Abstract
Background
Irritable bowel syndrome (IBS) is a common chronic gastrointestinal disorder. The role of pharmacotherapy for IBS is limited and focused mainly on symptom control.
Objectives
The objective of this systematic review was to evaluate the efficacy of bulking agents, antispasmodics and antidepressants for the treatment of irritable bowel syndrome.
Search methods
Computer assisted structured searches of MEDLINE, EMBASE, The Cochrane library, CINAHL and PsychInfo were conducted for the years 1966‐2009. An updated search in April 2011 identified 10 studies which will be considered for inclusion in a future update of this review.
Selection criteria
Randomized controlled trials comparing bulking agents, antispasmodics or antidepressants with a placebo treatment in patients with irritable bowel syndrome aged over 12 years were considered for inclusion. Only studies published as full papers were included. Studies were not excluded on the basis of language. The primary outcome had to include improvement of abdominal pain, global assessment or symptom score.
Data collection and analysis
Two authors independently extracted data from the selected studies. Risk Ratios (RR) and Standardized Mean Differences (SMD) with 95% confidence intervals (CI) were calculated. A proof of practice analysis was conducted including sub‐group analyses for different types of bulking agents, spasmolytic agents or antidepressant medication. This was followed by a proof of principle analysis where only the studies with adequate allocation concealment were included.
Main results
A total of 56 studies (3725 patients) were included in this review. These included 12 studies of bulking agents (621 patients), 29 of antispasmodics (2333 patients), and 15 of antidepressants (922 patients). The risk of bias was low for most items. However, selection bias is unclear for many of the included studies because the methods used for randomization and allocation concealment were not described. No beneficial effect for bulking agents over placebo was found for improvement of abdominal pain (4 studies; 186 patients; SMD 0.03; 95% CI ‐0.34 to 0.40; P = 0.87), global assessment (11 studies; 565 patients; RR 1.10; 95% CI 0.91 to 1.33; P = 0.32) or symptom score (3 studies; 126 patients SMD ‐0.00; 95% CI ‐0.43 to 0.43; P = 1.00). Subgroup analyses for insoluble and soluble fibres also showed no statistically significant benefit. Separate analysis of the studies with adequate concealment of allocation did not change these results. There was a beneficial effect for antispasmodics over placebo for improvement of abdominal pain (58% of antispasmodic patients improved compared to 46% of placebo; 13 studies; 1392 patients; RR 1.32; 95% CI 1.12 to 1.55; P < 0.001; NNT = 7), global assessment (57% of antispasmodic patients improved compared to 39% of placebo; 22 studies; 1983 patients; RR 1.49; 95% CI 1.25 to 1.77; P < 0.0001; NNT = 5) and symptom score (37% of antispasmodic patients improved compared to 22% of placebo; 4 studies; 586 patients; RR 1.86; 95% CI 1.26 to 2.76; P < 0.01; NNT = 3). Subgroup analyses for different types of antispasmodics found statistically significant benefits for cimteropium/ dicyclomine, peppermint oil, pinaverium and trimebutine. Separate analysis of the studies with adequate allocation concealment found a significant benefit for improvement of abdominal pain. There was a beneficial effect for antidepressants over placebo for improvement of abdominal pain (54% of antidepressants patients improved compared to 37% of placebo; 8 studies; 517 patients; RR 1.49; 95% CI 1.05 to 2.12; P = 0.03; NNT = 5), global assessment (59% of antidepressants patients improved compared to 39% of placebo; 11 studies; 750 patients; RR 1.57; 95% CI 1.23 to 2.00; P < 0.001; NNT = 4) and symptom score (53% of antidepressants patients improved compared to 26% of placebo; 3 studies; 159 patients; RR 1.99; 95% CI 1.32 to 2.99; P = 0.001; NNT = 4). Subgroup analyses showed a statistically significant benefit for selective serotonin releasing inhibitors (SSRIs) for improvement of global assessment and for tricyclic antidepressants (TCAs) for improvement of abdominal pain and symptom score. Separate analysis of studies with adequate allocation concealment found a significant benefit for improvement of symptom score and global assessment. Adverse events were not assessed as an outcome in this review.
Authors' conclusions
There is no evidence that bulking agents are effective for treating IBS. There is evidence that antispasmodics are effective for the treatment of IBS. The individual subgroups which are effective include: cimetropium/dicyclomine, peppermint oil, pinaverium and trimebutine. There is good evidence that antidepressants are effective for the treatment of IBS. The subgroup analyses for SSRIs and TCAs are unequivocal and their effectiveness may depend on the individual patient. Future research should use rigorous methodology and valid outcome measures.
Keywords: Humans, Abdominal Pain, Abdominal Pain/therapy, Antidepressive Agents, Antidepressive Agents/therapeutic use, Dietary Fiber, Dietary Fiber/therapeutic use, Irritable Bowel Syndrome, Irritable Bowel Syndrome/therapy, Parasympatholytics, Parasympatholytics/therapeutic use, Phytotherapy, Phytotherapy/methods, Plantago, Randomized Controlled Trials as Topic
Plain language summary
Bulking agents, antispasmodics and antidepressants for the treatment of irritable bowel syndrome
This review evaluates the effectiveness of medical therapies for patients with irritable bowel syndrome (IBS). We considered studies involving bulking agents (a fibre supplement), antispasmodics (smooth muscle relaxants) or antidepressants (drugs used to treat depression that can also change pain perceptions) that used outcome measures including improvement of abdominal pain, global assessment (overall relief of IBS symptoms) or symptom score. We found that bulking agents are not effective for treating IBS. There is evidence that antispasmodics including cimetropium/dicyclomine peppermint oil, pinaverium and trimebutine are effective for the treatment of IBS. Antidepressants are effective for the treatment of IBS. The side effects of these medications were not evaluated in this review. Physicians should be aware of the limitations of drug therapies and discuss these limitations with their patients before prescribing medication for IBS.
Background
Irritable bowel syndrome (IBS) is a chronic gastrointestinal disorder characterized by fluctuating complains of abdominal pain or discomfort and an altered bowel habit resulting in diarrhoea or constipation. The prevalence of IBS ranges from 5‐18 % depending on the clinical setting and the diagnostic criteria that are used. IBS is slightly more common in females (Hungin 2003; Hillila 2004). IBS is associated with depressive and anxiety disorders as well with somatic co‐morbidities including fibromyalgia, chronic fatigue syndrome and chronic pelvic pain (Riedl 2008).
Research shows that IBS can result in impaired health‐related quality of life and that IBS symptoms have a large impact on work productivity (Pare 2006; Creed 2001). IBS is also associated with increased health care utilization and costs (Longstreth 2003).
In the absence of a gold‐standard for diagnosing IBS, classification models have been developed including the Kruis scoring system, Manning criteria and Rome I, II, and III criteria (Manning 1978; Drossman 2006). Only the Rome I classification is validated (Ford 2010). These criteria are not widely used in clinical practice and the diagnosis of IBS is often made by a typical history, normal physical examination and the absence of alarm‐symptoms such as gastrointestinal bleeding, weight loss, or an abdominal mass (Jones 2000a).
The pathophysiology of IBS is still unclear. There are several putative mechanisms including visceral hypersensitivity, altered colonic motility, abnormal brain activation, serotine dysregulation, inflammation, abnormal colonic flora, stress, psychological factors and genetic factors (Talley 2006).
In the absence of a clear pathophysiology, explanation and reassurance are essential elements in the management of IBS (Jones 2000b). Pharmacotherapeutic interventions are limited and focus mostly on symptom control. High fibres diets and bulking agents are traditionally advised for their effect on stools and transit time (Burkitt 1972). Antispasmodics are given for their supposed effect on gastrointestinal motility. The more recent therapeutic options include the use of antidepressants, which are also given for other diseases associated with chronic pain (Verdu 2008).
Objectives
The objective of this systematic review was to evaluate the efficacy of bulking agents, antispasmodics and antidepressants for the treatment of irritable bowel syndrome.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials comparing bulking agents, antispasmodics or antidepressants with a placebo were considered for inclusion. Cross‐over studies were eligible if data from the first phase were reported separately. Only studies published as a full paper were included. Studies were not excluded on the basis of language.
Types of participants
Patients aged over 12 years with irritable bowel syndrome, diagnosed either using predefined diagnostic criteria (e.g. Manning or Rome) or on clinical grounds were considered for inclusion. Studies including patients with functional bowel disorders without separate data for IBS patients were also included if the proportion of IBS patients was more than 75% of the total included patients.
Types of interventions
Interventions including bulking agents, antispasmodics or antidepressants compared with a placebo treatment were considered for inclusion.
Types of outcome measures
Outcome measures for clinical trials of interventions in IBS have been discussed in several studies (Irvine 2006; Schoenfeld 2006). Primary outcome measures included:
Improvement of symptoms of abdominal pain;
Improvement of patients overall global assessment; and
Improvement of IBS‐symptom score.
Subgroup analyses included:
Soluble and insoluble bulking agents;
Individual antispasmodics; and
Selective serotonin releasing inhibitors (SSRIs) and tricyclic antidepressants (TCAs).
A sensitivity analysis excluded studies with poor methodological quality.
Search methods for identification of studies
Electronic searches
Computer assisted structured searches of MEDLINE, EMBASE, The Cochrane Library, CINAHL and PsychInfo were conducted, searching entries from 1966 to March 2009. The following search strategies were used:
Title/abstract search: spastic colon, irritable colon, irritable bowel, functional bowel, colonic disease, colonic diseases, IBS, gastrointestinal syndrome, gastrointestinal syndromes
Combined with title/abstract search: bulking agent, bulking agents, fiber, fibers, fibre, fibres, psyllium, plantago ovata, husk, bran, ispaghula, wheat, oat, sterculia, karaya gum
Or combined with title/abstract search: antispasmodic, antispasmodics, parasympatholytic, parasympatholytics. spasmolytic , spasmolytics, mebeverine, rociverine, pinaverium bromide, otilonium bromide, cimetropium bromide, trimebutine, pirenzipine, alverine, scopolamine, butylscopolamine, hyoscine , muscarinic antagonist, peppermint oil, mint oil
Or combined with title/abstract search: antidepressant, antidepressants, antidepressive agent, antidepressive agents, tricyclic, TCA, TCAs, selective serotonin reuptake inhibitor, selective serotonin reuptake inhibitors, SSRI, SSRIs
No limits or filters were used.
An updated search in April 2011 identified 10 studies which will be considered for inclusion in a future update of this review (See Characteristics of studies awaiting classification).
Searching other resources
The reference lists of the retrieved articles and reviews were hand searched to identify additional studies.
Data collection and analysis
Selection of studies
One author (LR) screened the title and abstract of all studies identified by the literature searches for eligibility . The full text articles were retrieved for all potentially eligible studies. The full text articles were screened according to predefined criteria by the same reviewer. All the doubtful articles were screened by a second reviewer (AOQ) and consensus on inclusions/ exclusion was achieved by discussion.
The predefined exclusion criteria included:
Not a randomised controlled trial
No placebo group
Inappropriate patient group
Patients younger than 12, diagnosis of functional bowel disorders not specified as IBS
Intervention not involving bulking agent, antispasmodic or antidepressant or mixed preparations
An outcome measurement other than abdominal pain, global assessment or IBS‐symptom score.
No extractable results or cross‐over trial with no report of first phase data
Duplicate trials
Data extraction and management
All studies were blinded for the reviewers in respect of authors, date of publication and journal or database of publication. Data were extracted independently by two authors for each study. A standardized data extraction form was used. Where necessary data were extracted from figures. If essential data were absent, the author of the article was contacted and requested to provide additional information.
Assessment of risk of bias in included studies
The methodological quality of included trials was independently assessed by two authors (AOQ and LR or NdW or GR).
Quality assessment criteria included: method of randomisation, concealment of allocation, blinding of patients and outcome measurers and description of lost to follow‐up.
Differences of opinion were resolved by discussion between two reviewers, and in case of disagreement by all the reviewers. A methodology expert (GvdH) was consulted for specific queries
In consultation with the Dutch Cochrane centre, concealment of allocation was used for additional analyses, since concealment of allocation is the only quality item that has proven to be associated with study outcome (Pidal 2007, Wood 2008).
Measures of treatment effect
The analyses were conducted using RevMan 5.0 software. For the dichotomous outcomes the risk ratio (RR) with 95% confidence intervals were calculated. For the continuous data standardized mean difference (SMD) with 95% confidence intervals were calculated.
A fixed‐ or random‐effects model was used, based on the heterogeneity between study data. Statistical heterogeneity was explored with the Chi square test with significance set at P <0.10. When statistically significant heterogeneity occurred, a random‐effects model was used for the analyses.
There were differences in the direction of the scales for the continuous data. To correct for this the data from scales increasing with disease severity were multiplied by ‐1.
First, a proof of practice analysis was conducted, including all available data. This was followed by a proof of principle analysis where only the studies with adequate allocation concealment were included.
Results
Description of studies
Bulking agents
The search identified 1118 studies, of which, after screening title and abstract 72 were potentially eligible. After applying the exclusion criteria to the full‐text publications of these 72 potentially eligible studies, 12 articles remained for review and meta‐analysis.
Sixty articles were excluded (table of characteristics of excluded studies), of which twenty‐six were not randomized controlled trials (exc1). Fourteen studies did not involve a placebo treatment (exc2). Four studies included patients with functional bowel disorders without providing extractable results for the patients with IBS (exc3 and exc4). Two studies involved an intervention with a mixed preparation (exc5). Two studies did not report the outcome of interest (exc6). Eleven studies were cross‐over trials with no report of the first phase data or did not provide extractable results (exc7). One study was a duplicate publication (exc8).
Twelve papers remained for review and meta‐analysis (Table of characteristics of included studies). Two studies had a cross‐over design (Jalihal 1990; Lucey 1987). The studies were published between 1976 and 2005 (Soltoft 1976; Rees 2005). The research setting was a GI out patients’ clinic in 11 studies, in the other three the setting was unclear (Arthurs 1983; Longstreth 1981; Soltoft 1976). Seven studies used a run‐in period of 1 to 4 weeks before beginning the actual trial (Fowlie 1992; Longstreth 1981; Prior 1987; Rees 2005; Soltoft 1976). The studies included between 20 and 168 participants (Jalihal 1990; Nigam 1984). The mean age of the participants ranged from 28 years to 46 years (Arthurs 1983; Aller 2004). The percentage of female participants included ranged from 20% to 83% (Jalihal 1990; Longstreth 1981). Six studies used insoluble fibres as intervention (Aller 2004; Fowlie 1992; Kruis 1986; Lucey 1987; Rees 2005; Soltoft 1976) and six studies used soluble fibres as intervention (Arthurs 1983; Jalihal 1990; Longstreth 1981; Nigam 1984; Prior 1987; Ritchie 1979). The intervention period lasted from 4 weeks (Ritchie 1979) to 16 weeks (Kruis 1986).
Antispasmodics
The search identified 444 studies, of which, after screening title and abstract, 144 were potentially eligible. After applying the exclusion criteria to the full‐text publications of these 144 potentially eligible studies, 29 articles remained for review and meta‐analysis.
One hundred and fifteen articles were excluded (table of characteristics of excluded studies). Thirty‐eight were not randomised controlled trials (exc1). Thirty four studies did not involve a placebo treatment (exc2). Six studies included patients with functional bowel disorders without providing extractable results for the patients with IBS (exc3 and exc4). Three studies involved an intervention with a mixed preparation (exc5). Twelve studies did not report the outcome of interest (exc6). Nineteen studies had no extractable results or were cross‐over trials with no report of the first phase data (exc7). Three were duplicate publications (exc8) (Baldi 1992; Glende 2002; Koch 1998).
Twenty‐nine papers remained review and meta‐analysis (Table of characteristics of included studies; Awad 1995; Baldi 1991; Battaglia 1998; Capanni 2005; Cappello 2007; Centonze 1988; Chen 2004; Czalbert 1990; d'Arienzo 1980; Delmont 1981; Dobrilla 1990; Dubarry 1977; Fielding 1980; Ghidini 1986; Gilvarry 1989; Kruis 1986; Lech 1988; Levy 1977; Liu 1997; Mitchell 2002; Moshal 1979; Nigam 1984; Page 1981; Passaretti 1989a; Piai 1979; Pulpeiro 2000; Ritchie 1979; Schafer 1990; Virat 1987). Two studies had a cross‐over design (Moshal 1979; Piai 1979). The studies were published between 1977 and 2007 (Levy 1977; Cappello 2007). The research setting was definitely defined as secondary care in 18 studies, none of the studies was definitely conducted in primary care. Fourteen studies used a run‐in period of 1 to 4 weeks before beginning the actual trial. Five of these used a placebo during the run‐in period and one used a high fibre diet (Gilvarry 1989). The studies included between 18 (Piai 1979) and 360 participants (Schafer 1990). The mean age of the participants ranged from 26 years (Fielding 1980) to 60.6 years (Baldi 1991). The percentage of female participants included ranged from 35% (Moshal 1979) to 100% (Awad 1995). The intervention period lasted from 1 week (Virat 1987) to 6 months (Centonze 1988). The antispasmodics are divided into ten pharmacological subgroups: Alverine (1 study), cimetropium/dicyclomine (4 studies), mebeverine (2 studies), Otilonium (6 studies), peppermint oil (5 studies), pinaverium (6 studies),pirenzepine (1 study), propinox (1 study), scopolamine derivates (4 studies), and trimebutine (3 studies).
Antidepressants
The search identified 419 studies, of which, after screening title and abstract 56 were potentially eligible. After applying the exclusion criteria on the full‐text publications of these 56 potentially eligible studies, 15 articles remained for review and meta‐analysis.
Forty articles were excluded (table of characteristics of excluded studies). Twenty were not randomized controlled trials (exc1). Six studies did not involve a placebo treatment (exc2). One study involved an intervention with a mixed preparation (exc5). Four studies did not report the outcome of interest (exc6). Three studies had no extractable results or were cross‐over trials with no report of the first phase data (exc7). Six studies were duplicate publications (exc8) (Kalpert 2005, Han 2009; Marks 2008; Block 1983; Tripathi 1983; Greenbaum 1987).
Fifteen studies remained for review and meta‐analysis (table of characteristics of included studies). One study had a cross‐over design (Tack 2006a). The studies were published between 1978 and 2009 (Heefner 1978; Masand 2009). Two studies were partly conducted in primary care (Myren 1982; Boerner 1988), the remainder in secondary care. Three studies used a run‐in period of 1 to 2 weeks before beginning the actual trial (Rajagopalan 1998; Tack 2006a; Talley 2008a). One study had a placebo run‐in period of unspecified duration (Masand 2009). One study randomised patients who had completed a 7 week open‐label high‐fibre trial (Tabas 2004). The studies included between 23 and 201 participants (Tack 2006a; Drossman 2003). The mean age of the participants ranged from 32 years to 49 years (Masand 2009). The percentage of female participants included ranged from 13% (Heefner 1978) to 100% (Drossman 2003). Five studies used a SSRI as the intervention (Kuiken 2003; Masand 2009; Tabas 2004; Tack 2006a; Vahedi 2005). Nine studies used a TCA the as intervention (Bahar 2008; Myren 1982; Boerner 1988; Drossman 2003; Heefner 1978; Rajagopalan 1998; Vahedi 2008; Vij 1991). One study compared an SSRI and a TCA with placebo treatment (Talley 2008a). The intervention period lasted from 4 weeks (Myren 1982) to 12 weeks (Vahedi 2005).
Risk of bias in included studies
The results of the risk of bias assessment are shown in Figure 1.The risk of bias was low for most items. However, selection bias is unclear for many of the included studies because the methods used for randomization and allocation concealment were not described.
1.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Bulking agents None of the twelve studies on bulking agents described the methods used for randomization and these studies were rated as unclear for this item. Five of the studies were rated as low risk for allocation concealment (Fowlie 1992; Jalihal 1990; Longstreth 1981; Ritchie 1979; Soltoft 1976). The other seven studies were rated as unclear for allocation concealment.
Antispasmodics Three studies reported on the methods used for randomization (Capanni 2005; Cappello 2007; Piai 1979) and were rated as low risk for this item. The other twenty‐six studies did not report on methods used for randomization and were rated as unclear. Four studies were rated as low risk for allocation concealment (Awad 1995; Chen 2004; Pulpeiro 2000; Ritchie 1979). The other studies were rated as unclear for this item.
Antidepressants Seven studies were reported the methods used for randomization and were rated as low risk for this item (Drossman 2003; Kuiken 2003; Tabas 2004; Talley 2008a; Vahedi 2005; Vahedi 2008; Vij 1991). The other studies were rated as unclear. Six studies were rated as low risk for allocation concealment (Drossman 2003; Kuiken 2003; Tabas 2004; Talley 2008a; Vahedi 2005; Vahedi 2008). The other studies were rated as unclear for this item.
Blinding
Bulking agents Six studies were rated as low risk for blinding (Fowlie 1992; Jalihal 1990; Longstreth 1981; Nigam 1984; Ritchie 1979; Soltoft 1976). Rees 2005 used a single blind design and was rated as a high risk of bias. The other studies did not describe methods used for blinding and were rated as unclear.
Antispasmodics Eleven studies were rated as low risk for blinding (Awad 1995; Fielding 1980; Ghidini 1986; Liu 1997; Mitchell 2002; Moshal 1979; Nigam 1984; Page 1981; Passaretti 1989a; Pulpeiro 2000; Ritchie 1979). The other studies did not describe methods used for blinding and were rated as unclear.
Antidepressants Nine studies were rated as low risk for blinding (Kuiken 2003; Myren 1982; Rajagopalan 1998; Tabas 2004; Tack 2006a; Talley 2008a; Vahedi 2005; Vahedi 2008; Vij 1991). Drossman 2003 used a single blind design and was rated as a high risk of bias. The other studies did not describe methods used for blinding and were rated as unclear.
Incomplete outcome data
Bulking agents Eleven studies were rated as low risk for incomplete outcome date (Aller 2004; Arthurs 1983; Fowlie 1992; Jalihal 1990; Kruis 1986; Longstreth 1981; Nigam 1984; Prior 1987; Rees 2005; Ritchie 1979; Soltoft 1976). One study was rated as unclear for this item (Lucey 1987).
Antispasmodics Twenty‐four studies were rated as low risk for incomplete outcome data (Awad 1995; Baldi 1991; Battaglia 1998; Capanni 2005; Cappello 2007; Centonze 1988; Delmont 1981; Dobrilla 1990; Dubarry 1977; Fielding 1980; Ghidini 1986; Gilvarry 1989; Kruis 1986; Lech 1988; Levy 1977; Liu 1997; Mitchell 2002; Moshal 1979; Nigam 1984; Page 1981; Passaretti 1989a; Piai 1979; Pulpeiro 2000; Ritchie 1979). Five studies were rated as unclear for this item (Chen 2004; Czalbert 1990; d'Arienzo 1980; Schafer 1990; Virat 1987).
Antidepressants Eleven studies were rated as low risk for incomplete outcome data (Bahar 2008; Drossman 2003; Heefner 1978; Kuiken 2003; Masand 2009; Myren 1982; Tabas 2004; Tack 2006a; Vahedi 2005; Vahedi 2008; Vij 1991). Four studies were rated as unclear for this item (Bergmann 1991; Boerner 1988; Rajagopalan 1998; Talley 2008a).
Selective reporting
Bulking agents All twelve studies were rated as low risk for selective reporting (Aller 2004; Arthurs 1983; Fowlie 1992; Jalihal 1990; Kruis 1986; Longstreth 1981; Lucey 1987; Nigam 1984; Prior 1987; Rees 2005; Ritchie 1979; Soltoft 1976).
Antispasmodics Twenty‐six studies were rated as low risk for selective reporting (Awad 1995; Baldi 1991; Battaglia 1998; Capanni 2005; Cappello 2007; Centonze 1988; Delmont 1981; Dobrilla 1990; Dubarry 1977; Fielding 1980; Ghidini 1986; Gilvarry 1989; Kruis 1986; Lech 1988; Levy 1977; Liu 1997; Mitchell 2002; Moshal 1979; Nigam 1984; Page 1981; Passaretti 1989a; Piai 1979; Pulpeiro 2000; Ritchie 1979; Schafer 1990; Virat 1987). Three studies were rated as unclear for this item (Chen 2004; Czalbert 1990; d'Arienzo 1980).
Antidepressants Fourteen studies were rated as low risk for selective reporting (Bahar 2008; Bergmann 1991; Drossman 2003; Heefner 1978; Kuiken 2003; Masand 2009; Myren 1982; Rajagopalan 1998; Tabas 2004; Tack 2006a; Talley 2008a; Vahedi 2005; Vahedi 2008; Vij 1991). One study was rated as unclear for this item (Boerner 1988).
