Abstract
The use of non-invasive respiratory strategies (NIRS) is crucial to improve oxygenation in COVID-19 patients with hypoxemia refractory to conventional oxygen therapy. However, the absence of respiratory symptoms may delay the start of NIRS. The aim of this study was to determine whether a simple bedside test such as single-breath counting test (SBCT) can predict the need for NIRS in the 24 h following the access to Emergency Department (ED). We performed a prospective observational study on 120 patients with COVID-19 pneumonia. ROC curves were used to analyze factors which might predict NIRS requirement. We found that 36% of patients had normal respiratory rate and did not experience dyspnea at rest. 65% of study population required NIRS in the 24 h following the access to ED. NIRS-requiring group presented lower PaO2/FiO2 (235.09 vs. 299.02), SpO2/FiO2 ratio (357.83 vs. 431.07), PaCO2 (35.12 vs. 40.08), and SBCT (24.46 vs. 30.36) and showed higher incidence of dyspnea at rest (57.7% vs. 28.6%). Furthermore, SBCT predicted NIRS requirement even in the subgroup of patients without respiratory symptoms (AUC = 0.882, cut-off = 30). SBCT might be a valuable tool for bedside assessment of respiratory function in patients with COVID-19 pneumonia and might be considered as an early clinical sign of impending respiratory deterioration.
Keywords: COVID-19, pneumonia, single breath count, high-flow nasal cannula, continuous positive airway pressure (CPAP)
1. Introduction
The coronavirus disease (COVID-19) pandemia has stressed worldwide healthcare systems requiring a tremendous increase of the capacity of Emergency Departments (ED) to handle the sharp rise of patients in critical situation [1,2]. Pneumonia is the most frequent complication of infection, evolving in some patients to acute respiratory distress syndrome (ARDS) [2,3,4,5]. Hypoxemia was shown to be an independent prognostic factor for the severe form of COVID-19 and it was associated with in-hospital mortality [6,7]. Hypoxemia unresponsive to oxygen therapy suggests that gas exchange impairment is due to intrapulmonary shunt. In these patients the use of non-invasive respiratory strategies (NIRS), which include high-flow nasal cannula (HFNC) and non-invasive ventilation (NIV), allows to apply a positive end-expiratory pressure (PEEP) to the airways, which may reopen collapsed alveoli improving oxygenation and reducing intubation rate [8,9,10,11].
Early identification of critical patients requiring NIRS is essential to plan hospital resources and to prioritize monitoring efforts. Many patients with COVID-19-associated pneumonia showed severe dyspnea, increased respiratory rate with shallow breaths that precede oxygen saturation drops [12,13,14]. On the other hand, some patients presented “silent” hypoxemia without experiencing overt respiratory symptoms and this clinical presentation may cause a delay in the start of NIRS [15,16].
Previous works demonstrated that single-breath counting test (i.e., how far an individual can count in a normal speaking voice after a maximal effort inhalation) has good correlation with the standard measures of pulmonary function and it has been proposed as an indicator of respiratory compromised patients both in acute and in chronic setting [17,18,19,20].
The purpose of the study was to determine whether single-breath counting test (SBCT) may be useful in patients with COVID-19 pneumonia to predict the need for NIRS in the 24 h following the access to the Emergency Department (ED). Furthermore, we would like to define the cut-off limit of SBCT associated to progression of acute respiratory failure (ARF).
2. Materials and Methods
After the approval of our IRB with code n.: ASO.Rian.Gen.21/01 on 23 February 2021, we performed a prospective observational cohort study in two Italian hospitals. Patients with COVID-19 treated in the ED, who met criteria for pneumonia between 21 April and 22 July 2021 were enrolled. COVID-19 pneumonia diagnosis was defined by positive PCR in nasopharyngeal swab and by the presence of radiological or ultrasound patterns suggestive of the disease. We included in the study only patients treated in the ED for less than two hours. All patients provided written authorization for the use of their medical records for research. The institutional protocol of the coordinating center (protocol No. 0014676/21) was approved by the Ethics Committee on Clinical Investigations.
Exclusion criteria of the study included (1) patients already initiated on NIV or HFNC, (2) patients with severe hypoxemia (i.e, a PaO2/FiO2 < 150), (3) patients with severe dyspnea unable to speak in complete sentences, (4) uncooperative patients and (5) patients with a ‘do not resuscitate or intubate’ order. We evaluated patient’s ability to speak in complete sentences asking all patients to say name, surname, place and date of birth.
