Abstract
Stool specimens from children (<4 years old) with diarrhea were collected over a 1-year period in Ticino (southern region of Switzerland). During the same period, environmental samples were collected from surface waters in the proximity of major water treatment plants. From treatment plants, samples were collected from the raw sewage and before the release of the treated water. From rivers, samples were collected before and after receiving the treated waters. A single-step reverse transcription (RT)-PCR amplification of the entire VP7 gene from extracted double-stranded RNA was developed. For the water samples, a further nested PCR was necessary to increase sensitivity. All amplified viral products were sequenced, and the sequence profile was compared to that of the VP7 genes of human and animal rotaviruses from GenBank. Rotavirus strains are characterized by outer capsid proteins G (glycoprotein) and P (protease-cleaved protein). Correct G genotyping of viral sequences from stool and water samples was possible by analyzing only 189 bp at the 5′ end of the VP7 gene. In the Ticino region, the most predominant G genotype among clinical and water samples was G1. Genotypes G2 and G4 were found only among clinical samples. We also detected rotavirus G1-type sequences in feces from a healthy adult. This finding corroborates the hypothesis that healthy adults act as potential reservoirs for the spread of rotavirus in the environment. In our experiments, this RT-PCR-based method for rotavirus genotyping has proven to be a useful tool for epidemiological investigations.
Group A rotaviruses are responsible for severe gastroenteritis in humans and animals (6). After replication in the gastrointestinal tract, they are excreted and may be dispersed in environmental waters (22). Rotaviruses have been implicated in waterborne gastroenteritis outbreaks in many countries. The stability of human rotaviruses in environmental waters and their resistance to physicochemical treatment processes used by sewage treatment plants may facilitate their transmission (1, 4, 12).
A number of surveys have been performed to examine the rotavirus strains in circulation (26). Rotaviruses are characterized by the outer capsid proteins that are important for virus neutralization: a glycoprotein (G) encoded by gene segment 7, 8, or 9 and the protease-cleaved protein (P), encoded by gene segment 4 (5). Furthermore, strains behave as if there is a local reservoir, since the same strains are found from year to year in the same location. It has been shown, primarily by direct typing of rotaviruses in fecal specimens with monoclonal antibodies, that the most prevalent type G rotavirus strains causing childhood diarrhea worldwide are G1 through G4 (7, 19, 24, 25, 26), although some unusual strains have been documented on rare occasions and confirmed by both G and P typing (3, 17, 22). Efforts to characterize the genetic diversity existing in rotavirus VP4 genes and to identify nontypeable strains observed in some G serotypes has stimulated the development of P and G genotyping methods as a substitute for serotyping (9, 10, 11, 14, 18).
In this study, we demonstrate the usefulness of a simple method for correctly determining the G genotypes circulating in a geographic region of interest, allowing rapid epidemiological surveys of rotaviruses in clinical and environmental samples. Our approach consists of simple concentration of rotaviruses from surface water samples, a single-step reverse transcription (RT)-PCR for amplification of the entire VP7 gene, a nested PCR (nPCR), and subsequent sequence analysis of the 189-bp amplification product.
MATERIALS AND METHODS
Rotavirus-positive stool specimens.
Stool specimens obtained from children who had gastroenteritis and were admitted to the hospitals in Ticino or obtained directly from pediatricians were collected over a 1-year period (from January to December 1998). A total of 36 stool specimens which had been found positive for rotavirus antigen by the latex agglutination method (Slidex Rota-Kit 2; BioMérieux, Geneva, Switzerland) were diluted to 20% in Dulbecco medium, clarified by vortexing and centrifugation, and stored at −20°C.
Collection of water samples.
One-liter samples of surface water and 1-ml samples of raw sewage were collected in sterile glass bottles and tubes, respectively, and stored at 4°C until concentration of the viruses was performed in the laboratory. Water samples were collected approximately each month over a 1-year period (from January to December 1998) from the rivers where major sewage treatment plants are located, i.e., in the three major effluents of Lake Lugano as well as the Ticino River. At the treatment plants, 31 samples were collected from raw sewage, 31 samples were collected from the treated water before release, and 34 samples each were collected from the rivers before and after receiving the treated water.
