the capsule because their ECM production remained decreased despite transfer to a stiff substrate (Figure 2). Thus, soft primed cells may deposit less ECM at the wound site compared to cells expanded on stiff substrates (e.g., TCP) and so have a greater potential to mitigate the fibrotic response after injury. Overall, consistent improvements in all parameters evaluating flexion-extension joint mechanics and morphology for INJ-GEL compared to INJ suggest that soft primed ASCs decreased the fibrotic wound healing response leading to enhanced joint function after injury.
Stem cells have been reported to influence the resident cell population by secreting bioactive, trophic factors which modulate the immune response and stimulate regeneration (23–25, 41, 60). Previously, ASC conditioned media was shown to contain elevated angiogenic markers like interleukin-6 (IL-6), vascular endothelial growth factor (VEGF), and MCP-1 (24). In this study, we expanded our investigation to an array of 30 well-characterized cytokines to investigate how soft priming alters ASC cytokine secretion. Of these 30 cytokines, only three exhibited increased expression in both soft culture conditions compared to stiff (Figures 5 and 7, Table 2, Supplemental Table 1). Interestingly, out of these three cytokines, only MCP-1 exhibited significantly increased expression in soft primed ASCs following transfer to a stiff substrate (Figure 5, Table 2). MCP-1 plays an important role in wound healing by influencing the immune response after injury (61). It is a chemotactic factor for monocytes, memory T cells, and dendritic cells (62). Some literature has shown that MCP-1 can activate macrophages and potentially alter their polarization from a pro-inflammatory (M1) to an anti-inflammatory (M2) phenotype (63, 64). Additional studies evaluating the immunomodulatory role of MCP-1 are warranted to determine if it is a primary factor driving the decreased fibrotic response and improved joint function after injury in our rat model of elbow contracture.
While in vitro comparisons were made between 1 and 120 kPa gels to isolate the effects of stiffness from other variables (e.g., gel topography), in vivo experiments utilized 1 kPa gels (a novel soft priming approach) and standard culture conditions (TCP) to enable comparison with existing literature. Most studies evaluating the efficacy of stem cell therapies first expanded cells on TCP prior to injection (26–28, 30–34, 39). Studies injecting BD-MSCs and ASCs into fibrotic models or after musculoskeletal injury reported variable results (26–34, 39). In our study, we similarly found mixed outcomes for INJ-TCP, where histological evidence for altered cell and tissue morphology did not translate into functional changes (Figures 3 and 4, Table 1). Interestingly, Balestrini et al. and Yang et al. showed that lung fibroblasts and BD-MSCs, respectively, were directed toward a pro-fibrotic phenotype when first cultured on a stiff substrate (40, 42). Hence, mechanical memory can also bias cells toward fibrotic activation. Therefore, the standard method to expand cell populations on TCP for therapeutic delivery has the potential to attenuate cell regenerative capacity and accentuate pro-fibrotic behavior. Our in vivo results suggest that soft priming is an effective method to avoid retention of TCP memory in stem cell-based therapies.
We designed the in vivo model with clinical translation in mind. ASCs were injected three days after injury to represent a more realistic patient time to treatment in clinical settings. Additionally, this aligns with the approximate time point at which fibroblasts infiltrate the wound site (12). These fibroblasts typically develop a pro-fibrotic phenotype after 8-9 days (12). Hence, we hypothesize that aligning ASC application with this phenotypic shift maximizes their potential to delay pro-fibrotic activation and enhance the regenerative potential of resident cell populations. We additionally chose to evaluate ASCs, rather than BD-MSCs, due to their improved efficiency of isolation and their increased use in clinical applications (21, 44). In this study, we demonstrated that human ASCs exhibit mechanical memory similar to rat ASCs and rat/human BD-MSCs (41, 42). Specifically, we found that human ASCs displayed decreased cell area and actin coherency on soft compared to stiff substrates and retained these changes at least a week longer than rat ASCs following exposure to a stiff substrate (Figures 6A–C). Human soft primed ASCs also exhibited persistently increased MCP-1 secretion following transfer to a stiff substrate, similar to rat ASCs (Figure 6D). Therefore, not only is mechanical memory a phenomenon that is conserved between species, but it also has the potential to enhance the therapeutic efficacy of stem cells in clinical settings. In addition, soft priming does not involve genetic or pharmacologic modification, which increases its translational potential not only for musculoskeletal applications but also for other organ systems commonly affected by fibrosis.