2.1. Protocol and Data Collection
Demographical characteristics (age, gender, body mass index), comorbidities and tobacco use were reported, SBTC, body temperature, pulse oximetry (SpO2), arterial blood gas (ABG) sampling, fraction of inspired oxygen (FiO2), respiratory rate (RR), heart rate (HR), presence of dyspnea at rest were collected upon inclusion into the study. In addition, blood tests were recorded.
Tachypnea in adults is defined as a respiratory rate greater than 20 breaths per minute. Dyspnea is defined as a subjective symptom described as an uncomfortable abnormal awareness of breathing, including a number of different sensations experienced by patients such as shortness of breath or inability to take a deep breath.
Investigators from each participating center were responsible for data collection; the protocol was explained and demonstrated during one specific educational meeting. The SBCT was performed by asking subjects to take a deep breath and count as far as possible in their normal voice at an approximate rate of two counts per second. Patients were instructed to stay in bed in the sitting position. We recorded two attempts, following a one-minute of rest between measurements.
Patients were followed up within the first 24 h of study inclusion and the level of respiratory support, such as low flow O2 therapy, HFNC, NIV, (i.e., continuous positive airway pressure—CPAP, or bi-level positive-pressure ventilation) or invasive mechanical ventilation (IMV) was reported.
We started conventional oxygen therapy when SpO2 was <92% on room air, using Venturi mask or nasal cannula and targeting SpO2 between 92–96% [8].
Patients with COVID-19 eligible for NIRS included subjects on conventional oxygen therapy having a PaO2/FiO2 ratio (P/F) < 250 or signs of increased work of breathing (i.e., RR > 30, accessory muscle use, abdominal paradox). In order to avoid that the decision to start NIRS was influenced by the SBCT value, this choice was left to the physician in charge who did not know the result of this test. The choice between CPAP, Bi-level Positive-Pressure ventilation or HFNC, as far as the decision to intubate and start invasive mechanical ventilation were based on clinical judgement of the physician in charge, who did not know the SBCT value.
2.2. Statistical Analysis
Quantitative variables were described with median and interquartile range (IQR), while qualitative variables were described with number and percentages. The statistical analysis was carried out using T student test for variables with normal distribution and as non-parametric test was used Mann-Whitney U test. The categorical data were tested with Chi2 test. The normal distribution was tested with Shapiro-Wilk test. The adjusted analysis was performed using penalized logistic regression models. The ROC curve was used to evaluate area under the curve (AUC) and cut-off. All tests were two sided and assumed a 5% significance level. Data analyses were performed using Addinsoft 2021 (XLSTAT Statistical and Data Analysis Solutions, New York, NY, USA).
3. Results
A total of 135 consecutive adults with COVID-19-associated pneumonia were treated during the study. We excluded 15 patients: ten for inability to perform SBCT, three for incompleteness of data and two for inability to obtain informed consent. Thus, the final number of patients enrolled in the study was 120 (mean age 66.9 ± 12.5 years old).
Demographic and clinical characteristics are shown in Table 1.
Table 1.
Baseline characteristics of patients with COVID-19 pneumonia collected at inclusion into the study.