Concentration of rotaviruses from surface water samples.
Viruses were concentrated from surface water with SiO2. One liter of water was acidified to pH 3.5 with acetic acid, and then 200 μl of autoclaved SiO2 (2, 8, 23; F. Baggi et al., submitted for publication) and AlCl3 (to a final concentration of 0.5 mM) were added. Samples were shaken at room temperature for about 30 min. The SiO2 was sedimented by centrifugation at 7,500 × g for 10 min; alternatively, it was allowed to settle and the supernatant was decanted. After removal of the supernatant, the pellet was transferred to a 1.5-ml tube; after the removal of excess liquid by a short centrifugation step, the viruses were eluted from the SiO2 pellet with 280 μl of glycine buffer (pH 9.5), homogenized, and incubated at 64°C for 10 min. After centrifugation at 12,000 × g for 2 min, the supernatant was collected in a new sterile tube and stored at −80°C until the extraction of nucleic acids was performed.
Concentration of rotaviruses from raw sewage samples.
One milliliter of raw sewage was centrifuged at 6,800 × g for 2 min. The supernatant was collected, and 20 μl of autoclaved SiO2 (2, 8, 23; Baggi et al., submitted) and 50 μl of acetic acid were added. The samples were shaken at room temperature for 10 min and centrifuged at 6,800 × g for 2 min. After removal of the supernatant, virus particles were eluted from the SiO2 pellet as previously described and stored at −80°C until the extraction of nucleic acids was performed.
Extraction of nucleic acids.
RNA was extracted from 140 μl of clarified stool samples or glycine buffer eluates by using the QIAgen viral RNA extraction kit (QIAGEN AG, Basel, Switzerland) and strictly following product manufacturer instructions. The 50-μl RNA eluates were stored at −20°C until the amplification of nucleic acids was performed.
Primers.
Primers END9, R1, R3, and Rp were used for the amplification of sequences from the VP7 gene of group A rotaviruses (16). The oligoprobe Rp corresponds to a conserved region located within the VP7 gene. The sequences, locations, and amplification products of the chosen primers within the rotavirus VP7 gene are given in Table 1.
TABLE 1.
Primers used for rotavirus RT-PCR and nPCR
| Reaction (product size, bp) | Primer | Nucleotide position in genome of human rotavirus Waa | Sequence (5′→3′) |
|---|---|---|---|
| RT-PCR (1,062) | R1 | 1–28 | GGC TTT AAA AGA GAG AAT TTC CGT CTG G |
| END9 | 1036–1062 | GGT CAC ATC ATA CAA TTC TAA TCT AAG | |
| nPCR (189) | R3 | 51–71 | GTA TGG TAT TGA ATA TAC CAC |
| Rp | 239–220 | TCC ATT GAT CCT GTT ATT GG |
Based on the National Center for Biotechnology Information (accession no. K02033).
Single-step RT-PCR.
In order to simplify the amplification procedure, a single-step RT-PCR was developed. RT and PCR were performed with a 100-μl reaction volume. For efficient denaturation of the double-stranded RNA (dsRNA), 7 μl of dimethyl sulfoxide was added to 5 μl of extracted nucleic acid. After denaturation at 97°C for 5 min, the samples were immediately cooled on ice. Following a short centrifugation, the samples were added to a mixture consisting of 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 0.2 mM each deoxyribonucleotide triphosphate (Boehringer), 1 μM primer R1, 1 μM primer END9, 5 U of Superscript-RTII reverse transcriptase (Gibco BRL), 2.5 U of Taq DNA polymerase (Boehringer Mannheim AG, Rotkreuz, Switzerland), and pyrocarbonic acid diethyl ester (DEPC)-treated H2O (Merck Schweiz AG, Dietikon, Switzerland) to a final volume of 100 μl. After incubation for 30 min at 42°C, the following cycling conditions were chosen: 1 min at 94°C, 2 min at 55°C, and 1 min at 72°C, for a total of 25 cycles. A final extension at 72°C for 7 min followed by storage at 4°C concluded the RT-PCR amplification step. A fragment of 1,062 bp was expected.