Other potential sources of bias
Bulking agents All twelve studies were rated as low risk for other potential sources of bias (Aller 2004; Arthurs 1983; Fowlie 1992; Jalihal 1990; Kruis 1986; Longstreth 1981; Lucey 1987; Nigam 1984; Prior 1987; Rees 2005; Ritchie 1979; Soltoft 1976).
Antispasmodics Twenty‐six studies were rated as low risk for other potential sources of bias Awad 1995; Baldi 1991; Battaglia 1998; Capanni 2005; Cappello 2007; Centonze 1988; Delmont 1981; Dobrilla 1990; Dubarry 1977; Fielding 1980; Ghidini 1986; Gilvarry 1989; Kruis 1986; Lech 1988; Levy 1977; Liu 1997; Mitchell 2002; Moshal 1979; Nigam 1984; Page 1981; Passaretti 1989a; Piai 1979; Pulpeiro 2000; Ritchie 1979; Schafer 1990; Virat 1987). Three studies were rated as unclear for this item (Chen 2004; Czalbert 1990; d'Arienzo 1980).
Antidepressants Fourteen studies were rated as low risk for other potential sources of bias (Bahar 2008; Bergmann 1991; Drossman 2003; Heefner 1978; Kuiken 2003;Masand 2009; Myren 1982; Rajagopalan 1998; Tabas 2004; Tack 2006a; Talley 2008a; Vahedi 2005; Vahedi 2008; Vij 1991). One study was rated as unclear for this item (Boerner 1988).
Effects of interventions
Bulking agents
Improvement of abdominal pain (outcome 1) One study with a total of 80 patients reported a dichotomous outcome for improvement of abdominal pain. The RR was 0.91 (95% CI 0.61 to 1.36) using a fixed‐effect model. A planned subgroup analysis for insoluble compared to soluble bulking agents could not be performed because there was only one study with soluble (Prior 1987) bulking agents.
Four studies with a total of 186 patients reported a continuous outcome for improvement of abdominal pain. Two of these studies did not report sufficient data to calculate a SMD (Fowlie 1992; Rees 2005). One study of an insoluble bulking agent with a total of 56 patients remained (Aller 2004). There was one study of a soluble fibre (Longstreth 1981). The pooled SMD was 0.03 (95% CI ‐0.34 to 0.40) using a fixed‐effect model.
Improvement of global assessment (outcome 2) Eleven studies, with a total of 565 patients reported a dichotomous outcome for improvement of global assessment. The chi‐square test for heterogeneity was not statistically significant (P = 0.12). The pooled relative risk was not statistically significant using a random‐effects model (RR 1.10; 95% CI 0.91 to 1.33). A RR of 0.95 (95% CI 0.76 to 1.19) was calculated for the studies using an insoluble bulking agent (244 patients) (Fowlie 1992; Kruis 1986; Lucey 1987; Rees 2005; Soltoft 1976). In the six studies with a soluble bulking agent the RR was 1.28 (95% CI 0.91 to 1.78; 321 patients) (Arthurs 1983; Jalihal 1990; Longstreth 1981; Nigam 1984; Prior 1987; Ritchie 1979).
One study of an insoluble bulking agent, comprising 56 patients, reported a continuous outcome for improvement of global assessment (Aller 2004). The standardized mean difference was not statistically significant (SMD ‐0.22; 95% CI ‐0.74 to 0.31).
Improvement of IBS symptom score (outcome 3) Three studies, with a total of 126 patients reported a continuous outcome for improvement of IBS symptom score. One study did not report sufficient data to calculate a SMD (Fowlie 1992). Two studies, both of insoluble fibre, with a total of 84 patients remained (Aller 2004; Fowlie 1992). The chi‐square test for heterogeneity was not statistically significant (P = 0.16). The pooled SMD was not statistically significant (SMD 0.00; 95%CI ‐0.43 to 0.43).
The main results for the bulking agents studies are summarized in additional Table 1.
1. Bulking agents: main results.
|
Dichotomous outcomes RR (95% CI) |
Continuous outcomes SMD (95% CI) |
|
| Abdominal pain | 0.91 (0.61 to 1.36) | 0.03 (‐0.34 to 0.40) |
| Global assessment | 1.11 (0.91 to 1.35) | |
| Symptom score | 0.00 (‐0.43 to 0.43) |
Antispasmodics
Improvement of abdominal pain (outcome 4) Thirteen studies with a total of 1392 patients reported a dichotomous outcome for improvement of abdominal pain. The chi‐square test for heterogeneity was statistically significant (P = 0.01). The pooled RR was 1.32 (95% CI 1.12 to 1.55) using a random‐effects model. Subgroup analyses showed statistically significant benefit for pinaverium bromide (RR 1.57; 95% CI 1.08 to 2.26; 158 patients) (Delmont 1981; Dubarry 1977; Virat 1987) and trimebutine (RR 1.32; 95% CI 1.07 to 1.64; 140 patients) (Fielding 1980; Ghidini 1986; Moshal 1979). There was no statistically significant benefit for scopolamine derivatives (RR 1.00; 95% CI 0.84 to 1.19; 360 patients) (Page 1981; Schafer 1990). The other subgroups contained only one study each. Eight studies comprising 455 patients reported a continuous outcome for improvement of abdominal pain. The chi‐square test for heterogeneity was statistically significant (P < 0.00001) The pooled SMD was 1.14 (95% CI 0.47 to 1.81) using a random‐effects model. Statistically significant benefit was present for the subgroup cimetropium/dicyclomine (SMD 1.08; 95% CI 0.73 to 1.43; 146 patients) (Centonze 1988; Dobrilla 1990; Passaretti 1989a). There was no statistically significant benefit for pinaverium (SMD 0.44; 95% CI ‐0.20 to 1.08; 114 patients) (Awad 1995; Virat 1987). The other subgroups contained none or only one study each.
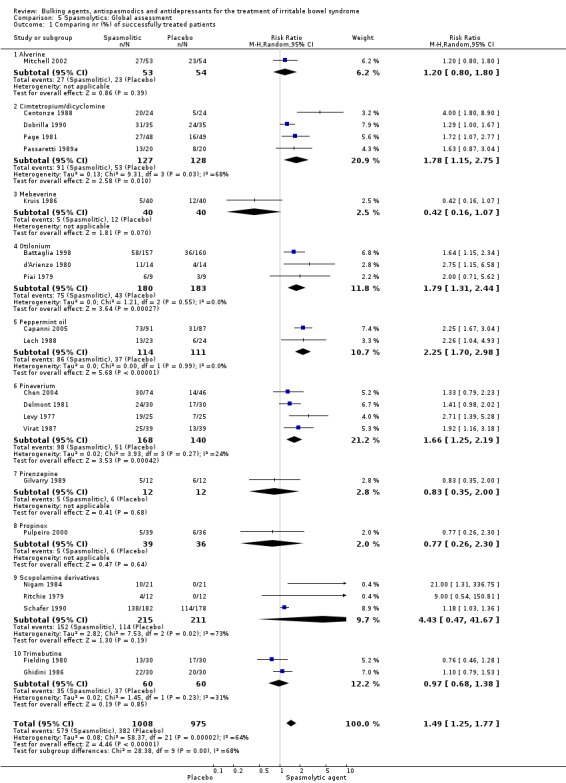
Improvement of global assessment (outcome 5) Twenty‐two studies with a total of 1983 patients reported a dichotomous outcome for improvement of global assessment. The chi‐square test for heterogeneity was statistically significant (P < 0.0001). The pooled relative risk was statistically significant (RR 1.49; 95% CI 1.25 to 1.77) using a random‐effects model. Statistically significant benefit was present for the subgroups cimetropium/dicyclomine (RR 1.78; 95% CI 1.15 to 2.75; 255 patients) (Centonze 1988; Dobrilla 1990; Page 1981; Passaretti 1989a), otilonium (RR 1.79; 95% CI 1.31 to 2.44; 363 patients) (Battaglia 1998; d'Arienzo 1980; Piai 1979); peppermint‐oil (RR 2.25; 95% CI 1.70 to 2.98; 225 patients) (Capanni 2005; Lech 1988) and pinaverium bromide (RR 1.66; 95% CI 1.25 to 2.19; 308 patients) (Chen 2004; Delmont 1981; Levy 1977; Virat 1987). There was no statistically significant benefit for alverine (RR 1.20; 95% CI 0.80 to 1.80; 107 patients) (Mitchell 2002); mebeverine (RR 0.42; 95% CI 0.16 to 1.07; 80 patients) (Kruis 1986), scolpamine derivates (RR 4.43; 95% CI 0.47 to 41.67; 426 patients) (Nigam 1984; Ritchie 1979; Schafer 1990) and trimebutine (RR 0.97; 95% CI 0.68 to 1.38; 120 patients) (Fielding 1980; Ghidini 1986).
Two studies comprising 331 patients reported a continuous outcome for improvement of global assessment. The pooled SMD could not be estimated because of lack of data provided by the studies (Battaglia 1998; Delmont 1981).
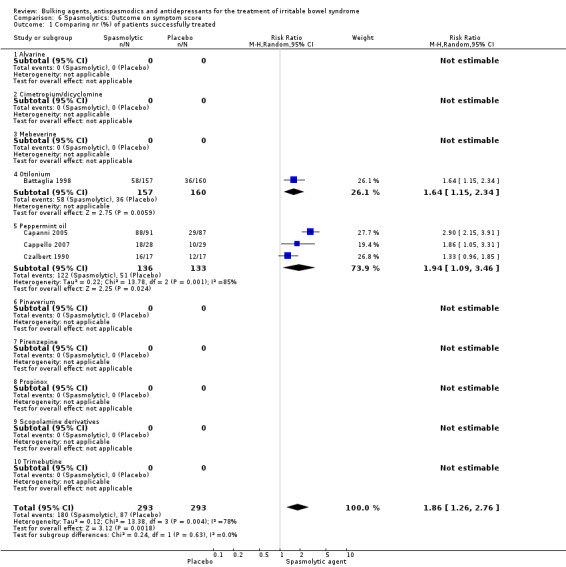
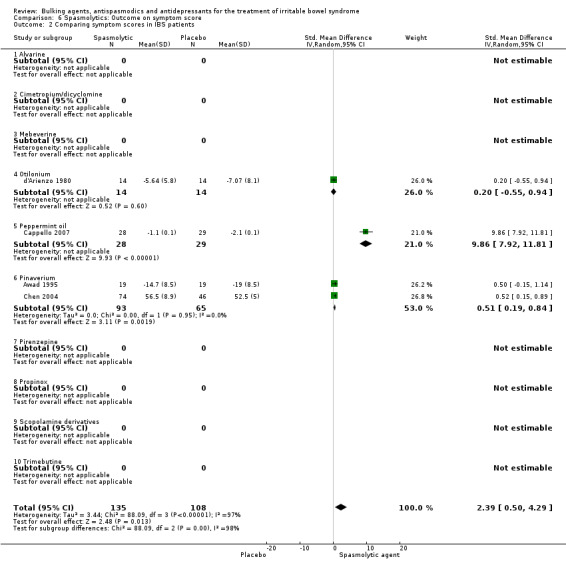
Improvement of IBS symptom score (outcome 6) Four studies with a total of 586 patients reported a dichotomous outcome for improvement of IBS symptom score. The chi‐square test for heterogeneity was statistically significant (P = 0.004). The pooled relative risk was statistically significant (RR 1.86; 95% CI 1.26 to 2.76) using a random‐effects model. Statistically significant benefit was present for the subgroups peppermint‐oil (RR 1.94; 95% CI 1.09 to 3.46; 269 patients) (Capanni 2005; Cappello 2007; Czalbert 1990) and otilonium (RR 1.64; 95% CI 1.15 to 2.34; 317 patients) (Battaglia 1998). Four studies comprising 243 patients reported a continuous outcome for improvement of global assessment. The chi‐square test for heterogeneity was statistically significant (P = 0.00001). The pooled SMD was statistically significant (SMD 2.39; 95% CI 0.50 to 4.29) using a random‐effects model. A statistically significant benefit was found for the pinaverium subgroup (SMD 0.51; 95% CI 0.19 to 0.84; 158 patients) (Awad 1995; Chen 2004). The other subgroups contained none or only one study each.
The main results for antispasmodics are summarized in additional Table 2.
2. Antispasmodics: main results.
|
Dichotomous outcomes RR (95% CI) |
Continuous outcomes SMD (95% CI) |
|
| Abdominal pain | 1.32 (1.12 to 1.55) | 1.14 (0.47 to 1.81) |
| Global assessment | 1.49 (1.25 to 1.77) | |
| Symptom score | 1.86 (1.26 to 2.76) | 2.39 (0.50 to 4.29) |
Individual spasmolytic agents: Cimetropium/dicyclomine No statistically significant effect for improvement of abdominal pain was found for Cimetropium/dicyclomine (SMD 1.08; 95% CI 0.73 to 1.43; 146 patients) (Centonze 1988; Dobrilla 1990; Passaretti 1989a). A statistically significant effect for improvement of global assessment was found for Cimetropium/dicyclomine (RR 1.88; 95% CI 1.04 to 3.42; 255 patients) (Centonze 1988; Dobrilla 1990; Page 1981; Passaretti 1989a). Mebeverine No statistically significant effect for improvement of global assessment was found for Mebeverine (RR 0.83; 95% CI 0.31 to 2.23; 149 patients) (Kruis 1986). Peppermint oil A statistically significant effect for improvement of global assessment was found for peppermint oil (RR 2.25; 95% CI 1.70 to 2.98; 225 patients) (Capanni 2005; Lech 1988). A statistically significant effect for improvement of IBS symptom score was found for peppermint oil (RR 1.94; 95% CI 1.09 to 3.46; 269 patients) (Capanni 2005; Cappello 2007; Czalbert 1990). Pinaverium Pinaverium provided a statistically significant benefit for improvement of abdominal pain, RR 1.57 (95% CI 1.08 to 2.26; 158 patients) (Delmont 1981; Dubarry 1977; Virat 1987) and SMD 0.44 (95% CI ‐0.20 to 1.08; 114 patients) (Awad 1995; Virat 1987). A statistically significant effect for improvement of global assessment, RR1.87 (95% CI1.41 to 2.48; 308 patients) (Chen 2004; Delmont 1981; Levy 1977; Virat 1987) and IBS‐symptom score was also found, SMD 0.51 (95% CI 0.19 to 0.84; 158 patients) (Awad 1995; Chen 2004). Scopolamine derivatives No statistically significant effect for improvement of global assessment was found for Scopolamine derivatives (RR 1.42; 95% CI 0.94 to 2.14; 442 patients) (Nigam 1984; Ritchie 1979; Schafer 1990).
Trimebutine A statistically significant effect for improvement of abdominal pain was found for Trimebutine (RR 1.32; 95% CI 1.07 to 1.64; 140 patients) (Fielding 1980; Ghidini 1986; Moshal 1979). No statistically significant effect for improvement of global assessment (RR 0.97; 95% CI 0.68 to 1.38; 120 patients) (Fielding 1980; Ghidini 1986). Other subgroups There was not enough data to calculate a pooled estimate effect.
Antidepressants
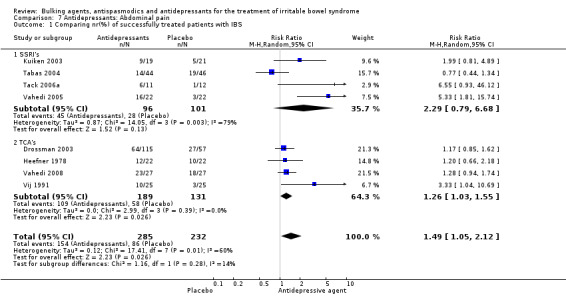
Improvement of abdominal pain (outcome 7) Eight studies with a total of 517 patients reported a dichotomous outcome for improvement of abdominal pain. The chi‐square test for heterogeneity was statistically significant (P = 0.01). The pooled relative risk was statistically significant (RR was 1.49; 95% CI 1.05 to 2.12) using a random‐effects model. Subgroup analyses showed no benefit for SSRIs (RR 2.29; 95% CI 0.79 to 6.68; 197 patients) (Kuiken 2003; Tabas 2004; Tack 2006a; Vahedi 2005), and a statistically significant benefit for TCAs (RR 1.26; 95% CI 1.03 to 1.55; 320 patients) (Drossman 2003; Heefner 1978; Vahedi 2008; Vij 1991).
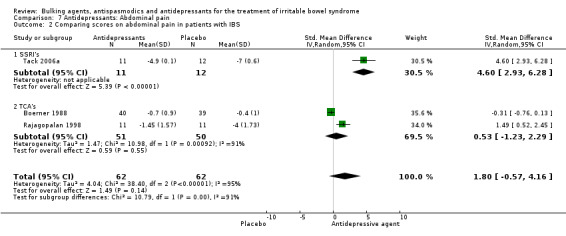
Three studies with a total of 124 patients reported a continuous outcome for improvement of abdominal pain. The chi‐square test for heterogeneity was statistically significant (P < 0.00001). The pooled RR was 1.80 (95% CI ‐0.57 to 4.16). Subgroup analyses showed no significant benefit for TCA’s (SMD was 0.53; 95% CI ‐1.23 to 2.29; 101 patients) (Boerner 1988; Drossman 2003; Rajagopalan 1998).
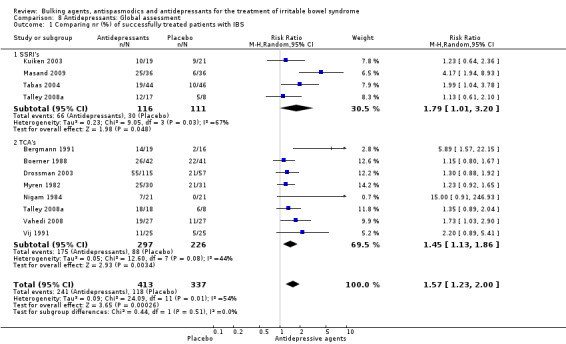
Improvement of global assessment (outcome 8) Twelve studies, with a total of 750 patients reported a dichotomous outcome for improvement of global assessment. The chi‐square test for heterogeneity was statistically significant (P = 0.01). The pooled relative risk was statistically significant (RR 1.57; 95% CI 1.23 to 2.00) using a random‐effects model. Subgroup analyses suggest a benefit for SSRIs (RR 1.79; 95% CI 1.01 to 3.20; P = 0.05; 227 patients) (Kuiken 2003; Masand 2009; Tabas 2004; Talley 2008a) and showed a statistically significant benefit for TCAs (RR 1.45; 95% CI 1.13 to 1.86; 523 patients) (Bergmann 1991; Boerner 1988; Drossman 2003; Myren 1982; Nigam 1984; Talley 2008a; Vahedi 2008; Vij 1991).
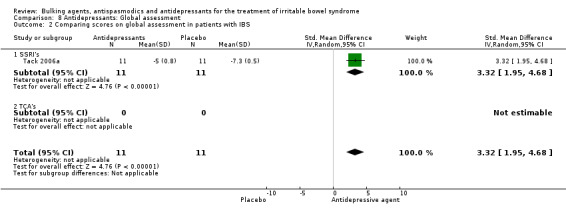
One study assessing an SSRI (Tack 2006a), with a total of 22 patients reported a continuous outcome for improvement of global assessment. The pooled SMD was 3.32 (95% CI 1.95 to 4.68).
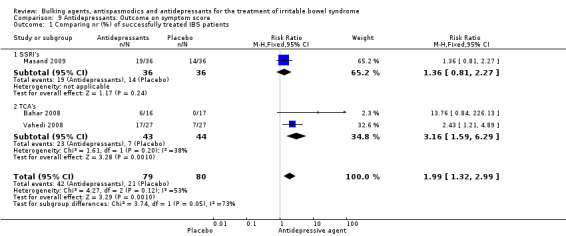
Improvement of IBS symptom score (outcome 9) Three studies with a total of 159 patients reported a dichotomous outcome for improvement of symptom score. The chi‐square test for heterogeneity was not statistically significant (P = 0.12). The pooled RR was 1.99 (95% CI 1.32 to 2.99) using a fixed‐effect model. Subgroup analyses showed a statistically significant benefit for TCAs (RR 3.16; 95% CI 1.59 to 6.29; 87 patients) (Bahar 2008; Vahedi 2008).
Two studies, with a total of 122 patients reported a continuous outcome for improvement of IBS symptom score. The chi‐square test for heterogeneity was statistically significant (P = 0.07). The pooled SMD was 0.38 (95% CI ‐0.30 to 1.06) using a random‐effects model.
The main results for antidepressants are summarized in Table 3.
3. Antidepressants: main results.
|
Dichotomous outcomes RR (95% CI) |
Continuous outcomes SMD (95% CI) |
|
| Abdominal pain | 1.49 (1.05 to 2.12) | 1.80 (‐0.57 to 4.16) |
| Global assessment | 1.57 (1.23 to 2.00) | 3.32 (1.95 to 4.68) |
| Symptom score | 1.99 (1.32 to 2.99) | 0.38 (‐0.30 to 1.06) |
Additional comparison: adequate concealment of allocation
Bulking agents abdominal pain (outcome 10.2) Two studies of bulking agents with adequate concealment of allocation reported a continuous outcome for improvement of abdominal pain (Longstreth 1981; Fowlie 1992). The chi‐square test for heterogeneity was not statistically significant (P = 0.88). The pooled SMD using a fixed‐effect model was ‐0.04 (95%CI ‐0.40 to 0.32; 119 patients).
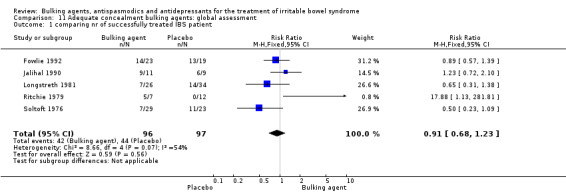
Bulking agents: global assessment (outcome 11.1) Five studies of bulking agents with adequate concealment of allocation reported a dichotomous outcome for improvement of abdominal pain (Fowlie 1992; Jalihal 1990; Longstreth 1981; Ritchie 1979; Soltoft 1976). Using a random‐effects model, the pooled RR was 0.91 (95% CI 0.68 to 1.23; 193 patients).
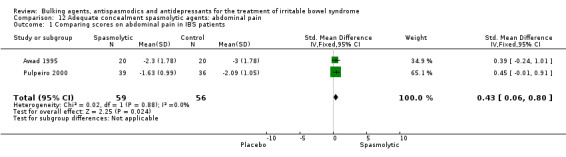
Antispasmodics: abdominal pain (outcome 12.1) Two studies of spasmolytic agents with adequate concealment of allocation reported a continuous outcome for improvement of abdominal pain (Awad 1995; Pulpeiro 2000). The chi‐square test for heterogeneity was not statistically significant (P = 0.88). Using a fixed‐effect model, the pooled SMD was 0.43 (95% CI 0.06 to 0.80; 115 patients).
Antispasmodics: global assessment (outcome 13.1) Three studies of spasmolytic agents with adequate concealment of allocation reported a dichotomous outcome for improvement of global assessment (Chen 2004; Pulpeiro 2000; Ritchie 1979). The chi‐square test for heterogeneity was not statistically significant (P= 0.25). Using a fixed‐effect model, the pooled RR was 1.35 (95% CI 0.85 to 2.12; 219 patients).
Antidepressants: abdominal pain (outcome 14.1) Five studies of antidepressant agents with adequate concealment of allocation reported a dichotomous outcome for improvement of abdominal pain. The chi‐square test for heterogeneity was statistically significant (P = 0.06). Using a random‐effects model, the pooled RR was 1.35 (95% CI 0.98 to 1.86; 364 patients) . Subgroup analyses showed no statistically significant benefit for SSRIs (RR 1.20; 95% CI 0.87 to 1.67) (Kuiken 2003; Tabas 2004; Vahedi 2005) or TCAs (RR 2.19; 95% CI 0.59 to 8.11) (Drossman 2003; Vahedi 2008).
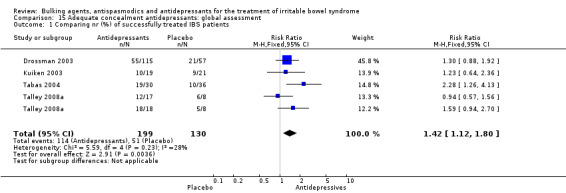
Antidepressants: global assessment (outcome 15.1) Four studies of antidepressant agents with adequate concealment of allocation reported a dichotomous outcome for global assessment. The chi‐square test for heterogeneity was not statistically significant (P = 0.23). Using a fixed‐effect model, the pooled RR was 1.42 (95% CI 1.12 to 1.80; 329 patients) (Drossman 2003; Kuiken 2003; Tabas 2004; Talley 2008a).
Antidepressants: IBS symptom score (outcome 16.1) One study of antidepressants with adequate concealment of allocation reported a continuous outcome for improvement of IBS symptom score (Vahedi 2008). Using a fixed‐effect model, the SMD was 0.75 (95% CI 0.17 to 1.32; 50 patients).