| NIRS Not Required (42) | NIRS Required (78) | p-Value | ||
|---|---|---|---|---|
| Age (years) | 68.14 ± 13.62 | 66.15 ± 11.66 | 0.431 | |
| Gender | F | 21 (50%) | 27 (34.6%) | 0.121 |
| M | 21 (50%) | 51 (65.4%) | ||
| BMI (kg/m2) | 25.67 ± 2.48 | 26.82 ± 5.79 | 0.449 | |
| Tobacco use | 3 (7.1%) | 3 (3.8%) | 0.700 | |
| Comorbidities: | ||||
| Hypertension | 30 (71.4%) | 36 (46.1%) | 0.007 | |
| Diabetes mellitus | 3 (7.1%) | 9 (11.5%) | 0.433 | |
| Chronic kidney disease | 6 (14.3%) | 2 (2.5%) | 0.017 | |
| Congestive heart failure | 15 (35.7%) | 12 (15.4%) | 0.013 | |
| Coronary heart disease | 12 (28.6%) | 15 (19.2%) | 0.248 | |
| Chronic respiratory disease | 6 (14.3%) | 12 (15.4%) | 0.872 | |
| Clinical characteristics: | ||||
| Onset of COVID-19 symptoms (days) | 5.3 ± 3.3 | 7.3 ± 3.8 | 0.01 | |
| Body Temperature (°C) | 36.8 ±0.8 | 37.2± 0.9 | 0.038 | |
| Heart rate (bpm) | 86.6 ± 7.4 | 85.8 ± 8.7 | 0.654 | |
| Respiratory rate (breaths/min) | 21.5 ± 8.1 | 23.1 ± 7.7 | 0.314 | |
| Dyspnea at rest (Nº of patients) | 12 (28.6%) | 45 (57.7%) | 0.02 | |
| SCBT | 30.4 ± 6.9 | 24.5 ± 6.4 | <0.0001 | |
| FiO2 | 0.2 ±0.02 | 0.3 ± 0.2 | 0.005 | |
| SpO2/FiO2 | 431.1 ± 39.1 | 357.8 ± 104.9 | 0.001 | |
| PaO2/FiO2 | 299.01 ± 95.1 | 235.1 ± 53.4 | 0.0001 | |
| PaCO2 (mmHg) | 40.1 ± 6.7 | 35.1 ± 4.1 | <0.0001 | |
| pH | 7.4 ± 0.02 | 7.5 ± 0.06 | 0.0001 | |
| D-dimer (mcg/mL) | 1.02 ± 0.8 | 0.9 ± 0.5 | 0.503 | |
| Ferritin (ng/mL) | 489.9 ± 440.5 | 993.9 ± 910.6 | 0.003 | |
| LDH (U/L) | 593.0 ± 107.2 | 698.9 ± 186.1 | 0.009 | |
Abbreviations: NIRS, non-invasive respiratory strategies; BMI, body mass index; FiO2, fraction of inspired oxygen; SpO2, pulse oximetry; SBCT, single-breath counting test, LDH, Lactate dehydrogenase.
At inclusion in the study patients presented a mean P/F of 258.7 (±77.9). None of them had a P/F lower than 150. 68% were treated with conventional oxygen therapy. The rest of the patients breathed on room air. Many patients had dyspnea at rest (57 patients) or tachypnea (42 patients). 36% of all subjects (43 patients) had a normal respiratory rate (i.e., lower or equal to 20 breaths/min) and did not experience dyspnea at rest.
Patients were divided in two groups: the first one included 42 patients (35%) who didn’t required NIRS during next 24 h and the second group included 78 patients (65%) which required NIRS. Demographic characteristics were similar in the two groups (Table 1). The group which required NIRS presented significantly lower P/F, SpO2/FiO2 ratio, PaCO2 and SBCT scores and showed higher body temperature, higher incidence of dyspnea at rest and higher FiO2 (Table 1). In addition, NIRS-requiring group had higher values of LDH and ferritin, and had COVID-19 symptoms for more days.
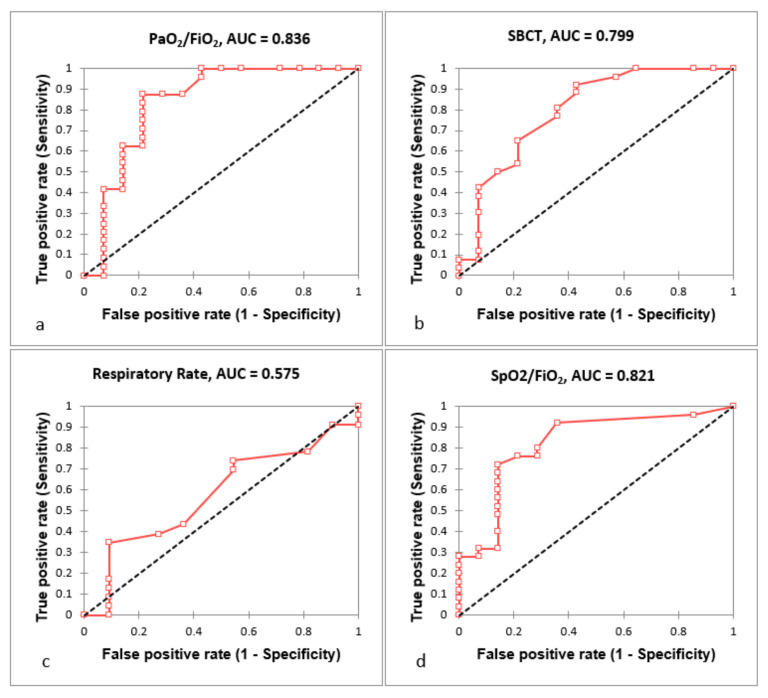
The ROC curves were performed to evaluate AUC and calculate cut-off value for SBCT, respiratory rate, PaO2/FiO2 and SpO2/FiO2 in order to predict NIRS requirement in the next 24 h (Figure 1). The respective AUC, cut-off, sensitivity and specificity values are presented in Table 2 and clearly indicated that all parameters, except RR, predicted the need of NIRS.