nPCR.
nPCR was performed with 5 μl of the RT-PCR amplification product under the following conditions: Taq Buffer(+MgCl2) (Boehringer), 0.1 mM each deoxyribonucleotide triphosphate (Boehringer), 1 U of Taq DNA polymerase, 1 μM primer R3, 1 μM primer Rp, and H2O-DEPC in a final volume of 50 μl. The cycling conditions were as follows: 30 s at 94°C, 30 s at 50°C, and 30 s at 72°C for 3 cycles, followed by 27 cycles of 15 s at 94°C, 15 s at 50°C, and 20 s at 72°C. A final extension at 72°C for 2 min followed by storage at 4°C concluded the nPCR amplification step. A fragment of 189 bp was expected.
Amplification control reactions.
Simian rotavirus SA11 dsRNA (kindly provided by M. Weitz, Institute of Clinical Microbiology and Immunology, St. Gallen, Switzerland) was extracted by using the QIAgen viral RNA extraction kit and following product manufacturer instructions. The extracted dsRNA was diluted 100-fold with H2O-DEPC and stored at −20°C until use. In order to detect false negatives due to RT-PCR inhibition, all samples that were found negative by nPCR were retested by RT-PCR after the addition of 5 μl of 100-fold-diluted simian rotavirus SA11 dsRNA as a control.
Visualization of RT-PCR and nPCR products.
RT-PCR fragments (1,062 bp) and nPCR fragments (189 bp) were analyzed by gel electrophoresis. Ten microliters of the amplification products was electrophoresed on 1.5% agarose gels in Tris-borate-EDTA buffer along with a 100-bp DNA ladder (Boehringer). Fragments were visualized after ethidium bromide staining.
Sequencing and analysis of PCR products.
Templates for sequencing were prepared by purification of the amplified products (1,062 or 189 bp) by using a QIAquick PCR purification kit (QIAgen) and following product manufacturer instructions. Sequencing reactions were performed with an ABI Prism dRhodamine dye terminator cycle sequencing ready reaction kit (Perkin-Elmer Applied Biosystems International, Inc.) on a Perkin-Elmer Cycler GeneAmp 9600 system. The cycling conditions followed product manufacturer instructions. Purification of sequencing products, necessary to remove excess dye terminator, was performed using columns from CENTRI SEP Princeton Separations, Inc., Adelphia, N.J., and 10 μl of the purified sequencing products was resuspended in 10 μl of template suppressor reagent. After denaturation at 94°C for 2 min, samples were rapidly cooled on ice, briefly vortexed, centrifuged, and electrophoresed on an ABI Prism 310 instrument (Perkin-Elmer Applied Biosystems) by using the Seq POP6 (1ml)E module. DNA sequences were aligned with the Lasergene program Megalign (DNAstar).
Phylogenetic VP7 sequence analysis and accession numbers.
Selected sequence data were aligned with the Lasergene program Megalign, and statistical and phylogenetic analyses were performed using Molecular Evolutionary Genetics Analysis version 1.01 (15). Phylogenetic trees were reconstructed by the neighbour joining method. The number of nucleotide substitutions per site was estimated by several distance methods, and the robustness of each node was tested by bootstrap analysis with 500 replications. Phylogenetic trees were drawn using TreeExplorer 2.01 (Koichiro Tamura).