Several questions from this study remain and provide motivation for future work. First, this study did not delineate the mechanism(s) underlying soft priming. While we showed that YAP is nuclear on stiff and cytoplasmic on soft substrates, YAP likely does not control long-term mechanical memory because it did not remain cytoplasmic in soft primed cells for either rat or human ASCs (Supplemental Figures 1 and 2) (56). Changes in miR-21 expression and epigenetics have been identified as potential factors which may contribute to long-term mechanical memory in stiff primed BD-MSCs; however, more work is needed to elucidate their roles (41, 59). Second, this work did not evaluate how long injected ASCs remained in the joint space or how long the functional improvements persisted. Future work will simultaneously answer these questions using longitudinal cell tracking techniques (e.g., Quantum Dots) over a longer time frame (65). These studies will also determine if additional cell injections are needed to maintain therapeutic benefits long-term. Third, it will be valuable to also examine biological changes in vivo at earlier time points following injection to determine if immunomodulation (e.g., altered macrophage activation and/or polarization) contributes to the decreased fibrotic response and improved joint function in our animal model. Fourth, this work did not compare culture expanded ASCs to naïve ASCs, injected immediately following isolation. Since ASCs are derived from a soft tissue, they may potentially be primed by their native environment in vivo. Borrelli et al. showed that fat grafting decreased dermal thickness and collagen production in a radiation-induced fibrotic skin mouse model (66). Therefore, injecting ASCs immediately post-isolation will determine if soft priming is necessary for this cell type to elicit an improved healing response. Finally, while translationally relevant, our model of elbow contracture is a highly specific wound environment. We believe that the soft primed ASCs could decrease the fibrotic response across a number of tissue injury and disease environments; however, more extensive experimentation will be required to determine the extent of future applications.
In conclusion, this study has shown that ASC mechanical memory can decrease soft tissue fibrosis and increase joint function after injury in the rat elbow. Specifically, we found (1) pre-conditioning ASCs on soft substrates delayed activation of a pro-fibrotic phenotype and (2) injection of soft primed ASCs into the elbow anterior capsule decreased capsular fibrosis and increased elbow total ROM in flexion-extension after injury. These findings suggest that modifying the culture substrate for stem cell expansion has the potential to improve the efficacy of stem cell-based therapies targeting fibrosis.
Supplementary Material
Acknowledgements
We thank Leanne Iannucci for her assistance harvesting and preparing cells for injection. Funding was provided by the Center of Regenerative Medicine at Washington University in St. Louis and the National Institutes of Health (R01 AR071444). Joints for histology were embedded, sectioned and stained by the Washington University Musculoskeletal Research Center (NIH P30 AR074992). A. Chamberlain has received support for work outside of the submitted manuscript from Zimmer-Biomet, DePuy, Arthrex, and Wright Medical. The remaining authors have no conflicts of interest.