Figure 1.
ROC curve analysis of PaO2/FiO2 (a), SBCT (b), Respiratory Rate (c) and SpO2/FiO2 (d) as predictors of NIRS requirement. Tests performed in all included patients. Abbreviations: NIRS, non-invasive respiratory strategies; SBCT, single-breath counting test.
Table 2.
ROC curves results of factors which might predict NIRS requirement. Tests performed in all included patients.
| AUC | Standard Error | Lower Bound (95%) | Upper Bound (95%) | Cut-Off | Sensitivity | Specificity | p-Value | |
|---|---|---|---|---|---|---|---|---|
| SBCT | 0.799 | 0.046 | 0.710 | 0.889 | 32 | 0.923 | 0.571 | <0.0001 |
| RR | 0.575 | 0.059 | 0.459 | 0.691 | 28 | 0.348 | 0.909 | 0.206 |
| PaO2/FiO2 | 0.836 | 0.057 | 0.725 | 0.948 | 280 | 0.875 | 0.786 | <0.0001 |
| SpO2/FiO2 | 0.821 | 0.052 | 0.720 | 0.923 | 438 | 0.720 | 0.857 | <0.0001 |
Abbreviations: NIRS, non-invasive respiratory strategies; SBCT, single-breath counting test; RR, respiratory rate.
In addition, we also evaluated the SBCT performance in predicting the use of NIRS analyzing subgroup of patients without respiratory signs or symptoms (Table 3).
Table 3.
ROC curve analysis of SBCT performance in predicting NIRS in different subgroups of patients.
| SBCT in Patients Not Requiring NIRS |
SBCT in Patients Requiring NIRS |
p-Value | |
|---|---|---|---|
| Patients without chronic respiratory disease | 30.7 ± 7.5 | 25.3 ± 5.1 | <0.0001 |
| Patients not needing supplemental oxygen therapy | 30.1 ± 7.5 | 26.7 ± 3.0 | 0.007 |
| Patients needing supplemental oxygen therapy | 32.0 ± 0.0 | 20.8 ± 8.6 | 0.003 |
| Patients without dyspnea at rest | 31.5 ± 4.2 | 24.5 ± 3.2 | <0.0001 |
| Patients with PaO2/FiO2 > 280 | 29.7 ± 7.9 | 20.0 ± 6.6 | 0.0001 |
| Patients with SpO2/FiO2 > 438 | 30.1 ± 7.9 | 27.3 ± 3.8 | 0.135 |
| Patients with normal respiratory rate | 30.2 ± 8.1 | 25.1 ± 3.2 | 0.0001 |
| Patients with normal respiratory rate and absence of dyspnea at rest | 31.9 ± 4.5 | 25.1 ± 3.3 | <0.0001 |
Abbreviations: NIRS, non-invasive respiratory strategies; SBCT, single-breath counting test.
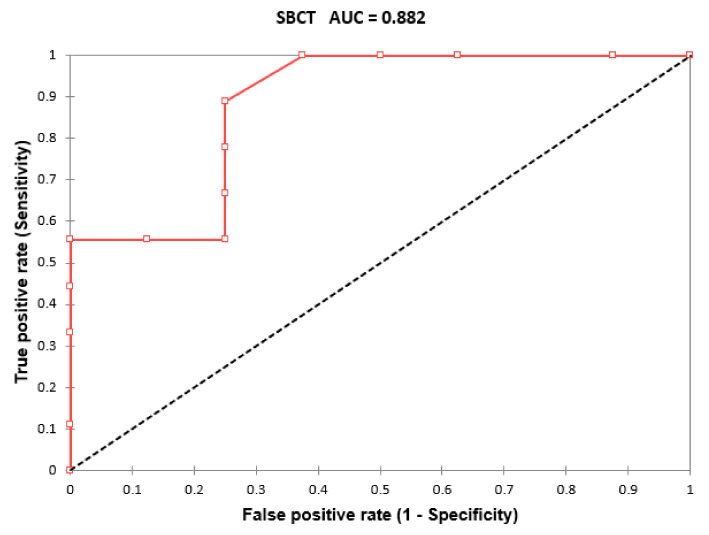
In particular, we analyzed separately patients without chronic respiratory disease, patients not receiving supplemental oxygen, subjects with normal respiratory rate (i.e., respiratory rate ≤ 20/min), without dyspnea at rest, with high P/F value (i.e., P/F > 280) or high SpO2/FiO2 ratio (i.e., SpO2/FiO2 ratio > 438). Both cut-off values were derived from ROC curves presented above. All subgroups except patients with SpO2/FiO2 ratio > 438 showed statistically significant difference in terms of SBTC between groups which required NIRS and group which didn’t required this therapy. Finally, we analyzed the group of patients without dyspnea at rest and with normal respiratory rate. In these population the SBCT was lower in patients requiring NIRS (25.11 ± 3.274 vs. 31.87 ± 4.52; p > 0.0001). The ROC curve analysis of this population highlighted the AUC equal to 0.882 [0.791–0.973], p-value < 0.0001 and cut-off values resulted to be 30 with sensitivity 0.889 and specificity 0.750 (Figure 2).