Comparisons were made with VP7 sequences of rotaviruses obtained from GenBank (human rotavirus serotype 1, accession numbers M93006 [HG1 1-41] and D16328 [HG1 1-49]; human rotavirus serotype 2, accession numbers D50125 [HG2 2-22] and D50114 [HG2 2-33]; human rotavirus serotype 3, accession numbers D86284 [HG3 1-12] and D86266 [HG3 1-30]; human rotavirus serotype 4, accession number A01321 [HG4 5-02]; human rotavirus serotype 8, accession number AF034852 [HG8 1-11]; rotavirus serotype 8 isolated from a river, accession number AF039524 [RG81-09]; human group C rotavirus, accession numbers D87544 [HGC 1-39] and D87543 [HGC 1-40]; avian rotavirus VP7 sequence, accession number L01098 [AVI 7-10]; canine rotavirus VP7 sequence, accession number U97199 [CAN 2-37]; chicken rotavirus VP7 sequence, accession number X56784 [CHI 5-01]; equine rotavirus VP7 sequence, accession numbers D13549, D00843, and X57785 [EQU 2-18]; lamb rotavirus VP7 sequence, accession number L11602 [LAM 7-30]; lapin rotavirus VP7 sequence, accession number U62153 [LAP 1-35]; murine rotavirus VP7 sequence, accession numbers AF039220 [MUR 1-10], D45216 [MUR 1-33], and U08425 [MUR 6-01]; porcine rotavirus VP7 sequence, accession numbers X04613 [POR 3-31] and U35850 [POR 3-32]; and porcine group C rotavirus, accession number M61101 [POR 7-02]).
Sensitivity of rotavirus concentration from water samples.
The sensitivity of virus concentration from water samples was determined as follows: six 1-liter sterile water samples were spiked with serial dilutions of simian rotavirus SA11 (final concentrations, 3.2 × 104, 3.2 × 102, 3.2 × 101, 3.2, 3.2 × 10−1, and 3.2 × 10−2 50% tissue culture infective doses [TCID50]/liter, respectively). Concentration, elution, extraction, and amplification were performed as previously described.
RESULTS
Evaluation of the sensitivity of rotavirus concentration from water samples.
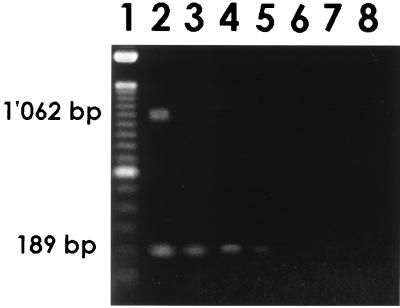
Only the sample seeded with 3.2 × 104 TCID50/liter displayed the 1,062-bp RT-PCR fragment; nPCR increased the sensitivity to 3.2 TCID50/liter (Fig. 1).
FIG. 1.
Sensitivity of rotavirus concentration from 1 liter of water, as analyzed by electrophoresis of amplification products. Lanes: 1, 100-bp DNA ladder; 2, 3.2 × 104 TCID50/liter; 3, 3.2 × 102 TCID50/liter; 4, 3.2 × 101 TCID50/liter; 5, 3.2 TCID50/liter; 6, 3.2 × 10−1 TCID50/liter; 7, 3.2 × 10−2 TCID50/liter; 8, RT-PCR and nPCR negative controls.
Phylogenetic analysis of rotavirus VP7 complete (1,062-bp) and partial (189-bp) sequences.