Discussion
Bulking agents The pooled data suggest that bulking agents do not provide any benefit for the treatment of IBS. No statistically significant differences between bulking agents and placebo were found for abdominal pain, global assessment or symptom score. Only 7 of the included studies had more than 30 patients and all studies had quality limitations (i.e. method of randomisation, double‐blinding, concealment of treatment allocation, description of withdrawals). There were five studies with adequate allocation concealment (Fowlie 1992; Jalihal 1990; Longstreth 1981; Ritchie 1979; Soltoft 1976). A sensitivity analysis of those studies with adequate allocation concealment, did not change the results. Subgroup analyses for the different type of bulking agents, soluble versus insoluble fibre, also gave no statistically significant findings.
We are aware of five systematic reviews of bulking agents for IBS. Jailwala 2000, who used less strict exclusion criteria than the present review, also concluded from an analysis of 13 studies that the efficacy of bulking agents is not clearly established. When Jailwala 2000 separately analysed high and low quality trials, the conclusion remained the same (Jailwala 2000). Akehurst 2001 included 7 studies on bulking agents in a review of IBS therapies and concluded that there was little reason to believe that bulking agents were effective for IBS (Akehurst 2001). Lesbros‐Pantoflickova 2004 included 13 studies in their meta‐analyses, of which 5 studies reported a statistically significant benefit of fibre treatment for the relief of global symptoms (OR 1.9; 95% CI 1.5 to 2.4). However, after exclusion of the low‐quality trials, this effect was not statistically significant. In conclusion, they found no evidence to recommend bulking agents for the treatment of IBS (Lesbros‐Pantoflickova 2004). Ford 2008 included 12 studies comparing fibre with placebo, and used persistent symptoms after treatment as an outcome measure. Ford 2008 calculated a RR of 0.87 (95% CI 0.76 to 1.00). A subgroup analysis identified a statistically significant benefit for ispaghula a soluble fibre (RR 0.78; 95% CI 0.63 to 0.96). Ford 2008 had almost the same strict inclusion criteria as our review but included different outcome analyses. Ford 2008 did not use an ITT‐analyses, only extracted dichotomous outcome and pooled all the outcomes (global assessment of symptoms and abdominal pain) as one. Bijkerk 2004 examined the separate effects of soluble and insoluble fibres, on global assessment and constipation. Bijkerk 2004 found a beneficial overall effect for fibre in general (RR 1.33; 95% CI 1.19 to 1.50) and soluble fibres for global assessment of IBS (RR 1.55; 95% CI 1.35 to 1.78). We could not reproduce these findings (outcomes 1.1 to 3.2). A possible explanation for this is that Bijkerk 2004 included cross‐over trials (7 of the 17 included studies), which were excluded in this review. Bijkerk 2004 also included two studies with no placebo comparison.
Antispasmodics Spasmolytic agents compared to placebo provided a statistically significant benefit for abdominal pain, global assessment and IBS‐symptom score. Spasmolytic agents are pharmacologically diverse and arbitrary choices were made regarding the pooling of results. We decided to treat peppermint‐oil as an anti‐spasmodic because of its known effect on smooth muscles. Trimebutine appears to be effective for abdominal pain, pinaverium for abdominal pain and global assessment, cimetropium/dicyclominand for global assessment and peppermint‐oil for global assessment and symptom score. Only four studies had adequate allocation concealment (Awad 1995; Chen 2004; Pulpeiro 2000; Ritchie 1979). It is important to note that none of the studies involving peppermint‐oil had adequate allocation concealment. When analysing the studies with adequate allocation concealment separately, the results get weaker and only improvement of abdominal pain has still a statistically significant benefit. Spasmolytics are extensively studied for their use in the treatment of IBS, however due to the diversity of types of spasmolytic agents, the number of studies for each compound are limited. Therefore most subgroups could not be pooled, and a type II error could have occurred.
Eight systematic reviews of antispasmodics for IBS have been published (Akehurst 2001; Brandt 2002; Ford 2008; Jailwala 2000; Lesbros‐Pantoflickova 2004; Poynard 1994; Poynard 2001; Tack 2006b). Jailwala 2000 included 13 studies and found that all of the 7 high‐quality trials demonstrated a benefit, mainly for abdominal pain, less so for constipation. Akehurst 2001 identified 12 studies and came to similar conclusions. Ford 2008 found consistent evidence of efficacy for otilonium (RR 0.55; 95% CI 0.31 to 0.97) and scopolamine (RR 0.63; 95% CI 0.51 to 0.78). Ford 2008 identified peppermint‐oil as an individual group, included 4 studies and calculated a RR of 0.43 (95% CI 0.33 to 0.59). These results are almost identical to our own. However, Ford 2008 used a different method to assess methodological quality (Jadad scale), and rated three studies as high quality, resulting in a greater effect than seen in this review. In an update of a 1994 meta‐analysis, Poynard 2001 included 23 trials comprising 6 types of drugs. Using a fixed‐effect model, there was a statistically significant benefit for global assessment (Peto OR 2.13; 95%CI 1.77 to 2.58) and pain (Peto OR 1.65; 95%CI 1.30 to 2.10) (Poynard 2001).This review provides similar evidence of the efficacy of spasmolytic agents for IBS. The reviews from Lesbros‐Pantoflickova 2004 and Tack 2006b concluded that there is some evidence that antispasmodic may improve symptoms of abdominal pain but are careful in recommending antispasmodics for the treatment of IBS due to the low methodological quality of the included RCTs.
Antidepressants
Antidepressants provide a statistically significant benefit over placebo for abdominal pain, global assessment and IBS‐symptom score. Subgroup analyses for SSRIs and TCAs, showed a statistically significant improvement in global assessment for SSRIs and a statistically significant improvement in abdominal pain and symptom score for TCAs. A sensitivity analysis of the six studies with adequate allocation concealment showed a statistically significant benefit for improvement of symptom score and global assessment (Drossman 2003; Kuiken 2003; Tabas 2004; Talley 2008a; Vahedi 2005; Vahedi 2008).
Given the significantly positive effects of antidepressant medication, the clinical indication of antidepressant medication in IBS needs to be discussed. Careful examination of the domain descriptions in the individual studies, shows no differences in patient population between studies investigating antidepressants, antispasmodics or bulking agents. Two studies performed a direct comparison of antidepressants with bulking agents or antispasmodics (Nigam 1984; Ritchie 1979), but found no proof of the superiority of either compound.
We are aware of eight systematic reviews of antidepressants for IBS (Akehurst 2001; Brandt 2002; Ford 2009; Jailwala 2000; Jackson 2000; Lesbros‐Pantoflickova 2004; Tack 2006b; Rahimi 2009). Most of these reviews are consistent with our results. Akehurst 2001 concluded from two studies that antidepressants were effective. Ford 2009 included 13 RCTs and found a RR of 0.66 (95% CI 0.57 to 0.78) for persistent symptoms after treatment, and no difference between SSRIs and TCAs. The Jailwala 2000 meta‐analysis included 7 studies, all reporting beneficial effect, and concluded that it was not clear whether this was due to resolving abdominal symptoms, or to improved psychological health. The Jackson 2000 review included 11 studies on functional gastro‐intestinal disorders, 8 of which were enrolled IBS patients exclusively. Jackson 2000 identified a statistically significant effect for overall assessment (7 studies; OR 4.2; 95% CI 2.3 to 7.9) and abdominal pain (9 studies; SMD 0.9; 95% CI 0.6 to 1.2). The Lesbros‐Pantoflickova 2004 review included 12 studies and found an OR: 2.6 (95% CI 1.9 to 3.5). They recommend antidepressant medication for the treatment of patients with severe IBS symptoms, i.e. patients with daily or persistent pain. Rahimi 2009 only investigated TCAs and found clinically and statistically significant control of IBS symptoms. They advised that treatment with TCAs should be limited to patients with moderate to severe IBS.
Brandt 2002 and Tack 2006b (a extended version of Brandt 2002) reported no beneficial effect for antidepressant medication. This difference may be due to less strict inclusion criteria: both included cross‐over studies with no report of the first phase data. They also failed to conduct a meta‐analysis of the data.
Authors' conclusions
Implications for practice.
The limitations of drug therapy should be discussed with the patient before deciding to prescribe medication for IBS. Agreement should be reached on treatment objectives, usually this will be relief of the most troublesome symptom. Our findings support the use of antispasmodics, although, it is not entirely clear whether one antispasmodic is more effective than another. Physicians will be limited to those antispasmodics which are locally available.
Antidepressants may also have a role for the treatment of IBS. Antidepressants could be used in patients who seek drug therapy and who have not responded to antispasmodics. The effectiveness of antidepressants may vary with individual patient features.
Implications for research.
It is likely that two different disease entities exist: constipation predominant IBS, and diarrhea predominant IBS. There may even be a third entity, patients with an alternating stool pattern. The pharmacological properties of bulking agents, spasmolytic agents and antidepressive medication suggest that different responses might be expected in these patient groups and this issue should be studied in future trials of “classic” drugs.
The variation in methods of outcome assessment in IBS studies is a validity problem. It is uncertain how precisely current outcome measures reflect the actual health status of the IBS patient. The need for more meaningful measures of response to treatment has led to the development of health‐related quality of life measures including stool frequency and consistency, social, daily, physical and sexual functioning, sleep, pain, emotion, and change of health. Future research should use validated outcome measures for IBS, such as the IBS Quality of Life Questionnaire (IBSQOL), the IBS Quality of Life Measure (IBS‐QOL), the Digestive Health Status Instrument (DHSI), the Functional Digestive Disorder Quality of Life questionnaire (FDDQOL), or the IBS‐Q.
The concept of the brain‐gut axis invites trials aimed at central and peripheral neural levels; apart from drug trials these may include cognitive behavioural therapy or other psychological interventions (e.g. hypnotherapy).
What's new
| Date | Event | Description |
|---|---|---|
| 21 February 2013 | Amended | Correction of minor errors in additional tables |
History
Protocol first published: Issue 1, 2002 Review first published: Issue 2, 2005
| Date | Event | Description |
|---|---|---|
| 29 September 2011 | Amended | Change in address for contact author |
| 26 April 2011 | New citation required and conclusions have changed | Change in authors, conclusions changed due to new data |
| 26 April 2011 | New search has been performed | New search, new studies included |
Acknowledgements
Funding for the IBD/FBD Review Group (September 1, 2010 ‐ August 31, 2015) has been provided by the Canadian Institutes of Health Research (CIHR) Knowledge Translation Branch (CON ‐ 105529) and the CIHR Institutes of Nutrition, Metabolism and Diabetes (INMD); and Infection and Immunity (III).
Miss Ila Stewart has provided support for the IBD/FBD Review Group through the Olive Stewart Fund.
Data and analyses
Comparison 1. Bulking agents: Abdominal pain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Comparing nr(%) of successfully treated IBS patients | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.61, 1.36] |
| 1.1 Insoluble fibres | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Soluble fibres | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.61, 1.36] |
| 2 Comparing scores on abdominal pain in IBS patients | 4 | 186 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.34, 0.40] |
| 2.1 Insoluble fibres | 3 | 126 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.52, 0.52] |
| 2.2 Soluble fibres | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.45, 0.57] |
1.1. Analysis.
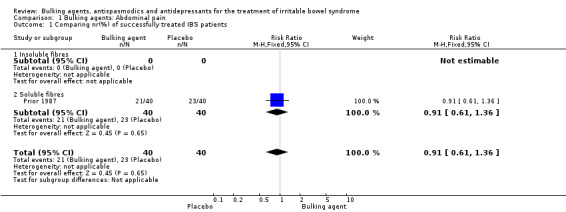
Comparison 1 Bulking agents: Abdominal pain, Outcome 1 Comparing nr(%) of successfully treated IBS patients.
1.2. Analysis.

Comparison 1 Bulking agents: Abdominal pain, Outcome 2 Comparing scores on abdominal pain in IBS patients.
Comparison 2. Bulking agents: Global assessment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Comparing nr(%) of successfully treated patients with IBS | 11 | 565 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.91, 1.33] |
| 1.1 Insoluble fibres | 5 | 244 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.76, 1.19] |
| 1.2 Soluble fibre | 6 | 321 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.91, 1.78] |
| 2 Comparing scores on global assessment in IBS patients | 1 | 56 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.22 [‐0.74, 0.31] |
| 2.1 Insoluble fibres | 1 | 56 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.22 [‐0.74, 0.31] |
| 2.2 Soluble fibres | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
2.1. Analysis.
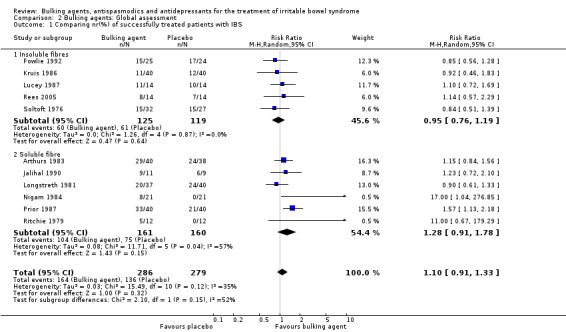
Comparison 2 Bulking agents: Global assessment, Outcome 1 Comparing nr(%) of successfully treated patients with IBS.
2.2. Analysis.

Comparison 2 Bulking agents: Global assessment, Outcome 2 Comparing scores on global assessment in IBS patients.
Comparison 3. Bulking agents: Outcome on symptom score.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Comparing symptom scores in IBS patients | 3 | 126 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.43, 0.43] |
| 1.1 Insoluble fibres | 3 | 126 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.43, 0.43] |
| 1.2 Soluble fibres | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.

Comparison 3 Bulking agents: Outcome on symptom score, Outcome 1 Comparing symptom scores in IBS patients.
Comparison 4. Spasmolytics: Abdominal pain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Comparing nr(%) of successfully treated IBS patients on Abdominal pain | 13 | 1392 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [1.12, 1.55] |
| 1.1 Alverine | 1 | 107 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.87, 1.62] |
| 1.2 Cimetropium/dicylomine | 1 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [1.06, 2.28] |
| 1.3 Mebeverine | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.38, 1.76] |
| 1.4 Otilonium | 1 | 325 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.96, 1.68] |
| 1.5 Peppermint oil | 1 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 2.15 [1.54, 3.00] |
| 1.6 Pinaverium | 3 | 158 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [1.08, 2.26] |
| 1.7 Pirenzepine | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.25, 1.78] |
| 1.8 Propinox | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.9 Scopolamine derivatives | 1 | 360 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.84, 1.19] |
| 1.10 Trimebutine | 3 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [1.07, 1.64] |
| 2 Comparing scores on abdominal pain in IBS patients | 8 | 455 | Std. Mean Difference (IV, Random, 95% CI) | 1.14 [0.47, 1.81] |
| 2.1 Alvarine | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Cimetropium/dicyclomine | 3 | 146 | Std. Mean Difference (IV, Random, 95% CI) | 1.08 [0.73, 1.43] |
| 2.3 Mebeverine | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Otilonium | 1 | 70 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [‐0.04, 0.91] |
| 2.5 Peppermint oil | 1 | 57 | Std. Mean Difference (IV, Random, 95% CI) | 3.88 [2.98, 4.79] |
| 2.6 Pinaverium | 2 | 114 | Std. Mean Difference (IV, Random, 95% CI) | 0.44 [‐0.20, 1.08] |
| 2.7 Pirenzepine | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.8 Propinox | 1 | 68 | Std. Mean Difference (IV, Random, 95% CI) | 0.45 [‐0.04, 0.93] |
| 2.9 Scopolamine derivatives | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.10 Trimebutine | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
4.1. Analysis.
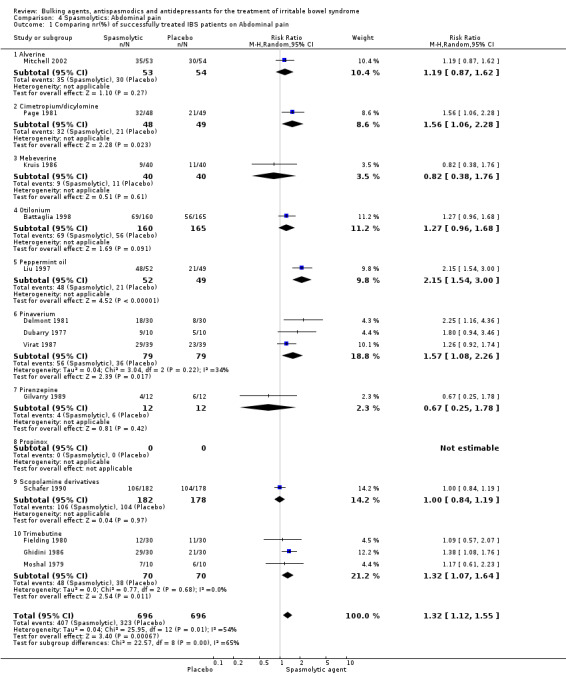
Comparison 4 Spasmolytics: Abdominal pain, Outcome 1 Comparing nr(%) of successfully treated IBS patients on Abdominal pain.
4.2. Analysis.
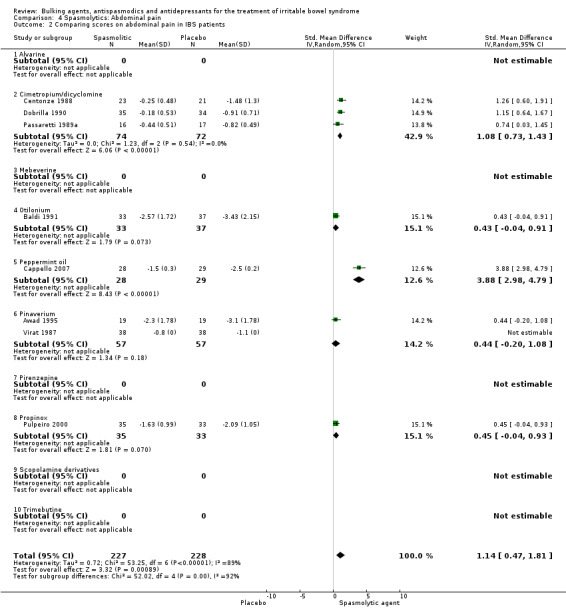
Comparison 4 Spasmolytics: Abdominal pain, Outcome 2 Comparing scores on abdominal pain in IBS patients.
Comparison 5. Spasmolytics: Global assessment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Comparing nr (%) of successfully treated patients | 22 | 1983 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [1.25, 1.77] |
| 1.1 Alverine | 1 | 107 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.80, 1.80] |
| 1.2 Cimtetropium/dicyclomine | 4 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [1.15, 2.75] |
| 1.3 Mebeverine | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.16, 1.07] |
| 1.4 Otilonium | 3 | 363 | Risk Ratio (M‐H, Random, 95% CI) | 1.79 [1.31, 2.44] |
| 1.5 Peppermint oil | 2 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 2.25 [1.70, 2.98] |
| 1.6 Pinaverium | 4 | 308 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [1.25, 2.19] |
| 1.7 Pirenzepine | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.35, 2.00] |
| 1.8 Propinox | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.26, 2.30] |
| 1.9 Scopolamine derivatives | 3 | 426 | Risk Ratio (M‐H, Random, 95% CI) | 4.43 [0.47, 41.67] |
| 1.10 Trimebutine | 2 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.68, 1.38] |
| 2 Comparing scores on global assessment in IBS patients | 2 | 331 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Alvarine | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Cimetropium/dicyclomine | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Mebeverine | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Otilonium | 1 | 271 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.5 Peppermint oil | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 Pinaverium | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.7 Pirenzepine | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.8 Propinox | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.9 Scopolamine derivatives | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.10 Trimebutine | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
5.1. Analysis.
Comparison 5 Spasmolytics: Global assessment, Outcome 1 Comparing nr (%) of successfully treated patients.
5.2. Analysis.

Comparison 5 Spasmolytics: Global assessment, Outcome 2 Comparing scores on global assessment in IBS patients.
Comparison 6. Spasmolytics: Outcome on symptom score.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Comparing nr (%) of patients successfully treated | 4 | 586 | Risk Ratio (M‐H, Random, 95% CI) | 1.86 [1.26, 2.76] |
| 1.1 Alvarine | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Cimetropium/dicyclomine | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Mebeverine | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Otilonium | 1 | 317 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [1.15, 2.34] |
| 1.5 Peppermint oil | 3 | 269 | Risk Ratio (M‐H, Random, 95% CI) | 1.94 [1.09, 3.46] |
| 1.6 Pinaverium | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.7 Pirenzepine | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.8 Propinox | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.9 Scopolamine derivatives | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.10 Trimebutine | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Comparing symptom scores in IBS patients | 4 | 243 | Std. Mean Difference (IV, Random, 95% CI) | 2.39 [0.50, 4.29] |
| 2.1 Alvarine | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Cimetropium/dicyclomine | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Mebeverine | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Otilonium | 1 | 28 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.55, 0.94] |
| 2.5 Peppermint oil | 1 | 57 | Std. Mean Difference (IV, Random, 95% CI) | 9.86 [7.92, 11.81] |
| 2.6 Pinaverium | 2 | 158 | Std. Mean Difference (IV, Random, 95% CI) | 0.51 [0.19, 0.84] |
| 2.7 Pirenzepine | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.8 Propinox | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.9 Scopolamine derivatives | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.10 Trimebutine | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
6.1. Analysis.
Comparison 6 Spasmolytics: Outcome on symptom score, Outcome 1 Comparing nr (%) of patients successfully treated.
6.2. Analysis.
Comparison 6 Spasmolytics: Outcome on symptom score, Outcome 2 Comparing symptom scores in IBS patients.
Comparison 7. Antidepressants: Abdominal pain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Comparing nr(%) of successfully treated patients with IBS | 8 | 517 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [1.05, 2.12] |
| 1.1 SSRI's | 4 | 197 | Risk Ratio (M‐H, Random, 95% CI) | 2.29 [0.79, 6.68] |
| 1.2 TCA's | 4 | 320 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [1.03, 1.55] |
| 2 Comparing scores on abdominal pain in patients with IBS | 3 | 124 | Std. Mean Difference (IV, Random, 95% CI) | 1.80 [‐0.57, 4.16] |
| 2.1 SSRI's | 1 | 23 | Std. Mean Difference (IV, Random, 95% CI) | 4.60 [2.93, 6.28] |
| 2.2 TCA's | 2 | 101 | Std. Mean Difference (IV, Random, 95% CI) | 0.53 [‐1.23, 2.29] |
7.1. Analysis.
Comparison 7 Antidepressants: Abdominal pain, Outcome 1 Comparing nr(%) of successfully treated patients with IBS.
7.2. Analysis.
Comparison 7 Antidepressants: Abdominal pain, Outcome 2 Comparing scores on abdominal pain in patients with IBS.
Comparison 8. Antidepressants: Global assessment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Comparing nr (%) of successfully treated patients with IBS | 11 | 750 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [1.23, 2.00] |
| 1.1 SSRI's | 4 | 227 | Risk Ratio (M‐H, Random, 95% CI) | 1.79 [1.01, 3.20] |
| 1.2 TCA's | 8 | 523 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [1.13, 1.86] |
| 2 Comparing scores on global assessment in patients with IBS | 1 | 22 | Std. Mean Difference (IV, Random, 95% CI) | 3.32 [1.95, 4.68] |
| 2.1 SSRI's | 1 | 22 | Std. Mean Difference (IV, Random, 95% CI) | 3.32 [1.95, 4.68] |
| 2.2 TCA's | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
8.1. Analysis.
Comparison 8 Antidepressants: Global assessment, Outcome 1 Comparing nr (%) of successfully treated patients with IBS.
8.2. Analysis.
Comparison 8 Antidepressants: Global assessment, Outcome 2 Comparing scores on global assessment in patients with IBS.
Comparison 9. Antidepressants: Outcome on symptom score.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Comparing nr (%) of successfully treated IBS patients | 3 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [1.32, 2.99] |
| 1.1 SSRI's | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.81, 2.27] |
| 1.2 TCA's | 2 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [1.59, 6.29] |
| 2 Comparing symptom scores of IBS patients | 2 | 122 | Std. Mean Difference (IV, Random, 95% CI) | 0.38 [‐0.30, 1.06] |
| 2.1 SSRI's | 1 | 72 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.41, 0.52] |
| 2.2 TCA's | 1 | 50 | Std. Mean Difference (IV, Random, 95% CI) | 0.75 [0.17, 1.32] |
9.1. Analysis.
Comparison 9 Antidepressants: Outcome on symptom score, Outcome 1 Comparing nr (%) of successfully treated IBS patients.
9.2. Analysis.

Comparison 9 Antidepressants: Outcome on symptom score, Outcome 2 Comparing symptom scores of IBS patients.