Figure 2.
ROC curve analysis of SBCT performance in predicting NIRS requirement in the subgroup of patients with normal respiratory rate and absence of dyspnea at rest. Abbreviations: NIRS, non-invasive respiratory strategies; SBCT, single-breath counting test.
Among patients who used NIRS most subjects underwent CPAP with helmet (73 patients) and only five patients used HFNC. All patients included in the study were hospitalized or transferred to other hospitals within 24 h of accessing to ED.
4. Discussion
This study, focused on non-severely dyspneic and non-severely hypoxemic patients provides three major findings: (1) SBCT is valuable, replicable, easy to perform for bedside assessment of respiratory function in patients with COVID-19 pneumonia; (2) SBCT may predict the requirement of NIRS in the 24 h following the access to the ED, both in patients with obvious respiratory distress and in those with “silent” hypoxemia; (3) the cut-off limit of SBCT in patients without dyspnea at rest and with normal respiratory rate is 30, showing a good sensitivity and specificity.
COVID-19 pneumonia is characterized by substantial clinical heterogeneity. It spans from mild forms to severe forms with ARF and high intubation rates [21,22]. NIRS can decrease the need for intubation in patients with ARF [9,10,11,23]. On the other hand, in these patients a delay in the start of respiratory support might be particularly harmful. Consequently, early estimation of severity of COVID-19 pneumonia may help clinicians to decide patients’ allocation and interventions, in particular when an overwhelming load of admissions exceed the capacity of the ED. The presence of low SpO2 associated with dyspnea at rest is an excellent predictor of mechanical ventilation requirement [13,14]. However, many reports have described a subset of COVID-19 patients with hypoxemia showing no obvious respiratory difficulties [24,25,26]. In one of the first largest studies on the clinical characteristics of COVID-19, shortness of breath has been reported in only 18.7% of 1099 hospitalized patients, despite hypoxemia requiring supplemental oxygen was described in 41% of patients [27]. This phenomenon is referred as “silent” or “happy” hypoxemia and may result in a missed early recognition of evolving respiratory failure leading to delayed institution of respiratory support. In particular for these patients a bedside test predicting the need of NIRS should be highly advisable. For this purpose, the use of spirometry could be considered. Indeed, in the ED measurement of peak expiratory flow (PEF) is used to assess the severity of asthma [28] and forced vital capacity (FVC) is utilized to monitor patients with myasthenic crisis [29]. In addition, spirometry is used to evaluate patients who survive ARDS [30,31]. Thus, these measurements could also be useful for the assessment of respiratory function in COVID-19 patients. However, spirometry is not readily available in the ED. Moreover, it has the potential for aerosol generation during forced exhalation or coughing provoked by the maneuver [32]. As a consequence, we chose SBCT as bedside test to assess respiratory function in COVID-19 patients. Indeed, it correlates well with standard measures of pulmonary function both in acute and chronic setting [18,19]. It is already used in patients with asthma attack [18,19,20] or in patients with myasthenic crisis treated in the ED [29]. Elsheikh and coauthors showed that SBCT ≥ 25 suggests normal respiratory muscle function in patients with myasthenia gravis (MG) [20]. On the other hand, in the same patients a SBCT ≤ 20 has been proposed as an indication of respiratory compromise [33].