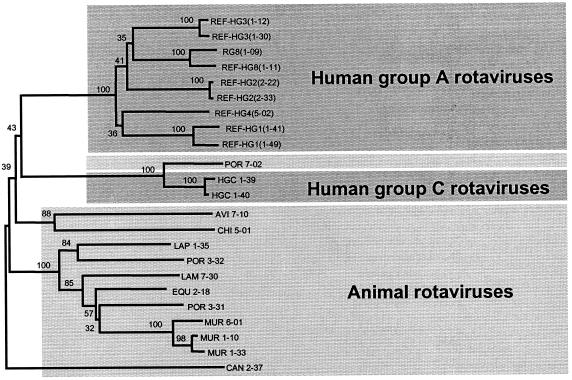
Sixty-two human VP7 sequences of group A rotavirus deposited in GenBank were selected to represent all the major VP7 serotypes (serotype G1, 12 sequences; serotype G2, 17 sequences; serotype G3, 20 sequences; serotype G4, 11 sequences; and serotype G8, 2 sequences). Two published human VP7 sequences of group C rotavirus were also included. Alignment and phylogenetic analysis of both the whole, 1,062-bp VP7 gene (RT-PCR product) and the 189-bp fragment (nPCR product) were performed as previously described in Materials and Methods. Results of both analyses were in agreement, allowing correct G genotyping of all sequences by examination of only the 189-bp nPCR product. In order to determine whether we could distinguish between animal VP7 sequences which could be found in environmental water samples and sequences of human origin, phylogenetic analysis of the 189-bp fragment included different published animal rotavirus VP7 sequences as well. All sequences of nonhuman animal origin generated distinct clusters clearly distinguishable from the human group A rotavirus sequences; therefore, it is possible to correctly discriminate between sequences of human and animal origins. Part of the phylogenetic sequence analysis is displayed in Fig. 2.
FIG. 2.
Phylogenetic sequence analysis of rotavirus VP7 sequences (189 bp) of human and animal origins (neighbor joining tree): human group A rotavirus sequences from different serotypes (REF strains); RG81-09 (rotavirus type 8 isolated from a river); HGC 1-39 and HGC 1-40 (human group C rotavirus); AVI 7-10 (avian rotavirus); CAN 2-37 (canine rotavirus); CHI 5-01 (chicken rotavirus); EQU 2-18 (equine rotavirus); LAM 7-30 (lamb rotavirus); LAP 1-35 (lapin rotavirus); MUR 1-10, MUR 1-33, and MUR 6-01 (murine rotavirus); POR 3-31 and POR 3-32 (porcine rotavirus); and POR 7-02 (porcine group C rotavirus). Numbers indicate percent similarity.
Phylogenetic analysis of rotavirus VP7 partial (189-bp) sequences from stool and water samples.
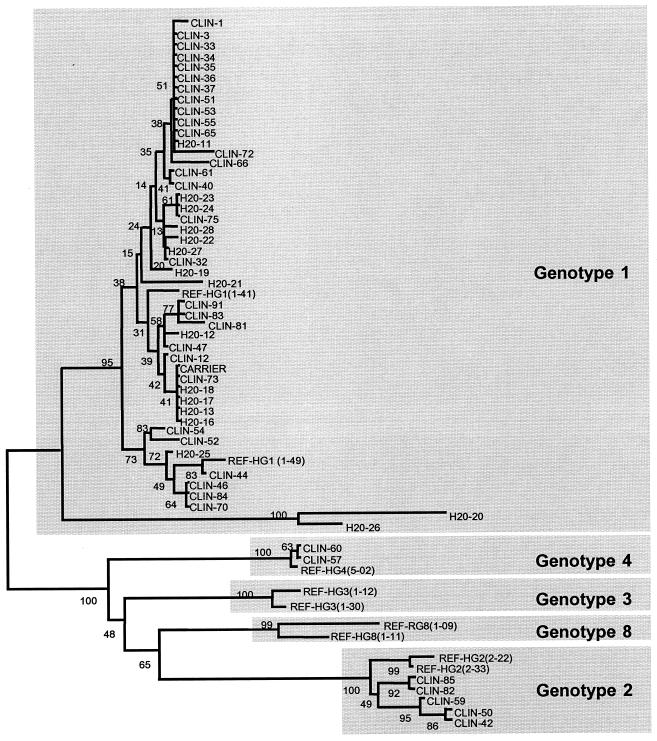
After RT-PCR amplification, all 36 clinical samples displayed the 1,062-bp fragment, and sequencing was performed with both R1 and END9 primers. After phylogenetic analysis of the corresponding 189-bp sequence, 29 patients displayed type G1 sequences, 5 patients exhibited type G2 sequences, and 2 patients had type G4 sequences. No sequences matching type G3 or G8 were found. For environmental water samples, the use of nPCR was indispensable in order to enhance the sensitivity of detection. In fact, all positive water samples were negative after RT-PCR but positive after nPCR. The absence of inhibitors during nucleic acid amplification was assayed for each nPCR-negative sample. A total of 16 positive water samples were found and were sequenced with both R3 and Rp primers. All samples were classified as G1. The positive water samples were found in January (seven positive samples), May (five positive samples), and June (four positive samples), in accordance with the observation that rotavirus infections have peak occurrences in winter and spring months. The results of the phylogenetic sequence analysis of all positive samples are displayed in Fig. 3.