Comparison 10. Adequate concealment bulking agents: abdominal pain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Comparing scores on abdominal pain | 2 | 119 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.32, 0.40] |
10.1. Analysis.

Comparison 10 Adequate concealment bulking agents: abdominal pain, Outcome 1 Comparing scores on abdominal pain.
Comparison 11. Adequate concealment bulking agents: global assessment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 comparing nr of successfully treated IBS patient | 5 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.68, 1.23] |
11.1. Analysis.
Comparison 11 Adequate concealment bulking agents: global assessment, Outcome 1 comparing nr of successfully treated IBS patient.
Comparison 12. Adequate concealment spasmolytic agents: abdominal pain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Comparing scores on abdominal pain in IBS patients | 2 | 115 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.43 [0.06, 0.80] |
12.1. Analysis.
Comparison 12 Adequate concealment spasmolytic agents: abdominal pain, Outcome 1 Comparing scores on abdominal pain in IBS patients.
Comparison 13. Adequate concealment spasmolytic agents: global assessment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 comparing nrs of successfully treated IBS patients with spasmolytic agents | 3 | 219 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.85, 2.12] |
13.1. Analysis.

Comparison 13 Adequate concealment spasmolytic agents: global assessment, Outcome 1 comparing nrs of successfully treated IBS patients with spasmolytic agents.
Comparison 14. Adequate concealment antidepressants: abdominal pain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Comparing nr (%) of successfully treated patients | 5 | 364 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.98, 1.86] |
| 1.1 SSRI | 3 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.87, 1.67] |
| 1.2 TCA | 2 | 208 | Risk Ratio (M‐H, Random, 95% CI) | 2.19 [0.59, 8.11] |
14.1. Analysis.

Comparison 14 Adequate concealment antidepressants: abdominal pain, Outcome 1 Comparing nr (%) of successfully treated patients.
Comparison 15. Adequate concealment antidepressants: global assessment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Comparing nr (%) of successfully treated IBS patients | 4 | 329 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [1.12, 1.80] |
15.1. Analysis.
Comparison 15 Adequate concealment antidepressants: global assessment, Outcome 1 Comparing nr (%) of successfully treated IBS patients.
Comparison 16. Adequate concealment antidepressants: Outcome on symptom score.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Comparing symptom scores in IBS patients | 1 | 50 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.75 [0.17, 1.32] |
16.1. Analysis.

Comparison 16 Adequate concealment antidepressants: Outcome on symptom score, Outcome 1 Comparing symptom scores in IBS patients.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aller 2004.
| Methods | RCT | |
| Participants | 56 patients Rome II criteria 53% diarrhea‐predominant Setting unclear Mean age 46 years 67% female |
|
| Interventions | Bulking agent 30 g fibre of which 25 g insoluble, over the day for 13 weeks |
|
| Outcomes | Abdominal pain, continuous Symptom score, continuous |
|
| Notes | Half a week run‐in | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single‐blind ‐ not described in detail |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients dropped out of the study |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Arthurs 1983.
| Methods | RCT | |
| Participants | 80 patients Setting unclear Diagnostic criteria not defined Mean age 27.7 years 78% female |
|
| Interventions | Bulking agent 4 weeks ispaghula husk 2 sachets/day | |
| Outcomes | Global assessment, dichotomous | |
| Notes | Unclear setting No run‐in | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind but procedures not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two dropouts |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Awad 1995.
| Methods | RCT | |
| Participants | 40 patients Tertiary care Rome criteria Mean age 31.3 years 100% female |
|
| Interventions | Spasmolytic pinaverium 50 mg od for 3 weeks | |
| Outcomes | Abdominal pain, continuous symptom score, continuous | |
| Notes | No run‐in | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical placebo, neither doctors nor patients knew which treatment was given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two dropouts one from each treatment group |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Bahar 2008.
| Methods | RCT | |
| Participants | 33 adolescent patients secondary care Rome criteria mean age 15 years 65% female |
|
| Interventions | Antidepressant amitriptyline 10 to 30 mg dd for 8 weeks |
|
| Outcomes | Abdominal pain, change score (not included global assessment, change score ( not included) and dichotomous |
|
| Notes | 2 weeks run in period adolescents |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 drop‐outs |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Baldi 1991.
| Methods | RCT | |
| Participants | 71 patients with IBS 8 GI centres Mean age 40 years 60.6% female |
|
| Interventions | Spasmolytic 4 weeks otilonium 40 mg tds | |
| Outcomes | abdominal pain | |
| Notes | 2 wk run‐in with placebo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, methods not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One drop‐out |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Battaglia 1998.
| Methods | RCT | |
| Participants | 325 patients multicentre GI outpatients Rome criteria Mean age 47.7 years 69% female |
|
| Interventions | Spasmolytic 15 weeks otilonium 40 mg tds | |
| Outcomes | abdominal pain, dichotomous global assessment, continuous | |
| Notes | 2 weeks run‐in, with placebo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, methods not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Bergmann 1991.
| Methods | RCT | |
| Participants | 35 patients Secondary care Clinical diagnosis and investigations |
|
| Interventions | Antidepressant 3 months Trimipramine 50 mg OD |
|
| Outcomes | Global assessment, dichotomous | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, methods not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Boerner 1988.
| Methods | RCT | |
| Participants | 79 IBS patients partly from primary care |
|
| Interventions | Antidepressive doxepine od 50 mg for 8 weeks | |
| Outcomes | Abdominal pain, continuous Global assessment, dichotomous | |
| Notes | No run‐in | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Not described |
| Other bias | Unclear risk | Not described |
Capanni 2005.
| Methods | RCT | |
| Participants | 178 patients secondary care Rome II criteria Mean age 42 years 75% female |
|
| Interventions | Spasmolytics peppermint oil for 3 months, dose not stated |
|
| Outcomes | Global assessment, dichotomous IBS‐symptoms, dichotomous |
|
| Notes | 4 weeks run‐in Dosage of intervention not clear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 drop‐outs from peppermint oil group and 2 drop‐outs from placebo |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Cappello 2007.
| Methods | RCT | |
| Participants | 57 patients Setting unclear Rome II criteria mean age 41 years 76% female |
|
| Interventions | Spasmodic peppermint oil capsules 500 mg for 4 weeks |
|
| Outcomes | Abdominal pain, continuous IBS‐symptom score, continuous and dichotomous |
|
| Notes | No run in | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double blind, methods not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Centonze 1988.
| Methods | RCT | |
| Participants | 48 patients Outclinic 50% female Mean duration of symptoms 4 years |
|
| Interventions | Spasmolytic cimetropium tds 50 mg for 6 months | |
| Outcomes | Abdominal pain, continuous Global assessment, dichotomous | |
| Notes | 3 weeks run‐in without placebo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, procedures not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Four patients (one in the drug group, three in the placebo group) did not complete the study, three because of noncompliance and one because he moved away. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Chen 2004.
| Methods | RCT | |
| Participants | 120 IBS patients Setting unclear Rome II criteria Mean age 43 years 48% female |
|
| Interventions | Spasmolytic pinaverium bromide 100 mg tds for 8 weeks |
|
| Outcomes | Global assessment, dichotomous Symptom score, continuous |
|
| Notes | No run‐in | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Not described |
| Other bias | Unclear risk | Not described |
Czalbert 1990.
| Methods | RCT | |
| Participants | 34 IBS Hospital out clinic Mean age 49.3 years |
|
| Interventions | Spasmolytic peppermint oil 0.2 ml tds for 10 days | |
| Outcomes | Symptom score, dichotomous | |
| Notes | No run‐in | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Not described |
| Other bias | Unclear risk | Not described |
d'Arienzo 1980.
| Methods | RCT | |
| Participants | 28 IBS patients Unclear setting Diagnostic criteria not defined 39% female |
|
| Interventions | Spasmolytic otilonium tds 20 mg 4 weeks | |
| Outcomes | Symptom score, continuous | |
| Notes | No run‐in | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double blind, procedures not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Not described |
| Other bias | Unclear risk | Not described |
Delmont 1981.
| Methods | RCT | |
| Participants | 60 IBS patients Setting unclear Mean age 57 years 67% female |
|
| Interventions | Spasmolytic pinaverium tds for 30 days | |
| Outcomes | abdominal pain, dichotomous global assessment, dichotomous | |
| Notes | unclear setting No run‐in | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double blind ‐ procedures not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 drop‐outs |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Dobrilla 1990.
| Methods | RCT | |
| Participants | 70 IBS patients Hospital out clinic Mean age 45 years 67% female Mean duration of symptoms 3 years |
|
| Interventions | Spasmolytic 13 weeks cimetropium tds 50mg | |
| Outcomes | Abdominal pain, continuous Global assessment, dichotomous | |
| Notes | 2 weeks run‐in without placebo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind ‐ procedures not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 drop‐out |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Drossman 2003.
| Methods | RCT | |
| Participants | 216 patients with functional bowel disorders, 80% with IBS Secondary care Rome criteria Mean age 39.9 years 100% female |
|
| Interventions | Antidepressant desipramine 50‐100‐150mg od for 12 weeks |
|
| Outcomes | Abdominal pain, continuous and dichotomous Global assessment, continuous and dichotomous Symptom score, continuous and dichotomous | |
| Notes | No run‐in; minimum duration of symptoms of 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Investigator‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Dubarry 1977.
| Methods | RCT | |
| Participants | 20 IBS patients Outpatient setting Diagnostic criteria not defined |
|
| Interventions | Spasmolytic 6 days pinaverium 50 mg tds | |
| Outcomes | Abdominal pain, dichotomous | |
| Notes | No run‐in | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, methods not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Fielding 1980.
| Methods | RCT | |
| Participants | 60 IBS patients Unclear setting Diagnostic criteria not defined Mean age 26 years 75% female mean duration of symptoms 2 years |
|
| Interventions | Spasmolytic trimebutine tds 200 mg for 6 months | |
| Outcomes | Abdominal pain, dichotomous Global assessment, dichotomous | |
| Notes | No run‐in | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 drop‐outs |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Fowlie 1992.
| Methods | RCT | |
| Participants | 49 patients Gastro intestinal outpatient clinic Mean age 40 years 65% female Mean duration of symptoms 3.8 years |
|
| Interventions | Bulking agent 3 months mixed cereal and fruit fibre 4.1 g/day | |
| Outcomes | Abdominal pain Global assessment Symptom score | |
| Notes | GI Outpatients 1 week run‐in | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Ghidini 1986.
| Methods | RCT | |
| Participants | 60 IBS patients Hospital setting Mean age 42 years 60% female |
|
| Interventions | Spasmolytic
rociverine tds 20 mg for 60 days trimebutine 3 dd 100 mg |
|
| Outcomes | Abdominal pain, dichotomous Global assessment, dichotomous | |
| Notes | No run‐in | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind design with preparations that were outwardly indistinguishable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs |
| Selective reporting (reporting bias) | Low risk | Expected outcomes are reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Gilvarry 1989.
| Methods | RCT | |
| Participants | 24 IBS patients Unclear setting Diagnostic criteria not defined Mean age 32 years 79% female |
|
| Interventions | Antispasmodic pirenzepine 100 mg for 4 weeks | |
| Outcomes | Abdominal pain, dichotomous Global assessment, dichotomous |
|
| Notes | 4 to 8 weeks run‐in with high fibre diet (> 30 g/day) continued high fibre diet during study period |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double blind, methods not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 drop outs |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Heefner 1978.
| Methods | RCT | |
| Participants | 31 patients Outpatients clinic Diagnostic criteria not defined Mean age 48 years 13% female |
|
| Interventions | Antidepressive desipramine od 150 mg, 2months | |
| Outcomes | Abdominal pain, dichotomous | |
| Notes | No run‐in | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double blind, methods not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Jalihal 1990.
| Methods | RCT with Cross‐over design | |
| Participants | 20 patients Gastro intestinal outpatients clinic Diagnoses on clinical grounds Mean age 38 years 20% female |
|
| Interventions | Bulking agent 4 weeks ispaghula husk 30 g/day | |
| Outcomes | Abdominal pain, dichotomous Global assessment, dichotomous | |
| Notes | GI Outpatients No run‐in | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, treatment was indistinguishable from the placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs. Two patients were eligible but dropped out before the study. |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Kruis 1986.
| Methods | RCT | |
| Participants | 80 patients Outpatients clinic Mean age 41 years 61% female |
|
| Interventions | Bulking agent 16 weeks wheat bran fibre 8 g/day Spasmolitic agent 16 weeks mebeverine 100 mg 4 dd | |
| Outcomes | Global assessment, dichotomous Abdomianl pain, dichotomous | |
| Notes | GI patients No run‐in | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Mebeverine and placebo arms were double‐blind. Methods not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Kuiken 2003.
| Methods | RCT | |
| Participants | 40 patients, GI out clinic Rome I criteria Mean age 40 years 55% female Mean duration of symptoms 5.9 years |
|
| Interventions | Antidepressive fluoxetine (SSRI) 20 mg od for 6 weeks |
|
| Outcomes | Abdominal pain, dichotomous Global assessment, dichotomous | |
| Notes | No run‐in | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Centralized randomization by pharmacy |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 patients dropped out (2 from treatment group and 4 from placebo) |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Lech 1988.
| Methods | RCT | |
| Participants | 47 IBS patients Hospitals outpatients clinic Mean age 42 years 76% female |
|
| Interventions | Spasmolytic 4 weeks peppermint oil 3 dd 200 mg | |
| Outcomes | Global assessment, dichotomous | |
| Notes | No run‐in | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, methods not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Levy 1977.
| Methods | RCT | |
| Participants | 50 IBS patients Unclear setting Diagnostic criteria not defined Mean age 48 years 46% female |
|
| Interventions | Spasmolytic Pinaverium tds 50 mg 15 days | |
| Outcomes | Global assessment, dichotomous | |
| Notes | No run‐in | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, methods not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop outs |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Liu 1997.
| Methods | RCT | |
| Participants | 101 IBS patients Unclear setting 40% females |
|
| Interventions | Spasmolytic 4 weeks peppermint oil tds‐qid 187 mg | |
| Outcomes | Abdominal pain, dichotomous | |
| Notes | No run‐in | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9 drop‐outs: 3 patients on Colpermin and 6 on placebo did not return for follow‐up |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Longstreth 1981.
| Methods | RCT | |
| Participants | 77 patients Setting unclear Mean age 38.4 years 83% female Mean duration of symptoms 7.9 years |
|
| Interventions | Bulking agent 8 weeks psyllium 19 g/day | |
| Outcomes | Abdominal pain, continuous Global assessment, dichotomous | |
| Notes | Unclear setting 2 weeks run‐in | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical sachets |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Lucey 1987.
| Methods | RCT with Cross‐over design | |
| Participants | 44 Patients Gastrointestinal outpatient clinic Manning criteria Mean age 32 years 68% female Mean duration of symptoms 60 months |
|
| Interventions | Bulking agent 3 months wheat bran fibre 13g/day | |
| Outcomes | IBS symptom score, continuous | |
| Notes | GI Outpatients No run‐in | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, methods not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 16 patients dropped out. Reasons for withdrawal not reported |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Masand 2009.
| Methods | RCT | |
| Participants | 72 patient Secondary care Rome II criteria Mean age 49 years 88% female |
|
| Interventions | Antidepressant paroxetine 12.5‐50 mg for 12 weeks |
|
| Outcomes | Global assessment, dichotomous IBS‐symptoms, continuous, dichotomous |
|
| Notes | run in with placebo, time period not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, methods not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Mitchell 2002.
| Methods | RCT | |
| Participants | 107 IBS patients Secondary care 3 different hospitals Rome criteria Mean age 53 years 80% female |
|
| Interventions | Antispasmodic Alverine citrate 360 mg for 12 weeks |
|
| Outcomes | Global assessment Abdominal pain |
|
| Notes | 2 weeks run‐in without placebo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Moshal 1979.
| Methods | RCT with cross‐over design | |
| Participants | 20 IBS patients Unclear setting Diagnostic criteria not defined Mean age 27 years 35% females Mean duration of symptoms 1 year |
|
| Interventions | Spasmolytic trimebutine tds 200 mg for 4 weeks | |
| Outcomes | Abdominal pain, dichotomous | |
| Notes | No run‐in | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Myren 1982.
| Methods | RCT | |
| Participants | 61 patients Primary care setting Mean age 38.9 years 54% female |
|
| Interventions | Antidepressive trimipramine 1 dd 50 mg 4 weeks | |
| Outcomes | Global assessment, dichotomous | |
| Notes | No run‐in | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Nigam 1984.
| Methods | RCT | |
| Participants | 168 patients Secondary care Mean age 34.5 years 45% female |
|
| Interventions | Bulking agents Ispaghula husk, unclear dose, 12 weeks Spasmolitic agent Hyoscinebutylbromide, unclear dose, 12 weeks |
|
| Outcomes | Global assessment, dichotomous | |
| Notes | Inclear dose of intervention No run in |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Page 1981.
| Methods | RCT | |
| Participants | 97 patients Unclear setting Mean age 36.7 years 83% females Mean duration of symptoms 2 years |
|
| Interventions | Spasmolytic dicyclomine qid 40 mg for 2 weeks | |
| Outcomes | Abdominal pain, dichotomous Global assessment, dichotomous | |
| Notes | 2 weeks placebo run‐in | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, methods not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Passaretti 1989a.
| Methods | RCT | |
| Participants | 40 patients Out patient clinic Mean age 39 years 60% females |
|
| Interventions | Spasmolytic 4 weeks cimetropium tds 50 mg | |
| Outcomes | Abdominal pain, continuous Global assessment, dichotomous | |
| Notes | 2 weeks run‐in without placebo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, methods not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Piai 1979.
| Methods | RCT with cross‐over design | |
| Participants | 18 patients Unclear setting Diagnostic criteria not defined 56% females |
|
| Interventions | Spasmolytic prifinium 30 mg tds for 3 weeks | |
| Outcomes | Global assessment, dichotomous | |
| Notes | No run‐in | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, methods not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs |
| Selective reporting (reporting bias) | Low risk | All expected outcomes are reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Prior 1987.
| Methods | RCT | |
| Participants | 80 patients Outpatients 90% female |
|
| Interventions | Bulking agents 12 weeks ispaghula husk 19 g/day | |
| Outcomes | Abdominal pain, dichotomous Global assessment, dichotomous | |
| Notes | Outpatients 2 weeks run‐in | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, methods not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Pulpeiro 2000.
| Methods | RCT | |
| Participants | 85 IBS patients Hospital GI department Mean age 45.2 years 69% female |
|
| Interventions | Spasmolytic Propinox 4 dd for 4 weeks | |
| Outcomes | Abdominal pain, continuous Global assessment, dichotomous | |
| Notes | No run‐in Dose unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Rajagopalan 1998.
| Methods | RCT | |
| Participants | 22 patients Outpatients clinic Rome criteria Mean age 35 years 50% female |
|
| Interventions | Antidepressive amitriptyline to 75 mg od for 12 weeks | |
| Outcomes | Abdominal pain, continuous | |
| Notes | 1 week run‐in | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical placebo, all investigators and patients were blind to the treatment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The same number of patients dropped out from each group. Reasons for drop‐out were not reported |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Rees 2005.
| Methods | RCT | |
| Participants | 28 patients GI out clinic Rome criteria Mean age 36.1 years 86% female |
|
| Interventions | Bulking agent 10‐20 g coarse wheat bran once daily for 8 to 12 weeks | |
| Outcomes | Abdominal pain, continuous Global assessment, dichotomous |
|
| Notes | No run in period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single‐blind (patients) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 patients dropped out from treatment group and 4 dropped out from placebo |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Ritchie 1979.
| Methods | RCT | |
| Participants | 24 patients Secondary setting Diagnostic criteria not defined Mean age 38 years 77% female |
|
| Interventions | Bulking agent 4 weeks ispaghula husk, 1 sachet 2 dd Spasmolytics hyoscine 4 dd 10 mg 4 weeks |
|
| Outcomes | Global assessment, dichotomous | |
| Notes | Hospital setting No run‐in | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Centralized randomization by hospital pharmacist |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, double‐dummy. Dummy preparations were identical to active |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 drop‐outs |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Schafer 1990.
| Methods | RCT | |
| Participants | 360 IBS pat from various origin (prim care, GI clinic, int med) | |
| Interventions | Spasmolytic 4 weeks butylscopamine 30 mg/day | |
| Outcomes | Abdominal pain Global assessment | |
| Notes | 0.5 wk run‐in | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, methods not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop outs not described |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Soltoft 1976.
| Methods | RCT | |
| Participants | 59 patients Setting unclear Mean age 40 years 64% female |
|
| Interventions | Bulking agent 6 weeks wheat bran 30 g/day | |
| Outcomes | Global assessment, dichotomous | |
| Notes | Unclear setting >1 week run‐in | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo of similar appearance |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Tabas 2004.
| Methods | RCT | |
| Participants | 90 patients Voluntary participants through advertisement Rome I criteria Mean age 46 years 74% female |
|
| Interventions | Antidepressant paroxetine (SSRI) 10 or 20 mg od for 12 weeks |
|
| Outcomes | Abdominal pain, dichotomous Global assessment, dichotomous |
|
| Notes | 69 of 81 took part in high‐fibre open label trial 7 weeks before | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Centralized randomization by hospital pharmacist |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, researchers and patients were unaware of assignment until the conclusion of the last visit |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Tack 2006a.
| Methods | Crossoveer with first phase data reported | |
| Participants | 23 IBS patients Tertiary setting Rome II criteria Mean age 40 years 78% female |
|
| Interventions | Antidepressant citalopram (SSRI) 20‐40mg for 6 weeks |
|
| Outcomes | Abdominal pain, continuous Global assessment, continuous |
|
| Notes | 2 weeks run‐in | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, matching placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Talley 2008a.
| Methods | RCT | |
| Participants | 51 patients Setting secondary care Rome criteria 60% female 70% diarrhea predominant |
|
| Interventions | Antidepressive Imipramine (TCA) 50 mg dd for 12 weeks Citalopram (SSRI) 40 mg dd for 12 weeks |
|
| Outcomes | Abdominal pain, changes score (not included) global assessment, dichotomous and change score (not included) |
|
| Notes | 2 weeks run‐in period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Centralized randomization by hospital pharmacy |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, double‐dummy, identical capsules |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reasons for drop‐out not provided |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Vahedi 2005.
| Methods | RCT | |
| Participants | 44 IBS patients GI clinic Rome II criteria Mean age 35 years 61% female |
|
| Interventions | Antidepressant fluoxetine(SSRI) 20mg od for 12 weeks | |
| Outcomes | Abdominal pain | |
| Notes | Only 44 of 64 eligible patients included. No run‐in No run in period |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical placebo. Both patients and researchers were unaware of the true identity of the prescribed medicine |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Vahedi 2008.
| Methods | RCT | |
| Participants | 50 diarrhoea‐predominant IBS patients Tertiary GI clinic Rome II criteria Mean age 36 years 42% female Mean duration of symptoms 39 months |
|
| Interventions | Antidepressant amitriptyline 10 mg for 2 months |
|
| Outcomes | Abdominal pain, dichotomous IBS‐symptom score, continuous and dichotomous |
|
| Notes | No run‐in Only diarrhoea‐predominant IBS |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical placebo. Both patients and researchers were unaware of the true identity of the prescribed medicine |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Vij 1991.
| Methods | RCT | |
| Participants | 50 IBS patient Unclear setting Mean age 32 Years 28% female |
|
| Interventions | Antidepressive doxepin od 75mg for 6 weeks | |
| Outcomes | Abdominal pain, dichotomous Global assessment, dichotomous | |
| Notes | No run‐un | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Virat 1987.