To our knowledge, this is the first study evaluating the use of SBCT in subjects with COVID-19 pneumonia. We found a good correlation between SBCT and the start of respiratory support in the next 24 h. In addition, SBCT appeared to perform consistently in discriminating between patients who required and who did not require NIRS. Good correlation was found also in the subgroup of subjects having a normal respiratory rate not experiencing dyspnea at rest. These results confirm that this measurement may be a useful tool for the assessment of patients with COVID-19 pneumonia who develop hypoxemia without showing first an obvious respiratory distress. In particular, in this subgroup a SBCT lower than 30 might identify patients requiring NIRS with good sensitivity and specificity. Even if we considered patients with P/F > 280 SBCT continued to be a good predictor of respiratory deterioration in the following 24 h.
All patients included in our study were at risk to devolve to severe hypoxemia and 65% of them were treated with NIRS in the 24 h following study inclusion. Of note, 36% of the enrolled patients had a normal respiratory rate and did not experiencing dyspnea at rest.
Our data confirms preceding studies which showed that in patients with COVID-19 pneumonia low P/F, low SpO2/FiO2 ratio, low PCO2 and the presence of dyspnea at rest predict the need of NIRS [12,13,14,34,35]. Of note, there is a slight discrepancy between PaO2/FiO2 and SpO2/FiO2 cut off, which predicts NIRS requirement (respectively 280 and 438). As far as the peculiar sigmoidal shape of the oxyhemoglobin dissociation curve is concerned, we can observe that in the higher range of arterial partial pressures of oxygen (PaO2) the upper part of the curve is flat. This feature prevents a significant decline in oxygen saturation when PaO2 starts to go down. Thus, SpO2 may underestimate an initial impairment of gas exchange [26].
This study has several limitations. The major limitation is that patients were only followed up for 24 h after study inclusion. Indeed, after the stabilization of clinical parameters, many patients were transferred to other hospitals due to the overwhelming inflow of COVID-19 patients. The second limitation is that patients during the 24 h of study period performed only one SBCT measurement. This limitation of the study protocol is aimed at reducing the exposure of investigators to the virus. Finally, we included in the study a relatively limited number of patients.
5. Conclusions
SBCT appears to be a promising tool in the early identification of patients with COVID-19 pneumonia who are at high risk of ARF requiring respiratory support. The simplicity of the SBCT makes this test appealing for rapid assessment of respiratory status also in patients with COVID-19 pneumonia treated in ED. More data from larger, prospective, multicenter studies are needed to confirm the efficacy of this test, possibly following SBCT trend for a longer time. In addition, SBCT could be evaluated in the assessment of COVID-19 outpatients to predict respiratory disfunction evolving to ARF, needing hospital admission.
Author Contributions
Conceptualization, Y.L. and C.Z.; methodology, T.R.; software A.S.; validation, T.P., M.L. and A.P.; formal analysis Y.L.; M.B.; investigation, A.M.; resources, I.P.; data curation, R.B.; writing—original draft preparation, J.R.; writing—review and editing, F.R., C.Z., Y.L., J.R.; visualization, F.F.; supervision, J.R., F.R.; funding acquisition, C.Z., Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of AON SS Antonio e Biagio e Cesare Arrigo, Alessandria, Italy and Policlinico Gemelli of Catholic of The Sacred Heart, Rome, Italy with code n. ASO.Rian.Gen.21/01 on 23 February 2021.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Huang C., Wang Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zanza C., Racca F., Longhitano Y., Piccioni A., Franceschi F., Artico M., Abenavoli L., Maiese A., Passaro G., Volonnino G., et al. Risk Management and Treatment of Coagulation Disorders Related to COVID-19 Infection. Int. J. Env. Res. Public Health. 2021;18:1268. doi: 10.3390/ijerph18031268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Longhitano Y., Racca F., Zanza C., Piccioni A., Audo A., Muncinelli M., Santi R., Kozel D., Geraci C., Taverna M., et al. Venous thromboembolism in critically ill patients affected by ARDS related to COVID-19 in Northern-West Italy. Eur. Rev. Med. Pharmacol. Sci. 2020;24:9154–9160. doi: 10.26355/eurrev_202009_22864. [DOI] [PubMed] [Google Scholar]