FIG. 3.
Phylogenetic sequence analysis (189 bp) of rotavirus-positive samples (neighbor joining tree) (CLIN, clinical stool samples [from children]; H2O, water samples; CARRIER, stool sample from a healthy adult; and REF, human group A rotavirus sequences from different serotypes [HG1, HG2, HG3, HG4, and HG8]).
Carriers of rotaviruses.
In order to assess if healthy adults could serve as reservoirs for rotaviruses found in environmental water or raw sewage, 20 stool samples from healthy, asymptomatic adults (collected for routine sanitary inquiries) were analyzed by both RT-PCR and nPCR. All samples but one were found negative by nPCR. Sequence analysis of the positive sample revealed type G1 (Fig. 3).
DISCUSSION
The present study demonstrates the efficiency of a single-step RT-PCR followed by nPCR and subsequent sequence analysis of a 189-bp product from the 5′ end of the VP7 gene for characterizing rotavirus contamination of environmental water samples and the usefulness of these techniques for studying epidemiological relationships between strains involved in human infections and those found in water. The single-step RT-PCR was developed in order to avoid reopening of the tubes and therefore to reduce the danger of cross contamination. Furthermore, this strategy reduced the technical steps necessary to perform the analysis while maintaining good sensitivity and specificity for detecting low viral loads in both human and water samples. Concentration of viruses from water using SiO2 is easy, inexpensive, and rapid and has good sensitivity and specificity. An nPCR result can be obtained 24 h after sampling, and a sequence profile can be obtained after a further 24 h. These properties allow rapid (within 48 h) detection of possible contamination sources, allowing appropriate preventive measures to be undertaken in time. Inhibitors were efficiently removed by RNA purification and extraction with the QIAgen viral RNA extraction kit.
Raw sewage and secondary effluent samples were found to be contaminated by rotaviruses despite the most efficient water treatment measures in use in sewage treatment plants (Baggi et al., submitted). Sequence analysis of the positive water samples revealed the presence of only rotavirus type G1, whereas among the clinical samples, type G1 predominated but types G2 and G4 also could be found. The fact that in healthy adults rotavirus could be detected by nPCR sustains a possible link between rotaviruses found in the environment and those found in clinical samples. In fact, our results support the presumption that healthy adults may serve as reservoirs for the spread of rotaviruses (12, 13, 20, 21). The knowledge of the sensitivity of detection of rotaviruses by RT-PCR and nPCR (3.2 × 104 and 3.2 TCID50/liter, respectively) makes it possible to gain useful information about the levels of rotavirus that one healthy adult may be shedding.
The phylogenetic analysis of different animal rotavirus VP7 sequences resulted in a clearly distinct cluster that did not match any of the human group A rotavirus sequences. This is a very fortuitous finding, because it allows the application of this molecular technique to environmental water samples to correctly discriminate between sequences of human and animal origins.
In conclusion, the present study demonstrates the efficiency of the nucleotide sequence analysis of 189 bp at the 5′ end of the VP7 gene for studying epidemiological relationships between strains involved in human infections and those found in water. From the sequence analysis, it was possible to predict the G genotype and to evaluate possible correlations between water contamination and viral sequences found among clinical samples.