| Methods | RCT | |
| Participants | 78 IBS patients GI Outpatients Diagnostic criteria not defined Mean age 44 years 67% female |
|
| Interventions | Spasmolytic pinaverium 50 mg tds 1 week | |
| Outcomes | Abdominal pain, dichotomous Global assessment, dichotomous | |
| Notes | No run‐in | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Low risk | Expected outcome reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Achord 1979 | Not an RCT |
| Alevizos 1989 | Not appropriate patients: depressive patients with IBS |
| Andre 1979 | Mixed intervention: composite of oxazepam and scopolamine butyl nitrate |
| Anonymous 1966 | Not an RCT |
| Anonymous 1976 | No extractable results |
| Anonymous 1982 | Not an RCT |
| Anonymous 1986 | Not an RCT; a review |
| Anonymous 1995 | Not an RCT |
| Anonymous 1998 | Not an RCT |
| Anonymous 2002a | Not an RCT |
| Anonymous 2002b | Not an RCT |
| Anonymous 2008 | Not an RCT; a report from Ford 2008 |
| Arffmann 1985 | Cross‐over study; no first phase data available |
| Awad 1997 | Not outcome of interest; post‐prandial motility |
| Baldi 1992 | Duplicate publication with Baldi 1991 |
| Barbier 1981 | Cross‐over study; no first phase data available |
| Bassotti 1988 | Not an RCT |
| Baume 1972 | Cross‐over study; no first phase data available |
| Bazzocchi 1992 | Not outcome of interest; anorectal function |
| Berthelot 1981 | Only 28% of patients had true IBS. Separate results were not available for these patients |
| Birt 1989 | Not available |
| Bixquert‐Jimenez 2005 | Not an RCT |
| Block 1983 | Duplicate publication with Myren 1982 |
| Bosaeus 2004 | Not an RCT; a review |
| Bouchoucha 2000 | Not outcome of interest; colonic response to food |
| Budavari 2002 | Not an RCT |
| Burden 2001 | Not an RCT; a review |
| Camarri 1981 | Cross‐over study; no first phase data available |
| Camarri 1986 | No placebo group; comparing fenoverine and trimebutine |
| Camatte 1966 | Not an RCT |
| Cann 1984 | Cross‐over study; no first phase data available |
| Capron 1981 | Mixed intervention; composite of bulk, sedative and laxative |
| Capurso 1984 | Cross over design with no parallel placebo administration in the first phase |
| Capurso 1992 | Mixed intervention; composite of octylonium bromide and diazepam |
| Carling 1989 | Cross‐over study with no reporting of outcomes before first cross‐over |
| Cerrato 2001 | Inappropriate patients; children |
| Chapman 1990 | No placebo; comparing mebeverine with high‐fibre dietary advice and mebeverine with ispaghula |
| Chassany 2007 | Inapproptiate patients; acute exacerbation |
| Chen 1999 | No placebo; comparing Alverine/dimeticone and nifedipine |
| Chen 2004a | No placebo |
| Chevrel 1978 | No placebo |
| Chicharro 2007 | Not an RCT |
| Christen 1990 | Not an RCT; a review |
| Clouse 1994 | Not an RCT; a chart review |
| Clouse 2003 | Not an RCT; a review |
| Cook 1990 | Cross‐over study; no first phase data available |
| Copé 1981 | No placebo; comparing clidiniumbromide‐chlordiazepoxide and trimebutine |
| Corazza 1983 | No placebo; comparing pinaverium bromide and trimebutine |
| Corazziari 1999 | Not an RCT; a review |
| Creed 2003 | No placebo; comparing SSRI and psychotherapy with usual care |
| Creed 2006 | Not an RCT; a review |
| Crowell 2004 | Not an RCT; a review |
| Curtiss 2008 | Not an RCT |
| Czimmer 2001 | Not outcome of interest; sensory thresholds and recto‐sigmoideal distention |
| Darnis 1980 | No placebo; comparing bran and kaologenis |
| De Groote 1968 | Not an RCT |
| de la Garoullaye 1991 | No placebo; comparing fenoverine with PVOO and karaya gum |
| Defrance 1991 | No placebo; comparing otilonium and pinaverium bromide |
| Delvaux 1997 | Not an RCT; a review |
| Dettmar 1998 | Not an RCT |
| Dettmar 1999 | No placebo; comparing ispaghula husk with mebeverine and mebeverine with high fibre diet |
| Dew 1984 | Cross‐over study; no first phase data available |
| Diaz‐Rubio 1985 | Not available |
| Dioguardi 1991 | No placebo; comparing octilonium bromide‐diazepam and propantheline bromide‐bromazepam |
| Dubinin 1987 | No placebo; comparing bran and bran with other drugs |
| Ehsanullah 1985 | Not outcome of interest; motility index |
| Eisenburg 1978 | Not an RCT |
| Evangelista 2004 | Not an RCT; a review |
| Evans 1996 | Not outcome of interest; motility index |
| Ferrari 1986 | No placebo; comparing octylonium bromide and cimetropium bromide |
| Fielding 1979 | Not an RCT |
| Fielding 1984 | No placebo; comparing different dietary fibres |
| Fioramonti 1988 | Not outcome of interest; colonic motility |
| Floch 1988 | Not an RCT |
| Francis 1994 | Not an RCT |
| Frexinos 1985 | Not outcome of interest; colonic motility |
| Fritz 1967 | Not an RCT |
| Galeone 1986a | No placebo; comparing tiropramide, trimebutine and octilonium bromide |
| Galeone 1986b | No placebo; comparing pinaverium bromide and otilonium bromide |
| Geismar 1971 | No extractable results; no clinically relevant data, only preferences |
| Geoffroy 1977 | No placebo |
| Giaccari 2001 | Not an RCT; patients divided in two groups bases on BMI |
| Giannini 2006 | Not an RCT |
| Gibbons 1979 | Not an RCT; a letter |
| Gilbody 2000 | No placebo; comparing two different formulations of mebeverine hydrochloride |
| Glende 2002 | Double‐publication with Battaglia 1998 |
| Gnauck 1977 | Not an RCT |
| Golechha 1982 | Cross‐over study; no first phase data available |
| Gorard 1994 | Not outcome of interest; whole gut and orocaecal transit times |
| Gorard 1995 | Not outcome of interest; small intestinal motility |
| Greenbaum 1981 | Not an RCT; a letter |
| Greenbaum 1984 | Duplicate publication with Greenbaum 1987 |
| Greenbaum 1987 | Cross‐over study; no first phase data available |
| Grigoleit 2005 | Not an RCT; a review |
| Guerre 1979 | No placebo; comparing karay gum and polyvinylpolypyrrolidone |
| Halpert 2005 | Duplicate publication; paper based on same patients as Drossman 2003 |
| Han 2009 | Duplicate publication; same patients as Masand 2009 |
| Hebden 2002 | Cross‐over study; no first phase data available |
| Herxheimer 1979 | Not an RCT; a letter |
| Hotz 1994 | No placebo |
| Houghton 1997 | Not outcome of interest; colonic motility |
| Inauen 1994 | No placebo; comparing mebeverine slow release and mebeverine tablets |
| Iwanaga 2002 | Not an RCT; a review |
| Jackson 1998 | Not an RCT |
| Jafri 2006 | Not an RCT |
| Jayanthi 1998 | Not an RCT |
| Ji 2007 | Inappropriate patients; patients with functional bowel disorder not specified as IBS |
| Jing 2004 | No placebo |
| Kaushik 2002 | Not an RCT |
| Kirsch 2000 | Not an RCT; a case report |
| Koch 1998 | Double publication with Liu 1997 |
| Koruda 1993 | Not an RCT |
| Kountouras 2002 | Inappropriate patients; patients with GERD, who also have IBS |
| Kumar 1987 | No placebo; comparing different doses of ispaghula husk |
| Kunze 1990 | No Placebo; comparing brief psychotherapy, acupuncture and papaverin |
| Lafon 1982 | No data extractable |
| Lambert 1989 | Not an RCT; a survey |
| Lambert 1991a | Not an RCT |
| Lambert 1991b | Inappropriate intervention; dietary advice |
| Lawson 1988 | Cross‐over study; no first phase data available |
| Levitan 1981 | Inappropriate patients; mixed IBS and diverticulosis |
| Lin 2003 | Not outcome of interest; anorectal visceral sensorimotor functions |
| Liu 2006 | Not an RCT |
| Locke 2004 | Not an RCT; a review article |
| Lu 2000 | No placebo; comparing pinaverlum bromide and mebeverine |
| Luttecke 1978 | Not outcome of interest; only preference |
| Luttecke 1980 | Cross‐over study; no first phase data available |
| Lydiard 2007 | Not an RCT |
| MacRae 1979 | Not an RCT; a letter |
| Manning 1976 | No placebo |
| Manning 1977 | No placebo; Comparing high and low‐fibre diet |
| Marks 2008 | Duplicate publication; same patients as Masand 2009 |
| Masamune 1998 | No placebo; comparing calcium polycarbophil and torimebutine maleate |
| Masand 2002 | Not an RCT |
| Masand 2005 | Not an RCT |
| Matts 1967 | No extractable data |
| Meier 1996 | Mixed intervention; psyllium and Cisapride versus placebo |
| Miller 2006 | Not an RCT |
| Misra 1989 | Not the appropriate patients; patients who had recovered completely after treatment |
| Modena 1993 | No placebo |
| Mollica 1992 | No placebo; comparing Otilonium bromide‐diazepam and otilonium bromide |
| Morgan 2005 | Cross‐over study; no first phase data available |
| Morrison 1987 | Not outcome of interest; the role of the dietitian |
| Mortensen 1987 | Not outcome of interest; short‐chain fatty acids (SCFA) in faeces |
| Nash 1986 | Cross‐over study; no first phase data available |
| Nedogoda 2000 | No placebo; comparing pinaverium bromide and otilonium bromide |
| Noel 1989 | Not an RCT |
| Olden 2005 | Not an RCT; a review |
| Pardell 1982 | No placebo; comparing clebopride and hyoscine N‐butylbromide |
| Parisi 2002 | No placebo; comparing guargum and wheat bran |
| Parisi 2005 | No placebo group; comparing different doses of guar gum |
| Passaretti 1985 | Not an RCT |
| Passaretti 1989b | Not outcome of interest; sigmoid‐rectal motility |
| Pearson 2000 | Not an RCT |
| Piai 1987a | Inappropriate patients; mixture of IBS and functional constipation |
| Piai 1987b | Inappropriate patients; IBS in remission |
| Pittler 1998 | Not an RCT; a meta‐analysis |
| Prior 1986a | Only abstract; full text not available |
| Prior 1986b | Duplicate publication; abstract publication of Prior 1987 |
| Prout 1983 | No placebo; comparing two doses of mebeverine |
| Quilici 1998 | Not an RCT |
| Quilici 2003 | Not an RCT |
| Rasmussen 1982 | Inappropriate intervention; diet |
| Rees 1979 | Cross‐over study; no first phase data available |
| Rhodes 1978 | Inaprporiate intervention; sedative‐anticholinergic drug combinations |
| Rhodes 1980 | No extractable results |
| Ritchie 1980 | No placebo; comparing psychotropic drug, smooth‐muscle relaxant, and bulking agent |
| Sagduyu 2002 | Not an RCT; a letter |
| Sato 2006 | Not an RCT; a review |
| Schaffstein 1990 | No placebo; comparing trimebutine and mebeverine |
| Schutz 1992 | Not an RCT |
| Secco 1983 | Not an RCT |
| Sharma 1987 | Not an RCT |
| Shaughnessy 2000 | Not an RCT |
| Shrivastava 1984 | Not outcome of interest |
| Singh 2007 | Not an RCT |
| Slawson 2002 | Not an RCT |
| Snook 1994 | Cross‐over study; no first phase data available |
| Soifer 1987 | Not an RCT |
| Soifer 1992 | Not outcome of interest; colon motility |
| Soriano 1992 | Not an RCT |
| Stiefelhagen 2008 | Not an RCT |
| Swiatczak 1998 | Not an RCT; comparing before and after treatment with bran |
| Talley 2003 | Not an RCT |
| Talley 2004 | Not an RCT |
| Talley 2008b | Not an RCT; a review |
| Tan 2007 | Not an RCT; a review |
| Tanum 1996 | Inappropriate patients; mixture of IBS and NUD patients |
| Tanum 2000 | Not outcome of interest; assessment of personality traits |
| Tarpila 2004 | No placebo; comparing flaxseed and psyllium |
| Tarquini 1984 | No placebo; comparing octilonium bromide and octilonium bromide with a benzodiazepine |
| Tasman‐Jones 1973 | Cross‐over study; no first phase data available |
| Tinozzi 1984 | No placebo; comparing tiropramide hydrochloride and octylonium bromide |
| Tomas‐Ridocci 1992 | Not the appropriate patients; patients with chronic constipation with or without IBS |
| Toussaint 1981 | No placebo; comparing trimebutine and mebeverine |
| Tripathi 1983 | Duplicate publication with Shrivastava 1984 |
| Tsuneoka 1987 | No placebo; comparing trimebutine maleate and mepenzolate bromide |
| Tudor 1986 | No placebo; comparing alverine citrate and mebeverine hydrochloride |
| van Kerkhoven 2007 | Not an RCT; a letter |
| Van Outryve 1995 | No placebo; comparing mebeverine and mebeverine sustained release |
| Van Steensel 1990 | Not an RCT |
| Villagrasa 1991 | No placebo; comparing fibre‐rich diet and otilonium bromide |
| Wald 1990 | Not an RCT |
| Wald 2002 | Not an RCT; a review |
| Wang 2003 | No placebo; comparing fluoxetine, paroxetine and doxepin |
| Wittmann 1999 | No RCT; healthy patients compared with IBS patients |
| Woolner 2000 | Not an RCT; preliminary before‐after study of low‐fibre diet |
| Yuan 2003 | Not an RCT |
| Yuan 2005 | No placebo; comparing trimebutine maleat and pinaveriumbromide |
| Zhang 2002 | No placebo; comparing otilonium bromide, collodal bismuth tartrate and compound diphenoxylate |
| Zhou 2002 | No placebo; comparing paroxetine and pinaverium bromide versus paroxetine versus pinaverium bromide |
| Zuckerman 2006 | Not an RCT; therapeutic recommendations |
Characteristics of studies awaiting assessment [ordered by study ID]
Abdul‐Baki 2009.
| Methods | RCT |
| Participants | 107 IBS patients |
| Interventions | Antidepressant imipramine 25 mg versus matched placebo for 12 weeks |
| Outcomes | Global symptom relief |
| Notes |
Bijkerk 2009.
| Methods | RCT |
| Participants | 275 patients with IBS General practice |
| Interventions | Bulking agent 12 weeks of treatment with: psyllium 10 g or bran 10 g or placebo 10 g (rice flour) |
| Outcomes | Adequate symptom relief IBS symptom severity score Severity of abdominal pain IBS quality of life scale |
| Notes |
Everitt 2010.
| Methods | RCT |
| Participants | 135 IBS patients (Rome III) |
| Interventions | Antispasmodic and bulking agent mebeverine methylcellulose placebo |
| Outcomes | IBS severity scale IBS‐QOL |
| Notes |
Khalif 2009.
| Methods | RCT |
| Participants | 118 IBS patients (Rome II) |
| Interventions | Antispasmodic oral buscopan (20 mg TID) buscopan suppository (30 mg OD) oral drotaverine (80 mg TID) calcium gluconate tablets (1 TID) calendula suppository (OD) |
| Outcomes | Pain score |
| Notes |
Ladabaum 2010.
| Methods | RCT |
| Participants | 54 non‐depressed IBS patients |
| Interventions | Antidepressant citalopram (20 mg/.day for 4 weeks, then 40 mg/day for 4 weeks) placebo |
| Outcomes | Adequate relief of IBS symptoms IBS‐QOL |
| Notes |
Merat 2010.
| Methods | RCT |
| Participants | 90 IBS outpatients |
| Interventions | Antispasmodic Peppermint oil, colpermin (1 capsule TID for 8 weeks) Placebo (1 capsule TID for 8 weeks) |
| Outcomes | Abdominal pain QOL |
| Notes |
Pace 2010.
| Methods | RCT |
| Participants | 186 IBS patients (Rome II) |
| Interventions | Antispasmodic octatropine (40 mg BID) and diazepam (2.5 mg BID) or placebo |
| Outcomes | Satisfactory relief of abdominal pain abdominal swelling abdominal pain and discomfort symptom severity number of bowel movements |
| Notes |
Reme 2010.
| Methods | RCT |
| Participants | 149 IBS patients |
| Interventions | Antispasmodic mebeverine or mebeverine and cognitive behavior therapy |
| Outcomes | Psychological distress (anxiety and depression) |
| Notes |
Saps 2009.
| Methods | RCT |
| Participants | 90 children with functional gastrointestinal disorders |
| Interventions | Antidepressant amitriptyline (dose based on weight) or placebo |
| Outcomes | Therapeutic response |
| Notes |
Wittmann 2010.
| Methods | RCT |
| Participants | 412 IBS patients (Rome III) |
| Interventions | Antispasmodic alverine citrate (60 mg) with simeticone (300 mg) BID or matching placebo |
| Outcomes | Abdominal pain |
| Notes |
Contributions of authors
Preparation of protocol Coordination of reviewers Data collection Data review Preparation of report
Declarations of interest
Greg Rubin owns shares in Glaxo Smith Kline and has received payment for consultancy from pharma companies. The other authors report no known declarations of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Aller 2004 {published data only}
- Aller R, Luis DA, Izaola O, Calle F, Olmo L, Fernandez L, et al. Dietary intake of a group of patients with irritable bowel syndrome; relation between dietary fiber and symptoms. Nutrition 2004;20:735‐7. [Google Scholar]
Arthurs 1983 {published data only}
- Arthurs Y, Fielding JF. Double blind trial of ispaghula/poloxamer in the irritable bowel syndrome. Ir Med J 1983;76(5):253. [PubMed] [Google Scholar]
Awad 1995 {published data only}
- Awad R, Dibildox M, Ortiz F. Irritable bowel syndrome treatment using pinaverium bromide as a calcium channel blocker. A randomized double‐blind placebo‐controlled trial. Acta Gastroenterol Latinoam 1995;25(3):137‐44. [PubMed] [Google Scholar]
Bahar 2008 {published data only}
- Bahar RJ, Collins BS, Steinmetz B, Ament ME. Double‐blind placebo‐controlled trial of amitriptyline for the treatment of irritable bowel syndrome in adolescents. J Pediatr 2008 Dec;153(6):872. [DOI] [PubMed] [Google Scholar]
Baldi 1991 {published data only}
- Baldi F, Longanesi A, Blasi A, Monello S, Cestari R, Missale G, et al. Clinical and functional evaluation of the efficacy of otilonium bromide: a multicenter study in Italy. Ital J Gastroenterol 1991;23(8 Suppl 1):60‐3. [PubMed] [Google Scholar]
Battaglia 1998 {published data only}
- Battaglia G, Morselli‐Labate AM, Camarri E, Francavilla A, Marco F, Mastropaolo G, et al. Otilonium bromide in irritable bowel syndrome: a double‐blind, placebo‐controlled, 15‐week study. Aliment Pharmacol Ther 1998;12(10):1003‐10. [DOI] [PubMed] [Google Scholar]
Bergmann 1991 {published data only}
- Bergmann M, Heddergott A, Schlosser T. Therapy of irritable colon with trimipramine (Herphonal) ‐ A controlled clinical study [Die therapie des colon irritabile mittrimipramin (Herphonal) ‐ Eine kontrollierte studie]. Z Klin Med 1991;46(23):1621‐8. [Google Scholar]
Boerner 1988 {published data only}
- Boerner D, Eberhardt R, Metz K, Schick E. Wirksamkeit ind vertraeglichkeit eines antidepressivums beim colon irritablie. Therapiewoche 1988;38:201‐8. [Google Scholar]
Capanni 2005 {published data only}
- Capanni M, Surrenti E, Biagini M R, Milani S, Surrenh C, Galli A. Efficacy of peppermint oil in the treatment of irritable bowel syndrome: A randomized, controlled trial. Gazz Med Ital Arch Sci Med 2005;164(2):119‐26. [Google Scholar]
Cappello 2007 {published data only}
- Cappello G, Spezzaferro M, Grossi L, Manzoli L, Marzio L. Peppermint oil (Mintoil) in the treatment of irritable bowel syndrome: a prospective double blind placebo‐controlled randomized trial. Dig Liver Dis 2007;39(6):530‐6. [DOI] [PubMed] [Google Scholar]
Centonze 1988 {published data only}
- Centonze V, Imbimbo BP, Campanozzi F, Attolini E, Daniotti S, Albano O. Oral cimetropium bromide, a new antimuscarinic drug, for long‐term treatment of irritable bowel syndrome. Am J Gastroenterol 1988;83(11):1262‐6. [PubMed] [Google Scholar]
Chen 2004 {published data only}
- Chen SJ, Li GX, Wang LJ, Sun LM, Si JM. SF‐36 quality of life in effectiveness assessment for irritable bowel syndrome. World Chin J Dig 2004;12(4):920‐3. [Google Scholar]
Czalbert 1990 {published data only}
- Czalbert HJ, Neder M, Feher K. Experiences with colpermintherapy (Tillots‐England) at patients of irritable colon syndrome. Gyogyszereszet 1990;34(5):251‐3. [Google Scholar]
d'Arienzo 1980 {published data only}
- d'Arienzo A, d'Agotino L. L'ottilonio bromuro nel trattamento della sindrome del colon irritabile. Rass Int Clin Ter 1980;60(10):649‐56. [Google Scholar]
Delmont 1981 {published data only}
- Delmont J. The value of adding an antispasmodic musculotropic agent in the treatment of painful constipation in functional colopathies with bran. Double‐blind study [Interet de l'adjonction d'un antispasmodique musculotrope au traitement des constipations douloureuses des colopathies fonctionnelles par le son. Essai en double insu]. Med Chir Dig 1981;10(4):365‐70. [PubMed] [Google Scholar]
Dobrilla 1990 {published data only}
- Dobrilla G, Imbimbo BP, Piazzi L, Bensi G. Longterm treatment of irritable bowel syndrome with cimetropium bromide: a double blind placebo controlled clinical trial. Gut 1990;31(3):355‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Drossman 2003 {published data only}
- Drossman DA, Toner BB, Whitehead WE, Diamant NE, Dalton CB, Duncan S, et al. Cognitive‐behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology 2003;125:19‐31. [DOI] [PubMed] [Google Scholar]
Dubarry 1977 {published data only}
- Dubarry JJ, Quinton A. Effet a court terme du bromure de pinaverium dans les oesophagites, gastro‐duodenites et colopathies fonctionnelles. Bordeaux Medical 1977;10(21):1457‐9. [Google Scholar]
Fielding 1980 {published data only}
- Fielding JF. Double blind trial of trimebutine in the irritable bowel syndrome. Ir Med J 1980;73(10):377‐9. [PubMed] [Google Scholar]
Fowlie 1992 {published data only}
- Fowlie S, Eastwood MA, Prescott R. Irritable bowel syndrome: assessment of psychological disturbance and its influence on the response to fibre supplementation. J Psychosom Res 1992;36(2):175‐80. [DOI] [PubMed] [Google Scholar]
Ghidini 1986 {published data only}
- Ghidini O, Saponati G, Intrieri L. Single drug treatment for irritable colon: Rociverine versus trimebutine maleate. Curr Ther Res Clin Exp 1986;39(4):541‐8. [Google Scholar]
Gilvarry 1989 {published data only}
- Gilvarry J, Kenny A, Fielding J F. The non‐effect of pirenzepine in dietary resistant irritable bowel syndrome. Ir J Med Sci 1989;158(10):262. [DOI] [PubMed] [Google Scholar]
Heefner 1978 {published data only}
- Heefner JD, Wilder RM, Wilson ID. Irritable colon and depression. Psychosomatics 1978;19(9):540‐7. [DOI] [PubMed] [Google Scholar]
Jalihal 1990 {published data only}
- Jalihal A, Kurian G. Ispaghula therapy in irritable bowel syndrome: improvement in overall well‐being is related to reduction in bowel dissatisfaction. J Gastroenterol Hepatol 1990;5(5):507‐13. [DOI] [PubMed] [Google Scholar]
Kruis 1986 {published data only}
- Kruis W, Weinzierl M, Schussler P, Holl J. Comparison of the therapeutic effect of wheat bran, mebeverine and placebo in patients with the irritable bowel syndrome. Digestion 1986;34(3):196‐201. [DOI] [PubMed] [Google Scholar]
Kuiken 2003 {published data only}
- Kuiken S D, Tytgat G N, Boeckxstaens G E. The selective serotonin reuptake inhibitor fluoxetine does not change rectal sensitivity and symptoms in patients with irritable bowel syndrome: a double blind, randomized, placebo‐controlled study. Clin Gastroenterol Hepatol 2003;1(3):219‐28. [DOI] [PubMed] [Google Scholar]
Lech 1988 {published data only}