- 5.Grasselli G., Tonetti T., Protti A., Langer T., Girardis M., Ballani G., Laffey J., Carrafiello G., Carsana L., Rizzuto C., et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: A multicentre prospective observational study. Lancet Respir. Med. 2020;8:1201–1208. doi: 10.1016/S2213-2600(20)30370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei Y.Y., Wang R.R., Zhang D.-W., Tu Y.-H., Chen C.-S., Ji S., Li C.-X., Li X.-Y., Zhou M.-X., Cao W.-S., et al. Risk factors for severe COVID-19: Evidence from 167 hospitalized patients in Anhui, China. J. Infect. 2020;81:e89–e92. doi: 10.1016/j.jinf.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie J., Covassin N., Fan Z., Singh P., Gao W., Li G., Kara T., Somers V.K. Association Between Hypoxemia and Mortality in Patients With COVID-19. Mayo Clin. Proc. 2020;95:1138–1147. doi: 10.1016/j.mayocp.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alhazzani W., Møller M.H., Arabi Y.M., Loeb M., Gong M.N., Fan E., Oczkowski S., Levy M.M., Derde L., Dzierba A., et al. Surviving Sepsis Campaign: Guidelines on the Management of Critically Ill Adults with Coronavirus Disease 2019 (COVID-19) Crit. Care Med. 2020;48:e440–e469. doi: 10.1097/CCM.0000000000004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frat J.P., Thille A.W., Mercat A., Girault C., Ragot S., Perbet S., Prat G., Boulain T., Morawiec E., Cottereau A., et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N. Engl. J. Med. 2015;372:2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 10.Antonelli M., Conti G., Esquinas A., Montini L., Maggiore S.M., Bello G., Rocco M., Maviglia R., Pennisi M.A., Gonzalez-Diaz G., et al. A multiple-center survey on the use in clinical practice of noninvasive ventilation as a first-line intervention for acute respiratory distress syndrome. Crit. Care Med. 2007;35:18–25. doi: 10.1097/01.CCM.0000251821.44259.F3. [DOI] [PubMed] [Google Scholar]
- 11.Carteaux G., Millán-Guilarte T., De Prost N., Razazi K., Abid S., Thille A.W., Schortgen F., Brochard L., Brun-Buisson C., Dessap A.M. Failure of noninvasive ventilation for de novo acute hypoxemic respiratory failure: Role of tidal volume. Crit. Care Med. 2016;44:282–290. doi: 10.1097/CCM.0000000000001379. [DOI] [PubMed] [Google Scholar]
- 12.Mukhtar A., Rady A., Hasanin A., Lotfy A., El Adawy A., Hussein A., El-Hefnawy I., Hassan M., Mostafa H. Admission SpO2 and ROX index predict outcome in patients with COVID-19. Am. J. Emerg. Med. 2021;50:106–110. doi: 10.1016/j.ajem.2021.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heo J., Han D., Kim H.-J., Kim D., Lee Y.-K., Lim D., Hong S.O., Park M.-J., Ha B., Seog W. Prediction of patients requiring intensive care for COVID-19: Development and validation of an integer-based score using data from Centers for Disease Control and Prevention of South Korea. J. Intensive Care. 2021;9:16. doi: 10.1186/s40560-021-00527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hao B., Sotudian S., Wang T., Xu T., Hu Y., Gaitanidis A., Breen K., Velmahos G.C., Paschalidis I.C. Early prediction of level-of-care requirements in patients with COVID-19. eLife. 2020;9:e60519. doi: 10.7554/eLife.60519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simonson T.S., Baker T.L., Banzett R.B., Bishop T., Dempsey J.A., Feldman J.L., Guyenet P.G., Hodson E.J., Mitchell G.S., Moya E.A., et al. Silent hypoxaemia in COVID-19 patients. J. Physiol. 2021;599:1057–1065. doi: 10.1113/JP280769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., Barnaby D.P., Becker L.B., Chelico J.D., Cohen S.L., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartfield J.M., Ushkow B.S., Rosen J.M., Dylong K. Single breath counting in the assessment of pulmonary function. Ann. Emerg. Med. 1994;24:256–259. doi: 10.1016/S0196-0644(94)70138-5. [DOI] [PubMed] [Google Scholar]