Nonstandard Abbreviations:
- αSMA
α-smooth muscle actin
- ASC
adipose-derived stem cell
- BD-MSC
bone-marrow derived stem cell
- ECM
extracellular matrix
- MCP-1
monocyte chemoattractant protein-1
- ROM
range-of-motion
- TCP
tissue culture plastic
- YAP
yes associated protein
References
- 1.Wynn TA (2008) Cellular and molecular mechanisms of fibrosis. J Pathol 214, 199–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clarke SA, Richardson WJ, and Holmes JW (2016) Modifying the mechanics of healing infarcts: is better the enemy of good? J Mol Cell Cardiol 93, 115–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isaka Y (2018) Targeting TGF-β signaling in kidney fibrosis. Int J Mol Sci 19, 2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manka P, Zeller A, and Syn W-K (2019) Fibrosis in Chronic Liver Disease: An Update on Diagnostic and Treatment Modalities. Drugs 79, 903–927 [DOI] [PubMed] [Google Scholar]
- 5.Cohen MS, Schimmel DR, Masuda K, Hastings H, and Muehleman C (2007) Structural and biochemical evaluation of the elbow capsule after trauma. J Shoulder Elb Surg 16, 484–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hildebrand KA, Sutherland C, and Zhang M (2004) Rabbit knee model of post-traumatic joint contractures: the long-term natural history of motion loss and myofibroblasts. J Orthop Res 22, 313–320 [DOI] [PubMed] [Google Scholar]
- 7.Dunham CL, Castile RM, Havlioglu N, Chamberlain AM, and Lake SP (2018) Temporal Patterns of Motion in Flexion-extension and Pronation-supination in a Rat Model of Posttraumatic Elbow Contracture. Clin Orthop Relat R 476, 1878–1889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anakwe RE, Middleton SD, Jenkins PJ, McQueen MM, and Court-Brown CM (2011) Patient-Reported Outcomes After Simple Dislocation of the Elbow. J Bone Joint Surg 93, 1220–1226 [DOI] [PubMed] [Google Scholar]
- 9.Schneider PS, Mohtadi N, Sajobi T, Wang M, Garven A, and Hildebrand KA (2017) Randomized, Placebo-controlled Clinical Trial Evaluating Ketotifen Fumarate in Reduction of Post-traumatic Elbow Joint Contracture: Level 2 Evidence. J Hand Surg Am 42, S50–S51 [Google Scholar]
- 10.Steplewski A, Fertala J, Beredjiklian PK, Abboud JA, Wang ML, Namdari S, Barlow J, Rivlin M, Arnold WV, and Kostas J (2017) Blocking collagen fibril formation in injured knees reduces flexion contracture in a rabbit model. J Orthop Res 35, 1038–1046 [DOI] [PubMed] [Google Scholar]
- 11.Morrey ME, Sanchez-Sotelo J, Lewallen EA, An KN, Grill DE, Steinmann SP, Yao JJ, Salib CG, Trousdale WH, and Reina N (2017) Intra-articular Injection of a Substance P Inhibitor Affects Gene Expression in a Joint Contracture Model. J Cell Biochem [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hinz B (2007) Formation and function of the myofibroblast during tissue repair. J Invest Dermatol 127, 526–537 [DOI] [PubMed] [Google Scholar]
- 13.Leask A, and Abraham DJ (2004) TGF-β signaling and the fibrotic response. FASEB 18, 816–827 [DOI] [PubMed] [Google Scholar]
- 14.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, and Brown RA (2002) Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Bio 3, 349–363 [DOI] [PubMed] [Google Scholar]
- 15.Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, and Tschumperlin DJ (2010) Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol 190, 693–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Györfi AH, Matei A-E, and Distler JH (2018) Targeting TGF-β signaling for the treatment of fibrosis. Matrix Biol 68, 8–27 [DOI] [PubMed] [Google Scholar]
- 17.Li X, Chen S, Yan L, Wang J, and Pei M (2019) Prospective application of stem cells to prevent post-operative skeletal fibrosis. J Orthop Res [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garg K, Corona BT, and Walters TJ (2014) Losartan administration reduces fibrosis but hinders functional recovery after volumetric muscle loss injury. J Appl Physiol 117, 1120–1131 [DOI] [PubMed] [Google Scholar]