- Lech Y, Olesen KM, Hey H, Rask‐Pedersen E, Vilien M, Ostergaard O. Treatment of irritable bowel syndrome with peppermint oil. A double‐blind study with a placebo [Behandling af colon irritabile med pebermynteolie. En dobbeltblind undersogelse med placebo]. Ugeskr Laeger 1988;150(40):2388‐9. [PubMed] [Google Scholar]
Levy 1977 {published data only}
- Levy C, Charbonnier A, Cachin M. Pinaverium bromide and functional colonic disease (double‐blind study) [Bromure de pinaverium et colopathie fonctionnelle (etude a double insu)]. Sem Hop Ther 1977;53(7‐8):372‐4. [PubMed] [Google Scholar]
Liu 1997 {published data only}
- Liu JH, Chen GH, Yeh HZ, Huang CK, Poon SK. Enteric‐coated peppermint‐oil capsules in the treatment of irritable bowel syndrome: a prospective, randomized trial. J Gastroenterol 1997;32(6):765‐8. [DOI] [PubMed] [Google Scholar]
Longstreth 1981 {published data only}
- Longstreth GF, Fox DD, Youkeles L, Forsythe AB, Wolochow DA. Psyllium therapy in the irritable bowel syndrome. A double‐blind trial. Ann Intern Med 1981;95(1):53‐6. [DOI] [PubMed] [Google Scholar]
Lucey 1987 {published data only}
- Lucey MR, Clark ML, Lowndes J, Dawson AM. Is bran efficacious in irritable bowel syndrome? A double blind placebo controlled crossover study. Gut 1987;28(2):221‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Masand 2009 {published data only}
- Masand PS, Pae CU, Krulewicz S, Peindl K, Mannelli P, Varia I M, et al. A double‐blind, randomized, placebo‐controlled trial of paroxetine controlled‐release in irritable bowel syndrome. Psychosomatics 2009;50(1):78‐86. [DOI] [PubMed] [Google Scholar]
Mitchell 2002 {published data only}
- Mitchell SA, Mee AS, Smith GD, Palmer KR, Chapman RW. Alverine citrate fails to relieve the symptoms of irritable bowel syndrome: results of a double‐blind, randomized, placebo‐controlled trial. Aliment Pharmacol Ther 2002;16(6):1187‐95. [DOI] [PubMed] [Google Scholar]
Moshal 1979 {published data only}
- Moshal MG, Herron M. A clinical trial of trimebutine (Mebutin) in spastic colon. J Int Med Res 1979;7(3):231‐4. [DOI] [PubMed] [Google Scholar]
Myren 1982 {published data only}
- Myren J, Groth H, Larssen SE, Larsen S. The effect of trimipramine in patients with the irritable bowel syndrome. A double‐blind study. Scand J Gastroenterol 1982;17(7):871‐5. [DOI] [PubMed] [Google Scholar]
Nigam 1984 {published data only}
- Nigam P, Kapoor KK, Rastog CK, Kumar A, Gupta AK. Different therapeutic regimens in irritable bowel syndrome. J Assoc Physicians India 1984;32(12):1041‐4. [PubMed] [Google Scholar]
Page 1981 {published data only}
- Page JG, Dirnberger GM. Treatment of the irritable bowel syndrome with Bentyl (dicyclomine hydrochloride). J Clin Gastroenterol 1981;3(2):153‐6. [DOI] [PubMed] [Google Scholar]
Passaretti 1989a {published data only}
- Passaretti S, Guslandi M, Imbimbo BP, Daniotti S, Tittobello A. Effects of cimetropium bromide on gastrointestinal transit time in patients with irritable bowel syndrome. Aliment Pharmacol Ther 1989;3(3):267‐76. [DOI] [PubMed] [Google Scholar]
Piai 1979 {published data only}
- Piai G, Mazzacca G. Prifinium bromide in the treatment of the irritable colon syndrome. Gastroenterology 1979;77(3):500‐2. [PubMed] [Google Scholar]
Prior 1987 {published data only}
- Prior A, Whorwell PJ. Double blind study of ispaghula in irritable bowel syndrome. Gut 1987;28(11):1510‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pulpeiro 2000 {published data only}
- Pulpeiro A, Marti ML, Santos AR, Girolamo G. Propinox in the treatment of irritable bowel syndrome [Propinox en sindrome de intestino irritable]. Prensa Medica Argentina 2000;87(3):299‐307. [Google Scholar]
Rajagopalan 1998 {published data only}
- Rajagopalan M, Kurian G, John J. Symptom relief with amitriptyline in the irritable bowel syndrome. J Gastroenterol Hepatol 1998;13(7):738‐41. [DOI] [PubMed] [Google Scholar]
Rees 2005 {published data only}
- Rees G, Davies J, Thompson R, Parker M, Liepins P. Randomised‐controlled trial of a fibre supplement on the symptoms of irritable bowel syndrome. J R Soc Health 2005;125(1):30‐4. [DOI] [PubMed] [Google Scholar]
Ritchie 1979 {published data only}
- Ritchie JA, Truelove SC. Treatment of irritable bowel syndrome with lorazepam, hyoscine butylbromide, and ispaghula husk. Br Med J 1979;1(6160):376‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schafer 1990 {published data only}
- Schafer E, Ewe K. The treatment of irritable colon. Efficacy and tolerance of buscopan plus, buscopan, paracetamol and placebo in ambulatory patients with irritable colon [Behandlung des Colon irritabile. Wirksamkeit und Vertraglichkeit von Buscopan plus, Buscopan, Paracetamol und Plazebo bei ambulanten Patienten mit Colon irritabile]. Fortschr Med 1990;108(25):488‐92. [PubMed] [Google Scholar]
Soltoft 1976 {published data only}
- Soltoft J, Krag B, Gudmand‐Hoyer E, Kristensen E, Wulff HR. A double‐blind trial of the effect of wheat bran on symptoms of irritable bowel syndrome. Lancet 1976;1(7954):270‐2. [DOI] [PubMed] [Google Scholar]
Tabas 2004 {published data only}
- Tabas G, Beaves M, Wang J, Friday P, Mardini H, Arnold G. Paroxetine to treat irritable bowel syndrome not responding to high‐fiber diet: A double‐blind, placebo‐controlled trial. Am J Gastroenterol 2004;99(5):914‐20. [DOI] [PubMed] [Google Scholar]
Tack 2006a {published data only}
- Tack J, Broekaert D, Fischler B, Oudenhove L, Gevers A M, Janssens J. A controlled crossover study of the selective serotonin reuptake inhibitor citalopram in irritable bowel syndrome. Gut 2006;55(8):1095‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Talley 2008a {published data only}
- Talley NJ, Kellow JE, Boyce P, Tennant C, Huskic S, Jones M. Antidepressant therapy (imipramine and citalopram) for irritable bowel syndrome: a double‐blind, randomized, placebo‐controlled trial. Dig Dis Sci 2008;53(1):108‐15. [DOI] [PubMed] [Google Scholar]
Vahedi 2005 {published data only}
- Vahedi H, Merat S, Rashidioon A, Ghoddoosi A, Malekzadeh R. The effect of fluoxetine in patients with pain and constipation‐predominant irritable bowel syndrome: a double‐blind randomized‐controlled study. Aliment Pharmacol Ther 2005;22(5):381‐5. [DOI] [PubMed] [Google Scholar]
Vahedi 2008 {published data only}
- Vahedi H, Merat S, Momtahen S, Kazzazi A S, Ghaffari N, Olfati G, et al. Clinical trial: the effect of amitriptyline in patients with diarrhoea‐predominant irritable bowel syndrome. Aliment Pharmacol Ther 2008;27(8):678‐84. [DOI] [PubMed] [Google Scholar]
Vij 1991 {published data only}
- Vij JG, Jiloha RC, Kumar N. Effect of antidepressant drug (doxepin) on irritable bowel syndrome patients. Indian J Psychiatry 1991;33:243‐6. [Google Scholar]
Virat 1987 {published data only}
- Virat J, Hueber D. Colopathy pain and dicetel. Prat Med 1987;43:32‐4. [Google Scholar]
References to studies excluded from this review
Achord 1979 {published data only}
- Achord JL. Irritable bowel syndrome and dietary fiber. J Am Diet Assoc 1979;75(4):452‐3. [PubMed] [Google Scholar]
Alevizos 1989 {published data only}
- Alevizos B, Christodoulou GN, Ioannidis C, Voulgari A, Mantidis A, Spiliadis C. The efficacy of amineptine in the treatment of depressive patients with irritable bowel syndrome. Clin Neuropharmacol 1989;12 Suppl 2:S66‐76. [DOI] [PubMed] [Google Scholar]
Andre 1979 {published data only}
- Andre PP, Windt J, Dony A. Basic treatment of spastic colon (multicenter trial with oxazepam plus scopolamine butyl nitrate). ARS MED INT TIJDSCHR PRAKT THER 1979;8(19):1673‐7. [Google Scholar]
Anonymous 1966 {published data only}
- Anonymous. Evaluation of an antispasmodic agent. Methixene hydrochloride (Trest). JAMA 1966;195(10):851. [DOI] [PubMed] [Google Scholar]
Anonymous 1976 {published data only}
- Anonymous. Irritable colon syndrome treated with an antispasmodic drug. Practitioner 1976;217(1298):276‐80. [PubMed] [Google Scholar]
Anonymous 1982 {published data only}
- Anonymous. Is pinaverium bromide suitable for treatment of spastic colon?. Ned Tijdschr Geneeskd 1982;126(38):1746. [Google Scholar]
Anonymous 1986 {published data only}
- Anonymous. Some antispasmodic drugs for the irritable bowel syndrome. Drug Ther Bull 1986;24(24):93‐5. [PubMed] [Google Scholar]
Anonymous 1995 {published data only}
- Anonymous. Effective and well‐tolerated spasmolytic agent in the treatment of irritable colon syndrome. Therapiewoche 1995;45(9):560, 562‐4. [Google Scholar]
Anonymous 1998 {published data only}
- Anonymous. Mebeverine suspension in childhood irritable bowel syndrome. Arztliche Prax Padiatr 1998;12:26‐7. [Google Scholar]
Anonymous 2002a {published data only}
- Anonymous. SSRIs to treat irritable bowel?. Pharm J 2002;268:755. [Google Scholar]
Anonymous 2002b {published data only}
- Anonymous. Trimebutine: Another therapeutic choice for irritable bowel syndrome. Chin J Gastroenterol 2002;7(4):206‐8. [Google Scholar]
Anonymous 2008 {published data only}
- Anonymous. Peppermint oil can relieve symptoms of irritable bowel syndrome. Dtsch Apoth 2008;148(50):41. [Google Scholar]
Arffmann 1985 {published data only}
- Arffmann S, Andersen JR, Hegnhoj J, Schaffalitzky de Muckadell OB, Mogensen NB, Krag E. The effect of coarse wheat bran in the irritable bowel syndrome. A double‐blind cross‐over study. Scand J Gastroenterol 1985;20(3):295‐8. [DOI] [PubMed] [Google Scholar]
Awad 1997 {published data only}
- Awad RA, Cordova VH, Dibildox M, Santiago R, Camacho S. Reduction of post‐prandial motility by pinaverium bromide a calcium channel blocker acting selectively on the gastrointestinal tract in patients with irritable bowel syndrome. Acta Gastroenterol Latinoam 1997;27(4):247‐51. [PubMed] [Google Scholar]
Baldi 1992 {published data only}
- Baldi F, Longanesi A, Blasi A, Monello S, Cestari R, Missale G, et al. Octylonium bromide in the treatment of the irritable bowel syndrome: a clinical‐functional study. Hepatogastroenterology 1992;39(5):392‐5. [PubMed] [Google Scholar]
Barbier 1981 {published data only}
- Barbier P. Double blind controlled trial of a new colonic antispasmodic agent, actylonium bromide [Etude controlee en double aveugle d'un nouvel antspasmodique colique]. Ars Medici 1981;36(21):1879‐80. [Google Scholar]
Bassotti 1988 {published data only}
- Bassotti G. Dietary fibers in gastroenterology. MED RIV ENCICL MED ITAL 1988;8(2):141‐5. [PubMed] [Google Scholar]
Baume 1972 {published data only}
- Baume P. Mebeverine, an effective agent in the irritable colon syndrome. Aust NZ J Med 1972;2(1):34‐6. [DOI] [PubMed] [Google Scholar]
Bazzocchi 1992 {published data only}
- Bazzocchi G, Difalco A, Bensi G, Lanfranchi GA. The effect of muscarinic blockade on anorectal function in patients with constipation‐predominant irritable bowel syndrome. Curr Ther Res Clin Exp 1992;52(1):135‐43. [Google Scholar]
Berthelot 1981 {published data only}
- Berthelot J, Centonze V. Etude controlee en double aveugle Duspatalin (Mebeverine) contre placebo, dans le traitement du colon irritable. Gaz Med Fr 1981;88(16):2341‐3. [Google Scholar]
Birt 1989 {published data only}
- Birt M, Dumitrascu D. Treatment with antidepressants in biliary dyskinesia and irritable colon. Rev Med Interna Neurol Psihiatr Neurochir Dermatovenerol Med Interna 1989;41(3):277‐82. [PubMed] [Google Scholar]
Bixquert‐Jimenez 2005 {published data only}
- Bixquert‐Jimenez M, Bixquert‐Pla L. Antidepressant therapy in functional gastrointestinal disorders. Gastroenterol Hepatol 2005;28(8):485‐92. [DOI] [PubMed] [Google Scholar]
Block 1983 {published data only}
- Block J, Myren J, Larssen SE, Larsen S. Trimipramin (Surmontil) and placebo in irritable colon syndrome. A double‐blind study [Trimipramin (Surmontil) og placebo ved irritable colon syndrom. En dobbeltblind studie]. Tidsskr Nor Laegeforen 1983;103(11):903‐5. [PubMed] [Google Scholar]
Bosaeus 2004 {published data only}
- Bosaeus I. Fiber effects on intestinal functions (diarrhoea, constipation and irritable bowel syndrome). Clin Nutr Suppl 2004;1(2):33‐8. [Google Scholar]
Bouchoucha 2000 {published data only}
- Bouchoucha M, Faye A, Devroede G, Arsac M. Effects of oral pinaverium bromide on colonic response to food in irritable bowel syndrome patients. Biomed Pharmacother 2000;54(7):381‐7. [DOI] [PubMed] [Google Scholar]
Budavari 2002 {published data only}
- Budavari AI, Olden KW. The use of antidepressants in irritable bowel syndrome. Pract Gastroenterol 2002;26(3):13‐27. [Google Scholar]
Burden 2001 {published data only}
- Burden S. Dietary treatment of irritable bowel syndrome: current evidence and guidelines for future practice. J Hum Nutr Diet 2001;14(3):231‐41. [DOI] [PubMed] [Google Scholar]
Camarri 1981 {published data only}
- Camarri E, Guidoni G, Marini G, Schiaroli G. La terapia del colon irritabile. Risultati del trattamento con un nuovo antispastico: l'ottilonio bromuro. Policlinico [Prat] 1981;88:174‐84. [Google Scholar]
Camarri 1986 {published data only}
- Camarri E. Fenoverine: Smooth muscle synchronizer for the management of gastro‐intestinal conditions. II. A trimebutine‐controlled, double‐blind, crossover clinical evaluation. Curr Med Res Opin 1986;10(1):52‐7. [DOI] [PubMed] [Google Scholar]
Camatte 1966 {published data only}
- Camatte R, Sarles H. Functional colonic diseases and Mebeverine. Sem Ther 1966;42(9):509‐10. [PubMed] [Google Scholar]
Cann 1984 {published data only}
- Cann PA, Read NW, Holdsworth CD. What is the benefit of coarse wheat bran in patients with irritable bowel syndrome?. Gut 1984;25(2):168‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Capron 1981 {published data only}
- Capron JP, Zeitoun P, Julien D. A multicenter controlled trial of a combination of kaolin, sterculia gum, meprobamate, and magnesium salts, in the irritable bowel syndrome (author's transl) [Effets d'un medicament associant kaolin, gomme sterculia, magnesium et meprobamate le traitement du colon irritable. Resultats d'une etude controlee multicentrique]. Gastroenterol Clin Biol 1981;5(1):67‐72. [PubMed] [Google Scholar]
Capurso 1984 {published data only}
- Capurso L, Koch M, Tarquini M, Dezi A, Papi C, Fracasso P. The irritable bowel syndrome. A cross‐over study of octilonium bromide, mebeverine and placebo. Clin Trials J 1984;21(5):285‐91. [Google Scholar]
Capurso 1992 {published data only}
- Capurso L, Sette F, Ferrario F, Tarquini M. The octylonium bromide‐benzodiazepine combination for management of the irritable bowel syndrome. Clin Ter 1992;141(8):121‐7. [PubMed] [Google Scholar]
Carling 1989 {published data only}
- Carling L, Svedberg LE, Hulten S. Short term treatment of the irritable bowel syndrome: A placebo‐controlled trial of peppermint oil against hyoscyamine. Opuscula Medica 1989;34(3):55‐7. [Google Scholar]
Cerrato 2001 {published data only}
- Cerrato PL. Altmed watch. Peppermint oil holds its own as a treatment for IBS. Contemp Ob Gyn 2001;46(5):128. [Google Scholar]
Chapman 1990 {published data only}
- Chapman ND, Grillage MG, Mazumder R, Atkinson SN. A comparison of mebeverine with high‐fibre dietary advice and mebeverine plus ispaghula in the treatment of irritable bowel syndrome: an open, prospectively randomised, parallel group study. Br J Clin Pract 1990;44(11):461. [PubMed] [Google Scholar]
Chassany 2007 {published data only}
- Chassany O, Bonaz B, Bruley Des Varannes S, Bueno L, Cargill G, Coffin B, et al. Acute exacerbation of pain in irritable bowel syndrome: efficacy of phloroglucinol/trimethylphloroglucinol. A randomized, double‐blind, placebo‐controlled study. Aliment Pharmacol Ther 2007;25(9):1115‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 1999 {published data only}
- Chen ZY, Yao JH. Alverine/dimeticone versus nifedipine in treating irritable bowel syndrome. Chinese Journal of New Drugs and Clinical Remedies 1999;18(2):94‐6. [Google Scholar]
Chen 2004a {published data only}
- Chen Y, Wang X, Shang J. Paroxetine and Polyethylene Glycol (PEG) 4000 Therapy in Irritable Bowel Syndrome. Chinese Mental Health Journal 2004;18(11):806‐9. [Google Scholar]
Chevrel 1978 {published data only}
- Chevrel B. Treatment of functional intestinal disorders with carbomucil [Traitement des troubles fonctionnels intestinaux par le carbomucil]. Med Chir Dig 1978;7(5):443‐5. [PubMed] [Google Scholar]
Chicharro 2007 {published data only}
- Chicharro Serrano L. Fiber in the treatment of intestinal diseases. Farm Hosp 2007;187:7‐13. [Google Scholar]
Christen 1990 {published data only}
- Christen MO. Action of pinaverium bromide, a calcium‐antagonist, on gastrointestinal motility disorders. Gen Pharmacol 1990;21(6):821‐5. [DOI] [PubMed] [Google Scholar]
Clouse 1994 {published data only}
- Clouse RE, Lustman PJ, Geisman RA, Alpers DH. Antidepressant therapy in 138 patients with irritable bowel syndrome: a five‐year clinical experience. Aliment Pharmacol Ther 1994;8(4):409‐16. [DOI] [PubMed] [Google Scholar]
Clouse 2003 {published data only}
- Clouse RE. Antidepressants for irritable bowel syndrome. Gut 2003;52(4):598‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cook 1990 {published data only}
- Cook IJ, Irvine EJ, Campbell D, Shannon S, Reddy SN, Collins SM. Effect of dietary fiber on symptoms and rectosigmoid motility in patients with irritable bowel syndrome. A controlled, crossover study. Gastroenterology 1990;98(1):66‐72. [DOI] [PubMed] [Google Scholar]
Copé 1981 {published data only}
- Copé R. Comparative clinical study of the combination of clidinium(br)‐chlordiazepoxide and trimebutine in the treatment of functional colopathies. Med Chir Dig 1981;10(8):713‐7. [PubMed] [Google Scholar]
Corazza 1983 {published data only}
- Corazza GR, Vaira D, Milletti S. Controlled clinical evaluation of pinaverium bromide and trimebutine in functional disorders of the colon. Acta Ther 1983;9(4):383‐9. [Google Scholar]
Corazziari 1999 {published data only}
- Corazziari E. Role of opioid ligands in the irritable bowel syndrome. Can J Gastroenterol 1999;13 Suppl A:71A‐5A. [DOI] [PubMed] [Google Scholar]
Creed 2003 {published data only}
- Creed F, Fernandes L, Guthrie E, Palmer S, Ratcliffe J, Read N, et al. The cost‐effectiveness of psychotherapy and paroxetine for severe irritable bowel syndrome. Gastroenterology 2003;124:303‐17. [DOI] [PubMed] [Google Scholar]
Creed 2006 {published data only}
- Creed F. How do SSRIs help patients with irritable bowel syndrome?. Gut 2006;55(8):1065‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crowell 2004 {published data only}
- Crowell MD, Jones MP, Harris LA, Dineen TN, Schettler VA, Olden KW. Antidepressants in the treatment of irritable bowel syndrome and visceral pain syndromes. Curr Opin Investig Drugs 2004;5(7):736‐42. [PubMed] [Google Scholar]
Curtiss 2008 {published data only}
- Curtiss FR. Irritable bowel syndrome and antidepressants. J Manag Care Pharm 2008;14(9):882‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Czimmer 2001 {published data only}
- Czimmer J, Suto G, Kiraly A, Mozsik G. Otilonium bromide enhances sensory thresholds of volume and pressure in patients with irritable bowel syndrome. J Physiol Paris 2001;95(1‐6):153‐6. [DOI] [PubMed] [Google Scholar]
Darnis 1980 {published data only}
- Darnis F. Clinical study of a new medication in the treatment of functional colopathies [Etude clinique d'une medication nouvelle dans le traitement des colopathies fonctionnelles]. Med Chir Dig 1981;10(5):461‐2. [PubMed] [Google Scholar]
Defrance 1991 {published data only}
- Defrance P, Casini A. A comparison of the action of otilonium bromide and pinaverium bromide: study conducted under clinical control. Ital J Gastroenterol 1991;23(8 Suppl 1):64‐6. [PubMed] [Google Scholar]
De Groote 1968 {published data only}
- Groote J, Standaert L. The effect of a new musculotropic subtance 9 (Mebeverine) on irritable colon. Tijdschr Gastroenterol 1968;11(5):524‐8. [PubMed] [Google Scholar]
de la Garoullaye 1991 {published data only}
- Garoullaye G, Bordet P. The combination fenoverine/PVPP + karaya gum in the treatment of irritable bowel. Comptes Rendus de Therapeutique et de Pharmacologie Clinique 1991;9(93):3‐10. [Google Scholar]
Delvaux 1997 {published data only}
- Delvaux M, Wingate D. Trimebutine: mechanism of action, effects on gastrointestinal function and clinical results. J Int Med Res 1997;25(5):225‐46. [DOI] [PubMed] [Google Scholar]
Dettmar 1998 {published data only}
- Dettmar PW, Sykes J. A comparison between a fixed combination of ispaghula husk and mebeverine hydrochloride or mebeverine hydrochloride plus high‐fibre dietary advice in the treatment of irritable bowel syndrome. J Clin Res 1998;1:453‐9. [Google Scholar]
Dettmar 1999 {published data only}
- Dettmar PW, Sykes J. A multicentre, general practice study of the effectiveness and acceptability of an ispaghula husk‐mebeverine hydrochloride combination product in irritable bowel syndrome. J Clin Res 1998;1:429‐38. [Google Scholar]
Dew 1984 {published data only}
- Dew MJ, Evans BK, Rhodes J. Peppermint oil for the irritable bowel syndrome: a multicentre trial. Br J Clin Pract 1984;38(11‐12):394, 398. [PubMed] [Google Scholar]
Diaz‐Rubio 1985 {published data only}
- Diaz‐Rubio M, Onis Sanz, Enriquez Gonzalez L, Esteban Bernaldez JM. Effects of wheat bran in the irritable bowel syndrome. Ann Med Interna 1985;2(9):420‐2. [Google Scholar]
Dioguardi 1991 {published data only}
- Dioguardi N, De FR, Agape D, Antoniozzi F, Ballarin E, Ferrara A, et al. Octilonium bromide‐diazepam versus propantheline bromide‐bromazepam in the treatment of irritable bowel‐syndrome: A multicenter, randomized, controlled, double‐blind trial. Ter Mod 1991;5(4):97‐104. [Google Scholar]
Dubinin 1987 {published data only}
- Dubinin AV, Kabanov AV, Kirkin BV, Kolkunova GK, Igorianova NA. Bran in the treatment of irritable bowel syndrome. Vopr Pitan 1987;1:13‐6. [PubMed] [Google Scholar]
Ehsanullah 1985 {published data only}
- Ehsanullah M, Lee DA, Williams T, Pollard P, Gazzard B. The effect of secoverine hydrochloride on stimulated sigmoid motility: a double‐blind, placebo controlled cross‐over study in irritable bowel syndrome. Br J Clin Pharmacol 1985;19(3):301‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eisenburg 1978 {published data only}