ACKNOWLEDGMENT
Special thanks are due to Reinhard Zbinden, Institute for Clinical Microbiology, University of Zurich, Zurich, Switzerland, for critically reading the manuscript.
REFERENCES
- 1.Ansari S A, Springthorpe V S, Sattar S A. Survival and vehicular spread of human rotaviruses: possible relation to seasonality of outbreaks. Rev Infect Dis. 1991;13:448–461. doi: 10.1093/clinids/13.3.448. [DOI] [PubMed] [Google Scholar]
- 2.Boom R, Sor C J A, Salilmans M M M, Jansen C L, Wertheim-Van Dillen P M E, Van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark H F, Hoshino Y, Bell L M, Groff J, Hess G, Bachman P, Offit P A. Rotavirus isolate W161 representing a presumptive new human serotype. J Clin Microbiol. 1987;25:1757–1762. doi: 10.1128/jcm.25.9.1757-1762.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook S M, Glass R I, LeBaron C W, Ho M S. Global seasonality of rotavirus infections. Bull W H O. 1990;68:171–177. [PMC free article] [PubMed] [Google Scholar]
- 5.Estes M K, Cohen J. Rotavirus gene structure and function. Microbiol Rev. 1989;53:410–449. doi: 10.1128/mr.53.4.410-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Estes M K, Palmer E L, Obijeski J F. Rotaviruses: a review. Curr Top Microbiol Immunol. 1983;105:123–184. doi: 10.1007/978-3-642-69159-1_3. [DOI] [PubMed] [Google Scholar]
- 7.Flores J, Taniguchi K, Green K, Perez-Schael I, Garcia D, Sears J, Urasawa S, Kapikian A Z. Relative frequencies of rotavirus serotypes 1, 2, 3, and 4 in Venezuelan infants with gastroenteritis. J Clin Microbiol. 1988;26:2092–2095. doi: 10.1128/jcm.26.10.2092-2095.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gakardo R, Diez J M, Jofre J, Bosch A. Adsorpion-elution with negatively and positively charged glass powder for the concentration of hepatitis A virus from water. J Virol Methods. 1991;31:345–352. doi: 10.1016/0166-0934(91)90172-v. [DOI] [PubMed] [Google Scholar]
- 9.Gerna G, Steele A D, Hoshino Y, Sereno M, Garcia D, Sarasini A, Flores J. A comparison of the VP7 gene sequences of human and bovine rotaviruses. J Gen Virol. 1994;75:1781–1784. doi: 10.1099/0022-1317-75-7-1781. [DOI] [PubMed] [Google Scholar]
- 10.Green K Y, Sears J F, Taniguchi K, Midthun K, Hoshino Y, Gorziglia M, Nishikawa K, Urasawa S, Kapikian A Z, Chanock R M. Prediction of human rotavirus serotype by nucleotide sequence analysis of the VP7 protein gene. J Virol. 1988;62:1819–1823. doi: 10.1128/jvi.62.5.1819-1823.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green K Y, Hoshino Y, Ikegami N. Sequence analysis of the gene encoding the serotype-specific glycoprotein (VP7) of two new human rotavirus serotypes. Virology. 1989;168:429–433. doi: 10.1016/0042-6822(89)90289-4. [DOI] [PubMed] [Google Scholar]
- 12.Hejkal T W, Smith E M, Gerba C P. Seasonal occurrence of rotavirus in sewage. Appl Environ Microbiol. 1984;47:588–590. doi: 10.1128/aem.47.3.588-590.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horst H, Kohlhase B. Asymptomatic excretors of rotavirus. Infection. 1986;14:163–166. doi: 10.1007/BF01645255. [DOI] [PubMed] [Google Scholar]