- 18.Ali S.S., O’Connell C., Kass L., Graff G. Single-breath counting: A pilot study of a novel technique for measuring pulmonary function in children. Am. J. Emerg. Med. 2011;29:33–36. doi: 10.1016/j.ajem.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Ushkow B.S., Bartfield J.M., Reicho P.R., Raccio-Robak N. Single-breath counting for the assessment of bronchospastic patients in the ED. Am. J. Emerg. Med. 1998;16:100–101. doi: 10.1016/S0735-6757(98)90081-X. [DOI] [PubMed] [Google Scholar]
- 20.Elsheikh B., Arnold W.D., Gharibshahi S., Reynolds J., Freimer M., Kissel J.T. Correlation of single-breath count test and neck flexor muscle strength with spirometry in myasthenia gravis. Muscle Nerve. 2016;53:134–136. doi: 10.1002/mus.24929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yadaw A.S., Li Y.-C., Bose S., Iyengar R., Bunyavanich S., Pandey G. Clinical features of COVID-19 mortality: Development and validation of a clinical prediction model. Lancet Digit. Health. 2020;2:e516–e525. doi: 10.1016/S2589-7500(20)30217-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu X.P., Zhang X.C., Hu S.L., Xu J.Y., Xie J.F., Liu S.Q., Liu L., Huang Y.Z., Guo F.M., Yang Y., et al. Noninvasive Ventilation in Acute Hypoxemic Nonhypercapnic Respiratory Failure: A Systematic Review and Meta-Analysis. Crit. Care Med. 2017;45:e727–e733. doi: 10.1097/CCM.0000000000002361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Couzin-Frankel J. The mystery of the pandemic’s ‘happy hypoxia’. Science. 2020;368:455–456. doi: 10.1126/science.368.6490.455. [DOI] [PubMed] [Google Scholar]
- 25.Tobin M.J., Laghi F., Jubran A. Why COVID-19 silent hypoxemia is baffling to physicians. Am. J. Respir. Crit. Care Med. 2020;202:356–360. doi: 10.1164/rccm.202006-2157CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allado E., Poussel M., Valentin S., Kimmoun A., Levy B., Nguyen D.T., Rumeau C., Chenuel B. The Fundamentals of Respiratory Physiology to Manage the COVID-19 Pandemic: An Overview. Front. Physiol. 2021;11:615690. doi: 10.3389/fphys.2020.615690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guan W.J., Ni Z.Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X., Liu L., Shan H., Lei C.-L., Hui D.S.C., et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Global Initiative for Asthma (GINA) Global Strategy for Asthma Management and Prevention. [(accessed on 18 May 2021)]. Available online: www.ginasthma.org.
- 29.Roper J., Fleming M.E., Long B., Koyfman A. Myasthenia Gravis and Crisis: Evaluation and Management in the Emergency Department. J. Emerg. Med. 2017;53:843–853. doi: 10.1016/j.jemermed.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 30.Herridge M.S., Cheung A.M., Tansey C.M., Matte-Martyn A., Diaz-Granados N., Al-Saidi F., Cooper A.B., Guest C.B., Mazer C.D., Mehta S., et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N. Engl. J. Med. 2003;348:683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 31.Heyland D.K., Groll D., Caeser M. Survivors of acute respiratory distress syndrome: Relationship between pulmonary dysfunction and long-term health-related quality of life. Crit. Care Med. 2005;33:1549–1556. doi: 10.1097/01.CCM.0000168609.98847.50. [DOI] [PubMed] [Google Scholar]
- 32.Kouri A., Gupta S., Yadollahi A., Ryan C., Gershon A.S., To T., Tarlo S.M., Goldstein R.S., Chapman K.R., Chow C.-W. Addressing Reduced Laboratory-Based Pulmonary Function Testing During a Pandemic. Chest. 2020;158:2502–2510. doi: 10.1016/j.chest.2020.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Juel V.C. Myasthenia gravis: Management of myasthenic crisis and perioperative care. Semin. Neurol. 2004;24:75–81. doi: 10.1055/s-2004-829595. [DOI] [PubMed] [Google Scholar]
- 34.Prediletto I., D’Antoni L., Carbonara P., Daniele F., Dongilli R., Flore R., Pacilli A.M.G., Pisani L., Tomsa C., Vega M.L., et al. Standardizing PaO2 for PaCO2 in P/F ratio predicts in-hospital mortality in acute respiratory failure due to COVID-19: A pilot prospective study. Eur. J. Intern. Med. 2021;92:48–54. doi: 10.1016/j.ejim.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zanza C., Tassi M.F., Romenskaya T., Piccolella F., Abenavoli L., Franceschi F., Piccioni A., Ojetti V., Saviano A., Canonico B., et al. Lock, Stock and Barrel: Role of Renin-Angiotensin-Aldosterone System in Coronavirus Disease 2019. Cells. 2021;10:1752. doi: 10.3390/cells10071752. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.