- Eisenburg J, Kruis W, Schussler P, Weinzierl M. The irritable colon syndrome. New therapeutic possibilities in the treatment of a frequent syndrome [Das irritable kolon‐syndrom. Neue therapeuische wege bei der behandlung eines haufigen krakheitsbildes]. Fortschr Med 1978;96(41):2064‐70. [PubMed] [Google Scholar]
Evangelista 2004 {published data only}
- Evangelista S. Quaternary ammonium derivatives as spasmolytics for irritable bowel syndrome. Curr Pharm Des 2004;10(28):3561‐8. [DOI] [PubMed] [Google Scholar]
Evans 1996 {published data only}
- Evans JM, Fleming KC, Talley NJ, Schleck CD, Zinsmeister AR, Melton LJ. Relation of colonic transit to functional bowel disease in older people: a population‐based study. J Am Geriatr Soc 1998;46(1):83‐7. [DOI] [PubMed] [Google Scholar]
Ferrari 1986 {published data only}
- Ferrari A, Cavallero M, Spandre M. Double‐blind study of a new antimuscarinic, cimetropium bromide, in patients with irritable bowel syndrome. Clin Ther 1986;8(3):320‐8. [PubMed] [Google Scholar]
Fielding 1979 {published data only}
- Fielding JF, Melvin K. Dietary fibre and the irritable bowel syndrome. J Hum Nutr 1979;33(4):243‐7. [DOI] [PubMed] [Google Scholar]
Fielding 1984 {published data only}
- Fielding JF, Kehoe M. Different dietary fibre formulations and the irritable bowel syndrome. Ir J Med Sci 1984;153(5):178‐80. [DOI] [PubMed] [Google Scholar]
Fioramonti 1988 {published data only}
- Fioramonti J, Frexinos J, Staumont G, Bueno L. Inhibition of the colonic motor response to eating by pinaverium bromide in irritable bowel syndrome patients. Fundam Clin Pharmacol 1988;2(1):19‐27. [DOI] [PubMed] [Google Scholar]
Floch 1988 {published data only}
- Floch MH. The irritable bowel syndrome: the possible link between dietary fiber deficiency and disturbed intestinal motility. Am J Gastroenterol 1988;83(9):963‐4. [PubMed] [Google Scholar]
Francis 1994 {published data only}
- Francis CY, Whorwell PJ. Bran and irritable bowel syndrome: time for reappraisal. Lancet 1994;344(8914):39‐40. [DOI] [PubMed] [Google Scholar]
Frexinos 1985 {published data only}
- Frexinos J, Fioramonti J, Bueno L. Effect of trimebutine on colonic myoelectrical activity in IBS patients. Eur J Clin Pharmacol 1985;28(2):181‐5. [DOI] [PubMed] [Google Scholar]
Fritz 1967 {published data only}
- Fritz A, Sardet M, Tendil H. Contribution to the treatment of colonic diseases. (Apropos of the uses of a new synthetic spasmolytic drug, mebeverine hydrochloride). Rev Corps Sante.Armees Terre Mer Air 1967;8(3):349‐58. [PubMed] [Google Scholar]
Galeone 1986a {published data only}
- Galeone M, Benazzi E, Bossi M. Clinical and instrumental evaluation by multiple colonic manometry of tiropramide, trimebutine and octilonium bromide in irritable colon: II. Multiple dose oral administration. Pharmatherapeutica 1986;4(8):496‐509. [PubMed] [Google Scholar]
Galeone 1986b {published data only}
- Galeone M, Stock F, Moise G. Pinaverium bromide versus otilonium bromide in patients with irritable bowel syndrome. Curr Ther Res Clin Exp 1986;39(4):613‐24. [Google Scholar]
Geismar 1971 {published data only}
- Geismar P. Investigation of the effect of a new spasmolytic preparation (Spasmeks) on spastic colon [Undersogelse over virkningen of et nyt spasmolytikum (Spasmeks) pa colon irritabile]. Ugeskr Laeger 1971;133(33):1610‐2. [PubMed] [Google Scholar]
Geoffroy 1977 {published data only}
- Geoffroy H. The treatment of the irritable colon syndrome by a musculotropic antispasmodic: Duspatalin. Gaz Med Fr 1977;84(12):1331‐3. [Google Scholar]
Giaccari 2001 {published data only}
- Giaccari S, Grasso G, Tronci S, Allegretta L, Sponziello G, Montefusco A, et al. Partially hyrdrolyzed guar gum: A fiber as coadiuvant in irritable bowel syndrome. Clinica Terapeutica 2001;152(1):21‐5. [PubMed] [Google Scholar]
Giannini 2006 {published data only}
- Giannini E G, Mansi C, Dulbecco P, Savarino V. Role of partially hydrolyzed guar gum in the treatment of irritable bowel syndrome. Nutrition 2006;22(3):334‐42. [DOI] [PubMed] [Google Scholar]
Gibbons 1979 {published data only}
- Gibbons D. Oral hyoscine butylbromide for irritable bowel syndrome?. Br Med J 1979;1(6165):752. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gilbody 2000 {published data only}
- Gilbody JS, Fletcher CP, Hughes IW, Kidman SP. Comparison of two different formulations of mebeverine hydrochloride in irritable bowel syndrome. Int J Clin Pract 2000;54(7):461‐4. [PubMed] [Google Scholar]
Glende 2002 {published data only}
- Glende M, Morselli‐Labate AM, Battaglia G, Evangelista S. Extended analysis of a double‐blind, placebo‐controlled, 15‐week study with otilonium bromide in irritable bowel syndrome. Eur J Gastroenterol Hepatol 2002;14(12):1331‐8. [DOI] [PubMed] [Google Scholar]
Gnauck 1977 {published data only}
- Gnauck R. Value of high fiber diet in treatment of diverticulosis, constipation and irritable colon. Therapiewoche 1977;27(37):6411‐2. [Google Scholar]
Golechha 1982 {published data only}
- Golechha AC, Chadda VS, Chadda S, Sharma SK, Mishra SN. Role of ispaghula husk in the management of irritable bowel syndrome (a randomized double‐blind crossover study). J Assoc Physicians India 1982;30(6):353‐5. [PubMed] [Google Scholar]
Gorard 1994 {published data only}
- Gorard DA, Libby GW, Farthing MJ. Influence of antidepressants on whole gut and orocaecal transit times in health and irritable bowel syndrome. Aliment Pharmacol Ther 1994;8(2):159‐66. [DOI] [PubMed] [Google Scholar]
Gorard 1995 {published data only}
- Gorard DA, Libby GW, Farthing MJ. Effect of a tricyclic antidepressant on small intestinal motility in health and diarrhea‐predominant irritable bowel syndrome. Dig Dis Sci 1995;40(1):86‐95. [DOI] [PubMed] [Google Scholar]
Greenbaum 1981 {published data only}
- Greenbaum DS, Stein GE. Psyllium and the irritable bowel syndrome. Ann Intern Med 1981;95(5):660. [DOI] [PubMed] [Google Scholar]
Greenbaum 1984 {published data only}
- Greenbaum DS. Preliminary report on antidepressant treatment of irritable bowel syndrome: comments on comparison with anxiolytic therapy. Psychopharmacol Bull 1984;20(4):622‐8. [PubMed] [Google Scholar]
Greenbaum 1987 {published data only}
- Greenbaum DS, Mayle JE, Vanegeren LE, Jerome JA, Mayor JW, Greenbaum RB, et al. Effects of desipramine on irritable bowel syndrome compared with atropine and placebo. Dig Dis Sci 1987;32(3):257‐66. [DOI] [PubMed] [Google Scholar]
Grigoleit 2005 {published data only}
- Grigoleit H, Grigoleit P. Peppermint oil in irritable bowel syndrome. Phytomedicine 2005;12(8):601‐6. [DOI] [PubMed] [Google Scholar]
Guerre 1979 {published data only}
- Guerre J, Neuman M. Treatment of chronic colonic diseases with a new topical digestive agent, mucilage (karaya gum) combined with polyvinylpolypyrrolidone (P.V.P.P.). Med Chir Dig 1979;8(7):679‐82. [PubMed] [Google Scholar]
Halpert 2005 {published data only}
- Halpert A, Dalton CB, Diamant NE, Toner BB, Hu Y, Morris CB, et al. Clinical response to tricyclic antidepressants in functional bowel disorders is not related to dosage. Am J Gastroenterol 2005;100:664‐71. [DOI] [PubMed] [Google Scholar]
Han 2009 {published data only}
- Han C, Masand PS, Krulewicz S, Peindl K, Mannelli P, Varia IM, et al. Childhood abuse and treatment response in patients with irritable bowel syndrome: a post‐hoc analysis of a 12‐week, randomized, double‐blind, placebo‐controlled trial of paroxetine controlled release. J Clin Pharm Ther 2009;34(1):79‐88. [DOI] [PubMed] [Google Scholar]
Hebden 2002 {published data only}
- Hebden JM, Blackshaw E, D'Amato M, Perkins AC, Spiller RC. Abnormalities of GI transit in bloated irritable bowel syndrome: effect of bran on transit and symptoms. Am J Gastroenterol 2002;97:2315‐20. [DOI] [PubMed] [Google Scholar]
Herxheimer 1979 {published data only}
- Herxheimer A, Misiewicz JJ. Oral hyoscine butylbromide for irritable bowel syndrome?. Br Med J 1979;1(6165):752. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hotz 1994 {published data only}
- Hotz J, Plein K. Effect of psyllium seeds compared to wheat bran on stool frequency and complaints in patients with irritable bowel syndrome and constipation. Medizinische Klinik 1994;89(12):645‐51. [PubMed] [Google Scholar]
Houghton 1997 {published data only}
- Houghton LA, Rogers J, Whorwell PJ, Campbell FC, Williams NS, Goka J. Zamifenacin (UK‐76, 654) a potent gut M3 selective muscarinic antagonist, reduces colonic motor activity in patients with irritable bowel syndrome. Aliment Pharmacol Ther 1997;11(3):561‐8. [DOI] [PubMed] [Google Scholar]
Inauen 1994 {published data only}
- Inauen W, Halter F. Clinical efficacy, safety and tolerance of mebeverine slow release (200 mg) vs mebeverine tablets in patients with irritable bowel syndrome. Drug Invest 1994;8(4):234‐40. [Google Scholar]
Iwanaga 2002 {published data only}
- Iwanaga Y. Physicochemical and pharmacological characteristic and clinical efficacy of an anti‐irritable bowel syndrome agent, polycarbophil calcium (Polyful). Nippon Yakurigaku Zasshi 2002;119(3):185‐90. [DOI] [PubMed] [Google Scholar]
Jackson 1998 {published data only}
- Jackson II A, Ofman J. Peppermint oil for irritable bowel syndrome: Remedy or herbal folklore. Integr Med 1998;1(4):163‐5. [Google Scholar]
Jafri 2006 {published data only}
- Jafri W, Yakoob J, Hussain S, Jafri N, Islam M. Phloroglucinol in irritable bowel syndrome. J Pak Med Assoc 2006;56(1):5‐8. [PubMed] [Google Scholar]
Jayanthi 1998 {published data only}
- Jayanthi V, Malathi S, Ramathilakam B, Dinakaran N, Balasubramanian V, Mathew S. Role of pinaverium bromide in south Indian patients with irritable bowel syndrome. J Assoc Physicians India 1998;46(4):369‐71. [PubMed] [Google Scholar]
Ji 2007 {published data only}
- Ji HZ, Wu XW, Xu XB, Sun Q, Liu Y, Zhang L, et al. Evaluating the clinical effect and quality of life in the elderly with functional bowel disorder after treatment with pinaverium bromide. J Clin Rehab Tissue Eng Res 2007;11(12):2275‐8. [Google Scholar]
Jing 2004 {published data only}
- Jing D, Xu M, Chen Z, Zhang J, Wang P, Jiang H, et al. Emotional disorders and treatment in patients with refractory irritable bowel syndrome. Chin J Gastroenterol 2004;9(2):90‐3. [Google Scholar]
Kaushik 2002 {published data only}
- Kaushik A, Pursnani ML, Kar P. Multicenter phase III clinical trial of otilonium bromide in irritable bowel syndrome. Indian Journal of Gastroenterology 2002;21:85‐6. [PubMed] [Google Scholar]
Kirsch 2000 {published data only}
- Kirsch MA, Louie AK. Paroxetine and irritable bowel syndrome. Am J Psychiatry 2000;157(9):1523‐4. [DOI] [PubMed] [Google Scholar]
Koch 1998 {published data only}
- Koch TR. Peppermint oil and irritable bowel syndrome. Am J Gastroenterol 1998;93(11):2304‐5. [DOI] [PubMed] [Google Scholar]
Koruda 1993 {published data only}
- Koruda M J. Dietary fiber and gastrointestinal disease. Surg Gynecol Obstet 1993;177(2):209‐14. [PubMed] [Google Scholar]
Kountouras 2002 {published data only}
- Kountouras J, Chatzopoulos D, Zavos C, Boura P, Venizelos J, Kalis A. Efficacy of trimebutine therapy in patients with gastroesophageal reflux disease and irritable bowel syndrome. Hepato‐Gastroenterology 2002;49:193‐7. [PubMed] [Google Scholar]
Kumar 1987 {published data only}
- Kumar A, Kumar N, Vij JC, Sarin SK, Anand BS. Optimum dosage of ispaghula husk in patients with irritable bowel syndrome: correlation of symptom relief with whole gut transit time and stool weight. Gut 1987;28(2):150‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kunze 1990 {published data only}
- Kunze M, Seidel HJ, Stube G. Comparative studies of the effectiveness of brief psychotherapy, acupuncture and papaverin therapy in patients with irritable bowel syndrome. Z Gesamte Inn Med 1990;45(20):625‐7. [PubMed] [Google Scholar]
Lafon 1982 {published data only}
- Lafon J, Cougard A, Pin G, Julien D. Is the combination of kaolin, sterculia gum and magnesium superior to sound in the treatment of irritable colon? Controlled clinical trial [L'association kaolin, gomme sterculia,magnesium est‐elle suerieure au son dans le traitement symptomatique du colon irritable? Etude clinique controlee]. Rev Proct 1982;1:67‐76. [Google Scholar]
Lambert 1989 {published data only}
- Lambert JP, Dickerson JW. A survey of high‐fibre diet‐sheets used in the treatment of irritable bowel syndrome in Great Britain. J Hum Nutr Diet 1989;2(6):429‐35. [Google Scholar]
Lambert 1991a {published data only}
- Lambert JP, Brunt PW, Mowat NA, Khin CC, Lai CK, Morrison V, et al. The value of prescribed 'high‐fibre' diets for the treatment of the irritable bowel syndrome. Eur J Clin Nutr 1991;45(12):601‐9. [PubMed] [Google Scholar]
Lambert 1991b {published data only}
- Lambert JP, Morrison V, Brunt PW, Mowat NA, Eastwood MA, Dickerson JW. Dietary fibre intake of irritable bowel patients prescribed a high fibre diet. J Hum Nutr Diet 1991;4(3):155‐64. [PubMed] [Google Scholar]
Lawson 1988 {published data only}
- Lawson MJ, Knight RE, Tran K, Walker G, Roberts‐Thomson IC. Failure of enteric‐coated peppermint oil in the irritable bowel syndrome: A randomized, double‐blind crossover study. J Gastroenterol Hepatol 1988;3(3):235‐8. [Google Scholar]
Levitan 1981 {published data only}
- Levitan MK, Dubinin AV, Beiul EA, Iurkov M, Shechovskaia AK. Treatment of colonic diverticulosis and irritable colon with wheat bran. Sov Med 1981;8(8):109‐12. [PubMed] [Google Scholar]
Lin 2003 {published data only}
- Lin JK, Hu PJ, Wang WA, Chen Q, Chen MH. Effects of otilonium bromide on anorectal visceral sensorimotor functions of patients with diarrhea‐predominant irritable bowel syndrome. World Chin J Dig 2003;11(12):1926‐8. [Google Scholar]
Liu 2006 {published data only}
- Liu DL, Huo JR, Wu XP. Effect of subclincal dosage of antidepressants on refractory irritable bowel syndrome. Chinese Journal of Clinical Psychology 2006;14(4):424‐5. [Google Scholar]
Locke 2004 {published data only}
- Locke GR III. Review: soluble fibre improves overall symptoms and constipation but not abdominal pain in irritable bowel syndrome. Evid Based Med 2004;9(6):172. [Google Scholar]
Lu 2000 {published data only}
- Lu CL, Chen CY, Chang FY, Chang SS, Kang LJ, Lu RH, et al. Effect of a calcium channel blocker and antispasmodic in diarrhoea‐predominant irritable bowel syndrome. J Gastroenterol Hepatol 2000;15(8):925‐30. [DOI] [PubMed] [Google Scholar]
Luttecke 1978 {published data only}
- Luttecke K. A trial of trimebutine in spastic colon. J Int Med Res 1978;6(2):86‐8. [DOI] [PubMed] [Google Scholar]
Luttecke 1980 {published data only}
- Luttecke K. A three‐part controlled study of trimebutine in the treatment of irritable colon syndrome. Curr Med Res Opin 1980;6(6):437‐43. [DOI] [PubMed] [Google Scholar]
Lydiard 2007 {published data only}
- Lydiard RB. Psychopharmacology in the treatment of irritable bowel syndrome. Prim Psychiatry 2007;14(4):40‐50. [Google Scholar]
MacRae 1979 {published data only}
- MacRae KD. Oral hyoscine butylbromide for irritable bowel syndrome?. Br Med J 1979;1(6165):752. [DOI] [PMC free article] [PubMed] [Google Scholar]
Manning 1976 {published data only}
- Manning AP, Heaton KW, Harvey RF, Uglow P. Cereal fibre and the irritable bowel: a controlled trial. Gut 1976;17(10):822‐3. [Google Scholar]
Manning 1977 {published data only}
- Manning AP, Heaton KW, Harvey RF. Wheat fibre and irritable bowel syndrome. A controlled trial. Lancet 1977;2(8035):417‐8. [DOI] [PubMed] [Google Scholar]
Marks 2008 {published data only}
- Marks DM, Han C, Krulewicz S, Pae CU, Peindl K, Patkar AA, et al. History of depressive and anxiety disorders and paroxetine response in patients with irritable bowel syndrome: post hoc analysis from a placebo‐controlled study. Prim Care Companion J Clin Psychiatry 2008;10(5):368‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Masamune 1998 {published data only}
- Masamune O, Miwa T, Fukutomi H, Matsuo Y, Sasaki D, Chiba M, et al. Clinical phase III comparative study of calcium polycarbophil tablet on irritable bowel syndrome ‐ A double‐blind study in comparison with torimebutine maleate. Jpn Pharmacol Ther 1998;26:63‐92. [Google Scholar]
Masand 2002 {published data only}
- Masand PS, Gupta S, Schwartz TL, Virk S, Lockwood K, Hameed A, et al. Paroxetine in patients with irritable bowel syndrome: A pilot open‐label study. Prim Care Companion J Clin Psychiatry 2002;4(1):12‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Masand 2005 {published data only}
- Masand PS, Gupta S, Schwartz TL, Virk S, Hameed A, Kaplan DS. Open‐label treatment with citalopram in patients with irritable bowel syndrome: a pilot study. Prim Care Companion J Clin Psychiatry 2005;7(4):162‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matts 1967 {published data only}
- Matts SG. An assessment of dicyclomine hydrochloride ('Merbentyl') in the irritable colon syndrome. Br J Clin Pract 1967;21(11):549‐51. [PubMed] [Google Scholar]
Meier 1996 {published data only}
- Meier R, Beglinger Ch, Brignoli R, the IBS‐CIS study group. Symptoms and colonic transit time in the irritable bowel syndrome treated with psyllium and cisapride or placebo (abstract). Gut 1996;39 Suppl 3:A33. [Google Scholar]
Miller 2006 {published data only}
- Miller V, Lea R, Agrawal A, Whorwell PJ. Bran and irritable bowel syndrome: the primary‐care perspective. Dig Liver Dis 2006;38(10):737‐40. [DOI] [PubMed] [Google Scholar]
Misra 1989 {published data only}
- Misra SP, Thorat VK, Sachdev GK, Anand BS. Long‐term treatment of irritable bowel syndrome: results of a randomized controlled trial. Q J Med 1989;73(270):931‐9. [PubMed] [Google Scholar]
Modena 1993 {published data only}
- Modena L. Single drug treatment for irritable colon syndrome: Rociverine 20 mg t.i.d. Minerva Med 1993;84(5):263‐8. [PubMed] [Google Scholar]
Mollica 1992 {published data only}
- Mollica G, Manno G. Otilonium bromide‐diazepam in the treatment of the irritable colon. A controlled study versus otilonium bromide. Clin Ter 1992;141(8):129‐34. [PubMed] [Google Scholar]
Morgan 2005 {published data only}
- Morgan V, Pickens D, Gautam S, Kessler R, Mertz H. Amitriptyline reduces rectal pain related activation of the anterior cingulate cortex in patients with irritable bowel syndrome. Gut 2005;54(5):601‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morrison 1987 {published data only}
- Morrison V, Lai C KW, Khin CC. High fibre diets and irritable bowel syndrome ‐ The role of the dietitian. Scott Med J 1987;32(4):124. [Google Scholar]
Mortensen 1987 {published data only}
- Mortensen PB, Andersen JR, Arffmann S, Krag E. Short‐chain fatty acids and the irritable bowel syndrome: the effect of wheat bran. Scand J Gastroenterol 1987;22(2):185‐92. [DOI] [PubMed] [Google Scholar]
Nash 1986 {published data only}
- Nash P, Gould SR, Bernardo DE. Peppermint oil does not relieve the pain of irritable bowel syndrome. Br J Clin Pract 1986;40(7):292‐3. [PubMed] [Google Scholar]
Nedogoda 2000 {published data only}
- Nedogoda SV, Parshev VV. Treatment of irritable bowel syndrome with dicetelium and spasmomen. Klin Med 2000;78(10):42‐6. [PubMed] [Google Scholar]
Noel 1989 {published data only}
- Noel B. A multicentric study of pinaverium bromide on irritable bowel syndrome, performed in Mexico. Investigacion Medica Internacional 1989;15(4):190‐6. [Google Scholar]
Olden 2005 {published data only}
- Olden KW. The use of antidepressants in functional gastrointestinal disorders: new uses for old drugs. CNS Spectr 2005;10(11):891‐6. [DOI] [PubMed] [Google Scholar]
Pardell 1982 {published data only}
- Pardell H, Marcillas J, Celdran E. Double‐blind parallel study of clebopride and hyoscine N‐butylbromide in patients with irritable colon syndrome. Curr Ther Res Clin Exp 1982;31(1):S74‐9. [Google Scholar]
Parisi 2002 {published data only}
- Parisi GC, Zilli M, Miani MP, Carrara M, Bottona E, Verdianelli G, et al. High‐fiber diet supplementation in patients with irritable bowel syndrome (IBS): a multicenter, randomized, open trial comparison between wheat bran diet and partially hydrolyzed guar gum (PHGG). Dig Dis Sci 2002;47(8):1697‐1704. [DOI] [PubMed] [Google Scholar]
Parisi 2005 {published data only}
- Parisi G, Bottona E, Carrara M, Cardin F, Faedo A, Goldin D, et al. Treatment effects of partially hydrolyzed guar gum on symptoms and quality of life of patients with irritable bowel syndrome. A multicenter randomized open trial. Dig Dis Sci 2005;50(6):1107‐12. [DOI] [PubMed] [Google Scholar]
Passaretti 1985 {published data only}
- Passaretti S, Fesce E, Fanti L. Effect of tiropramide hydrochloride on the intestinal transit time in patients with irritable bowel syndrome. Minerva Med 1985;76(19‐20):923‐6. [PubMed] [Google Scholar]
Passaretti 1989b {published data only}
- Passaretti S, Sorghi M, Colombo E, Mazzotti G, Tittobello A, Guslandi M. Motor effects of locally administered pinaverium bromide in the sigmoid tract of patients with irritable bowel syndrome. Int J Clin Pharmacol Ther Toxicol 1989;27(1):47‐50. [PubMed] [Google Scholar]
Pearson 2000 {published data only}
- Pearson RL. How effective are antidepressant medications in the treatment of irritable bowel syndrome and nonulcer dyspepsia?. J Fam Pract 2000;49(5):396. [PubMed] [Google Scholar]
Piai 1987a {published data only}
- Piai G, Visconti M, d'Angelo V, Sauro A, Sparano R, Mazzacca G. Glucomannan in treatment of irritable colon. Results of a controlled clinical trial [Il glucomannano nel trattamento del colon irritabile; risultati di uno studio clinico controllato]. Quaderni di Medicina e Chirurgia 1987;3(1):83‐6. [Google Scholar]
Piai 1987b {published data only}
- Piai G, Visconti M, Imbimbo BP, Minieri M, Sollazzo R, Mazzacca G. Long‐term treatment of irritable bowel syndrome with cimetropium bromide, a new antimuscarinic compound. Curr Ther Res Clin Exp 1987;41(6):967‐77. [Google Scholar]
Pittler 1998 {published data only}
- Pittler MH, Ernst E. Peppermint oil for irritable bowel syndrome: a critical review and metaanalysis. Am J Gastroenterol 1998;93(7):1131‐5. [DOI] [PubMed] [Google Scholar]
Prior 1986a {published data only}
- Prior A, Whorwell PJ. Management of irritable bowel syndrome. Biomed Pharmacother 1986;40(1):4‐5. [PubMed] [Google Scholar]