- 19.Gumucio JP, Flood MD, Phan AC, Brooks SV, and Mendias CL (2013) Targeted inhibition of TGF-β results in an initial improvement but long-term deficit in force production after contraction-induced skeletal muscle injury. J Appl Physiol 115, 539–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kocaoglu B, Agir I, Nalbantoglu U, Karahan M, and Türkmen M (2010) Effect of mitomycin-C on post-operative adhesions in tendon surgery: an experimental study in rats. J Bone Joint Surg Br 92, 889–893 [DOI] [PubMed] [Google Scholar]
- 21.Mizuno H, Tobita M, and Uysal AC (2012) Concise review: adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cell 30, 804–810 [DOI] [PubMed] [Google Scholar]
- 22.Spees JL, Lee RH, and Gregory CA (2016) Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther 7, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caplan AI (2017) Mesenchymal stem cells: time to change the name! Stem Cell Transl Med 6, 1445–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.da Silva Meirelles L, Fontes AM, Covas DT, and Caplan AI (2009) Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth F R 20, 419–427 [DOI] [PubMed] [Google Scholar]
- 25.Murphy MB, Moncivais K, and Caplan AI (2013) Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med 45, e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Breitbach M, Bostani T, Roell W, Xia Y, Dewald O, Nygren JM, Fries JW, Tiemann K, Bohlen H, and Hescheler J (2007) Potential risks of bone marrow cell transplantation into infarcted hearts. Blood 110, 1362–1369 [DOI] [PubMed] [Google Scholar]
- 27.Papazova DA, Oosterhuis NR, Gremmels H, Van Koppen A, Joles JA, and Verhaar MC (2015) Cell-based therapies for experimental chronic kidney disease: a systematic review and meta-analysis. Dis Model Mech 8, 281–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Álvarez D, Levine M, and Rojas M (2015) Regenerative medicine in the treatment of idiopathic pulmonary fibrosis: current position. Stem cells and cloning: advances and applications 8, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vilar JM, Cuervo B, Rubio M, Sopena J, Domínguez JM, Santana A, and Carrillo JM (2016) Effect of intraarticular inoculation of mesenchymal stem cells in dogs with hip osteoarthritis by means of objective force platform gait analysis: concordance with numeric subjective scoring scales. BMC Vet Res 12, 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Degen RM, Carbone A, Carballo C, Zong J, Chen T, Lebaschi A, Ying L, Deng X-H, and Rodeo SA (2016) The effect of purified human bone marrow–derived mesenchymal stem cells on rotator cuff tendon healing in an athymic rat. Arthroscopy 32, 2435–2443 [DOI] [PubMed] [Google Scholar]
- 31.Xing D, Kwong J, Yang Z, Hou Y, Zhang W, Ma B, and Lin J (2018) Intra-articular injection of mesenchymal stem cells in treating knee osteoarthritis: a systematic review of animal studies. Osteoarthr Cartilage 26, 445–461 [DOI] [PubMed] [Google Scholar]
- 32.Jo CH, Lee YG, Shin WH, Kim H, Chai JW, Jeong EC, Kim JE, Shim H, Shin JS, and Shin IS (2014) Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A proof-of-concept clinical trial. Stem Cell 32, 1254–1266 [DOI] [PubMed] [Google Scholar]
- 33.Pers Y-M, Rackwitz L, Ferreira R, Pullig O, Delfour C, Barry F, Sensebe L, Casteilla L, Fleury S, and Bourin P (2016) Adipose mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: A phase i dose-escalation trial. Stem Cell Transl Med 5, 847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Najar M, Khalil H, Al-Ajlouni J, Al-Antary E, Hamdan M, Rahmeh R, Alhattab D, Samara O, Yasin M, and A1 Abdullah A (2017) Intra-articular injection of expanded autologous bone marrow mesenchymal cells in moderate and severe knee osteoarthritis is safe: a phase I/II study. J Orthop Surg Res 12, 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wakitani S, Imoto K, Yamamoto T, Saito M, Murata N, and Yoneda M (2002) Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthr Cartilage 10, 199–206 [DOI] [PubMed] [Google Scholar]