- 14.Husain M, Pradeep S, Dar L, Broor S. Classification of rotavirus into G and P types with specimens from children with acute diarrhea in New Delhi, India. J Clin Microbiol. 1996;34:1592–1594. doi: 10.1128/jcm.34.6.1592-1594.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar S, Tamura K, Nei M. MEGA: molecular evolutionary genetic analysis software for microcomputers. Comput Appl Biosci. 1994;10:189–199. doi: 10.1093/bioinformatics/10.2.189. [DOI] [PubMed] [Google Scholar]
- 16.Le Guyader F, Dubois E, Menard D, Pommepuy M. Detection of hepatitis A virus, rotavirus, and enterovirus in naturally contaminated shellfish and sediment by reverse transcription-seminested PCR. Appl Environ Microbiol. 1994;60:3665–3671. doi: 10.1128/aem.60.10.3665-3671.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li B, Clark H F, Gouvea V. Nucleotide sequence of the VP4-encoding gene of an unusual human rotavirus (HCR3) Virology. 1993;196:825–830. doi: 10.1006/viro.1993.1540. [DOI] [PubMed] [Google Scholar]
- 18.Mason B B, Dheer S K, Hsiao C L, Zandle G, Kostek B, Rosanoff E I, Hung P P, Davis A R. Sequence of the serotype-specific glycoprotein of the human rotavirus Wa strain and comparison with other human rotavirus serotypes. Virus Res. 1985;2:291–299. doi: 10.1016/0168-1702(85)90026-7. [DOI] [PubMed] [Google Scholar]
- 19.Matson D O, Estes M K, Burns J W, Greenberg H B, Taniguchi K, Urasawa S. Serotype variation of human group A rotaviruses in two regions of the USA. J Infect Dis. 1990;162:605–614. doi: 10.1093/infdis/162.3.605. [DOI] [PubMed] [Google Scholar]
- 20.Menhert D U, Stewien K E. Detection and distribution of rotavirus in raw sewage and creeks in Sao Paulo, Brazil. Appl Environ Microbiol. 1993;59:140–143. doi: 10.1128/aem.59.1.140-143.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richardson S, Grimwood K, Gorrell R, Palombo E, Barnes G, Bishop R. Extended excretion of rotavirus after severe diarrhoea in young children. Lancet. 1998;351:1844–1848. doi: 10.1016/S0140-6736(97)11257-0. [DOI] [PubMed] [Google Scholar]
- 22.Santos N, Riepenhoff-Talty M, Clark H F, Offit P, Gouvea V. VP4 genotyping of human rotavirus in the United States. J Clin Microbiol. 1994;32:205–208. doi: 10.1128/jcm.32.1.205-208.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartzbrot L, Lucerna-Gutierrez F. Concentration des entérovirus dans les eaux par absorption sur poudre de verre: proposition d'un appareillage simplifié. Microbia. 1978;4:55–68. [Google Scholar]
- 24.Taniguchi K, Urasawa T, Morita Y, Greenberg H B, Urasawa S. Direct serotyping of human rotavirus in stools by an enzyme-linked immunosorbent assay using serotype 1-, 2-, 3-, and 4-specific monoclonal antibodies to VP7. J Infect Dis. 1987;155:1159–1166. doi: 10.1093/infdis/155.6.1159. [DOI] [PubMed] [Google Scholar]
- 25.Urasawa S, Urasawa T, Taniguchi K, Wakasugi F, Kobayashi N, Chiba S, Sakurada N, Morita M, Morita O, Tokieda M, et al. Survey of human rotavirus serotypes in different locales in Japan by enzyme-linked immunosorbent assay with monoclonal antibodies. J Infect Dis. 1989;160:44–51. doi: 10.1093/infdis/160.1.44. [DOI] [PubMed] [Google Scholar]
- 26.Woods P A, Gentsch J, Gouvea V, Mata L, Santosham M, Urasawa S, Glass R I. Distribution of serotypes of human rotavirus in different populations. J Clin Microbiol. 1992;30:781–785. doi: 10.1128/jcm.30.4.781-785.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]