Prior 1986b {published data only}
- Prior A, Akbar FA, Shroff NE, Whorwell PJ. A double blind study of ispaghula in irritable bowel syndrome (abstract). Gut 1986;27(Suppl 5):A625. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prout 1983 {published data only}
- Prout B J. The treatment of irritable bowel syndrome. Two doses of mebeverine compared. Practitioner 1983;227(1384):1607‐8. [PubMed] [Google Scholar]
Quilici 1998 {published data only}
- Quilici FA, Dos Reis Neto, Cordeiro F, Ciquini S, Dos Reis J. Treatment of irritable bowel syndrome with pinaverium bromide. GED Gastrentologia Endosc Dig 1998;17(3):79‐86. [Google Scholar]
Quilici 2003 {published data only}
- Quilici FA, Cordeiro F, Ciquini SA, Cunha HA, Bertocello LC, Sugahara R, et al. Treatment of irritable bowel syndrome: Single drug therapy with mebeverine. GED Gastrentologia Endosc Dig 2003;22(6):219‐26. [Google Scholar]
Rasmussen 1982 {published data only}
- Rasmussen SN, Bondesen S, Edmund C, Frandsen I, Andersen I, Kempel K, et al. Treatment of irritable bowel with dietary fiber. A controlled clinical study [Behandling af colon irritabile med kostfiberrig diaet. En kontrolleret klinisk undersogelse]. Ugeskr Laeger 1982;144(33):2415‐7. [PubMed] [Google Scholar]
Rees 1979 {published data only}
- Rees WD, Evans BK, Rhodes J. Treating irritable bowel syndrome with peppermint oil. Br Med J 1979;2(6194):835‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rhodes 1978 {published data only}
- Rhodes JB, Abrams JH, Manning RT. Controlled clinical trial of sedative‐anticholinergic drugs in patients with the irritable bowel syndrome. J Clin Pharmacol 1978;18(7):340‐5. [DOI] [PubMed] [Google Scholar]
Rhodes 1980 {published data only}
- Rhodes J, Evans BK, Rees WD. Peppermint oil in enteric coated capsules for the treatment of irritable bowel syndrome: A double blind controlled trial. Hepatogastroenterology 1980;27(Suppl):E31‐6. [Google Scholar]
Ritchie 1980 {published data only}
- Ritchie JA, Truelove SC. Comparison of various treatments for irritable bowel syndrome. Br Med J 1980;281(6251):1317‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sagduyu 2002 {published data only}
- Sagduyu K. Peppermint oil for irritable bowel syndrome. Psychosomatics 2002;43(6):508‐9. [DOI] [PubMed] [Google Scholar]
Sato 2006 {published data only}
- Sato M, Murakami M. Treatment for irritable bowel syndrome‐‐psychotropic drugs, antidepressants and so on. Nippon Rinsho 2006;64(8):1495‐500. [PubMed] [Google Scholar]
Schaffstein 1990 {published data only}
- Schaffstein W, Panijel M, Luttecke K. Comparative safety and efficacy of trimebutine versus mebeverine in the treatment of irritable bowel syndrome. A multicenter double‐blind study. Curr Ther Res Clin Exp 1990;47(1):136‐45. [Google Scholar]
Schutz 1992 {published data only}
- Schutz E, Peters‐Hartel W, Mauersberger H, Munster U. The treatment of irritable colon with mebeverine. Pain relief most important. Therapiewoche Schweiz 1992;8(10):759‐760, 762. [Google Scholar]
Secco 1983 {published data only}
- Secco G B, Somma C, Arnulfo G, Ricci C. Controlled clinical study of the action of mebeverine hydrochloride in the treatment of irritable colon. Minerva Med. 1983;74(13):699‐702. [PubMed] [Google Scholar]
Sharma 1987 {published data only}
- Sharma S, Saini RK, Goswami SK, Sharma A, Singh S. Role of dietary fibre in irritable bowel syndrome: a clinical study. Indian J Med Sci 1987;41(2):29‐33. [PubMed] [Google Scholar]
Shaughnessy 2000 {published data only}
- Shaughnessy A. Are antidepressants effective in the treatment of the functional gastrointestinal (GI) disorders of irritable bowel syndrome and nonulcer dyspepsia?. Evid Based Pract 2000;3(5):4, insert 2p. [Google Scholar]
Shrivastava 1984 {published data only}
- Shrivastava R K, Siegel H. The role of tricyclics and benzodiazepine compounds in the treatment of irritable gut syndrome and peptic ulcer disease. Psychopharmacol Bull 1984;20(4):616‐21. [PubMed] [Google Scholar]
Singh 2007 {published data only}
- Singh B. Psyllium as therapeutic and drug delivery agent. Int J Pharm 2007;334(1‐2):1‐14. [DOI] [PubMed] [Google Scholar]
Slawson 2002 {published data only}
- Slawson D. How do high‐fiber supplements compare with guar gum for irritable bowel syndrome?. Evid Based Pract 2002;5(11):3‐4. [Google Scholar]
Snook 1994 {published data only}
- Snook J, Shepherd HA. Bran supplementation in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther 1994;8(5):511‐4. [DOI] [PubMed] [Google Scholar]
Soifer 1987 {published data only}
- Soifer LO, Paula JA, Caruso P. Effects of medicinal fiber on colonic transit in patients with irritable colon syndrome. Acta Gastroenterol Latinoam 1987;17(4):317‐23. [PubMed] [Google Scholar]
Soifer 1992 {published data only}
- Soifer L, Varela E, Olmos J. Manometric effects of pinaverium bromide in irritable bowel syndrome [Efectos manometricos del bromuro de pinaverio en el sindrome de intestino irritable]. Acta Gastroenterol Latinoam 1992;22(1):37‐43. [PubMed] [Google Scholar]
Soriano 1992 {published data only}
- Soriano C, Espejo H, Garcia L. Treatment of irritable bowel syndrome with a calcium antagonist (pinaverium bromide): experience with 40 cases. Rev Gastroenterol Peru 1992;12(1):36‐8. [PubMed] [Google Scholar]
Stiefelhagen 2008 {published data only}
- Stiefelhagen P. Peppermint oil is helpful in irritable bowel syndrome. MMW‐Fortschr Med 2008;150(47):25. [Google Scholar]
Swiatczak 1998 {published data only}
- Swiatczak C, Jawien A, Swiatkowski M, Slominski JM, Kowianski Z, Zasieczny W. Duodenogastric reflux in patients with irritable bowel syndrome treated with wheat bran. Pol Merkuriusz Lek 1998;4(22):196‐8. [PubMed] [Google Scholar]
Talley 2003 {published data only}
- Talley NJ. SSRIs in IBS: sensing a dash of disappointment. Clin Gastroenterol Hepatol 2003;1(3):155‐9. [DOI] [PubMed] [Google Scholar]
Talley 2004 {published data only}
- Talley NJ. Antidepressants in IBS: are we deluding ourselves?. Am J Gastroenterol 2004;99(5):921‐3. [DOI] [PubMed] [Google Scholar]
Talley 2008b {published data only}
- Talley NJ. Newer antidepressants in irritable bowel syndrome: What is the evidence?. Arch Med Sci 2008;4(1):77‐8. [Google Scholar]
Tan 2007 {published data only}
- Tan KY, Seow‐Choen F. Fiber and colorectal diseases: Separating fact from fiction. World J Gastroenterol 2007;13(31):4161‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tanum 1996 {published data only}
- Tanum L, Malt UF. A new pharmacologic treatment of functional gastrointestinal disorder. A double‐blind placebo‐controlled study with mianserin. Scand J Gastroenterol 1996;31(4):318‐25. [DOI] [PubMed] [Google Scholar]
Tanum 2000 {published data only}
- Tanum L, Malt UF. Personality traits predict treatment outcome with an antidepressant in patients with functional gastrointestinal disorder. Scand J Gastroenterol 2000;35(9):935‐41. [DOI] [PubMed] [Google Scholar]
Tarpila 2004 {published data only}
- Tarpila S, Tarpila A, Grohn P, Silvennoinen T, Lindberg L. Efficacy of ground flaxseed on constipation in patients with irritable bowel syndrome. Current Topics in Nutraceuticals Research 2002;2(2):119‐25. [Google Scholar]
Tarquini 1984 {published data only}
- Tarquini M, Sannino L, Bazuro G. The irritable bowel syndrome: therapeutic effect of octilonium bromide alone and combined with a benzodiazepine. A double‐blind study. Clinica Terapeutica 1984;109(6):525‐31. [PubMed] [Google Scholar]
Tasman‐Jones 1973 {published data only}
- Tasman‐Jones C. Mebeverine in patients with the irritable colon syndrome: double blind study. N Z Med J 1973;77(491):232‐5. [PubMed] [Google Scholar]
Tinozzi 1984 {published data only}
- Tinozzi S, Valesi MG. Controlled clinical trial of the effectiveness of tiropramide hydrochloride versus octylonium bromide in the treatment of irritable colon syndrome. Minerva Med 1984;75(1‐2):23‐31. [PubMed] [Google Scholar]
Tomas‐Ridocci 1992 {published data only}
- Tomas‐Ridocci M, Anon R, Minguez M, Zaragoza A, Ballester J, Benages A. The efficacy of Plantago ovata as a regulator of intestinal transit. A double‐blind study compared to placebo. Rev Esp Enferm Dig 1992;82(1):17‐22. [PubMed] [Google Scholar]
Toussaint 1981 {published data only}
- Toussaint J, Cremer M, Pintens H. Single blind study of trimebutine and mebeverine in irritable colon and dyspepsia. Acta Ther 1981;7(3):261‐8. [Google Scholar]
Tripathi 1983 {published data only}
- Tripathi BM, Misra NP, Gupta AK. Evaluation of tricyclic compound (trimipramine) vis‐a‐vis placebo in irritable bowel syndrome. (Double blind randomised study). J Assoc Physicians India 1983;31(4):201‐3. [PubMed] [Google Scholar]
Tsuneoka 1987 {published data only}
- Tsuneoka K, Miyoshi A, Kawakami K, Sekiguchi T, Nakazawa S. Clinical evaluation of TM 906 (trimebutine maleate) in the treatment of irritable bowel syndrome. Multi center double blind study in comparison with mepenzolate bromide. Rinsho Hyoka (Clinical Evaluation) 1987;15:307‐34. [Google Scholar]
Tudor 1986 {published data only}
- Tudor GJ. A general practice study to compare alverine citrate with mebeverine hydrochloride in the treatment of irritable bowel syndrome. Br J Clin Pract 1986;40(7):276‐8. [PubMed] [Google Scholar]
van Kerkhoven 2007 {published data only}
- Kerkhoven LA, Laheij RJ, Jansen JB. The role of the selective serotonin reuptake inhibitor citalopram in irritable bowel syndrome. Gut 2007;56(5):733; author reply 733. [PMC free article] [PubMed] [Google Scholar]
Van Outryve 1995 {published data only}
- Outryve M, Mayeur S, Meeus MA, Rosillon D, Hendrickx B, Ceuppens M. A double‐blind crossover comparison study of the safety and efficacy of mebeverine with mebeverine sustained release in the treatment of irritable bowel syndrome. J Clin Pharm Ther 1995;20(5):277‐82. [DOI] [PubMed] [Google Scholar]
Van Steensel 1990 {published data only}
- Steensel CJ. Effect of dietary fiber on symptoms and rectosigmoid motility in patients with irritable bowel syndrome. Gastroenterology 1990;99(5):1538. [DOI] [PubMed] [Google Scholar]
Villagrasa 1991 {published data only}
- Villagrasa M, Boix J, Humbert P, Quer JC. Aleatory clinical study comparing otilonium bromide with a fiber‐rich diet in the treatment of irritable bowel syndrome. Ital J Gastroenterol 1991;8 Suppl 1:67‐70. [PubMed] [Google Scholar]
Wald 1990 {published data only}
- Wald A. Fiber supplements for irritable bowel syndrome: do they really make a difference?. Am J Gastroenterol 1990;85(12):1652‐3. [PubMed] [Google Scholar]
Wald 2002 {published data only}
- Wald A. Psychotropic agents in irritable bowel syndrome. J Clin Gastroenterol 2002;35(1 Suppl):S53‐7. [DOI] [PubMed] [Google Scholar]
Wang 2003 {published data only}
- Wang WA, Qian JM, Pan GZ. Treatment of refractory irritable bowel syndrome with subclinical dosage of antidepressants. Chung‐Kuo I Hsueh Ko Hsueh Yuan Hsueh Pao 2003;25(1):74‐8. [PubMed] [Google Scholar]
Wittmann 1999 {published data only}
- Wittmann T, Feher A, Rosztoczy A, Janosi J. Effectiveness of pinaverium bromide therapy on colonic motility disorders in irritable bowel syndrome. Orv Hetil 1999;140(9):469‐73. [PubMed] [Google Scholar]
Woolner 2000 {published data only}
- Woolner JT, Kirby GA. Clinical audit of the effects of low‐fibre diet on irritable bowel syndrome. J Hum Nutr Diet 2000;13(4):249‐53. [Google Scholar]
Yuan 2003 {published data only}
- Yuan Y, Xu B, Ke M, Liu Y, Fu L, Li Z, et al. Efficacy and safety of otilonium bromide for irritable bowel syndrome. Chin J Gastroenterol 2003;8(5):279‐82. [Google Scholar]
Yuan 2005 {published data only}
- Yuan Y, Xu B, Mo J, Wang J, Li Z. Efficacy and safety use of trimebutine maleate in treatment of irritable bowel syndrome. Chin.J.Gastroenterol. 2005;10(3):143‐7. [Google Scholar]
Zhang 2002 {published data only}
- Zhang HB, Han GH, Zhou XM, Guo XG, Wang BL, Wu KC, et al. Effects of treatments with otilonium bromide, collodal bismuth tartrate and compound diphenoxylate in the irritable bowel syndrome: a double‐blind random controlled study. The Chinese Journal of Clinical Pharmacology 2002;18(5):328‐32. [Google Scholar]
Zhou 2002 {published data only}
- Zhou HQ, Li DG, Sogn GH. Effect of paroxetine hydrochloride and pinaverium bromide therapy in patients with irritable bowel syndrome. Chin J Clin Rehab 2002;6(23):3576‐7. [Google Scholar]
Zuckerman 2006 {published data only}
- Zuckerman MJ. The role of fiber in the treatment of irritable bowel syndrome: therapeutic recommendations. J Clin Gastroenterol 2006;40(2):104‐8. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Abdul‐Baki 2009 {published data only}
- Abdul‐Baki H, Hajj II, Elzahabi L, Azar C, Aoun E, Skoury A, et al. A randomized controlled trial of imipramine in patients with irritable bowel syndrome. World J Gastroenterol 2009;15(29):3636‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bijkerk 2009 {published data only}
- Bijkerk CJ, Wit NJ, Muris JW, Whorwell PJ, Knottnerus JA, Hoes AW. Soluble or insoluble fibre in irritable bowel syndrome in primary care? Randomised placebo controlled trial. BMJ 2009;339:b3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Everitt 2010 {published data only}
- Everitt HA, Moss‐Morris RE, Sibelli A, Tapp L, Coleman NS, Yardley L, et al. Management of irritable bowel syndrome in primary care: feasibility randomised controlled trial of mebeverine, methylcellulose, placebo and a patient self‐management cognitive behavioural therapy website. (MIBS trial). BMC Gastroenterol 2010;10:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khalif 2009 {published data only}
- Khalif IL, Quigley EM, Makarchuk PA, Golovenko OV, Podmarenkova LF, Dzhanayev YA. Interactions between symptoms and motor and visceral sensory responses of irritable bowel syndrome patients to spasmolytics (antispasmodics). J Gastrointestin Liver Dis 2009;18(1):17‐22. [PubMed] [Google Scholar]
Ladabaum 2010 {published data only}
- Ladabaum U, Sharabidze A, Levin TR, Zhao WK, Chung E, Bacchetti P, et al. Citalopram provides little or no benefit in nondepressed patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 2010;8(1):42‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Merat 2010 {published data only}
- Merat S, Khalili S, Mostajabi P, Ghorbani A, Ansari R, Malekzadeh R. The effect of enteric‐coated, delayed‐release peppermint oil on irritable bowel syndrome. Dig Dis Sci 2010;55(5):1385‐90. [DOI] [PubMed] [Google Scholar]
Pace 2010 {published data only}
- Pace F, Maurano A, Ciacci C, Savarino V, Attili A, Iaquinto G, et al. Octatropine methyl bromide and diazepam combination (Valpinax) in patients with irritable bowel syndrome: a multicentre, randomized, placebo‐controlled trial. Eur Rev Med Pharmacol Sci 2010;14(3):155‐62. [PubMed] [Google Scholar]
Reme 2010 {published data only}
- Reme SE, Kennedy T, Jones R, Darnley S, Chalder T. Predictors of treatment outcome after cognitive behavior therapy and antispasmodic treatment for patients with irritable bowel syndrome in primary care. J Psychosom Res 2010;68(4):385‐8. [DOI] [PubMed] [Google Scholar]
Saps 2009 {published data only}
- Saps M, Youssef N, Miranda A, Nurko S, Hyman P, Cocjin J, et al. Multicenter, randomized, placebo‐controlled trial of amitriptyline in children with functional gastrointestinal disorders. Gastroenterology 2009;137(4):1261‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wittmann 2010 {published data only}
- Wittmann T, Paradowski L, Ducrotté P, Bueno L, Andro Delestrain MC. Clinical trial: the efficacy of alverine citrate/simeticone combination on abdominal pain/discomfort in irritable bowel syndrome‐‐a randomized, double‐blind, placebo‐controlled study. Aliment Pharmacol Ther 2010;31(6):615‐24. [DOI] [PubMed] [Google Scholar]
Additional references
Akehurst 2001
- Akehurst R, Kaltenthaler E. Treatment of irritable bowel syndrome: A review of randomised controlled trials. Gut 2001;48(2):272‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bijkerk 2004
- Bijkerk CJ, Muris JW, Knottnerus JA, Hoes AW, Wit NJ. Systematic review: the role of different types of fibre in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther 2004;19(3):245‐51. [DOI] [PubMed] [Google Scholar]
Brandt 2002
- Brandt LJ, Bjorkman D, Fennerty MB, Locke GR, Olden K, Peterson W, et al. Systematic review on the management of irritable bowel syndrome in North America. Am J Gastroenterol 2002;97(11 Suppl):S7‐26. [DOI] [PubMed] [Google Scholar]
Burkitt 1972
- Burkitt DP, Walker AR, Painter NS. Effect of dietary fibre on stools and the transit‐times, and its role in the causation of disease. Lancet 1972;2(7792):1408‐12. [DOI] [PubMed] [Google Scholar]
Creed 2001
- Creed F, Ratcliffe J, Fernandez L, Tomenson B, Palmer S, Rigby C, et al. Health‐related quality of life and health care costs in severe, refractory irritable bowel syndrome. Ann Intern Med 2001;134(9 Pt 2):860‐8. [DOI] [PubMed] [Google Scholar]
Drossman 2006
- Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology 2006;130(5):1377‐90. [DOI] [PubMed] [Google Scholar]
Ford 2008
- Ford AC, Talley NJ, Spiegel BM, Foxx‐Orenstein AE, Schiller L, Quigley EM, et al. Effect of fibre, antispasmodics, and peppermint oil in the treatment of irritable bowel syndrome: systematic review and meta‐analysis. BMJ (Clinical research ed) 2008;337:a2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ford 2009
- Ford A C, Talley N J, Schoenfeld P S, Quigley E M, Moayyedi P. Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: systematic review and meta‐analysis. Gut 2009;58(3):367‐78. [DOI] [PubMed] [Google Scholar]
Ford 2010
- Ford AC, Guyatt GH, Talley NJ, Moayyedi P. Errors in the conduct of systematic reviews of pharmacological interventions for irritable bowel syndrome. The American Journal of Gastroenterology 2010;105(2):280‐8. [DOI] [PubMed] [Google Scholar]
Hillila 2004
- Hillila MT, Farkkila MA. Prevalence of irritable bowel syndrome according to different diagnostic criteria in a non‐selected adult population. Aliment Pharmacol Ther 2004;20(3):339‐45. [DOI] [PubMed] [Google Scholar]
Hungin 2003
- Hungin AP, Whorwell PJ, Tack J, Mearin F. The prevalence, patterns and impact of irritable bowel syndrome: an international survey of 40,000 subjects. Aliment Pharmacol Ther 2003;17(5):643‐50. [DOI] [PubMed] [Google Scholar]
Irvine 2006
- Irvine EJ, Whitehead WE, Chey WD, Matsueda K, Shaw M, et al. Design of treatment trials for functional gastrointestinal disorders. Gastroenterology 2006;130(5):1538‐51. [DOI] [PubMed] [Google Scholar]
Jackson 2000
- Jackson JL, O'Malley PG, Tomkins G, Balden E, Santoro J, Kroenke K. Treatment of functional gastrointestinal disorders with antidepressant medications: a meta‐analysis. Am J Med 2000;108(1):65‐72. [DOI] [PubMed] [Google Scholar]
Jailwala 2000
- Jailwala J, Imperiale TF, Kroenke K. Pharmacologic treatment of the irritable bowel syndrome: a systematic review of randomized, controlled trials. Ann Intern Med 2000;133(2):136‐47. [DOI] [PubMed] [Google Scholar]
Jones 2000a
- Jones J, Boorman J, Cann P, Forbes A, Gomborone J, Heaton K, et al. British Society of Gastroenterology guidelines for the management of the irritable bowel syndrome. Gut 2000;47 Suppl 2:ii1‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2000b
- Jones R. IBS: prime problem in primary care. Gut 2000;46(1):7‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lesbros‐Pantoflickova 2004
- Lesbros‐Pantoflickova D, Michetti P, Fried M, Beglinger C, Blum A L. Meta‐analysis: The treatment of irritable bowel syndrome. Aliment Pharmacol Ther 2004;20(11‐12):1253‐69. [DOI] [PubMed] [Google Scholar]
Longstreth 2003
- Longstreth GF, Wilson A, Knight K, Wong J, Chiou CF, Barghout V, et al. Irritable bowel syndrome, health care use, and costs: a U.S. managed care perspective. Am J Gastroenterol 2003;98(3):600‐7. [DOI] [PubMed] [Google Scholar]
Manning 1978
- Manning AP, Thompson WG, Heaton KW, Morris AF. Towards positive diagnosis of the irritable bowel. Br Med J 1978;2(6138):653‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pare 2006
- Pare P, Gray J, Lam S, Balshaw R, Khorasheh S, Barbeau M, et al. Health‐related quality of life, work productivity, and health care resource utilization of subjects with irritable bowel syndrome: baseline results from LOGIC (Longitudinal Outcomes Study of Gastrointestinal Symptoms in Canada), a naturalistic study. Clin Ther 2006;28(10):1726‐35; discussion 1710‐1. [DOI] [PubMed] [Google Scholar]
Poynard 1994
- Poynard T, Naveau S, Mory B, Chaput JC. Meta‐analysis of smooth muscle relaxants in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther 1994;8(5):499‐510. [DOI] [PubMed] [Google Scholar]
Poynard 2001
- Poynard T, Regimbeau C, Benhamou Y. Meta‐analysis of smooth muscle relaxants in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther 2001;15(3):355‐61. [DOI] [PubMed] [Google Scholar]
Rahimi 2009
- Rahimi R, Nikfar S, Rezaie A, Abdollahi M. Efficacy of tricyclic antidepressants in irritable bowel syndrome: a meta‐analysis. World J Gastroenterol 2009;15(13):1548‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Riedl 2008
- Riedl A, Schmidtmann M, Stengel A, Goebel M, Wisser AS, Klapp BF, et al. Somatic comorbidities of irritable bowel syndrome: a systematic analysis. J Psychosom Res 2008;64(6):573‐82. [DOI] [PubMed] [Google Scholar]
Schoenfeld 2006
- Schoenfeld P, Talley NJ. Measuring successful treatment of irritable bowel syndrome: is "satisfactory relief " enough?. Am J Gastroenterol 2006;101(5):1066‐8. [DOI] [PubMed] [Google Scholar]
Tack 2006b
- Tack J, Fried M, Houghton LA, Spicak J, Fisher G. Systematic review: the efficacy of treatments for irritable bowel syndrome‐‐a European perspective. Aliment Pharmacol Ther 2006;24(2):183‐205. [DOI] [PubMed] [Google Scholar]
Talley 2006
- Talley NJ. A unifying hypothesis for the functional gastrointestinal disorders: really multiple diseases or one irritable gut?. Rev Gastroenterol Disord 2006;6(2):72‐8. [PubMed] [Google Scholar]
Verdu 2008
- Verdu B, Decosterd I, Buclin T, Stiefel F, Berney A. Antidepressants for the treatment of chronic pain. Drugs 2008;68(18):2611‐32. [DOI] [PubMed] [Google Scholar]