- 36.Wong KL, Lee KBL, Tai BC, Law P, Lee EH, and Hui JH (2013) Injectable cultured bone marrow–derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: a prospective, randomized controlled clinical trial with 2 Years’ follow-up. Arthroscopy 29, 2020–2028 [DOI] [PubMed] [Google Scholar]
- 37.Vangsness CT Jr., Farr J 2nd, Boyd J, Dellaero DT, Mills CR, and LeRoux-Williams M (2014) Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy: a randomized, double-blind, controlled study. J Bone Joint Surg 96, 90–98 [DOI] [PubMed] [Google Scholar]
- 38.Dallari D, Savarino L, Stagni C, Cenni E, Cenacchi A, Fornasari PM, Albisinni U, Rimondi E, Baldini N, and Giunti A (2007) Enhanced tibial osteotomy healing with use of bone grafts supplemented with platelet gel or platelet gel and bone marrow stromal cells. J Bone Joint Surg 89, 2413–2420 [DOI] [PubMed] [Google Scholar]
- 39.Kim YS, Sung CH, Chung SH, Kwak SJ, and Koh YG (2017) Does an Injection of Adipose-Derived Mesenchymal Stem Cells Loaded in Fibrin Glue Influence Rotator Cuff Repair Outcomes? A Clinical and Magnetic Resonance Imaging Study. Am J Sport Med [DOI] [PubMed] [Google Scholar]
- 40.Balestrini JL, Chaudhry S, Sarrazy V, Koehler A, and Hinz B (2012) The mechanical memory of lung myofibroblasts. Integr Biol 4, 410–421 [DOI] [PubMed] [Google Scholar]
- 41.Li CX, Talele NP, Boo S, Koehler A, Knee-Walden E, Balestrini JL, Speight P, Kapus A, and Hinz B (2016) MicroRNA-21 preserves the fibrotic mechanical memory of mesenchymal stem cells. Nat Mater [DOI] [PubMed] [Google Scholar]
- 42.Yang C, Tibbitt MW, Basta L, and Anseth KS (2014) Mechanical memory and dosing influence stem cell fate. Nat Mater 13, 645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang H, Nguyen KT, Leong DT, Tan NS, and Tay CY (2016) Soft material approach to induce oxidative stress in mesenchymal stem cells for functional tissue repair. ACS Appl Mater Inter 8, 26591–26599 [DOI] [PubMed] [Google Scholar]
- 44.Baer PC, and Geiger H (2012) Adipose-derived mesenchymal stromal/stem cells: tissue localization, characterization, and heterogeneity. Stem Cell Internat 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A, Di Halvorsen Y, Storms RW, Goh B, Kilroy G, Wu X, and Gimble JM (2006) Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cell 24, 376–385 [DOI] [PubMed] [Google Scholar]
- 46.Tse JR, and Engler AJ (2010) Preparation of hydrogel substrates with tunable mechanical properties. Curr Protoc Cell Biol, 10.16.11–10.16.16 [DOI] [PubMed] [Google Scholar]
- 47.Talele NP, Fradette J, Davies JE, Kapus A, and Hinz B (2015) Expression of α-smooth muscle actin determines the fate of mesenchymal stromal cells. Stem Cell Rep 4, 1016–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McLeod CM, and Mauck RL (2016) High fidelity visualization of cell-to-cell variation and temporal dynamics in nascent extracellular matrix formation. Sci Rep 6, 38852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loebel C, Mauck RL, and Burdick JA (2019) Local nascent protein deposition and remodelling guide mesenchymal stromal cell mechanosensing and fate in three-dimensional hydrogels. Nat Mater, 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lake SP, Castile RM, Borinsky S, Dunham CL, Havlioglu N, and Galatz LM (2016) Development and use of an animal model to study post-traumatic stiffness and contracture of the elbow. J Orthop Res 34, 354–364 [DOI] [PubMed] [Google Scholar]
- 51.Dunham CL, Castile RM, Havlioglu N, Chamberlain AM, Galatz LM, and Lake SP (2017) Persistent motion loss after free joint mobilization in a rat model of post-traumatic elbow contracture. J Shoulder Elb Surg 26, 611–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Engler AJ, Sen S, Sweeney HL, and Discher DE (2006) Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 [DOI] [PubMed] [Google Scholar]
- 53.Nardone G, Oliver-De La Cruz J, Vrbsky J, Martini C, Pribyl J, Skládal P, Pešl M, Caluori G, Pagliari S, and Martino F (2017) YAP regulates cell mechanics by controlling focal adhesion assembly. Nat Commun 8, 15321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Syed S, Schober J, Blanco A, and Zustiak SP (2017) Morphological adaptations in breast cancer cells as a function of prolonged passaging on compliant substrates. PLOS One 12, e0187853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nasrollahi S, Walter C, Loza AJ, Schimizzi GV, Longmore GD, and Pathak A (2017) Past matrix stiffness primes epithelial cells and regulates their future collective migration through a mechanical memory. Biomaterials 146, 146–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dupont S, Morsut L, Aragona E, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, and Bicciato S (2011) Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 [DOI] [PubMed] [Google Scholar]
- 57.Elosegui-Artola A, Andreu I, Beedle AE, Lezamiz A, Uroz M, Kosmalska AJ, Oria R, Kechagia JZ, Rico-Lastres P, and Le Roux A-L (2017) Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell [DOI] [PubMed] [Google Scholar]
- 58.Caliari SR, Perepelyuk M, Cosgrove BD, Tsai SJ, Lee GY, Mauck RL, Wells RG, and Burdick JA (2016) Stiffening hydrogels for investigating the dynamics of hepatic stellate cell mechanotransduction during myofibroblast activation. Sci Rep 6, 21387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heo S-J, Thorpe SD, Driscoll TP, Duncan RL, Lee DA, and Mauck RL (2015) Biophysical regulation of chromatin architecture instills a mechanical memory in mesenchymal stem cells. Sci Rep 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barrachina L, Remacha AR, Romero A, Vázquez FJ, Albareda J, Prades M, Ranera B, Zaragoza P, Martín-Burriel I, and Rodellar C (2017) Inflammation affects the viability and plasticity of equine mesenchymal stem cells: possible implications in intra-articular treatments. J Vet Sci 18, 39–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Low QE, Drugea IA, Duffner LA, Quinn DG, Cook DN, Rollins BJ, Kovacs EJ, and DiPietro LA (2001) Wound healing in MIP-1α−/− and MCP-1−/− mice. Am J Pathol 159, 457–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Solovyeva VV, Salafutdinov II, Tazetdinova LG, Khaiboullina SF, Masgutov RF, and Rizvanov AA (2014) Genetic modification of adipose derived stem cells with recombinant plasmid DNA pBud-VEGF-FGF2 results in increased of IL-8 and MCP-1 secretion. J Pure Appl Microbiol 8, 523–528 [Google Scholar]
- 63.Wood S, Jayaraman V, Huelsmann EJ, Bonish B, Burgad D, Sivaramakrishnan G, Qin S, DiPietro LA, Zloza A, and Zhang C (2014) Pro-inflammatory chemokine CCL2 (MCP-1) promotes healing in diabetic wounds by restoring the macrophage response. PLOS One 9, e91574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chow JP, Simionescu DT, Carter AL, and Simionescu A (2016) Immunomodulatory effects of adipose tissue-derived stem cells on elastin scaffold remodeling in diabetes. Tissue Eng Regen Med 13, 701–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yukawa H, Watanabe M, Kaji N, Okamoto Y, Tokeshi M, Miyamoto Y, Noguchi H, Baba Y, and Hayashi S (2012) Monitoring transplanted adipose tissue-derived stem cells combined with heparin in the liver by fluorescence imaging using quantum dots. Biomaterials 33, 2177–2186 [DOI] [PubMed] [Google Scholar]
- 66.Borrelli MR, Diaz Deleon NM, Adem S, Patel RA, Mascharak S, Shen AH, Irizarry D, Nguyen D, Momeni A, and Longaker MT (2020) Fat grafting rescues radiation-induced joint contracture. Stem Cell 38, 382–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


